The Pharmacokinetics, Dosage, Preparation Forms, and Efficacy of Orally Administered Melatonin for Non-Organic Sleep Disorders in Autism Spectrum Disorder During Childhood and Adolescence: A Systematic Review
Abstract
1. Introduction
1.1. Sleep Disorders in Children and Adolescents with ASD
1.2. Objectives
1.3. Preliminary Considerations on the Dosage, Pharmaceutical Formulation and Pharmacokinetics of Orally Administered Melatonin Preparations
- -
- In 13 children with Angelman syndrome aged 2–10 years, low evening oral doses of 0.3 mg were associated with relatively high blood melatonin concentrations and improvements in actigraphic parameters, such as total sleep time (TST) [38].
- -
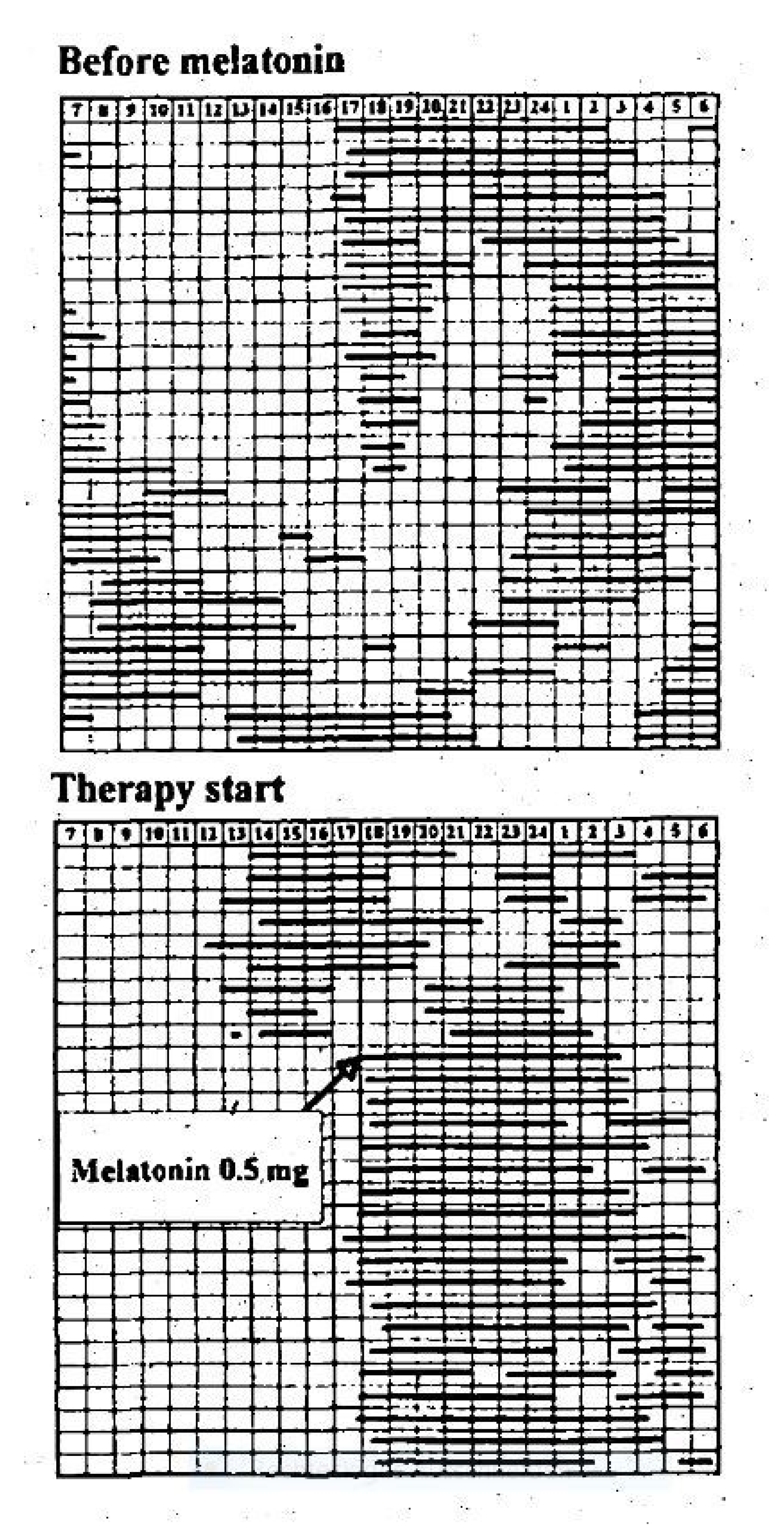
- Niederhofer et al. showed in 2003 that 0.3 mg of melatonin in the non-delayed form led to an improvement in sleep parameters in adolescents aged 14 to 18 years with insomnia and intellectual disabilities in a placebo-controlled RCT, in which blood was taken every 15–60 min within 24 h via a venous vascular access to determine the melatonin concentration and polysomnographic controls were carried out [34].
- -
- -
- In 74 children with insomnia in ASD in an open-label setting, Yuge et al. found that 1 mg of an orally administered rapid-release non-delayed melatonin preparation was associated with a significant reduction in SOL (mean 37%; 95% CI 26 to 48%; p < 0.0001) [41].
- -
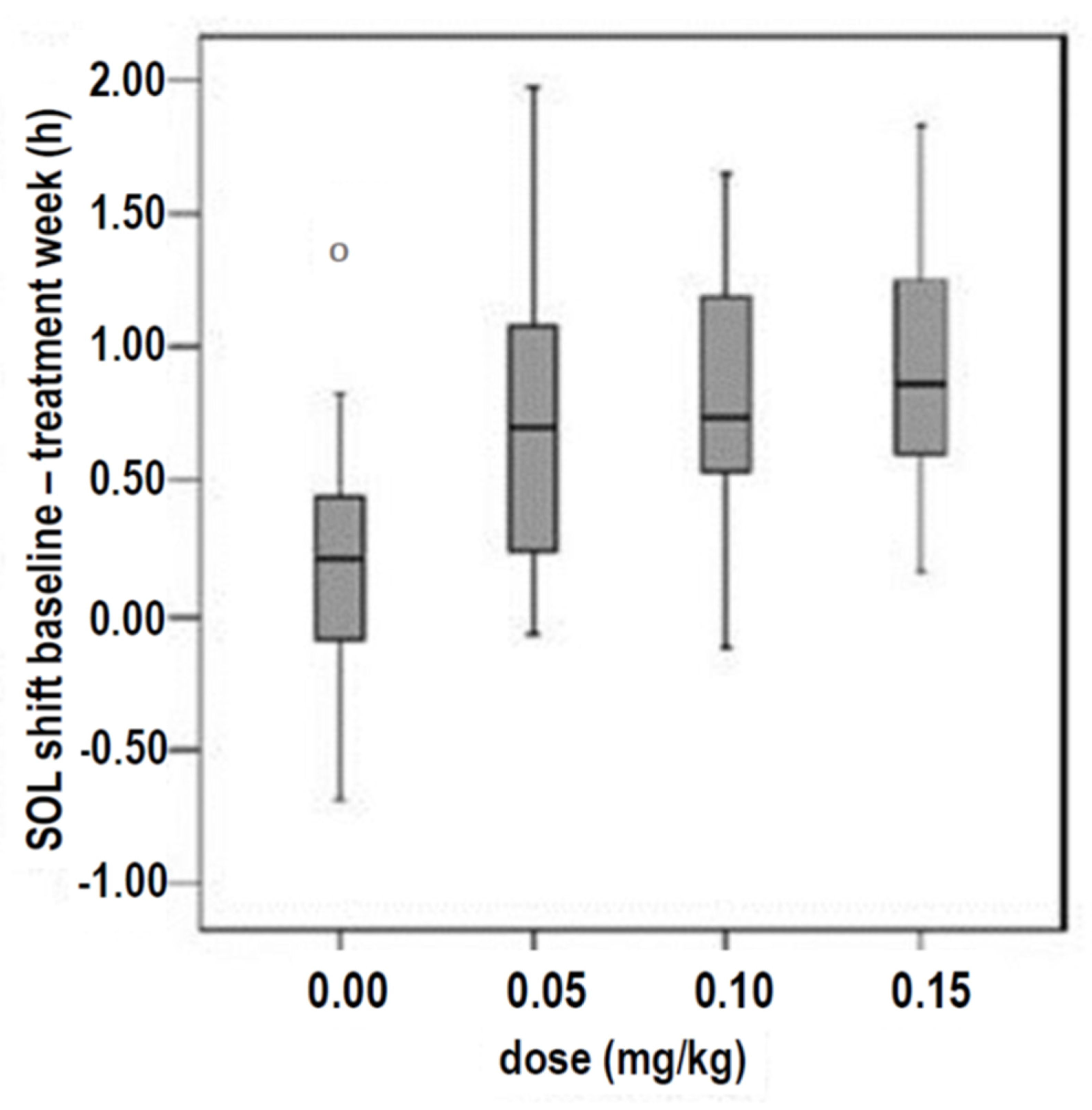
- Van Geijlswijk et al. studied 72 children and adolescents with delayed sleep phase disorder (DSPD), who were treated in parallel in four groups for 1 week with 0.05, 0.1, or 0.15 mg/kg body weight (1.6 mg, 2.91 mg, or 4.39 mg, respectively) of melatonin or with a placebo. The results showed that no dose–response correlations were observed for these three doses, as several sleep parameters (sleep onset, SO; sleep onset latency, SOL; evening melatonin increase = dim light melatonin onset, DLMO) improved independently of the doses investigated (Figure 2) [42].
- -
- In 18 premature infants within their first week of life, the melatonin elimination half-life was between 16.9 and 21.0 h after intravenous melatonin administration [43]. In a further 15 premature infants, an elimination half-life of between 6.2 and 15.5 h was measured after intragastric melatonin administration via a nasogastric tube [44]. In nine children with ASD aged 3–8 years, the elimination half-life after the oral administration of 1 mg of melatonin was 1.3 ± 0.42 h (range: 0.68–2.0 h) [45]. Nine prepubertal adolescents had a slightly shorter elimination half-life than sixteen young adults (0.67 ± 0.12 vs. 0.79 ± 0.10 h, corresponding to a mean of 40.2 and 47.4 min, respectively) [46]. In young adults, the elimination half-life after the oral or intravenous administration of melatonin was less than one hour (53.7 + 7.0 min and 39.4 + 3.6 min, respectively) [15]. Other authors measured comparable values after the oral administration of melatonin in young adults aged 21 to 32 years (47 + 3 min) [47].
- -
- Pharmacokinetic data on melatonin sustained-release preparations have not yet been published (except for the reference by Lalanne et al. in 2021 to unpublished data in an assessment report for the EMA from 2007 (https://www.ema.europa.eu/en/documents/scientific-discussion/circadin-epar-scientific-discussion_en.pdf (accessed on 9 May 2025), p. 16), which were measured by Ruth Kitzes-Cohen et al. in eight healthy male volunteers after the administration of a 2 mg sustained-release preparation for adults, and these would represent the only data to date on the pharmacokinetics of a melatonin sustained-release preparation with an active ingredient content of 2 mg [48]).
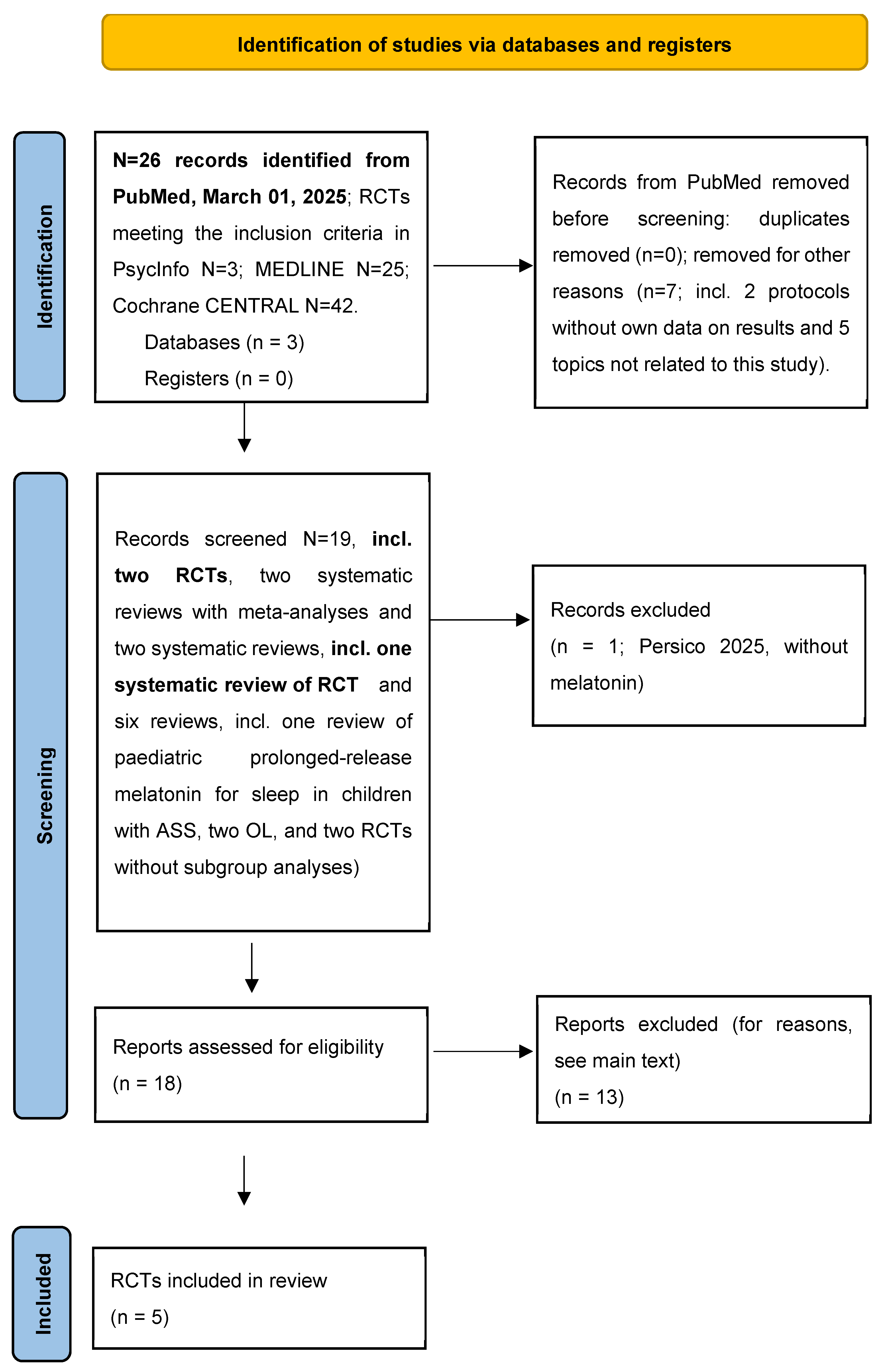
2. Method
3. Results
3.1. The Results of the Five RCTs Representing the Outcome of the Present Systematic Review
| Author, Country | Diagnosis | N, Age | Melatonin (Dose, Preparation) | Result | Conclusion | ||
|---|---|---|---|---|---|---|---|
| Garstang, 2006 [75] UK | ASS with insomnia. | N = 7, 5–15 years. | Five-milligram oral capsules, immediate release, 4 weeks vs. placebo, crossover after washout for 1 week. | TST ↑, SOL ↓, WASO ↓ | This non-delayed preparation was effective in terms of falling asleep, sleeping through the night, and TST. | ||
| Wright, 2011 [76] UK | ASS with insomnia. | N = 17, 9 ± 2.9 (4–16) years. | Two-milligram oral non-delayed, 30–40 min before the expected sleep; increased every 3 days by two milligrams to max. ten milligrams (average seven milligrams). | SOL ↓, TST ↑, WASO ↓ Improvement in behaviour, communication, and dyssomnia. | This non-delayed preparation was effective in terms of falling asleep, sleeping through the night, TST, and improvement in behaviour and communication. | ||
| Cortesi, 2012 [7] Italy | ASS with insomnia. Comparison of melatonin alone and in combination with CBT (cognitive behavioural therapy; four sessions). | N = 134, 6.8 ± 0.9 years. | Three milligrams of a combined preparation (one milligrams rapid release, two milligrams sustained release (6 h)) oral administration at 9:00 p.m. Four randomised groups in parallel; 3 months for each of the following: ● Melatonin (N = 34); ● CBT (N = 33); ● Melat. + CBT (N = 35); ● Placebo (N = 32). | SOL ↓ to 44% with melatonin alone, to 23% after CBT, and to 61% with melatonin + CBT. TST ↑, WASO ↓, sleep anxiety ↓ after KVT to 17%, after melatonin to 14%, and after both to 34%. | This combined 3 mg preparation alone was more effective than CBT alone. The combination of both was more effective than either method alone. | ||
| Hayashi, 2022 [49] Japan | ASS with insomnia. ADHD at 55% (108/196): comparable effects regarding improvement in SOL by melatonin in both doses. | N = 196, aged 11.2 ± 2.5 (6–15) years. | Three parallel randomised, double-blind groups: 1 milligram or 4 milligrams non-delayed melatonin vs. placebo for 2 weeks, administered 45 min before bedtime. | SOL ↓, SE ↑. Sleep hygiene alone in the prephase with lower effects compared to both melatonin doses. | The administration of this non-delayed preparation with 4 mg 45 min before bedtime was associated with comparable effects to 1 mg, but more frequent AEs. In the OL phase with up to 10 mg, a further increase in AEs was documented. | ||
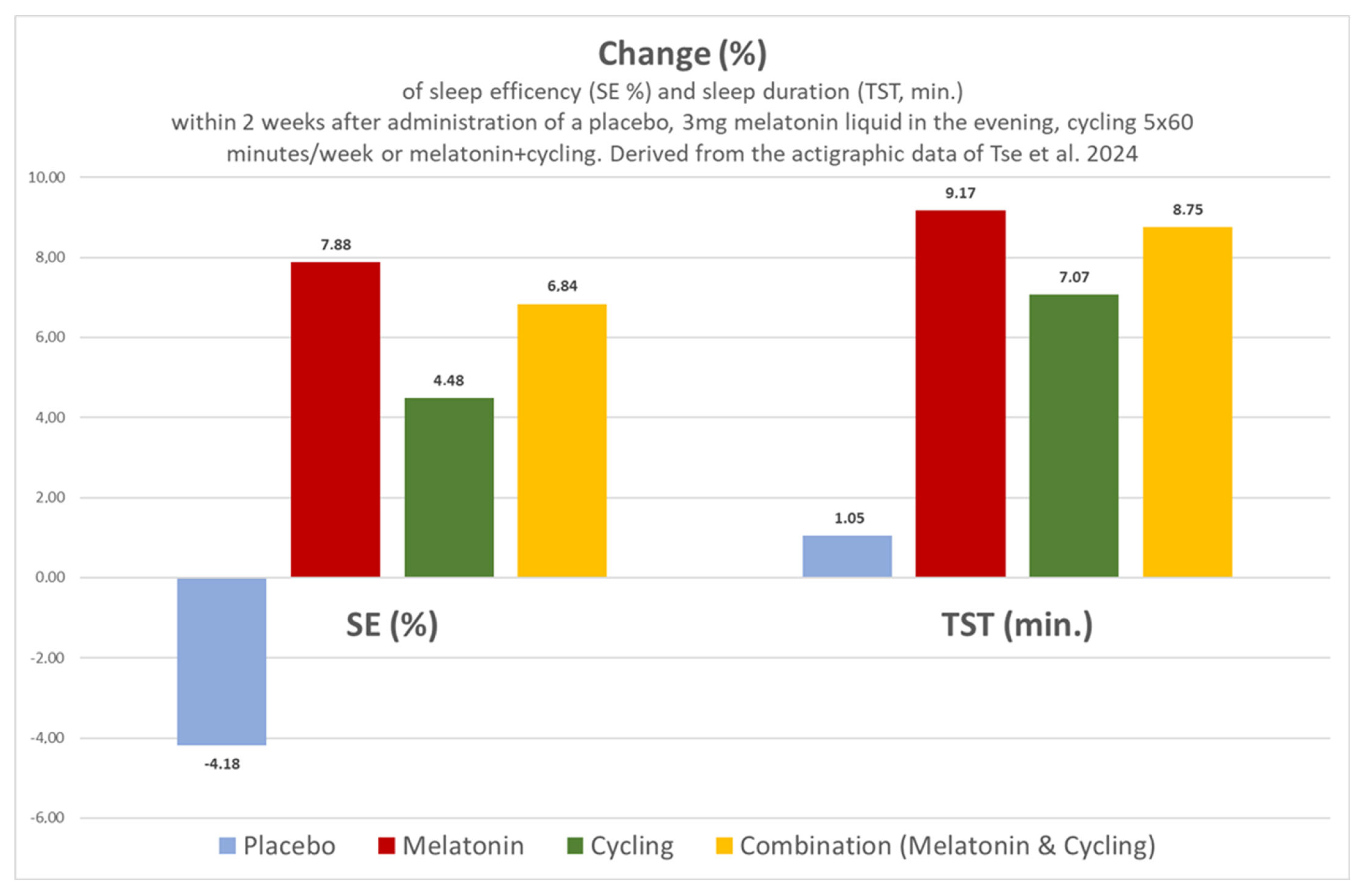
| Tse, 2024 [6] Hong Kong | ASS with insomnia | N = 62, aged 9.6 to 10.4 years | Four randomised arms: ● Three milligrams of liquid Melatonin 30 min before bedtime (N = 14); ● Cycling in the morning only (N = 18); ● Melatonin and cycling (N = 12); ● Placebo alone (N = 18). | SOL ↓, TST ↑, SE ↑, WASO ↑. Melatonin or cycling alone or melatonin with cycling exerted better effects compared to placebo. | The administration of this non-delayed liquid preparation with 3 mg 30 min before bedtime was associated with comparable effects to cyling. The combination of both methods was just as effective as each method on its own. | ||
| Author, Country | Diagnosis | N, Age, Dropouts, Missing Data | Target Values | Melatonin (Dose, Preparation) | Result | Conclusion | Side Effects |
| Garstang, 2006 [75] UK | ASS with insomnia *. | N = 7 (6 ♂), 5–15 years. * Dropouts: 36.4% (4/11). Missing data: none. | SOL; TST; WASO; waking-up activity in the morning via a sleep log written by parents. | Five-milligram oral capsules, immediate release ** 4 weeks vs. placebo, crossover after washout for 1 week. Instructions on sleep hygiene showed no effect before the start of the study and were maintained during the study. | Signif. # TST ↑ 8.05 to 9.84 h; SOL ↓ 2.60 to 1.06 h; awakenings ↓ from 0.35 to 0.08. | This non-delayed preparation was effective in terms of falling asleep, sleeping through the night, and TST. | No data available. |
| Wright, 2011 [76] UK | ASS with insomnia ***. | N = 17 (10 ♂), 9 ± 2.9 (4–16) years. Dropouts: 15% (3/20). Missing data: 5.9% (1/17). Previous statistical case number planning and power analysis. | As above; also, four standardised questionnaires on sleep, behaviour, health, and complications ##. | Two-milligram oral non-delayed §, 30–40 min before the expected sleep; increased by the parents every 3 days by two milligrams to max. ten milligrams (average seven milligrams) until “good sleep” with an “improvement of ≥50%” was achieved. Instructions on sleep hygiene showed no effect before the start of the study and were maintained during the study after further instruction of the parents. | SOL ↓ 135 to 82 min (baseline vs. melatonin at bedtime), 130 vs. 78 min on melatonin application vs. placebo. TST ↑ 500 to 556 min. Number of awakenings ↓ 0.5 to 0.43/night. Improvement in behaviour, communication, and dyssomnia. | This non-delayed preparation was effective in terms of falling asleep, sleeping through the night, TST, and improvement in behaviour and communication. | No statistically significant differences between verum and placebo with regard to all characteristics such as headache, vomiting, reduced appetite, and reduced attention, according to SEQ ##. |
| Cortesi, 2012 [7] Italy | ASS with insomnia +. Comparison of melatonin alone and in combination with CBT (cognitive behavioural therapy; four sessions). | N = 134 (82% ♂ in the melatonin group with a total of 34 children) aged 6.8 ± 0.9 years. Dropouts: 10% (16/160). Missing data: 6.9% (10/144). | SOL; TST; WASO (sleep diary and actigraphy); two standardised questionnaires on sleep and behaviour ++. | Three milligrams of a combined preparation (one milligram rapid release, two milligrams sustained release (6 h)) §§ oral administration at 9:00 p.m. Four randomised groups in parallel; 3 months each of following: ● Melatonin (N = 34); ● CBT (N = 33); ● Melat. + CBT (N = 35); ● Placebo (N = 32). | SOL ↓ to 44% with melatonin alone, to 23% after CBT, and to 61% with melatonin + CBT. TST ↑. Number of awakenings ↓. Sleep anxiety ↓ after KVT to 17%, after Melat. to 14%, and after both to 34%. | This combined 3 mg preparation alone was more effective than CBT alone. The combination of both was more effective than either method alone. | No side effects were observed. |
| Hayashi, 2022 [49] Japan | ASS with insomnia. ADHD at 55% (108/196): comparable effects regarding improvement in SOL by melatonin in both doses. No differences regarding the improvement in SOL after 1 mg or 4 mg of melatonin in children with a height <145 cm. | N = 196, (62% ♂) aged 11.2 ± 2.5 (6–15) years. Dropouts: 14.4% (33/229). Missing data: 4.6% (9/196). Preliminary statistical case number planning and power analysis. | SOL; TST; SE; WASO; sleep diary; actigraphy; standardised recording of five characteristics for irregular behaviour (ABC-J); height; weight; standardised checklist for recording adverse events (AEs)) +++. | Three parallel randomised, double-blind groups: 1 mg or 4 mg non-delayed melatonin vs. placebo for 2 weeks, administered 45 min before bedtime. Then, 42 days OL with dose increase if required after 7 days, up to max. 4 mg. Then, 14-day follow-up to exclude rebounds and withdrawal symptoms. Compliance with defined instructions on sleep hygiene before and during the study. | SOL ↓ to 21/20/1 min after 1 mg mel./4 mg mel./placebo, respectively (actigraphy, p < 0.0001). SE ↑/= to 2.35 resp. 2.07% after 4 mg resp. 1 mg (p = 0.04 resp. 0.13 n.s.). TST unchanged. WASO unchanged. Sleep hygiene alone in the prephase with lower effects compared to both MEL doses. | The administration of this non-delayed preparation with 4 mg 45 min before bedtime was associated with comparable effects to 1 mg, but more frequent AEs. In the OL phase with up to 10 mg, a further increase in AEs was documented. | Discontinuation at 4 mg during the RCT phase in one child due to AEs. No change in the five characteristics for aberrant behaviours in the remaining children during the RCT phase according to parents and physicians. Drug-related AEs 0/5/3 = 0%/7.7%/4.5% for 1 mg/4 mg/placebo during RCT. AEs total incl. the OL phase: RCT 1 mg: 13.8%; RCT 4 mg: 29.2%; RCT Plac.: 18.2%; OL 1–10 mg: 36.3%. |
| Tse 2024 [6] Hong Kong | ASS with insomnia | N = 62 (81% ♂; nine boys and five girls in the melatonin group) aged 9.61 to 10.36 years. Dropouts: 22.5% (18/80). Missing data: none. Preliminary statistical case number planning and power analysis. | SOL; TST; SE; WASO; sleep diary; actigraphy. $ | Four randomised arms for 2 weeks: 3 mg liquid melatonin 30 min before bedtime (N = 14); cycling 5 × 60 min/week (N = 18); melatonin and cycling (N = 12); placebo (N = 18). | SOL ↓, TST ↑, SE ↑, WASO ↑. Melatonin or cycling alone or Melatonin with cycling with better effects compared to placebo (Figure 4). | The administration of this non-delayed liquid preparation with 3 mg 30 min before bedtime was associated with comparable effects to cycling. The combination of both methods was just as effective as each method on its own (Figure 4). | No side effects were observed after the administration of melatonin. |
3.2. Critical Comparison with the Results of Reviews by Other Authors
3.3. Missing Subgroup Analyses in RCTs on Patients with Multiple Diagnoses
- -
- Gringras et al. reported on 121 children and adolescents with ASD and 4 children and adolescents with Smith–Magenis syndrome without differentiating the subgroups, in which 28.8% of the patients participating in the randomisation had ADHD and 12.8% had epilepsy [72]. Only 46.8% (125/267) of the originally recruited patients were included in the randomisation. With dropout rates of 15% (9/60) and 32.3% (21/65) in the placebo group, data from 51 verum and 44 placebo “cases” were finally analysed. This study of the sustained-release formulation was financed by the manufacturer and statistically analysed under its responsibility.
- -
- Schröder et al. published an analysis of some of the above data in 2019 [67]. Malow et al. analysed the data of the same(?) 51 verum vs. 44 placebo “cases” again in 2021, again without differentiating between the above-mentioned subgroups [79]. In 2012, Malow et al. had previously reported on different doses of melatonin in 24 children and adolescents with difficulty falling asleep and ASD, but without a randomised setting [71].
3.4. Differences in Circadian Rhythmicity in Patients with ASD or Smith–Magenis Syndrome
- In patients with SMS, no or very low melatonin concentrations are spontaneously detectable in the saliva between 23:00 and 07:00 without prior melatonin input, while, during the day, the paradoxical melatonin releases typical of SMS are detectable [80].
- ASD, on the other hand, is associated with endogenous melatonin release at night between 22:00 and 04:00 [45].
- The joint biostatistical evaluation of the circadian rhythm of peripheral melatonin concentrations in the blood, saliva, or urine of patients with ASD and SMS can therefore lead to the assumption of a nocturnal melatonin deficiency in ASD which, however, would only have to be analysed for this subgroup alone in order to obtain comprehensible conclusions.
- There was a reciprocal difference between patients with ASD and SMS in terms of the frequency of disorders of social communication behaviour (4:1 vs. 1:3) [83].
- -
- Wirojanan et al. also reported on a small group of patients in which no subgroup analyses were performed (five patients with ASD alone, three patients with ASD with fragile X syndrome, and four patients with fragile X syndrome) [73].
- -
- Wasdell et al. analysed the data of 16 patients with ASD without subgroup analysis, as part of an unspecified group of 47 patients with neurodevelopmental disorders [84].
3.5. Pharmacokinetics of Melatonin in Children and Adolescents with ASD
4. Discussion
4.1. Importance of Diagnosis-Related RCTs with Subgroup Analyses
4.2. Quality Heterogeneity of Existing RCTs with Regard to the Use of Adequate Parameters and Methods for the Assessment of Sleep and the Different Types of Sleep Disorders
4.3. Physiological and Pathophysiological Basics
- (a)
- The pineal gland appears to be involved in the pathogenesis of autism spectrum disorder (ASD) in the following two ways:
- -
- Based on the retrospective comparison of numerous data from children with ASD compared to control groups, Miike et al. (2020) assumed that the development of chronobiological rhythms in children with ASD can be disturbed by the following factors: maternal bed rest only after midnight, prematurity, irritability, and a tendency towards disturbed sleep in early infancy [91].
- -
- Shomrat and Nesher (2019) summarised the results of numerous studies on detailed questions of ASD pathogenesis [92]. As endogenous N,N-dimethyltryptamine (DMT) is formed and secreted in the pineal gland, in addition to the pulsatile circadian nocturnal synthesis and secretion of melatonin, synaptogenesis and neuroplasticity could be disturbed via altered DMT activity and the reduced melatonin concentrations in ASD, thereby resulting in the development of ASD [92]. DMT is also formed from tryptophan and classified as a neurotransmitter [93].
- (b)
- Hayashi et al. (2022) identified seven further pathogenetic factors in relation to melatonin in ASD, as follows: abnormalities in synthesis, concentration, secretion patterns, metabolism (such as polymorphisms of genes involved in the formation of pineal enzymes for the synthesis of melatonin = polymorphisms of the AOMT gene = the acetylserotonin-O-methyltransferase gene); impaired signalling to melatonin 1A receptors; the dysregulation of immunological signalling; and the inflammation of the central and peripheral immune system (Hayashi 2022) [49]. These factors have also been considered in several whole-genome association studies and comparable studies in which associations with such pathways have been demonstrated [94,95,96,97,98].
- (c)
- The “disruption of nocturnal melatonin synthesis and secretion” observed in children with autism is associated with measurable interleukin-6 and tumour necrosis factor activations during sleep, which are detectable in ASD but not in healthy controls (Figure 6); for a recent review of these neuroimmunological features in ASD, see Hughes et al. (2023) [99]. To our knowledge, clinical studies have not yet investigated whether the anti-inflammatory and immunomodulatory effect of melatonin [100,101,102] is of clinical significance in children and adolescents with ASD.
- (d)
- Goldman et al. (2014) showed that, in pharmacokinetic studies on endogenous melatonin concentrations in the blood of children with ASD, in therapeutic terms, it is not a question of replacing reduced melatonin concentrations, as there are no simple dose–response relationships. After the oral administration of 1 mg of melatonin, the measured melatonin concentrations were significantly higher than endogenous melatonin concentrations: “suggest[ing] that supplemental melatonin is not replacing a deficiency state but has other mechanisms of action” (Figure 4; Goldman et al. 2014, p. 9) [45].

4.4. Strengths and Limitations of the Present Systematic Review
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| AE | Adverse event |
| AOMT | Acetylserotonin-O-methyltransferase |
| ASD | Autism spectrum disorder |
| CBT | Cognitive behavioural therapy |
| CSF | Cerebrospinal Fluid |
| DLMO | Dim light melatonin onset |
| DMT | N,N-dimethyltryptamine |
| DSPD | Delayed sleep phase disorder |
| EMA | European Medicines Agency |
| G-BA | Joint Federal Committee (Gemeinsamer Bundesausschuss) |
| Non24 | Non-24 syndrome; deviation from 24 h circadian rhythmicity |
| OL | Off-label |
| RCT | Randomised controlled trial |
| SE | Sleep efficiency |
| SMS | Smith–Magenis syndrome |
| SO | Sleep onset |
| SOL | Sleep onset latency |
| TST | Total sleep time |
| WASO | Wake after sleep onset = awakenings; number and duration |
References
- Mutluer, T.; Aslan Genç, H.; Özcan Morey, A.; Yapici Eser, H.; Ertinmaz, B.; Can, M.; Munir, K. Population-Based Psychiatric Comorbidity in Children and Adolescents with Autism Spectrum Disorder: A Meta-Analysis. Front. Psychiatry 2022, 13, 856208. [Google Scholar] [CrossRef] [PubMed]
- Baldini, V.; Gnazzo, M.; Maragno, M.; Biagetti, R.; Stefanini, C.; Canulli, F.; Varallo, G.; Donati, C.; Neri, G.; Fiorillo, A.; et al. Suicidal risk among adolescent psychiatric inpatients: The role of insomnia, depression, and social-personal factors. Eur. Psychiatry J. Assoc. Eur. Psychiatr. 2025, 68, e42. [Google Scholar] [CrossRef] [PubMed]
- Vasa, R.A.; Kalari, V.K.; Kitchen, C.A.; Kharrazi, H.; Campo, J.V.; Wilcox, H.C. Suicide Risk Screening in Children and Adolescents with Autism Spectrum Disorder Presenting to the Emergency Department. Jt. Comm. J. Qual. Patient Saf. 2025, 51, 192–198. [Google Scholar] [CrossRef] [PubMed]
- Ipsiroglu, O.S.; Klösch, G.; Stein, M.; Blunden, S.; Brand, S.; Clemens, S.; Cortese, S.; Dück, A.; Dye, T.; Gringras, P.; et al. Phenotyping sleep disturbances in ADHD and identifying harmonised outcome measures. Somnologie 2024, 28, 189–200. [Google Scholar] [CrossRef]
- Schlarb, A.A.; Landwehr, J.; Prehn-Kristensen, A.; Paditz, E.; Quante, M.; Schneider, B. 33 Praxis-Tipps für müde Eltern, um über den Tag zu kommen. Somnologie 2024, 28, 131–137. [Google Scholar] [CrossRef]
- Tse, A.C.Y.; Lee, P.H.; Sit, C.H.P.; Poon, E.T.; Sun, F.; Pang, C.L.; Cheng, J.C.H. Comparing the Effectiveness of Physical Exercise Intervention and Melatonin Supplement in Improving Sleep Quality in Children with ASD. J. Autism Dev. Disord. 2024, 54, 4456–4464. [Google Scholar] [CrossRef]
- Cortesi, F.; Giannotti, F.; Sebastiani, T.; Panunzi, S.; Valente, D. Controlled-release melatonin, singly and combined with cognitive behavioural therapy, for persistent insomnia in children with autism spectrum disorders: A randomized placebo-controlled trial. J. Sleep Res. 2012, 21, 700–709. [Google Scholar] [CrossRef]
- Baglioni, C.; Altena, E.; Bjorvatn, B.; Blom, K.; Bothelius, K.; Devoto, A.; Espie, C.A.; Frase, L.; Gavriloff, D.; Tuuliki, H.; et al. The European Academy for Cognitive Behavioural Therapy for Insomnia: An initiative of the European Insomnia Network to promote implementation and dissemination of treatment. J. Sleep Res. 2020, 29, e12967. [Google Scholar] [CrossRef]
- Baglioni, C.; Espie, C.A.; Altena, E.; Gavriloff, D.; Jernelöv, S.; Holzinger, B.; Schlarb, A.; Riemann, D. Cognitive behavioural therapy for insomnia disorder: Extending the stepped care model. J. Sleep Res. 2023, 32, e14016. [Google Scholar] [CrossRef]
- Kirchhoff, F.; Paditz, E.; Erler, T.; Kerzel, S.; Eichholz, S.; Schlarb, A.; Schneider, B. Einsatz von Melatonin bei Kindern mit Schlafstörungen—Stellungnahme der Arbeitsgruppe Pädiatrie der Deutschen Gesellschaft für Schlafforschung und Schlafmedizin e. V. (DGSM). In Zeit Alter Schlaf. Aktuelle Kinderschlafmedizin 2018; Erler, T., Paditz, E., Eds.; kleanthes: Dresden, Germany, 2018; pp. 68–82. [Google Scholar]
- Paditz, E. Melatonin bei Schlafstörungen im Kindes- und Jugendalter. Monatsschrift Kinderheilkd. 2024, 172, 44–51. [Google Scholar] [CrossRef]
- Bruni, O.; Biggio, G.; Malorgio, E.; Nobili, L. Insomnia in children affected by autism spectrum disorder: The role of melatonin in treatment. Sleep Med. 2024, 119, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Gail Williams, P.; Sears, L.L.; Allard, A. Sleep problems in children with autism. J. Sleep Res. 2004, 13, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.; Liu, Z.; Xu, G.; Jiang, F.; Lu, N.; Baylor, A.; Owens, J. Sleep Disturbances and Associated Factors in Chinese Children with Autism Spectrum Disorder: A Retrospective and Cross-Sectional Study. Child Psychiatry Hum. Dev. 2016, 47, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Lerner, A.B.; Case, J.D.; Takahashi, Y.; Lee, T.H.; Mori, W. Isolation of Melatonin, the Pineal Gland Factor That Lightens Melanocytes1. J. Am. Chem. Soc. 1958, 80, 2587. [Google Scholar] [CrossRef]
- Vaughan, G.M.; Pelham, R.W.; Pang, S.F.; Loughlin, L.L.; Wilson, K.M.; Sandock, K.L.; Vaughan, M.K.; Koslow, S.H.; Reiter, R.J. Nocturnal elevation of plasma melatonin and urinary 5-hydroxyindoleacetic acid in young men: Attempts at modification by brief changes in environmental lighting and sleep and by autonomic drugs. J. Clin. Endocrinol. Metab. 1976, 42, 752–764. [Google Scholar] [CrossRef]
- Quay, W.B. Circadian Rhythm in Rat Pineal Serotonin and Its Modifications by Estrous Cycle and Photoperiod. Gen. Comp. Endocrinol. 1963, 3, 473–479. [Google Scholar] [CrossRef]
- Quay, W.B. Circadian and Estrous Rhythms in Pineal Melatonin and 5-Hydroxy Indole-3-Acetic Acid. Proc. Soc. Exp. Biol. Med. 1964, 115, 710–713. [Google Scholar] [CrossRef]
- Quay, W.B. 24-hour rhythms in pineal 5-hydroxytryptamine and hydroxyindole-o-methyl transferase activity in the macaque. Proc. Soc. Exp. Biol. Med. 1966, 121, 946–948. [Google Scholar] [CrossRef]
- Brown, G.M. Chronopharmacological actions of the pineal gland. Drug Metab. Drug Interact. 1990, 8, 189–201. [Google Scholar]
- Kumar, V. Melatonin: A master hormone and a candidate for universal panacea. Indian J. Exp. Biol. 1996, 34, 391–402. [Google Scholar]
- Coon, S.L.; Del Olmo, E.; Young, W.S., 3rd; Klein, D.C. Melatonin synthesis enzymes in Macaca mulatta: Focus on arylalkylamine N-acetyltransferase (EC 2.3.1.87). J. Clin. Endocrinol. Metab. 2002, 87, 4699–4706. [Google Scholar] [CrossRef] [PubMed]
- Klein, D.C. Evolution of the vertebrate pineal gland: The AANAT hypothesis. Chronobiol. Int. 2006, 23, 5–20. [Google Scholar] [CrossRef] [PubMed]
- Falcón, J.; Besseau, L.; Fuentès, M.; Sauzet, S.; Magnanou, E.; Boeuf, G. Structural and functional evolution of the pineal melatonin system in vertebrates. Ann. N. Y. Acad. Sci. 2009, 1163, 101–111. [Google Scholar] [CrossRef] [PubMed]
- Hedlund, L.; Lischko, M.; Rollag, M.D.; Niswender, G. Melatonin in Calf serum and cerebrospinal fluid: A daily cycle. Am. Zool. 1976, 16, 234. [Google Scholar]
- Hedlund, L.; Lischko, M.M.; Rollag, M.D.; Niswender, G.D. Melatonin: Daily cycle in plasma and cerebrospinal fluid of calves. Science 1977, 195, 686–687. [Google Scholar] [CrossRef]
- Hardeland, R.; Poeggeler, B. Non-vertebrate melatonin. J. Pineal Res. 2003, 34, 233–241. [Google Scholar] [CrossRef]
- Migliori, M.L.; Romanowski, A.; Simonetta, S.H.; Valdez, D.; Guido, M.; Golombek, D.A. Daily variation in melatonin synthesis and arylalkylamine N-acetyltransferase activity in the nematode Caenorhabditis elegans. J. Pineal Res. 2012, 53, 38–46. [Google Scholar] [CrossRef]
- Maciel, F.E.; Geihs, M.A.; Vargas, M.A.; Cruz, B.P.; Ramos, B.P.; Vakkuri, O.; Meyer-Rochow, V.B.; Maia Nery, L.E.; Allodi, S. Daily variation of melatonin content in the optic lobes of the crab Neohelice granulata. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2008, 149, 162–166. [Google Scholar] [CrossRef]
- Itoh, M.T.; Hattori, A.; Nomura, T.; Sumi, Y.; Suzuki, T. Melatonin and arylalkylamine N-acetyltransferase activity in the silkworm, Bombyx mori. Mol. Cell. Endocrinol. 1995, 115, 59–64. [Google Scholar] [CrossRef]
- Itoh, M.T.; Hattori, A.; Sumi, Y.; Suzuki, T. Day-night changes in melatonin levels in different organs of the cricket (Gryllus bimaculatus). J. Pineal Res. 1995, 18, 165–169. [Google Scholar] [CrossRef]
- McArthur, A.J.; Budden, S.S. Sleep dysfunction in Rett syndrome: A trial of exogenous melatonin treatment. Dev. Med. Child Neurol. 1998, 40, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Goldouzi, H.R.; Akhondian, J.; Beiraghi Toosi, M.; Mehrad Majd, H.; Shekari, S.; Babaei, M. The Effect of Melatonin on Sleep Disorders in Children with Cerebral Palsy A Randomized Clinical Trial. Iran. J. Child Neurol. 2024, 18, 51–59. [Google Scholar] [PubMed]
- Niederhofer, H.; Staffen, W.; Mair, A.; Pittschieler, K. Brief report: Melatonin facilitates sleep in individuals with mental retardation and insomnia. J. Autism Dev. Disord. 2003, 33, 469–472. [Google Scholar] [CrossRef] [PubMed]
- Parinas, M.L.C.; Pandher, P.K.; Klösch, G.; Paditz, E.; Spruyt, K.; Ipsiroglu, O.S. Analysis of Melatonin RCTs in Children with NDDs: Do We Need to Harmonize Sleep Research? In Proceedings of the 8th Congress of the International Pediatric Sleep Association (IPSA), Glasgow, Scotland, 26–28 April 2024; pp. 171–172. [Google Scholar]
- Paditz, E. Postnatal Development of the Circadian Rhythmicity of Human Pineal Melatonin Synthesis and Secretion (Systematic Review). Children 2024, 11, 1197. [Google Scholar] [CrossRef]
- Paditz, E. Melatonin in infants—Physiology, pathophysiology and intervention options. Somnologie 2024, 28, 103–109. [Google Scholar] [CrossRef]
- Zhdanova, I.V.; Wurtman, R.J.; Morabito, C.; Piotrovska, V.R.; Lynch, H.J. Effects of Low Oral Doses of Melatonin, Given 2–4 Hours Before Habitual Bedtime, On Sleep in Normal Young Humans. Sleep 1996, 19, 423–431. [Google Scholar] [CrossRef]
- Palm, L.; Blennow, G.; Wetterberg, L. Correction of non-24-hour sleep/wake cycle by melatonin in a blind retarded boy. Ann. Neurol. 1991, 29, 336–339. [Google Scholar] [CrossRef]
- Palm, L.; Blennow, G.; Wetterberg, L. Long-term melatonin treatment in blind children and young adults with circadian sleep-wake disturbances. Dev. Med. Child Neurol. 1997, 39, 319–325. [Google Scholar] [CrossRef]
- Yuge, K.; Nagamitsu, S.; Ishikawa, Y.; Hamada, I.; Takahashi, H.; Sugioka, H.; Yotsuya, O.; Mishima, K.; Hayashi, M.; Yamashita, Y. Long-term melatonin treatment for the sleep problems and aberrant behaviors of children with neurodevelopmental disorders. BMC Psychiatry 2020, 20, 445. [Google Scholar] [CrossRef]
- van Geijlswijk, I.M.; van der Heijden, K.B.; Egberts, A.C.; Korzilius, H.P.; Smits, M.G. Dose finding of melatonin for chronic idiopathic childhood sleep onset insomnia: An RCT. Psychopharmacology 2010, 212, 379–391. [Google Scholar] [CrossRef]
- Merchant, N.M.; Azzopardi, D.V.; Hawwa, A.F.; McElnay, J.C.; Middleton, B.; Arendt, J.; Arichi, T.; Gressens, P.; Edwards, A.D. Pharmacokinetics of melatonin in preterm infants. Br. J. Clin. Pharmacol. 2013, 76, 725–733. [Google Scholar] [CrossRef]
- Carloni, S.; Proietti, F.; Rocchi, M.; Longini, M.; Marseglia, L.; D’Angelo, G.; Balduini, W.; Gitto, E.; Buonocore, G. Melatonin Pharmacokinetics Following Oral Administration in Preterm Neonates. Molecules 2017, 22, 2115. [Google Scholar] [CrossRef]
- Goldman, S.E.; Adkins, K.W.; Calcutt, M.W.; Carter, M.D.; Goodpaster, R.L.; Wang, L.; Shi, Y.; Burgess, H.J.; Hachey, D.L.; Malow, B.A. Melatonin in children with autism spectrum disorders: Endogenous and pharmacokinetic profiles in relation to sleep. J. Autism Dev. Disord. 2014, 44, 2525–2535. [Google Scholar] [CrossRef]
- Cavallo, A.; Ritschel, W.A. Pharmacokinetics of melatonin in human sexual maturation. J. Clin. Endocrinol. Metab. 1996, 81, 1882–1886. [Google Scholar]
- Di, W.L.; Kadva, A.; Johnston, A.; Silman, R. Variable bioavailability of oral melatonin. N. Engl. J. Med. 1997, 336, 1028–1029. [Google Scholar] [CrossRef]
- Lalanne, S.; Fougerou-Leurent, C.; Anderson, G.M.; Schroder, C.M.; Nir, T.; Chokron, S.; Delorme, R.; Claustrat, B.; Bellissant, E.; Kermarrec, S.; et al. Melatonin: From Pharmacokinetics to Clinical Use in Autism Spectrum Disorder. Int. J. Mol. Sci. 2021, 22, 1490. [Google Scholar] [CrossRef]
- Hayashi, M.; Mishima, K.; Fukumizu, M.; Takahashi, H.; Ishikawa, Y.; Hamada, I.; Sugioka, H.; Yotsuya, O.; Yamashita, Y. Melatonin Treatment and Adequate Sleep Hygiene Interventions in Children with Autism Spectrum Disorder: A Randomized Controlled Trial. J. Autism Dev. Disord. 2022, 52, 2784–2793. [Google Scholar] [CrossRef]
- Persico, A.M.; Asta, L.; Chehbani, F.; Mirabelli, S.; Parlatini, V.; Cortese, S.; Arango, C.; Vitiello, B. The pediatric psychopharmacology of autism spectrum disorder: A systematic review—Part II: The future. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2025, 136, 111176. [Google Scholar] [CrossRef]
- Parker, A.; Beresford, B.; Dawson, V.; Elphick, H.; Fairhurst, C.; Hewitt, C.; Scantlebury, A.; Spiers, G.; Thomas, M.; Wright, K.; et al. Oral melatonin for non-respiratory sleep disturbance in children with neurodisabilities: Systematic review and meta-analyses. Dev. Med. Child Neurol. 2019, 61, 880–890. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Melatonin in autism spectrum disorders: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2011, 53, 783–792. [Google Scholar] [CrossRef]
- Tse, A.C.Y.; Lee, P.H.; Lau, E.Y.Y.; Cheng, J.C.H.; Ho, A.W.Y.; Lai, E.W.H. Study protocol for a randomized controlled trial comparing the effectiveness of physical exercise and melatonin supplement on treating sleep disturbance in children with autism spectrum disorders. PLoS ONE 2022, 17, e0270428. [Google Scholar] [CrossRef]
- Ayuse, T.; Ozaki-Honda, Y.; Kurata, S.; Mishima, G.; Kiriishi, K.; Magata, N.; Kawasaki, H.; Yamaguchi-Komeyama, K.; Tanoue, N.; Ayuse, T. Study on the preventive effect of ramelteon on the onset of sleep disorder after general anesthesia in patients with autism spectrum disorder: A study protocol. Medicine 2020, 99, e22826. [Google Scholar] [CrossRef]
- Parvataneni, T.; Srinivas, S.; Shah, K.; Patel, R.S. Perspective on Melatonin Use for Sleep Problems in Autism and Attention-Deficit Hyperactivity Disorder: A Systematic Review of Randomized Clinical Trials. Cureus 2020, 12, e8335. [Google Scholar] [CrossRef]
- McDonagh, M.S.; Holmes, R.; Hsu, F. Pharmacologic Treatments for Sleep Disorders in Children: A Systematic Review. J. Child Neurol. 2019, 34, 237–247. [Google Scholar] [CrossRef]
- Guénolé, F.; Godbout, R.; Nicolas, A.; Franco, P.; Claustrat, B.; Baleyte, J.M. Melatonin for disordered sleep in individuals with autism spectrum disorders: Systematic review and discussion. Sleep Med. Rev. 2011, 15, 379–387. [Google Scholar] [CrossRef]
- Rossignol, D.A. Novel and emerging treatments for autism spectrum disorders: A systematic review. Ann. Clin. Psychiatry Off. J. Am. Acad. Clin. Psychiatr. 2009, 21, 213–236. [Google Scholar]
- Liang, X.; Haegele, J.A.; Tse, A.C.; Li, M.; Zhang, H.; Zhao, S.; Li, S.X. The impact of the physical activity intervention on sleep in children and adolescents with autism spectrum disorder: A systematic review and meta-analysis. Sleep Med. Rev. 2024, 74, 101913. [Google Scholar] [CrossRef]
- Raghavan, K.; Dedeepiya, V.D.; Yamamoto, N.; Ikewaki, N.; Sonoda, T.; Iwasaki, M.; Kandaswamy, R.S.; Senthilkumar, R.; Preethy, S.; Abraham, S.J.K. Benefits of Gut Microbiota Reconstitution by Beta 1,3-1,6 Glucans in Subjects with Autism Spectrum Disorder and Other Neurodegenerative Diseases. J. Alzheimer’s Dis. 2023, 94, S241–S252. [Google Scholar] [CrossRef]
- Goltz, J.; Ivanov, I.; Rice, T.R. Second generation antipsychotic-induced weight gain in youth with autism spectrum disorders: A brief review of mechanisms, monitoring practices, and indicated treatments. Int. J. Dev. Disabil. 2019, 67, 159–167. [Google Scholar] [CrossRef]
- Klein, N.; Kemper, K.J. Integrative Approaches to Caring for Children with Autism. Curr. Probl. Pediatr. Adolesc. Health Care 2016, 46, 195–201. [Google Scholar] [CrossRef]
- Anagnostou, E.; Hansen, R. Medical treatment overview: Traditional and novel psycho-pharmacological and complementary and alternative medications. Curr. Opin. Pediatr. 2011, 23, 621–627. [Google Scholar] [CrossRef]
- Schröder, C.M.; Broquère, M.A.; Claustrat, B.; Delorme, R.; Franco, P.; Lecendreux, M.; Tordjman, S. Therapeutic approaches for sleep and rhythms disorders in children with ASD. L’Encephale 2022, 48, 294–303. [Google Scholar] [CrossRef]
- Zisapel, N. Assessing the potential for drug interactions and long term safety of melatonin for the treatment of insomnia in children with autism spectrum disorder. Expert Rev. Clin. Pharmacol. 2022, 15, 175–185. [Google Scholar] [CrossRef]
- Cortese, S.; Wang, F.; Angriman, M.; Masi, G.; Bruni, O. Sleep Disorders in Children and Adolescents with Autism Spectrum Disorder: Diagnosis, Epidemiology, and Management. CNS Drugs 2020, 34, 415–423. [Google Scholar] [CrossRef]
- Schroder, C.M.; Malow, B.A.; Maras, A.; Melmed, R.D.; Findling, R.L.; Breddy, J.; Nir, T.; Shahmoon, S.; Zisapel, N.; Gringras, P. Pediatric Prolonged-Release Melatonin for Sleep in Children with Autism Spectrum Disorder: Impact on Child Behavior and Caregiver’s Quality of Life. J. Autism Dev. Disord. 2019, 49, 3218–3230. [Google Scholar] [CrossRef]
- Rossignol, D.A.; Frye, R.E. Melatonin in autism spectrum disorders. Curr. Clin. Pharmacol. 2014, 9, 326–334. [Google Scholar] [CrossRef]
- Tordjman, S.; Najjar, I.; Bellissant, E.; Anderson, G.M.; Barburoth, M.; Cohen, D.; Jaafari, N.; Schischmanoff, O.; Fagard, R.; Lagdas, E.; et al. Advances in the research of melatonin in autism spectrum disorders: Literature review and new perspectives. Int. J. Mol. Sci. 2013, 14, 20508–20542. [Google Scholar] [CrossRef]
- Maras, A.; Schroder, C.M.; Malow, B.A.; Findling, R.L.; Breddy, J.; Nir, T.; Shahmoon, S.; Zisapel, N.; Gringras, P. Long-Term Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children with Autism Spectrum Disorder. J. Child Adolesc. Psychopharmacol. 2018, 28, 699–710. [Google Scholar] [CrossRef]
- Malow, B.; Adkins, K.W.; McGrew, S.G.; Wang, L.; Goldman, S.E.; Fawkes, D.; Burnette, C. Melatonin for sleep in children with autism: A controlled trial examining dose, tolerability, and outcomes. J. Autism Dev. Disord. 2012, 42, 1729–1737, author reply 1738. [Google Scholar] [CrossRef]
- Gringras, P.; Nir, T.; Breddy, J.; Frydman-Marom, A.; Findling, R.L. Efficacy and Safety of Pediatric Prolonged-Release Melatonin for Insomnia in Children With Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2017, 56, 948–957.e944. [Google Scholar] [CrossRef]
- Wirojanan, J.; Jacquemont, S.; Diaz, R.; Bacalman, S.; Anders, T.F.; Hagerman, R.J.; Goodlin-Jones, B.L. The efficacy of melatonin for sleep problems in children with autism, fragile X syndrome, or autism and fragile X syndrome. J. Clin. Sleep Med. Off. Publ. Am. Acad. Sleep Med. 2009, 5, 145–150. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Garstang, J.; Wallis, M. Randomized controlled trial of melatonin for children with autistic spectrum disorders and sleep problems. Child Care Health Dev. 2006, 32, 585–589. [Google Scholar] [CrossRef]
- Wright, B.; Sims, D.; Smart, S.; Alwazeer, A.; Alderson-Day, B.; Allgar, V.; Whitton, C.; Tomlinson, H.; Bennett, S.; Jardine, J.; et al. Melatonin versus placebo in children with autism spectrum conditions and severe sleep problems not amenable to behaviour management strategies: A randomised controlled crossover trial. J. Autism Dev. Disord. 2011, 41, 175–184. [Google Scholar] [CrossRef]
- Schroder, C.M.; Banaschewski, T.; Fuentes, J.; Hill, C.M.; Hvolby, A.; Posserud, M.B.; Bruni, O. Pediatric prolonged-release melatonin for insomnia in children and adolescents with autism spectrum disorders. Expert Opin. Pharmacother. 2021, 22, 2445–2454. [Google Scholar] [CrossRef]
- Kulman, G.; Lissoni, P.; Rovelli, F.; Roselli, M.G.; Brivio, F.; Sequeri, P. Evidence of pineal endocrine hypofunction in autistic children. Neuro Endocrinol. Lett. 2000, 21, 31–34. [Google Scholar]
- Malow, B.A.; Findling, R.L.; Schroder, C.M.; Maras, A.; Breddy, J.; Nir, T.; Zisapel, N.; Gringras, P. Sleep, Growth, and Puberty After 2 Years of Prolonged-Release Melatonin in Children With Autism Spectrum Disorder. J. Am. Acad. Child Adolesc. Psychiatry 2021, 60, 252–261.e253. [Google Scholar] [CrossRef]
- Spruyt, K.; Braam, W.; Smits, M.; Curfs, L.M. Sleep Complaints and the 24-h Melatonin Level in Individuals with Smith-Magenis Syndrome: Assessment for Effective Intervention. CNS Neurosci. Ther. 2016, 22, 928–935. [Google Scholar] [CrossRef]
- Rinaldi, B.; Villa, R.; Sironi, A.; Garavelli, L.; Finelli, P.; Bedeschi, M.F. Smith-Magenis Syndrome-Clinical Review, Biological Background and Related Disorders. Genes 2022, 13, 335. [Google Scholar] [CrossRef]
- Girirajan, S.; Vlangos, C.N.; Szomju, B.B.; Edelman, E.; Trevors, C.D.; Dupuis, L.; Nezarati, M.; Bunyan, D.J.; Elsea, S.H. Genotype-phenotype correlation in Smith-Magenis syndrome: Evidence that multiple genes in 17p11.2 contribute to the clinical spectrum. Genet. Med. Off. J. Am. Coll. Med. Genet. 2006, 8, 417–427. [Google Scholar] [CrossRef]
- Nag, H.E.; Nordgren, A.; Anderlid, B.M.; Nærland, T. Reversed gender ratio of autism spectrum disorder in Smith-Magenis syndrome. Mol. Autism 2018, 9, 1. [Google Scholar] [CrossRef]
- Wasdell, M.B.; Jan, J.E.; Bomben, M.M.; Freeman, R.D.; Rietveld, W.J.; Tai, J.; Hamilton, D.; Weiss, M.D. A randomized, placebo-controlled trial of controlled release melatonin treatment of delayed sleep phase syndrome and impaired sleep maintenance in children with neurodevelopmental disabilities. J. Pineal Res. 2008, 44, 57–64. [Google Scholar] [CrossRef]
- Carmassi, C.; Palagini, L.; Caruso, D.; Masci, I.; Nobili, L.; Vita, A.; Dell’Osso, L. Systematic Review of Sleep Disturbances and Circadian Sleep Desynchronization in Autism Spectrum Disorder: Toward an Integrative Model of a Self-Reinforcing Loop. Front. Psychiatry 2019, 10, 366. [Google Scholar] [CrossRef]
- Nir, I.; Meir, D.; Zilber, N.; Knobler, H.; Hadjez, J.; Lerner, Y. Brief report: Circadian melatonin, thyroid-stimulating hormone, prolactin, and cortisol levels in serum of young adults with autism. J. Autism Dev. Disord. 1995, 25, 641–654. [Google Scholar] [CrossRef]
- Tordjman, S.; Anderson, G.M.; Pichard, N.; Charbuy, H.; Touitou, Y. Nocturnal excretion of 6-sulphatoxymelatonin in children and adolescents with autistic disorder. Biol. Psychiatry 2005, 57, 134–138. [Google Scholar] [CrossRef]
- Tang, K.W.; Toh, Q.C.; Teo, B.W. Normalisation of urinary biomarkers to creatinine for clinical practice and research—When and why. Singap. Med. J. 2015, 56, 7–10. [Google Scholar] [CrossRef]
- Bartakovicova, K.; Kemenyova, P.; Belica, I.; Janik Szapuova, Z.; Stebelova, K.; Waczulikova, I.; Ostatnikova, D.; Babinska, K. Sleep Problems and 6-Sulfatoxymelatonin as Possible Predictors of Symptom Severity, Adaptive and Maladaptive Behavior in Children with Autism Spectrum Disorder. Int. J. Environ. Res. Public Health 2022, 19, 7594. [Google Scholar] [CrossRef]
- de Souza, A.L.D.M.; Giacheti, C.M.; Do Couto, M.C.H.; Galina Spilla, C.S.; da Silva, N.C.; Proença, M.; Pinato, L. Sleep disturbance in children with attention-deficit hyperactivity disorder: Relationship with melatonin and behavior. Neurol. Res. 2024, 46, 803–811. [Google Scholar] [CrossRef]
- Miike, T.; Toyoura, M.; Tonooka, S.; Konishi, Y.; Oniki, K.; Saruwatari, J.; Tajima, S.; Kinoshita, J.; Nakai, A.; Kikuchi, K. Neonatal irritable sleep-wake rhythm as a predictor of autism spectrum disorders. Neurobiol. Sleep Circadian Rhythm. 2020, 9, 100053. [Google Scholar] [CrossRef]
- Shomrat, T.; Nesher, N. Updated View on the Relation of the Pineal Gland to Autism Spectrum Disorders. Front. Endocrinol. 2019, 10, 37. [Google Scholar] [CrossRef]
- Gheban, B.A.; Rosca, I.A.; Crisan, M. The morphological and functional characteristics of the pineal gland. Med. Pharm. Rep. 2019, 92, 226–234. [Google Scholar] [CrossRef]
- Lin, P.I.; Masi, A.; Moni, M.A.; Kummerfeld, S.; Eapen, V. Genetic Pathways Associated With Sleep Problems in Children with Autism Spectrum Disorder. Front. Psychiatry 2022, 13, 904091. [Google Scholar] [CrossRef]
- Yap, C.X.; Alvares, G.A.; Henders, A.K.; Lin, T.; Wallace, L.; Farrelly, A.; McLaren, T.; Berry, J.; Vinkhuyzen, A.A.E.; Trzaskowski, M.; et al. Analysis of common genetic variation and rare CNVs in the Australian Autism Biobank. Mol. Autism 2021, 12, 12. [Google Scholar] [CrossRef]
- Benabou, M.; Rolland, T.; Leblond, C.S.; Millot, G.A.; Huguet, G.; Delorme, R.; Leboyer, M.; Pagan, C.; Callebert, J.; Maronde, E.; et al. Heritability of the melatonin synthesis variability in autism spectrum disorders. Sci. Rep. 2017, 7, 17746. [Google Scholar] [CrossRef]
- Han, P.P.; Zou, M.Y.; Yang, X.L.; Liu, X.C.; Liang, S.; Sun, C.H.; Xia, W.; Wu, L.J. Sleep problems and the association with the levels of 6-sulfatoxymelatonin in children with autism spectrum disorder. Zhonghua Er Ke Za Zhi = Chin. J. Pediatr. 2017, 55, 911–915. [Google Scholar]
- Braam, W.; Keijzer, H.; Struijker Boudier, H.; Didden, R.; Smits, M.; Curfs, L. CYP1A2 polymorphisms in slow melatonin metabolisers: A possible relationship with autism spectrum disorder? J. Intellect. Disabil. Res. 2013, 57, 993–1000. [Google Scholar] [CrossRef]
- Hughes, H.K.; Moreno, R.J.; Ashwood, P. Innate immune dysfunction and neuroinflammation in autism spectrum disorder (ASD). Brain Behav. Immun. 2023, 108, 245–254. [Google Scholar] [CrossRef]
- Galano, A.; Reiter, R.J. Melatonin and its metabolites vs oxidative stress: From individual actions to collective protection. J. Pineal Res. 2018, 65, e12514. [Google Scholar] [CrossRef]
- Maestroni, G.J.; Conti, A.; Pierpaoli, W. Role of the pineal gland in immunity. III. Melatonin antagonizes the immunosuppressive effect of acute stress via an opiatergic mechanism. Immunology 1988, 63, 465–469. [Google Scholar]
- Carrillo-Vico, A.; Guerrero, J.M.; Lardone, P.J.; Reiter, R.J. A review of the multiple actions of melatonin on the immune system. Endocrine 2005, 27, 189–200. [Google Scholar] [CrossRef]
- Claustrat, B.; Leston, J. Melatonin: Physiological effects in humans. Neurochirurgie 2015, 61, 77–84. [Google Scholar] [CrossRef]
- da Silveira Cruz-Machado, S.; Guissoni Campos, L.M.; Fadini, C.C.; Anderson, G.; Markus, R.P.; Pinato, L. Disrupted nocturnal melatonin in autism: Association with tumor necrosis factor and sleep disturbances. J. Pineal Res. 2021, 70, e12715. [Google Scholar] [CrossRef]
- Pinato, L.; Galina Spilla, C.S.; Markus, R.P.; da Silveira Cruz-Machado, S. Dysregulation of Circadian Rhythms in Autism Spectrum Disorders. Curr. Pharm. Des. 2019, 25, 4379–4393. [Google Scholar] [CrossRef]
- Martinez-Cayuelas, E.; Merino-Andreu, M.; Losada-Del Pozo, R.; Gavela-Pérez, T.; Garcés, C.; Soriano-Guillén, L. Response to Melatonin Treatment in Children With Autism spectrum Disorder and Relationship to Sleep Parameters and Melatonin Levels. J. Child Neurol. 2023, 38, 253–262. [Google Scholar] [CrossRef]
- Almirall, D.; Compton, S.N.; Gunlicks-Stoessel, M.; Duan, N.; Murphy, S.A. Designing a pilot sequential multiple assignment randomized trial for developing an adaptive treatment strategy. Stat. Med. 2012, 31, 1887–1902. [Google Scholar] [CrossRef]
- Debus, O.M.; Lerchl, A.; Bothe, H.W.; Bremer, J.; Fiedler, B.; Franssen, M.; Koehring, J.; Steils, M.; Kurlemann, G. Spontaneous central melatonin secretion and resorption kinetics of exogenous melatonin: A ventricular CSF study. J. Pineal Res. 2002, 33, 213–217. [Google Scholar] [CrossRef]
- Longatti, P.; Perin, A.; Rizzo, V.; Comai, S.; Giusti, P.; Costa, C.V. Ventricular cerebrospinal fluid melatonin concentrations investigated with an endoscopic technique. J. Pineal Res. 2007, 42, 113–118. [Google Scholar] [CrossRef]
- Legros, C.; Chesneau, D.; Boutin, J.A.; Barc, C.; Malpaux, B. Melatonin from cerebrospinal fluid but not from blood reaches sheep cerebral tissues under physiological conditions. J. Neuroendocrinol. 2014, 26, 151–163. [Google Scholar] [CrossRef]
- Kanematsu, N.; Mori, Y.; Hayashi, S.; Hoshino, K. Presence of a distinct 24-hour melatonin rhythm in the ventricular cerebrospinal fluid of the goat. J. Pineal Res. 1989, 7, 143–152. [Google Scholar] [CrossRef]
- Shaw, P.F.; Kennaway, D.J.; Seamark, R.F. Evidence of high concentrations of melatonin in lateral ventricular cerebrospinal fluid of sheep. J. Pineal Res. 1989, 6, 201–208. [Google Scholar] [CrossRef]
- Rollag, M.D.; Morgan, R.J.; Niswender, G.D. Utilization of the convolution integral to calculate rates of melatonin secretion into blood and CSF of sheep. ISA Trans. 1977, 16, 91–96. [Google Scholar] [PubMed]
- Skinner, D.C.; Malpaux, B. High Melatonin Concentrations in Third Ventricular Cerebrospinal Fluid Are Not due to Galen Vein Blood Recirculating through the Choroid Plexus1. Endocrinology 1999, 140, 4399–4405. [Google Scholar] [CrossRef] [PubMed]
- Tricoire, H.; Locatelli, A.; Chemineau, P.; Malpaux, B. Melatonin enters the cerebrospinal fluid through the pineal recess. Endocrinology 2002, 143, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Tricoire, H.; Malpaux, B.; Møller, M. Cellular lining of the sheep pineal recess studied by light-, transmission-, and scanning electron microscopy: Morphologic indications for a direct secretion of melatonin from the pineal gland to the cerebrospinal fluid. J. Comp. Neurol. 2003, 456, 39–47. [Google Scholar] [CrossRef]
- Reiter, R.J.; Tan, D.X.; Kim, S.J.; Cruz, M.H. Delivery of pineal melatonin to the brain and SCN. role of canaliculi, cerebrospinal fluid, tanycytes and Virchow-Robin perivascular spaces. Brain Struct. Funct. 2014, 219, 1873–1887. [Google Scholar] [CrossRef]
- Paditz, E. Chronobiologische Besonderheiten der frühkindlichen Ernährung. In Aktuelle Kinderschlafmedizin 2024. Kinderschlafmedizin Interdisziplinär; Hübler, A., Lobstein, S., Strobel, K., Eds.; Kleanthes: Dresden, Germany, 2024; pp. 58–99. [Google Scholar]
- Tricoire, H.; Møller, M.; Chemineau, P.; Malpaux, B. Origin of cerebrospinal fluid melatonin and possible function in the integration of photoperiod. Reprod. Suppl. 2003, 61, 311–321. [Google Scholar] [CrossRef] [PubMed]
- Hardeland, R. Divergent Importance of Chronobiological Considerations in High- and Low-dose Melatonin Therapies. Diseases 2021, 9, 18. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Paditz, E.; Renner, B.; Koch, R.; Schneider, B.M.; Schlarb, A.A.; Ipsiroglu, O.S. The Pharmacokinetics, Dosage, Preparation Forms, and Efficacy of Orally Administered Melatonin for Non-Organic Sleep Disorders in Autism Spectrum Disorder During Childhood and Adolescence: A Systematic Review. Children 2025, 12, 648. https://doi.org/10.3390/children12050648
Paditz E, Renner B, Koch R, Schneider BM, Schlarb AA, Ipsiroglu OS. The Pharmacokinetics, Dosage, Preparation Forms, and Efficacy of Orally Administered Melatonin for Non-Organic Sleep Disorders in Autism Spectrum Disorder During Childhood and Adolescence: A Systematic Review. Children. 2025; 12(5):648. https://doi.org/10.3390/children12050648
Chicago/Turabian StylePaditz, Ekkehart, Bertold Renner, Rainer Koch, Barbara M. Schneider, Angelika A. Schlarb, and Osman S. Ipsiroglu. 2025. "The Pharmacokinetics, Dosage, Preparation Forms, and Efficacy of Orally Administered Melatonin for Non-Organic Sleep Disorders in Autism Spectrum Disorder During Childhood and Adolescence: A Systematic Review" Children 12, no. 5: 648. https://doi.org/10.3390/children12050648
APA StylePaditz, E., Renner, B., Koch, R., Schneider, B. M., Schlarb, A. A., & Ipsiroglu, O. S. (2025). The Pharmacokinetics, Dosage, Preparation Forms, and Efficacy of Orally Administered Melatonin for Non-Organic Sleep Disorders in Autism Spectrum Disorder During Childhood and Adolescence: A Systematic Review. Children, 12(5), 648. https://doi.org/10.3390/children12050648









