Abstract
Background/Objectives: CMV (cytomegalovirus) is associated with several developmental disorders. The incidence of congenital cytomegalovirus infection is around 1%, depending on the region. Previous prospective studies have shown that certain ultrasound findings are predictive factors for prenatal CMV infection. Methods: During this systematic review, we searched PubMed and Embas. Out of 569 results, 19 met our search criteria (we included cases where prenatally positive amniocentesis PCR for CMV was performed or autopsy confirmed the CMV diagnosis). A total of 237 cases were reported from 19 studies. Results: In 64 cases, abortion or perinatal death occurred. The most common prenatal abnormalities were small for gestational age (n = 47), ventriculomegaly (n = 51), and hyperechogenic bowels (n = 39). A subependymal cyst was the most common prenatal MRI abnormality (n = 20). Hearing loss was observed in 61 cases (42 mild, 19 severe). Among prenatal signs, we found a correlation between hearing loss and ventriculomegaly (Fisher’s exact test, p = 0.0052). The most common neurological complication was speech delay. We were able to demonstrate a prenatal association with neurological complications and subependymal cyst (Fisher’s exact test, p = 0.00003547), but this pattern could only be reliably seen with MRI. Conclusions: In prenatally diagnosed CMV infection, ultrasound signals may be suitable for estimating the outcome. Conducting a prospective study and establishing a score would be worthwhile for its clinical application. In cases of ultrasound abnormalities and suspicion of CMV, it is worth performing a prenatal MRI, even in everyday practice.
1. Introduction
Cytomegalovirus (CMV) is a member of the herpesvirus family. It primarily attacks endothelial cells and is a neurotropic virus. It has a potentially lifelong incubation period; therefore, an active infection may arise from a primary infection or from a non-primary infection caused by reinfection and/or reactivation. It is asymptomatic in immunocompetent individuals, while in immunocompromised individuals and pregnant women it can cause serious infection. Also, it is associated with the development of numerous developmental disorders [1]. Congenital CMV infection occurs via vertical transmission across the placenta, with a transmission rate of 30–40% for primary infection and around 1% for non-primary maternal infection [2].
Congenital cytomegalovirus infection occurs in 0.4–0.6% of live births in developed countries, and over 1% in developing countries [3]. The risk of perinatal death is increased in both symptomatic and asymptomatic cases, although different studies report different levels of risk (0.4–0.8%) [4].
As cytomegalovirus primarily attacks endothelial cells [5,6], the organ systems affected are diverse.
The most characteristic ultrasound signals were summarized by Benoist et al. and Malinger et al. [7,8]. The most characteristic intracranial abnormalities are ventriculomegaly, increased periventricular echogenicity or halo, periventricular pseudocysts, fetal intracranial calcification (especially periventricular calcification), heterogeneous brain parenchyma, microcephaly, and intraventricular adhesions. Other “minor” abnormalities may also occur, such as polar temporal lesions (dilation of temporal horns, WM T2-weighted signal hyperintensity, and cystic lesions), subependymal cysts, and intraventricular septa [9]. Extracerebral abnormalities are generally nonspecific, but with appropriate history and clinical presentation, they may indicate infection. These include IUGR/SGR (intrauterine growth restriction/retardation, small for gestational age), hepatosplenomegaly, hyperechogenic intestines, pericardial or pleural fluid, ascites or hydrops, and cardiomegaly.
With the spread of prenatal MRI, increasingly accurate examinations have become possible, so prenatal brain MRI suggests CMV infection by white matter lesions, late myelination, periventricular and temporal horn cysts, and migration disorders (lissencephaly, polymicrogyria, etc.) [10]. MRI can be the gold standard in postnatal diagnosis, but it is also increasingly used in prenatal diagnosis. MRI is better at detecting temporal polar lesions and polymicrogyria. However, the clinical significance of these minor signs is questionable, as there is no reliable study of the relationship between minor lesions and long-term follow-up [9,11]. With the development of radiology, prenatal MRI is now as informative as neonatal MRI [10].
Neurotropic viruses primarily affect the central nervous system, resulting in varying degrees of mental retardation, epilepsy, and cerebral palsy, and varying degrees and sides of hearing impairment or loss (uni- or bilateral) [12].
The timing of infection and seroconversion has a major impact on the course of infection and the development of malformations. The current position is that infection in the first trimester carries the highest risk. The results are more uncertain regarding later infection [11,13,14,15].
After the neonatal period, prenatal CMV detection by serological and virological methods is very difficult, and an alternative is retrospective examination of CMV DNA on dried blood spots collected in the first few days of life [16]. In postnatally symptomatic cases, neonatal cranial MRI is recommended to assess the outcome. A correlation between intracranial calcification and white matter abnormalities and lower intelligence has been demonstrated, but there are also case reports to the contrary [17,18,19].
Although the association between prenatal imaging findings and CMV is well-known and documented, there is relatively little literature on prognosis and its association with prenatal testing. Our aim in this study was to compile and review studies that specifically address prenatal imaging findings and postnatal outcome.
2. Materials and Methods
This study was a systematic review and was conducted following PRISMA 2020 guidelines [20].
During the systematic review, we searched PubMed, Web of Science, and Embase. We examined all available scientific articles, case studies, and retrospective and prospective cohort studies published after 1995. Those that matched the search terms “cytomegalovirus”, “ultrasound and/or MRI”, “prenatal”, “congenital”, and/or “infant” were reviewed. After a detailed analysis of the abstracts, we excluded those that were not written in English or whose English transcripts were not available, summary studies, meta-analyses, experimental descriptions, or descriptions of animal models.
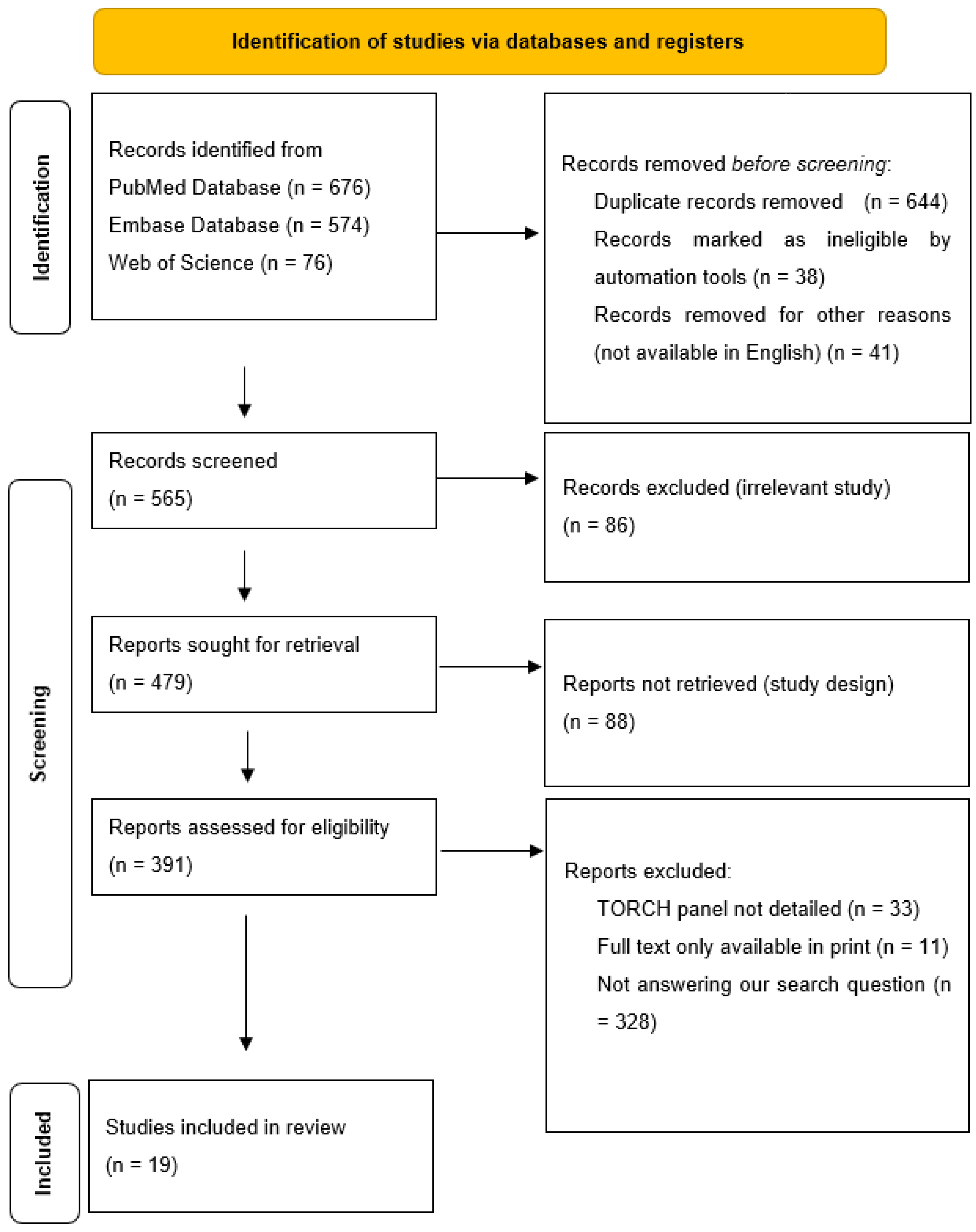
While processing the abstracts, we excluded all articles that did not adequately answer our research question, i.e., what prenatal ultrasound abnormalities are associated with congenital cytomegalovirus, and what postnatal abnormalities are associated with these? We excluded all articles from the processing where cytomegalovirus and other TORCH (“toxoplasmosis, rubella cytomegalovirus, herpes simplex, and HSV”) infections were described together. The decision process is described in Figure 1.
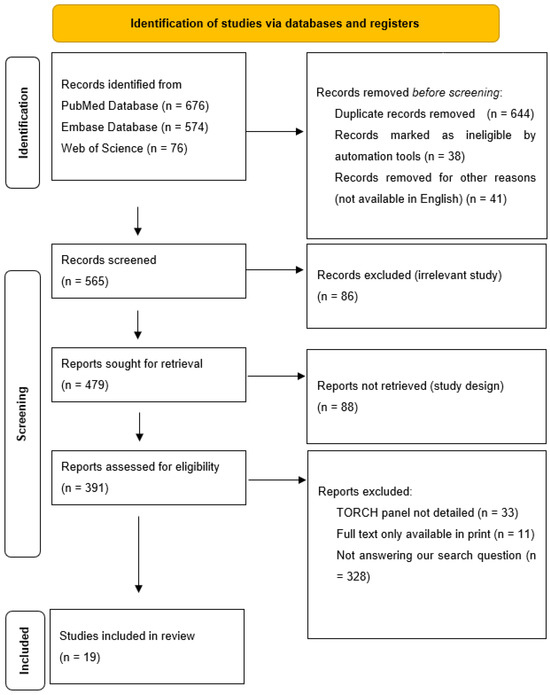
Figure 1.
Elimination process.
The last day of the search was the 20 December 2024. The search and the elimination process was carried out by both authors. The data collection was conducted by Virag Bartek. The elimination process was performed using Rayyan (https://www.rayyan.ai/, last accessed on 5 January 2025).
The data were collected and analyzed in Excel (v. 16.89.1 (24091630)). The collected data were as follows: number of patients, author, publication year and place, ultrasound abnormalities prenatally (hydrops, hyperechogenic bowels, intracranial calcification, microcephaly, ventriculomegaly, hepatosplenomegaly, placentomegaly, oligohydramnios, IUGR/SGR, other), MRI abnormalities (pseudocyst, periventricular cyst, subependymal cyst, hypoplastic cerebellum, other), TOP (termination of pregnancy)/perinatal exitus, postnatal complication—deafness, neurological postnatal complication, postnatal imaging results, and long-term follow up.
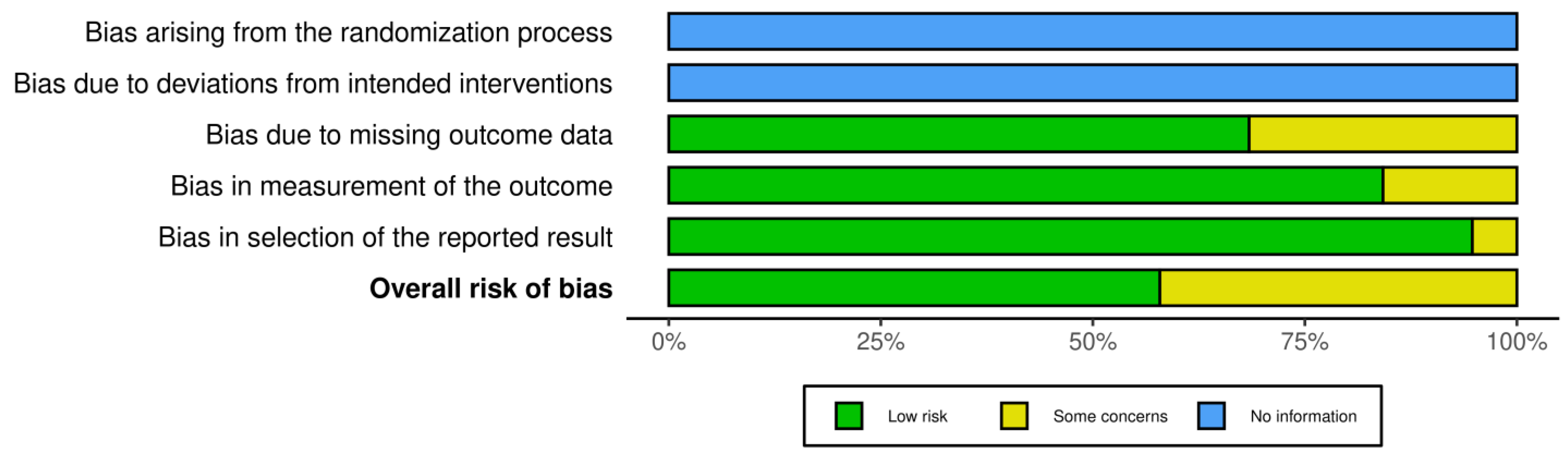
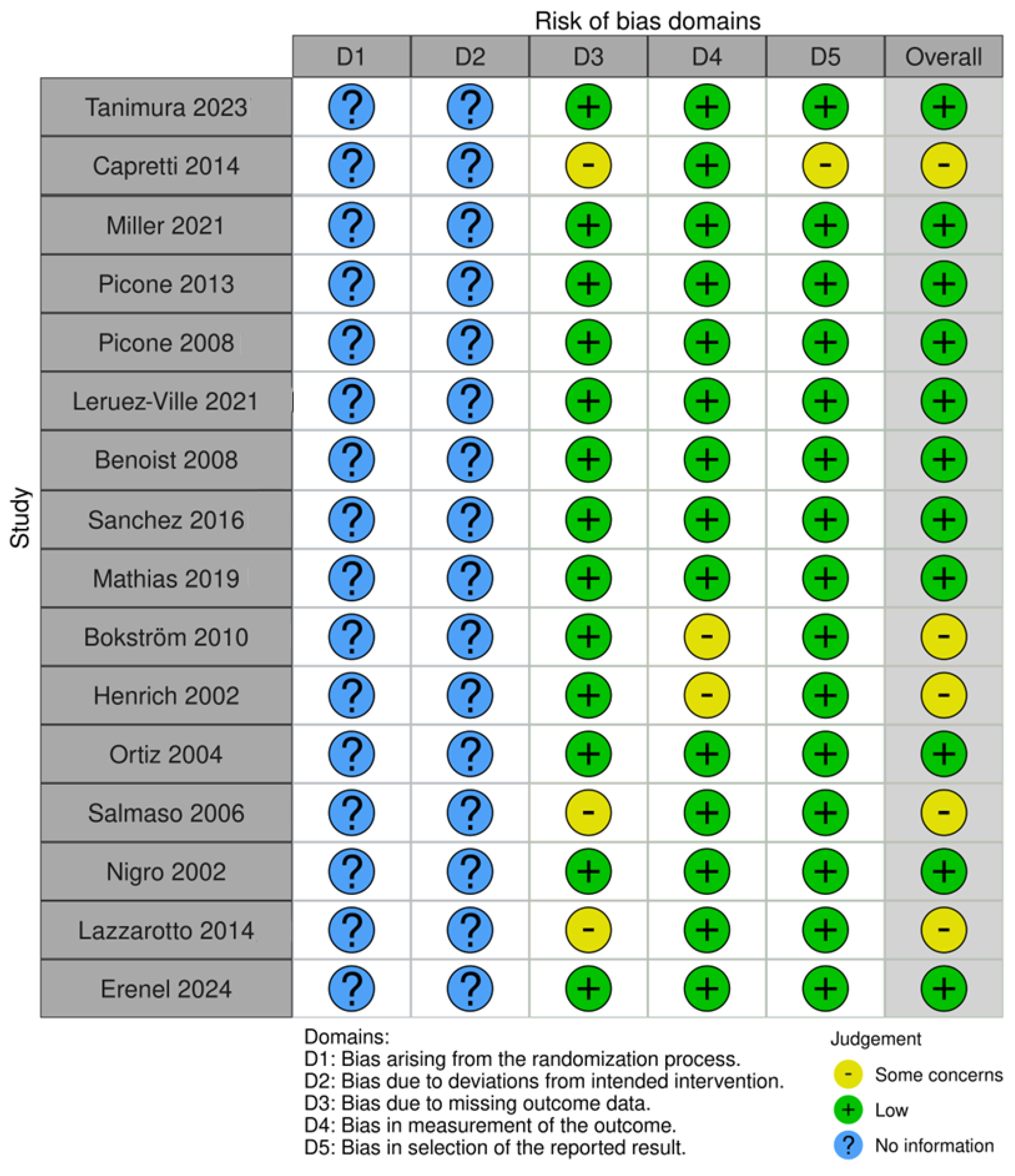
Risk bias was carried out via robvis (RobVis 2008, McGuinness, LA, Higgins, JPT. Risk-of-bias VISualization (robvis)), accessed on 14 April 2025. There was no bias arising from the randomization or the deviations of intended interventions, as there was not any randomization in our systematic review. Also, we selected studies with the same design (mostly individual case reports). The risk bias was performed by both authors. The risk was considered low if there was a detailed prenatal examination report with ultrasound and MRI report, and when the follow-up was available. Also, if there was a case of perinatal exitus, the autopsy report was available. When the ultrasound report or the MRI report was not fully available, or the follow-up was available only for the perinatal period, and in cases where the autopsy report was not fully available, and the authors only described the findings, the study was categorized as “some concerns”. Cases where the ultrasound or MRI report was completely missing, and neither a follow up nor an autopsy had been conducted, were excluded from the study. The risk bias is described in Figure 2.
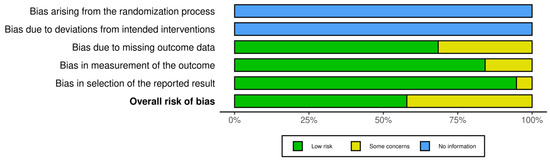
Figure 2.
Risk bias for the statistical analysis due to the low number of data we used for Fisher’s exact test.
3. Results
A total of 237 cases from 19 studies were included. Cases were included where prenatally positive amniocentesis PCR (polymerase chain reaction) for CMV was performed, or autopsy confirmed the CMV diagnosis.
Of the 237 cases, 64 resulted in termination of pregnancy or perinatal death.
The most common ultrasound abnormalities were tabulated and their association with outcome was examined (Table 1). The ultrasound abnormalities are summarized in Table 2.

Table 1.
Summary of data obtained during the literature review. For ease of interpretation, cases where there was only MRI abnormality but no ultrasound abnormality are not presented separately.

Table 2.
Ultrasound abnormalities summarized.
The risk bias of all included studies is presented in Figure 3.
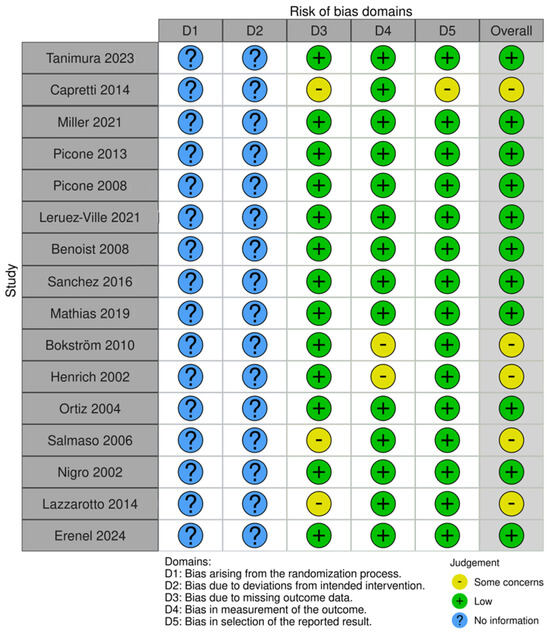
Figure 3.
Risk bias of all studies with ultrasound anomalies.
Among other abnormalities, cardiomegaly was described in two cases, hyperechogenic kidneys in two cases, and corpus callosum abnormalities in one case.
Among the MRI abnormalities, we found the following (Table 3):

Table 3.
MRI abnormalities.
We examined ENT (ear, nose, and throat) complications. We considered these to be mild if the hearing loss was unilateral or bilateral, and mild, and severe if the hearing loss was bilateral or unilateral, and severe or accompanied by complete deafness (Table 4). All data are given on 173 cases, as 64 cases ended in TOP or perinatal death.

Table 4.
Complications with hearing loss.
We used Fisher’s exact test to examine whether there was a correlation between prenatal ultrasound abnormalities and deafness. We examined the five most common prenatal abnormalities. Since we did not obtain significant results even in the case of hydrops, we did not examine the others due to the low number of cases. We obtained a significant result for ventriculomegaly, meaning that there was a correlation between prenatally diagnosed ventriculomegaly and deafness (Table 5).

Table 5.
Association between prenatal ultrasound signals and postnatal hearing loss.
We examined whether there was a correlation between the severity of ventriculomegaly and the severity of deafness (Fisher’s exact test), but this could not be confirmed (0.11076711). The severity was based on the following: mild, the ventricles were between 10 mm and 12 mm; moderate, the ventricles were between 13 mm and 15 mm; and severe, the ventricles were more than 15 mm wide. As we did not get a significant result, we did not carry out any more tests or investigations related to severity.
We investigated whether there was a correlation between the prenatally indicated abnormality and later neurological complications (Table 6). We found a small number of cases with postnatal neurological complications and examined these together.

Table 6.
Summary of neurological complications.
Due to the small number of cases, we examined the relationship between ventriculomegaly and subependymal cyst, where we obtained a significant result with Fisher’s exact test for the subependymal cyst (Table 7). However, due to the small number of cases, the clinical relevance of this is questionable.

Table 7.
Association between prenatal ultrasound signals and postnatal neurological complications.
4. Discussion
Cytomegalovirus infection is a worldwide problem in prenatal screening and care. With the spread of imaging methods, adequate prenatal screening and treatment can be performed, thus preventing the development of later complications.
As can be seen from the literature review, although prenatal cytomegalovirus infection imposes a great burden on both healthcare and pregnant mothers, few of the studies dealing with it are clinically relevant. This is because prospective studies are difficult to design and blinded studies are ethically unfeasible. Therefore, our current knowledge is mainly limited to retrospective and descriptive studies. Our review aimed to summarize the available scientific papers from the past twenty years, specifically focusing on long-term outcomes. Since prenatal imaging signs of cytomegalovirus have been well documented, we aimed to determine what findings and relationships could be discovered regarding prognosis.
Tanimura et al. in 2017 prospectively determined that specific ultrasound signs were independently predictive factors (odds ratio [OR], 31.9; 95% confidence interval [CI], 8.5–120.3; p < 0.001) of congenital cytomegalovirus infection in IgM-positive pregnant women [35]. Because ultrasound is highly operator- and device-dependent, it is difficult to determine the exact specificity and sensitivity. Previous studies have shown that diagnostic sensitivity is much lower for screening ultrasounds than for targeted testing after a confirmed infection [20,36]. This may be because CMV infection has nonspecific ultrasound signs, and in cases of confirmed infection, it is easier for the sonographer to look for common ultrasound abnormalities [24].
Among the specific (intracranial) and nonspecific (extracranial) ultrasound signs, the most common in our study were ventriculomegaly (n = 51), IUGR/SGR (n = 47), and hyperechogenic intestines (n = 39), which is consistent with the literature data [7,8].
The most common complications were hearing loss and neurological complications. In the case of hearing loss, the degree of hearing loss was mild in 42 cases, while in 19 cases the degree of hearing loss was severe. Among the neurological complications, epilepsy developed in 4 cases, cerebral palsy in 6 cases, motor delay in 8 cases, cognitive delay in 8 cases, and verbal delay in 15 cases. ADHD (attention-deficit/hyperactivity disorder) was described in four cases.
Prenatal CMV infection is the leading enviromental cause of sensorineural hearing loss in the United States. Although the association between prenatal CMV infection and postnatal hearing loss has been known for more than fifty years, it is still not possible to assess prenatally whether, and to what extent, the affected child will be affected after birth [37]. Craeghs et al. investigated whether there was a link between postnatal neurological abnormalities confirmed by neonatal imaging, and postnatal hearing loss. They included 411 patients, 40% of whom were symptomatic at birth. They found a significant association between newborns with ultrasound and/or MRI abnormalities and symptomatic hearing loss, but not between late-onset hearing loss and imaging abnormalities [38]. Corazzi and colleagues found a correlation between postnatal brain MRI abnormalities and sensorineural hearing loss [39]. There is little literature available on the relationship between prenatal imaging abnormalities and hearing loss. Lipitz et al. found no significant association between prenatal imaging abnormalities and hearing loss (p = 0.084, 0.109, and 0.176, respectively) [40].
Attention-deficit/hyperactivity disorder (ADHD) is a heterogeneous disorder with both predetermined, genetic and environmental causes. Its exact pathomechanism is unknown. It has shown an increasing trend in recent years, and is therefore—understandably—an area of active research [41]. In their meta-analysis, Chun-yan Zhu et al. found that prenatal infection slightly increased the risk of ADHD (OR, 1.25; 95% CI, 1.09, 1.44; p < 0.0001; I2 = 92.9%, p < 0.0001), but the results were not significant in studies examining siblings, so prenatal infection can only partially explain the increased risk. Chun-yan Zhu et al. did not specifically examine cytomegalovirus [42]. Due to the small number of cases (four cases), we were unable to significantly examine the relationship between ADHD and prenatal imaging studies. Nis Borbye-Lorenzen and colleagues found that high anti-CMV levels increased the risk of later ADHD development [43].
The relationship between prenatal ultrasound signals and postnatal outcome is currently unclear. In our study, we found a significant association between ventriculomegaly and deafness, but the sample size was small, so we could only perform statistical testing with Fisher’s exact test, which did not show a strong statistical association. Letouzey et al., in their 2017 retrospective study, found no association between neurological outcome and the degree of isolated ventriculomegaly (n = 21) [44]. In their 2013 cohort study (n = 23) Alacron et al. examined several prenatal factors and found that combining prenatal imaging studies, CSF (cerebrospinal fluid) β2-m levels, and head circumference could identify a prognostic factor for neurological outcome [45].
As can be seen from the above, doing prenatal studies is very difficult, both from an ethical and from a research design perspective. As can be seen from the above descriptions, the case numbers were generally very low, and there was great heterogeneity in the studies described regarding which prenatal factors were analyzed (imaging studies, antibodies, prenatal treatment) and what type and length of follow-up was performed. Interruption of follow-up is also common since, to achieve significant results, follow-up should be carried out at least until school age. Nevertheless, this field is worth addressing. In particular, due to the heterogeneity of the sample, and the examination of several different factors, it would be worthwhile to develop a scoring system based on a large, multicenter, prospective study, if possible, but at least on a retrospective study with long follow-up, which summarizes all the factors described above. Based on these factors, an estimate of the expected prognosis could be given in the prenatal period. The current literature on this topic also supports the recommendation for a multimodal, multifactorial prognostic factor [23,46]. This is not only important for the pregnant woman to make adequate decisions but also provides guidance for the team providing postnatal care as to what professionals need to be involved and what improvements can be introduced. It would also give a more accurate screening method.
5. Conclusions
In the case of prenatally diagnosed CMV infection, the prognosis can be estimated based on ultrasound signs. Since it is a phenotypically diverse disease, it would be worthwhile to develop a score system for risk estimation and appropriate clinical application by examining several different factors, possibly with a prospective study.
Certain ultrasound signs, especially in combination, suggest the diagnosis of prenatal TORCH infection, especially CMV, in which case it may be worth recommending amniocentesis to the pregnant woman even in the case of doubtful serological findings. If the ultrasound abnormalities are unclear, prenatal MRI can also be recommended as an additional diagnosis, as it provides a much clearer picture of the extent of the disease.
There is probably a correlation between abnormalities affecting the nervous system (ventriculomegaly and subependymal cyst) and prognosis, which we could confirm, but only with weak statistical tests due to the small number of cases. We plan to investigate this further with a larger number of cases.
Proper prenatal diagnosis not only contributes to adequate prenatal treatment, but also it also contributes to the pregnant woman being able to make the right decision regarding the outcome of her pregnancy. If delivery occurs, proper diagnosis and risk assessment contribute to the newborn being treated by an interdisciplinary team experienced in CMV infection. Early treatment is essential for a positive outcome.
Author Contributions
Conceptualization and methodology, V.B.; validation, A.B.; data curation and writing—original draft preparation, V.B.; writing—review and editing, A.B.; visualization, V.B.; supervision, A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Our work complies with the principles of the Declaration of Helsinki, and was approved by the Ethics Committee of the institution (Scientific Research Ethics Committee permission number: SE-TUKEB 231), ethics approval date: 31 August 2018.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available upon request.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| CMV | Cytomegalovirus |
| MRI | Magnetic resonance imaging |
| CT | Computer tomography |
| TOP | Termination of pregnancy |
| IUGR | Intrauterine growth restriction/retardation |
| SGR | Small for gestational age |
References
- Pontes, K.F.M.; Nardozza, L.M.M.; Peixoto, A.B.; Werner, H.; Tonni, G.; Granese, R.; Araujo Júnior, E. Cytomegalovirus and Pregnancy: A Narrative Review. J. Clin. Med. 2024, 13, 640. [Google Scholar] [CrossRef] [PubMed]
- Yinon, Y.; Farine, D.; Yudin, M.H. No. 240-Cytomegalovirus Infection in Pregnancy. J. Obstet. Gynaecol. Can. 2018, 40, e134–e141. [Google Scholar] [CrossRef] [PubMed]
- Ssentongo, P.; Hehnly, C.; Birungi, P.; Roach, M.A.; Spady, J.; Fronterre, C.; Wang, M.; Murray-Kolb, L.E.; Al-Shaar, L.; Chinchilli, V.M.; et al. Congenital Cytomegalovirus Infection Burden and Epidemiologic Risk Factors in Countries with Universal Screening: A Systematic Review and Meta-analysis. JAMA Netw. Open. 2021, 4, e2120736. [Google Scholar] [CrossRef] [PubMed]
- Grosse, S.D.; Fleming, P.; Pesch, M.H.; Rawlinson, W.D. Estimates of congenital cytomegalovirus-attributable infant mortality in high-income countries: A review. Rev. Med. Virol. 2024, 34, e2502. [Google Scholar] [CrossRef]
- Rahbar, A.; Söderberg-Nauclér, C. Human cytomegalovirus infection of endothelial cells triggers platelet adhesion and aggregation. J. Virol. 2005, 79, 2211–2220. [Google Scholar] [CrossRef]
- Capretti, M.G.; Marsico, C.; Guidelli Guidi, S.; Ciardella, A.; Simonazzi, G.; Galletti, S.; Gabrielli, L.; Lazzarotto, T.; Faldella, G. Neonatal and long-term ophthalmological findings in infants with symptomatic and asymptomatic congenital cytomegalovirus infection. J. Clin. Virol. 2017, 97, 59–63. [Google Scholar] [CrossRef]
- Benoist, G.; Leruez-Ville, M.; Magny, J.F.; Jacquemard, F.; Salomon, L.J.; Ville, Y. Management of pregnancies with confirmed cytomegalovirus fetal infection. Fetal. Diagn. Ther. 2013, 33, 203–214. [Google Scholar] [CrossRef]
- Malinger, G.; Lev, D.; Lerman-Sagie, T. Imaging of fetal cytomegalovirus infection. Fetal. Diagn. Ther. 2011, 29, 117–126. [Google Scholar] [CrossRef]
- Doneda, C.; Scelsa, B.; Introvini, P.; Zavattoni, M.; Orcesi, S.; Lombardi, G.; Pugni, L.; Fumagalli, M.; Rustico, M.; Vola, E.; et al. Congenital Cytomegalovirus Infection with Isolated "Minor" Lesions at Fetal Magnetic Resonance Imaging: Long-Term Neurological Outcome. Pediatr. Neurol. 2024, 155, 104–113. [Google Scholar] [CrossRef]
- Kyriakopoulou, A.; Papaevangelou, V.; Argyropoulou, M.; Papathanasiou, M.; Xydis, V.; Giorgi, M.; Ntorkou, A.; Chlapoutaki, C.; Alexopoulou, E. Fetal brain imaging provides valuable information in cCMV infected infants. J. Matern. Fetal. Neonatal Med. 2023, 36, 2220564. [Google Scholar] [CrossRef]
- Miller, T.E.; Weisz, B.; Yinon, Y.; Weissbach, T.; De Castro, H.; Avnet, H.; Hoffman, C.; Katorza, E.; Lipitz, S. Congenital Cytomegalovirus Infection Following Second and Third Trimester Maternal Infection Is Associated with Mild Childhood Adverse Outcome Not Predicted by Prenatal Imaging. J. Pediatr. Infect. Dis. Soc. 2021, 10, 562–568. [Google Scholar] [CrossRef] [PubMed]
- Capretti, M.G.; Lanari, M.; Tani, G.; Ancora, G.; Sciutti, R.; Marsico, C.; Lazzarotto, T.; Gabrielli, L.; Guerra, B.; Corvaglia, L.; et al. Role of cerebral ultrasound and magnetic resonance imaging in newborns with congenital cytomegalovirus infection. Brain Dev. 2014, 36, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Lipitz, S.; Yinon, Y.; Malinger, G.; Yagel, S.; Levit, L.; Hoffman, C.; Rantzer, R.; Weisz, B. Risk of cytomegalovirus-associated sequelae in relation to time of infection and findings on prenatal imaging. Ultrasound Obstet. Gynecol. 2013, 41, 508–514. [Google Scholar] [CrossRef]
- Faure-Bardon, V.; Millischer, A.E.; Deloison, B.; Sonigo, P.; Grévent, D.; Salomon, L.; Stirnemann, J.; Nicloux, M.; Magny, J.F.; Leruez-Ville, M.; et al. Refining the prognosis of fetuses infected with Cytomegalovirus in the first trimester of pregnancy by serial prenatal assessment: A single-centre retrospective study. Bjog 2020, 127, 355–362. [Google Scholar] [CrossRef] [PubMed]
- Faure-Bardon, V.; Magny, J.F.; Parodi, M.; Couderc, S.; Garcia, P.; Maillotte, A.M.; Benard, M.; Pinquier, D.; Astruc, D.; Patural, H.; et al. Sequelae of Congenital Cytomegalovirus Following Maternal Primary Infections Are Limited to Those Acquired in the First Trimester of Pregnancy. Clin. Infect. Dis. 2019, 69, 1526–1532. [Google Scholar] [CrossRef]
- Uematsu, M.; Haginoya, K.; Kikuchi, A.; Hino-Fukuyo, N.; Ishii, K.; Shiihara, T.; Kato, M.; Kamei, A.; Kure, S. Asymptomatic congenital cytomegalovirus infection with neurological sequelae: A retrospective study using umbilical cord. Brain Dev. 2016, 38, 819–826. [Google Scholar] [CrossRef]
- Boppana, S.B.; Fowler, K.B.; Vaid, Y.; Hedlund, G.; Stagno, S.; Britt, W.J.; Pass, R.F. Neuroradiographic findings in the newborn period and long-term outcome in children with symptomatic congenital cytomegalovirus infection. Pediatrics 1997, 99, 409–414. [Google Scholar] [CrossRef]
- Inaba, Y.; Motobayashi, M.; Nishioka, M.; Kaneko, T.; Yamauchi, S.; Kawasaki, Y.; Shiba, N.; Nishio, S.Y.; Moteki, H.; Miyagawa, M.; et al. Correlation Between White Matter Lesions and Intelligence Quotient in Patients with Congenital Cytomegalovirus Infection. Pediatr. Neurol. 2016, 55, 52–57. [Google Scholar] [CrossRef]
- Suganuma, E.; Oka, A.; Sakata, H.; Adachi, N.; Asanuma, S.; Oguma, E.; Yamaguchi, A.; Furuichi, M.; Uejima, Y.; Sato, S.; et al. 10-year follow-up of congenital cytomegalovirus infection complicated with severe neurological findings in infancy: A case report. BMC Pediatr. 2018, 18, 369. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Tanimura, K.; Uchida, A.; Uenaka, M.; Imafuku, H.; Tairaku, S.; Hashimura, H.; Ueno, Y.; Kido, T.; Fujioka, K. Fetal Ultrasound and Magnetic Resonance Imaging Abnormalities in Congenital Cytomegalovirus Infection Associated with and without Fetal Growth Restriction. Diagnostics 2023, 13, 306. [Google Scholar] [CrossRef] [PubMed]
- Picone, O.; Vauloup-Fellous, C.; Cordier, A.G.; Guitton, S.; Senat, M.V.; Fuchs, F.; Ayoubi, J.M.; Grangeot Keros, L.; Benachi, A. A series of 238 cytomegalovirus primary infections during pregnancy: Description and outcome. Prenat. Diagn. 2013, 33, 751–758. [Google Scholar] [CrossRef] [PubMed]
- Picone, O.; Simon, I.; Benachi, A.; Brunelle, F.; Sonigo, P. Comparison between ultrasound and magnetic resonance imaging in assessment of fetal cytomegalovirus infection. Prenat. Diagn. 2008, 28, 753–758. [Google Scholar] [CrossRef] [PubMed]
- Leruez-Ville, M.; Ren, S.; Magny, J.F.; Jacquemard, F.; Couderc, S.; Garcia, P.; Maillotte, A.M.; Benard, M.; Pinquier, D.; Minodier, P.; et al. Accuracy of prenatal ultrasound screening to identify fetuses infected by cytomegalovirus which will develop severe long-term sequelae. Ultrasound Obs. Gynecol. 2021, 57, 97–104. [Google Scholar] [CrossRef]
- Benoist, G.; Salomon, L.J.; Jacquemard, F.; Daffos, F.; Ville, Y. The prognostic value of ultrasound abnormalities and biological parameters in blood of fetuses infected with cytomegalovirus. Bjog 2008, 115, 823–829. [Google Scholar] [CrossRef]
- Sanchez, T.R.; Datlow, M.D.; Nidecker, A.E. Diffuse periventricular calcification and brain atrophy: A case of neonatal central nervous system cytomegalovirus infection. Neuroradiol. J. 2016, 29, 314–316. [Google Scholar] [CrossRef]
- Mathias, C.R.; Joung, S.J.S. Diagnostic challenges in congenital cytomegalovirus infection in pregnancy: A case report. Case Rep. Women’s Health 2019, 22, e00119. [Google Scholar] [CrossRef]
- Bokström, H.; Holst, R.-M.; Mattsson, L.Å.; Fyhr, I.-M. Congenital cytomegalovirus infection presenting with echogenic bowel and oligohydramnios. Acta Obstet. Gynecol. Scandi. 2007, 86, 248–250. [Google Scholar] [CrossRef]
- Henrich, W.; Meckies, J.; Dudenhausen, J.W.; Vogel, M.; Enders, G. Recurrent cytomegalovirus infection during pregnancy: Ultrasonographic diagnosis and fetal outcome. Ultrasound Obstet. Gynecol. 2002, 19, 608–611. [Google Scholar] [CrossRef]
- Ortiz, J.U.; Ostermayer, E.; Fischer, T.; Kuschel, B.; Rudelius, M.; Schneider, K.T. Severe fetal cytomegalovirus infection associated with cerebellar hemorrhage. Ultrasound Obstet. Gynecol. 2004, 23, 402–406. [Google Scholar] [CrossRef]
- Salmaso, R.; De Santis, M.; Franco, R.; Paternoster, D.; Carollo, C.; Suma, V.; Righini, A.; Manara, R. Maternal Seropositivity for Cytomegalovirus with Severe Foetal Hydrops: Foetal Analysis Focusing on Cerebral Changes. Riv. Neuroradiol. 2006, 19, 176–179. [Google Scholar] [CrossRef]
- Nigro, G.; La Torre, R.; Sali, E.; Auteri, M.; Mazzocco, M.; Maranghi, L.; Cosmi, E. Intraventricular haemorrhage in a fetus with cerebral cytomegalovirus infection. Prenat. Diagn. 2002, 22, 558–561. [Google Scholar] [CrossRef] [PubMed]
- Lazzarotto, T.; Gabrielli, L.; Guerra, B.; Cervi, F.; Piccirilli, G.; Simonazzi, G.; Chiereghin, A.; Bellini, F.; Landini, M.P. Diagnosis and prognosis of congenital CMV infection: A case report and review of the literature. Scand. J. Clin. Lab. Investig. Suppl. 2014, 244, 34–40; discussion 39. [Google Scholar] [CrossRef] [PubMed]
- Erenel, H.; Tuna, G.; Alpay, V.; Polat, İ. Fetal Cytomegalovirus Infection in the Absence of Maternal Cytomegalovirus-IgM Seropositivity. Reprod. Sci. 2024, 31, 1533–1540. [Google Scholar] [CrossRef]
- Tanimura, K.; Tairaku, S.; Ebina, Y.; Morioka, I.; Nagamata, S.; Deguchi, K.; Morizane, M.; Deguchi, M.; Minematsu, T.; Yamada, H. Prediction of Congenital Cytomegalovirus Infection in High-Risk Pregnant Women. Clin. Infect. Dis. 2017, 64, 159–165. [Google Scholar] [CrossRef]
- Leyder, M.; Vorsselmans, A.; Done, E.; Van Berkel, K.; Faron, G.; Foulon, I.; Naessens, A.; Jansen, A.; Foulon, W.; and Gucciardo, L. Primary maternal cytomegalovirus infections: Accuracy of etal ultrasound for predicting sequelae in offspring. Am. J. Obstet. Gynecol. 2016, 215, 638.e1–638.e8. [Google Scholar] [CrossRef]
- Fowler, K.B. Congenital cytomegalovirus infection: Audiologic outcome. Clin. Infect. Dis. 2013, 57 (Suppl. 4), S182–S184. [Google Scholar] [CrossRef]
- Craeghs, L.; Goderis, J.; Acke, F.; Keymeulen, A.; Smets, K.; Van Hoecke, H.; De Leenheer, E.; Boudewyns, A.; Desloovere, C.; Kuhweide, R.; et al. Congenital CMV-Associated Hearing Loss: Can Brain Imaging Predict Hearing Outcome? Ear Hear. 2021, 42, 373–380. [Google Scholar] [CrossRef]
- Corazzi, V.; Musumano, L.B.; Migliorelli, A.; Negossi, L.; Bianchini, C.; Stomeo, F.; Pelucchi, S.; Ciorba, A. Predictive Factors for Hearing Loss in Congenital Cytomegalovirus Infection. Audiol. Res. 2025, 15, 2. [Google Scholar] [CrossRef]
- Lipitz, S.; Elkan Miller, T.; Yinon, Y.; Weissbach, T.; De-Castro, H.; Hoffman, C.; Katorza, E.; Weisz, B. Revisiting short- and long-term outcome after fetal first-trimester primary cytomegalovirus infection in relation to prenatal imaging findings. Ultrasound Obstet. Gynecol. 2020, 56, 572–578. [Google Scholar] [CrossRef]
- Olusanya, B.O.; Davis, A.C.; Wertlieb, D.; Boo, N.-Y.; Nair, M.K.C.; Halpern, R.; Kuper, H.; Breinbauer, C.; de Vries, P.J.; Gladstone, M.; et al. Developmental disabilities among children younger than 5 years in 195 countries and territories, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Glob. Health 2018, 6, e1100–e1121. [Google Scholar] [CrossRef] [PubMed]
- Zhu, C.-y.; Jiang, H.-y.; Sun, J.-j. Maternal infection during pregnancy and the risk of attention-deficit/hyperactivity disorder in the offspring: A systematic review and meta-analysis. Asian J. Psychiatry 2022, 68, 102972. [Google Scholar] [CrossRef] [PubMed]
- Borbye-Lorenzen, N.; Holmgaard, S.; Ottosson, F.; Nudel, R.; Appadurai, V.; Laursen, T.M.; Bækvad-Hansen, M.; Bybjerg-Grauholm, J.; Nordentoft, M.; Børglum, A.D.; et al. High level of immunoglobulin G targeting mycoplasma or cytomegalovirus in the newborn increases risk of ADHD. Brain Behav. Immun 2025, 123, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Letouzey, M.; Chadie, A.; Brasseur-Daudruy, M.; Proust, F.; Verspyck, E.; Boileau, P.; and Marret, S. Severe apparently isolated fetal ventriculomegaly and neurodevelopmental outcome. Prenat. Diagn. 2017, 37, 820–826. [Google Scholar] [CrossRef]
- Alarcon, A.; Martinez-Biarge, M.; Cabañas, F.; Hernanz, A.; Quero, J.; Garcia-Alix, A. Clinical, biochemical, and neuroimaging findings predict long-term neurodevelopmental outcome in symptomatic congenital cytomegalovirus infection. J. Pediatr. 2013, 163, 828–834. [Google Scholar] [CrossRef]
- Van den Eede, E.; De Keersmaecker, B.; Lagrou, K.; Van der Veeken, L.; Vanwinkel, S.; Vangoitsenhoven, M.; Aertsen, M.; De Catte, L. Prevalence and timing of prenatal ultrasound findings in cytomegalovirus-infected pregnancies. Acta Obstet. Gynecol. Scandi. 2025, 104, 302–308. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).