Predictive Value of Maternal HbA1c Levels for Fetal Hypertrophic Cardiomyopathy in Pregestational Diabetic Pregnancies
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Outcomes
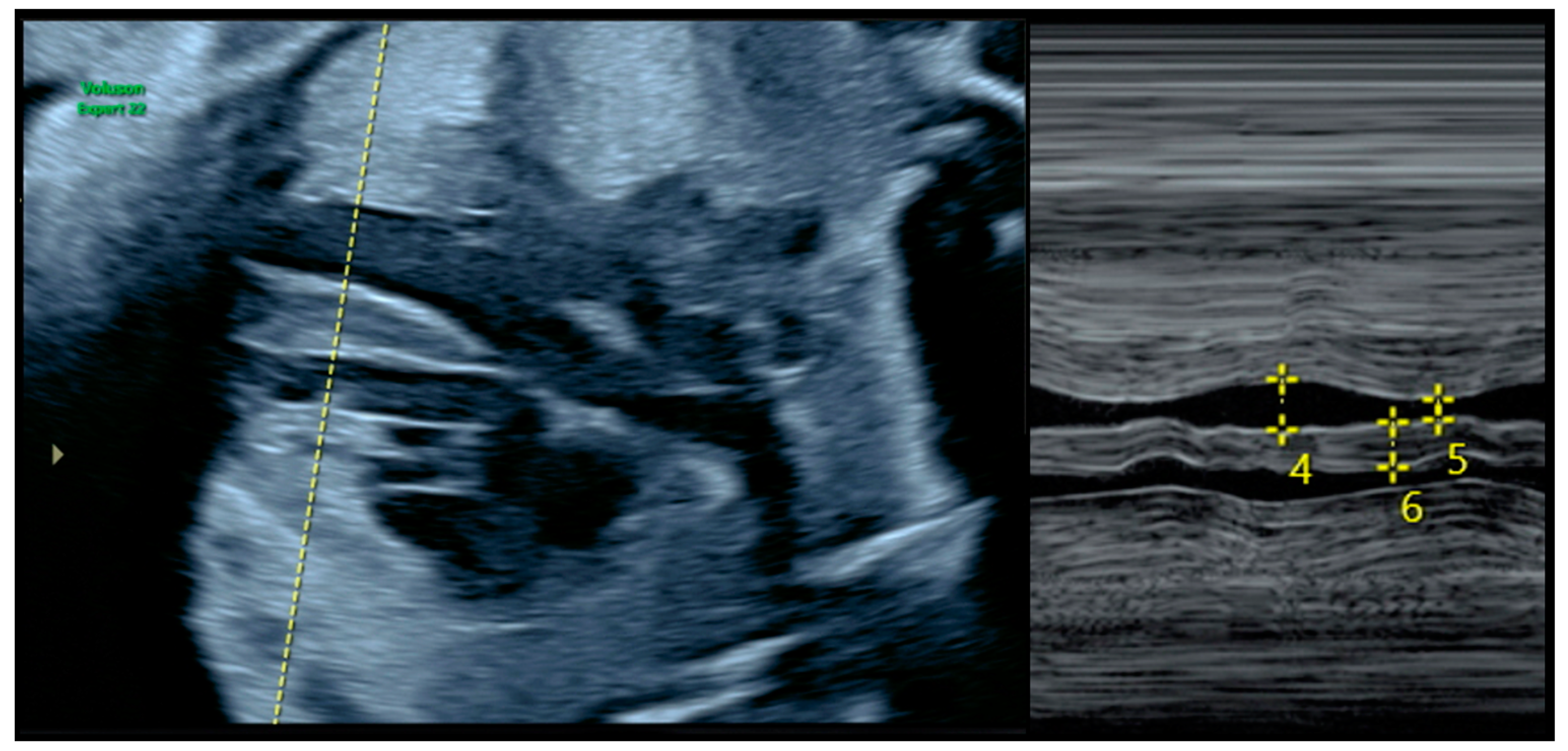
2.2. Diagnosis of FHCM and Echocardiographic Parameters
2.3. Participants and Eligibility Criteria
2.4. Data Compilation and Analysis
3. Results
3.1. Baseline Characteristics
3.2. Maternal Biometric, Analytical, and Diabetological Parameters
3.3. Fetal Biometric Outcomes
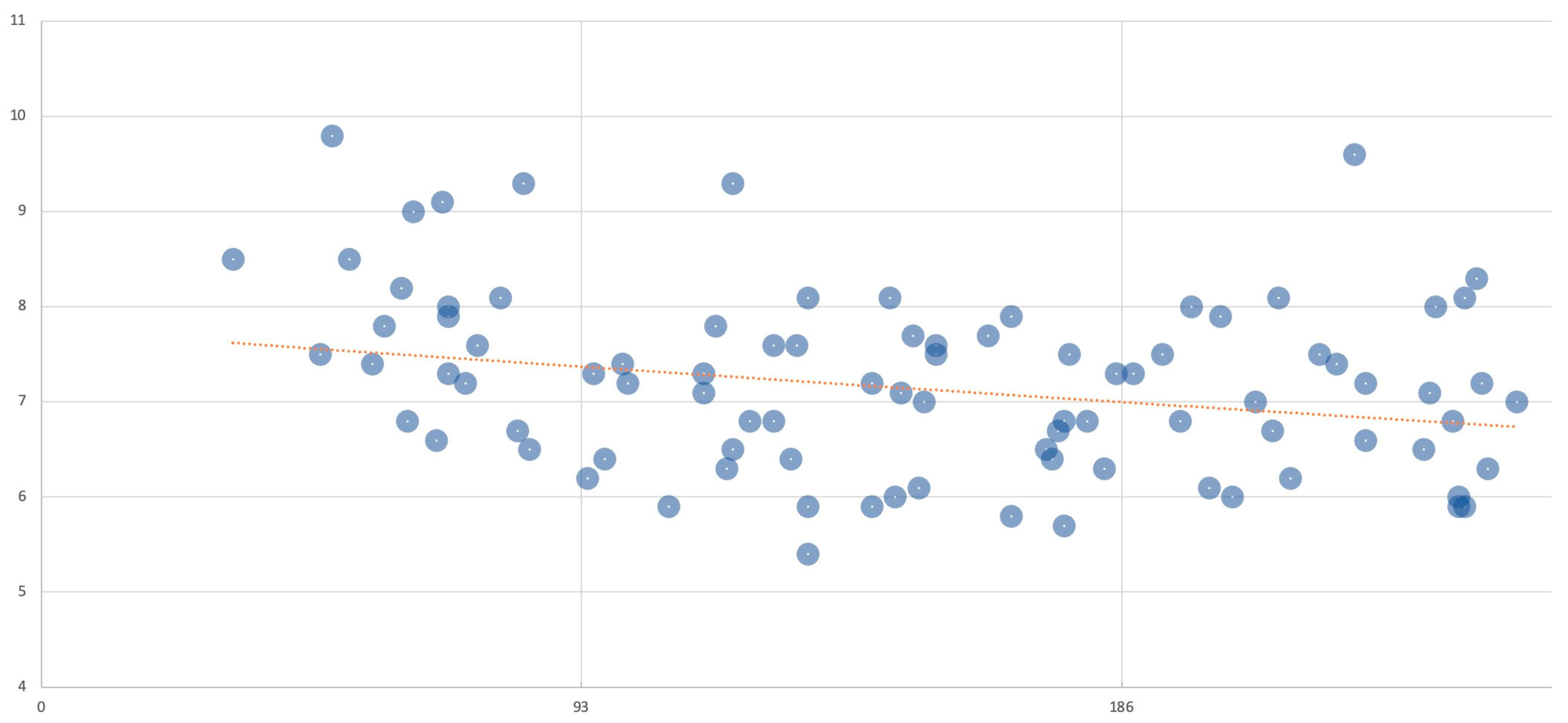
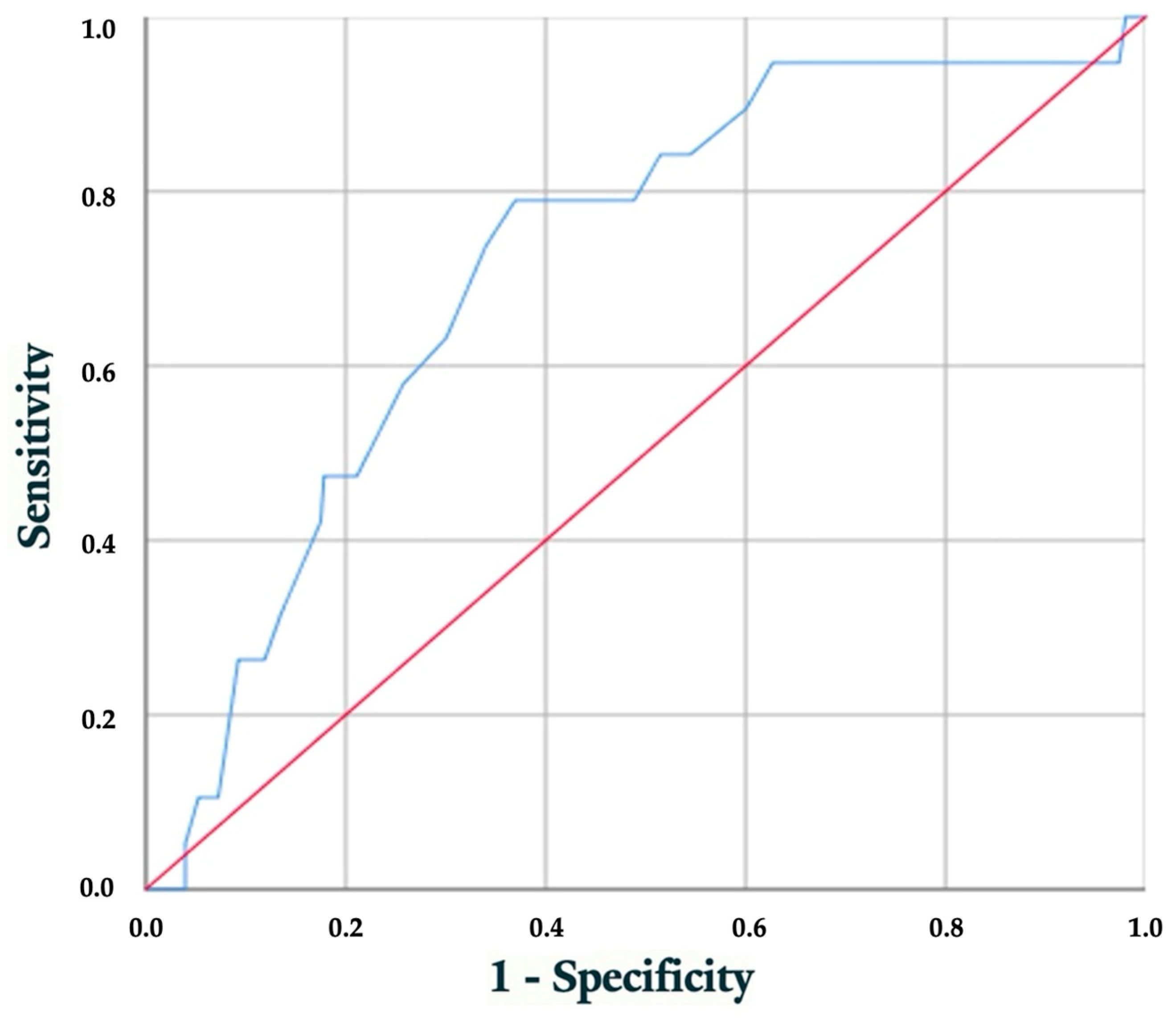
3.4. Performance Evaluation of Early HbA1c Level for Predicting Hypertrophic Cardiomyopathy
4. Discussion
4.1. Main Findings
4.2. Impact of Poor Glycemic Control on Fetal Hypertrophic Cardiomyopathy
4.3. Overall Performance of HbA1c at First Visit for Predicting FHCM
4.4. Strengths and Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Rosenberg, T.J.; Garbers, S.; Lipkind, H.; Chiasson, M.A. Maternal obesity and diabetes as risk factors for adverse pregnancy outcomes: Differences among 4 racial/ethnic groups. Am. J. Public. Health 2005, 95, 1545–1551. [Google Scholar] [CrossRef]
- Shefali, A.K.; Kavitha, M.; Deepa, R.; Mohan, V. Pregnancy outcomes in pre-gestational and gestational diabetic women in comparison to non-diabetic women—A prospective study in Asian Indian mothers (CURES-35). J. Assoc. Physicians India 2006, 54, 613–618. [Google Scholar] [PubMed]
- Cormier, C.M.; Martinez, C.A.; Refuerzo, J.S.; Monga, M.; Ramin, S.M.; Saade, G.; Blackwell, S.C. White’s classification of diabetes in pregnancy in the 21st century: Is it still valid? Am. J. Perinatol. 2010, 27, 349–352. [Google Scholar] [CrossRef]
- Wahabi, H.A.; Esmaeil, S.A.; Fayed, A.; Al-Shaikh, G.; Alzeidan, R.A. Pre-existing diabetes mellitus and adverse pregnancy outcomes. BMC Res. Notes 2012, 5, 496. [Google Scholar] [CrossRef] [PubMed]
- Cnattingius, S.; Lindam, A.; Persson, M. Risks of asphyxia-related neonatal complications in offspring of mothers with type 1 or type 2 diabetes: The impact of maternal overweight and obesity. Diabetologia 2017, 60, 1244–1251. [Google Scholar] [CrossRef]
- Mackin, S.T.; Nelson, S.M.; Kerssens, J.J.; Wood, R.; Wild, S.; Colhoun, H.M.; Leese, G.P.; Philip, S.; Lindsay, R.S.; SDRN Epidemiology Group. Diabetes and pregnancy: National trends over a 15 year period. Diabetologia 2018, 61, 1081–1088. [Google Scholar] [CrossRef]
- Stogianni, A.; Lendahls, L.; Landin-Olsson, M.; Thunander, M. Obstetric and perinatal outcomes in pregnancies complicated by diabetes, and control pregnancies, in Kronoberg, Sweden. BMC Pregnancy Childbirth 2019, 19, 15. [Google Scholar] [CrossRef] [PubMed]
- Gabbay-Benziv, R.; Reece, E.A.; Wang, F.; Yang, P. Birth defects in pregestational diabetes: Defect range, glycemic threshold and pathogenesis. World J. Diabetes 2015, 6, 481–488. [Google Scholar] [CrossRef]
- Tinker, S.C.; Gilboa, S.M.; Moore, C.A.; Waller, D.K.; Simeone, R.M.; Kim, S.Y.; Jamieson, D.J.; Botto, L.D.; Reefhuis, J.; National Birth Defects Prevention Study. Specific birth defects in pregnancies of women with diabetes: National Birth Defects Prevention Study, 1997-2011. Am J Obstet Gynecol 2020, 222, 176.e1–176.e11. [Google Scholar] [CrossRef]
- Quaresima, P.; Saccone, G.; Morelli, M.; Interlandi, F.; Votino, C.; Zuccalà, V.; Di Carlo, C.; Zullo, F.; Venturella, R. Stillbirth, potentially preventable cases: An Italian retrospective study. J. Obstet. Gynaecol. 2022, 34, 89–102. [Google Scholar] [CrossRef]
- Engineer, A.; Saiyin, T.; Greco, E.R.; Feng, Q. Say NO to ROS: Their Roles in Embryonic Heart Development and Pathogenesis of Congenital Heart Defects in Maternal Diabetes. Antioxidants 2019, 8, 436. [Google Scholar] [CrossRef]
- Ayerza-Casas, A.; Dios-Javierre, B.; Galve-Pradel, Z.; Jiménez-Montañés, L.; Lerma-Puertas, D.; López-Ramón, M.; Palanca-Arias, D.; Pérez-Pérez, P.; Rite-Gracia, S.; Samper-Villagrasa, P. Hallazgos cardiológicos en hijos de madre con diabetes durante el embarazo tratada con insulina y ecografías prenatales normales [Cardiological findings in children of mothers with diabetes during pregnancy treated with insulin and normal prenatal ultrasounds]. Bol. Pediatr. Arag Rioj Sor. 2014, 44, 57–63. [Google Scholar]
- Elliott, P.; Andersson, B.; Arbustini, E.; Bilinska, Z.; Cecchi, F.; Charron, P.; Dubourg, O.; Kühl, U.; Maisch, B.; McKenna, W.J.; et al. Classification of the cardiomyopathies: A position statement from the European Society Of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2008, 29, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Paauw, N.D.; Stegeman, R.; de Vroede, M.A.M.J.; Termote, J.U.M.; Freund, M.W.; Breur, J.M.P.J. Neonatal cardiac hypertrophy: The role of hyperinsulinism-a review of literature. Eur. J. Pediatr. 2020, 179, 39–50. [Google Scholar] [CrossRef] [PubMed]
- Ornoy, A.; Becker, M.; Weinstein-Fudim, L.; Ergaz, Z. Diabetes during Pregnancy: A Maternal Disease Complicating the Course of Pregnancy with Long-Term Deleterious Effects on the Offspring. A Clinical Review. Int. J. Mol. Sci. 2021, 22, 2965. [Google Scholar] [CrossRef]
- Díaz-Duran, S. Diagnóstico prenatal de miocardiopatía hipertrófica septal en hijos de madres diabéticas a través de estudio ecocardiógráfico fetal y su relación con los valores de hemoglobina glicosilada maternal [Prenatal diagnosis of septal hypertrophic cardiomyopathy in children of diabetic mothers through fetal echocardiographic study and its relationship with maternal glycosylated hemoglobin values]. Rev. Guatem. Cardiol. 2014, 24, 12–14. [Google Scholar]
- Asoglu, M.R.; Gabbay-Benziv, R.; Turan, O.M.; Turan, S. Exposure of the developing heart to diabetic environment and early cardiac assessment: A review. Echocardiography 2018, 35, 244–257. [Google Scholar] [CrossRef]
- Peixoto, A.B.; Bravo-Valenzuela, N.J.M.; Martins, W.P.; Słodki, M.; Mattar, R.; Moron, A.F.; Araujo Júnior, E. Impact of type I and type II maternal diabetes mellitus on fetal cardiac function assessment parameters using spectral and tissue Doppler. Int. J. Cardiovasc. Imaging 2020, 36, 1237–1247. [Google Scholar] [CrossRef]
- Schneider, C.; McCrindle, B.W.; Carvalho, J.S.; Hornberger, L.K.; McCarthy, K.P.; Daubeney, P.E. Development of Z-scores for fetal cardiac dimensions from echocardiography. Ultrasound Obstet. Gynecol. 2005, 26, 599–605. [Google Scholar] [CrossRef]
- Fetal Medicine Barcelona [Internet]. Available online: https://fetalmedicinebarcelona.org/calc/ (accessed on 29 January 2025).
- Depla, A.L.; De Wit, L.; Steenhuis, T.J.; Slieker, M.G.; Voormolen, D.N.; Scheffer, P.G.; De Heus, R.; Van Rijn, B.B.; Bekker, M.N. Effect of maternal diabetes on fetal heart function on echocardiography: Systematic review and meta-analysis. Ultrasound Obstet. Gynecol. 2021, 57, 539–550. [Google Scholar] [CrossRef]
- Patey, O.; Carvalho, J.S.; Thilaganathan, B. Perinatal changes in fetal cardiac geometry and function in diabetic pregnancy at term. Ultrasound Obstet. Gynecol. 2019, 54, 634–642. [Google Scholar] [CrossRef]
- Bayoumy, S.; Habib, M.; Abdelmageed, R. Impact of maternal diabetes and obesity on fetal cardiac functions. Egypt. Heart J. 2020, 72, 46. [Google Scholar] [CrossRef]
- Lin, X.; Yang, P.; Reece, E.A.; Yang, P. Pregestational type 2 diabetes mellitus induces cardiac hypertrophy in the murine embryo through cardiac remodeling and fibrosis. Am. J. Obstet. Gynecol. 2017, 217, 216.e1–216.e13. [Google Scholar] [CrossRef] [PubMed]
- Chimenea, A.; Calderón, A.M.; Antiñolo, G.; Moreno-Reina, E.; García-Díaz, L. Assessing the impact of pregnancy planning on obstetric and perinatal outcomes in women with pregestational diabetes mellitus. Diabetes Res. Clin. Pract. 2024, 209, 111599. [Google Scholar] [CrossRef]
- Russell, N.E.; Foley, M.; Kinsley, B.T.; Firth, R.G.; Coffey, M.; McAuliffe, F.M. Effect of pregestational diabetes mellitus on fetal cardiac function and structure. Am. J. Obstet. Gynecol. 2008, 199, 312.e1–312.e7. [Google Scholar] [CrossRef] [PubMed]
- Topcuoglu, S.; Karatekin, G.; Yavuz, T.; Arman, D.; Kaya, A.; Gursoy, T.; Ovalı, F. The relationship between the oxidative stress and the cardiac hypertrophy in infants of diabetic mothers. Diabetes Res. Clin. Pract. 2015, 109, 104–109. [Google Scholar] [CrossRef]
- Rizzo, G.; Arduini, D.; Romanini, C. Cardiac function in fetuses of type I diabetic mothers. Am. J. Obstet. Gynecol. 1991, 164, 837–843. [Google Scholar] [CrossRef] [PubMed]
- Weiner, Z.; Zloczower, M.; Lerner, A.; Zimmer, E.; Itskovitz-Eldor, J. Cardiac compliance in fetuses of diabetic women. Obstet. Gynecol. 1999, 93, 948–951. [Google Scholar] [CrossRef]
- Jaeggi, E.T.; Fouron, J.C.; Proulx, F. Fetal cardiac performance in uncomplicated and well-controlled maternal type I diabetes. Ultrasound Obstet. Gynecol. 2001, 17, 311–315. [Google Scholar] [CrossRef]
- Wong, M.L.; Wong, W.H.; Cheung, Y.F. Fetal myocardial performance in pregnancies complicated by gestational impaired glucose tolerance. Ultrasound Obstet. Gynecol. 2007, 29, 395–400. [Google Scholar] [CrossRef]
- Garcia-Flores, J.; Jañez, M.; Gonzalez, M.C.; Martinez, N.; Espada, M.; Gonzalez, A. Fetal myocardial morphological and functional changes associated with well-controlled gestational diabetes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2011, 154, 24–26. [Google Scholar] [CrossRef]
- Garg, S.; Sharma, P.; Sharma, D.; Behera, V.; Durairaj, M.; Dhall, A. Use of fetal echocardiography for characterization of fetal cardiac structure in women with normal pregnancies and gestational diabetes mellitus. J. Ultrasound Med. 2014, 33, 1365–1369. [Google Scholar] [CrossRef] [PubMed]
- National Institute for Health and Care Excellence (NICE). Diabetes in Pregnancy: Management from Preconception to the Postnatal Period (NG3); NICE: London, UK, 2015; Available online: https://www.nice.org.uk/guidance/ng3 (accessed on 29 January 2025).
- El-Ganzoury, M.M.; El-Masry, S.A.; El-Farrash, R.A.; Anwar, M.; Abd Ellatife, R.Z. Infants of diabetic mothers: Echocardiographic measurements and cord blood IGF-I and IGFBP-1. Pediatr. Diabetes 2012, 13, 189–196. [Google Scholar] [CrossRef] [PubMed]
- Hernández-Del Río, J.E.; Magaña-Cárdenas, M.T.; Hernández-Flores, M.S. Miocardiopatía hipertrófica en el hijo de madre con diabetes [Hypertrophic cardiomyopathy in the child of a mother with diabetes]. Rev. Medica MD 2013, 4, 152–157. [Google Scholar]
- Pauliks, L.B. The effect of pregestational diabetes on fetal heart function. Expert. Rev. Cardiovasc. Ther. 2015, 13, 67–74. [Google Scholar] [CrossRef]
- Gonzalez, A.B.; Young, L.; Doll, J.A.; Morgan, G.M.; Crawford, S.E.; Plunkett, B.A. Elevated neonatal insulin-like growth factor I is associated with fetal hypertrophic cardiomyopathy in diabetic women. Am. J. Obstet. Gynecol. 2014, 211, 290.e1–290.e7. [Google Scholar] [CrossRef]
| Gestational Week/Time | Clinical Setting | Main Actions |
|---|---|---|
| Preconception Visit | Endocrinology Clinic |
|
| First Visit (confirmation of pregnancy) | Diabetes/Pregnancy Clinic and Endocrinology |
|
| 11.0–13.6 weeks | Obstetrics and Diabetes/Pregnancy Clinic |
|
| 18.0–21.6 weeks | Fetal Medicine and Diabetes/Pregnancy Clinic |
|
| From 28.0 weeks (visit schedule depends on metabolic control: well controlled: weeks 28, 32, 36, 37, 38; poor control: weekly visits) | Diabetes/Pregnancy Clinic and Endocrinology |
|
| Delivery | Hospital |
|
| Baseline Characteristics | Patients Enrolled N = 329 |
|---|---|
| Maternal age, yrs | 40 ± 5.22 |
| Maternal weight at the onset of pregnancy, kg | 72.4 ± 16.35 |
| Height, cm | 162 ± 6.00 |
| Body Mass Index at the onset of pregnancy, kg/m2 | 26.0 ± 5.48 |
| Ethnicity, n (%) | |
| Caucasian | 313 (95.1%) |
| Black | 8 (2.4%) |
| Maghrebi | 7 (2.1%) |
| Asian | 1 (0.3%) |
| Smoker, n (%) | 64 (19.5%) |
| ART, n (%) | 23 (7.0%) |
| Nulliparous, n (%) | 111 (33.7%) |
| Previous cesarean section, n (%) | 85 (25.8%) |
| Type of PGDM, n (%) | |
| Type 1 PGDM | 237 (72.0%) |
| Type 2 PGDM | 90 (27.4%) |
| MODY PGDM | 1 (0.3%) |
| LADA PGDM | 1 (0.3%) |
| Pregestational hypertension, n (%) | 44 (13.4%) |
| FHCM N = 19 | No FHCM N = 310 | p-Value | |
|---|---|---|---|
| Maternal weight at the onset of pregnancy, kg | 70 ± 13.21 | 72 ± 16.26 | 0.526 |
| Maternal weight at the end of pregnancy, kg | 85 ± 1.05 | 84 ± 15.71 | 0.689 |
| Body Mass Index at the onset of pregnancy, kg/m2 | 26.6 ± 5.24 | 27.6 ± 6.33 | 0.615 |
| Body Mass Index at the end of pregnancy, kg/m2 | 32.5 ± 5.48 | 32.4 ± 6.05 | 0.721 |
| Pregestational HbA1c (%) | 8.2 ± 1.00 | 7.3 ± 1.32 | 0.003 |
| HbA1c at first visit (%) | 7.6 ± 0.92 | 6.9 ± 1.12 | 0.001 |
| HbA1c at last visit (%) | 6.9 ± 1.03 | 6.7 ± 0.78 | 0.003 |
| HbA1c increment (%) | −0.74 ± 0.78 | −0.73 ± 1.03 | 0.677 |
| Postgestational HbA1c (%) | 7.5 ± 0.60 | 6.6 ± 1.13 | 0.008 |
| Planned pregnancy | 1 (5.26%) | 84 (27.1%) | 0.035 |
| White’s Classification | 0.602 | ||
| Insulin dosage at first visit (Ud) | 42 ± 15.08 | 42 ± 21.53 | 0.783 |
| Insulin dosage at last visit (Ud) | 69 ± 28.91 | 67 ± 32.17 | 0.425 |
| Insulin increment (Ud) | 29 ± 24.78 | 28 ± 23.97 | 0.961 |
| FHCM N = 19 | No FHCM N = 310 | p-Value | OR (95% CI) | |
|---|---|---|---|---|
| Birth weight, grams | 3893 ± 1006 | 3389 ± 651 | 0.001 | - |
| Weight > 4000 g | 12 (63.2%) | 55 (17.7%) | <0.001 | 9.20 (3.31–25.58) |
| Weight > 4500 g | 6 (31.6%) | 5 (1.6%) | <0.001 | 30.3 (8.10–113.38) |
| Weight percentile | 88 ± 29.51 | 73 ± 31.15 | 0.001 | - |
| Gestational age at birth, weeks | 36.5 ± 2.11 | 37.6 ± 1.66 | 0.001 | - |
| Birth < 37 weeks | 7/18 (38.9%) | 61/307 (19.9%) | 0.054 | 2.57 (0.96–6.89) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chimenea, A.; Calderón, A.M.; Antiñolo, G.; Moreno-Reina, E.; García-Díaz, L. Predictive Value of Maternal HbA1c Levels for Fetal Hypertrophic Cardiomyopathy in Pregestational Diabetic Pregnancies. Children 2025, 12, 312. https://doi.org/10.3390/children12030312
Chimenea A, Calderón AM, Antiñolo G, Moreno-Reina E, García-Díaz L. Predictive Value of Maternal HbA1c Levels for Fetal Hypertrophic Cardiomyopathy in Pregestational Diabetic Pregnancies. Children. 2025; 12(3):312. https://doi.org/10.3390/children12030312
Chicago/Turabian StyleChimenea, Angel, Ana María Calderón, Guillermo Antiñolo, Eduardo Moreno-Reina, and Lutgardo García-Díaz. 2025. "Predictive Value of Maternal HbA1c Levels for Fetal Hypertrophic Cardiomyopathy in Pregestational Diabetic Pregnancies" Children 12, no. 3: 312. https://doi.org/10.3390/children12030312
APA StyleChimenea, A., Calderón, A. M., Antiñolo, G., Moreno-Reina, E., & García-Díaz, L. (2025). Predictive Value of Maternal HbA1c Levels for Fetal Hypertrophic Cardiomyopathy in Pregestational Diabetic Pregnancies. Children, 12(3), 312. https://doi.org/10.3390/children12030312






