Autism Spectrum Disorder, Oral Implications, and Oral Microbiota
Abstract
1. Introduction
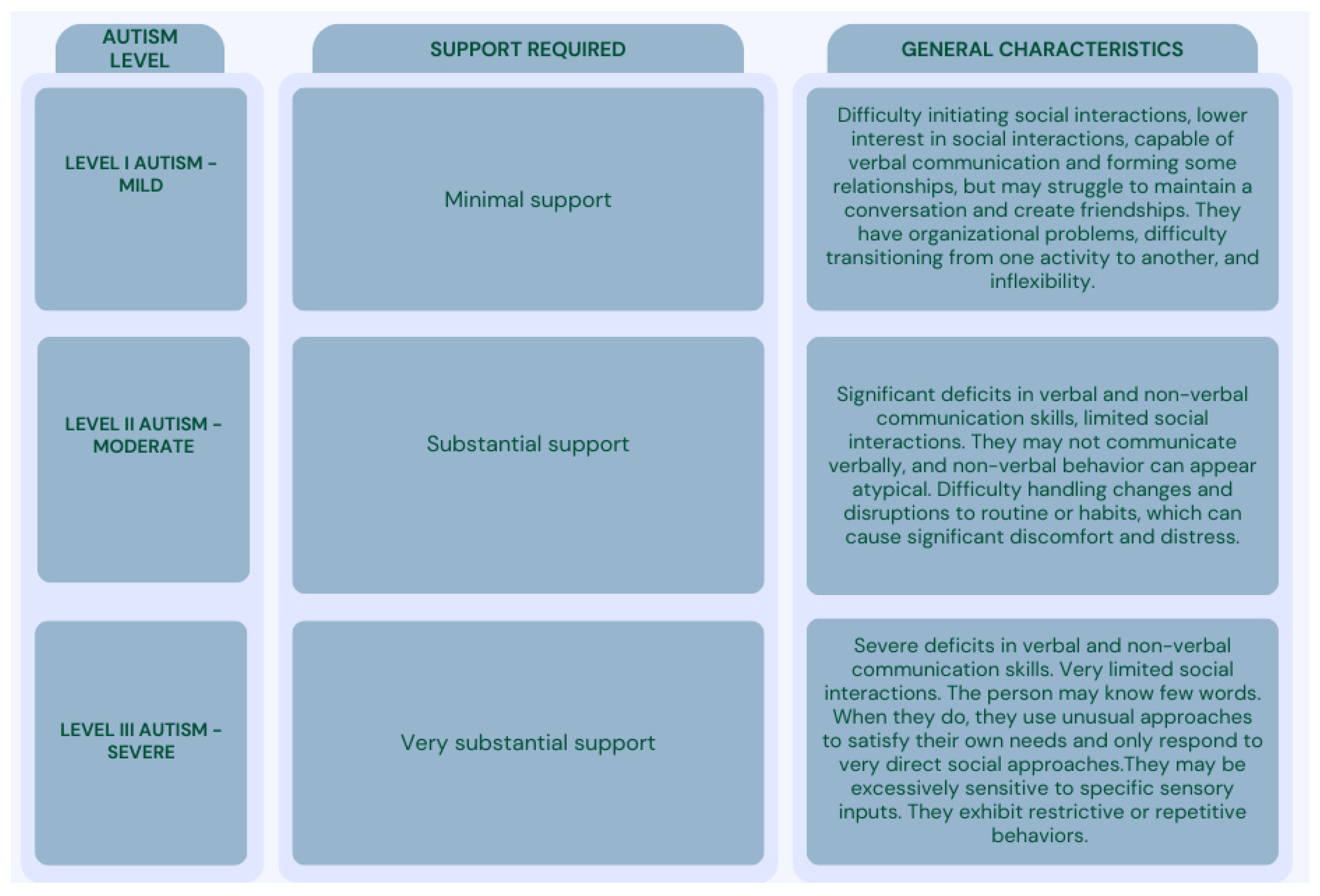
1.1. Autism Spectrum Disorder (ASD) and Associated Characteristics
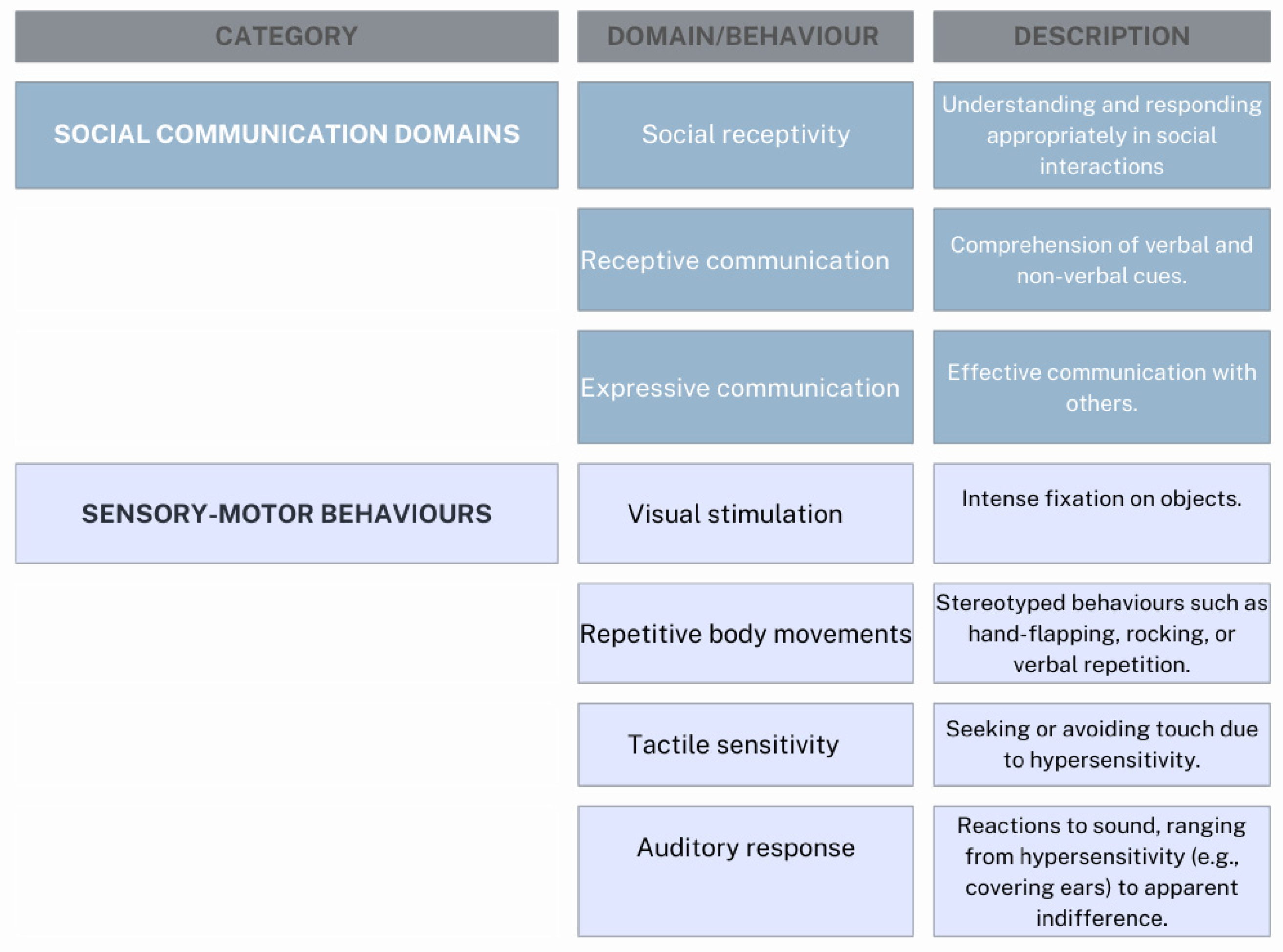
1.2. Pathogenetic Hypothesis
- Proteins involved in synaptic communication, which enable interactions between nerve cells;
- Factors regulating gene expression to determine which genes are activated;
- Neurotransmitters and receptors, which transmit nerve signals between cells via synapses;
- Genes related to brain development, which influence the growth and organization of the brain.
1.3. Aims
2. Materials and Methods
3. Results
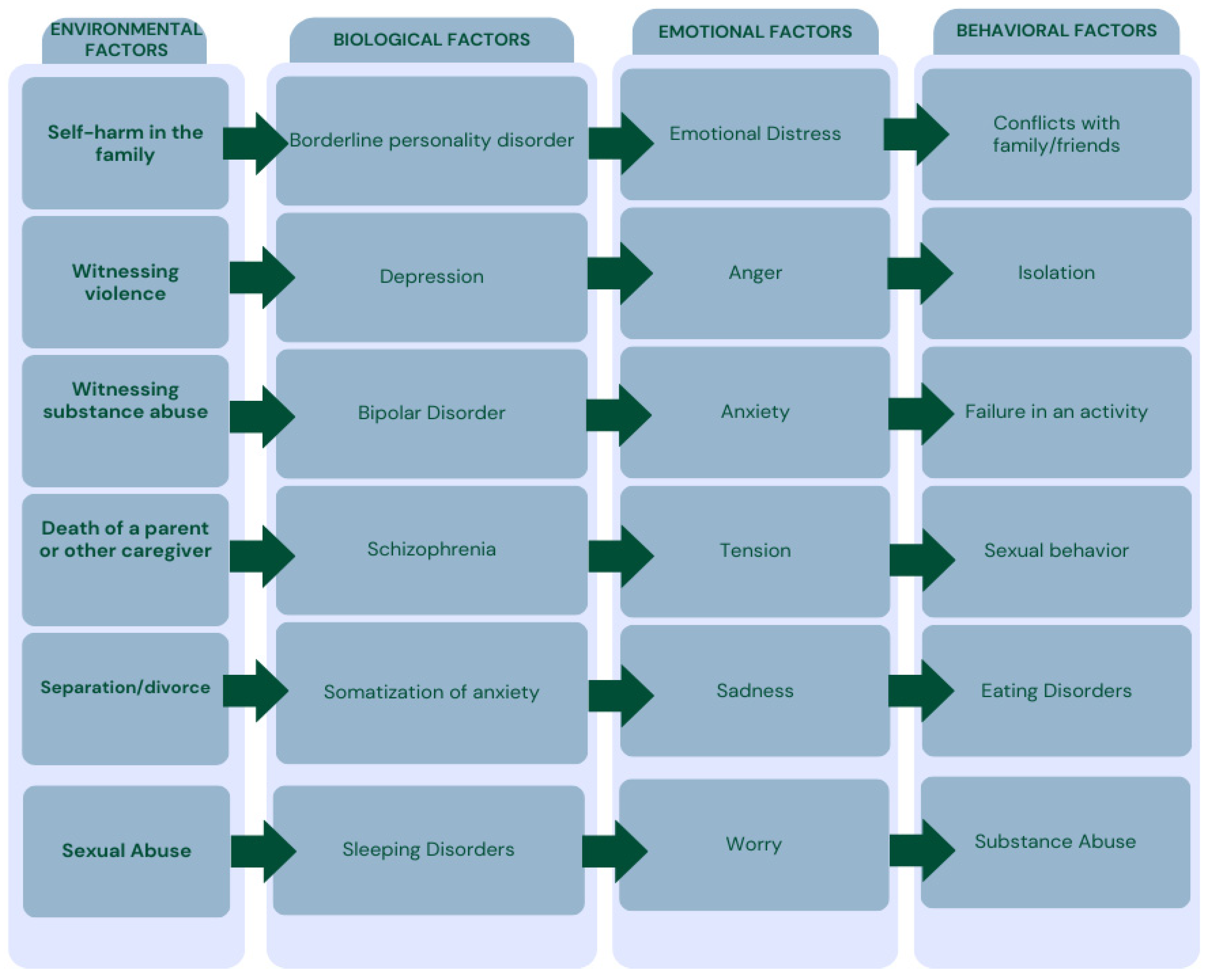
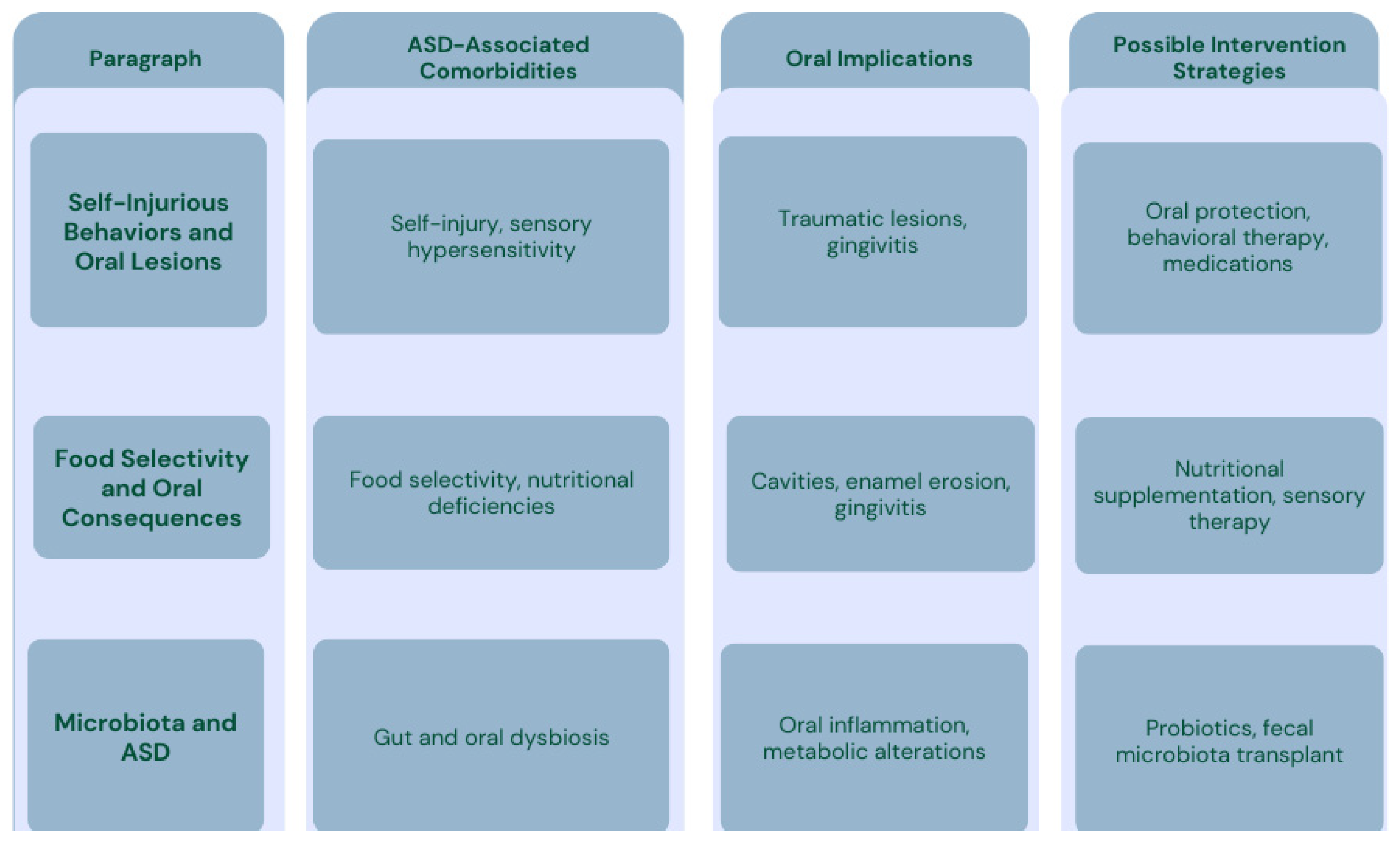
3.1. Self-Injurious Behavior (SIB) and Oral Lesions
- Stereotypic, including head-banging, self-hitting, biting, and scratching.
- Major, often associated with psychosis and causing significant harm, such as self-enucleation, self-castration, and self-amputation.
- Compulsive, referring to behaviors like hair-pulling, skin-picking, and nail-biting, often linked to conditions such as trichotillomania, stereotypic movement disorder, or obsessive-compulsive disorders.
- Impulsive, encompassing skin-cutting and burning, associated with borderline personality disorder, antisocial personality disorder, post-traumatic stress disorder (PTSD), and eating disorders.
3.2. Consequences of Dermatillomania in the Oral Cavity
- Recurrent skin picking that results in lesions;
- Repeated attempts to reduce or stop the behavior;
- Significant clinical distress or impairment in social, occupational, or other important areas of functioning caused by the behavior;
- The skin picking is not attributable to the physiological effects of a substance or another medical condition;
- The behavior is not better explained by the symptoms of another mental disorder.
- Skin lesions, such as abrasions and ulcerations on the lips, mouth, or fingers;
- Infections, as damaged skin becomes more vulnerable to bacterial or fungal infections;
- Scarring, particularly in areas where the skin is thin, like the lips, potentially leading to permanent marks;
- Pain and discomfort, which can affect the individual’s quality of life;
- Dental complications, if oral tissues are involved, such as irritation or cuts from scratching or picking;
- Oral health issues, possibly stemming from frequent contact with teeth or oral structures during the behavior.
3.3. Food Selectivity in Patients with ASD and Impacts on the Oral Cavity
3.3.1. Oral Consequences of Food Selectivity
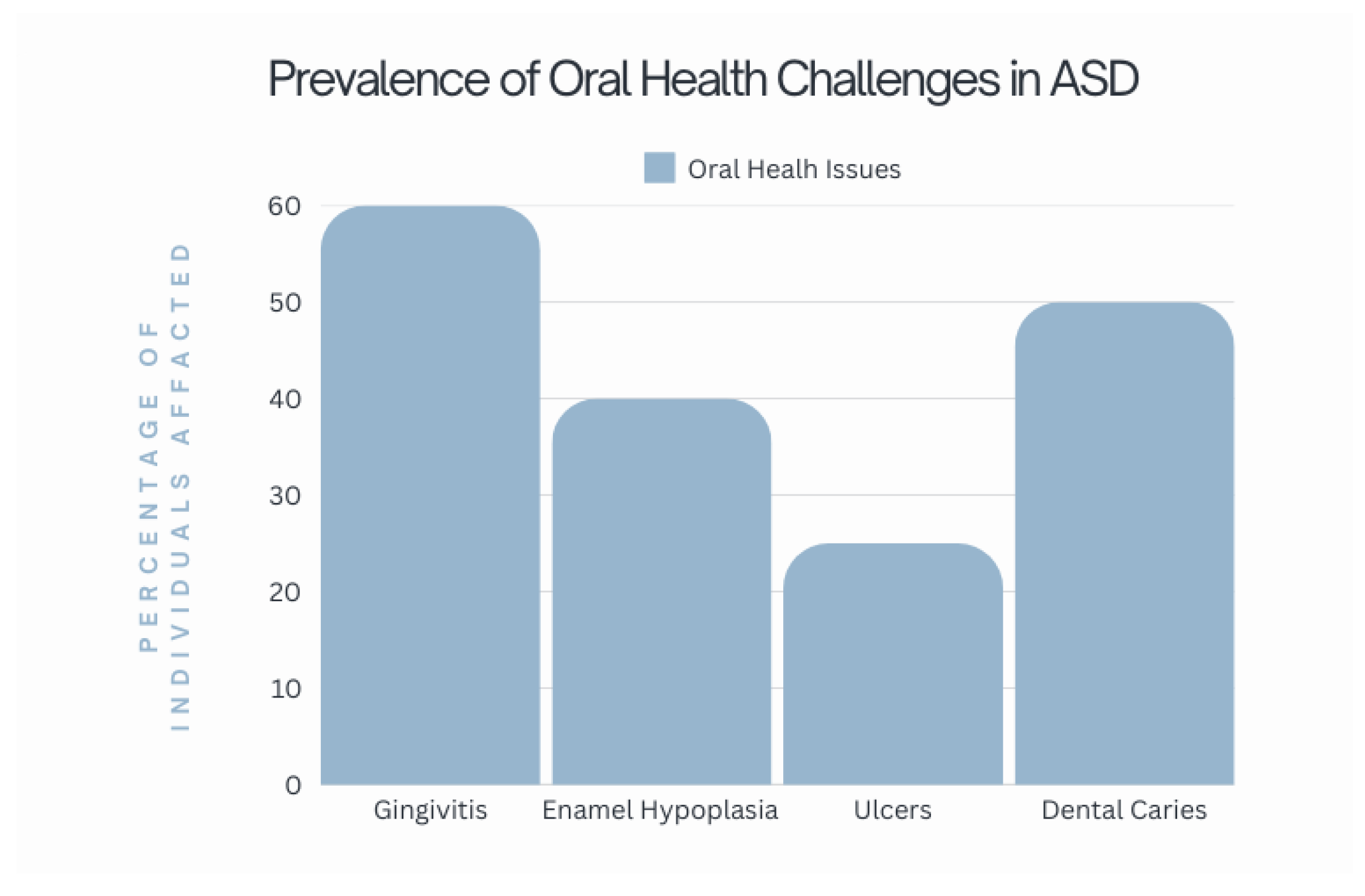
Vitamin A Deficiency
B Vitamins Deficiencies
Vitamin C Deficiency
Vitamin D Deficiency
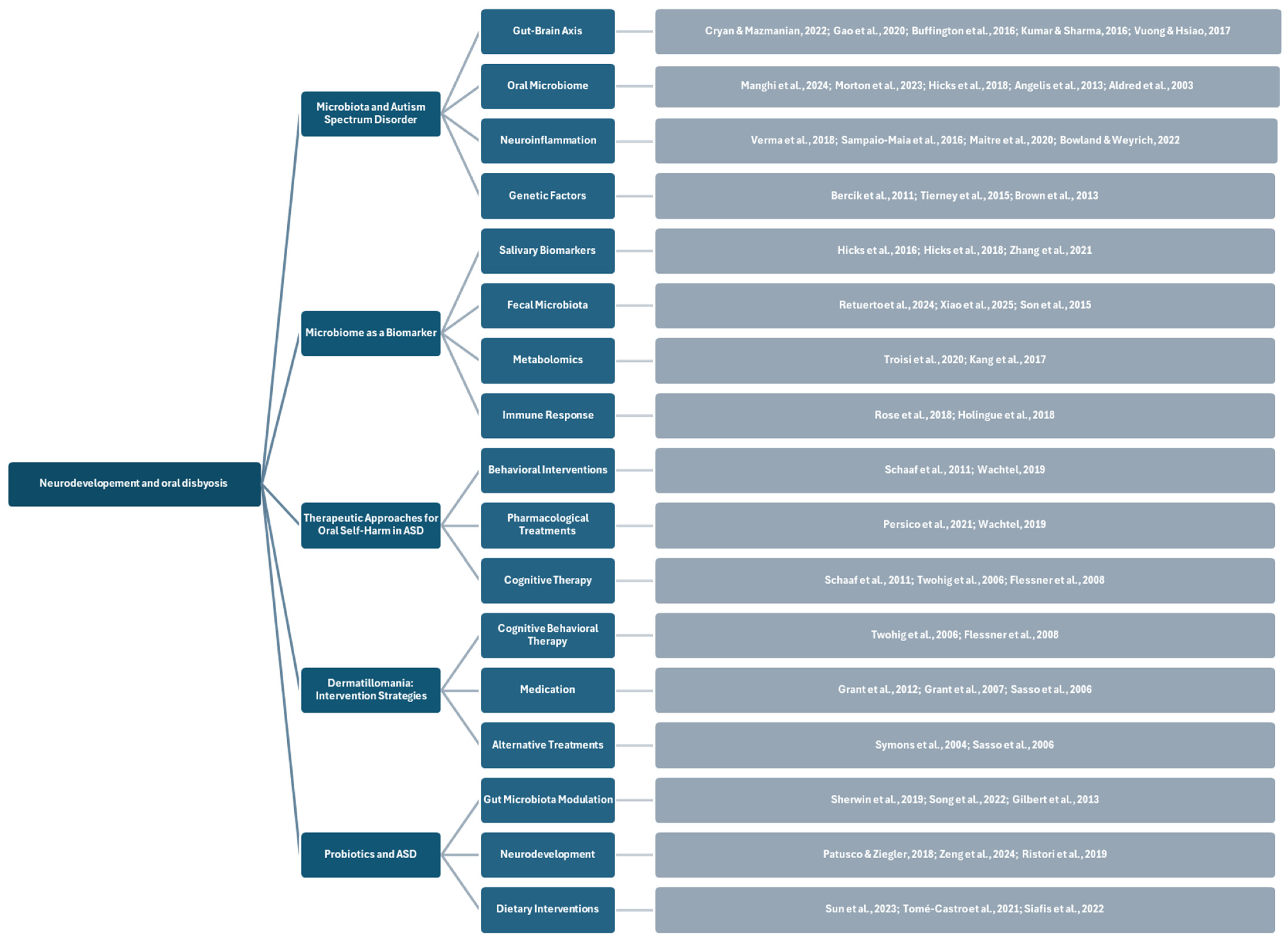
3.4. Microbiota and Microbial Diversity in Patients with Autism Spectrum Disorders
3.4.1. A Hypothesized Correlation Between Microbiota and Autism Spectrum Disorder
3.4.2. Microbiome as a Biomarker
3.5. Analysis of Existing Therapeutic Approaches to Manage Oral Self-Harm in Patients with ASD
3.5.1. Dermatillomania: Targeted Intervention Strategies
- Cognitive-behavioral therapy (CBT): This approach involves psychoeducation, cognitive restructuring, and relapse prevention through self-esteem enhancement, along with clearly defined strategies to prevent or manage potential relapses [156].
- Habit reversal training (HRT): Previously used to treat a variety of repetitive behavior problems, such as cheek biting, oral-digital habits, and trichotillomania (TTM19) [157]. It includes the following:
- ○
- Awareness training (self-monitoring): Teaching the patient to recognize skin-picking triggers and behavior.
- ○
- Competing response training: The patient learns to replace skin-picking with an incompatible action, such as clenching their fist.
- ○
- Decoupling (DC): The patient is trained to “unlearn” skin-picking by replacing it with a harmless behavior that mimics the central movements of the problematic behavior, such as bringing the hand close to the face without picking and then redirecting it to a different location, such as the ear, where picking does not occur.
3.5.2. Probiotics
Challenges
4. Discussion and Conclusions
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASD | autism spectrum disorder |
| SIB | self-injurious behavior |
| PDD-NOS | pervasive developmental disorder not otherwise specified |
| GEMMA | Genomic, Environmental, Microbiome, and Metabolomic Assessment |
| ADHD | attention-deficit/hyperactivity disorder |
| NDD | neurodevelopmental disorders |
References
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association Publishing: Washington, DC, USA, 2022. [Google Scholar]
- Kanner, L. Autistic Disturbances of Affective Contact. Acta Paedopsychiatr. 1968, 35, 100–136. [Google Scholar]
- Harris, J. Leo Kanner and Autism: A 75-Year Perspective. Int. Rev. Psychiatry 2018, 30, 3–17. [Google Scholar] [CrossRef] [PubMed]
- Pearson, N.; Charman, T.; Happé, F.; Bolton, P.F.; McEwen, F.S. Regression in Autism Spectrum Disorder: Reconciling Findings from Retrospective and Prospective Research. Autism Res. 2018, 11, 1602–1620. [Google Scholar] [CrossRef]
- Rosen, N.E.; Lord, C.; Volkmar, F.R. The Diagnosis of Autism: From Kanner to DSM-III to DSM-5 and Beyond. J. Autism Dev. Disord. 2021, 51, 4253–4270. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.P.; Myers, S.M.; Lipkin, P.H.; Cartwright, J.D.; Desch, L.W.; Duby, J.C.; Elias, E.R.; Levey, E.B.; Liptak, G.S.; Murphy, N.A.; et al. Identification and Evaluation of Children with Autism Spectrum Disorders. Pediatrics 2007, 120, 1183–1215. [Google Scholar] [CrossRef] [PubMed]
- Gupta, V.; Ben-Mahmoud, A.; Idris, A.B.; Hottenga, J.-J.; Habbab, W.; Alsayegh, A.; Kim, H.-G.; Al-Mamari, W.; Stanton, L.W. Genetic Variant Analyses Identify Novel Candidate Autism Risk Genes from a Highly Consanguineous Cohort of 104 Families from Oman. Int. J. Mol. Sci. 2024, 25, 13700. [Google Scholar] [CrossRef]
- Sandin, S.; Lichtenstein, P.; Kuja-Halkola, R.; Hultman, C.; Larsson, H.; Reichenberg, A. The Heritability of Autism Spectrum Disorder. JAMA 2017, 318, 1182–1184. [Google Scholar] [CrossRef]
- Gaugler, T.; Klei, L.; Sanders, S.J.; Bodea, C.A.; Goldberg, A.P.; Lee, A.B.; Mahajan, M.; Manaa, D.; Pawitan, Y.; Reichert, J.; et al. Most Genetic Risk for Autism Resides with Common Variation. Nat. Genet. 2014, 46, 881. [Google Scholar] [CrossRef]
- Scattoni, M.L.; Fatta, L.M.; Micai, M.; Sali, M.E.; Bellomo, M.; Salvitti, T.; Fulceri, F.; Castellano, A.; Molteni, M.; Gambino, G.; et al. Autism Spectrum Disorder Prevalence in Italy: A Nationwide Study Promoted by the Ministry of Health. Child. Adolesc. Psychiatry Ment. Health 2023, 17, 125. [Google Scholar] [CrossRef]
- Masiran, R. Stimming Behaviour in a 4-Year-Old Girl with Autism Spectrum Disorder. BMJ Case Rep. 2018, 2018, bcr-2017. [Google Scholar] [CrossRef]
- Lord, C.; Elsabbagh, M.; Baird, G.; Veenstra-Vanderweele, J. Autism Spectrum Disorder. Lancet 2018, 392, 508–520. [Google Scholar] [CrossRef] [PubMed]
- Jaber, M.A. Dental Caries Experience, Oral Health Status and Treatment Needs of Dental Patients with Autism. J. Appl. Oral Sci. 2011, 19, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Bejjani, A.; O’Neill, J.; Kim, J.A.; Frew, A.J.; Yee, V.W.; Ly, R.; Kitchen, C.; Salamon, N.; McCracken, J.T.; Toga, A.W.; et al. Elevated Glutamatergic Compounds in Pregenual Anterior Cingulate in Pediatric Autism Spectrum Disorder Demonstrated by 1H MRS and 1H MRSI. PLoS ONE 2012, 7, e38786. [Google Scholar] [CrossRef]
- Evenepoel, M.; Daniels, N.; Moerkerke, M.; Van de Vliet, M.; Prinsen, J.; Tuerlinckx, E.; Steyaert, J.; Boets, B.; Alaerts, K.; Joossens, M. Oral microbiota in autistic children: Diagnosis-related differences and associations with clinical characteristics. Brain Behav. Immun. Health 2024, 38, 100801. [Google Scholar] [CrossRef]
- Cryan, J.F.; O’Mahony, S.M. The Microbiome-Gut-Brain Axis: From Bowel to Behavior. Neurogastroenterol. Motil. 2011, 23, 187–192. [Google Scholar] [CrossRef] [PubMed]
- Careaga, M.; Rogers, S.; Hansen, R.L.; Amaral, D.G.; Van de Water, J.; Ashwood, P. Immune Endophenotypes in Children with Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 434–441. [Google Scholar] [CrossRef]
- Blumenthal, I.; Ragavendran, A.; Erdin, S.; Klei, L.; Sugathan, A.; Guide, J.R.; Manavalan, P.; Zhou, J.Q.; Wheeler, V.C.; Levin, J.Z.; et al. Transcriptional Consequences of 16p11.2 Deletion and Duplication in Mouse Cortex and Multiplex Autism Families. Am. J. Hum. Genet. 2014, 94, 870–883. [Google Scholar] [CrossRef]
- Gupta, A.R.; Westphal, A.; Yang, D.Y.J.; Sullivan, C.A.W.; Eilbott, J.; Zaidi, S.; Voos, A.; Vander Wyk, B.C.; Ventola, P.; Waqar, Z.; et al. Neurogenetic Analysis of Childhood Disintegrative Disorder. Mol. Autism 2017, 8, 19. [Google Scholar] [CrossRef]
- Nava, C.; Lamari, F.; Héron, D.; Mignot, C.; Rastetter, A.; Keren, B.; Cohen, D.; Faudet, A.; Bouteiller, D.; Gilleron, M.; et al. Analysis of the Chromosome X Exome in Patients with Autism Spectrum Disorders Identified Novel Candidate Genes, Including TMLHE. Transl. Psychiatry 2012, 2, e179. [Google Scholar] [CrossRef]
- Furukawa, S.; Kushima, I.; Aleksic, B.; Ozaki, N. Case Reports of Two Siblings with Autism Spectrum Disorder and 15q13.3 Deletions. Neuropsychopharmacol. Rep. 2023, 43, 462–466. [Google Scholar] [CrossRef]
- Parenti, M.; Shoff, S.; Sotelo-Orozco, J.; Hertz-Picciotto, I.; Slupsky, C.M. Metabolomics of Mothers of Children with Autism, Idiopathic Developmental Delay, and Down Syndrome. Sci. Rep. 2024, 14, 31981. [Google Scholar] [CrossRef]
- Cernigliaro, F.; Santangelo, A.; Nardello, R.; Lo Cascio, S.; D’Agostino, S.; Correnti, E.; Marchese, F.; Pitino, R.; Valdese, S.; Rizzo, C.; et al. Prenatal Nutritional Factors and Neurodevelopmental Disorders: A Narrative Review. Life 2024, 14, 1084. [Google Scholar] [CrossRef]
- Instanes, J.T.; Solberg, B.S.; Kvalvik, L.G.; Klungsøyr, K.; Posserud, M.-B.R.; Hartman, C.A.; Haavik, J. Organic Food Consumption during Pregnancy and Symptoms of Neurodevelopmental Disorders at 8 Years of Age in the Offspring: The Norwegian Mother, Father and Child Cohort Study (MoBa). BMC Med. 2024, 22, 482. [Google Scholar] [CrossRef]
- Sousamli, A.; Dragioti, E.; Metallinou, D.; Lykeridou, A.; Dourou, P.; Athanasiadou, C.R.; Anagnostopoulos, D.; Sarantaki, A. Perinatal and Demographic Risk Factors Associated with Autism Spectrum Disorder: A National Survey of Potential Predictors and Severity. Healthcare 2024, 12, 2057. [Google Scholar] [CrossRef] [PubMed]
- Abdelkader, A.; AlRadini, F.; Alosaimi, A.; Abbas, A.; Judeh, Z.; Emy Abu Esaid, T.; Saleh, A.; Shah, J.; Amer, S. Unveiling the Influences of Prenatal and Maternal Factors on the Journey of an Autistic Child. Front. Psychiatry 2024, 15, 1467821. [Google Scholar] [CrossRef] [PubMed]
- Inamdar, A.; Gurupadayya, B.; Sharma, H. The Role of Glial Cells in Autism Spectrum Disorder: Molecular Mechanisms and Therapeutic Approaches. CNS Neurol. Disord. Drug Targets 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, E.F.; Russell, K.M.; Licona, S.J.; Cai, R.Y.; Frazier, T.W.; Vivanti, G.; Gengoux, G.W.; Hardan, A.Y.; Uljarević, M. Toward Improved Understanding and Treatment of Self-Injurious Behaviors in Autistic Individuals with Profound Intellectual Disability. Autism Res. 2024, 18, 261–272. [Google Scholar] [CrossRef]
- Schwartzman, J.M.; Rubin, A.; Fox, K.R.; Hedley, D.; Bettis, A.H. Type, Content, and Triggers for Self-Injurious Thoughts and Behaviors in Autistic Youth and Their Disclosure to Caregivers. Autism 2024, in press. [Google Scholar] [CrossRef]
- Simeon, D.; Favazza, A.R. Self-Injurious Behaviors: Phenomenology and Assessment. In Self-Injurious Behaviors: Assessment and Treatment; Simeon, D., Hollander, E., Eds.; American Psychiatric Publishing, Inc.: Washington, DC, USA, 2001; pp. 1–28. [Google Scholar]
- Gulsrud, A.; Lin, C.E.; Park, M.N.; Hellemann, G.; McCracken, J. Self-Injurious Behaviours in Children and Adults with Autism Spectrum Disorder (ASD). J. Intellect. Disabil. Res. 2018, 62, 1030–1042. [Google Scholar] [CrossRef]
- Naidoo, M.; Singh, S. The Oral Health Status of Children with Autism Spectrum Disorder in KwaZulu-Nata, South Africa. BMC Oral Health 2018, 18, 165. [Google Scholar] [CrossRef]
- Medina, A.C.; Sogbe, R.; Gómez-Rey, A.M.; Mata, M. Factitial Oral Lesions in an Autistic Paediatric Patient. Int. J. Paediatr. Dent. 2003, 13, 130–137. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, D.; Matt, M. Autoextraction in an Autistic Dental Patient: A Case Report. Spec. Care Dent. 1999, 19, 72–74. [Google Scholar] [CrossRef]
- Ross-Russell, M.; Sloan, P. Autoextraction in a Child with Autistic Spectrum Disorder. Br. Dent. J. 2005, 198, 473–474. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.C. Autoextraction of Twelve Permanent Teeth in a Child with Autistic Spectrum Disorder. Int. J. Paediatr. Dent. 2016, 26, 157–159. [Google Scholar] [CrossRef]
- Cooper, A.; Weyman, J.R.; Kahng, S. An Evaluation of Contingent Gum Chewing on Rumination Exhibited by an Adolescent with Autism Spectrum Disorder. Behav. Anal. Pract. 2023, 16, 1211–1215. [Google Scholar] [CrossRef] [PubMed]
- Leader, G.; Tuohy, E.; Chen, J.L.; Mannion, A.; Gilroy, S.P. Feeding Problems, Gastrointestinal Symptoms, Challenging Behavior and Sensory Issues in Children and Adolescents with Autism Spectrum Disorder. J. Autism Dev. Disord. 2020, 50, 1401–1410. [Google Scholar] [CrossRef]
- Khan, A.J.; Afrose, T.; Nuha, F.A.; Islam, M.A.; Ahmad, M.S.B. Bruxism Management in Individuals with Autism Spectrum Disorder and down Syndrome—A Systematic Review. Spec. Care Dent. 2024, 44, 645–658. [Google Scholar] [CrossRef]
- George, S.S.; Elenjickal, M.G.; Naik, S.; Thomas, N.G.; Vellappally, S.; Varghese, N.; Mathew, A.; Narayan, V.; Varughese, R.P.; Anil, S. Oral Health Status and Dental Treatment Needs in Children with Autism Spectrum Disorder. Heliyon 2024, 10, e37728. [Google Scholar] [CrossRef]
- Alfahaad, H.; Aldehri, M.; Alsaiari, S.A.; Asiri, F.; Alfataih, M.; Alahmari, S. Exploring Skin Picking Disorder: Aetiology, Treatment, and Future Directions. Postep. Dermatol. Alergol. 2024, 41, 545–551. [Google Scholar] [CrossRef]
- Parsa, L.; Pixley, J.N.; Fried, R.G. “Pick” Wisely: An Approach to Diagnosis and Management of Pathologic Skin Picking. Clin. Dermatol. 2023, 41, 41–48. [Google Scholar] [CrossRef]
- Lochner, C.; Maré, K.T.; Stein, D.J. Structured Clinical Interview for Diagnosing Obsessive-Compulsive Spectrum Disorders. Compr. Psychiatry 2024, 133, 152494. [Google Scholar] [CrossRef]
- Al-Beltagi, M. Nutritional Management and Autism Spectrum Disorder: A Systematic Review. World J. Clin. Pediatr. 2024, 13, 99649. [Google Scholar] [CrossRef]
- Gicchino, M.F.; Romano, A.; Cioffi, S.; Fiori, F.; Miraglia Del Giudice, E.; Lucchese, A.; Olivieri, A.N.; Serpico, R. Oral Manifestations in Scurvy Pediatric Patients: A Systematic Review and a Case Report. Appl. Sci. 2021, 11, 8323. [Google Scholar] [CrossRef]
- Alsada, F.; Sebastian, T.; Alzayer, Z.; Alabbas, H.; Alhaddad, N.; Shahin, H.A.; Alghamdi, A.; Alhmly, H.F.; Baassiri, M.J.; Alkhalifa, B.; et al. Determinants of Infants and Young Children Feeding Practices among Mothers Living in Saudi Arabia: A Cross-Sectional Study. BMC Public Health 2025, 25, 388. [Google Scholar] [CrossRef] [PubMed]
- Pinhas, L.; Nicholls, D.; Crosby, R.D.; Morris, A.; Lynn, R.M.; Madden, S. Classification of Childhood Onset Eating Disorders: A Latent Class Analysis. Int. J. Eat. Disord. 2017, 50, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Abu, B.A.Z.; Morrissey, A.; Wu, Y.; Castillo, D.A.; Becker, R.; Wu, T.; Fiscella, K.; Gill, S.; Xiao, J. Pica Practices, Anemia, and Oral Health Outcomes: A Systemic Review. BMC Oral Health 2025, 25, 13. [Google Scholar] [CrossRef] [PubMed]
- Bryant-Waugh, R. Avoidant/Restrictive Food Intake Disorder. Child. Adolesc. Psychiatr. Clin. N. Am. 2019, 28, 557–565. [Google Scholar] [CrossRef]
- Fields, V.L.; Soke, G.N.; Reynolds, A.; Tian, L.H.; Wiggins, L.; Maenner, M.; DiGuiseppi, C.; Kral, T.V.E.; Hightshoe, K.; Schieve, L.A. Pica, Autism, and Other Disabilities. Pediatrics 2021, 147, e20200462. [Google Scholar] [CrossRef]
- Cermak, S.A.; Curtin, C.; Bandini, L.G. Food Selectivity and Sensory Sensitivity in Children with Autism Spectrum Disorders. J. Am. Diet. Assoc. 2010, 110, 238–246. [Google Scholar] [CrossRef]
- Aponte, C.A.; Romanczyk, R.G. Assessment of Feeding Problems in Children with Autism Spectrum Disorder. Res. Autism Spectr. Disord. 2016, 21, 61–72. [Google Scholar] [CrossRef]
- Lockner, D.W.; Crowe, T.K.; Skipper, B.J. Dietary Intake and Parents’ Perception of Mealtime Behaviors in Preschool-Age Children with Autism Spectrum Disorder and in Typically Developing Children. J. Am. Diet. Assoc. 2008, 108, 1360–1363. [Google Scholar] [CrossRef] [PubMed]
- Arija, V.; Esteban-Figuerola, P.; Morales-Hidalgo, P.; Jardí, C.; Canals-Sans, J. Nutrient Intake and Adequacy in Children with Autism Spectrum Disorder: EPINED Epidemiological Study. Autism 2023, 27, 371–388. [Google Scholar] [CrossRef]
- Esteban-Figuerola, P.; Canals, J.; Fernández-Cao, J.C.; Arija Val, V. Differences in Food Consumption and Nutritional Intake between Children with Autism Spectrum Disorders and Typically Developing Children: A Meta-Analysis. Autism 2019, 23, 1079–1095. [Google Scholar] [CrossRef] [PubMed]
- Harris, H.A.; Bowling, A.; Santos, S.; Greaves-Lord, K.; Jansen, P.W. Child ADHD and Autistic Traits, Eating Behaviours and Weight: A Population-Based Study. Pediatr. Obes. 2022, 17, e12951. [Google Scholar] [CrossRef]
- Metwally, A.M.; Helmy, M.A.; Aboulghate, A.; Hassan, N.A.M.; Mahmoud, W.S.; Ismail, A.S.; El Shebini, S.M.; Ahmed, N.H.; Mabrok, H.B.; Mahmoud, M.H.; et al. The Odds of Having Obesity in Egyptian Children with Autism Spectrum Disorders Is Higher than Stunting Compared to Healthy Developing Peers: A National Survey. BMC Pediatr. 2024, 24, 465. [Google Scholar] [CrossRef]
- Vissoker, R.E.; Latzer, Y.; Gal, E. Eating and Feeding Problems and Gastrointestinal Dysfunction in Autism Spectrum Disorders. Res. Autism Spectr. Disord. 2015, 12, 10–21. [Google Scholar] [CrossRef]
- Attia, M.; Lavoie-Forrest, A.A.; Langius, P.; Melnitsky, L.; Lopez, S.; Boccio, E. Gastroduodenal Obstruction Secondary to Pica-Associated Bezoar: A Case Report. Clin. Pract. Cases Emerg. Med. 2025, 9, 53–56. [Google Scholar] [CrossRef]
- Bachmeyer, M.H. Treatment of Selective and Inadequate Food Intake in Children: A Review and Practical Guide. Behav. Anal. Pract. 2009, 2, 43–50. [Google Scholar] [CrossRef] [PubMed]
- Ma, B.; Liang, J.; Dai, M.; Wang, J.; Luo, J.; Zhang, Z.; Jing, J. Altered Gut Microbiota in Chinese Children with Autism Spectrum Disorders. Front. Cell Infect. Microbiol. 2019, 9, 40. [Google Scholar] [CrossRef]
- Plaza-Díaz, J.; Gómez-Fernández, A.; Chueca, N.; de la Torre-Aguilar, M.J.; Gil, Á.; Perez-Navero, J.L.; Flores-Rojas, K.; Martín-Borreguero, P.; Solis-Urra, P.; Ruiz-Ojeda, F.J.; et al. Autism Spectrum Disorder (ASD) with and without Mental Regression Is Associated with Changes in the Fecal Microbiota. Nutrients 2019, 11, 337. [Google Scholar] [CrossRef]
- Katikar, M.S.; Devi, A.; Prabhu, P. Sensory Processing in Autism Spectrum Disorder: Insights into Misophonia, and Hyperacusis in a Pediatric Population. Int. J. Pediatr. Otorhinolaryngol. 2025, 189, 112241. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.D.C.S.; Cândido, F.G.; Filgueiras, M.D.S.; Rosa, C.D.O.B.; Novaes, J.F.D.; Araujo, R.M.A. Problematic Behaviors at Mealtimes and the Nutritional Status of Brazilian Children with Autism Spectrum Disorder. Front. Public Health 2024, 12, 1392478. [Google Scholar] [CrossRef] [PubMed]
- Ayres, A.J. Tactile Functions. Their Relation To Hyperactive And Perceptual Motor Behavior. Am. J. Occup. Ther. 1964, 18, 6–11. [Google Scholar] [PubMed]
- Calisan Kinter, R.; Ozbaran, B.; Inal Kaleli, I.; Kose, S.; Bildik, T.; Ghaziuddin, M. The Sensory Profiles, Eating Behaviors, and Quality of Life of Children with Autism Spectrum Disorder and Avoidant/Restrictive Food Intake Disorder. Psychiatr. Q. 2024, 95, 85–106. [Google Scholar] [CrossRef]
- Talib, M.; Rachdi, M.; Papazova, A.; Nicolis, H. The Role of Dietary Patterns and Nutritional Supplements in the Management of Mental Disorders in Children and Adolescents: An Umbrella Review of Meta-Analyses. Can. J. Psychiatry 2024, 69, 567–589. [Google Scholar] [CrossRef]
- Postorino, V.; Sanges, V.; Giovagnoli, G.; Fatta, L.M.; De Peppo, L.; Armando, M.; Vicari, S.; Mazzone, L. Clinical Differences in Children with Autism Spectrum Disorder with and without Food Selectivity. Appetite 2015, 92, 126–132. [Google Scholar] [CrossRef]
- Curtin, C.; Hubbard, K.; Anderson, S.E.; Mick, E.; Must, A.; Bandini, L.G. Food Selectivity, Mealtime Behavior Problems, Spousal Stress, and Family Food Choices in Children with and without Autism Spectrum Disorder. J. Autism Dev. Disord. 2015, 45, 3308–3315. [Google Scholar] [CrossRef]
- Groden, J.; Diller, A.; Bausman, M.; Velicer, W.; Norman, G.; Cautela, J. The Development of a Stress Survey Schedule for Persons with Autism and Other Developmental Disabilities. J. Autism Dev. Disord. 2001, 31, 207–217. [Google Scholar] [CrossRef]
- della Vella, F.; Lauritano, D.; Lajolo, C.; Lucchese, A.; Di Stasio, D.; Contaldo, M.; Serpico, R.; Petruzzi, M. The Pseudolesions of the Oral Mucosa: Differential Diagnosis and Related Systemic Conditions. Appl. Sci. 2019, 9, 2412. [Google Scholar] [CrossRef]
- Adams, J.B.; Johansen, L.J.; Powell, L.D.; Quig, D.; Rubin, R.A. Gastrointestinal Flora and Gastrointestinal Status in Children with Autism—Comparisons to Typical Children and Correlation with Autism Severity. BMC Gastroenterol. 2011, 11, 22. [Google Scholar] [CrossRef]
- Blanchard, A.; Chihuri, S.; DiGuiseppi, C.G.; Li, G. Risk of Self-Harm in Children and Adults with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. JAMA Netw. Open 2021, 4, E2130272. [Google Scholar] [CrossRef] [PubMed]
- Dixon, C.J.; Ethridge, B.A.; Tillman, A.P.; Sugg, J.H. Self-Restricted Diet in Pediatric Autism Leading to Vitamin A Deficiency and Severe Photophobia. Cureus 2024, 16, e54618. [Google Scholar] [CrossRef]
- Kacimi, F.E.; Didou, L.; Ed Day, S.; Azzaoui, F.Z.; Ramchoun, M.; Berrougui, H.; Khalki, H.; Boulbaroud, S. Gut Microbiota, Vitamin A Deficiency and Autism Spectrum Disorder: An Interconnected Trio—A Systematic Review. Nutr. Neurosci. 2024, 13, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lv, P.; jun Gao, X. Phenotype Analysis and the Molecular Mechanism of Enamel Hypoplasia. Beijing Da Xue Xue Bao Yi Xue Ban J. Peking Univ. Health Sci. 2009, 41, 121–123. [Google Scholar]
- Contaldo, M.; della Vella, F.; Raimondo, E.; Minervini, G.; Buljubasic, M.; Ogodescu, A.; Sinescu, C.; Serpico, R. Early Childhood Oral Health Impact Scale (ECOHIS): Literature Review and Italian Validation. Int. J. Dent. Hyg. 2020, 18, 396–402. [Google Scholar] [CrossRef]
- Zheng, L.; Jiao, Y.; Zhong, H.; Tan, Y.; Yin, Y.; Liu, Y.; Liu, D.; Wu, M.; Wang, G.; Huang, J.; et al. Human-Derived Fecal Microbiota Transplantation Alleviates Social Deficits of the BTBR Mouse Model of Autism through a Potential Mechanism Involving Vitamin B6 Metabolism. mSystems 2024, 9, e0025724. [Google Scholar] [CrossRef] [PubMed]
- Sawada, Y.; Sakamoto, K.; Tsukamura, A.; Sawai, C. Vitamin B12 Deficiency-Induced Megaloblastic Anemia in a Pediatric Patient with Autism Spectrum Disorder with a Chronically Unbalanced Diet. Int. J. Hematol. 2024, 119, 613–616. [Google Scholar] [CrossRef]
- Al-Maweri, S.A.; Halboub, E.S.; Al-Soneidar, W.A.; Al-Sufyani, G.A. Oral Lesions and Dental Status of Autistic Children in Yemen: A Case-Control Study. J. Int. Soc. Prev. Community Dent. 2014, 4, S199–S203. [Google Scholar] [CrossRef]
- Maxfield, L.; Daley, S.F.; Crane, J.S. Vitamin C Deficiency; StatPearls: Treasure Island, FL, USA, 2025. [Google Scholar]
- Firth, N.; Marvan, E. Oral Lesions in Scurvy. Aust. Dent. J. 2001, 46, 298–300. [Google Scholar] [CrossRef]
- Sethi, N.K.; Kratunova, E.; Hill, B.; Reilly, P. Oral Manifestations of Vitamin C Deficiency in a Toddler. J Dent Child (Chic) 2024, 91, 167–172. [Google Scholar]
- Pancheva, R.; Toneva, A.; Bocheva, Y.; Georgieva, M.; Koleva, K.; Yankov, I. Prevalence of Vitamin D Deficiency in Children with Cerebral Palsy and Autism Spectrum Disorder: A Comparative Pilot Study. Folia Med. 2024, 66, 787–794. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Di Stasio, D.; Vella, F.D.; Lauritano, D.; Serpico, R.; Santoro, R.; Lucchese, A. Real Time in Vivo Confocal Microscopic Analysis of the Enamel Remineralization by Casein Phosphopeptide-Amorphous Calcium Phosphate (CPP-ACP): A Clinical Proof-of-Concept Study. Appl. Sci. 2020, 10, 4155. [Google Scholar] [CrossRef]
- Schmidt, R.J.; Hansen, R.L.; Hartiala, J.; Allayee, H.; Sconberg, J.L.; Schmidt, L.C.; Volk, H.E.; Tassone, F. Selected Vitamin D Metabolic Gene Variants and Risk for Autism Spectrum Disorder in the CHARGE Study. Early Hum. Dev. 2015, 91, 483–489. [Google Scholar] [CrossRef]
- Saechua, C.; Sarachana, T.; Chonchaiya, W.; Trairatvorakul, P.; Yuwattana, W.; Poolcharoen, C.; Sangritdech, M.; Saeliw, T.; van Erp, M.L.; Sangsuthum, S.; et al. Impact of Gene Polymorphisms Involved in the Vitamin D Metabolic Pathway on the Susceptibility to and Severity of Autism Spectrum Disorder. Sci. Rep. 2024, 14, 28333. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, M.; Elfeky, O.; Ertugrul, H.; Chela, H.K.; Daglilar, E. Scurvy: Rediscovering a Forgotten Disease. Diseases 2023, 11, 78. [Google Scholar] [CrossRef]
- Contaldo, M. Use of Probiotics for Oral Candidiasis: State of the Art and Perspective. A Further Step Toward Personalized Medicine? Front. Biosci. 2023, 15, 6. [Google Scholar] [CrossRef]
- Contaldo, M.; Lucchese, A.; Romano, A.; Vella, F.D.; Di Stasio, D.; Serpico, R.; Petruzzi, M. Oral Microbiota Features in Subjects with down Syndrome and Periodontal Diseases: A Systematic Review. Int. J. Mol. Sci. 2021, 22, 9251. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Blaser, M.J.; Caporaso, J.G.; Jansson, J.K.; Lynch, S.V.; Knight, R. Current Understanding of the Human Microbiome. Nat. Med. 2018, 24, 392–400. [Google Scholar] [CrossRef]
- Preidis, G.A.; Versalovic, J. Targeting the Human Microbiome with Antibiotics, Probiotics, and Prebiotics: Gastroenterology Enters the Metagenomics Era. Gastroenterology 2009, 136, 2015–2031. [Google Scholar] [CrossRef]
- Mayer, E.A.; Nance, K.; Chen, S. The Gut–Brain Axis. Annu. Rev. Med. 2022, 73, 439–453. [Google Scholar] [CrossRef]
- Almohmadi, N.H. Brain-Gut-Brain Axis, Nutrition, and Autism Spectrum Disorders: A Review. Transl. Pediatr. 2024, 13, 1652–1670. [Google Scholar] [CrossRef] [PubMed]
- de Theije, C.G.M.; Wopereis, H.; Ramadan, M.; van Eijndthoven, T.; Lambert, J.; Knol, J.; Garssen, J.; Kraneveld, A.D.; Oozeer, R. Altered Gut Microbiota and Activity in a Murine Model of Autism Spectrum Disorders. Brain Behav. Immun. 2014, 37, 197–206. [Google Scholar] [CrossRef]
- Mayer, E.A.; Padua, D.; Tillisch, K. Altered Brain-Gut Axis in Autism: Comorbidity or Causative Mechanisms? Bioessays 2014, 36, 933–939. [Google Scholar] [CrossRef]
- Horvath, K.; Perman, J.A. Autism and Gastrointestinal Symptoms. Curr. Gastroenterol. Rep. 2002, 4, 251–258. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Molitoris, D.; Song, Y.; Liu, C.; Vaisanen, M.L.; Bolte, E.; McTeague, M.; Sandler, R.; Wexler, H.; Marlowe, E.M.; et al. Gastrointestinal Microflora Studies in Late-Onset Autism. Clin. Infect. Dis. 2002, 35, S6–S16. [Google Scholar] [CrossRef] [PubMed]
- O’Mahony, S.M.; Clarke, G.; Borre, Y.E.; Dinan, T.G.; Cryan, J.F. Serotonin, Tryptophan Metabolism and the Brain-Gut-Microbiome Axis. Behav. Brain Res. 2015, 277, 32–48. [Google Scholar] [CrossRef]
- Yirmiya, N.; Pilowsky, T.; Nemanov, L.; Arbelle, S.; Feinsilver, T.; Fried, I.; Ebstein, R.P. Evidence for an Association with the Serotonin Transporter Promoter Region Polymorphism and Autism. Am. J. Med. Genet. 2001, 105, 381–386. [Google Scholar] [CrossRef]
- Reigstad, C.S.; Salmonson, C.E.; Rainey, J.F.; Szurszewski, J.H.; Linden, D.R.; Sonnenburg, J.L.; Farrugia, G.; Kashyap, P.C. Gut Microbes Promote Colonic Serotonin Production through an Effect of Short-Chain Fatty Acids on Enterochromaffin Cells. FASEB J. 2015, 29, 1395–1403. [Google Scholar] [CrossRef]
- Yano, J.M.; Yu, K.; Donaldson, G.P.; Shastri, G.G.; Ann, P.; Ma, L.; Nagler, C.R.; Ismagilov, R.F.; Mazmanian, S.K.; Hsiao, E.Y. Indigenous Bacteria from the Gut Microbiota Regulate Host Serotonin Biosynthesis. Cell 2015, 161, 264–276. [Google Scholar] [CrossRef]
- Dicks, L.M.T. Our Mental Health Is Determined by an Intrinsic Interplay between the Central Nervous System, Enteric Nerves, and Gut Microbiota. Int. J. Mol. Sci. 2023, 25, 38. [Google Scholar] [CrossRef]
- Frye, R.E.; Rose, S.; Slattery, J.; MacFabe, D.F. Gastrointestinal Dysfunction in Autism Spectrum Disorder: The Role of the Mitochondria and the Enteric Microbiome. Microb. Ecol. Health Dis. 2015, 26, 27458. [Google Scholar] [CrossRef] [PubMed]
- Parracho, H.M.R.T.; Bingham, M.O.; Gibson, G.R.; McCartney, A.L. Differences between the Gut Microflora of Children with Autistic Spectrum Disorders and That of Healthy Children. J. Med. Microbiol. 2005, 54, 987–991. [Google Scholar] [CrossRef] [PubMed]
- Finegold, S.M.; Dowd, S.E.; Gontcharova, V.; Liu, C.; Henley, K.E.; Wolcott, R.D.; Youn, E.; Summanen, P.H.; Granpeesheh, D.; Dixon, D.; et al. Pyrosequencing Study of Fecal Microflora of Autistic and Control Children. Anaerobe 2010, 16, 444–453. [Google Scholar] [CrossRef] [PubMed]
- Tomova, A.; Husarova, V.; Lakatosova, S.; Bakos, J.; Vlkova, B.; Babinska, K.; Ostatnikova, D. Gastrointestinal Microbiota in Children with Autism in Slovakia. Physiol. Behav. 2015, 138, 179–187. [Google Scholar] [CrossRef]
- Williams, B.L.; Hornig, M.; Buie, T.; Bauman, M.L.; Paik, M.C.; Wick, I.; Bennett, A.; Jabado, O.; Hirschberg, D.L.; Lipkin, W.I. Impaired Carbohydrate Digestion and Transport and Mucosal Dysbiosis in the Intestines of Children with Autism and Gastrointestinal Disturbances. PLoS ONE 2011, 6, e24585. [Google Scholar] [CrossRef]
- Vuong, H.E.; Hsiao, E.Y. Emerging Roles for the Gut Microbiome in Autism Spectrum Disorder. Biol. Psychiatry 2017, 81, 411–423. [Google Scholar] [CrossRef]
- Tang, J.W.-Y.; Hau, C.C.-F.; Tong, W.-M.; Watt, R.M.; Yiu, C.K.Y.; Shum, K.K.-M. Alterations of Oral Microbiota in Young Children with Autism: Unraveling Potential Biomarkers for Early Detection. J. Dent. 2025, 152, 105486. [Google Scholar] [CrossRef]
- Wan, L.; Wang, H.; Liang, Y.; Zhang, X.; Yao, X.; Zhu, G.; Cai, J.; Liu, G.; Liu, X.; Niu, Q.; et al. Effect of Oral Faecal Microbiota Transplantation Intervention for Children with Autism Spectrum Disorder: A Randomised, Double-Blind, Placebo-Controlled Trial. Clin. Transl. Med. 2024, 14, e70006. [Google Scholar] [CrossRef]
- Manghi, P.; Filosi, M.; Zolfo, M.; Casten, L.G.; Garcia-Valiente, A.; Mattevi, S.; Heidrich, V.; Golzato, D.; Perini, S.; Thomas, A.M.; et al. Large-Scale Metagenomic Analysis of Oral Microbiomes Reveals Markers for Autism Spectrum Disorders. Nat. Commun. 2024, 15, 9743. [Google Scholar] [CrossRef]
- Morton, J.T.; Jin, D.M.; Mills, R.H.; Shao, Y.; Rahman, G.; McDonald, D.; Zhu, Q.; Balaban, M.; Jiang, Y.; Cantrell, K.; et al. Multi-Level Analysis of the Gut-Brain Axis Shows Autism Spectrum Disorder-Associated Molecular and Microbial Profiles. Nat. Neurosci. 2023, 26, 1208–1217. [Google Scholar] [CrossRef]
- Sharon, G.; Cruz, N.J.; Kang, D.W.; Gandal, M.J.; Wang, B.; Kim, Y.M.; Zink, E.M.; Casey, C.P.; Taylor, B.C.; Lane, C.J.; et al. Human Gut Microbiota from Autism Spectrum Disorder Promote Behavioral Symptoms in Mice. Cell 2019, 177, 1600–1618.e17. [Google Scholar] [CrossRef] [PubMed]
- Yap, C.X.; Henders, A.K.; Alvares, G.A.; Wood, D.L.A.; Krause, L.; Tyson, G.W.; Restuadi, R.; Wallace, L.; McLaren, T.; Hansell, N.K.; et al. Autism-Related Dietary Preferences Mediate Autism-Gut Microbiome Associations. Cell 2021, 184, 5916–5931.e17. [Google Scholar] [CrossRef]
- Cryan, J.F.; Mazmanian, S.K. Microbiota-Brain Axis: Context and Causality. Science 2022, 376, 938–939. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the Human Oral Microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef]
- Sampaio-Maia, B.; Caldas, I.M.; Pereira, M.L.; Pérez-Mongiovi, D.; Araujo, R. The Oral Microbiome in Health and Its Implication in Oral and Systemic Diseases. Adv. Appl. Microbiol. 2016, 97, 171–210. [Google Scholar] [CrossRef]
- Maitre, Y.; Micheneau, P.; Delpierre, A.; Mahalli, R.; Guerin, M.; Amador, G.; Denis, F. Did the Brain and Oral Microbiota Talk to Each Other? A Review of the Literature. J. Clin. Med. 2020, 9, 3876. [Google Scholar] [CrossRef] [PubMed]
- Bowland, G.B.; Weyrich, L.S. The Oral-Microbiome-Brain Axis and Neuropsychiatric Disorders: An Anthropological Perspective. Front. Psychiatry 2022, 13, 810008. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Milioli, G.; Elias, S.G.; Teixeira, A.L. Pathophysiology of Bacterial Infection of the Central Nervous System and Its Putative Role in the Pathogenesis of Behavioral Changes. Braz. J. Psychiatry 2013, 35, 81–87. [Google Scholar] [CrossRef]
- Mussap, M.; Noto, A.; Fanos, V. Metabolomics of Autism Spectrum Disorders: Early Insights Regarding Mammalian-Microbial Cometabolites. Expert. Rev. Mol. Diagn. 2016, 16, 869–881. [Google Scholar] [CrossRef]
- Gao, K.; Mu, C.L.; Farzi, A.; Zhu, W.Y. Tryptophan Metabolism: A Link Between the Gut Microbiota and Brain. Adv. Nutr. 2020, 11, 709–723. [Google Scholar] [CrossRef]
- Buffington, S.A.; Di Prisco, G.V.; Auchtung, T.A.; Ajami, N.J.; Petrosino, J.F.; Costa-Mattioli, M. Microbial Reconstitution Reverses Maternal Diet-Induced Social and Synaptic Deficits in Offspring. Cell 2016, 165, 1762–1775. [Google Scholar] [CrossRef] [PubMed]
- Kumar, H.; Sharma, B. Minocycline Ameliorates Prenatal Valproic Acid Induced Autistic Behaviour, Biochemistry and Blood Brain Barrier Impairments in Rats. Brain Res. 2016, 1630, 83–97. [Google Scholar] [CrossRef]
- Urbano, M.; Okwara, L.; Manser, P.; Hartmann, K.; Herndon, A.; Deutsch, S.I. A Trial of D-Cycloserine to Treat Stereotypies in Older Adolescents and Young Adults with Autism Spectrum Disorder. Clin. Neuropharmacol. 2014, 37, 69–72. [Google Scholar] [CrossRef] [PubMed]
- Sandler, R.H.; Finegold, S.M.; Bolte, E.R.; Buchanan, C.P.; Maxwell, A.P.; Väisänen, M.L.; Nelson, M.N.; Wexler, H.M. Short-Term Benefit from Oral Vancomycin Treatment of Regressive-Onset Autism. J. Child. Neurol. 2000, 15, 429–435. [Google Scholar] [CrossRef]
- Kang, D.W.; Adams, J.B.; Gregory, A.C.; Borody, T.; Chittick, L.; Fasano, A.; Khoruts, A.; Geis, E.; Maldonado, J.; McDonough-Means, S.; et al. Microbiota Transfer Therapy Alters Gut Ecosystem and Improves Gastrointestinal and Autism Symptoms: An Open-Label Study. Microbiome 2017, 5, 10. [Google Scholar] [CrossRef]
- Tierney, C.; Mayes, S.; Lohs, S.R.; Black, A.; Gisin, E.; Veglia, M. How Valid Is the Checklist for Autism Spectrum Disorder When a Child Has Apraxia of Speech? J. Dev. Behav. Pediatr. 2015, 36, 569–574. [Google Scholar] [CrossRef] [PubMed]
- Hicks, S.D.; Ignacio, C.; Gentile, K.; Middleton, F.A. Salivary MiRNA Profiles Identify Children with Autism Spectrum Disorder, Correlate with Adaptive Behavior, and Implicate Autism Candidate Genes Involved in Neurodevelopment. BMC Pediatr. 2016, 16, 52. [Google Scholar] [CrossRef]
- Bercik, P.; Park, A.J.; Sinclair, D.; Khoshdel, A.; Lu, J.; Huang, X.; Deng, Y.; Blennerhassett, P.A.; Fahnestock, M.; Moine, D.; et al. The Anxiolytic Effect of Bifidobacterium Longum NCC3001 Involves Vagal Pathways for Gut-Brain Communication. Neurogastroenterol. Motil. 2011, 23, 1132–1139. [Google Scholar] [CrossRef]
- Hicks, S.D.; Uhlig, R.; Afshari, P.; Williams, J.; Chroneos, M.; Tierney-Aves, C.; Wagner, K.; Middleton, F.A. Oral Microbiome Activity in Children with Autism Spectrum Disorder. Autism Res. 2018, 11, 1286–1299. [Google Scholar] [CrossRef]
- De Angelis, M.; Piccolo, M.; Vannini, L.; Siragusa, S.; De Giacomo, A.; Serrazzanetti, D.I.; Cristofori, F.; Guerzoni, M.E.; Gobbetti, M.; Francavilla, R. Fecal Microbiota and Metabolome of Children with Autism and Pervasive Developmental Disorder Not Otherwise Specified. PLoS ONE 2013, 8, e76993. [Google Scholar] [CrossRef]
- Aldred, S.; Moore, K.M.; Fitzgerald, M.; Waring, R.H. Plasma Amino Acid Levels in Children with Autism and Their Families. J. Autism Dev. Disord. 2003, 33, 93–97. [Google Scholar] [CrossRef] [PubMed]
- Shinohe, A.; Hashimoto, K.; Nakamura, K.; Tsujii, M.; Iwata, Y.; Tsuchiya, K.J.; Sekine, Y.; Suda, S.; Suzuki, K.; Sugihara, G.; et al. Increased Serum Levels of Glutamate in Adult Patients with Autism. Prog. Neuropsychopharmacol. Biol. Psychiatry 2006, 30, 1472–1477. [Google Scholar] [CrossRef] [PubMed]
- Brown, M.S.; Singel, D.; Hepburn, S.; Rojas, D.C. Increased Glutamate Concentration in the Auditory Cortex of Persons with Autism and First-Degree Relatives: A (1)H-MRS Study. Autism Res. 2013, 6, 1–10. [Google Scholar] [CrossRef]
- Wu, T.; Wang, H.; Lu, W.; Zhai, Q.; Zhang, Q.; Yuan, W.; Gu, Z.; Zhao, J.; Zhang, H.; Chen, W. Potential of Gut Microbiome for Detection of Autism Spectrum Disorder. Microb. Pathog. 2020, 149, 104568. [Google Scholar] [CrossRef]
- Erkosar, B.; Dupuis, C.; Savary, L.; Kawecki, T.J. Shared Genetic Architecture Links Energy Metabolism, Behavior and Starvation Resistance along a Power-Endurance Axis. Evol. Lett. 2025, 9, 150–162. [Google Scholar] [CrossRef]
- Retuerto, M.; Al-Shakhshir, H.; Herrada, J.; McCormick, T.S.; Ghannoum, M.A. Analysis of Gut Bacterial and Fungal Microbiota in Children with Autism Spectrum Disorder and Their Non-Autistic Siblings. Nutrients 2024, 16, 3004. [Google Scholar] [CrossRef]
- Xiao, H.-L.; Zhu, H.; Zeng, T.-A.; Xu, F.; Yu, S.-H.; Yang, C.-J. Potential Similarities in Gut Microbiota Composition between Autism Spectrum Disorder and Neurotypical Siblings: Insights from a Comprehensive Meta-Analysis. Neuroscience 2025, 567, 172–181. [Google Scholar] [CrossRef]
- Son, J.S.; Zheng, L.J.; Rowehl, L.M.; Tian, X.; Zhang, Y.; Zhu, W.; Litcher-Kelly, L.; Gadow, K.D.; Gathungu, G.; Robertson, C.E.; et al. Comparison of Fecal Microbiota in Children with Autism Spectrum Disorders and Neurotypical Siblings in the Simons Simplex Collection. PLoS ONE 2015, 10, e0137725. [Google Scholar] [CrossRef] [PubMed]
- Faraone, S.V.; Larsson, H. Genetics of Attention Deficit Hyperactivity Disorder. Mol. Psychiatry 2019, 24, 562–575. [Google Scholar] [CrossRef]
- McElhanon, B.O.; McCracken, C.; Karpen, S.; Sharp, W.G. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Meta-Analysis. Pediatrics 2014, 133, 872–883. [Google Scholar] [CrossRef]
- Holingue, C.; Newill, C.; Lee, L.C.; Pasricha, P.J.; Fallin, M.D. Gastrointestinal Symptoms in Autism Spectrum Disorder: A Review of the Literature on Ascertainment and Prevalence. Autism Res. 2018, 11, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Carissimi, C.; Laudadio, I.; Palone, F.; Fulci, V.; Cesi, V.; Cardona, F.; Alfonsi, C.; Cucchiara, S.; Isoldi, S.; Stronati, L. Functional Analysis of Gut Microbiota and Immunoinflammation in Children with Autism Spectrum Disorders. Dig. Liver Dis. 2019, 51, 1366–1374. [Google Scholar] [CrossRef]
- Rose, D.R.; Yang, H.; Serena, G.; Sturgeon, C.; Ma, B.; Careaga, M.; Hughes, H.K.; Angkustsiri, K.; Rose, M.; Hertz-Picciotto, I.; et al. Differential Immune Responses and Microbiota Profiles in Children with Autism Spectrum Disorders and Co-Morbid Gastrointestinal Symptoms. Brain Behav. Immun. 2018, 70, 354–368. [Google Scholar] [CrossRef] [PubMed]
- Özyurt, G.; Öztürk, Y.; Appak, Y.Ç.; Arslan, F.D.; Baran, M.; Karakoyun, İ.; Tufan, A.E.; Pekcanlar, A.A. Increased Zonulin Is Associated with Hyperactivity and Social Dysfunctions in Children with Attention Deficit Hyperactivity Disorder. Compr. Psychiatry 2018, 87, 138–142. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sun, Z.; Zhang, Q.; Sun, Z.; Su, Y.; Song, J.; Wang, B.; Gao, R. Preliminary Evidence for an Influence of Exposure to Polycyclic Aromatic Hydrocarbons on the Composition of the Gut Microbiota and Neurodevelopment in Three-Year-Old Healthy Children. BMC Pediatr. 2021, 21, 86. [Google Scholar] [CrossRef]
- Troisi, J.; Autio, R.; Beopoulos, T.; Bravaccio, C.; Carraturo, F.; Corrivetti, G.; Cunningham, S.; Devane, S.; Fallin, D.; Fetissov, S.; et al. Genome, Environment, Microbiome and Metabolome in Autism (GEMMA) Study Design: Biomarkers Identification for Precision Treatment and Primary Prevention of Autism Spectrum Disorders by an Integrated Multi-Omics Systems Biology Approach. Brain Sci. 2020, 10, 743. [Google Scholar] [CrossRef]
- Salenius, K.; Väljä, N.; Thusberg, S.; Iris, F.; Ladd-Acosta, C.; Roos, C.; Nykter, M.; Fasano, A.; Autio, R.; Lin, J.; et al. Exploring autism spectrum disorder and co-occurring trait associations to elucidate multivariate genetic mechanisms and insights. BMC Psychiatry 2024, 24, 934. [Google Scholar] [CrossRef]
- Zeng, P.; Zhang, C.Z.; Fan, Z.X.; Yang, C.J.; Cai, W.Y.; Huang, Y.F.; Xiang, Z.J.; Wu, J.Y.; Zhang, J.; Yang, J. Effect of Probiotics on Children with Autism Spectrum Disorders: A Meta-Analysis. Ital. J. Pediatr. 2024, 50, 120. [Google Scholar] [CrossRef]
- Mazzone, L.; Dooling, S.W.; Volpe, E.; Uljarević, M.; Waters, J.L.; Sabatini, A.; Arturi, L.; Abate, R.; Riccioni, A.; Siracusano, M.; et al. Precision microbial intervention improves social behavior but not autism severity: A pilot double-blind randomized placebo-controlled trial. Cell Host Microbe 2024, 32, 106–116.e6. [Google Scholar] [CrossRef]
- Persico, A.M.; Ricciardello, A.; Lamberti, M.; Turriziani, L.; Cucinotta, F.; Brogna, C.; Vitiello, B.; Arango, C. The Pediatric Psychopharmacology of Autism Spectrum Disorder: A Systematic Review—Part I: The Past and the Present. Prog. Neuropsychopharmacol. Biol. Psychiatry 2021, 110, 110326. [Google Scholar] [CrossRef]
- Schaaf, R.C.; Toth-Cohen, S.; Johnson, S.L.; Outten, G.; Benevides, T.W. The Everyday Routines of Families of Children with Autism: Examining the Impact of Sensory Processing Difficulties on the Family. Autism 2011, 15, 373–389. [Google Scholar] [CrossRef] [PubMed]
- Wachtel, L.E. Treatment of Catatonia in Autism Spectrum Disorders. Acta Psychiatr. Scand. 2019, 139, 46–55. [Google Scholar] [CrossRef]
- Twohig, M.P.; Hayes, S.C.; Masuda, A. A Preliminary Investigation of Acceptance and Commitment Therapy as a Treatment for Chronic Skin Picking. Behav. Res. Ther. 2006, 44, 1513–1522. [Google Scholar] [CrossRef] [PubMed]
- Flessner, C.A.; Busch, A.M.; Heideman, P.W.; Woods, D.W. Acceptance-Enhanced Behavior Therapy (AEBT) for Trichotillomania and Chronic Skin Picking: Exploring the Effects of Component Sequencing. Behav. Modif. 2008, 32, 579–594. [Google Scholar] [CrossRef]
- Grant, J.E.; Odlaug, B.L.; Chamberlain, S.R.; Keuthen, N.J.; Lochner, C.; Stein, D.J. Skin Picking Disorder. Am. J. Psychiatry 2012, 169, 1143–1149. [Google Scholar] [CrossRef]
- Grant, J.E.; Odlaug, B.L.; Suck, W.K. Lamotrigine Treatment of Pathologic Skin Picking: An Open-Label Study. J. Clin. Psychiatry 2007, 68, 1384–1391. [Google Scholar] [CrossRef]
- Sasso, D.A.; Kalanithi, P.S.A.; Trueblood, K.V.; Pittenger, C.; Kelmendi, B.; Wayslink, S.; Malison, R.T.; Krystal, J.H.; Coric, V. Beneficial Effects of the Glutamate-Modulating Agent Riluzole on Disordered Eating and Pathological Skin-Picking Behaviors. J. Clin. Psychopharmacol. 2006, 26, 685–687. [Google Scholar] [CrossRef] [PubMed]
- Symons, F.J.; Thompson, A.; Rodriguez, M.C. Self-Injurious Behavior and the Efficacy of Naltrexone Treatment: A Quantitative Synthesis. Ment. Retard. Dev. Disabil. Res. Rev. 2004, 10, 193–200. [Google Scholar] [CrossRef]
- Sanders, M.E.; Heimbach, J.T.; Pot, B.; Tancredi, D.; Lenoir-Wijnkoop, I.; Lähteenmäki-Uutela, A.; Gueimonde, M.; Bañares, S. Health Claims Substantiation for Probiotic and Prebiotic Products. Gut Microbes 2011, 2, 127–133. [Google Scholar] [CrossRef]
- Gibson, G.R.; Roberfroid, M.B. Dietary Modulation of the Human Colonic Microbiota: Introducing the Concept of Prebiotics. J. Nutr. 1995, 125, 1401–1412. [Google Scholar] [CrossRef]
- Gibson, G.R.; Hutkins, R.; Sanders, M.E.; Prescott, S.L.; Reimer, R.A.; Salminen, S.J.; Scott, K.; Stanton, C.; Swanson, K.S.; Cani, P.D.; et al. Expert Consensus Document: The International Scientific Association for Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Prebiotics. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 491–502. [Google Scholar] [CrossRef]
- Markowiak, P.; Ślizewska, K. Effects of Probiotics, Prebiotics, and Synbiotics on Human Health. Nutrients 2017, 9, 1021. [Google Scholar] [CrossRef]
- Sharma, M.; Shukla, G. Metabiotics: One Step Ahead of Probiotics; an Insight into Mechanisms Involved in Anticancerous Effect in Colorectal Cancer. Front. Microbiol. 2016, 7, 1940. [Google Scholar] [CrossRef] [PubMed]
- Sherwin, E.; Bordenstein, S.R.; Quinn, J.L.; Dinan, T.G.; Cryan, J.F. Microbiota and the Social Brain. Science 2019, 366, eaar2016. [Google Scholar] [CrossRef]
- Song, W.; Zhang, M.; Teng, L.; Wang, Y.; Zhu, L. Prebiotics and Probiotics for Autism Spectrum Disorder: A Systematic Review and Meta-Analysis of Controlled Clinical Trials. J. Med. Microbiol. 2022, 71, 001510. [Google Scholar] [CrossRef] [PubMed]
- Perrin, S.; Warchol, M.; Grill, J.P.; Schneider, F. Fermentations of Fructo-Oligosaccharides and Their Components by Bifidobacterium Infantis ATCC 15697 on Batch Culture in Semi-Synthetic Medium. J Appl Microbiol 2001, 90, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.A.; Krajmalnik-Brown, R.; Porazinska, D.L.; Weiss, S.J.; Knight, R. Toward Effective Probiotics for Autism and Other Neurodevelopmental Disorders. Cell 2013, 155, 1446–1448. [Google Scholar] [CrossRef]
- Patusco, R.; Ziegler, J. Role of Probiotics in Managing Gastrointestinal Dysfunction in Children with Autism Spectrum Disorder: An Update for Practitioners. Adv. Nutr. 2018, 9, 637–650. [Google Scholar] [CrossRef]
- Sun, N.; Xue, Y.; Wei, S.; Wu, B.; Wang, H.; Zeng, D.; Zhao, Y.; Khalique, A.; Pan, K.; Zeng, Y.; et al. Compound Probiotics Improve Body Growth Performance by Enhancing Intestinal Development of Broilers with Subclinical Necrotic Enteritis. Probiot. Antimicrob. Proteins 2023, 15, 558–572. [Google Scholar] [CrossRef]
- Ristori, M.V.; Quagliariello, A.; Reddel, S.; Ianiro, G.; Vicari, S.; Gasbarrini, A.; Putignani, L. Autism, Gastrointestinal Symptoms and Modulation of Gut Microbiota by Nutritional Interventions. Nutrients 2019, 11, 2812. [Google Scholar] [CrossRef]
- Tomé-Castro, X.M.; Rodriguez-Arrastia, M.; Cardona, D.; Rueda-Ruzafa, L.; Molina-Torres, G.; Roman, P. Probiotics as a Therapeutic Strategy in Obesity and Overweight: A Systematic Review. Benef. Microbes 2021, 12, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Siafis, S.; Çıray, O.; Wu, H.; Schneider-Thoma, J.; Bighelli, I.; Krause, M.; Rodolico, A.; Ceraso, A.; Deste, G.; Huhn, M.; et al. Pharmacological and Dietary-Supplement Treatments for Autism Spectrum Disorder: A Systematic Review and Network Meta-Analysis. Mol. Autism 2022, 13, 10. [Google Scholar] [CrossRef] [PubMed]
- Iglesias-Vázquez, L.; Van Ginkel Riba, G.; Arija, V.; Canals, J. Composition of Gut Microbiota in Children with Autism Spectrum Disorder: A Systematic Review and Meta-Analysis. Nutrients 2020, 12, 792. [Google Scholar] [CrossRef] [PubMed]
- Balian, A.; Cirio, S.; Salerno, C.; Wolf, T.G.; Campus, G.; Cagetti, M.G. Is Visual Pedagogy Effective in Improving Cooperation Towards Oral Hygiene and Dental Care in Children with Autism Spectrum Disorder? A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 789. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
D’Angelo, E.; Fiori, F.; Ferraro, G.A.; Tessitore, A.; Nazzaro, L.; Serpico, R.; Contaldo, M. Autism Spectrum Disorder, Oral Implications, and Oral Microbiota. Children 2025, 12, 368. https://doi.org/10.3390/children12030368
D’Angelo E, Fiori F, Ferraro GA, Tessitore A, Nazzaro L, Serpico R, Contaldo M. Autism Spectrum Disorder, Oral Implications, and Oral Microbiota. Children. 2025; 12(3):368. https://doi.org/10.3390/children12030368
Chicago/Turabian StyleD’Angelo, Emiliana, Fausto Fiori, Giuseppe A. Ferraro, Assunta Tessitore, Luca Nazzaro, Rosario Serpico, and Maria Contaldo. 2025. "Autism Spectrum Disorder, Oral Implications, and Oral Microbiota" Children 12, no. 3: 368. https://doi.org/10.3390/children12030368
APA StyleD’Angelo, E., Fiori, F., Ferraro, G. A., Tessitore, A., Nazzaro, L., Serpico, R., & Contaldo, M. (2025). Autism Spectrum Disorder, Oral Implications, and Oral Microbiota. Children, 12(3), 368. https://doi.org/10.3390/children12030368








