Highlights
What are the main findings?
- •
- Antegrade continence enemas (ACE) and Botulinum Toxin (BT) both improved oral laxative use and constipation-related symptoms at one year postoperatively from baseline.
- •
- ACE recipients had a greater reduction in oral laxative use, while BT was associated with fewer complications and healthcare utilization visits at 1 year postoperatively.
What is the implication of the main finding?
- •
- Decision-making for surgical interventions in pediatric CIC should be individualized and balanced between effectiveness and healthcare utilization.
Abstract
Introduction: Chronic Idiopathic Constipation (CIC) is a common pediatric gastrointestinal disorder (GI) characterized by persistent difficulty in defecation, with no identifiable underlying cause. Although most patients are successfully treated with medical therapies, surgical intervention is often needed for refractory disease. We evaluated the impact of Antegrade Continence Enemas (ACE) and Botulinum Toxin (BT) injection to the internal anal sphincter on laxative use, symptom resolution, and healthcare utilization. Methods: A retrospective chart review was conducted to identify patients ≤ 18 years old presenting to a pediatric surgery clinic with a chief complaint of CIC between 1 March 2014 and 1 March 2024. Patients meeting the Rome IV criteria for idiopathic constipation and fecal incontinence were included. Surgical procedures were categorized into BT injection or ACE channel creation. The primary outcome was change in daily oral laxative use at 1 year, and secondary outcomes included symptom resolution and CIC-healthcare utilization at 1 year postoperatively. Results: Of the 125 children who presented with CIC, 47 (37.6%) underwent surgery. Mean age was 6 years at the time of surgery. 17 (36.2%) had ACE channel creation, while 30 (63.8%) received BT injections. At 1 year, daily oral laxative polypharmacy decreased from 60.2% to 41.0%, p < 0.001, with a greater reduction in ACE than BT (adjusted mean difference: −1.05, 95% CI: −1.75 to 0.34, p = 0.004) after adjusting for demographics and baseline clinical factors. Overall, symptom resolution of encopresis (79.1% to 39.5%, p = 0.001), abdominal pain (88.4% to 27.9%, p < 0.001), and abdominal distension (67.4% to 27.9%, p < 0.001) was observed with no significant difference between groups at 1 year. ACE patients had significantly more postoperative outpatient CIC-related visits and no change in ED visits compared to fewer visits in BT patients. Conclusions: Both ACE and BT recipients had improvements in constipation-related symptoms and laxative use. However, ACE resulted in a significantly greater reduction in daily laxative use and more postoperative CIC-healthcare visits than BT alone.
1. Introduction
Chronic constipation is one of the most common gastrointestinal disorders, affecting up to 30% of children and adolescents [1,2,3]. It is defined as infrequent or difficult stool passage, or a sensation of incomplete evacuation, persisting for at least three months [2,4,5]. Although structural, inflammatory, and metabolic abnormalities can cause constipation, the majority of pediatric cases lack an identifiable etiology and are classified as chronic idiopathic constipation (CIC) [6,7]. CIC significantly impairs quality of life and incurs a substantial economic burden on the healthcare system [8,9,10,11,12,13]. In the United States alone, CIC-related encounters account for more than 2.5 million hospital and physical visits per year, resulting in $3.9 billion in annual healthcare costs. First-line management includes medical and behavioral therapies such as laxatives, enemas, and dietary modification. However, children with moderate to severe forms of CIC, particularly those with encopresis or soiling, intractable abdominal pain, distension, or fecal impaction, often remain refractory to medical therapy and may require surgical intervention [7,14].
Surgical management of pediatric constipation includes anal procedures, antegrade continence enemas (ACE), and, in refractory cases, intestinal diversion or resection [7,15,16]. Among anal procedures, botulinum toxin (BT) injection into the internal anal sphincter (IAS) is preferred over myotomy, myectomy, or anal dilatation due to its minimally invasive nature, safety, and effectiveness [15,17]. ACE procedures, including Malone appendicostomy and cecostomy, provide direct colonic access for irrigation and are effective in improving symptoms, though complications such as infection, leakage, or stenosis may require revision or reversal [18,19]. More invasive options, including diversion (ileostomy or colostomy) or colorectal resection, are typically reserved for the most severe and refractory cases [15]. No single method has been established as the best practice for CIC, and surgical approach often varies by patient and provider.
BT injections and ACE channels are common surgical treatments for medically refractory CIC; however, there is limited comparative data on their outcomes [15,16,17,20]. To date, no studies have directly compared ACE and BT in children, and there are no guidelines to inform treatment selection. We conducted a retrospective cohort study of pediatric patients with CIC who had surgery at a single institution over a 10-year period to compare the effectiveness of BT and ACE in clinically important treatment outcomes: symptom resolution, change in laxative use, and healthcare utilization. Our findings demonstrate the effectiveness of ACE and BT in symptom resolution and oral laxative use reduction. Despite a greater reduction in laxative use in ACE recipients, healthcare utilization and complication rates were higher at one year postoperatively.
2. Materials and Methods
With permission from our institutional review board (IRB), we performed a retrospective review of patients presenting with a chief complaint of chronic idiopathic constipation (International Classification of Diseases, 10th revision code: K59.04) to the pediatric surgery clinic at the University of Virginia between 1 March 2014 and 1 March 2024. Informed consent was not required for this study. The study was conducted in accordance with the Declaration of Helsinki, and the protocol was approved by the IRB of the University of Virginia (HSR230261) on 7 September 2023. Inclusion criteria included patients ages 0–18 years with symptoms consistent with chronic idiopathic constipation according to the Rome IV criteria [21,22], lasting at least three months. Exclusion criteria included Hirschsprung disease, anorectal malformations, congenital colorectal disease, spina bifida, inflammatory bowel disease, metabolic abnormalities, and other known causes of constipation. Patients with other comorbidities or history of prior abdominal surgery not directly related to constipation or the outcomes of interest were not excluded. Among eligible patients, those who underwent surgical intervention, specifically ACE channel placement or BT injection, were identified and categorized by procedure type. The ACE group included patients who underwent Malone appendicostomy or cecostomy button placement as previously described in the literature [23,24]. The BT group comprised patients who received 100 units of BT injected into the IAS, as previously described [25]. Patients who underwent ACE channel placement after BT injection were categorized as the ACE group. Patients who received multiple BT injections were analyzed based on their response to their first BT injection.
Data collected included demographics (age, sex, race/ethnicity), insurance provider, home-to-hospital travel distance, and Area Deprivation Index (ADI) [26,27] percentile as a measure of socioeconomic status. Clinical characteristics, such as diagnostic testing for constipation, constipation-related symptoms, and the presence of a neurodiverse disorder were also obtained. Neurodiverse disorders were defined as autism spectrum disorder (ASD), anxiety, attention-deficit/hyperactivity disorder (ADHD), or intellectual and developmental disabilities (IDD). The authors included neurodiverse disorders given the well-documented association between neurodiversity and constipation [28,29,30,31]. Constipation-related variables assessed included abdominal pain, abdominal distension, bowel movement frequency, encopresis, and oral laxative use. Bowel movement frequency was reported as the number of bowel movements per week (BM/wk). Oral laxatives were categorized by mechanism (osmotics, stimulants, secretagogues) and total number of laxative agents taken daily (e.g., polyethylene glycol, senna, milk of magnesia).
Among patients who underwent surgical intervention, including both ACE and BT, data collected included age at surgery, frequency of GI and constipation-related healthcare visits per year before and one year after surgery, and 30-day postoperative complications. The primary outcome was the change in the total number of daily oral laxative agents used from baseline to one year postoperatively. Secondary outcomes included resolution of abdominal pain, distension, and encopresis, if present. The frequency of BM/wk and changes in GI-related healthcare visits were also examined over the same period. These data were abstracted from patients’ electronic medical records including doctors’ notes from GI-related healthcare visits. Patients who were lost to follow-up were excluded from the analysis of outcomes at the corresponding evaluation time points.
Numeric variables were summarized as mean with standard deviation (SD). Categorical variables were reported as frequency and percentage. Between-group comparisons were performed using McNemar for paired and Chi-square or Fisher’s exact tests for unpaired categorical variables. A paired Student’s t-test was used for continuous variables. Univariate and multivariate linear regression analyses were used to evaluate the outcomes of interest while accounting for potential confounders. Statistical analyses were conducted using R Studio (version 2024.04.2+764; Boston, MA, USA). A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Study Population Characteristics
A total of 125 patients met criteria for chronic idiopathic constipation, of whom 47 (37.6%) underwent surgery (Figure 1). Demographics, including sex and race/ethnicity, did not differ between surgical and non-surgical groups, nor did insurance type, and ADI percentile (Table 1). Surgical patients were significantly more likely to have a neurodiverse diagnosis (68.1% vs. 41.0%, p = 0.003). Constipation-related symptoms were common: abdominal pain was reported in 82% of patients, and abdominal distension in 67.2%, with no differences between surgical and nonsurgical groups. Surgical patients were more likely to present with encopresis (79.2% vs. 39.7%, p < 0.001). Twenty-one patients underwent lower gastrointestinal endoscopy for diagnostic evaluation, with 12 in the nonsurgical group and 9 in the surgical group. Only one patient, from the surgical group, had an abnormal finding of colonic redundancy. Laxative use was common (99.2%), with no significant difference in surgical and nonsurgical patients (100% vs. 98.7%, p = 1.0).
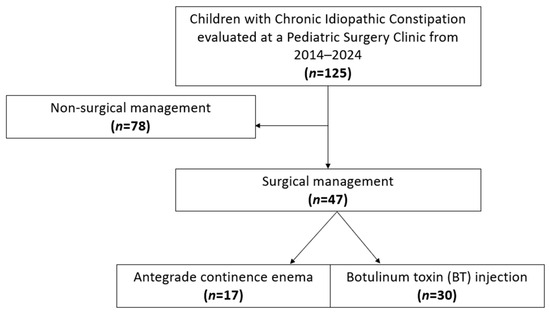
Figure 1.
STROBE flowchart of study cohort.

Table 1.
Baseline characteristics of pediatric patients with Chronic Idiopathic Constipation.
3.2. Characteristics of Surgical Patients
Among the 47 patients who underwent surgery, 17 (36.2%) received an ACE channel and 30 (63.8%) underwent BT injections alone. Ten ACE patients (58.8%) had a prior history of BT injection. The median interval between BT and ACE placement was 7.9 months (IQR 2.7–19.6 months). For this study, these patients were categorized within the ACE group, as they demonstrated no symptom improvement prior to ACE placement. Choice of treatment was determined through shared decision-making between patient and physician. The mean age at the time of surgery was 6.02 ± 2.60 years old. Within the ACE group, 9 patients (52.9%) had a Malone antegrade continence enema (MACE) and 8 (47.1%) underwent cecostomy. ACE reversal was performed in 8 patients (47.1%), with two cases due to treatment failure, from no improvement in abdominal distension, pain, and encopresis, and six following symptomatic improvement. The mean duration of ACE prior to reversal was 49.5 ± 22.6 months.
Of those treated with BT alone, most (90.0%) received a single injection, while 3 patients received two injections. In patients who received multiple BT injections, only the treatment response from the first BT treatment was included in our analysis. Compared with BT patients, ACE patients were significantly older at the time of presenting to a pediatric surgery clinic (7.72 ± 2.35 vs. 5.02 ± 1.93, p = 0.0001) and at surgery (9.10 ± 1.67 vs. 5.22 ± 1.85 years, p < 0.01). ACE patients also reported more frequent weekly bowel movements preoperatively (11.32 ± 10.76 vs. 4.37 ± 4.07 BM/wk, p = 0.003) and had a higher occurrence of encopresis prior to surgery (100% vs. 70.0%, p = 0.034). Demographics, neurodiverse disorder diagnosis, distance to hospital, ADI percentile, insurance, and daily laxative use did not differ significantly between groups at baseline (Table 2). Contrast enema was performed in 47.1% of ACE patients and 53.3% of BT patients, with abnormal findings in 0% and 12.5%, respectively (p = 0.536). Other preoperative symptoms were similar, including abdominal pain (94.1% vs. 86.7%, p = 0.761) and abdominal distension (52.9% vs. 70.0%, p = 0.393). Yearly CIC-related healthcare encounters before surgery were comparable between groups (5.33 ± 3.01 vs. 6.22 ± 5.17, p = 0.524).

Table 2.
Baseline characteristics of surgical pediatric patients with Chronic Idiopathic Constipation.
3.3. Daily Oral Laxative Use in Surgical Patients
Oral laxative use was expectedly common among patients undergoing surgery, with 97.9% taking them at the time of surgery. Laxatives were categorized by mechanism of action (osmotic, stimulant, bulk-forming, stool softeners, secretagogues). Most patients (61.7%) were prescribed two or more classes, while 36.2% received a single class, and only 2.1% received none. Among specific laxative classes, osmotic agents were the most frequently prescribed (95.7%), followed by stimulants (56.5%). Fewer patients received secretagogues, including lubiprostone (4.3%) and linaclotide (2.2%), while none were prescribed stool softeners or bulk-forming agents. In the ACE group (n = 17), 58.8% of patients were prescribed two or more classes of laxatives, 35.3% received a single class, and one (5.9%) received none. Among the 16 patients prescribed laxatives at the time of surgery, osmotic agents were most frequently used in 93.8% of patients, followed by stimulants (56.3%) and secretagogues (25%). In the BT group (n = 30), 63.3% of patients were prescribed two or more classes and 36.7% a single class. Similarly, osmotic agents were the most common (96.7%), with stimulants (56.7%) and secretagogues (3.3%) prescribed less often.
For paired analysis before and after surgery, patients with missing documentation of daily oral laxative use at one year postoperatively were excluded. This resulted in a total of 44 patients out of the 47 who underwent surgery, including 17 in the ACE group and 27 in the BT group. Laxative use was significantly reduced in both groups at 1 year. Overall, 26 patients (59.1%) required two or more laxatives preoperatively, compared with only 8 patients (18.2%) postoperatively (McNemar’s χ2 = 14.0, p < 0.001). In the ACE group, 10 patients (58.8%) required polypharmacy (two or more laxatives) prior to surgery, whereas none required more than one laxative at 1 year (McNemar’s χ2 = 8.0, p = 0.004). In the BT group, polypharmacy decreased from 16 patients (59.3%) at baseline to 8 patients (29.6%) postoperatively (McNemar’s χ2 = 5.0, p = 0.030) (Figure 2A). While a higher percentage of patients in the ACE group showed improvement in polypharmacy compared to those who received BT, it was not statistically significant (58.8% vs. 29.6%, χ2 = 3.3, p = 0.068).
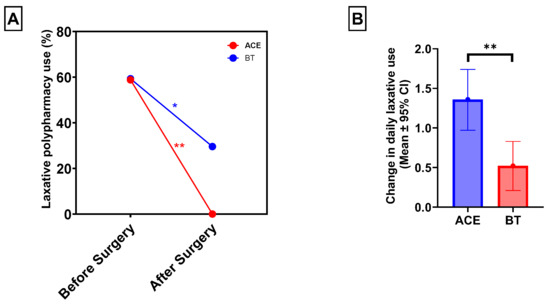
Figure 2.
ACE and BT resulted in significantly reduced oral laxative polypharmacy use at one year postoperatively (A). ACE recipients had a significantly greater reduction in the number of daily oral laxative medications at one year postoperatively: 1.36 (95% CI: 0.97–1.75) vs. 0.52 (95% CI: 0.21–0.83) (B). * p < 0.05; ** p < 0.01.
Among the 44 patients with 1-year follow-up data, the mean number of daily oral laxatives before surgery decreased significantly from 1.64 ± 0.67 to 0.77 ± 0.74 at 1 year postoperatively (p < 0.001). In the ACE group, daily laxative use decreased from 1.54 ± 0.79 preoperatively to 0.17 ± 0.39 at 1 year, p < 0.01). In the BT group, daily laxative use decreased from 1.70 ± 0.60 to 1.15 ± 0.66, 0.52; p = 0.002. After adjusting for age, sex, race/ethnicity, neurodiverse disorder, distance from hospital, and frequency of CIC-related visits, patients who underwent ACE experienced a significantly greater reduction in daily laxative use compared with those who received BT alone (adjusted mean difference: 0.94; 95% CI: −1.56 to −1.32; p = 0.005) (Figure 2B).
3.4. Chronic Constipation-Related Symptoms in Surgical Patients
We evaluated the impact of surgical intervention on constipation-related symptoms, including bowel movement frequency, encopresis, abdominal pain, and abdominal distension. Symptom data at 1 year were available for 43 patients (91.4%) among the surgical patients (n = 47). Bowel movement frequency did not significantly change (6.88 ± 7.88 BM/wk preoperatively vs. 7.99 ± 5.20 BM/wk at 1 year, p = 0.735). There were no differences in bowel movement frequency in ACE (11.53 ± 10.76 to 10.82 ± 5.38 BM/wk, p = 0.835) and BT (4.06 ± 3.95 to 5.58 ± 3.67 BM/wk, p = 0.336).
In contrast, encopresis improved markedly, decreasing from 34 patients (79.1%) preoperatively to 17 patients (39.5%) at 1 year (McNemar’s χ2 = 15.1, p = 0.001; Figure 3A). Abdominal pain was also significantly reduced, from 38 patients (88.4%) before surgery to 12 patients (27.9%) at 1 year (McNemar’s χ2 = 24.1, p < 0.001). Similarly, abdominal distension decreased from 29 patients (67.4%) to 12 patients (27.9%) (McNemar’s χ2 = 15.1, p < 0.001). Subgroup analyses demonstrated comparable resolution rates between ACE and BT groups for encopresis (47.1% vs. 52.9%, χ2 = 0.0, p = 1.00), abdominal pain (75.0% vs. 63.6%, χ2 = 0.2, p = 0.70), and abdominal distension (66.7% vs. 55.0%, χ2 = 0.03, p = 0.70; Figure 3B).
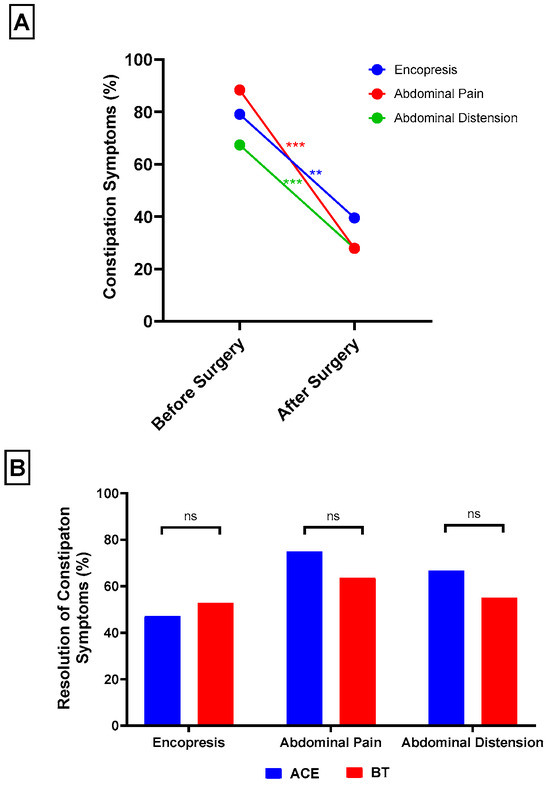
Figure 3.
Overall, surgical patients experienced significant reduction in encopresis, abdominal pain, and abdominal distension at one year postoperatively (A). ACE and BT patients showed similar rates of resolution in encopresis, abdominal pain, and abdominal distension (B). ** p < 0.01; *** p < 0.001.
3.5. Chronic Idiopathic Constipation-Related Healthcare Visits in Surgical Patients
We evaluated healthcare utilization related to constipation before and after surgery. CIC-related visits were defined as encounters with any healthcare provider in which constipation was documented as a problem or in the encounter note. Among all surgical patients, the mean number of yearly outpatient CIC-related visits was similar before and after surgery (6.48 ± 6.02 vs. 6.83 ± 6.26, p = 0.70). Subgroup analyses revealed divergent trends: ACE patients had a significant increase in visits (5.33 ± 3.01 to 13.5 ± 5.09, p < 0.001), whereas BT patients had a significant decrease (6.52 ± 5.39 to 3.38 ± 2.58, p = 0.01). On univariate comparison, ACE patients had a mean increase of 8.2 visits per year, while BT recipients and a mean decrease of 3.2 visits (difference = −11.37 [95% CI: −14.7 to −8.1], p < 0.001). This difference remained significant in multivariate analysis adjusting for age, sex, race, neurodiversity, insurance type and distance from hospital (adjusted difference = −13.76 [95% CI: −18.85 to −8.67, p < 0.001]). No other covariates were significantly associated with change in visit frequency.
We also assessed constipation-related emergency department (ED) encounters. Overall, ED utilization did not differ significantly before and after surgery (0.55 ± 1.02 vs. 0.32 ± 0.84, p = 0.10). By surgery type, BT patients experienced a significant reduction (0.65 ± 1.02 to 0.15 ± 0.61, p = 0.003), while ACE patients had no significant change (0.53 ± 1.12 vs. 0.65 ± 1.11, p = 0.70).
Four patients (8.5%) experienced complications, including three in the ACE group (two with cecostomies and one with an MACE) and one in the BT group, within 30 days postoperatively. Two of the three patients in the ACE group with complications had a stoma surgical site infection, and the other experienced peri-stoma pain. The sole patient in the BT group had a complication of minor perianal bleeding.
4. Discussion
We found that a significant percentage of children (37.6%) referred to a surgeon received surgical intervention, including ACE channel creation and BT injection. Encopresis and neurodiversity were both associated with increased need for surgery. Our retrospective study demonstrates that both ACE and BT are effective surgical treatments for children with CIC. At one year postoperatively, daily laxative polypharmacy was reduced in 40.9% of patients, while encopresis, abdominal pain, and distension resolved in 39.6%, 60.5%, and 39.5% of patients, respectively. Notably, ACE recipients achieved a greater reduction in daily laxative use but experienced higher complication rates and more frequent CIC-related visits compared with patients treated with BT alone. These findings may help guide individualized clinical decision-making by identifying which patients are most likely to benefit from each intervention.
Pediatric CIC presents a challenge for surgeons, as evidence to guide surgical selection and predict response to treatment remains limited. In our study, treatment success was defined as reduced daily oral laxative use and symptom resolution at one year. ACE recipients achieved greater reductions in laxative use (58.5%) than BT recipients (29.6%), consistent with prior reports of effective long-term outcomes with ACE. Vriesman et al. reported that 73.9% and 52.2% of ACE patients discontinued laxatives at 6 and 8 months, respectively [20]. No prior studies have examined the effect of BT on oral laxative reduction. These differences in efficacy between ACE and BT may be in part explained by the distinct mechanisms of the two procedures. ACE channels provide direct access for antegrade delivery of irrigation with saline and the option of adding polyethylene glycol or stimulant-containing solutions, enabling mechanical emptying of the colon. BT injections, by contrast, act through temporary relaxation of the IAS, thereby reducing outlet obstruction and facilitating defecation [15,32,33]. However, because the pharmacologic effect of BT typically lasts only three to six months, its long-term impact on laxative reduction is variable. This transient effect of BT was observed in our cohort, where 10% of BT patients received more than one injection. The difference in efficacy between ACE and BT is also likely due to differences in patient selection between groups. In our cohort, ACE patients had more severe and longer duration of symptoms. Most (58.8%) of ACE patients had previously received BT injection and presumably had incomplete relief of symptoms, prompting escalation of surgical care. However, our observation of symptomatic improvement in BT recipients at 1 year is notable and suggests some patients with CIC may have long-lasting benefits from a single BT injection.
In addition to reducing laxative use, both ACE and BT improved constipation-related symptoms. Although bowel movement frequency did not significantly change from baseline to one year, other symptoms—including encopresis, abdominal pain, and distension—showed notable improvement. Encopresis, which is commonly due to overflow incontinence, is often a sign of severe constipation. It is particularly burdensome for children with CIC and is strongly associated with social stigma and reduced health-related quality of life [34,35]. In our cohort, encopresis was highly prevalent at baseline, especially among ACE recipients. One year after surgery, encopresis resolved with comparable improvement between ACE (47.1%) and BT (52.9%) recipients. Prior studies have also reported reductions in refractory encopresis following ACE, whereas the effect of BT has been more variable, with some reports showing improved stool frequency but limited impact on continence [36,37]. Abdominal pain and distension, reported by 88.4% in ACE and 67.4% in BT patients preoperatively, decreased to 27.9% at one year, with similar rates of relief between groups. Together, these findings highlight the effectiveness of both surgical interventions in not only reducing laxative use but also achieving meaningful symptom resolution.
Children with chronic constipation have higher healthcare utilization, including outpatient consultations, ED visits, and drug prescription costs, with overall expenditures estimated to be threefold greater than those of unaffected children [38]. Therefore, in addition to laxative use and symptom resolution, we examined healthcare utilization as an outcome of surgical treatment. In our cohort, the overall frequency of constipation-related encounters remained unchanged at one year compared with baseline. Subgroup analyses, however, revealed divergent trends: ACE recipients had significantly more outpatient visits at one year compared to baseline, whereas BT recipients had significantly fewer visits. ED encounters decreased among BT patients but remained unchanged in ACE patients. These findings suggest that although ACE effectively improves constipation-related symptoms and reduces laxative use, the need for maintenance care may contribute to greater postoperative healthcare utilization. ACE patients had a higher rate of complications than BT patients in our cohort, including peri-stoma pain and surgical site infection. Additionally, while nearly half of ACE patients ultimately underwent reversal, with the majority due to symptomatic improvement, some were due to treatment failure. Prior studies have reported mixed results on healthcare utilization after ACE, including decreased or unchanged outpatient visits but increased ED visits, largely related to device complications [34,39,40]. The increased need for healthcare utilization after ACE may also reflect greater disease severity in the ACE group, who likely required closer postoperative monitoring and medication adjustments, particularly within the first year.
This study has several strengths, including the direct comparison of two common surgical treatments for CIC, ACE and BT, and the assessment of laxative use, symptom resolution, and healthcare utilization over a ten-year period. However, several limitations should also be noted. First, the retrospective, single-center design and relatively small sample size limit the general application of our study. Similarly, nearly all surgical patients in this cohort presented with encopresis at baseline, which may not represent the broader CIC population. Next, few patients in our cohort underwent preoperative motility testing, such as anorectal or colonic manometry, which may help to guide and predict surgical treatment and response. Additionally, our analytic approach grouped patients who received ACE regardless of prior BT as ACE recipients because they demonstrated no symptom improvement prior to ACE placement. For BT analyses, outcomes were assessed after each patient’s first injection; these categorizations may have introduced bias. Another limitation of our study is that, given the relatively small size of the ACE cohort, we lacked the power to compare outcomes between ACE techniques (Malone appendicostomy vs. cecostomy). Finally, our study was limited to only postoperative outcomes in one year, and data accuracy was dependent on of doctors’ notes in the electronic medical record. Future prospective multicenter studies comparing the effects of surgical options on relieving constipation symptoms and healthcare costs are necessary, especially in children with neurodiverse diagnoses, who have consistently shown higher rates of constipation that are refractory to medical therapies [30,41].
5. Conclusions
In summary, both ACE and BT injections were effective surgical options for pediatric chronic idiopathic constipation. ACE resulted in greater reduction in laxative burden and resolution of constipation symptoms at the cost of increased postoperative healthcare utilization. These findings highlight the importance of an individualized approach in selecting the most appropriate management and examining opportunities to optimize postoperative care in this population.
Author Contributions
Conceptualization, L.S.C. and P.C.O.; methodology, L.S.C. and P.C.O.; software, P.C.O. and T.C.J.; validation, L.S.C., P.C.O. and T.C.J.; formal analysis, P.C.O.; investigation, L.S.C., P.C.O., T.C.J., C.M., A.B., L.W., A.J. and Z.H.; resources, L.S.C.; data curation, P.C.O., T.C.J., A.B., L.W., A.J. and Z.H.; writing—original draft preparation, P.C.O. and T.C.J.; writing—review and editing, L.S.C., P.C.O., T.C.J., C.M., A.B., L.W., A.J. and Z.H.; visualization, P.C.O. and T.C.J.; supervision, L.S.C., P.C.O. and C.M.; project administration, L.S.C. and P.C.O.; funding acquisition, L.S.C. and P.C.O. All authors have read and agreed to the published version of the manuscript.
Funding
P.C.O. is supported by a training grant from the NIH/NHLBI (T32HL007849). L.S.C. is supported by NIH/NIDDK K08DK133673.
Institutional Review Board Statement
This study was approved by the University of Virginia Institutional Review Board (protocol code HSR230261 and date of approval: 9 July 2023).
Informed Consent Statement
This retrospective study was deemed not to require informed consent according to our institution’s review board.
Data Availability Statement
In accordance with data sharing guidelines and to facilitate transparency in research, the data upon which this study was based is available upon request.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| CIC | Chronic Idiopathic Constipation |
| BT | Botulinum Toxin |
| ACE | Antegrade Continence Enema |
| MACE | Malone Antegrade Continence Enema |
| IAS | Internal Anal Sphincter |
| GI | Gastrointestinal |
| BM/wk | Bowel movement per week |
| ASD | Autism spectrum disorder |
| IDD | Intellectual and developmental disability |
| ADHD | Attention-deficit/hyperactivity |
| SD | Standard deviation |
References
- Madani, S.; Tsang, L.; Kamat, D. Constipation in Children: A Practical Review. Pediatr. Ann. 2016, 45, e189–e196. [Google Scholar] [CrossRef] [PubMed]
- Bischoff, A.; Brisighelli, G.; Dickie, B.; Frischer, J.; Levitt, M.A.; Peña, A. Idiopathic constipation: A challenging but manageable problem. J. Pediatr. Surg. 2018, 53, 1742–1747. [Google Scholar] [CrossRef]
- Reppucci, M.L.; Nolan, M.M.; Cooper, E.; Wehrli, L.A.; Schletker, J.; Ketzer, J.; Peña, A.; Bischoff, A.; De la Torre, L. The success rate of antegrade enemas for the management of idiopathic constipation. Pediatr. Surg. Int. 2022, 38, 1729–1736. [Google Scholar] [CrossRef]
- Mutyala, R.; Sanders, K.; Bates, M.D. Assessment and management of pediatric constipation for the primary care clinician. Curr. Probl. Pediatr. Adolesc. Health Care 2020, 50, 100802. [Google Scholar] [CrossRef] [PubMed]
- Gray, J.R. What is chronic constipation? Definition and diagnosis. Can. J. Gastroenterol. Hepatol. 2011, 25 (Suppl. B), 7B–10B. [Google Scholar] [CrossRef]
- Levitt, M.A.; Martin, C.A.; Falcone, R.A.; Peña, A. Transanal rectosigmoid resection for severe intractable idiopathic constipation. J. Pediatr. Surg. 2009, 44, 1285–1291. [Google Scholar] [CrossRef]
- Kuizenga-Wessel, S.; Koppen, I.J.N.; Zwager, L.W.; Di Lorenzo, C.; De Jong, J.R.; Benninga, M.A. Surgical management of children with intractable functional constipation; experience of a single tertiary children’s hospital. Neurogastroenterol. Motil. 2017, 29, e13005. [Google Scholar] [CrossRef]
- Nellesen, D.; Yee, K.; Chawla, A.; Lewis, B.E.; Carson, R.T. A systematic review of the economic and humanistic burden of illness in irritable bowel syndrome and chronic constipation. J. Manag. Care Pharm. 2013, 19, 755–764. [Google Scholar] [CrossRef]
- Dik, V.K.; Siersema, P.D.; Joseph, A.; Hodgkins, P.; Smeets, H.M.; van Oijen, M.G.H. Constipation-related direct medical costs in 16 887 patients newly diagnosed with chronic constipation. Eur. J. Gastroenterol. Hepatol. 2014, 26, 1260–1266. [Google Scholar] [CrossRef]
- Nag, A.; Martin, S.A.; Mladsi, D.; Olayinka-Amao, O.; Purser, M.; Vekaria, R.M. The Humanistic and Economic Burden of Chronic Idiopathic Constipation in the USA: A Systematic Literature Review. Clin. Exp. Gastroenterol. 2020, 13, 255–265. [Google Scholar] [CrossRef] [PubMed]
- Black, C.J.; Ford, A.C. Chronic idiopathic constipation in adults: Epidemiology, pathophysiology, diagnosis and clinical management. Med. J. Aust. 2018, 209, 86–91. [Google Scholar] [CrossRef]
- Martin, B.C.; Barghout, V.; Cerulli, A. Direct medical costs of constipation in the United States. Manag. Care Interface 2006, 19, 43–49. [Google Scholar]
- Oh, S.J.; Fuller, G.; Patel, D.; Khalil, C.; Spalding, W.; Nag, A.; Spiegel, B.M.; Almario, C.V. Chronic Constipation in the United States: Results From a Population-Based Survey Assessing Healthcare Seeking and Use of Pharmacotherapy. Am. J. Gastroenterol. 2020, 115, 895–905. [Google Scholar] [CrossRef]
- Christison-Lagay, E.R.; Rodriguez, L.; Kurtz, M.; St Pierre, K.; Doody, D.P.; Goldstein, A.M. Antegrade colonic enemas and intestinal diversion are highly effective in the management of children with intractable constipation. J. Pediatr. Surg. 2010, 45, 213–219. [Google Scholar] [CrossRef]
- Cheng, L.; Goldstein, A. Surgical Management of Idiopathic Constipation in Pediatric Patients. Clin. Colon. Rectal Surg. 2018, 31, 089–098. [Google Scholar] [CrossRef] [PubMed]
- Siminas, S.; Losty, P.D. Current Surgical Management of Pediatric Idiopathic Constipation: A Systematic Review of Published Studies. Ann. Surg. 2015, 262, 925–933. [Google Scholar] [CrossRef]
- Wood, R.J.; Yacob, D.; Levitt, M.A. Surgical options for the management of severe functional constipation in children. Curr. Opin. Pediatr. 2016, 28, 370–379. [Google Scholar] [CrossRef] [PubMed]
- Svetanoff, W.J.; Dekonenko, C.; Dorman, R.M.; Osuchukwu, O.; Carrasco, A.; Gatti, J.M., Jr.; Rentea, R.M. Optimization of Pediatric Bowel Management Using an Antegrade Enema Troubleshooting Algorithm. J. Surg. Res. 2020, 254, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Kuizenga-Wessel, S.; Mousa, H.M.; Benninga, M.A.; Di Lorenzo, C. Lack of Agreement on How to Use Antegrade Enemas in Children. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 71–79. [Google Scholar] [CrossRef]
- Vriesman, M.H.; Wang, L.; Park, C.; Diefenbach, K.A.; Levitt, M.A.; Wood, R.J.; Alpert, S.A.; Benninga, M.A.; Vaz, K.; Yacob, D.; et al. Comparison of antegrade continence enema treatment and sacral nerve stimulation for children with severe functional constipation and fecal incontinence. Neurogastroenterol. Motil. 2020, 32, e13809. [Google Scholar] [CrossRef]
- Lacy, B.; Patel, N. Rome Criteria and a Diagnostic Approach to Irritable Bowel Syndrome. J. Clin. Med. 2017, 6, 99. [Google Scholar] [CrossRef]
- Drossman, D.A.; Hasler, W.L. Rome IV—Functional GI Disorders: Disorders of Gut-Brain Interaction. Gastroenterology 2016, 150, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Cascio, S.; Flett, M.E.; De La Hunt, M.; Barrett, A.M.; Jaffray, B. MACE or caecostomy button for idiopathic constipation in children: A comparison of complications and outcomes. Pediatr. Surg. Int. 2004, 20, 484–487. [Google Scholar] [CrossRef]
- Saikaly, S.K.; Rich, M.A.; Swana, H.S. Assessment of pediatric Malone antegrade continence enema (MACE) complications: Effects of variations in technique. J. Pediatr. Urol. 2016, 12, e1–e246. [Google Scholar] [CrossRef]
- Ahmadi, J.; Azary, S.; Ashjaei, B.; Paragomi, P.; Khalifeh-Soltani, A. Intrasphincteric botulinum toxin injection in treatment of chronic idiopathic constipation in children. Iran. J. Pediatr. 2013, 23, 574–578. [Google Scholar] [PubMed]
- University of Wisconsin School of Medicine and Public Health. Available online: https://www.neighborhoodatlas.medicine.wisc.edu/ (accessed on 24 February 2025).
- Kind, A.J.H.; Buckingham, W.R. Making Neighborhood-Disadvantage Metrics Accessible—The Neighborhood Atlas. N. Engl. J. Med. 2018, 378, 2456–2458. [Google Scholar] [CrossRef]
- Lee, Y.F.; Wu, M.C.; Ma, K.S.K.; Huang, J.Y.; Wei, J.C.C. Association of early childhood constipation with the risk of autism spectrum disorder in Taiwan: Real-world evidence from a nationwide population-based cohort study. Front. Psychiatry 2023, 14, 1116239. [Google Scholar] [CrossRef] [PubMed]
- Mulay, K.V.; Karthik, S.V. Managing constipation in children with ASD—A challenge worth tackling. Pediatr. Neonatol. 2022, 63, 211–219. [Google Scholar] [CrossRef]
- Short, S.S.; Reeder, R.W.; Lewis, K.E.; Dickie, B.; Grabowski, J.; Sepuha, T.; Durham, M.M.; Frischer, J.; Badillo, A.; Calkins, C.M.; et al. The presence of a neurodiverse disorder is associated with increased use of antegrade enema therapy in children with severe constipation: A study from the Pediatric Colorectal and Pelvic Learning Consortium (PCPLC). J. Pediatr. Surg. 2022, 57, 1676–1680. [Google Scholar] [CrossRef]
- Sotelo-Orozco, J.; Hertz-Picciotto, I. The Association Between Gastrointestinal Issues and Psychometric Scores in Children with Autism Spectrum Disorder, Developmental Delays, Down Syndrome, and Typical Development. J. Autism. Dev. Disord. 2025, 55, 2452–2462. [Google Scholar] [CrossRef]
- Radwan, A.B.; Gadallah, M.A.; Shahawy, M.R.; Albagdady, A.A.; Talaat, A.A. Can botulinum toxin help in managing children with functional constipation and obstructed defecation? J. Pediatr. Surg. 2021, 56, 750–753. [Google Scholar] [CrossRef]
- Kilgore, A.L.; Rogers Boruta, M.K.; Ambartsumyan, L.; Suarez, R.G.; Patel, D.; Wood, R.J.; Darbari, A.; Rodriguez, L. Evaluation and management of pediatric refractory constipation: Recommendations from the NASPGHAN neurogastroenterology and motility committee. J. Pediatr. Gastroenterol. Nutr. 2025, 80, 353–373. [Google Scholar] [CrossRef]
- Ayub, S.S.; Zeidan, M.; Larson, S.D.; Islam, S. Long-term outcomes of antegrade continence enema in children with chronic encopresis and incontinence: What is the optimal flush to use? Pediatr. Surg. Int. 2019, 35, 431–438. [Google Scholar] [CrossRef] [PubMed]
- Rajindrajith, S.; Devanarayana, N.M.; Weerasooriya, L.; Hathagoda, W.; Benninga, M.A. Quality of Life and Somatic Symptoms in Children with Constipation: A School-Based Study. J. Pediatr. 2013, 163, 1069–1072.e1. [Google Scholar] [CrossRef] [PubMed]
- Patel, D.; Saikumar, P.; Jayaraman, M.; Desai, C.; Rosen, J.; Rodriguez, L. Efficacy of anal botulinum toxin injection in children with functional constipation. J. Pediatr. Gastroenterol. Nutr. 2025, 80, 956–962. [Google Scholar] [CrossRef] [PubMed]
- Davis, T.A.; Turner, A.; Wilson, C.; Wang, J.; Joerger, S.; Utterson, E.C.; Shakhsheer, B.A. Botulinum Toxin Improves Quality of Life and Clinical Outcomes in Pediatric Defecation Disorders. Neurogastroenterol. Motil. 2025, 37, e70005. [Google Scholar] [CrossRef]
- Liem, O.; Harman, J.; Benninga, M.; Kelleher, K.; Mousa, H.; Di Lorenzo, C. Health Utilization and Cost Impact of Childhood Constipation in the United States. J. Pediatr. 2009, 154, 258–262. [Google Scholar] [CrossRef]
- Soyer, T. Prevention and management of complications in various antegrade enema procedures in children: A review of the literature. Pediatr. Surg. Int. 2020, 36, 657–668. [Google Scholar] [CrossRef]
- Gould, M.J.; Marcon, M.A.; Nguyen, G.C.; Benchimol, E.I.; Moineddin, R.; Swayze, S.; Kopp, A.; Ratcliffe, E.M.; Merritt, N.; Davidson, J.; et al. Impact of antegrade enema initiation on healthcare utilization in pediatric patients: A population-based cohort study. Neurogastroenterol. Motil. 2023, 35, e14495. [Google Scholar] [CrossRef]
- Smith, C.A.; Kwon, E.G.; Nicassio, L.; Glazer, D.; Avansino, J.; Durham, M.M.; Frischer, J.; Calkins, C.; Rentea, R.M.; Ralls, M.; et al. Fecal continence disparities in patients with idiopathic constipation treated at referral institutions for pediatric colorectal surgery. J. Pediatr. Surg. 2023, 58, 56–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).