Abstract
Background: Guided-growth modulation is a first-line treatment widely adopted to correct lower-limb angular deformities and limb-length discrepancies (LLD) in the paediatric population. Methods: We conducted a retrospective study to evaluate the safety and performance of a new construct (8-Plate Plus or Guided-Growth Plate System Plus, Orthofix S.r.l) used to correct angular deformities and LLD in non-skeletally mature children. The primary endpoint was safety (from plate implantation to removal). The secondary endpoint was performance; patients treated for LLD achieved complete correction if a pre- and post-surgery difference of <0 was observed; angular deformities performance was measured in terms of IMD, ICD, mMPTA, and mLDFA. Results: We performed 69 procedures in 41 patients. A total of 10 patients had an LLD, and 31 had an angular deformity. We observed nine minor complications in the hemiepiphysiodesis group. One patient experienced rebound. All 10 LLD patient treatments were successful. A total of 30/31 patients with an angular deformity had a successful treatment; the remaining patient had a partial correction. Conclusions: Guided-growth by temporary epiphysiodesis or hemiepiphysiodesis was safe and effective for angular deformities and limb-length discrepancies. Further prospective and/or randomized controlled trial studies assessing more significant cohorts of patients and a comparison group could add evidence to our findings.
1. Introduction
Paediatric orthopaedists state that lower-limb angular deformities are one of the most frequently non-traumatic conditions being researched [1,2,3]. While lower limb position tends to change during growth, from slight varus to slight valgus between 1 and 7 years of age, past this threshold, a paediatric subject is considered to have a neutral alignment [3,4,5]. When this is not the case, lower-limb deformities may compromise gait, produce pain, give instability, and limit functionality [3,4]. Lower-limb deformities may include both idiopathic and pathological malalignment and limb-length discrepancies. Obesity is known to be a risk factor, but other factors may not be that predictable [5].
Guided growth or modulation of the growing bone is widely approved as the first-line approach when conservative treatment has failed [4,6]. This is mainly because it is simple to technically assimilate and perform, is minimally invasive and reversible, gives rise to lesser evident scars, and has fewer complications compared to other more invasive treatments such as osteotomy with acute correction and stabilization, physeal bar resection, or multi-planar dynamic external fixation [3,4,6].
Although these procedures are deemed to be safe and effective, proper indication and meticulous application of the standard technique are essential to good outcomes [4]. Timely intervention is paramount for a successful outcome, as late indication may lead to incomplete corrections [3,4,7]. The treatment aims to restore limb alignment, arrest progression, and prevent recurrence after the intervention [6,8].
Guided-growth plating has shown positive results in different settings, from multiple osteochondromas to idiopathic cases [7].
There are several 8-shaped plates on the market. The Guided-Growth Plate System Plus (GGPSP) produced by Orthofix, Bussolengo, Italy—also known as 8-Plate Plus and released in 2021—is a new construct that differs from its predecessor, the Guided-Growth System or 8-Plate, in the dimensional range of the implantable components (plates and screws). The new construct also allows for a higher degree of motion, which may provide a better outcome in patients needing to stay on the plate longer and might generate fewer device-related complications. In particular, the total height of the system (screw plus plate) is inferior to the old version (3.5 mm vs. 4.1 mm) in the early post-operative construct, adding a “low profile” feature that could be linked to better patient tolerance. Furthermore, the screws have a higher time for reaching maximum angulation. This arrangement, where the total height of the system is 4.5 mm, like in the first generation, could lead to a better outcome in terms of daily pain. To our knowledge, this study is the first one reporting clinical data on this new device. It aims to evaluate the safety and performance of its use in non-skeletally mature children diagnosed with a lower-limb angular deformity or leg-length discrepancy.
2. Materials and Methods
2.1. Study Design and Participants
We conducted a retrospective study on non-skeletally mature children with deformities of femur and tibia (most frequent sites of applications), admitted at our institution—a national referral centre for rare skeletal disorders (Rizzoli Orthopedic Institute, Bologna, Italy).
Patients were eligible if they (1) had been treated with an 8-Plate Plus to correct deformities of the femur and/or tibia; (2) were not skeletally mature (according to the pre-surgical X-ray, the growth plates of the treated limb(s) were still evident); (3) had completed therapy with 8-Plate Plus and had already attended at least one post-plate removal check-up; (4) had an accessible dataset for the assessment of safety and clinical efficacy of treatment.
On the contrary, patients were not eligible if they (1) had been treated with an 8-Plate Plus for an off-label anatomical location; (2) had been treated with concomitant devices other than the 8-Plate Plus (unless these did not compromise the safety and performance of the primary treatment); (3) were still being treated with 8-Plate Plus or had not attended any post-plate removal check-ups; (4) did not have an accessible dataset for the assessment of safety and the clinical efficacy of treatment.
The institutional review board approved the study protocol (ID: 663/2021/Oss/IOR—n. 0012656) according to Italian regulations (art. 110bis of decree n.196/2003). Explicit consent was not required for retrospective chart reviews and publication of the aggregated data. The study followed the Declaration of Helsinki (Fortaleza, October 2013) and the Good Clinical Practice (ICH-GCP) guidelines.
2.2. Surgical Procedure
The surgical procedure, conducted as part of the hospital’s routine practice, was planned and performed by a team of 11 paediatric orthopaedists, following the standard treatment at our institution.
Under general or local anaesthesia, the patient was placed, as standard technique required, in supine position on a radiolucent operating table with a thigh tourniquet. Using fluoroscopy, femoral and/or tibial physes were exhibited, the skin marked and incised about 2–3 cm from the level of the identified area and dissected down to the periosteum.
8-Plate Plus (Guided-Growth Plate System Plus, Orthofix Srl, Bussolengo, Italy) was placed, centred, over the growth plates. Implant size was chosen according to the size of the knee. Sutures of the fascia and subcutaneous planes with final closure of the skin using running absorbable wire were then performed.
After surgery, children could fully move around and walk with full weight bearing and were usually discharged by the second or third day after the intervention. Clinical assessments were conducted at regular intervals post-operatively at one and three months to check the status of the deformity correction. The plate was removed once the corrected alignment was reached or after a slight overcorrection following the surgeon’s post-operative planning.
2.3. Assessment of Baseline Variables and Outcomes
At baseline, demographic data included race, gender, and age, while anthropometric parameters including body weight, height, body mass index (BMI), and related z-scores were evaluated according to the CDC calculator (https://www.cdc.gov/healthyweight/bmi/calculator.html, accessed on 1 December 2021).
Radiographic parameters at baseline, obtained from standard standing X-rays that involve the segments from pelvis to foot (standard long leg view) included mechanical hip–knee–ankle angle (HKA) and mechanical axis of deviation (MAD); anatomical lateral distal femoral angle (aLDFA) and mechanical distal lateral femoral angle (mLDFA); mechanical medial proximal tibial angle (mMPTA); distal lateral tibial angle (LDTA); the tibia–fibula ratio (tibial length/fibular length), and femur–tibia ratio (femur length/tibial length) [9,10].
The severity of the angular deformity of the knee was evaluated using the intermalleolar distance (IMD) and intercondylar distance (ICD) scores [5,11,12].
During and after surgery, the following parameters were recorded: age at the time of surgery; surgery details (duration, complications, post-operative X-rays) and data on the device(s) implanted (i.e., quantity, type, and size); medications and concomitant treatments; treatment time (from plate implantation to removal).
We measured safety endpoints as expected, and unexpected serious adverse events and hardware complications (breakage, bending, etc.) were recorded from plate implantation to removal. Once the device was removed from the application site, whether the correction had been maintained was evaluated during the post-plate removal visit. The objective was to verify whether rebound cases, typical of these conditions, had occurred.
Performance endpoints were recorded at plate removal and six months past plate removal. Performance was measured in terms of complete correction (full response to treatment). Performance parameters are shown in Figure 1.
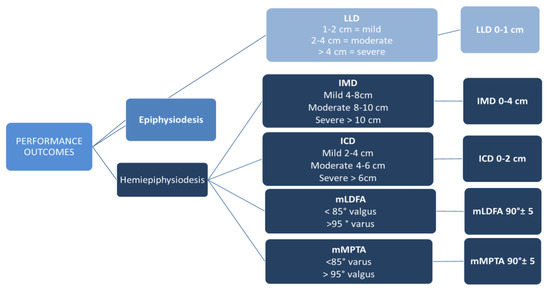
Figure 1.
Performance outcome parameters and definition of complete correction. mMPTA: mechanical medial proximal tibial angle; mLDFA: mechanical lateral distal femoral angle; IMC: intermalleolar distance; ICD: intercondylar distance.
2.4. Sample Size Estimation
Because the growth-plate technique is a consolidated treatment and its performance widely proven in the scientific literature, the safety of this reversible treatment was considered for the calculation of the sample size.
The scientific literature reports that the percentage of patients treated with a tensioning plate for a lower-limb deformity who faced at least one device-related complication was between 3% and 12%, depending on many factors, mainly the heterogeneity of the prevalent cause [13]. Assuming that the expected percentage of patients with at least one complication for the 8 Plate Plus was to be 3%, the sample size calculation showed that the upper limit of the 95% confidence interval for the primary endpoint based on the exact binomial method must remain below 12 per cent (i.e., 9.94%); thus, 70 procedures (performed on approximately 40 patients) were needed.
2.5. Statistical Analysis
The clinical data were analysed using descriptive statistics: the primary endpoint is presented as a percentage value and the corresponding 95% confidence interval; the secondary endpoint is presented as a percentage value and the corresponding 95% confidence interval.
3. Results
3.1. Patients’ Characteristics at Baseline
Between January 2019 to September 2020, 141 children underwent guided growth by temporary hemiepiphysiodesis or epiphysiodesis. Of them, 42 children (with 70 knees) were treated using the 8-Plate. One patient was lost to follow up, leaving 41 children (69 knees) available for the analysis. The mean age at surgery was 12.3 ± 1 years, and the overall treatment time was 618.5 days (499.0–928.0). Patients’ characteristics at baseline are reported in Table 1.

Table 1.
Patients’ characteristics at baseline.
A total of 10 patients were obese and 27 patients had comorbidities. Some patients presented more than one comorbidity. See Table 2.

Table 2.
Patients’ comorbidities.
3.2. Surgery
The procedure was carried out in 24 patients (58.5%) under local anaesthesia and in 6 (14.6%) cases under general anaesthesia; in the remaining patients, other types of anaesthesia were used (infiltration + local, sedation + local, total + local).
We used one plate in 59 knees that underwent hemiepiphysiodesis (28 bilateral) (85.5%), and 10 cases received epiphysiodesis (14.5%). The plate application site was predominantly in the distal femoral region in 57 knees (82.65%), while, in 12 (17.4%), the application was carried out in the proximal tibial region.
In nine patients, in addition to the 8-Plate Plus, a screw was applied to the patient’s heel to correct flat foot. One patient sustained combined intramedullary nailing to treat an impending fracture of the femur due to monostotic fibrous dysplasia.
In 40 patients, antibiotic and anti-inflammatory treatments were administered in the post-surgery phase following standard clinical practice.
Surgical data are reported in Table 3.

Table 3.
Surgical data considering procedures.
3.3. Safety
No complications occurred during surgery.
Nine minor complications were recorded in the hemiepiphysiodesis group during follow-up. Detailed information is presented in Table 4. None of the epiphysiodesis cases experienced any complication. All complications required just some minimal treatment, like painkillers, physiotherapy, oral antibiotics (for pain, stiffness, and superficial infection), or none, and they were deemed as not clinically relevant (Grade I Modified Clavien–Dindo–Sink Classification). No complications regarding the device used, like screw breakage, failure of correction, screw pull-out, screw/plate migration, growth disturbance, or physeal injury, were experienced.

Table 4.
Complications per patient.
3.4. Performance
Regarding the evaluation of the clinical benefit of treatment, the 69 procedures included in the study were divided as follows:
- −
- 10 (14.5%) cases of epiphysiodesis in 10 patients
- −
- 59 (85.5%) cases of hemiepiphysiodesis in 31 patients
One patient did not maintain the degree of correction (the only case of rebound complication) due to the increase in weight and thigh fat, which caused a rise in IMD compared to the previous visit.
3.4.1. Epiphysiodesis
Ten patients were treated with epiphysiodesis for LLD. All of them were classified as “low” or “moderate” LLD (from 1 to 4 cm). There were not “severe” LLD cases due to our current clinical practice of favouring a limb lengthening in these cases to avoid a final short height.
The median duration of treatment (calculated as the interval between the application of the plate and its removal) was 656.8 days (range: 499 to 928 days).
In seven patients, the LLD was of the femur, while, in three patients, it was of the tibia. In subject 7, the procedure was carried out in the femur, even if the tibia was the bone segment in question, due to pathological growth plates (Metachondromatosis/Ollier disease). At final follow-up, the complete (femur + tibia) LLD was nullified. All the patients treated reached complete correction (as the performance outcome stated, LLD lower than 1 cm). The mean correction rate for distal femur epiphysiodesis was 1.43 (SD 0.82) mm/month, and it was 0.75 (SD 0.35) mm/month for proximal tibia. See Table 5.

Table 5.
Results of the cases treated for LLD.
3.4.2. Hemiepiphysiodesis
Of the 41 patients included in the study, 31 were treated with hemiepiphysiodesis, corresponding to 59 procedures.
The median duration of treatment (calculated as the interval between the application of the plate and its removal) was 453.8 days (range: 241 to 774 days).
For all 59 procedures (31 patients), pre- and post-operative mLDFA and mMPTA values were recorded. For the 49 treatments of valgus deformity at the distal femur, the mean mLDFA increased from 85.3° ± 3.1° (range, 78° to 91°) pre-operatively to 93.2° ± 4.2° (range, 86° to 103°) post-operatively, with a mean angle variation of 7.9°. In these cases, the mean rate of mLDFA angle correction was 0.57°/month. For the three treatments of valgus deformity at the proximal tibia, the mean mMPTA decreased from 97.3° ± 5.1° (range, 93° to 103°) pre-operatively to 87.7° ± 1.5° (range, 86° to 89°) post-operatively, with a mean angle variation of −9.7°. In these cases, the mean rate of mMPTA angle correction was −0.14°/month. For the seven treatments of varus deformity at the proximal tibia, the mean mMPTA increased from 84.1° ± 3.8° (range, 79° to 88°) pre-operatively to 90.4° ± 3.9° (range, 84° to 97°) post-operatively, with a mean angle variation of 6.3°. In these cases, the mean rate of mMPTA angle correction was 0.09°/month. Data are summarized in the table below (Table 6).

Table 6.
Evaluation of the improvement of hemiepiphysiodesis: pre- and post-surgery mean values through the assessment of mLDFA and mMPTA angles and severity of the IMD or the ICD.
Radiological parameters were not fully obtained at the final follow-up because, considering the retrospective nature of the study, X-rays were not deemed necessary in the outpatient clinic visit. In our current clinical practice, we tend to avoid radiological hazards if they are not indispensable, especially on paediatric patients; for that reason, we used IMD and ICD as indicators for possible rebound effect, even though we acknowledge that they could not be as precise as radiological standards.
Out of a total of 31 patients, in 6 patients (12 knees), it was not possible to evaluate the intermalleolar or intercondylar distance (IMD or ICD) and, therefore, for the evaluation, mLDFA and mMPTA parameters were used to assess the difference before and after application of the plates. A total of 11 knees (91.7%) achieved complete correction, according to the criteria for the femoral or tibial angle variation. One knee (8.3%) did not achieve correction. Nevertheless, according to the investigator’s opinion, the case had reached partial or complete correction, as there had been an improvement in stature and gait at the time of the clinical evaluation, probably due to weight loss.
One patient was evaluated with both criteria and achieved a full correction as expected.
In the remaining 24 patients (77.4%), it was possible to evaluate the intermalleolar or intercondylar distance (IMD or ICD), and the change in score before and after application of the plate(s) was therefore assessed. All 24 achieved a full correction with a significant change in deformation (100%). See Table 6.
4. Discussion
This observational retrospective study analysed a cohort of patients undergoing guided growth with an 8-Plate Plus to correct genu valgus, genu varus, or limb-length discrepancy.
Timely intervention is paramount for a successful outcome [14], as late or incorrect indication may lead to incomplete corrections, risk of secondary deformities, and rebound effect, as several authors have consistently stated [3,4,7]. The precise timing is really the main aspect to fully understand in order to accomplish a satisfactory outcome and eliminate or minimize the risk of complications. For example, in our current practice, growth-plate modulation is advised in pre-adolescent subjects, regarding typically idiopathic cases, to exploit the puberal spurt and achieve correction in relatively lesser time. Also, in our cohort, the average age at surgery was 12.3 years old. In the case of males, delaying intervention may be possible, whereas, in the case of females, it is generally advisable to intervene at an earlier stage, as bone age correlates with chronological age for both sexes at the beginning of puberty but not at the end [15]. Our patient population is comparable to that of previous and recent studies [16,17,18,19,20]. Besides, if the patient has associated pathologies, it is also advisable to intervene at an earlier stage. This is probably connected to a not fully functional growth plate, differently from what is seen in the idiopathic cases. Furthermore, the increased risk of relative shorter height in these kinds of conditions could be another factor in favour of forerunning the surgical treatment [7].
All our patients treated for LLD of the femur or tibia with this new construct (8-Plate Plus or GGPSP) reached full correction. Borbas and colleagues published similar results using a previous version of the GGPSP (the Guided-Growth Plate, manufactured by Orthofix Srl., Bussolengo Verona, Italy) in 2019 [17], after a study comparing definitive percutaneous epiphysiodesis versus temporary epiphysiodesis with this device. The reduction of the LLD in 12 months was 5.7 mm in patients treated with the tension band plate and 8.4 mm with definitive treatment. This difference was, however, statistically not significant (38 patients were included overall, which does not allow for clear significant analysis). Demirel et al. [18] reached limb-length discrepancy correction in 54.5% of the patients studied (11 patients) using a different 8-shaped plate.
Regarding angular correction, our study shows that this technique is safe and effective in correcting angular deformities during growth, achieving 97.6% (40 out of 41 patients) success. Similar results with different guided-growth plates have been reported by Park and colleagues [19]: 97.1% of patients achieved full corrections (a retrospective study including 35 patients treated with the R-plate). But, previous studies did not report such encouraging results: Quintero and colleagues [20] reported an 81.7% achievement of correction on 115 plates; Ellsworth et al. [21] reported 82.4% on 17 patients; and Ghaznavi and colleagues [22] reported 95.45% on 109 patients. In the end, in terms of safety and effectiveness, our results on the GGPSP are comparable to the recent literature regarding guided-growth techniques using 8-shape plates. This kind of construct is currently one of the most (if not the most) used system for temporary hemiepiphysiodesis/epiphysiodesis. For this reason, future studies with larger series may disclose possible advantages in using 8-Plate Plus in comparison to other 8-shape plates.
The guided-growth technique is easy to perform and is not influenced by the surgeons’ experience, as seen from the present study, in which a team of 11 different surgeons, with different years of experience, operated on the whole sample. And, overall, the body of literature suggests that it is an advisable first approach, given the fact that it is reversible [17,18,19,20,21,22,23,24].
Limitations of the current study include the small number of patients, which may not provide a statistically significant result and may have limited power to detect an actual effect due to the small sample size. It also increases the likelihood of chance effects or errors in the results. Limitations also include the study’s observational nature, which lacked a comparator. Thus, the effect of treatment cannot be attributed solely to the intervention. Finally, our study was conducted in a single centre and may not be representative of other settings or populations. That is why broader multicentre studies regarding this matter could add important evidence in terms of safety and performance in wide and varied populations. The relative simplicity of the surgical technique and expansive indication from idiopathic to pathological deformities could be helpful for reaching strong results.
With the emerging technologies of assisted pre-planning with software and 3D printing, the field of guided-growth systems is expected to see significant developments in the future. These technologies allow doctors to create a customised treatment plan for each patient based on individual needs and unique anatomical considerations [23]. These systems are already used with alternative yet more invasive treatment approaches.
5. Conclusions
In conclusion, guided growth by temporary epiphysiodesis or hemiepiphysiodesis was safe and effective in correcting femoral and tibial axial deformities and limb-length discrepancy in idiopathic and pathologic children, with results visible after 6 months past the removal of the plates. Further prospective and/or randomized control trial studies assessing bigger cohorts of patients and with a comparison group could add evidence to our findings.
Author Contributions
Conceptualization, G.L.D.G. and G.G.; methodology, S.S.; software, G.G.; validation, G.R., G.G. and G.L.D.G.; formal analysis, G.T.; investigation, G.L.D.G.; resources, M.R.; data curation, S.S.; writing—original draft preparation, G.G.; writing—review and editing, G.G.; visualization, M.R.; supervision, G.R.; project administration, G.L.D.G.; funding acquisition, G.L.D.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Orthofix S.r.l., as the study sponsor. The APC was funded by Orthofix S.r.l.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Ethics Committee of Istituto Ortopedico Rizzoli of Bologna (protocol code 0012656 and date of approval) on 25 August 2021.
Informed Consent Statement
Patient consent was waived due the retrospective nature of the study, in which charts were reviewed and aggregated data were analysed. Istituto Ortopedico Rizzoli is an institution that comprises treatment and research, and, according to Italian regulations (art. 110bis of D.lgs. n.196—30/6/2003), we can analyse and publish aggregated anonymous data.
Data Availability Statement
Data available on request due to restrictions. The data presented in this study could be available on request from the corresponding author. The data are not publicly available due to national privacy regulations.
Acknowledgments
We would like to thank all the patients and their families for their participation in this study. Editorial assistance in the preparation of this article was provided by Laura C. Collada Ali (Medical Writing Consultant).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Dovris, D.; Mavrogenis, A.F.; Christogiannis, I.; Nomikos, G.; Papaparaskeva, K.; Koulalis, D.; Papalois, A.; Karameris, A.; Babis, G.C. Hemiepiphysiodeses for guided growth in children. J. Long. Term. Eff. Med. Implant. 2014, 24, 121–129. [Google Scholar] [CrossRef] [PubMed]
- Ekwedigwe, H.C.; Enweani, U.N.; Madu, K.A.; Nwadinigwe, C.U.; Okwesili, I.C.; Ekwunife, R.T. Clinical measurement of angular profile of the knee and correlation with intermalleolar distance in children in Enugu metropolis. Niger. J. Clin. Pract. 2020, 23, 7–11. [Google Scholar] [CrossRef] [PubMed]
- Gupta, P.; Gupta, V.; Patil, B.; Verma, V. Angular deformities of lower limb in children: Correction for whom, when and how? J. Clin. Orthop. Trauma 2020, 11, 196–201. [Google Scholar] [CrossRef] [PubMed]
- Braga, S.R.; Santili, C.; Rodrigues, N.V.M.; Soni, J.F.; Green, D.W. Growth modulation for angular knee deformities: A practical guideline. Curr. Opin. Pediatr. 2023, 35, 110–117. [Google Scholar] [CrossRef] [PubMed]
- Jankowicz-Szymanska, A.; Mikolajczyk, E. Genu Valgum and Flat Feet in Children with Healthy and Excessive Body Weight. Pediatr. Phys. Ther. 2016, 28, 200–206. [Google Scholar] [CrossRef] [PubMed]
- Robbins, C.A. Deformity Reconstruction Surgery for Blount’s Disease. Children 2021, 8, 566. [Google Scholar] [CrossRef]
- Trisolino, G.; Boarini, M.; Mordenti, M.; Evangelista, A.; Gallone, G.; Stallone, S.; Zarantonello, P.; Antonioli, D.; Di Gennaro, G.L.; Stilli, S.; et al. Outcomes of Temporary Hemiepiphyseal Stapling for Correcting Genu Valgum in Children with Multiple Osteochondromas: A Single Institution Study. Children 2021, 8, 287. [Google Scholar] [CrossRef]
- Joseph, B.; Nayagam, S.; Loder, R.T.; Torode, I. Paediatric Orthopaedics: A System of Decision-Making; CRC Press: Boca Raton, FL, USA, 2016; p. 600. [Google Scholar]
- Paley, D. Normal Lower Limb Alignment and Joint Orientation. In Principles of Deformity Correction; Paley, D., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 1–18. [Google Scholar]
- Cho, T.J.; Choi, I.H.; Chung, C.Y.; Yoo, W.J.; Park, M.S.; Lee, D.Y. Hemiepiphyseal stapling for angular deformity correction around the knee joint in children with multiple epiphyseal dysplasia. J. Pediatr. Orthop. 2009, 29, 52–56. [Google Scholar] [CrossRef]
- Heath, C.H.; Staheli, L.T. Normal limits of knee angle in white children—genu varum and genu valgum. J. Pediatr. Orthop. 1993, 13, 259–262. [Google Scholar]
- Sabharwal, S.; Zhao, C.; Edgar, M. Lower Limb Alignment in Children: Reference Values Based on a Full-Length Standing Radiograph. J. Pediatr. Orthop. 2008, 28, 740–746. [Google Scholar] [CrossRef]
- Saran, N.; Rathjen, K.E. Guided growth for the correction of pediatric lower limb angular deformity. J. Am. Acad. Orthop. Surg. 2010, 18, 528–536. [Google Scholar] [CrossRef] [PubMed]
- Artioli, E.; Mazzotti, A.; Ramacci, V.; Zielli, S.O.; Digennaro, V.; Ruffilli, A.; Faldini, C. Indications and timing in isolated medial femoral hemiepiphysiodesis for idiopathic genu valgum: A systematic review. Knee 2023, 40, 52–62. [Google Scholar] [CrossRef] [PubMed]
- García Cuartero, B.; Gónzalez Vergaz, A.; Frías García, E.; Arana Cañete, C.; Díaz Martínez, E.; Tolmo, M.D. Assessment of the secular trend in puberty in boys and girls. An. Pediatr. 2010, 73, 320–326. [Google Scholar] [CrossRef] [PubMed]
- Schagemann, J.; Kudernatsch, N.; Russlies, M.; Mittelstädt, H.; Götze, M.; Horter, M.; Paech, A.; Behnke, B. Prediction of loss of correction after hemiepiphysiodesis for the alignment of lower limb angular deformities. Medicine 2022, 101, e28626. [Google Scholar] [CrossRef] [PubMed]
- Borbas, P.; Agten, C.A.; Rosskopf, A.B.; Hingsammer, A.; Eid, K.; Ramseier, L.E. Guided growth with tension band plate or definitive epiphysiodesis for treatment of limb length discrepancy? J. Orthop. Surg. Res. 2019, 14, 99. [Google Scholar] [CrossRef] [PubMed]
- Demirel, M.; Sağlam, Y.; Yıldırım, A.M.; Bilgili, F.; Şeker, A.; Şen, C. Temporary Epiphysiodesis Using the Eight-Plate in the Management of Children with Leg Length Discrepancy: A Retrospective Case Series. JOIO 2022, 5, 56874–56882. [Google Scholar] [CrossRef] [PubMed]
- Park, K.H.; Oh, C.W.; Kim, J.W.; Park, I.H.; Kim, H.J.; Choi, Y.S. Angular deformity correction by guided growth in growing children: Eight-plate versus 3.5-mm reconstruction plate. J. Orthop. Sci. 2017, 22, 919–923. [Google Scholar] [CrossRef]
- Quintero, D.; Galbán, M.; Gil, C. Correction of pediatric angular deformities in lower limbs through guided growth using a novel flexible plate system. Orthop. Traumatol. Surg. Res. 2022, 109, 103406. [Google Scholar] [CrossRef]
- Ellsworth, B.K.; Aitchison, A.H.; Fabricant, P.D.; Green, D.W. Use of Implant-Mediated Guided Growth with Tension Band Plate in Skeletally Immature Patients with Knee Pathology: A Retrospective Review. HSS J. 2022, 18, 18399–18407. [Google Scholar] [CrossRef]
- Ghaznavi, A.; Baghdadi, T.; Bagherifard, A.; Fakoor, S.; Shirvani, S.; Kiaei, S.M.S.; Mohammadpour, M. Comparison of eight-plate with reconstruction plate in temporary hemiepiphysiodesis to correct the idiopathic genu valgum in pediatrics. ABJS 2022, 10, 585–591. [Google Scholar] [CrossRef]
- Fan, M.; Wang, Y.; Pang, H.; Wang, Y.; Xu, P.; Lou, Y.; Zheng, P.; Tang, K. Application of three-dimensional printed navigation templates to correct lower limb deformities in children by the guided growth technique. World J. Pediatr. Surg. 2022, 5, e000349. [Google Scholar] [CrossRef] [PubMed]
- Mare, P.H.; Marais, L.C. Gradual Deformity Correction with a Computer-assisted Hexapod External Fixator in Blount’s Disease. Strateg. Trauma. Limb Reconstr. 2022, 17, 32–37. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).