Imaging of Acute Complications of Community-Acquired Pneumonia in the Paediatric Population—From Chest Radiography to MRI
Abstract
1. Introduction
2. Imaging Modalities
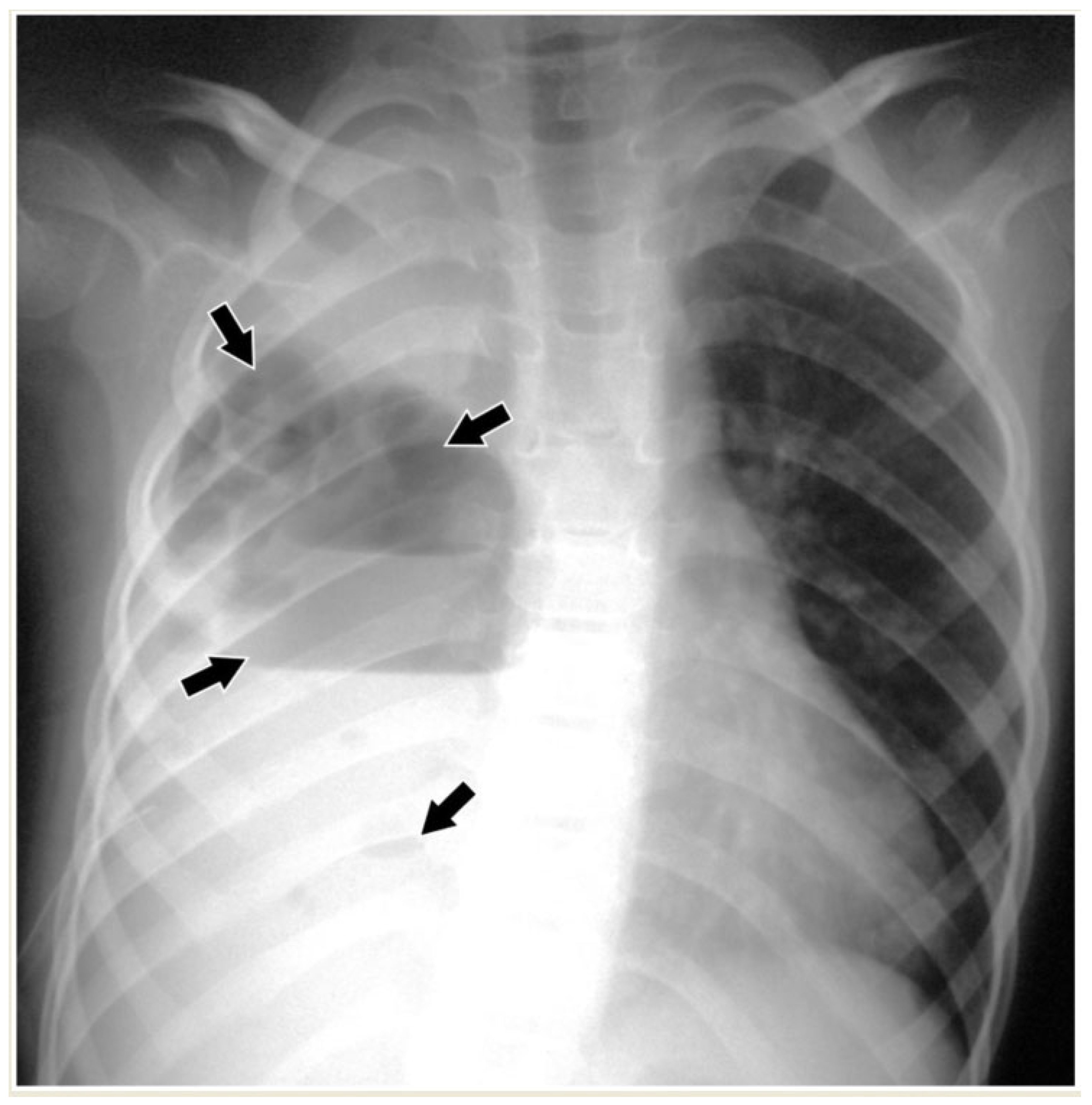
2.1. Chest Radiography
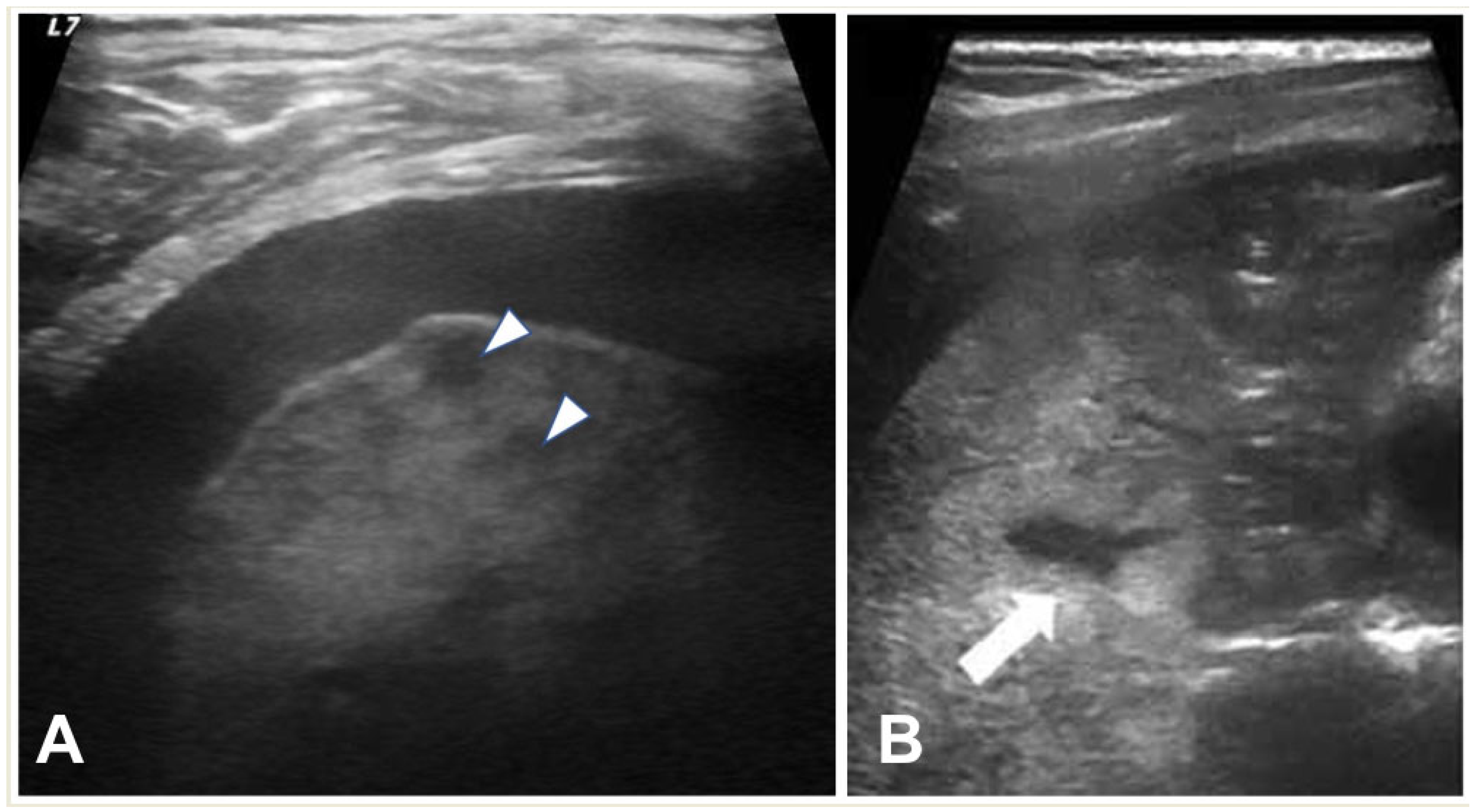
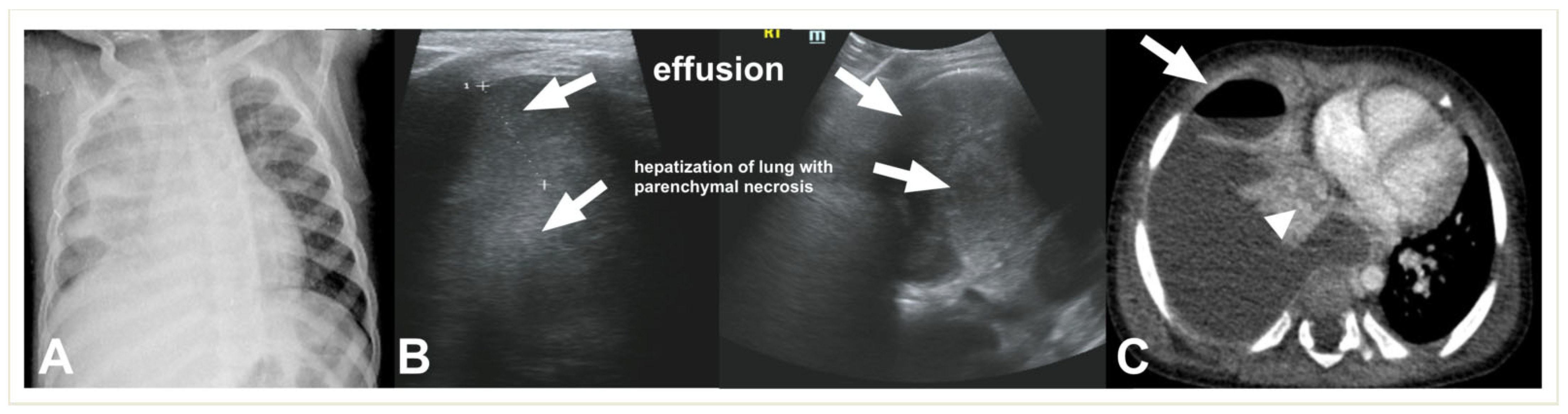
2.2. Lung Ultrasound
2.3. Computed Tomography
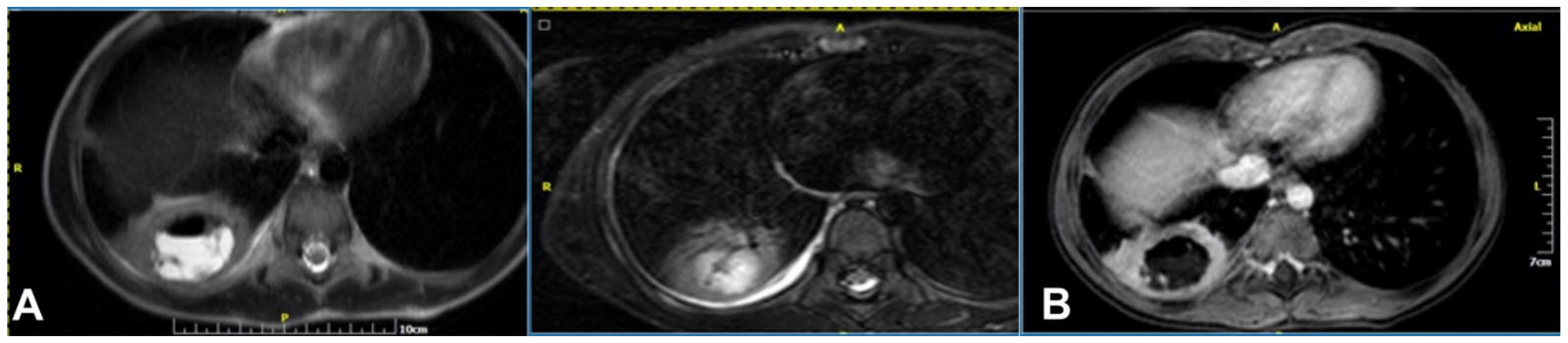
2.4. Magnetic Resonance Imaging
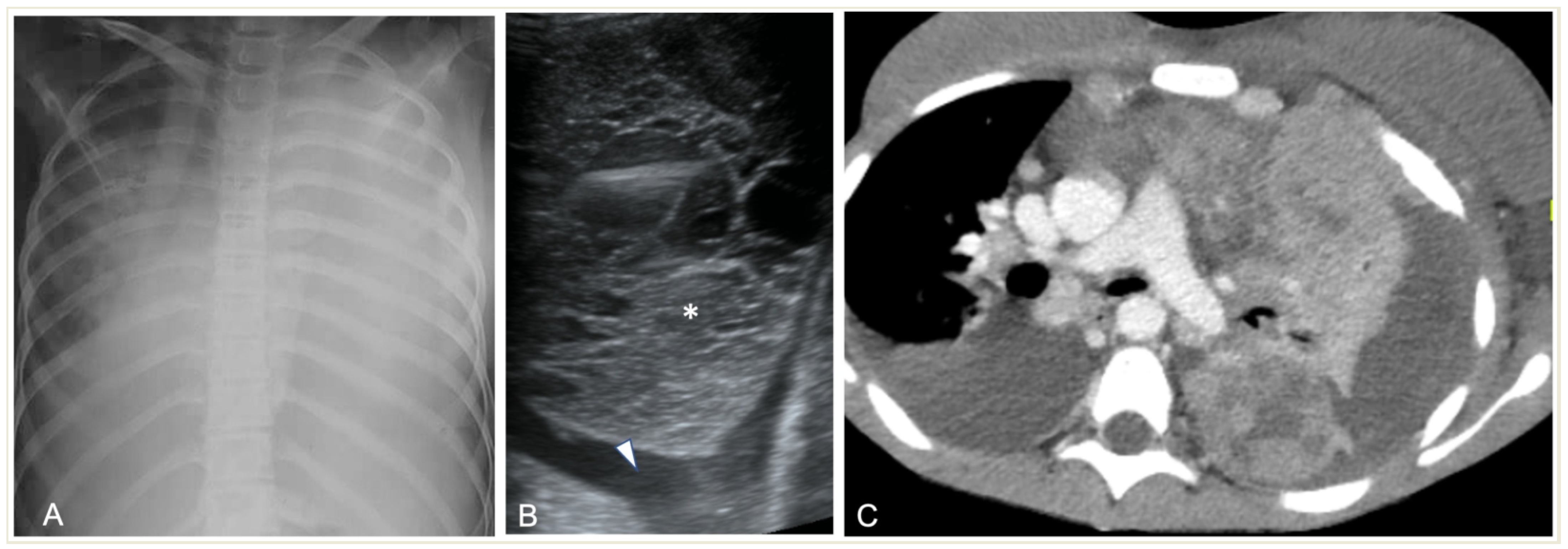
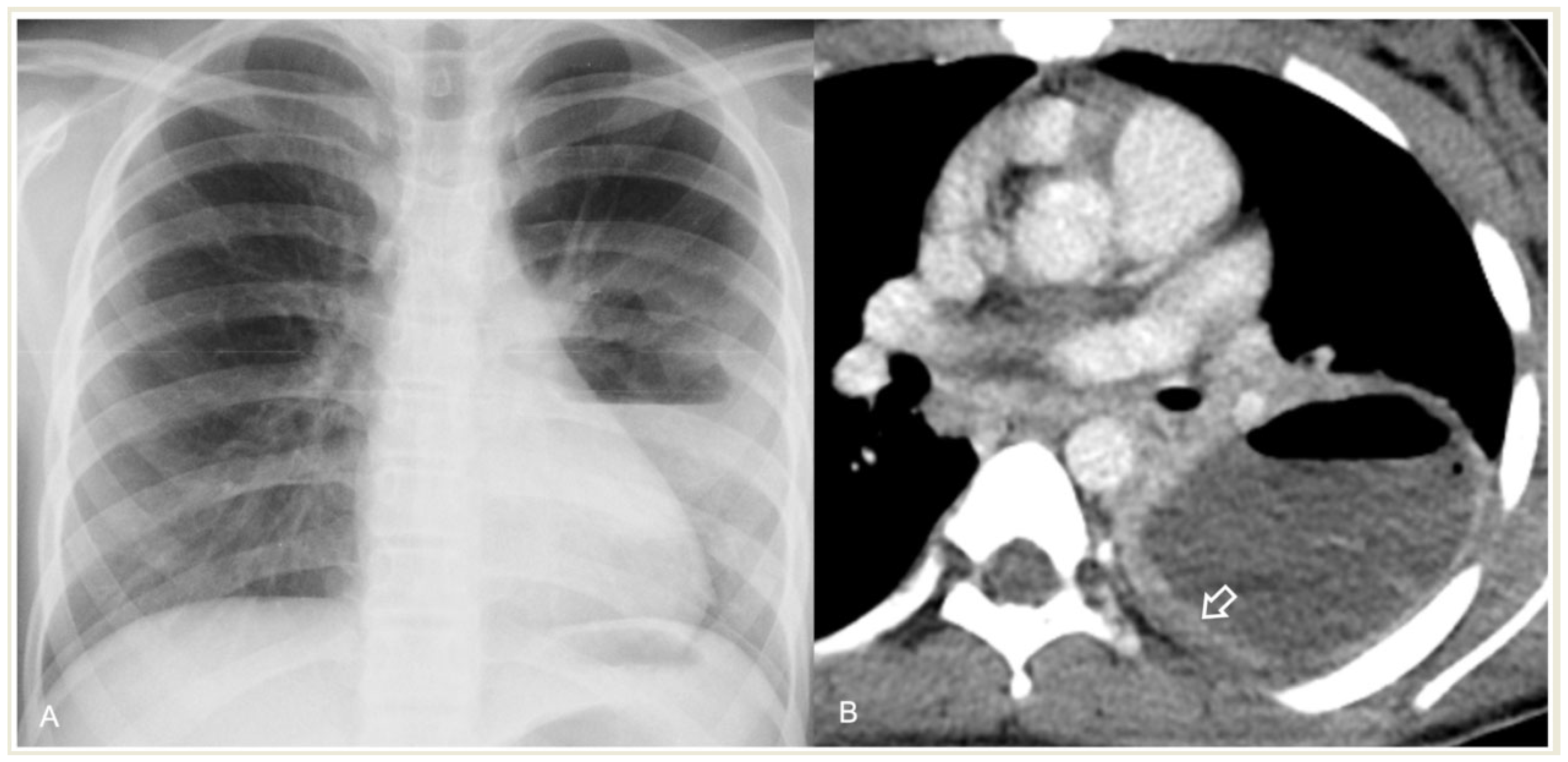
2.4.1. Pleural Effusion—Empyema
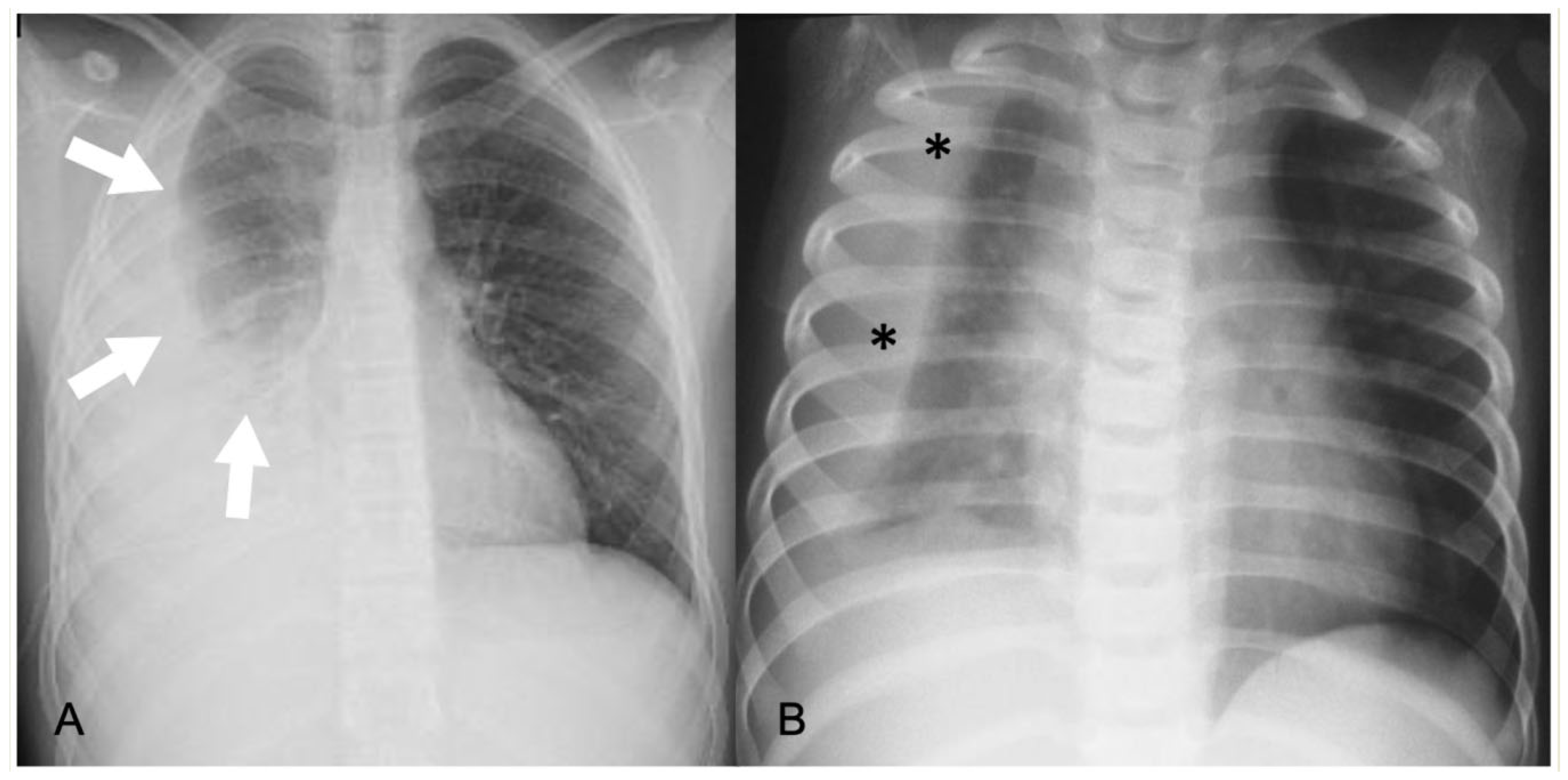
2.4.2. Acute Lung Parenchyma Complications
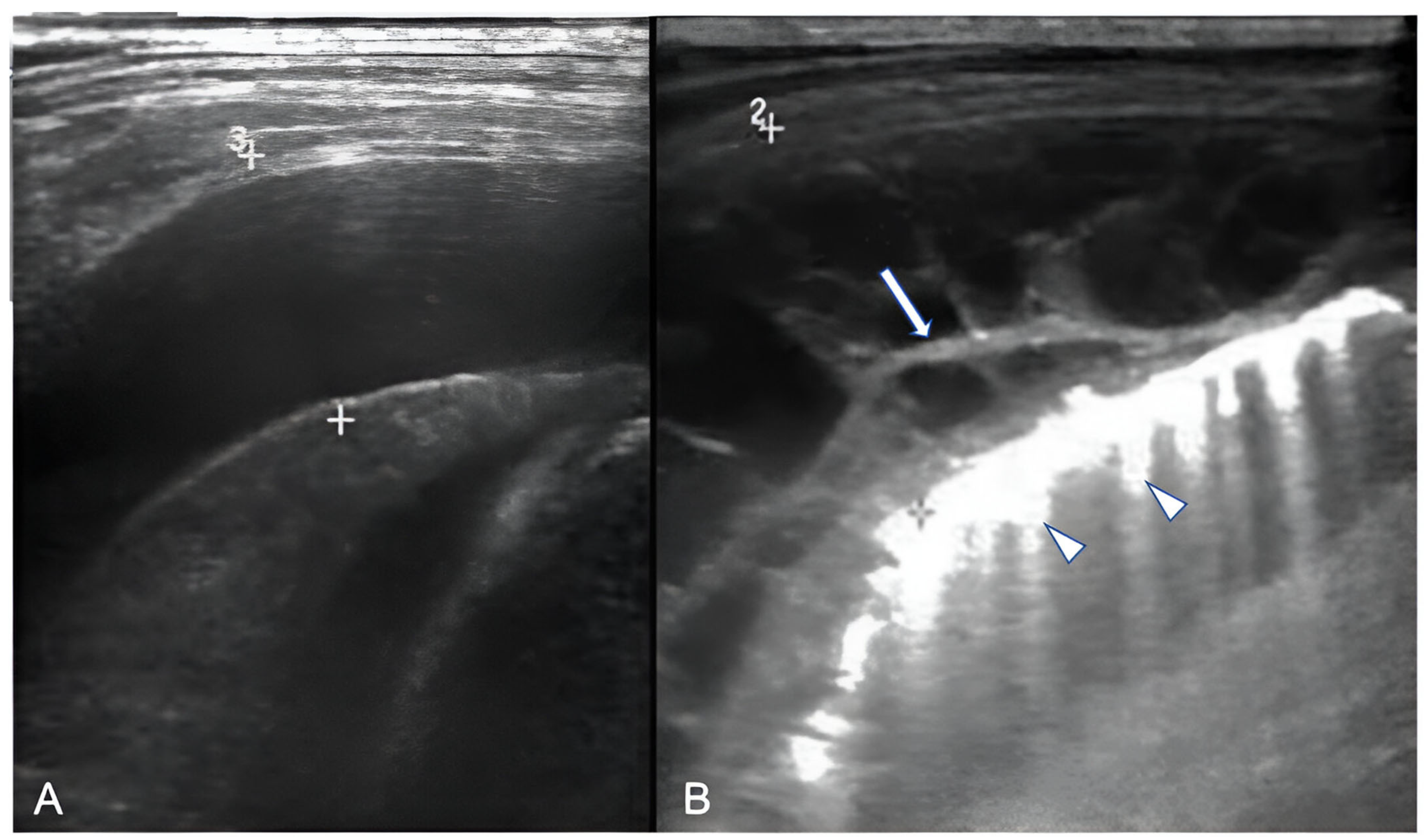
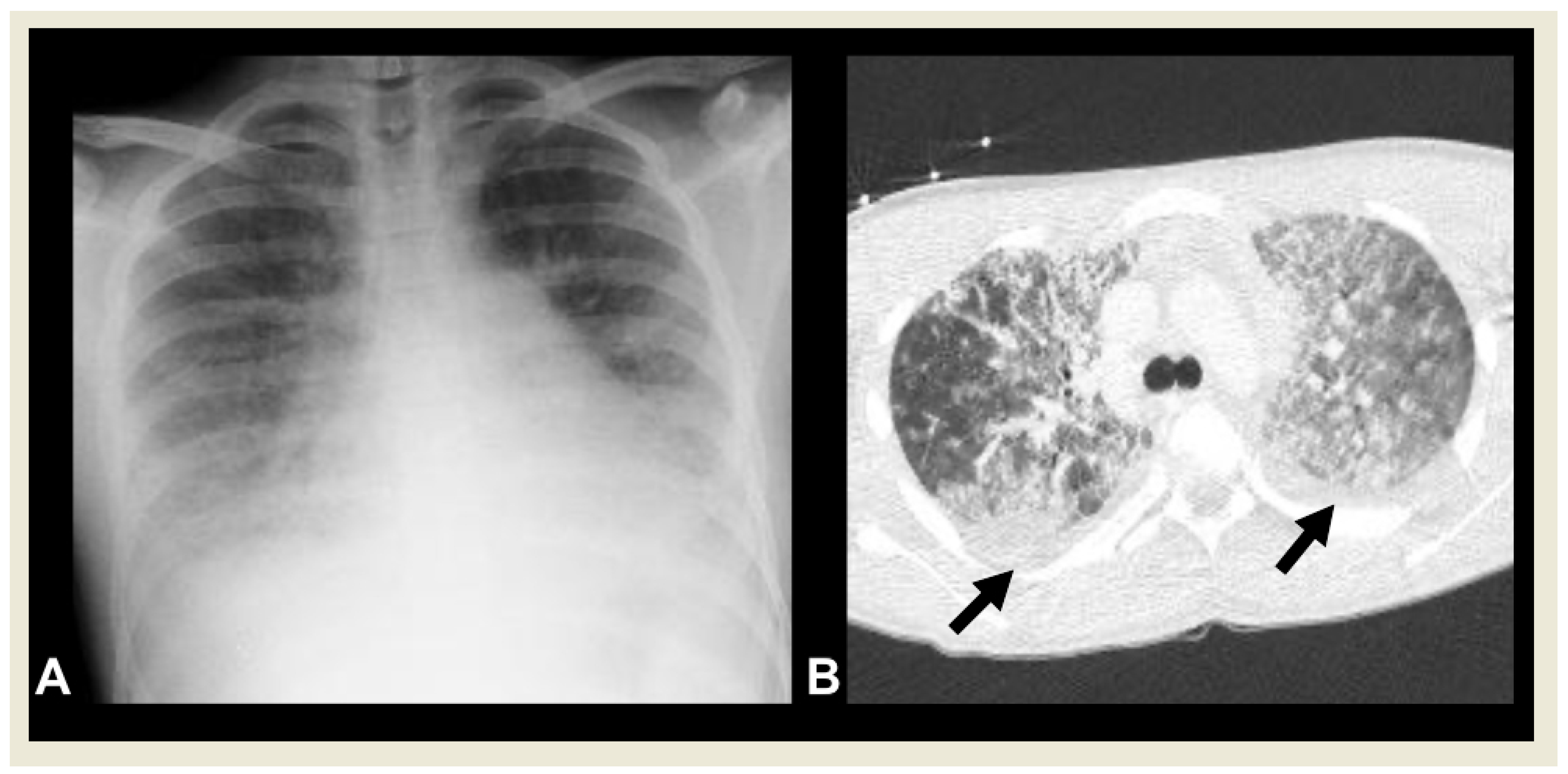
2.4.3. Necrotizing Pneumonia
2.4.4. Abscess
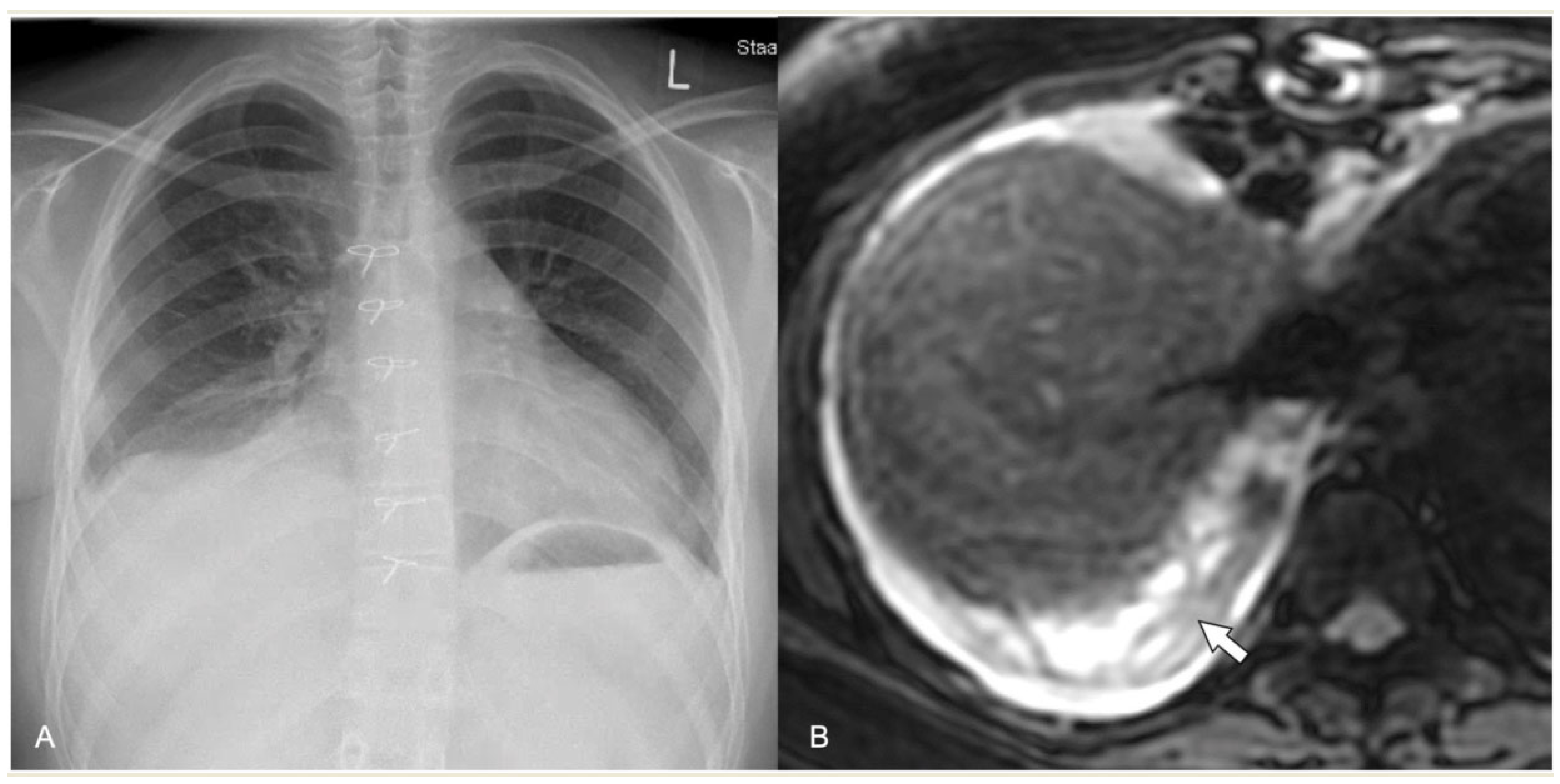
2.4.5. Pneumatocele
2.4.6. Pleural Fistulas
2.4.7. Pediatric Acute Respiratory Distress Syndrome (PARDS)
3. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wardlaw, T.; Salama, P.; Johansson, E.W.; Mason, E. Pneumonia: The leading killer of children. Lancet 2006, 368, 1048–1050. [Google Scholar] [CrossRef] [PubMed]
- Pneumonia in Children. Available online: https://www.who.int/news-room/fact-sheets/detail/pneumonia (accessed on 7 January 2024).
- Bradley, J.S.; Byington, C.L.; Shah, S.S.; Alverson, B.; Carter, E.R.; Harrison, C.; Kaplan, S.L.; Mace, S.E.; McCracken, G.H.; Moore, M.R.; et al. The Management of Community-Acquired Pneumonia in Infants and Children Older than 3 Months of Age: Clinical Practice Guidelines by the Pediatric Infectious Diseases Society and the Infectious Diseases Society of America. Clin. Infect. Dis. 2011, 53, e25–e76. [Google Scholar] [CrossRef] [PubMed]
- Harris, M.; Clark, J.; Coote, N.; Fletcher, P.; Harnden, A.; Mckean, M.; Thomson, A. British Thoracic Society guidelines for the management of community acquired pneumonia in children: Update 2011. Thorax 2011, 66, ii1–ii23. [Google Scholar] [CrossRef]
- Edis, E.C.; Hatipoglu, O.N.; Yilmam, I.; Eker, A.; Tansel, O.; Sut, N. Hospital-Acquired Pneumonia Developed in Non-Intensive Care Units. Respiration 2009, 78, 416–422. [Google Scholar] [CrossRef]
- De Benedictis, F.M.; Kerem, E.; Chang, A.B.; Colin, A.; Zar, H.J.; Bush, A. Complicated pneumonia in children. Lancet 2020, 396, 786–798. [Google Scholar] [CrossRef] [PubMed]
- Roh, E.J.; Shim, J.Y.; Chung, E.H. Epidemiology and surveillance implications of community-acquired pneumonia in children. Clin. Exp. Pediatr. 2022, 65, 563–573. [Google Scholar] [CrossRef] [PubMed]
- Von Mollendorf, C.; Berger, D.; Gwee, A.; Duke, T.; Graham, S.M.; Russell, F.M.; Mulholland, E.K. ARI review group Aetiology of childhood pneumonia in low- and middle-income countries in the era of vaccination: A systematic review. J. Glob. Health 2022, 12, 10009. [Google Scholar] [CrossRef]
- Cilla, G.; Oñate, E.; Perez-Yarza, E.G.; Montes, M.; Vicente, D.; Perez-Trallero, E. Viruses in community-acquired pneumonia in children aged less than 3 years old: High rate of viral coinfection. J. Med. Virol. 2008, 80, 1843–1849. [Google Scholar] [CrossRef]
- Chan, S.S.; Kotecha, M.K.; Rigsby, C.K.; Iyer, R.S.; Alazraki, A.L.; Anupindi, S.A.; Bardo, D.M.; Brown, B.P.; Chandra, T.; Dorfman, S.R.; et al. ACR Appropriateness Criteria® Pneumonia in the Immunocompetent Child. J. Am. Coll. Radiol. 2020, 17, S215–S225. [Google Scholar] [CrossRef]
- Cherian, T.; Mulholland, E.K.; Carlin, J.B.; Ostensen, H.; Amin, R.; De Campo, M.; Greenberg, D.; Lagos, R.; Lucero, M.; Madhi, S.A.; et al. Standardized interpretation of paediatric chest radiographs for the diagnosis of pneumonia in epidemiological studies. Bull. World Health Organ. 2005, 83, 353–359. [Google Scholar] [PubMed]
- Andronikou, S.; Lambert, E.; Halton, J.; Hilder, L.; Crumley, I.; Lyttle, M.D.; Kosack, C. Guidelines for the use of chest radiographs in community-acquired pneumonia in children and adolescents. Pediatr. Radiol. 2017, 47, 1405–1411. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.-H.; Yu, N.; Wang, Y.-H.; Gao, Y.-B.; Pan, L. Lung ultrasound vs chest radiography in the diagnosis of children pneumonia. Medicine 2020, 99, e23671. [Google Scholar] [CrossRef]
- Ibitoye, B.O.; Idowu, B.M.; Ogunrombi, A.B.; Afolabi, B.I. Ultrasonographic quantification of pleural effusion: Comparison of four formulae. Ultrasonography 2018, 37, 254–260. [Google Scholar] [CrossRef] [PubMed]
- Brogna, B.; Bignardi, E.; Brogna, C.; Volpe, M.; Lombardi, G.; Rosa, A.; Gagliardi, G.; Capasso, P.F.M.; Gravino, E.; Maio, F.; et al. A Pictorial Review of the Role of Imaging in the Detection, Management, Histopathological Correlations, and Complications of COVID-19 Pneumonia. Diagnostics 2021, 11, 437. [Google Scholar] [CrossRef]
- Kogias, C.; Prountzos, S.; Alexopoulou, E.; Douros, K. Lung ultrasound systematic review shows its prognostic and diagnostic role in acute viral bronchiolitis. Acta Paediatr. 2023, 112, 222–232. [Google Scholar] [CrossRef]
- Liu, J.; Copetti, R.; Sorantin, E.; Lovrenski, J.; Rodriguez-Fanjul, J.; Kurepa, D.; Feng, X.; Cattaross, L.; Zhang, H.; Hwang, M.; et al. Protocol and Guidelines for Point-of-Care Lung Ultrasound in Diagnosing Neonatal Pulmonary Diseases Based on International Expert Consensus. J. Vis. Exp. 2019, 2019, e58990. [Google Scholar] [CrossRef]
- Bhalla, D.; Naranje, P.; Jana, M.; Bhalla, A.S. Pediatric lung ultrasonography: Current perspectives. Pediatr. Radiol. 2022, 52, 2038–2050. [Google Scholar] [CrossRef]
- Lovrenski, J. Pediatric lung ultrasound cons—Are they really strong enough? Pediatr. Radiol. 2020, 50, 321–322. [Google Scholar] [CrossRef]
- Lovrenski, J. Pediatric lung ultrasound—Pros and potentials. Pediatr. Radiol. 2020, 50, 306–313. [Google Scholar] [CrossRef]
- Laya, B.F.; Concepcion, N.D.P.; Garcia-Peña, P.; Naidoo, J.; Kritsaneepaiboon, S.; Lee, E.Y. Pediatric Lower Respiratory Tract Infections: Imaging Guidelines and Recommendations. Radiol. Clin. N. Am. 2022, 60, 15–40. [Google Scholar] [CrossRef]
- Dietrich, C.F.; Buda, N.; Ciuca, I.M.; Dong, Y.; Fang, C.; Feldkamp, A.; Jüngert, J.; Kosiak, W.; Mentzel, H.J.; Pienar, C.; et al. Lung ultrasound in children, WFUMB review paper (part 2). Med. Ultrason. 2021, 23, 443–452. [Google Scholar] [CrossRef]
- Chidini, G.; Raimondi, F. Lung ultrasound for the sick child: Less harm and more information than a radiograph. Eur. J. Pediatr. 2023. online ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Hansell, L.; Milross, M.; Delaney, A.; Tian, D.H.; Ntoumenopoulos, G. Lung ultrasound has greater accuracy than conventional respiratory assessment tools for the diagnosis of pleural effusion, lung consolidation and collapse: A systematic review. J. Physiother. 2021, 67, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Tschauner, S.; Schramek, C.; Sorantin, E. Paediatric CT made easy. Pediatr. Radiol. 2023, 53, 581–588. [Google Scholar] [CrossRef] [PubMed]
- Worrall, M.; Holubinka, M.; Havariyoun, G.; Hodgson, K.; Edyvean, S.; Holroyd, J.; Davis, A.; Dunn, M.; Gardiner, A. Analysis and results from a UK national dose audit of paediatric CT examinations. Br. J. Radiol. 2022, 95, 20210796. [Google Scholar] [CrossRef]
- Liszewski, M.C.; Görkem, S.; Sodhi, K.S.; Lee, E.Y. Lung magnetic resonance imaging for pneumonia in children. Pediatr. Radiol. 2017, 47, 1420–1430. [Google Scholar] [CrossRef]
- Jaffe, A.; Calder, A.D.; Owens, C.M.; Stanojevic, S.; Sonnappa, S. Role of routine computed tomography in paediatric pleural empyema. Thorax 2008, 63, 897–902. [Google Scholar] [CrossRef]
- Odev, K.; Guler, I.; Altinok, T.; Pekcan, S.; Batur, A.; Ozbiner, H. Cystic and Cavitary Lung Lesions in Children: Radiologic Findings with Pathologic Correlation. J. Clin. Imaging Sci. 2013, 3, 60. [Google Scholar] [CrossRef]
- Hirsch, F.W.; Sorge, I.; Vogel-Claussen, J.; Roth, C.; Gräfe, D.; Päts, A.; Voskrebenzev, A.; Anders, R.M. The current status and further prospects for lung magnetic resonance imaging in pediatric radiology. Pediatr. Radiol. 2020, 50, 734–749. [Google Scholar] [CrossRef]
- Hirsch, F.W.; Sorge, I.; Voit, D.; Frahm, J.; Prenzel, F.; Wachowiak, R.; Anders, R.; Roth, C.; Gräfe, D. Chest examinations in children with real-time magnetic resonance imaging: First clinical experience. Pediatr. Radiol. 2023, 53, 12–20. [Google Scholar] [CrossRef]
- Wielpütz, M.O.; Triphan, S.M.F.; Ohno, Y.; Jobst, B.J.; Kauczor, H.-U. Outracing Lung Signal Decay—Potential of Ultrashort Echo Time MRI. RoFo Fortschritte Auf Dem Geb. Der Rontgenstrahlen Und Der Bildgeb. Verfahr. 2019, 191, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Liszewski, M.C.; Ciet, P.; Winant, A.J.; Lee, E.Y. Magnetic Resonance Imaging of Pediatric Lungs and Airways. J. Thorac. Imaging 2023, 39, 57–66. [Google Scholar] [CrossRef] [PubMed]
- Konietzke, P.; Mueller, J.; Wuennemann, F.; Wagner, W.L.; Schenk, J.-P.; Alrajab, A.; Kauczor, H.-U.; Stahl, M.; Mall, M.A.; Wielpütz, M.O.; et al. The value of chest magnetic resonance imaging compared to chest radiographs with and without additional lung ultrasound in children with complicated pneumonia. PLoS ONE 2020, 15, e0230252. [Google Scholar] [CrossRef]
- Peltola, V.; Ruuskanen, O.; Svedström, E. Magnetic resonance imaging of lung infections in children. Pediatr. Radiol. 2008, 38, 1225–1231. [Google Scholar] [CrossRef]
- Attenberger, U.; Morelli, J.; Henzler, T.; Buchheidt, D.; Fink, C.; Schoenberg, S.; Reichert, M. 3Tesla proton MRI for the diagnosis of pneumonia/lung infiltrates in neutropenic patients with acute myeloid leukemia: Initial results in comparison to HRCT. Eur. J. Radiol. 2014, 83, e61–e66. [Google Scholar] [CrossRef]
- Sodhi, K.S.; Khandelwal, N.; Saxena, A.K.; Singh, M.; Agarwal, R.; Bhatia, A.; Lee, E.Y. Rapid lung MRI in children with pulmonary infections: Time to change our diagnostic algorithms. J. Magn. Reson. Imaging 2016, 43, 1196–1206. [Google Scholar] [CrossRef]
- Calder, A.; Owens, C.M. Imaging of parapneumonic pleural effusions and empyema in children. Pediatr. Radiol. 2009, 39, 527–537. [Google Scholar] [CrossRef]
- Feller-Kopman, D.; Light, R. Pleural Disease. N. Engl. J. Med. 2018, 378, 740–751. [Google Scholar] [CrossRef]
- Lai-Fook, S.J. Pleural Mechanics and Fluid Exchange. Physiol. Rev. 2004, 84, 385–410. [Google Scholar] [CrossRef]
- Eslamy, H.K.; Newman, B. Pneumonia in Normal and Immunocompromised Children: An Overview and Update. Radiol. Clin. N. Am. 2011, 49, 895–920. [Google Scholar] [CrossRef]
- Tracy, M.C.; Mathew, R. Complicated pneumonia: Current concepts and state of the art. Curr. Opin. Pediatr. 2018, 30, 384–392. [Google Scholar] [CrossRef] [PubMed]
- Fischer, G.B.; Mocelin, H.T.; Andrade, C.F.; Sarria, E.E. When should parapneumonic pleural effusions be drained in children? Paediatr. Respir. Rev. 2018, 26, 27–30. [Google Scholar] [CrossRef]
- James, C.A.; Lewis, P.S.; Moore, M.B.; Wong, K.; Rader, E.K.; Roberson, P.K.; Ghaleb, N.A.; Jensen, H.K.; Pezeshkmehr, A.H.; Stroud, M.H.; et al. Efficacy of standardizing fibrinolytic therapy for parapneumonic effusion. Pediatr. Radiol. 2022, 52, 2413–2420. [Google Scholar] [CrossRef] [PubMed]
- Deganello, A.; Rafailidis, V.; Sellars, M.E.; Ntoulia, A.; Kalogerakou, K.; Ruiz, G.; Cosgrove, D.O.; Sidhu, P.S. Intravenous and Intracavitary Use of Contrast-Enhanced Ultrasound in the Evaluation and Management of Complicated Pediatric Pneumonia. J. Ultrasound Med. 2017, 36, 1943–1954. [Google Scholar] [CrossRef] [PubMed]
- Tomà, P. Lung ultrasound in pediatric radiology—Cons. Pediatr. Radiol. 2020, 50, 314–320. [Google Scholar] [CrossRef]
- Chen, H.-J.; Yu, Y.-H.; Tu, C.-Y.; Chen, C.-H.; Hsia, T.-C.; Tsai, K.-D.; Shih, C.-M.; Hsu, W.-H. Ultrasound in Peripheral Pulmonary Air-Fluid Lesions. Chest 2009, 135, 1426–1432. [Google Scholar] [CrossRef]
- Kapur, S.; Bhalla, A.S.; Jana, M. Pediatric Chest MRI: A Review. Indian J. Pediatr. 2019, 86, 842–853. [Google Scholar] [CrossRef]
- Chiu, C.-Y.; Cheng, M.-L.; Wong, K.-S.; Lai, S.-H.; Chiang, M.-H.; Tsai, M.-H.; Lin, G. Metabolomics Reveals Anaerobic Bacterial Fermentation and Hypoxanthine Accumulation for Fibrinous Pleural Effusions in Children with Pneumonia. J. Proteome Res. 2019, 18, 1248–1254. [Google Scholar] [CrossRef]
- Musolino, A.M.; Tomà, P.; Supino, M.C.; Scialanga, B.; Mesturino, A.; Scateni, S.; Battaglia, M.; Pirozzi, N.; Bock, C.; Buonsenso, D. Lung ultrasound features of children with complicated and noncomplicated community acquired pneumonia: A prospective study. Pediatr. Pulmonol. 2019, 54, 1479–1486. [Google Scholar] [CrossRef]
- Rafailidis, V.; Andronikou, S.; Mentzel, H.-J.; Piskunowicz, M.; Squires, J.H.; Barnewolt, C.E. Contrast-enhanced ultrasound of pediatric lungs. Pediatr. Radiol. 2021, 51, 2340–2350. [Google Scholar] [CrossRef]
- Eber Fabio, E.M. (Ed.) ERS Handbook of Paediatric Respiratory Medicine; The European Respiratory Society: Lausanne, Switzerland, 2021. [Google Scholar] [CrossRef]
- Gorkem, S.B.; Coskun, A.; Yikilmaz, A.; Zurakowski, D.; Mulkern, R.V.; Lee, E.Y. Evaluation of Pediatric Thoracic Disorders: Comparison of Unenhanced Fast-Imaging-Sequence 1.5-T MRI and Contrast-Enhanced MDCT. Am. J. Roentgenol. 2013, 200, 1352–1357. [Google Scholar] [CrossRef] [PubMed]
- Baez, J.C.; Ciet, P.; Mulkern, R.; Seethamraju, R.T.; Lee, E.Y. Pediatric Chest MR Imaging. Magn. Reson. Imaging Clin. N. Am. 2015, 23, 337–349. [Google Scholar] [CrossRef] [PubMed]
- Ciet, P.; Tiddens, H.A.W.M.; Wielopolski, P.A.; Wild, J.M.; Lee, E.Y.; Morana, G.; Lequin, M.H. Magnetic resonance imaging in children: Common problems and possible solutions for lung and airways imaging. Pediatr. Radiol. 2015, 45, 1901–1915. [Google Scholar] [CrossRef] [PubMed]
- Al-Saleh, S.; Grasemann, H.; Cox, P. Necrotizing Pneumonia Complicated by Early and Late Pneumatoceles. Can. Respir. J. 2008, 15, 129–132. [Google Scholar] [CrossRef]
- Andronikou, S.; Goussard, P.; Sorantin, E. Computed tomography in children with community-acquired pneumonia. Pediatr. Radiol. 2017, 47, 1431–1440. [Google Scholar] [CrossRef]
- Kapoor, H.; Gulati, V.; Gulati, A.; Donuru, A.; Parekh, M. Comprehensive Imaging Review of Pleural Fistulas from Diagnosis to Management. RadioGraphics 2022, 42, 1940–1955. [Google Scholar] [CrossRef]
- Shein, S.L.; Maddux, A.B.; Klein, M.J.; Bhalla, A.; Briassoulis, G.; Dahmer, M.K.; Emeriaud, G.; Flori, H.R.; Gedeit, R.; Ilia, S.; et al. Epidemiology and Outcomes of Critically Ill Children at Risk for Pediatric Acute Respiratory Distress Syndrome: A Pediatric Acute Respiratory Distress Syndrome Incidence and Epidemiology Study. Crit. Care Med. 2022, 50, 363–374. [Google Scholar] [CrossRef]
- Kohne, J.G.; Flori, H.R. Risk Factors and Etiologies of Pediatric Acute Respiratory Distress Syndrome. In Pediatric Acute Respiratory Distress Syndrome; Springer: Cham, Switzerland, 2019; pp. 33–46. [Google Scholar] [CrossRef]





| Modality | Pros | Cons |
|---|---|---|
| CR | Widely available, very quick examination | Radiation exposure, prone to misinterpretation |
| LUS | Widely available, no ionizing radiation, point-of-care examination | User-dependent and time-consuming examination, findings are dependent on the location |
| CT | Widely available, quick examination; excellent spatial resolution for chest examinations, with high sensitivity | Radiation exposure, patient cooperation needed |
| MRI | Excellent soft tissue contrast, no ionizing radiation | Not widely available, patient cooperation needed, time-consuming examination, newer techniques and software needed for lung examinations to improve spatial and temporal resolution |
| Complicated CAP | Uncomplicated CAP | ||||||
|---|---|---|---|---|---|---|---|
| Pleural Effusion | Empyema | Necrotising Pneumonia | Abscess | Pneumatocele | Pleural Fistula | PARDS | |
| US: usually appropriate | US/CT: usually appropriate | US/CT: usually appropriate | US/CT: usually appropriate | CT: usually appropriate | CT: usually appropriate | CR: usually appropriate | No imaging is necessary |
| CR/CT: may be appropriate | MRI: may be appropriate | MRI: may be appropriate | MRI: may be appropriate | CR: may be appropriate | US/CT: may be appropriate | ||
| MRI: usually not appropriate | CR: usually not appropriate | CR: usually not appropriate | CR: usually not appropriate | US/MRI: usually not appropriate | CR/US/MRI: usually not appropriate | MRI: usually not appropriate | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alexopoulou, E.; Prountzos, S.; Raissaki, M.; Mazioti, A.; Caro-Dominguez, P.; Hirsch, F.W.; Lovrenski, J.; Ciet, P. Imaging of Acute Complications of Community-Acquired Pneumonia in the Paediatric Population—From Chest Radiography to MRI. Children 2024, 11, 122. https://doi.org/10.3390/children11010122
Alexopoulou E, Prountzos S, Raissaki M, Mazioti A, Caro-Dominguez P, Hirsch FW, Lovrenski J, Ciet P. Imaging of Acute Complications of Community-Acquired Pneumonia in the Paediatric Population—From Chest Radiography to MRI. Children. 2024; 11(1):122. https://doi.org/10.3390/children11010122
Chicago/Turabian StyleAlexopoulou, Efthymia, Spyridon Prountzos, Maria Raissaki, Argyro Mazioti, Pablo Caro-Dominguez, Franz Wolfgang Hirsch, Jovan Lovrenski, and Pierluigi Ciet. 2024. "Imaging of Acute Complications of Community-Acquired Pneumonia in the Paediatric Population—From Chest Radiography to MRI" Children 11, no. 1: 122. https://doi.org/10.3390/children11010122
APA StyleAlexopoulou, E., Prountzos, S., Raissaki, M., Mazioti, A., Caro-Dominguez, P., Hirsch, F. W., Lovrenski, J., & Ciet, P. (2024). Imaging of Acute Complications of Community-Acquired Pneumonia in the Paediatric Population—From Chest Radiography to MRI. Children, 11(1), 122. https://doi.org/10.3390/children11010122







