Impact of Nutritional Status on Total Brain Tissue Volumes in Preterm Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Data Collection
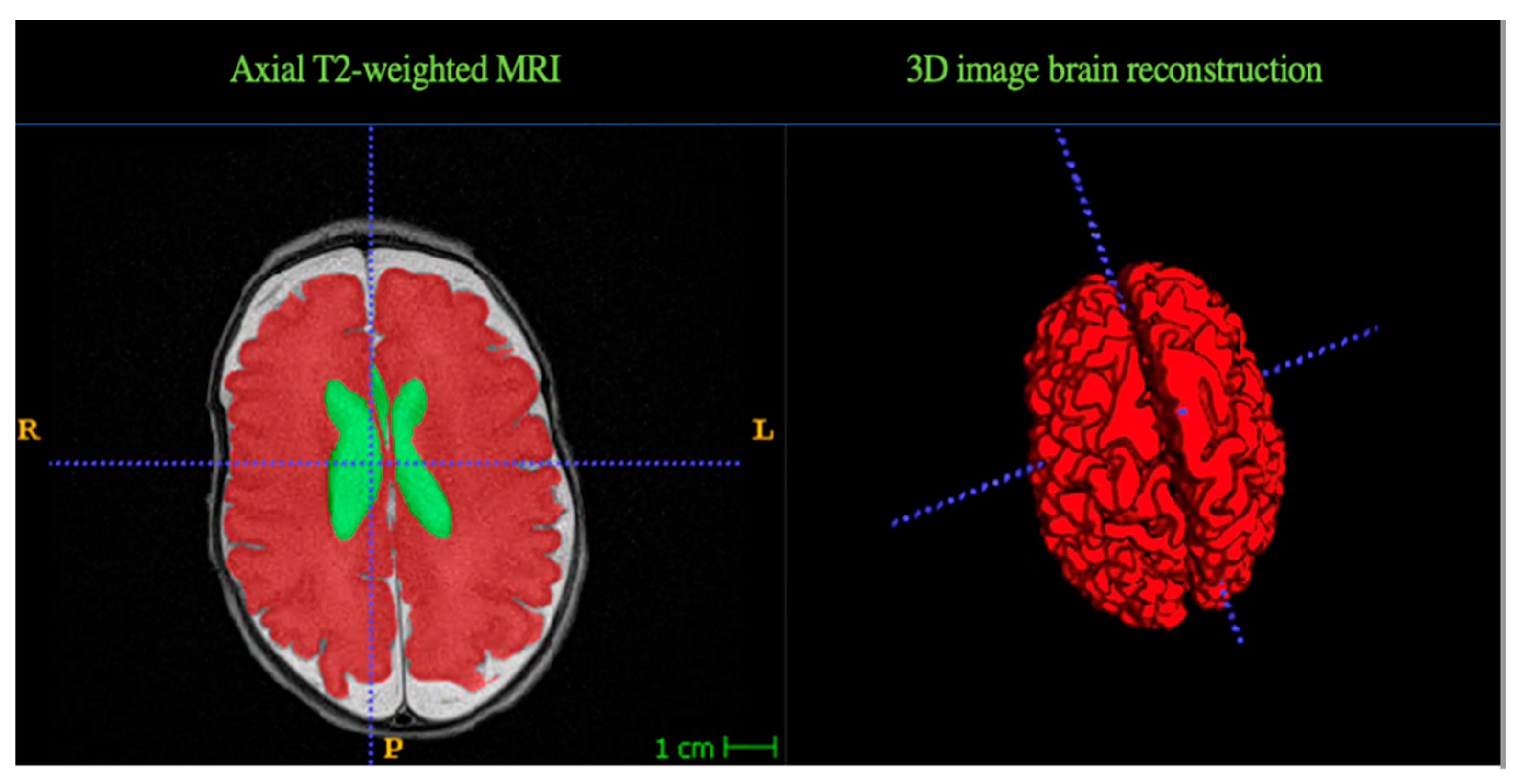
2.3. MRI Data Acquisition
2.4. Nutritional Status
2.5. Data Analysis
3. Results
3.1. Subject Demographics and Characteristics

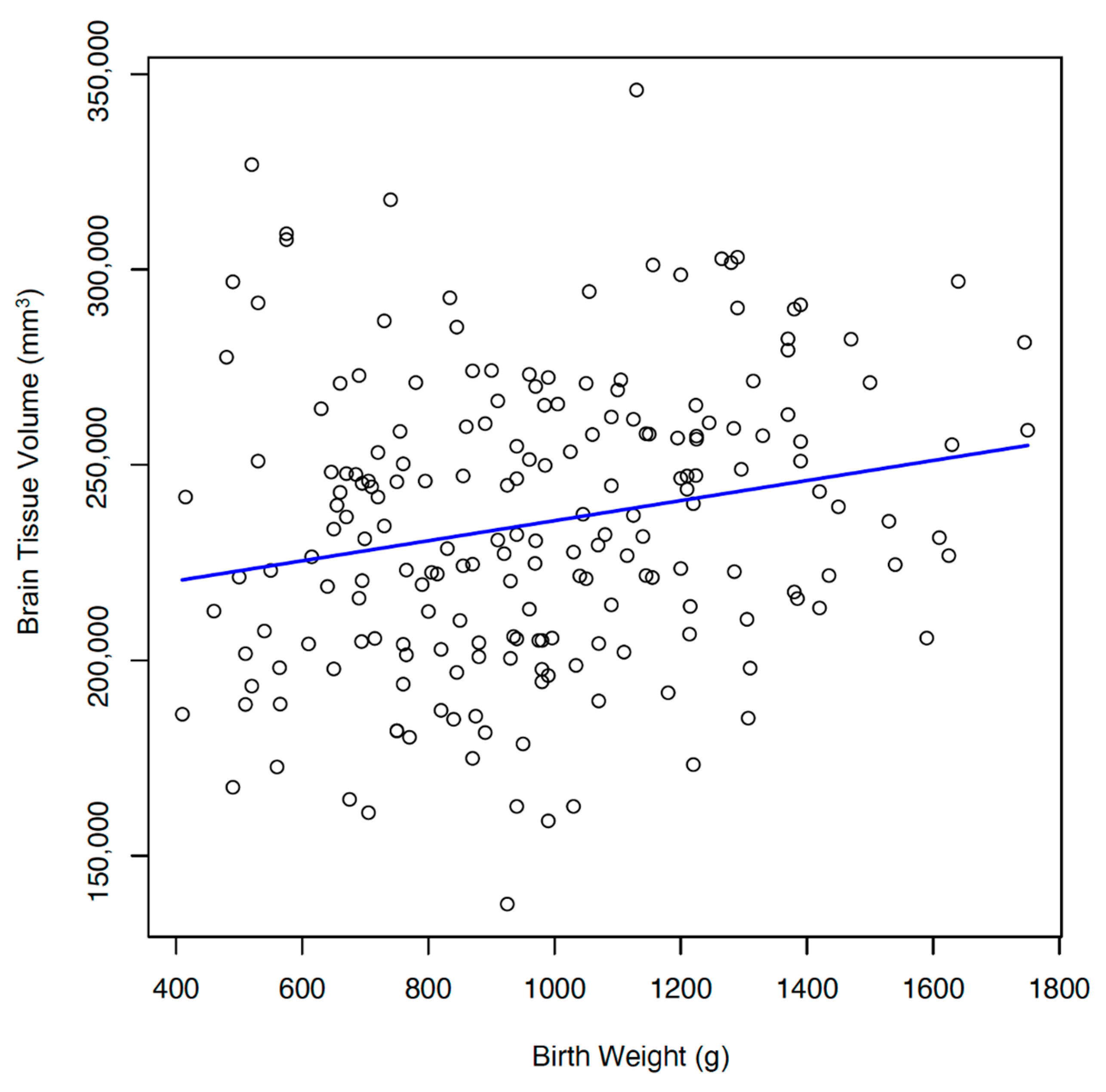
3.2. Relationship with Brain Tissue Volumes and Malnutrition
3.3. Association between Type of Enteral Feed and Brain Tissue Volumes
3.4. Intracranial Hemorrhage and Brain Tissue Volumes Association
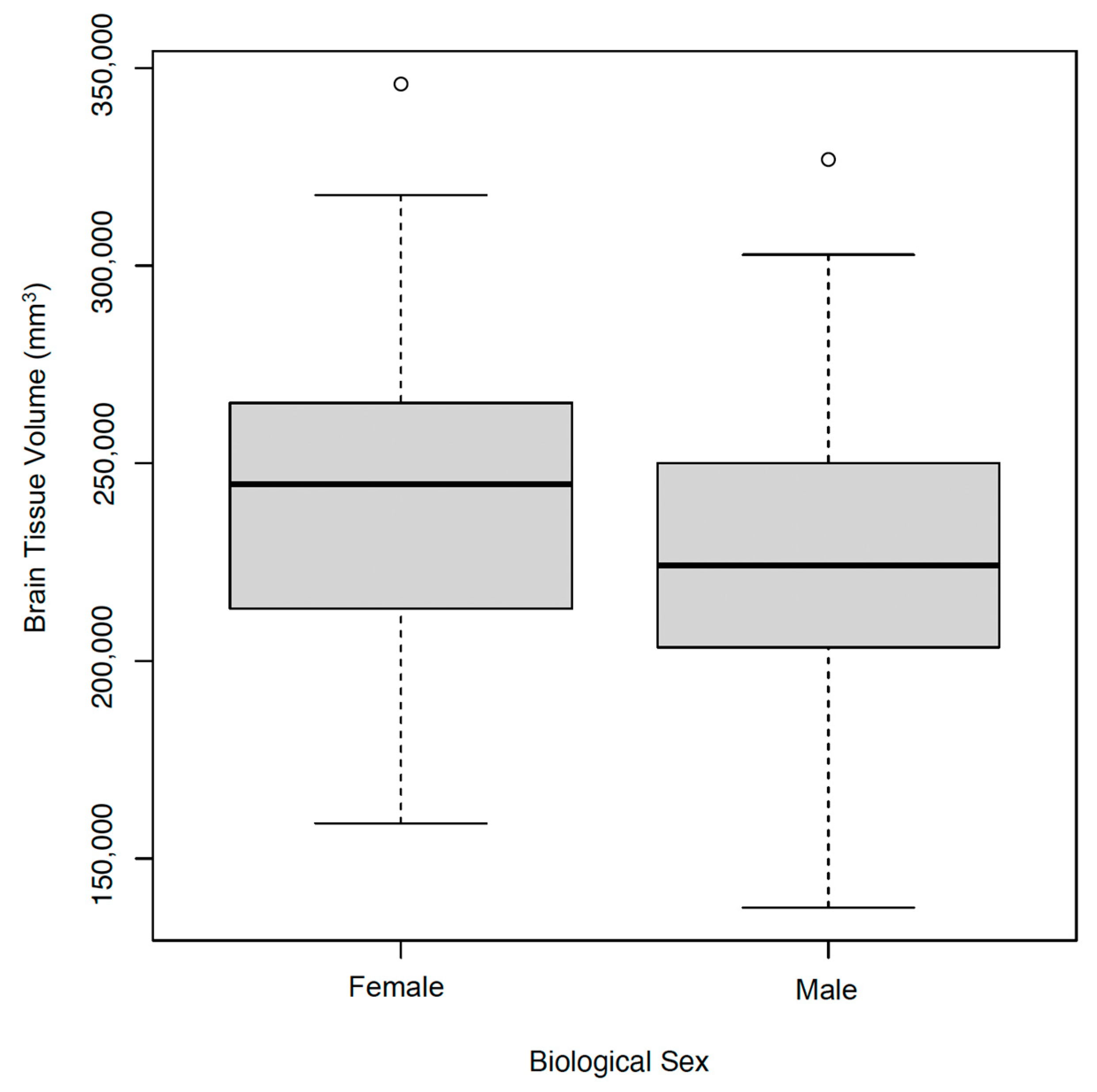
3.5. Demographics and Brain Tissue Volumes
3.6. Associations between Multiple Clinical Variables and Brain Tissue Volumes
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ramel, S.E.; Georgieff, M.K. Preterm nutrition and the brain. World Rev. Nutr. Diet 2014, 110, 190–200. [Google Scholar]
- Power, V.A.; Spittle, A.J.; Lee, K.J.; Anderson, P.J.; Thompson, D.K.; Doyle, L.W.; Cheong, J.L.Y. Nutrition, growth, brain volume, and neurodevelopment in very preterm children. J. Pediatr. 2019, 215, 50–55 e53. [Google Scholar] [CrossRef] [PubMed]
- Bell, K.A.; Matthews, L.G.; Cherkerzian, S.; Prohl, A.K.; Warfield, S.K.; Inder, T.E.; Onishi, S.; Belfort, M.B. Associations of body composition with regional brain volumes and white matter microstructure in very preterm infants. Arch. Dis. Child Fetal Neonatal Ed. 2022, 107, 533–538. [Google Scholar]
- Goldberg, D.L.; Becker, P.J.; Brigham, K.; Carlson, S.; Fleck, L.; Gollins, L.; Sandrock, M.; Fullmer, M.; Van Poots, H.A. Identifying malnutrition in preterm and neonatal populations: Recommended indicators. J. Acad. Nutr. Diet 2018, 118, 1571–1582. [Google Scholar] [CrossRef] [PubMed]
- Reber, E.; Gomes, F.; Vasiloglou, M.F.; Schuetz, P.; Stanga, Z. Nutritional risk screening and assessment. J. Clin. Med. 2019, 8, 1065. [Google Scholar] [PubMed]
- Martin, C.R.; Brown, Y.F.; Ehrenkranz, R.A.; O'Shea, T.M.; Allred, E.N.; Belfort, M.B.; McCormick, M.C.; Leviton, A.; Extremely Low Gestational Age Newborns Study Investigators. Nutritional practices and growth velocity in the first month of life in extremely premature infants. Pediatrics 2009, 124, 649–657. [Google Scholar] [CrossRef] [PubMed]
- Tan, M.J.; Cooke, R.W. Improving head growth in very preterm infants--a randomised controlled trial i: Neonatal outcomes. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F337–F341. [Google Scholar] [CrossRef]
- Clouchoux, C.; Guizard, N.; Evans, A.C.; du Plessis, A.J.; Limperopoulos, C. Normative fetal brain growth by quantitative in vivo magnetic resonance imaging. Am. J. Obstet Gynecol. 2012, 206, 173.e1-8. [Google Scholar]
- Matthews, L.G.; Walsh, B.H.; Knutsen, C.; Neil, J.J.; Smyser, C.D.; Rogers, C.E.; Inder, T.E. Brain growth in the nicu: Critical periods of tissue-specific expansion. Pediatr. Res. 2018, 83, 976–981. [Google Scholar] [CrossRef]
- Keunen, K.; Kersbergen, K.J.; Groenendaal, F.; Isgum, I.; de Vries, L.S.; Benders, M.J. Brain tissue volumes in preterm infants: Prematurity, perinatal risk factors and neurodevelopmental outcome: A systematic review. J. Matern. Fetal Neonatal Med. 2012, 25 (Suppl. S1), 89–100. [Google Scholar]
- Cormack, B.E.; Harding, J.E.; Miller, S.P.; Bloomfield, F.H. The influence of early nutrition on brain growth and neurodevelopment in extremely preterm babies: A narrative review. Nutrients 2019, 11, 2029. [Google Scholar] [PubMed]
- Huppi, P.S.; Warfield, S.; Kikinis, R.; Barnes, P.D.; Zientara, G.P.; Jolesz, F.A.; Tsuji, M.K.; Volpe, J.J. Quantitative magnetic resonance imaging of brain development in premature and mature newborns. Ann. Neurol. 1998, 43, 224–235. [Google Scholar] [CrossRef] [PubMed]
- Gousias, I.S.; Edwards, A.D.; Rutherford, M.A.; Counsell, S.J.; Hajnal, J.V.; Rueckert, D.; Hammers, A. Magnetic resonance imaging of the newborn brain: Manual segmentation of labelled atlases in term-born and preterm infants. Neuroimage 2012, 62, 1499–1509. [Google Scholar]
- Romberg, J.; Wilke, M.; Allgaier, C.; Nagele, T.; Engel, C.; Poets, C.F.; Franz, A. Mri-based brain volumes of preterm infants at term: A systematic review and meta-analysis. Arch. Dis. Child. Fetal Neonatal Ed. 2022, 107, 520–526. [Google Scholar]
- Coviello, C.; Keunen, K.; Kersbergen, K.J.; Groenendaal, F.; Leemans, A.; Peels, B.; Isgum, I.; Viergever, M.A.; de Vries, L.S.; Buonocore, G.; et al. Effects of early nutrition and growth on brain volumes, white matter microstructure, and neurodevelopmental outcome in preterm newborns. Pediatr. Res. 2018, 83, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Moeskops, P.; Viergever, M.A.; Mendrik, A.M.; de Vries, L.S.; Benders, M.J.; Isgum, I. Automatic segmentation of mr brain images with a convolutional neural network. IEEE Trans. Med. Imaging 2016, 35, 1252–1261. [Google Scholar] [CrossRef] [PubMed]
- van Beek, P.E.; Claessens, N.H.P.; Makropoulos, A.; Groenendaal, F.; de Vries, L.S.; Counsell, S.J.; Benders, M. Increase in brain volumes after implementation of a nutrition regimen in infants born extremely preterm. J. Pediatr. 2020, 223, 57–63 e55. [Google Scholar]
- Lemmers, P.M.; Benders, M.J.; D'Ascenzo, R.; Zethof, J.; Alderliesten, T.; Kersbergen, K.J.; Isgum, I.; de Vries, L.S.; Groenendaal, F.; van Bel, F. Patent ductus arteriosus and brain volume. Pediatrics 2016, 137, e20153090. [Google Scholar] [CrossRef]
- Kumar, J.; Mukhopadhyay, K. Longitudinal growth of extremely low birth weight infants at 5 years. Indian J. Pediatr. 2019, 86, 1139–1141. [Google Scholar]
- Becker, P.; Carney, L.N.; Corkins, M.R.; Monczka, J.; Smith, E.; Smith, S.E.; Spear, B.A.; White, J.V.; Academy of Nutrition and Dietetics; American Society for Parenteral and Enteral Nutrition. Consensus statement of the academy of nutrition and dietetics/american society for parenteral and enteral nutrition: Indicators recommended for the identification and documentation of pediatric malnutrition (undernutrition). Nutr. Clin. Pract. 2015, 30, 147–161. [Google Scholar] [CrossRef]
- Inder, T.E.; Warfield, S.K.; Wang, H.; Huppi, P.S.; Volpe, J.J. Abnormal cerebral structure is present at term in premature infants. Pediatrics 2005, 115, 286–294. [Google Scholar] [CrossRef] [PubMed]
- Jensen, E.A.; Dysart, K.; Gantz, M.G.; McDonald, S.; Bamat, N.A.; Keszler, M.; Kirpalani, H.; Laughon, M.M.; Poindexter, B.B.; Duncan, A.F.; et al. The diagnosis of bronchopulmonary dysplasia in very preterm infants. An evidence-based approach. Am. J. Respir. Crit. Care Med. 2019, 200, 751–759. [Google Scholar] [CrossRef] [PubMed]
- Rutherford, M. Mri of the neonatal brain atlas.
- Rochow, N.; Raja, P.; Liu, K.; Fenton, T.; Landau-Crangle, E.; Gottler, S.; Jahn, A.; Lee, S.; Seigel, S.; Campbell, D.; et al. Physiological adjustment to postnatal growth trajectories in healthy preterm infants. Pediatr. Res. 2016, 79, 870–879. [Google Scholar] [PubMed]
- Taylor-Rodriguez, D.; Womack, A.; Bliznyuk, N. Bayesian variable selection on model spaces constrained by heredity conditions. J. Comput. Graph Stat. 2016, 25, 515–535. [Google Scholar] [CrossRef]
- Papile, L.A.; Burstein, J.; Burstein, R.; Koffler, H. Incidence and evolution of subependymal and intraventricular hemorrhage: A study of infants with birth weights less than 1500 gm. J. Pediatr. 1978, 92, 529–534. [Google Scholar]
- Belfort, M.B.; Rifas-Shiman, S.L.; Sullivan, T.; Collins, C.T.; McPhee, A.J.; Ryan, P.; Kleinman, K.P.; Gillman, M.W.; Gibson, R.A.; Makrides, M. Infant growth before and after term: Effects on neurodevelopment in preterm infants. Pediatrics 2011, 128, e899–e906. [Google Scholar] [CrossRef] [PubMed]
- Simon, L.; Theveniaut, C.; Flamant, C.; Frondas-Chauty, A.; Darmaun, D.; Roze, J.C. In preterm infants, length growth below expected growth during hospital stay predicts poor neurodevelopment at 2 years. Neonatology 2018, 114, 135–141. [Google Scholar] [PubMed]
- Scharf, R.J.; Stroustrup, A.; Conaway, M.R.; DeBoer, M.D. Growth and development in children born very low birthweight. Arch. Dis. Child. Fetal Neonatal Ed. 2016, 101, F433–F438. [Google Scholar] [CrossRef]
- Skullerud, K. Variations in the size of the human brain. Influence of age, sex, body length, body mass index, alcoholism, alzheimer changes, and cerebral atherosclerosis. Acta Neurol. 1985, 102, 1–94. [Google Scholar]
- Noori, S. Patent ductus arteriosus in the preterm infant: To treat or not to treat? J. Perinatol. 2010, 30, S31–S37. [Google Scholar]
- Benitz, W.E. Learning to live with patency of the ductus arteriosus in preterm infants. J. Perinatol. 2011, 31 (Suppl. S1), S42–S48. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kersbergen, K.J.; de Vries, L.S.; van Kooij, B.J.; Isgum, I.; Rademaker, K.J.; van Bel, F.; Huppi, P.S.; Dubois, J.; Groenendaal, F.; Benders, M.J. Hydrocortisone treatment for bronchopulmonary dysplasia and brain volumes in preterm infants. J. Pediatr. 2013, 163, 666–671 e661. [Google Scholar] [CrossRef] [PubMed]
- Tam, E.W.; Chau, V.; Ferriero, D.M.; Barkovich, A.J.; Poskitt, K.J.; Studholme, C.; Fok, E.D.; Grunau, R.E.; Glidden, D.V.; Miller, S.P. Preterm cerebellar growth impairment after postnatal exposure to glucocorticoids. Sci. Transl. Med. 2011, 3, 105ra105. [Google Scholar] [CrossRef] [PubMed]
- Beauport, L.; Schneider, J.; Faouzi, M.; Hagmann, P.; Huppi, P.S.; Tolsa, J.F.; Truttmann, A.C.; Fischer Fumeaux, C.J. Impact of early nutritional intake on preterm brain: A magnetic resonance imaging study. J. Pediatr. 2017, 181, 29–36 e21. [Google Scholar] [PubMed]
- Embleton, N.E.; Pang, N.; Cooke, R.J. Postnatal malnutrition and growth retardation: An inevitable consequence of current recommendations in preterm infants? Pediatrics 2001, 107, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Stephens, B.E.; Walden, R.V.; Gargus, R.A.; Tucker, R.; McKinley, L.; Mance, M.; Nye, J.; Vohr, B.R. First-week protein and energy intakes are associated with 18-month developmental outcomes in extremely low birth weight infants. Pediatrics 2009, 123, 1337–1343. [Google Scholar] [CrossRef] [PubMed]
- Franz, A.R.; Pohlandt, F.; Bode, H.; Mihatsch, W.A.; Sander, S.; Kron, M.; Steinmacher, J. Intrauterine, early neonatal, and postdischarge growth and neurodevelopmental outcome at 5.4 years in extremely preterm infants after intensive neonatal nutritional support. Pediatrics 2009, 123, e101–e109. [Google Scholar] [CrossRef]
- Ehrenkranz, R.A.; Dusick, A.M.; Vohr, B.R.; Wright, L.L.; Wrage, L.A.; Poole, W.K. Growth in the neonatal intensive care unit influences neurodevelopmental and growth outcomes of extremely low birth weight infants. Pediatrics 2006, 117, 1253–1261. [Google Scholar]
| Characteristic | Mean + SD | n | % |
|---|---|---|---|
| Gestational age at birth, wk. | 26.8 ± 1.8 | 198 | |
| Birth weight, g | 973 + 294 | ||
| Male | 103 | 52 | |
| Female | 95 | 48 | |
| Brain volume, mm3 | 235,059 ± 37,045 | ||
| Max change in weight, z-score | −1.3 ± 0.6 | ||
| Max change in length, z-score | −1.5 ± 1.3 | ||
| SGA | 22 | 11 | |
| AGA | 171 | 86 | |
| LGA | 5 | 2.5 | |
| IVH low grade | 74 | 37 | |
| IVH high grade | 13 | 7 |
| Characteristic | 23 wk. n = 10 | 24 wk. n = 17 | 25 wk. n = 19 | 26 wk. n = 29 | 27 wk. n = 34 | 28 wk. n = 47 | 29 wk. n = 42 |
|---|---|---|---|---|---|---|---|
| Birth weight (g), mean (SD) | 573 (115) | 683 (100) | 729 (125) | 787 (178) | 971 (171) | 1128 (229) | 1252 (259) |
| Max change weight(z-score), mean (SD) | −1.2 (0.8) | −1.7 (0.8) | −1.7 (0.8) | −1.3 (0.7) | −1.4 (0.6) | −1.2 (0.6) | −1.1 (0.5) |
| Max change length (z-score), mean (SD) | −1.8 (1.0) | −1.9 (0.9) | −2.0 (0.7) | −1.2 (2.7) | −1.7 (0.8) | −1.5 (0.8) | −1.1 (1.0) |
| Total brain volume (mm3), mean (SD) | 234,350 (43,762) | 231,847 (25,145) | 244,605 (38,345) | 239,696 (38,767) | 205,882 (38,136) | 242,304 (29,398) | 244,519 (34,573) |
| Size | n | Brain Volume (mm3), Mean (SD) | Max Change Weight, (z-Score), Mean (SD) | Max Change Length, (z-Score), Mean (SD) |
|---|---|---|---|---|
| SGA | 22 | 227,595.5 (44,160) | −1.3 (1.0) | −1.6 (1.1) |
| AGA | 17 | 1236,135.1 (36,179) | −1.3 (0.6) | −1.5 (1.4) |
| LGA | 5 | 231,100.0 (36,708) | −1.9 (0.2) | −1.4 (0.1) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdes, C.; Nataraj, P.; Kisilewicz, K.; Simenson, A.; Leon, G.; Kang, D.; Nguyen, D.; Sura, L.; Bliznyuk, N.; Weiss, M. Impact of Nutritional Status on Total Brain Tissue Volumes in Preterm Infants. Children 2024, 11, 121. https://doi.org/10.3390/children11010121
Valdes C, Nataraj P, Kisilewicz K, Simenson A, Leon G, Kang D, Nguyen D, Sura L, Bliznyuk N, Weiss M. Impact of Nutritional Status on Total Brain Tissue Volumes in Preterm Infants. Children. 2024; 11(1):121. https://doi.org/10.3390/children11010121
Chicago/Turabian StyleValdes, Cyndi, Parvathi Nataraj, Katherine Kisilewicz, Ashley Simenson, Gabriela Leon, Dahyun Kang, Dai Nguyen, Livia Sura, Nikolay Bliznyuk, and Michael Weiss. 2024. "Impact of Nutritional Status on Total Brain Tissue Volumes in Preterm Infants" Children 11, no. 1: 121. https://doi.org/10.3390/children11010121
APA StyleValdes, C., Nataraj, P., Kisilewicz, K., Simenson, A., Leon, G., Kang, D., Nguyen, D., Sura, L., Bliznyuk, N., & Weiss, M. (2024). Impact of Nutritional Status on Total Brain Tissue Volumes in Preterm Infants. Children, 11(1), 121. https://doi.org/10.3390/children11010121







