Advancing the Assessment and Treatment of Comorbid Pediatric Chronic Functional Abdominal Pain (CFAP) and Restrictive Eating Disorders
Abstract
:1. Introduction
2. Method
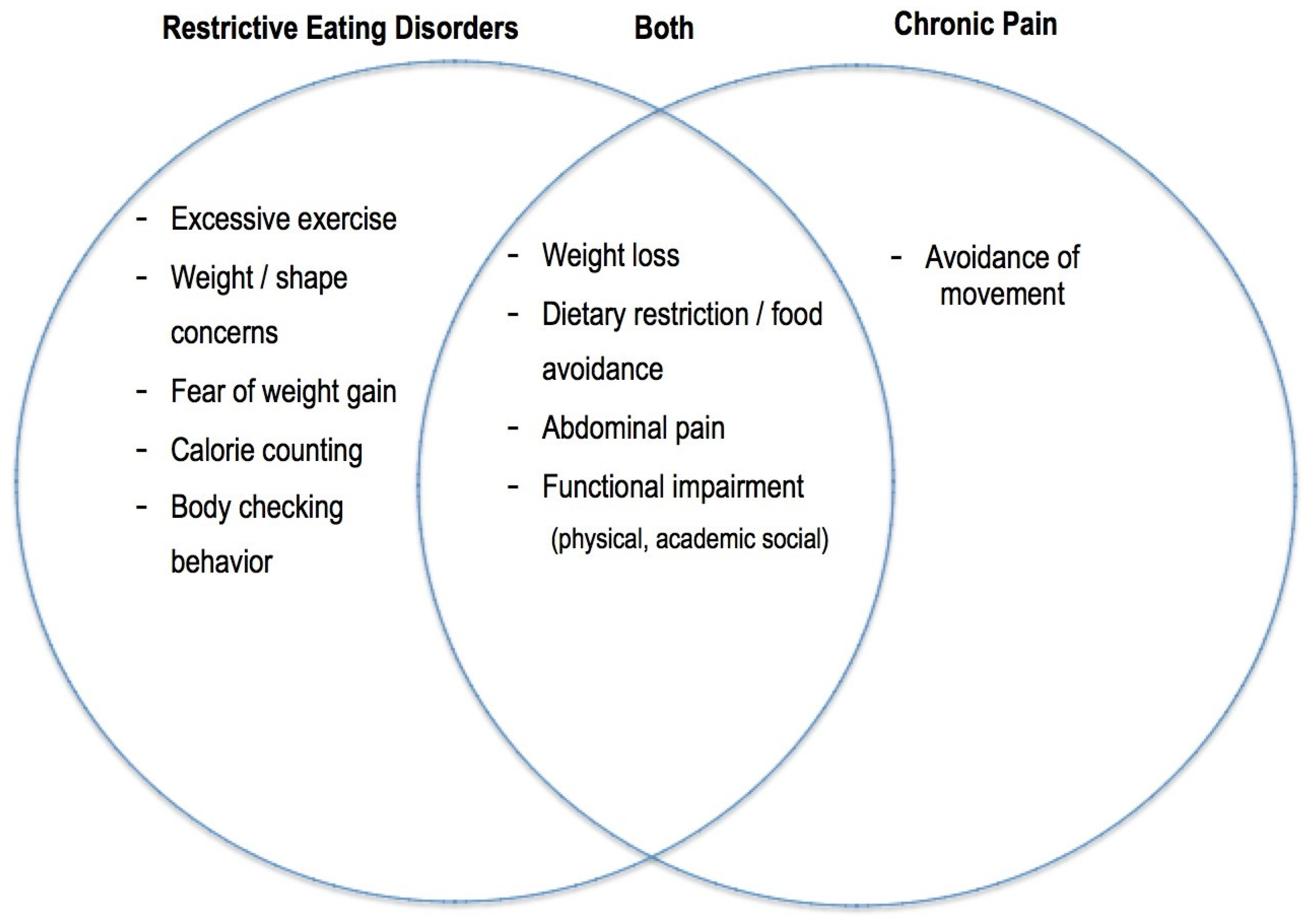
3. Restrictive EDs in Adolescents with Chronic Functional Abdominal Pain (CFAP)
4. Current Assessment Practices for CFAP and Restrictive EDs
5. Recommendations for Assessment of CFAP and Restrictive EDs
5.1. Current Challenges
5.2. Improve Identification of Comorbidity through Standardized Screening and Assessment Protocols
6. Current Treatment Practices for CFAP and Restrictive EDs
6.1. Treatment for CFAP
6.2. Treatment for Restrictive EDs
7. Recommendations for Treatment of CFAP and Restrictive EDs
7.1. Special Considerations: Dietary Changes
7.2. Importance of Interdisciplinary Care
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Drossman, D.A. Functional abdominal pain syndrome. IFFGD 2013, 141, 1–3. [Google Scholar] [CrossRef]
- Korterink, J.; Benninga, M.; Tabbers, M. Epidemiology of pediatric functional abdominal pain disorders: A meta-analysis. PLoS ONE 2015, 10, e0126982. [Google Scholar] [CrossRef] [PubMed]
- Becker, S.; Gandhi, W.; Schweinhardt, P. Cerebral interactions of pain and reward and their relevance for chronic pain. Neurosci. Lett. 2012, 520, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Bushnell, M.C.; Ceko, M.; Low, L.A. Cognitive and emotional control of pain and its disruption in chronic pain. Nat. Rev. Neurosci. 2013, 14, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Leknes, S.; Tracey, I. A common neurobiology for pain and pleasure. Nat. Rev. Neurosci. 2008, 9, 314–320. [Google Scholar] [CrossRef]
- Jensen, K.B.; Regenbogen, C.; Ohse, M.C.; Frasnelli, J.; Freiherr, J.; Lundström, J.N. Brain activations during pain: A neuroimaging meta-analysis of patients with pain and healthy controls. Pain 2016, 157, 1279–1286. [Google Scholar] [CrossRef]
- Johnson, M.K.; Nolen-Hoeksema, S.; Mitchell, K.J.; Levin, Y. Medial cortex activity, self-reflection and depression. Soc. Cogn. Affect. Neurosci. 2009, 4, 313–327. [Google Scholar] [CrossRef]
- Barbara, G.; Cremon, C.; Carini, G.; Bellacosa, L.; Zecchi, L.; De Giorgio, R.; Corinaldesi, R.; Stanghellini, V. The immune system in irritable bowel syndrome. J. Neurogastroenterol. Motil. 2011, 17, 349–359. [Google Scholar] [CrossRef]
- Woolf, C.J. Central sensitization: Implications for the diagnosis and treatment of pain. Pain 2011, 152, S2–S15. [Google Scholar] [CrossRef]
- Gibler, R.C.; Jastrowski Mano, K.E. Systematic review of autonomic nervous system functioning in pediatric chronic pain. Clin. J. Pain 2021, 37, 281–294. [Google Scholar] [CrossRef]
- Harte, S.E.; Harris, R.E.; Clauw, D.J. The neurobiology of central sensitization. J. Appl. Behav. Res. 2018, 23, e12137. [Google Scholar] [CrossRef]
- Volcheck, M.M.; Graham, S.M.; Fleming, K.C.; Mohabbat, A.B.; Luedtke, C.A. Central Sensitization, chronic pain, and other symptoms: Better understanding, better management. Clevel. Clinic. J. Med. 2023, 90, 245–254. [Google Scholar] [CrossRef] [PubMed]
- Sim, L.A.; Lebow, J.; Weiss, K.; Harrison, T.; Bruce, B. Eating disorders in adolescents with chronic pain. J. Pediatr. Health Care 2017, 31, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Liossi, C.; Howard, R.F. Pediatric chronic pain: Biopsychosocial assessment and formulation. Pediatrics 2016, 138, e20160331. [Google Scholar] [CrossRef] [PubMed]
- Riddell, R.P.; Racine, N.; Craig, K.D.; Campbell, L. Psychological theories and biopsychosocial models in paediatric pain. In Oxford Textbook of Paediatric Pain; Oxford University Press: Oxford, UK, 2014; pp. 85–94. [Google Scholar]
- American Psychiatric Association. Feeding and Eating Disorders. In Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Washington, DC, USA, 2013. [Google Scholar]
- Smink, F.R.E.; Van Hoeken, D.; Hoek, H.W. Epidemiology of eating disorders: Incidence, prevalence and mortality rates. Curr. Psychiatry Rep. 2012, 14, 406–414. [Google Scholar] [CrossRef]
- Arcelus, J.; Mitchell, A.J.; Wales, J.; Nielsen, S. Mortality rates in patients with anorexia nervosa and other eating disorders. A meta-analysis of 36 studies. Arch. Gen. Psychiatry 2011, 68, 724–731. [Google Scholar] [CrossRef]
- Baker, J.H.; Schaumberg, K.; Munn-Chernoff, M.A. Genetics of anorexia nervosa. Curr. Psychiatry Rep. 2017, 19, 84. [Google Scholar] [CrossRef]
- Bulik, C.M.; Flatt, R.; Abbaspour, A.; Carroll, I. Reconceptualizing anorexia nervosa. Psychiatry Clin. Neurosci. 2019, 73, 518–525. [Google Scholar] [CrossRef]
- Phillipou, A.; Rossell, S.L.; Castle, D.J. The neurobiology of anorexia nervosa: A systematic review. Aust. N. Z. J. Psychiatry 2014, 48, 128–152. [Google Scholar] [CrossRef]
- Werlang, M.E.; Sim, L.A.; Lebow, J.R.; Lacy, B.E. Assessing for eating disorders: A primer for gastroenterologists. Am. J. Gastroenterol. 2021, 116, 68–76. [Google Scholar] [CrossRef]
- Allison, S.; Warin, M.; Bastiampillai, T.; Looi, J.C.L.; Strand, M. Recovery from anorexia nervosa: The influence of women’s sociocultural milieux. Australas. Psychiatry 2021, 29, 513–515. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, S.M.; Whitelaw, M.; Le Grange, D.; Yeo, M.; Hughes, E.K. Physical and psychological morbidity in adolescents with atypical anorexia nervosa. Pediatrics 2016, 137, e20154080. [Google Scholar] [CrossRef] [PubMed]
- Zimmerman, J.; Fisher, M. Avoidant/Restrictive Food Intake Disorder (ARFID). Curr. Problems Pediatric Adol Health Care 2017, 47, 95–103. [Google Scholar] [CrossRef]
- Rowe, E. Early detection of eating disorders in general practice. Aust. Fam. Physician 2017, 46, 833–838. [Google Scholar] [PubMed]
- Sato, Y.; Fukudo, S. Gastrointestinal symptoms and disorders in patients with eating disorders. Clin. J. Gastroenterol. 2015, 8, 255–263. [Google Scholar] [CrossRef]
- Hilbert, A.; Pike, K.M.; Goldschmidt, A.B.; Wilfley, D.E.; Fairburn, C.G.; Dohm, F.-A.; Walsh, B.T.; Weissman, R.S. Risk factors across the eating disorders. Psychiatry Res. 2014, 220, 500–506. [Google Scholar] [CrossRef]
- Fletcher, B.C.; Kupshik, G.A.; Uprichard, S.; Shah, S.; Nash, A.S. Eating disorders and concurrent psychopathology: A reconceptualisation of clinical need through Rasch analysis. Eur. Eat. Disord. 2008, 16, 191–198. [Google Scholar] [CrossRef]
- Leal, A.M.O.; Moreira, A.C. Food and the circadian activity of the hypothalamic-pituitary-adrenal axis. Braz. J. Med. Biologic Res. 1997, 30, 1391–1405. [Google Scholar] [CrossRef]
- Nitsch, A.; Knopf, E.; Manwaring, J.; Mehler, P.S. Avoidant/restrictive food intake disorder (ARFID): Its medical complications and their treatment—An emerging area. Pediatr. Rep. 2021, 9, 21–29. [Google Scholar] [CrossRef]
- Strigo, I.A.; Matthews, S.C.; Simmons, A.N.; Oberndorfer, T.; Klabunde, M.; Reinhardt, L.E.; Kaye, W.H. Altered insula activation during pain anticipation in individuals recovered from anorexia nervosa: Evidence of interoceptive dysregulation. Int. J. Eat. Disord. 2013, 46, 23–33. [Google Scholar] [CrossRef]
- Lin, Y.; De Araujo, I.; Stanley, G.; Small, D.; Geha, P. Chronic pain precedes disrupted eating behavior in low-back pain patients. PLoS ONE 2022, 17, e0263527. [Google Scholar] [CrossRef] [PubMed]
- Souza, S.P.; Antequerdds, R.; Aratangy, E.W.; Siqueira, S.; Cordas, T.A.; Siqueira, J.T.T. Pain and temporomandibular disorders in patients with eating disorders. Brazilian Oral. Res. 2018, 32, e51. [Google Scholar] [CrossRef] [PubMed]
- Thapar, N.; Benninga, M.A.; Crowell, M.D.; Di Lorenzo, C.; Mack, I.; Nurko, S.; Saps, M.; Shulman, R.J.; Szajewska, H.; van Tilburg, M.A.L.; et al. Paediatric functional abdominal pain disorders. Nat. Rev. Dis. Primers 2020, 6, 89. [Google Scholar] [CrossRef]
- Carter, B.D.; Threlkeld, B.M. Psychosocial perspectives in the treatment of pediatric chronic pain. Pediatr. Rheumatol. 2012, 10, 15. [Google Scholar] [CrossRef] [PubMed]
- Johnston, J.; Shu, C.Y.; Hoiles, K.J.; Clarke, P.J.F.; Watson, H.J.; Dunlop, P.D.; Egan, S.J. Perfectionism is associated with higher eating disorder symptoms and lower remission in children and adolescents diagnosed with eating disorders. Eat. Behav. 2018, 30, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Crombez, G.; Eccleston, C.; Van Damme, S.; Vlaeyen, J.W.S.; Karoly, P. Fear-avoidance model of chronic pain. Clin. J. Pain 2012, 28, 475–483. [Google Scholar] [CrossRef]
- Zale, E.L.; Ditre, J.W. Pain-related fear, disability, and the fear-avoidance model of chronic pain. Curr. Opinion Psychol. 2015, 5, 24–30. [Google Scholar] [CrossRef]
- Jastrowski Mano, K.E.; O’Bryan, E.M.; Gibler, R.C.; Beckmann, E.A. The co-occurrence of pediatric chronic pain and anxiety: A theoretical review of a developmentally informed shared vulnerability model. Clin. J. Pain 2019, 35, 989–1002. [Google Scholar] [CrossRef]
- Witte, M.A.; Weber, C.H.; Lebow, J.; LeMahieu, A.; Geske, J.; Witte, N.; Whiteside, S.; Loth, K.P.; Sim, L. Lifetime prevalence of psychiatric disorders in adolescents with unexplained weight loss, underweight, or poor appetite. J. Dev. Behav. Pediatr. 2023, 44, e277–e283. [Google Scholar] [CrossRef]
- Sim, L.; Harbeck Weber, C.; Harrison, T.; Peterson, C. Central sensitization in chronic pain and eating disorders: A potential shared pathogenesis. J. Clin. Psychol. Med. Settings 2021, 28, 40–52. [Google Scholar] [CrossRef]
- Guillaume, S.; Jaussent, I.; Maimoun, L.; Ryst, A.; Seneque, M.; Villain, L.; Hamroun, D.; Lefebvre, P.; Renard, E.; Courtet, P. Associations between adverse childhood experiences and clinical characteristics of eating disorders. Sci. Rep. 2016, 6, 35761. [Google Scholar] [CrossRef] [PubMed]
- Groenewald, C.B.; Murray, C.B.; Palermo, T.M. Adverse childhood experiences and chronic pain among children and adolescents in the United States. Pain Rep. 2020, 5, e839. [Google Scholar] [CrossRef]
- Sood, M.R.; Di Lorenzo, C.; Hyams, J.; Miranda, A.; Simpson, P.; Mousa, H.; Nurko, S. Beliefs and attitudes of general pediatricians and pediatric gastroenterologists regarding functional gastrointestinal disorders: A survey study. Clin. Pediatr. 2011, 50, 891–896. [Google Scholar] [CrossRef] [PubMed]
- Rouster, A.S.; Karpinski, A.C.; Silver, D.; Monagas, J.; Hyman, P.E. Functional gastrointestinal disorders dominate pediatric gastroenterology outpatient practice. J. Pediatr. Gastroenterol. Nutr. 2016, 62, 847–851. [Google Scholar] [CrossRef] [PubMed]
- Gieteling, M.J.; Lisman-van Leeuwen, Y.; van der Wouden, J.C.; Schellevis, F.G.; Berger, M.Y. Childhood nonspecific abdominal pain in family practice: Incidence, associated factors, and management. Ann. Fam. Med. 2011, 9, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Benninga, M.A.; Faure, C.; Hyman, P.E.; St James Roberts, I.; Schechter, N.L.; Nurko, S. Childhood functional gastrointestinal disorders: Neonate/toddler. J. Gastroenterol. 2016, 50, 443–1455. [Google Scholar] [CrossRef]
- Collins, A.B. Chronic pain in children interdisciplinary management. Pediatr. Clin. 2023, 70, 575–588. [Google Scholar]
- Eccleston, Z.; Eccleston, C. Interdisciplinary management of adolescent chronic pain: Developing the role of physiotherapy. Physiotherapy 2004, 90, 77–81. [Google Scholar] [CrossRef]
- Morgan, J.F.; Reid, F.; Lacey, J.H. The SCOFF questionnaire: Assessment of a new screening tool for eating disorders. BMJ 1999, 319, 1467–1468. [Google Scholar] [CrossRef]
- Stice, E.; Telch, C.F.; Rizvi, S.L. Development and validation of the Eating Disorder Diagnostic Scale: A brief self-report measure of anorexia, bulimia, and binge-eating disorder. Psychol Assess. 2002, 12, 123–131. [Google Scholar] [CrossRef]
- Fairburn, C.G. Cognitive Behavior Therapy and Eating Disorders; Guilford Press: New York, NY, USA, 2008. [Google Scholar]
- DeSocio, J.E.; O’Toole, J.K.; Nemirow, S.J.; Lukach, M.E.; Magee, M.G. Screening for childhood eating disorders in primary care. Prim. Care Companion J. Clin. Psych. 2007, 9, 16. [Google Scholar] [CrossRef] [PubMed]
- Pritts, S.D.; Susman, J. Diagnosis of eating disorders in primary care. Am. Fam. Physician 2003, 67, 297–304. [Google Scholar] [PubMed]
- Sim, L.A.; McAlpine, D.E.; Grothe, K.B.; Himes, S.M.; Cockerill, R.G.; Clark, M.M. Identification and treatment of eating disorders in the primary care setting. Mayo Clinic Proc. 2010, 85, 746–751. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Luscombe, G.M.; Boyd, C.; Kellow, J.; Abraham, S. Functional gastrointestinal disorders in eating disorder patients: Altered distribution and predictors using ROME III compared to ROME II criteria. World J. Gastroenterol. 2014, 20, 16293–16299. [Google Scholar] [CrossRef]
- Williams, S.E.; Zahka, N.E. Treating Somatic Symptoms in Children and Adolescents; Guilford Press: New York, NY, USA, 2017. [Google Scholar]
- Malas, N.; Ortiz-Aguayo, R.; Giles, L.; Ibeziako, P. Pediatric somatic symptom disorders. Curr. Psychiatry Rep. 2017, 19, 11. [Google Scholar] [CrossRef]
- Guthrie, E.A.; Creed, F.H.; Whorwell, P.J.; Tomenson, B. Outpatients with irritable bowel syndrome: A comparison of first time and chronic attenders. Gut 1992, 33, 361–363. [Google Scholar] [CrossRef]
- Lalouni, M.; Hesser, H.; Bonnert, M.; Hedman-Lagerlöf, E.; Serlachius, E.; Olén, O.; Ljótsson, B. Breaking the vicious circle of fear and avoidance in children with abdominal pain: A mediation analysis. J. Psychosom. Res. 2021, 140, 110287. [Google Scholar] [CrossRef]
- Bucchianeri, M.M.; Neumark-Sztainer, D. Body dissatisfaction: An overlooked public health concern. J. Public. Ment. Health 2014, 13, 64–69. [Google Scholar] [CrossRef]
- Van der Veek, S.M.C.; Derkx, B.H.; Benninga, M.A.; Boer, F.; de Haan, E. Cognitive behavior therapy for pediatric functional abdominal pain: A randomized controlled trial. Pediatrics 2013, 132, e1163–e1172. [Google Scholar] [CrossRef]
- Warschburger, P.; Calvano, C.; Becker, S.; Ebinger, F.; Hudert, C.; Iven, E.; Posovszky, C.; Winter, S.-M.; Daubmann, A.; Ozga, A.-K.; et al. Do children with functional abdominal pain benefit more from a pain-specific cognitive-behavioral intervention than from an unspecific attention control intervention? results of a randomized controlled trial. Am. J. Gastroenterol. 2021, 116, 1322–1335. [Google Scholar] [CrossRef]
- Baranguán Castro, M.L.; Ros Arnal, I.; García Romero, R.; Rodríguez Martínez, G.; Ubalde Sainz, E. Implantación de la dieta baja en FODMAP para el dolor abdominal funcional [Implementation of a low FODMAP diet for functional abdominal pain]. An. Pediatr. 2019, 90, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Brands, M.M.; Purperhart, H.; Deckers-Kocken, J.M. A pilot study of yoga treatment in children with functional abdominal pain and irritable bowel syndrome. Complement. Ther. Med. 2011, 19, 109–114. [Google Scholar] [CrossRef] [PubMed]
- van Tilburg, M.A.; Chitkara, D.K.; Palsson, O.S.; Turner, M.; Blois-Martin, N.; Ulshen, M.; Whitehead, W.E. Audio-recorded guided imagery treatment reduces functional abdominal pain in children: A pilot study. Pediatrics 2009, 124, e890–e897. [Google Scholar] [CrossRef]
- Madden, S.; Hay, P.; Touyz, S. Systematic review of evidence for different treatment settings in anorexia nervosa. World J. Psychiatry 2015, 5, 147–153. [Google Scholar] [CrossRef]
- Yager, J.; Devlin, M.J.; Halmi, K.A.; Herzog, D.B.; Mitchell, J.E.; Powers, P.; Zerbe, K.J. APA Practice Guidelines for the Treatment of Psychiatric Disorders: Comprehensive Guidelines and Guideline Watches; American Psychiatric Association: Washington, DC, USA, 2010. [Google Scholar]
- Touyz, S.; Le Grange, D.; Lacey, H.; Hay, P.; Smith, R.; Maguire, S.; Bamford, B.; Pike, K.M.; Crosby, R.D. Treating severe and enduring anorexia nervosa: A randomized controlled trial. Psychol. Med. 2013, 43, 2501–2511. [Google Scholar] [CrossRef]
- Lock, J.; Le Grange, D. Family-based treatment: Where are we and where should we be going to improve recovery in child and adolescent eating disorders. Int. J. Eat. Disord. 2019, 52, 481–487. [Google Scholar] [CrossRef]
- Linardon, J.; Wade, T.D.; de la Piedad Garcia, X.; Brennan, L. The efficacy of cognitive-behavioral therapy for eating disorders: A systematic review and meta-analysis. J. Consult. Clin. Psychol. 2017, 85, 1080–1094. [Google Scholar] [CrossRef]
- Gowers, S.G.; Clark, A.; Roberts, C.; Griffiths, A.; Edwards, V.; Bryan, C.; Smethurst, N.; Byford, S.; Barrett, B. Clinical effectiveness of treatments for anorexia nervosa in adolescents. Brit. J. Psych. 2007, 19, 427–435. [Google Scholar] [CrossRef]
- Thompson-Brenner, H.; Brooks, G.E.; Boswell, J.F.; Espel-Huynh, H.; Dore, R.; Franklin, D.R.; Gonçalves, A.; Smith, M.; Ortiz, S.; Ice, S.; et al. Evidence-based implementation practices applied to the intensive treatment of eating disorders: Summary of research and illustration of principles using a case example. Clin. Psychol. Sci. Pract. 2018, 25, e12221. [Google Scholar] [CrossRef]
- Couturier, J.; Kimber, M.; Szatmari, P. Efficacy of family-based treatment for adolescents with eating disorders: A systematic review and meta-analysis. Int. J. Eat. Disord. 2012, 46, 3–11. [Google Scholar] [CrossRef]
- Hay, P.J.; Touyz, S.; Claudino, A.M.; Lujic, S.; Smith, C.A.; Madden, S. Inpatient versus outpatient care, partial hospitalisation and waiting list for people with eating disorders. Cochrane Database Syst. Rev. 2019, 1, CD010827. [Google Scholar] [CrossRef]
- Atwood, M.E.; Friedman, A. A systematic review of enhanced cognitive behavioral therapy (CBT-E) for eating disorders. Int. J. Eat. Disord. 2019, 53, 311–330. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.J.; Wons, O.; Eddy, K. Cognitive-behavioral treatment of avoidant/restrictive food intake disorder. Curr. Opin. Psychiatry 2018, 31, 425. [Google Scholar] [CrossRef] [PubMed]
- Vlaeyen, J.W.S.; Crombez, G.; Linton, S.J. The fear-avoidance model of pain. Pain 2016, 157, 1588–1589. [Google Scholar] [CrossRef] [PubMed]
- Zucker, N.L.; Bulik, C.M. On bells, saliva, and abdominal pain or discomfort: Early aversive visceral conditioning and vulnerability for anorexia nervosa. Int. J. Eat. Disord. 2020, 53, 508–512. [Google Scholar] [CrossRef]
- Vlaeyen, J.W.S.; Morley, S.; Linton, S.J.; Boersma, K.; de Jong, J. Pain-Related Fear: Exposure-Based Treatment of Chronic Pain; IASP Press: Washington, DC, USA, 2012. [Google Scholar]
- King, S.; Boutilier, J.; Chambers, C.T. Cognitive behavioural therapy for abdominal pain. In Paediatric Gastrointestinal Disorders; CRC Press: Boca Raton, FL, USA, 2019; pp. 51–61. [Google Scholar]
- Tregarthen, J.P.; Lock, J.; Darcy, A.M. Development of a smartphone application for eating disorder self-monitoring. Int. J. Eat. Disord. 2015, 48, 972–982. [Google Scholar] [CrossRef]
- Stróżyk, A.; Horvath, A.; Szajewska, H. FODMAP dietary restrictions in the management of children with functional abdominal pain disorders: A systematic review. Neurogastroenterol. Motil. 2022, 34, e14345. [Google Scholar] [CrossRef]
- Brusaferro, A.; Farinelli, E.; Zenzeri, L.; Cozzali, R.; Esposito, S. The management of paediatric functional abdominal pain disorders: Latest evidence. Pediatr. Drugs 2018, 20, 235–247. [Google Scholar] [CrossRef]
- Monteleone, A.M.; Fernandez-Aranda, F.; Voderholzer, U. Evidence and perspectives in eating disorders: A paradigm for a multidisciplinary approach. World Psych. 2019, 18, 369. [Google Scholar] [CrossRef]
- Deacy, A.D.; Friesen, C.A.; Staggs, V.S.; Schurman, J.V. Evaluation of clinical outcomes in an interdisciplinary abdominal pain clinic: A retrospective, exploratory review. World J. Gastroenterol. 2019, 25, 3079–3090. [Google Scholar] [CrossRef]
- Luzier, J.L.; Sondike, S.B.; Linton, J.C.; Mizes, J.S. Interdisciplinary treatment of adolescent eating disorders in West Virginia. West. Va. Med. J. 2012, 108, 36–41. [Google Scholar]
- Cushing, C.C.; Friesen, C.A.; Schurman, J.V. Collaboration with medical professionals in clinical practice: Pediatric abdominal pain as a case example. Fam. Syst. Health 2012, 30, 279–290. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Beckmann, E.A.; Aarnio-Peterson, C.M.; Jastrowski Mano, K.E. Advancing the Assessment and Treatment of Comorbid Pediatric Chronic Functional Abdominal Pain (CFAP) and Restrictive Eating Disorders. Children 2023, 10, 1539. https://doi.org/10.3390/children10091539
Beckmann EA, Aarnio-Peterson CM, Jastrowski Mano KE. Advancing the Assessment and Treatment of Comorbid Pediatric Chronic Functional Abdominal Pain (CFAP) and Restrictive Eating Disorders. Children. 2023; 10(9):1539. https://doi.org/10.3390/children10091539
Chicago/Turabian StyleBeckmann, Emily A., Claire M. Aarnio-Peterson, and Kristen E. Jastrowski Mano. 2023. "Advancing the Assessment and Treatment of Comorbid Pediatric Chronic Functional Abdominal Pain (CFAP) and Restrictive Eating Disorders" Children 10, no. 9: 1539. https://doi.org/10.3390/children10091539
APA StyleBeckmann, E. A., Aarnio-Peterson, C. M., & Jastrowski Mano, K. E. (2023). Advancing the Assessment and Treatment of Comorbid Pediatric Chronic Functional Abdominal Pain (CFAP) and Restrictive Eating Disorders. Children, 10(9), 1539. https://doi.org/10.3390/children10091539




