Cannabidiol Selectively Binds to the Voltage-Gated Sodium Channel Nav1.4 in Its Slow-Inactivated State and Inhibits Sodium Current
Abstract
1. Introduction
2. Experimental Section
2.1. Wild-Type (WT) Nav1.4 Channel CDNA Constructs
2.2. Transient Transfection of Chinese Hamster Ovary (CHO-K1) Cells
2.3. Whole-Cell Patch-Clamp Recording
2.4. Chemical Drugs
2.5. Molecular Docking of CBD to the Nav1.4 Channel
2.6. Data Analysis
3. Results
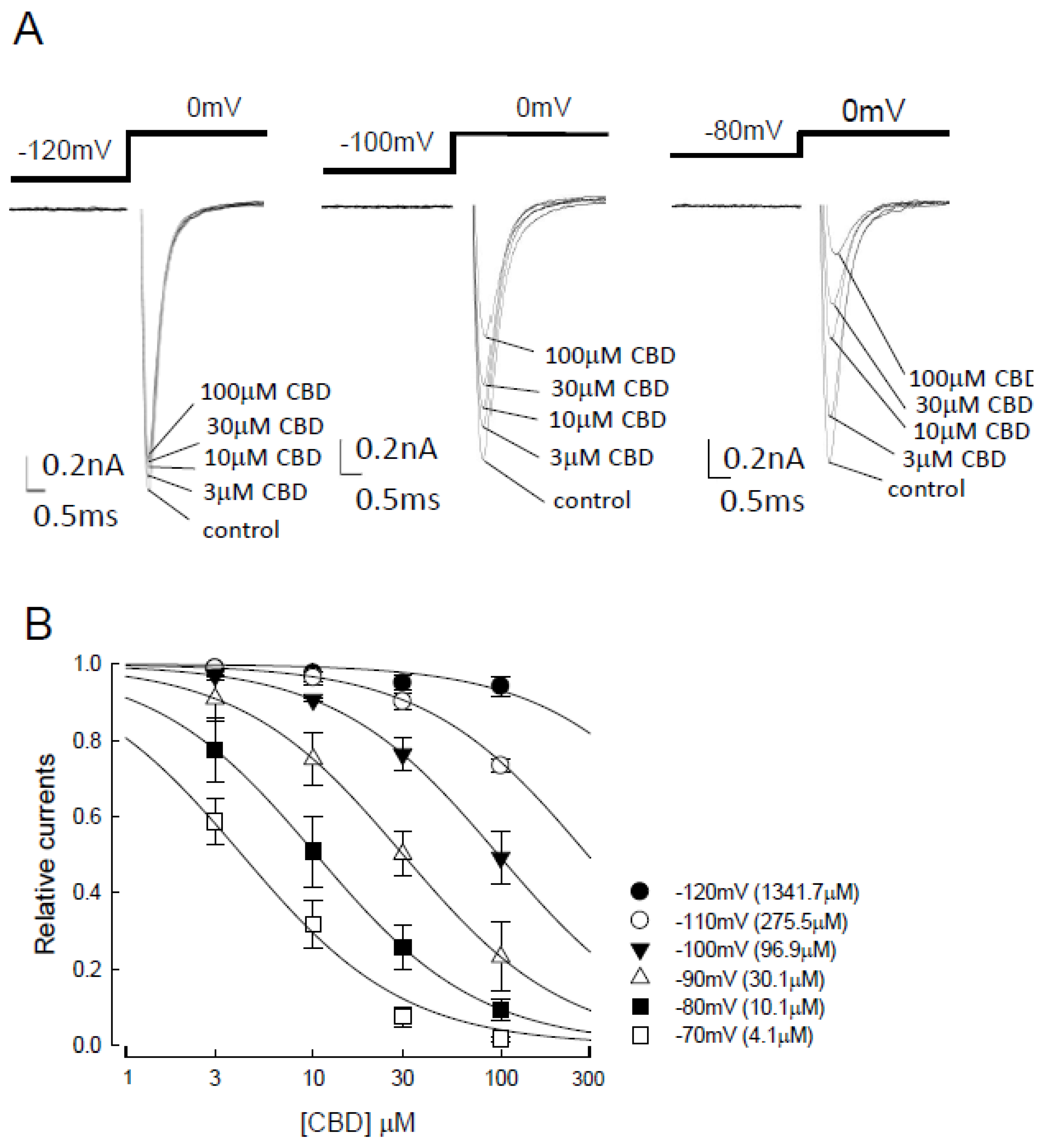
3.1. The Assessment of Transient Sodium Current Inhibition by CBD with Variable Holding Potentials on the CHO Cells Expressing Nav1.4 Channel
3.2. The Influence of CBD on the Inactivated Curve of Nav1.4 Channels Following a Short Depolarizing Pulse
3.3. Exploration of Whether CBD Can Bind to the Slow-Inactivated Nav1.4 Channel with an Elongation of the Depolarizing Prepulse
3.4. Determination of Nav1.4 Channel Slow Inactivated State
3.5. Evaluation of Different Depolarizing Potentials Influence on the CBD Inhibitory Effect
3.6. Evaluation of CBD Inhibition by the Time Elapse for Depolarization in Nav1.4 Channel
3.7. Molecular Mechanism of the Nav1.4 Channel with Cannabidiol
4. Discussion
4.1. The Binding Kinetic Analysis of Cannabidiol with Nav1.4 Channel
4.2. Clinical Implications
4.3. Same Molecular Structure but Different Biological Functions in Cannabidiol and Tetrahydrocannabinol
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Catterall, W.A. Voltage-gated sodium channels at 60: Structure, function and pathophysiology. J. Physiol. 2012, 590, 2577–2589. [Google Scholar] [CrossRef]
- Ghovanloo, M.R.; Aimar, K.; Ghadiry-Tavi, R.; Yu, A.; Ruben, P.C. Physiology and Pathophysiology of Sodium Channel Inactivation. Curr. Top. Membr. 2016, 78, 479–509. [Google Scholar] [CrossRef]
- Ghovanloo, M.R.; Atallah, J.; Escudero, C.A.; Ruben, P.C. Biophysical Characterization of a Novel SCN5A Mutation Associated with an Atypical Phenotype of Atrial and Ventricular Arrhythmias and Sudden Death. Front. Physiol. 2020, 11, 610436. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.R.; Ruben, P.C. Say Cheese: Structure of the Cardiac Electrical Engine Is Captured. Trends Biochem. Sci. 2020, 45, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Fouda, M.A.; Ghovanloo, M.R.; Ruben, P.C. Cannabidiol protects against high glucose-induced oxidative stress and cytotoxicity in cardiac voltage-gated sodium channels. Br. J. Pharmacol. 2020, 177, 2932–2946. [Google Scholar] [CrossRef] [PubMed]
- Kol, S.; Turrell, B.R.; de Keyzer, J.; van der Laan, M.; Nouwen, N.; Driessen, A.J. YidC-mediated membrane insertion of assembly mutants of subunit c of the F1F0 ATPase. J. Biol. Chem. 2006, 281, 29762–29768. [Google Scholar] [CrossRef] [PubMed]
- David, M.; Martinez-Marmol, R.; Gonzalez, T.; Felipe, A.; Valenzuela, C. Differential regulation of Na(v)beta subunits during myogenesis. Biochem. Biophys. Res. Commun. 2008, 368, 761–766. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Goodchild, S.J.; Ahern, C.A. Local anesthetic inhibition of a bacterial sodium channel. J. Gen. Physiol. 2012, 139, 507–516. [Google Scholar] [CrossRef]
- Gamal El-Din, T.M.; Lenaeus, M.J.; Zheng, N.; Catterall, W.A. Fenestrations control resting-state block of a voltage-gated sodium channel. Proc. Natl. Acad. Sci. USA 2018, 115, 13111–13116. [Google Scholar] [CrossRef]
- Pan, X.; Li, Z.; Zhou, Q.; Shen, H.; Wu, K.; Huang, X.; Chen, J.; Zhang, J.; Zhu, X.; Lei, J.; et al. Structure of the human voltage-gated sodium channel Nav1.4 in complex with beta1. Science 2018, 362, eaau2486. [Google Scholar] [CrossRef]
- Ghovanloo, M.R.; Choudhury, K.; Bandaru, T.S.; Fouda, M.A.; Rayani, K.; Rusinova, R.; Phaterpekar, T.; Nelkenbrecher, K.; Watkins, A.R.; Poburko, D.; et al. Cannabidiol inhibits the skeletal muscle Nav1.4 by blocking its pore and by altering membrane elasticity. J. Gen. Physiol. 2021, 153, e202012701. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.R.; Abdelsayed, M.; Peters, C.H.; Ruben, P.C. A Mixed Periodic Paralysis & Myotonia Mutant, P1158S, Imparts pH-Sensitivity in Skeletal Muscle Voltage-gated Sodium Channels. Sci. Rep. 2018, 8, 6304. [Google Scholar] [CrossRef] [PubMed]
- Ghovanloo, M.R.; Shuart, N.G.; Mezeyova, J.; Dean, R.A.; Ruben, P.C.; Goodchild, S.J. Inhibitory effects of cannabidiol on voltage-dependent sodium currents. J. Biol. Chem. 2018, 293, 16546–16558. [Google Scholar] [CrossRef]
- Lossin, C.; George, A.L., Jr. Myotonia congenita. Adv. Genet. 2008, 63, 25–55. [Google Scholar] [CrossRef]
- Huang, S.; Zhang, W.; Chang, X.; Guo, J. Overlap of periodic paralysis and paramyotonia congenita caused by SCN4A gene mutations two family reports and literature review. Channels 2019, 13, 110–119. [Google Scholar] [CrossRef] [PubMed]
- Holzherr, B.; Lehmann-Horn, F.; Kuzmenkina, E.; Fan, C.; Jurkat-Rott, K. A gating model for wildtype and R1448H Nav1.4 channels in paramyotonia. Acta Myol. 2014, 33, 22–33. [Google Scholar] [PubMed]
- Hsu, W.C.; Huang, Y.C.; Wang, C.W.; Hsueh, C.H.; Lai, L.P.; Yeh, J.H. Paralysis periodica paramyotonica caused by SCN4A Arg1448Cys mutation. J. Formos. Med. Assoc. 2006, 105, 503–507. [Google Scholar] [CrossRef][Green Version]
- Ke, Q.; Ye, J.; Tang, S.; Wang, J.; Luo, B.; Ji, F.; Zhang, X.; Yu, Y.; Cheng, X.; Li, Y. N1366S mutation of human skeletal muscle sodium channel causes paramyotonia congenita. J. Physiol. 2017, 595, 6837–6850. [Google Scholar] [CrossRef] [PubMed]
- Mazon, M.J.; Barros, F.; De la Pena, P.; Quesada, J.F.; Escudero, A.; Cobo, A.M.; Pascual-Pascual, S.I.; Gutierrez-Rivas, E.; Guillen, E.; Arpa, J.; et al. Screening for mutations in Spanish families with myotonia. Functional analysis of novel mutations in CLCN1 gene. Neuromuscul. Disord. 2012, 22, 231–243. [Google Scholar] [CrossRef] [PubMed]
- Lehmann-Horn, F.; Jurkat-Rott, K.; Rudel, R.; Ulm Muscle, C. Diagnostics and therapy of muscle channelopathies—Guidelines of the Ulm Muscle Centre. Acta Myol. 2008, 27, 98–113. [Google Scholar] [PubMed]
- Cannon, S.C. Pathomechanisms in channelopathies of skeletal muscle and brain. Annu. Rev. Neurosci. 2006, 29, 387–415. [Google Scholar] [CrossRef] [PubMed]
- Loussouarn, G.; Sternberg, D.; Nicole, S.; Marionneau, C.; Le Bouffant, F.; Toumaniantz, G.; Barc, J.; Malak, O.A.; Fressart, V.; Pereon, Y.; et al. Physiological and Pathophysiological Insights of Nav1.4 and Nav1.5 Comparison. Front. Pharmacol. 2015, 6, 314. [Google Scholar] [CrossRef] [PubMed]
- Beard, J.M.; Shockett, P.E.; O’Reilly, J.P. Substituted cysteine scanning in D1-S6 of the sodium channel hNav1.4 alters kinetics and structural interactions of slow inactivation. Biochim. Biophys. Acta Biomembr. 2020, 1862, 183129. [Google Scholar] [CrossRef] [PubMed]
- Vite, C.H. Myotonia and disorders of altered muscle cell membrane excitability. Vet. Clin. N. Am. Small Anim. Pract. 2002, 32, 169–187. [Google Scholar] [CrossRef]
- Pagliarani, S.; Lucchiari, S.; Scarlato, M.; Redaelli, E.; Modoni, A.; Magri, F.; Fossati, B.; Previtali, S.C.; Sansone, V.A.; Lecchi, M.; et al. Sodium Channel Myotonia Due to Novel Mutations in Domain I of Nav1.4. Front. Neurol. 2020, 11, 255. [Google Scholar] [CrossRef]
- Devinsky, O.; Marsh, E.; Friedman, D.; Thiele, E.; Laux, L.; Sullivan, J.; Miller, I.; Flamini, R.; Wilfong, A.; Filloux, F.; et al. Cannabidiol in patients with treatment-resistant epilepsy: An open-label interventional trial. Lancet. Neurol. 2016, 15, 270–278. [Google Scholar] [CrossRef]
- Patel, R.R.; Barbosa, C.; Brustovetsky, T.; Brustovetsky, N.; Cummins, T.R. Aberrant epilepsy-associated mutant Nav1.6 sodium channel activity can be targeted with cannabidiol. Brain 2016, 139, 2164–2181. [Google Scholar] [CrossRef]
- Petrovici, A.R.; Simionescu, N.; Sandu, A.I.; Paraschiv, V.; Silion, M.; Pinteala, M. New Insights on Hemp Oil Enriched in Cannabidiol: Decarboxylation, Antioxidant Properties and In Vitro Anticancer Effect. Antioxidants 2021, 10, 738. [Google Scholar] [CrossRef]
- Campos, A.C.; Moreira, F.A.; Gomes, F.V.; Del Bel, E.A.; Guimaraes, F.S. Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3364–3378. [Google Scholar] [CrossRef]
- Black, N.; Stockings, E.; Campbell, G.; Tran, L.T.; Zagic, D.; Hall, W.D.; Farrell, M.; Degenhardt, L. Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: A systematic review and meta-analysis. Lancet Psychiatry 2019, 6, 995–1010. [Google Scholar] [CrossRef]
- VanDolah, H.J.; Bauer, B.A.; Mauck, K.F. Clinicians’ Guide to Cannabidiol and Hemp Oils. Mayo Clin. Proc. 2019, 94, 1840–1851. [Google Scholar] [CrossRef] [PubMed]
- Itin, C.; Barasch, D.; Domb, A.J.; Hoffman, A. Prolonged oral transmucosal delivery of highly lipophilic drug cannabidiol. Int. J. Pharm. 2020, 581, 119276. [Google Scholar] [CrossRef] [PubMed]
- Itin, C.; Domb, A.J.; Hoffman, A. A meta-opinion: Cannabinoids delivered to oral mucosa by a spray for systemic absorption are rather ingested into gastro-intestinal tract: The influences of fed/fasting states. Expert Opin. Drug Deliv. 2019, 16, 1031–1035. [Google Scholar] [CrossRef]
- Devinsky, O.; Cross, J.H.; Wright, S. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 377, 699–700. [Google Scholar] [CrossRef] [PubMed]
- Devinsky, O.; Cross, J.H.; Laux, L.; Marsh, E.; Miller, I.; Nabbout, R.; Scheffer, I.E.; Thiele, E.A.; Wright, S.; Cannabidiol in Dravet Syndrome Study Group. Trial of Cannabidiol for Drug-Resistant Seizures in the Dravet Syndrome. N. Engl. J. Med. 2017, 376, 2011–2020. [Google Scholar] [CrossRef]
- Devinsky, O.; Patel, A.D.; Cross, J.H.; Villanueva, V.; Wirrell, E.C.; Privitera, M.; Greenwood, S.M.; Roberts, C.; Checketts, D.; VanLandingham, K.E.; et al. Effect of Cannabidiol on Drop Seizures in the Lennox-Gastaut Syndrome. N. Engl. J. Med. 2018, 378, 1888–1897. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.W.; Lai, H.J.; Lin, P.C.; Lee, M.J. Changes of Resurgent Na(+) Currents in the Nav1.4 Channel Resulting from an SCN4A Mutation Contributing to Sodium Channel Myotonia. Int. J. Mol. Sci. 2020, 21, 2593. [Google Scholar] [CrossRef]
- Huang, C.W.; Lai, H.J.; Lin, P.C.; Lee, M.J. Changes in Resurgent Sodium Current Contribute to the Hyperexcitability of Muscles in Patients with Paramyotonia Congenita. Biomedicines 2021, 9, 51. [Google Scholar] [CrossRef]
- Sait, L.G.; Sula, A.; Ghovanloo, M.R.; Hollingworth, D.; Ruben, P.C.; Wallace, B.A. Cannabidiol interactions with voltage-gated sodium channels. eLife 2020, 9, 9. [Google Scholar] [CrossRef]
- Kuo, C.C.; Bean, B.P. Na+ channels must deactivate to recover from inactivation. Neuron 1994, 12, 819–829. [Google Scholar] [CrossRef]
- Peng, Y.S.; Wu, H.T.; Lai, Y.C.; Chen, J.L.; Yang, Y.C.; Kuo, C.C. Inhibition of neuronal Na(+) currents by lacosamide: Differential binding affinity and kinetics to different inactivated states. Neuropharmacology 2020, 179, 108266. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Chen, R.S.; Lu, L.; Chen, R.C. Carbamazepine inhibition of neuronal Na+ currents: Quantitative distinction from phenytoin and possible therapeutic implications. Mol. Pharmacol. 1997, 51, 1077–1083. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C.; Lu, L. Characterization of lamotrigine inhibition of Na+ channels in rat hippocampal neurones. Br. J. Pharmacol. 1997, 121, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Kuo, C.C. A common anticonvulsant binding site for phenytoin, carbamazepine, and lamotrigine in neuronal Na+ channels. Mol. Pharmacol. 1998, 54, 712–721. [Google Scholar] [PubMed]
- Hebeisen, S.; Pires, N.; Loureiro, A.I.; Bonifacio, M.J.; Palma, N.; Whyment, A.; Spanswick, D.; Soares-da-Silva, P. Eslicarbazepine and the enhancement of slow inactivation of voltage-gated sodium channels: A comparison with carbamazepine, oxcarbazepine and lacosamide. Neuropharmacology 2015, 89, 122–135. [Google Scholar] [CrossRef]
- Kuo, C.C.; Bean, B.P. Slow binding of phenytoin to inactivated sodium channels in rat hippocampal neurons. Mol. Pharmacol. 1994, 46, 716–725. [Google Scholar]
- Kuo, C.C.; Huang, R.C.; Lou, B.S. Inhibition of Na(+) current by diphenhydramine and other diphenyl compounds: Molecular determinants of selective binding to the inactivated channels. Mol. Pharmacol. 2000, 57, 135–143. [Google Scholar]
- Keating, G.M. Eslicarbazepine acetate: A review of its use as adjunctive therapy in refractory partial-onset seizures. CNS Drugs 2014, 28, 583–600. [Google Scholar] [CrossRef]
- Zaccara, G.; Perucca, P.; Loiacono, G.; Giovannelli, F.; Verrotti, A. The adverse event profile of lacosamide: A systematic review and meta-analysis of randomized controlled trials. Epilepsia 2013, 54, 66–74. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (CBD) and its analogs: A review of their effects on inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Devinsky, O.; Cilio, M.R.; Cross, H.; Fernandez-Ruiz, J.; French, J.; Hill, C.; Katz, R.; Di Marzo, V.; Jutras-Aswad, D.; Notcutt, W.G.; et al. Cannabidiol: Pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders. Epilepsia 2014, 55, 791–802. [Google Scholar] [CrossRef] [PubMed]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and brain pharmacokinetic profile of cannabidiol (CBD), cannabidivarine (CBDV), Delta(9)-tetrahydrocannabivarin (THCV) and cannabigerol (CBG) in rats and mice following oral and intraperitoneal administration and CBD action on obsessive-compulsive behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [CrossRef] [PubMed]
- Pertwee, R.G. The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: Delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 2008, 153, 199–215. [Google Scholar] [CrossRef] [PubMed]
- Straiker, A.; Dvorakova, M.; Zimmowitch, A.; Mackie, K. Cannabidiol Inhibits Endocannabinoid Signaling in Autaptic Hippocampal Neurons. Mol. Pharmacol. 2018, 94, 743–748. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, C.-W.; Lin, P.-C.; Chen, J.-L.; Lee, M.-J. Cannabidiol Selectively Binds to the Voltage-Gated Sodium Channel Nav1.4 in Its Slow-Inactivated State and Inhibits Sodium Current. Biomedicines 2021, 9, 1141. https://doi.org/10.3390/biomedicines9091141
Huang C-W, Lin P-C, Chen J-L, Lee M-J. Cannabidiol Selectively Binds to the Voltage-Gated Sodium Channel Nav1.4 in Its Slow-Inactivated State and Inhibits Sodium Current. Biomedicines. 2021; 9(9):1141. https://doi.org/10.3390/biomedicines9091141
Chicago/Turabian StyleHuang, Chiung-Wei, Pi-Chen Lin, Jian-Lin Chen, and Ming-Jen Lee. 2021. "Cannabidiol Selectively Binds to the Voltage-Gated Sodium Channel Nav1.4 in Its Slow-Inactivated State and Inhibits Sodium Current" Biomedicines 9, no. 9: 1141. https://doi.org/10.3390/biomedicines9091141
APA StyleHuang, C.-W., Lin, P.-C., Chen, J.-L., & Lee, M.-J. (2021). Cannabidiol Selectively Binds to the Voltage-Gated Sodium Channel Nav1.4 in Its Slow-Inactivated State and Inhibits Sodium Current. Biomedicines, 9(9), 1141. https://doi.org/10.3390/biomedicines9091141






