Rapid Fabrication of MgNH4PO4·H2O/SrHPO4 Porous Composite Scaffolds with Improved Radiopacity via 3D Printing Process
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Synthesis of SrHPO4 Powder
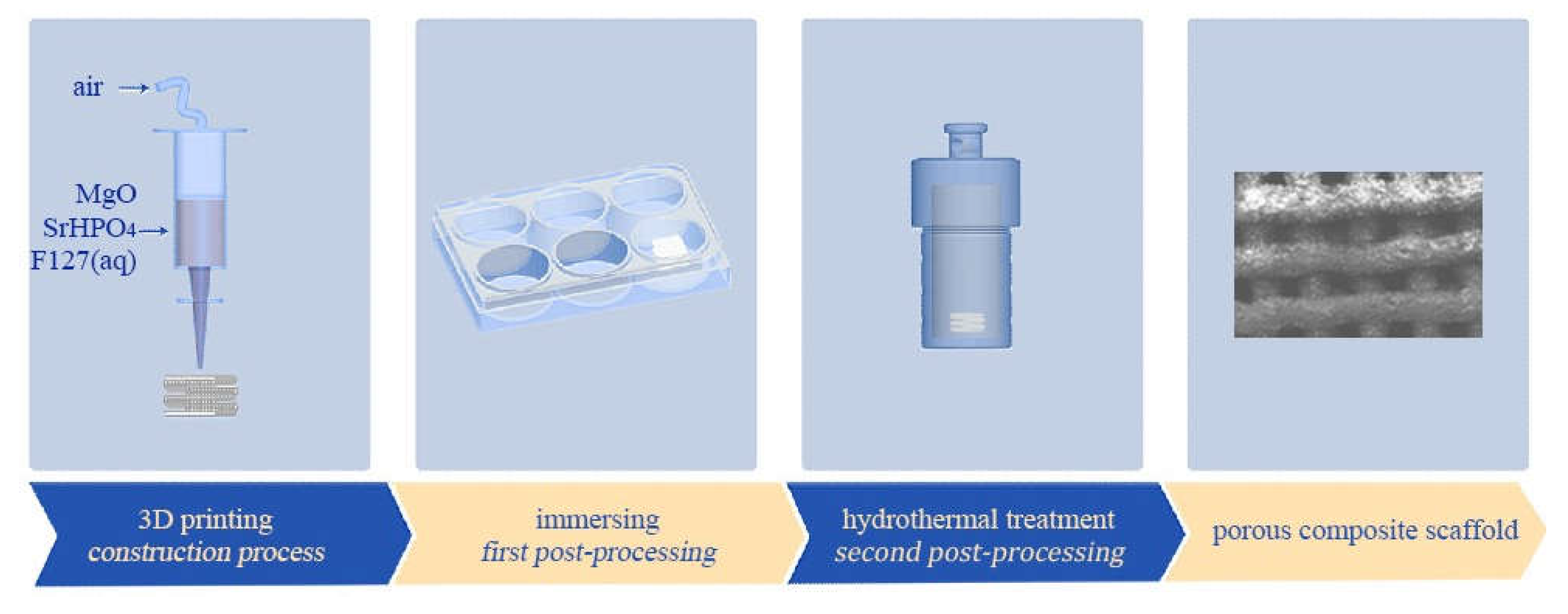
2.3. Fabrication of MgP/SrP 3D Porous Composite Scaffolds
2.4. Characterization of the Products
2.5. Porosity Measurement
2.6. X-ray Radiopacity
2.7. Degradation In Vitro
2.8. In Vitro Cytotoxicity Assay
2.9. Cell Adhesion on the Scaffolds
2.10. Statistical Analysis
3. Results
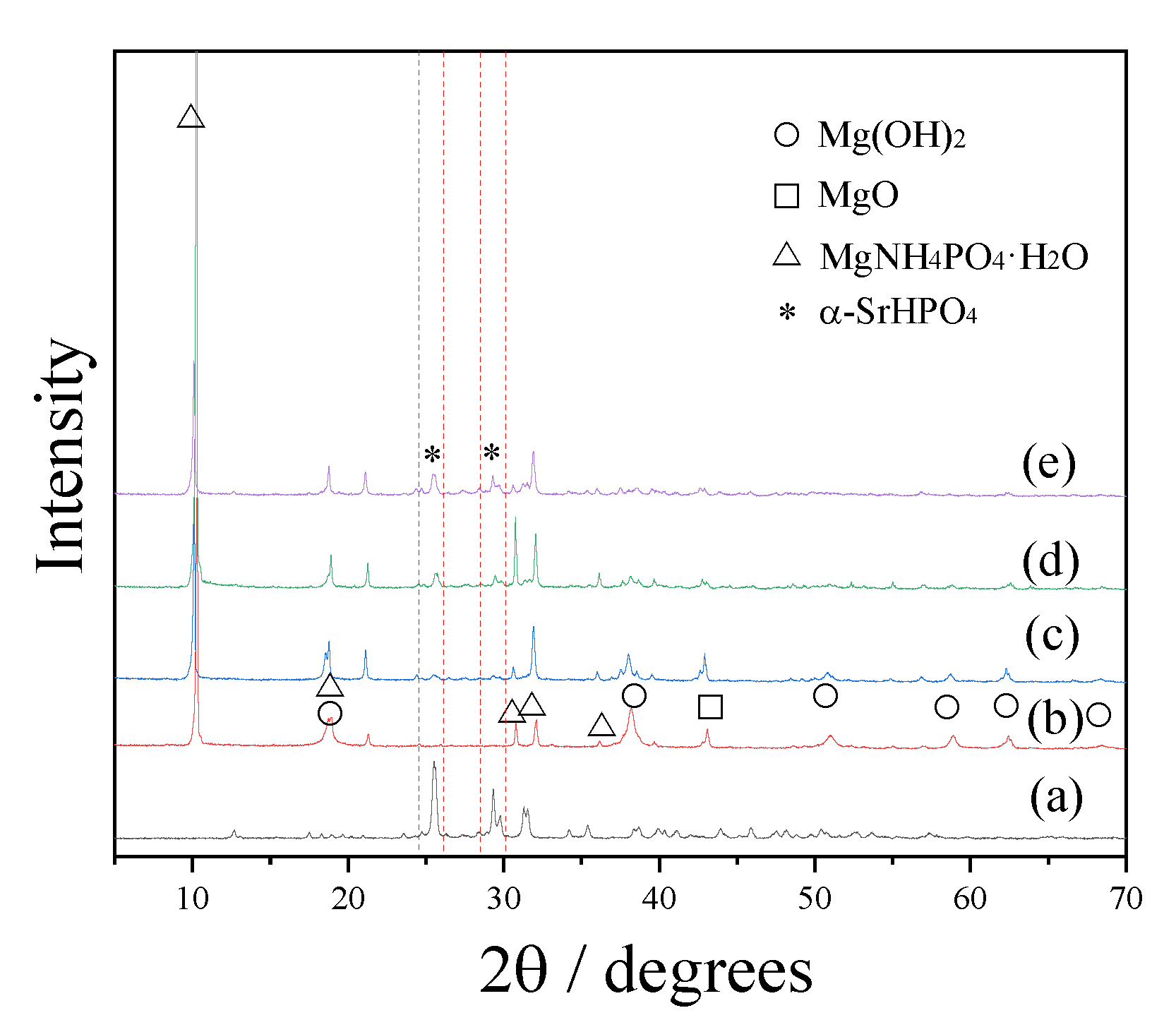
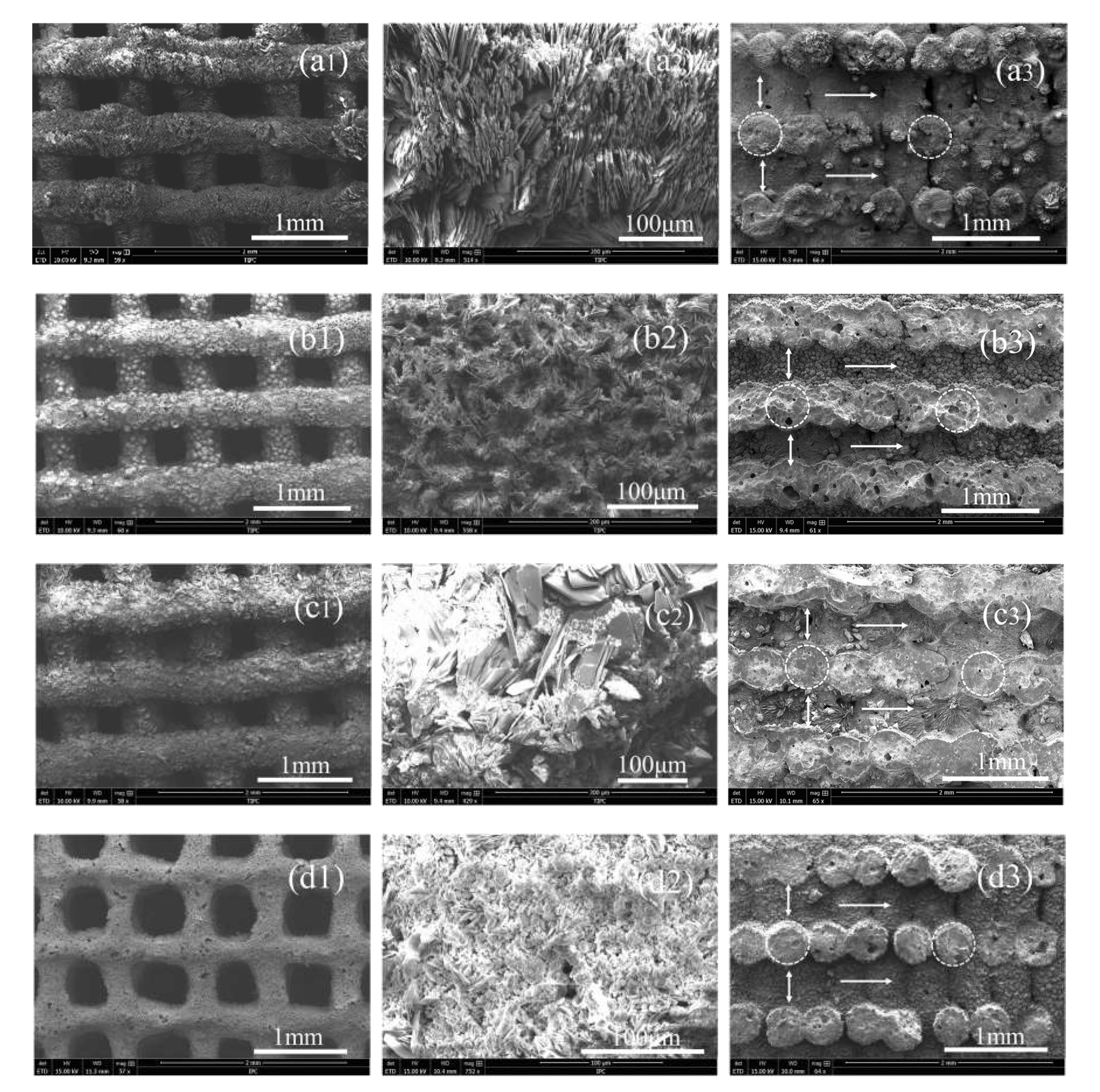
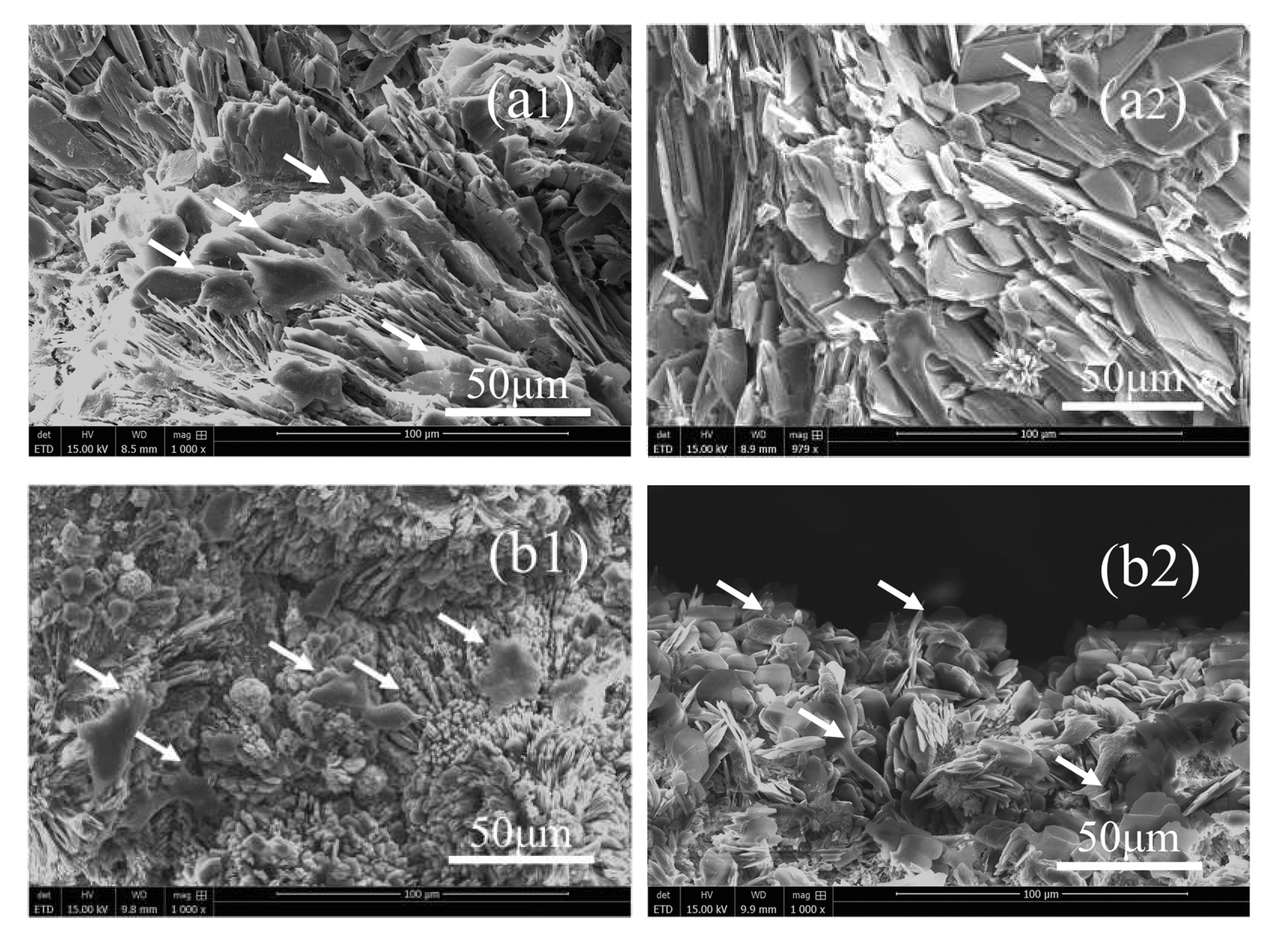
3.1. Phases, Components, Morphologies, and Porosities of the Scaffolds
3.2. X-ray Radiopacity
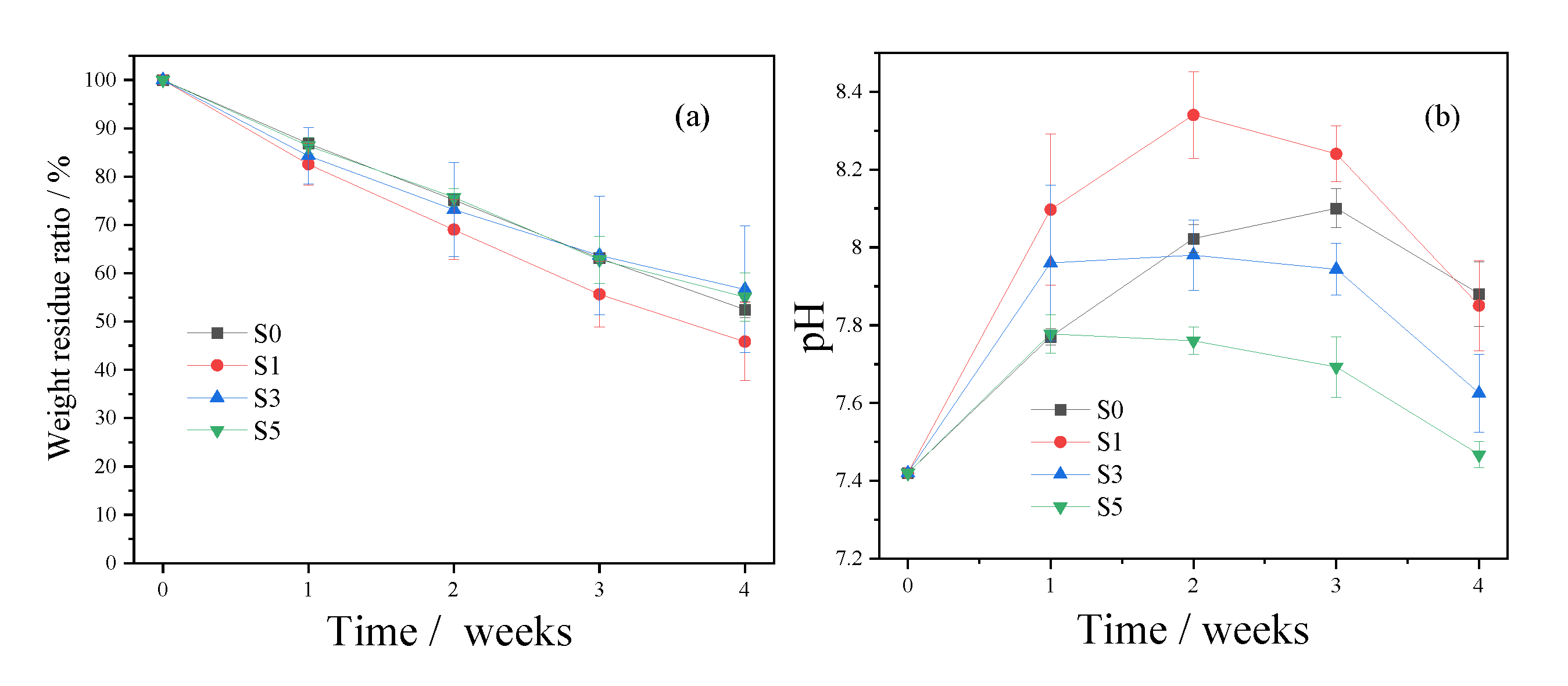
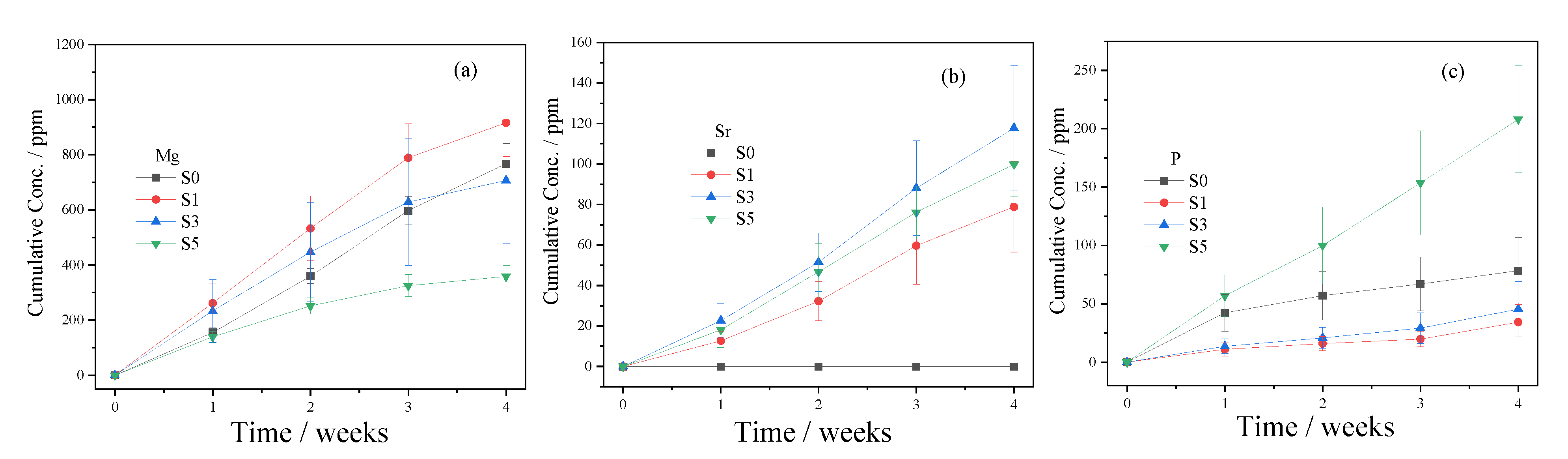
3.3. In Vitro Degradation
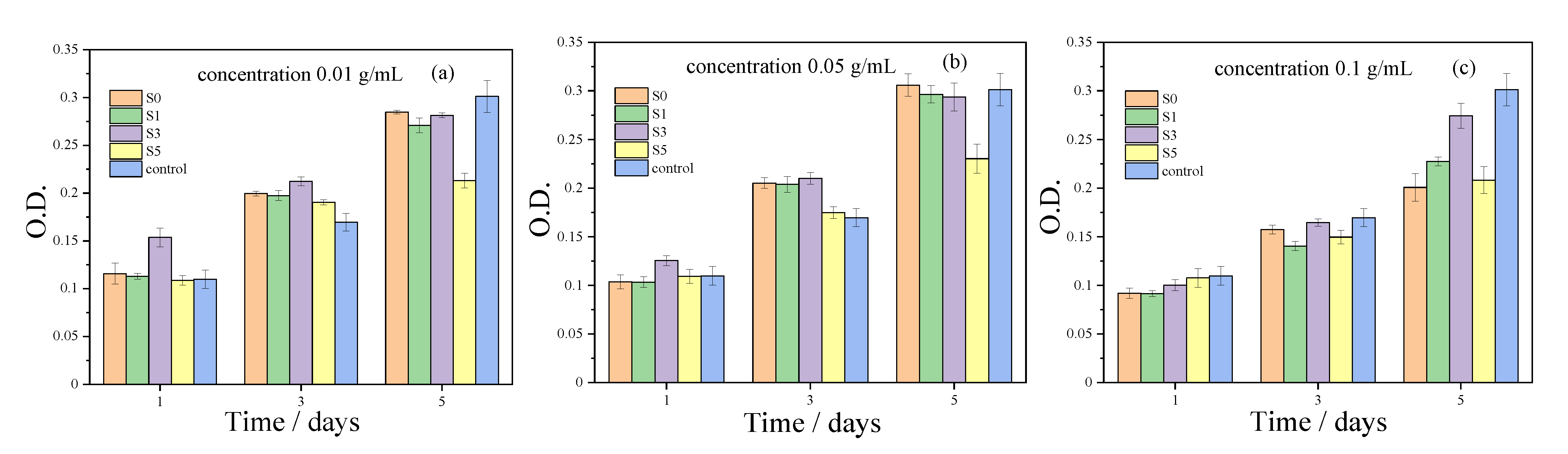
3.4. In Vitro Cytotoxicity Assay
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stevens, M.M. Biomaterials for bone tissue engineering. Mater. Today 2008, 11, 18–25. [Google Scholar] [CrossRef]
- Yu, X.H.; Tang, X.Y.; Gohil, S.V.; Laurencin, C.T. Biomaterials for Bone Regenerative Engineering. Adv. Healthc. Mater. 2015, 4, 1268–1285. [Google Scholar] [CrossRef] [PubMed]
- Rosetia, L.; Parisia, V.; Petrettaa, M.; Cavalloa, C.; Desandoa, G.; Bartolottia, I.; Grigolo, B. Scaffolds for Bone Tissue Engineering: State of the art and new perspectives. Mater. Sci. Eng. C 2017, 78, 1246–1262. [Google Scholar] [CrossRef] [PubMed]
- Koons, G.L.; Diba, M.; Mikos, A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020, 5, 584–603. [Google Scholar] [CrossRef]
- Tang, G.K.; Liu, Z.Q.; Liu, Y.; Yu, J.M.; Wang, X.; Tan, Z.H.; Ye, X.J. Recent Trends in the Development of Bone Regenerative Biomaterials. Front. Cell Dev. Biol. 2021, 9, 665813. [Google Scholar] [CrossRef]
- Collins, M.N.; Ren, G.; Young, K.; Pina, S.; Reis, R.L.; Oliveira, J.M. Scaffold Fabrication Technologies and Structure/Function Properties in Bone Tissue Engineering. Adv. Funct. Mater. 2021, 31, 2010609. [Google Scholar] [CrossRef]
- Bose, S.; Vahabzadeh, S.; Bandyopadhyay, A. Bone tissue engineering using 3D printing. Mater. Today 2013, 16, 496–504. [Google Scholar] [CrossRef]
- Ma, H.S.; Feng, C.; Chang, J.; Wu, C.T. 3D-printed bioceramic scaffolds: From bone tissue engineering to tumor therapy. Acta Biomater. 2018, 79, 37–59. [Google Scholar] [CrossRef]
- Peng, Z.L.; Zhao, T.S.; Zhou, Y.Q.; Li, S.H.; Li, J.J.; Leblanc, R.M. Bone Tissue Engineering via Carbon-Based Nanomaterials. Adv. Healthc. Mater. 2020, 9, 1901495. [Google Scholar] [CrossRef]
- Pavek, A.; Nartker, C.; Saleh, M.; Kirkham, M.; Khajeh Pour, S.; Aghazadeh-Habashi, A.; Barrott, J.J. Tissue Engineering Through 3D Bioprinting to Recreate and Study Bone Disease. Biomedicines 2021, 9, 551. [Google Scholar] [CrossRef]
- Mohd Roslan, M.R.; Mohd Kamal, N.L.; Abdul Khalid, M.F.; Mohd Nasir, N.F.; Cheng, E.M.; Beh, C.Y.; Tan, J.S.; Mohamed, M.S. The State of Starch/Hydroxyapatite Composite Scaffold in Bone Tissue Engineering with Consideration for Dielectric Measurement as an Alternative Characterization Technique. Materials 2021, 14, 1960. [Google Scholar] [CrossRef]
- Wang, Q.F.; Yan, J.H.; Yang, J.L.; Li, B.Y. Nanomaterials promise better bone repair. Mater. Today 2016, 19, 451–463. [Google Scholar] [CrossRef]
- Neto, A.S.; Ferreira, J.M.F. Synthetic and Marine-Derived Porous Scaffolds for Bone Tissue Engineering. Materials 2018, 11, 1702. [Google Scholar] [CrossRef] [Green Version]
- Wang, N.Y.; Dheen, S.T.; Fuh, J.Y.H.; Kumara, A.S. A review of multi-functional ceramic nanoparticles in 3D printed bone tissue engineering. Bioprinting 2021, 23, e00146. [Google Scholar] [CrossRef]
- Alizadeh-Osgouei, M.; Li, Y.C.; Wen, C.E. A comprehensive review of biodegradable synthetic polymer-ceramic composites and their manufacture for biomedical applications. Bioact. Mater. 2019, 4, 22–36. [Google Scholar] [CrossRef]
- Yang, G.Y.; Liu, J.L.; Li, F.; Pan, Z.Y.; Ni, X.; Shen, Y.; Xu, H.Z.; Huang, Q. Bioactive calcium sulfate/magnesium phosphate cement for bone substitute applications. Mater. Sci. Eng. C 2014, 35, 70–76. [Google Scholar] [CrossRef]
- Ji, M.Z.; Ding, Z.W.; Chen, H.; Peng, H.T.; Yan, Y.G. Design of novel organic–inorganic composite bone cements with high compressive strength, in vitro bioactivity and cytocompatibility. J. Biomed. Mater. Res. Part. B 2019, 107, 2365–2377. [Google Scholar] [CrossRef]
- Zhu, W.; Li, W.; Chen, K.Y.; Feng, B.; Zhou, L.Z.; Weng, X.S.; Cui, S.; Engqvist, H.; Xia, W. Injectable and assembled 3D solid structure for free-to-fixed shape in bone reconstruction. Appl. Mater. Today 2020, 21, 100823. [Google Scholar] [CrossRef]
- Liu, W.J.; Zhai, D.; Huan, Z.G.; Wu, C.T.; Chang, J. Novel tricalcium silicate/magnesium phosphate composite bone cement having high compressive strength, in vitro bioactivity and cytocompatibility. Acta Biomater. 2015, 21, 217–227. [Google Scholar] [CrossRef]
- Sikder, P.; Coomar, P.P.; Mewborn, J.M.; Bhaduri, S.B. Antibacterial calcium phosphate composite cements reinforced with silver-doped magnesium phosphate (newberyite) micro-platelets. J. Mech. Behav. Biomed. Mater. 2020, 110, 103934. [Google Scholar] [CrossRef]
- Gong, C.T.; Fang, S.; Xia, K.Z.; Chen, J.T.; Guo, L.Y.; Guo, W.C. Enhancing the mechanical properties and cytocompatibility of magnesium potassium phosphate cement by incorporating oxygen-carboxymethyl chitosan. Regener. Biomater. 2020, 8, rbaa048. [Google Scholar] [CrossRef]
- Fang, C.; Hou, R.X.; Zhou, K.F.; Hua, F.B.; Cong, Y.; Zhang, J.F.; Fu, J.; Cheng, Y.J. Surface functionalized barium sulfate nanoparticles: Controlled in situ synthesis and application in bone cement. J. Mater. Chem. B 2014, 2, 1264–1274. [Google Scholar] [CrossRef]
- Liu, H.L.; Zhang, Z.Y.; Gao, C.X.; Bai, Y.J.; Liu, B.; Wang, W.H.; Ma, Y.X.; Fu, S.; Yang, H.L.; Li, Y.D.; et al. Enhancing effects of radiopaque agent BaSO4 on mechanical and biocompatibility properties of injectable calcium phosphate composite cement. Mater. Sci. Eng. C 2020, 116, 110904. [Google Scholar] [CrossRef]
- Tallia, F.; Gallo, M.; Pontiroli, L.; Baino, F.; Fiorilli, S.; Onida, B.; Anselmetti, G.C.; Manca, A.; Vitale-Brovarone, C. Zirconia-containing radiopaque mesoporous bioactive glasses. Mater. Lett. 2014, 130, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Martin, J.T.; Milby, A.H.; Ikuta, K.; Poudel, S.; Pfeifer, C.G.; Elliott, D.M.; Smith, H.E.; Mauck, R.L. A radiopaque electrospun scaffold for engineering fibrous musculoskeletal tissues: Scaffold characterization and in vivo applications. Acta Biomater. 2015, 26, 97–104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- No, Y.J.; Holzmeister, I.; Lu, Z.F.; Prajapati, S.; Shi, J.; Gbureck, U.; Zreiqat, H. Effect of Baghdadite Substitution on the Physicochemical Properties of Brushite Cements. Materials 2019, 12, 1719. [Google Scholar] [CrossRef] [Green Version]
- Wu, T.T.; Yang, S.E.; Shi, H.S.; Ye, J.D. Preparation and cytocompatibility of a novel bismuth aluminate/calcium phosphate cement with high radiopacity. J. Mater. Sci.: Mater. Med. 2018, 29, 149. [Google Scholar] [CrossRef] [PubMed]
- Medkov, M.A.; Grishchenko, D.N.; Klimo, M.A.; Kudryavyi, V.G.; Apanasevich, V.I. Calcium-Phosphate X-ray Contrast Cements for Bone Repair. Theor. Found. Chem. Eng. 2020, 54, 693–698. [Google Scholar] [CrossRef]
- Bartoli, M.; Jagdale, P.; Tagliaferro, A. A Short Review on Biomedical Applications of Nanostructured Bismuth Oxide and Related Nanomaterials. Materials 2020, 13, 5234. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.P.; Ye, J.D.; Wang, Y.J. Influence of a novel radiopacifier on the properties of an injectable calcium phosphate cement. Acta Biomater. 2007, 3, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Romieu, G.; Garric, X.; Munier, S.; Vert, M.; Boudeville, P. Calcium-strontium mixed phosphate as novel injectable and radio-opaque hydraulic cement. Acta Biomater. 2010, 6, 3208–3215. [Google Scholar] [CrossRef]
- López, A.; Montazerolghaem, M.; Engqvist, H.; Ott, M.K.; Persson, C. Calcium phosphate cements with strontium halides as radiopacifiers. J. Biomed. Mater. Res. Part. B 2014, 102, 250–259. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.P.; Liu, C.S.; Mao, Y.H. Bismuth-doped injectable calcium phosphate cement with improved radiopacity and potent antimicrobial activity for root canal filling. Acta Biomater. 2010, 6, 3199–3207. [Google Scholar] [CrossRef]
- Wu, I.T.; Chiang, T.Y.; Chen, C.C.; Chen, Y.C.; Ding, S.J. Dopant-dependent tailoring of physicochemical and biological properties of calcium silicate bone cements. Bio-Med. Mater. Eng. 2018, 29, 773–785. [Google Scholar] [CrossRef]
- Carvalho, E.V.; de Paula, D.M.; Neto, D.M.A.; Costa, L.S.; Dias, D.F.; Feitosa, V.P.; Fechine, P.B.A. Radiopacity and mechanical properties of dental adhesives with strontium hydroxyapatite nanofillers. J. Mech. Behav. Biomed. Mater. 2020, 101, 103447. [Google Scholar] [CrossRef]
- Dai, J.W.; Fu, Y.F.; Chen, D.M.; Sun, Z.Y. A novel and injectable strontium-containing hydroxyapatite bone cement for bone substitution: A systematic evaluation. Mater. Sci. Eng. C 2021, 124, 112052. [Google Scholar] [CrossRef]
- Wu, T.T.; Yang, S.E.; Lu, T.L.; He, F.P.; Zhang, J.; Shi, H.S.; Lin, Z.F.; Ye, J.D. Strontium ranelate simultaneously improves the radiopacity and osteogenesis of calcium phosphate cement. Biomed. Mater. 2019, 14, 035005. [Google Scholar] [CrossRef]
- Schumacher, M.; Gelinsky, M. Strontium modified calcium phosphate cements–approaches towards targeted stimulation of bone turnover. J. Mater. Chem. B 2015, 3, 4626–4640. [Google Scholar] [CrossRef] [Green Version]
- Ginebra, M.P.; Canal, C.; Espanol, M.; Pastorino, D.; Montufar, E.B. Calcium phosphate cements as drug delivery materials. Adv. Drug Deliv. Rev. 2012, 64, 1090–1110. [Google Scholar] [CrossRef]
- Lin, S.H.; Zhang, W.J.; Jiang, X.Q. Applications of Bioactive Ions in Bone Regeneration. Chin. J. Dent. Res. 2019, 22, 93–104. [Google Scholar]
- Roming, M.; Feldmann, C. Selective synthesis of α- and β-SrHPO4 nanoparticles. J. Mater. Sci. 2008, 43, 5504–5507. [Google Scholar] [CrossRef]
- Lu, Z.Z.; Chu, W.W.; Tan, R.Q.; Tang, S.H.; Xu, F.; Song, W.J.; Zhao, J.H. Facile Synthesis of β-SrHPO4 with Wide Applications in the Effective Removal of Pb2+ and Methyl Blue. J. Chem. Eng. Data 2017, 62, 3501–3511. [Google Scholar] [CrossRef]
- Shi, H.S.; Wu, T.T.; Zhang, J.; Ye, X.L.; Zeng, S.H.; Liu, X.; Yu, T.; Ye, J.D.; Zhou, C.R. Biocompatible β-SrHPO4 clusters with dandelion-like structure as an alternative drug carrier. Mater. Sci. Eng. C 2017, 81, 8–12. [Google Scholar] [CrossRef]
- Gashti, M.P.; Stir, M.; Hulliger, J. Growth of strontium hydrogen phosphate/gelatin composites: A biomimetic approach. New J. Chem. 2016, 40, 5495–5500. [Google Scholar] [CrossRef] [Green Version]
- Farrar, D.F. Bone adhesives for trauma surgery: A review of challenges and developments. Int. J. Adhes. Adhes. 2012, 33, 89–97. [Google Scholar] [CrossRef]
- Ostrowski, N.; Roy, A.; Kumta, P.K. Magnesium Phosphate Cement Systems for Hard Tissue Applications: A Review. ACS Biomater. Sci. Eng. 2016, 2, 1067–1083. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.L.; Wang, J.; Liu, C.S.; Zhang, B.W.; Chen, H.H.; Guo, H.; Zhong, G.R.; Qu, W.D.; Jiang, S.H.; Huang, H.Y. Evaluation of inherent toxicology and biocompatibility of magnesium phosphate bone cement. Colloids Surf. B 2010, 76, 496–504. [Google Scholar] [CrossRef]
- Tamimi, F.; Nihouannen, D.L.; Bassett, D.C.; Ibasco, S.; Gbureck, U.; Knowles, J.; Wright, A.; Flynn, A.; Komarova, S.V.; Barralet, J.E. Biocompatibility of magnesium phosphate minerals and their stability under physiological conditions. Acta Biomater. 2011, 7, 2678–2685. [Google Scholar] [CrossRef]
- Ostrowski, N.; Lee, B.; Hong, D.; Enick, P.N.; Roy, A.; Kumta, P.N. Synthesis, Osteoblast, and Osteoclast Viability of Amorphous and Crystalline Tri-Magnesium Phosphate. ACS Biomater. Sci. Eng. 2015, 1, 52–63. [Google Scholar] [CrossRef]
- Wang, Z.W.; Ma, Y.H.; Wei, J.; Chen, X.; Cao, L.H.; Weng, W.Z.; Li, Q.; Guo, H.; Su, J.C. Effects of sintering temperature on surface morphology/microstructure, in vitro degradability, mineralization and osteoblast response to magnesium phosphate as biomedical material. Sci. Rep. 2017, 7, 823. [Google Scholar] [CrossRef] [Green Version]
- Zhou, H.; Luchini, T.J.F.; Bhaduri, S.B. Microwave assisted synthesis of amorphous magnesium phosphate nanospheres. J. Mater. Sci. Mater. Med. 2012, 23, 2831–2837. [Google Scholar] [CrossRef] [PubMed]
- Sikder, P.; Bhaduri, S.B. Microwave assisted synthesis and characterization of single-phase tabular hexagonal newberyite, an important bioceramic. J. Am. Ceram. Soc. 2018, 101, 2537–2544. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics, 1st ed.; Elsevier: Oxford, UK, 2004; pp. 19–21. [Google Scholar]
- Meininger, S.; Moseke, C.; Spatz, K.; Märza, E.; Blum, C.; Ewald, A.; Vorndran, E. Effect of strontium substitution on the material properties and osteogenic potential of 3D powder printed magnesium phosphate scaffolds. Mater. Sci. Eng. C 2019, 98, 1145–1158. [Google Scholar] [CrossRef]
- Yang, N.; Shi, C.J.; Yang, J.M.; Chang, Y. Research Progresses in Magnesium Phosphate Cement-Based Materials. J. Mater. Civ. Eng. 2014, 26, 04014071. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, B. Research progresses on magnesium phosphate cement: A review. Constr. Build. Mater. 2019, 211, 885–898. [Google Scholar] [CrossRef]
- Snow, M.R.; Pring, A.; Allen, N. Minerals of the Wooltana Cave, Flinders Ranges, South Australia. Trans. R. Soc. South. Aust. 2014, 138, 214–230. [Google Scholar] [CrossRef]
- Afaj, A.H.; Sultan, M. Mineralogical Composition of the Urinary Stones from Different Provinces in Iraq. Sci. World J. 2005, 5, 24–38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayuseno, A.P.; Schmahl, W.W. Hydrothermal synthesis of struvite and its phase transition: Impacts of pH, heating and subsequent cooling methods. J. Cryst. Growth 2018, 498, 336–345. [Google Scholar] [CrossRef]
- Cao, X.F.; Zhang, L.L.; Chen, Q.F.; Zhang, B.; Guo, L.; Guo, Y.C. Rapid preparation of novel MgNH4PO4·H2O porous scaffolds via 3D-printing combined with a hydrothermal-process-assisted post treatment. Ceram. Int. 2020, 46, 19792–19798. [Google Scholar] [CrossRef]
- Fischer, J.; Prosenc, M.H.; Wolff, M.; Hort, N.; Willumeit, R.; Feyerabend, F. Interference of magnesium corrosion with tetrazolium-based cytotoxicity assays. Acta Biomater. 2010, 6, 1813–1823. [Google Scholar] [CrossRef] [Green Version]
- Huang, J.S.; Huang, Z.L.; Yao, D.H.; Wu, C.S.; Cheng, Y.L.; Wu, F.J. Crystallization process and growth mechanism for hexagonal prism of strontium hydroxyapatite by urea hydrolysis. J. Cryst. Growth 2019, 512, 105–111. [Google Scholar] [CrossRef]
- Cao, X.F.; Lu, H.J.; Liu, J.L.; Lu, W.P.; Guo, L.; Ma, M.; Zhang, B.; Guo, Y.C. 3D plotting in the preparation of newberyite, struvite, and brushite porous scaffolds: Using magnesium oxide as a starting material. J. Mater. Sci.: Mater. Med. 2019, 30, 88. [Google Scholar] [CrossRef] [PubMed]
- Decuzzi, P.; Ferrari, M. Modulating cellular adhesion through nanotopography. Biomaterials 2010, 31, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Mandal, S.; Barui, S.; Vasireddi, R.; Gbureck, U.; Gelinsky, M.; Basu, B. Low temperature additive manufacturing of three dimensional scaffolds for bone-tissue engineering applications: Processing related challenges and property assessment. Mater. Sci. Eng. R 2016, 103, 1–39. [Google Scholar] [CrossRef]
- Chattopadhyay, N.; Quinn, S.J.; Kifor, O.; Ye, C.P.; Brown, E.M. The calcium-sensing receptor (CaR) is involved in strontium ranelate-induced osteoblast proliferation. Biochem. Pharmacol. 2007, 74, 438–447. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.S.; Roy, A.; Lee, B.; Parekh, S.; Kumta, P.N. Murine osteoblastic and osteoclastic differentiation on strontium releasing hydroxyapatite forming cements. Mater. Sci. Eng. C 2016, 63, 429–438. [Google Scholar] [CrossRef] [PubMed] [Green Version]




| Scaffold | SrHPO4 wt% | MgO wt% | Sr:Mg a | Sr:Mg b | Porosity% |
|---|---|---|---|---|---|
| S0 | 0 | 100 | 0:100 | 0:100 | 50.85 ± 3.78% |
| S1 | 10 | 90 | 2.42:100 | 2.49:100 | 51.52 ± 4.77% |
| S3 | 30 | 70 | 9.34:100 | 9.06:100 | 50.29 ± 3.71% |
| S5 | 50 | 50 | 21.79:100 | 21.05:100 | 52.15 ± 3.73% |
| Sample | Strut a | Pore a | Tunnel a | Cross-Section a |
|---|---|---|---|---|
| S0 | 0.35–0.52 | 0.28–0.45 | 0.30–0.45 | 0.35–0.48 |
| S1 | 0.35–0.48 | 0.29–0.45 | 0.31–0.42 | 0.42–0.49 |
| S3 | 0.43–0.53 | 0.26–0.38 | 0.28–0.34 | 0.43–0.48 |
| S5 | 0.28–0.41 | 0.42–0.61 | 0.38–0.45 | 0.38–0.43 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cao, X.; Ge, W.; Wang, Y.; Ma, M.; Wang, Y.; Zhang, B.; Wang, J.; Guo, Y. Rapid Fabrication of MgNH4PO4·H2O/SrHPO4 Porous Composite Scaffolds with Improved Radiopacity via 3D Printing Process. Biomedicines 2021, 9, 1138. https://doi.org/10.3390/biomedicines9091138
Cao X, Ge W, Wang Y, Ma M, Wang Y, Zhang B, Wang J, Guo Y. Rapid Fabrication of MgNH4PO4·H2O/SrHPO4 Porous Composite Scaffolds with Improved Radiopacity via 3D Printing Process. Biomedicines. 2021; 9(9):1138. https://doi.org/10.3390/biomedicines9091138
Chicago/Turabian StyleCao, Xiaofeng, Wufei Ge, Yihu Wang, Ming Ma, Ying Wang, Bing Zhang, Jianing Wang, and Yanchuan Guo. 2021. "Rapid Fabrication of MgNH4PO4·H2O/SrHPO4 Porous Composite Scaffolds with Improved Radiopacity via 3D Printing Process" Biomedicines 9, no. 9: 1138. https://doi.org/10.3390/biomedicines9091138
APA StyleCao, X., Ge, W., Wang, Y., Ma, M., Wang, Y., Zhang, B., Wang, J., & Guo, Y. (2021). Rapid Fabrication of MgNH4PO4·H2O/SrHPO4 Porous Composite Scaffolds with Improved Radiopacity via 3D Printing Process. Biomedicines, 9(9), 1138. https://doi.org/10.3390/biomedicines9091138






