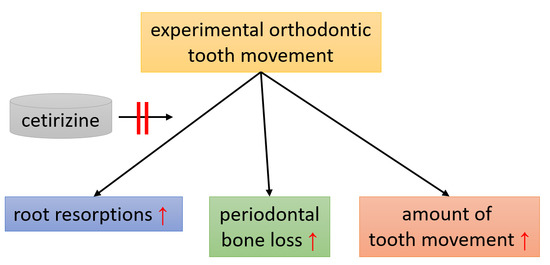
Effects of Histamine Receptor Antagonist Cetirizine on Orthodontic Tooth Movement
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Animals and Housing
2.2. Study Design, Sample Size, and Allocation
2.3. Medication and Monitoring
2.4. Orthodontic Treatment
2.5. Preparation of Histological Slides
2.6. TRAP (Tartrate-Resistant Acid Phosphatase) Staining
2.7. Hematoxylin-Eosin (HE) Staining
2.8. Cranial Growth, Root Torque and Anterior Movement
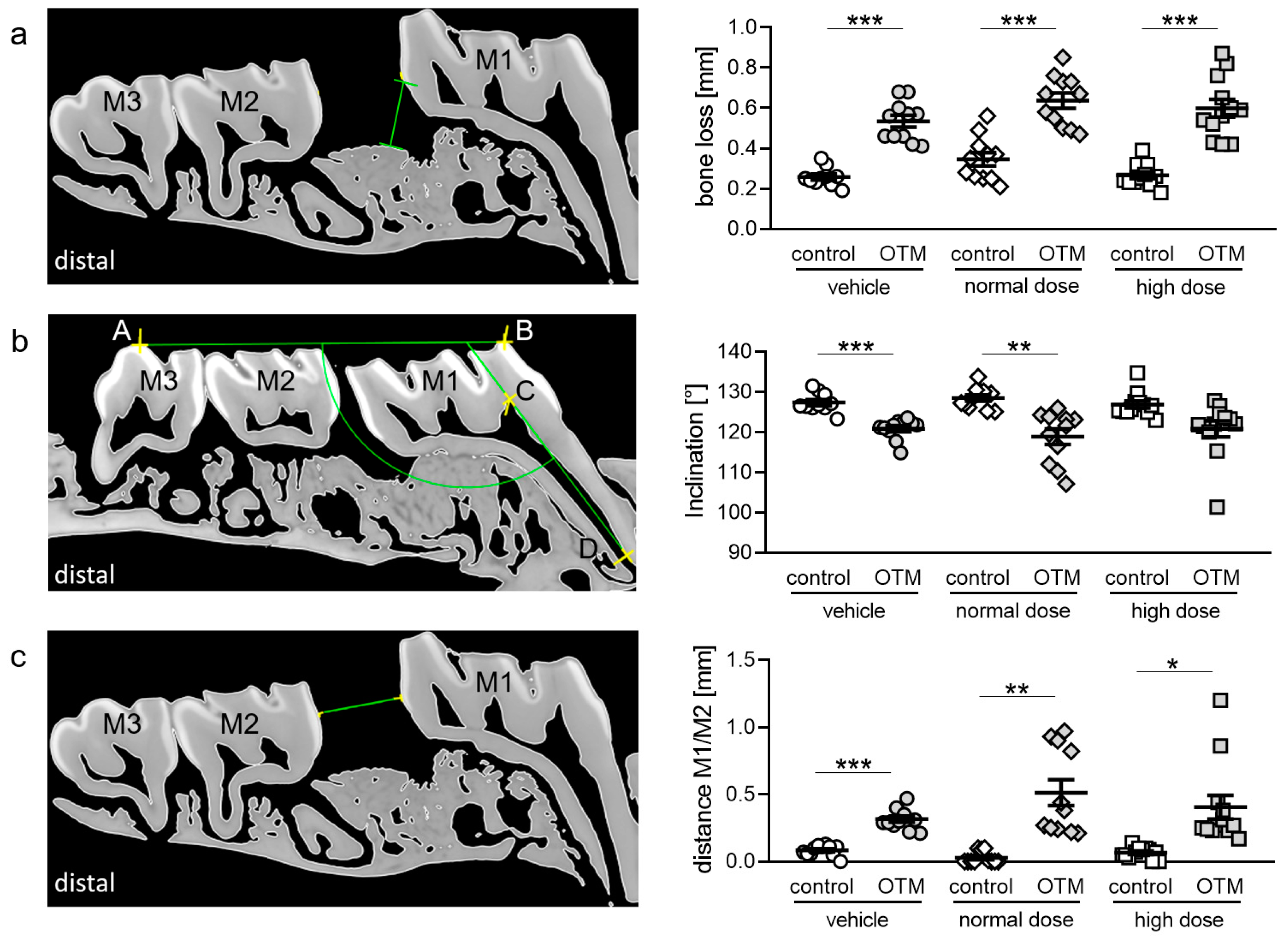
2.9. Assessment of Distal Periodontal Bone Loss, Molar Inclination and Distance M1/M2 (µCT)
2.10. Statistical Methods
3. Results
3.1. Time Flow Chart of Animal Experiment and Effects of Cetirizine and OTM on Animal Welfare
3.2. Effects of Cetirizine and OTM on Osteoclastogenesis and Root Resorption.
3.3. Effects of Cetirizine and OTM on Cranial Growth, Root Torque and Anterior Movement.
3.4. Effects of Cetirizine and OTM on Periodontal Bone Loss, Molar Inclination and Anterior Movement.
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Katzung, B.G. Basic & Clinical Pharmacology, 4th ed.; McGraw-Hill: New York, NY, USA, 2018; ISBN 978-1-259-64115-2. [Google Scholar]
- Thangam, E.B.; Jemima, E.A.; Singh, H.; Baig, M.S.; Khan, M.; Mathias, C.B.; Church, M.K.; Saluja, R. The Role of Histamine and Histamine Receptors in Mast Cell-Mediated Allergy and Inflammation: The Hunt for New Therapeutic Targets. Front. Immunol. 2018, 9, 1873. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biosse-Duplan, M.; Baroukh, B.; Dy, M.; de Vernejoul, M.-C.; Saffar, J.-L. Histamine promotes osteoclastogenesis through the differential expression of histamine receptors on osteoclasts and osteoblasts. Am. J. Pathol. 2009, 174, 1426–1434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aasarød, K.M.; Stunes, A.K.; Mosti, M.P.; Ramezanzadehkoldeh, M.; Viggaklev, B.I.; Reseland, J.E.; Skallerud, B.H.; Fossmark, R.; Syversen, U. Effects of the Histamine 1 Receptor Antagonist Cetirizine on the Osteoporotic Phenotype in H(+)/K(+) ATPase Beta Subunit KO Mice. J. Cell. Biochem. 2016, 117, 2089–2096. [Google Scholar] [CrossRef] [PubMed]
- Deyama, Y.; Kikuiri, T.; Ohnishi, G.-i.; Feng, Y.-G.; Takeyama, S.; Hatta, M.; Yoshimura, Y.; Suzuki, K. Histamine stimulates production of osteoclast differentiation factor/receptor activator of nuclear factor-κB ligand by osteoblasts. Biochem. Biophys. Res. Commun. 2002, 298, 240–246. [Google Scholar] [CrossRef]
- Ikawa, Y.; Yonekawa, T.; Ohkuni, Y.; Kuribayashi, M.; Fukino, K.; Ueno, K. A comparative study of histamine activities on differentiation of osteoblasts and osteoclasts. J. Toxicol. Sci. 2007, 32, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, P. The basics of histamine biology. Ann. Allergy Asthma Immunol. 2011, 106, S2–S5. [Google Scholar] [CrossRef]
- Freissmuth, M.; Offermanns, S.; Böhm, S. Pharmakologie & Toxikologie. Von den molekularen Grundlagen zur Pharmakotherapie; Springer: Berlin/Heidelberg, Germany, 2012; ISBN 9783642123535. [Google Scholar]
- Fehr, A.; Lange, C.; Fuchs, J.; Neuhauser, H.; Schmitz, R. Gesundheitsmonitoring und Gesundheitsindikatoren in Europa. J. Health Monit. 2017, 3–23. [Google Scholar] [CrossRef]
- Proffit, W.R.; Fields, H.W.; Larson, B.E.; Sarver, D.M. Contemporary Orthodontics, 6th ed.; Elsevier: Philadelphia, PA, USA, 2019; ISBN 9780323543873. [Google Scholar]
- Newman, M.G.; Takei, H.; Klokkevold, P.R.; Carranza, F.A. Newman and Carranza’s Clinical Periodontology, 13th ed.; Elsevier-Health Sciences Division: Philadelphia, PA, USA, 2018; ISBN 9780323523004. [Google Scholar]
- Nanci, A.; Bosshardt, D.D. Structure of periodontal tissues in health and disease. Periodontol. 2000 2006, 40, 11–28. [Google Scholar] [CrossRef]
- Krishnan, V.; Davidovitch, Z. (Eds.) Biological Mechanisms of Tooth Movement, 2nd ed.; John Wiley & Sons Inc.: Chichester, UK; Ames, IA, USA, 2015; ISBN 9781118916148. [Google Scholar]
- Niisato, N.; Ogata, Y.; Furuyama, S.; Sugiya, H. Histamine H1 receptor-stimulated Ca2+ signaling pathway in human periodontal ligament cells. J. Periodont. Res. 1996, 31, 113–119. [Google Scholar] [CrossRef]
- Groeger, M.; Spanier, G.; Wolf, M.; Deschner, J.; Proff, P.; Schröder, A.; Kirschneck, C. Effects of histamine on human periodontal ligament fibroblasts under simulated orthodontic pressure. PLoS ONE 2020, 15, e0237040. [Google Scholar] [CrossRef]
- Makrygiannakis, M.A.; Kaklamanos, E.G.; Athanasiou, A.E. Medication and orthodontic tooth movement. J. Orthod. 2019, 46, 39–44. [Google Scholar] [CrossRef] [PubMed]
- Meh, A.; Sprogar, S.; Vaupotic, T.; Cör, A.; Drevenšek, G.; Marc, J.; Drevenšek, M. Effect of cetirizine, a histamine (H(1)) receptor antagonist, on bone modeling during orthodontic tooth movement in rats. Am. J. Orthod. Dentofacial Orthop. 2011, 139, e323-9. [Google Scholar] [CrossRef] [PubMed]
- Kriznar, I.; Sprogar, S.; Drevensek, M.; Vaupotic, T.; Drevensek, G. Cetirizine, a histamine H1 receptor antagonist, decreases the first stage of orthodontic tooth movement in rats. Inflamm. Res. 2008, 57 (Suppl. 1), S29–S30. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.; Chung, C.J.; Choi, Y.J.; Kim, T.; Kim, K.-H. The effect of cetirizine, a histamine 1 receptor antagonist, on bone remodeling after calvarial suture expansion. Korean J. Orthod. 2020, 50, 42–51. [Google Scholar] [CrossRef]
- Folwarczna, J.; Konarek, N.; Freier, K.; Karbowniczek, D.; Londzin, P.; Janas, A. Effects of loratadine, a histamine H1 receptor antagonist, on the skeletal system of young male rats. Drug Des. Devel. Ther. 2019, 13, 3357–3367. [Google Scholar] [CrossRef] [Green Version]
- Brezniak, N.; Wasserstein, A. Orthodontically induced inflammatory root resorption. Part I: The basic science aspects. Angle Orthod. 2002, 72, 175–179. [Google Scholar] [CrossRef]
- Maués, C.P.R.; do Nascimento, R.R.; Vilella, O.d.V. Severe root resorption resulting from orthodontic treatment: Prevalence and risk factors. Dental Press J. Orthod. 2015, 20, 52–58. [Google Scholar] [CrossRef]
- Amaro, E.R.S.; Ortiz, F.R.; Dorneles, L.S.; Santos, M.d.S.; Barrioni, B.R.; Miranda, R.M.; Garlet, G.P.; Teixeira, M.M.; Szawka, R.E.; Silva, T.A.; et al. Estrogen protects dental roots from orthodontic-induced inflammatory resorption. Arch. Oral Biol. 2020, 117, 104820. [Google Scholar] [CrossRef]
- Yang, F.; Wang, X.X.; Li, J.; Nie, F.J.; Cui, Q.; Fu, Y.J.; Zhang, J. The effects of binge alcohol exposure on tooth movement and associated root resorption in rats. Alcohol 2020. [Google Scholar] [CrossRef]
- Kirschneck, C.; Meier, M.; Bauer, K.; Proff, P.; Fanghänel, J. Meloxicam medication reduces orthodontically induced dental root resorption and tooth movement velocity: A combined in vivo and in vitro study of dental-periodontal cells and tissue. Cell Tissue Res. 2017, 368, 61–78. [Google Scholar] [CrossRef]
- Kirschneck, C.; Wolf, M.; Reicheneder, C.; Wahlmann, U.; Proff, P.; Roemer, P. Strontium ranelate improved tooth anchorage and reduced root resorption in orthodontic treatment of rats. Eur. J. Pharmacol. 2014, 744, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Kirschneck, C.; Maurer, M.; Wolf, M.; Reicheneder, C.; Proff, P. Regular nicotine intake increased tooth movement velocity, osteoclastogenesis and orthodontically induced dental root resorptions in a rat model. Int. J. Oral Sci. 2017, 9, 174–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krishnan, V.; Ambili, R.; Davidovitch, Z.E.; Murphy, N.C. Gingiva and Orthodontic Treatment. Semin. Orthod. 2007, 13, 257–271. [Google Scholar] [CrossRef]
- Talic, N.F. Adverse effects of orthodontic treatment: A clinical perspective. Saudi Dent. J. 2011, 23, 55–59. [Google Scholar] [CrossRef] [Green Version]
- Teodora, C.; Ionescu, E.; Preoteas, E. Risks and Complications Associated with Orthodontic Treatment. In Orthodontic Treatment Need: An Epidemiological Approach; Bourzgui, F., Ed.; INTECH Open Access Publisher: London, UK, 2012; ISBN 978-953-51-0143-7. [Google Scholar]
- Meikle, M.C. The tissue, cellular, and molecular regulation of orthodontic tooth movement: 100 years after Carl Sandstedt. Eur. J. Orthod. 2006, 28, 221–240. [Google Scholar] [CrossRef]
- Kirschneck, C.; Küchler, E.C.; Wahlmann, U.; Proff, P.; Schröder, A. Effects of the highly COX-2-selective analgesic NSAID etoricoxib on the rate of orthodontic tooth movement and cranial growth. Ann. Anat. 2018, 220, 21–28. [Google Scholar] [CrossRef]
- Simons, F.E.; Simons, K.J. Clinical pharmacology of new histamine H1 receptor antagonists. Clin. Pharmacokinet. 1999, 36, 329–352. [Google Scholar] [CrossRef]
- Turner, P.V.; Brabb, T.; Pekow, C.; Vasbinder, M.A. Administration of substances to laboratory animals: Routes of administration and factors to consider. J. Am. Assoc. Lab. Anim. Sci. 2011, 50, 600–613. [Google Scholar]
- White, R.; Bradnam, V. Handbook of Drug Administration via Enteral Feeding Tubes, 3th ed.; Pharmaceutical Press: London, UK, 2015; ISBN 9780857112224. [Google Scholar]
- Reagan-Shaw, S.; Nihal, M.; Ahmad, N. Dose translation from animal to human studies revisited. FASEB J. 2008, 22, 659–661. [Google Scholar] [CrossRef] [Green Version]
- Kirschneck, C.; Proff, P.; Fanghaenel, J.; Behr, M.; Wahlmann, U.; Roemer, P. Differentiated analysis of orthodontic tooth movement in rats with an improved rat model and three-dimensional imaging. Ann. Anat. 2013, 195, 539–553. [Google Scholar] [CrossRef]
- Lin, L.I. A concordance correlation coefficient to evaluate reproducibility. Biometrics 1989, 45, 255–268. [Google Scholar] [CrossRef] [PubMed]
- Kirschneck, C.; Fanghänel, J.; Wahlmann, U.; Wolf, M.; Roldán, J.C.; Proff, P. Interactive effects of periodontitis and orthodontic tooth movement on dental root resorption, tooth movement velocity and alveolar bone loss in a rat model. Ann. Anat. 2017, 210, 32–43. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P. The Laboratory Rat: Relating Its Age With Human’s. Int. J. Prev. Med. 2013, 4, 624–630. [Google Scholar] [PubMed]
- Tsichlaki, A.; Chin, S.Y.; Pandis, N.; Fleming, P.S. How long does treatment with fixed orthodontic appliances last? A systematic review. Am. J. Orthod. Dentofacial Orthop. 2016, 149, 308–318. [Google Scholar] [CrossRef]
- Walpole, S.C.; Prieto-Merino, D.; Edwards, P.; Cleland, J.; Stevens, G.; Roberts, I. The weight of nations: An estimation of adult human biomass. BMC Public Health 2012, 12, 439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wilkinson, I.B.; Raine, T.; Wiles, K. Oxford Handbook of Clinical Medicine, 10th ed.; Oxford University Press: Oxford, UK, 2017; ISBN 978-0-19-968990-3. [Google Scholar]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sperl, G.; Gattner, J.; Deschner, J.; Wolf, M.; Proff, P.; Schröder, A.; Kirschneck, C. Effects of Histamine Receptor Antagonist Cetirizine on Orthodontic Tooth Movement. Biomedicines 2020, 8, 583. https://doi.org/10.3390/biomedicines8120583
Sperl G, Gattner J, Deschner J, Wolf M, Proff P, Schröder A, Kirschneck C. Effects of Histamine Receptor Antagonist Cetirizine on Orthodontic Tooth Movement. Biomedicines. 2020; 8(12):583. https://doi.org/10.3390/biomedicines8120583
Chicago/Turabian StyleSperl, Gregor, Johanna Gattner, James Deschner, Michael Wolf, Peter Proff, Agnes Schröder, and Christian Kirschneck. 2020. "Effects of Histamine Receptor Antagonist Cetirizine on Orthodontic Tooth Movement" Biomedicines 8, no. 12: 583. https://doi.org/10.3390/biomedicines8120583
APA StyleSperl, G., Gattner, J., Deschner, J., Wolf, M., Proff, P., Schröder, A., & Kirschneck, C. (2020). Effects of Histamine Receptor Antagonist Cetirizine on Orthodontic Tooth Movement. Biomedicines, 8(12), 583. https://doi.org/10.3390/biomedicines8120583