The Effect of Probiotics on Preterm Birth Rates in Pregnant Women After a Threatened Preterm Birth Episode (The PROPEV Trial)
Abstract
1. Introduction
2. Material and Methods
2.1. Study Design and Participants
2.2. Sample Size
2.3. Procedures, Randomization, and Masking
2.4. Outcomes
2.5. Statistical Analysis
2.6. DNA Extraction and 16S rRNA Sequencing
2.7. Microbiota Analysis
3. Results
3.1. Microbiota Analysis
3.2. Safety Management
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PTB | preterm birth |
| CSTs | composted tannery sludge |
| sPTB | spontaneous preterm birth |
| AEs | adverse events |
| SUSAR | serious unexpected adverse event reaction |
| BMI | body mass index |
References
- WHO. Low Birth Weight. Available online: https://www.who.int/data/nutrition/nlis/info/low-birth-weight (accessed on 20 May 2024).
- Lawn, J.E.; Gravett, M.G.; Nunes, T.M.; Rubens, C.E.; Stanton, C.; the GAPPS Review Group. Global report on preterm birth and stillbirth (1 of 7): Definitions, description of the burden and opportunities to improve data. BMC Pregnancy Childbirth 2010, 10, S1. [Google Scholar] [CrossRef] [PubMed]
- Mwaniki, M.K.; Atieno, M.; Lawn, J.E.; Newton, C.R. Long-term neurodevelopmental outcomes after intrauterine and neonatal insults: A systematic review. Lancet 2012, 379, 445–452. [Google Scholar] [CrossRef] [PubMed]
- WHO. Born Too Soon: The Global Action Report on Preterm Birth. Available online: https://www.who.int/publications/i/item/9789241503433 (accessed on 20 May 2024).
- Blencowe, H.; Cousens, S.; Oestergaard, M.Z.; Chou, D.; Moller, A.B.; Narwal, R.; Adler, A.; Garcia, C.V.; Rohde, S.; Say, L.; et al. National, regional, and worldwide estimates of preterm birth rates in the year 2010 with time trends since 1990 for selected countries: A systematic analysis and implications. Lancet 2012, 379, 2162–2172. [Google Scholar] [CrossRef] [PubMed]
- Slattery, M.M.; Morrison, J.J. Preterm delivery. Lancet 2002, 360, 1489–1497. [Google Scholar] [CrossRef]
- Goldenberg, R.L.; Culhane, J.F.; Iams, J.D.; Romero, R. Epidemiology and causes of preterm birth. Lancet 2008, 371, 75–84. [Google Scholar] [CrossRef]
- Iams, J.D.; Romero, R.; Culhane, J.F.; Goldenberg, R.L. Primary, secondary, and tertiary interventions to reduce the morbidity and mortality of preterm birth. Lancet 2008, 371, 164–175. [Google Scholar] [CrossRef]
- France, M.; Alizadeh, M.; Brown, S.; Ravel, J. Towards a deeper understanding of the vaginal microbiota. Nat. Microbiol. 2022, 7, 367–378. [Google Scholar] [CrossRef]
- Kwon, M.S.; Lee, H.K. Host and Microbiome Interplay Shapes the Vaginal Microenvironment. Front. Immunol. 2022, 13, 919728. [Google Scholar] [CrossRef]
- Plesniarski, A.; Siddik, A.B.; Su, R.C. The Microbiome as a Key Regulator of Female Genital Tract Barrier Function. Front. Cell. Infect. Microbiol. 2021, 11, 790627. [Google Scholar] [CrossRef]
- Bayar, E.; Bennett, P.R.; Chan, D.; Sykes, L.; MacIntyre, D.A. The pregnancy microbiome and preterm birth. Semin. Immunopathol. 2020, 42, 487–499. [Google Scholar] [CrossRef]
- Fettweis, J.M.; Serrano, M.G.; Edwards, D.J.; Girerd, P.H.; Parikh, H.I.; Huang, B.; Arodz, T.J.; Edupuganti, L.; Glascock, A.L.; Xu, J.; et al. The vaginal microbiome and preterm birth. Nat. Med. 2019, 25, 1012–1021. [Google Scholar] [CrossRef] [PubMed]
- Gudnadottir, U.; Debelius, J.W.; Du, J.; Hugerth, L.W.; Danielsson, H.; Schuppe-Koistinen, I.; Fransson, E.; Brusselaers, N. The vaginal microbiome and the risk of preterm birth: A systematic review and network meta-analysis. Sci. Rep. 2022, 12, 7926. [Google Scholar] [CrossRef] [PubMed]
- Serrano, M.G.; Parikh, H.I.; Edwards, D.J.; Arodz, T.J.; Edupuganti, L.; Huang, B.; Girerd, P.H.; Bokhari, Y.A.; Bradley, S.P.; Brooks, J.L. Racioethnic diversity in the dynamics of the vaginal microbiome during pregnancy. Nat. Med. 2019, 25, 1001–1011. [Google Scholar] [CrossRef] [PubMed]
- Mls, J.; Stráník, J.; Kacerovský, M. Lactobacillus iners-dominated vaginal microbiota in pregnancy. Ceska Gynekol. 2019, 84, 463–467. [Google Scholar]
- Petricevic, L.; Domig, K.J.; Nierscher, F.J.; Sandhofer, M.J.; Fidesser, M.; Krondorfer, I.; Husslein, P.; Kneifel, W.; Kiss, H. Characterisation of the vaginal Lactobacillus microbiota associated with preterm delivery. Sci. Rep. 2014, 4, 5136. [Google Scholar] [CrossRef]
- Zhou, Q.; Yu, Y.; Zhou, J.; Liu, J.; Gao, J. Relationship of Lactobacillus Vaginal Microbiota Changes and the Risk of Preterm Birth: A Systematic Review and Meta-Analysis. J. Womens Health 2024, 33, 228–238. [Google Scholar] [CrossRef]
- Biggs, W.S.; Williams, R.M. Common Gynecologic Infections. Prim. Care Clin. Off. Pract. 2009, 36, 33–51. [Google Scholar] [CrossRef]
- Boris, S.; Suárez, J.E.; Vázquez, F.; Barbés, C. Adherence of Human Vaginal lactobacilli to Vaginal epithelial Cells and Interaction with Uropathogens. Infect. Immun. 1998, 66, 1985–1989. [Google Scholar] [CrossRef]
- Mastromarino, P.; Brigidi, P.; Macchia, S.; Maggi, L.; Pirovano, F.; Trinchieri, V.; Conte, U.; Matteuzzi, D. Characterization and selection of vaginal Lactobacillus strains for the preparation of vaginal tablets. J. Appl. Microbiol. 2002, 93, 884–893. [Google Scholar] [CrossRef]
- Atassi, F.; Brassart, D.; Grob, P.; Graf, F.; Servin, A.L. Lactobacillus strains isolated from the vaginal microbiota of healthy women inhibit Prevotella bivia and Gardnerella vaginalis in coculture and cell culture. FEMS Immunol. Med. Microbiol. 2006, 48, 424–432. [Google Scholar] [CrossRef]
- Coudeyras, S.; Jugie, G.; Vermerie, M.; Forestier, C. Adhesion of Human Probiotic Lactobacillus rhamnosus to Cervical and Vaginal Cells and Interaction with Vaginosis-Associated Pathogens. Infect. Dis. Obs. Gynecol. 2008, 2008, 549640. [Google Scholar]
- Phukan, N.; Parsamand, T.; Brooks, A.E.S.; Nguyen, T.N.M.; Simoes-Barbosa, A. The adherence of Trichomonas vaginalis to host ectocervical cells is influenced by lactobacilli. Sex. Transm. Infect. 2013, 89, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Juárez Tomás, M.S.; Ocaña, V.S.; Wiese, B.; Nader-Macías, M.E. Growth and lactic acid production by vaginal Lactobacillus acidophilus CRL 1259, and inhibition of uropathogenic Escherichia coli. J. Med. Microbiol. 2003, 52, 1117–1124. [Google Scholar] [CrossRef] [PubMed]
- Vielfort, K.; Sjölinder, H.; Roos, S.; Jonsson, H.; Aro, H. Adherence of clinically isolated lactobacilli to human cervical cells in competition with Neisseria gonorrhoeae. Microbes Infect. 2008, 10, 1325–1334. [Google Scholar] [CrossRef] [PubMed]
- Malik, S.; Petrova, M.I.; Claes, I.J.J.; Verhoeven, T.L.A.; Busschaert, P.; Vaneechoutte, M.; Lievens, B.; Lambrichts, I.; Siezen, R.J.; Balzarini, J.; et al. The Highly Autoaggregative and Adhesive Phenotype of the Vaginal Lactobacillus plantarum Strain CMPG5300 Is Sortase Dependent. Appl. Env. Microbiol. 2013, 79, 4576–4585. [Google Scholar] [CrossRef]
- Rossi, A.; Rossi, T.; Bertini, M.; Caccia, G. The use of Lactobacillus rhamnosus in the therapy of bacterial vaginosis. Evaluation of clinical efficacy in a population of 40 women treated for 24 months. Arch. Gynecol. Obstet. 2010, 281, 1065–1069. [Google Scholar] [CrossRef]
- Marcone, V.; Calzolari, E.; Bertini, M. Effectiveness of vaginal administration of Lactobacillus rhamnosus following conventional metronidazole therapy: How to lower the rate of bacterial vaginosis recurrences. New Microbiol. 2008, 31, 429–433. [Google Scholar]
- Marcone, V.; Rocca, G.; Lichtner, M.; Calzolari, E. Long-term vaginal administration of Lactobacillus rhamnosus as a complementary approach to management of bacterial vaginosis. Int. J. Gynaecol. Obs. Off. Organ. Int. Fed. Gynaecol. Obstet. 2010, 110, 223–226. [Google Scholar] [CrossRef]
- Larsson, P.G.; Brandsborg, E.; Forsum, U.; Pendharkar, S.; Andersen, K.K.; Nasic, S.; Hammarström, L.; Marcotte, H. Extended antimicrobial treatment of bacterial vaginosis combined with human lactobacilli to find the best treatment and minimize the risk of relapses. BMC Infect. Dis. 2011, 11, 223. [Google Scholar] [CrossRef]
- Rautava, S.; Kalliomäki, M.; Isolauri, E. Probiotics during pregnancy and breast-feeding might confer immunomodulatory protection against atopic disease in the infant. J. Allergy Clin. Immunol. 2002, 109, 119–121. [Google Scholar] [CrossRef]
- Huurre, A.; Laitinen, K.; Rautava, S.; Korkeamäki, M.; Isolauri, E. Impact of maternal atopy and probiotic supplementation during pregnancy on infant sensitization: A double-blind placebo-controlled study. Clin. Exp. Allergy. 2008, 38, 1342–1348. [Google Scholar] [CrossRef] [PubMed]
- Schulz, K.F.; Altman, D.G.; Moher, D.; CONSORT Group. CONSORT 2010 statement: Updated guidelines for reporting parallel group randomised trials. BMJ 2010, 340, c332. [Google Scholar] [CrossRef] [PubMed]
- Effect of Probiotics on the Preterm Delivery Rate in Pregnant Women at High Risk for Preterm Birth (PROPEV). Available online: https://clinicaltrials.gov/study/NCT03689166 (accessed on 16 September 2022).
- International Human Microbiome Standards (IHMS). International Human Microbiome Standards (IHMS). Available online: http://www.human-microbiome.org (accessed on 16 March 2023).
- Costea, P.I.; Zeller, G.; Sunagawa, S.; Pelletier, E.; Alberti, A.; Levenez, F.; Tramontano, M.; Driessen, M.; Hercog, R.; Jung, F.E.; et al. Towards standards for human fecal sample processing in metagenomic studies. Nat. Biotechnol. 2017, 35, 1069–1076. [Google Scholar] [CrossRef] [PubMed]
- Cuenca, S.; Soler, Z.; Serrano-Gómez, G.; Xie, Z.; Barquinero, J.; Roca, J.; Sirvent, J.M.; Manichanh, C. Dysbiosis: An Indicator of COVID-19 Severity in Critically Ill Patients. Int. J. Mol. Sci. 2022, 23, 15808. [Google Scholar] [CrossRef]
- Husain, S.; Wilks, M.; Mupita, M.; Reddy, S.; Hennessy, E.; Macfarlane, A.; Millar, M. Diversity and stability of cultured vaginal lactobacilli in pregnant women from a multi-ethnic urban UK population. J. Appl. Microbiol. 2014, 117, 258–265. [Google Scholar] [CrossRef]
- Vargas, M.; Yañez, F.; Elias, A.; Bernabeu, A.; Goya, M.; Xie, Z.; Farrás, A.; Sánchez, O.; Soler, Z.; Blasquez, C.; et al. Cervical pessary and cerclage placement for preterm birth prevention and cervicovaginal microbiome changes. Acta Obstet. Gynecol. Scand. 2022, 101, 1403–1413. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F.; et al. Reproducible, interactive, scalable and extensible microbiome data science using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-resolution sample inference from Illumina amplicon data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]
- Molano, L.A.G.; Vega-Abellaneda, S.; Manichanh, C. GSR-DB: A manually curated and optimized taxonomical database for 16S rRNA amplicon analysis. mSystems 2024, 9, e0095023. [Google Scholar] [CrossRef]
- Mallick, H.; Rahnavard, A.; McIver, L.J.; Ma, S.; Zhang, Y.; Nguyen, L.H.; Tickle, T.L.; Weingart, G.; Ren, B.; Schwager, E.H.; et al. Multivariable association discovery in population-scale meta-omics studies. PLoS Comput. Biol. 2021, 17, e1009442. [Google Scholar] [CrossRef]
- Lin, H.; Peddada, S.D. Multigroup analysis of compositions of microbiomes with covariate adjustments and repeated measures. Nat. Methods 2024, 21, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Nearing, J.T.; Douglas, G.M.; Hayes, M.G.; MacDonald, J.; Desai, D.K.; Allward, N.; Jones, C.M.A.; Wright, R.J.; Dhanani, A.S.; Comeau, A.M.; et al. Microbiome differential abundance methods produce different results across 38 datasets. Nat. Commun. 2022, 13, 342. [Google Scholar] [CrossRef] [PubMed]
- Oksanen, J.; Blanchet, F.G.; Friendly, M.; Kindt, R.; Legendre, P.; McGlinn, D.; Minchin, P.R.; O’Hara, R.B.; Simpson, G.L.; Solymos, P.; et al. Vegan: Community Ecology Package. Available online: https://github.com/vegandevs/vegan (accessed on 20 May 2024).
- Jarde, A.; Lewis-Mikhael, A.-M.; Moayyedi, P.; Stearns, J.C.; Collins, S.M.; Beyene, J.; McDonald, S.D. Pregnancy outcomes in women taking probiotics or prebiotics: A systematic review and meta-analysis. BMC Pregnancy Childbirth 2018, 18, 14. [Google Scholar] [CrossRef] [PubMed]
- Vanda, R.; Dastani, T.; Taghavi, S.-A.; Sadeghi, H.; Lambert, N.; Bazarganipour, F. Pregnancy outcomes in pregnant women taking oral probiotic undergoing cerclage compared to placebo: Two blinded randomized controlled trial. BMC Pregnancy Childbirth 2024, 24, 311. [Google Scholar] [CrossRef]
- Petricevic, L.; Rosicky, I.; Kiss, H.; Janjic, N.; Kaufmann, U.; Holzer, I.; Farr, A. Effect of vaginal probiotics containing Lactobacillus casei rhamnosus (Lcr regenerans) on vaginal dysbiotic microbiota and pregnancy outcome, prospective, randomized study. Sci. Rep. 2023, 13, 7129. [Google Scholar] [CrossRef]
- Yefet, E.; Colodner, R.; Strauss, M.; Letova, Y.G.Z.; Nachum, Z. A Randomized Controlled Open Label Crossover Trial to Study Vaginal Colonization of Orally Administered Lactobacillus reuteri RC-14 and Rhamnosus GR-1 in Pregnant Women at High Risk for Preterm Labor. Nutrients. 2020, 12, 1141. [Google Scholar] [CrossRef]
- McDougall, A.; Nguyen, R.; Nguyen, P.Y.; Allen, C.; Cheang, S.; Makama, M.; Mills, K.; Hastie, R.; Ammerdorffer, A.; Gulmezoglu, A.M.; et al. The effects of probiotics administration during pregnancy on preeclampsia and associated maternal, fetal, and newborn outcomes: A systematic review and meta-analysis. Am. J. Obstet. Gynecol. MFM 2024, 6, 101322. [Google Scholar] [CrossRef]
- Husain, S.; Allotey, J.; Drymoussi, Z.; Wilks, M.; Fernandez-Felix, B.; Whiley, A.; Dodds, J.; Thangaratinam, S.; McCourt, C.; Prosdocimi, E.; et al. Effects of oral probiotic supplements on vaginal microbiota during pregnancy: A randomised, double-blind, placebo-controlled trial with microbiome analysis. BJOG Int. J. Obstet. Gynaecol. 2020, 127, 275–284. [Google Scholar] [CrossRef]




| Active Treatment (n = 101) | Placebo (n = 99) | |||
|---|---|---|---|---|
| Age (years) [mean (SD)] | 32.08 (5.70) | 31.66 (6.28) | ||
| BMI (kg/m2) [mean (SD)] | 24.93 (6.51) | 23.34 (4.06) | ||
| Gestational age at inclusion [mean (SD)] | 28.43 (2.71) | 28.77 (2.66) | ||
| Ethnicity | White | 65 (65%) | 63 (63.6%) | |
| Hispanic | 21 (21%) | 19 (19.8%) | ||
| Black | 3 (3%) | 2 (2.1%) | ||
| Asian | 3 (3%) | 2 (2.1%) | ||
| Other | 8 (8%) | 3 (3%) | ||
| Current smoking | Yes | 17 (17%) | 5 (5.1%) | |
| No | 83 (83%) | 93 (94.9%) | ||
| Parity | Term | 0 | 66 (65.3%) | 61 (61.6%) |
| 1 | 25 (24.8%) | 32 (32.3%) | ||
| >2 | 10 (9.9%) | 6 (6.1%) | ||
| Preterm | 0 | 77 (76.2%) | 86 (86.9%) | |
| 1 | 20 (19.8%) | 11 (11.1%) | ||
| >2 | 4 (4%) | 2 (2%) | ||
| Abortions | 0 | 52 (51.5%) | 53 (53.5%) | |
| 1 | 20 (19.8%) | 11 (11.1%) | ||
| >2 | 29 (28.7%) | 16 (16.2%) | ||
| Live | 0 | 50 (49.5%) | 52 (52.5%) | |
| 1 | 32 (31.7%) | 38 (38.4%) | ||
| >2 | 15 (14.9%) | 9 (9.1%) | ||
| Active Treatment (n = 91) | Placebo (n = 90) | OR (95% CI) | p-Value | |
|---|---|---|---|---|
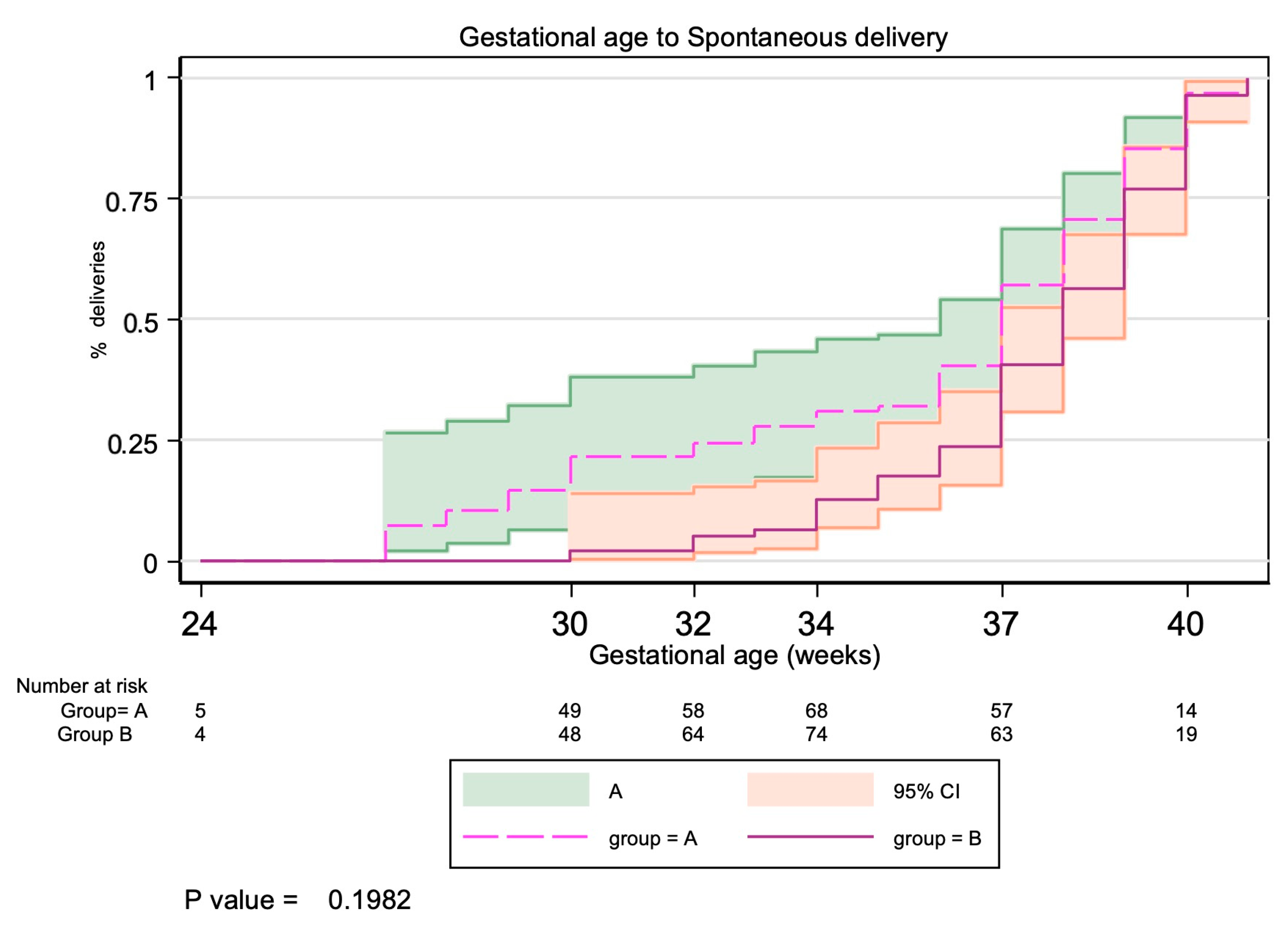
| Gestational age at delivery (weeks) [mean (SD)] | 36.33 (3.57) | 37.20 (2.73) | 0.1379 | |
| Preterm birth < 37 weeks | 28 (32.9%) | 22 (25.9%) | 1.37 (0.71 to 2.65) | 0.3125 |
| Preterm birth < 34 weeks | 16 (17.8%) | 8 (9%) | 2.19 (0.88 to 5.40) | 0.0844 |
| Preterm birth < 32 weeks | 11 (12.2%) | 3 (3.3%) | 4.07 (1.10 to 15.15) | 0.0260 |
| Preterm birth < 28 weeks | 6 (6.7%) | 1 (1.1%) | 6.28 (0.74 to 53.28) | 0.1177 |
| Preterm birth < 24 weeks | 2 (2.2%) | 0 (0%) | - | 0.4972 |
| Active Treatment (n = 91) | Placebo (n = 90) | OR (95% CI) | p-Value | |
|---|---|---|---|---|
| Weight at birth (mean ± SD) | 2799.87 (751.78) | 2874.67 (661.72) | - | 0.6699 |
| Composite outcome | 32 (35.3%) | 29 (32.2%) | 1.14 (0.62 to 2.11) | 0.6754 |
| Low birth weight (<2500 g) | 8 (8.9%) | 4 (4.4%) | 2.07 (0.60 to 7.14) | 0.2320 |
| NICU admission | 18 (22.5%) | 15 (17.4%) | 1.23 (0.58 to 2.63) | 0.4145 |
| Hyaline membrane | 10 (55.6%) | 3 (21.4%) | 3.58 (0.95 to 13.47) | 0.0512 |
| Brain hemorrhage | 1 (5.6%) | 2 (14.3%) | 0.49 (0.04 to 5.49) | 0.5681 |
| Neonatal sepsis | 4 (22.2%) | 1 (7.1%) | 4.09 (0.45 to 37.35) | 0.3547 |
| Necrotizing enterocolitis | 0 (0%) | 2 (14.3%) | - | 0.1835 |
| Treatment Received | |||||
|---|---|---|---|---|---|
| Characteristic | n | Overall, n = 55 1 | Probiotics, n = 30 | Placebo, n = 25 | p-Value 2 |
| CST Before, n (%) | 51 | 0.69 | |||
| CST-I | 13 (25%) | 8 (29%) | 5 (22%) | ||
| CST-II | 5 (9.8%) | 2 (7.1%) | 3 (13%) | ||
| CST-III | 23 (45%) | 14 (50%) | 9 (39%) | ||
| CST-IV | 9 (18%) | 4 (14%) | 5 (22%) | ||
| CST-V | 1 (2 %) | 0 (0%) | 1 (4.3%) | ||
| CST After, n (%) | 41 | 0.80 | |||
| CST-I | 8 (20%) | 5 (21%) | 3 (18%) | ||
| CST-II | 4 (9.8%) | 2 (8.3%) | 2 (12%) | ||
| CST-III | 17 (41%) | 11 (46%) | 6 (35%) | ||
| CST-IV | 12 (29%) | 6 (25%) | 6 (35%) | ||
| CST-V | 0 (0%) | 0 (0%) | 0 (0%) | ||
| CST shift, n (%) | 37 | >0.99 | |||
| No | 31 (84%) | 18 (82%) | 13 (87%) | ||
| Yes | 6 (16%) | 4 (18%) | 2 (13%) | ||
| Transition from specific CST, n (%) | 6 | 0.20 | |||
| CST-I. Yes | 2 (33%) | 2 (50%) | 0 (0%) | ||
| CST-II. Yes | 1 (17%) | 0 (0%) | 1 (50%) | ||
| CST-III. Yes | 2 (33%) | 2 (50%) | 0 (0%) | ||
| CST-V. Yes | 1 (17%) | 0 (0%) | 1 (50%) | ||
| Transition to specific CST, n (%) | 6 | >0.99 | |||
| CST-I. Yes | 1 (17%) | 1 (25%) | 0 (0%) | ||
| CST-IV. Yes | 5 (83%) | 3 (75%) | 2 (100%) | ||
| Adverse Effect Number | Group | PT Name | PT Code | SOC Name | SOC Code | Intensity | Causality | SAE? |
|---|---|---|---|---|---|---|---|---|
| 1 | Placebo | Gastric ulcer | 10017822 | Gastrointestinal disorders | 10017947 | Moderate | Not related | Yes |
| 2 | Active treatment | Hospitalization | 10054112 | Surgical and medical procedures | 10042613 | Mild | Not related | Yes |
| 3 | Active treatment | Amniotic cavity infection | 10060937 | Infections and infestations | 10021881 | Mild | Not related | No |
| 4 | Placebo | Gestational diabetes | 10018209 | Pregnancy, puerperium, and perinatal conditions | 10036585 | Mild | Not related | No |
| 5 | Placebo | Abdominal Pain | 10000081 | Gastrointestinal disorders | 10017947 | Mild | Possible | No |
| 6 | Placebo | Constipation | 10010774 | Gastrointestinal disorders | 10017947 | Mild | Possible | No |
| 7 | Placebo | Uterine contractions during pregnancy | 10049975 | Pregnancy, puerperium, and perinatal conditions | 10036585 | Mild | Possible | No |
| 8 | Placebo | Genital discharge | 10056740 | Reproductive system and breast disorders | 10038604 | Mild | Possible | No |
| 9 | Placebo | Photopsia | 10034962 | Eye disorders | 10015919 | Mild | Unlikely | No |
| 10 | Placebo | Edema peripheral | 10030124 | General disorders and administration site conditions | 10018065 | Mild | Unlikely | No |
| 11 | Placebo | Suprapubic pain | 10050822 | General disorders and administration site conditions | 10018065 | Mild | Possible | No |
| 12 | Active treatment | Dyspepsia | 10013946 | Gastrointestinal disorders | 10017947 | Mild | Not related | No |
| 13 | Active treatment | Abdominal Pain upper | 10000087 | Gastrointestinal disorders | 10017947 | Moderate | Probable | No |
| 14 | Active treatment | Fetal growth restriction | 10070531 | Pregnancy, puerperium, and perinatal conditions | 10036585 | Mild | Not related | No |
| 15 | Placebo | Premature delivery | 10036595 | Pregnancy, puerperium, and perinatal conditions | 10036585 | Moderate | Unlikely | No |
| 16 | Placebo | Threatened labor | 10043508 | Pregnancy, puerperium, and perinatal conditions | 10036585 | Mild | Unlikely | No |
| 17 | Active treatment | Preeclampsia | 10036485 | Pregnancy, puerperium, and perinatal conditions | 10036585 | Mild | Not related | No |
| 18 | Active treatment | Urinary tract infection | 10046571 | Infections and infestations | 10021881 | Mild | Not related | No |
| 19 | Active treatment | Gestational diabetes | 10018209 | Pregnancy, puerperium, and perinatal conditions | 10036585 | Mild | Not related | No |
| 20 | Active treatment | COVID-19 | 10084268 | Infections and infestations | 10021881 | Mild | Not related | No |
| 21 | Active treatment | Threatened labor | 10043508 | Pregnancy, puerperium, and perinatal conditions | 10036585 | Mild | Not related | No |
| 22 | Placebo | Threatened labor | 10043508 | Pregnancy, puerperium, and perinatal conditions | 10036585 | Mild | Not related | No |
| 23 | Placebo | Adverse drug reaction | 10061623 | General disorders and administration site conditions | 10018065 | Mild | Not related | No |
| 24 | Placebo | COVID-19 | 10084268 | Infections and infestations | 10021881 | Mild | Unlikely | No |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
del Barco, E.; Molano, L.-A.G.; Vargas, M.; Miserachs, M.; Puerto, L.; Garrido-Giménez, C.; Soler, Z.; Muñoz, B.; Pratcorona, L.; Rimbaut, S.; et al. The Effect of Probiotics on Preterm Birth Rates in Pregnant Women After a Threatened Preterm Birth Episode (The PROPEV Trial). Biomedicines 2025, 13, 1141. https://doi.org/10.3390/biomedicines13051141
del Barco E, Molano L-AG, Vargas M, Miserachs M, Puerto L, Garrido-Giménez C, Soler Z, Muñoz B, Pratcorona L, Rimbaut S, et al. The Effect of Probiotics on Preterm Birth Rates in Pregnant Women After a Threatened Preterm Birth Episode (The PROPEV Trial). Biomedicines. 2025; 13(5):1141. https://doi.org/10.3390/biomedicines13051141
Chicago/Turabian Styledel Barco, Ester, Leidy-Alejandra G. Molano, Mireia Vargas, Marta Miserachs, Linda Puerto, Carmen Garrido-Giménez, Zaida Soler, Begoña Muñoz, Laia Pratcorona, Sonia Rimbaut, and et al. 2025. "The Effect of Probiotics on Preterm Birth Rates in Pregnant Women After a Threatened Preterm Birth Episode (The PROPEV Trial)" Biomedicines 13, no. 5: 1141. https://doi.org/10.3390/biomedicines13051141
APA Styledel Barco, E., Molano, L.-A. G., Vargas, M., Miserachs, M., Puerto, L., Garrido-Giménez, C., Soler, Z., Muñoz, B., Pratcorona, L., Rimbaut, S., Vidal, M., Dalmau, M., Casellas, A., Carreras, E., Manichanh, C., & Goya, M. (2025). The Effect of Probiotics on Preterm Birth Rates in Pregnant Women After a Threatened Preterm Birth Episode (The PROPEV Trial). Biomedicines, 13(5), 1141. https://doi.org/10.3390/biomedicines13051141






