Characteristics of Patients with Newly Diagnosed High-Grade Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer in 2018–2023 and the Impact of Molecular Diagnostics on Chemotherapy in Clinical Practice
Abstract
1. Introduction
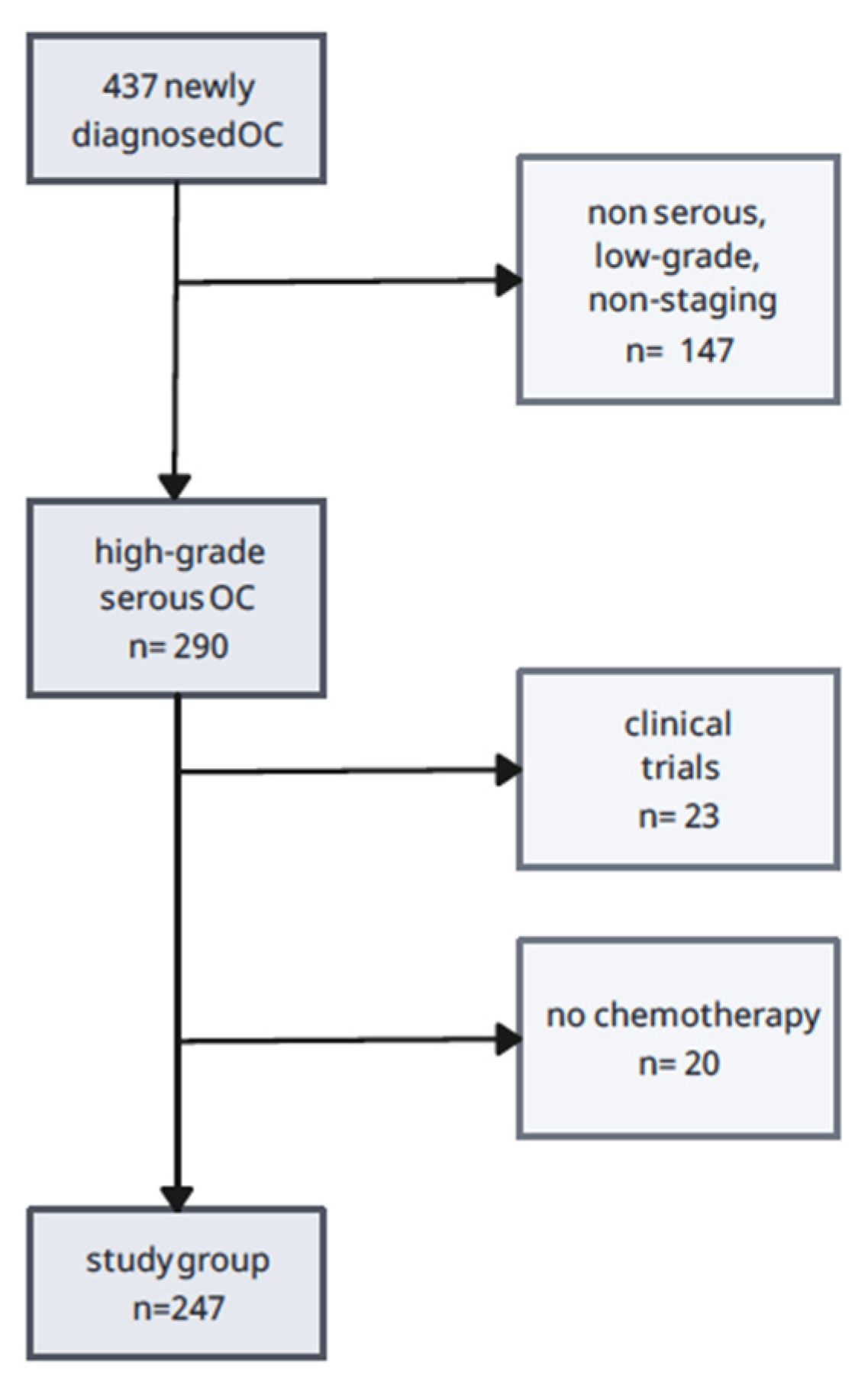
2. Materials and Methods
2.1. Study Design
- (1)
- high-grade ovarian, primary peritoneal, and fallopian tube cancer,
- (2)
- serous histological type,
- (3)
- FIGO stage III, IV.
- (1)
- lack of chemotherapy due to the patient’s overall poor clinical condition or refusal of treatment,
- (2)
- participation in clinical trials using another chemotherapeutic, e.g., pembrolizumab,
- (3)
- incomplete documentation, including the non-staging type of OC.
2.2. Statistical Analysis
3. Results
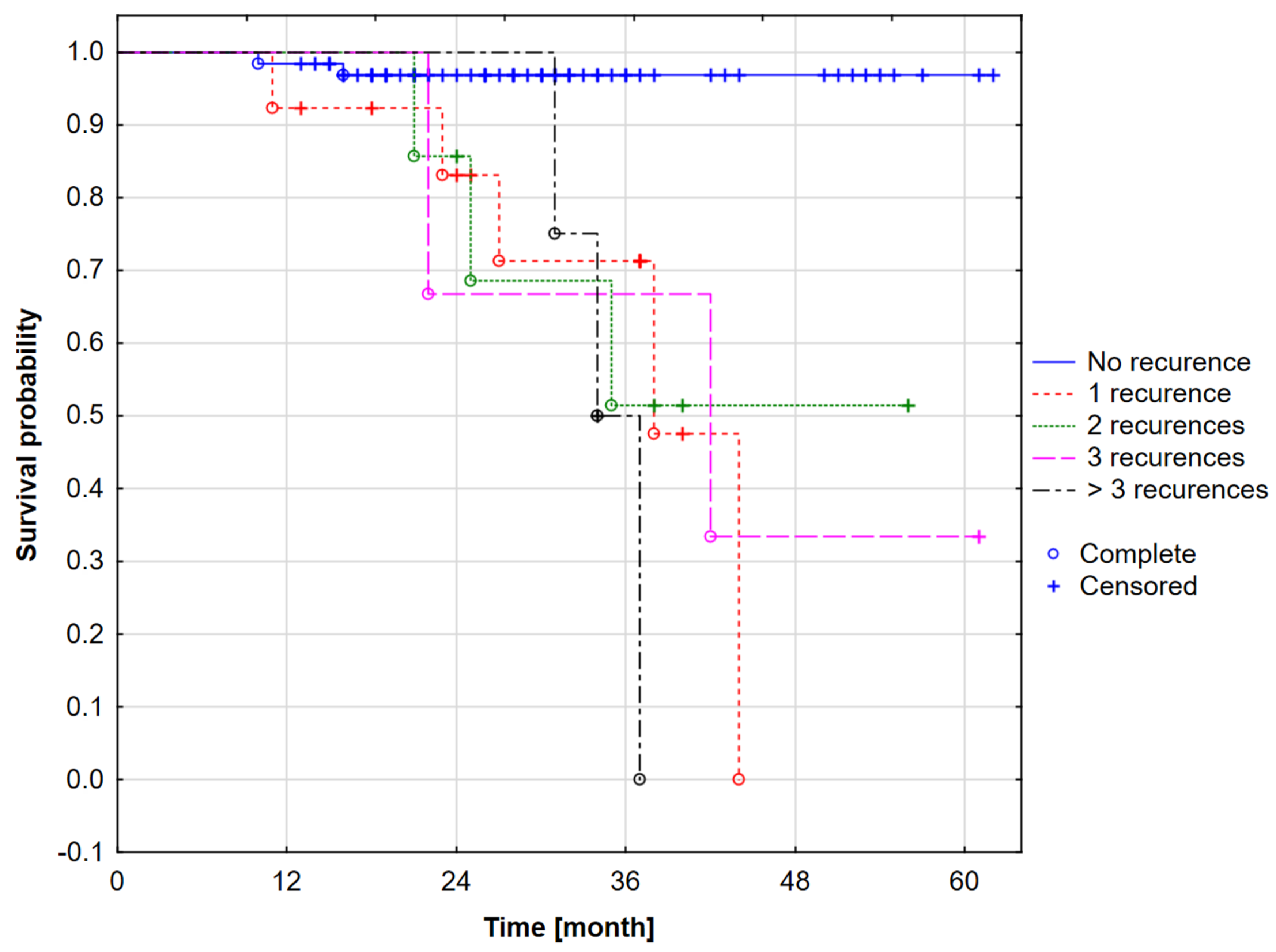
- 25% of patients from the analyzed group died within 27 months (exactly 27.11 months) from the beginning of observation. This means that one-quarter of the patients had a survival time of less than 27 months.
- 50th percentile (median): 50% of patients died within 38 months (37.81 months, to be exact). The median means that half of the patients survived less than 38 months, and the other half lived longer.
- 75th percentile: 75% of patients died within 44 months (43.89 months, to be exact). This means that three-quarters of patients had a survival time of less than 44 months, and only 25% of patients survived beyond this period.
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- The Global Cancer Observatory. GLOBOCAN 2020: International Agency Research on Cancer. Int. Agency Res. Cancer 2020, 509, 336–343. [Google Scholar]
- Ferlay, J.; Colombet, M.; Soerjomataram, I.; Mathers, C.; Parkin, D.M.; Piñeros, M.; Znaor, A.; Bray, F. Estimating the global cancer incidence and mortality in 2018: GLOBOCAN sources and methods. Int. J. Cancer 2019, 144, 1941–1953. [Google Scholar] [CrossRef] [PubMed]
- McGuire, W.P.; Hoskins, W.J.; Brady, M.F.; Kucera, P.R.; Partridge, E.E.; Look, K.Y.; Clarke-Pearson, D.L.; Davidson, M. Cyclophosphamide and Cisplatin Compared with Paclitaxel and Cisplatin in Patients with Stage III and Stage IV Ovarian Cancer. N. Engl. J. Med. 1996, 334, 1–6. [Google Scholar] [CrossRef] [PubMed]
- du Bois, A.; Lück, H.J.; Meier, W.; Adams, H.P.; Mobus, V.; Costa, S.; Bauknecht, T.; Richter, B.; Warm, M.; Schroder, W.; et al. A randomized clinical trial of cisplatin/paclitaxel versus carboplatin/paclitaxel as first-line treatment of ovarian cancer. J. Natl. Cancer Inst. 2003, 95, 1320–1329. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. N. Engl. J. Med. 2011, 365, 2473–2483. [Google Scholar] [CrossRef] [PubMed]
- Oza, A.M.; Cook, A.D.; Pfisterer, J.; Embleton, A.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; et al. Standard chemotherapy with or without bevacizumab for women with newly diagnosed ovarian cancer (ICON7): Overall survival results of a phase 3 randomised trial. Lancet Oncol. 2015, 16, 928–936. [Google Scholar] [CrossRef] [PubMed]
- Tewari, K.S.; Burger, R.A.; Enserro, D.; Norquist, B.M.; Swisher, E.M.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Huang, H.; Homesley, H.D.; et al. Final Overall Survival of a Randomized Trial of Bevacizumab for Primary Treatment of Ovarian Cancer. J. Clin. Oncol. 2019, 37, 2317–2328. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- du Bois, A.; Combe, M.; Rochon, J.; Jackisch, C.; Malaurie, E.; Lueck, H.J.; Loibl, S.; Schroeder, W.; Burges, A.; Weber, B. Epirubicin/paclitaxel/carboplatin (TEC) vs paclitaxel/carboplatin (TC) in first-line treatment of ovarian cancer (OC) FIGO stages IIB–IV. An AGO-GINECO Intergroup phase III trial. J. Clin. Oncol. 2004, 22 (Suppl. 14), 5007. [Google Scholar] [CrossRef]
- Kristensen, G.B.; Vergote, I.; Stuart, G.; del Campo, J.; Kaern, J.; Baekelandt, M.; Lopez, A.; Hirte, H.; Aavall-Lundqvist, E.; Lorenz, E.; et al. First-line treatment of ovarian/tubal/peritoneal cancer FIGO stage IIB-IV with paclitaxel/carboplatin with or without epirubicin (TEC vs TC). A gynecologic cancer intergroup study of the NSGO, EORTC GCG, and NCIC CTG. Results on progression-free survival. Int. J. Gynecol. Cancer 2005, 15, 221. [Google Scholar] [CrossRef]
- Bookman, M.A.; Brady, M.F.; McGuire, W.P.; Harper, P.G.; Alberts, D.S.; Friedlander, M.; Colombo, N.; Fowler, J.M.; Argenta, P.A.; De Geest, K.; et al. Evaluation of new platinum-based treatment regimens in advanced-stage ovarian cancer: A phase III trial of the gynecologic cancer intergroup. J. Clin. Oncol. 2009, 27, 1419–1425. [Google Scholar] [CrossRef]
- Burger, R.A.; Brady, M.F.; Bookman, M.A.; Fleming, G.F.; Monk, B.J.; Huang, H.; Mannel, R.S.; Homesley, H.D.; Fowler, J.; Greer, B.E.; et al. Incorporation of bevacizumab in the primary treatment of ovarian cancer. Obstet. Gynecol. Surv. 2012, 67, 289–290. [Google Scholar] [CrossRef]
- Perren, T.J.; Swart, A.M.; Pfisterer, J.; Ledermann, J.A.; Pujade-Lauraine, E.; Kristensen, G.; Carey, M.S.; Beale, P.; Cervantes, A.; Kurzeder, C.; et al. A Phase 3 Trial of Bevacizumab in Ovarian Cancer. N. Engl. J. Med. 2011, 365, 2484–2496. [Google Scholar] [CrossRef]
- Verheijen, R.H.; Massuger, L.F.; Benigno, B.B.; Epenetos, A.A.; Lopes, A.; Soper, J.T.; Markowska, J.; Vyzula, R.; Jobling, T.; Stamp, G.; et al. Phase III trial of intraperitoneal therapy with yttrium-90-labeled HMFG1 murine monoclonal antibody in patients with epithelial ovarian cancer after a surgically defined complete remission. J. Clin. Oncol. 2006, 24, 571–578. [Google Scholar] [CrossRef]
- Piccart, M.J.; Floquet, A.; Scarfone, G.; Willemse, P.H.B.; Emerich, J.; Vergote, I.; Giurgea, L.; Coens, C.; Awada, A.; Vermorken, J.B. Intraperitoneal cisplatin versus no further treatment: 8-Year results of EORTC 55875, a randomized phase III study in ovarian cancer patients with a pathologically complete remission after platinum-based intravenous chemotherapy. Int. J. Gynecol. Cancer 2003, 13, 196–203. [Google Scholar] [CrossRef] [PubMed]
- Ragupathi, A.; Singh, M.; Perez, A.M.; Zhang, D. Targeting the BRCA1/2 deficient cancer with PARP inhibitors: Clinical outcomes and mechanistic insights. Front. Cell Dev. Biol. 2023, 11, 1133472. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Bryant, H.E.; Schultz, N.; Thomas, H.D.; Parker, K.M.; Flower, D.; Lopez, E.; Kyle, S.; Meuth, M.; Curtin, N.J.; Helleday, T. Specific killing of BRCA2-deficient tumours with inhibitors of poly(ADP-ribose) polymerase. Nature 2005, 434, 913–917. [Google Scholar] [CrossRef] [PubMed]
- Farmer, H.; McCabe, H.; Lord, C.J.; Tutt, A.N.J.; Johnson, D.A.; Richardson, T.B.; Santarosa, M.; Dillon, K.J.; Hickson, I.; Knights, C.; et al. Targeting the DNA repair defect in BRCA mutant cells as a therapeutic strategy. Nature 2005, 434, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Audeh, M.W.; Carmichael, J.; Penson, R.T.; Friedlander, M.; Powell, B.; Bell-McGuinn, K.M.; Scott, C.; Weitzel, J.N.; Oaknin, A.; Loman, N.; et al. Oral poly(ADP-ribose) polymerase inhibitor olaparib in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer: A proof-of-concept trial. Lancet 2010, 376, 245–251. [Google Scholar] [CrossRef] [PubMed]
- Kaye, S.B.; Lubinski, J.; Matulonis, U.; Ang, J.E.; Gourley, C.; Karlan, B.Y.; Amnon, A.; Bell-McGuinn, K.M.; Chen, L.-M.; Friedlander, M.; et al. Phase II, open-label, randomized, multicenter study comparing the efficacy and safety of olaparib, a poly (ADP-ribose) polymerase inhibitor, and pegylated liposomal doxorubicin in patients with BRCA1 or BRCA2 mutations and recurrent ovarian cancer. J. Clin. Oncol. 2012, 30, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.; Colombo, N.; Scambia, G.; Kim, B.-G.; Oaknin, A.; Friedlander, M.; Lisyanskaya, A.; Floquet, A.; Leary, A.; Sonke, G.S.; et al. Maintenance Olaparib in Patients with Newly Diagnosed Advanced Ovarian Cancer. N. Engl. J. Med. 2018, 379, 2495–2505. [Google Scholar] [CrossRef] [PubMed]
- González-Martín, A.; Pothuri, B.; Vergote, I.; Graybill, W.; Lorusso, D.; McCormick, C.C.; Freyer, G.; Backes, F.; Heitz, F.; Redondo, A.; et al. Progression-free survival and safety at 3.5years of follow-up: Results from the randomised phase 3 PRIMA/ENGOT-OV26/GOG-3012 trial of niraparib maintenance treatment in patients with newly diagnosed ovarian cancer. Eur. J. Cancer 2023, 189, 112908. [Google Scholar] [CrossRef] [PubMed]
- Ray-Coquard, I.; Pautier, P.; Pignata, S.; Pérol, D.; González-Martín, A.; Berger, R.; Fujiwara, K.; Vergote, I.; Colombo, N.; Mäenpää, J.; et al. Olaparib plus Bevacizumab as First-Line Maintenance in Ovarian Cancer. N. Engl. J. Med. 2019, 381, 2416–2428. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.F.; Zhang, J.; Zhang, C.; Yu, J.; Shi, Y.; Xu, S.Q.; Fan, Y.; Zhou, F.Z.; Song, S.Q.; Liu, H.; et al. [Real-world clinical data analysis of PARPi as first-line maintenance therapy in newly diagnosed epithelial ovarian cancer patients]. Zhonghua Fu Chan Ke Za Zhi 2022, 57, 641–652. (In Chinese) [Google Scholar] [CrossRef] [PubMed]
- Obwieszczenia Ministra Zdrowia—Lista Leków Refundowanych. Available online: https://www.gov.pl/web/zdrowie/obwieszczenia-ministra-zdrowia-lista-lekow-refundowanych (accessed on 6 July 2024).
- Nguyen, L.; WMMartens, J.; Van Hoeck, A.; Cuppen, E. Pan-cancer landscape of homologous recombination deficiency. Nat. Commun. 2020, 11, 5584. [Google Scholar] [CrossRef]
- Lheureux, S.; Braunstein, M.; Oza, A.M. Epithelial ovarian cancer: Evolution of management in the era of precision medicine. CA Cancer J. Clin. 2019, 69, 280–304. [Google Scholar] [CrossRef] [PubMed]
- Enshaei, A.; Robson, C.; Edmondson, R. Artificial intelligence systems as prognostic and predictive tools in ovarian cancer. Ann. Surg. Oncol. 2015, 22, 3970–3975. [Google Scholar] [CrossRef]
- Zhang, B.; Shi, H.; Wang, H. Machine Learning and AI in Cancer Prognosis, Prediction, and Treatment Selection: A Critical Approach. J. Multidiscip. Health 2023, 16, 1779–1791. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Vergote, I.; Ray-Coquard, I.; Anderson, D.M.; Cantuaria, G.; Colombo, N.; Garnier-Tixidre, C.; Gilbert, L.; Harter, P.; Hettle, R.; Lorusso, D.; et al. Population-adjusted indirect treatment comparison of the SOLO1 and PAOLA-1/ENGOT-ov25 trials evaluating maintenance olaparib or bevacizumab or the combination of both in newly diagnosed, advanced BRCA-mutated ovarian cancer. Eur. J. Cancer 2021, 157, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Millert-Kalińska, S.; Przybylski, M.; Pruski, D.; Stawicka-Niełacna, M.; Mądry, R. Epithelial Ovarian Cancer-Varied Treatment Results. Healthcare 2023, 11, 2043. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Millert-Kalińska, S.; Pruski, D.; Przybylski, M.; Stawicka-Niełacna, M.; Mądry, E.; Mądry, R. High-Volume Hospitals’ Ovarian Cancer Care-Less Individual Approach or Better Treatment Results? Curr. Oncol. 2022, 29, 5278–5294. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]

| Period | I/January 2018–April 2021 (40 Months) | II/May 2021–October 2022 (17 Months) | III/November 2022–December 2023 (13 Months) | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n | % of a Specific Group | % of the Group from the Whole Period | n | % of a Specific Group | % of the Group from the Whole Period | n | % of a Specific Group | % of the Group from the Whole Period | ||||
| BRCAmut | ||||||||||||
| PC | - | - | - | 7 | 24.1% | 6.6% | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% |
| PC | BEV | - | - | 13 | 44.8% | 12.3% | 1 | 4.8% | 1.2% | 3 | 15.0% | 5.0% |
| PC | BEV | OLA | - | 5 | 17.2% | 4.7% | 13 | 61.9% | 16.0% | 10 | 50.0% | 16.7% |
| PC | BEV | - | NIR | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% | 1 | 5.0% | 1.7% |
| PC | - | OLA | - | 4 | 13.9% | 3.8% | 7 | 33.3% | 8.7% | 5 | 25.0% | 8.3% |
| PC | - | - | NIR | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% | 1 | 5.0% | 1.7% |
| 29 | 100.0% | 27.4% | 21 | 100.0% | 25.9% | 20 | 100.0% | 33.4% | ||||
| nonBRCAmut | ||||||||||||
| PC | - | - | - | 21 | 42.9% | 19.9% | 6 | 10.5% | 7.4% | 7 | 20.0% | 11.7% |
| PC | BEV | - | - | 27 | 55.1% | 25.5% | 27 | 47.4% | 33.3% | 13 | 37.1% | 21.7% |
| PC | BEV | OLA | - | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% | 4 | 11.4% | 6.6% |
| PC | BEV | - | NIR | 0 | 0.0% | 0.0% | 9 | 15.8% | 11.1% | 3 | 8.6% | 5.0% |
| PC | - | OLA | - | 1 | 2.0% | 0.9% | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% |
| PC | - | - | NIR | 0 | 0.0% | 0.0% | 15 | 26.3% | 18.6% | 8 | 22.9% | 13.3% |
| 49 | 100.0% | 46.3% | 57 | 100.0% | 70.4% | 35 | 100.0% | 58.3% | ||||
| no data | ||||||||||||
| PC | - | - | - | 15 | 53.6% | 14.1% | 2 | 66.7% | 2.5% | 2 | 40.0% | 3.3% |
| PC | BEV | - | - | 12 | 42.9% | 11.3% | 1 | 33.3% | 1.2% | 3 | 60.0% | 5.0% |
| PC | BEV | OLA | - | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% |
| PC | BEV | - | NIR | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% |
| PC | - | OLA | - | 1 | 3.5% | 0.9% | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% |
| PC | - | - | NIR | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% | 0 | 0.0% | 0.0% |
| 28 | 100.0% | 26.3% | 3 | 100.0% | 3.7% | 5 | 100.0% | 8.3% | ||||
| total | 106 | 81 | 60 | |||||||||
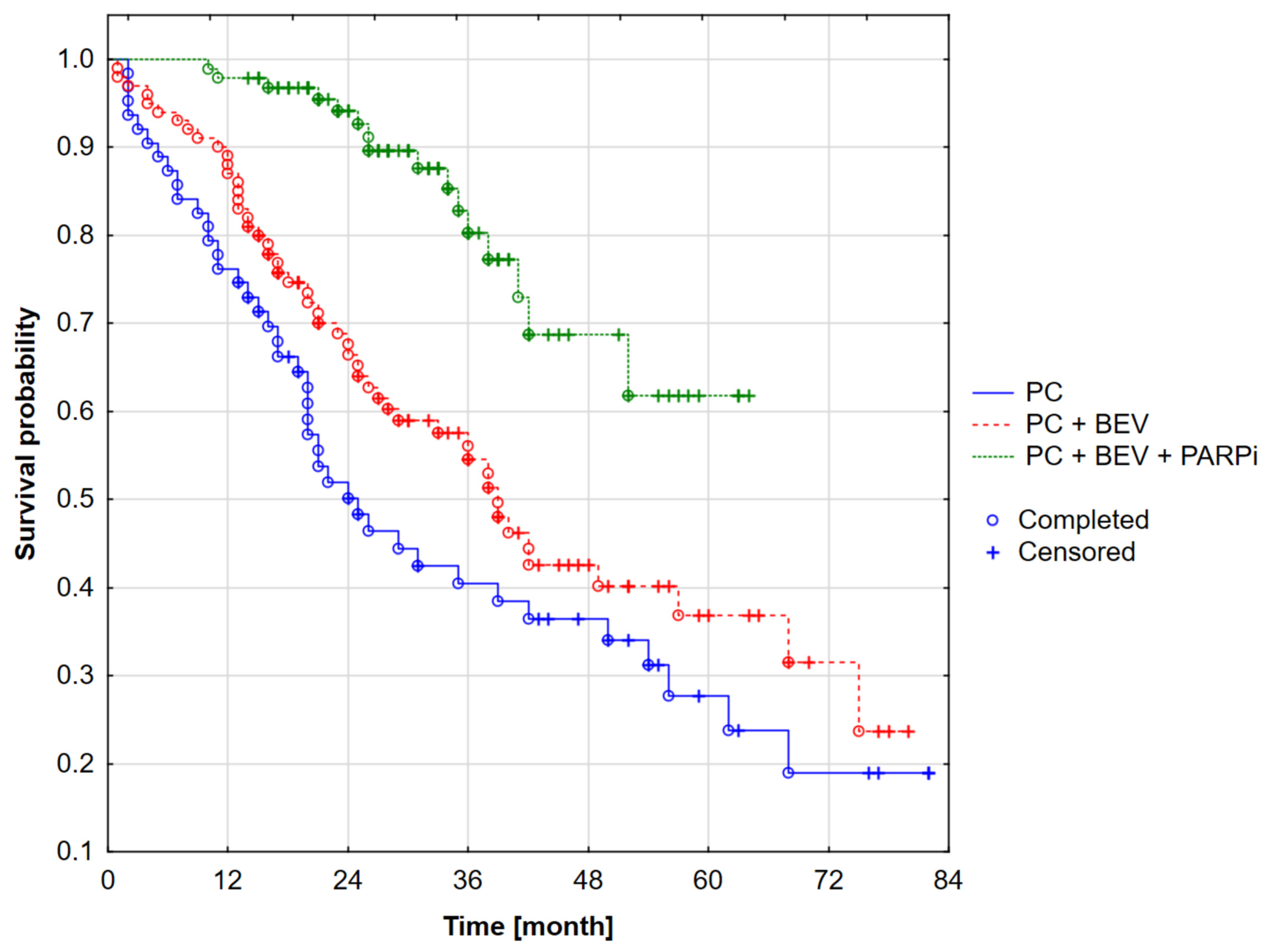
| BRCA Status | Period I | Period II | Period III | All | χ2 | p | Vc | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | % | N | % | N | % | N | % | |||||
| BRCAmut | PC | 7 | 24.1% | 0 | 0.0% | 0 | 0.0% | 7 | 10.0% | 26.86 * | <0.001 | 0.45 |
| PC + BEV | 13 | 44.8% | 1 | 4.8% | 3 | 15.0% | 17 | 24.3% | ||||
| PC + PARPi | 4 | 13.8% | 7 | 33.3% | 6 | 30.0% | 17 | 24.3% | ||||
| PC + BEV + PARPi | 5 | 17.3% | 13 | 61.9% | 11 | 55.0% | 29 | 41.4% | ||||
| all | 29 | 100.0% | 21 | 100.0% | 20 | 100.0% | 70 | 100.0% | ||||
| PARPi together | 9 | 31.0% | 20 | 95.2% | 17 | 85.0% | ||||||
| PC | 21 | 42.9% | 6 | 10.5% | 7 | 20.0% | 34 | 24.1% | 36.13 * | <0.001 | 0.34 | |
| PC + BEV | 27 | 55.1% | 27 | 47.4% | 13 | 37.1% | 67 | 47.5% | ||||
| non-BRCA-mut | PC + PARPi | 1 | 2.0% | 15 | 26.3% | 8 | 22.9% | 24 | 17.0% | |||
| PC + BEV + PARPi | 0 | 0.0% | 9 | 15.8% | 7 | 20.0% | 16 | 11.4% | ||||
| all | 49 | 100.0% | 57 | 100.0% | 35 | 100.0% | 141 | 100.0% | ||||
| PARPi together | 1 | 2.0% | 24 | 42.1% | 15 | 42.9% | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Millert-Kalińska, S.; Stawicka-Niełacna, M.; Tomczak, L.; Pruski, D.; Przybylski, M.; Jaszczyńska-Nowinka, K.; Poprawski, G.; Mądry, R. Characteristics of Patients with Newly Diagnosed High-Grade Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer in 2018–2023 and the Impact of Molecular Diagnostics on Chemotherapy in Clinical Practice. Biomedicines 2025, 13, 483. https://doi.org/10.3390/biomedicines13020483
Millert-Kalińska S, Stawicka-Niełacna M, Tomczak L, Pruski D, Przybylski M, Jaszczyńska-Nowinka K, Poprawski G, Mądry R. Characteristics of Patients with Newly Diagnosed High-Grade Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer in 2018–2023 and the Impact of Molecular Diagnostics on Chemotherapy in Clinical Practice. Biomedicines. 2025; 13(2):483. https://doi.org/10.3390/biomedicines13020483
Chicago/Turabian StyleMillert-Kalińska, Sonja, Małgorzata Stawicka-Niełacna, Lidia Tomczak, Dominik Pruski, Marcin Przybylski, Karolina Jaszczyńska-Nowinka, Grzegorz Poprawski, and Radosław Mądry. 2025. "Characteristics of Patients with Newly Diagnosed High-Grade Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer in 2018–2023 and the Impact of Molecular Diagnostics on Chemotherapy in Clinical Practice" Biomedicines 13, no. 2: 483. https://doi.org/10.3390/biomedicines13020483
APA StyleMillert-Kalińska, S., Stawicka-Niełacna, M., Tomczak, L., Pruski, D., Przybylski, M., Jaszczyńska-Nowinka, K., Poprawski, G., & Mądry, R. (2025). Characteristics of Patients with Newly Diagnosed High-Grade Advanced Ovarian, Fallopian Tube, and Primary Peritoneal Cancer in 2018–2023 and the Impact of Molecular Diagnostics on Chemotherapy in Clinical Practice. Biomedicines, 13(2), 483. https://doi.org/10.3390/biomedicines13020483








