SIRT1 Mediates the Effects of Sera from Athletes Who Engage in Aerobic Exercise Training in Activating Cells for Wound Healing
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethical Approval
2.2. The Study Population
2.3. Blood Samples
2.4. Cell Cultures and Treatments
2.5. Western Blotting
2.6. In Vitro Wound Healing and Invasion Assays
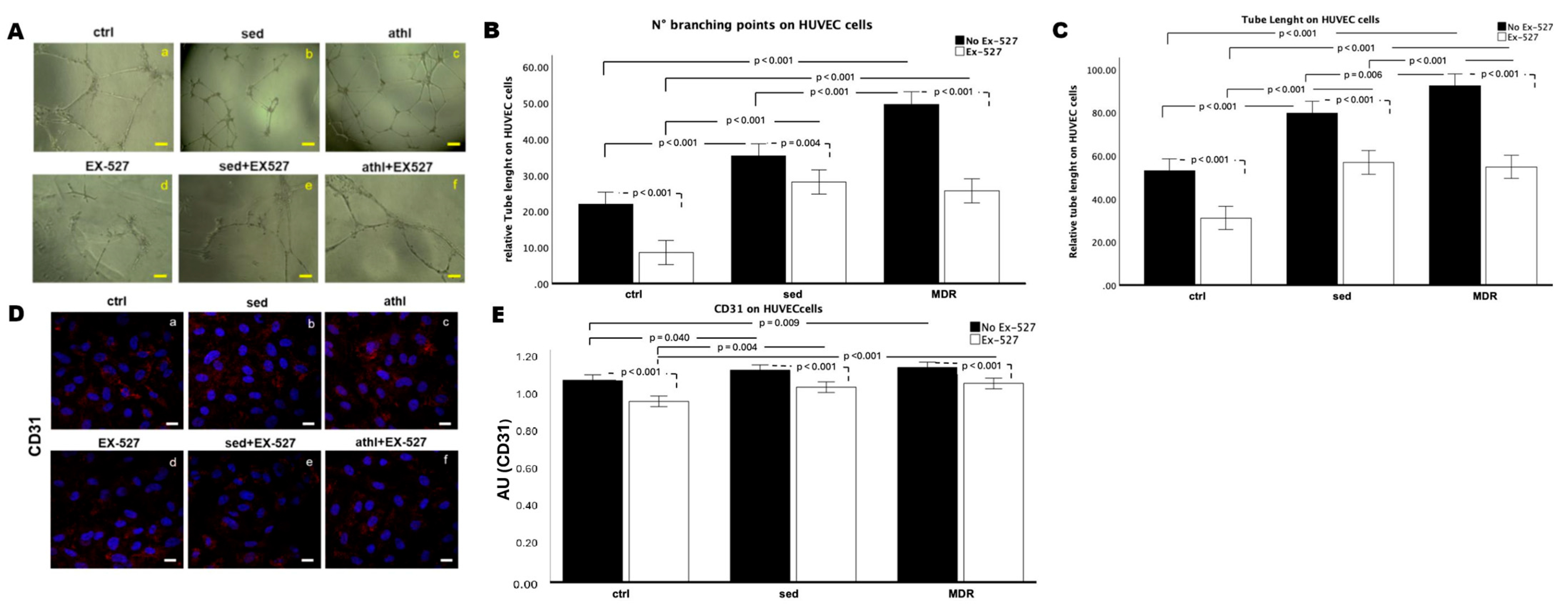
2.7. The Tube Formation Assay
2.8. Confocal Microscopy
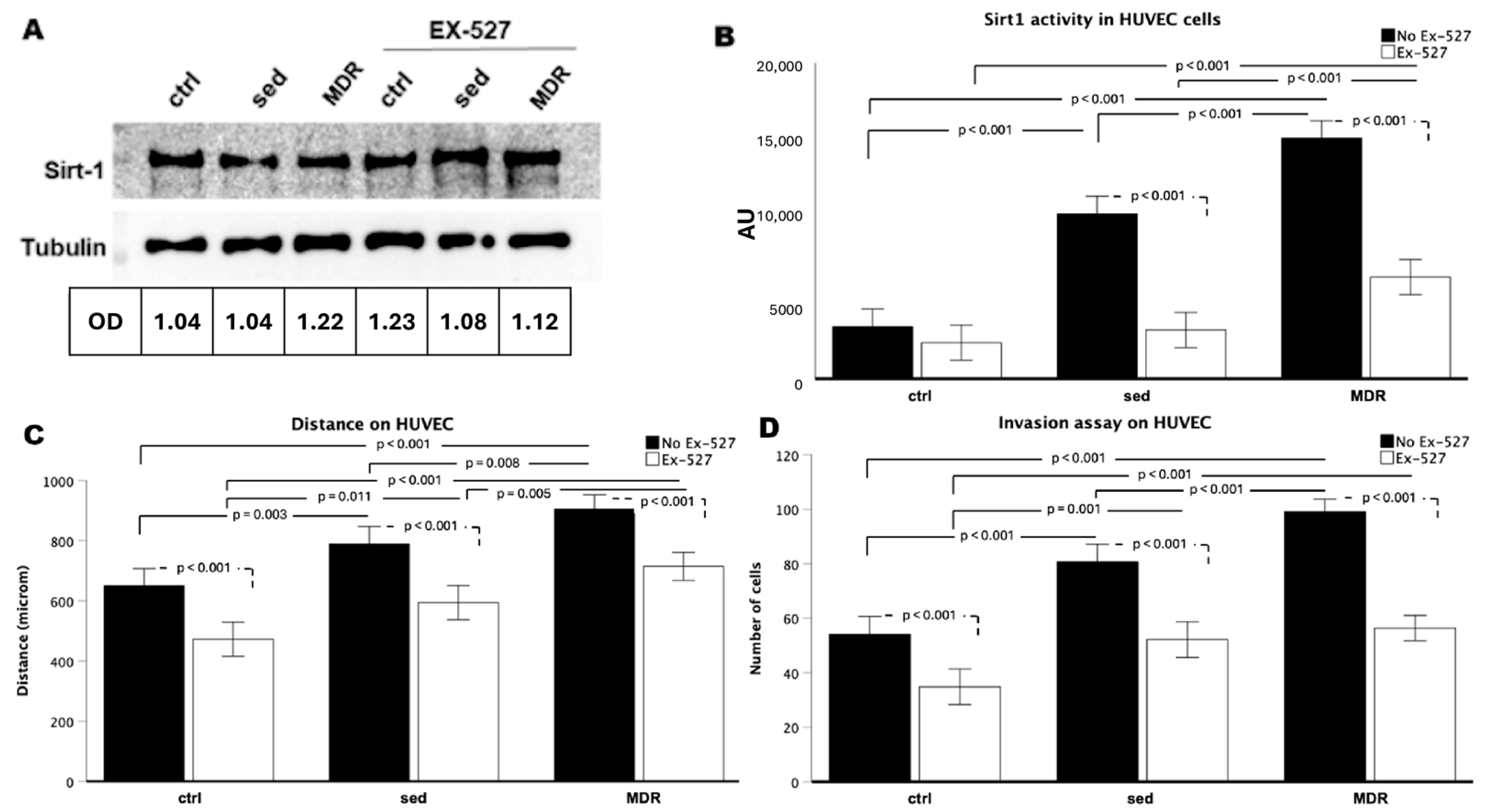
2.9. SIRT1 Activity
2.10. Statistical Analysis
3. Results
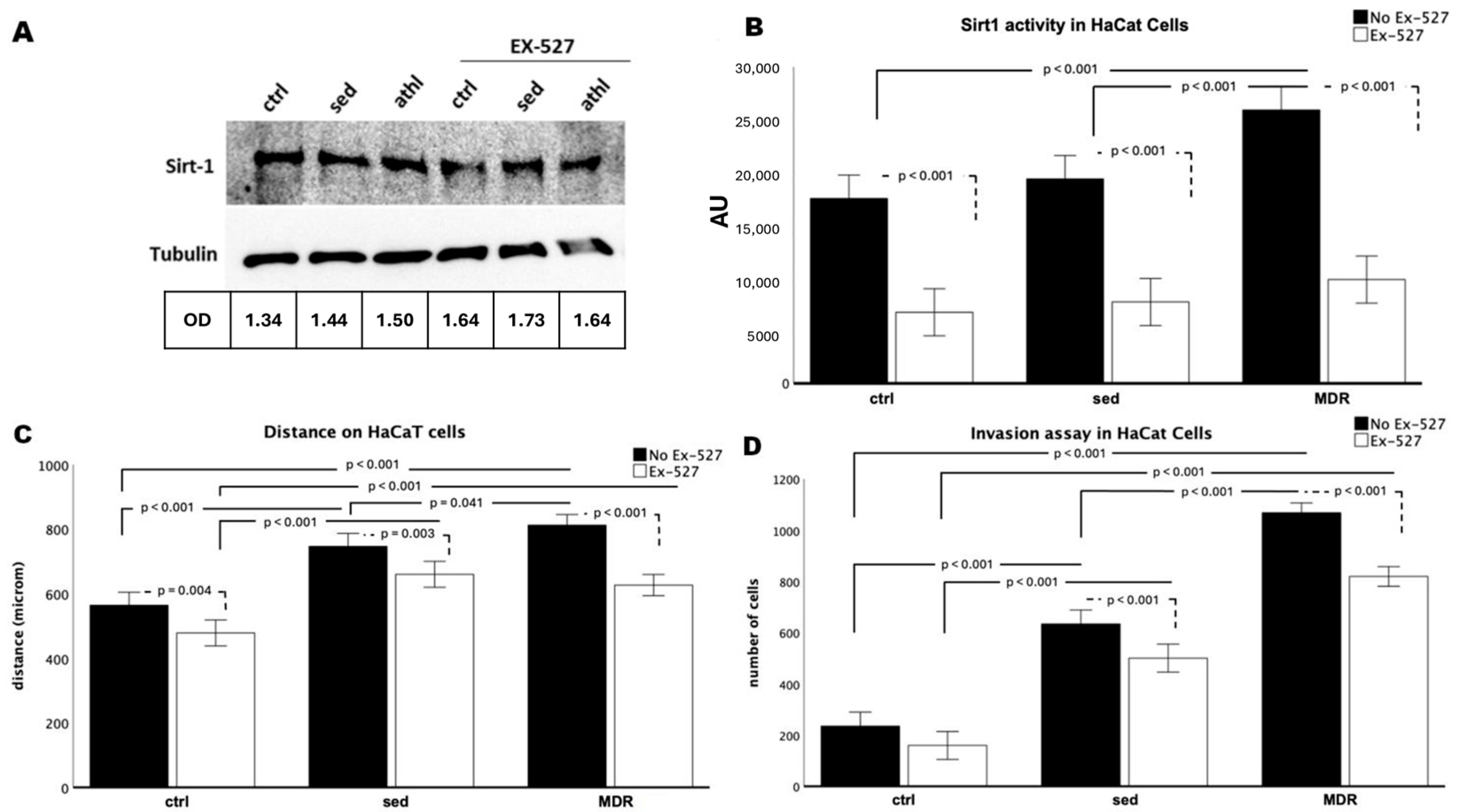
3.1. The MDR Group Sera Increased the Migration and Invasion of the HaCaT Cells by Activating SIRT1
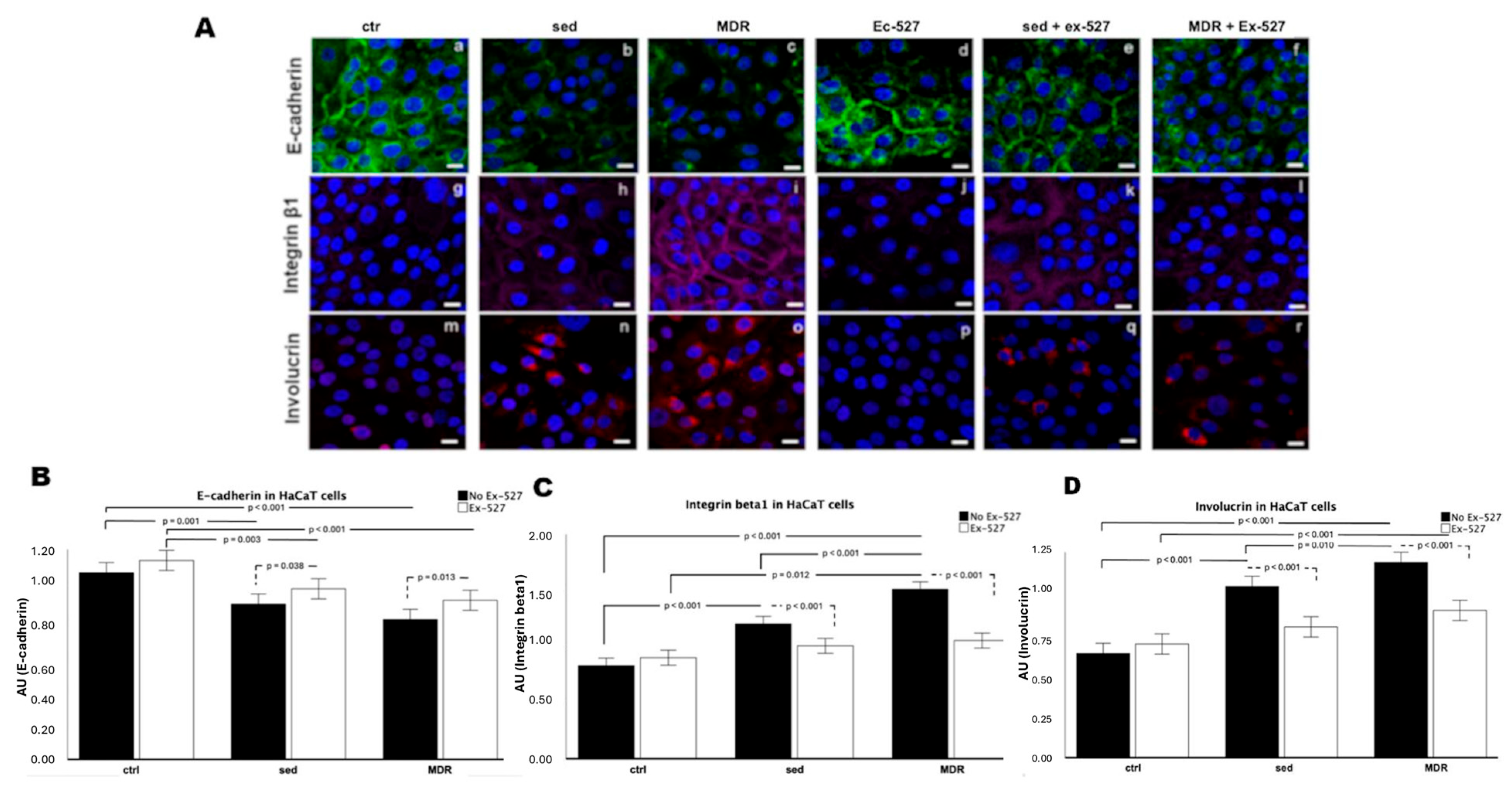
3.2. The Levels of Motility and Differentiation Markers in the Human Keratinocytes Were Reversed in the Presence of the Selective SIRT1 Inhibitor
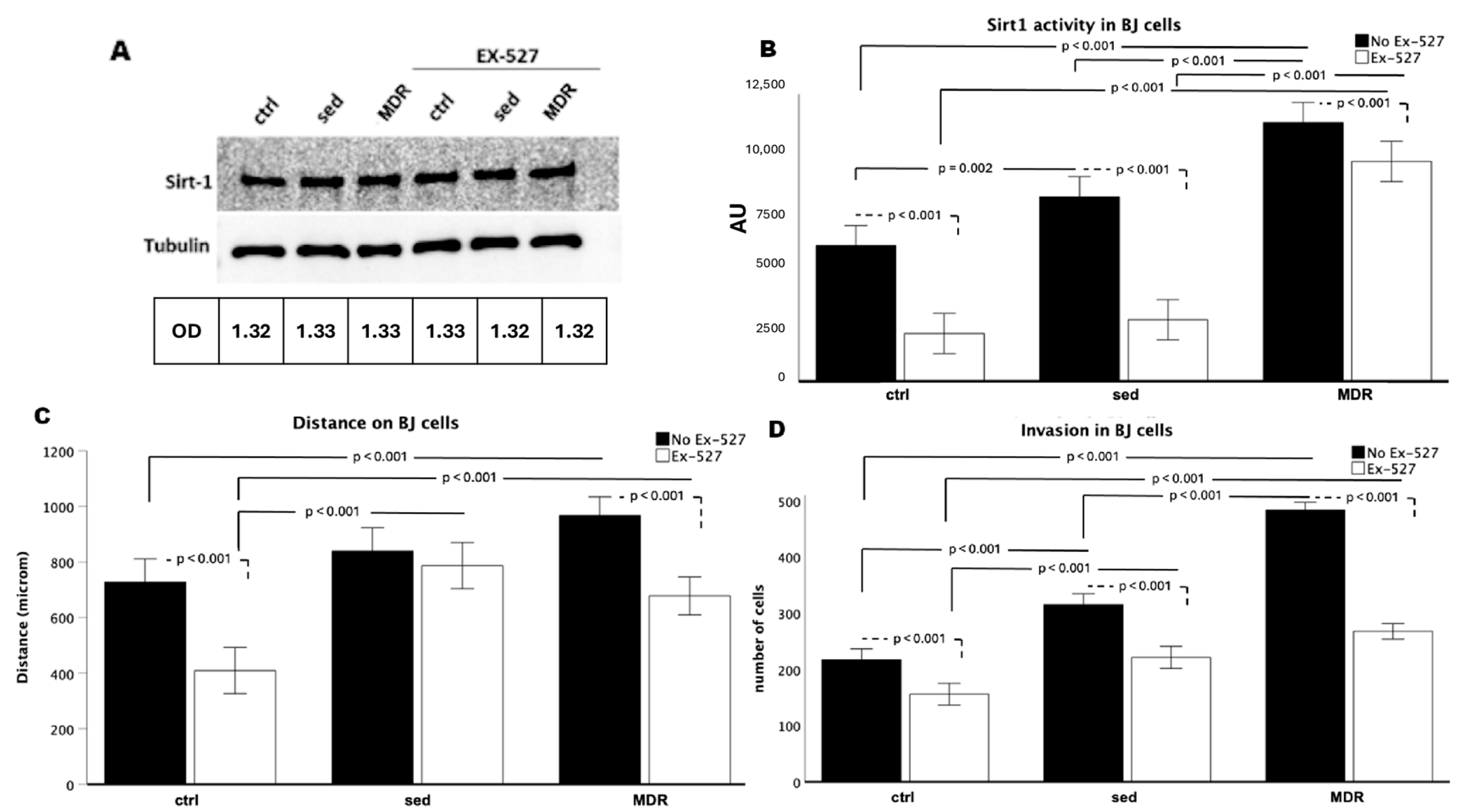
3.3. Human Fibroblasts Acquired a Higher Rate of Migration and Invasion Speed When Treated with the MDR Group Sera, Depending on the Activity of SIRT1
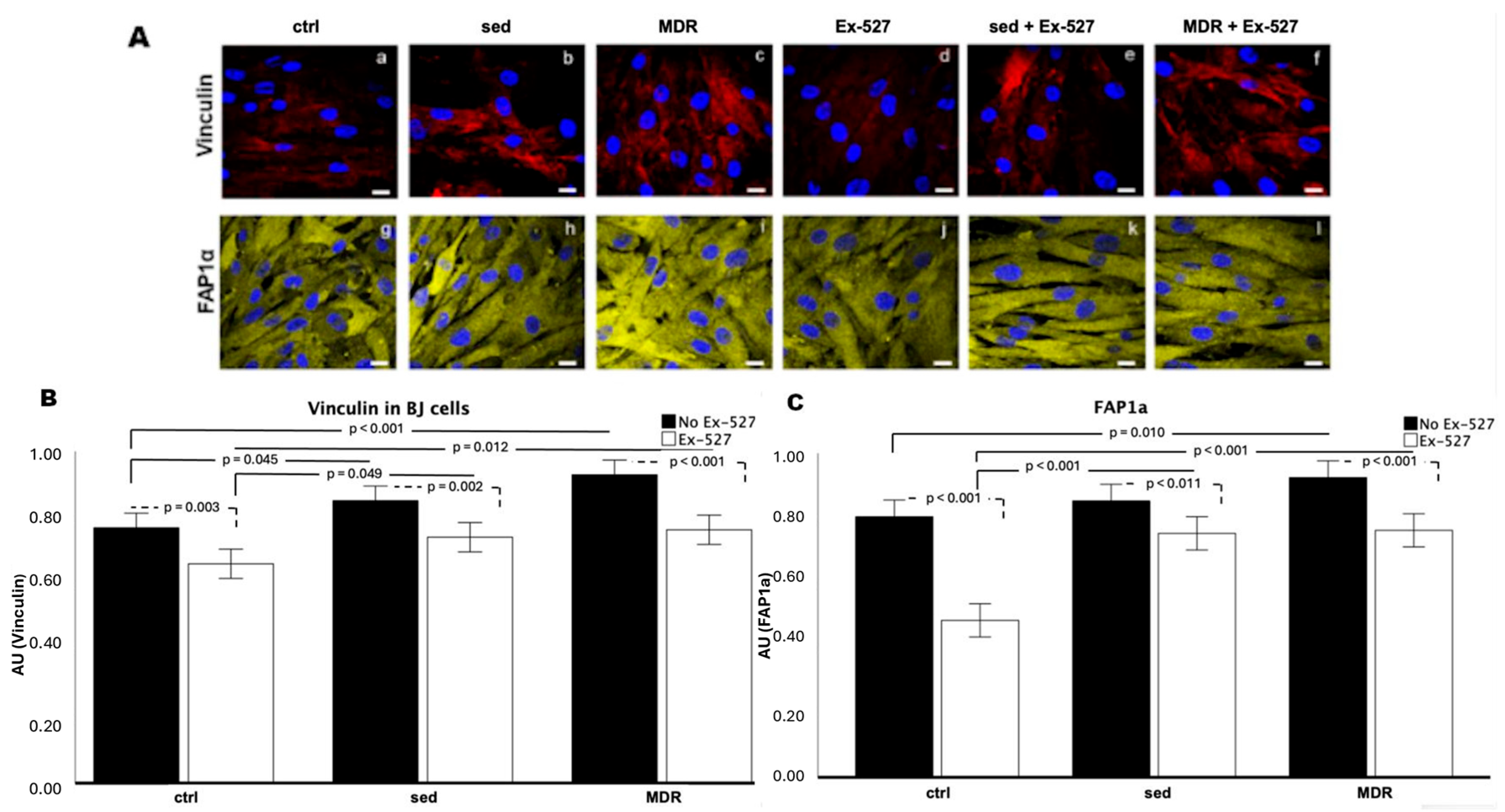
3.4. BJ Cell Activation Is Promoted by the MDR Group Sera and Inhibited by EX-527
3.5. Motility of Human Endothelial Cells Is Enhanced in the Presence of MDR Sera if SIRT1 Is Not Inhibited
3.6. In Vitro Angiogenesis and CD31 Expression Are Amplified by MDR Group Sera but Not in the Presence of EX-527
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ET | exercise training |
| NAD+ | nicotinamide adenine dinucleotide |
| SIRT1 | sirtuin 1 |
| MDR | middle-distance running |
| sed | sedentary volunteers |
| HUVEC | human umbilical vein endothelial cells |
| ctrl | control |
| BIO-RAD | Bio-Rad Dye Reagent Concentrate |
| BSA | bovine serum albumin |
| SD | standard deviation |
References
- Annibalini, G.; Contarelli, S.; Lucertini, F.; Guescini, M.; Maggio, S.; Ceccaroli, P.; Gervasi, M.; Ferri Marini, C.; Fardetti, F.; Grassi, E.; et al. Muscle and Systemic Molecular Responses to a Single Flywheel Based Iso-Inertial Training Session in Resistance-Trained Men. Front. Physiol. 2019, 10, 554. [Google Scholar] [CrossRef] [PubMed]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand J. Med. Sci. Sports 2015, 25 (Suppl. S3), 1–72. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhou, R.; Feng, Y.; Cheng, L. Molecular mechanisms of exercise contributing to tissue regeneration. Signal Transduct. Target. Ther. 2022, 7, 383. [Google Scholar] [CrossRef] [PubMed]
- Emery, C.F.; Kiecolt-Glaser, J.K.; Glaser, R.; Malarkey, W.B.; Frid, D.J. Exercise accelerates wound healing among healthy older adults: A preliminary investigation. J. Gerontol. A Biol. Sci. Med. Sci. 2005, 60, 1432–1436. [Google Scholar] [CrossRef]
- Smith, D.; Lane, R.; McGinnes, R.; O’Brien, J.; Johnston, R.; Bugeja, L.; Team, V.; Weller, C. What is the effect of exercise on wound healing in patients with venous leg ulcers? A systematic review. Int. Wound J. 2018, 15, 441–453. [Google Scholar] [CrossRef]
- Walzik, D.; Jonas, W.; Joisten, N.; Belen, S.; Wüst, R.C.I.; Guillemin, G.; Zimmer, P. Tissue-specific effects of exercise as NAD+-boosting strategy: Current knowledge and future perspectives. Acta Physiol. 2023, 237, e13921. [Google Scholar] [CrossRef]
- White, A.T.; Schenk, S. NAD(+)/NADH and skeletal muscle mitochondrial adaptations to exercise. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E308–E321. [Google Scholar] [CrossRef]
- Chen, M.; Tan, J.; Jin, Z.; Jiang, T.; Wu, J.; Yu, X. Research progress on Sirtuins (SIRTs) family modulators. Biomed. Pharmacother. 2024, 174, 116481. [Google Scholar] [CrossRef]
- Russomanno, G.; Corbi, G.; Manzo, V.; Ferrara, N.; Rengo, G.; Puca, A.A.; Latte, S.; Carrizzo, A.; Calabrese, M.C.; Andriantsitohaina, R.; et al. The anti-ageing molecule sirt1 mediates beneficial effects of cardiac rehabilitation. Immun. Ageing 2017, 14, 7. [Google Scholar] [CrossRef]
- Conti, V.; Corbi, G.; Polito, M.V.; Ciccarelli, M.; Manzo, V.; Torsiello, M.; De Bellis, E.; D’Auria, F.; Vitulano, G.; Piscione, F.; et al. Sirt1 Activity in PBMCs as a Biomarker of Different Heart Failure Phenotypes. Biomolecules 2020, 10, 1590. [Google Scholar] [CrossRef]
- Juan, C.G.; Matchett, K.B.; Davison, G.W. A systematic review and meta-analysis of the SIRT1 response to exercise. Sci. Rep. 2023, 13, 14752. [Google Scholar] [CrossRef] [PubMed]
- Serravallo, M.; Jagdeo, J.; Glick, S.A.; Siegel, D.M.; Brody, N.I. Sirtuins in dermatology: Applications for future research and therapeutics. Arch. Dermatol. Res. 2013, 305, 269–282. [Google Scholar] [CrossRef]
- Su, S.; Ndiaye, M.; Singh, C.K.; Ahmad, N. Mitochondrial Sirtuins in Skin and Skin Cancers. Photochem. Photobiol. 2020, 96, 973–980. [Google Scholar] [CrossRef]
- Qiang, L.; Sample, A.; Liu, H.; Wu, X.; He, Y.Y. Epidermal SIRT1 regulates inflammation, cell migration, and wound healing. Sci. Rep. 2017, 7, 14110. [Google Scholar] [CrossRef] [PubMed]
- Wahedi, H.M.; Chae, J.K.; Subedi, L.; Kang, M.C.; Cho, H.; Kim, S.; Kim, S.Y. NED416, a novel synthetic Sirt1 activator, promotes cutaneous wound healing via the MAPK/Rho pathway. Int. J. Mol. Med. 2020, 46, 149–158. [Google Scholar] [CrossRef]
- Słuczanowska-Głabowska, S.; Salmanowicz, M.; Staniszewska, M.; Pawlik, A. The Role of Sirtuins in the Pathogenesis of Psoriasis. Int. J. Mol. Sci. 2023, 24, 10782. [Google Scholar] [CrossRef]
- Zhang, P.; He, L.; Zhang, J.; Mei, X.; Zhang, Y.; Tian, H.; Chen, Z. Preparation of novel berberine nano-colloids for improving wound healing of diabetic rats by acting Sirt1/NF-κB pathway. Colloids Surf. B Biointerfaces 2020, 187, 110647. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.M.; Mathes, S.C.; Mahajan, A.S.; Rohan, C.A.; Travers, J.B.; Thyagarajan, A. The role of sirtuins in dermal fibroblast function. Front. Med. 2023, 10, 1021908. [Google Scholar] [CrossRef] [PubMed]
- Christovam, A.C.; Theodoro, V.; Mendonça, F.A.S.; Esquisatto, M.A.M.; Dos Santos, G.M.T.; do Amaral, M.E.C. Activators of SIRT1 in wound repair: An animal model study. Arch. Dermatol. Res. 2019, 311, 193–201. [Google Scholar] [CrossRef]
- Belvedere, R.; Bizzarro, V.; Parente, L.; Petrella, F.; Petrella, A. Effects of Prisma® Skin dermal regeneration device containing glycosaminoglycans on human keratinocytes and fibroblasts. Cell Adhes. Migr. 2018, 12, 168–183. [Google Scholar] [CrossRef]
- Belvedere, R.; Novizio, N.; Pessolano, E.; Tosco, A.; Eletto, D.; Porta, A.; Campiglia, P.; Perretti, M.; Filippelli, A.; Petrella, A. Heparan sulfate binds the extracellular Annexin A1 and blocks its effects on pancreatic cancer cells. Biochem. Pharmacol. 2020, 182, 114252. [Google Scholar] [CrossRef] [PubMed]
- Conti, V.; Corbi, G.; Russomanno, G.; Simeon, V.; Ferrara, N.; Filippelli, W.; Limongelli, F.; Canonico, R.; Grasso, C.; Stiuso, P.; et al. Oxidative stress effects on endothelial cells treated with different athletes’ sera. Med. Sci. Sports Exerc. 2012, 44, 39–49. [Google Scholar] [CrossRef]
- Kim, B.S.; Lee, C.H.; Chang, G.E.; Cheong, E.; Shin, I. A potent and selective small molecule inhibitor of sirtuin 1 promotes differentiation of pluripotent P19 cells into functional neurons. Sci. Rep. 2016, 6, 34324. [Google Scholar] [CrossRef] [PubMed]
- Belvedere, R.; Novizio, N.; Palazzo, M.; Pessolano, E.; Petrella, A. The pro-healing effects of heparan sulfate and growth factors are enhanced by the heparinase enzyme: New association for skin wound healing treatment. Eur. J. Pharmacol. 2023, 960, 176138. [Google Scholar] [CrossRef] [PubMed]
- Grose, R.; Hutter, C.; Bloch, W.; Thorey, I.; Watt, F.M.; Fässler, R.; Brakebusch, C.; Werner, S. A crucial role of beta 1 integrins for keratinocyte migration in vitro and during cutaneous wound repair. Development 2002, 129, 2303–2315. [Google Scholar] [CrossRef]
- Li, E.R.; Owens, D.M.; Djian, P.; Watt, F.M. Expression of involucrin in normal, hyperproliferative and neoplastic mouse keratinocytes. Exp. Dermatol. 2000, 9, 431–438. [Google Scholar] [CrossRef]
- Bays, J.L.; DeMali, K.A. Vinculin in cell-cell and cell-matrix adhesions. Cell. Mol. Life Sci. 2017, 74, 2999–3009. [Google Scholar] [CrossRef]
- Kelly, T.; Huang, Y.; Simms, A.E.; Mazur, A. Fibroblast activation protein-α: A key modulator of the microenvironment in multiple pathologies. Int. Rev. Cell. Mol. Biol. 2012, 297, 83–116. [Google Scholar]
- Belvedere, R.; Novizio, N.; Morello, S.; Petrella, A. The combination of mesoglycan and VEGF promotes skin wound repair by enhancing the activation of endothelial cells and fibroblasts and their cross-talk. Sci. Rep. 2022, 12, 11041. [Google Scholar] [CrossRef]
- Corbi, G.; Conti, V.; Troisi, J.; Colucci, A.; Manzo, V.; Di Pietro, P.; Calabrese, M.C.; Carrizzo, A.; Vecchione, C.; Ferrara, N.; et al. Cardiac Rehabilitation Increases SIRT1 Activity and β-Hydroxybutyrate Levels and Decreases Oxidative Stress in Patients with HF with Preserved Ejection Fraction. Oxidative Med. Cell. Longev. 2019, 2019, 7049237. [Google Scholar] [CrossRef]
- Augustine, R.; Hasan, A.; Patan, N.K.; Dalvi, Y.B.; Varghese, R.; Antony, A.; Unni, R.N.; Sandhyarani, N.; Moustafa, A.A. Cerium Oxide Nanoparticle Incorporated Electrospun Poly(3-hydroxybutyrate-co-3-hydroxyvalerate) Membranes for Diabetic Wound Healing Applications. ACS Biomater. Sci. Eng. 2020, 6, 58–70. [Google Scholar] [CrossRef]
- Nasiri, S.S.; Ahmadi, Z.; Afshar-Taromi, F. Design and characterization of Poly(glycerol sebacate)/Poly(3-hydroxybutyrate)/bioglass/curcumin nanocomposite scaffold for wound healing application. Int. J. Biol. Macromol. 2023, 245, 125521. [Google Scholar] [CrossRef]
- Anderson, K.A.; Madsen, A.S.; Olsen, C.A.; Hirschey, M.D. Metabolic control by sirtuins and other enzymes that sense NAD+, NADH, or their ratio. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 991–998. [Google Scholar] [CrossRef]
- Cerutti, R.; Pirinen, E.; Lamperti, C.; Marchet, S.; Sauve, A.A.; Li, W.; Leoni, V.; Schon, E.A.; Dantzer, F.; Auwerx, J.; et al. NAD(+)-dependent activation of Sirt1 corrects the phenotype in a mouse model of mitochondrial disease. Cell Metab. 2014, 19, 1042–1049. [Google Scholar] [CrossRef]
- Ji, L.L.; Yeo, D. Maintenance of NAD+ Homeostasis in Skeletal Muscle during Aging and Exercise. Cells 2022, 11, 710. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Wu, G.; Han, F.; Wang, K.; Bai, X.; Jia, Y.; Li, Z.; Cai, W.; Zhang, W.; Su, L.; et al. SIRT1 activation promotes angiogenesis in diabetic wounds by protecting endothelial cells against oxidative stress. Arch. Biochem. Biophys. 2019, 661, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.M.; Fernandez, A.F.; Fraga, M.F. Role of sirtuins in stem cell differentiation. Genes Cancer 2013, 4, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Hisahara, S.; Chiba, S.; Matsumoto, H.; Tanno, M.; Yagi, H.; Shimohama, S.; Sato, M.; Horio, Y. Histone deacetylase SIRT1 modulates neuronal differentiation by its nuclear translocation. Proc. Natl. Acad. Sci. USA 2008, 105, 15599–15604. [Google Scholar] [CrossRef]
- Saini, A.; Al-Shanti, N.; Sharples, A.P.; Stewart, C.E. Sirtuin 1 regulates skeletal myoblast survival and enhances differentiation in the presence of resveratrol. Exp. Physiol. 2012, 97, 400–418. [Google Scholar] [CrossRef]
- Buhrmann, C.; Busch, F.; Shayan, P.; Shakibaei, M. Sirtuin-1 (SIRT1) is required for promoting chondrogenic differentiation of mesenchymal stem cells. J. Biol. Chem. 2014, 289, 22048–22062. [Google Scholar] [CrossRef]
- Koltai, E.; Szabo, Z.; Atalay, M.; Boldogh, I.; Naito, H.; Goto, S.; Nyakas, C.; Radak, Z. Exercise alters SIRT1, SIRT6, NAD and NAMPT levels in skeletal muscle of aged rats. Mech. Ageing Dev. 2010, 131, 21–28. [Google Scholar] [CrossRef] [PubMed]
- Lamb, D.A.; Moore, J.H.; Mesquita, P.H.C.; Smith, M.A.; Vann, C.G.; Osburn, S.C.; Fox, C.D.; Lopez, H.L.; Ziegenfuss, T.N.; Huggins, K.W.; et al. Resistance training increases muscle NAD+ and NADH concentrations as well as NAMPT protein levels and global sirtuin activity in middle-aged, overweight, untrained individuals. Aging 2020, 12, 9447–9460. [Google Scholar] [CrossRef] [PubMed]
- Gurd, B.J.; Perry, C.G.; Heigenhauser, G.J.; Spriet, L.L.; Bonen, A. High-intensity interval training increases SIRT1 activity in human skeletal muscle. Appl. Physiol. Nutr. Metab. 2010, 35, 350–357. [Google Scholar] [CrossRef] [PubMed]
| MDR (N = 23) | SED (N = 12) | p-Value | |
|---|---|---|---|
| Age, years (mean ± SD) | 55.35 ± 7.21 | 49.83 ± 9.75 | 0.07 |
| Height (m) | 1.72 | 1.67 | 0.12 |
| Weight (kg) | 69.17 | 77.75 | 0.033 |
| BMI (mean ± SD) | 23.47 ± 2.37 | 27.28 ± 3.77 | 0.00083 |
| Weekly frequency of training, times/week (mean ± SD) | 3.32 ± 0.89 | - | - |
| Kilometers travelled per week (mean ± SD) | 38.21 ± 16 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Belvedere, R.; Novizio, N.; Stefanelli, B.; Sellitto, C.; Palazzo, M.; Trucillo, M.; De Luca, A.; De Bellis, E.; Corbi, G.; Filippelli, A.; et al. SIRT1 Mediates the Effects of Sera from Athletes Who Engage in Aerobic Exercise Training in Activating Cells for Wound Healing. Biomedicines 2025, 13, 1041. https://doi.org/10.3390/biomedicines13051041
Belvedere R, Novizio N, Stefanelli B, Sellitto C, Palazzo M, Trucillo M, De Luca A, De Bellis E, Corbi G, Filippelli A, et al. SIRT1 Mediates the Effects of Sera from Athletes Who Engage in Aerobic Exercise Training in Activating Cells for Wound Healing. Biomedicines. 2025; 13(5):1041. https://doi.org/10.3390/biomedicines13051041
Chicago/Turabian StyleBelvedere, Raffaella, Nunzia Novizio, Berenice Stefanelli, Carmine Sellitto, Mariangela Palazzo, Marta Trucillo, Antonio De Luca, Emanuela De Bellis, Graziamaria Corbi, Amelia Filippelli, and et al. 2025. "SIRT1 Mediates the Effects of Sera from Athletes Who Engage in Aerobic Exercise Training in Activating Cells for Wound Healing" Biomedicines 13, no. 5: 1041. https://doi.org/10.3390/biomedicines13051041
APA StyleBelvedere, R., Novizio, N., Stefanelli, B., Sellitto, C., Palazzo, M., Trucillo, M., De Luca, A., De Bellis, E., Corbi, G., Filippelli, A., Conti, V., & Petrella, A. (2025). SIRT1 Mediates the Effects of Sera from Athletes Who Engage in Aerobic Exercise Training in Activating Cells for Wound Healing. Biomedicines, 13(5), 1041. https://doi.org/10.3390/biomedicines13051041








