Photobiomodulation in Medication-Related Osteonecrosis of the Jaw: Outcomes in Stage I and Its Adjunctive Role in Advanced Cases
Abstract
1. Introduction
2. Materials and Methods
Statistical Analysis
- The assessment of the compliance of the empirical distributions of continuous quantitative variables (age and time to necrosis) and discrete variables (pain level on VAS scale) with theoretical normal distributions was performed using the Shapiro–Wilk test [51]. The critical significance level was p < 0.05.
- For quantitative variables, mean values (M), standard deviations (SD), medians (Me), lower (Q1) and upper (Q3) quartiles, extreme values, and smallest (Min) and largest (Max) values were calculated. In tables and graphs, variables with a distribution close to normal were presented using the mean and standard deviation: M (SD), while variables with a distribution different from normal were presented as medians and quartiles: Me [Q1; Q3] [52].
- For qualitative (nominal, e.g., sex, osteoporosis, and healing of the mucous membrane and gums) and ordinal (e.g., the degree of necrosis) variables, counts (n) and percentages (%) were calculated and collected in contingency tables [52].
- Hypotheses about the lack of correlation between qualitative characteristics were verified using the Pearson chi-square test (χ2) [53,54] or Fisher’s exact test [55]. In the case of four-box tables (with dimensions of 2 × 2), the values of the odds ratio (OR) and their 95% confidence intervals (95% CI) were estimated.
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ARDs | Antiresorptive Drugs |
| CBCT | Cone-Beam Computed Tomography |
| CW | Continuous Wave |
| Er:YAG | Erbium-Doped Yttrium Aluminum Garnet |
| J/cm2 | Joules per Square Centimeter |
| MRONJ | Medication-Related Osteonecrosis of the Jaw |
| mW | Milliwatt |
| nm | Nanometer |
| OR | Odds Ratio |
| PBM | Photobiomodulation |
| PRGF | Plasma Rich in Growth Factors |
| QoL | Quality of Life |
| SD | Standard Deviation |
| VAS | Visual Analog Scale |
| W/cm2 | Watts per Square Centimeter |
References
- Kendler, D.L.; Cosman, F.; Stad, R.K.; Ferrari, S. Denosumab in the Treatment of Osteoporosis: 10 Years Later: A Narrative Review. Adv. Ther. 2022, 39, 58–74. [Google Scholar] [CrossRef] [PubMed]
- Ayers, C.; Kansagara, D.; Lazur, B.; Fu, R.; Kwon, A.; Harrod, C. Effectiveness and Safety of Treatments to Prevent Fractures in People with Low Bone Mass or Primary Osteoporosis: A Living Systematic Review and Network Meta-Analysis for the American College of Physicians. Ann. Intern. Med. 2023, 176, 182–195. [Google Scholar] [CrossRef]
- Gehrke, B.; Coelho, M.C.A.; D’alva, C.B.; Madeira, M. Long-Term Consequences of Osteoporosis Therapy with Bisphosphonates. Arch. Endocrinol. Metab. 2023, 68, e220334. [Google Scholar] [CrossRef] [PubMed]
- Reid, I.R.; Sharma, S.; Kalluru, R.; Eagleton, C. Treatment of Paget’s Disease of Bone with Denosumab: Case Report and Literature Review. Calcif. Tissue Int. 2016, 99, 322–325. [Google Scholar] [CrossRef]
- Corral-Gudino, L.; Tan, A.J.H.; del Pino-Montes, J.; Ralston, S.H. Bisphosphonates for Paget’s Disease of Bone in Adults. Cochrane Database Syst. Rev. 2017, 2017, CD004956. [Google Scholar] [CrossRef]
- Lipton, A. Denosumab in Breast Cancer. Curr. Oncol. Rep. 2011, 13, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Goldvaser, H.; Amir, E. Role of Bisphosphonates in Breast Cancer Therapy. Curr. Treat. Options Oncol. 2019, 20, 26. [Google Scholar] [CrossRef]
- Galvano, A.; Scaturro, D.; Badalamenti, G.; Incorvaia, L.; Rizzo, S.; Castellana, L.; Cusenza, S.; Cutaia, S.; Santini, D.; Guadagni, F.; et al. Denosumab for Bone Health in Prostate and Breast Cancer Patients Receiving Endocrine Therapy? A Systematic Review and a Meta-Analysis of Randomized Trials. J. Bone Oncol. 2019, 18, 100252. [Google Scholar] [CrossRef]
- Jakob, T.; Tesfamariam, Y.M.; Macherey, S.; Kuhr, K.; Adams, A.; Monsef, I.; Heidenreich, A.; Skoetz, N. Bisphosphonates or RANK-Ligand-Inhibitors for Men with Prostate Cancer and Bone Metastases: A Network Meta-Analysis. Cochrane Database Syst. Rev. 2020, 2020, CD013020. [Google Scholar]
- Gavaldá, C.; Bagan, J.V. Concept, Diagnosis and Classification of Bisphosphonate-Associated Osteonecrosis of the Jaws. A Review of the Literature. Med. Oral Patol. Oral Cir. Bucal 2016, 21, e260–e270. [Google Scholar] [CrossRef]
- Ruggiero, S.L. Reply: AAOMS Position Paper on Medication-Related Osteonecrosis of the Jaws—2022 Update. Diagnostic Milestones, Doubts, and Perspectives on MRONJ. J. Oral Maxillofac. Surg. 2022, 80, 1724. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Kohn, N. Disease Stage and Mode of Therapy Are Important Determinants of Treatment Outcomes for Medication-Related Osteonecrosis of the Jaw. J. Oral Maxillofac. Surg. 2015, 73, S94–S100. [Google Scholar] [CrossRef]
- Marx, R.E. Pamidronate (Aredia) and Zoledronate (Zometa) Induced Avascular Necrosis of the Jaws: A Growing Epidemic. J. Oral Maxillofac. Surg. 2003, 61, 1115–1117. [Google Scholar] [CrossRef]
- Fleisch, H. Bisphosphonates in Bone Disease from the Laboratory to the Patient; Academic Press: Cambridge, MA, USA, 2000; ISBN 978-0-12-260371-6. [Google Scholar]
- Tenenbaum, H.C.; Shelemay, A.; Girard, B.; Zohar, R.; Fritz, P.C. Bisphosphonates and Periodontics: Potential Applications for Regulation of Bone Mass in the Periodontium and Other Therapeutic/Diagnostic Uses. J. Periodontol. 2002, 73, 813–822. [Google Scholar] [CrossRef]
- Frith, J.C.; Mönkkönen, J.; Blackburn, G.M.; Russell, R.G.G.; Rogers, M.J. Clodronate and Liposome-Encapsulated Clodronate Are Metabolized to a Toxic ATP Analog, Adenosine 5′-(β,γ-Dichloromethylene) Triphosphate, by Mammalian Cells in Vitro. J. Bone Miner. Res. 1997, 12, 1358–1367. [Google Scholar] [CrossRef] [PubMed]
- Jiang, A.; Zhang, Z.; Qiu, X.; Guo, Q. Medication-Related Osteonecrosis of the Jaw (MRONJ): A Review of Pathogenesis Hypothesis and Therapy Strategies. Arch Toxicol 2024, 98, 689–708. [Google Scholar] [CrossRef]
- Campisi, G.; Fedele, S.; Fusco, V.; Pizzo, G.; Di Fede, O.; Bedogni, A. Epidemiology, Clinical Manifestations, Risk Reduction and Treatment Strategies of Jaw Osteonecrosis in Cancer Patients Exposed to Antiresorptive Agents. Future Oncol. 2014, 10, 257–275. [Google Scholar] [CrossRef] [PubMed]
- AlRowis, R.; Aldawood, A.; AlOtaibi, M.; Alnasser, E.; AlSaif, I.; Aljaber, A.; Natto, Z. Medication-Related Osteonecrosis of the Jaw (MRONJ): A Review of Pathophysiology, Risk Factors, Preventive Measures and Treatment Strategies. Saudi Dent. J. 2022, 34, 202–210. [Google Scholar] [CrossRef]
- Lo, J.C.; O’Ryan, F.S.; Gordon, N.P.; Yang, J.; Hui, R.L.; Martin, D.; Hutchinson, M.; Lathon, P.V.; Sanchez, G.; Silver, P.; et al. Prevalence of Osteonecrosis of the Jaw in Patients With Oral Bisphosphonate Exposure. J. Oral Maxillofac. Surg. 2010, 68, 243–253. [Google Scholar] [CrossRef]
- Rugani, P.; Walter, C.; Kirnbauer, B.; Acham, S.; Begus-Nahrman, Y.; Jakse, N. Prevalence of Medication-Related Osteonecrosis of the Jaw in Patients with Breast Cancer, Prostate Cancer, and Multiple Myeloma. Dent. J. 2016, 4, 32. [Google Scholar] [CrossRef]
- Carlos, A.C.A.M.; Coelho, L.M.C.; Malta, C.E.N.; Magalhães, I.A.; Borges, M.M.F.; da Silva, J.E.; Silva, L.F.G.; de Barros Silva, P.G. Risk Factors for Bisphosphonate-Related Osteonecrosis of the Jaws in Bone Metastatic Breast and Prostate Cancer under Zoledronate Treatment: A Retrospective Analysis from 10 Years of Evaluation. Asian Pac. J. Cancer Prev. 2023, 24, 2681–2689. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, S. Management of Cancer Treatment-Induced Bone Loss (CTIBL) in Patients with Breast Cancer or Prostate Cancer. J. Bone Min. Metab. 2023, 41, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Kawahara, M.; Kuroshima, S.; Sawase, T. Clinical Considerations for Medication-Related Osteonecrosis of the Jaw: A Comprehensive Literature Review. Int. J. Implant. Dent. 2021, 7, 47. [Google Scholar] [CrossRef]
- Schwech, N.; Nilsson, J.; Gabre, P. Incidence and Risk Factors for Medication-Related Osteonecrosis after Tooth Extraction in Cancer Patients—A Systematic Review. Clin. Exp. Dent. Res. 2023, 9, 55–65. [Google Scholar] [CrossRef]
- Soutome, S.; Otsuru, M.; Hayashida, S.; Murata, M.; Yanamoto, S.; Sawada, S.; Kojima, Y.; Funahara, M.; Iwai, H.; Umeda, M.; et al. Relationship between Tooth Extraction and Development of Medication-Related Osteonecrosis of the Jaw in Cancer Patients. Sci. Rep. 2021, 11, 17226. [Google Scholar] [CrossRef]
- Michalak, F.; Dominiak, M.; Kiryk, J.; Popecki, P.; Kubicki, D.; Matys, J.; Grzech-Leśniak, K. The Influence of Vitamin D Levels and Supplementation on the Treatment of Patients Affected by MRONJ. Appl. Sci. 2025, 15, 670. [Google Scholar] [CrossRef]
- Hallmer, F.; Bjarnadottir, O.; Götrick, B.; Malmström, P.; Andersson, G. Incidence of and Risk Factors for Medication-Related Osteonecrosis of the Jaw in Women with Breast Cancer with Bone Metastasis: A Population-Based Study. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 252–257. [Google Scholar] [CrossRef]
- Momesso, G.A.C.; Lemos, C.A.A.; Santiago-Júnior, J.F.; Faverani, L.P.; Pellizzer, E.P. Laser Surgery in Management of Medication-Related Osteonecrosis of the Jaws: A Meta-Analysis. Oral Maxillofac. Surg. 2020, 24, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Govaerts, D.; Piccart, F.; Ockerman, A.; Coropciuc, R.; Politis, C.; Jacobs, R. Adjuvant Therapies for MRONJ: A Systematic Review. Bone 2020, 141, 115676. [Google Scholar] [CrossRef]
- Vescovi, P.; De Francesco, P.; Giovannacci, I.; Leão, J.C.; Barone, A. Piezoelectric Surgery, Er:YAG Laser Surgery and Nd:YAG Laser Photobiomodulation: A Combined Approach to Treat Medication-Related Osteonecrosis of the Jaws (MRONJ). Dent. J. 2024, 12, 261. [Google Scholar] [CrossRef]
- Kiryk, J.; Matys, J.; Grzech-Leśniak, K.; Dominiak, M.; Małecka, M.; Kuropka, P.; Wiglusz, R.J.; Dobrzyński, M. Sem Evaluation of Tooth Surface after a Composite Filling Removal Using Er:Yag Laser, Drills with and without Curettes, and Optional Edta or Naocl Conditioning. Materials 2021, 14, 4469. [Google Scholar] [CrossRef]
- Matys, J.; Grzech-Leśniak, K.; Flieger, R.; Dominiak, M. Assessment of an Impact of a Diode Laser Mode with Wavelength of 980 Nm on a Temperature Rise Measured by Means of K-02 Thermocouple: Preliminary Results. Dent. Med. Probl. 2016, 53, 345–351. [Google Scholar] [CrossRef]
- Kiryk, J.; Kiryk, S.; Kensy, J.; Świenc, W.; Palka, B.; Zimoląg-Dydak, M.; Dobrzyński, W.; Matys, J.; Dobrzyński, M. Effectiveness of Laser-Assisted Teeth Bleaching: A Systematic Review. Appl. Sci. 2024, 14, 9219. [Google Scholar] [CrossRef]
- Ahrari, F.; Shafaee, H.; Haghpanahi, M.; Bardideh, E. Low-Level Laser Therapy and Laser Acupuncture Therapy for Pain Relief after Initial Archwire Placement. J. Orofac. Orthop./Fortschritte Der Kieferorthopädie 2024, 85, 198–207. [Google Scholar] [CrossRef] [PubMed]
- Chhabrani, A.; Avinash, B.S.; Bharadwaj, R.S.; Gupta, M. Laser Light: Illuminating the Path to Enhanced Periodontal Care. Photodiagnosis Photodyn. Ther. 2024, 46, 104036. [Google Scholar] [CrossRef] [PubMed]
- Matys, J.; Flieger, R.; Gedrange, T.; Janowicz, K.; Kempisty, B.; Grzech-Leśniak, K.; Domin, M. Effect of 808 Nm Semiconductor Laser on the Stability of Orthodontic Micro-Implants: A Split-Mouth Study. Materials 2020, 13, 2265. [Google Scholar] [CrossRef]
- Kocherova, I.; Bryja, A.; Błochowiak, K.; Kaczmarek, M.; Stefańska, K.; Matys, J.; Grzech-Leśniak, K.; Dominiak, M.; Mozdziak, P.; Kempisty, B.; et al. Photobiomodulation with Red and Near-Infrared Light Improves Viability and Modulates Expression of Mesenchymal and Apoptotic-Related Markers in Human Gingival Fibroblasts. Materials 2021, 14, 3427. [Google Scholar] [CrossRef] [PubMed]
- Karu, T.I. Mitochondrial Signaling in Mammalian Cells Activated by Red and Near-IR Radiation. Photochem. Photobiol. 2008, 84, 1091–1099. [Google Scholar] [CrossRef]
- Karu, T.I.; Pyatibrat, L.V.; Afanasyeva, N.I. Cellular Effects of Low Power Laser Therapy Can Be Mediated by Nitric Oxide. Lasers Surg. Med. 2005, 36, 307–314. [Google Scholar] [CrossRef]
- Lipko, N.B. Photobiomodulation: Evolution and Adaptation. Photobiomodul Photomed. Laser Surg. 2022, 40, 213–233. [Google Scholar] [CrossRef]
- Vescovi, P.; Meleti, M.; Merigo, E.; Manfredi, M.; Fornaini, C.; Guidotti, R.; Nammour, S. Case Series of 589 Tooth Extractions in Patients under Bisphosphonates Therapy. Proposal of a Clinical Protocol Supported by Nd: YAG Low-Level Laser Therapy. Med. Oral Patol. Oral Cir. Bucal 2013, 18, e680–e685. [Google Scholar] [CrossRef] [PubMed]
- El Mobadder, M.; Grzech-Lesniak, Z.; El Mobadder, W.; Rifai, M.; Ghandour, M.; Nammour, S. Management of Medication-Related Osteonecrosis of the Jaw with Photobiomodulation and Minimal Surgical Intervention. Dent. J. 2023, 11, 127. [Google Scholar] [CrossRef]
- Tenore, G.; Zimbalatti, A.; Rocchetti, F.; Graniero, F.; Gaglioti, D.; Mohsen, A.; Caputo, M.; Lollobrigida, M.; Lamazza, L.; De Biase, A.; et al. Management of Medication-Related Osteonecrosis of the Jaw (MRONJ) Using Leukocyte-and Platelet-Rich Fibrin (l-PRF) and Photobiomodulation: A Retrospective Study. J. Clin. Med. 2020, 9, 3505. [Google Scholar] [CrossRef]
- Razavi, P.; Jafari, A.; Vescovi, P.; Fekrazad, R. Efficacy of Adjunctive Photobiomodulation in the Management of Medication-Related Osteonecrosis of the Jaw: A Systematic Review. Photobiomodul Photomed. Laser Surg. 2022, 40, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Ruggiero, S.L.; Dodson, T.B.; Fantasia, J.; Goodday, R.; Aghaloo, T.; Mehrotra, B.; O’Ryan, F. American Association of Oral and Maxillofacial Surgeons Position Paper on Medication-Related Osteonecrosis of the Jaw—2014 Update. J. Oral Maxillofac. Surg. 2014, 72, 1938–1956. [Google Scholar] [CrossRef]
- Sterczała, B.; Grzech-Leśniak, K.; Michel, O.; Trzeciakowski, W.; Dominiak, M.; Jurczyszyn, K. Assessment of Human Gingival Fibroblast Proliferation after Laser Stimulation in Vitro Using Different Laser Types and Wavelengths (1064, 980, 635, 450, and 405 Nm)—Preliminary Report. J. Pers. Med. 2021, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Manley, B.J.; Neal, T.W.; Indrakanti, S.; Schlieve, T. Spontaneous Medication-Related Osteonecrosis of the Jaws in a 23-Year-Old. Oral Maxillofac. Surg. Cases 2023, 9, 100289. [Google Scholar] [CrossRef]
- D’Agostino, S.; Valentini, G.; Dolci, M.; Ferrara, E. Potential Relationship between Poor Oral Hygiene and MRONJ: An Observational Retrospective Study. Int. J. Environ. Res. Public Health 2023, 20, 5402. [Google Scholar] [CrossRef]
- Wei, J. The Adoption of Repeated Measurement of Variance Analysis and Shapiro—Wilk Test. Front. Med. 2022, 16, 659–660. [Google Scholar] [CrossRef]
- Mishra, P.; Pandey, C.M.; Singh, U.; Gupta, A.; Sahu, C.; Keshri, A. Descriptive Statistics and Normality Tests for Statistical Data. Ann. Card. Anaesth. 2019, 22, 67–72. [Google Scholar] [CrossRef] [PubMed]
- Shih, J.H.; Fay, M.P. Pearson’s Chi-Square Test and Rank Correlation Inferences for Clustered Data. Biometrics 2017, 73, 822–834. [Google Scholar] [CrossRef]
- Lydersen, S.; Fagerland, M.W.; Laake, P. Pearsons Khikvadrattest. Tidsskr. Den. Nor. Laegeforening 2019, 139. [Google Scholar] [CrossRef]
- Jung, S.H. Stratified Fisher’s Exact Test and Its Sample Size Calculation. Biom. J. 2014, 56, 129–140. [Google Scholar] [CrossRef]
- Kim, S.; Lee, W. Does McNemar’s Test Compare the Sensitivities and Specificities of Two Diagnostic Tests? Stat. Methods Med. Res. 2017, 26, 142–154. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y. Weighted McNemar’s Test for the Comparison of Two Screening Tests in the Presence of Verification Bias. Stat. Med. 2022, 41, 3149–3163. [Google Scholar] [CrossRef]
- Wadhwa, R.R.; Marappa-Ganeshan, R. T Test. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2025. [Google Scholar]
- Mishra, P.; Singh, U.; Pandey, C.M.; Mishra, P.; Pandey, G. Application of Student’s t-Test, Analysis of Variance, and Covariance. Ann. Card. Anaesth. 2019, 22, 407–411. [Google Scholar] [CrossRef]
- Di Fede, O.; Canepa, F.; Panzarella, V.; Mauceri, R.; Del Gaizo, C.; Bedogni, A.; Fusco, V.; Tozzo, P.; Pizzo, G.; Campisi, G.; et al. The Treatment of Medication-Related Osteonecrosis of the Jaw (Mronj): A Systematic Review with a Pooled Analysis of Only Surgery versus Combined Protocols. Int. J. Environ. Res. Public Health 2021, 18, 8432. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Rabié, C.; Gaêta-Araujo, H.; Ferreira-Leite, A.; Coucke, W.; Gielen, E.; Van den Wyngaert, T.; Jacobs, R. Local Radiographic Risk Factors for MRONJ in Osteoporotic Patients Undergoing Tooth Extraction. Oral Dis. 2024, 30, 1632–1642. [Google Scholar] [CrossRef]
- Şahin, O.; Akan, E.; Tatar, B.; Ekmekcioğlu, C.; Ünal, N.; Odabaşı, O. Combined Approach to Treatment of Advanced Stages of Medication-Related Osteonecrosis of the Jaw Patients. Braz. J. Otorhinolaryngol. 2022, 88, 613–620. [Google Scholar] [CrossRef]
- Petrovic, M.; Jelovac, D.B.; Antic, S.; Antunovic, M.; Lukic, N.; Sabani, M.; Mudrak, J.; Jezdic, Z.; Pucar, A.; Stefanovic, A.; et al. Medication-Related Osteonecrosis of the Jaws: Two Center Retrospective Cohort Studies. Biomed. Res. Int. 2019, 2019, 8345309. [Google Scholar] [CrossRef] [PubMed]
- Goldman, L.; Gray, J.A.; Goldman, J.; Goldman, B.; Meyer, R. Effect of Laser Beam Impacts on Teeth. J. Am. Dent. Assoc. 1965, 70, 601–606. [Google Scholar] [CrossRef]
- Maiman, T.H. Stimulated Optical Radiation in Ruby. Nature 1960, 187, 493–494. [Google Scholar] [CrossRef]
- Deeb, J.G.; Smith, J.; Belvin, B.R.; Grzech-Leśniak, K.; Lewis, J. Er:YAG Laser Irradiation Reduces Microbial Viability When Used in Combination with Irrigation with Sodium Hypochlorite, Chlorhexidine, and Hydrogen Peroxide. Microorganisms 2019, 7, 612. [Google Scholar] [CrossRef] [PubMed]
- Verma, S.; Maheshwari, S.; Singh, R.; Chaudhari, P. Laser in Dentistry: An Innovative Tool in Modern Dental Practice. Natl. J. Maxillofac. Surg. 2012, 3, 124–132. [Google Scholar] [CrossRef]
- Wigdor, H.A.; Walsh, J.T.; Featherstone, J.D.B.; Visuri, S.R.; Fried, D.; Waldvogel, J.L. Lasers in Dentistry. Lasers Surg. Med. 1995, 16, 103–133. [Google Scholar] [CrossRef]
- Carroll, J.D.; Milward, M.R.; Cooper, P.R.; Hadis, M.; Palin, W.M. Developments in Low Level Light Therapy (LLLT) for Dentistry. Dent. Mater. 2014, 30, 465–475. [Google Scholar] [CrossRef]
- Sourvanos, D.; Lander, B.; Sarmiento, H.; Carroll, J.; Hall, R.D.; Zhu, T.C.; Fiorellini, J.P. Photobiomodulation in Dental Extraction Therapy: Postsurgical Pain Reduction and Wound Healing. J. Am. Dent. Assoc. 2023, 154, 567–579. [Google Scholar] [CrossRef]
- Michalak, F.; Hnitecka, S.; Dominiak, M.; Grzech-Leśniak, K. Schemes for Drug-Induced Treatment of Osteonecrosis of Jaws with Particular Emphasis on the Influence of Vitamin d on Therapeutic Effects. Pharmaceutics 2021, 13, 354. [Google Scholar] [CrossRef]
- Haviv, Y.; Geller, Z.; Mazor, S.; Sharav, Y.; Keshet, N.; Zadik, Y. Pain Characteristics in Medication-Related Osteonecrosis of the Jaws. Support. Care Cancer 2021, 29, 1073–1080. [Google Scholar] [CrossRef]
- Li, F.L.; Wu, C.B.; Sun, H.J.; Zhou, Q. Effectiveness of Laser-Assisted Treatments for Medication-Related Osteonecrosis of the Jaw: A Systematic Review. Br. J. Oral Maxillofac. Surg. 2020, 58, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Del Vecchio, A.; Tenore, G.; Pergolini, D.; Rocchetti, F.; Palaia, G.; Romeo, U. The Role of the Laser Photobiomodulation (PBM) in the Management of Patients at Risk or Affected by MRONJ. Oral 2022, 2, 7–15. [Google Scholar] [CrossRef]
- Ciobanu, G.A.; Camen, A.; Ionescu, M.; Vlad, D.; Munteanu, C.M.; Gheorghiță, M.I.; Lungulescu, C.V.; Staicu, I.E.; Sin, E.C.; Chivu, L.; et al. Risk Factors for Medication-Related Osteonecrosis of the Jaw—A Binomial Analysis of Data of Cancer Patients from Craiova and Constanta Treated with Zoledronic Acid. J. Clin. Med. 2023, 12, 3747. [Google Scholar] [CrossRef]
- Campisi, G.; Mauceri, R.; Bertoldo, F.; Bettini, G.; Biasotto, M.; Colella, G.; Consolo, U.; Di Fede, O.; Favia, G.; Fusco, V.; et al. Medication-Related Osteonecrosis of Jaws (MRONJ) Prevention and Diagnosis: Italian Consensus Update 2020. Int. J. Environ. Res. Public Health 2020, 17, 5998. [Google Scholar] [CrossRef]
- Avishai, G.; Muchnik, D.; Masri, D.; Zlotogorski-Hurvitz, A.; Chaushu, L. Minimizing MRONJ after Tooth Extraction in Cancer Patients Receiving Bone-Modifying Agents. J. Clin. Med. 2022, 11, 1807. [Google Scholar] [CrossRef]
- Coello-Suanzes, J.A.; Rollon-Ugalde, V.; Castaño-Seiquer, A.; Lledo-Villar, E.; Herce-Lopez, J.; Infante-Cossio, P.; Rollon-Mayordomo, A. Preventive Dental Management of Osteonecrosis of the Jaws Related to Zoledronic Acid Treatment. Oral Dis. 2018, 24, 1029–1036. [Google Scholar] [CrossRef]
- Hasegawa, T.; Hayashida, S.; Kondo, E.; Takeda, Y.; Miyamoto, H.; Kawaoka, Y.; Ueda, N.; Iwata, E.; Nakahara, H.; Kobayashi, M.; et al. Medication-Related Osteonecrosis of the Jaw after Tooth Extraction in Cancer Patients: A Multicenter Retrospective Study. Osteoporos. Int. 2019, 30, 231–239. [Google Scholar] [CrossRef]
- Beth-Tasdogan, N.H.; Mayer, B.; Hussein, H.; Zolk, O. Interventions for Managing Medication-Related Osteonecrosis of the Jaw. Cochrane Database Syst. Rev. 2017, 2017, CD012432. [Google Scholar] [CrossRef]
- Patel, V.; Mansi, J.; Ghosh, S.; Kwok, J.; Burke, M.; Reilly, D.; Nizarali, N.; Sproat, C.; Chia, K. MRONJ Risk of Adjuvant Bisphosphonates in Early Stage Breast Cancer. Br. Dent. J. 2018, 224, 74–79. [Google Scholar] [CrossRef]
- Limones, A.; Sáez-Alcaide, L.M.; Díaz-Parreño, S.A.; Helm, A.; Bornstein, M.M.; Molinero-Mourelle, P. Medication-Related Osteonecrosis of the Jaws (MRONJ) in Cancer Patients Treated with Denosumab VS. Zoledronic Acid: A Systematic Review and Meta-Analysis. Med. Oral Patol. Oral Cir. Bucal 2020, 25, e326–e336. [Google Scholar] [CrossRef]
- Hayashida, S.; Soutome, S.; Yanamoto, S.; Fujita, S.; Hasegawa, T.; Komori, T.; Kojima, Y.; Miyamoto, H.; Shibuya, Y.; Ueda, N.; et al. Evaluation of the Treatment Strategies for Medication-Related Osteonecrosis of the Jaws (MRONJ) and the Factors Affecting Treatment Outcome: A Multicenter Retrospective Study with Propensity Score Matching Analysis. J. Bone Miner. Res. 2017, 32, 2022–2029. [Google Scholar] [CrossRef] [PubMed]
- Lesclous, P.; Grabar, S.; Abi Najm, S.; Carrel, J.P.; Lombardi, T.; Saffar, J.L.; Samson, J. Relevance of Surgical Management of Patients Affected by Bisphosphonate-Associated Osteonecrosis of the Jaws. A Prospective Clinical and Radiological Study. Clin. Oral Investig. 2014, 18, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer Statistics, 2024. CA Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef]

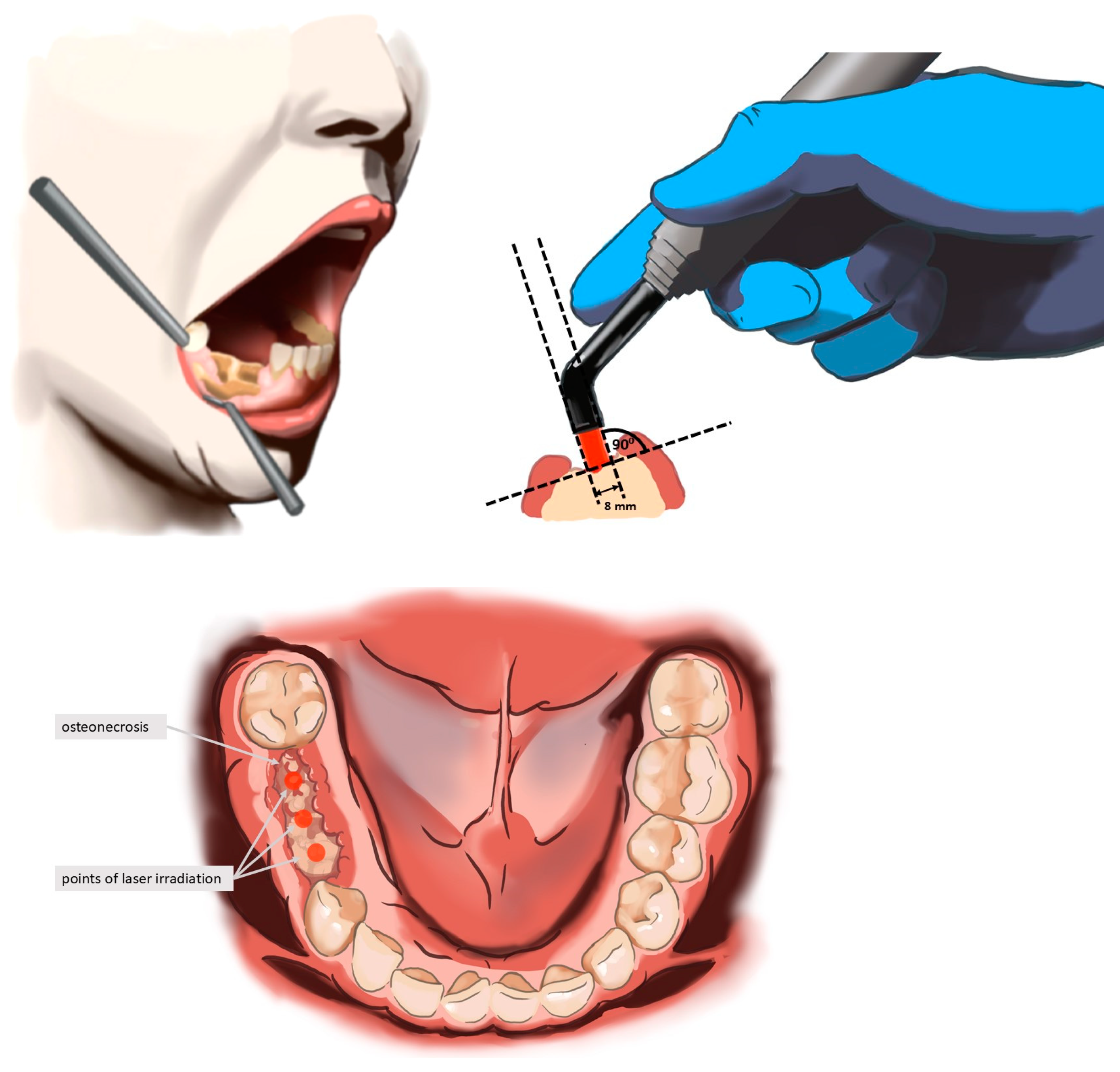


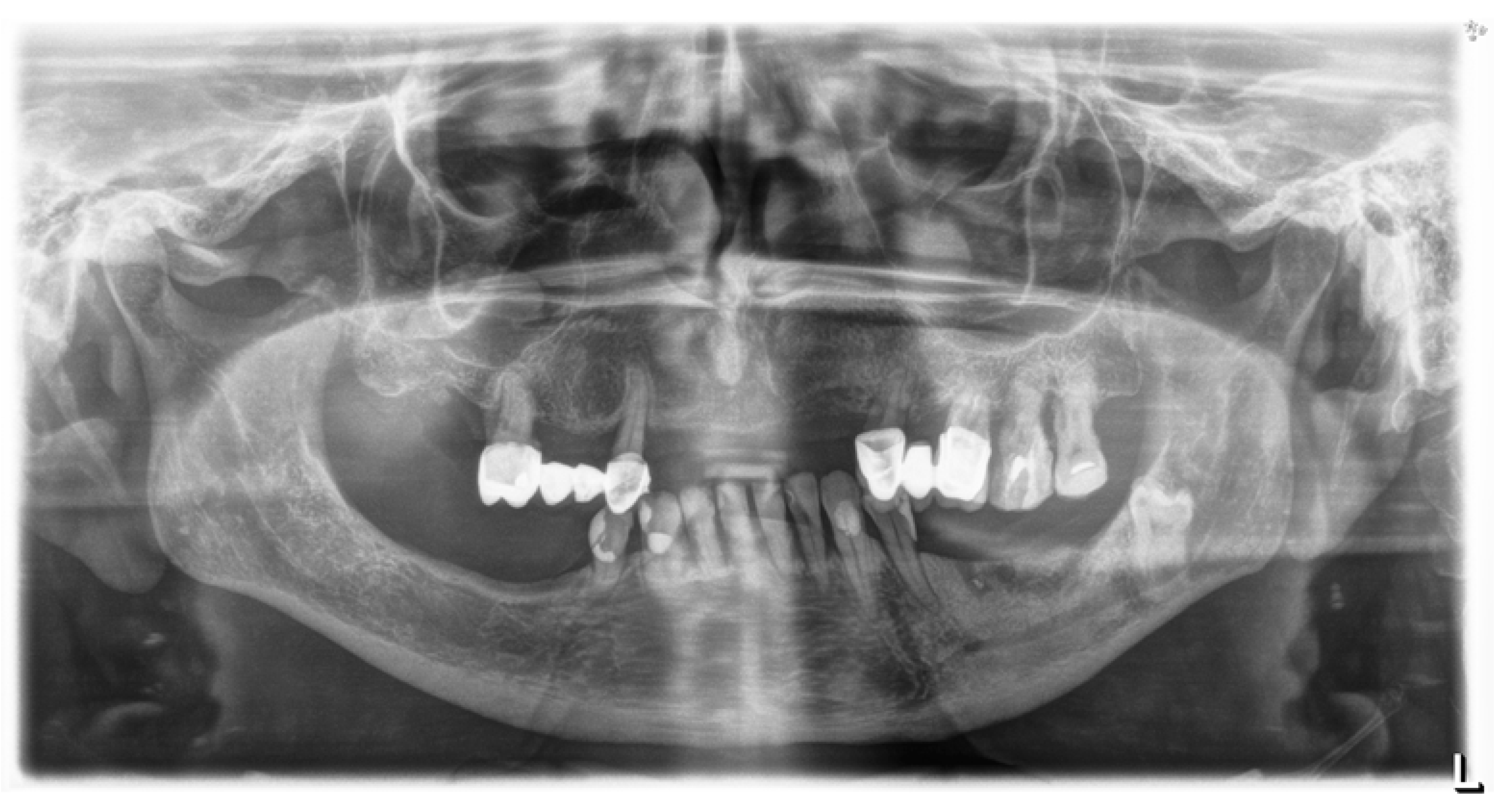
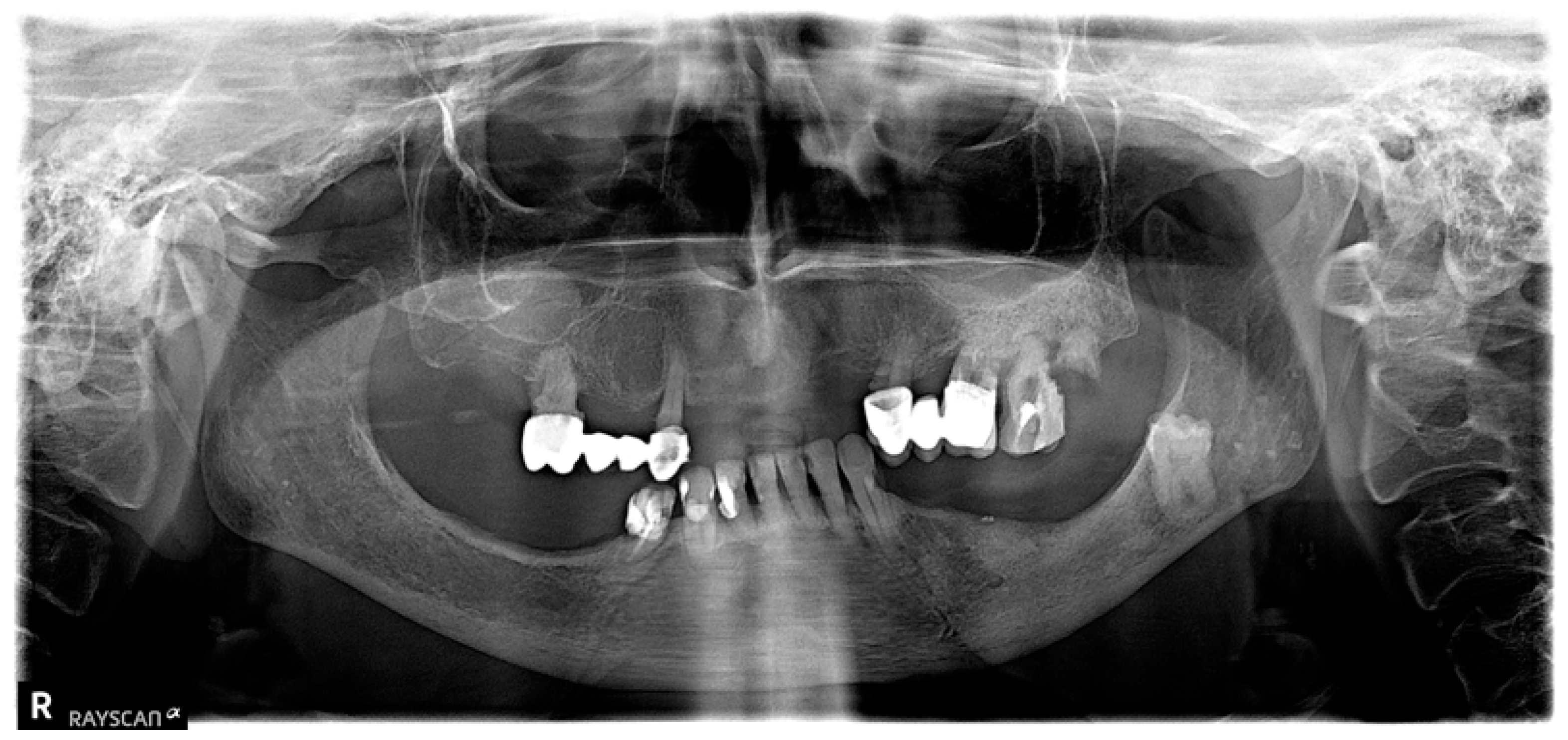
| Variable | Group I n = 14 | Group II n = 17 | p-Value |
|---|---|---|---|
| Pain level before treatment on VAS scale | <0.001 d | ||
| M ± SD | 3.9 ± 0.9 | 6.1 ± 1.0 | |
| Me [Q1; Q3] | 4 [3; 4] | 6 [6; 7] | |
| Min–Max | 3–6 | 4–8 | |
| Pain level 3 months after treatment on VAS scale | 0.004 d | ||
| M ± SD | 0.1 ± 0.3 | 1.1 ± 0.9 | |
| Me [Q1; Q3] | 0 [0; 0] | 1 [0; 2] | |
| Min–Max | 0–1 | 0–2 | |
| Pain level 6 months after treatment on VAS scale | 0.067 d | ||
| M ± SD | 0.1 ± 0.3 | 0.5 ± 0.8 | |
| Me [Q1; Q3] | 0 [0; 0] | 0 [0; 1] | |
| Min–Max | 0–1 | 0–2 |
| Pain Reduction Between 3 and 6 Months of Treatment | Test Results | OR [95% CI] | ||
|---|---|---|---|---|
| Yes | No | |||
| Group II | 8 (47.1) | 9 (52.9) | p = 0.003 b | 25.9 [1.33; 504] |
| Group I | 0 (0.0) | 14 (100.0) | 1.00 (Ref.) | |
| Healed After 6 Months | Healed After 3 Months | Chi-Square McNemar’s A/D | |
|---|---|---|---|
| Yes (0°) n = 14 | No (I° or II°) n = 17 | ||
| Yes (0°) n = 15 | 14 (45.2%) | 1 (3.2%) | p = 0.855 e |
| No (I° or II°) n = 16 | 0 (0.0%) | 16 (51.6%) | |
| Group I | Group II | Test Result | |
|---|---|---|---|
| Improved by one degree, n (%) | 14 (100.0) | 16 (94.1) | p = 1.000 b |
| Improved by two degrees, n (%) | 0 (0.0) | 1 (5.9) |
| The Degree of Necrosis | Healing of the Mucous Membrane and Gums | Test Results | OR [95% CI] | |
|---|---|---|---|---|
| Yes (n = 18) | No (n = 13) | |||
| I, n (%) | 14 (77.8) | 0 (0.0) | p < 0.001 b | 87.0 [4.27; 1773] |
| II and III, n (%) | 4 (22.2) | 13 (100.0) | 1.00 (Ref.) | |
| Need for Further Treatment | Test Results | OR [95% CI] | ||
|---|---|---|---|---|
| No (n = 15) | Yes (n = 16) | |||
| Group I | 13 (86.7) | 1 (6.2) | p < 0.001 b | 97.5 [7.90; 1203] |
| Group II | 2 (13.3) | 15 (93.8) | 1.00 (Ref.) | |
| Initial Grade of Necrosis | Osteoporosis | p | |
|---|---|---|---|
| Yes (n = 9) | No (n = 12) | ||
| I (n = 9) | 5 (55.6%) | 4 (33.3%) | 0.465 c |
| II (n = 10) | 4 (44.4%) | 6 (50.0%) | |
| III (n = 2) | 0 (0.0%) | 2 (16.7%) | |
| Initial Grade of Necrosis | Breast Cancer | p | |
|---|---|---|---|
| Yes (n = 9) | No (n = 12) | ||
| I (n = 9) | 3 (33.3%) | 6 (50.0%) | 0.820 c |
| II (n = 10) | 5 (55.6%) | 5 (41.7%) | |
| III (n = 2) | 1 (11.1%) | 1 (8.3%) | |
| Initial Grade of Necrosis | Prostate Cancer | p | |
|---|---|---|---|
| Yes (n = 6) | No (n = 4) | ||
| I (n = 5) | 2 (33.3%) | 3 (75.0%) | 0.714 c |
| II (n = 4) | 3 (50.0%) | 1 (25.0%) | |
| III (n = 1) | 1 (16.7%) | 0 (0.0%) | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michalak, F.; Dominiak, M.; Grzech-Leśniak, Z.; Kiryk, J.; Grzech-Leśniak, K. Photobiomodulation in Medication-Related Osteonecrosis of the Jaw: Outcomes in Stage I and Its Adjunctive Role in Advanced Cases. Biomedicines 2025, 13, 1042. https://doi.org/10.3390/biomedicines13051042
Michalak F, Dominiak M, Grzech-Leśniak Z, Kiryk J, Grzech-Leśniak K. Photobiomodulation in Medication-Related Osteonecrosis of the Jaw: Outcomes in Stage I and Its Adjunctive Role in Advanced Cases. Biomedicines. 2025; 13(5):1042. https://doi.org/10.3390/biomedicines13051042
Chicago/Turabian StyleMichalak, Filip, Marzena Dominiak, Zuzanna Grzech-Leśniak, Jan Kiryk, and Kinga Grzech-Leśniak. 2025. "Photobiomodulation in Medication-Related Osteonecrosis of the Jaw: Outcomes in Stage I and Its Adjunctive Role in Advanced Cases" Biomedicines 13, no. 5: 1042. https://doi.org/10.3390/biomedicines13051042
APA StyleMichalak, F., Dominiak, M., Grzech-Leśniak, Z., Kiryk, J., & Grzech-Leśniak, K. (2025). Photobiomodulation in Medication-Related Osteonecrosis of the Jaw: Outcomes in Stage I and Its Adjunctive Role in Advanced Cases. Biomedicines, 13(5), 1042. https://doi.org/10.3390/biomedicines13051042








