Abstract
Background: The radial forearm free flap (RFFF) is a common technique in head and neck reconstructive surgery. This study aimed to compare the clinical and biochemical outcomes of wound healing following ulnar-based transposition flap (UBTF) versus split-thickness skin grafting (STSG) for donor site closure, with a particular emphasis on tissue regeneration. Materials and Methods: A total of 24 patients (6 women, 18 men), underwent RFFF reconstruction. The donor site was closed using the UBTF technique in 10 cases, while STSG was performed in 14 cases. Postoperative complications—including necrosis, edema, hematoma, infection, and wound dehiscence—along with healing times were assessed daily during the first seven postoperative days and at monthly follow-ups over six months. Pre- and postoperative biochemical analyses included hemoglobin (HB), white blood cell count (WBC), platelets (PLT), albumin, and C-reactive protein (CRP) levels. An aesthetic evaluation of the flap was also performed. Results: The two groups were homogeneous. Postoperative complications occurred more frequently in the STSG group, which also demonstrated significantly longer healing times (p = 0.0004). In contrast, the UBTF group showed significantly better aesthetic outcomes in terms of skin color (p = 0.000021), skin texture (p = 0.000018), and flap stability (p = 0.0398). Additionally, pre- and postoperative PLT counts were significantly higher in the UBTF group (p = 0.001 and p = 0.043, respectively). Conclusions: While STSG remains a well-established method for forearm donor site closure following RFFF harvest, this study demonstrates that UBTF is a viable alternative associated with better clinical and aesthetic outcomes.
1. Introduction
Wound healing is a fundamental aspect of reconstructive surgery, particularly in the treatment of patients with burns, traumatic injuries, and surgical defects [1]. Its importance extends well beyond aesthetic restoration, with primary emphasis placed on functional tissue recovery and the restoration of skin integrity [2]. In parallel, regenerative medicine has emerged as a rapidly evolving field, introducing innovative approaches such as bioengineered skin substitutes, growth factor delivery, 3D-bioprinted scaffolds and cell-based therapies that are designed to enhance and accelerate the healing process [3,4,5]. As regenerative approaches continue to evolve, they promise to bridge critical gaps left by conventional therapies, ultimately advancing personalized and durable clinical solutions.
Recent innovations, such as dermal regeneration templates and autologous spray-on cell therapies, have expanded the therapeutic landscape, offering alternatives to traditional grafts by promoting vascularization and reducing donor site morbidity [6,7]. Bioengineered skin grafts, composed of biocompatible scaffolds that support cellular proliferation and tissue regeneration, are specifically designed to mimic the structure and function of native skin [8]. Conversely, spray-on cell therapies—commonly used in the treatment of acute and chronic wounds, including burns of various etiologies, diabetic and venous ulcers, post-cancer surgical wounds, and hypopigmentation disorders—offer the advantage of significantly reducing the donor site area required compared to conventional autologous skin grafting [9,10]. In addition, advances in skin 3D bioprinting, which uses bioprintable materials known as bioinks—including natural polymers such as collagen, alginate, chitosan, hyaluronic acid, and cellulose—have introduced new possibilities in regenerative medicine [11,12]. This technology enables precise spatial control over the distribution of different skin components, allowing for the fabrication of complex structures such as the epidermis, dermis, and vascular networks [12]. Furthermore, 3D bioprinting holds the potential for personalized wound coverage, offering customized and more effective treatment options for complex skin defects [13].
However, despite these advancements, traditional clinical techniques, including autologous skin grafting and flap reconstruction, remain essential in surgical practice, offering biologically compatible and clinically validated methods for effective wound closure and long-term tissue regeneration [6,14,15].
The current practice of reconstructive surgery and wound care still relies heavily on skin grafting techniques and flap-based reconstruction [16,17]. Although often associated with plastic and aesthetic surgery, these techniques are equally vital in surgical oncology, particularly in reconstructions following breast cancer treatment and head and neck cancer resections [18,19].
One of the fasciocutaneous flaps most widely used in head and neck reconstruction is the radial forearm free flap (RFFF), especially for defects involving the tongue and oral cavity [20,21]. Its versatility and high survival rate—exceeding 95%—make it an excellent choice for addressing a wide range of complex defects in this region [22]. However, despite its effectiveness, donor site closure remains a significant clinical challenge, often accompanied by complications such as partial or total graft necrosis, infection, and delayed healing [23,24]. A variety of closure techniques have been described in the literature, including direct closure, local flaps, skin grafting, tissue expansion, and the use of artificial dermal substitutes [25,26,27]. Among these, skin grafting remains the most commonly employed method; however, it is frequently associated with suboptimal aesthetic and functional outcomes and prolonged healing times [28].
Numerous modifications of skin grafting have been proposed, including the use of both split-thickness (STSG) and full-thickness skin grafts (FTSG) [27,29]. In a study by Al-Aroomi et al., STSG and FTSG were compared for donor site closure. While functional outcomes were similar, FTSG resulted in superior aesthetic results [30]. However, their evaluation was limited to clinical complications, without addressing biochemical or regenerative parameters. Similarly, a study by Mashrah et al. evaluated the use of a bilobed flap for donor site reconstruction but focused solely on functional outcomes, omitting an analysis of biological or regenerative aspects [31].
An alternative technique described in the literature is the ulnar-based transposition flap (UBTF), which presents a promising solution for forearm donor site closure [32]. This method may allow for shorter healing times and avoids the need for tissue harvest from a secondary site, potentially reducing overall morbidity [33]. However, existing studies primarily assess aesthetic and functional outcomes, with limited investigation into biochemical or regenerative markers of tissue healing.
Therefore, the objective of the present study was to evaluate and compare donor site morbidity, healing times, aesthetic outcomes, and biochemical markers of wound healing between patients undergoing RFFF donor site closure with either the UBTF or STGS. This single-institution clinical experience aims to contribute novel insights into optimizing donor site management strategies, integrating both clinical and regenerative perspectives in head and neck reconstruction.
2. Materials and Methods
2.1. Subjects
We conducted a monocentric retrospective case-control study from January 2024 to January 2025 at the Head and Neck Cancer Department of the Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw, Poland. The choice between STSG and UBTF was determined based on the size and precise location of the cancerous lesion. The study included 24 patients, with 10 undergoing the ulnar-based closure technique and 14 receiving STSG. Patient sociodemographic data were extracted from medical records using patient identifiers and hospital numbers. Pre-existing factors such as age, gender, smoking history, diabetes mellitus (DM), and atherosclerosis were documented. Additionally, clinical and pathological tumor characteristics including tumor location, histological type, and the size of the harvested RFFF were evaluated. Subsequently, a detailed analysis was performed on post-surgical complications based on follow-up data and potential contributing patient features, aimed at assessing flap morbidity. The following criteria were used in patient selection:
The inclusion criteria were:
- Patients aged ≥ 18 years.
- Histologically confirmed diagnosis of head and neck cancer requiring RFFF reconstruction.
- No contraindications for microsurgical reconstruction.
The exclusion criteria were:
- Severe systemic comorbidities contraindicating free flap surgery, including uncontrolled DM or severe peripheral vascular disease.
- Prior surgery or radiotherapy to the forearm affecting flap harvest feasibility.
- Incomplete medical records or lack of postoperative follow-up.
2.2. Biochemical Analysis
Several blood parameters with potential relevance to wound healing were assessed preoperatively and one day after surgery. We evaluated white blood cell (WBC) count, which is a key indicator of immune function and may reflect the presence of infection or inflammation, both of which can adversely affect the wound healing process. Hemoglobin (HB), which plays a critical role in oxygen transport to tissues, was also measured; decreased levels may impair tissue oxygenation and delay healing. Platelet (PLT) count was evaluated as well, since PLTs are essential for clot formation and the initiation of tissue repair, making their levels important for assessing the body’s capacity to initiate healing. In addition, albumin was measured as a well-established marker of nutritional and inflammatory status; reduced albumin levels are associated with poor wound healing and an increased risk of postoperative complications. Finally, C-reactive protein (CRP)—measured only in the postoperative period—is a sensitive biomarker of systemic inflammation and may assist in the early identification of infection or an excessive inflammatory response. Blood samples were collected preoperatively from a peripheral venous access using standard procedures, and postoperatively from a central venous line. No complications were reported during the sample collection process.
2.3. Flap Morbidity Assessment
Flap assessments were conducted daily during the initial 7-day postoperative hospital stay, with a focus on identifying potential complications. Each flap was evaluated three times per day. The assessed complications included edema, flap necrosis, wound dehiscence, hematoma formation, and infection. Each variable was scored on a binary scale: 0, indicating normal findings, and 1, indicating the presence of a confirmed complication. The scores were then summarized and reported as mean values with corresponding standard deviations (SD). Follow-up evaluations were subsequently performed postoperatively at regular one-month intervals throughout the first six months. Additionally, healing time was assessed for patients in both groups using the following formula:
Healing Time (days) = Date of Complete Healing − Date of Surgery.
2.4. Aesthetic Flap Evaluation
An aesthetic evaluation of all donor sites was conducted during the 7-day postoperative hospital stay and at monthly clinical follow-up appointments. The assessment included skin color, skin texture, and flap stability, which were compared to the surrounding tissue. Each parameter was rated on a three-point scale, where 1 indicated a poor match, 2 represented a moderate match, and 3 signified an excellent match. The scores for each evaluated variable within both groups were summarized and presented as mean values with corresponding SD.
2.5. Surgical Technique
The donor site following the RFFF harvest was closed using two different techniques. The first method involved harvesting an STSG from the patient’s groin using a size 20 blade. The harvest site was pre-marked with a surgical marker after measuring the donor site dimensions with a surgical ruler. An incision was then made with a size 20 blade, and the graft was carefully elevated with minimal traction until fully excised. The donor site was subsequently sutured using only Dermalon skin suture (3-0) (Covidien Ilc®, Mansfield, MA, USA) (Figure 1). The ulnar-based transposition flap technique (Figure 2) began with an incision along the ulnar side of the forearm, carefully preserving the subcutaneous tissue. The flap was then elevated with minimal tension and rotated to cover the RFFF donor site. A 16.0 surgical drain (B.Braun®, Melsungen, Germany) was inserted, and the flap was secured using interrupted cutaneous and subcutaneous sutures (3-0) (Covidien Ilc®, Mansfield, MA, USA) (Figure 3). All patients received postoperative prophylaxis with Enoxaparin sodium (40 mg subcutaneously) once daily for seven days during their post-surgical hospitalization. Additionally, the standard intravenous antibiotic regimen consisted of 2 g of Ceftriaxone and 1.5 g of Metronidazole, administered intravenously on a daily basis for four days post-operation.
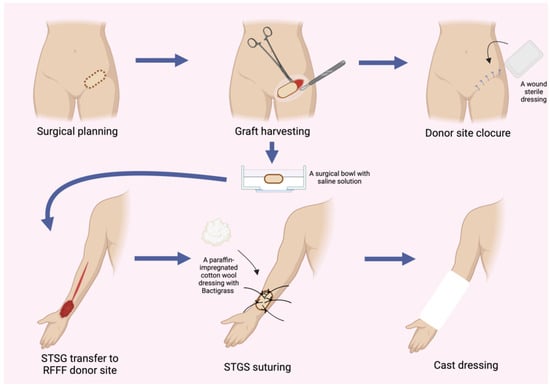
Figure 1.
Schematic representation illustrating the step-by-step procedure for harvesting an STSG and subsequent closure of the RFFF donor site. Abbreviations: RFFF—radial forearm free flap; STSG—split-thickness skin graft.
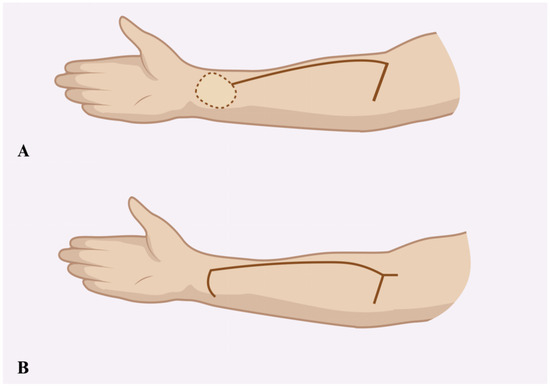
Figure 2.
Schematic illustration showing the incision design for the ulnar-based transposition flap (A) and the typical appearance of donor site closure following flap transfer (B).
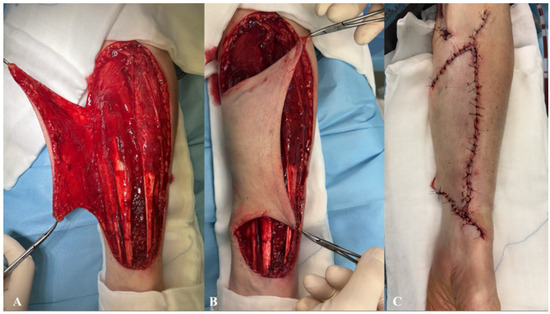
Figure 3.
Schematic intra-operative illustration of the ulnar-based transposition flap technique. (A) Elevated view of the ulnar-based flap; (B) flap transposition to cover the radial forearm free flap (RFFF) donor site; (C) final closure with skin sutures and insertion of a drainage tube.
2.6. Statistical Analysis
Categorical variables were presented as frequencies and percentages and compared between groups using the Chi-square test or Fisher’s exact test as appropriate. Continuous variables were expressed as means with SD, and group comparisons were conducted using either the independent samples t-test or the Mann–Whitney U test depending on data distribution. All statistical analyses were performed using RStudio (Version 1.4.1564) on macOS 10.15.7. A p-value < 0.05 was considered statistically significant.
3. Results
3.1. Patients Characteristics
A total of 24 patients (18 males and 6 females) diagnosed with head and neck cancer were included in the study. The majority of cases (20 patients) were diagnosed with squamous cell carcinoma (SCC), while one patient was diagnosed with mucoepidermoid carcinoma, one with melanoma, one with sarcoma, and one with basal cell carcinoma (BCC) (Figure 4). The most common tumor location within the head and neck region was the tongue (9 patients, 37.5%), followed by the cheek mucosa (4 patients, 16.7%) (Figure 5). Patients were divided into two groups: the STGS group (14 patients, 62.3%) and the UBTF group (10 patients, 41.7%). The mean age of patients in the UBTF group was 57.8 ± 14.1 years, while in the STSG group, it was 63.2 ± 13.8 years. There were no significant differences between the two groups in terms of gender, age, tumor histopathology, tumor location, smoking history, DM2 or atherosclerosis, and the size of harvested RFFF. A detailed overview of patient demographics and clinical characteristics is presented in Table 1.
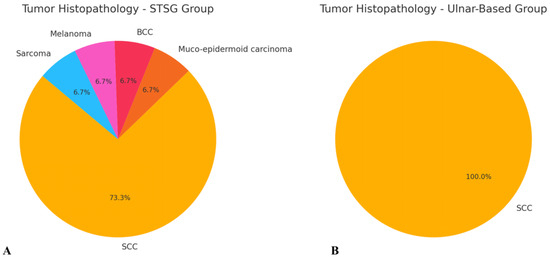
Figure 4.
Schematic representation of tumor histopathology in both evaluated groups: (A) STSG group and (B) ulnar-based flap group. Abbreviations: BCC—basal cell carcinoma; SCC—squamous cell carcinoma.
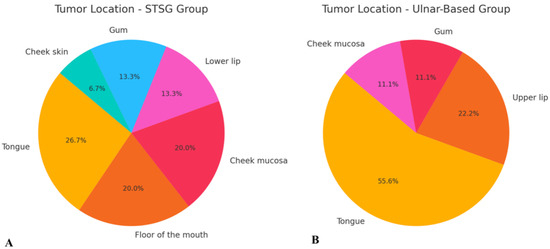
Figure 5.
Schematic representation of tumor location distribution in both evaluated groups: (A) STSG group and (B) ulnar-based flap group. Abbreviation: STSG—split-thickness skin graft.

Table 1.
Patients characteristic.
3.2. Biochemical Results Evaluation
The biochemical analysis was conducted using the mean values of HB, WBC count, PLT count, and albumin before and after surgery. Among these parameters, the only statistically significant difference between the UBTF and STGS groups was observed in PLT levels, both preoperatively (p = 0.0018) and postoperatively (p = 0.0439) (Table 2). Additionally, postoperative CRP evaluation revealed higher CRP levels in the STSG group compared to the UBTF group (64.28 mg/L vs. 47.58 mg/L, respectively), although the difference was not statistically significant (p = 0.747).

Table 2.
Biochemical parameters analysis.
3.3. Aesthetic Flap Assessment
The analysis revealed that, in terms of aesthetic flap assessment, all evaluated parameters—including skin color, skin texture, and flap stability—were significantly better in the UBTF group compared to the STSG group. The most pronounced differences were observed in skin color (p = 0.000021) and skin texture (p = 0.000018), where the UBTF flap group demonstrated superior aesthetic outcomes (Table 3). A postoperative follow-up image taken three months after surgery demonstrated satisfactory aesthetic outcomes following both the STSG and UBTF techniques (Figure 6).

Table 3.
Aesthetic flap assessment.
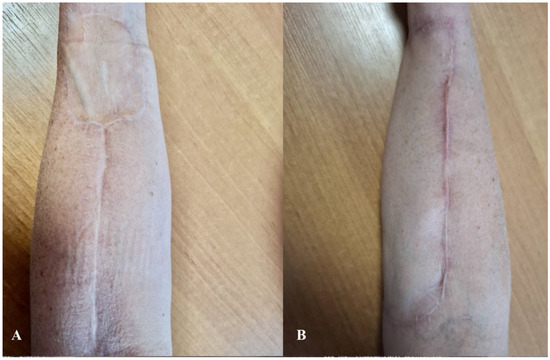
Figure 6.
Schematic representation of aesthetic outcomes three months post-surgery using (A) the STSG technique and (B) the UBTF technique. Abbreviations: STSG—split-thickness skin graft; UBTF—ulnar-based transposition flap.
3.4. Post-Surgical Complications Assessment
The analysis of post-surgical complications revealed no cases of flap necrosis in either group. However, edema, infection, and wound dehiscence were observed more frequently in the STSG group compared to the UBTF group. Despite this trend, the differences were not statistically significant (p > 0.05). In contrast, the evaluation of healing times demonstrated a significantly shorter healing duration in the UBTF group compared to the STSG group (p = 0.0004), indicating a clear advantage in regenerative efficiency (Table 4). Additionally, the analysis did not reveal any significant correlation between patient comorbidities and postoperative complications (Table 5).

Table 4.
Post-surgical complications analysis.

Table 5.
Analysis of patient comorbidities in relation to postoperative complications.
4. Discussion
Efficient, rapid, and complication-free wound healing is a central goal of regenerative medicine. Optimizing the healing process—particularly by reducing healing time—not only accelerates overall patient recovery but also reduces healthcare costs and enhances healthcare system efficiency [34,35]. By leveraging the body’s intrinsic regenerative capacity—often via biologically active materials, growth factor delivery, or stem cell-based therapies—regenerative approaches aim to restore tissue structure and function more effectively than traditional methods [36,37].
In the era of regenerative medicine, a wide array of wound dressings, including artificial skin substitutes, has been developed. Although artificial wound dressings, such as synthetic skin substitutes and bioengineered scaffolds, have advanced significantly and contribute to wound healing by promoting moisture retention, infection control, and even cellular stimulation, they typically serve as temporary covers that facilitate secondary intention healing rather than providing immediate, definitive tissue replacement [38,39,40]. In contrast, skin grafting techniques such as STSG remain the gold standard for donor site closure following RFFF harvest. STSG offers definitive coverage, biological integration, and durable mechanical strength, qualities that artificial dressings have yet to fully replicate [41]. Moreover, skin grafts achieve full dermal–epidermal barrier restoration and are particularly suitable for covering larger or surgically created defects like RFFF donor sites, where rapid, stable closure is essential to minimize morbidity and functional impairment.
Therefore, while advanced technologies in tissue healing are increasingly adopted in daily clinical practice, surgical techniques focused on minimizing healing time still remain foundational to successful patient care [42]. The synergistic integration of regenerative technologies with time-tested surgical approaches holds the greatest promise for optimizing both functional recovery and cost-effective treatment, thereby advancing standards in modern wound management.
Given the high risk of complications and morbidity associated with head and neck cancer reconstructive surgery, approaches that promote faster healing are critically important [43]. In this study, we evaluated STSG and UBTF as two distinct strategies for donor site closure following RFFF harvest. Although a range of closure techniques have been described in the literature, STSG remains the most commonly used method [44]. The main advantage of STSG lies in its ability to be precisely tailored to the donor site dimensions; however, it requires advanced surgical skills and creates an additional donor site wound [45]. Conversely, UBTF is relatively easy to perform but may be unsuitable for larger defects where the flap may not fully cover the donor area [46].
Our findings showed comparable postoperative complication rates between the two groups, with no instances of flap failure and no influence of patient comorbidities on healing outcomes. However, healing times were significantly shorter in the UBTF group. Despite requiring an additional incision, UBTF demonstrated favorable wound healing, likely due to its reliable vascular supply [32,47]. In contrast, STSG healing is typically slower and carries a higher risk of complications due to its reliance on a multi-phase biological process involving plasmatic imbibition, inosculation, and revascularization [48,49] (Figure 7). During the plasmatic imbibition phase, essential nutrients—including oxygen, glucose, and electrolytes—passively diffuse from the wound bed into the skin graft to maintain cellular viability. This is followed by the inosculation phase, during which initial vascular connections begin to form between the graft and the recipient site. Subsequently, revascularization occurs, characterized by active angiogenesis and the establishment of a functional microvascular network. Ultimately, the healing process culminates in tissue integration and remodeling, leading to the restoration of structural and functional integrity [50,51]. The results of our study are also consistent with those reported by Loeffelbein et al., reinforcing the regenerative advantages of vascularized local flaps over grafts for donor site reconstruction [52].
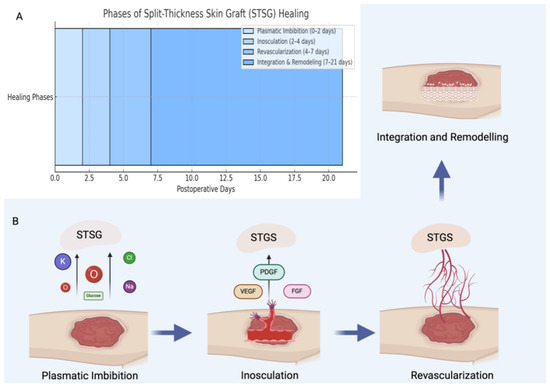
Figure 7.
Schematic illustration showing the approximate timeline of the STSG healing process (A), followed by a step-by-step depiction of the biological phases involved (B). The process begins with plasmatic imbibition, during which basic nutrients and oxygen diffuse passively from the wound bed into the graft. This is followed by the phase of inosculation, where initial vascular connections begin to form between the graft and the recipient site. Subsequently, revascularization occurs, marked by active angiogenesis that restores a functional blood supply. Finally, the healing process concludes with integration and remodeling, during which the grafted tissue matures and reorganizes structurally to achieve long-term stability and functionality. Abbreviations: STSG—split-thickness skin graft; PDGF—platelet-derived growth factor; VEGF—vascular endothelial growth factor; FGF—fibroblast growth factor.
Importantly, the factors influencing wound healing extend beyond flap design. Our biochemical analysis found no significant differences in HB, WBC, or albumin levels between the two groups. Low infection rates may explain the only slightly elevated WBC values, likely attributed to the surgical intervention itself. However, PLT counts were significantly higher in the UBTF group. Given the role of PLT in clot formation and angiogenesis—mediated by the release of PDGF, VEGF, and FGF—their elevated levels may contribute to enhanced neovascularization and the reduced healing times observed in the UBTF group [53]. Lacci et al. emphasized the growing role of platelet-rich preparations in enhancing wound closure [54], while Margolis et al. demonstrated the superior efficacy of platelet releasate over standard care in treating diabetic foot ulcers [55]. High PLT counts may therefore represent a biomarker of regenerative capacity, potentially guiding flap selection in surgical planning.
The aesthetic outcomes further favored the UBTF group, which showed superior skin color, texture, and flap stability. These improvements are likely due to the use of native forearm skin, which better matches surrounding tissue. In contrast, STSG is typically harvested from other anatomical areas such as the groin, thigh, or abdomen [56], where differences in texture and pigmentation can result in less favorable aesthetic outcomes. The superior flap stability observed in the UBTF group may also be attributed to the use of tissue with similar biomechanical properties and shorter healing durations.
The primary limitations of this study include its small sample size and relatively short follow-up period. Nevertheless, most major postoperative complications occur within the first few days following surgery, aligning with the timeline of this study. Future research should involve larger cohorts and deeper investigations into the molecular and biochemical mechanisms underlying wound healing.
This study lays important groundwork and provides novel insights into regenerative and surgical approaches for donor site closure following RFFF harvest. Elevated PLT levels may serve as a predictive marker of wound healing efficacy. Compared to the widely used STSG method, UBTF demonstrates significant advantages in healing time, aesthetic outcomes, and potential regenerative benefits, establishing it as a compelling alternative in clinical practice.
5. Conclusions
Regenerative medicine plays a pivotal role in advancing modern clinical practice by enhancing tissue repair strategies. This study demonstrates that the use of UBTF offers significant advantages over STSG for donor site closure following RFFF harvest. Specifically, UBTF was associated with shorter healing times, superior aesthetic outcomes (including better skin color, texture, and flap stability), and a lower incidence of postoperative complications. Additionally, elevated PLT levels in the UBTF group may have contributed to enhanced angiogenesis and improved wound healing. These findings suggest that the UBTF technique may represent a superior and more efficient alternative to traditional STSG closure methods. Nevertheless, further studies with larger patient cohorts and molecular-level evaluations are warranted to validate these promising results and to refine regenerative surgical strategies for clinical application.
Author Contributions
A.G.: Conceptualization, formal analysis, investigation, methodology, writing—original draft, writing—review & editing. K.S.: Investigation, methodology, formal analysis, writing—review & editing, writing—original draft. K.B.-P.: Conceptualization, data curation, investigation, methodology, supervision, visualization, writing—review & editing. M.P.: Conceptualization, investigation, methodology, supervision, writing—review & editing. M.M.-G.: Investigation, methodology, writing—review & editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The Ethics Committee of the Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw waived the need for ethics approval and patient consent for the collection, analysis, and publication of the retrospectively obtained and anonymised data for this study.
Informed Consent Statement
Informed consent for the information published in this article was not required, as it was waived by the Ethics Committee of the Maria Sklodowska-Curie National Research Institute of Oncology in Warsaw. The study involved the retrospective collection, analysis, and publication of anonymized data from a non-interventional study.
Data Availability Statement
All data supporting the findings of this study are available upon reasonable request from the corresponding author.
Acknowledgments
The authors would like to acknowledge BioRender software (Science Suite Inc., Toronto, ON, Canada) for its use in creating figures. The citation is as follows: “Created with BioRender.” Publications, P. (2025). Available at: https://BioRender.com/j57d385 accessed on 3 October 2025.
Conflicts of Interest
The authors declare that they have no potential conflicts of interest related to this work. Dr. Magdalena Misiak-Gałązka is affiliated with both the Evimed Medical Center and the Maria Sklodowska-Curie National Research Institute of Oncology; however, this dual affiliation has not influenced the content or outcomes of the study.
Abbreviations
The following abbreviations are used in this manuscript:
| BCC | Basal cell carcinoma |
| CRP | C-reactive protein |
| DM2 | Diabetes mellitus type 2 |
| FGF | Fibroblast growth factor |
| FTSG | Full-thickness skin graft |
| HB | Hemoglobin |
| PDGF | Platelet-derived growth factor |
| PLT | Platelets |
| RFFF | Radial free forearm flap |
| SCC | Squamous cell carcinoma |
| STGS | Split-thickness skin graft |
| UBTF | Ulnar-based transposition flap |
| VEGF | Vascular endothelial growth factor |
| WBC | White blood cells |
References
- Markiewicz-Gospodarek, A.; Kozioł, M.; Tobiasz, M.; Baj, J.; Radzikowska-Büchner, E.; Przekora, A. Burn Wound Healing: Clinical Complications, Medical Care, Treatment, and Dressing Types: The Current State of Knowledge for Clinical Practice. Int. J. Environ. Res. Public Health 2022, 19, 1338. [Google Scholar] [CrossRef]
- Tottoli, E.M.; Dorati, R.; Genta, I.; Chiesa, E.; Pisani, S.; Conti, B. Skin Wound Healing Process and New Emerging Technologies for Skin Wound Care and Regeneration. Pharmaceutics 2020, 12, 735. [Google Scholar] [CrossRef] [PubMed]
- Pang, C.; Ibrahim, A.; Bulstrode, N.W.; Ferretti, P. An overview of the therapeutic potential of regenerative medicine in cutaneous wound healing. Int. Wound J. 2017, 14, 450–459. [Google Scholar] [CrossRef] [PubMed]
- Dutra Alves, N.S.; Reigado, G.R.; Santos, M.; Caldeira, I.D.S.; Hernandes, H.D.S.; Freitas-Marchi, B.L.; Zhivov, E.; Chambergo, F.S.; Nunes, V.A. Advances in regenerative medicine-based approaches for skin regeneration and rejuvenation. Front. Bioeng. Biotechnol. 2025, 13, 1527854. [Google Scholar] [CrossRef]
- Kumar, R.; Kumar, V.; Mohan, A.; Gupta, G.; Kashyap, V. Translational research in the generation of therapeutic medicine for wound healing: A review. Discov. Med. 2024, 1, 158. [Google Scholar] [CrossRef]
- Dean, J.; Hoch, C.; Wollenberg, B.; Navidzadeh, J.; Maheta, B.; Mandava, A.; Knoedler, S.; Sherwani, K.; Baecher, H.; Schmitz, A.; et al. Advancements in bioengineered and autologous skin grafting techniques for skin reconstruction: A comprehensive review. Front. Bioeng. Biotechnol. 2025, 12, 1461328. [Google Scholar] [CrossRef]
- Šuca, H.; Čoma, M.; Tomšů, J.; Sabová, J.; Zajíček, R.; Brož, A.; Doubková, M.; Novotný, T.; Bačáková, L.; Jenčová, V.; et al. Current Approaches to Wound Repair in Burns: How far Have we Come from Cover to Close? A Narrative Review. J. Surg. Res. 2024, 296, 383–403. [Google Scholar] [CrossRef]
- Kaur, A.; Midha, S.; Giri, S.; Mohanty, S. Functional Skin Grafts: Where Biomaterials Meet Stem Cells. Stem Cells Int. 2019, 2019, 1286054. [Google Scholar] [CrossRef]
- Motamedi, S.; Esfandpour, A.; Babajani, A.; Jamshidi, E.; Bahrami, S.; Niknejad, H. The Current Challenges on Spray-Based Cell Delivery to the Skin Wounds. Tissue Eng. Part C Methods 2021, 27, 543–558. [Google Scholar] [CrossRef]
- Gerlach, J.C.; Johnen, C.; Ottomann, C.; Bräutigam, K.; Plettig, J.; Belfekroun, C.; Münch, S.; Hartmann, B. Method for autologous single skin cell isolation for regenerative cell spray transplantation with non-cultured cells. Int. J. Artif. Organs 2011, 34, 271–279. [Google Scholar] [CrossRef]
- Carvalho, L.N.; Peres, L.C.; Alonso-Goulart, V.; Santos, B.J.; Braga, M.F.; Campos, F.D.; Palis, G.D.; Quirino, L.S.; Guimarães, L.D.; Lafetá, S.A.; et al. Recent advances in the 3D skin bioprinting for regenerative medicine: Cells, biomaterials, and methods. J. Biomater. Appl. 2024, 39, 421–438. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Park, S.A.; Kim, W.D.; Ha, T.; Xin, Y.-Z.; Lee, J.; Lee, D. Current Advances in 3D Bioprinting Technology and Its Applications for Tissue Engineering. Polymers 2020, 12, 2958. [Google Scholar] [CrossRef]
- Weng, T.; Zhang, W.; Xia, Y.; Wu, P.; Yang, M.; Jin, R.; Xia, S.; Wang, J.; You, C.; Han, C.; et al. 3D bioprinting for skin tissue engineering: Current status and perspectives. J. Tissue Eng. 2021, 12, 20417314211028574. [Google Scholar] [CrossRef]
- Kianian, S.; Zhao, K.; Kaur, J.; Lu, K.W.; Rathi, S.; Ghosh, K.; Rogoff, H.; Hays, T.R.; Park, J.; Rafailovich, M.; et al. Autologous Skin Grafts, versus Tissue-engineered Skin Constructs: A Systematic Review and Meta-analysis. Plast. Reconstr. Surg. Glob. Open 2023, 11, e5100. [Google Scholar] [CrossRef] [PubMed]
- Kozak, G.M.; Hsu, J.Y.; Broach, R.B.; Shakir, S.; Calvert, C.; Stranix, J.T.; Messa C4th Levin, L.S.; Serletti, J.M.; Kovach, S.J.; Fischer, J.P. Comparative Effectiveness Analysis of Complex Lower Extremity Reconstruction: Outcomes and Costs for Biologically Based, Local Tissue Rearrangement, and Free Flap Reconstruction. Plast Reconstr. Surg. 2020, 145, 608e–616e. [Google Scholar] [CrossRef]
- Kolimi, P.; Narala, S.; Nyavanandi, D.; Youssef, A.A.A.; Dudhipala, N. Innovative Treatment Strategies to Accelerate Wound Healing: Trajectory and Recent Advancements. Cells 2022, 11, 2439. [Google Scholar] [CrossRef]
- Norman, G.; Wong, J.K.; Amin, K.; Dumville, J.C.; Pramod, S. Reconstructive surgery for treating pressure ulcers. Cochrane Database Syst. Rev. 2022, 10, CD012032. [Google Scholar] [PubMed]
- Vindigni, V.; Marena, F.; Zanettin, C.; Bassetto, F. Breast Reconstruction: The Oncoplastic Approach. J. Clin. Med. 2024, 13, 4718. [Google Scholar] [CrossRef]
- Thariat, J.; Carsuzaa, F.; Beddok, A.; Deneuve, S.; Marcy, P.Y.; Merlotti, A.; Dejean, C.; Devauchelle, B. Reconstructive flap surgery in head and neck cancer patients: An interdisciplinary view of the challenges encountered by radiation oncologists in postoperative radiotherapy. Front. Oncol. 2024, 14, 1379861. [Google Scholar] [CrossRef]
- Ibrahim, B.; Rahal, A.; Bissada, E.; Christopoulos, A.; Guertin, L.; Ayad, T. Reconstruction of medium-size defects of the oral cavity: Radial forearm free flap vs facial artery musculo-mucosal flap. J. Otolaryngol. Head Neck Surg. 2021, 50, 67. [Google Scholar] [CrossRef]
- Gałązka, A.; Bieńkowska-Pluta, K.; Kalecińska, Z.; Krupa, Z.; Woźniczko, K. Thumb-up modification of the radial forearm free flap in oral cavity reconstruction: A description of a surgical technique. Pol. Otorhino Rev. 2024, 13, 36–39. [Google Scholar] [CrossRef]
- Wood, J.W.; Broussard, K.C.; Burkey, B. Preoperative Testing for Radial Forearm Free Flaps to Reduce Donor Site Morbidity. JAMA Otolaryngol. Head Neck Surg. 2013, 139, 183–186. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.K.; Lee, K.T. Coverage of radial forearm free flap donor site defect using another free flap. Microsurgery 2023, 43, 775–781. [Google Scholar] [CrossRef]
- Wirthmann, A.; Finke, J.C.; Giovanoli, P.; Lindenblatt, N. Long-term follow-up of donor site morbidity after defect coverage with Integra following radial forearm flap elevation. Eur. J. Plast Surg. 2014, 37, 159–166. [Google Scholar] [CrossRef]
- Shah, R.; Rodrigues, R.; Phillips, V.; Khatib, M. The use of artificial dermal substitutes for repair of the donor site following harvesting of a radial forearm free flap: A systematic review. J. Plast Reconstr. Aesthet. Surg. 2024, 88, 501–516. [Google Scholar] [CrossRef]
- Giordano, L.; Bondi, S.; Ferrario, F.; Fabiano, B.; Bussi, M. Radial forearm free flap surgery: A modified skin-closure technique improving donor-site aesthetic appearance. Acta Otorhinolaryngol. Ital. 2012, 32, 158–163. [Google Scholar] [PubMed]
- Ho, T.; Couch, M.; Carson, K.; Schimberg, A.; Manley, K.; Byrne, P.J. Radial forearm free flap donor site outcomes comparison by closure methods. Otolaryngol. Head Neck Surg. 2006, 134, 309–315. [Google Scholar] [CrossRef]
- Hong, S. Risk Factors for Postoperative Donor Site Complications in Radial Forearm Free Flaps. Medicina 2024, 60, 1487. [Google Scholar] [CrossRef]
- Zhang, C.; Pandya, S.; Alessandri Bonetti, M.; Costantino, A.; Egro, F.M. Comparison of split thickness skin graft versus full thickness skin graft for radial forearm flap donor site closure: A systematic review and Meta-analysis. Am. J. Otolaryngol. 2024, 45, 104156. [Google Scholar] [CrossRef]
- Al-Aroomi, M.A.; Mashrah, M.A.; Al-Worafi, N.A.; Zhou, W.; Sun, C.; Pan, C. Biomechanical and aesthetic outcomes following radial forearm free flap transfer: Comparison of ipsilateral full-thickness skin graft and traditional split-thickness skin graft. Int. J. Oral Maxillofac. Surg. 2024, 53, 109–116. [Google Scholar] [CrossRef]
- Mashrah, M.A.; Lingjian, Y.; Handley, T.P.; Pan, C.; Weiliang, C. Novel technique for the direct closure of the radial forearm flap donor site defect with a local bilobed flap. Head Neck 2019, 41, 3282–3289. [Google Scholar] [CrossRef] [PubMed]
- Jaquet, Y.; Enepekides, D.J.; Torgerson, C.; Higgins, K.M. Radial forearm free flap donor site morbidity: Ulnar-based transposition flap vs split-thickness skin graft. Arch. Otolaryngol. Head Neck Surg. 2012, 138, 38–43. [Google Scholar] [CrossRef]
- Marchesi, A.; Gatto, A.; Cavalli, E.M.; Del Bene, M. Free-style propeller ulnar artery perforator flaps for radial forearm flap donor site repair. Microsurgery 2024, 44, e31074. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, G.K.; Martinez-Rodriguez, S.; Md Fadilah, N.I.; Looi Qi Hao, D.; Markey, G.; Shukla, P.; Fauzi, M.B.; Panetsos, F. Progress in Wound-Healing Products Based on Natural Compounds, Stem Cells, and MicroRNA-Based Biopolymers in the European, USA, and Asian Markets: Opportunities, Barriers, and Regulatory Issues. Polymers 2024, 16, 1280. [Google Scholar] [CrossRef] [PubMed]
- Olteanu, G.; Neacșu, S.M.; Joița, F.A.; Musuc, A.M.; Lupu, E.C.; Ioniță-Mîndrican, C.-B.; Lupuliasa, D.; Mititelu, M. Advancements in Regenerative Hydrogels in Skin Wound Treatment: A Comprehensive Review. Int. J. Mol. Sci. 2024, 25, 3849. [Google Scholar] [CrossRef]
- Velikic, G.; Maric, D.M.; Maric, D.L.; Supic, G.; Puletic, M.; Dulic, O.; Vojvodic, D. Harnessing the Stem Cell Niche in Regenerative Medicine: Innovative Avenue to Combat Neurodegenerative Diseases. Int. J. Mol. Sci. 2024, 25, 993. [Google Scholar] [CrossRef]
- Hussen, B.M.; Taheri, M.; Yashooa, R.K.; Abdullah, G.H.; Abdullah, S.R.; Kheder, R.K.; Mustafa, S.A. Revolutionizing medicine: Recent developments and future prospects in stem-cell therapy. Int. J. Surg. 2024, 110, 8002–8024. [Google Scholar] [CrossRef]
- Urciuolo, F.; Casale, C.; Imparato, G.; Netti, P.A. Bioengineered Skin Substitutes: The Role of Extracellular Matrix and Vascularization in the Healing of Deep Wounds. J. Clin. Med. 2019, 8, 2083. [Google Scholar] [CrossRef]
- Vecin, N.M.; Kirsner, R.S. Skin substitutes as treatment for chronic wounds: Current and future directions. Front. Med. 2023, 10, 1154567. [Google Scholar] [CrossRef]
- Zhang, M.; Xing, J.; Zhong, Y.; Zhang, T.; Liu, X.; Xing, D. Advanced function, design and application of skin substitutes for skin regeneration. Mater. Today Bio 2023, 24, 100918. [Google Scholar] [CrossRef]
- Saleki, M.; Noor, M.A.; Hurt, P.; Abul, A. Full-Thickness Skin Graft Versus Split-Thickness Skin Graft for Radial Forearm Free Flap Transfer in Oral Cavity Reconstruction: A Systematic Review and Meta-Analysis. Cureus 2023, 15, e49279. [Google Scholar] [CrossRef] [PubMed]
- Niederstätter, I.M.; Schiefer, J.L.; Fuchs, P.C. Surgical Strategies to Promote Cutaneous Healing. Med. Sci. 2021, 9, 45. [Google Scholar] [CrossRef] [PubMed]
- Mulvey, C.L.; Brant, J.A.; Bur, A.M.; Chen, J.; Fischer, J.P.; Cannady, S.B.; Newman, J.G. Complications Associated with Mortality after Head and Neck Surgery. Otolaryngol. Head Neck Surg. 2017, 156, 504–510. [Google Scholar] [CrossRef] [PubMed]
- Zuidam, J.M.; Coert, J.H.; Hofer, S.O. Closure of the donor site of the free radial forearm flap: A comparison of full-thickness graft and split-thickness skin graft. Ann. Plast Surg. 2005, 55, 612–616. [Google Scholar] [CrossRef]
- Manek, Y.B.; Jajoo, S.; Mahakalkar, C. A Comprehensive Review of Evaluating Donor Site Morbidity and Scar Outcomes in Skin Transfer Techniques. Cureus 2024, 16, e53433. [Google Scholar] [CrossRef]
- Benanti, E.; De Santis, G.; Leti Acciaro, A.; Colzani, G.; Baccarani, A.; Starnoni, M. Soft tissue coverage of the upper limb: A flap reconstruction overview. Ann. Med. Surg. 2020, 60, 338–343. [Google Scholar] [CrossRef]
- Patel, R. Reducing morbidity in radial forearm free flap donor site: A review of closure techniques. Curr. Opin. Otolaryngol. Head Neck Surg. 2022, 30, 363–367. [Google Scholar] [CrossRef]
- Converse, J.M.; Uhlschmid, G.K.; Ballantyne, D.L. “Plasmatic circulation” in skin grafts. The phase of serum imbibition. Plast Reconstr. Surg. 1969, 43, 495–499. [Google Scholar] [CrossRef]
- Converse, J.M.; Smahel, J.; Ballantyne, D.L.; Harper, A.D. Inosculation of vessels of skin graft and host bed: A fortuitous encounter. Br. J. Plast Surg. 1975, 28, 274–282. [Google Scholar] [CrossRef]
- Williams, Z.J.; Pezzanite, L.M.; Hendrickson, D.A. Review of skin grafting in equine wounds: Indications and techniques. Equine Vet. Educ. 2024, 36, 484–493. [Google Scholar] [CrossRef]
- Kanapathy, M.; Hachach-Haram, N.; Bystrzonowski, N.; Connelly, J.T.; O’Toole, E.A.; Becker, D.L.; Mosahebi, A.; Richards, T. Epidermal grafting for wound healing: A review on the harvesting systems, the ultrastructure of the graft and the mechanism of wound healing. Int. Wound J. 2017, 14, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Loeffelbein, D.J.; Al-Benna, S.; Steinsträßer, L.; Satanovskij, R.M.; Rohleder, N.H.; Mücke, T.; Wolff, K.D.; Kesting, M.R. Reduction of donor site morbidity of free radial forearm flaps: What level of evidence is available? Eplasty 2012, 12, e9. [Google Scholar] [PubMed]
- Locatelli, L.; Colciago, A.; Castiglioni, S.; Maier, J.A. Platelets in Wound Healing: What Happens in Space? Front. Bioeng. Biotechnol. 2021, 9, 716184. [Google Scholar] [CrossRef]
- Lacci, K.M.; Dardik, A. Platelet-rich plasma: Support for its use in wound healing. Yale J. Biol. Med. 2010, 83, 1–9. [Google Scholar] [PubMed]
- Margolis, D.J.; Kantor, K.; Santanna, J.; Strom, B.L.; Berlin, J.A. Effectiveness of platelet releasate for the treatment of diabetic neuropathic foot ulcers. Diabetes Care 2001, 24, 483–488. [Google Scholar] [CrossRef]
- Aleman Paredes, K.; Selaya Rojas, J.C.; Flores Valdés, J.R.; Castillo, J.L.; Montelongo Quevedo, M.; Mijangos Delgado, F.J.; de la Cruz Durán, H.A.; Nolasco Mendoza, C.L.; Nuñez Vazquez, E.J. A Comparative Analysis of the Outcomes of Various Graft Types in Burn Reconstruction Over the Past 24 Years: A Systematic Review. Cureus 2024, 16, e54277. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).