Inhibition of Th2 Differentiation Accelerates Chronic Wound Healing by Facilitating Lymphangiogenesis
Abstract
1. Introduction
2. Materials and Methods
3. Results
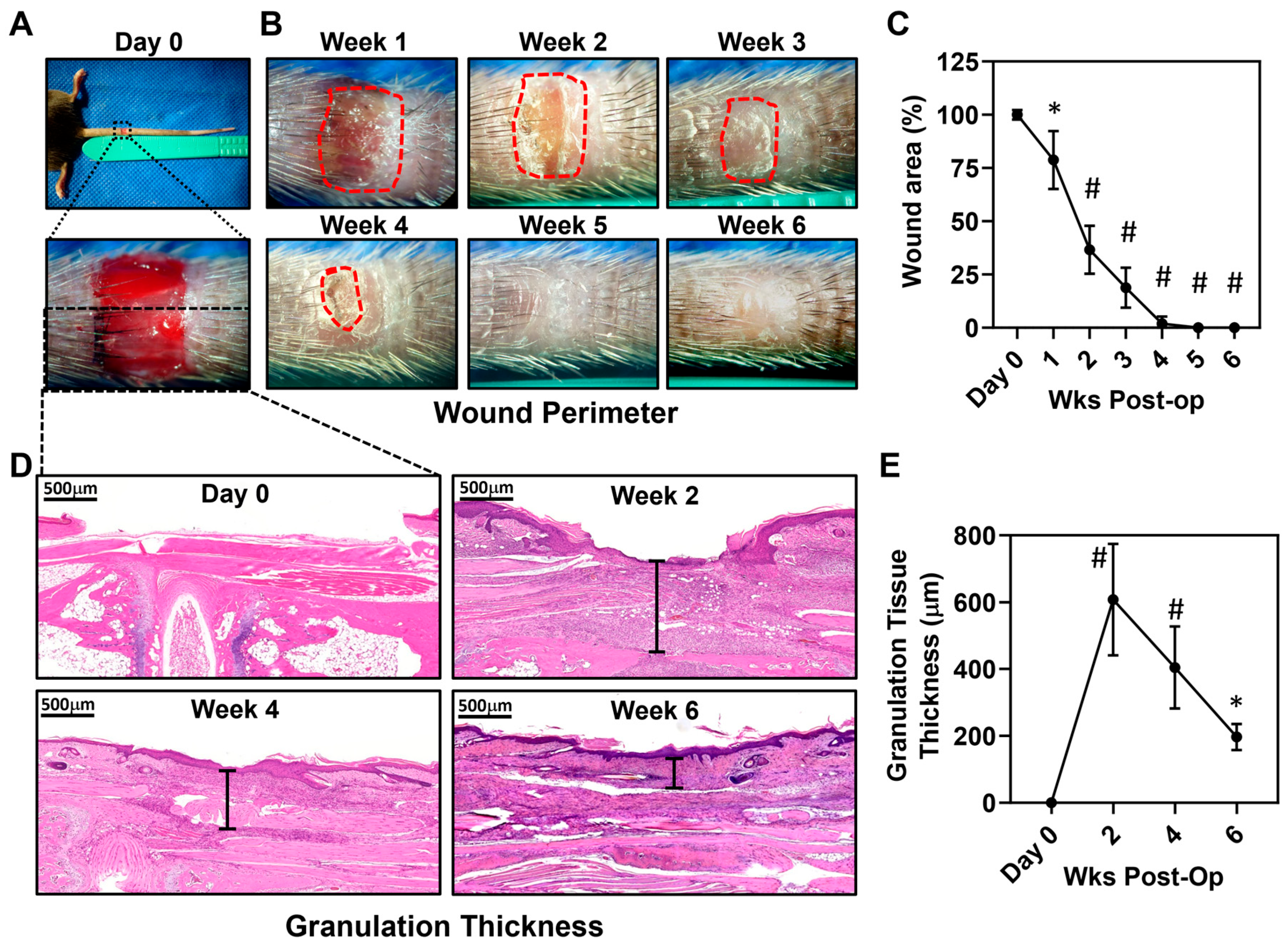
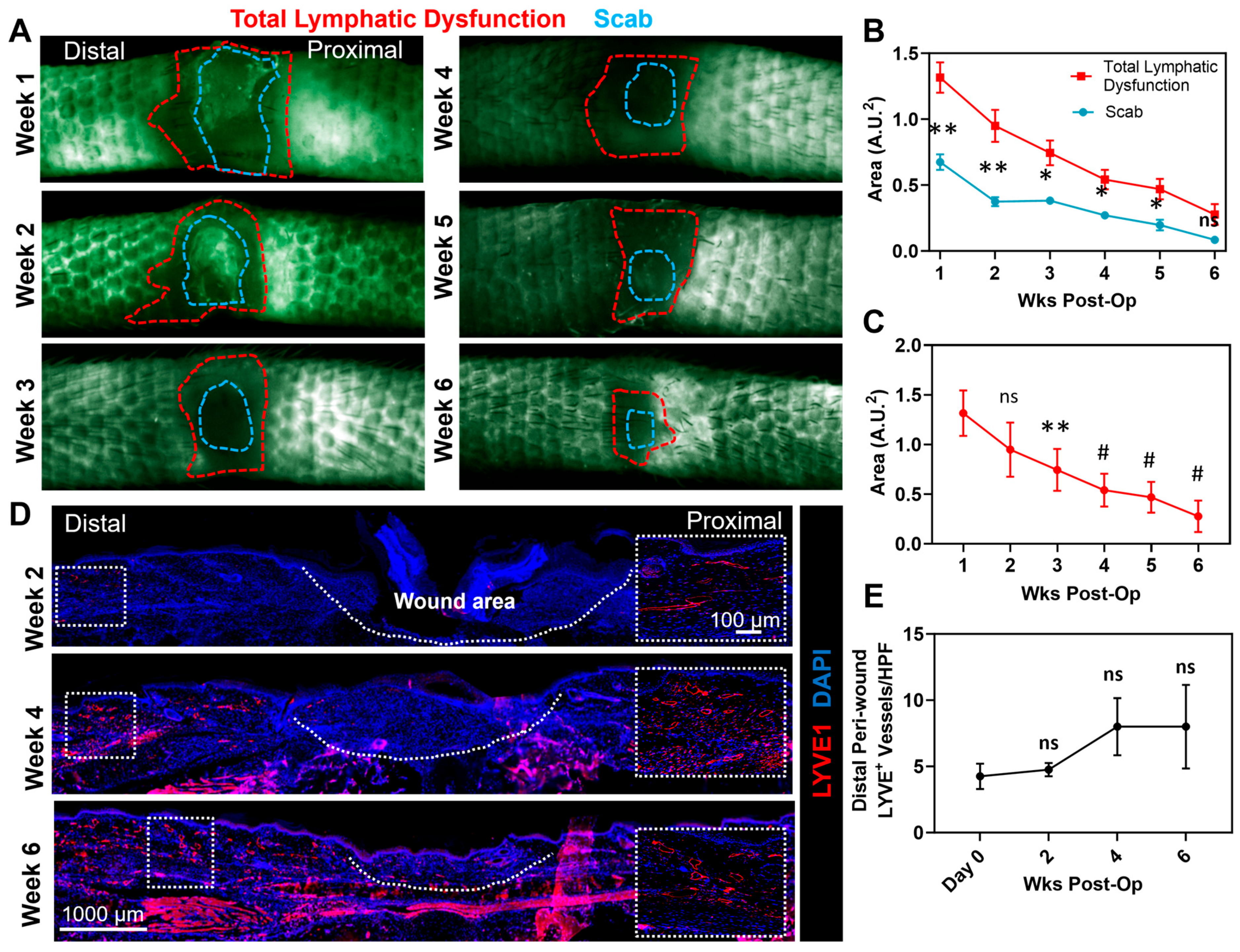
3.1. Full-Thickness Tail Excisions Result in Chronic Wounds That Persist for 4 Weeks and Are Surrounded by a Zone of Dysfunctional Lymphatics
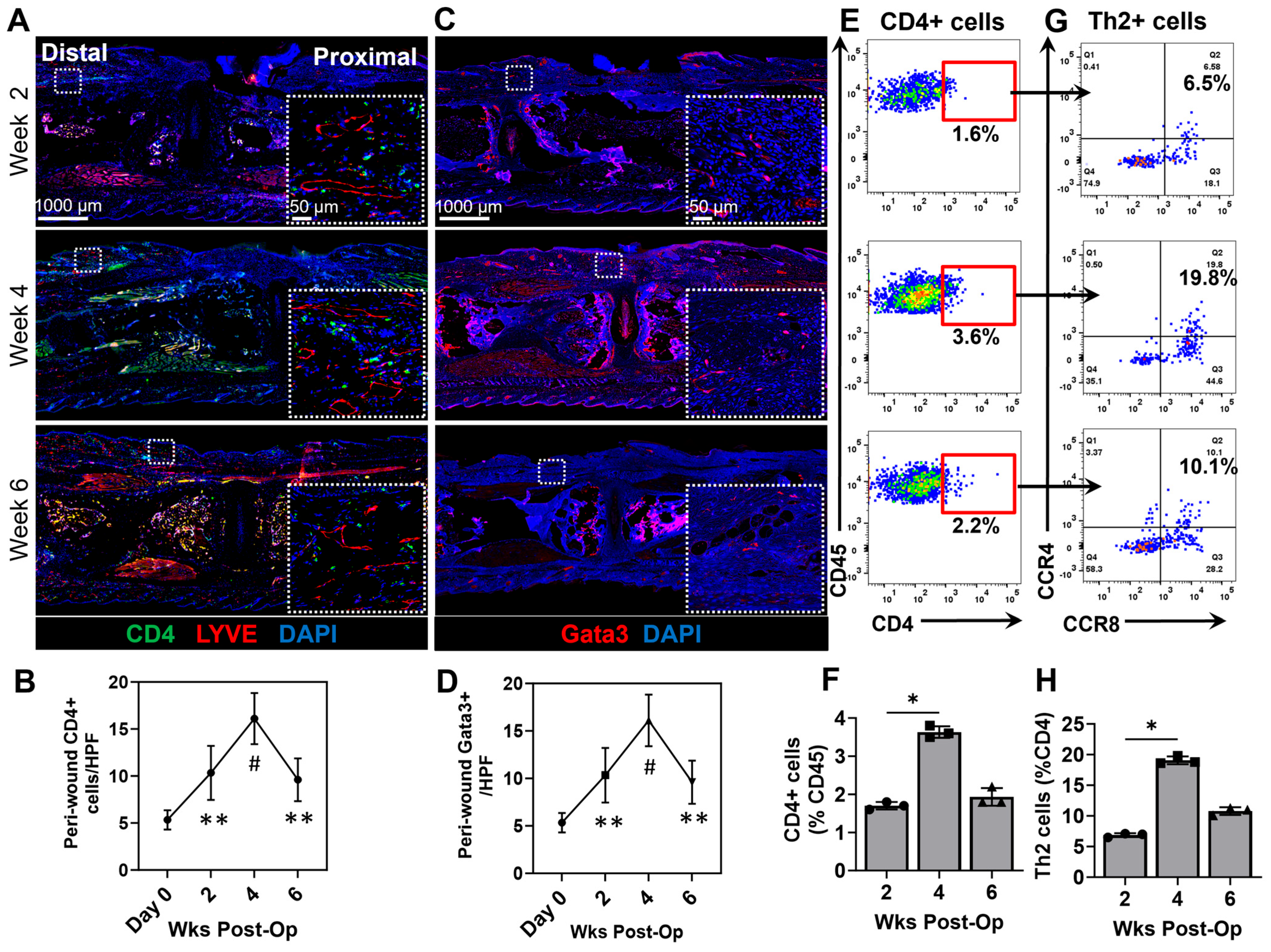
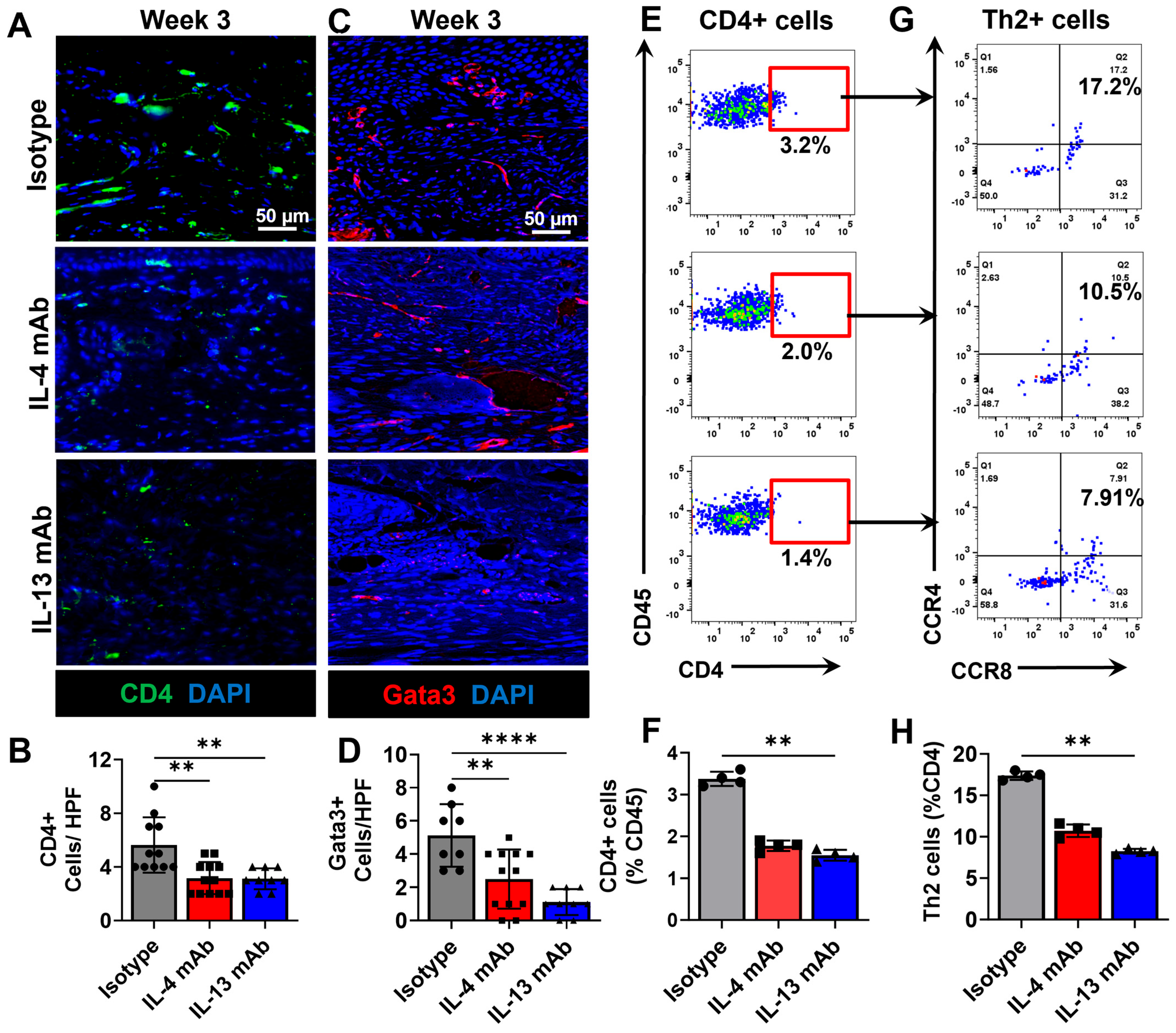
3.2. Chronic Wounds Result in a T-Helper Cell Infiltration and Th2 Differentiation
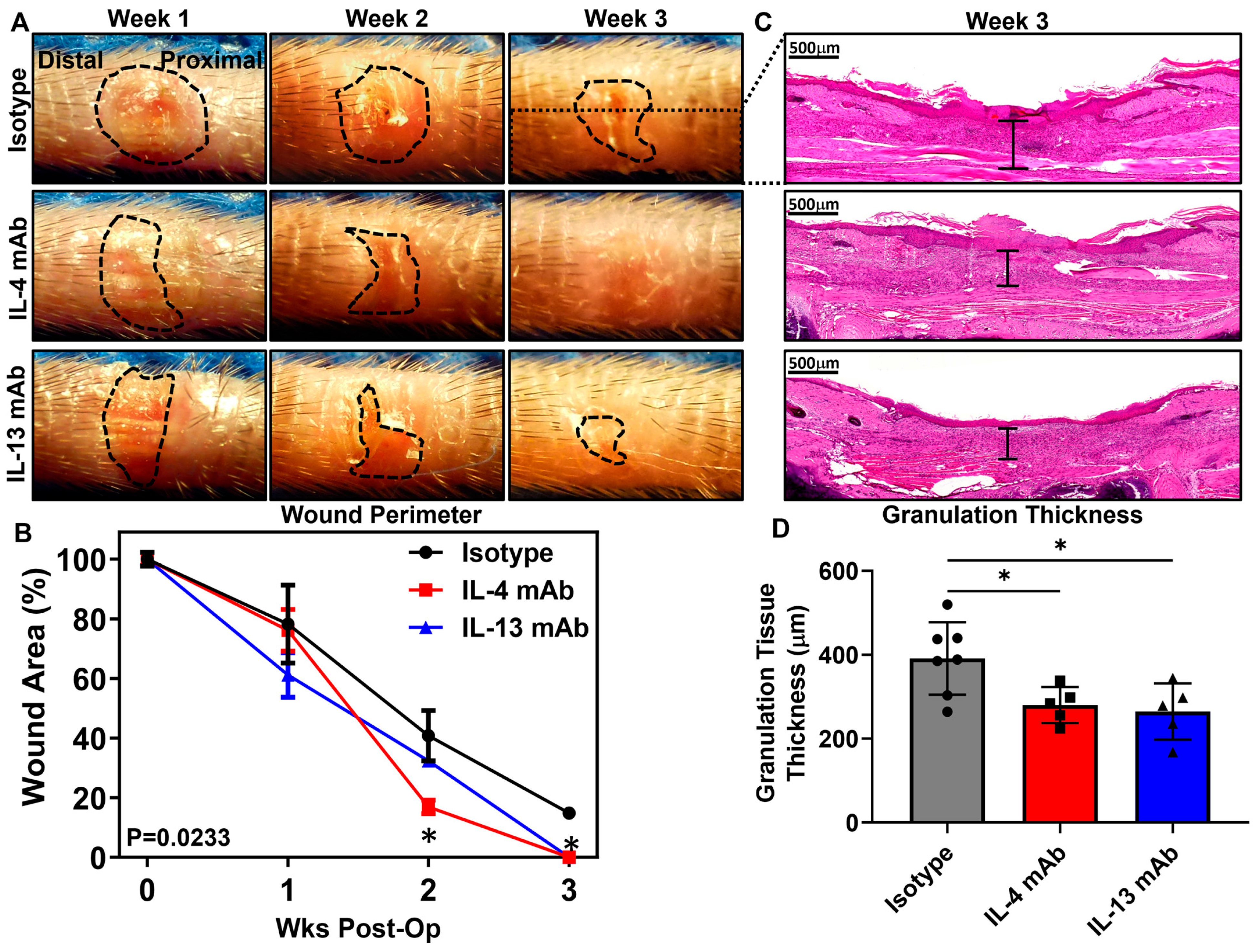
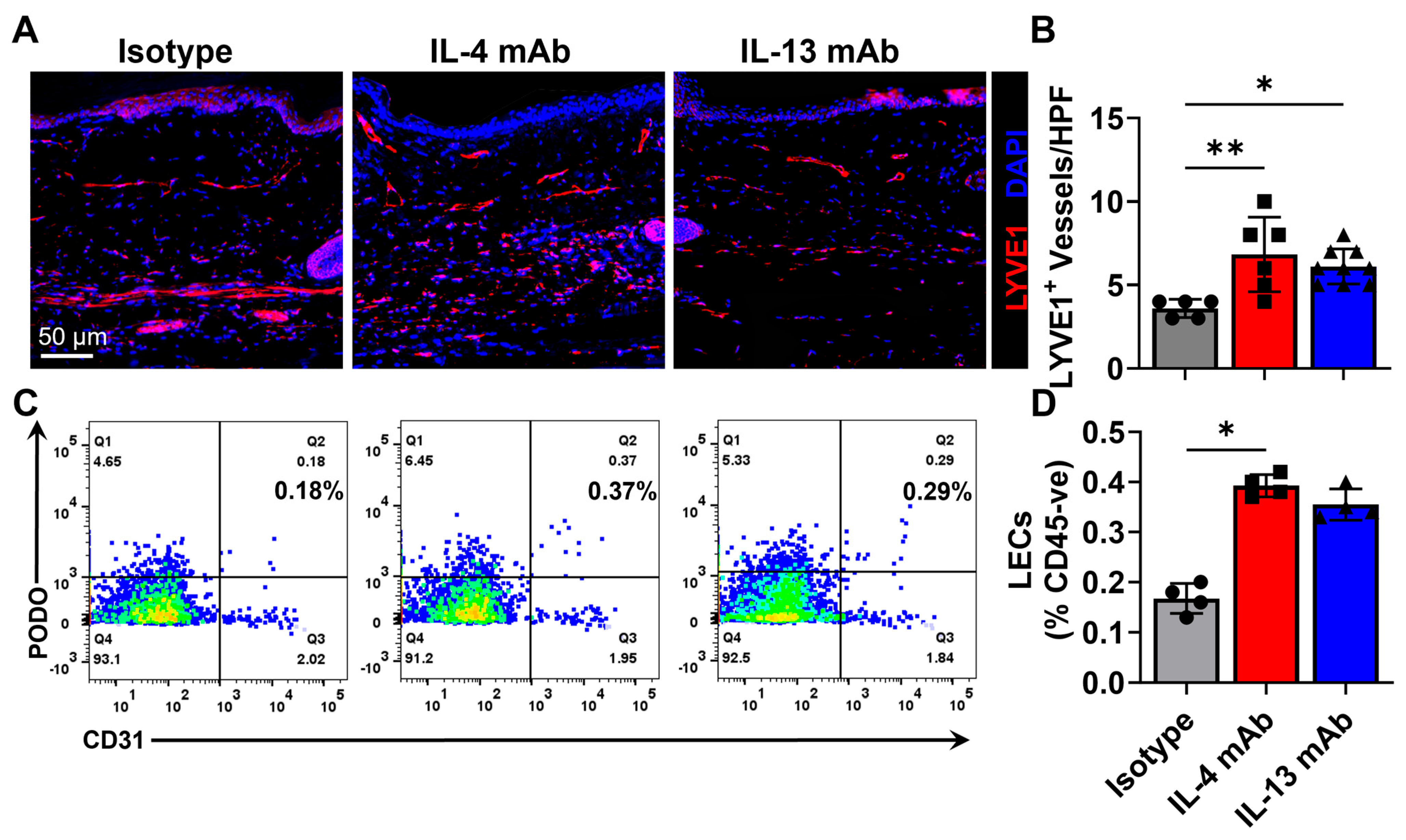
3.3. Healing of Excisional Wounds and Lymphangiogenesis Is Accelerated by Inhibition of Th2 Cytokines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| LEC | Lymphatic endothelial cell |
| Th2 | T-helper-2 |
| IACUC | Institutional Animal Care and Use Committee |
| MSKCC | Memorial Sloan Kettering Cancer Center |
| mAB | Monoclonal antibody |
| FITC | Fluorescein isothiocyanate |
| kDa | Kilodalton |
| EDTA | Ethylenediaminetetraacetic acid |
| H2O2 | Hydrogen peroxide |
| HPF | High-power field |
| ANOVA | Analysis of variance |
| VEGF-C | Vascular endothelial growth factor C |
| LYVE-1 | Lymphatic vessel endothelial hyaluronan receptor-1 |
References
- Siddiqui, A.R.; Bernstein, J.M. Chronic wound infection: Facts and controversies. Clin. Dermatol. 2010, 28, 519–526. [Google Scholar] [CrossRef] [PubMed]
- Bickers, D.R.; Lim, H.W.; Margolis, D.; Weinstock, M.A.; Goodman, C.; Faulkner, E.; Gould, C.; Gemmen, E.; Dall, T. The burden of skin diseases: 2004 a joint project of the American Academy of Dermatology Association and the Society for Investigative Dermatology. J. Am. Acad. Dermatol. 2006, 55, 490–500. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum, S.R.; Carter, M.J.; Fife, C.E.; DaVanzo, J.; Haught, R.; Nusgart, M.; Cartwright, D. An Economic Evaluation of the Impact, Cost, and Medicare Policy Implications of Chronic Nonhealing Wounds. Value Health 2018, 21, 27–32. [Google Scholar] [CrossRef]
- Leaper, D.J.; Durani, P. Topical antimicrobial therapy of chronic wounds healing by secondary intention using iodine products. Int. Wound J. 2008, 5, 361–368. [Google Scholar] [CrossRef]
- Nunan, R.; Harding, K.G.; Martin, P. Clinical challenges of chronic wounds: Searching for an optimal animal model to recapitulate their complexity. Dis. Models Mech. 2014, 7, 1205–1213. [Google Scholar] [CrossRef]
- Eriksson, E.; Liu, P.Y.; Schultz, G.S.; Martins-Green, M.M.; Tanaka, R.; Weir, D.; Gould, L.J.; Armstrong, D.G.; Gibbons, G.W.; Wolcott, R.; et al. Chronic wounds: Treatment consensus. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2022, 30, 156–171. [Google Scholar] [CrossRef]
- Caley, M.; Wall, I.B.; Peake, M.; Kipling, D.; Giles, P.; Thomas, D.W.; Stephens, P. Development and Characterisation of a Human Chronic Skin Wound Cell Line-Towards an Alternative for Animal Experimentation. Int. J. Mol. Sci. 2018, 19, 1001. [Google Scholar] [CrossRef]
- Hofmann, E.; Fink, J.; Pignet, A.L.; Schwarz, A.; Schellnegger, M.; Nischwitz, S.P.; Holzer-Geissler, J.C.J.; Kamolz, L.P.; Kotzbeck, P. Human In Vitro Skin Models for Wound Healing and Wound Healing Disorders. Biomedicines 2023, 11, 1056. [Google Scholar] [CrossRef]
- Saeed, S.; Martins-Green, M. Assessing Animal Models to Study Impaired and Chronic Wounds. Int. J. Mol. Sci. 2024, 25, 3837. [Google Scholar] [CrossRef]
- Zindle, J.K.; Wolinsky, E.; Bogie, K.M. A review of animal models from 2015 to 2020 for preclinical chronic wounds relevant to human health. J. Tissue Viability 2021, 30, 291–300. [Google Scholar] [CrossRef]
- Dantzer, D.; Ferguson, P.; Hill, R.P.; Keating, A.; Kandel, R.A.; Wunder, J.S.; O’Sullivan, B.; Sandhu, J.; Waddell, J.; Bell, R.S. Effect of radiation and cell implantation on wound healing in a rat model. J. Surg. Oncol. 2003, 83, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Tattini, C.; Manchio, J.; Zaporojan, V.; Carderelli, G.; Bonassar, L.; Spangenberger, A.; Weinzweig, J. Role of TGF-β and FGF in the Treatment of Radiation-Impaired Wounds Using a Novel Drug Delivery System. Plast. Reconstr. Surg. 2008, 122, 1036–1045. [Google Scholar] [CrossRef]
- Bian, X.; Piipponen, M.; Liu, Z.; Luo, L.; Geara, J.; Chen, Y.; Sangsuwan, T.; Maselli, M.; Diaz, C.; Bain, C.A.; et al. Epigenetic memory of radiotherapy in dermal fibroblasts impairs wound repair capacity in cancer survivors. Nat. Commun. 2024, 15, 9286. [Google Scholar] [CrossRef]
- Volk, S.W.; Radu, A.; Zhang, L.; Liechty, K.W. Stromal progenitor cell therapy corrects the wound-healing defect in the ischemic rabbit ear model of chronic wound repair. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2007, 15, 736–747. [Google Scholar] [CrossRef]
- Xia, Y.-P.; Zhao, Y.; Marcus, J.; Jimenez, P.A.; Ruben, S.M.; Moore, P.A.; Khan, F.; Mustoe, T.A. Effects of keratinocyte growth factor-2 (KGF-2) on wound healing in an ischaemia-impaired rabbit ear model and on scar formation. J. Pathol. 1999, 188, 431–438. [Google Scholar] [CrossRef]
- Badillo, A.T.; Chung, S.; Zhang, L.; Zoltick, P.; Liechty, K.W. Lentiviral Gene Transfer of SDF-1α to Wounds Improves Diabetic Wound Healing. J. Surg. Res. 2007, 143, 35–42. [Google Scholar] [CrossRef]
- Steinberg, J.P.; Hong, S.J.; Geringer, M.R.; Galiano, R.D.; Mustoe, T.A. Equivalent Effects of Topically-Delivered Adipose-Derived Stem Cells and Dermal Fibroblasts in the Ischemic Rabbit Ear Model for Chronic Wounds. Aesthetic Surg. J. 2012, 32, 504–519. [Google Scholar] [CrossRef]
- Sullivan, S.R.; Underwood, R.A.; Gibran, N.S.; Sigle, R.O.; Usui, M.L.; Carter, W.G.; Olerud, J.E. Validation of a Model for the Study of Multiple Wounds in the Diabetic Mouse (db/db). Plast. Reconstr. Surg. 2004, 113, 953–960. [Google Scholar] [CrossRef]
- Stadler, I.; Lanzafame, R.J.; Evans, R.; Narayan, V.; Dailey, B.; Buehner, N.; Naim, J.O. 830-nm irradiation increases the wound tensile strength in a diabetic murine model. Lasers Surg. Med. 2001, 28, 220–226. [Google Scholar] [CrossRef]
- Hardwicke, J.T.; Hart, J.; Bell, A.; Duncan, R.; Thomas, D.W.; Moseley, R. The effect of dextrin–rhEGF on the healing of full-thickness, excisional wounds in the (db/db) diabetic mouse. J. Control. Release 2011, 152, 411–417. [Google Scholar] [CrossRef]
- Yu, W.; Naim, J.O.; Lanzafame, R.J. Effects of photostimulation on wound healing in diabetic mice. Lasers Surg. Med. 1997, 20, 56–63. [Google Scholar] [CrossRef]
- Zhao, G.; Hochwalt, P.C.; Usui, M.L.; Underwood, R.A.; Singh, P.K.; James, G.A.; Stewart, P.S.; Fleckman, P.; Olerud, J.E. Delayed wound healing in diabetic (db/db) mice with Pseudomonas aeruginosa biofilm challenge: A model for the study of chronic wounds. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2010, 18, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Rai, V.; Moellmer, R.; Agrawal, D.K. Clinically relevant experimental rodent models of diabetic foot ulcer. Mol. Cell Biochem. 2022, 477, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Demidova-Rice, T.N.; Hamblin, M.R.; Herman, I.M. Acute and impaired wound healing: Pathophysiology and current methods for drug delivery, part 1: Normal and chronic wounds: Biology, causes, and approaches to care. Adv. Skin. Wound Care 2012, 25, 304–314. [Google Scholar] [CrossRef]
- Icli, B.; Nabzdyk, C.S.; Lujan-Hernandez, J.; Cahill, M.; Auster, M.E.; Wara, A.K.M.; Sun, X.; Ozdemir, D.; Giatsidis, G.; Orgill, D.P.; et al. Regulation of impaired angiogenesis in diabetic dermal wound healing by microRNA-26a. J. Mol. Cell. Cardiol. 2016, 91, 151–159. [Google Scholar] [CrossRef]
- Galiano, R.D.; Tepper, O.M.; Pelo, C.R.; Bhatt, K.A.; Callaghan, M.; Bastidas, N.; Bunting, S.; Steinmetz, H.G.; Gurtner, G.C. Topical vascular endothelial growth factor accelerates diabetic wound healing through increased angiogenesis and by mobilizing and recruiting bone marrow-derived cells. Am. J. Pathol. 2004, 164, 1935–1947. [Google Scholar] [CrossRef]
- Peña, O.A.; Martin, P. Cellular and molecular mechanisms of skin wound healing. Nat. Rev. Mol. Cell Biol. 2024, 25, 599–616. [Google Scholar] [CrossRef]
- Maruyama, K.; Ii, M.; Cursiefen, C.; Jackson, D.G.; Keino, H.; Tomita, M.; Van Rooijen, N.; Takenaka, H.; D’Amore, P.A.; Stein-Streilein, J.; et al. Inflammation-induced lymphangiogenesis in the cornea arises from CD11b-positive macrophages. J. Clin. Investig. 2005, 115, 2363–2372. [Google Scholar] [CrossRef]
- Hosono, K.; Isonaka, R.; Kawakami, T.; Narumiya, S.; Majima, M. Signaling of Prostaglandin E Receptors, EP3 and EP4 Facilitates Wound Healing and Lymphangiogenesis with Enhanced Recruitment of M2 Macrophages in Mice. PLoS ONE 2016, 11, e0162532. [Google Scholar] [CrossRef]
- Okizaki, S.-i.; Ito, Y.; Hosono, K.; Oba, K.; Ohkubo, H.; Kojo, K.; Nishizawa, N.; Shibuya, M.; Shichiri, M.; Majima, M. Vascular Endothelial Growth Factor Receptor Type 1 Signaling Prevents Delayed Wound Healing in Diabetes by Attenuating the Production of IL-1β by Recruited Macrophages. Am. J. Pathol. 2016, 186, 1481–1498. [Google Scholar] [CrossRef]
- Renò, F.; Sabbatini, M. Breaking a Vicious Circle: Lymphangiogenesis as a New Therapeutic Target in Wound Healing. Biomedicines 2023, 11, 656. [Google Scholar] [CrossRef] [PubMed]
- Avraham, T.; Clavin, N.W.; Daluvoy, S.V.; Fernandez, J.; Soares, M.A.; Cordeiro, A.P.; Mehrara, B.J. Fibrosis is a key inhibitor of lymphatic regeneration. Plast. Reconstr. Surg. 2009, 124, 438–450. [Google Scholar] [CrossRef] [PubMed]
- Clavin, N.W.; Avraham, T.; Fernandez, J.; Daluvoy, S.V.; Soares, M.A.; Chaudhry, A.; Mehrara, B.J. TGF-beta1 is a negative regulator of lymphatic regeneration during wound repair. Am. J. Physiol. Heart Circ. Physiol. 2008, 295, H2113–H2127. [Google Scholar] [CrossRef]
- Romani, L.; Mencacci, A.; Grohmann, U.; Mocci, S.; Mosci, P.; Puccetti, P.; Bistoni, F. Neutralizing antibody to interleukin 4 induces systemic protection and T helper type 1-associated immunity in murine candidiasis. J. Exp. Med. 1992, 176, 19–25. [Google Scholar] [CrossRef]
- Yang, G.; Volk, A.; Petley, T.; Emmell, E.; Giles-Komar, J.; Shang, X.; Li, J.; Das, A.M.; Shealy, D.; Griswold, D.E.; et al. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine 2004, 28, 224–232. [Google Scholar] [CrossRef]
- Avraham, T.; Zampell, J.C.; Yan, A.; Elhadad, S.; Weitman, E.S.; Rockson, S.G.; Bromberg, J.; Mehrara, B.J. Th2 differentiation is necessary for soft tissue fibrosis and lymphatic dysfunction resulting from lymphedema. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2013, 27, 1114–1126. [Google Scholar] [CrossRef]
- Bollinger, A.; Jäger, K.; Sgier, F.; Seglias, J. Fluorescence microlymphography. Circulation 1981, 64, 1195–1200. [Google Scholar] [CrossRef]
- Swartz, M.A.; Berk, D.A.; Jain, R.K. Transport in lymphatic capillaries. I. Macroscopic measurements using residence time distribution theory. Am. J. Physiol. 1996, 270, H324–H329. [Google Scholar] [CrossRef]
- Avraham, T.; Daluvoy, S.; Zampell, J.; Yan, A.; Haviv, Y.S.; Rockson, S.G.; Mehrara, B.J. Blockade of transforming growth factor-beta1 accelerates lymphatic regeneration during wound repair. Am. J. Pathol. 2010, 177, 3202–3214. [Google Scholar] [CrossRef]
- Zampell, J.C.; Yan, A.; Elhadad, S.; Avraham, T.; Weitman, E.; Mehrara, B.J. CD4(+) cells regulate fibrosis and lymphangiogenesis in response to lymphatic fluid stasis. PLoS ONE 2012, 7, e49940. [Google Scholar] [CrossRef]
- Ly, C.L.; Nores, G.D.G.; Kataru, R.P.; Mehrara, B.J. T helper 2 differentiation is necessary for development of lymphedema. Transl. Res. 2019, 206, 57–70. [Google Scholar] [CrossRef] [PubMed]
- Gousopoulos, E.; Proulx, S.T.; Scholl, J.; Uecker, M.; Detmar, M. Prominent Lymphatic Vessel Hyperplasia with Progressive Dysfunction and Distinct Immune Cell Infiltration in Lymphedema. Am. J. Pathol. 2016, 186, 2193–2203. [Google Scholar] [CrossRef] [PubMed]
- Savetsky, I.L.; Ghanta, S.; Gardenier, J.C.; Torrisi, J.S.; Garcia Nores, G.D.; Hespe, G.E.; Nitti, M.D.; Kataru, R.P.; Mehrara, B.J. Th2 cytokines inhibit lymphangiogenesis. PLoS ONE 2015, 10, e0126908. [Google Scholar] [CrossRef]
- Shin, K.; Kataru, R.P.; Park, H.J.; Kwon, B.I.; Kim, T.W.; Hong, Y.K.; Lee, S.H. TH2 cells and their cytokines regulate formation and function of lymphatic vessels. Nat. Commun. 2015, 6, 6196. [Google Scholar] [CrossRef]
- Durand, B.; Pouget, C.; Magnan, C.; Molle, V.; Lavigne, J.P.; Dunyach-Remy, C. Bacterial Interactions in the Context of Chronic Wound Biofilm: A Review. Microorganisms 2022, 10, 1500. [Google Scholar] [CrossRef]
- Jian, Y.; Li, Y.; Zhang, Y.; Tang, M.; Deng, M.; Liu, C.; Cheng, M.; Xiao, S.; Deng, C.; Wei, Z. Lymphangiogenesis: Novel strategies to promote cutaneous wound healing. Burn. Trauma 2024, 12, tkae040. [Google Scholar] [CrossRef]
- Labanaris, A.P.; Polykandriotis, E.; Horch, R.E. The effect of vacuum-assisted closure on lymph vessels in chronic wounds. J. Plast. Reconstr. Aesthetic Surg. 2009, 62, 1068–1075. [Google Scholar] [CrossRef]
- Kurashige, C.; Hosono, K.; Matsuda, H.; Tsujikawa, K.; Okamoto, H.; Majima, M. Roles of receptor activity-modifying protein 1 in angiogenesis and lymphangiogenesis during skin wound healing in mice. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2014, 28, 1237–1247. [Google Scholar] [CrossRef]
- Paavonen, K.; Puolakkainen, P.; Jussila, L.; Jahkola, T.; Alitalo, K. Vascular Endothelial Growth Factor Receptor-3 in Lymphangiogenesis in Wound Healing. Am. J. Pathol. 2000, 156, 1499–1504. [Google Scholar] [CrossRef]
- Cho, C.H.; Sung, H.K.; Kim, K.T.; Cheon, H.G.; Oh, G.T.; Hong, H.J.; Yoo, O.J.; Koh, G.Y. COMP-angiopoietin-1 promotes wound healing through enhanced angiogenesis, lymphangiogenesis, and blood flow in a diabetic mouse model. Proc. Natl. Acad. Sci. USA 2006, 103, 4946–4951. [Google Scholar] [CrossRef]
- Rasmussen, J.C.; Zhu, B.; Morrow, J.R.; Aldrich, M.B.; Sahihi, A.; Harlin, S.A.; Fife, C.E.; O’Donnell, T.F., Jr.; Sevick-Muraca, E.M. Degradation of lymphatic anatomy and function in early venous insufficiency. J. Vasc. Surg. Venous Lymphat. Disord. 2021, 9, 720–730.e722. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Corral, I.; Olmeda, D.; Diéguez-Hurtado, R.; Tammela, T.; Alitalo, K.; Ortega, S. In vivo imaging of lymphatic vessels in development, wound healing, inflammation, and tumor metastasis. Proc. Natl. Acad. Sci. USA 2012, 109, 6223–6228. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Wei, T.; He, Z. ADSCs enhance VEGFR3-mediated lymphangiogenesis via METTL3-mediated VEGF-C m(6)A modification to improve wound healing of diabetic foot ulcers. Mol. Med. 2021, 27, 146. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Liu, Q.; Yu, Z.; Karvar, M.; Aoki, S.; Hamaguchi, R.; Ma, C.; Orgill, D.P.; Panayi, A.C. Negative-Pressure Wound Therapy Induces Lymphangiogenesis in Murine Diabetic Wound Healing. Plast. Reconstr. Surg. 2023, 151, 779–790. [Google Scholar] [CrossRef]
- Maruyama, K.; Asai, J.; Ii, M.; Thorne, T.; Losordo, D.W.; D’Amore, P.A. Decreased Macrophage Number and Activation Lead to Reduced Lymphatic Vessel Formation and Contribute to Impaired Diabetic Wound Healing. Am. J. Pathol. 2007, 170, 1178–1191. [Google Scholar] [CrossRef]
- Saaristo, A.; Tammela, T.; Fārkkilā, A.; Kärkkäinen, M.; Suominen, E.; Yla-Herttuala, S.; Alitalo, K. Vascular Endothelial Growth Factor-C Accelerates Diabetic Wound Healing. Am. J. Pathol. 2006, 169, 1080–1087. [Google Scholar] [CrossRef]
- Brunner, L.M.; He, Y.; Cousin, N.; Scholl, J.; Albin, L.K.; Schmucki, B.; Supersaxo, S.; Restivo, G.; Hafner, J.; Neri, D.; et al. Promotion of Lymphangiogenesis by Targeted Delivery of VEGF-C Improves Diabetic Wound Healing. Cells 2023, 12, 472. [Google Scholar] [CrossRef]
- Oliver, G.; Kipnis, J.; Randolph, G.J.; Harvey, N.L. The Lymphatic Vasculature in the 21(st) Century: Novel Functional Roles in Homeostasis and Disease. Cell 2020, 182, 270–296. [Google Scholar] [CrossRef]
- Petrova, T.V.; Koh, G.Y. Biological functions of lymphatic vessels. Science 2020, 369, eaax4063. [Google Scholar] [CrossRef]
- Leach, R.M.; Treacher, D.F. Oxygen transport2. Tissue hypoxia. BMJ 1998, 317, 1370–1373. [Google Scholar] [CrossRef]
- Jiang, X.; Tian, W.; Granucci, E.J.; Tu, A.B.; Kim, D.; Dahms, P.; Pasupneti, S.; Peng, G.; Kim, Y.; Lim, A.H.; et al. Decreased lymphatic HIF-2α accentuates lymphatic remodeling in lymphedema. J. Clin. Investig. 2020, 130, 5562–5575. [Google Scholar] [CrossRef] [PubMed]
- Chávez, M.N.; Fuchs, B.; Moellhoff, N.; Hofmann, D.; Zhang, L.; Selão, T.T.; Giunta, R.E.; Egaña, J.T.; Nickelsen, J.; Schenck, T.L. Use of photosynthetic transgenic cyanobacteria to promote lymphangiogenesis in scaffolds for dermal regeneration. Acta Biomater. 2021, 126, 132–143. [Google Scholar] [CrossRef] [PubMed]
- Aldrich, M.B.; Rasmussen, J.C.; Fife, C.E.; Shaitelman, S.F.; Sevick-Muraca, E.M. The Development and Treatment of Lymphatic Dysfunction in Cancer Patients and Survivors. Cancers 2020, 12, 2280. [Google Scholar] [CrossRef] [PubMed]
- Smith, P.C.; Sarin, S.; Hasty, J.; Scurr, J.H. Sequential gradient pneumatic compression enhances venous ulcer healing: A randomized trial. Surgery 1990, 108, 871–875. [Google Scholar]
- Milic, D.J.; Zivic, S.S.; Bogdanovic, D.C.; Perisic, Z.D.; Milosevic, Z.D.; Jankovic, R.J.; Visnjic, A.M.; Jovanovic, B.M. A randomized trial of the Tubulcus multilayer bandaging system in the treatment of extensive venous ulcers. J. Vasc. Surg. 2007, 46, 750–755. [Google Scholar] [CrossRef][Green Version]
- Schwager, S.; Detmar, M. Inflammation and Lymphatic Function. Front. Immunol. 2019, 10, 308. [Google Scholar] [CrossRef]
- Diegelmann, R.F.; Evans, M.C. Wound healing: An overview of acute, fibrotic and delayed healing. Front. Biosci. A J. Virtual Libr. 2004, 9, 283–289. [Google Scholar] [CrossRef]
- Mast, B.A.; Schultz, G.S. Interactions of cytokines, growth factors, and proteases in acute and chronic wounds. Wound Repair Regen. Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 1996, 4, 411–420. [Google Scholar] [CrossRef]
- Güç, E.; Briquez, P.S.; Foretay, D.; Fankhauser, M.A.; Hubbell, J.A.; Kilarski, W.W.; Swartz, M.A. Local induction of lymphangiogenesis with engineered fibrin-binding VEGF-C promotes wound healing by increasing immune cell trafficking and matrix remodeling. Biomaterials 2017, 131, 160–175. [Google Scholar] [CrossRef]
- Kasuya, A.; Sakabe, J.-i.; Tokura, Y. Potential application of in vivo imaging of impaired lymphatic duct to evaluate the severity of pressure ulcer in mouse model. Sci. Rep. 2014, 4, 4173. [Google Scholar] [CrossRef]
- Abdreshov, S.N.; Demchenko, G.A.; Kozhaniyazova, U.N.; Yeshmukhanbet, A.N.; Yessenova, M.A.; Nurmakhanova, B.A.; Karjaubaev, R.M.; Koibasova, L.U. Lymph flow, ionic and biochemical indicators of lymph and blood during hypoxia. Braz. J. Biol. 2025, 84, e284264. [Google Scholar] [CrossRef] [PubMed]
- Garcia Nores, G.D.; Ly, C.L.; Cuzzone, D.A.; Kataru, R.P.; Hespe, G.E.; Torrisi, J.S.; Huang, J.J.; Gardenier, J.C.; Savetsky, I.L.; Nitti, M.D.; et al. CD4(+) T cells are activated in regional lymph nodes and migrate to skin to initiate lymphedema. Nat. Commun. 2018, 9, 1970. [Google Scholar] [CrossRef]
- Ly, C.L.; Cuzzone, D.A.; Kataru, R.P.; Mehrara, B.J. Small Numbers of CD4+ T Cells Can Induce Development of Lymphedema. Plast. Reconstr. Surg. 2019, 143, 518e–526e. [Google Scholar] [CrossRef] [PubMed]
- Azhar, S.H.; Lim, H.Y.; Tan, B.K.; Angeli, V. The Unresolved Pathophysiology of Lymphedema. Front. Physiol. 2020, 11, 137. [Google Scholar] [CrossRef] [PubMed]
- Fu, A.; Liu, C. The Function of T Cell Immunity in Lymphedema: A Comprehensive Review. Lymphat. Res. Biol. 2023, 21, 556–564. [Google Scholar] [CrossRef]
- Serezani, A.P.M.; Bozdogan, G.; Sehra, S.; Walsh, D.; Krishnamurthy, P.; Sierra Potchanant, E.A.; Nalepa, G.; Goenka, S.; Turner, M.J.; Spandau, D.F.; et al. IL-4 impairs wound healing potential in the skin by repressing fibronectin expression. J. Allergy Clin. Immunol. 2017, 139, 142–151.e145. [Google Scholar] [CrossRef]
- Ahdieh, M.; Vandenbos, T.; Youakim, A. Lung epithelial barrier function and wound healing are decreased by IL-4 and IL-13 and enhanced by IFN-γ. Am. J. Physiol. Cell Physiol. 2001, 281, C2029–C2038. [Google Scholar] [CrossRef]
- Hong, Y.K.; Chang, Y.H.; Lin, Y.C.; Chen, B.; Guevara, B.E.K.; Hsu, C.K. Inflammation in Wound Healing and Pathological Scarring. Adv. Wound Care 2023, 12, 288–300. [Google Scholar] [CrossRef]
- Mehrara, B.J.; Park, H.J.; Kataru, R.P.; Bromberg, J.; Coriddi, M.; Baik, J.E.; Shin, J.; Li, C.; Cavalli, M.R.; Encarnacion, E.M.; et al. Pilot Study of Anti-Th2 Immunotherapy for the Treatment of Breast Cancer-Related Upper Extremity Lymphedema. Biology 2021, 10, 934. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pollack, B.L.; Torrisi, J.S.; Hespe, G.E.; Ashokan, G.; Shin, J.; Mehrara, B.J.; Kataru, R.P. Inhibition of Th2 Differentiation Accelerates Chronic Wound Healing by Facilitating Lymphangiogenesis. Biomedicines 2025, 13, 1026. https://doi.org/10.3390/biomedicines13051026
Pollack BL, Torrisi JS, Hespe GE, Ashokan G, Shin J, Mehrara BJ, Kataru RP. Inhibition of Th2 Differentiation Accelerates Chronic Wound Healing by Facilitating Lymphangiogenesis. Biomedicines. 2025; 13(5):1026. https://doi.org/10.3390/biomedicines13051026
Chicago/Turabian StylePollack, Bracha L., Jeremy S. Torrisi, Geoffrey E. Hespe, Gopika Ashokan, Jinyeon Shin, Babak J. Mehrara, and Raghu P. Kataru. 2025. "Inhibition of Th2 Differentiation Accelerates Chronic Wound Healing by Facilitating Lymphangiogenesis" Biomedicines 13, no. 5: 1026. https://doi.org/10.3390/biomedicines13051026
APA StylePollack, B. L., Torrisi, J. S., Hespe, G. E., Ashokan, G., Shin, J., Mehrara, B. J., & Kataru, R. P. (2025). Inhibition of Th2 Differentiation Accelerates Chronic Wound Healing by Facilitating Lymphangiogenesis. Biomedicines, 13(5), 1026. https://doi.org/10.3390/biomedicines13051026






