One-Dimensional Nanomaterials in Resistive Gas Sensor: From Material Design to Application
Abstract
:1. Introduction
2. Introduction to Resistive Gas Sensor
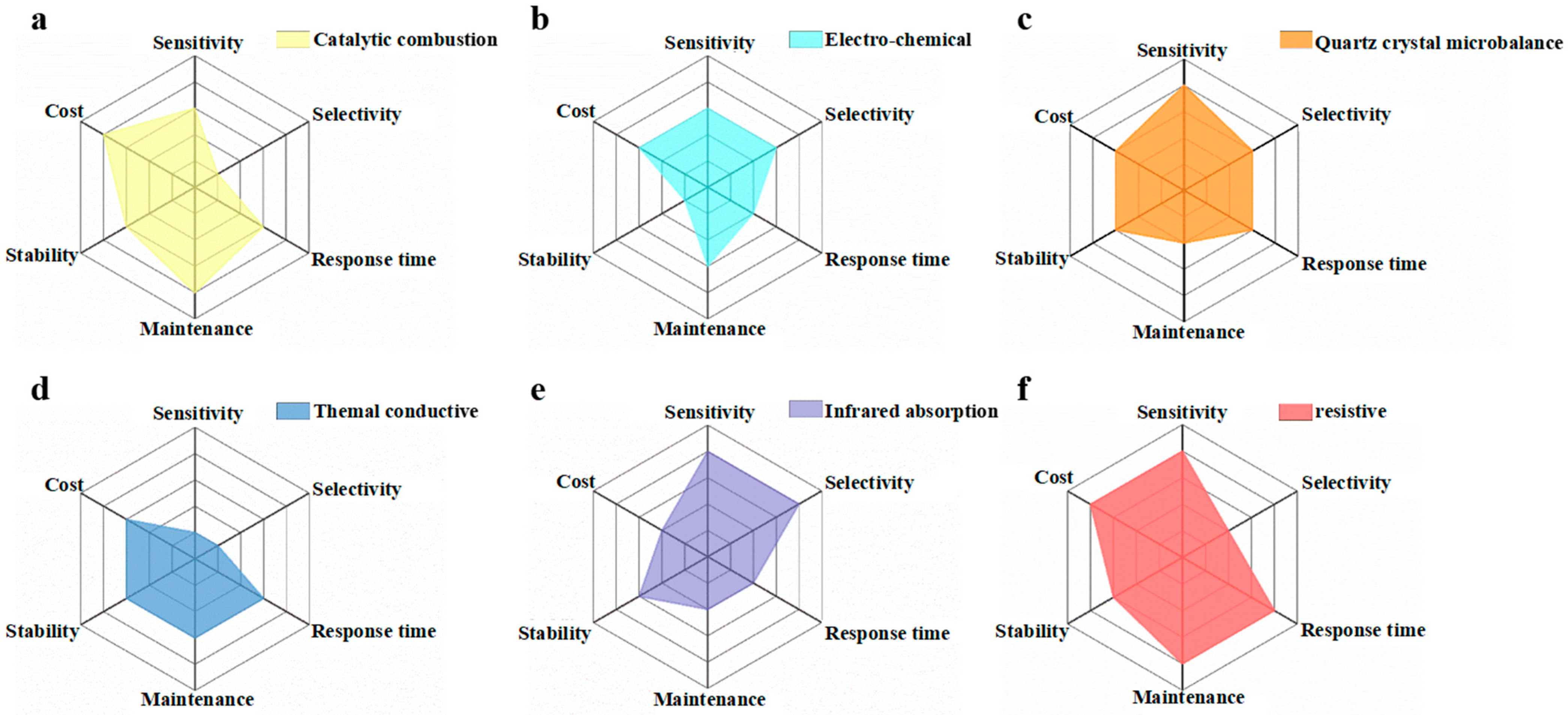
2.1. Performance of Resistive Gas Sensor
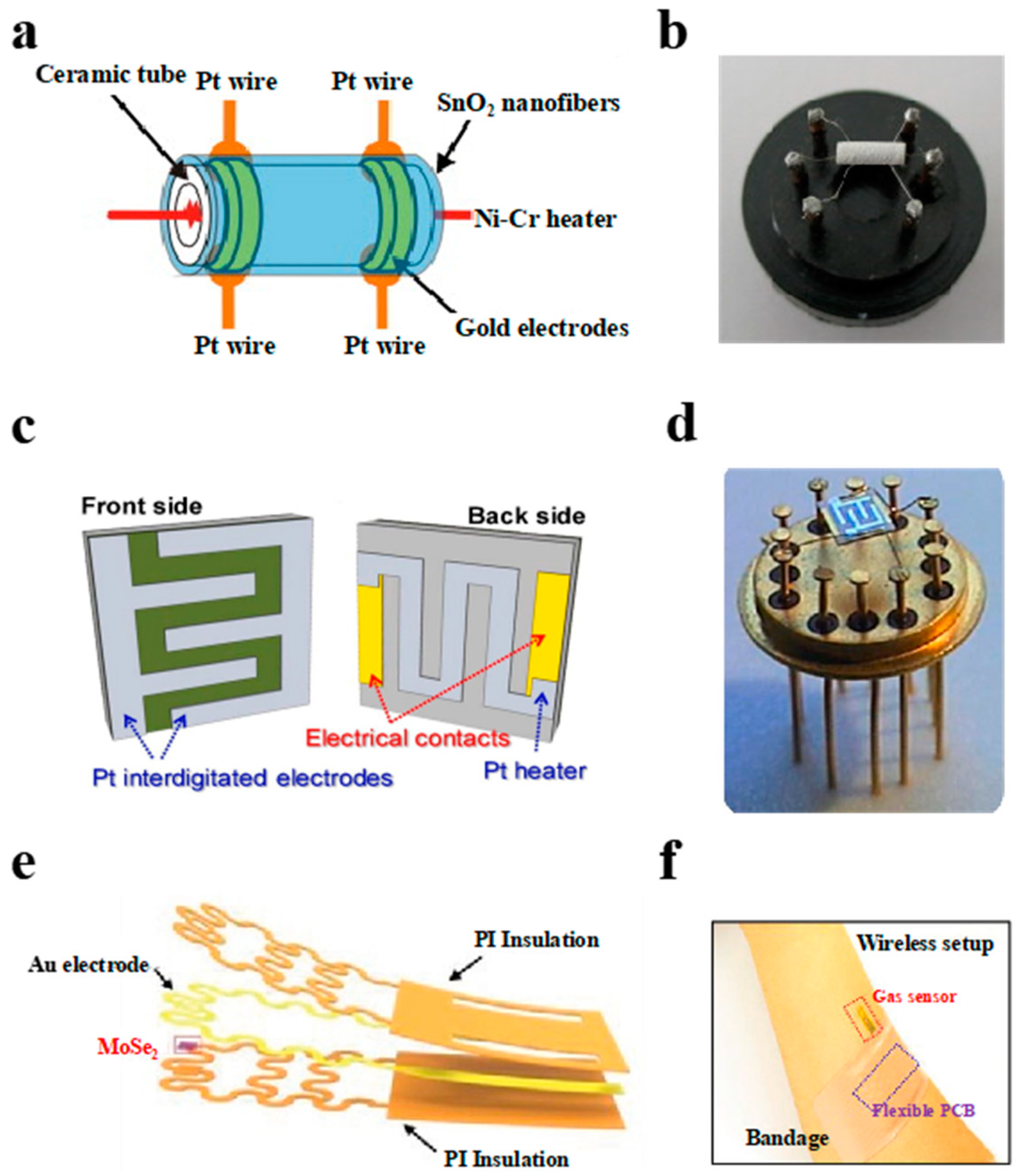
2.2. The Configuration Type of Resistive Gas Sensor
2.3. Sensing Mechanism of Resistive Gas Sensor
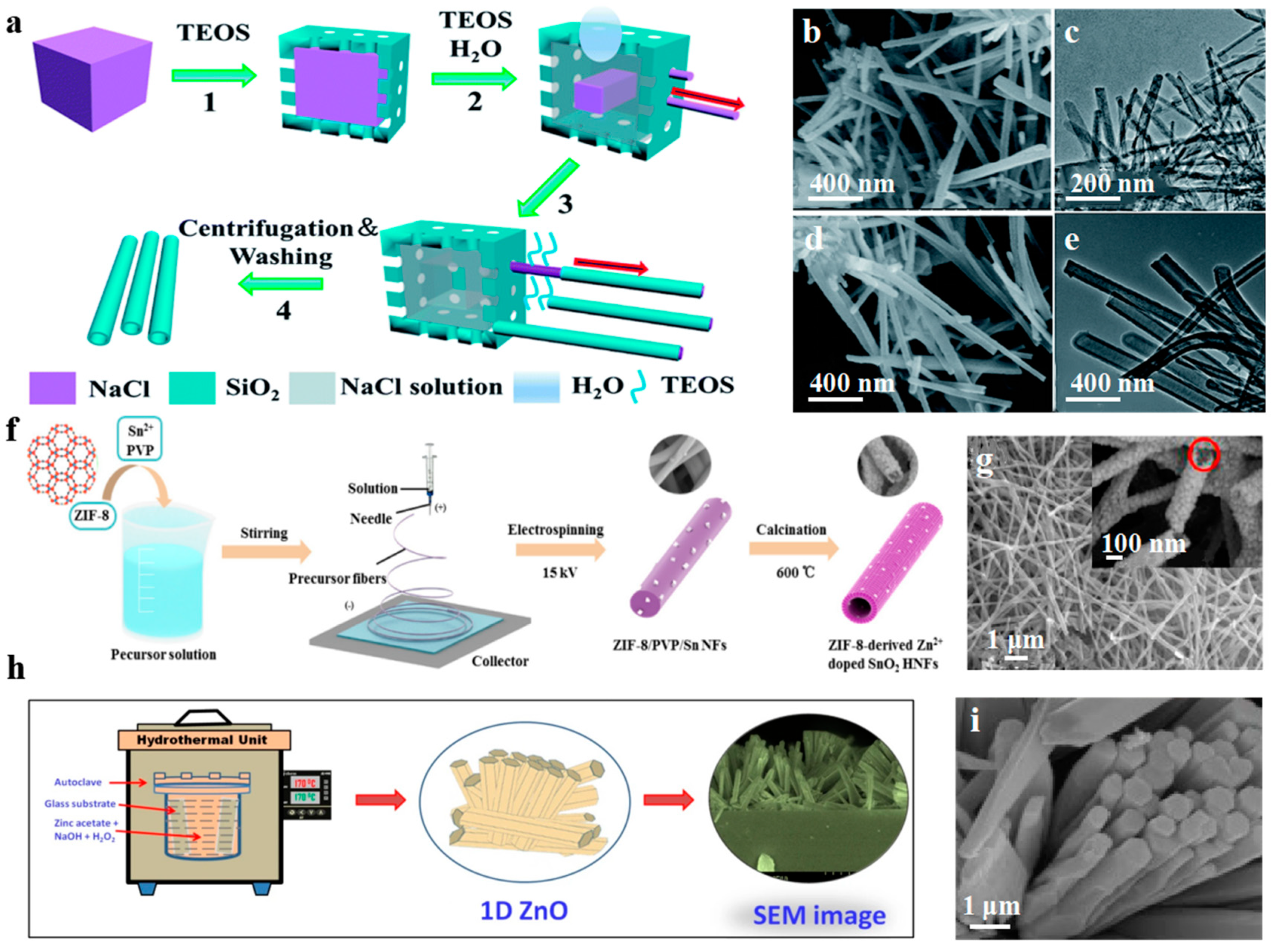
2.4. 1-D Nanomaterials
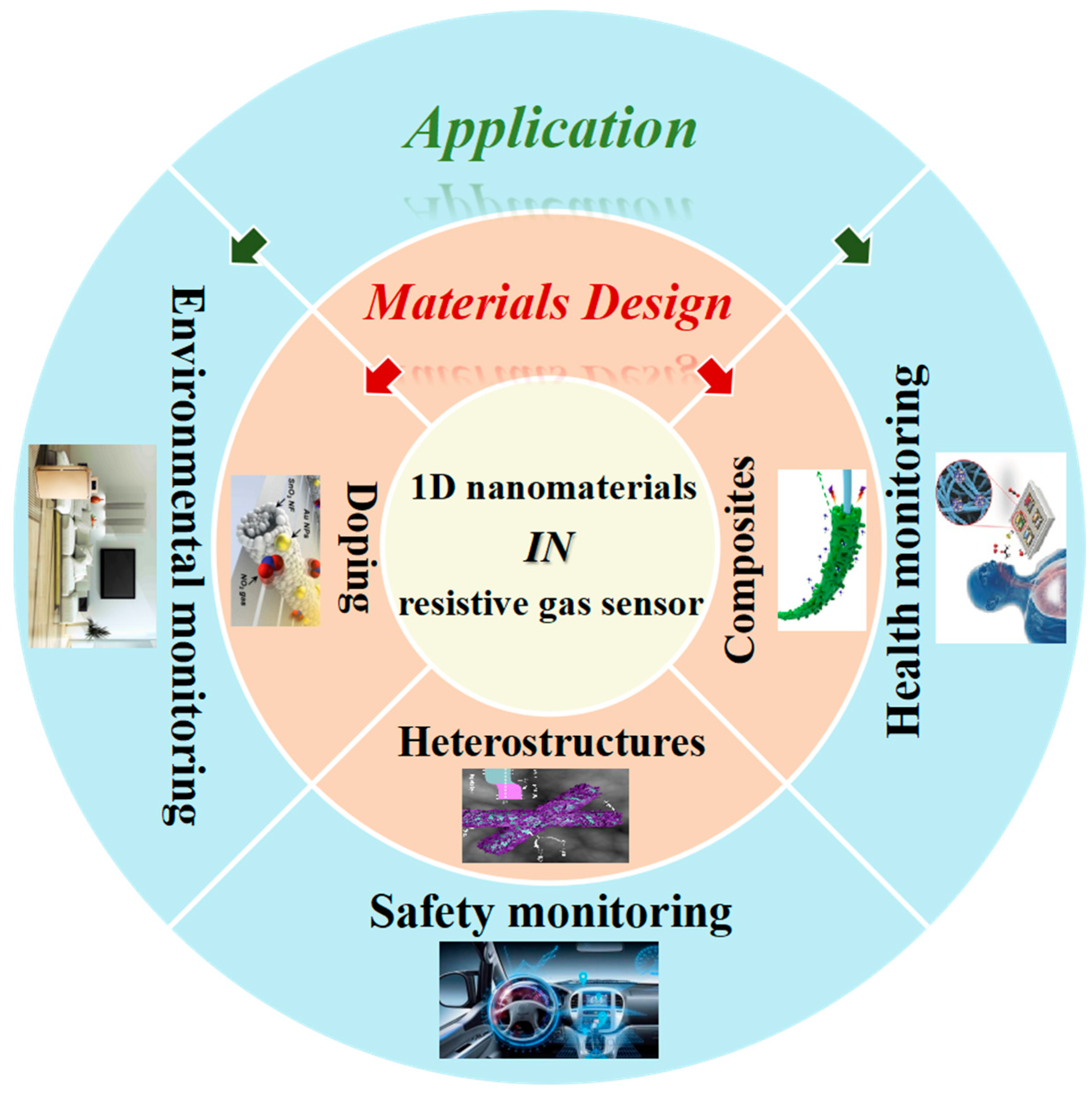
3. Materials Design
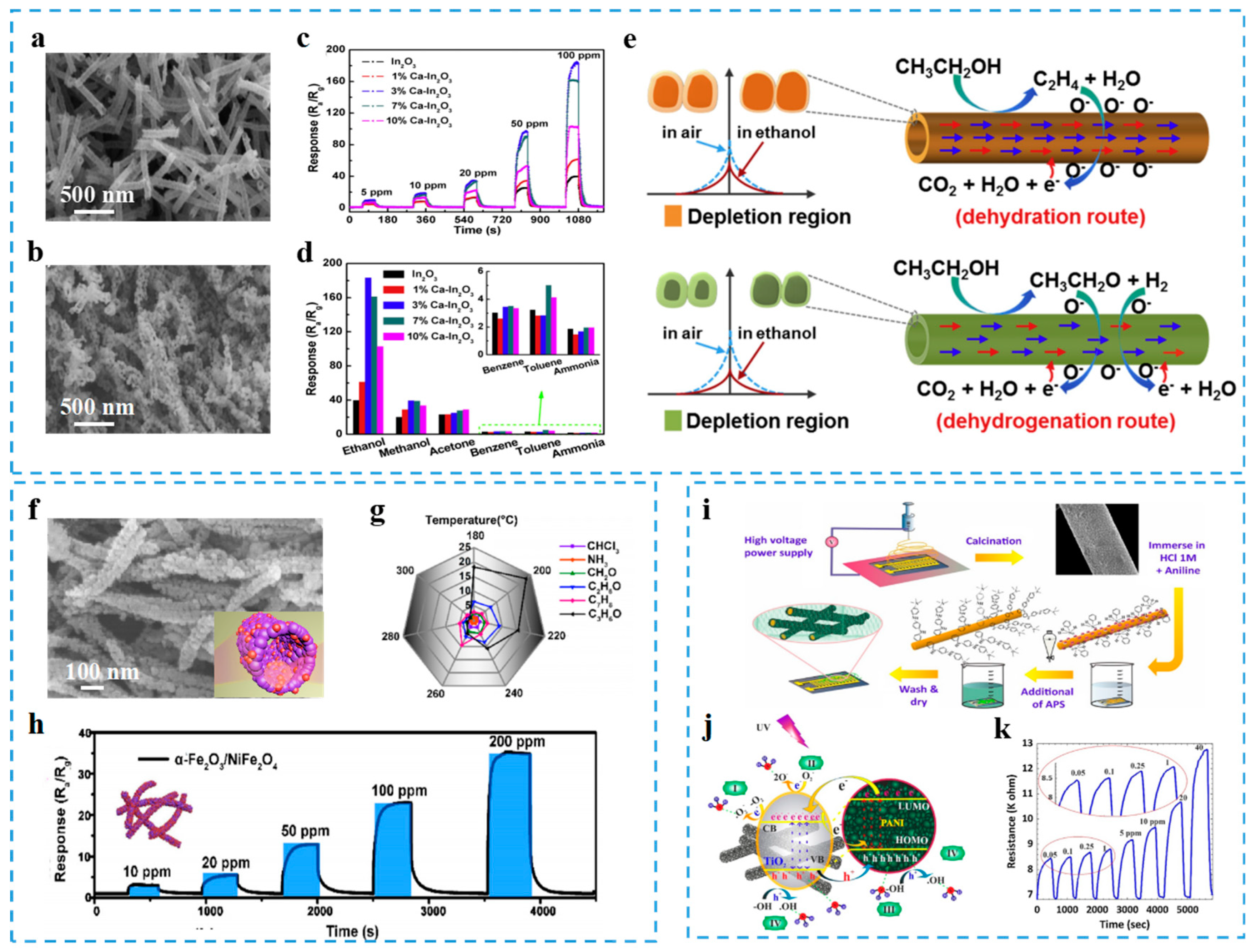
3.1. Doping
3.2. Heterostructures
3.3. Composites
4. Application
4.1. Environmental Monitoring
4.2. Safety Monitoring
4.3. Health Monitoring
5. Summary and Perspective
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Korotcenkov, G. Gas response control through structural and chemical modification of metal oxide films: State of the art and approaches. Sens. Actuators B 2005, 107, 209–232. [Google Scholar] [CrossRef]
- Qu, J.; Chai, Y.; Yang, S.X. A real-time de-noising algorithm for e-noses in a wireless sensor network. Sensors 2009, 9, 895–908. [Google Scholar] [CrossRef] [Green Version]
- Su, P.G.; Chuang, T.Y. Simple and rapid differentiation of toxic gases using a quartz crystal microbalance sensor array coupled with principal component analysis. Sens. Actuators A 2017, 263, 1–7. [Google Scholar] [CrossRef]
- Dong, Q.; Xiao, M.; Chu, Z.Y.; Li, G.C.; Zhang, Y. Recent progress of toxic gas sensors based on 3D graphene frameworks. Sensors 2021, 21, 3386. [Google Scholar] [CrossRef] [PubMed]
- Cruz-Martinez, H.; Rojas-Chavez, H.; Montejo-Alvaro, F.; Pena-Castaneda, Y.A.; Matadamas-Ortiz, P.T.; Medina, D.I. Recent developments in graphene-based toxic gas sensors: A theoretical overview. Sensors 2021, 21, 1992. [Google Scholar] [CrossRef]
- Long, L.S.; Ying, X.Y.; Yang, Y.; Wang, L.P. Tuning the infrared absorption of SiC metasurfaces by electrically gating monolayer graphene with solid polymer electrolyte for dynamic radiative thermal management and sensing applications. ACS Appl. Nano Mater. 2019, 2, 4810–4817. [Google Scholar] [CrossRef]
- Hollerbach, A.; Fedick, P.W.; Cooks, R.G. Ion mobility-mass spectrometry using a dual-gated 3D printed ion mobility spectrometer. Anal. Chem. 2018, 90, 13265–13272. [Google Scholar] [CrossRef] [PubMed]
- Yoon, D.; Lee, J.; Lim, J.S. Detachable trap preconcentrator with a gas chromatograph-mass spectrometer for the analysis of trace halogenated greenhouse gases. Anal. Chem. 2019, 91, 3342–3349. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro- and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [Green Version]
- Majhi, S.M.; Mirzaei, A.; Kim, H.W.; Kim, S.S.; Kim, T.W. Recent advances in energy-saving chemiresistive gas sensors: A review. Nano Energy 2021, 79, 105369. [Google Scholar] [CrossRef]
- Fan, Y.F.; Zhu, X.D.; Sui, H.L.; Sun, H.T.; Wang, Z.M. Design and application of toxic and harmful gas monitoring system in fire fighting. Sensors 2019, 19, 369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Khan, M.A.H.; Rao, M.V.; Li, Q. Recent advances in electrochemical sensors for detecting toxic gases: NO2, SO2 and H2S. Sensors 2019, 19, 905. [Google Scholar] [CrossRef] [Green Version]
- Koo, W.T.; Cho, H.J.; Kim, D.H.; Kim, Y.H.; Shin, H.; Penner, R.M.; Kim, I.D. Chemiresistive hydrogen sensors: Fundamentals, recent advances, and challenges. ACS Nano 2020, 14, 14284–14322. [Google Scholar] [CrossRef]
- Zhou, X.; Lee, S.Y.; Xu, Z.C.; Yoon, J. Recent progress on the development of chemosensors for gases. Chem. Rev. 2015, 115, 7944–8000. [Google Scholar] [CrossRef]
- Del Orbe Henriquez, D.; Cho, I.; Yang, H.; Choi, J.; Kang, M.; Chang, K.S.; Jeong, C.B.; Han, S.W.; Park, I. Pt nanostructures fabricated by local hydrothermal synthesis for low-power catalytic-combustion hydrogen sensors. ACS Appl. Nano Mater. 2020, 4, 7–12. [Google Scholar] [CrossRef]
- Kim, E.B.; Seo, H.K. Highly sensitive formaldehyde detection using well-aligned Zinc oxide nanosheets synthesized by chemical bath deposition technique. Materials 2019, 12, 250. [Google Scholar] [CrossRef] [Green Version]
- Pérez, R.L.; Ayala, C.E.; Park, J.Y.; Choi, J.W.; Warner, I.M. Coating-based quartz crystal microbalance detection methods of environmentally relevant volatile organic compounds. Chemosensors 2021, 9, 153. [Google Scholar] [CrossRef]
- Zhang, H.Q.; Shen, B.; Hu, W.Q.; Liu, X.L. Research on a fast-response thermal conductivity sensor based on carbon nanotube modification. Sensors 2018, 18, 2191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, Y.H.; Hasan, D.; Dong, B.W.; Wei, J.X.; Ma, Y.M.; Zhou, G.Y.; Ang, K.W.; Lee, C.K. All-dielectric surface-enhanced infrared absorption-based gas sensor using guided resonance. ACS Appl. Mater. Interfaces 2018, 10, 38272–38279. [Google Scholar] [CrossRef] [PubMed]
- Mikołajczyk, J.; Bielecki, Z.; Stacewicz, T.; Smulko, J.; Wojtas, J.; Szabra, D.; Lentka, Ł.; Prokopiuk, A.; Magryta, P. Detection of gaseous compounds with different techniques. Metro. Meas. Syst. 2016, 23, 205–224. [Google Scholar] [CrossRef]
- Jian, Y.Y.; Hu, W.W.; Zhao, Z.H.; Cheng, P.F.; Haick, H.; Yao, M.S.; Wu, W.W. Gas sensors based on chemi-resistive hybrid functional nanomaterials. Nano-Micro Lett. 2020, 12, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Hosoya, A.; Tamura, S.; Imanaka, N. A new catalytic combustion-type carbon monoxide gas sensor employing precious metal-free CO oxidizing catalyst. ISIJ Int. 2015, 55, 1699–1701. [Google Scholar] [CrossRef] [Green Version]
- Hosoya, A.; Tamura, S.; Imanaka, N. A catalytic combustion-type carbon monoxide gas sensor incorporating an apatite-type oxide. ISIJ Int. 2016, 56, 1634–1637. [Google Scholar] [CrossRef] [Green Version]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Zhang, G.J.; Li, Y.P.; Li, Q.B. A miniaturized carbon dioxide gas sensor based on infrared absorption. Opt. Lasers Eng. 2010, 48, 1206–1212. [Google Scholar] [CrossRef]
- Kang, Z.J.; Zhang, D.Z.; Li, T.T.; Liu, X.H.; Song, X.S. Polydopamine-modified SnO2 nanofiber composite coated QCM gas sensor for high-performance formaldehyde sensing. Sens. Actuators B 2021, 345, 130299. [Google Scholar] [CrossRef]
- Koo, W.T.; Jang, J.S.; Kim, I.D. Metal-organic frameworks for chemiresistive sensors. Chem 2019, 5, 1938–1963. [Google Scholar] [CrossRef]
- Zhao, J.J.; Losego, M.D.; Lemaire, P.C.; Williams, P.S.; Gong, B.; Atanasov, S.E.; Blevins, T.M.; Oldham, C.J.; Walls, H.J.; Shepherd, S.D.; et al. Highly adsorptive, MOF-functionalized nonwoven fiber mats for hazardous gas capture enabled by atomic layer deposition. Adv. Mater. Interfaces 2014, 1. [Google Scholar] [CrossRef]
- Chludziński, T.K.; Kwiatkowski, A. Exhaled breath analysis by resistive gas sensors. Metro. Meas. Syst. 2020, 27, 87–89. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Low power-consumption CO gas sensors based on Au-functionalized SnO2-ZnO core-shell nanowires. Sens. Actuators B 2018, 267, 597–607. [Google Scholar] [CrossRef]
- Huang, J.; Wan, Q. Gas sensors based on semiconducting metal oxide one-dimensional nanostructures. Sensors 2009, 9, 9903–9924. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.H.; Kim, H.W.; Kim, S.S. Resistive-based gas sensors for detection of benzene, toluene and xylene (BTX) gases: A review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Mirzaei, A.; Lee, J.H.; Majhi, S.M.; Weber, M.; Bechelany, M.; Kim, H.W.; Kim, S.S. Resistive gas sensors based on metal-oxide nanowires. J. Appl. Phys. 2019, 126, 241102. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.L.; Kumar, P.; Yao, D.J. Printed Resistive sensor array combined with a flexible substrate for ethanol and methane detection. ECS J. Solid State Sci. Technol. 2020, 9, 115008. [Google Scholar] [CrossRef]
- Lee, J.H. Gas sensors using hierarchical and hollow oxide nanostructures: Overview. Sens. Actuators B 2009, 140, 319–336. [Google Scholar] [CrossRef]
- Xiao, X.Y.; Zhou, X.R.; Ma, J.H.; Zhu, Y.H.; Cheng, X.W.; Luo, W.; Deng, Y.H. Rational synthesis and gas sensing performance of ordered mesoporous semiconducting WO3/NiO composites. ACS Appl. Mater. Interfaces 2019, 11, 26268–26276. [Google Scholar] [CrossRef]
- Wang, D.; Chu, X.F.; Gong, M.L. Gas-sensing properties of sensors based on single-crystalline SnO2 nanorods prepared by a simple molten-salt method. Sens. Actuators B 2006, 117, 183–187. [Google Scholar] [CrossRef]
- Zhou, X.R.; Zou, Y.D.; Ma, J.D.; Cheng, X.W.; Li, Y.Y.; Deng, Y.H.; Zhao, D.Y. Cementing mesoporous ZnO with silica for controllable and switchable gas sensing selectivity. Chem. Mater. 2019, 31, 8112–8120. [Google Scholar] [CrossRef]
- Tiwari, J.N.; Tiwari, R.N.; Kim, K.S. Zero-dimensional, one-dimensional, two-dimensional and three-dimensional nanostructured materials for advanced electrochemical energy devices. Prog. Mater. Sci. 2012, 57, 724–803. [Google Scholar] [CrossRef]
- Korotcenkov, G. Metal oxides for solid-state gas sensors: What determines our choice? Mater. Sci. Eng. B 2007, 139, 1–23. [Google Scholar] [CrossRef]
- Xu, H.Y.; Ju, D.X.; Chen, Z.R.; Han, R.; Zhai, T.; Yu, H.Q.; Liu, C.Y.; Wu, X.W.; Wang, J.Q.; Cao, B.Q. A novel hetero-structure sensor based on Au/Mg-doped TiO2/SnO2 nanosheets directly grown on Al2O3 ceramic tubes. Sens. Actuators B 2018, 273, 328–335. [Google Scholar] [CrossRef]
- Chen, Z.R.; Xu, H.Y.; Liu, C.Y.; Cao, D.Q.; Ye, Q.; Wu, X.W.; Wang, J.Q.; Cao, B.Q. Good triethylamine sensing properties of Au@MoS2 nanostructures directly grown on ceramic tubes. Mater. Chem. Phys. 2020, 245, 122683. [Google Scholar] [CrossRef]
- Wang, C.; Wang, Y.L.; Cheng, P.F.; Xu, L.P.; Dang, F.; Wang, T.L.; Lei, Z.H. In-situ generated TiO2/α-Fe2O3 heterojunction arrays for batch manufacturing of conductometric acetone gas sensors. Sens. Actuators B 2021, 340, 129926. [Google Scholar] [CrossRef]
- Meng, D.; Si, J.P.; Wang, M.Y.; Wang, G.S.; Shen, Y.B.; San, X.G.; Meng, F.L. In-situ growth of V2O5 flower-like structures on ceramic tubes and their trimethylamine sensing properties. Chin. Chem. Lett. 2020, 31, 2133–2136. [Google Scholar] [CrossRef]
- Du, H.F.; Xie, G.Z.; Su, Y.J.; Tai, H.L.; Du, X.S.; Yu, H.; Zhang, Q.P. A new model and its application for the dynamic response of RGO resistive gas sensor. Sensors 2019, 19, 889. [Google Scholar] [CrossRef] [Green Version]
- Filippidou, M.K.; Chatzichristidi, M.; Chatzandroulis, S. A fabrication process of flexible IDE capacitive chemical sensors using a two step lift-off method based on PVA patterning. Sens. Actuators B 2019, 284, 7–12. [Google Scholar] [CrossRef]
- Li, X.Y.; Ni, L.; Chen, N.; Liu, J.L.; Li, W.J.; Xian, Y. Enhanced sensitivity of humidity sensor using Nafion/graphene oxide quantum dot nanocomposite. Measurement 2021, 181, 109566. [Google Scholar] [CrossRef]
- Rivadeneyra, A.; Fernández-Salmerón, J.; Banqueri, J.; López-Villanueva, J.A.; Capitan-Vallvey, L.F.; Palma, A.J. A novel electrode structure compared with interdigitated electrodes as capacitive sensor. Sens. Actuators B 2014, 204, 552–560. [Google Scholar] [CrossRef]
- Afsarimanesh, N.; Nag, A.; Alahi, M.E.E.; Han, T.; Mukhopadhyay, S.C. Interdigital sensors: Biomedical, environmental and industrial applications. Sens. Actuators A 2020, 305, 111923. [Google Scholar] [CrossRef]
- Zhao, D.W.; Zhu, Y.Z.; Cheng, W.K.; Chen, W.S.; Wu, Y.Q.; Yu, H.P. Cellulose-based flexible functional materials for emerging intelligent electronics. Adv. Mater. 2020, 33, e2000619. [Google Scholar] [CrossRef]
- Zhao, Z.J.; Ko, J.; Ahn, J.; Bok, M.; Gao, M.; Hwang, S.H.; Kang, H.J.; Jeon, S.; Park, I.; Jeong, J.H. 3D layer-by-layer Pd-containing nanocomposite platforms for enhancing the performance of hydrogen sensors. ACS Sens. 2020, 5, 2367–2377. [Google Scholar] [CrossRef]
- Li, W.W.; Chen, R.S.; Qi, W.Z.; Cai, L.; Sun, Y.L.; Sun, M.X.; Li, C.; Yang, X.K.; Xiang, L.; Xie, D.; et al. Reduced graphene oxide/mesoporous ZnO NSs hybrid fibers for flexible, stretchable, twisted, and wearable NO2 E-textile gas sensor. ACS Sens. 2019, 4, 2809–2818. [Google Scholar] [CrossRef] [PubMed]
- Singh, E.; Meyyappan, M.; Nalwa, H.S. Flexible graphene-based wearable gas and chemical sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Z.Q.; Yao, J.D.; Wang, B.; Yang, G.W. Light-controlling, flexible and transparent ethanol gas sensor based on ZnO nanoparticles for wearable devices. Sci. Rep. 2015, 5, 11070. [Google Scholar] [CrossRef] [PubMed]
- Yun, Y.J.; Hong, W.G.; Choi, N.J.; Kim, B.H.; Jun, Y.; Lee, H.K. Ultrasensitive and highly selective graphene-based single yarn for use in wearable gas sensor. Sci. Rep. 2015, 5, 10904. [Google Scholar] [CrossRef] [PubMed]
- Punetha, D.; Kar, M.; Pandey, S.K. A new type low-cost, flexible and wearable tertiary nanocomposite sensor for room temperature hydrogen gas sensing. Sci. Rep. 2020, 10, 2151. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.Y.; Xu, X.R.; Liu, M.D.; Chen, C.; Cui, J.; Chen, X.; Wu, K.; Sun, D.P. Wearable and flexible bacterial cellulose/polyaniline ammonia sensor based on a synergistic doping strategy. Sens. Actuators B 2021, 334, 129647. [Google Scholar] [CrossRef]
- Nair, K.G.; Vishnuraj, R.; Pullithadathil, B. Highly sensitive, flexible H2 gas sensors based on less platinum bimetallic Ni–Pt nanocatalyst-functionalized carbon nanofibers. ACS Appl. Electron. Mater. 2021, 3, 1621–1633. [Google Scholar] [CrossRef]
- Zhu, Z.Y.; Liu, C.C.; Jiang, F.X.; Liu, J.; Liu, G.Q.; Ma, X.M.; Liu, P.P.; Huang, R.; Xu, J.K.; Wang, L. Flexible fiber-shaped hydrogen gas sensor via coupling palladium with conductive polymer gel fiber. J. Hazard. Mater. 2021, 411, 125008. [Google Scholar] [CrossRef]
- Qi, Q.; Zhang, T.; Liu, L.; Zheng, X.J. Synthesis and toluene sensing properties of SnO2 nanofibers. Sens. Actuators B 2009, 137, 471–475. [Google Scholar] [CrossRef]
- Bigiani, L.; Zappa, D.; Maccato, C.; Comini, E.; Barreca, D.; Gasparotto, A. Quasi-1D MnO2 nanocomposites as gas sensors for hazardous chemicals. Appl. Surf. Sci. 2020, 512, 145667. [Google Scholar] [CrossRef]
- Guo, S.Q.; Yang, D.; Zhang, S.; Dong, Q.; Li, B.C.; Tran, N.; Li, Z.Y.; Xiong, Y.J.; Zaghloul, M.E. Development of a cloud-based epidermal MoSe2 Device for hazardous gas sensing. Adv. Funct. Mater. 2019, 29. [Google Scholar] [CrossRef]
- Kim, H.J.; Lee, J.H. Highly sensitive and selective gas sensors using p-type oxide semiconductors: Overview. Sens. Actuators B 2014, 192, 607–627. [Google Scholar] [CrossRef]
- Li, Z.J.; Li, H.; Wu, Z.L.; Wang, M.K.; Luo, J.T.; Torun, H.; Hu, P.A.; Yang, C.; Grundmann, M.; Liu, X.T.; et al. Advances in designs and mechanisms of semiconducting metal oxide nanostructures for high-precision gas sensors operated at room temperature. Mater. Horiz. 2019, 6, 470–506. [Google Scholar] [CrossRef] [Green Version]
- Nasri, A.; Pétrissans, M.; Fierro, V.; Celzard, A. Gas sensing based on organic composite materials: Review of sensor types, progresses and challenges. Mater. Sci. Semicond. Process. 2021, 128. [Google Scholar] [CrossRef]
- Wang, C.X.; Yin, L.W.; Zhang, L.W.; Xiang, D.; Gao, R. Metal oxide gas sensors: Sensitivity and influencing factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef] [Green Version]
- Katoch, A.; Choi, S.W.; Kim, H.W.; Kim, S.S. Highly sensitive and selective H2 sensing by ZnO nanofibers and the underlying sensing mechanism. J. Hazard. Mater. 2015, 286, 229–235. [Google Scholar] [CrossRef]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Chatterjee, S.G.; Chatterjee, S.; Ray, A.K.; Chakraborty, A.K. Graphene–metal oxide nanohybrids for toxic gas sensor: A review. Sens. Actuators B 2015, 221, 1170–1181. [Google Scholar] [CrossRef]
- Yuan, W.J.; Shi, G.Q. Graphene-based gas sensors. J. Mater. Chem. A 2013, 1, 10078–10091. [Google Scholar] [CrossRef]
- Zhang, H.J.; Meng, F.N.; Liu, L.Z.; Chen, Y.J.; Wang, P.J. Highly sensitive H2S sensor based on solvothermally prepared spinel ZnFe2O4 nanoparticles. J. Alloys Compd. 2018, 764, 147–154. [Google Scholar] [CrossRef]
- Kim, M.; Lim, H.J.; Kwon, S.; Lee, M.; Jeong, T.Y.; Kwon, Y.K. Synthesis of highly-energetic nanoparticles based on monodisperse hollow mesoporous nanospheres. Microporous Mesoporous Mater. 2019, 277, 295–300. [Google Scholar] [CrossRef]
- Ratnesh, R.K.; Mehata, M.S. Investigation of biocompatible and protein sensitive highly luminescent quantum dots/nanocrystals of CdSe, CdSe/ZnS and CdSe/CdS. Spectrochim. Acta Part A 2017, 179, 201–210. [Google Scholar] [CrossRef]
- Trung, D.D.; Cuong, N.D.; Trung, K.Q.; Nguyen, T.-D.; Van Toan, N.; Hung, C.M.; Hieu, N.V. Controlled synthesis of manganese tungstate nanorods for highly selective NH3 gas sensor. J. Alloys Compd. 2018, 735, 787–794. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, Z.H.; Zou, H.F.; Ma, M. Fabrication of electrospun LaFeO3 nanotubes via annealing technique for fast ethanol detection. Mater. Lett. 2018, 215, 58–61. [Google Scholar] [CrossRef]
- Al-Hadeethi, Y.; Umar, A.; Ibrahim, A.A.; Al-Heniti, S.H.; Kumar, R.; Baskoutas, S.; Raffah, B.M. Synthesis, characterization and acetone gas sensing applications of Ag-doped ZnO nanoneedles. Ceram. Int. 2017, 43, 6765–6770. [Google Scholar] [CrossRef]
- Liu, X.H.; Ma, T.T.; Xu, Y.S.; Sun, L.; Zheng, L.L.; Schmidt, O.G.; Zhang, J. Rolled-up SnO2 nanomembranes: A new platform for efficient gas sensors. Sens. Actuators B 2018, 264, 92–99. [Google Scholar] [CrossRef]
- Wang, D.; Cheng, Y.; Wan, K.C.; Yang, J.L.; Xu, J.C.; Wang, X.Y. High efficiency xylene detection based on porous MoO3 nanosheets. Vacuum 2020, 179, 109487. [Google Scholar] [CrossRef]
- Xu, Y.Y.; Tian, X.; Liu, P.; Sun, Y.Q.; Du, G.X. In2O3 nanoplates with different crystallinity and porosity: Controllable synthesis and gas-sensing properties investigation. J. Alloys Compd. 2019, 787, 1063–1073. [Google Scholar] [CrossRef]
- Singh, N.; Kumar, A.; Kaur, D. Hydrogen gas sensing properties of platinum decorated silicon carbide (Pt/SiC) Nanoballs. Sens. Actuators B 2018, 262, 162–170. [Google Scholar] [CrossRef]
- Song, Y.; Chen, F.; Zhang, Y.Y.; Zhang, S.M.; Liu, F.M.; Sun, P.; Yan, X.; Lu, G.Y. Fabrication of highly sensitive and selective room-temperature nitrogen dioxide sensors based on the ZnO nanoflowers. Sens. Actuators B 2019, 287, 191–198. [Google Scholar] [CrossRef]
- Zhang, S.D.; Yang, M.J.; Liang, K.Y.; Turak, A.; Zhang, B.X.; Meng, D.; Wang, C.X.; Qu, F.D.; Cheng, W.L.; Yang, M.H. An acetone gas sensor based on nanosized Pt-loaded Fe2O3 nanocubes. Sens. Actuators B 2019, 290, 59–67. [Google Scholar] [CrossRef]
- Jang, J.S.; Choi, S.J.; Koo, W.T.; Kim, S.J.; Cheong, J.Y.; Kim, I.D. Elaborate manipulation for Sub-10 nm hollow catalyst sensitized heterogeneous oxide nanofibers for room temperature chemical sensors. ACS Appl. Mater. Interfaces 2017, 9, 24821–24829. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, J.; Zhang, Y.; Tian, F.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J.; Pinna, N. Edge-enriched WS2 nanosheets on carbon nanofibers boosts NO2 detection at room temperature. J. Hazard. Mater. 2021, 411, 125120. [Google Scholar] [CrossRef]
- Sen, S.; Nilabh, A.; Kundu, S. Room temperature acetone sensing performance of Pt/Sb2O3 impregnated Fe2O3 thin film: Noninvasive diabetes detection. Microchem. J. 2021, 165, 106111. [Google Scholar] [CrossRef]
- Sohal, M.K.; Mahajan, A.; Gasso, S.; Bedi, R.K.; Singh, R.C.; Debnath, A.K.; Aswal, D.K. Ultrasensitive yttrium modified tin oxide thin film based sub-ppb level NO2 detector. Sens. Actuators B 2021, 329, 129169. [Google Scholar] [CrossRef]
- Lou, C.; Wang, K.; Mei, H.; Xie, J.; Zheng, W.; Liu, X.; Zhang, J. ZnO nanoarrays via a thermal decomposition–deposition method for sensitive and selective NO2 detection. CrystEngComm 2021, 23, 3654–3663. [Google Scholar] [CrossRef]
- Li, Z.; Yi, J. Drastically enhanced ammonia sensing of Pt/ZnO ordered porous ultra-thin films. Sens. Actuators B 2020, 317, 128217. [Google Scholar] [CrossRef]
- Lei, G.; Lou, C.; Liu, X.; Xie, J.; Li, Z.; Zheng, W.; Zhang, J. Thin films of tungsten oxide materials for advanced gas sensors. Sens. Actuators B 2021, 341, 129996. [Google Scholar] [CrossRef]
- Schneider, K.; Maziarz, W. V2O5 thin films as nitrogen dioxide sensors dagger. Sensors 2018, 18, 4177. [Google Scholar] [CrossRef] [Green Version]
- Wilson, R.L.; Simion, C.E.; Blackman, C.S.; Carmalt, C.J.; Stanoiu, A.; Di Maggio, F.; Covington, J.A. The effect of film thickness on the gas sensing properties of ultra-thin TiO(2) films deposited by atomic layer deposition. Sensors 2018, 18, 735. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hermawan, A.; Asakura, Y.; Inada, M.; Yin, S. One-step synthesis of micro-/mesoporous SnO2 spheres by solvothermal method for toluene gas sensor. Ceram. Int. 2019, 45, 15435–15444. [Google Scholar] [CrossRef]
- Wales, D.J.; Grand, J.; Ting, V.P.; Burke, R.D.; Edler, K.J.; Bowen, C.R.; Mintova, S.; Burrows, A.D. Gas sensing using porous materials for automotive applications. Chem Soc. Rev. 2015, 44, 4290–4321. [Google Scholar] [CrossRef] [Green Version]
- Thong, L.V.; Hoa, N.D.; Le, D.T.T.; Viet, D.T.; Tam, P.D.; Le, A.T.; Hieu, N.V. On-chip fabrication of SnO2-nanowire gas sensor: The effect of growth time on sensor performance. Sens. Actuators B 2010, 146, 361–367. [Google Scholar] [CrossRef]
- Zhu, J.T.; Wang, B.B.; Jin, P. Synthesis and formation mechanism of 1D hollow SiO2 nanomaterials using in situ formed 1D NaCl crystal templates. RSC Adv. 2015, 5, 92004–92007. [Google Scholar] [CrossRef]
- Zhu, L.; Wang, J.N.; Liu, J.W.; Nasir, M.S.; Zhu, J.W.; Li, S.S.; Liang, J.D.; Yan, W. Smart formaldehyde detection enabled by metal organic framework-derived doped electrospun hollow nanofibers. Sens. Actuators B 2021, 326, 128819. [Google Scholar] [CrossRef]
- Liu, J.W.; Wang, J.N.; Zhu, L.; Chen, X.; Ma, Q.Y.; Wang, L.; Wang, X.; Yan, W. A high-safety and multifunctional MOFs modified aramid nanofiber separator for lithium-sulfur batteries. Chem. Eng. J. 2021, 411, 128540. [Google Scholar] [CrossRef]
- Wang, J.N.; Yang, G.R.; Chen, J.; Liu, Y.P.; Wang, Y.K.; Lao, C.Y.; Xi, K.; Yang, D.W.; Harris, C.J.; Yan, W.; et al. Flexible and high-loading lithium–sulfur batteries enabled by integrated three-in-one fibrous membranes. Adv. Energy Mater. 2019, 9. [Google Scholar] [CrossRef]
- Wang, J.N.; Yang, G.R.; Wang, L.; Yan, W. Synthesis of one-dimensional NiFe2O4 nanostructures: Tunable morphology and high-performance anode materials for Li ion batteries. J. Mater. Chem. A 2016, 4, 8620–8629. [Google Scholar] [CrossRef]
- Lupan, O.; Chai, G.Y.; Chow, L. Novel hydrogen gas sensor based on single ZnO nanorod. Microelectron. Eng. 2008, 85, 2220–2225. [Google Scholar] [CrossRef]
- Godse, P.R.; Mane, A.T.; Navale, Y.H.; Navale, S.T.; Mulik, R.N.; Patil, V.B. Hydrothermally grown 1D ZnO nanostructures for rapid detection of NO2 gas. SN Appl. Sci. 2021, 3. [Google Scholar] [CrossRef]
- Lu, X.F.; Yin, L.W. Porous indium oxide nanorods: Synthesis, characterization and gas sensing properties. J. Mater. Sci. Technol. 2011, 27, 680–684. [Google Scholar] [CrossRef]
- Wang, L.; Deng, J.; Lou, Z.; Zhang, T. Nanoparticles-assembled Co3O4 nanorods p-type nanomaterials: One-pot synthesis and toluene-sensing properties. Sens. Actuators B 2014, 201, 1–6. [Google Scholar] [CrossRef]
- Zhao, C.H.; Gong, H.M.; Niu, G.Q.; Wang, F. Electrospun Ca-doped In2O3 nanotubes for ethanol detection with enhanced sensitivity and selectivity. Sens. Actuators B 2019, 299. [Google Scholar] [CrossRef]
- Seif, A.M.; Nikfarjam, A.; Hajghassem, H. UV enhanced ammonia gas sensing properties of PANI/TiO2 core-shell nanofibers. Sens. Actuators B 2019, 298. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Wang, L.; Yan, W.; Wei, W. C@CoFe2O4 fiber-in-tube mesoporous nanostructure: Formation mechanism and high electrochemical performance as an anode for lithium-ion batteries. J. Alloys Compd. 2017, 693, 110–117. [Google Scholar] [CrossRef]
- Wang, J.; Yang, G.; Wang, L.; Yan, W. Fabrication of one-dimensional CdFe2O4 yolk/shell flat nanotubes as a high-performance anode for lithium-ion batteries. J. Mater. Sci. 2016, 52, 4096–4108. [Google Scholar] [CrossRef]
- Jiang, Z.Q.; Yin, M.Y.; Wang, C. Facile synthesis of Ca2+/Au co-doped SnO2 nanofibers and their application in acetone sensor. Mater. Lett. 2017, 194, 209–212. [Google Scholar] [CrossRef]
- Xu, X.L.; Chen, Y.; Zhang, G.H.; Yan, S.H.; Bian, H.Q.; Chen, Q.; Lu, Y.; Ma, S.Y. Fabrication of Pr-doped SnO2 spherical core-shell nanostructure with wrinkly shell and the gas sensing properties. Mater. Lett. 2017, 195, 159–163. [Google Scholar] [CrossRef]
- Kou, X.; Xie, N.; Chen, F.; Wang, T.; Guo, L.; Wang, C.; Wang, Q.; Ma, J.; Sun, Y.; Zhang, H.; et al. Superior acetone gas sensor based on electrospun SnO2 nanofibers by Rh doping. Sens. Actuators B 2018, 256, 861–869. [Google Scholar] [CrossRef]
- Yu, A.; Li, Z.; Yi, J. Selective detection of parts-per-billion H2S with Pt-decorated ZnO nanorods. Sens. Actuators B 2021, 333. [Google Scholar] [CrossRef]
- Shen, J.L.; Li, F.; Yin, B.; Sun, L.; Chen, C.; Wen, S.P.; Chen, Y.; Ruan, S.P. Enhanced ethyl acetate sensing performance of Al-doped In2O3 microcubes. Sens. Actuators B 2017, 253, 461–469. [Google Scholar] [CrossRef]
- Wang, Z.H.; Hou, C.L.; De, Q.M.; Gu, F.B.; Han, D.M. One-step synthesis of co-doped In2O3 nanorods for high response of formaldehyde sensor at low temperature. ACS Sens. 2018, 3, 468–475. [Google Scholar] [CrossRef]
- Wang, J.; Su, J.; Chen, H.; Zou, X.X.; Li, G.D. Oxygen vacancy-rich, Ru-doped In2O3 ultrathin nanosheets for efficient detection of xylene at low temperature. J. Mater. Chem. C 2018, 6, 4156–4162. [Google Scholar] [CrossRef]
- Chen, H.; Zhao, Y.; Shi, L.; Li, G.D.; Sun, L.; Zou, X. Revealing the relationship between energy level and gas sensing performance in heteroatom-doped semiconducting nanostructures. ACS Appl. Mater. Interfaces 2018, 10, 29795–29804. [Google Scholar] [CrossRef]
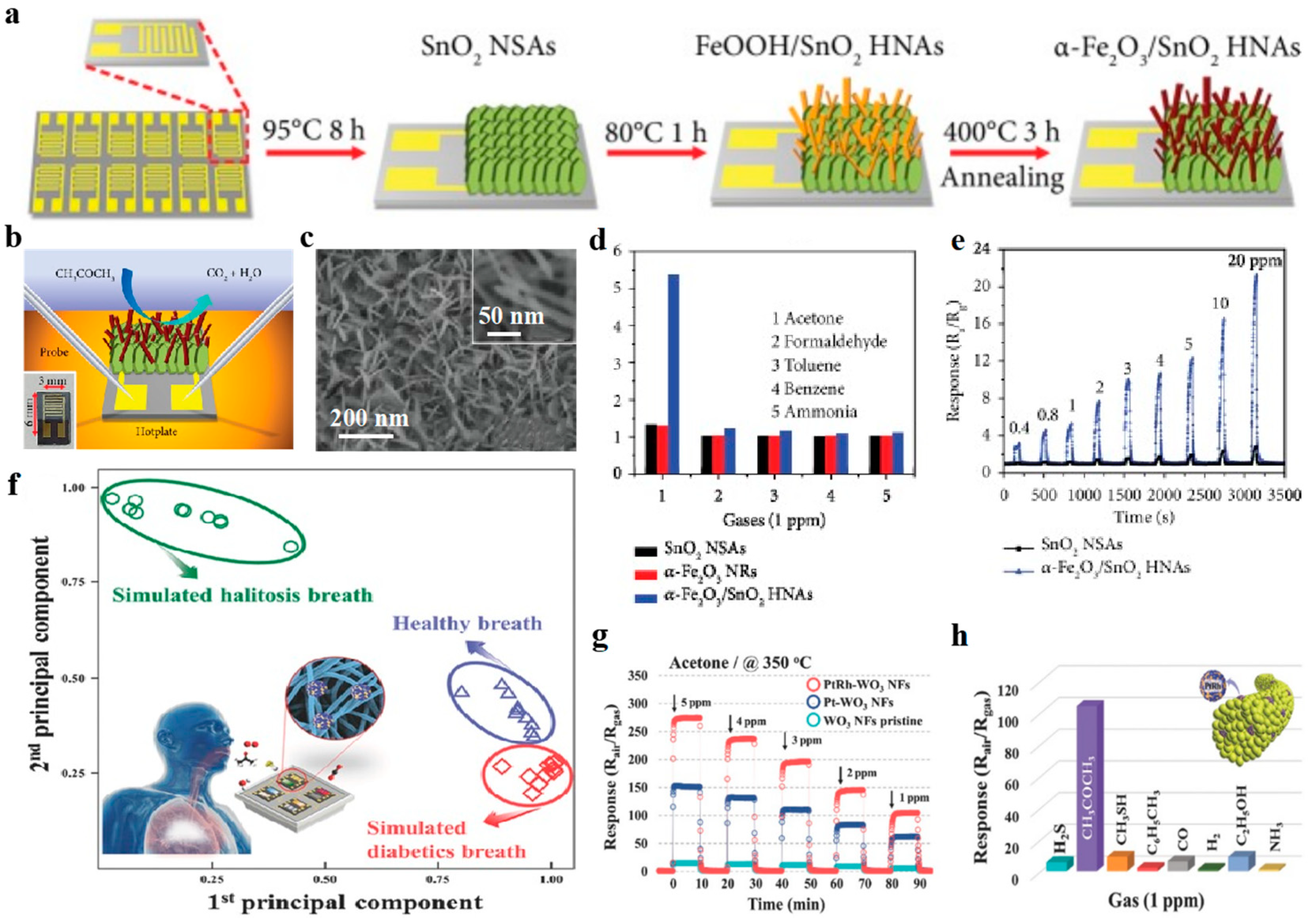
- Zhou, T.T.; Zhang, R.; Wang, Y.B.; Zhang, T. MOF–Derived 1 D α–Fe2O3/NiFe2O4 heterojunction as efficient sensing materials of acetone vapors. Sens. Actuators B 2019, 281, 885–892. [Google Scholar] [CrossRef]
- Walker, J.M.; Akbar, S.A.; Morris, P.A. Synergistic effects in gas sensing semiconducting oxide nano-heterostructures: A review. Sens. Actuators B 2019, 286, 624–640. [Google Scholar] [CrossRef]
- Ueda, T.; Boehme, I.; Hyodo, T.; Shimizu, Y.; Weimar, U.; Barsan, N. Enhanced NO2-sensing properties of Au-loaded porous In2O3 Gas sensors at low operating temperatures. Chemosensors 2020, 8, 72. [Google Scholar] [CrossRef]
- Yang, C.; Yang, Y.; Zhang, C.; Yu, H.; Wang, T.; Shi, K.; Zhang, Z.; Wang, D.; Dong, X. High selectivity of Ag-doped Fe2O3 hollow nanofibers in H2S detection at room operating temperature. Sens. Actuators B 2021, 341, 129919. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Oxygen vacancies enabled porous SnO2 thin films for highly sensitive detection of triethylamine at room temperature. ACS Appl. Mater. Interfaces 2020, 12, 20704–20713. [Google Scholar] [CrossRef]
- Bai, X.; Lv, H.; Liu, Z.; Chen, J.; Wang, J.; Sun, B.; Zhang, Y.; Wang, R.; Shi, K. Thin-layered MoS2 nanoflakes vertically grown on SnO2 nanotubes as highly effective room-temperature NO2 gas sensor. J. Hazard. Mater. 2021, 416, 125830. [Google Scholar] [CrossRef]
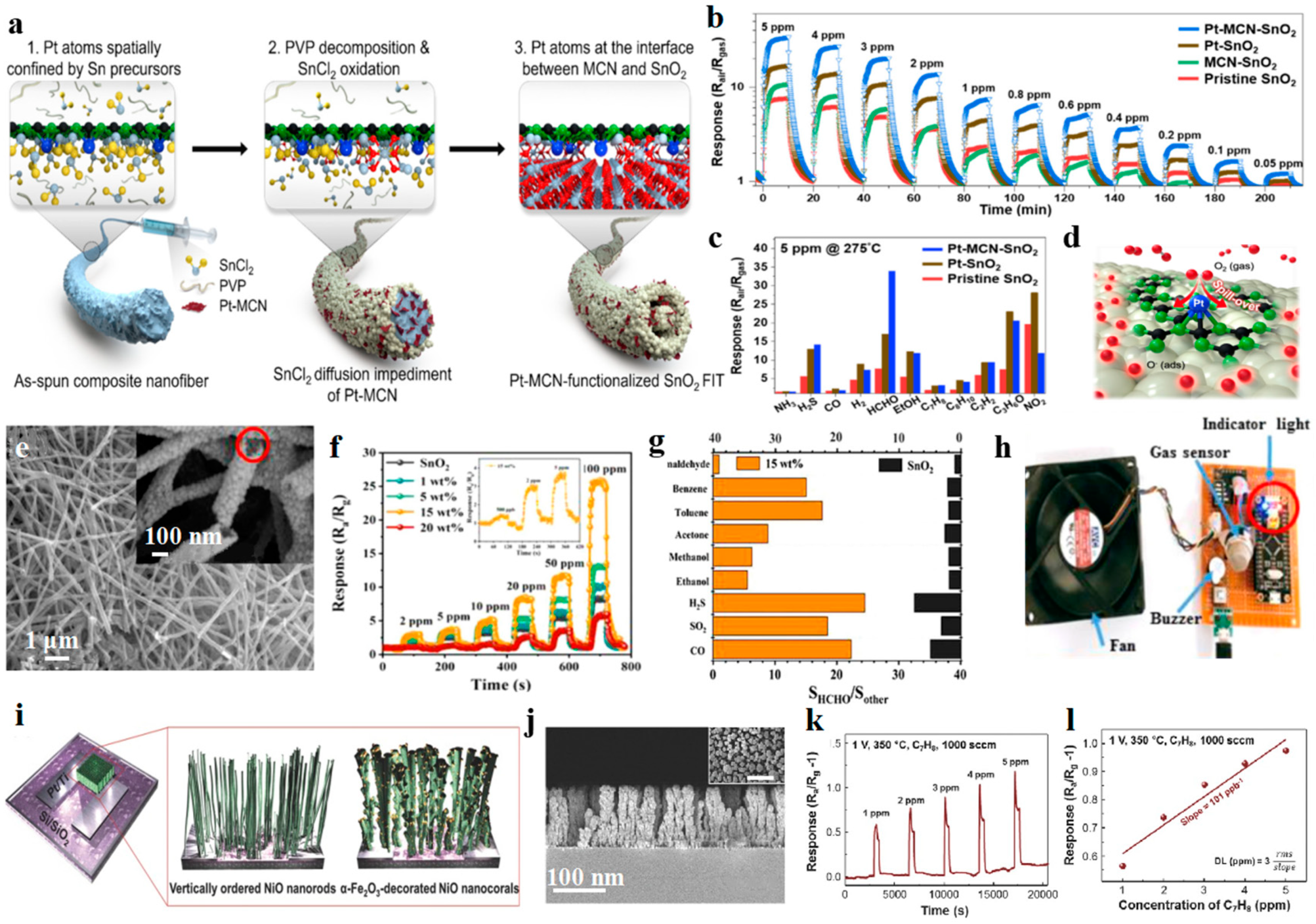
- Xu, Y.; Zheng, W.; Liu, X.; Zhang, L.; Zheng, L.; Yang, C.; Pinna, N.; Zhang, J. Platinum single atoms on tin oxide ultrathin films for extremely sensitive gas detection. Mater. Horiz. 2020, 7, 1519–1527. [Google Scholar] [CrossRef]
- Xu, Y.; Lou, C.; Zheng, L.; Zheng, W.; Liu, X.; Kumar, M.; Zhang, J. Highly sensitive and selective detection of acetone based on platinum sensitized porous tungsten oxide nanospheres. Sens. Actuators B 2020, 307, 127616. [Google Scholar] [CrossRef]
- Ma, A.; Baek, S.Y.; Seo, J.H.; Abbas, S.A.; Kwon, J.-H.; Ahn, S.J.; Nam, K.M. Photodeposition of Pt nanoparticles on Co3O4 nanocubes for detection of acetone at part-per-billion levels. ACS Appl. Nano Mater. 2021, 4, 2752–2759. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Zhu, B.; Yu, J. Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sens. Actuators B 2021, 331, 129425. [Google Scholar] [CrossRef]
- Zheng, L.; Xie, J.; Liu, X.; Yang, C.; Zheng, W.; Zhang, J. Unveiling the electronic interaction in ZnO/PtO/Pt nanoarrays for catalytic detection of triethylamine with ultrahigh sensitivity. ACS Appl. Mater. Interfaces 2020, 12, 46267–46276. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.Y.; Chi, Z.T.; Liu, J.; Li, D.H.; Sun, X.J.; Yan, C.; Wang, Y.C.; Li, H.; Wang, X.D.; Xie, W.F. Enhanced gas sensing performance based on p-NiS/n-In2O3 heterojunction nanocomposites. Sens. Actuators B 2020, 304, 127305. [Google Scholar] [CrossRef]
- Xu, Y.; Zheng, L.; Yang, C.; Zheng, W.; Liu, X.; Zhang, J. Chemiresistive sensors based on core-shell ZnO@TiO2 nanorods designed by atomic layer deposition for n-butanol detection. Sens. Actuators B 2020, 310, 127846. [Google Scholar] [CrossRef]
- Miller, D.R.; Akbar, S.A.; Morris, P.A. Nanoscale metal oxide-based heterojunctions for gas sensing: A review. Sens. Actuators B 2014, 204, 250–272. [Google Scholar] [CrossRef]
- Karnati, P.; Akbar, S.; Morris, P.A. Conduction mechanisms in one dimensional core-shell nanostructures for gas sensing: A review. Sens. Actuators B 2019, 295, 127–143. [Google Scholar] [CrossRef]
- Park, K.R.; Cho, H.B.; Lee, J.; Song, Y.; Kim, W.B.; Choa, Y.H. Design of highly porous SnO2-CuO nanotubes for enhancing H2S gas sensor performance. Sens. Actuators B 2020, 302, 127179. [Google Scholar] [CrossRef]
- Yang, S.; Lei, G.; Xu, H.; Lan, Z.; Wang, Z.; Gu, H. Metal oxide based heterojunctions for gas sensors: A review. Nanomaterials 2021, 11, 1026. [Google Scholar] [CrossRef]
- Hermawan, A.; Asakura, Y.; Inada, M.; Yin, S. A facile method for preparation of uniformly decorated-spherical SnO2 by CuO nanoparticles for highly responsive toluene detection at high temperature. J. Mater. Sci. Technol. 2020, 51, 119–129. [Google Scholar] [CrossRef]
- Li, Y.; Ban, H.T.; Yang, M.J. Highly sensitive NH3 gas sensors based on novel polypyrrole-coated SnO2 nanosheet nanocomposites. Sens. Actuators B 2016, 224, 449–457. [Google Scholar] [CrossRef]
- Zang, X.N.; Zhou, Q.; Chang, J.Y.; Liu, Y.M.; Lin, L.W. Graphene and carbon nanotube (CNT) in MEMS/NEMS applications. Microelectron. Eng. 2015, 132, 192–206. [Google Scholar] [CrossRef]
- Luo, G.F.; Xie, L.L.; He, M.; Jaisutti, R.; Zhu, Z.G. Flexible fabric gas sensors based on reduced graphene-polyaniline nanocomposite for highly sensitive NH3 detection at room temperature. Nanotechnology 2021, 32, 305501. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.L.; Sun, J.Y.; Hao, J.Y.; Sun, Q.; Wan, P.; Li, Y.; Zhou, X.; Yuan, Y.; Zhang, X.; Wang, Y. Engineering SnO2 nanorods/ethylenediamine-modified graphene heterojunctions with selective adsorption and electronic structure modulation for ultrasensitive room-temperature NO2 detection. Nanotechnology 2021, 32, 155505. [Google Scholar] [CrossRef]
- Reddy, C.S.; Zhang, L.W.; Qiu, Y.J.; Chen, Y.N.; Reddy, A.S.; Reddy, P.S.; Dugasani, S.R. Investigation of hydrogen sensing properties of graphene/Al–SnO2 composite nanotubes derived from electrospinning. J. Ind. Eng. Chem. 2018, 63, 411–419. [Google Scholar] [CrossRef]
- Kim, J.H.; Zheng, Y.F.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Synthesis and selective sensing properties of rGO/Metal-coloaded SnO2 nanofibers. J. Electron. Mater. 2017, 46, 3531–3541. [Google Scholar] [CrossRef]
- Liu, W.; Gu, D.; Zhang, J.W.; Li, X.G.; Rumyantseva, M.N.; Gaskov, A.M. ZnSe/NiO heterostructure-based chemiresistive-type sensors for low-concentration NO2 detection. Rare Met. 2020, 40, 1632–1641. [Google Scholar] [CrossRef]
- Wang, L.; Wang, L.H.; Li, G.J.; Zhu, Y.Y.; Liu, C.; Zeng, L.J.; Zhong, S.; Wang, L.J. Three-dimensional CuPc films decorated with well-ordered PVA parallel nanofiber arrays for low concentration detecting NO2 sensor. Sens. Actuators B 2021, 337, 129781. [Google Scholar] [CrossRef]
- Lim, K.; Jo, Y.M.; Yoon, J.W.; Kim, J.S.; Lee, D.J.; Moon, Y.K.; Yoon, J.W.; Kim, J.H.; Choi, H.J.; Lee, J.H. A transparent nanopatterned chemiresistor: Visible-light plasmonic sensor for trace-level NO2 detection at room temperature. Small 2021, e2100438. [Google Scholar] [CrossRef] [PubMed]
- Suh, J.M.; Kwon, K.C.; Lee, T.H.; Kim, C.; Lee, C.W.; Song, Y.G.; Choi, M.J.; Choi, S.; Cho, S.H.; Kim, S.; et al. Edge-exposed WS2 on 1D nanostructures for highly selective NO2 sensor at room temperature. Sens. Actuators B 2021, 333, 129566. [Google Scholar] [CrossRef]
- Cui, S.M.; Pu, H.H.; Lu, G.H.; Wen, Z.H.; Mattson, E.C.; Hirschmugl, C.; Gajdardziska-Josifovska, M.; Weinert, M.; Chen, J.H. Fast and selective room-temperature ammonia sensors using silver nanocrystal-functionalized carbon nanotubes. ACS Appl. Mater. Interfaces 2012, 4, 4898–4904. [Google Scholar] [CrossRef] [PubMed]
- Han, P.; Song, X.D.; Hao, C. Excited-state hydrogen bonding: Detecting ammonia using an HHTP-DPB covalent organic framework. Chem. Phys. 2020, 536, 110822. [Google Scholar] [CrossRef]
- He, M.; Xie, L.L.; Luo, G.F.; Li, Z.H.; Wright, J.; Zhu, Z.G. Flexible fabric gas sensors based on PANI/WO3 p−n heterojunction for high performance NH3 detection at room temperature. Sci. China Mater. 2020, 63, 2028–2039. [Google Scholar] [CrossRef]
- Li, S.Q.; Liu, A.; Yang, Z.J.; He, J.M.; Wang, J.; Liu, F.M.; Lu, H.Y.; Yan, X.; Sun, P.; Liang, X.S.; et al. Room temperature gas sensor based on tin dioxide@ polyaniline nanocomposite assembled on flexible substrate: Ppb-level detection of NH3. Sens. Actuators B 2019, 299. [Google Scholar] [CrossRef]
- Zhang, K.; Huang, S.Y.; Lu, H.B.; Li, F.; Shao, L. Experimental study of the light source characteristics for the NH3 concentration detection. Optik 2020, 209, 164608. [Google Scholar] [CrossRef]
- Zhang, W.Q.; Li, G.S.; She, C.K.; Liu, A.Y.; Cheng, J.N.; Li, H.K.; Liu, S.H.; Jing, C.B.; Cheng, Y.; Chu, J.H. High performance tube sensor based on PANI/Eu(3+) nanofiber for low-volume NH3 detection. Anal. Chim. Acta 2020, 1093, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Singh, A.; Singh, R.; Singh, S.; Kumar, P.; Kumar, R. Facile h-MoO3 synthesis for NH3 gas sensing application at moderate operating temperature. Sens. Actuators B 2020, 325, 128974. [Google Scholar] [CrossRef]
- Zheng, J.Z.; Pang, K.L.; Liu, X.; Li, S.X.; Song, R.; Liu, Y.L.; Tang, Z.Y. Integration and synergy of organic single crystals and metal–organic frameworks in core–shell heterostructures enables outstanding gas selectivity for detection. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef]
- Seifaddini, P.; Ghasempour, R.; Ramezannezhad, M.; Nikfarjam, A. Room temperature ammonia gas sensor based on Au/graphene nanoribbon. Mater. Res. Express 2019, 6, 045054. [Google Scholar] [CrossRef]
- Bindra, P.; Hazra, A. Dielectric sensor system using TiO2 nanotubes for real-time detection of methanol contamination in alcoholic beverages. IEEE Trans. Instrum. Meas. 2020, 69, 6621–6629. [Google Scholar] [CrossRef]
- Chinh, N.D.; Hung, N.M.; Majumder, S.; Kim, C.; Kim, D. Hole-supply-rate-controlled methanol-gas-sensing reaction over p-type Co3O4/single-walled carbon nanotube hybrid structures. Sens. Actuators B 2021, 326, 128956. [Google Scholar] [CrossRef]
- Du, B.B.; Ruan, Y.N.; Ly, T.T.; Jia, P.P.; Sun, Q.T.; Feng, Q.L.; Yang, D.X.; Ebendorff-Heidepriem, H. MoS2-enhanced epoxy-based plasmonic fiber-optic sensor for selective and sensitive detection of methanol. Sens. Actuators B 2020, 305, 127513. [Google Scholar] [CrossRef]
- Urfa, Y.; Çorumlu, V.; Altındal, A. Gamma ray irradiation dose dependent methanol sensing with ZnO nanoparticles. Mater. Chem. Phys. 2021, 264, 124473. [Google Scholar] [CrossRef]
- Liang, Y.; Liu, W.H.; Hu, W.; Zhou, Q.H.; He, K.R.; Xu, K.; Yang, Y.; Yu, T.; Yuan, C.L. Synthesis and gas-sensing properties of ZnO@NiCo2O4 core@shell nanofibers. Mater. Res. Bull. 2019, 114, 1–9. [Google Scholar] [CrossRef]
- Hu, Q.; Huang, B.Y.; Li, Y.; Zhang, S.M.; Zhang, Y.X.; Hua, X.H.; Liu, G.; Li, B.S.; Zhou, J.Y.; Xie, E.Q.; et al. Methanol gas detection of electrospun CeO2 nanofibers by regulating Ce3+/Ce4+ mole ratio via Pd doping. Sens. Actuators B 2020, 307, 127638. [Google Scholar] [CrossRef]
- Sinha, M.; Neogi, S.; Mahapatra, R.; Krishnamurthy, S.; Ghosh, R. Material dependent and temperature driven adsorption switching (p- to n- type) using CNT/ZnO composite-based chemiresistive methanol gas sensor. Sens. Actuators B 2021, 336, 129729. [Google Scholar] [CrossRef]
- Ai, L.; Wang, J.; Li, T.T.; Zhao, C.; Tang, Y.H.; Wang, W.S.; Zhao, S.J.; Jiang, W.J.; Di, Y.L.; Fei, X.C.; et al. A rapid and sensitive fluorescence method for detecting urine formaldehyde in patients with Alzheimer’s disease. Ann. Clin. Biochem. 2019, 56, 210–218. [Google Scholar] [CrossRef]
- Chen, W.; Yang, M.; Luo, N.; Wang, F.; Yu, R.Q.; Jiang, J.H. An intramolecular charge transfer and excited state intramolecular proton transfer based fluorescent probe for highly selective detection and imaging of formaldehyde in living cells. Analyst 2019, 144, 6922–6927. [Google Scholar] [CrossRef]
- Nie, X.M.; Chen, Z.Y.; Tian, Y.P.; Chen, S.; Qu, L.; Fan, M.B. Rapid detection of trace formaldehyde in food based on surface-enhanced Raman scattering coupled with assembled purge trap. Food Chem. 2021, 340, 127930. [Google Scholar] [CrossRef] [PubMed]
- Shin, H.; Jung, W.G.; Kim, D.H.; Jang, J.S.; Kim, Y.H.; Koo, W.T.; Bae, J.; Park, C.; Cho, S.H.; Kim, B.J.; et al. Single-atom Pt stabilized on one-dimensional nanostructure support via carbon nitride/SnO2 heterojunction trapping. ACS Nano 2020, 14, 11394–11405. [Google Scholar] [CrossRef]
- Suh, J.M.; Shim, Y.S.; Kim, D.H.; Sohn, W.; Jung, Y.M.; Lee, S.Y.; Choi, S.; Kim, Y.H.; Jeon, J.M.; Hong, K.; et al. Synergetically selective toluene sensing in hematite-decorated nickel oxide nanocorals. Adv. Mater. Technol. 2017, 2. [Google Scholar] [CrossRef]
- Zeng, X.G.; Wang, Z.; Li, Y.; Li, H.Y.; Xu, S.Y.; Liu, L.; Gong, Y.M.; Liang, Q.C.; Duan, H.J.; Cheng, Y.L.; et al. Highly sensitive and selective formaldehyde gas sensor based on CdO–In2O3 beaded porous nanotubes at low temperature. J. Mater. Sci. Mater. Electron. 2018, 29, 17533–17541. [Google Scholar] [CrossRef]
- Ma, H.; Xu, Y.M.; Rong, Z.M.; Cheng, X.L.; Gao, S.; Zhang, X.F.; Zhao, H.; Huo, L.H. Highly toluene sensing performance based on monodispersed Cr2O3 porous microspheres. Sens. Actuators B 2012, 174, 325–331. [Google Scholar] [CrossRef]
- Wang, L.L.; Zhou, T.T.; Zhang, R.; Lou, Z.; Deng, J.N.; Zhang, T. Comparison of toluene sensing performances of zinc stannate with different morphology-based gas sensors. Sens. Actuators B 2016, 227, 448–455. [Google Scholar] [CrossRef]
- Bang, J.H.; Choi, M.S.; Mirzaei, A.; Han, S.; Lee, H.Y.; Choi, S.W.; Kim, S.S.; Kim, H.W. Hybridization of silicon nanowires with TeO2 branch structures and Pt nanoparticles for highly sensitive and selective toluene sensing. Appl. Surf. Sci. 2020, 525, 146620. [Google Scholar] [CrossRef]
- Wang, J.N.; Yi, S.S.; Liu, J.W.; Sun, S.Y.; Liu, Y.P.; Yang, D.W.; Xi, K.; Gao, G.X.; Abdelkader, A.; Yan, W.; et al. Suppressing the shuttle effect and dendrite growth in lithium-sulfur batteries. ACS Nano 2020, 14, 9819–9831. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.Q.; Kang, Y.Q.; Zhao, Y.; Wang, L.; Liu, J.L.; Li, Y.X.; Liang, Z.; He, X.M.; Li, X.; Tavajohi, N.; et al. A review of lithium-ion battery safety concerns: The issues, strategies, and testing standards. J. Energy Chem. 2021, 59, 83–99. [Google Scholar] [CrossRef]
- Huang, L.L.; Liu, L.S.; Lu, L.G.; Feng, X.N.; Han, X.B.; Li, W.H.; Zhang, M.X.; Li, D.S.; Liu, X.B.; Sauer, D.U.; et al. A review of the internal short circuit mechanism in lithium-ion batteries: Inducement, detection and prevention. Int. J. Energy Res. 2021. [Google Scholar] [CrossRef]
- Nair, K.G.; Ramakrishnan, V.; Unnathpadi, R.; Karuppanan, K.K.; Pullithadathil, B. Unraveling hydrogen adsorption kinetics of bimetallic Au–Pt nanoisland-functionalized carbon nanofibers for room-temperature Gas sensor applications. J. Phys. Chem. C 2020, 124, 7144–7155. [Google Scholar] [CrossRef]
- Wang, L.L.; Chai, R.Q.; Lou, Z.; Shen, G.Z. Highly sensitive hybrid nanofiber-based room-temperature CO sensors: Experiments and density functional theory simulations. Nano Res. 2017, 11, 1029–1037. [Google Scholar] [CrossRef]
- Hittini, W.; Greish, Y.E.; Qamhieh, N.N.; Alnaqbi, M.A.; Zeze, D.; Mahmoud, S.T. Ultrasensitive and low temperature gas sensor based on electrospun organic-inorganic nanofibers. Org. Electron. 2020, 81, 105659. [Google Scholar] [CrossRef]
- Zhang, S.Y.; Yin, C.B.; Yang, L.; Zhang, Z.L.; Han, Z.J. Investigation of the H2 sensing properties of multilayer mesoporous pure and Pd-doped SnO2 thin film. Sens. Actuators B 2019, 283, 399–406. [Google Scholar] [CrossRef]
- Huang, J.R.; Hsu, W.C.; Chen, Y.J.; Wang, T.B.; Lin, K.W.; Chen, H.I.; Liu, W.C. Comparison of hydrogen sensing characteristics for Pd/GaN and Pd/Al0.3Ga0.7As Schottky diodes. Sens. Actuators B 2006, 117, 151–158. [Google Scholar] [CrossRef]
- Gu, H.H.; Wang, Z.; Hu, Y.M. Hydrogen gas sensors based on semiconductor oxide nanostructures. Sensors 2012, 12, 5517–5550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hübert, T.; Boon-Brett, L.; Palmisano, V.; Bader, M.A. Developments in gas sensor technology for hydrogen safety. Int. J. Hydrog. Energy 2014, 39, 20474–20483. [Google Scholar] [CrossRef]
- Wadell, C.; Nugroho, F.A.; Lidstrom, E.; Iandolo, B.; Wagner, J.B.; Langhammer, C. Hysteresis-free nanoplasmonic Pd-Au alloy hydrogen sensors. Nano Lett. 2015, 15, 3563–3570. [Google Scholar] [CrossRef]
- Hermawan, A.; Asakura, Y.; Kobayashi, M.; Kakihana, M.; Yin, S. High temperature hydrogen gas sensing property of GaN prepared from α-GaOOH. Sens. Actuators B 2018, 276, 388–396. [Google Scholar] [CrossRef]
- Raza, M.H.; Kaur, N.; Comini, E.; Pinna, N. Toward optimized radial modulation of the space-charge region in one-dimensional SnO2-NiO core-shell nanowires for hydrogen sensing. ACS Appl. Mater. Interfaces 2020, 12, 4594–4606. [Google Scholar] [CrossRef]
- Nikfarjam, A.; Hosseini, S.; Salehifar, N. Fabrication of a highly sensitive single aligned TiO2 and gold nanoparticle embedded TiO2 nano-fiber Gas sensor. ACS Appl. Mater. Interfaces 2017, 9, 15662–15671. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.L.; Yan, J.; Lian, J.B.; Pu, W.J.; Jing, L.Q.; Xu, H.; Li, H.M. Graphene oxide-loaded SnO2 quantum wires with Sub-4 nanometer diameters for low-temperature H2S gas sensing. ACS Appl. Nano Mater. 2020, 3, 6385–6393. [Google Scholar] [CrossRef]
- Tsai, Y.S.; Tsai, S.C.; Kuo, C.C.; Chan, W.L.; Lin, W.H.; Wu, Y.S.; Lin, Y.S.; Li, M.H.; Kuo, M.Y.; Chen, H. Organic/inorganic hybrid nanostructures of polycrystalline perylene diimide decorated ZnO nanorods highly enhanced dual sensing performance of UV light/CO gas sensors. Results Phys. 2021, 24, 104173. [Google Scholar] [CrossRef]
- Weng, T.F.; Ho, M.S.; Sivakumar, C.; Balraj, B.; Chung, P.F. VLS growth of pure and Au decorated β-Ga2O3 nanowires for room temperature CO gas sensor and resistive memory applications. Appl. Surf. Sci. 2020, 533, 147476. [Google Scholar] [CrossRef]
- Kumar, R.; Jaiswal, M.; Singh, O.; Gupta, A.; Ansari, M.S.; Mittal, J. Selective and reversible sensing of low concentration of carbon monoxide gas using Nb-doped OMS-2 nanofibers at room temperature. IEEE Sens. J. 2019, 19, 7201–7206. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, F.; Gan, J.L.; Cui, Y.J.; Li, B.; Yang, Y.; Qian, G.D. Metal-organic framework film for fluorescence turn-on H2S gas sensing and anti-counterfeiting patterns. Sci. China Mater. 2019, 62, 1445–1453. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gao, P.; Sha, L.N.; Chi, Q.Q.; Yang, L.; Zhang, J.J.; Chen, Y.J.; Zhang, M.L. Spatial separation of electrons and holes for enhancing the gas-sensing property of a semiconductor: ZnO/ZnSnO3 nanorod arrays prepared by a hetero-epitaxial growth. Nanotechnology 2018, 29, 175501. [Google Scholar] [CrossRef]
- Güntner, A.T.; Abegg, S.; Wegner, K.; Pratsinis, S.E. Zeolite membranes for highly selective formaldehyde sensors. Sens. Actuators B 2018, 257, 916–923. [Google Scholar] [CrossRef]
- Kim, N.H.; Choi, S.J.; Yang, D.J.; Bae, J.; Park, J.; Kim, I.D. Highly sensitive and selective hydrogen sulfide and toluene sensors using Pd functionalized WO3 nanofibers for potential diagnosis of halitosis and lung cancer. Sens. Actuators B 2014, 193, 574–581. [Google Scholar] [CrossRef]
- Chuang, M.Y.; Chen, C.C.; Zan, H.W.; Meng, H.F.; Lu, C.J. Organic gas sensor with an improved lifetime for detecting breath ammonia in hemodialysis patients. ACS Sens. 2017, 2, 1788–1795. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.; Choi, S.J.; Park, K.M.; Lee, S.S.; Choi, S.; Kim, I.D.; Park, C.O. The stability, sensitivity and response transients of ZnO, SnO2 and WO3 sensors under acetone, toluene and H2S environments. Sens. Actuators B 2014, 197, 300–307. [Google Scholar] [CrossRef]
- Van den Broek, J.; Guntner, A.T.; Pratsinis, S.E. Highly selective and rapid breath isoprene sensing enabled by activated alumina filter. ACS Sens. 2018, 3, 677–683. [Google Scholar] [CrossRef] [Green Version]
- Rydosz, A. A negative correlation between blood glucose and acetone measured in healthy and type 1 diabetes mellitus patient breath. J. Diabetes Sci. Technol. 2015, 9, 881–884. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.X.; Zhao, X.M.; Yin, H.J.; Wang, Z.N.; Jiang, C.Y.; Liu, W.C.; Chen, Z.Y.; Yuan, Y.; Li, Y.X.; Wang, C.J. Study of breath acetone and its correlations with blood glucose and blood beta-hydroxybutyrate using an animal model with lab-developed type 1 diabetic rats. RSC Adv. 2015, 5, 71002–71010. [Google Scholar] [CrossRef]
- Ama, O.; Sadiq, M.; Johnson, M.; Zhang, Q.F.; Wang, D.L. Novel 1D/2D KWO/Ti3C2Tx nanocomposite-based acetone sensor for diabetes prevention and monitoring. Chemosensors 2020, 8, 102. [Google Scholar] [CrossRef]
- Gong, H.M.; Zhao, C.H.; Niu, G.Q.; Zhang, W.; Wang, F. Construction of 1D/2D alpha-Fe2O3/SnO2 hybrid nanoarrays for sub-ppm acetone detection. Research (Wash D C) 2020, 2020, 2196063. [Google Scholar] [CrossRef] [Green Version]
- Kim, D.H.; Jang, J.S.; Koo, W.T.; Kim, I.D. Graphene oxide templating: Facile synthesis of morphology engineered crumpled SnO2 nanofibers for superior chemiresistors. J. Mater. Chem. A 2018, 6, 13825–13834. [Google Scholar] [CrossRef]
- Shingange, K.; Swart, H.; Mhlongo, G.H. Ultrafast detection of low acetone concentration displayed by Au-loaded LaFeO3 nanobelts owing to synergetic effects of porous 1D morphology and catalytic activity of Au nanoparticles. ACS Omega 2019, 4, 19018–19029. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Choi, S.J.; Jang, J.S.; Cho, H.J.; Koo, W.T.; Tuller, H.L.; Kim, I.D. Exceptional high-performance of Pt-based bimetallic catalysts for exclusive detection of exhaled biomarkers. Adv. Mater. 2017, 29. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.P.; Li, Y.L.; Pionteck, J.; Zhou, Z.; Weng, W.; Luo, X.G.; Qin, Z.Y.; Voit, B.; Zhu, M.F. Flexible poly(styrene-butadiene-styrene)/carbon nanotube fiber based vapor sensors with high sensitivity, wide detection range, and fast response. Sens. Actuators B 2018, 256, 896–904. [Google Scholar] [CrossRef]
- Hu, Z.D.; Sun, X.Y.; Li, H.F.; Kang, Y.R.; Song, X.Q.; Wang, P.; Luan, Q.T.; Wang, X.D.; Chi, Z.T.; Xie, W.F. Cobalt monosulfide nanofibers: Ethanol sensing and magnetic properties. Rare Met. 2021, 40, 1554–1560. [Google Scholar] [CrossRef]
- Burris, A.J.; Tran, K.; Cheng, Q. Tunable enhancement of a graphene/polyaniline/poly(ethylene oxide) composite electrospun nanofiber gas sensor. J. Anal. Test. 2017, 1, 12. [Google Scholar] [CrossRef]
- Bai, J.L.; Luo, Y.B.; Chen, C.; Deng, Y.; Cheng, X.; An, B.X.; Wang, Q.; Li, J.P.; Zhou, J.Y.; Wang, Y.R.; et al. Functionalization of 1D In2O3 nanotubes with abundant oxygen vacancies by rare earth dopant for ultra-high sensitive ethanol detection. Sens. Actuators B 2020, 324, 128755. [Google Scholar] [CrossRef]
- Han, D.; Yang, J.; Gu, F.; Wang, Z. Effects of rare earth element doping on the ethanol gas-sensing performance of three-dimensionally ordered macroporous In2O3. RSC Adv. 2016, 6, 45085–45092. [Google Scholar] [CrossRef]
- Anand, K.; Kaur, J.; Singh, R.C.; Thangaraj, R. Effect of terbium doping on structural, optical and gas sensing properties of In2O3 nanoparticles. Mater. Sci. Semicond. Process. 2015, 39, 476–483. [Google Scholar] [CrossRef]
- Wei, D.; Huang, Z.; Wang, L.; Chuai, X.; Zhang, S.; Lu, G. Hydrothermal synthesis of Ce-doped hierarchical flower-like In2O3 microspheres and their excellent gas-sensing properties. Sens. Actuators B 2018, 255, 1211–1219. [Google Scholar] [CrossRef]
- Anand, K.; Kaur, J.; Singh, R.C.; Thangaraj, R. Temperature dependent selectivity towards ethanol and acetone of Dy3+-doped In2O3 nanoparticles. Chem. Phys. Lett. 2017, 670, 37–45. [Google Scholar] [CrossRef]
- Liu, X.; Jiang, L.; Jiang, X.; Tian, X.; Sun, X.; Wang, Y.; He, W.; Hou, P.; Deng, X.; Xu, X. Synthesis of Ce-doped In2O3 nanostructure for gas sensor applications. Appl. Surf. Sci. 2018, 428, 478–484. [Google Scholar] [CrossRef]



| Application | Target Gas | Material | Design Strategy | Performance | Ref. | ||||
|---|---|---|---|---|---|---|---|---|---|
| Concentration | Response | Tresponse/Trecovery (s) | Temperature (°C) | Limit of Detection | |||||
| Environmental monitoring | NO2 | Au–SnO2 NFs | Doping | 5 ppm | 180 | 500/223 | RT | 6 ppb | [142] |
| WS2–SiO2 NRs | Composites | 5 ppm | 151.2 | - | RT | 13.726 ppb | [143] | ||
| ZnO NRs | - | 5 ppm | 70 | 16/200 | 150 | 1 ppm | [101] | ||
| NH3 | h-MoO3 NRs | - | 5 ppm | 36 | 230/267 | 200 | - | [150] | |
| CuPc–MOF-3 | Heterostructures | 5 ppm | 45 | - | RT | 52 ppb | [151] | ||
| AuGNR | Composites | 25 ppm | 34 | 224/178 | RT | - | [152] | ||
| Ag NC−MWCNTs | Composites | 100 ppm | 9 | 15/7 | RT | - | [144] | ||
| Methanol | ZnO–NiCo2O4 NFs | Heterostructures | 100 ppm | 6.67 | 37/175 | 250 | - | [157] | |
| Pd–CeO2 NFs | Doping | 100 ppm | 6.95 | - | 200 | 402 ppb | [158] | ||
| CNT–ZnO | Composites | 25 ppm | 72.6 | 3.15/3.45 | 250 | - | [159] | ||
| HCHO | Pt–MCN–SnO2 | Doping/Heterostructures | 5 ppm | 33.9 | - | 275 | 50 ppb | [163] | |
| ZZS HNFs | Doping | 100 ppm | 25.7 | 12/45 | 400 | 500 ppb | [96] | ||
| CdO–In2O3 NTs | Heterostructures | 50 ppm | 72 | 6/12 | 132 | 100 ppb | [165] | ||
| Toluene | Pt–TeO2–Si NW | Doping/Composites | 50 ppm | 45 | 20/500 | 200 | - | [168] | |
| α-Fe2O3–NiO nanocorals | Heterostructures | 50 ppm | 45.4 | - | 350 | 22 ppb | [164] | ||
| Safety monitoring | H2 | Au-Pt-CNFs | Doping/Composites | 500 ppm | 33 | 6.6/18 | RT | - | [172] |
| SnO2/NiO CSNWs | Heterostructures | 500 ppm | 114 | 120/660 | 500 | 0.9 ppm | [181] | ||
| CO | Au–β–Ga2O3 NWs | Doping | 100 ppm | 4.8 | 21.14/21.34 | RT | - | [185] | |
| Nb–OMS–2 NFs | Doping | 2 ppm | 22 | 25/40 | RT | - | [186] | ||
| NiO–TiO2 HNFs | Heterostructures | 50 ppm | 2.07 | 10/20 | RT | 1 ppm | [173] | ||
| GNP–TiO2 NFs | Doping | 30 ppb | 75 | 3/4 | 250 | 700 ppt | [182] | ||
| H2S | SnO2–GO | Composites | 10 ppm | 17.9 | 12/137 | 70 | 61 ppb | [177] | |
| ZnO–ZnSnO3 NRs | Heterostructures | 30 ppm | 137.9 | 14/26 | 165 | 700 ppb | [188] | ||
| Health monitoring | Acetone | KWO–Ti3C2TX | Composites | 2.86 ppm | 2.5 | - | RT | - | [196] |
| α-Fe2O3–SnO2 | Heterostructures | 1 ppm | 5.37 | 14/70 | 340 | 0.4 ppm | [197] | ||
| Pt–SnO2 NFs | Doping | 5 ppm | 245.2 | 12.7/- | 350 | 100 ppb | [198] | ||
| Au–LaFeO3 NBs | Doping | 40 ppm | 125 | 26/20 | 100 | 267 ppb | [199] | ||
| PtPd–WO3 NFs | Doping | 1 ppm | 97.5 | 4.2/204 | 300 | 1.07 ppb | [200] | ||
| Ethanol | GO–PANi–PEO | Composites | 200 ppm | 2.1 | 30/252 | RT | 15 ppm | [203] | |
| Tb–In2O3 NTs | Doping | 100 ppm | 159.8 | 1/60 | 220 | - | [204] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Z.; Zhu, L.; Sun, S.; Wang, J.; Yan, W. One-Dimensional Nanomaterials in Resistive Gas Sensor: From Material Design to Application. Chemosensors 2021, 9, 198. https://doi.org/10.3390/chemosensors9080198
Wang Z, Zhu L, Sun S, Wang J, Yan W. One-Dimensional Nanomaterials in Resistive Gas Sensor: From Material Design to Application. Chemosensors. 2021; 9(8):198. https://doi.org/10.3390/chemosensors9080198
Chicago/Turabian StyleWang, Ze, Lei Zhu, Shiyi Sun, Jianan Wang, and Wei Yan. 2021. "One-Dimensional Nanomaterials in Resistive Gas Sensor: From Material Design to Application" Chemosensors 9, no. 8: 198. https://doi.org/10.3390/chemosensors9080198
APA StyleWang, Z., Zhu, L., Sun, S., Wang, J., & Yan, W. (2021). One-Dimensional Nanomaterials in Resistive Gas Sensor: From Material Design to Application. Chemosensors, 9(8), 198. https://doi.org/10.3390/chemosensors9080198