Abstract
The preparation and testing of a colour-based prototype indicator for high-pressure processing (HPP) are described. The indicator is a layered structure comprising a pressed disc of a mixture of silica gel, which has been previously loaded with a set wt% of acidified water, and polytetrafluoroethylene, PTFE, powders, a water-permeable barrier layer, and a Congo-Red-based pH indicator layer, all vacuum-sealed in a water impermeable plastic film. The value of the wt% is calculated from the ratio of the mass of acidified water added to the mass of originally dry silica gel. The high pressures associated with HPP drive the release of the acidified water from the silica gel and its subsequent transport through the water-permeable barrier layer to the pH indicator, thereby producing a striking red-to-blue colour change. The response of the HPP indicator can be tuned to different HPP conditions by varying the wt% of acidified water used to load the silica gel powder. Indicators, with 61, 63, and 65 wt% acidified water loaded silica gel, are prepared and found to require, respectively, the application of at least, 600, 400, and 300 MPa pressure for 3 min to effect a change colour. To our knowledge, this is the first reported example of a prototype HPP indicator that can be tuned to respond to the very different pressure and time conditions used in HPP to sterilise such very different products as milk, apple and orange juice, and aloe vera gel.
1. Introduction
Pasteurisation is a common thermal processing technique used in the food industry, which, in many cases, induces undesired changes in product quality, such as loss of smell, colour, flavour, texture, and nutritional value []. Consumer demands for fresh, safe, and nutritional food products are driving the development of alternative food pasteurisation technologies, such as high-pressure processing (i.e., HPP) [,]. In HPP, the products are usually already sealed (often vacuum-sealed) in their final flexible, plastic packaging before being placed in the HPP reactor and subjected to pressure, generally in the range of 100–800 MPa, over an exposure time that can range from a few seconds to over 20 min, depending on the desired treatment []. HPP inactivates microbes by damaging their outer cell membrane and essential proteins in cells [,,].
In many cases, HPP allows the food to retain its organoleptic and nutritional properties and extend its shelf-life through its pasteurisation action. It is often claimed that, in comparison with thermal-processed foods, HPP foods have “better texture, fresher taste, improved appearance and increased retention of nutrients” [], and it is reported that “HPP usually results in minimal or no permanent change in textural characteristics” []. In HPP, the pressure is transmitted to food products equally from all sides and, as a result, the foods are not crushed during treatment and thus many foodstuffs can be treated using HPP []. However, under typical conditions, HPP cannot be used to make shelf-stable, low-acid products such as vegetables, milk, or soups, because of its inability to destroy spores, although it can be used to extend the refrigerated shelf life of such products []. HPP is not effective for foods with a very low water content, such as dried fruits and spices, nor is it appropriate for use on solid foods with a high air content, such as bread and mousses. In addition, a major barrier to the use of HPP is the initial cost of a reactor, typically $0.3 to over $3 M, depending on the equipment capacity and the extent of automation. However, the cost of HP processing can be comparable to the cost of thermal processing, ca 2–5 cents per kg [,], and the significant beneficial features over thermal pasteurisation are such that the number of installed commercial HPP reactor units has risen markedly since the mid-90s [] so that the global HPP products market is now valued at >$15.5 billion []. At present, fruit and vegetable products such as juices and purees are the largest product market for HPP, followed by meat, seafood, and fish products [].
With the growing use of HPP, there is a need for an inexpensive indicator to check and validate the pressure and time profiles used in HPP [,]. To date, various different types of HPP indicators have been developed, examples of which are given in Table 1. However, all the HPP indicators listed in Table 1 fail to give a simple, easy-to-use, clear, and instant visible indication that the HPP process has been carried out at the desired pressure and time. A colourimetric HPP indicator that exhibits such features would appear to present an attractive alternative to the HPP indicators developed to date, and there are some commercial, colour-based HPP indicators promoted online that claim to do this.

Table 1.
Types of reported HPP indicators.
For example, Multi-Color CorporationTM has reported a label, based on pressure-sensitive microcapsules, that can inform the consumer whether the product has been protected by HPP technology []. When the label is subjected to a pressure greater than the compressive bursting strength of the micro-capsule, an indicator material is released and a check mark appears; the ink colour change is irreversible []. By altering the microcapsule wall thickness, the trigger pressure can be customised to respond to different high pressures, although it does not appear possible to tune them to respond to a particular high pressure and time applied, which is essential in an ideal HPP indicator. Chromatic Technologies Inc. has also developed a similar, micro-capsule based HPP indicator that can be delivered through commercial printing inks and responds to pressures of ca. 480 MPa, producing an increasingly striking colour change up to 600 MPa []; however, once again, the technology does not appear able to respond to a set high pressure and application time.
Neither of the two on-line promoted indicators described above appear commercially available as yet. In addition, both indicators do not appear to exhibit a response that is tuneable with respect to different sets of applied high pressure and time conditions, despite the fact that the exact HPP process conditions that are required for processing different foods can vary considerably. Some of the key food groups and the usual time, pressure, and temperature conditions used in their HP processing are listed in Section S1, Table S1, in the Electronic Supplementary Information folder, i.e., ESI, associated with this paper. An ideal HPP indicator should be inexpensive, capable of measuring and integrating the combined effects of time and pressure within the HPP system, and exhibit a striking colour change, all at the desired operating temperature, and this paper describes a novel prototype HPP indicator that appears to show promise in this regard.
2. Materials and Methods
2.1. Materials
Silica gel (CAS: 7631-86-9, pore size 60 Angstrom, particle size: 40–63 µm) was purchased from Fluorochem (Glossop, UK), and all other chemicals were purchased from Merck Life Science UK Limited (Gillingham, UK) in the highest purity available. All aqueous solutions were made using double-distilled and deionised water. The water-impermeable, clear plastic polymer film (110 µm thick), used to encapsulate the indicator components, was a laminate of polyethylene terephthalate (PET) with an aluminium coating, low-density polyethylene (LDPE), and a peelable co-extruded film of proprietary composition; this film was provided by Sensor Indicator Products (Seattle, WA, USA) []. A schematic cross section of the latter laminate film is given in Section S2, Figure S1, in the ESI. The water-permeable barrier film layers used in this work, i.e., Whatman Nuclepore™ track-etched polycarbonate (hydrophilic) membranes (1 μm pore size, 13 mm diameter), were purchased from Scientific laboratory Supplies (Nottingham, UK).
2.2. Methods
2.2.1. The HPP Indicator
The novel, layered HPP indicator used in this work had the following components, (i) a brightly coloured, pH-indicating film, (ii) a water-permeable barrier layer, and (iii) a pressed disc that, when subjected to very high pressures, released acidified water that flowed through (ii) and produced a striking colour change in (i). All of the above components were vacuum-sealed in a water-impermeable clear plastic film (iv). A schematic illustration of the architecture of the HPP indicator is given below in Figure 1.
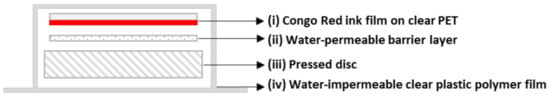
Figure 1.
Schematic of the layered HPP indicator, comprising a bright red colour Congo Red ink film (i), a water-permeable barrier layer (ii), and an acidified-water releasing pressed disc (iii), all vacuum-sealed in a water-impermeable clear plastic polymer film (iv).
The preparations of the individual components of the HPP indicator illustrated in Figure 1 are described below.
- The pH indicator film disc
The Congo Red (i.e., CR; pKa = 4.0) [] ink film, i.e., (i) in Figure 1, was generated by adding 7 mL of a 30 mg/mL Congo Red aqueous solution to 20 g of a 15 wt% aqueous solution of polyvinyl alcohol, PVA (MW 146,00–186,000). A total of 3 mL of water was then added to the mixture, and the final solution was stirred for 1 h. The CR-based pH indicator film was then created by coating the ink onto a ca. 50 μm thick clear PET sheet using a K-bar (No. 3), producing a wet film thickness of ca. 24 µm that dried to a bright red, pH indicator film, ca. 3.0 µm thick. Once dried, the pH indicator film was cut into 12 mm (diameter) discs, using a computer controlled cutting machine (Cricut Explore Air 2, Amazon, London, UK) [], for subsequent incorporation into the HPP indicator, illustrated in Figure 1, as a pH-indicator film (on PET) disc.
- The water-permeable barrier layer
A water-permeable barrier layer, i.e., layer (ii) in Figure 1, is required to prevent direct contact between the acidified water-containing pressed disc (iii) and the underlying pH indicator layer (i) and also to provide a conduit for the acidified water generated by this disc to the pH indicator layer, (i). In most of this work, a Whatman Nuclepore™ [] track-etched polycarbonate (hydrophilic) membrane (1 μm pore size, 13 mm diameter) was used as the water-permeable barrier layer. A scanning electron micrograph image of the membrane is illustrated in Figure 2, revealing its microporous nature and an average pore size of 1 μm.
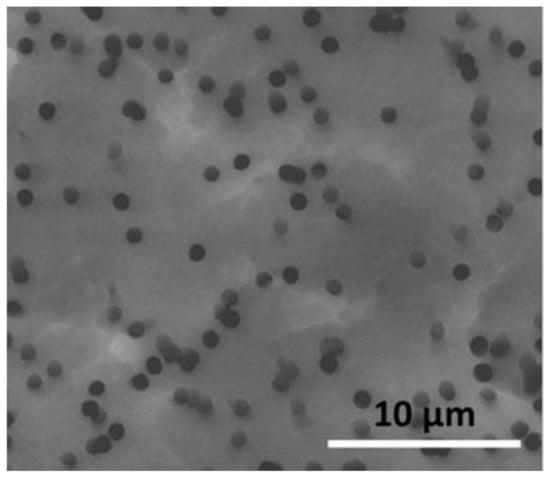
Figure 2.
Scanning electron micrograph showing the surface of a Whatman Nuclepore™ track-etched polycarbonate (Hydrophilic) membrane. The average pore size is measured as ca. 1 µm.
- The acidified water-releasing pressed disc
The acidified water-releasing discs used in this work comprised a mixture of poly tetrafluoro ethylene (PTFE) powder and silica gel powder, where the latter had been loaded with a defined wt% of water, acidified with a non-volatile acid, camphor sulfonic acid (CSA). In all this work, the value of the wt% was calculated from the ratio of the mass of acidified water added to the mass of originally dry silica gel. A typical pressed disc was made by dissolving 664 mg of camphor sulfonic acid (CSA) in 6.1 g of water placed in a 250 mL glass jar to yield an acidified aqueous solution with a pH of ca. 0.45. Once fully dissolved, the acidified water was added to 10 g of silica gel and the resulting fluffy powder mixed continuously for 1 h using an overhead shaker (Heidolph Reax 2, Merck Life Science UK Limited, Gillingham, UK). The final form of the silica powder had 61 wt% of acidified water in it. A total of 4 g of PTFE powder (particle size 1 µm) was then added and the product mixed for a further 30 min to produce a homogeneous white powder. Finally, 500 mg of the mixed powder was pressed into a 12 mm diameter, 3 mm thick disc using a KBr Press (Specac® Atlas 15T manual hydraulic press, Merck Life Science UK Limited, Gillingham, UK) by applying 1 tonne via a hydraulic ram (equivalent to an applied pressure of 74 MPa) for 10 s. Note that no loss of water was observed when the powder was pressed using the latter conditions. Since this disc contained silica gel loaded with 6.1 g of acidified water, it is referred to throughout as a 61 wt% acidified water-loaded silica gel/PTFE disc, or a “61 wt% disc” for short. Other wt% discs were made using the same procedure by altering the amount of acidified water used to load the originally dry silica gel.
- Assembling the HPP indicator
In the HPP indicator, the 12 mm diameter Congo Red pH indicator film (on PET) disc (layer (i) in Figure 1) was placed on the water-permeable membrane (layer (ii) in Figure 1) with the coloured ink film side (not the clear, colourless, supporting PET film substrate side) facing the water barrier layer so as to receive and respond to any acidified water that might be made to flow through the membrane. These combined two films were then placed on the pressed disc, i.e., layer (iii), to create the layered structure illustrated in Figure 1, which was then vacuum-sealed in a 110 µm thick, water-impermeable, plastic encapsulating film, i.e., (iv) in Figure 1. Unless stated otherwise, all HPP indicators were stored in a fridge (or freezer) at 5 °C (or −20 °C) until use.
2.2.2. High-Pressure Processing/Pasteurisation (HPP)
All HPP work was carried out using an Avure [] QUITUS™ type vertical HPP reactor, (QFP 35 L-600 (purchased from Quintus Food Press, Sweden, Västerås), which has a cylindrical pressure vessel with a volume of 35 L, a maximum filling capacity = 70%, a maximum pressure = 600 MPa, and a maximum vessel temperature of 50 °C and uses water as the high-pressure-transmitting medium []. The unit can process up to 25 kg per cycle and was installed and operated at the Agri Food and Biosciences Institute, Belfast, UK. The system allows precise control of the key process parameters, namely, pressure, temperature, and times, and a typical profile for 3 min at 600 MPa is illustrated in Section S3, Figure S2, in the ESI section. The temperature profile in Figure S2 shows that before pressurisation, the vessel temperature is 21 °C (which was the case for all the HPP work reported here), and 32 °C when it reached 600 MPa. This temperature increase is due to adiabatic heating and is a typical feature of HP processing, i.e., ca. 2 °C rise per 100 MPa pressure []. Typically, it takes ca. 2.2 min to reach the set pressure, and after the application of this pressure for the set time (for example, 3 min in Figure S2), the final release and return to atmospheric pressure and the original temperature (always 21 °C) is almost instantaneous (<0.5 min).
In the batch HPP reactor used in this work, samples for HPP processing are routinely placed in a polyethene plastic bag (10 × 15 cm2), which is then heat-sealed and placed in the reactor’s cylindrical load basket. The latter is loaded into the 35 L pressure vessel, which is then filled with water and brought up to the desired pressure within ca. 2 min. The vacuum-sealed bagging of goods is necessary to ensure that if any of the product packaging breaks, no contamination of the water used to pressurise the cylinder occurs. In all of this work, the HPP indicators were treated as a typical product and so were heat-sealed in the usual HPP containment plastic bag described above, placed in the load basket, and then loaded into the pressure vessel. Thus, the indicators were used to probe the typical pressure and application time conditions in the pressure vessel during an HPP cycle. However, there is no reason why the indicator could not also be placed inside the packaged product itself and so provide a valuable insight into the actual pressure/time conditions experienced by the packaged food product itself.
2.2.3. Other Methods
UV/vis spectrophotometry was carried out using a CARY60 UV/vis spectrophotometer (Agilent, Craven Arms, UK), and all digital photography was taken using a Canon 7D digital camera (Canon Ltd., Uxbridge, UK).
3. Results and Discussion
3.1. The pH Indicator Film Disc
The role of the Congo Red ink/PVA pH indicator film (on PET) disc is to produce a striking colour change, from red to blue, when placed in contact with water with a pH < 2, i.e., two pH units below its pKa of 4.0. The UV/vis absorption spectrum of the CR pH indicator film in its usual red deprotonated form is illustrated in Figure 3. The absorption spectrum of the blue-coloured acidified form of the CR pH indicator film is also illustrated in Figure 3 and was generated by simply exposing it to the acidic fumes from a bottle of concentrated hydrochloric acid. Details of the structures of the deprotonated and protonated forms of CR are given in Section S4 in the ESI. Photographic images of the indicator film in its deprotonated and protonated forms are also illustrated in Figure 3.
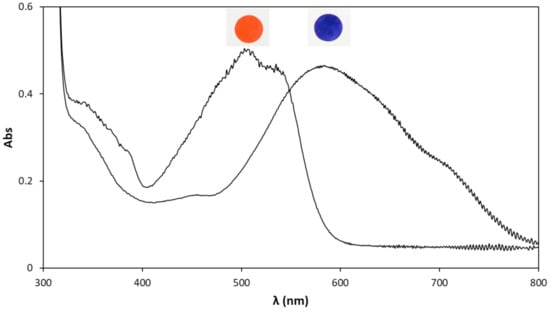
Figure 3.
UV/vis absorption spectra and digital photographic images of the Congo Red ink film in ambient air (red, λmax = 586 nm), and after being placed in contact with an acidic (HCl) vapour (blue, λmax = 505 nm).
A careful examination of both spectra illustrated in Figure 3 reveals a series of ripples in the measured absorbance between 700–800 nm, which are due to the interference pattern created by the incident light beam from the spectrophotometer on the combination of the thin pH indicator ink film coated on the thick (ca. 50 μm) clear PET supporting substrate. Swanepoel analyses [,] of this pattern, details of which are given in Section S5 in the ESI, exhibited in the UV/Vis spectrum of the PET film, with and without the CR ink coating, revealed overall thicknesses of ca. 54.8 and 51.8 μm, respectively, indicating that the CR ink film thickness is ca. 3.0 μm.
3.2. The Acidified Water-Releasing Pressed Disc
The key component and unique feature of the HPP indicator reported here is the acidified-water-releasing pressed disc, highlighted in the schematic of the HPP indicator, see Figure 1, and described in detail in Section 2.2.1. Its uniqueness lies in that the amount of acidified water it delivers to the water-permeable barrier layer and so the pH indicator on the other side, see Figure 1, which depends upon both the (high) pressure employed and the exposure time. The component of the pressed disc that is responsible for the above features is the silica gel loaded with acidified water.
Silica gel is an amorphous, porous form of silicon dioxide (silica) []. The high surface area (usually around 750–800 m2/g) [] of silica gel allows it to absorb water readily, making it useful as a desiccant. Silica gel is non-toxic, non-flammable, and chemically inert and is often used as a drying agent in many products where contact with water would cause damage or spoilage, such as many foodstuffs, medicines, and types of electrical equipment []. The water that is so readily absorbed by silica gel can usually be expelled from the silica gel by heating it up to ca. 120–140 °C for 2–3 h [].
In terms of the use of silica gel in the HPP indicator reported here, the work of Costa et al. is highly relevant, since these workers were able to show that high-pressure treatment (4.5 GPa) of silica gel loaded with water causes it to expel ca. 60% of its adsorbed water []. In this work, a demonstration of this feature was effected by placing exactly 500 mg of a powder comprising 61 wt% water-loaded silica gel mixed in with PTFE powder in an infra-red disc press and subjecting each sample to a different high pressure, spanning the range 100–600 MPa for different application times (i.e., 1, 2, and 3 min). In each case, the mass of the final pressed disc was measured, and the loss of mass by the disc, due to high-pressure-induced loss of water from the water-loaded silica gel, was calculated. The results of this work, in the form of plots of mass of water loss versus applied pressure, exhibited by the pressed 61 wt% water-loaded silica gel/PTFE powder for different applied pressure times, are illustrated in Figure 4.
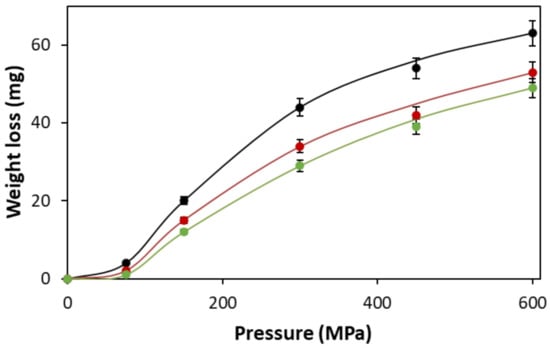
Figure 4.
Measured mass of water loss vs. applied pressure plots for 500 mg of a 61 wt% water-loaded silica gel/PTFE powder placed under different high pressures for 3 min (black dots), 2 min (red dots), or 1 min (green dots). All work was carried out at 20 °C.
The results, illustrated in Figure 4, show that the higher the applied pressure and exposure time used to press the 500 mg sample of the 61 wt% silica gel/PTFE powder, the greater the loss of the loaded water. A brief inspection of the x-axis in Figure 4 reveals that the applied pressures and times used in this work are similar to those used in the HPP processing of foodstuffs, see Table S1 in ESI.
It follows from the above that the water loss feature exhibited by the 61 wt% silica gel/PTFE powder could be used to create a unique HPP indicator that is both high-pressure- and exposure-time-sensitive, assuming a simple method can be found to indicate the loss of water. A simple way this might be achieved is through a change in colour of a pH indicator placed next to the pressed disc. However, initial experiments revealed, not surprisingly, that with the simple silica gel/PTFE powder used above for generating the data in Figure 4, the pH of the released water was ca. 6.0, which makes pH detection non-trivial.
The above problem was readily resolved by loading the silica gel with water acidified using a small amount of a non-volatile, strong organic acid, such as camphor sulfonic acid, CSA, which has a pKa of ca. 1.2 []. In order to test the system with this new additive, the same mass loss vs. applied pressure experiments as described above for the 61 wt% silica gel/PTFE powder mixture were then carried out using instead a silica gel powder loaded with 61wt% of water acidified with CSA and PTFE powder mix, i.e., the same mix as used to prepare the typical pressed discs described in Section 2.2.1 and used in the HPP indicator illustrated in Figure 1. The measured mass of water loss vs. applied pressure plots for 500 mg of a 61 wt% acidified water-loaded silica gel/PTFE powder described above were found to be virtually identical to those illustrated in Figure 4 for the 61 wt% water-loaded silica gel/PTFE powder, with the exception that the water released by each disc had a pH of ca. 1.0 instead of 6.0. The high acidity of the pressure-induced released acidified water generated by the 61 wt% acidified water-loaded silica gel/PTFE powder should, therefore, produce a striking colour change (red to blue) in a Congo Red indicator film (pKa = 4.0) placed next to the pressed disc. However, initial experiments showed that the simple combination of a 61 wt% acidified-water-releasing pressed disc placed in direct contact with a CR pH indicator film could not be used as a HPP indicator because, when the combined layers were stored, the pH indicator layer slowly (over days) changed from red to dark blue due to the slow diffusion of some of the acidified water from the pressed disc to the pH indicator film. Thus, to make a shelf-life stable HPP indicator based on the above technology, a water-permeable barrier layer needed to be added to separate the pressed disc and the pH indicator film and so prevent their direct contact.
3.3. The Water-Permeable Barrier Layer
The water-permeable barrier layer noted above had to be such that it prevented the diffusion of small amounts of the acidified water in the pressed disc making their way to the pH indicator film under ambient conditions and yet did provide a ready conduit for any significant amount released due to the application of a high pressure associated with HPP. As noted earlier, the material selected for this purpose was a Whatman Nuclepore™ track-etched polycarbonate membrane, the porous features of which are illustrated in Figure 2. Although the latter was used in all the HPP indicators reported here, other work showed that it could be readily replaced with a number of different water-permeable, non-absorbing thin film materials such as nylon mesh and glass micro-fibre filter paper.
3.4. The HPP Indicator
The silica-gel-based HPP indicators used in this work were prepared using three different acidified water loadings, i.e., 61, 63, and 65 wt%. The colour changes exhibited by each of these indicators when exposed to a wide variety of different pressures (100–600 MPa) and times (1–3 min), delivered using the HPP reactor, were recorded using digital photography, and the results of this work are illustrated in Figure 5a–c, respectively. A brief examination of these results reveals that the HPP indicator loaded with 61 wt% water only gave the expected colour change due to acidification of the pH indicator film, from red to blue, when exposed to the highest pressure and longest time used in this work, namely, 600 MPa for 3 min. As noted earlier, see Table S1 in ESI, the latter HPP conditions are those typically employed to pasteurise milk and soy smoothies [], orange juice [], and apple juice []. In contrast, the 63 wt% acidified water-loaded HPP indicator first produced the expected red-to-blue colour change when exposed to 400 MPa for 3 min, which are the typical HPP pressure/time conditions used to pasteurise cashew apple juice [] and aloe vera gel []. Finally, the 65 wt% acidified water-loaded HPP indicator first produced the expected red-to-blue colour change when exposed to 300 MPa for 3 min, which are the HPP conditions used in the HP pasteurisation of rabbit meat sausages [] and Cape gooseberry pulp []. The results illustrated in Figure 5 are typical for the three different HPP indicators used in this work and were reproduced using at least five different batches of each indicator. These results indicate that the indicator technology can be used to identify specific pressure (±<50 MPa) and application time (±<1 min) conditions associated with three commonly employed commercial HPP conditions, and so, under such conditions, could be used as a quality control tool for the HPP operator and/or the customer. Most importantly, this work shows that these HPP indicators are both high-pressure- and application-time-dependent, which are features largely absent from most if not all HPP indicators reported to date, see Table 1.
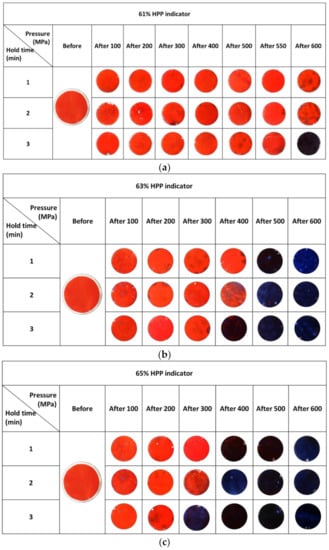
Figure 5.
Digital images of three different HPP indicators before and after exposure to a series of different HPP pressures, ranging from 100–600 MPa, and times, ranging from 1–3 min. The three types of HPP indicators tested were all constructed as described in the experiment, but with the silica gel loaded with (a) 61, (b) 63, and (c) 65 wt% acidified water, respectively. The initial and final temperatures during pressurisation were 21 and 32 °C, respectively, see Figure S2 in ESI.
3.5. HPP Indicator Shelf-Life
Not surprisingly, given the key role played by water in this system, the HPP indicators illustrated in Figure 5 appeared to lose their ability to respond to high pressures if stored at room temperature, i.e., ca. 20 °C, for long periods of time (i.e., several weeks). This deterioration in performance of the HPP indicators with storage time appeared to be due to the loss of water by the silica, which was slightly surprising given the use of a highly (water) impermeable plastic film to seal in the indicator components, see Figure 1. The results of further work suggest that the performance characteristics of the HPP indicators remain largely unchanged for at least 9 months if stored in a fridge at 5 °C or freezer at −20 °C. Clearly, a better water barrier film is required if a long-shelf life HPP indicator that can be stored at room temperature is required.
4. Conclusions
A new type of HPP indicator is described, based on the release of acidified water from silica gel due to the application of the high pressures associated with HPP. To our knowledge, this is the first reported colourimetric HPP indicator that can quickly, and accurately, show that the minimum high-pressure and application time required for the pasteurisation of a particular food stuff have been used. The HPP indicators are easy to use, rapid, and tuneable and offer a level of quality assurance to the HPP industry that is not available currently. However, clearly, the results illustrated in Figure 5 provide just an early guide to the performance characteristics of the HPP indicator technology, and further work is required. For example, the accuracy of the pressure and time responses exhibited by each indicator needs to be more clearly defined by using intermediate pressures, such as 575 MPa, and times, such as 2.5 min. In addition, the effect of the wt% of acidified water on the pressure–time response exhibited by the HPP indicator needs to be more clearly defined using intermediate wt% values such as 62 and 64 wt%. The indicator also needs to be made smaller, by reducing the existing three layers into one, so that it can be more easily placed on the outside, or inside, of the HPP package. Finally, a more water-impermeable plastic encapsulating film needs to be identified so that the indicator can be stored under ambient conditions. As a consequence, it is appropriate to consider this work as a proof-of-concept study and the indicators as initial prototypes.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/chemosensors9070164/s1, Table S1: Examples of the different HPP conditions used to treat various foodstuffs, Figure S1: Cross section schematic of the water-impermeable clear plastic polymer film, Figure S2: A typical pressure vs. time, and vessel temperature vs. time profile of HPP reactor when pressure is set at 600 MPa and hold time is 3 min, where before pressurisation the vessel temperature is 21 °C, and 32 °C when it reached 600 MPa, Figure S3: Structures of the deprotonated (red) and protonated (blue) congo red, Figure S4: UV/vis absorption spectra of the CR ink film in ambient air and interference bands, α = 0 to 6 are numbered and highlighted by the broken red lines, Figure S5: Plot of interference data from Figure S4 in the form of α/2 vs. nf/λ. The gradient (=2b) of the line of best fit is 109.5 μm, thus, b = 54.8 μm for the CR ink film, Figure S6: UV/vis absorption spectra of the clear PET film and interference bands, α = 0 to 4 are numbered and highlighted by the broken red lines, Figure S7: Plot of interference data from Figure S6 in the form of α/2 vs. nf/λ. The gradient (=2b) of the line of best fit is 103.6 μm, thus, b = 51.8 μm for the clear PET film.
Author Contributions
Conceptualization, A.M.; methodology, A.M. and D.Y.; validation, D.Y. and M.B.; formal analysis, D.Y. and M.B.; investigation, D.Y. and M.B.; writing—original draft preparation, A.M. and D.Y.; writing—review and editing, A.M., D.Y. and M.B.; supervision, A.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Sensor Indicator Products Inc and the EPSRC through an Impact Acceleration Award.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author. The data are not publicly available due to privacy concerns.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abera, G. Review on high-pressure processing of foods. Cogent Food Agric. 2019, 5, 1568725. [Google Scholar] [CrossRef]
- Queiroz, C.; Moreira, C.F.; Lavinas, F.C.; Lopes, M.L.; Fialho, E.; Valente-Mesquita, V.L. Effect of high hydrostatic pressure on phenolic compounds, ascorbic acid and antioxidant activity in cashew apple juice. High Press. Res. 2010, 30, 507–513. [Google Scholar] [CrossRef]
- Farkas, D.F.; Hoover, D.G. High pressure processing. J. Food Sci. 2000, 65, 47–64. [Google Scholar] [CrossRef]
- Sun, D.-W. Emerging Technologies for Food Processing; Elsevier: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Monteagudo, S.M.; Balasubramaniam, V.M. Fundamentals and Applications of High-Pressure Processing Technology. In Engineering Foods for Bioactives Stability and Delivery; Springer: Berlin, Germany, 2016; pp. 3–17. [Google Scholar]
- Pal, M.; Devrani, M. Application of Various Techniques for Meat Preservation. J. Exp. Food Chem. 2018, 4, 1–6. [Google Scholar] [CrossRef]
- Hogan, E.; Kelly, A.L.; Sun, D.-W. High Pressure Processing of Foods: An Overview. In Emerging Technologies for Food Pro-cessing; Elsevier: Amsterdam, The Netherlands, 2005; pp. 3–32. [Google Scholar]
- Rastogi, N.K.; Knorr, D. Recent Developments in High Pressure Processing of Foods; Springer: Berlin, Germany, 2013. [Google Scholar]
- Koutchma, T. Adapting High Hydrostatic Pressure (HPP) for Food Processing Operations; Academic Press: Cambridge, MA, USA, 2014. [Google Scholar]
- Elamin, W.; Endan, J.B.; Yosuf, Y.A.; Shamsudin, R.; Ahmedov, A.A. High Pressure Processing Technology and Equipment Evolution: A Review. J. Eng. Sci. Technol. Rev. 2015, 8, 75–83. [Google Scholar] [CrossRef]
- Global High Pressure Processing (HPP) Food Market—Analysis By Product Type, Distribution Channel, By Region, By Country (2020 Edition): Market Insights, Outlook Post Covid-19 Pandemic (2020–2025). Available online: https://www.reportlinker.com/p05993453/Global-High-Pressure-Processing-HPP-Food-Market-Analysis-By-Product-Type-Distribution-Channel-By-Region-By-Country-Edition-Market-Insights-Outlook-Post-Covid-19-Pandemic.html?utm_source=GNW (accessed on 30 June 2021).
- Minerich, P.L.; Labuza, T.P. Development of a pressure indicator for high hydrostatic pressure processing of foods. Innov. Food Sci. Emerg. Technol. 2003, 4, 235–243. [Google Scholar] [CrossRef]
- Bauer, B.; Knorr, D. The impact of pressure, temperature and treatment time on starches: Pressure-induced starch gelatinisation as pressure time temperature indicator for high hydrostatic pressure processing. J. Food Eng. 2005, 68, 329–334. [Google Scholar] [CrossRef]
- García, A.F.; Butz, P.; Corrales, M.; Lindauer, R.; Picouet, P.; Rodrigo, G.; Tauscher, B. A simple coloured indicator for moni-toring ultra high pressure processing conditions. J. Food Eng. 2009, 92, 410–415. [Google Scholar] [CrossRef]
- Koutchma, T.; Guo, B.; Patazca, E.; Parisi, B. High pressure-high temperature sterilization: From kinetic analysis to process verification+. J. Food Process. Eng. 2005, 28, 610–629. [Google Scholar] [CrossRef]
- Multi-Color Corporation: High Pressure Processing (HPP) Labels that Can Withstand the High-Pressure Process. Available online: https://www.mcclabel.com/en/food-dairy/innovations/functional-labels/high-pressure-processing-hpp (accessed on 30 June 2021).
- Bushman, A.C. High Pressure Processing Indicator. U.S. Patent 2020/0037639 A1, 6 February 2020. [Google Scholar]
- McCann, B. HPP Indicator Technology Helps Food Brands know for Certain. Available online: https://www.ctiinks.com/post/2019/05/30/hpp-indicator-technology-for-food (accessed on 30 June 2021).
- Sensor Indicator Products. Available online: http://sensorindicator.com/ (accessed on 30 June 2021).
- Wu, C.; Scott, J.; Shea, J.-E. Binding of Congo Red to Amyloid Protofibrils of the Alzheimer Aβ9–40 Peptide Probed by Molecular Dynamics Simulations. Biophys. J. 2012, 103, 550–557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cricut Explore Air 2. Available online: https://cricut.com/en_us/machines/cricut-explore-air-2.html (accessed on 30 June 2021).
- Whatman Nuclepore Membrane. Available online: https://www.sigmaaldrich.com/catalog/product/aldrich/wha110410?lang=en®ion=GB (accessed on 30 June 2021).
- Avure. Available online: www.avure.com (accessed on 30 June 2021).
- Swanepoel, R. Determination of the thickness and optical constants of amorphous silicon. J. Phys. E Sci. Instrum. 1983, 16, 1214–1222. [Google Scholar] [CrossRef]
- Mergel, D.; Buschendorf, D.; Eggert, S.; Grammes, R.; Samset, B.H. Density and refractive index of TiO2 films prepared by reactive evaporation. Thin Solid Film. 2000, 371, 218–224. [Google Scholar] [CrossRef]
- Bergna, H.E. Colloid Chemistry of Silica: An Overview; ACS Publications: Washington, DC, USA, 1994. [Google Scholar]
- Greenwood, N.; Earnshaw, A. Chemistry of the Elements; Elsevier: Amsterdam, The Netherlands, 1984; p. 911. [Google Scholar]
- Sillanpää, M.; Bhatnagar, A. NOM Removal by Adsorption. Nat. Org. Matter Water 2015, 213–238. [Google Scholar] [CrossRef]
- Bathen, D.; Breitbach, M. Adsorptionstechnik; Springer: Berlin, Germany, 2013. [Google Scholar]
- Costa, T.; Gallas, M.; Benvenutti, E.; da Jornada, J. Infrared and thermogravimetric study of high pressure consolidation in alkoxide silica gel powders. J. Non-Cryst. Solids 1997, 220, 195–201. [Google Scholar] [CrossRef]
- Tran, A.T.; Tomlin, J.; Lam, P.H.; Stinger, B.L.; Miller, A.D.; Walczyk, D.J.; Cruz, O.; Vaden, T.D.; Yu, L. Conductivity, Vis-cosity, Spectroscopic Properties of Organic Sulfonic Acid solutions in Ionic Liquids. ChemEngineering 2019, 3, 81. [Google Scholar] [CrossRef] [Green Version]
- Andrés, V.; Villanueva, M.-J.; Tenorio, M.-D. Influence of high pressure processing on microbial shelf life, sensory profile, soluble sugars, organic acids, and mineral content of milk- and soy-smoothies. LWT 2016, 65, 98–105. [Google Scholar] [CrossRef]
- Daher, D.; Le Gourrierec, S.; Pérez-Lamela, C. Effect of high pressure processing on the microbial inactivation in fruit prepa-rations and other vegetable based beverages. Agriculture 2017, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Yi, J.; Kebede, B.T.; Dang, D.N.H.; Buvé, C.; Grauwet, T.; Van Loey, A.; Hu, X.; Hendrickx, M. Quality change during high pressure processing and thermal processing of cloudy apple juice. LWT 2017, 75, 85–92. [Google Scholar] [CrossRef]
- Reyes, J.E.; Guanoquiza, M.I.; Tabilo-Munizaga, G.; Vega-Galvez, A.; Miranda, M.; Pérez-Won, M. Microbiological stabilization of Aloe vera (Aloe barbadensis Miller) gel by high hydrostatic pressure treatment. Int. J. Food Microbiol. 2012, 158, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Vega-Galvez, A.; López, J.; Torres-Ossandón, M.J.; Galotto, M.J.; Puente-Díaz, L.; Quispe-Fuentes, I.; Di Scala, K. High hy-drostatic pressure effect on chemical composition, color, phenolic acids and antioxidant capacity of Cape gooseberry pulp (Physalis peruviana L.). LWT 2014, 58, 519–526. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).