Thin Films Sensor Devices for Mycotoxins Detection in Foods: Applications and Challenges
Abstract
1. Introduction
2. Conventional Analytical Methods for Mycotoxins Detection
3. Thin Film Based Sensors
3.1. Optical Sensors
3.1.1. Fluorescence Sensors
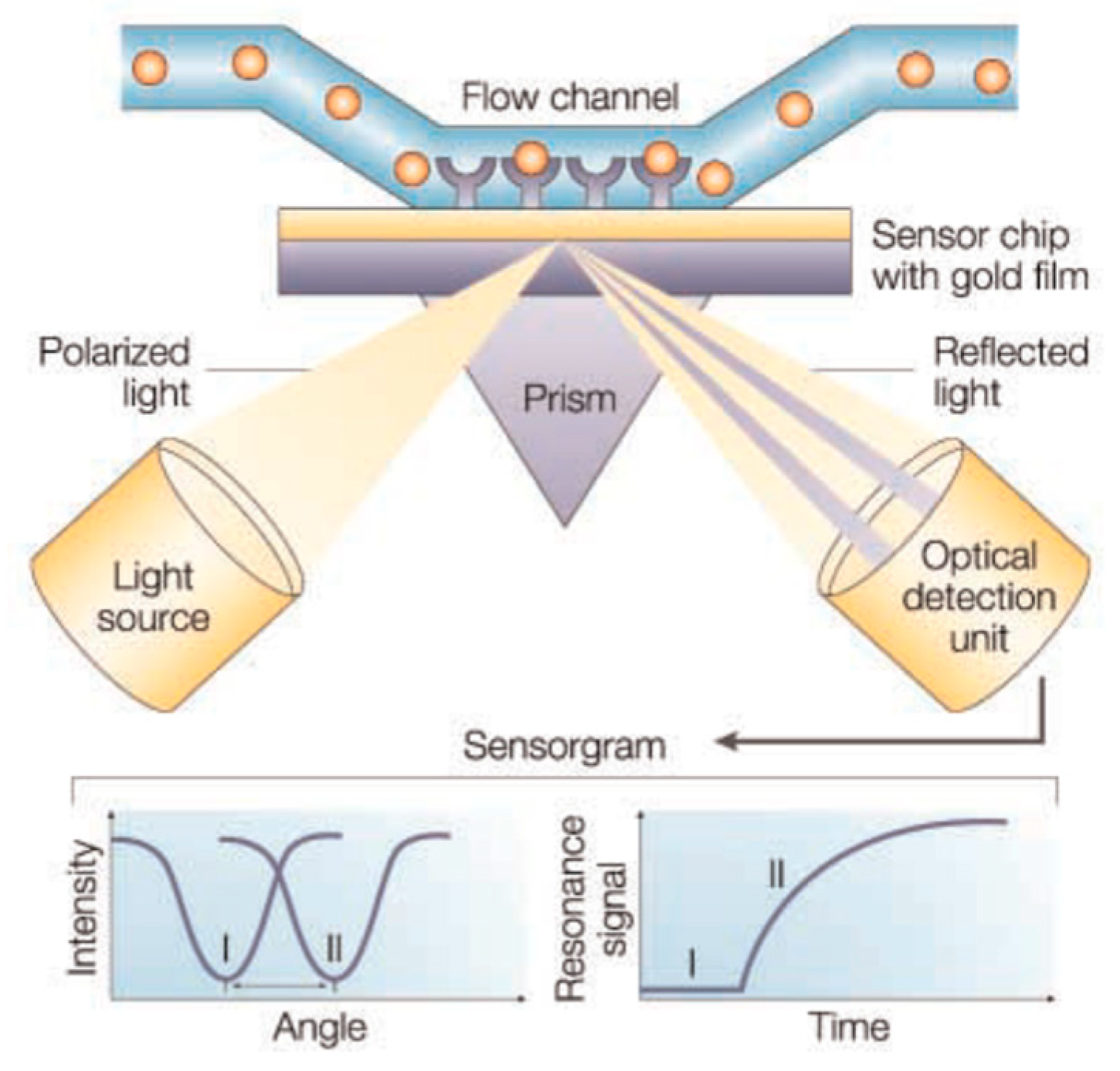
3.1.2. Surface Plasmon Resonance (SPR) Sensors
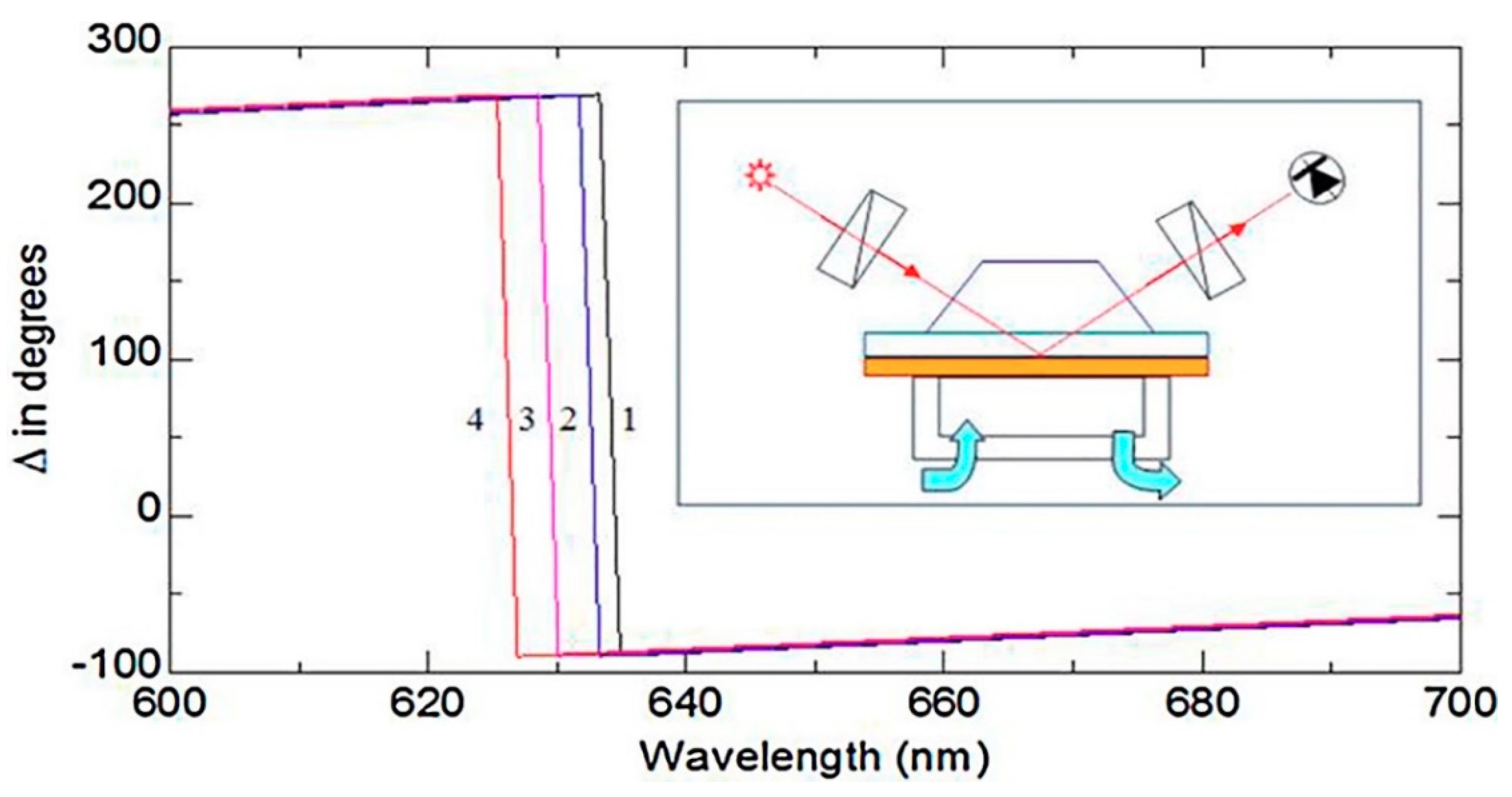
3.1.3. Total Internal Reflection Ellipsometry (TIRE)
3.2. Electrochemical Biosensors
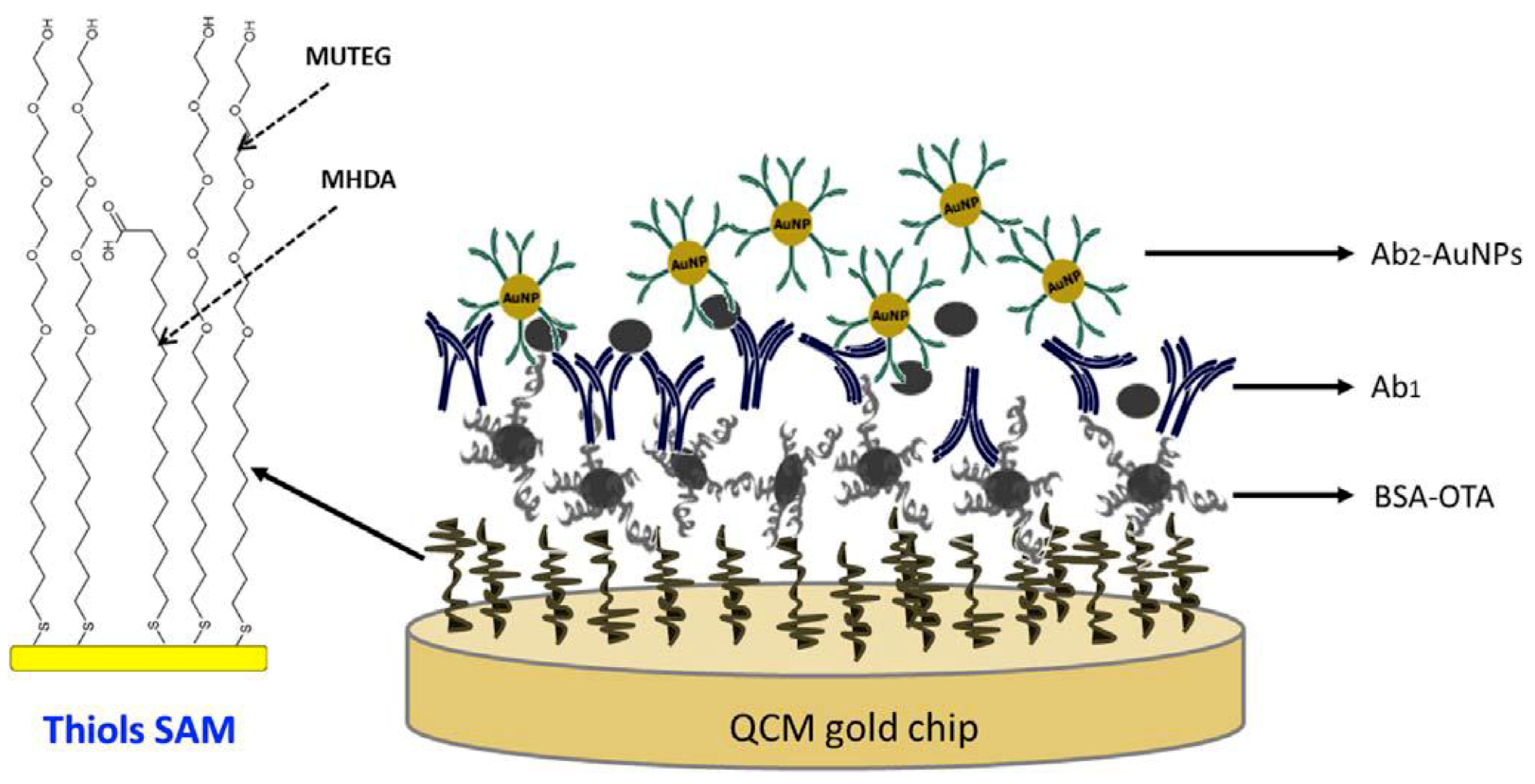
3.3. Mass-Based Piezoelectric Biosensors (Quartz Crystal Microbalance)
4. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Bennett, J.W.; Klich, M. Mycotoxins. Clin. Microbiol. Rev. 2003. [Google Scholar] [CrossRef]
- Creppy, E.E. Update of survey, regulation and toxic effects of mycotoxins in Europe. Toxicol. Lett. 2002, 127, 19–28. [Google Scholar] [CrossRef]
- Hussein, H. Toxicity, metabolism, and impact of mycotoxins on humans and animals. Toxicology 2001, 167, 101–134. [Google Scholar] [CrossRef]
- Turner, N.W.; Subrahmanyam, S.; Piletsky, S.A. Analytical methods for determination of mycotoxins: A review. Anal. Chim. Acta 2009, 632, 168–180. [Google Scholar] [CrossRef] [PubMed]
- Amirahmadi, M.; Shoeibi, S.; Rastegar, H.; Elmi, M.; Mousavi Khaneghah, A. Simultaneous analysis of mycotoxins in corn flour using LC/MS-MS combined with a modified QuEChERS procedure. Toxin Rev. 2017, 37, 187–195. [Google Scholar] [CrossRef]
- Rodrigues, P.; Venâncio, A.; Lima, N. Mycobiota and mycotoxins of almonds and chestnuts with special reference to aflatoxins. Food Res. Int. 2012, 48, 76–90. [Google Scholar] [CrossRef]
- Bryden, W.L. Mycotoxin contamination of the feed supply chain: Implications for animal productivity and feed security. Anim. Feed Sci. Technol. 2012, 173, 134–158. [Google Scholar] [CrossRef]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Abrunhosa, L.; Morales, H.; Soares, C.; Calado, T.; Vila-Chã, A.S.; Pereira, M.; Venâncio, A. A Review of Mycotoxins in Food and Feed Products in Portugal and Estimation of Probable Daily Intakes. Crit. Rev. Food Sci. Nutr. 2014, 56, 249–265. [Google Scholar] [CrossRef]
- Smith, M.C.; Madec, S.; Coton, E.; Hymery, N. Natural Co-Occurrence of Mycotoxins in Foods and Feeds and Their in vitro Combined Toxicological Effects. Toxins 2016, 8, 94. [Google Scholar] [CrossRef]
- Vipotnik, Z.; Rodríguez, A.; Rodrigues, P. Aspergillus westerdijkiae as a major ochratoxin A risk in dry-cured ham based-media. Int. J. Food Microbiol. 2017, 241, 244–251. [Google Scholar] [CrossRef]
- Fernández, H.; Arévalo, F.J.; Granero, A.M.; Robledo, S.N.; Nieto, C.H.D.; Riberi, W.I.; Zon, M.A. Electrochemical Biosensors for the Determination of Toxic Substances Related to Food Safety Developed in South America: Mycotoxins and Herbicides. Chemosensors 2017, 5, 23. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Commission Regulation (EU) No 1881/2006, Setting Maximum Levels for certain Contaminants in Foodstuffs. Off. J. Eur. Union 2006, L364, 5–24. [Google Scholar]
- The Commission of the European Communities. Commission Regulation (EC) No 1126/2007 of 28 September 2007 amending Regulation (EC) No 1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards Fusarium toxins in maize and maize products (Text with EEA relevance). Off. J. Eur. Union 2007, L255, 14–17. [Google Scholar]
- The Commission of the European Communities. Commission Regulation (EU) No 165/2010 of 26 February 2010 amending Regulation (EC) No1881/2006 setting maximum levels for certain contaminants in foodstuffs as regards aflatoxins (Text with EEA relevance). Off. J. Eur. Union 2010, L50, 8–12. [Google Scholar]
- The Commission of the European Communities. Commission Regulation (EU) No 212/2014 of 6 March 2014 amending Regulation (EC) No 1881/2006 as regards maximum levels of the contaminant citrinin in food supplements based on rice fermented with red yeast Monascus purpureus. Off. J. Eur. Union 2014, L67, 3–4. [Google Scholar]
- Pascale, M.; Visconti, A. Overview of Detection Methods for Mycotoxins. In Mycotoxins: Detection Methods, Management; Leslie, J.F., Bandyopadhyay, R., Visconti, A., Eds.; Public Health and Agricultural Trade, CAB International: Oxfordshire, UK, 2008; pp. 171–183. [Google Scholar]
- Williams, J.H.; Phillips, T.D.; Jolly, P.E.; Stiles, J.K.; Jolly, C.M.; Aggarwal, D. Human aflatoxicosis in developing countries: A review of toxicology, exposure, potential health consequences, and interventions. Am. J. Clin. Nutr. 2004, 80, 1106–1122. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Commission Regulation (EU) No 2015/1005 of 25 June 2015 amending Regulation (EC) No 1881/2006 as regards maximum levels of lead in certain foodstuffs (Text with EEA relevance). Off. J. Eur. Union 2015, L161, 9–13. [Google Scholar]
- Gong, Y.Y.; Watson, S.; Routledge, M.N. Aflatoxin Exposure and Associated Human Health Effects, a Review of Epidemiological Studies. Food Saf. 2016, 4, 14–27. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Recent advances in Nanomaterial-mediated Bio and immune sensors for detection of aflatoxin in food products. TrAC Trends Anal. Chem. 2017, 87, 112–128. [Google Scholar] [CrossRef]
- Duarte, S.C.; Lino, C.M.; Pena, A. Mycotoxin food and feed regulation and the specific case of ochratoxin A: A review of the worldwide status. Food Addit. Contam. Part A 2010, 27, 1440–1450. [Google Scholar] [CrossRef] [PubMed]
- Meulenberg, E.P. Immunochemical Methods for Ochratoxin A Detection: A Review. Toxins 2012, 4, 244–266. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Calderara, M.; Baggiani, C.; Giovannoli, C.; Arletti, E.; Giraudi, G. Development and application of a quantitative lateral flow immunoassay for fumonisins in maize. Anal. Chim. Acta 2010, 682, 104–109. [Google Scholar] [CrossRef] [PubMed]
- Visconti, A.; Lattanzio, V.M.T.; Pascale, M.; Haidukowski, M. Analysis of T-2 and HT-2 toxins in cereal grains by immunoaffinity clean-up and liquid chromatography with fluorescence detection. J. Chromatogr. A 2005, 1075, 151–158. [Google Scholar] [CrossRef]
- De Melo, F.T.; de Oliveira, I.M.; Greggio, S.; Dacosta, J.C.; Guecheva, T.N.; Saffi, J.; Rosa, R.M. DNA damage in organs of mice treated acutely with patulin, a known mycotoxin. Food Chem. Toxicol. 2012 50, 3548–3555. [CrossRef]
- Hueza, I.; Raspantini, P.; Raspantini, L.; Latorre, A.; Górniak, S. Zearalenone, an Estrogenic Mycotoxin, Is an Immunotoxic Compound. Toxins 2014, 6, 1080–1095. [Google Scholar] [CrossRef]
- Bovdisova, I.; Zbynovska, K.; Kalafova, A.; Capcarova, M. Toxicological properties of mycotoxin Citrinin. J. Microbiol. Biotechnol. Food Sci. 2016, 5, 10–13. [Google Scholar] [CrossRef]
- Shephard, G.S. Current Status of Mycotoxin Analysis: A Critical Review. J. AOAC Int. 2016, 99, 842–848. [Google Scholar] [CrossRef]
- Shephard, G.S. Chromatographic separation techniques for determination of mycotoxins in food and feed. In Determining Mycotoxins and Mycotoxigenic Fungi in Food and Feed; Saeger, S., Ed.; Woodhead Publishing Limited: Philadelphia, PA, USA, 2011; pp. 71–89. ISBN 978-0-85709-097-3. [Google Scholar]
- Filazi, A.; İnce, S.; Temamoğulları, F. Survey of the occurrence of aflatoxin M1 in cheeses produced by dairy ewe’s milk in Urfa city, Turkey. Ankara Üniv. Vet. Fak. Derg. 2010, 57, 197–199. [Google Scholar]
- Li, Q.; Lu, Z.; Tan, X.; Xiao, X.; Wang, P.; Wu, L.; Shao, K.; Yin, W.; Han, H. Ultrasensitive detection of aflatoxin B1 by SERS aptasensor based on exonuclease-assisted recycling amplification. Biosens. Bioelectron. 2017, 97, 59–64. [Google Scholar] [CrossRef]
- Rahmani, A.; Jinap, S.; Soleimany, F. Qualitative and Quantitative Analysis of Mycotoxins. Compr. Rev. Food Sci. Food Saf. 2009, 8, 202–251. [Google Scholar] [CrossRef]
- Pereira, V.L.; Fernandes, J.O.; Cunha, S.C. Mycotoxins in cereals and related foodstuffs: A review on occurrence and recent methods of analysis. Trends Food Sci. Technol. 2014, 36, 96–136. [Google Scholar] [CrossRef]
- Orata, F. Derivatization reactions and reagents for gas chromatography analysis. In Advanced Gas Chromatography—Progress in Agricultural, Biomedical and Industrial Applications, 1st ed.; Mohd, M.A., Ed.; InTech: Rijeka, Croatia, 2012; pp. 83–108. ISBN 978-953-51-0298-4. [Google Scholar]
- Anfossi, L.; Giovannoli, C.; Baggiani, C. Mycotoxin detection. Curr. Opin. Biotechnol. 2016, 37, 120–126. [Google Scholar] [CrossRef]
- Alshannaq, A.; Yu, J.H. Occurrence, Toxicity, and Analysis of Major Mycotoxins in Food. Int. J. Environ. Res. Public Health 2017, 14, 632. [Google Scholar] [CrossRef]
- Man, Y.; Liang, G.; Li, A.; Pan, L. Recent Advances in Mycotoxin Determination for Food Monitoring via Microchip. Toxins 2017, 9, 324. [Google Scholar] [CrossRef] [PubMed]
- Dzuman, Z.; Vaclavikova, M.; Polisenska, I.; Veprikova, Z.; Fenclova, M.; Zachariasova, M.; Hajslova, J. Enzyme-linked immunosorbent assay in analysis of deoxynivalenol: Investigation of the impact of sample matrix on results accuracy. Anal. Bioanal. Chem. 2013, 406, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Soares, R.R.G.; Novo, P.; Azevedo, A.M.; Fernandes, P.; Chu, V.; Conde, J.P.; Aires-Barros, M.R. Aqueous two-phase systems for enhancing immunoassay sensitivity: Simultaneous concentration of mycotoxins and neutralization of matrix interference. J. Chromatogr. A 2014, 1361, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Turner, N.W.; Bramhmbhatt, H.; Szabo-Vezse, M.; Poma, A.; Coker, R.; Piletsky, S.A. Analytical methods for determination of mycotoxins: An update (2009–2014). Anal. Chim. Acta 2015, 901, 12–33. [Google Scholar] [CrossRef] [PubMed]
- Goryacheva, I.Y.; Saeger, S.D.; Eremin, S.A.; Peteghem, C.V. Immunochemical methods for rapid mycotoxin detection: Evolution from single to multiple analyte screening: A review. Food Addit. Contam. 2007, 24, 1169–1183. [Google Scholar] [CrossRef]
- Neogen Veratox®. Available online: http://foodsafety.neogen.com/en/veratox#mycotoxins (accessed on 27 September 2018).
- De Cesare, G.; Nascetti, A.; Scipinotti, R.; Fanelli, C.; Ricelli, A.; Caputo, D. Optoelectronic System for Mycotoxin Detection in Food Quality Control. IEEE Trans. Compon. Packag. Manuf. Technol. 2018, 8, 1195–1202. [Google Scholar] [CrossRef]
- Kirsch, J.; Siltanen, C.; Zhou, Q.; Revzin, A.; Simonian, A. Biosensor technology: Recent advances in threat agent detection and medicine. Chem. Soc. Rev. 2013, 42, 8733. [Google Scholar] [CrossRef] [PubMed]
- Stroka, J.; Maragos, C.M. Challenges in the analysis of multiple mycotoxins. World Mycotoxin J. 2016, 9, 847–861. [Google Scholar] [CrossRef]
- Mishra, G.; Barfidokht, A.; Tehrani, F.; Mishra, R. Food Safety Analysis Using Electrochemical Biosensors. Foods 2018, 7, 141. [Google Scholar] [CrossRef] [PubMed]
- Tothill, I.E.; Turner, A.P.F. Biosensors. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Trugo, L., Finglas, P., Eds.; Academic Press: London, UK, 2003; pp. 489–499. ISBN 978-0-12-227055-0. [Google Scholar]
- Song, S.; Liu, N.; Zhao, Z.; Njumbe Ediage, E.; Wu, S.; Sun, C.; Saeger, S.; Wu, A. Multiplex Lateral Flow Immunoassay for Mycotoxin Determination. Anal. Chem. 2014, 86, 4995–5001. [Google Scholar] [CrossRef] [PubMed]
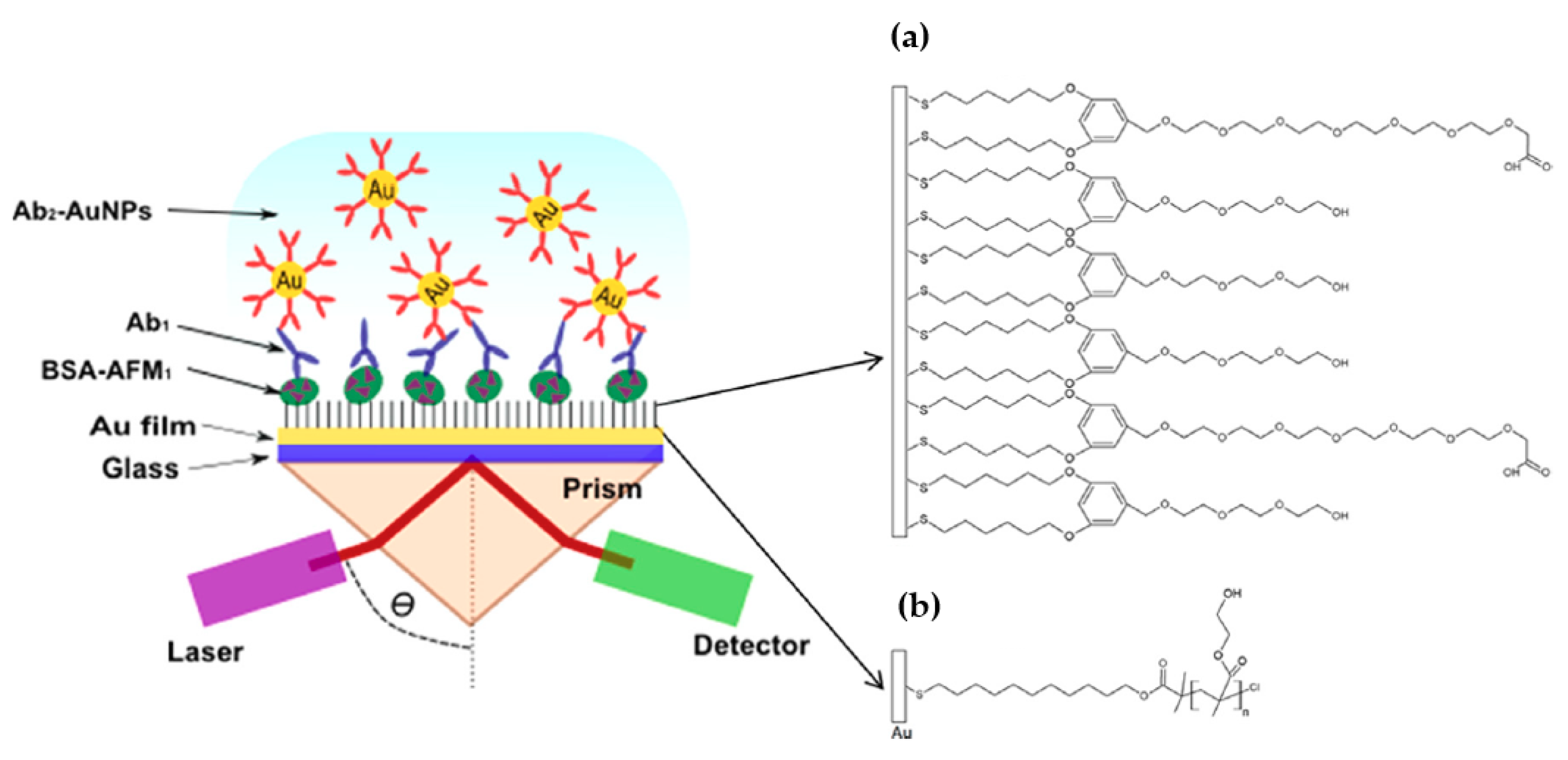
- Li, Y.; Liu, X.; Lin, Z. Recent developments and applications of surface plasmon resonance biosensors for the detection of mycotoxins in foodstuffs. Food Chem. 2012, 132, 1549–1554. [Google Scholar] [CrossRef] [PubMed]
- Al Rubaye, A.; Nabok, A.; Catanante, G.; Marty, J.L.; Takács, E.; Székács, A. Label-Free Optical Detection of Mycotoxins Using Specific Aptamers Immobilized on Gold Nanostructures. Toxins 2018, 10, 291. [Google Scholar] [CrossRef]
- Zhang, L.; Dou, X.W.; Zhang, C.; Logrieco, A.; Yang, M.H. A Review of Current Methods for Analysis of Mycotoxins in Herbal Medicines. Toxins 2018, 10, 65. [Google Scholar] [CrossRef] [PubMed]
- Rocha, D.F.D.L.; Oliveira, M.D.S.; Furlong, E.B.; Junges, A.; Paroul, N.; Valduga, E.; Cansian, R.L. Evaluation of the TLC quantification method and occurrence of deoxynivalenol in wheat flour of southern Brazil. Food Addit. Contam. Part A 2017, 34, 2220–2229. [Google Scholar] [CrossRef]
- Kong, W.; Wei, R.; Logrieco, A.F.; Wei, J.; Wen, J.; Xiao, X.; Yang, M. Occurrence of toxigenic fungi and determination of mycotoxins by HPLC-FLD in functional foods and spices in China markets. Food Chem. 2014, 146, 320–326. [Google Scholar] [CrossRef]
- Juan, C.; Covarelli, L.; Beccari, G.; Colasante, V.; Mañes, J. Simultaneous analysis of twenty-six mycotoxins in durum wheat grain from Italy. Food Control 2016, 62, 322–329. [Google Scholar] [CrossRef]
- Spanjer, M.C.; Rensen, P.M.; Scholten, J.M. LC–MS/MS multi-method for mycotoxins after single extraction, with validation data for peanut, pistachio, wheat, maize, cornflakes, raisins and figs. Food Addit. Contam. Part A 2008, 25, 472–489. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, J.; Graziani, G.; Gaspari, A.; Chianese, D.; Ferrer, E.; Mañes, J.; Ritieni, A. Multi-Mycotoxin Analysis in Durum Wheat Pasta by Liquid Chromatography Coupled to Quadrupole Orbitrap Mass Spectrometry. Toxins 2017, 9, 59. [Google Scholar] [CrossRef]
- Bouafifssa, Y.; Manyes, L.; Rahouti, M.; Mañes, J.; Berrada, H.; Zinedine, A.; Fernández-Franzón, M. Multi-Occurrence of Twenty Mycotoxinsin Pasta and a Risk Assessment in the Moroccan Population. Toxins 2018, 10, 432. [Google Scholar] [CrossRef] [PubMed]
- Urusov, A.; Zherdev, A.; Petrakova, A.; Sadykhov, E.; Koroleva, O.; Dzantiev, B. Rapid Multiple Immunoenzyme Assay of Mycotoxins. Toxins 2015, 7, 238–254. [Google Scholar] [CrossRef] [PubMed]
- Levasseur-Garcia, C. Updated Overview of Infrared Spectroscopy Methods for Detecting Mycotoxins on Cereals (Corn, Wheat, and Barley). Toxins 2018, 10, 38. [Google Scholar] [CrossRef] [PubMed]
- Stasiewicz, M.J.; Falade, T.D.O.; Mutuma, M.; Mutiga, S.K.; Harvey, J.J.W.; Fox, G.; Pearson, T.C.; Muthomi, J.W.; Nelson, R.J. Multi-spectral kernel sorting to reduce aflatoxins and fumonisins in Kenyan maize. Food Control 2017, 78, 203–214. [Google Scholar] [CrossRef]
- Eslamian, M. Inorganic and Organic Solution-Processed Thin Film Devices. Nano-Micro Lett. 2017, 9, 1–23. [Google Scholar] [CrossRef]
- Mitzi, D.B. Thin-Film Deposition of Organic−Inorganic Hybrid Materials. Chem. Mater. 2001, 13, 3283–3298. [Google Scholar] [CrossRef]
- Edwards, G.A.; Bergren, A.J.; Porter, M.D. Chemically Modified Electrodes. In Handbook of Electrochemistry, 1st ed.; Zoski, C., Ed.; Elsevier Science: New York, NY, USA, 2007; pp. 295–327. ISBN 9780080469300. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Gerard, M.; Chaubey, A.; Malhotra, B. Application of conducting polymers to biosensors. Biosens. Bioelectron. 2002, 17, 345–359. [Google Scholar] [CrossRef]
- Pandey, P.; Datta, M.; Malhotra, B.D. Prospects of Nanomaterials in Biosensors. Anal. Lett. 2008, 41, 159–209. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Agrawal, V.V.; Malhotra, B.D. Nanostructured metal oxide-based biosensors. NPG Asia Mater. 2011, 3, 17–24. [Google Scholar] [CrossRef]
- Ansari, A.A.; Kaushik, A.; Solanki, P.; Malhotra, B. Sol–gel derived nanoporous cerium oxide film for application to cholesterol biosensor. Electrochem Commun. 2008, 10, 1246–1249. [Google Scholar] [CrossRef]
- Arya, S.K.; Solanki, P.R.; Datta, M.; Malhotra, B.D. Recent advances in self-assembled monolayers based biomolecular electronic devices. Biosens. Bioelectron. 2009, 24, 2810–2817. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Jun, D.; Kuca, K. Mycotoxin Assays Using Biosensor Technology: A Review. Drug Chem. Toxicol. 2007, 30, 253–261. [Google Scholar] [CrossRef]
- Bhand, S.; Kanungo, L.; Pal, S. Chemiluminescence and fluorescence optical biosensor for the detection of aflatoxin in food. In Food Biosensors; Ahmed, M.U., Zourob, M., Tamiya, E., Eds.; The Royal Society of Chemistry: Cambridge, UK, 2017; pp. 161–181. ISBN 978-1-78262-390-8. [Google Scholar]
- Lin, X.; Guo, X. Advances in Biosensors, Chemosensors and Assays for the Determination of Fusarium Mycotoxins. Toxins 2016, 8, 161. [Google Scholar] [CrossRef]
- Evtugyn, G.; Subjakova, V.; Melikishvili, S.; Hianik, T. Affinity Biosensors for Detection of Mycotoxins in Food. Adv. Food Nutr. Res. 2018, 263–310. [Google Scholar] [CrossRef]
- Jiang, C.; Lan, L.; Yao, Y.; Zhao, F.; Ping, J. Recent progress in application of nanomaterial-enabled biosensors for ochratoxin A detection. TrAC Trends Anal. Chem. 2018, 102, 236–249. [Google Scholar] [CrossRef]
- Xu, L.; Zhang, Z.; Zhang, Q.; Li, P. Mycotoxin Determination in Foods Using Advanced Sensors Based on Antibodies or Aptamers. Toxins 2016, 8, 239. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Chen, Y.; Wu, Y.; Weng, B.; Liu, Y.; Lu, Z.; Li, C.M.; Yu, C. Aptamer induced assembly of fluorescent nitrogen-doped carbon dots on gold nanoparticles for sensitive detection of AFB 1. Biosens. Bioelectron. 2016, 78, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Tothill, I. Biosensors and nanomaterials and their application for mycotoxin determination. World Mycotoxin J. 2011, 4, 361–374. [Google Scholar] [CrossRef]
- Beloglazova, N.V.; Speranskaya, E.S.; Wu, A.; Wang, Z.; Sanders, M.; Goftman, V.V.; De Saeger, S. Novel multiplex fluorescent immunoassays based on quantum dot nanolabels for mycotoxins determination. Biosens. Bioelectron. 2014, 62, 59–65. [Google Scholar] [CrossRef]
- Wu, S.; Duan, N.; Ma, X.; Xia, Y.; Wang, H.; Wang, Z.; Zhang, Q. Multiplexed Fluorescence Resonance Energy Transfer Aptasensor between Upconversion Nanoparticles and Graphene Oxide for the Simultaneous Determination of Mycotoxins. Anal. Chem. 2012, 84, 6263–6270. [Google Scholar] [CrossRef] [PubMed]
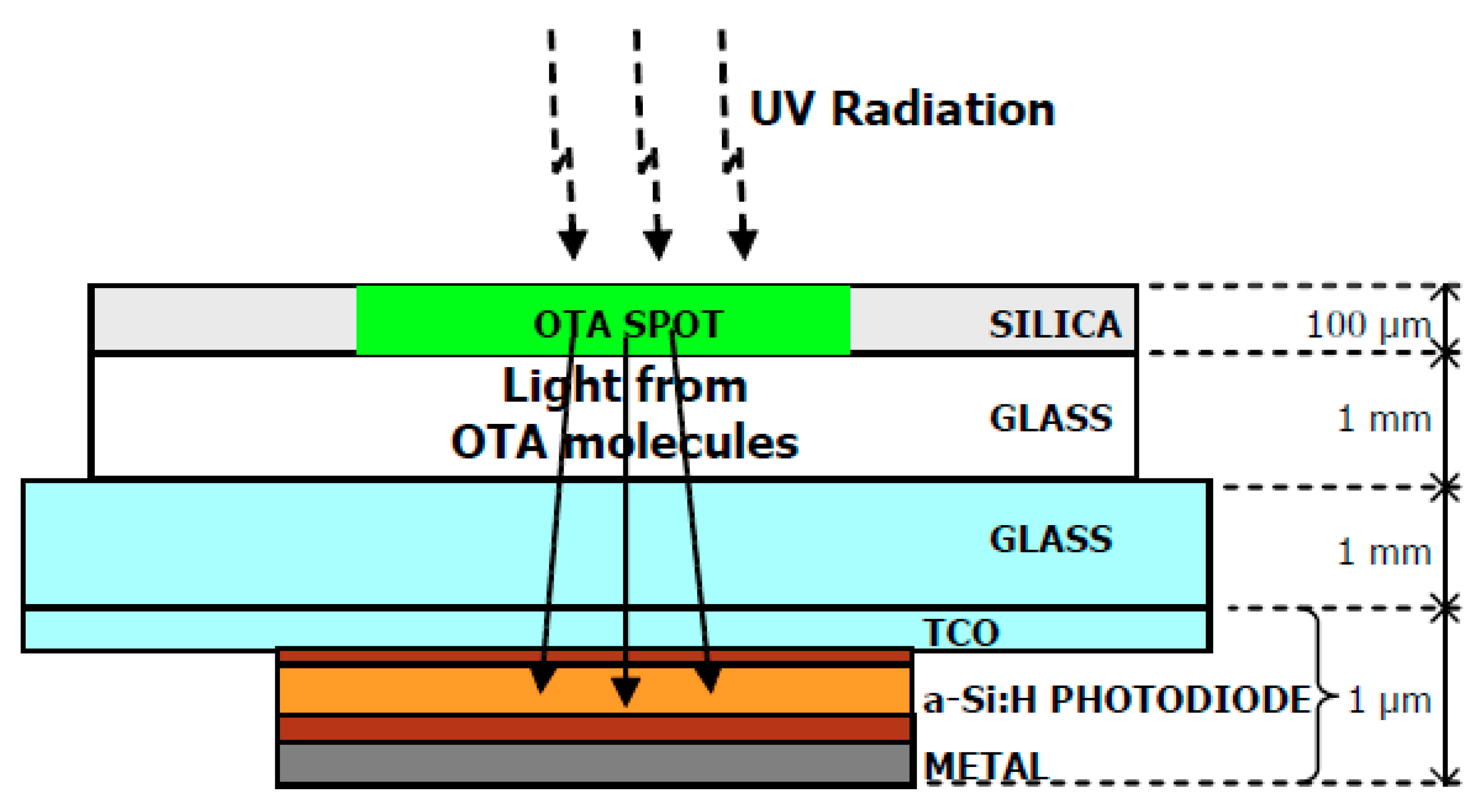
- Costantini, F.; Sberna, C.; Petrucci, G.; Reverberi, M.; Domenici, F.; Fanelli, C.; Caputo, D. Aptamer-based sandwich assay for on chip detection of Ochratoxin A by an array of amorphous silicon photosensors. Sens. Actuators B Chem. 2016, 230, 31–39. [Google Scholar] [CrossRef]
- Al Rubaye, A.; Nabok, A.; Catanante, G.; Marty, J.L.; Takacs, E.; Szekacs, A. Detection of ochratoxin A in aptamer assay using total internal reflection ellipsometry. Sens. Actuators B Chem. 2018, 263, 248–251. [Google Scholar] [CrossRef]
- Danesh, N.M.; Bostan, H.B.; Abnous, K.; Ramezani, M.; Youssefi, K.; Taghdisi, S.M.; Karimi, G. Ultrasensitive detection of aflatoxin B1 and its major metabolite aflatoxin M1 using aptasensors: A review. TrAC Trends Anal. Chem. 2018, 99, 117–128. [Google Scholar] [CrossRef]
- Caputo, D.; de Cesare, G.; Fanelli, C.; Nascetti, A.; Ricelli, A.; Scipinotti, R. Innovative Detection System of Ochratoxin A by Thin Film Photodiodes. Sensors 2007, 7, 1317–1322. [Google Scholar] [CrossRef]
- Caputo, D.; de Cesare, G.; Fanelli, C.; Nascetti, A.; Ricelli, A.; Scipinotti, R. Amorphous Silicon Photosensors for Detection of Ochratoxin a in Wine. IEEE Sens. J. 2012, 12, 2674–2679. [Google Scholar] [CrossRef]
- The Commission of the European Communities. Commission Regulation (EC) No 123/2005 of 26 January 2005 amending Regulation (EC) No 466/2001 as regards ochratoxin A (Text with EEA relevance). Off. J. Eur. Union 2005, L25, 3–5. [Google Scholar]
- Sabet, F.S.; Hosseini, M.; Khabbaz, H.; Dadmehr, M.; Ganjali, M.R. FRET-based aptamer biosensor for selective and sensitive detection of aflatoxin B1 in peanut and rice. Food Chem. 2017, 220, 527–532. [Google Scholar] [CrossRef]
- Tang, X.; Zhang, Z.; Li, P.; Zhang, Q.; Jiang, J.; Wang, D.; Lei, J. Sample-pretreatment-free based high sensitive determination of aflatoxin M1 in raw milk using a time-resolved fluorescent competitive immunochromatographic assay. RSC Adv. 2015, 5, 558–564. [Google Scholar] [CrossRef]
- Hodnik, V.; Anderluh, G. Toxin Detection by Surface Plasmon Resonance. Sensors 2009, 9, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Present and future of surface plasmon resonance biosensors. Anal. Bioanal. Chem. 2003, 377, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Cooper, M.A. Optical biosensors in drug discovery. Nat. Rev. Drug Discov. 2002, 1, 515–528. [Google Scholar] [CrossRef] [PubMed]
- Mitchell, J. Small Molecule Immunosensing Using Surface Plasmon Resonance. Sensors 2010, 10, 7323–7346. [Google Scholar] [CrossRef] [PubMed]
- Homola, J. Surface Plasmon Resonance Sensors for Detection of Chemical and Biological Species. Chem. Rev. 2008, 108, 462–493. [Google Scholar] [CrossRef] [PubMed]
- Babu, D.; Muriana, P.M. Immunomagnetic bead-based recovery and real time quantitative PCR (RT iq-PCR) for sensitive quantification of aflatoxin B1. J. Microbiol. Methods 2011, 86, 188–194. [Google Scholar] [CrossRef] [PubMed]
- Gnedenko, O.V.; Mezentsev, Y.V.; Molnar, A.A.; Lisitsa, A.V.; Ivanov, A.S.; Archakov, A.I. Highly sensitive detection of human cardiac myoglobin using a reverse sandwich immunoassay with a gold nanoparticle-enhanced surface plasmon resonance biosensor. Anal. Chim. Acta 2013, 759, 105–109. [Google Scholar] [CrossRef] [PubMed]
- Dieringer, J.A.; McFarland, A.D.; Shah, N.C.; Stuart, D.A.; Whitney, A.V.; Yonzon, C.R.; Young, M.A.; Zhang, X.; Van Duyne, R.P. Surface enhanced Raman spectroscopy: New materials, concepts, characterization tools, and applications. Faraday Discuss. 2006, 132, 9–26. [Google Scholar] [CrossRef] [PubMed]
- Fu, X. Surface Plasmon Resonance Immunoassay for Ochratoxin A Based on Nanogold Hollow Balls with Dendritic Surface. Anal. Lett. 2007, 40, 2641–2652. [Google Scholar] [CrossRef]
- Todescato, F.; Antognoli, A.; Meneghello, A.; Cretaio, E.; Signorini, R.; Bozio, R. Sensitive detection of Ochratoxin A in food and drinks using metal-enhanced fluorescence. Biosens. Bioelectron. 2014, 57, 125–132. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Reiner-Rozman, C.; Hageneder, S.; Dubiak-Szepietowska, M.; Dostálek, J.; Feller, K.H. Fast and sensitive detection of ochratoxin A in red wine by nanoparticle-enhanced SPR. Anal. Chim. Acta 2016, 937, 143–150. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Dubiak-Szepietowska, M.; Vorobii, M.; Rodriguez-Emmenegger, C.; Dostálek, J.; Feller, K.H. Sensitive and rapid detection of aflatoxin M1 in milk utilizing enhanced SPR and p(HEMA) brushes. Biosens. Bioelectron. 2016, 81, 159–165. [Google Scholar] [CrossRef] [PubMed]
- Dinçkaya, E.; Kınık, Ö.; Sezgintürk, M.K.; Altuğ, Ç.; Akkoca, A. Development of an impedimetric aflatoxin M1 biosensor based on a DNA probe and gold nanoparticles. Biosens. Bioelectron. 2011, 26, 3806–3811. [Google Scholar] [CrossRef] [PubMed]
- Radoi, A.; Targa, M.; Prieto-Simon, B.; Marty, J.L. Enzyme-Linked Immunosorbent Assay (ELISA) based on superparamagnetic nanoparticles for aflatoxin M1 detection. Talanta 2008, 77, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Karakouz, T.; Tesler, A.B.; Bendikov, T.A.; Vaskevich, A.; Rubinstein, I. Highly Stable Localized Plasmon Transducers Obtained by Thermal Embedding of Gold Island Films on Glass. Adv. Mater. 2008, 20, 3893–3899. [Google Scholar] [CrossRef]
- Lopatynskyi, A.M.; Lopatynska, O.G.; Guo, L.J.; Chegel, V.I. Localized surface plasmon resonance biosensor—Part I: Theoretical study of sensitivity—Extended Mie approach. IEEE Sens. 2011, 11, 361–369. [Google Scholar] [CrossRef]
- Nabok, A.V.; Tsargorodskaya, A.; Hassan, A.K.; Starodub, N.F. Total internal reflection ellipsometry and SPR detection of low molecular weight environmental toxins. Appl. Surf. Sci. 2005, 246, 381–386. [Google Scholar] [CrossRef]
- Viswanathan, S.; Radecka, H.; Radecki, J. Electrochemical biosensors for food analysis. Monatshefte fur Chemie 2009, 140, 891–899. [Google Scholar] [CrossRef]
- Vidal, J.C.; Bonel, L.; Ezquerra, A.; Hernández, S.; Bertolín, J.R.; Cubel, C.; Castillo, J.R. Electrochemical affinity biosensors for detection of mycotoxins: A review. Biosens. Bioelectron. 2013, 49, 146–158. [Google Scholar] [CrossRef]
- Campàs, M.; Garibo, D.; Prieto-Simón, B. Novel nanobiotechnological concepts in electrochemical biosensors for the analysis of toxins. Analyst 2012, 137, 1055. [Google Scholar] [CrossRef] [PubMed]
- Palchetti, I.; Mascini, M. Electroanalytical biosensors and their potential for food pathogen and toxin detection. Anal. Bioanal. Chem. 2008, 391, 455–471. [Google Scholar] [CrossRef] [PubMed]
- Grieshaber, D.; MacKenzie, R.; Vörös, J.; Reimhult, E. Electrochemical Biosensors—Sensor Principles and Architectures. Sensors 2008, 8, 1400–1458. [Google Scholar] [CrossRef] [PubMed]
- Pohanka, M.; Skladal, P. Electrochemical biosensors-principles and applications. J. Appl. Biomed. 2008, 6, 57–64. [Google Scholar]
- Laschi, S.; Centi, S.; Mascini, M. Electrochemical arrays coupled with magnetic separators for immunochemistry. Bioanal. Rev. 2010, 3, 11–25. [Google Scholar] [CrossRef]
- Rhouati, A.; Bulbul, G.; Latif, U.; Hayat, A.; Li, Z.H.; Marty, J. Nano-Aptasensing in Mycotoxin Analysis: Recent Updates and Progress. Toxins 2017, 9, 349. [Google Scholar] [CrossRef]
- Sharma, A.; Goud, K.; Hayat, A.; Bhand, S.; Marty, J. Recent Advances in Electrochemical-Based Sensing Platforms for Aflatoxins Detection. Chemosensors 2016, 5, 1. [Google Scholar] [CrossRef]
- Goud, K.Y.; Kalisa, S.K.; Kumar, V.; Tsang, Y.F.; Lee, S.; Gobi, K.V.; Kim, K.H. Progress on nanostructured electrochemical sensors and their recognition elements for detection of mycotoxins: A review. Biosens. Bioelectron. 2018, 121, 205–222. [Google Scholar] [CrossRef]
- Arévalo, F.J.; Granero, A.M.; Fernández, H.; Raba, J.; Zón, M. ACitrinin (CIT) determination in rice samples using a micro fluidic electrochemical immunosensor. Talanta 2011, 83, 966–973. [Google Scholar] [CrossRef]
- Parker, C.O.; Tothill, I.E. Development of an electrochemical immunosensor for aflatoxin M1 in milk with focus on matrix interference. Biosens. Bioelectron. 2009, 24, 2452–2457. [Google Scholar] [CrossRef]
- Liu, L.; Chao, Y.; Cao, W.; Wang, Y.; Luo, C.; Pang, X.; Fan, D.; Wei, Q. A label-free amperometric immunosensor for detection of zearalenone based on trimetallic Au-core/AgPt-shell nanorattles and mesoporous carbon. Anal. Chim. Acta 2014, 847, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Ma, H.; Sun, J.; Zhang, Y.; Xia, S. Disposable amperometric immunosensor for simple and sensitive determination of aflatoxin B1 in wheat. Biochem. Eng. J. 2016, 115, 38–46. [Google Scholar] [CrossRef]
- Dridi, F.; Marrakchi, M.; Gargouri, M.; Garcia-Cruz, A.; Dzyadevych, S.; Vocanson, F.; Saulnierb, J.; Renault, N.J.; Lagarde, F. Thermolysin entrapped in a gold nanoparticles/polymer composite for direct and sensitive conductometric biosensing of ochratoxin A in olive oil. Sens. Actuators B Chem. 2015, 221, 480–490. [Google Scholar] [CrossRef]
- Soldatkin, O.O.; Burdak, O.S.; Sergeyeva, T.A.; Arkhypova, V.M.; Dzyadevych, S.V.; Soldatkin, A.P. Acetylcholinesterase-based conductometric biosensor for determination of aflatoxin B1. Sens. Actuators B Chem. 2013, 188, 999–1003. [Google Scholar] [CrossRef]
- Solanki, P.R.; Kaushik, A.; Manaka, T.; Pandey, M.K.; Iwamoto, M.; Agrawal, V.V.; Malhotra, B.D. Self-assembled monolayer based impedimetric platform for food borne mycotoxin detection. Nanoscale 2010, 2, 2811–2817. [Google Scholar] [CrossRef] [PubMed]
- Mishra, R.K.; Hayat, A.; Catanante, G.; Ocaña, C.; Marty, J.L. A label free aptasensor for Ochratoxin A detection in cocoa beans: An application to chocolate industries. Anal. Chim. Acta 2015, 889, 106–112. [Google Scholar] [CrossRef] [PubMed]
- Istamboulié, G.; Paniel, N.; Zara, L.; Granados, L.R.; Barthelmebs, L.; Noguer, T. Development of an impedimetric aptasensor for the determination of aflatoxin M1 in milk. Talanta 2016, 146, 464–469. [Google Scholar] [CrossRef]
- Bacher, G.; Pal, S.; Kanungo, L.; Bhand, S. A label-free silver wire based impedimetric immunosensor for detection of aflatoxin M1 in milk. Sens. Actuators B Chem. 2012, 168, 223–230. [Google Scholar] [CrossRef]
- Wei, M.; Zhang, W. A novel impedimetric aptasensor based on AuNPs–carboxylic porous carbon for the ultrasensitive detection of ochratoxin A. RSC Adv. 2017, 7, 28655–28660. [Google Scholar] [CrossRef]
- Evtugyn, G.; Porfireva, A.; Stepanova, V.; Kutyreva, M.; Gataulina, A.; Ulakhovich, N.; Evtugyn, V.; Hianik, T. Impedimetric Aptasensor for Ochratoxin A Determination Based on Au Nanoparticles Stabilized with Hyper-Branched Polymer. Sensors 2013, 13, 16129–16145. [Google Scholar] [CrossRef]
- Rivas, L.; Mayorga-Martinez, C.C.; Quesada-González, D.; Zamora-Gálvez, A.; de la Escosura-Muñiz, A.; Merkoçi, A. Label-Free Impedimetric Aptasensor for Ochratoxin-A Detection Using Iridium Oxide Nanoparticles. Anal. Chem. 2015, 87, 5167–5172. [Google Scholar] [CrossRef] [PubMed]
- Sunday, C.; Masikini, M.; Wilson, L.; Rassie, C.; Waryo, T.; Baker, P.; Iwuoha, E. Application on Gold Nanoparticles-Dotted 4-Nitrophenylazo Graphene in a Label-Free Impedimetric Deoxynivalenol Immunosensor. Sensors 2015, 15, 3854–3871. [Google Scholar] [CrossRef] [PubMed]
- Foguel, M.; Furlan Giordano, G.; de Sylos, C.; Carlos, I.; Pupim Ferreira, A.; Benedetti, A.; Yamanaka, H. A Low-Cost Label-Free AFB1 Impedimetric Immunosensor Based on Functionalized CD-Trodes. Chemosensors 2016, 4, 17. [Google Scholar] [CrossRef]
- Badea, M.; Floroian, L.; Restani, P.; Cobzac, S.C.A.; Moga, M. Ochratoxin A Detection on Antibody- Immobilized on BSA-Functionalized Gold Electrodes. PLoS ONE 2016, 11, e0160021. [Google Scholar] [CrossRef] [PubMed]
- Rameil, S.; Schubert, P.; Grundmann, P.; Dietrich, R.; Märtlbauer, E. Use of 3-(4-hydroxyphenyl)propionic acid as electron donating compound in a potentiometric aflatoxin M1-immunosensor. Anal. Chim. Acta 2010, 661, 122–127. [Google Scholar] [CrossRef] [PubMed]
- Shadjou, R.; Hasanzadeh, M.; Heidar-poor, M.; Shadjou, N. Electrochemical monitoring of aflatoxin M1 in milk samples using silver nanoparticles dispersed on α-cyclodextrin-GQDs nanocomposite. J. Mol. Recognit. 2018, 31, e2699. [Google Scholar] [CrossRef]
- Huang, K.J.; Shuai, H.L.; Chen, Y.X. Layered molybdenum selenide stacking flower-like nanostructure coupled with guanine-rich DNA sequence for ultrasensitive ochratoxin A aptasensor application. Sens. Actuators B Chem. 2016, 225, 391–397. [Google Scholar] [CrossRef]
- Qing, Y.; Li, C.; Yang, X.; Zhou, X.; Xue, J.; Luo, M.; Xu, X.; Chen, S.; Qiu, J.F. Electrochemical immunosensor using single-walled carbon nanotubes/chitosan for ultrasensitive detection of deoxynivalenol in food samples. J. Appl. Electrochem. 2016, 46, 1049–1057. [Google Scholar] [CrossRef]
- Lu, L.; Seenivasan, R.; Wang, Y.C.; Yu, J.H.; Gunasekaran, S. An Electrochemical Immunosensor for Rapid and Sensitive Detection of Mycotoxins Fumonisin B1 and Deoxynivalenol. Electrochim. Acta 2016, 213, 89–97. [Google Scholar] [CrossRef]
- Liu, X.; Yang, Z.; Zhang, Y.; Yu, R. A novel electrochemical immunosensor for ochratoxin A with hapten immobilization on thionine/gold nanoparticle modified glassy carbon electrode. Anal. Methods 2013, 5, 1481. [Google Scholar] [CrossRef]
- Pacheco, J.G.; Castro, M.; Machado, S.; Barroso, M.F.; Nouws, H.P.A.; Delerue-Matos, C. Molecularly imprinted electrochemical sensor for ochratoxin A detection in food samples. Sens. Actuators B Chem. 2015, 215, 107–112. [Google Scholar] [CrossRef]
- Zhang, S.; Shen, Y.; Shen, G.; Wang, S.; Shen, G.; Yu, R. Electrochemical immunosensor based on Pd–Au nanoparticles supported on functionalized PDDA-MWCNT nanocomposites for aflatoxin B1 detection. Anal. Biochem. 2016, 494, 10–15. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Baeumner, A.J.; Feller, K.H. Rapid and sensitive inhibition-based assay for the electrochemical detection of Ochratoxin A and Aflatoxin M1 in red wine and milk. Electrochim. Acta 2017, 243, 82–89. [Google Scholar] [CrossRef]
- Castillo, G.; Spinella, K.; Poturnayová, A.; Šnejdárková, M.; Mosiello, L.; Hianik, T. Detection of aflatoxin B 1 by aptamer-based biosensor using PAMAM dendrimers as immobilization platform. Food Control 2015, 52, 9–18. [Google Scholar] [CrossRef]
- Pirinçci, Ş.; Ertekin, Ö.; Laguna, D.; Özen, F.; Öztürk, Z.; Öztürk, S. Label-Free QCM Immunosensor for the Detection of Ochratoxin A. Sensors 2018, 18, 1161. [Google Scholar] [CrossRef] [PubMed]
- Karczmarczyk, A.; Haupt, K.; Feller, K.H. Development of a QCM-D biosensor for Ochratoxin A detection in red wine. Talanta 2017, 166, 193–197. [Google Scholar] [CrossRef] [PubMed]
- Tang, Y.; Tang, D.; Zhang, J.; Tang, D. Novel quartz crystal microbalance immunodetection of aflatoxin B1 coupling cargo-encapsulated liposome with indicator-triggered displacement assay. Anal. Chim. Acta 2018, 1031, 161–168. [Google Scholar] [CrossRef]
| Mycotoxin | Abbreviation | Food Matrix | Toxic effect | Limit EU * | Fungal Species | References |
|---|---|---|---|---|---|---|
| Aflatoxins | AFM1, AFM2, AFB1, AFB2, AFG1, AFG2 | Peanuts, maize, milk and derived, cereals, oilseeds | Hepatotoxic, carcinogenic, probable immune suppression and childhood stunting reduced growth | AFB1: 2–12 μg/kg AFB1: 0.1 μg/kg for cereal-based foods for children and medicinal purposes AFM1: 0.05 μg/kg in milk and 0.025 μg/kg in foods for infants Total aflatoxin: 4–15 μg/kg | Aspergillus flavus, A. parasiticus | [15,18,19,20,21] |
| Ochratoxins | OTA, OTB, OTC | Cereals, coffee, cocoa, wine, beer, grapes, dried fruits | Nephrotoxic, hepatotoxic, neurotoxic, teratogenic, immunotoxic | 0.5–10 μg/kg | Aspergillus ochraceus, A. carbonarius, Penicillium verrucosum | [12,13,22,23] |
| Fumonisins | FB1 e FB2 | Maize and maize based food, rice, sorghum, soybeans | Neurotoxic, genotoxic, immunotoxic, carcinogenic, hepatotoxic, nephrotoxic | 200–4000 μg/kg | Fusarium verticillioides, F. proliferatum | [14,24] |
| Trichothecenes | Type A: HT-2, T-2, Type B: DON | Wheat, barley, and maize and less often in oats, rice, rye, sorghum and triticale | Inhibition of protein synthesis, immunosuppressive and cytotoxic effect | DON: 200–1750 μg/kg | Fusarium sporotrichioides, F. graminearum, F. culmorum | [2,13,14,16,25] |
| Patulin | PAT | Apples and apple products, fruit juice | Genotoxic, embryotoxic, immunotoxic, teratogenic, carcinogenic | 10–50 μg/kg | Penicillium expansum | [13,26] |
| Zearalenone | ZEA | Corn, oats | Hepatotoxic, genotoxic, immunotoxic, carcinogenic | 20–400 μg/kg | Fusarium graminearum | [14,27] |
| Citrinin | CIT | Various commodities of plant origin, cereals, namely fermented rice | Nephrotoxic, neurotoxic, genotoxic, embryotoxic, immunotoxic | 2000 μg/kg for rice | P. citrinum, P. expansum, P. verrucosum, Aspergillus carneus, A. niveus, Monascus purpureus | [12,16,28] |
| Method | Advantages | Disadvantages | Mycotoxin/Matrix | LOD | LOQ | References |
|---|---|---|---|---|---|---|
| TLC | Simple and inexpensive Can be used as a rapid screening method | Poor sensitivity Poor precision Quantitative approach only if coupled with a densitometer | DON/Wheat flour | 30 ng·mL−1 | 100 ng·mL−1 | [30,52,53] |
| HPLC-FLD | Good selectivity Accurate identification Short analysis time Automatic analysis (autosampler) Official methods available | Expensive equipment Specialist expertise required Derivatization may be required | AFB1/Spices | 0.04 ng·mL−1 | 0.15 ng·mL−1 | [54,55] |
| LC-MS | Selective and sensitive detection Capability to generate structural information of the target analyte Low detection limits Simultaneous analysis of multiple mycotoxins Minimum sample pre-treatment steps | Expensive equipment Specialist expertise required Sensitivity depends on ionization technique | AFB1/Wheat grain | 2 µg·kg−1 | 3.5 µg·kg−1 | [55,56,57] |
| GC | Simultaneous analysis of multiple mycotoxins Selective and sensitive detection | Expensive equipment Specialist expertise required Derivatization required Non-linear calibration curve Carry over effects from previous sample | DON/Pasta | 0.5 µg·kg−1 | 1 µg·kg−1 | [4,35,58] |
| ELISA | Convenient and sensitive detection Ease of operation Rapid sample screening Simultaneous analysis of multiple mycotoxins Low use of organic solvents | Matrix interference problems Cross-reactivity with related mycotoxins Possible false positive/negative results Narrow operating range | OTA/Corn | 4.0 ng·mL−1 | Not specified | [59] |
| Spectral analysis technology | Rapid screening of a large number of samples Qualitative and quantitative information about the structure of mycotoxins Can be used in situ | Complicated interpretation of spectral data Spectra overlapping Possible false positive/negative results | Fumonisin/Corn | 100 μg·kg−1 | Not specified | [60,61] |
| Method | Mycotoxin | Bioreceptor | Interface Material | Sample Type | Limit of Detection (LOD) | Linearity Range | Reference |
|---|---|---|---|---|---|---|---|
| Amperometric | CIT | Antibody | Au surface electrodeposited on a GCE | Rice | 0.1 ng·mL−1 | 0.5–50 ng·mL−1 | [116] |
| AFM1 | Antibody | SPCE | Milk | 0.039·ng·mL−1 | 1–10.000 ng·L−1 | [117] | |
| ZEA | Antibody | Au@AgPt | Milk | 0.0017 ng·mL−1 | 0.005–15 ng·mL−1 | [118] | |
| AFB1 | Antibody | Chitosan-AuNPs modified gold microelectrode | Wheat | 0.15 ng·mL−1 | 1.6–32 ng·mL−1 | [119] | |
| Conductometric | OTA | Enzyme | TLN/AuNPs/(PVA/PEI) | Olive oil | 1 nM | 2–100 nM | [120] |
| AFB1 | Enzyme | Au + Pyroceramic + Cr | Standard solution | 50 ng·mL−1 | 0.25–1 mM | [121] | |
| Impedimetric | OTA | Antibody | SAM (AUT/Au) | Coffee | 0.0008 ng·mL−1 | 0.5–6.0 ng·dL−1 | [122] |
| OTA | Aptamers | SPCE | Cocoa beans | 0.15 ng·mL−1 | 0.15–2.5 ng·mL−1 | [123] | |
| AFM1 | Aptamers | SPCE | Milk | Not specified | 20–1000 ng·kg−1 | [124] | |
| AFM1 | Aptamers | SPCE | Buffer | 1.15 ng·L−1 | 2–150 ng·L−1 | [124] | |
| AFM1 | Antibody | Ag wire | Milk | 0.001 ng·mL−1 | 6.25–100 pg·mL−1 | [125] | |
| OTA | Aptamers | AuNPs–cPC | Soybean | 10−8 ng·mL−1 | 10−8–0.1 ng·mL−1 | [126] | |
| OTA | Aptamers | Au electrode + AuNPs/Boltorn H30® | Beer | 0.02 nM | 0.1–100 nM | [127] | |
| OTA | Aptamers | SPCE/PTH + IrO2 NPs | White wine | 0.014 nM; 5.65 ng·kg−1 | 0.01–100 nM | [128] | |
| DON | Antibody | GCE + AuNPs/G/PhNO2 | Cereals | 0.3 ng·mL−1 | 6–30 ng·mL−1 | [129] | |
| AFB1 | Antibody | CD-trodes modified with lipoic acid SAM | Peanut | 0.11 ng·mL−1 | 1.56–31.2 ng·mL−1 | [130] | |
| OTA | Antibody | AuSPCE/BSA | Plant extracts | Not specified | 2.5–100 ng·mL−1 | [131] | |
| Potentiometric | AFM1 | Antibody | Ag/AgCl | Standard solution | 0.04 ng·mL−1 | 0.25–2 ng·mL−1 | [132] |
| AFM1 | Antibody | Ag/AgCl | Milk | 0.5 ng·mL−1 | Not specified | [132] | |
| AFM1 | - | GCE + GQDs-α-CD + AgNPs | Milk | 2000 nM | 0.015–25 mM | [133] | |
| OTA | Aptamers | GCE + AuNPs/MoSe2 | Red wine | 0.00008 nM | 0.0001–1 nM | [134] | |
| Voltammetry | DON | Antibody | GCE + SWNTs/CS | Sorghum | 0.005 ng·mL−1 | 0.01–1000 ng·mL−1 | [135] |
| AFB1 | Antibody | SPCE modified with AuNPs and PPy/ErGO film | Corn | 4.2 ng·mL−1 | 200–4500 ng·mL−1 | [136] | |
| DON | Antibody | SPCE modified with AuNPs and PPy/ErGO film | Corn | 8.6 ng·mL−1 | 50–1000 ng·mL−1 | [136] | |
| OTA | Antibody | GCE + PTH/AuNPs | Corn | 0.2 ng·mL−1 | 1–1000 ng·mL−1 | [137] | |
| OTA | - | GCE + MIP/MWCNT | Beer and wine | 4.1 nM (1.7 ng·mL−1) | 50–1000 nM | [138] | |
| AFB1 | Antibody | Au electrode + CNTs/PDDA/PdeAu | Standard solution | 0.03 ng·mL−1 | 0.05–25 ng·mL−1 | [139] | |
| AFB1 | Antibody | Au electrode + CNTs/PDDA/PdeAu | Rice | 1250 ng·kg−1 | Not specified | [139] | |
| OTA | Antibody + enzyme | AuSPE | Red wine | 15 ng·mL−1 | 10−2–103 ng·mL−1 | [140] | |
| AFM1 | Antibody + enzyme | AuSPE | Milk | 0.037 ng·mL−1 | 10−2–103 ng·mL−1 | [140] | |
| AFB1 | Aptamers | PAMAM G4 + Au electrode + cystamine | Peanuts | 0.40 nM | 0.1–10 nM | [141] |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, A.O.; Vaz, A.; Rodrigues, P.; Veloso, A.C.A.; Venâncio, A.; Peres, A.M. Thin Films Sensor Devices for Mycotoxins Detection in Foods: Applications and Challenges. Chemosensors 2019, 7, 3. https://doi.org/10.3390/chemosensors7010003
Santos AO, Vaz A, Rodrigues P, Veloso ACA, Venâncio A, Peres AM. Thin Films Sensor Devices for Mycotoxins Detection in Foods: Applications and Challenges. Chemosensors. 2019; 7(1):3. https://doi.org/10.3390/chemosensors7010003
Chicago/Turabian StyleSantos, Andréia O., Andreia Vaz, Paula Rodrigues, Ana C. A. Veloso, Armando Venâncio, and António M. Peres. 2019. "Thin Films Sensor Devices for Mycotoxins Detection in Foods: Applications and Challenges" Chemosensors 7, no. 1: 3. https://doi.org/10.3390/chemosensors7010003
APA StyleSantos, A. O., Vaz, A., Rodrigues, P., Veloso, A. C. A., Venâncio, A., & Peres, A. M. (2019). Thin Films Sensor Devices for Mycotoxins Detection in Foods: Applications and Challenges. Chemosensors, 7(1), 3. https://doi.org/10.3390/chemosensors7010003






