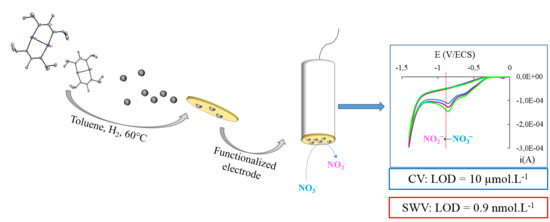
In Situ Metalorganic Deposition of Silver Nanoparticles on Gold Substrate and Square Wave Voltammetry: A Highly Efficient Combination for Nanomolar Detection of Nitrate Ions in Sea Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Synthesis of N,N′-Diisopropylacetamidinate Silver Precursor ([Ag(Amd)])
2.3. Gold Substrates (EAu): Gold Electrodes Fabrication
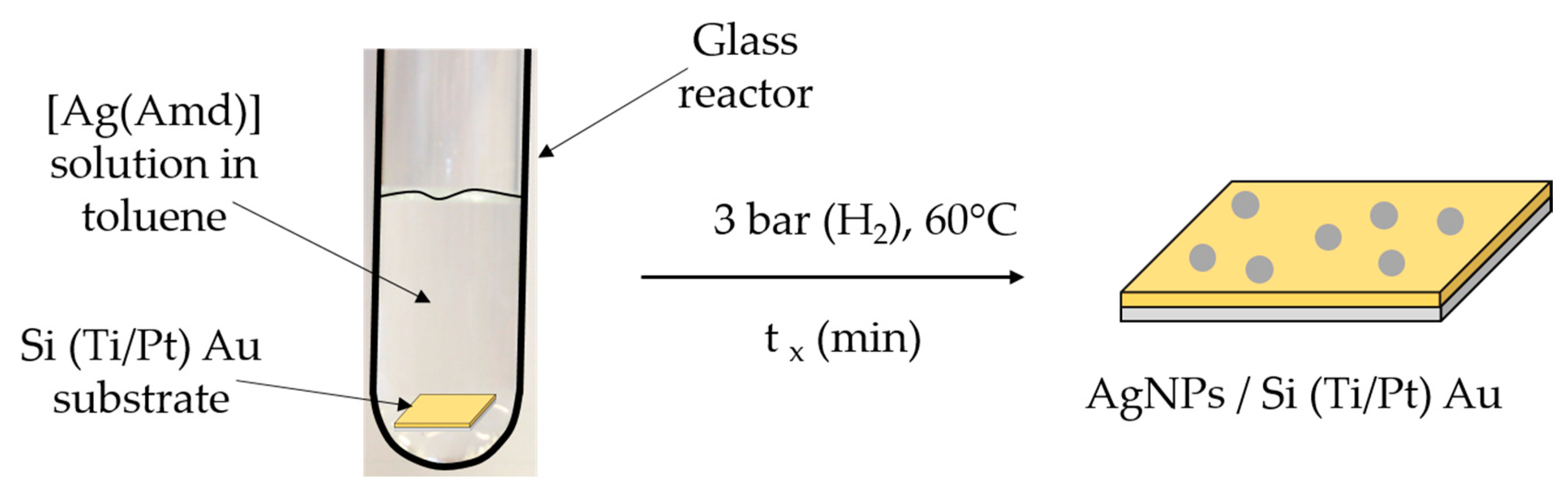
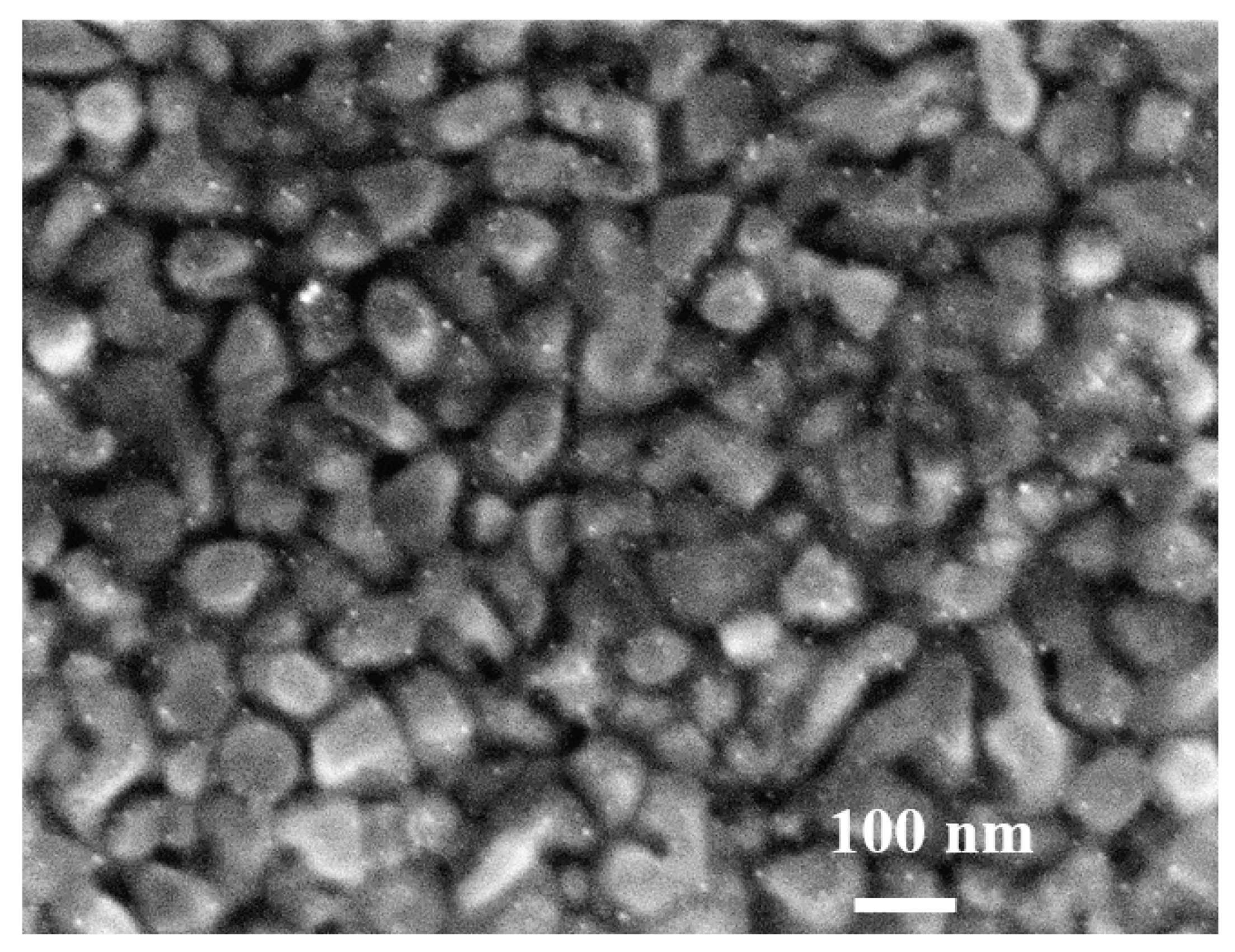
2.4. In Situ Functionalization of Gold Substrate by AgNPs (EAu/AgNPs) and Characterization of EAu/AgNPs
2.5. Characterization of Gold Electrode Modified by Silver Nanoparticles (EAu/AgNPs)
2.6. Electrochemical Measurements
3. Results and Discussion
3.1. Characterization of the Gold Electrode Functionalized by Silver Nanoparticles (EAu/AgNPs)
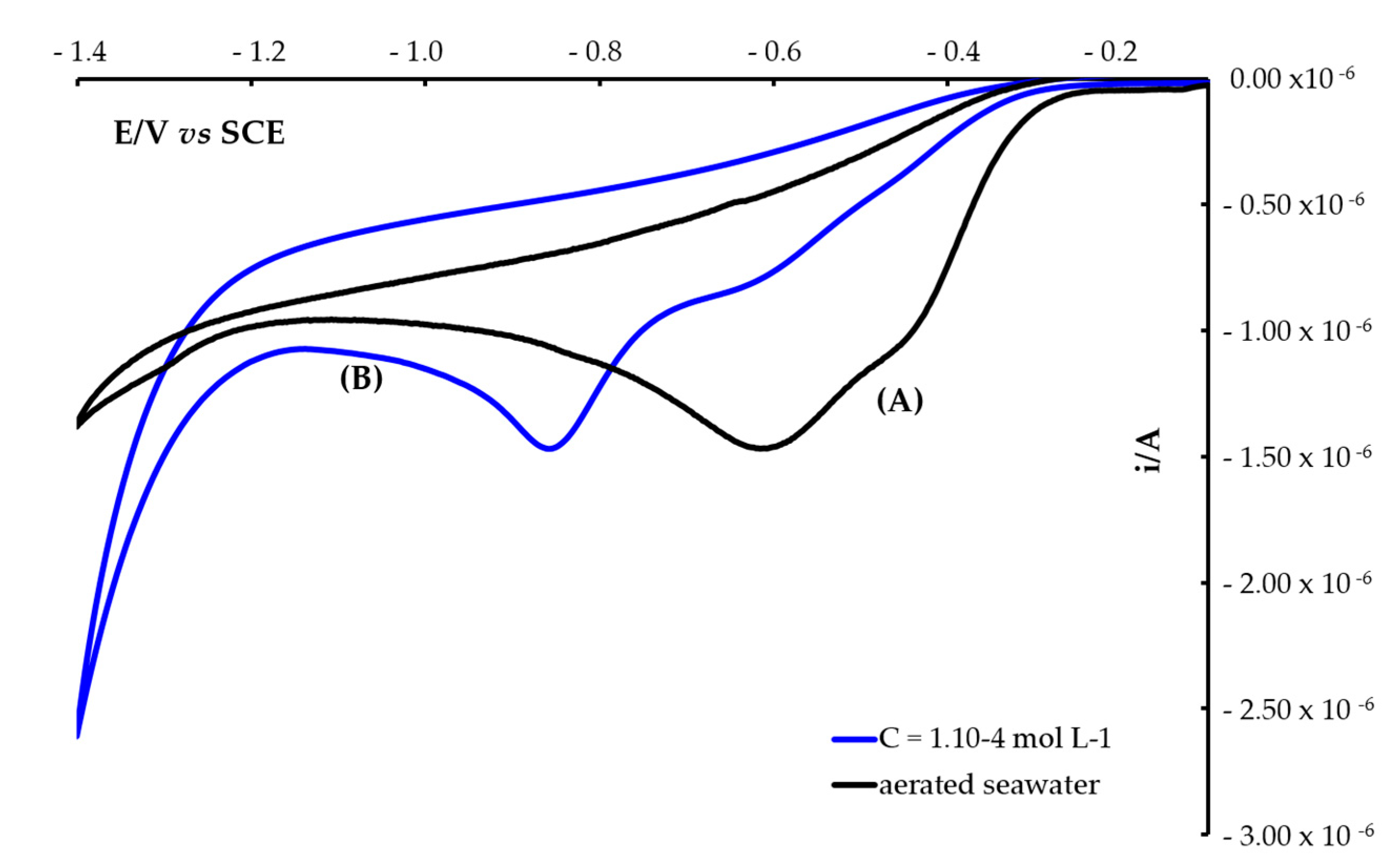
3.2. Cyclic Voltammetry for the Oxygen Reduction Reaction ORR and the Electroreduction of NO3− Ions
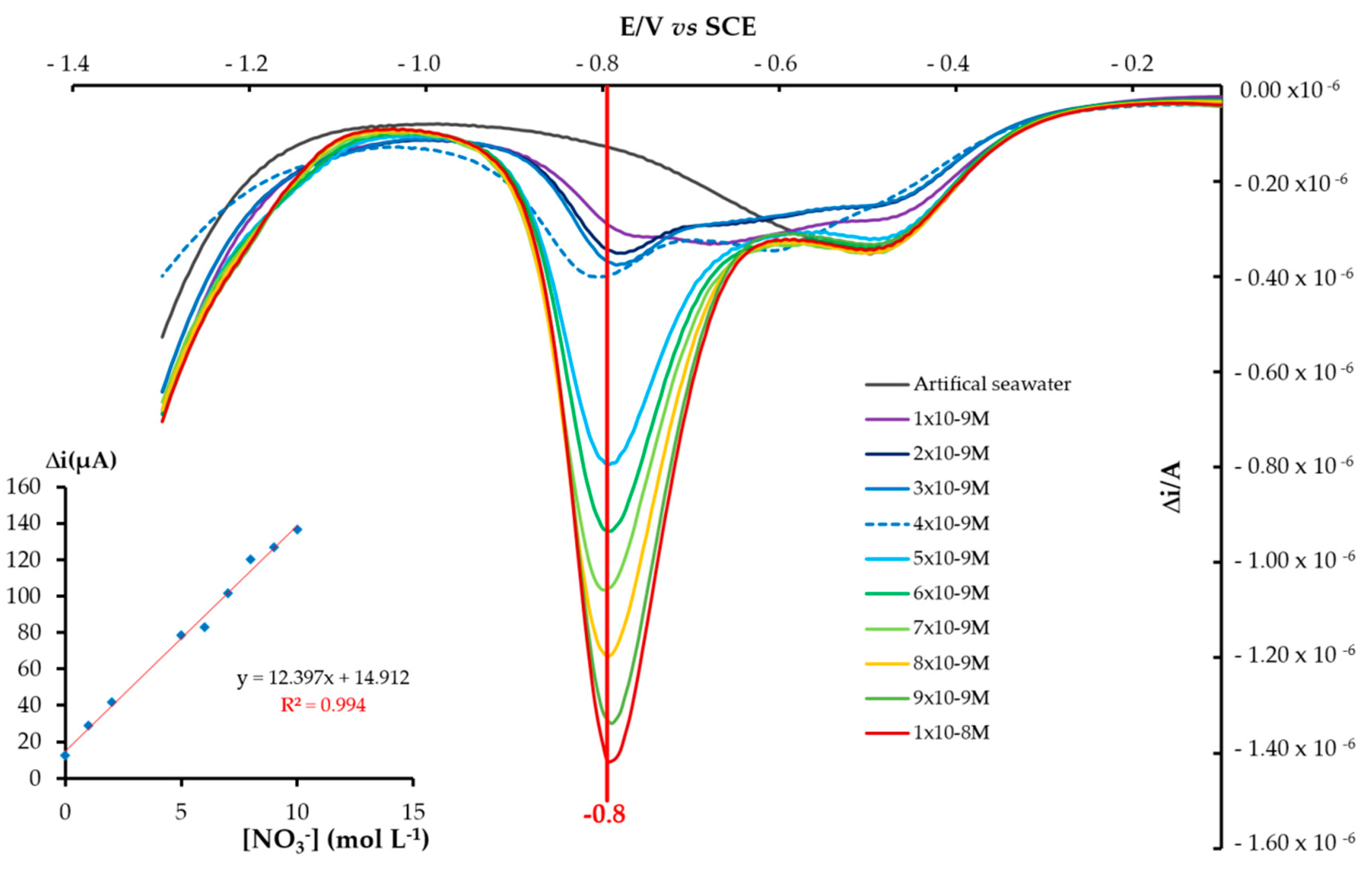
3.3. Analytical Performances towards Nitrate Ions NO3− Concentration by Square Wave Voltammetry (SWV)
4. Conclusions
Supplementary Materials
Author Contributions
Acknowledgments
Conflicts of Interest
References
- Moorcroft, M.J.; Davis, J.; Compton, R.G. Detection and determination of nitrate and nitrite: A review. Talanta 2001, 54, 785–803. [Google Scholar] [CrossRef]
- Solail, M.; Adeloju, S.B. Nitrate biosensors and biological methods for nitrate determination. Talanta 2016, 153, 83–98. [Google Scholar] [CrossRef] [PubMed]
- Patey, M.J.; Rijkenberg, M.J.A.; Statham, P.J.; Stinchcombe, M.C.; Achterberg, E.P.; Mowlen, M. Determination of nitrate and phosphate in sea water at nanomolar concentrations. Trends Anal. Chem. 2008, 27, 169–182. [Google Scholar] [CrossRef]
- Wang, Q.-H.; Yu, L.-J.; Liu, Y.; Lin, L.; Lu, R.; Zhu, J.; He, L.; Lu, Z.-L. Methods for the detection and determination of nitrite and nitrate: A review. Talanta 2017, 165, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.D.; Barus, C.; Garçon, V. Square Wave Voltammetry Measurements of Low Concentrations of Nitrate Using Au/AgNPs Electrode in Chloride Solutions. Electroanalysis 2017, 29, 2882–2887. [Google Scholar] [CrossRef]
- Rosca, V.; Duca, M.; de Groot, M.T.; Koper, M.T.M. Nitrogen cycle electrocatalysis. Chem. Rev. 2009, 109, 2209–2244. [Google Scholar] [CrossRef] [PubMed]
- Hezard, T.; Fajerwerg, K.; Evrard, D.; Collière, V.; Behra, P.; Gros, P. Influence of the gold nanoparticles electrodeposition method on Hg(II) trace electrochemical detection. Electrochim. Acta 2012, 73, 15–22. [Google Scholar] [CrossRef]
- Liu, Y.; Su, G.; Zhang, B.; Jiang, G.; Yan, B. Nanoparticle-based strategies for detection and remediation of environmental pollutants. Analyst 2011, 136, 872–877. [Google Scholar] [CrossRef] [PubMed]
- Welch, C.M.; Compton, R.G. The use of nanoparticles in electroanalysis: A review. Anal. Bioanal. Chem. 2006, 384, 601–619. [Google Scholar] [CrossRef] [PubMed]
- Campbell, F.W.; Compton, R.G. The use of nanoparticles in electroanalysis: An updated review. Anal. Bioanal. Chem. 2010, 396, 241–259. [Google Scholar] [CrossRef] [PubMed]
- Tashkhourian, J.; Hormozi Nezhad, M.R.; Khodavesi, J.; Javadi, S. Silver nanoparticles modified carbon nanotube paste electrode for simultaneous determination of dopamine and ascorbic acid. J. Electroanal. Chem. 2009, 633, 85–91. [Google Scholar] [CrossRef]
- Baghayeri, M.; Mahdavi, B.; Hosseinpor, Z.; Abadi, M.; Farhadi, S. Green synthesis of silver nanoparticles using water extract of Salvia leriifolia: Antibacterial studies and applications as catalysts in the electrochemical detection of nitrite. Appl. Organomet. Chem. 2018, 32, 4057. [Google Scholar] [CrossRef]
- Liu, M.; Chen, W.W. Green synthesis of silver nanoclusters supported on carbon nanodots: Enhanced photoluminescence and high catalytic activity for oxygen reduction reaction. Nanoscale 2013, 5, 12558–12564. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.P.; Zhao, Y.; Qiu, K.Q.; Chu, J.; Yu, H.Q.; Liu, G.; Tian, Y.C.; Xiong, Y. Fabrication of dendritic silver nanostructure using an integration of holographic lithography and electrochemical deposition. Electrochim. Acta 2011, 56, 9088–9094. [Google Scholar] [CrossRef]
- Hu, J.; Sun, I.; Bian, C.; Tong, J.; Shanhong, X. 3D Dendritic Nanostructure of Silver-Array: Preparation, Growth Mechanism and Application in Nitrate Sensor. Electroanalysis 2013, 25, 546–556. [Google Scholar] [CrossRef]
- Lotfi, H.R.; Zhad, Z.; Lai, R.Y. Comparison of nanostructured silver-modified silver and carbon ultramicroelectrodes for electrochemical detection of nitrate. Anal. Chim. Acta 2015, 892, 153–159. [Google Scholar] [CrossRef] [PubMed]
- Guadagnini, L.; Tonelli, D. Carbon electrodes unmodified and decorated with silver nanoparticles for the determination of nitrite, nitrate and iodate. Sens. Actuators B 2013, 188, 8069–8814. [Google Scholar] [CrossRef]
- Ghanbari, K. Silver Nanoparticles Dispersed in Polypyrrole Matrixes Coated on Glassy Carbon Electrode as a Nitrate Sensor. Anal. Bioanal. Electrochem. 2013, 5, 46–58. [Google Scholar]
- Fajerwerg, K.; Ynam, V.; Chaudret, B.; Garçon, V.; Thouron, D.; Comtat, M. An original nitrate sensor based on silver nanoparticles electrodeposited on a gold electrode. Electrochem. Commun. 2010, 12, 1439–1441. [Google Scholar] [CrossRef]
- Bonyani, M.; Mirzaei, A.; Leonardi, S.G.; Bonavita, A.; Neri, G. Electrochemical Properties of Ag@iron Oxide Nanocomposite for Application as Nitrate Sensor. Electroanalysis 2015, 27, 2654–2662. [Google Scholar] [CrossRef]
- Chaudret, B. Organometallic approach to nanoparticles synthesis and self organization. C. R. Acad. Sci Phys. 2005, 6, 117–131. [Google Scholar] [CrossRef]
- Debouttiere, P.J.; Coppel, Y.; Behra, P.; Chaudret, B.; Fajerwerg, K. One pot organometallic synthesis of well-controlled gold nanoparticles by gas reduction of Au(I) precursor. A spectroscopic NMR study. Gold Bull. 2013, 46, 291–398. [Google Scholar] [CrossRef]
- Amiens, C.; Chaudret, B.; Ciuculescu-Pradines, D.; Collière, V.; Fajerwerg, K.; Khan, M.L.; Maisonnat, A.; Soulantica, K.; Philippot, K. Organometallic approach for the synthesis of nanostructures. New J. Chem. 2013, 37, 3374–3401. [Google Scholar] [CrossRef]
- Cure, J.; Coppel, Y.; Dammak, T.; Fazzini, P.F.; Mlayah, A.; Chaudret, B. Monitoring the Coordination of Amine Ligands on Silver Nanoparticles Using NMR and SERS. Langmuir 2015, 31, 1362–1367. [Google Scholar] [CrossRef] [PubMed]
- Lim, B.S.; Rahtu, A.; Park, J.S.; Gordon, R.G. Synthesis and characterization of volatile,thermally stable, reactive transition metal amidinates. Inorg. Chem. 2003, 42, 7951–7958. [Google Scholar] [CrossRef] [PubMed]
- Cure, J.; Piettre, K.; Sournia-Saquet, A.; Coppel, Y.; Esvan, J.; Chaudret, B.; Fau, P. A novel method for the metallization of 3D silicon induced by metastable copper nanoparticles. ACS Appl. Mater. Interfaces 2018, 10, 32838–32848. [Google Scholar] [CrossRef] [PubMed]
- Mirčeski, V.; Komorsky-Lovrić, S.; Lovrić, M. Square-Wave Voltammetry: Theory and Application; Scholz, F., Ed.; Springer: Berlin, Germany, 2007. [Google Scholar]
- Bard, A.J.; Faulkner, L.R. Electrochemical Methods: Fundamental and Applications, 2nd ed.; Wiley: New York, NY, USA, 2001; ISBN 978-0-471-04372-0. [Google Scholar]
- Raj, C.R.; Abdelrahman, A.J.; Ohsaka, J. Gold nanoparticle-assisted electroreduction of oxygen. Electrochem. Commun. 2005, 7, 888–893. [Google Scholar] [CrossRef]
- Rodriguez, P.; Koper, M.T.M. Electrocatalysis on gold. Phys. Chem. Chem. Phys. 2014, 16, 13583–13594. [Google Scholar] [CrossRef] [PubMed]
- Cleve, T.V.; Gibara, E.; Linic, S. Electrochemical Oxygen Reduction Reaction on Ag Nanoparticles of Different Shapes. ChemCatChem 2016, 8, 256–261. [Google Scholar] [CrossRef]
- Singh, P.; Buttry, D.A. Comparison of Oxygen Reduction Reaction at Silver Nanoparticles and Polycrystalline Silver Electrodes in Alkaline Solution. J. Phys. Chem. C 2012, 116, 10656–10663. [Google Scholar] [CrossRef]
- Mott, D.M.; Dao, T.N.A.; Singh, P.; Shankar, C.; Maenosono, S. Electronic transfer as a route to increase the chemical stability in gold and silver core–shell nanoparticles. Adv. Colloid Interface Sci. 2012, 185, 14–33. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deming, C.P.; Peng, Y.; Hu, P.; Stofan, J.; Chen, S. Gold core@silver semishell Janus nanoparticles prepared by interfacial etching. Nanoscale 2016, 30, 14565–14572. [Google Scholar] [CrossRef] [PubMed]
- Käthelhön, E.; Compton, R.G. Nanoparticles in sensing applications: On what timescale do analyte species adsorb on the particle surface? Analyst 2014, 139, 2411–2415. [Google Scholar] [CrossRef] [PubMed]
- Calle-Vallejo, F.; Huang, M.; Henry, J.B.; Koper, M.T.M.; Bandarenka, A.S. Theoretical design and experimental implementation of Ag/Au electrodes for the electrochemical reduction of nitrate. Phys. Chem. Chem. Phys. 2013, 15, 3196–3202. [Google Scholar] [CrossRef] [PubMed]
- Lebon, E.; JCure, J.; Fau, P.; Kahn, M.L.; Lepetit, C.; Fajerwerg, K. Micromolar nitrate electrochemical sensors for sea water analysis with silver nanoparticles modified gold electrode. In Proceedings of the IEEE Nanotechnology Materials and Devices Conference, Toulouse, France, 9–12 October 2016. [Google Scholar] [CrossRef]
| Nanostructure | Preparation | Electrolyte | pH | Method | Linear Range (µM) | Sensitivity (µA∙mM−1∙cm−2) | LOD LOQ (µM) | References |
|---|---|---|---|---|---|---|---|---|
| 3D Ag Array | Electrodeposition | 0.5M NaCl | 7.0 | SWV | 2–1000 | 28.2 | 2 | [15] |
| Ag NPs/C | Electrodeposition | 0.1M Na2SO4 | 6.5 | Amp. | 4–1000 | Not indicated | 3.2 | [16] |
| Ag NPs/Graphite sheets | Electrodeposition | Phosphate buffer | 6.7 | Amp. | 20–5000 | 1700 | 10 | [17] |
| Ag NPs/GC | Electrodeposition | Phosphate buffer | 6.7 | Amp. | 10–5000 | 3400 | 4 | [17] |
| Ag NPs/Au | Electrodeposition | 0.6M NaCl | 6.0 | CV | 10–1000 | 3000 | 10 | [19] |
| Ag NP/polypyrrole/GC | Electrodeposition | 0.1M KCl | 7.0 | DPV | 1–10000 | 2500 | 2 | [18] |
| Ag NPs/Iron oxide | Chemical | Phosphate buffer | 7.0 | Amp. | 0–1000 | 660 | 30 | [19] |
| Ag NPs/Au | Electrodeposition | 0.6M NaCl | 6.0 | SWV | 0.39–50 | Not indicated | 0.39 | [5] |
| Ag NPs/Au | Metalorganic | 0.6M NaCl | 6.0 | SWV | 0.9 × 10−3–1000 | 5000 | 0.9 × 10−3 | This work |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebon, E.; Fau, P.; Comtat, M.; Kahn, M.L.; Sournia-Saquet, A.; Temple-Boyer, P.; Dubreuil, B.; Behra, P.; Fajerwerg, K. In Situ Metalorganic Deposition of Silver Nanoparticles on Gold Substrate and Square Wave Voltammetry: A Highly Efficient Combination for Nanomolar Detection of Nitrate Ions in Sea Water. Chemosensors 2018, 6, 50. https://doi.org/10.3390/chemosensors6040050
Lebon E, Fau P, Comtat M, Kahn ML, Sournia-Saquet A, Temple-Boyer P, Dubreuil B, Behra P, Fajerwerg K. In Situ Metalorganic Deposition of Silver Nanoparticles on Gold Substrate and Square Wave Voltammetry: A Highly Efficient Combination for Nanomolar Detection of Nitrate Ions in Sea Water. Chemosensors. 2018; 6(4):50. https://doi.org/10.3390/chemosensors6040050
Chicago/Turabian StyleLebon, Emilie, Pierre Fau, Maurice Comtat, Myrtil L. Kahn, Alix Sournia-Saquet, Pierre Temple-Boyer, Brigitte Dubreuil, Philippe Behra, and Katia Fajerwerg. 2018. "In Situ Metalorganic Deposition of Silver Nanoparticles on Gold Substrate and Square Wave Voltammetry: A Highly Efficient Combination for Nanomolar Detection of Nitrate Ions in Sea Water" Chemosensors 6, no. 4: 50. https://doi.org/10.3390/chemosensors6040050
APA StyleLebon, E., Fau, P., Comtat, M., Kahn, M. L., Sournia-Saquet, A., Temple-Boyer, P., Dubreuil, B., Behra, P., & Fajerwerg, K. (2018). In Situ Metalorganic Deposition of Silver Nanoparticles on Gold Substrate and Square Wave Voltammetry: A Highly Efficient Combination for Nanomolar Detection of Nitrate Ions in Sea Water. Chemosensors, 6(4), 50. https://doi.org/10.3390/chemosensors6040050