1. Introduction
Recent advances in science and technology have increased the demand for highly accurate, durable, sustainable, reproducible, and cheap humidity sensors. These sensors find many industrial applications ranging from environmental and meteorological monitoring, soil water content determination in agriculture, air conditioning systems, food quality monitoring, medical equipment, and other fields [
1,
2]. Humidity sensors can be classified as capacitive, resistive, or thermal conductive ones [
3]. Usually, humidity sensors are based on metal oxide semiconductors, conducting polymers, carbon nanotubes, and/or graphene oxides. At room temperature, the adsorption of water vapor/molecules changes the resistance or the capacitance of the sensing film [
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15].
Carbon-based humidity sensors have attracted great attention since a couple of decades because of their large sensing area and high chemical inertness [
16,
17,
18,
19,
20]. Biomass is a qualified carbon source, it is available in high quality and huge amount, and it is considered as an environmental friendly renewable resource [
21]. In recent years, biochar applications invested in many fields [
22], though the main application of this material remains field amendment in agriculture [
22,
23]. In addition, different biochars (residues of biomass pyrolysis) are becoming available from pilot plants producing biogas and energy [
24,
25]. Besides, in the last few years, biochar has been extensively studied as a substitute for more expensive carbon materials like carbon nanotubes, graphene, and others.
In a previous work, pyrolyzed bamboo was proposed as a sensing material with promising results, showing an excellent response toward humidity starting from 10% relative humidity (RH) [
26]. However, the adhesion onto alumina substrates was minimal, and these films could be easily damaged when testing, preventing their use in commercial products. Thus, in this research, the room temperature humidity sensing properties of two commercial biochars derived from different precursors [
24,
25] were investigated. First, the biochars were drop coated onto commercial alumina substrates with platinum interdigitated electrodes. Then, polyvinylpyrrolidone (PVP), acting as an organic binder was added to improve the adhesion onto the ceramic substrates. This polymer is used in many industrial applications, such as adhesives to enhance strength and toughness, paper manufacture for increasing strength and as a coating resin, as well as in synthetic fibers to accentuate dye receptivity. PVP polymers are also widely employed in inks, imaging, lithography, detergents, and soaps, in textile, ceramic, electrical, and metallurgical industries, and as a polymerization additive. Only in few studies was PVP used as humidity sensor in composites [
27,
28]. The sensing materials and the films were studied by means of Field Emission-Scanning Electron Microscopy (FESEM), X-Ray diffraction (XRD), Raman spectroscopy, X-ray photoelectron spectroscopy (XPS), and laser-granulometry measurements. The sensors were tested toward relative humidity at room temperature.
2. Materials and Methods
2.1. Materials
Two types of certified biochar were purchased from the UK Biochar Research Centre (BRC) (Edinburgh, UK) and used as innovative humidity sensing materials. The two biochars were selected among many available from BRC, based on the highest surface area and on the highest electrical conductivity. SWP700 is made from pyrolyzed mixed softwood pellets, obtained at a maximum treatment temperature of 700 °C with a heating rate of 87 °C/min. Its biochar yield is of 17.34%. OSR700 is produced from oil seed rape, by a pyrolysis process at 700 °C and a heating rate of 103 °C/min. In this case, a biochar yield of 22.62% is achieved.
Table 1 reports the specific surface area (SSA) and the conductivity of both materials, as reported on their datasheets [
24,
25].
Both biochars in the form of pellets were first mixed in a mechanical grinder for 10 min and later manually ground in order to attain a homogeneous powder.
Polyvinylpyrrolidone (PVP, average Mw~13,000,000 by LS, Sigma Aldrich, Milano, Italy) was used as a polymeric binder and mixed with biochars in ethanol (for residual analysis, Sigma Aldrich ≥ 99.8% v/v).
2.2. Characterization of Biochars
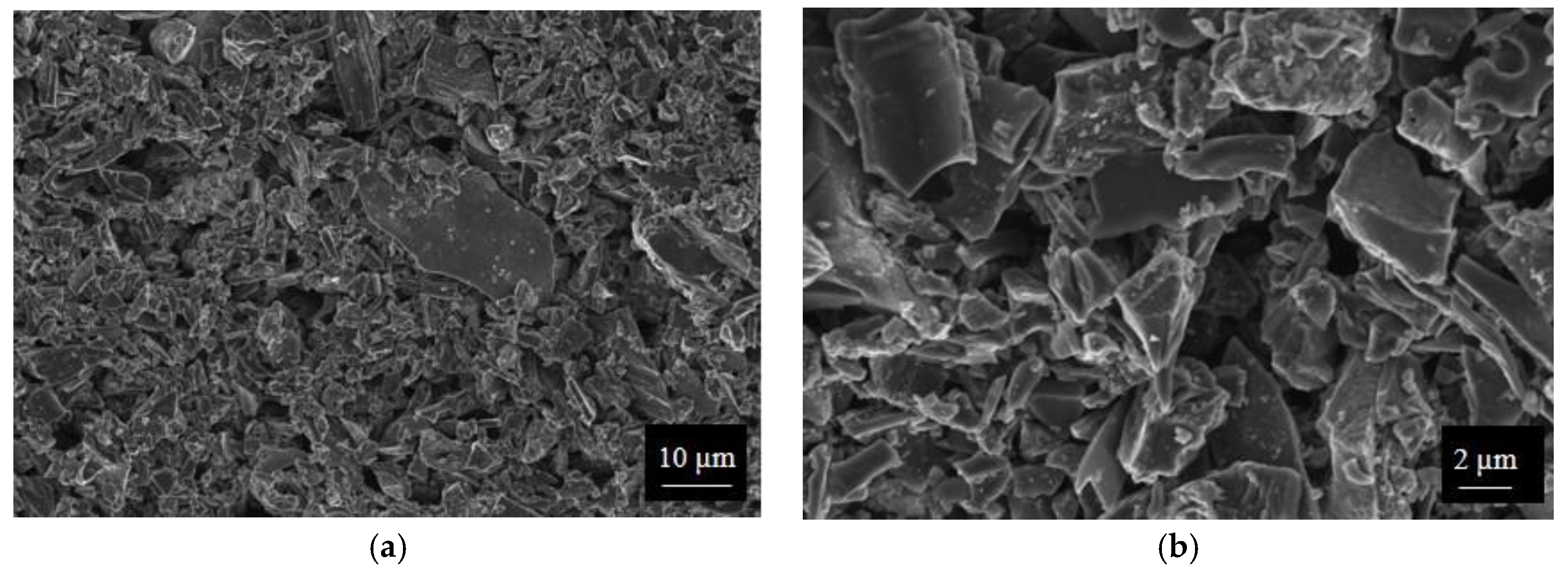
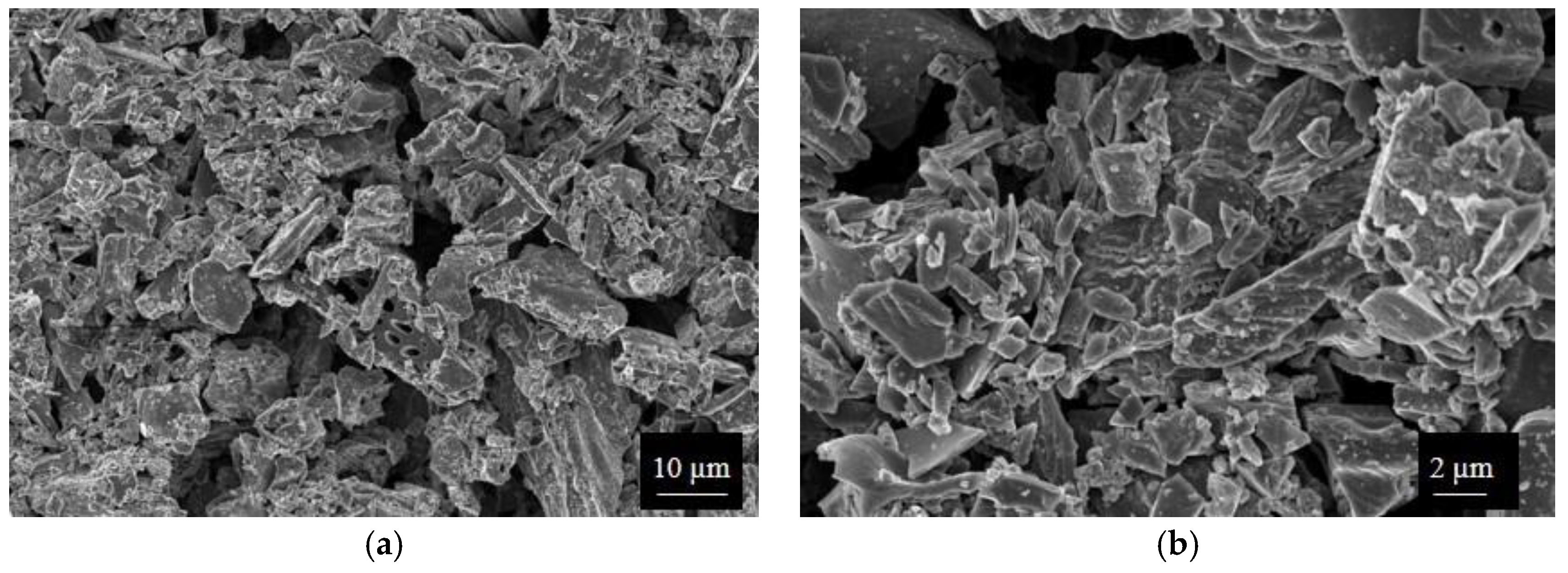
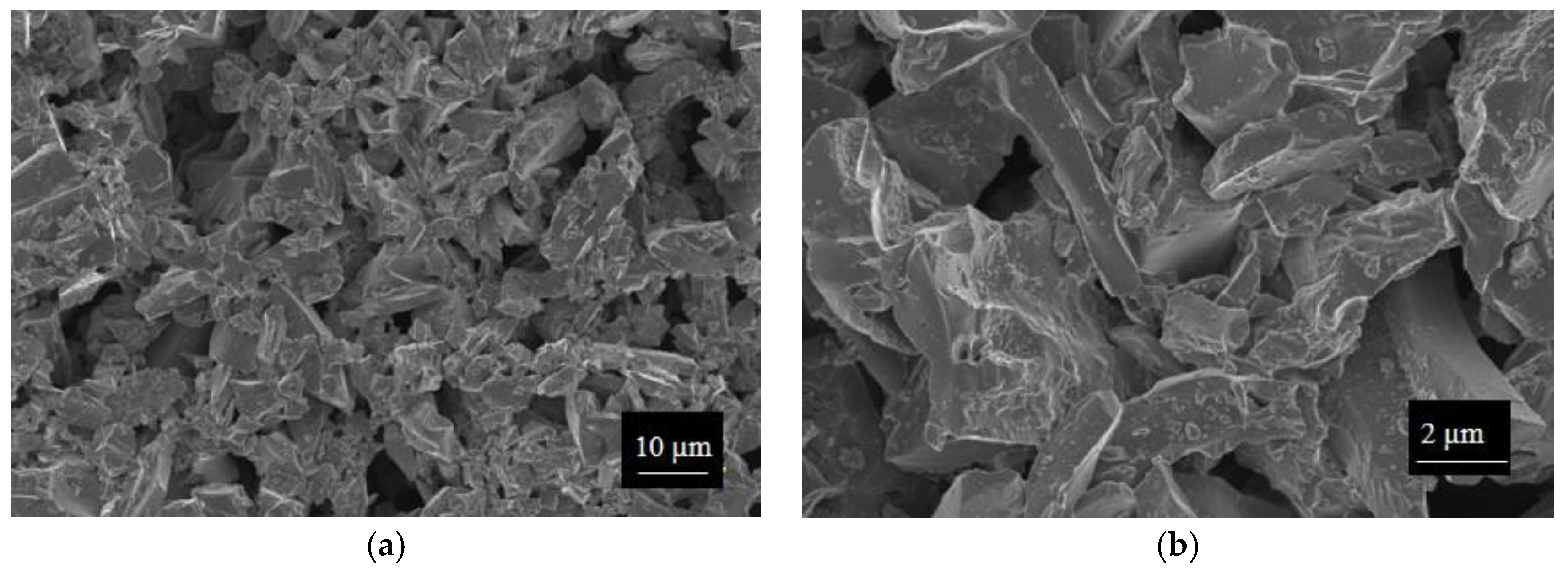
SWP700 and OSR700 biochar powders were characterized by laser granulometry, X-Ray Diffraction (XRD), Raman Spectroscopy, X-Ray Photoelectron Spectroscopy (XPS), and Field Emission Scanning Electron Microscopy (FESEM) observations.
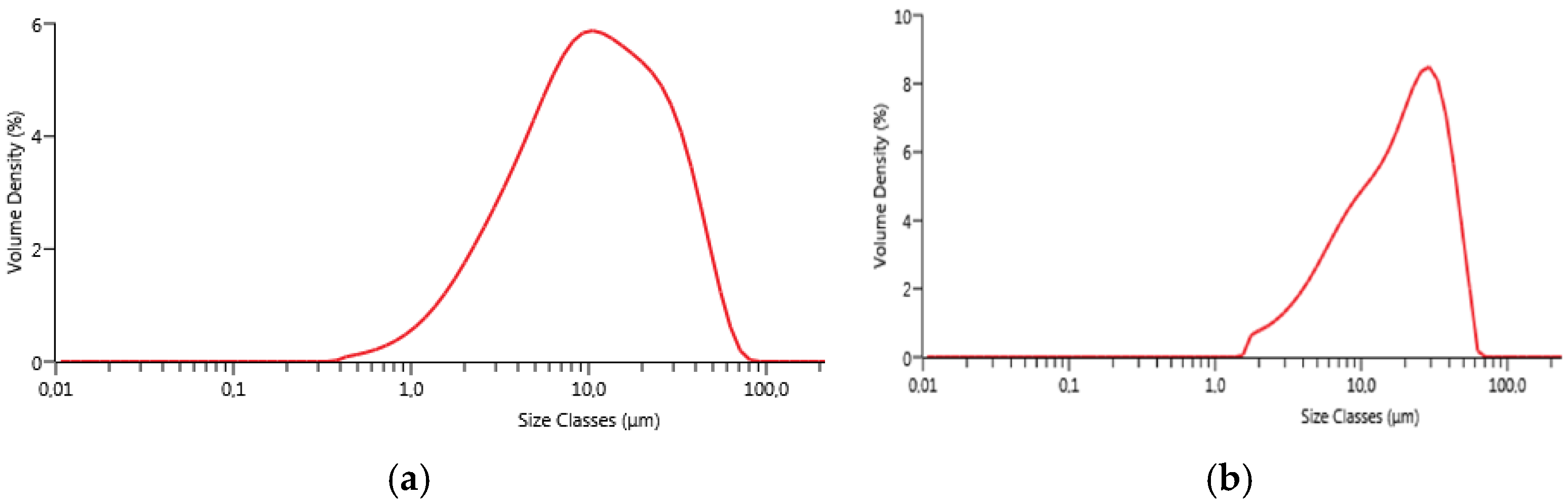
Particle size distribution was evaluated by laser granulometry (Mastersizer 3000, Malvern, Worcestershire, UK). The measurements were performed after manual grinding of the powders by means of an agate mortar and an agate pestle and sieving with a sieve having apertures of 45 µm. Laser granulometry measurements were carried out after dispersion in ethanol (500 ppm in volume) and sonication for 5 min.
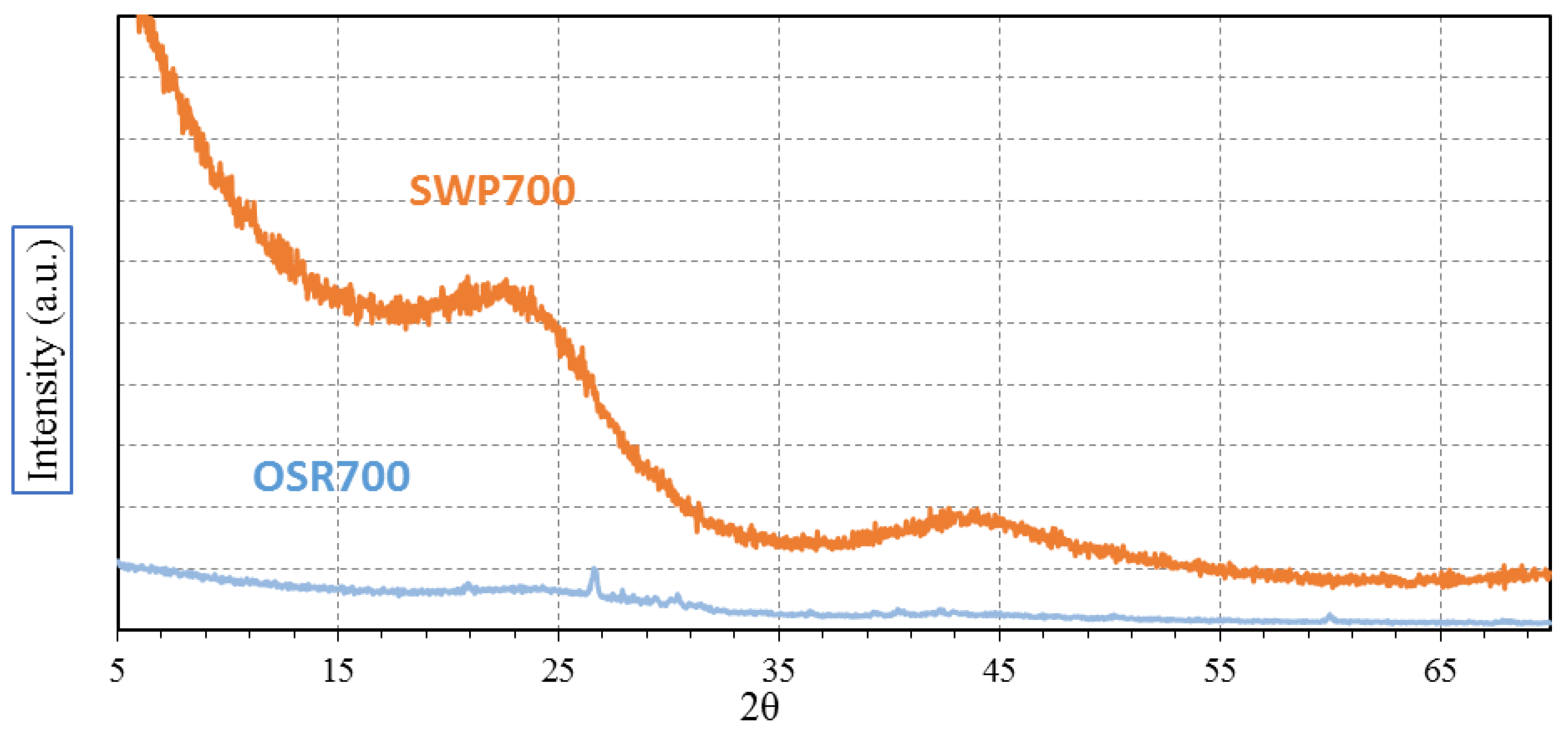
XRD analysis was carried out with the aim of evidencing any possible crystallization peak among OSR700 and SWP700 raw materials. Spectra were recorded on a Pan’Analytical X’Pert Pro instrument (Pan’Analytical, Almelo, The Netherlands) working with Cu Kα radiation (0.154056 nm) in the range 5–70° 2θ, with a step size of 0.05° in 2θ and an acquisition time per step of 5 s. Diffraction patterns were indexed by means of the Powder Data File database (P.D.F. 2000, International Centre of Diffraction Data, Newtown Square, PA, USA).
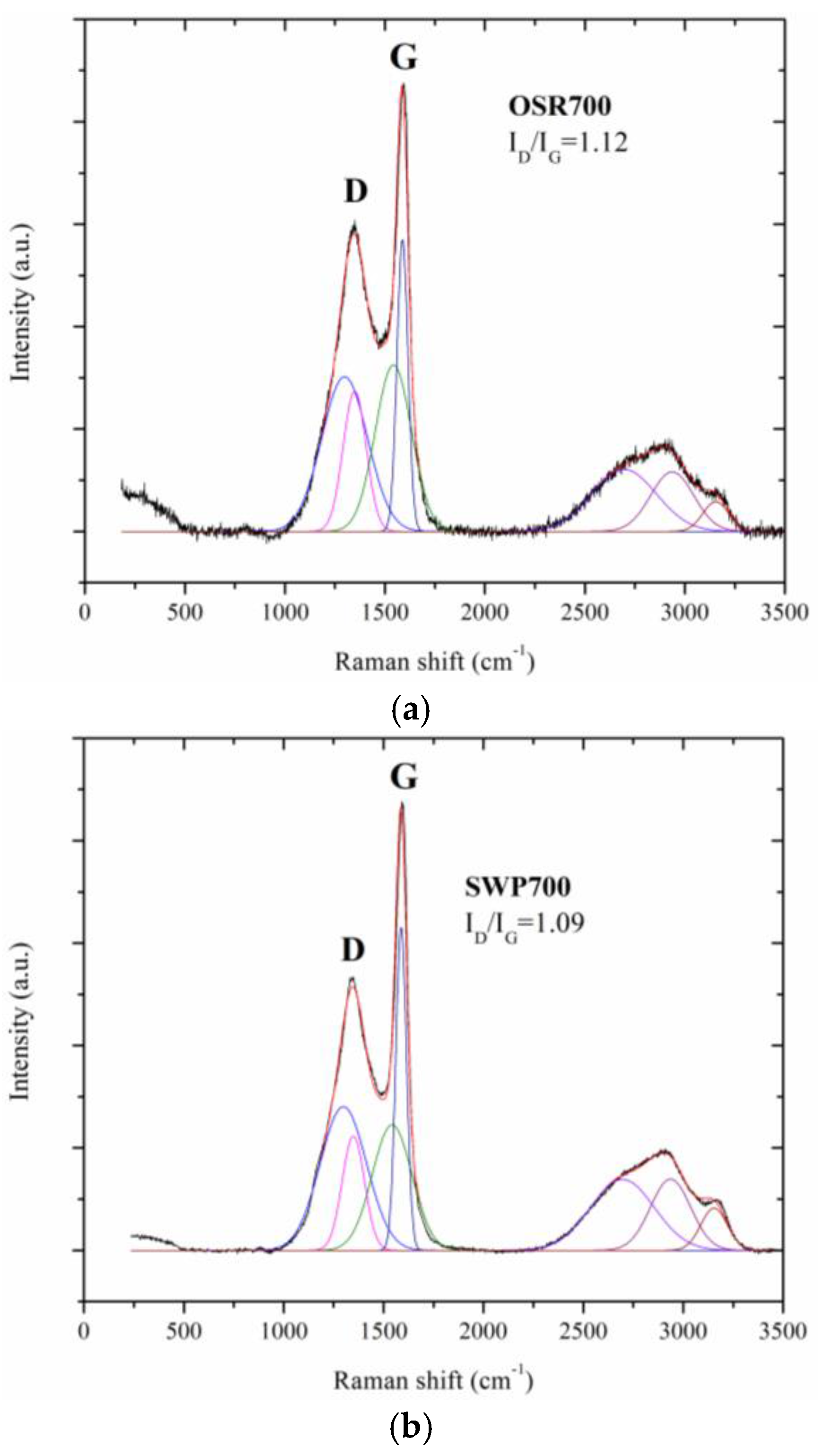
A Renishaw micro Raman (Renishaw, New Mills, UK) equipped with a green laser (514 nm) and a 50× objective was used to study the disorder and graphitization grade of the biochars under test. Raman characterization were performed at room temperature. The samples, in powder form, were exposed, without any pretreatment, to the micro laser beam (2 μm in diameter) for 10 s.
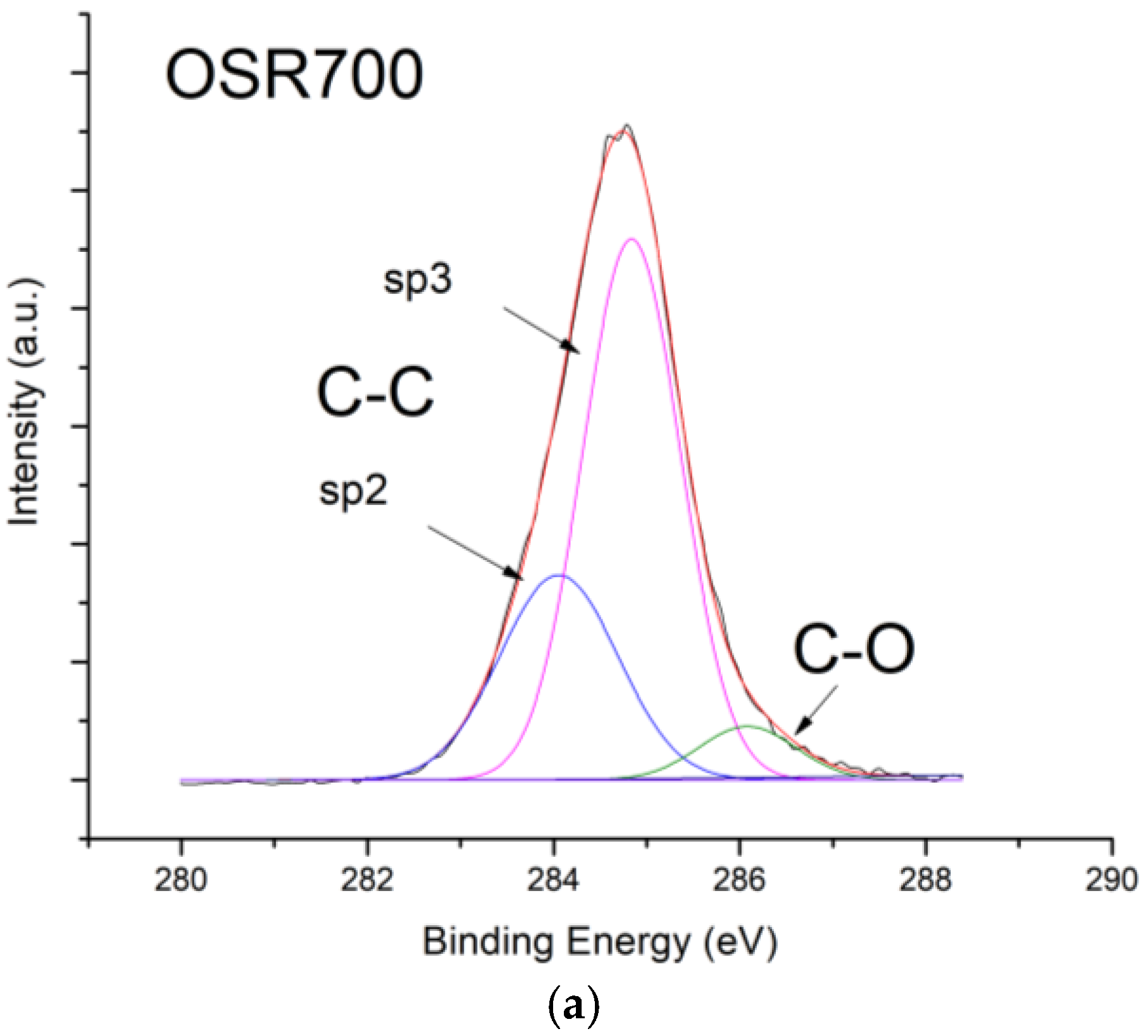
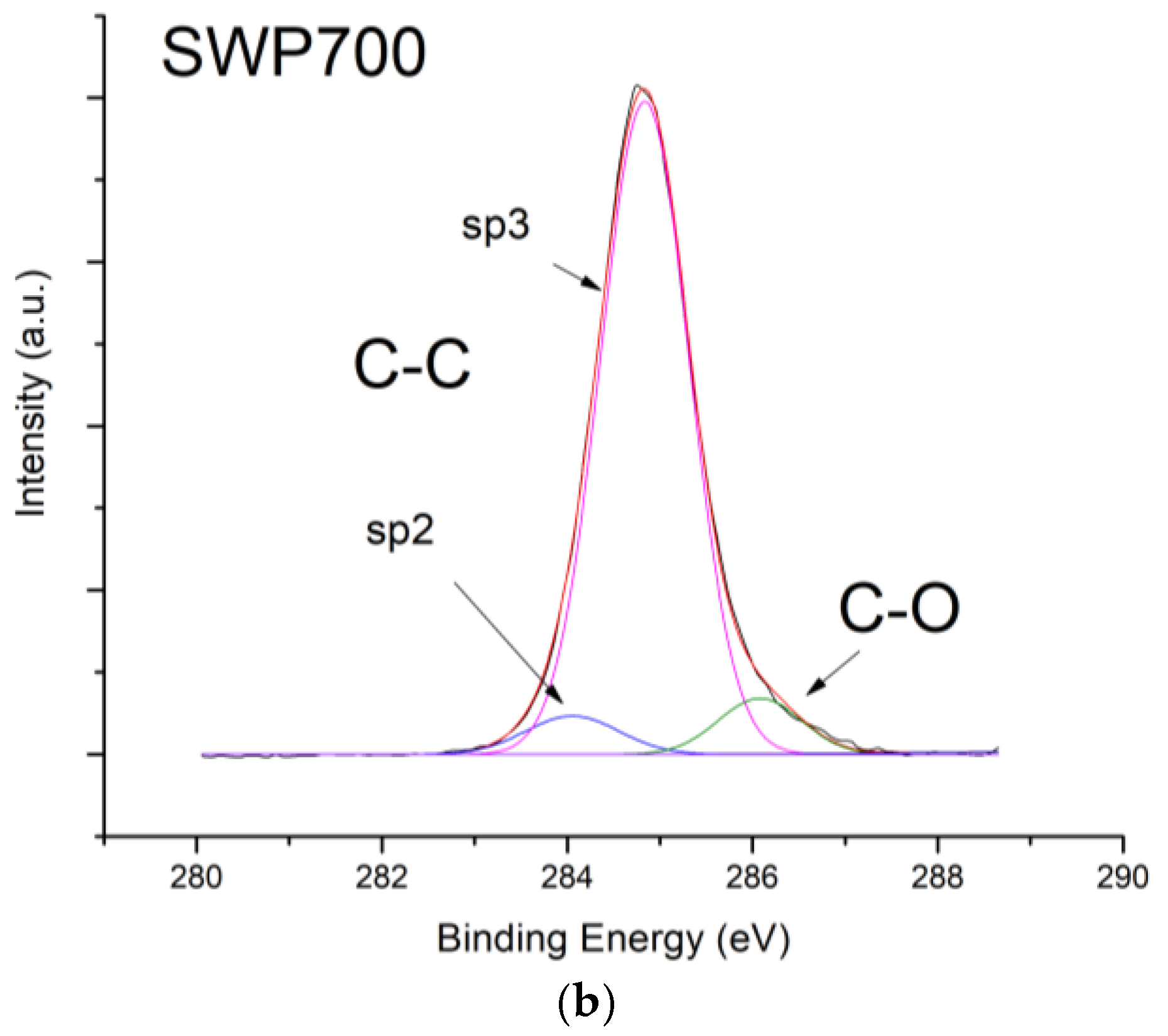
X-ray photoelectron spectroscopy (XPS) investigations were carried out by means of a PHI 5000 Versaprobe (Physical Electronics, Chanhassen, MN, USA) scanning X-ray photoelectron spectrometer (monochromatic Al K-alpha X-ray source with 1486.6 eV energy, 15 kV voltage, and 1 mA anode current) to investigate surface chemical composition.
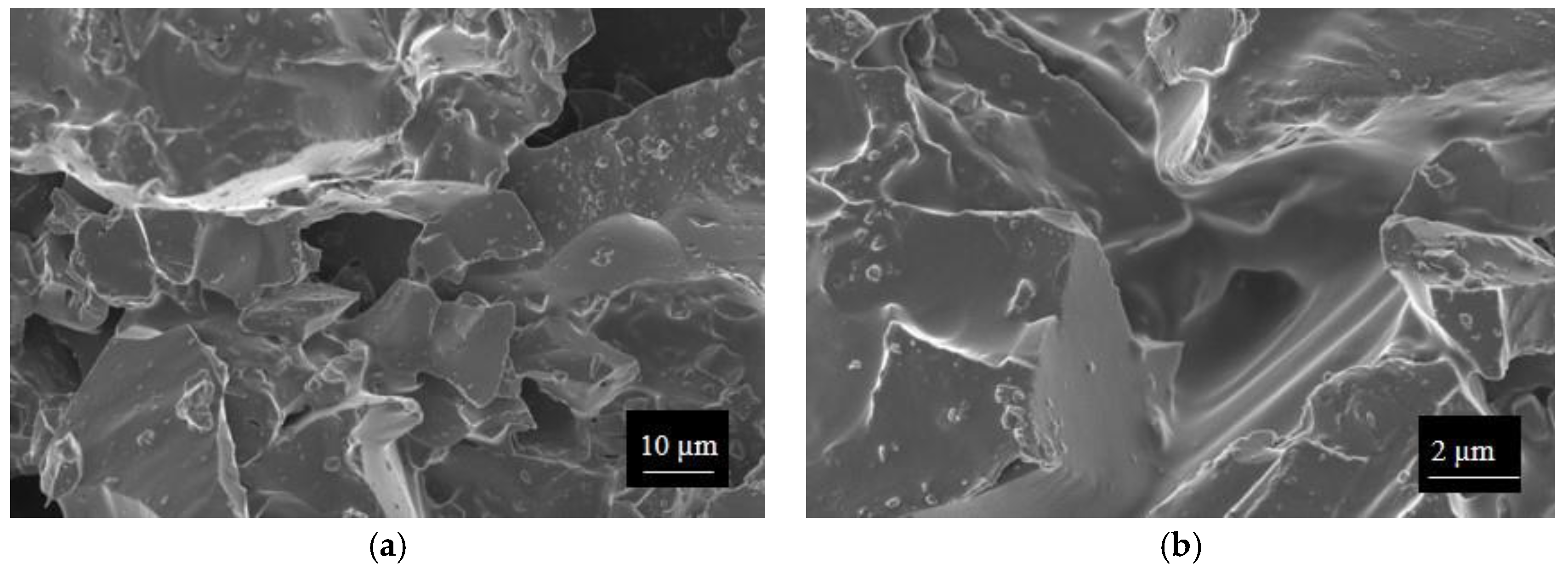
Finally, morphologies of both kinds of powders and sensors were examined by means of an FESEM (Zeiss Supra-40, Oberkochen, Germany) equipped with an Oxford Energy Dispersive X-ray detector after the Cr sputtering process.
2.3. Sensor Fabrication
Platinum (5545-LS, ESL, King of Prussia, PA, USA) interdigitated electrodes were screen-printed on commercial α-alumina substrates (Coors Tek, Golden, CO, USA, ADS-96, 96% alumina, 0.85 cm × 1.7 cm). These electrodes were then fired at 980 °C (2 °C/min heating and cooling ramps) for 18 min, obtaining a high-grade adhesion and optimizing their electrical conductivity, as per the ink manufacturer’s recommendations. Electrodes present a thickness of 400 µm, and their spacing is equal to 450 µm. In addition, the edge of an electrode belonging to one comb and the vertical connection of the second comb are separated by 400 µm.
To obtain biochar, PVP and biochar-PVP sensors, a drop coating technique was adopted by mixing with a magnetic stirrer 0.1 g of material (SWP700, OSR700, PVP, or biochar-PVP, respectively, in four different preparations) with 10 mL of ethanol in a beaker. After 1 h of mixing, solvent was evaporated at 80 °C, and sensors were realized by drop coating technique with a volume of 40 µL of this suspension onto α-alumina substrates with Pt electrodes by means of a micropipette.
Sensors were finally dried overnight at 80 °C (3 mg of material deposited) (
Figure 1).
Subsequently, the sensors were tested toward relative humidity and cross-sensitivity tests were carried out toward ozone, ammonia, carbon dioxide, and methane at room temperature (SIAD, Bergamo, Italy; research quality gases).
2.4. Electrical Characterization
Humidity sensors were inserted into a laboratory apparatus made of a hermetic chamber in which relative humidity could be varied between 0 and 99% by steps, each one of 3 min. In fact, in this system, compressed air is separated into two flows: one is dehydrated over a chromatography alumina bed, while the second one is directed through two water bubblers, generating, respectively, a dry and a humid flow. Two precision microvalves recombine the two fluxes into one by means of a mixer and adjust the RH content [
4]. All humidity measurements were carried out under a constant air flow of 1700 sccm (standard cubic centimeters). A commercial humidity and temperature probe was used as reference for temperature and RH values (Delta Ohm DO9406, Caselle di Selvazzano (PD), Italy). All the measurements were done at room temperature (25 ± 0.5 °C). Relative humidity is at all temperatures and pressures defined as the ratio of the water vapor pressure (
Pw) to the saturation water vapor pressure (over water,
Pws) at the gas temperature (
T), according to Equation (1) and Ref. [
29].
The water vapor saturation pressure (in hPa) over water in the temperature range −20 °C–50 °C is given by Equation (2).
Thus, the water vapor pressure (in hPa) can be determined as follows (Equation (3)):
During tests in a dynamic flow under variable amounts of relative humidity (RH), the sensors impedances were determined by means of a LCR meter (Hioki 3533-01, Nagano, Japan). The sensors were alimented by an AC voltage of 1 V at 1 kHz. The sensor response (SR), expressed in %, is defined as the relative variation of the starting impedance under dry air (Z
0), compared with the impedance measured under gas/humidity (Z
g) exposure (Equation (4)).
Moreover, cross-sensitivity measurements were carried out toward NH3 (50 ppm in air), CO2 (500 and 600 ppm in dry air and in air with 40% of relative humidity), CH4 (100 ppm in air), and ozone (0.5 ppm in air) at room temperature under a constant flow of 1000 sccm, and sensors were put in another chamber of 0.25 L volume (in fact a three-neck glass flask). Target gas was diluted with air by means of flow meters (Teledyne Hastings Instruments HFM 300 controller and flow meters HFC 302, Teledyne Hastings, Hampton, VA, USA). Ozone was generated by a UV lamp (SOG-01, UVP-LLC Cambridge, UK) from a constant air flow of compressed air (1000 sccm). Ozone concentration was determined by the length of lamp exposed, according to calibration curves given by the lamp manufacturer. When performing measurements under both gas and humidity, a water bubbler was added to the flow of synthetic air used for the dilution of target gas.
The response times (the time taken by a sensor to achieve 90% of the total impedance change in the case of gas adsorption) together with the recovery times (the time necessary to reach 90% of the total impedance changes in the case of gas desorption) were also determined in the present work.
4. Discussion
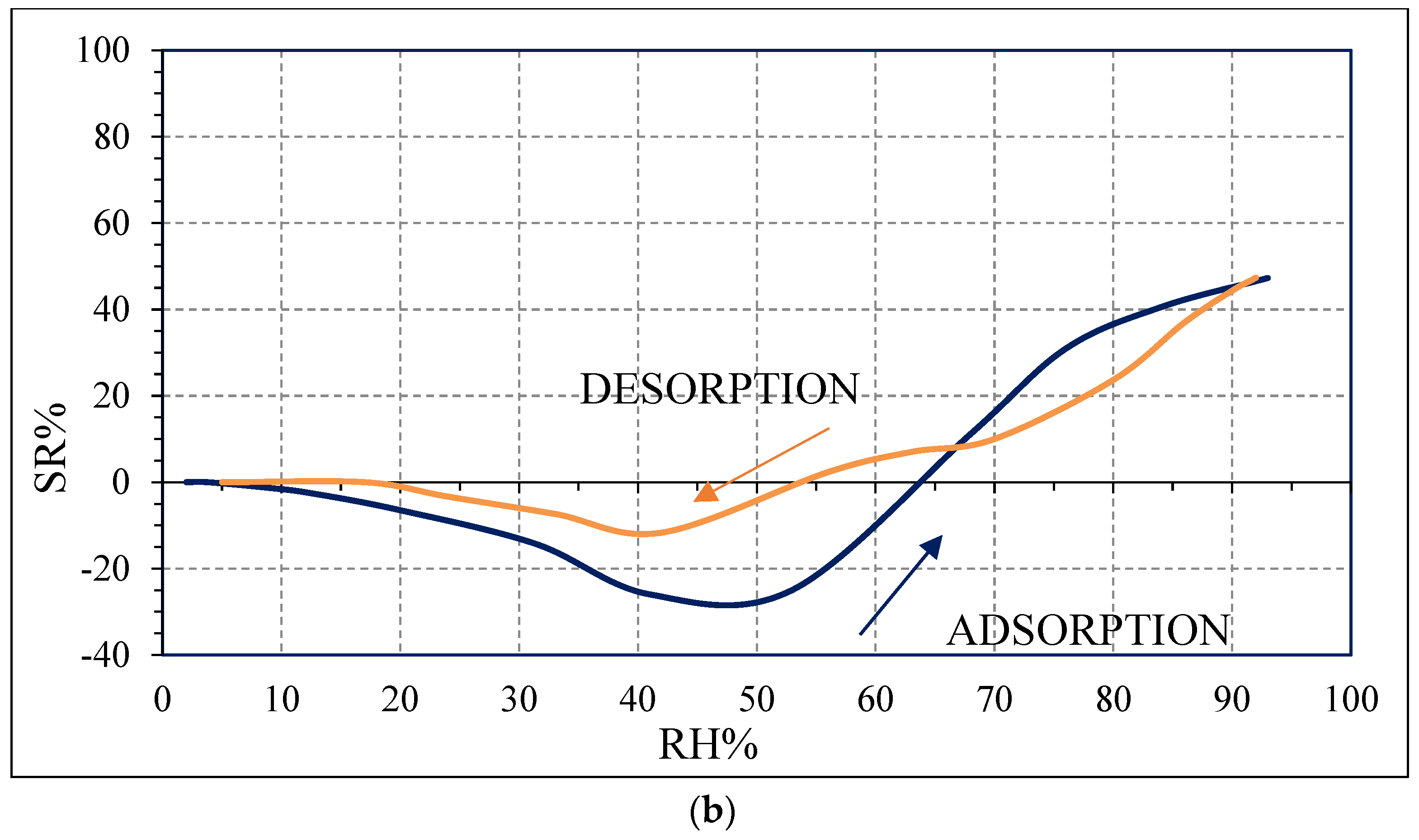
Water molecules can adsorb on the surface of carbon films due to a weak binding of water hydrogens to biochar surface carbon atoms [
20]. Moreover, the sensing performance can be improved by the presence of defects in carbon grains, as evidenced by Raman spectroscopy results (
Figure 5), which could create favorable adsorption sites to water molecules. These defects also favor water molecules adsorption by creating favorable adsorption sites [
20]. Oxygen derivatives on carbon surface make the carbon surface hydrophilic and increase water vapor adsorption [
20]. Thus, at low RH values, a few water vapor molecules chemically adsorb on the biochar grain surfaces. Pati et al. [
33] suggested a possible charge transfer between the adsorbate and the carbon film. This, probably leads to electrons exchange with a p-type semiconductor and their transfer to the valence band, leading to an increase of the impedance [
20]. However, for higher RH values (above 40 RH% for OSR700 and above 25 RH% for SWP700), more water molecules are adsorbed on the surface, and operating at room temperature, water molecules will cluster to form a liquid-like multilayer of hydrogen-bonded water molecules [
34]. Moreover, these physisorbed water molecules can condense into pores having a size in the range 1–250 nm. Since the formation of clusters of H
2O and the hydration of H
+ into H
3O
+ are energetically favored in liquid water, H
+ are the dominant charge carriers in the water adsorbed in the mesopores. As the amount of H
+ increases when increasing the moisture content, H
+ can move freely in liquid water, leading to a decrease of grain surface impedance with increasing RH values [
34]. This mechanism explains why Z
g, defined in Equation (1), tends to decrease with increasing RH values, leading to an enhancement of the sensor response, as observed in
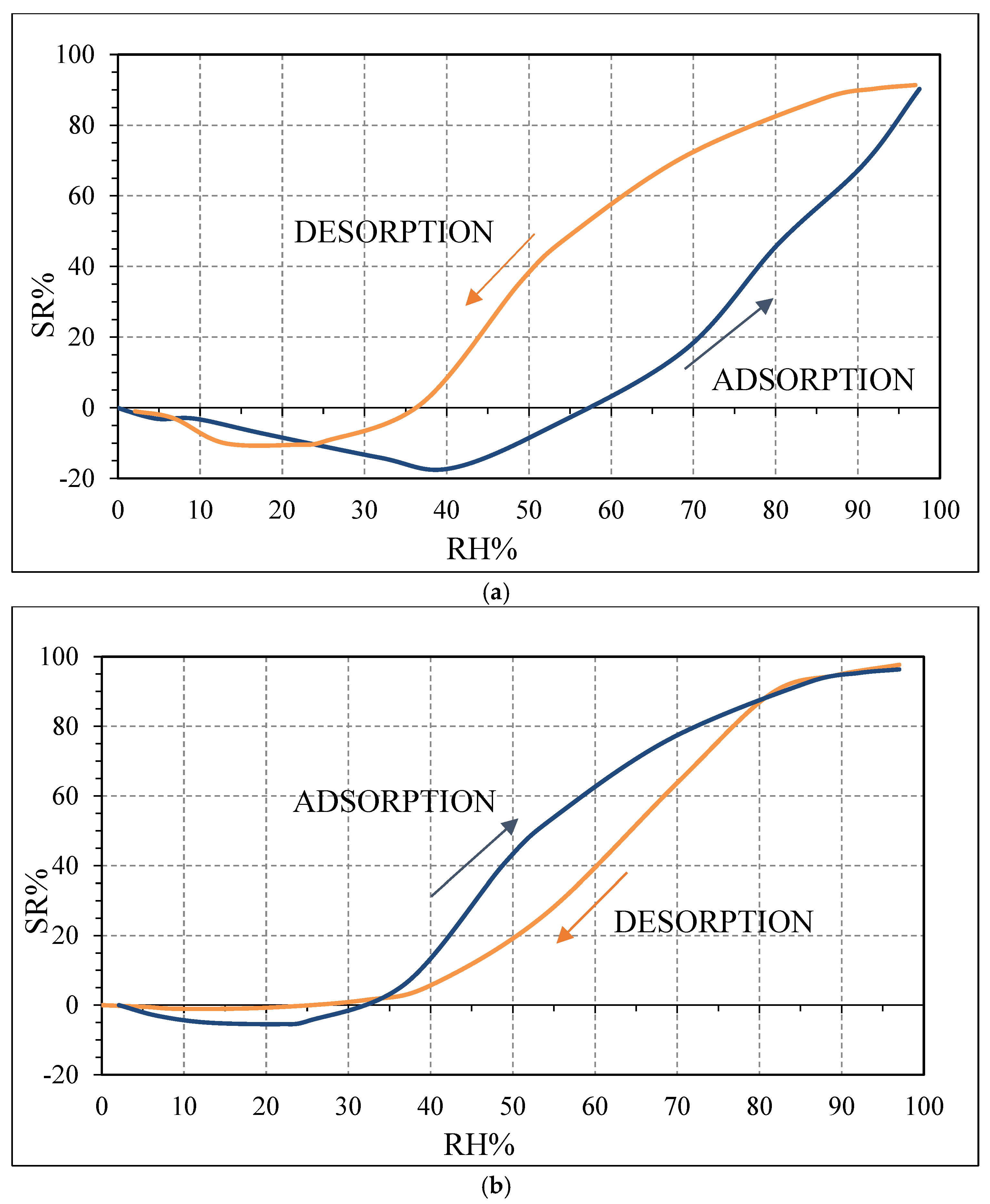
Figure 10, contrarily to what was expected from biochars as p-type semiconductors. Furthermore, the sp2/sp3 ratio determined by XPS measurements could also affect the sensor response at low relative humidity values (5–30%). In fact, the hydrophilic character is evidenced by a higher sp2/sp3 ratio, like in OSR700 film (sp2/sp3 = 0.45) compared to SWP700 one (sp2/sp3 = 0.06), in accordance with the literature [
35]. As a consequence, the former biochar exhibits an enhanced affinity for the adsorption of water molecules at lower amounts of RH with respect to the latter one.
The difference in the hysteresis between the two biochars (
Figure 10) may be probably explained by the different pore size distribution among the samples: in the case of OSR700, the hysteresis is higher because water molecules desorption is more difficult and takes longer time due to smaller pores or more tortuous ones. However, this feature was not investigated in this work and should be further studied. The effect of specific surface area on sensitivity of a quartz crystal microbalance-based polyacrylic acid ammonia sensor is reported in ref. [
36]. The results of this study confirmed that the ammonia sensitivity was enhanced rapidly by the continuous increase of SSA and showed a logarithmic growth trend [
36]. In our case, the higher SSA of SWP700 biochar probably favors water molecules adsorption too, leading to a faster decrease of the impedance value from 25 RH%, with respect to 40 RH% for OSR700 sensor.
Pristine PVP is a highly hydrophilic polymer due to the presence of a nitrogen atom in its molecule [
37,
38]. Thus, it can easily adsorb water molecules. As previously explained, at low relative humidity values, only a few water molecules can be adsorbed on the surface of PVP to generate a few H
+ and H
3O
+ ions. Then, the intrinsic electrons of PVP are the main responsible for the conduction of PVP which behaves as a n-type semiconductor as confirmed in
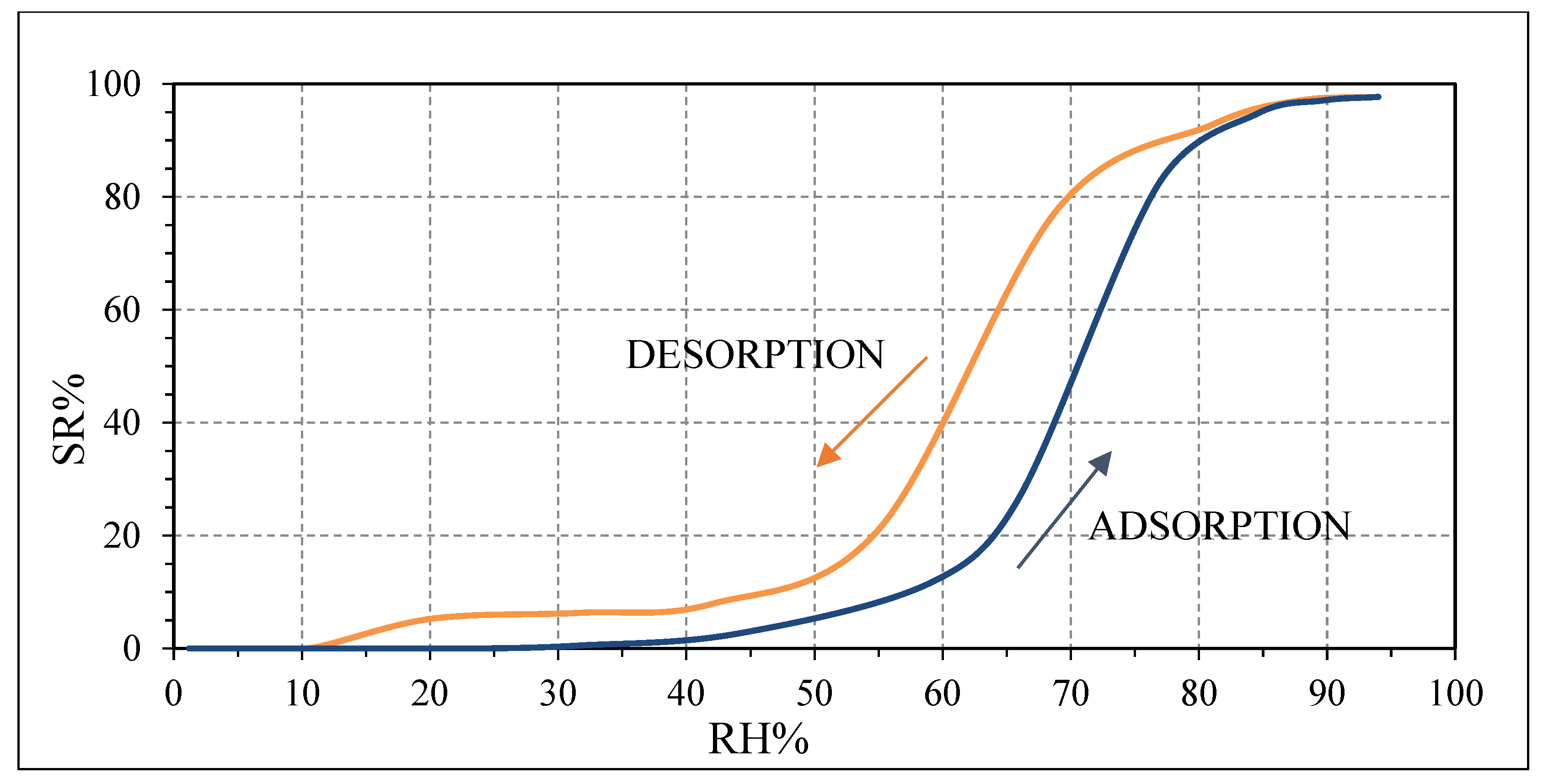
Figure 11 [
37,
38]. When increasing RH values, the water molecules on the surface of PVP provide more carriers (H
+ and H
3O
+) which mainly contribute to the conduction of PVP [
39]. Above 94 RH%, the pristine PVP can be swelled by water, generating many conductive ions [
40].
The decrease in impedance for the SWP700-10% PVP sensor compared to the SWP700 is probably due to the enhanced connection between biochar grains in the polymeric medium compared to biochar as it is. Moreover, when n-p heterojunctions are formed at nanoscale level between two semiconductors, i.e., PVP and biochar, electrons are transferred from n-type PVP to p-type biochar, while holes are moved in the opposite direction, to equate the Fermi levels of both materials [
41]. Then, a potential barrier with the bending of energy levels is formed at the heterojunctions. Thus, the impedance of the n-p composite increases, compared to the pure biochar [
41]. With increasing initial impedance, the variation of impedance due to the presence of few water molecules becomes more evident, leading to a higher sensitivity, as experimentally observed for the sensors SWP700-10% PVP and SWP700-20% PVP (
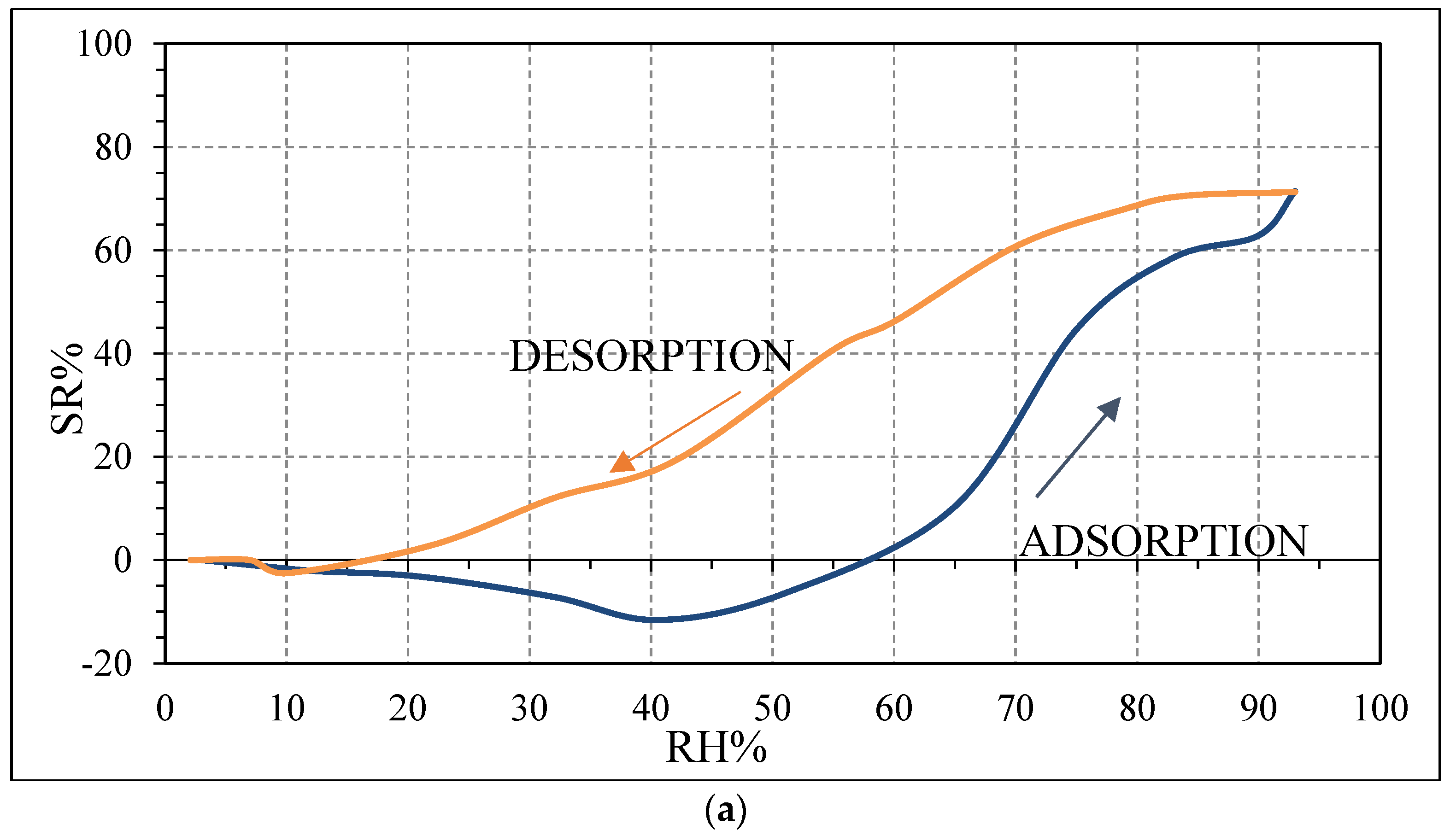
Figure 12a,b), compared to the sensor SWP700 (
Figure 10b). This larger initial impedance leads to a stronger initial response [
41].
Table 7 compares the results presented in this paper and literature data when possible because the initial resistance value under dry air is not always mentioned or determinable from experimental curves. The obtained results are rather promising.
Future prospective involves monitoring sensors’ response in function of the temperature, since a preliminary evaluation of carbonaceous materials change resistance values with temperature was performed only under dry air. Moreover, ageing phenomena will be investigated too, and verifying how their response is eventually affected by storage must be investigated.
5. Conclusions
In this work, two commercial biochars were evaluated as new humidity sensing materials.
The sensors were prepared by a simple and low-cost process, and their humidity sensing properties were investigated at room temperature in the relative humidity range from 0.0 to almost 100%. Both pristine materials are sensitive toward humidity starting from low RH values. In fact, impedance begins to increase from 5 RH%, and an increment in impedance was noticed until about 40 RH% for the OSR700 sensor, and until 25 RH% for the SWP700 sensor. In both cases, biochar materials behave as p-type semiconductors under low amounts of humidity. On the contrary, for higher RH values, the impedance decreased due to a higher amount of water molecules adsorption.
When PVP is added as a polymeric binder to SWP700 biochar, n-p heterojunctions are formed between the two semiconductors, leading to a higher sensitivity at low RH values for the sensors SWP700-10% PVP and SWP700-20% PVP with respect to the pure SWP700 sensor. This behavior makes these sensors potentially interesting for industrial applications where low RH values are required (drying of clothes (0–40 RH%), pharmaceuticals (20–40 RH%), cereal stocking (0–45 RH%), electronic parts, ceramic powders, humidity control in factories, dryers, dried foodstuffs (0–50 RH%), and film desiccation (0–30 RH%)…) [
34]. Thus, they are rather devoted to niche markets rather than the mass market.
Finally, the response and recovery times were reasonably fast (in the order of 1 min), and the cross-sensitivity to methane, carbon dioxide, ammonia, and ozone was negligible.