Recent Advances in the Detection of Neurotransmitters
Abstract
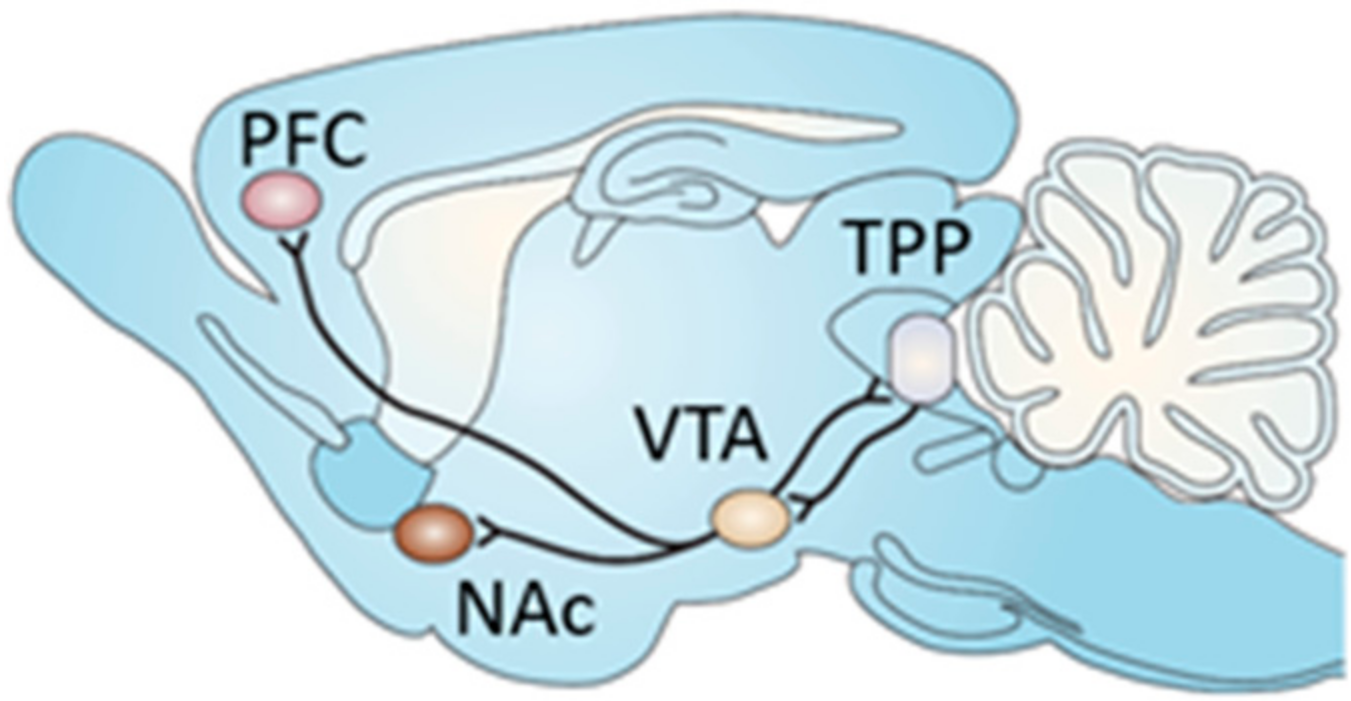
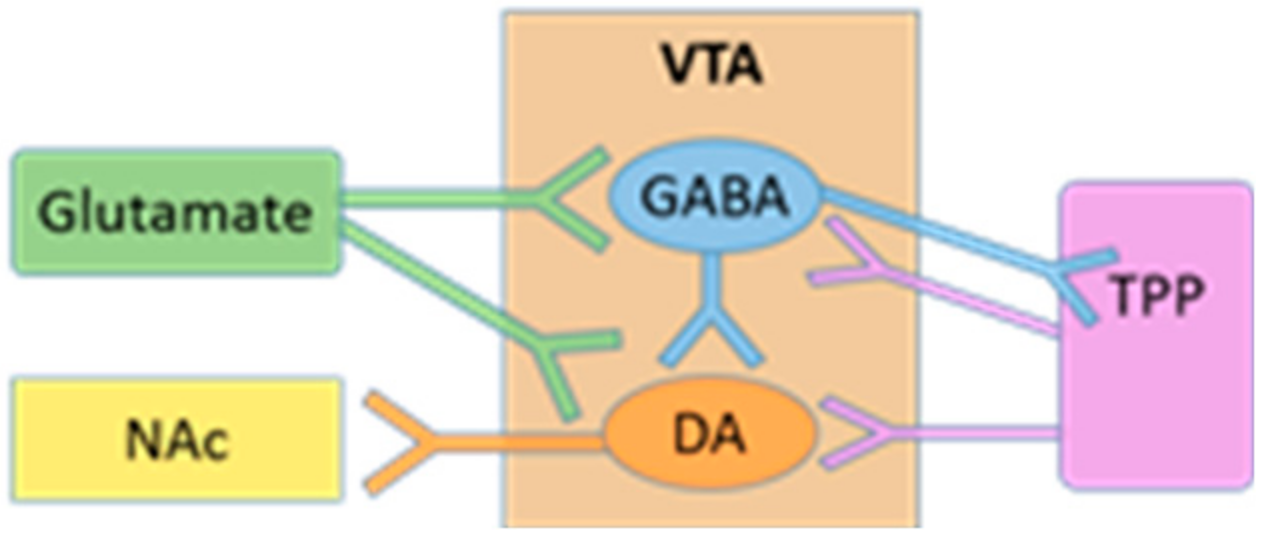
:1. Introduction
2. Materials for Biosensor Development
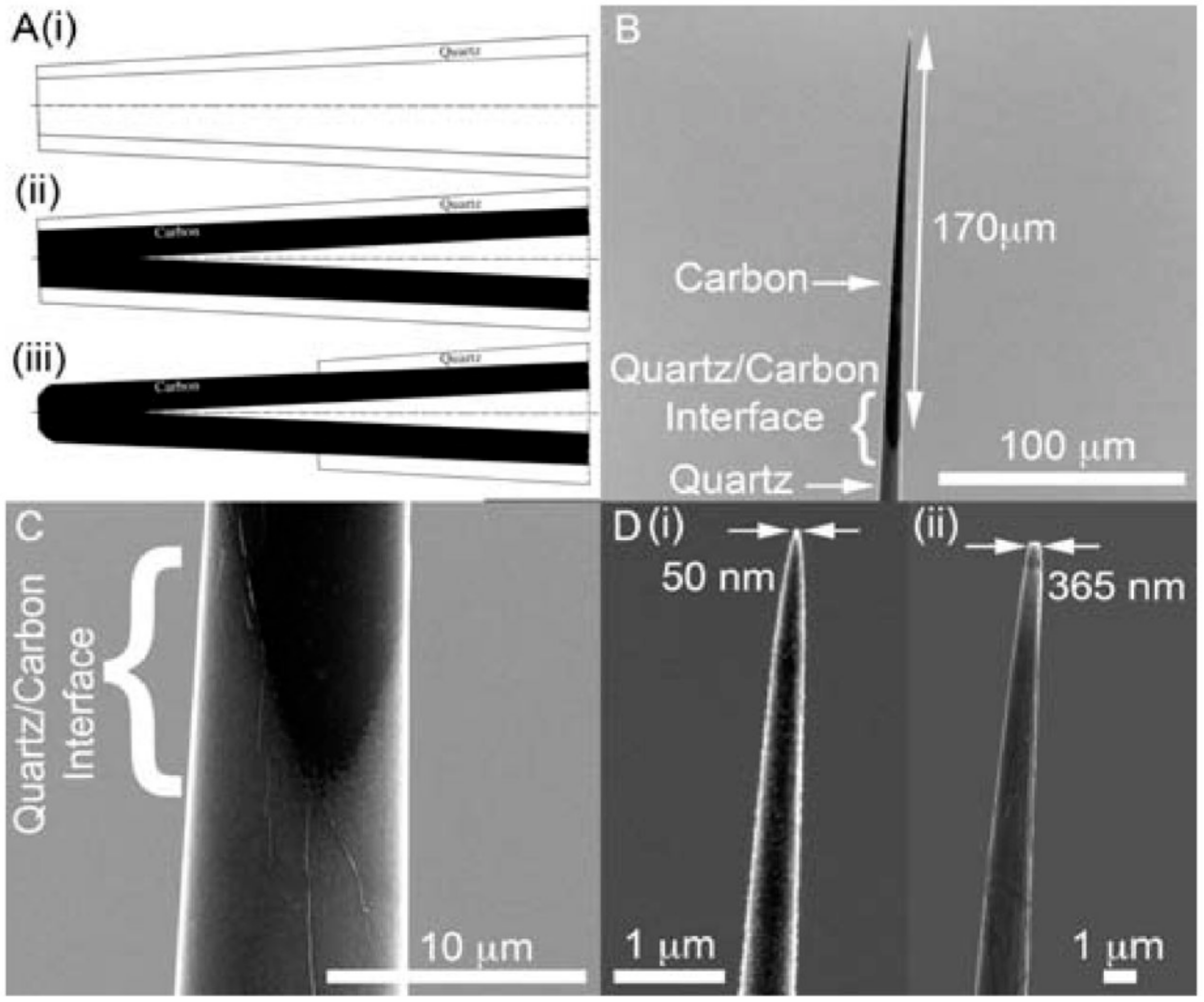
2.1. Carbon-Based Sensor
2.2. Polymer-Based Sensor
2.3. Aptamer-Based Sensor
2.4. Enzyme-Based Sensor
3. Sensor Modification
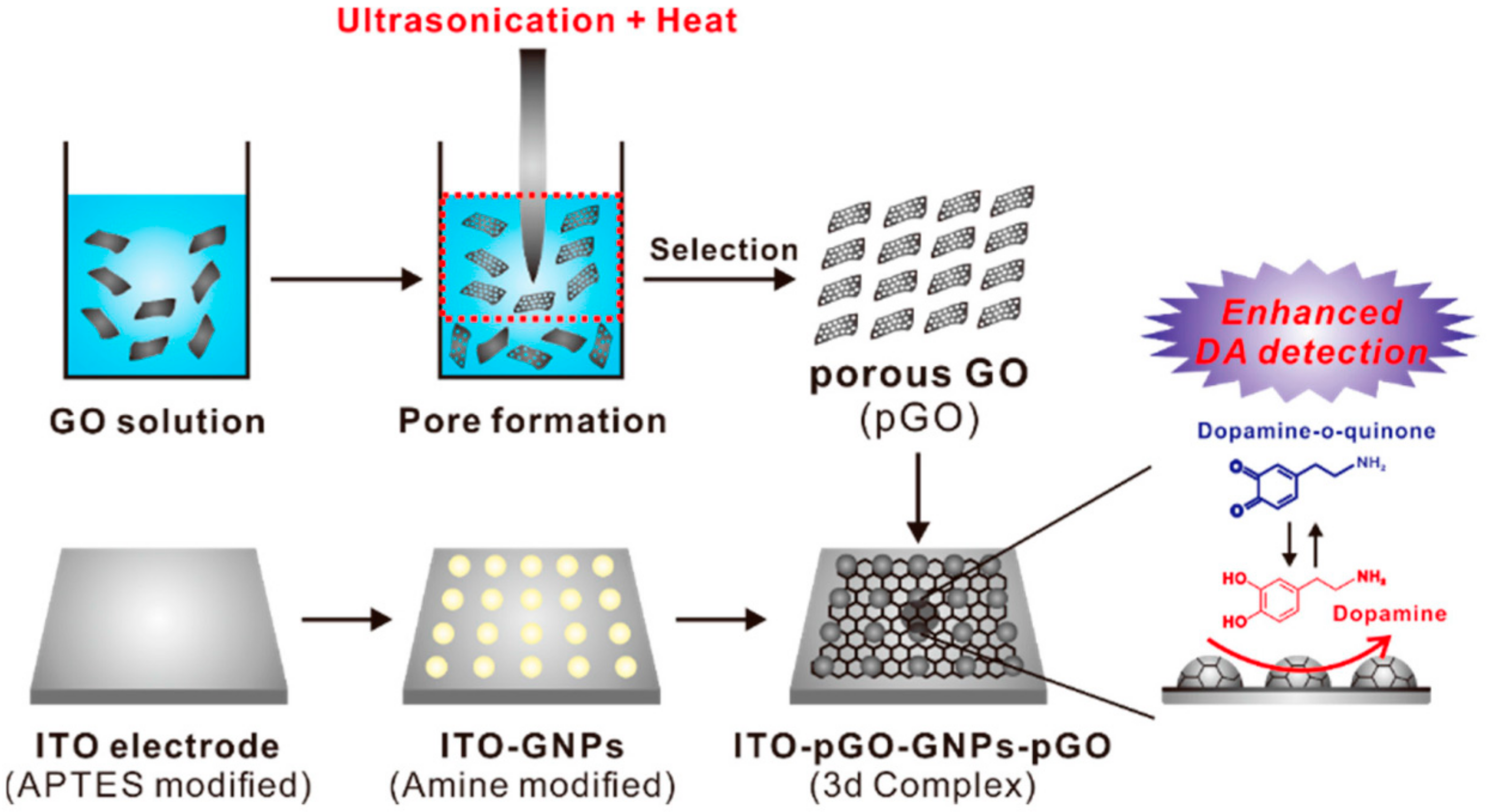
3.1. Nanostructure
3.2. Catalytic Nanoparticles
3.3. Molecular Imprinting Technique
4. Simultaneous Detection of Multiple Species
5. Optical Sensing of Neurotransmitters
6. Conclusions
Conflicts of Interest
References
- Pradhan, T.; Jung, H.S.; Jang, J.H.; Kim, T.W.; Kang, C.K.; Kim, J.S. Chemical sensing of neurotransmitters. Chem. Soc. Rev. 2014, 43, 4684–4713. [Google Scholar] [CrossRef] [PubMed]
- Jankovic, J. Parkinson’s disease: Clinical features and diagnosis. J. Neurol. Neurosurg. Psychiatry 2008, 79, 368–376. [Google Scholar] [CrossRef] [PubMed]
- Fearnley, J.M.; Lees, A.J. Ageing and Parkinson’s disease: Substantia nigra regional selectivity. Brain 1991, 114, 2283–2301. [Google Scholar] [CrossRef] [PubMed]
- Gröger, A.; Kolb, R.; Schäfer, R.; Klose, U. Dopamine reduction in the substantia nigra of Parkinson’s disease patients confirmed by in vivo magnetic resonance spectroscopic imaging. PLoS ONE 2014, 9, e84081. [Google Scholar] [CrossRef] [PubMed]
- Braak, H.; Tredici, K.D.; Rüb, U.; de Vos, R.A.I.; Steur, E.N.H.J.; Braak, E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging 2003, 24, 197–211. [Google Scholar] [CrossRef]
- Bezard, E.; Gross, C.E.; Brotchie, J.M. Presymptomatic compensation in Parkinson’s disease is not dopamine-mediated. Trends Neurosci. 2003, 26, 215–221. [Google Scholar] [CrossRef]
- Rodriguez-Oroz, M.C.; Jahanshahi, M.; Krack, P.; Litvan, I.; Macias, R.; Bezard, E.; Obeso, J.A. Initial clinical manifestations of Parkinson’s disease: Features and pathophysiological mechanisms. Lancet Neurol. 2009, 8, 1128–1139. [Google Scholar] [CrossRef]
- Aosaki, T.; Miura, M.; Suzuki, T.; Nishimura, K.; Masuda, M. Acetylcholine–dopamine balance hypothesis in the striatum: An update. Geriatr. Gerontol. Int. 2010, 10, S148–S157. [Google Scholar] [CrossRef] [PubMed]
- Calabresi, P.; Picconi, B.; Parnetti, L.; Di Filippo, M. A convergent model for cognitive dysfunctions in Parkinson’s disease: The critical dopamine–acetylcholine synaptic balance. Lancet Neurol. 2006, 5, 974–983. [Google Scholar] [CrossRef]
- DeBoer, P.; Heeringa, M.J.; Abercrombie, E.D. Spontaneous release of acetylcholine in striatum is preferentially regulated by inhibitory dopamine D2 receptors. Eur. J. Pharmacol. 1996, 317, 257–262. [Google Scholar] [CrossRef]
- Braak, H.; Ghebremedhin, E.; Rüb, U.; Bratzke, H.; Tredici, K.D. Stages in the development of Parkinson’s disease-related pathology. Cell Tissue Res. 2004, 318, 121–134. [Google Scholar] [CrossRef] [PubMed]
- Millan, M.J. The neurobiology and control of anxious states. Prog. Neurobiol. 2003, 70, 83–244. [Google Scholar] [CrossRef]
- Rocha, S.M.; Pires, J.; Esteves, M.; Graça, B.; Bernardino, L. Histamine: A new immunomodulatory player in the neuron-glia crosstalk. Front. Cell. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [PubMed]
- Rinne, J.O.; Anichtchik, O.V.; Eriksson, K.S.; Kaslin, J.; Tuomisto, L.; Kalimo, H.; Röyttä, M.; Panula, P. Increased brain histamine levels in Parkinson’s disease but not in multiple system atrophy. J. Neurochem. 2002, 81, 954–960. [Google Scholar] [CrossRef] [PubMed]
- Laviolette, S.R.; van der Kooy, D. The neurobiology of nicotine addiction: Bridging the gap from molecules to behaviour. Nat. Rev. Neurosci. 2004, 5, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Koob, G.F. Drugs of abuse: Anatomy, pharmacology and function of reward pathways. Trends Pharmacol. Sci. 1992, 13, 177–184. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Wang, H.-L.; Li, X.; Ng, T.H.; Morales, M. Mesocorticolimbic glutamatergic pathway. J. Neurosci. 2011, 31, 8476–8490. [Google Scholar] [CrossRef] [PubMed]
- Cameron, D.L.; Williams, J.T. Cocaine inhibits GABA release in the VTA through endogenous 5-HT. J. Neurosci. 1994, 14, 6763–6767. [Google Scholar] [PubMed]
- Nisell, M.; Nomikos, G.G.; Svensson, T.H. Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse 1994, 16, 36–44. [Google Scholar] [CrossRef] [PubMed]
- Nader, K.; van der Kooy, D. Deprivation state switches the neurobiological substrates mediating opiate reward in the ventral tegmental area. J. Neurosci. 1997, 17, 383–390. [Google Scholar] [PubMed]
- Venton, B.J.; Wightman, R.M. Psychoanalytical electrochemistry: Dopamine and behavior. Anal. Chem. 2003, 75, 414A–421A. [Google Scholar] [CrossRef]
- Perry, M.; Li, Q.; Kennedy, R.T. Review of recent advances in analytical techniques for the determination of neurotransmitters. Anal. Chim. Acta 2009, 653, 1–22. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, R.T.; Watson, C.J.; Haskins, W.E.; Powell, D.H.; Strecker, R.E. In vivo neurochemical monitoring by microdialysis and capillary separations. Curr. Opin. Chem. Biol. 2002, 6, 659–665. [Google Scholar] [CrossRef]
- Chuang, M.C.; Lai, H.Y.; Ho, J.A.A.; Chen, Y.Y. Multifunctional microelectrode array (mMEA) chip for neural-electrical and neural-chemical interfaces: Characterization of comb interdigitated electrode towards dopamine detection. Biosens. Bioelectron. 2013, 41, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Xu, B.; Tokranova, N.; Feng, X.; Castracane, J. Novel microfabricated device to measure hormone/neurotransmitter release with millisecond temporal resolution. Proc. SPIE Int. Soc. Opt. Eng. 2002, 4626, 378–382. [Google Scholar] [CrossRef]
- Vaisocherová, H. Functionalized ultra-low fouling surface platforms for biosensing in real-world media. In Bragg Gratings, Photosensitivity, and Poling in Glass Waveguides; Optical Society of America: Washington, DC, USA, 2014. [Google Scholar]
- Hanssen, B.L.; Siraj, S.; Wong, D.K.Y. Recent strategies to minimise fouling in electrochemical detection systems. Rev. Anal. Chem. 2016, 35, 1–28. [Google Scholar] [CrossRef]
- Rees, H.R.; Anderson, S.E.; Privman, E.; Bau, H.H.; Venton, B.J. Carbon nanopipette electrodes for dopamine detection in drosophila. Anal. Chem. 2015, 87, 3849–3855. [Google Scholar] [CrossRef] [PubMed]
- Zestos, A.G.; Yang, C.; Jacobs, C.B.; Hensley, D.; Jill Venton, B. Carbon nanospikes grown on metal wires as microelectrode sensors for dopamine. Analyst 2015, 140, 7283–7292. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.C.; Wang, X.; Zhu, Y.; Sombers, L.A. Carbon nanotube yarn electrodes for enhanced detection of neurotransmitter dynamics in live brain tissue. ACS Nano 2013, 7, 7864–7873. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Wang, Y.; Jacobs, C.B.; Ivanov, I.N.; Venton, B.J. O2 plasma etching and antistatic gun surface modifications for CNT yarn microelectrode improve sensitivity and antifouling properties. Anal. Chem. 2017, 89, 5605–5611. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Trikantzopoulos, E.; Nguyen, M.D.; Jacobs, C.B.; Wang, Y.; Mahjouri-Samani, M.; Ivanov, I.N.; Venton, B.J. Laser treated carbon nanotube yarn microelectrodes for rapid and sensitive detection of dopamine in vivo. ACS Sens. 2016, 1, 508–515. [Google Scholar] [CrossRef] [PubMed]
- Allen, M.J.; Tung, V.C.; Kaner, R.B. Honeycomb carbon: A review of graphene. Chem. Rev. 2010, 110, 132–145. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Du, D.; Yang, F.; Liang, Z.; Ning, Y.; Wang, H.; Zhang, G.-J. Preparation of graphene-modified acupuncture needle and its application in detecting neurotransmitters. Sci. Rep. 2015, 5, 11627. [Google Scholar] [CrossRef] [PubMed]
- Atta, N.F.; El-Ads, E.H.; Ahmed, Y.M.; Galal, A. Determination of some neurotransmitters at cyclodextrin/ionic liquid crystal/graphene composite electrode. Electrochim. Acta 2016, 199, 319–331. [Google Scholar] [CrossRef]
- Choo, S.-S.; Kang, E.-S.; Song, I.; Lee, D.; Choi, J.-W.; Kim, T.-H. Electrochemical detection of dopamine using 3D porous graphene oxide/gold nanoparticle composites. Sensors 2017, 17, 861. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Yue, R.; Ren, F.; Yao, Z.; Jiang, F.; Yang, P.; Du, Y. Novel graphene flowers modified carbon fibers for simultaneous determination of ascorbic acid, dopamine and uric acid. Biosens. Bioelectron. 2014, 53, 220–224. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Du, J.; Wang, H.; Zou, C.; Jiang, F.; Yang, P.; Du, Y. A facile electrochemical sensor based on reduced graphene oxide and Au nanoplates modified glassy carbon electrode for simultaneous detection of ascorbic acid, dopamine and uric acid. Sens. Actuators B Chem. 2014, 204, 302–309. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, H.; Hu, S.; Li, F.; Weng, W.; Chen, J.; Wang, Q.; He, Y.; Zhang, W.; Bao, X. A sensitive and reliable dopamine biosensor was developed based on the Au@carbon dots–chitosan composite film. Biosens. Bioelectron. 2014, 52, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.J.; Li, W. CTAB functionalized graphene oxide/multiwalled carbon nanotube composite modified electrode for the simultaneous determination of ascorbic acid, dopamine, uric acid and nitrite. Biosens. Bioelectron. 2014, 56, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Yuan, D.; Chen, S.; Yuan, R.; Zhang, J.; Liu, X. An ECL sensor for dopamine using reduced graphene oxide/multiwall carbon nanotubes/gold nanoparticles. Sens. Actuators B Chem. 2014, 191, 415–420. [Google Scholar] [CrossRef]
- Numan, A.; Shahid, M.M.; Omar, F.S.; Ramesh, K.; Ramesh, S. Facile fabrication of cobalt oxide nanograin-decorated reduced graphene oxide composite as ultrasensitive platform for dopamine detection. Sens. Actuators B Chem. 2017, 238, 1043–1051. [Google Scholar] [CrossRef]
- Al-Graiti, W.; Yue, Z.; Foroughi, J.; Huang, X.-F.; Wallace, G.; Baughman, R.; Chen, J. Probe sensor using nanostructured multi-walled carbon nanotube yarn for selective and sensitive detection of dopamine. Sensors 2017, 17, 884. [Google Scholar] [CrossRef] [PubMed]
- Dong, W.; Ren, Y.; Bai, Z.; Jiao, J.; Chen, Y.; Han, B.; Chen, Q. Synthesis of tetrahexahedral Au-Pd core-shell nanocrystals and reduction of graphene oxide for the electrochemical detection of epinephrine. J. Colloid Interface Sci. 2018, 512, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Mphuthi, N.G.; Adekunle, A.S.; Ebenso, E.E. Electrocatalytic oxidation of epinephrine and norepinephrine at metal oxide doped phthalocyanine/MWCNT composite sensor. Sci. Rep. 2016, 6, 26938. [Google Scholar] [CrossRef] [PubMed]
- Ding, M.; Zhou, Y.; Liang, X.; Zou, H.; Wang, Z.; Wang, M.; Ma, J. An electrochemical sensor based on graphene/poly(brilliant cresyl blue) nanocomposite for determination of epinephrine. J. Electroanal. Chem. 2016, 763, 25–31. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, S.; Tao, L.; Min, Q.; Xiang, J.; Wang, Q.; Xie, J.; Yue, Y.; Wu, S.; Li, X.; et al. A disposable electrochemical sensor for simultaneous determination of norepinephrine and serotonin in rat cerebrospinal fluid based on MWNTs-ZnO/chitosan composites modified screen-printed electrode. Biosens. Bioelectron. 2015, 65, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Mukdasai, S.; Langsi, V.; Pravda, M.; Srijaranai, S.; Glennon, J.D. A highly sensitive electrochemical determination of norepinephrine using l-cysteine self-assembled monolayers over gold nanoparticles/multi-walled carbon nanotubes electrode in the presence of sodium dodecyl sulfate. Sens. Actuators B Chem. 2016, 236, 126–135. [Google Scholar] [CrossRef]
- Moghaddam, H.M.; Beitollahi, H.; Tajik, S.; Maleh, H.K.; Noudeh, G.D. Simultaneous determination of norepinephrine, acetaminophen and tryptophan using a modified graphene nanosheets paste electrode. Res. Chem. Intermed. 2015, 41, 6885–6896. [Google Scholar] [CrossRef]
- Dalkıran, B.; Erden, P.E.; Kılıç, E. Graphene and tricobalt tetraoxide nanoparticles based biosensor for electrochemical glutamate sensing. Artif. Cells Nanomed. Biotechnol. 2017, 45, 340–348. [Google Scholar] [CrossRef] [PubMed]
- Thanh, T.D.; Balamurugan, J.; Hien, H.V.; Kim, N.H.; Lee, J.H. A novel sensitive sensor for serotonin based on high-quality of AuAg nanoalloy encapsulated graphene electrocatalyst. Biosens. Bioelectron. 2017, 96, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Sadanandhan, N.K.; Cheriyathuchenaaramvalli, M.; Devaki, S.J.; Ravindranatha Menon, A.R. PEDOT-reduced graphene oxide-silver hybrid nanocomposite modified transducer for the detection of serotonin. J. Electroanal. Chem. 2017, 794, 244–253. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Ebenso, E.E. Electrochemical determination of serotonin in urine samples based on metal oxide nanoparticles/MWCNT on modified glassy carbon electrode. Sens. Bio-Sens. Res. 2017, 13, 17–27. [Google Scholar] [CrossRef]
- Stojanović, Z.S.; Mehmeti, E.; Kalcher, K.; Guzsvány, V.; Stanković, D.M. SWCNT-modified carbon paste electrode as an electrochemical sensor for histamine determination in alcoholic beverages. Food Anal. Methods 2016, 9, 2701–2710. [Google Scholar] [CrossRef]
- Wang, L.; Chen, X.; Liu, C.; Yang, W. Non-enzymatic acetylcholine electrochemical biosensor based on flower-like NiAl layered double hydroxides decorated with carbon dots. Sens. Actuators B Chem. 2016, 233, 199–205. [Google Scholar] [CrossRef]
- Qian, J.; Yang, X.; Jiang, L.; Zhu, C.; Mao, H.; Wang, K. Facile preparation of Fe3O4 nanospheres/reduced graphene oxide nanocomposites with high peroxidase-like activity for sensitive and selective colorimetric detection of acetylcholine. Sens. Actuators B Chem. 2014, 201, 160–166. [Google Scholar] [CrossRef]
- Barsan, M.M.; Ghica, M.E.; Brett, C.M.A. Electrochemical sensors and biosensors based on redox polymer/carbon nanotube modified electrodes: A review. Anal. Chim. Acta 2015, 881, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Li, H.; Xue, X.; Fan, H.; Xin, Q.; Wei, Q. Sensitive and selective determination of dopamine by electrochemical sensor based on molecularly imprinted electropolymerization of o-phenylenediamine. Anal. Methods 2013, 5, 1469–1473. [Google Scholar] [CrossRef]
- Chauhan, N.; Chawla, S.; Pundir, C.S.; Jain, U. An electrochemical sensor for detection of neurotransmitter-acetylcholine using metal nanoparticles, 2D material and conducting polymer modified electrode. Biosens. Bioelectron. 2017, 89, 377–383. [Google Scholar] [CrossRef] [PubMed]
- Sangamithirai, D.; Munusamy, S.; Narayanan, V.; Stephen, A. Fabrication of neurotransmitter dopamine electrochemical sensor based on poly(o-anisidine)/CNTs nanocomposite. Surf. Interfaces 2016, 4, 27–34. [Google Scholar] [CrossRef]
- Tseng, T.T.-C.; Monbouquette, H.G. Implantable microprobe with arrayed microsensors for combined amperometric monitoring of the neurotransmitters, glutamate and dopamine. J. Electroanal. Chem. 2012, 682, 141–146. [Google Scholar] [CrossRef] [PubMed]
- Rui, Z.; Huang, W.; Chen, Y.; Zhang, K.; Cao, Y.; Tu, J. Facile synthesis of graphene/polypyrrole 3D composite for a high-sensitivity non-enzymatic dopamine detection. J. Appl. Polym. Sci. 2017, 134. [Google Scholar] [CrossRef]
- Li, X.; Lu, X.; Kan, X. 3D electrochemical sensor based on poly(hydroquinone)/gold nanoparticles/nickel foam for dopamine sensitive detection. J. Electroanal. Chem. 2017, 799, 451–458. [Google Scholar] [CrossRef]
- Khudaish, E.A.; Al-Nofli, F.; Rather, J.A.; Al-Hinaai, M.; Laxman, K.; Kyaw, H.H.; Al-Harthy, S. Sensitive and selective dopamine sensor based on novel conjugated polymer decorated with gold nanoparticles. J. Electroanal. Chem. 2016, 761, 80–88. [Google Scholar] [CrossRef]
- Mao, H.; Zhang, H.; Jiang, W.; Liang, J.; Sun, Y.; Zhang, Y.; Wu, Q.; Zhang, G.; Song, X.-M. Poly(ionic liquid) functionalized polypyrrole nanotubes supported gold nanoparticles: An efficient electrochemical sensor to detect epinephrine. Mater. Sci. Eng. C 2017, 75, 495–502. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Huang, H.; Zeng, Y.; Tang, H.; Li, L. A novel composite of molecularly imprinted polymer-coated PdNPs for electrochemical sensing norepinephrine. Biosens. Bioelectron. 2015, 65, 366–374. [Google Scholar] [CrossRef] [PubMed]
- Ganesh, P.S.; Swamy, B.E.K. Simultaneous electroanalysis of norepinephrine, ascorbic acid and uric acid using poly(glutamic acid) modified carbon paste electrode. J. Electroanal. Chem. 2015, 752, 17–24. [Google Scholar] [CrossRef]
- Batra, B.; Kumari, S.; Pundir, C.S. Construction of glutamate biosensor based on covalent immobilization of glutmate oxidase on polypyrrole nanoparticles/polyaniline modified gold electrode. Enzyme Microb. Technol. 2014, 57, 69–77. [Google Scholar] [CrossRef] [PubMed]
- Batra, B.; Yadav, M.; Pundir, C.S. l-Glutamate biosensor based on l-glutamate oxidase immobilized onto ZnO nanorods/polypyrrole modified pencil graphite electrode. Biochem. Eng. J. 2016, 105, 428–436. [Google Scholar] [CrossRef]
- Ran, G.; Chen, X.; Xia, Y. Electrochemical detection of serotonin based on a poly(bromocresol green) film and Fe3O4 nanoparticles in a chitosan matrix. RSC Adv. 2017, 7, 1847–1851. [Google Scholar] [CrossRef]
- Tertiș, M.; Cernat, A.; Lacatiș, D.; Florea, A.; Bogdan, D.; Suciu, M.; Săndulescu, R.; Cristea, C. Highly selective electrochemical detection of serotonin on polypyrrole and gold nanoparticles-based 3D architecture. Electrochem. Commun. 2017, 75, 43–47. [Google Scholar] [CrossRef]
- Akhoundian, M.; Rüter, A.; Shinde, S. Ultratrace detection of histamine using a molecularly-imprinted polymer-based voltammetric sensor. Sensors 2017, 17, 645. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Guo, W.; Qin, X.; Liu, Y.; Zhu, X.; Pei, M.; Wang, L. A molecularly imprinted electrochemical sensor based on gold nanoparticles and multiwalled carbon nanotube–chitosan for the detection of tryptamine. RSC Adv. 2014, 4, 38649–38654. [Google Scholar] [CrossRef]
- Liu, J.; Wagan, S.; Dávila Morris, M.; Taylor, J.; White, R.J. Achieving reproducible performance of electrochemical, folding aptamer-based sensors on microelectrodes: Challenges and prospects. Anal. Chem. 2014, 86, 11417–11424. [Google Scholar] [CrossRef] [PubMed]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef] [PubMed]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef] [PubMed]
- Lakhin, A.V.; Tarantul, V.Z.; Gening, L.V. Aptamers: Problems, solutions and prospects. Acta Naturae 2013, 5, 34–43. [Google Scholar] [PubMed]
- Baker, B.R.; Lai, R.Y.; Wood, M.S.; Doctor, E.H.; Heeger, A.J.; Plaxco, K.W. An electronic, aptamer-based small-molecule sensor for the rapid, label-free detection of cocaine in adulterated samples and biological fluids. J. Am. Chem. Soc. 2006, 128, 3138–3139. [Google Scholar] [CrossRef] [PubMed]
- Chávez, J.L.; Hagen, J.A.; Kelley-Loughnane, N. Fast and selective plasmonic serotonin detection with aptamer-gold nanoparticle conjugates. Sensors 2017, 17, 681. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Hun, X.; Liu, F.; Wen, X.; Luo, X. Aptamer biosensor for dopamine based on a gold electrode modified with carbon nanoparticles and thionine labeled gold nanoparticles as probe. Microchim. Acta 2015, 182, 1797–1802. [Google Scholar] [CrossRef]
- Azadbakht, A.; Roushani, M.; Abbasi, A.R.; Derikvand, Z. Design and characterization of electrochemical dopamine–aptamer as convenient and integrated sensing platform. Anal. Biochem. 2016, 507, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Xia, N.; Meng, J.-J.; Zhou, B.-B.; Li, S.-J. An electrochemical aptasensor for sensitive and selective detection of dopamine based on signal amplification of electrochemical-chemical redox cycling. J. Electroanal. Chem. 2016, 775, 58–63. [Google Scholar] [CrossRef]
- Bahrami, S.; Abbasi, A.R.; Roushani, M.; Derikvand, Z.; Azadbakht, A. An electrochemical dopamine aptasensor incorporating silver nanoparticle, functionalized carbon nanotubes and graphene oxide for signal amplification. Talanta 2016, 159, 307–316. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Wang, W.; Davis, J.J.; Luo, X. Ultrasensitive and selective voltammetric aptasensor for dopamine based on a conducting polymer nanocomposite doped with graphene oxide. Microchim. Acta 2015, 182, 1123–1129. [Google Scholar] [CrossRef]
- Saraf, N.; Bosak, A.; Willenberg, A.; Das, S.; Willenberg, B.J.; Seal, S. Colorimetric detection of epinephrine using an optimized paper-based aptasensor. RSC Adv. 2017, 7, 49133–49143. [Google Scholar] [CrossRef]
- Li, H.; Dauphin-Ducharme, P.; Arroyo-Currás, N.; Tran, C.H.; Vieira, P.A.; Li, S.; Shin, C.; Somerson, J.; Kippin, T.E.; Plaxco, K.W. A biomimetic phosphatidylcholine-terminated monolayer greatly improves the in vivo performance of electrochemical aptamer-based sensors. Angew. Chem. 2017, 129, 7492–7495. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.; Yu, P.; Mao, L. A multi-enzyme microreactor-based online electrochemical system for selective and continuous monitoring of acetylcholine. Analyst 2015, 140, 3781–3787. [Google Scholar] [CrossRef] [PubMed]
- Okon, S.L.; Ronkainen, N.J. Enzyme-based electrochemical glutamate biosensors. In Electrochemical Sensors Technology; InTech: Rijeka, Croatia, 2017. [Google Scholar] [CrossRef]
- Wassum, K.; Tolosa, V.; Tseng, T.; Balleine, B.; Monbouquette, H.; Maidment, N. Transient extracellular glutamate events in the basolateral amygdala track reward seeking actions. J. Neurosci. Off. J. Soc. Neurosci. 2012, 32, 2734–2746. [Google Scholar] [CrossRef] [PubMed]
- Burmeister, J.J.; Pomerleau, F.; Huettl, P.; Gash, C.R.; Werner, C.E.; Bruno, J.P.; Gerhardt, G.A. Ceramic-based multisite microelectrode arrays for simultaneous measures of choline and acetylcholine in CNS. Biosens. Bioelectron. 2008, 23, 1382–1389. [Google Scholar] [CrossRef] [PubMed]
- Tolosa, V.M.; Wassum, K.M.; Maidment, N.T.; Monbouquette, H.G. Electrochemically deposited iridium oxide reference electrode integrated with an electroenzymatic glutamate sensor on a multi-electrode array microprobe. Biosens. Bioelectron. 2013, 42, 256–260. [Google Scholar] [CrossRef] [PubMed]
- Tseng, T.T.; Chang, C.F.; Chan, W.C. Fabrication of implantable, enzyme-immobilized glutamate sensors for the monitoring of glutamate concentration changes in vitro and in vivo. Molecules 2014, 19, 7341–7355. [Google Scholar] [CrossRef] [PubMed]
- Xiao, G.; Song, Y.; Zhang, S.; Yang, L.; Xu, S.; Zhang, Y.; Xu, H.; Gao, F.; Li, Z.; Cai, X. A high-sensitive nano-modified biosensor for dynamic monitoring of glutamate and neural spike covariation from rat cortex to hippocampal sub-regions. J. Neurosci. Methods 2017, 291, 122–130. [Google Scholar] [CrossRef] [PubMed]
- Deng, Y.; Wang, W.; Ma, C.; Li, Z. Fabrication of an electrochemical biosensor array for simultaneous detection of l-glutamate and acetylcholine. J. Biomed. Nanotechnol. 2013, 9, 1378–1382. [Google Scholar] [CrossRef] [PubMed]
- Joshi, S.; Pellacani, P.; van Beek, T.A.; Zuilhof, H.; Nielen, M.W.F. Surface characterization and antifouling properties of nanostructured gold chips for imaging surface plasmon resonance biosensing. Sens. Actuators B Chem. 2015, 209, 505–514. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Yang, C.; Trikantzopoulos, E.; Jacobs, C.B.; Venton, B.J. Evaluation of carbon nanotube fiber microelectrodes for neurotransmitter detection: Correlation of electrochemical performance and surface properties. Anal. Chim. Acta 2017, 965, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-H.; Wang, H.-H.; Li, W.-T.; Fang, X.-X.; Guo, X.-C.; Zhou, W.-H.; Cao, X.; Kou, D.-X.; Zhou, Z.-J.; Wu, S.-X. A novel electrochemical sensor for epinephrine based on three dimensional molecularly imprinted polymer arrays. Sens. Actuators B Chem. 2016, 222, 1127–1133. [Google Scholar] [CrossRef]
- Song, E.; Choi, J.-W. A selective hydrogen peroxide sensor based on chemiresistive polyaniline nanowires modified with silver catalytic nanoparticles. J. Micromech. Microeng. 2014, 24, 065004. [Google Scholar] [CrossRef]
- Li, W.; Li, D.; Xiao, H.; He, B. Facile preparation of gold nanoparticles-decorated poly(o-phenylenediamine) hollow microspheres and their application for the detection of dopamine. High Perform. Polym. 2016, 28, 993–1002. [Google Scholar] [CrossRef]
- Manica, D.P.; Mitsumori, Y.; Ewing, A.G. Characterization of electrode fouling and surface regeneration for a platinum electrode on an electrophoresis microchip. Anal. Chem. 2003, 75, 4572–4577. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Miao, Y.-E.; Ji, S.; Tjiu, W.W.; Liu, T. Electrospun carbon nanofibers decorated with Ag–Pt bimetallic nanoparticles for selective detection of dopamine. ACS Appl. Mater. Interfaces 2014, 6, 12449–12456. [Google Scholar] [CrossRef] [PubMed]
- Song, E.; Choi, J.-W. Multi-analyte detection of chemical species using a conducting polymer nanowire-based sensor array platform. Sens. Actuators B Chem. 2015, 215, 99–106. [Google Scholar] [CrossRef]
- Gao, G.; Zhang, Z.; Wang, K.; Yuan, Q.; Wang, X. One-pot synthesis of dendritic Pt 3 Ni nanoalloys as nonenzymatic electrochemical biosensors with high sensitivity and selectivity for dopamine detection. Nanoscale 2017, 9, 10998–11003. [Google Scholar] [CrossRef] [PubMed]
- Jiang, G.; Gu, X.; Jiang, G.; Chen, T.; Zhan, W.; Tian, S. Application of a mercapto-terminated binuclear Cu(II) complex modified Au electrode to improve the sensitivity and selectivity for dopamine detection. Sens. Actuators B Chem. 2015, 209, 122–130. [Google Scholar] [CrossRef]
- Salamon, J.; Sathishkumar, Y.; Ramachandran, K.; Lee, Y.S.; Yoo, D.J.; Kim, A.R.; Gnana kumar, G. One-pot synthesis of magnetite nanorods/graphene composites and its catalytic activity toward electrochemical detection of dopamine. Biosens. Bioelectron. 2015, 64, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Raj, D.R.; Prasanth, S.; Vineeshkumar, T.V.; Sudarsanakumar, C. Surface plasmon resonance based fiber optic dopamine sensor using green synthesized silver nanoparticles. Sens. Actuators B Chem. 2016, 224, 600–606. [Google Scholar] [CrossRef]
- Baraneedharan, P.; Alexander, S.; Ramaprabhu, S. One-step in situ hydrothermal preparation of graphene–SnO2 nanohybrid for superior dopamine detection. J. Appl. Electrochem. 2016, 46, 1187–1197. [Google Scholar] [CrossRef]
- Karim-Nezhad, G.; Khorablou, Z. Selective analysis of epinephrine in the presence of uric acid by using an amplified electrochemical sensor employing a gold nanoparticle decorated cysteic acid film. Anal. Methods 2017. [Google Scholar] [CrossRef]
- Lavanya, N.; Sekar, C. Electrochemical sensor for simultaneous determination of epinephrine and norepinephrine based on cetyltrimethylammonium bromide assisted SnO2 nanoparticles. J. Electroanal. Chem. 2017, 801, 503–510. [Google Scholar] [CrossRef]
- Anithaa, A.C.; Asokan, K.; Sekar, C. Highly sensitive and selective serotonin sensor based on gamma ray irradiated tungsten trioxide nanoparticles. Sens. Actuators B Chem. 2017, 238, 667–675. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Chen, C.-H.; Lin, M.S. Enzyme-free amperometric method for rapid determination of histamine by using surface oxide regeneration behavior of copper electrode. Sens. Actuators B Chem. 2018, 255, 2838–2843. [Google Scholar] [CrossRef]
- Sattarahmady, N.; Heli, H.; Vais, R.D. An electrochemical acetylcholine sensor based on lichen-like nickel oxide nanostructure. Biosens. Bioelectron. 2013, 48, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Zhao, J.; Wei, X. A sensitive and selective sensor for dopamine determination based on a molecularly imprinted electropolymer of o-aminophenol. Sens. Actuators B Chem. 2009, 140, 663–669. [Google Scholar] [CrossRef]
- Tadi, K.K.; Motghare, R.V.; Ganesh, V. Electrochemical detection of epinephrine using a biomimic made up of hemin modified molecularly imprinted microspheres. RSC Adv. 2015, 5, 99115–99124. [Google Scholar] [CrossRef]
- Sacramento, A.S.; Moreira, F.T.C.; Guerreiro, J.L.; Tavares, A.P.; Sales, M.G.F. Novel biomimetic composite material for potentiometric screening of acetylcholine, a neurotransmitter in Alzheimer’s disease. Mater. Sci. Eng. C 2017, 79, 541–549. [Google Scholar] [CrossRef] [PubMed]
- Vandenryt, T.; van Grinsven, B.; Eersels, K.; Cornelis, P.; Kholwadia, S.; Cleij, T.J.; Thoelen, R.; De Ceuninck, W.; Peeters, M.; Wagner, P. Single-shot detection of neurotransmitters in whole-blood samples by means of the heat-transfer method in combination with synthetic receptors. Sensors 2017, 17, 2701. [Google Scholar] [CrossRef] [PubMed]
- Qian, T.; Yu, C.; Zhou, X.; Ma, P.; Wu, S.; Xu, L.; Shen, J. Ultrasensitive dopamine sensor based on novel molecularly imprinted polypyrrole coated carbon nanotubes. Biosens. Bioelectron. 2014, 58, 237–241. [Google Scholar] [CrossRef] [PubMed]
- Teng, Y.; Liu, F.; Kan, X. Voltammetric dopamine sensor based on three-dimensional electrosynthesized molecularly imprinted polymers and polypyrrole nanowires. Microchim. Acta 2017, 184, 2515–2522. [Google Scholar] [CrossRef]
- Li, B.; Zhou, Y.; Wu, W.; Liu, M.; Mei, S.; Zhou, Y.; Jing, T. Highly selective and sensitive determination of dopamine by the novel molecularly imprinted poly(nicotinamide)/CuO nanoparticles modified electrode. Biosens. Bioelectron. 2015, 67, 121–128. [Google Scholar] [CrossRef] [PubMed]
- Pakchin, P.S.; Nakhjavani, S.A.; Saber, R.; Ghanbari, H.; Omidi, Y. Recent advances in simultaneous electrochemical multi-analyte sensing platforms. TrAC Trends Anal. Chem. 2017, 92, 32–41. [Google Scholar] [CrossRef]
- Van Schoors, J.; Viaene, J.; Van Wanseele, Y.; Smolders, I.; Dejaegher, B.; Vander Heyden, Y.; Van Eeckhaut, A. An improved microbore UHPLC method with electrochemical detection for the simultaneous determination of low monoamine levels in in vivo brain microdialysis samples. J. Pharm. Biomed. Anal. 2016, 127, 136–146. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Lei, W.; Xu, Y.; Xia, X.; Hao, Q. Simultaneous detection of dopamine and uric acid using a poly(l-lysine)/graphene oxide modified electrode. Nanomaterials 2016, 6. [Google Scholar] [CrossRef] [PubMed]
- Sun, H.; Chao, J.; Zuo, X.; Su, S.; Liu, X.; Yuwen, L.; Fan, C.; Wang, L. Gold nanoparticle-decorated MoS2 nanosheets for simultaneous detection of ascorbic acid, dopamine and uric acid. RSC Adv. 2014, 4, 27625–27629. [Google Scholar] [CrossRef]
- Jafarinejad, S.; Ghazi-Khansari, M.; Ghasemi, F.; Sasanpour, P.; Hormozi-Nezhad, M.R. Colorimetric fingerprints of gold nanorods for discriminating catecholamine neurotransmitters in urine samples. Sci. Rep. 2017, 7, 8266. [Google Scholar] [CrossRef] [PubMed]
- Wojnicz, A.; Ortiz, J.A.; Casas, A.I.; Freitas, A.E.; López, G.M.; Ruiz-Nuño, A. Simultaneous determination of 8 neurotransmitters and their metabolite levels in rat brain using liquid chromatography in tandem with mass spectrometry: Application to the murine Nrf2 model of depression. Clin. Chim. Acta 2016, 453, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Fang, C.; Smagin, G. Derivatization for the simultaneous LC/MS quantification of multiple neurotransmitters in extracellular fluid from rat brain microdialysis. J. Pharm. Biomed. Anal. 2014, 100, 357–364. [Google Scholar] [CrossRef] [PubMed]
- Barman, K.; Jasimuddin, S. Simultaneous electrochemical detection of dopamine and epinephrine in the presence of ascorbic acid and uric acid using a AgNPs–penicillamine–Au electrode. RSC Adv. 2016, 6, 99983–99988. [Google Scholar] [CrossRef]
- Tezerjani, M.D.; Benvidi, A.; Dehghani Firouzabadi, A.; Mazloum-Ardakani, M.; Akbari, A. Epinephrine electrochemical sensor based on a carbon paste electrode modified with hydroquinone derivative and graphene oxide nano-sheets: Simultaneous determination of epinephrine, acetaminophen and dopamine. Measurement 2017, 101, 183–189. [Google Scholar] [CrossRef]
- Soleymani, J. Advanced materials for optical sensing and biosensing of neurotransmitters. Trac. Trends Anal. Chem. 2015, 72, 27–44. [Google Scholar] [CrossRef]
- Polo, E.; Kruss, S. Nanosensors for neurotransmitters. Anal. Bioanal. Chem. 2016, 408, 2727–2741. [Google Scholar] [CrossRef] [PubMed]
- Kruss, S.; Hilmer, A.J.; Zhang, J.; Reuel, N.F.; Mu, B.; Strano, M.S. Carbon nanotubes as optical biomedical sensors. Adv. Drug Deliv. Rev. 2013, 65, 1933–1950. [Google Scholar] [CrossRef] [PubMed]
- Kruss, S.; Landry, M.P.; Vander Ende, E.; Lima, B.M.A.; Reuel, N.F.; Zhang, J.; Nelson, J.; Mu, B.; Hilmer, A.; Strano, M. Neurotransmitter detection using corona phase molecular recognition on fluorescent single-walled carbon nanotube sensors. J. Am. Chem. Soc. 2014, 136, 713–724. [Google Scholar] [CrossRef] [PubMed]
- Kruss, S.; Salem, D.P.; Vuković, L.; Lima, B.; Ende, E.V.; Boyden, E.S.; Strano, M.S. High-resolution imaging of cellular dopamine efflux using a fluorescent nanosensor array. Proc. Natl. Acad. Sci. USA 2017, 114, 1789–1794. [Google Scholar] [CrossRef] [PubMed]
- López-Valenzuela, C.L.; Morales-Villagrán, A.; Medina-Ceja, L. A novel method for simultaneous glutamate and extracellular activity measurement in brain slices with high temporal resolution. Talanta 2015, 144, 1231–1238. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.H.; Yoon, H.; Choi, S.H.; Zhao, F.; Kim, J.; Song, K.D.; Lee, U. Miniaturized and wireless optical neurotransmitter sensor for real-time monitoring of dopamine in the brain. Sensors 2016, 16, 1894. [Google Scholar] [CrossRef] [PubMed]
- Baluta, S.; Cabaj, J.; Malecha, K. Neurotransmitters detection using a fluorescence-based sensor with graphene quantum dots. Opt. Appl. 2017, 47. [Google Scholar] [CrossRef]
- Huang, Y.; Ding, M.; Guo, T.; Hu, D.; Cao, Y.; Jin, L.; Guan, B.O. A fiber-optic sensor for neurotransmitters with ultralow concentration: near-infrared plasmonic electromagnetic field enhancement using raspberry-like meso-SiO2 nanospheres. Nanoscale 2017, 9, 14929. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Nandi, C.K. PC12 live cell ultrasensitive neurotransmitter signaling using high quantum yield sulphur doped carbon dots and its extracellular Ca2+ ion dependence. Sens. Actuators B Chem. 2017, 245, 137–145. [Google Scholar] [CrossRef]
- Guan, B.O.; Sun, L.P.; Ding, M.; Guo, T.; Huang, Y. Mesoporous nanospheres functionalized optical microfiber biosensor for low concentration neurotransmitter detection. Opt. Express 2016, 24, 27152. [Google Scholar] [CrossRef]

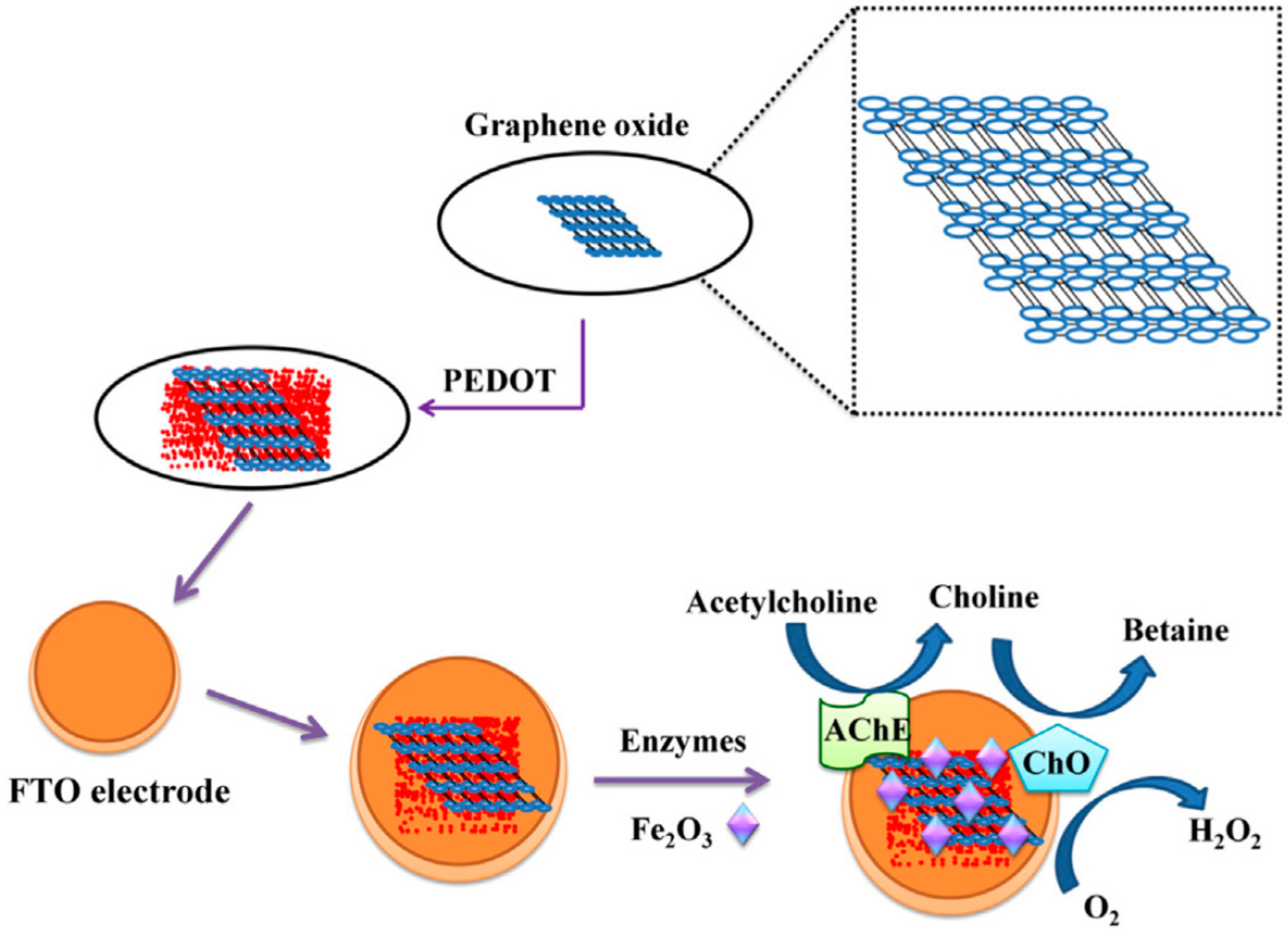
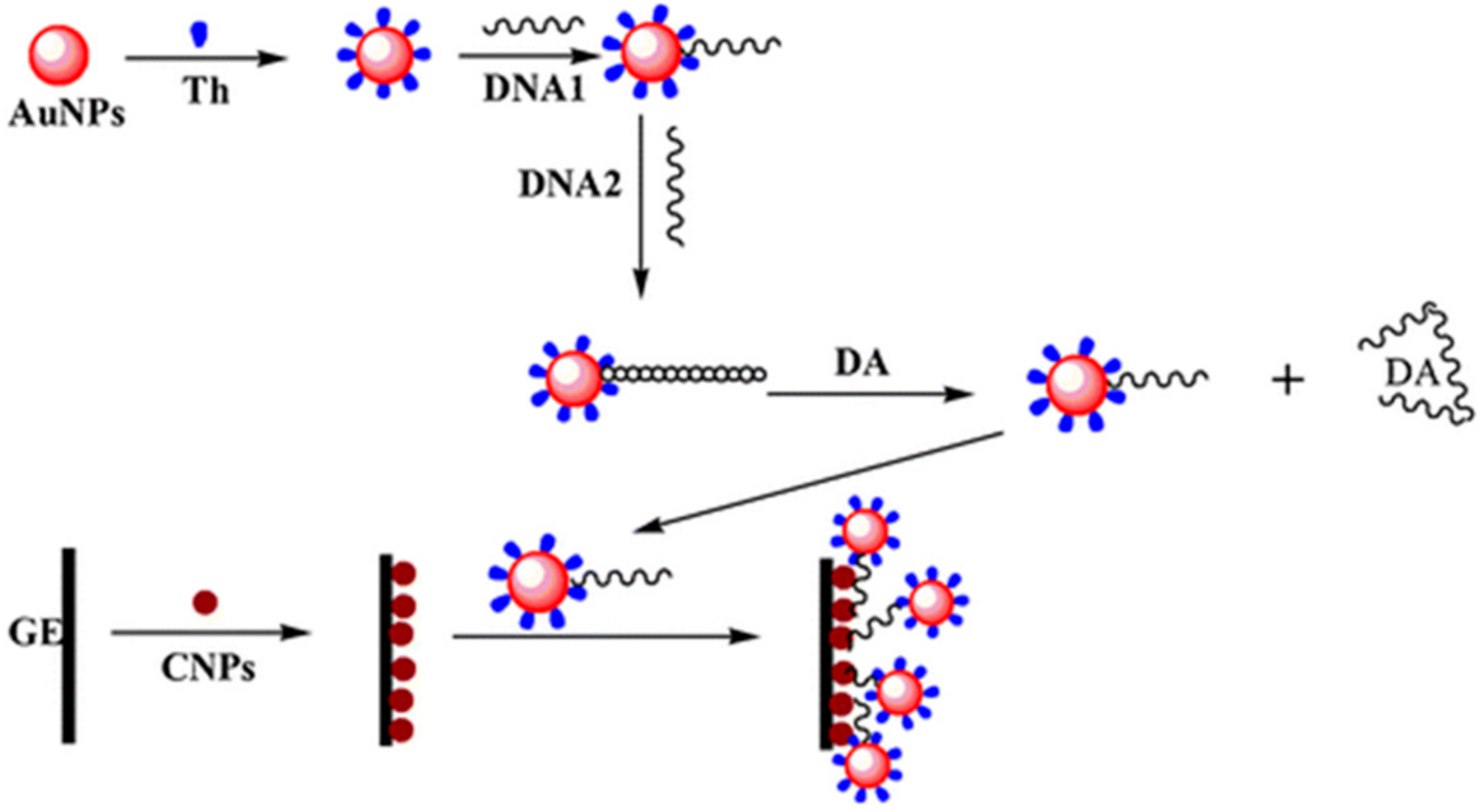
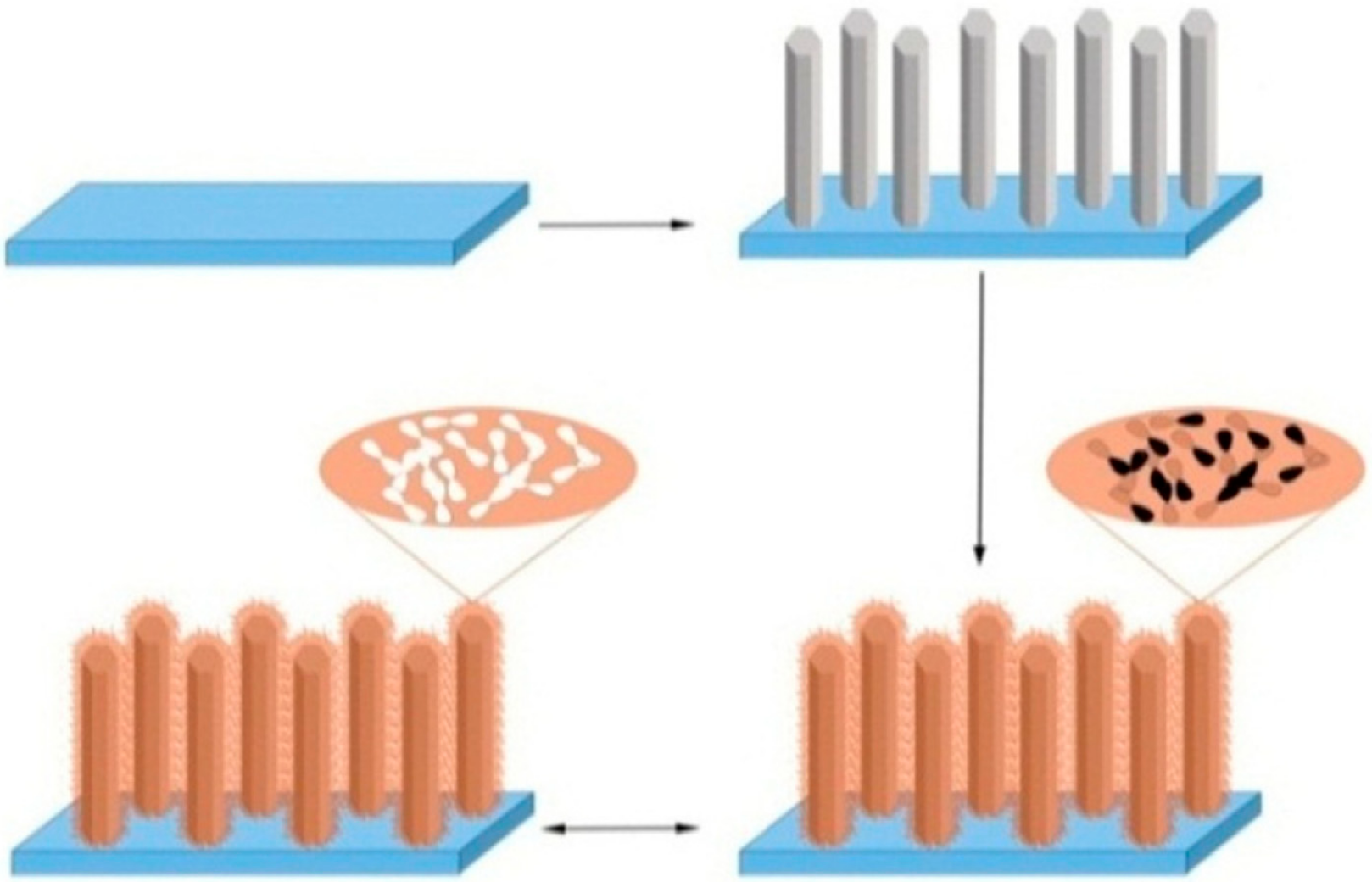
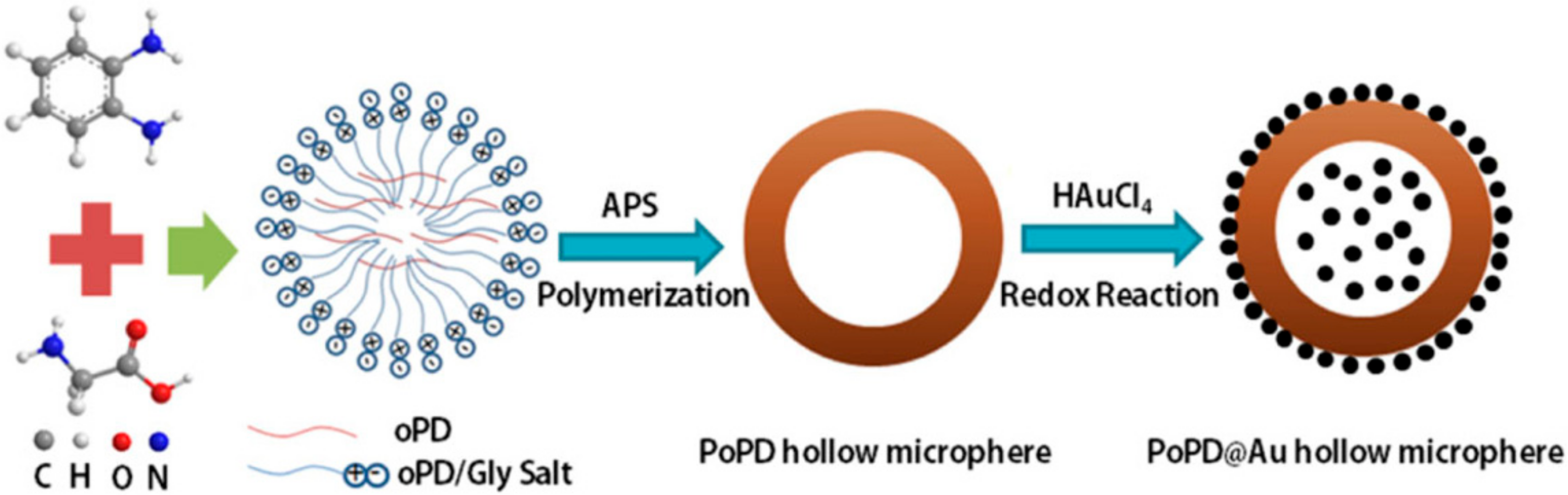
| Material | Analyte | Measurement | LOD | Detection Range | References |
|---|---|---|---|---|---|
| Graphene, Carbon fiber | DA | DPV | 1.36 μM | 1.36–125.69 μM | [37] |
| Reduced Graphene oxide | DA | DPV | 1.4 μM | 6.8–41 μM | [38] |
| Carbon dots | DA | DPV | 10 nM | 0.01–100 μM | [39] |
| Graphene oxide, MWCNT | DA | DPV | 1.5 μM | 5–500 μM | [40] |
| Reduced Graphene oxide, MWCNT | DA | ECL | 0.067 μM | 0.2–70 μM | [41] |
| Graphene oxide | DA | Amperometry | 0.277 μM | 1–30 μM | [42] |
| Nafion-coated MWNT yarn | DA | DPV | 0.01 µM | 0.01–5 μM | [43] |
| Reduced Graphene oxide | EP | DPV | 0.0012 μM | 0.001–1000 μM | [44] |
| MWCNT | EP, NE | DPV | 4.6 μM, 1.7 μM | 7.5–48 μM | [45] |
| Graphene | EP | CV | 0.24 μM | 1–1000 μM | [46] |
| MWCNT | NE, 5-HT | SWV | 0.2 μM, 0.01 μM | 0.5–30 μM, 0.05–1 μM | [47] |
| MWCNT | NE | DPV | 0.03 μM | 0.2–100 μM | [48] |
| Graphene | NE | SWV | 30 nM | 0.08–600 μM | [49] |
| Graphene | Glu | Amperometry | 2 μM | 4–600 μM | [50] |
| Graphene | 5-HT | Amperometry | 1.6 nM | 0.0027–4.82 μM | [51] |
| Reduced Graphene oxide | 5-HT | DPV | 0.1 nM | 0.001–500 μM | [52] |
| MWCNT | 5-HT | SWV | 118 nM | 0.006–62.8 μM | [53] |
| SWCNT | His | DPV | 1.26 μM | 4.5–720 μM | [54] |
| Carbon dots | ACh | Amperometry | 1.7 μM | 5–6885 μM | [55] |
| Reduced Graphene oxide | ACh | Colorimetric | 39 nM | 0.1–10000 μM | [56] |
| Material | Analyte | Measurement | LOD | References |
|---|---|---|---|---|
| Overoxidized polypyrrole | Glu/DA | Amperometry | 2.1 µM/62 nM | [61] |
| Graphene/polypyrrole | DA | Amperometry | 2.3 µM | [62] |
| Poly(hydroquinone) | DA | DPV | 41.9 nM | [63] |
| Poly(2,4,6-triaminopyrmidine) | DA | DPV | 0.017 μM | [64] |
| Polypyrrole | EP | DPV | 298.9 nM | [65] |
| Poly(brilliant cresyl blue) | EP | CV | 0.24 μM | [46] |
| Poly(phenyl trimethoxysilane) | NE | CV | 0.1 μM | [66] |
| Poly(glutamic acid) | NE | DPV | 0.43 μM | [67] |
| Polypyrrole/polyaniline | Glu | Amperometry | 0.1 nM | [68] |
| Polypyrrole | Glu | Photochlorometric | 0.18 nM | [69] |
| Poly(bromocresol green) | 5-HT | DPV | 80 nM | [70] |
| Polypyrrole | 5-HT | SWV | 33.22 nM | [71] |
| Methacrylic acid | His | CV | 0.074 nM | [72] |
| Poly(3-thiophenemalonic acid) | tryptamine | Amperometry | 41.7 nM | [73] |
| Analyte | Measurement | LOD | References |
|---|---|---|---|
| DA | DPV | 10 nM | [80] |
| DA | DPV | 0.22 nM | [81] |
| DA | Amperometry | 1.8 nM | [82] |
| DA | DPV | 700 ± 19.23 pM | [83] |
| DA | DPV | 78 fM | [84] |
| EP | UV-Vis | 0.9 nM | [85] |
| 5-HT | Colorimetric | 300 nM | [79] |
| Analyte | Measurement | LOD | References |
|---|---|---|---|
| Glu | CV | 0.1 nM | [68] |
| Glu | Amperometry | 0.32 μM | [91] |
| Glu | Amperometry | 2.5 μM | [92] |
| Glu | CV | 0.56 μM | [93] |
| Glu, ACh | CV | 0.25 μM, 0.15 μM | [94] |
| ACh | Amperometry | 0.2 μM | [90] |
| Catalyst | Analyte | Measurement | LOD | References |
|---|---|---|---|---|
| Co3O4 | DA | Amperometry | 0.277 μM | [42] |
| Gold nanoparticles | DA | DPV | 41.9 nM | [63] |
| Gold nanoparticles | DA | DPV | 0.017 μM | [64] |
| Ag–Pt Bimetallic Nanoparticles | DA | DPV | 0.11 μM | [102] |
| CuO/Mn2O3/Silver nanoparticles | DA | Chemiresistive | - | [103] |
| Pt3Ni nanoalloys | DA | Amperometry | 10 nM | [104] |
| Cu(II) | DA | DPV | 0.08 μM | [105] |
| Fe3O4 nanorods | DA | Amperometry | 7 nM | [106] |
| Silver nanoparticles | DA | UV–Vis | 0.2 μM | [107] |
| SnO2 | DA | CV | 6.3 nM | [108] |
| Au-Pd nanocrystals | EP | DPV | 0.0012 μM | [44] |
| Gold nanoparticles | EP | DPV | 80 nM | [109] |
| Gold nanoparticles | EP | DPV | 298.9 nM | [65] |
| SiO2 | EP, NE | DPV | 10 nM, 6 nM | [110] |
| Fe3O4, ZnO | EP, NE | DPV | 4.6 μM, 1.7 μM | [45] |
| ZnO | NE,5-HT | SWV | 0.2 μM, 0.01 μM | [47] |
| Palladium nanoparticles | NE | CV | 0.1 μM | [66] |
| Gold nanoparticles | NE | DPV | 0.03 μM | [48] |
| WO3 nanoparticles | 5-HT | DPV | 1.42 nM | [111] |
| Fe3O4 nanoparticles | 5-HT | DPV | 80 nM | [70] |
| CuO | His | Amperometry | 0.33 μM | [112] |
| Nickel oxide | ACh | Amperometry | 26.7 μM | [113] |
| Polymer | Analyte | Measurement | LOD | References |
|---|---|---|---|---|
| Poly(o-aminophenol) | DA | DPV | 1.98 nM | [114] |
| Polyaniline | ACh | Potentiometry | 34.5 µM | [116] |
| Polypyrrole | DA | DPV | 10 nM | [118] |
| Polypyrrole | DA | DPV | 33 nM | [119] |
| Poly(nicotinamide) | DA | CV | 8 nM | [120] |
| Poly(2,4,6-trisacrylamido-1,3,5-triazine) | EP | DPV | 12 nM | [115] |
| Poly(phenyl trimethoxysilane) | NE | CV | 0.1 μM | [66] |
| EGDMA/MAA | His | CV | 0.074 nM | [72] |
| EGDMA/AIBN/MAA/AM | 5-HT | Thermal Resistance | 100 nM | [117] |
| Analyte | Measurement | LOD | References |
|---|---|---|---|
| ACh | Colorimetric | 39 nM | [56] |
| Glu | Photochlorometric | 0.18 nM | [69] |
| 5-HT | Colorimetric | 300 nM | [79] |
| EP | UV-Vis | 0.9 nM | [85] |
| DA | UV–Vis | 0.2 μM | [107] |
| DA, NE, EP | Colorimetric | 5 µg/mL, 1 µg/mL, 1 µg/mL | [125] |
| DA | Near-IR fluorescence | 11 nM | [133,134] |
| Glu | Fluorescence | 1 μM | [135] |
| DA | Fluorescence | 100 nM | [136] |
| DA | Fluorescence | 22 nM | [137] |
| GABA | Silica microfiber interferometry | M | [138] |
| DA | Fluorescence | 47 pM | [139] |
| 5-HT | Tapered microfiber interferometer | 84 fM | [140] |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Si, B.; Song, E. Recent Advances in the Detection of Neurotransmitters. Chemosensors 2018, 6, 1. https://doi.org/10.3390/chemosensors6010001
Si B, Song E. Recent Advances in the Detection of Neurotransmitters. Chemosensors. 2018; 6(1):1. https://doi.org/10.3390/chemosensors6010001
Chicago/Turabian StyleSi, Bo, and Edward Song. 2018. "Recent Advances in the Detection of Neurotransmitters" Chemosensors 6, no. 1: 1. https://doi.org/10.3390/chemosensors6010001
APA StyleSi, B., & Song, E. (2018). Recent Advances in the Detection of Neurotransmitters. Chemosensors, 6(1), 1. https://doi.org/10.3390/chemosensors6010001






