From Volatile Profiling to Sensory Prediction: Recent Advances in Wine Aroma Modeling Using Chemometrics and Sensor Technologies
Abstract
1. Introduction
2. Profiling of Volatile Compounds in Wine
2.1. Origins and Chemical Diversity of Volatile Compounds
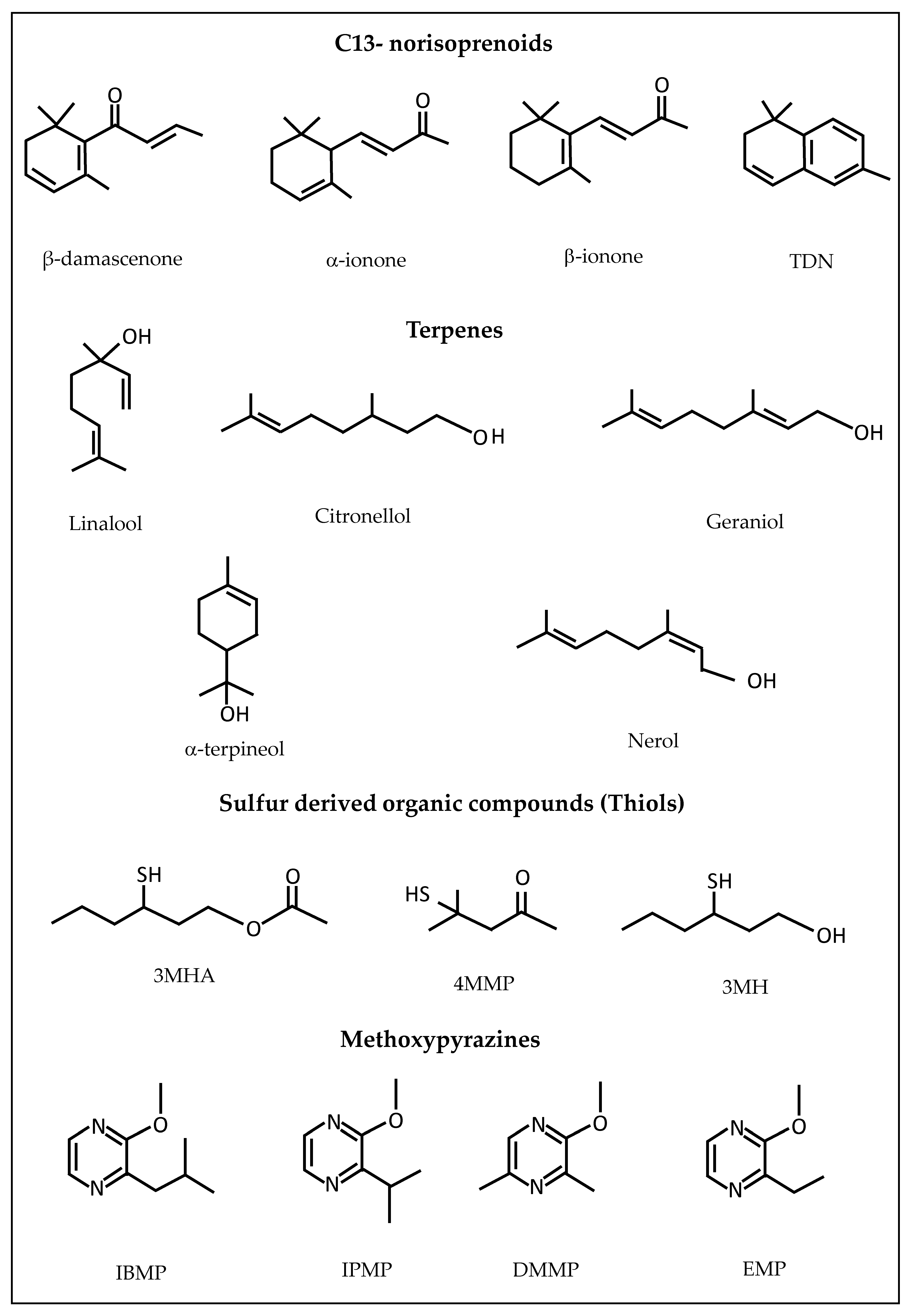
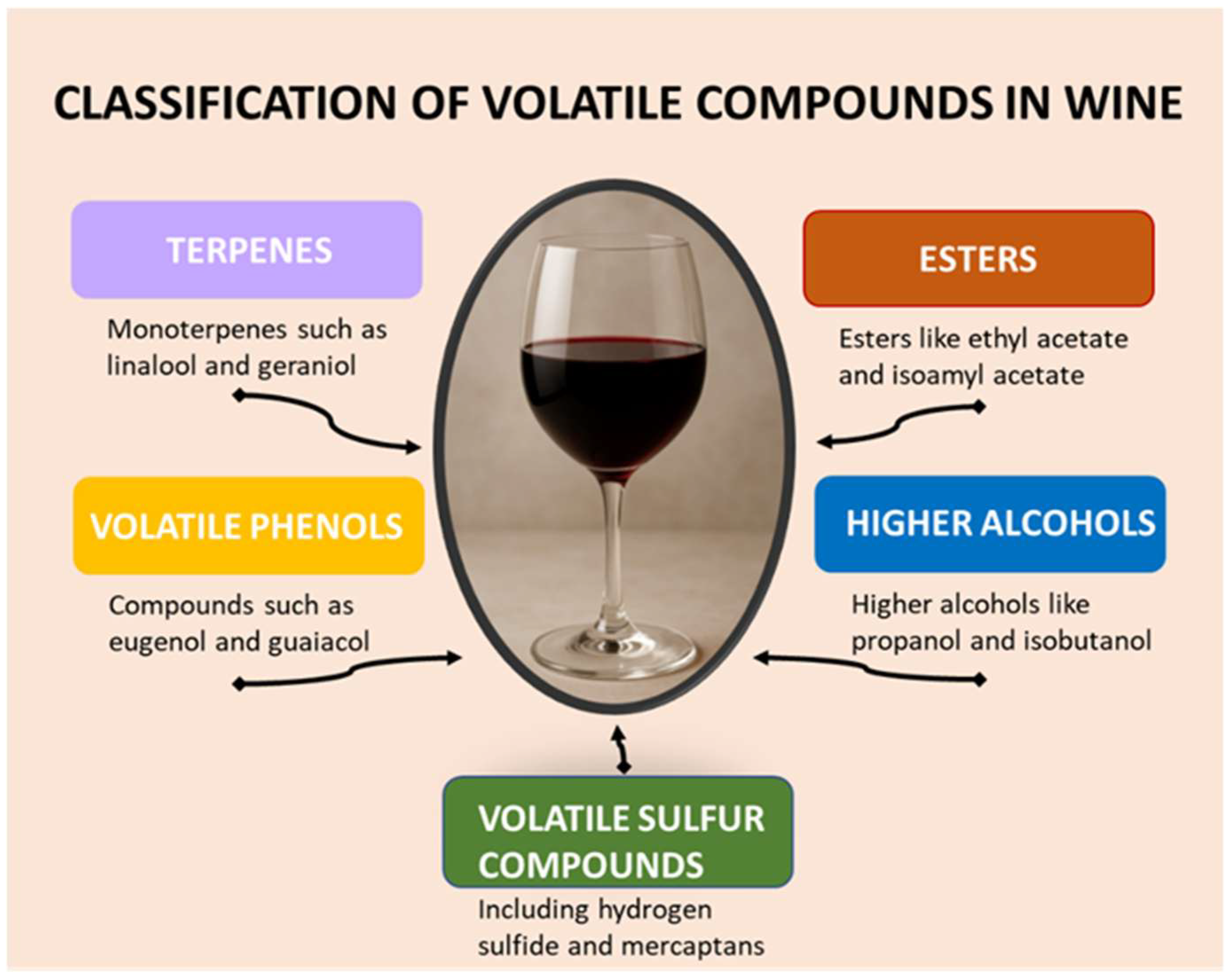
2.2. Classification of Volatile Compounds
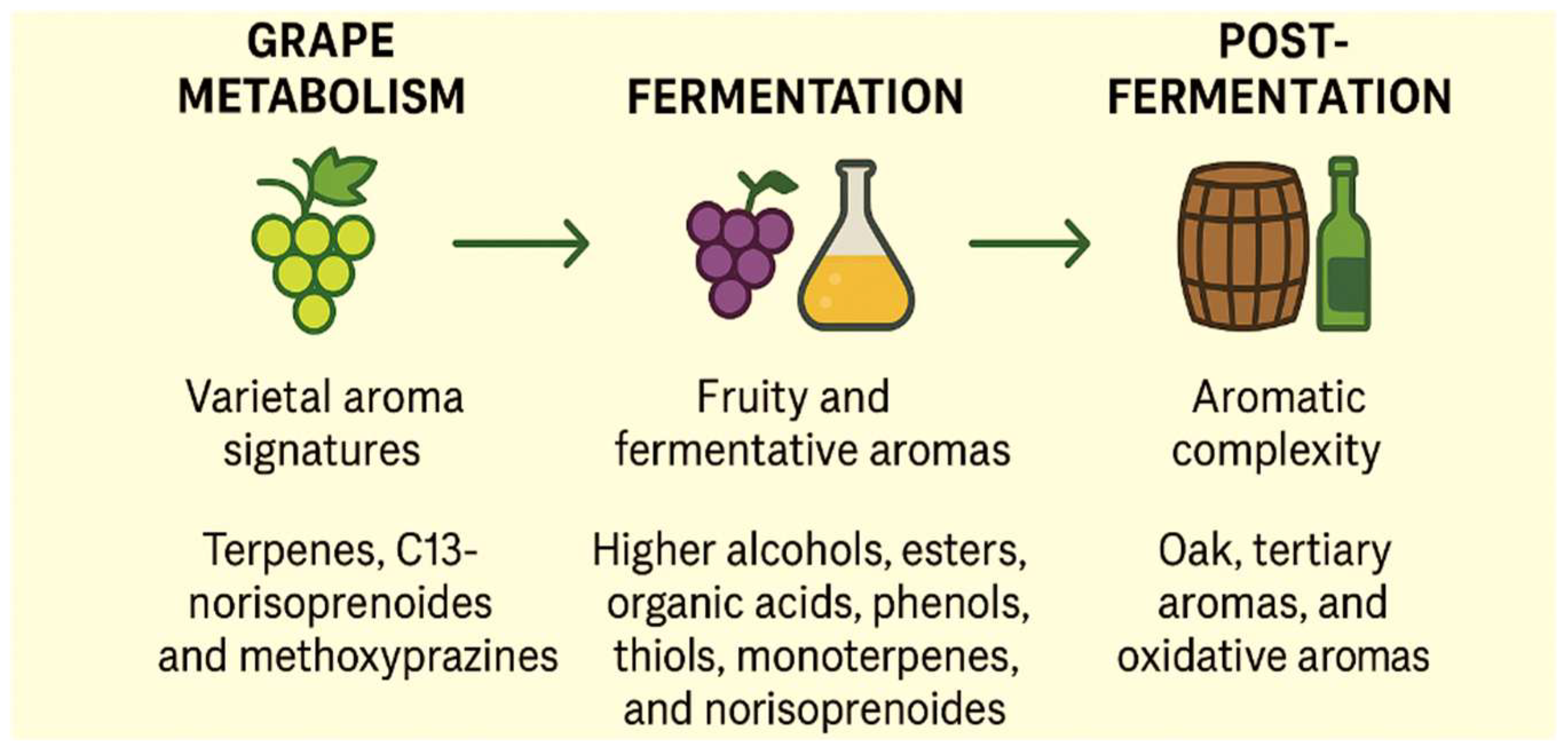
2.3. Evolution of Volatile Compounds During Winemaking
2.4. Chemical and Sensory Modifications of Wine During Aging
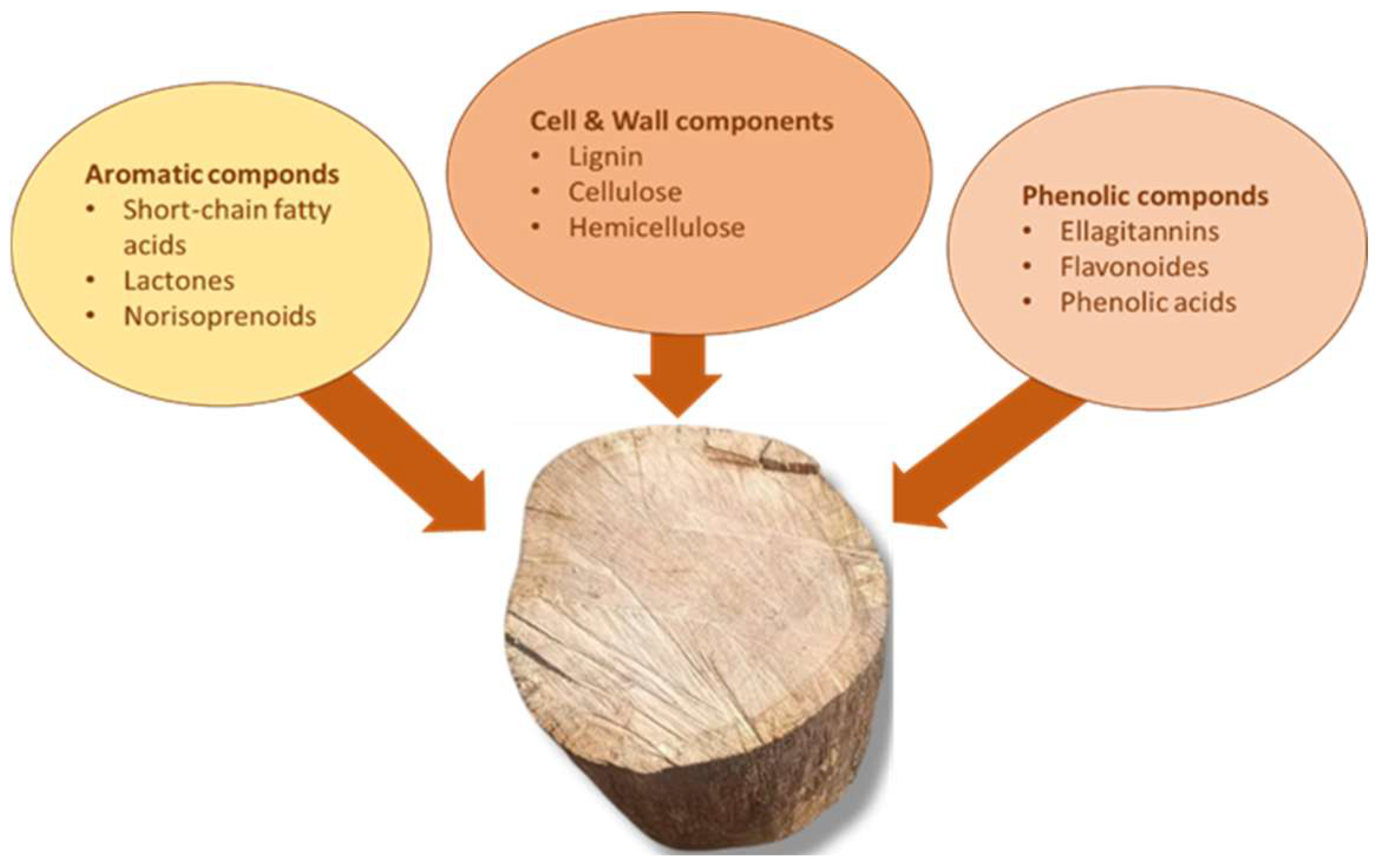
2.4.1. The Influence of Barrels, Wood Fragments, and Alternative Wood Species on Wine Quality
2.4.2. Influence of Extractable Wood Compounds on the Chemical and Sensory Profile of Wine
2.4.3. Influence of Contact Time, Wood Type, and Barrel Condition on Wine Aging
2.5. Varietal and Regional Aromatic Fingerprinting in Wine: The Role of Volatile Compounds in Defining Aroma, Authenticity, and “Terroir”
3. Integration of Analytical Data with Sensory Analysis and Sensomics
3.1. Applications and Benefits
| Application Area | Analytical Tools Used | Sensory Integration Method | Key Benefit | References |
|---|---|---|---|---|
| Dairy and Bakery | GC × GC, LC-MS, NMR | Sensory reconstitution, omission | Flavor optimization, blueprinting | [165,167] |
| Cocoa/Chocolate | UHPLC-HRMS, Chemometrics | Sensory prediction, origin mapping | Quality control, origin screening | [169] |
| Beer | Video, IRTI, EEG, ANN | Biometric + sensory data | Consumer acceptability modeling, sensory pleasantness | [172] |
| General Food Products | GC-O-MS, Chemometrics | Machine perception, artificial intelligence (AI) | Rapid aroma profiling, automation | [166,168] |
| Wine | GC-MS, GC × GC-MS, HPLC-MS | Trained panels, MRATA, and volatile profile analysis | Marker identification, quality prediction | [170,175,176] |
| Wine | Spectrofluorometry, SHS-GC-IMS | Rate-all-that-apply, sensory prediction | Sensory trait prediction, classification | [6,177,178,179] |
| Wine | Video, FaceReader, ANN | Biometric + sensory data | Consumer acceptability, sensory pleasantness | [173,174] |
3.2. Chemometrics Methods for Flavor Prediction
3.2.1. Multivariate Regression and Machine Learning
3.2.2. Pattern Recognition and Classification
3.2.3. Data Fusion and Sensor Integration
3.3. Can Combining Chemometrics and Sensomics Improve Flavor Prediction Accuracy?
3.4. Comparative Analysis: Chemometrics vs. Sensory Panels in Flavor Evaluation
4. Advances in Wine Aroma Profiling: Integrating Sensor Technologies, Chemometrics, and Machine Learning for Quality Prediction
5. Detection of Off-Flavors in Wine Using Integrated Sensor Technologies
| Application | Sensor Array | Chemometrics | Reference |
|---|---|---|---|
| Detection of Wine Spoilage Thresholds Using an Electronic Nose System: Focus on Acetic Acid | Metal Oxide Semiconductors | Principal Component Analysis, Support Vector Machines | [252] |
| Electronic Nose for Early Detection of Wine Spoilage | Metal Oxide Semiconductors | [266] | |
| Enhancing Electronic Nose Performance for Wine Defect Evaluation | Metal Oxide Semiconductors | Deep Learning, Support Vector Machines | [267] |
| Rapid Detection of TCA (2,4,6-Trichloroanisole) | Metal Oxide Semiconductors | Principal Component Analysis | [268] |
| Portable Electronic Nose for TCA Detection in Wines | Metal Oxide Semiconductors | Principal Component Analysis | [269] |
| Artificial Diagnosis of Brettanomyces spp. | Quartz Crystal Microbalance | Principal Component Analysis | [270] |
| Detection of Phenolic Derivatives (e.g., 4-Ethylphenol, 4-Ethylguaiacol) | [260] |
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- García-Carpintero, E.G.; Sánchez-Palomo, E.; Gómez-Gallego, M.A.; González-Viñas, M.A. Volatile and sensory characterization of red wines from cv. Moravia Agria minority grape variety cultivated in La Mancha region over five consecutive vintages. Food Res. Int. 2011, 44, 1549–1560. [Google Scholar] [CrossRef]
- Jackson, R.S. Wine Science: Principles and Applications; Academic Press: London, UK, 2014. [Google Scholar]
- Buratti, S.; Benedetti, S.; Scampicchio, M.; Pangerod, E.C. Characterization and classification of Italian Barbera wines by using an electronic nose and an amperometric electronic tongue. Anal. Chim. Acta 2004, 525, 133–139. [Google Scholar] [CrossRef]
- Buratti, S.; Ballabio, D.; Benedetti, S.; Cosio, M.S. Prediction of Italian red wine sensorial descriptors from electronic nose, electronic tongue and spectrophotometric measurements by means of Genetic Algorithm regression models. Food Chem. 2007, 100, 211–218. [Google Scholar] [CrossRef]
- Ouyang, Q.; Zhao, J.; Chen, Q. Instrumental intelligent test of food sensory quality as mimic of human panel test combining multiple cross-perception sensors and data fusion. Anal. Chim. Acta 2014, 841, 68–76. [Google Scholar] [CrossRef]
- Muñoz-Redondo, J.; Puertas, B.; Pereira-Caro, G.A.; Ordóñez-Díaz, J.; Ruiz-Moreno, M.; Cantos-Villar, E.; Moreno-Rojas, J. Statistical Workflow to Evaluate the Modulation of Wine Metabolome and Its Contribution to the Sensory Attributes. Fermentation 2021, 7, 72. [Google Scholar] [CrossRef]
- Aznar, M.; López, R.; Cacho, J.; Ferreira, V. Prediction of aged red wine aroma properties from aroma chemical composition. Partial least squares regression models. J. Agric. Food Chem. 2003, 51, 2700–2707. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, R.; Tudu, B.; Bandyopadhyay, R.; Bhattacharyya, N. A review on combined odor and taste sensor systems. J. Food Eng. 2016, 190, 10–21. [Google Scholar] [CrossRef]
- Aguilera, T.; Lozano, J.; Paredes, J.A.; Alvarez, F.J.; Suárez, J.I. Electronic nose based on independent component analysis combined with partial least squares and artificial neural networks for wine prediction. Sensors 2012, 12, 8055–8072. [Google Scholar] [CrossRef]
- Mafata, M.; Brand, J.; Medvedovici, A.; Buica, A. Chemometric and sensometric techniques in enological data analysis. Crit. Rev. Food Sci. Nutr. 2023, 32, 10995–11009. [Google Scholar] [CrossRef]
- Gamboa, J.C.R.; da Silva, A.J.; de Andrade Lima, L.L.; Ferreira, T.A.E. Wine quality rapid detection using a compact electronic nose system: Application focused on spoilage thresholds by acetic acid. LWT 2019, 108, 377–384. [Google Scholar] [CrossRef]
- González Viejo, C.; Torrico, D.D.; Dunshea, F.R.; Fuentes, S. Emerging Technologies Based on Artificial Intelligence to Assess the Quality and Consumer Preference of Beverages. Beverages 2019, 5, 62. [Google Scholar] [CrossRef]
- Loutfi, A.; Coradeschi, S.; Mani, G.K.; Shankar, P.; Rayappan, J.B.B. Electronic Noses for Food Quality: A Review. J. Food Eng. 2015, 144, 103–111. [Google Scholar] [CrossRef]
- Rodríguez-Méndez, M.L.; De Saja, J.A.; González-Antón, R.; García-Hernández, C.; Medina-Plaza, C.; García-Cabezón, C.; Martín-Pedrosa, F. Electronic Noses and Tongues in Wine Industry. Front. Bioeng. Biotechnol. 2016, 4, 81. [Google Scholar] [CrossRef] [PubMed]
- Han, F.; Zhang, D.; Aheto, J.H.; Feng, F.; Duan, T. Integration of a low-cost electronic nose and a voltammetric electronic tongue for red wines identification. Food Sci. Nutr. 2020, 8, 4330–4339. [Google Scholar] [CrossRef]
- Di Rosa, A.R.; Leone, F.; Chiofalo, V. Electronic noses and tongues. In Chemical Analysis of Food, 2nd ed.; Pico, Y., Ed.; Academic Press: London, UK, 2020; Chapter 7; pp. 353–389. [Google Scholar] [CrossRef]
- Zwaardemaker, H.; Hogewind, F. On spray-electricity and waterfall electricity. KNAW Proc. 1919, 22, 429–437. [Google Scholar]
- Hartman, J. A possible objective method for the rapid estimation of flavors in vegetables. Proc. Am. Soc. Hort. Sci. 1954, 64, 335–342. [Google Scholar]
- Moncrieff, R.W. The characterization of odours. J. Physiol. 1954, 125, 453–465. [Google Scholar] [CrossRef]
- Moncrieff, R.W. An instrument for measuring and classifying odors. J. Appl. Physiol. 1961, 16, 742–749. [Google Scholar] [CrossRef]
- Abdelkhalek, M.; Alfayad, S.; Benouezdou, F.; Fayek, M.B.; Chassagne, L. Compact and embedded electronic nose for volatile and non-volatile odor classification for robot applications. IEEE Access 2019, 7, 98267–98276. [Google Scholar] [CrossRef]
- Persaud, K.; Dodd, G. Analysis of discrimination mechanisms in the mammalian olfactory system using a model nose. Nature 1982, 299, 352–355. [Google Scholar] [CrossRef]
- Ikegami, A.; Kaneyasu, M. Olfactory detection using integrated sensors. In Proceedings of the 3rd International Conference on Solid-State Sensors and Actuators, Philadelphia, PA, USA, 1–14 June 1985; pp. 136–139. [Google Scholar]
- Otto, M.; Thomas, J.D.R. Model studies on multiple channel analysis of free magnesium, calcium, sodium, and potassium at physiological concentration levels with ion-selective electrodes. Anal. Chem. 1985, 57, 2647–2651. [Google Scholar] [CrossRef]
- Gardner, J.W.; Bartlett, P.N. A brief history of electronic noses. Sens. Actuators B Chem. 1994, 18, 211–220. [Google Scholar] [CrossRef]
- Toko, K. Taste sensor with global selectivity. Mater. Sci. Eng. C 1996, 4, 69–82. [Google Scholar] [CrossRef]
- Hayashi, K.; Yamanaka, M.; Toko, K.; Yamafuji, K. Multichannel taste sensor using lipid membranes. Sens. Actuators B Chem. 1990, 2, 205–213. [Google Scholar] [CrossRef]
- Vlasov, Y.; Legin, A.; Rudnitskaya, A.; Di Natale, C.; D’Amico, A. Multisensor system with an array of chemical sensors and artificial neural networks (electronic tongue) for quantitative analysis of multicomponent aqueous solutions. Russ. J. Appl. Chem. 1996, 69, 848–853. [Google Scholar]
- Vlasov, Y.; Andrey, L. Non-selective chemical sensors in analytical chemistry: From “electronic nose” to “electronic tongue”. Fresenius. J. Anal. Chem. 1998, 361, 255–260. [Google Scholar] [CrossRef]
- Pearce, T.C.; Schiffman, S.S.; Nagle, H.T.; Gardner, J.W. Handbook of Machine Olfaction: Electronic Nose Technology; Pearce, T.C., Schiffman, S.S., Nagle, H.T., Gardner, J.W., Eds.; VCH: New York, NY, USA, 2003. [Google Scholar] [CrossRef]
- Schwarzböck, T. Market Review on Available Instruments for Odour Measurement Berlin; Kompetenzzentrum Wasser Berlin gGmbH: Berlin, Germany, 2012. [Google Scholar]
- Winquist, F.; Wide, P.; Lundström, I. An electronic tongue based on voltammetry. Anal. Chim. Acta 1997, 357, 21–31. [Google Scholar] [CrossRef]
- Winquist, F.; Holmin, S.; Krantz-Rülcker, C.; Wide, P.; Lundström, I. A hybrid electronic tongue. Anal. Chim. Acta 2000, 406, 147–157. [Google Scholar] [CrossRef]
- Ciosek, P.; Wróblewski, W. Sensor arrays for liquid sensing—Electronic tongue systems. Analyst 2007, 132, 963–978. [Google Scholar] [CrossRef]
- Escuder-Gilabert, L.; Peris, M. Review: Highlights in recent applications of electronic tongues in food analysis. Anal. Chim. Acta 2010, 665, 15–25. [Google Scholar] [CrossRef]
- Hilding-Ohlsson, A.; Fauerbach, J.A.; Sacco, N.J.; Bonetto, M.C.; Cortón, E. Voltamperometric discrimination of urea and melamine adulterated skimmed milk powder. Sensors 2012, 12, 12220–12234. [Google Scholar] [CrossRef]
- Wilson, A.D.; Baietto, M. Applications and advances in electronic-nose technologies. Sensors 2009, 9, 5099–5148. [Google Scholar] [CrossRef]
- Riul, A.; Malmegrim, R.; Fonseca, F.; Mattoso, L. An artificial taste sensor based on conducting polymers. Biosens. Bioelectron. 2003, 18, 1365–1369. [Google Scholar] [CrossRef]
- Kiani, S.; Minaei, S.; Ghasemi-Varnamkhasti, M. Fusion of Artificial Senses as a Robust Approach to Food Quality Assessment. J. Food Eng. 2016, 171, 230–239. [Google Scholar] [CrossRef]
- Qiu, S.; Wang, J.; Gao, L. Qualification and quantisation of processed strawberry juice based on electronic nose and tongue. LWT Food Sci. Technol. 2015, 60, 115–123. [Google Scholar] [CrossRef]
- Wasilewski, T.; Gębicki, J. Emerging strategies for enhancing detection of explosives by artificial olfaction. Microchem. J. 2021, 164, 106025. [Google Scholar] [CrossRef]
- Dung, T.T.; Oh, Y.; Choi, S.J.; Kim, I.D.; Oh, M.K.; Kim, M. Applications and advances in bioelectronic noses for odour sensing. Sensors 2018, 18, 103. [Google Scholar] [CrossRef]
- Son, M.; Lee, J.Y.; Ko, H.J.; Park, T.H. Bioelectronic Nose: An Emerging Tool for Odor Standardization. Trends Biotechnol. 2017, 35, 301–307. [Google Scholar] [CrossRef]
- Cheng, H.; Qin, Z.; Guo, X.; Hu, X.; Wu, J. Geographical origin identification of propolis using GC-MS and electronic nose combined with principal component analysis. Food Res. Int. 2013, 51, 813–822. [Google Scholar] [CrossRef]
- Delahunty, C.M.; Eyres, G.; Dufour, J.P. Gas chromatography-olfactometry. J. Sep. Sci. 2006, 29, 2107–2125. [Google Scholar] [CrossRef]
- Röck, F.; Barsan, N.; Weimar, U. Electronic Nose: Current Status and Future. Trends. Chem. Rev. 2008, 108, 705–725. [Google Scholar] [CrossRef]
- Zampolli, S.; Elmi, I.; Mancarella, F.; Betti, P.; Dalcanale, E.; Cardinali, G.C.; Severi, M. Real-time monitoring of sub-ppb concentrations of aromatic volatiles with a MEMS-enabled miniaturized gas-chromatograph. Sens. Actuators B Chem. 2009, 141, 322–328. [Google Scholar] [CrossRef]
- Chung, N.; Ameer, K.; Jo, Y.; Kwon, J.-H. Comparison of electronic sensing techniques for screening dried shrimps irradiated using three types of approved radiation with standard analytical methods. Food Chem. 2019, 286, 395–404. [Google Scholar] [CrossRef]
- King, E.S.; Osidacz, P.; Curtin, C.; Bastian, S.E.P.; Francis, I.L. Assessing desirable levels of sensory properties in Sauvignon Blanc wines—consumer preferences and contribution of key aroma compounds. Aust. J. Grape Wine Res. 2011, 17, 169–180. [Google Scholar] [CrossRef]
- Liu, S.; Lou, Y.; Li, Y.; Zhao, Y.; Laaksonen, O.; Li, P.; Zhang, J.; Battino, M.; Yang, B.; Gu, Q. Aroma characteristics of volatile compounds brought by variations in microbes in winemaking. Food Chem. 2023, 420, 136075. [Google Scholar] [CrossRef] [PubMed]
- Kuchen, B.; Maturano, Y.P.; Mestre, M.V.; Combina, M.; Toro, M.E.; Vazquez, F. Selection of Native Non-Saccharomyces Yeasts with Biocontrol Activity against Spoilage Yeasts in Order to Produce Healthy Regional Wines. Fermentation 2019, 5, 60. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, C.; Câmara, J.S. Madeira Wine Volatile Profile. A Platform to Establish Madeira Wine Aroma Descriptors. Molecules 2019, 24, 3028. [Google Scholar] [CrossRef]
- van Leeuwen, C.; Barbe, J.-C.; Darriet, P.; Geffroy, O.; Gomès, E.; Guillaumie, S.; Thibon, C. Recent advancements in understand-ing the terroir effect on aromas in grapes and wines. OENO One 2020, 54, 985–1006. [Google Scholar] [CrossRef]
- Wang, H.; Hopfer, H.; Cockburn, D.W.; Wee, J. Characterization of microbial dynamics and volatile metabolome changes during fermentation of Chambourcin hybrid grapes from two Pennsylvania regions. Front. Microbiol. 2021, 11, 614278. [Google Scholar] [CrossRef]
- Ferreira, V.; Lopez, R. The Actual and Potential Aroma of Winemaking Grapes. Biomolecules 2019, 9, 818. [Google Scholar] [CrossRef]
- Torres, N.; Martínez-Lüscher, J.; Porte, E.; Yu, R.; Kurtural, S.K. Impacts of leaf removal and shoot thinning on cumulative daily light intensity and thermal time and their cascading effects of grapevine (Vitis vinifera L.) berry and wine chemistry in warm climates. Food Chem. 2021, 343, 128447. [Google Scholar] [CrossRef]
- Belda, I.; Ruiz, J.; Esteban-Fernández, A.; Navascués, E.; Marquina, D.; Santos, A.; Moreno-Arribas, M.V. Microbial Contribution to Wine Aroma and Its Intended Use for Wine Quality Improvement. Molecules 2017, 22, 189. [Google Scholar] [CrossRef]
- Carpena, M.; Fraga-Corral, M.; Otero, P.; Nogueira, R.A.; Garcia-Oliveira, P.; Prieto, M.A.; Simal-Gandara, J. Secondary aroma: Influence of wine microorganisms in their aroma profile. Foods 2021, 10, 51. [Google Scholar] [CrossRef]
- Romano, P.; Braschi, G.; Siesto, G.; Patrignani, F.; Lanciotti, R. Role of yeasts on the sensory component of wines. Foods 2022, 11, 1921. [Google Scholar] [CrossRef] [PubMed]
- Bosman, R.N.; Vervalle, J.A.; November, D.L.; Burger, P.; Lashbrooke, J.G. Grapevine Genome Analysis Demonstrates the Role of Gene Copy Number Variation in the Formation of Monoterpenes. Front. Plant Sci. 2023, 14, 1112214. [Google Scholar] [CrossRef] [PubMed]
- Gomez, H.A.G.; Niederauer, G.F.; Minatel, I.O.; Antunes, E.R.M.; Carneiro, M.J.; Sawaya, A.C.H.F.; Zanus, M.C.; Ritschel, P.S.; Quecini, V.; Pereira Lima, G.P.; et al. Metabolite profiling reveals the influence of grapevine genetic distance on the chemical signature of juices. J. Sci. Food Agric. 2024, 104, 2383–2397. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Massonnet, M.; Cantu, D. The Genetic Basis of Grape and Wine Aroma. Hortic. Res. 2019, 6, 81. [Google Scholar] [CrossRef]
- Rienth, M.; Vigneron, N.; Darriet, P.; Sweetman, C.; Burbidge, C.; Bonghi, C.; Walker, R.P.; Famiani, F.; Castellarin, S.D. Grape berry secondary metabolites and their modulation by abiotic factors in a climate change scenario—A review. Front. Plant Sci. 2021, 12, 643258. [Google Scholar] [CrossRef]
- Delić, K.; Milinčić, D.D.; Pešić, M.B.; Lević, S.; Nedović, V.A.; Gancel, A.-L.; Jourdes, M.; Teissedre, P.-L. Grape, wine and pomace anthocyanins: Winemaking biochemical transformations, application and potential benefits. OENO One 2024, 58, 1–25. [Google Scholar] [CrossRef]
- Koundouras, S. Environmental and Viticultural Effects on Grape Composition and Wine Sensory Properties. Elements 2018, 14, 173–178. [Google Scholar] [CrossRef]
- Baltazar, M.; Castro, I.; Gonçalves, B. Adaptation to Climate Change in Viticulture: The Role of Varietal Selection—A Review. Plants 2025, 14, 104. [Google Scholar] [CrossRef]
- Álvarez, R.; Garces, F.; Louis, E.J.; Dequin, S.; Camarasa, C. Beyond S. cerevisiae for Winemaking: Fermentation-Related Trait Diversity in the Genus Saccharomyces. Food Microbiol. 2023, 113, 104270. [Google Scholar] [CrossRef] [PubMed]
- Godoy, L.; Acuña-Fontecilla, A.; Catrileo, D. Formation of Aromatic and Flavor Compounds in Wine: A Perspective of Positive and Negative Contributions of Non-Saccharomyces Yeasts. In Chemistry and Biochemistry of Winemaking, Wine Stabilization and Aging; IntechOpen: Rijeka, Croatia, 2021. [Google Scholar] [CrossRef]
- Mota, J.; Vilela, A. Exploring Microbial Dynamics: The Interaction between Yeasts and Acetic Acid Bacteria in Port Wine Vinegar and Its Implications on Chemical Composition and Sensory Acceptance. Fermentation 2024, 10, 421. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, Q.; Liu, X.; Bian, X.; Li, J.; Meng, N.; Liu, M.; Huang, M.; Sun, B.; Li, J. Flavor Interactions in Wine: Current Status and Future Directions from Interdisciplinary and Crossmodal Perspectives. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70199. [Google Scholar] [CrossRef] [PubMed]
- Lasik-Kurdyś, M.; Majcher, M.; Nowak, J. Effects of Different Techniques of Malolactic Fermentation Induction on Diacetyl Metabolism and Biosynthesis of Selected Aromatic Esters in Cool-Climate Grape Wines. Molecules 2018, 23, 2549. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Teissedre, P.-L.; Chira, K. Impact of Oak Wood Ageing Modalities on the (Non)-Volatile Composition and Sensory Attributes of Red Wines. OENO One 2021, 55, 285–299. [Google Scholar] [CrossRef]
- Tarko, T.; Krankowski, F.; Duda-Chodak, A. The Impact of Compounds Extracted from Wood on the Quality of Alcoholic Beverages. Molecules 2023, 28, 620. [Google Scholar] [CrossRef]
- Echave, J.; Barral, M.; Fraga-Corral, M.; Prieto, M.A.; Simal-Gandara, J. Bottle Aging and Storage of Wines: A Review. Molecules 2021, 26, 713. [Google Scholar] [CrossRef]
- Zhang, D.; Wei, Z.; Han, Y.; Duan, Y.; Shi, B.; Ma, W. A Review on Wine Flavour Profiles Altered by Bottle Aging. Molecules 2023, 28, 6522. [Google Scholar] [CrossRef]
- Tarko, T.; Duda, A. Volatilomics of Fruit Wines. Molecules 2024, 29, 2457. [Google Scholar] [CrossRef]
- Chigo-Hernandez, M.M.; DuBois, A.; Tomasino, E. Aroma Perception of Rose Oxide, Linalool and α-Terpineol Combinations in Gewürztraminer Wine. Fermentation 2022, 8, 30. [Google Scholar] [CrossRef]
- Buican, B.-C.; Luchian, C.E.; Colibaba, L.C.; Niculaua, M.; Bordean, M.-E.; Kallithraka, S.; Cotea, V.V. Aroma Compounds from Grape Pomace: Investigation of Key Winemaking Factors for Future Extraction Applications—A Review. Horticulturae. 2025, 11, 302. [Google Scholar] [CrossRef]
- Black, C.; Parker, M.; Siebert, T.; Capone, D.; Francis, I. Terpenoids and Their Role in Wine Flavour: Recent Advances. Aust. J. Grape Wine Res. 2015, 21, 582–600. [Google Scholar] [CrossRef]
- Sumby, K.M.; Grbin, P.R.; Jiranek, V. Microbial Modulation of Aromatic Esters in Wine: Current Knowledge and Future Prospects. Food Chem. 2010, 121, 1–16. [Google Scholar] [CrossRef]
- Cordente, A.G.; Curtin, C.D.; Varela, C.; Pretorius, I.S. Flavour-Active Wine Yeasts. Appl. Microbiol. Biotechnol. 2012, 96, 601–618. [Google Scholar] [CrossRef]
- Saerens, S.; Delvaux, F.; Verstrepen, K.; Thevelein, J. Production and Biological Function of Volatile Esters in Saccharomyces cerevisiae. Microb. Biotechnol. 2010, 3, 165–177. [Google Scholar] [CrossRef]
- Pittari, E.; Moio, L.; Piombino, P. Interactions between Polyphenols and Volatile Compounds in Wine: A Literature Review on Physicochemical and Sensory Insights. Appl. Sci. 2021, 11, 1157. [Google Scholar] [CrossRef]
- Zhao, F.; Martínez-Lapuente, L.; Ayestarán, B.; Guadalupe, Z. Volatile and Sensory Characterization of Tempranillo Wines Aged in Quercus alba Oak Barrels of Different Geographical Origins in USA. LWT Food Sci. Technol. 2023, 173, 114328. [Google Scholar] [CrossRef]
- Yang, C.; Li, Y.; He, L.; Song, Y.; Zhang, P.; Liu, S. Metabolomic and transcriptomic analyses of monoterpene biosynthesis in Muscat and Neutral grape hybrids. Sci. Hortic. 2024, 336, 113434. [Google Scholar] [CrossRef]
- Carien, C.; Wessel, J. A comprehensive review on Sauvignon Blanc aroma with a focus on certain positive volatile thiols. Food Res. Int. 2012, 45, 287–298. [Google Scholar] [CrossRef]
- Davis, P.M.; Qian, M.C. Progress on Volatile Sulfur Compound Analysis in Wine. In Volatile Sulfur Compounds in Food; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 2011; Volume 1068, pp. 93–115. [Google Scholar] [CrossRef]
- Jimenez-Lorenzo, R.; Bloem, A.; Farines, V.; Sablayrolles, J.M.; Camarasa, C. How to Modulate the Formation of Negative Volatile Sulfur Compounds during Wine Fermentation? FEMS Yeast Res. 2021, 21, foab038. [Google Scholar] [CrossRef]
- De-La-Fuente-Blanco, A.; Sáenz-Navajas, M.-P.; Ferreira, V. On the effects of higher alcohols on red wine aroma. Food Chem. 2016, 210, 107–114. [Google Scholar] [CrossRef]
- Cordente, A.G.; Espinase Nandorfy, D.; Solomon, M.; Schulkin, A.; Kolouchova, R.; Francis, I.L.; Schmidt, S.A. Aromatic higher alcohols in wine: Implication on aroma and palate attributes during Chardonnay aging. Molecules 2021, 26, 4979. [Google Scholar] [CrossRef]
- Nguyen, T.H.; Zimmermann, D.; Durner, D. Aroma Potential of German Riesling Winegrapes during Late-Stage Ripening. Beverages 2024, 10, 77. [Google Scholar] [CrossRef]
- Gregan, S.M.; Jordan, B. Methoxypyrazine Accumulation and O-Methyltransferase Gene Expression in Sauvignon blanc Grapes: The Role of Leaf Removal, Light Exposure, and Berry Development. J. Agric. Food Chem. 2016, 64, 2200–2208. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Li, X.; Guo, X.; Wang, N.; Geng, K.; Li, D.; Wang, Z. Comparison of Methoxypyrazine Content and Expression Pattern of O-Methyltransferase Genes in Grape Berries and Wines from Six Cultivars (Vitis vinifera L.) in the Eastern Foothill of the Helan Mountain. Plants 2022, 11, 1613. [Google Scholar] [CrossRef] [PubMed]
- Barbará, J.A.; Nicolli, K.P.; Souza-Silva, É.A.; Biasoto, A.C.T.; Welke, J.E.; Zini, C.A. Volatile profile and aroma potential of tropical Syrah wines elaborated in different maturation and maceration times using comprehensive two-dimensional gas chromatography and olfactometry. Food Chem. 2020, 308, 125552. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Jiménez, M.; Sherman, E.; Pozo-Bayón, M.A.; Pinu, F.R. Application of untargeted volatile profiling and data driven approaches in wine flavoromics research. Food Res. Int. 2021, 145, 110392. [Google Scholar] [CrossRef]
- Lanza, C.; Mazzaglia, A.; Scacco, A.; Tripodi, G.; Dima, G.; Verzera, A. Correlation between aroma compounds and sensory properties of Malvasia wines produced in Sicily. Am. J. Enol. Vitic. 2010, 61, 260–268. [Google Scholar] [CrossRef]
- Bergo, A.M.; Taglieri, I.; Venturi, F.; Matak, L.; Gondas, P.; Kloucek, P.; Havlik, J. 1H NMR spectrum carries signatures of white wine aroma. LWT Food Sci. Technol. 2025, 223, 117786. [Google Scholar] [CrossRef]
- Álvarez-Pérez, J.M.; Campo, E.; San-Juan, F.; Coque, J.J.R.; Ferreira, V.; Hernández-Orte, P. Sensory and chemical characterisation of the aroma of Prieto Picudo rosé wines: The differential role of autochthonous yeast strains on aroma profiles. Food Chem. 2012, 133, 284–292. [Google Scholar] [CrossRef]
- Amores-Arrocha, A.; Sancho-Galán, P.; Jiménez-Cantizano, A.; Palacios, V. A Comparative Study on Volatile Compounds and Sensory Profile of White and Red Wines Elaborated Using Bee Pollen versus Commercial Activators. Foods 2021, 10, 1082. [Google Scholar] [CrossRef]
- Li, S.-Y.; Duan, C.-Q. Astringency, bitterness and color changes in dry red wines before and during oak barrel aging: An updated phenolic perspective review. Crit. Rev. Food Sci. Nutr. 2019, 59, 1840–1867. [Google Scholar] [CrossRef]
- Rollero, S.; Bloem, A.; Ortiz-Julien, A.; Camarasa, C.; Divol, B. Altered fermentation performances, growth, and metabolic footprints reveal competition for nutrients between yeast species inoculated in synthetic grape juice-like medium. Front. Microbiol. 2018, 9, 196. [Google Scholar] [CrossRef]
- Wang, X.; Bohlscheid, J.; Edwards, C. Fermentative activity and production of volatile compounds by Saccharomyces grown in synthetic grape juice media deficient in assimilable nitrogen and/or pantothenic acid. J. Appl. Microbiol. 2003, 94, 349–359. [Google Scholar] [CrossRef]
- Bohlscheid, J.; Fellman, J.; Wang, X.; Ansen, D.; Edwards, C. The influence of nitrogen and biotin interactions on the performance of Saccharomyces in alcoholic fermentations. J. Appl. Microbiol. 2007, 102, 390–400. [Google Scholar] [CrossRef] [PubMed]
- Kotarska, K.; Czupryński, B.; Kłosowski, G. Effect of various activators on the course of alcoholic fermentation. J. Food Eng. 2006, 77, 965–971. [Google Scholar] [CrossRef]
- Garde-Cerdán, T.; Ancín-Azpilicueta, C. Effect of the addition of different quantities of amino acids to nitrogen-deficient must on the formation of esters, alcohols, and acids during wine alcoholic fermentation. LWT Food Sci. Technol. 2008, 41, 501–510. [Google Scholar] [CrossRef]
- Lee, P.; Toh, M.; Yu, B.; Curran, P.; Liu, S. Manipulation of volatile compound transformation in durian wine by nitrogen supplementation. Int. J. Food Sci. Technol. 2013, 48, 650–662. [Google Scholar] [CrossRef]
- Walker, G.; Stewart, G. Saccharomyces cerevisiae in the Production of Fermented Beverages. Beverages 2016, 2, 30. [Google Scholar] [CrossRef]
- Wang, X.; Glawe, D.A.; Kramer, E.; Weller, D.; Okubara, P.A. Biological control of Botrytis cinerea: Interactions with native vineyard yeasts from Washington State. Phytopathology 2018, 108, 691–701. [Google Scholar] [CrossRef]
- Jiménez-Lorenzo, R.; Farines, V.; Sablayrolles, J.-M.; Camarasa, C.; Bloem, A. New Insights into the Origin of Volatile Sulfur Compounds during Wine Fermentation and Their Evolution during Aging. Fermentation 2022, 8, 139. [Google Scholar] [CrossRef]
- Carpena, M.; Pereira, A.G.; Prieto, M.A.; Simal-Gandara, J. Wine Aging Technology: Fundamental Role of Wood Barrels. Foods 2020, 9, 1160. [Google Scholar] [CrossRef]
- Jordão, A.M.; Cosme, F. The Application of Wood Species in Enology: Chemical Wood Composition and Effect on Wine Quality. Appl. Sci. 2022, 12, 3179. [Google Scholar] [CrossRef]
- Pérez-Magariño, S.; Cano-Mozo, E.; Bueno-Herrera, M.; Fernández-Fernández, E.; Orden, D.; Albors, C.; López, L.; Navascués, E. Aromatic Evolution of Red Wine Aged in Oak Vats and Barrels. Relationship between Volatile Compounds and Sensory Analysis. Am. J. Enol. Vitic. 2024, 75, 0750008. [Google Scholar] [CrossRef]
- Resolution OENO 4/2005 of Organization of Vine and Wine. Available online: https://www.oiv.int/public/medias/776/oeno-4-2005-en.pdf (accessed on 26 June 2025).
- De Simón, B.F.; Sanz, M.; Cadahía, E.; Martínez, J.; Esteruelas, E.; Muñoz, A. Polyphenolic compounds as chemical markers of wine ageing in contact with cherry, chestnut, false acacia, ash and oak wood. Food Chem. 2014, 143, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gil, A.M.; del Alamo-Sanza, M.; Gutiérrez-Gamboa, G.; Moreno-Simunovic, Y.; Nevares, I. Volatile composition and sensory characteristics of Carménère wines macerating with Colombian (Quercus Humboldtii) oak chips compared to wines macerated with American (Q. alba) and European (Q. petraea) oak chips. Food Chem. 2018, 266, 90–100. [Google Scholar] [CrossRef]
- De Simón, B.F.; Esteruelas, E.; Muñoz, Á.M.; Cadahía, E.; Sanz, M. Volatile compounds in acacia, chestnut, cherry, ash, and oak woods, with a view to their use in cooperage. J. Agric. Food Chem. 2009, 57, 3217–3227. [Google Scholar] [CrossRef]
- Peynaud, E. Enología Práctica: Conocimiento y Elaboración del Vino; Ediciones Mundi Prensa: Madrid, Spain, 2006. [Google Scholar]
- Alañón, M.E.; Castro-Vázquez, L.; Díaz-Maroto, M.C.; Hermosín-Gutiérrez, I.; Gordon, M.H.; Pérez-Coello, M.S. Antioxidant capacity and phenolic composition of different woods used in cooperage. Food Chem. 2011, 129, 1584–1590. [Google Scholar] [CrossRef]
- De Simón, B.F.; Cadahía, E.; Muiño, I.; del Álamo, M.; Nevares, I. Volatile composition of toasted oak chips and staves and of red wine aged with them. Am. J. Enol. Vitic. 2010, 61, 157–165. [Google Scholar] [CrossRef]
- Gallego, L.; Del Alamo, M.; Nevares, I.; Fernández, J.A.; De Simón, B.F.; Cadahía, E. Phenolic compounds and sensorial characterization of wines aged with alternative to barrel products made of Spanish oak wood (Quercus pyrenaica Willd.). Food Sci. Technol. Int. 2012, 18, 151–165. [Google Scholar] [CrossRef]
- De Simón, B.F.; Cadahía, E.; Sanz, M.; Poveda, P.; Perez-Magariño, S.; Ortega-Heras, M.; González-Huerta, C. Volatile compounds and sensorial characterization of wines from four spanish denominations of origin, aged in Spanish Rebollo (Quercus pyrenaica Willd.) oak wood barrels. J. Agric. Food Chem. 2008, 56, 9046–9055. [Google Scholar] [CrossRef]
- Martínez-Gil, A.; del Alamo-Sanza, M.; Sánchez-Gómez, R.; Nevares, I. Alternative woods in enology: Characterization of tannin and low molecular weight phenol compounds with respect to traditional oak woods. A review. Molecules 2020, 25, 1474. [Google Scholar] [CrossRef] [PubMed]
- Miranda, I.; Sousa, V.; Ferreira, J.; Pereira, H. Chemical characterization and extractives composition of heartwood and sapwood from Quercus faginea. PLoS ONE 2017, 12, 0179268. [Google Scholar] [CrossRef] [PubMed]
- Vivas, N.; Hueso Oñate, J.A. Manual de Tonelería: Destinado a Usuarios de Toneles; Mundi Prensa: Madrid, Spain, 2005. [Google Scholar]
- Martínez-Gil, A.; Cadahía, E.; de Simón, B.F.; Gutiérrez-Gamboa, G.; Nevares, I.; del Álamo-Sanza, M. Quercus Humboldtii (Colombian Oak): Characterisation of wood phenolic composition with respect to traditional oak wood used in oenology. Cienc. Tec. Vitivinic. 2017, 32, 93–101. [Google Scholar] [CrossRef]
- De Rosso, M.; Panighel, A.; Vedova, A.D.; Stella, L.; Flamini, R. Changes in chemical composition of a red wine aged in acacia, cherry, chestnut, mulberry, and oak wood barrels. J. Agric. Food Chem. 2009, 57, 1915–1920. [Google Scholar] [CrossRef]
- Canas, S.; Caldeira, I.; Belchior, A.P.; Spranger, M.I.; Clímaco, M.C.; Bruno-de-Sousa, R. Chestnut Wooden Barrels for the Ageing of Wine Spirits. Organisation Internationale de la Vigne et du Vin (OIV) 2018. Available online: https://www.oiv.int/sites/default/files/2022-10/oiv-collective-expertise-chestnut-wooden-barrels-for-the-age2_EN.pdf (accessed on 24 June 2025).
- De Simón, B.F.; Martínez, J.; Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, A. Volatile compounds and sensorial characterisation of red wine aged in cherry, chestnut, false acacia, ash and oak wood barrels. Food Chem. 2014, 147, 346–356. [Google Scholar] [CrossRef]
- Kozlovic, G.; Jeromel, A.; Maslov, L.; Pollnitz, A.; Orlić, S. Use of acacia barrique barrels—Influence on the quality of Malvazija from Istria wines. Food Chem. 2010, 120, 698–702. [Google Scholar] [CrossRef]
- Pasheva, M.; Nashar, M.; Pavlov, D.; Slavova, S.; Ivanov, D.; Ivanova, D. Antioxidant Capacity of Different Woods Traditionally Used for Coloring Hard Alcoholic Beverages in Bulgaria. Med. Baltim. 2013, 3, 123–127. [Google Scholar]
- Tavares, M.; Jordão, A.M.; Ricardo-da-Silva, J.M. Impact of cherry, acacia and oak chips on red wine phenolic parameters and sensory profile. OENO One 2017, 51, 329–342. [Google Scholar] [CrossRef]
- Soares, B.; Garcia, R.; Freitas, A.M.C.; Cabrita, M.J. Phenolic compounds released from oak, cherry, chestnut and robinia chips into a syntethic wine: Influence of toasting level. Cienc. Tec. Vitivinic. 2012, 27, 17–26. [Google Scholar]
- Nunes, P.; Muxagata, S.; Correia, A.C.; Nunes, F.M.; Cosme, F.; Jordão, A.M. Effect of oak wood barrel capacity and utilization time on phenolic and sensorial profile evolution of an Encruzado white wine. J. Sci. Food Agric. 2017, 97, 4847–4856. [Google Scholar] [CrossRef]
- European Commission, E. Commission Regulation (EC) No 1881/2006 of 19 December 2006 setting maximum levels for certain contaminants in foodstuffs. Off. J. Eur. Union. 2006, 364, 5–24. [Google Scholar]
- Laqui-Estaña, J.; López-Solís, R.; Peña-Neira, Á.; Medel-Marabolí, M.; Obreque-Slier, E. Wines in contact with oak wood: The impact of the variety (Carménère and Cabernet Sauvignon), format (barrels, chips and staves), and aging time on the phenolic composition. J. Sci. Food Agric. 2018, 99, 436–448. [Google Scholar] [CrossRef] [PubMed]
- Caldeira, I.; Anjos, O.; Vitória, C.; Oliveira-Alves, S.; Fernandes, T.A.; Canas, S.; Catarino, S. The Interplay of Bottle Storage and Wood Ageing Technology: Volatile and Sensory Profiles of Wine Spirits Aged with Chestnut Wood. Food Bioprocess. Technol. 2025, 18, 1983–1996. [Google Scholar] [CrossRef]
- Cejudo-Bastante, M.J.; Hermosin-Gutierrez, I.; Perez-Coello, M.S. Accelerated aging against conventional storage: Effects on the volatile composition of Chardonnay white wines. J. Food Sci. 2013, 78, C507–C5013. [Google Scholar] [CrossRef] [PubMed]
- Spillman, P.J.; Sefton, M.A.; Gawel, R. The effect of oak Wood source, location of seasoning and coopering on the composition of volatile compounds in oak-matured wines. Aust. J. Grape Wine Res. 2004, 10, 216–226. [Google Scholar] [CrossRef]
- Obreque-Slier, E.; Molina-Tejada, N.; Medel-Marabolí, M.; López-Solís, R. Diverse phenolic responses of six varietal wines (Vitis vinifera L.) throughout ageing in contact with a single type of oak barrels. Int. J. Food Sci. Technol. 2023, 58, 2949–2961. [Google Scholar] [CrossRef]
- Prida, A.; Chatonnet, P. Impact of oak-derived compounds on the olfactory perception of barrel-aged wines. Am. J. Enol. Vitic. 2010, 61, 408–413. [Google Scholar] [CrossRef]
- Swan, J.S.; Burtles, S.M. V The development of flavour in potable spirits. Chem. Soc. Rev. 1978, 7, 201–211. [Google Scholar] [CrossRef]
- Masson, G.; Guichard, E.; Fournier, N.; Puech, J.L. Teneurs en stéréo-isomeres de la β-metil γ-octolactone des bois de chêne européens et amé ricains. Application aux vins et aux eaux-de-vie. J. Sci. Tech. Tonn. 1997, 3, 1–18. [Google Scholar]
- Guerrero-Chanivet, M.; Ortega-Gavilán, F.; Bagur-González, M.G.; Valcárcel-Muñoz, M.J.; García-Moreno, M.V.; Guillén-Sánchez, D.A. Influence of Oak Species, Toasting Degree, and Aging Time on the Differentiation of Brandies Using a Chemometrics Approach Based on Phenolic Compound UHPLC Fingerprints. J. Agric. Food Chem. 2024, 72, 1959–1968. [Google Scholar] [CrossRef]
- Morata, A. Red Wine Technology; Elsevier: London, UK, 2018; ISBN 9780128144008. [Google Scholar]
- Navarro, M.; Kontoudakis, N.; Giordanengo, T.; Gómez-Alonso, S.; García-Romero, E.; Fort, F.; Canals, J.M.; Hermosín-Gutíerrez, I.; Zamora, F. Oxygen consumption by oak chips in a model wine solution; Influence of the botanical origin, toast level and ellagitannin content. Food Chem. 2016, 199, 822–827. [Google Scholar] [CrossRef]
- Sanz, M.; de Simón, B.F.; Cadahía, E.; Esteruelas, E.; Muñoz, Á.M.; Hernández, M.T.; Estrella, I. Polyphenolic profile as a useful tool to identify the wood used in wine aging. Anal. Chim. Acta 2012, 732, 33–45. [Google Scholar] [CrossRef]
- Chinnici, F.; Natali, N.; Bellachioma, A.; Versari, A.; Riponi, C. Changes in phenolic composition of red wines aged in cherry wood. LWT-Food Sci. Technol. 2015, 60, 977–984. [Google Scholar] [CrossRef]
- Stadler, E.; Fischer, U. Sanitization of Oak Barrels for Wine—A Review. J. Agric. Food Chem. 2020, 68, 5283–5295. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Panighel, A.; De Marchi, F. Mass spectrometry in the study of wood compounds released in the barrel-aged wine and spirits. Mass. Spectrom. Rev. 2023, 42, 1174–1220. [Google Scholar] [CrossRef] [PubMed]
- Jarauta, I.; Cacho, J.; Ferreira, V. Concurrent phenomena contributing to the formation of the aroma of wine during aging in oak wood: An analytical study. J. Agric. Food Chem. 2005, 53, 4166–4177. [Google Scholar] [CrossRef]
- Sanz, M.; Cadahía, E.; Esteruelas, E.; Muñoz, Á.M.; De Simón, B.F.; Hernández, T.; Estrella, I. Phenolic compounds in cherry (Prunus avium) heartwood with a view to their use in cooperage. J. Agric. Food Chem. 2010, 58, 4907–4914. [Google Scholar] [CrossRef]
- Milheiro, J.; Filipe-Ribeiro, L.; Vilela, A.; Cosme, F.; Nunes, F.M. 4-Ethylphenol, 4-ethylguaiacol and 4-ethylcatechol in red wines: Microbial formation, prevention, remediation and overview of analytical approaches. Crit. Rev. Food Sci. Nutr. 2019, 59, 1367–1391. [Google Scholar] [CrossRef]
- Yang, W.; Zheng, Z.; Shi, Y.; Reynolds, A.G.; Duan, C.; Lan, Y. Volatile phenols in wine: Overview of origin, formation, analysis, and sensory expression. Crit. Rev. Food Sci. Nutr. 2024, 65, 3001–3026. [Google Scholar] [CrossRef]
- Cao, W.; Shu, N.; Wen, J.; Yang, Y.; Jin, Y.; Lu, W. Characterization of the Key Aroma Volatile Compounds in Nine Different Grape Varieties Wine by Headspace Gas Chromatography–Ion Mobility Spectrometry (HS-GC-IMS), Odor Activity Values (OAV) and Sensory Analysis. Foods 2022, 11, 2767. [Google Scholar] [CrossRef]
- Šikuten, I.; Štambuk, P.; Tomaz, I.; Marchal, C.; Kontić, J.K.; Lacombe, T.; Maletić, E.; Preiner, D. Discrimination of Genetic and Geographical Groups of Grape Varieties (Vitis vinifera L.) Based on Their Volatile Organic Compounds. Front. Plant Sci. 2022, 13, 942148. [Google Scholar] [CrossRef] [PubMed]
- Cassino, C.; Tsolakis, C.; Bonello, F.; Gianotti, V.; Osella, D. Wine evolution during bottle aging, studied by 1H NMR spectroscopy and multivariate statistical analysis. Food Res. Int. 2019, 116, 566–577. [Google Scholar] [CrossRef] [PubMed]
- Philippidis, A.; Poulakis, E.; Kontzedaki, R.; Orfanakis, E.; Symianaki, A.; Zoumi, A.; Velegrakis, M. Application of Ultraviolet-Visible Absorption Spectroscopy with Machine Learning Techniques for the Classification of Cretan Wines. Foods 2021, 10, 9. [Google Scholar] [CrossRef] [PubMed]
- Gilmore, A.M.; Sui, Q.; Blair, B.; Pan, B.S. Accurate varietal classification and quantification of key quality compounds of grape extracts using the absorbance-transmittance fluorescence excitation emission matrix (A-TEEM) method and machine learning. OENO One 2022, 56, 107–115. [Google Scholar] [CrossRef]
- Koljančić, N.; Furdíková, K.; Gomes, A.A.; Špánik, I. Wine authentication: Current progress and state of the art. Trends Food Sci. Technol. 2024, 150, 104598. [Google Scholar] [CrossRef]
- Vanzo, A.; Janeš, L.; Požgan, F.; Bolta, S.V.; Silvilotti, P.; Lisjak, K. UHPLC-MS/MS determination of varietal thiol precursors in Sauvignon Blanc grapes. Sci. Rep. 2017, 7, 13122. [Google Scholar] [CrossRef]
- Siebert, T.E.; Wood, C.; Elsey, G.M.; Pollnitz, A.P. Determination of rotundone, the pepper aroma impact compound, in grapes and wine. J. Agric. Food Chem. 2008, 56, 3745–3748. [Google Scholar] [CrossRef]
- Peirano-Bolelli, P.; Heller-Fuenzalida, F.; Cuneo, I.F.; Peña-Neira, Á.; Cáceres-Mella, A. Changes in the Composition of Flavonols and Organic Acids during Ripening for Three cv. Sauvignon Blanc Clones Grown in a Cool-Climate Valley. Agronomy 2022, 12, 1357. [Google Scholar] [CrossRef]
- Kwasniewski, M.T.; Vanden Heuvel, J.E.; Pan, B.S.; Sacks, G.L. Timing of cluster light environment manipulation during grape development affects C13 norisoprenoid and carotenoid concentrations in Riesling. J. Agric. Food Chem. 2010, 58, 6841–6849. [Google Scholar] [CrossRef]
- Mateus, N.; Machado, J.M.; de Freitas, V. Development changes of anthocyanins in Vitis vinifera grapes grown in the Douro Valley and concentration in respective wines. J. Sci. Food Agric. 2002, 82, 1689–1695. [Google Scholar] [CrossRef]
- Utz, F.; Kreissl, J.; Stark, T.D.; Schmid, C.; Tanger, C.; Kulozik, U.; Hofmann, T.; Dawid, C. Sensomics-Assisted Flavor Decoding of Dairy Model Systems and Flavor Reconstitution Experiments. J. Agric. Food Chem. 2021, 69, 6588–6600. [Google Scholar] [CrossRef]
- Moon, H.; Yu, S.; Ban, Y.; Park, H.; Hong, S.; Kim, K.; Shin, E.; Kim, H.; Jeong, E.; Shin, E.C. Sensomics combined with Chemometrics approaches of enzymatically hydrolyzed animal by-product proteins using biomimetic sensory-based machine perception techniques and gas chromatography-Olfactometry-mass spectrometry (GC-O-MS). Food Chem. X 2025, 26, 102343. [Google Scholar] [CrossRef] [PubMed]
- Merlino, M.; Arena, E.; Cincotta, F.; Condurso, C.; Brighina, S.; Grasso, A.; Fallico, B.; Verzera, A. Fat type and baking conditions for cookies recipe: A sensomic approach. Int. J. Food Sci. Technol. 2022, 57, 5943–5953. [Google Scholar] [CrossRef]
- Nicolotti, L.; Mall, V.; Schieberle, P. Characterization of Key Aroma Compounds in a Commercial Rum and an Australian Red Wine by Means of a New Sensomics-Based Expert System (SEBES)-An Approach To Use Artificial Intelligence in Determining Food Odor Codes. J. Agric. Food Chem. 2019, 67, 4011–4022. [Google Scholar] [CrossRef] [PubMed]
- Spataro, F.; Rosso, F.; Peraino, A.; Arese, C.; Caligiani, A. Key molecular compounds for simultaneous origin discrimination and sensory prediction of cocoa: An UHPLC-HRMS sensomics approach. Food Chem. 2025, 463, 141201. [Google Scholar] [CrossRef]
- Welke, J.; Hernandes, K.C.; Lago, L.O.; Silveira, R.D.; Marques, A.T.B.; Zini, C.A. Flavoromic analysis of wines using gas chromatography, mass spectrometry and sensory techniques. J. Chromatogr. A 2024, 1734, 465264. [Google Scholar] [CrossRef]
- Darnal, A.; Poggesi, S.; Longo, E.; Arbore, A.; Boselli, E. Decoding the Identity of Pinot Gris and Pinot Noir Wines: A Comprehensive Chemometric Fusion of Sensory (from Dual Panel) and Chemical Analysis. Foods 2024, 13, 18. [Google Scholar] [CrossRef]
- Viejo, C.; Fuentes, S.; Howell, K.; Torrico, D.; Dunshea, F. Integration of non-invasive biometrics with sensory analysis techniques to assess acceptability of beer by consumers. Physiol. Behav. 2019, 200, 139–147. [Google Scholar] [CrossRef]
- Marques, C.; Dinis, L.-T.; Modesti, M.; Bellincontro, A.; Correia, E.; Vilela, A. Exploring the influence of terroir on Douro white and red wines characteristics: A study of human perception and electronic analysis. Eur. Food Res. Technol. 2024, 250, 3011–3027. [Google Scholar] [CrossRef]
- Marques, C.; Vilela, A. FaceReader Insights into the Emotional Response of Douro Wines. Appl. Sci. 2024, 14, 10053. [Google Scholar] [CrossRef]
- Poggesi, S.; Darnal, A.; Ceci, A.; Longo, E.; Vanzo, L.; Mimmo, T.; Boselli, E. Fusion of 2DGC-MS, HPLC-MS and Sensory Data to Assist Decision-Making in the Marketing of International Monovarietal Chardonnay and Sauvignon Blanc Wines. Foods 2022, 11, 3458. [Google Scholar] [CrossRef]
- Chen, L.; Darriet, P. Strategies for the identification and sensory evaluation of volatile constituents in wine. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4549–4583. [Google Scholar] [CrossRef] [PubMed]
- Souza Gonzaga, L.; Bastian, S.E.P.; Capone, D.L.; Ranaweera, R.K.R.; Jeffery, D.W. Modelling Cabernet-Sauvignon wine sensory traits from spectrofluorometric data. OENO One 2021, 55, 19–33. [Google Scholar] [CrossRef]
- Zhu, W.; Benkwitz, F.; Kilmartin, P. Volatile-Based Prediction of Sauvignon Blanc Quality Gradings with Static Headspace-Gas Chromatography-Ion Mobility Spectrometry (SHS-GC-IMS) and Interpretable Machine Learning Techniques. J. Agric. Food Chem. 2021, 69, 3255–3265. [Google Scholar] [CrossRef]
- Schwindt, V.; Coletto, M.; Díaz, M.; Ponzoni, I. Could QSOR Modelling and Machine Learning Techniques Be Useful to Predict Wine Aroma? Food Bioprocess. Technol. 2023, 16, 24–42. [Google Scholar] [CrossRef]
- Ribeiro, J.; Ferreira, M.; Salva, T. Chemometric models for the quantitative descriptive sensory analysis of Arabica coffee beverages using near infrared spectroscopy. Talanta 2011, 83, 1352–1358. [Google Scholar] [CrossRef]
- Ferreira, M.; Augusto, F.; Ribeiro, J.; Salva, T. Prediction models for Arabica coffee beverage quality based on aroma analyses and chemometrics. Talanta 2012, 101, 253–260. [Google Scholar] [CrossRef]
- Yu, S.; Huang, X.; Wang, L.; Chang, X.; Ren, Y.; Zhang, X.; Wang, Y. Qualitative and quantitative assessment of flavor quality of Chinese soybean paste using multiple sensor technologies combined with chemometrics and a data fusion strategy. Food Chem. 2023, 405, 134859. [Google Scholar] [CrossRef]
- Yu, S.; Huang, X.; Wang, L.; Ren, Y.; Zhang, X.; Wang, Y. Characterization of selected Chinese soybean paste based on flavor profiles using HS-SPME-GC/MS, E-nose and E-tongue combined with chemometrics. Food Chem. 2022, 375, 131840. [Google Scholar] [CrossRef]
- Yu, S.; Huang, X.; Wang, L.; Wang, Y.; Jiao, X.; Zhang, X.; Tian, X.; Ren, Y.; Chang, X. Characterization of the volatile flavor profiles of black garlic using nanomaterial-based colorimetric sensor array, HS-SPME-GC/MS coupled with chemometrics strategies. Food Chem. 2024, 458, 140213. [Google Scholar] [CrossRef]
- Shen, C.; Cai, Y.; Wu, X.; Gai, S.; Wang, B.; Liu, D. Characterization of selected commercially available grilled lamb shashliks based on flavor profiles using GC-MS, GC × GC-TOF-MS, GC-IMS, E-nose and E-tongue combined with chemometrics. Food Chem. 2023, 423, 136257. [Google Scholar] [CrossRef] [PubMed]
- McCune, J.; Riley, A.; Chen, B. Clustering in Wineinformatics with Attribute Selection to Increase Uniqueness of Clusters. Fermentation 2021, 7, 27. [Google Scholar] [CrossRef]
- Vilela, A.; Marques, C.; Correia, E. Structural Equation Modelling (SEM) applied to sensory profile of Vinho Verde monovarietal wines. Food Res. Int. 2018, 111, 650–660. [Google Scholar] [CrossRef] [PubMed]
- Meléndez, F.; Arroyo, P.; Gómez-Suárez, J.; Palomeque-Mangut, S.; Suárez, J.I.; Lozano, J. Portable Electronic Nose Based on Digital and Analog Chemical Sensors for 2,4,6-Trichloroanisole Discrimination. Sensors 2022, 22, 3453. [Google Scholar] [CrossRef] [PubMed]
- Hasan, M.; Alev, O.; Skrabanek, P.; Cheffena, M. Molecularly Imprinted Polymer-Based Electronic Nose for Ultrasensitive, Selective Detection, and Concentration Estimation of VOC Mixtures. IEEE Sens. J. 2025, 25, 18277–18290. [Google Scholar] [CrossRef]
- Alfieri, G.; Modesti, M.; Riggi, R.; Bellincontro, A. Recent Advances and Future Perspectives in the E-Nose Technologies Addressed to the Wine Industry. Sensors 2024, 24, 2293. [Google Scholar] [CrossRef]
- Cho, S.; Moazzem, M. Recent Applications of Potentiometric Electronic Tongue and Electronic Nose in Sensory Evaluation. Prev. Nutr. Food Sci. 2022, 27, 354–364. [Google Scholar] [CrossRef]
- Xi, Y.; Yang, Y.; Chi, X.; Wang, W.; Sun, B.; Ai, N. Characterization of the flavor profile of UHT milk during shelf-life via volatile metabolomics fingerprinting combined with chemometrics. Food Res. Int. 2024, 191, 114705. [Google Scholar] [CrossRef]
- Ranaweera, R.K.R.; Capone, D.L.; Bastian, S.E.P.; Cozzolino, D.; Jeffery, D.W. A Review of Wine Authentication Using Spectroscopic Approaches in Combination with Chemometrics. Molecules 2021, 26, 4334. [Google Scholar] [CrossRef]
- Barátossy, G.; Berinkeiné Donkó, M.; Csikorné Vásárhelyi, H.; Héberger, K.; Rácz, A. Comprehensive Classification and Regression Modeling of Wine Samples Using 1H NMR Spectra. Foods 2021, 10, 64. [Google Scholar] [CrossRef]
- Uttl, L.; Bechynska, K.; Ehlers, M.; Kadlec, V.; Navratilova, K.; Džuman, Z.; Fauhl-Hassek, C.; Hajšlová, J. Critical assessment of chemometric models employed for varietal authentication of wine based on UHPLC-HRMS data. Food Control 2022, 143, 109336. [Google Scholar] [CrossRef]
- Leong, Y.X.; Lee, Y.H.; Koh, C.S.L.; Phan-Quang, G.C.; Han, X.; Phang, I.Y.; Ling, X.Y. Surface-Enhanced Raman Scattering (SERS) Taster: A Machine-Learning-Driven Multireceptor Platform for Multiplex Profiling of Wine Flavors. Nano Lett. 2021, 21, 2642–2649. [Google Scholar] [CrossRef] [PubMed]
- Niimi, J.; Liland, K.H.; Tomic, O.; Jeffery, D.W.; Bastian, S.E.P.; Boss, P.K. Prediction of wine sensory properties using mid-infrared spectra of Cabernet Sauvignon and Chardonnay grape berries and wines. Food Chem. 2021, 344, 128634. [Google Scholar] [CrossRef] [PubMed]
- Baqueta, M.; Coqueiro, A.; Valderrama, P. Brazilian Coffee Blends: A Simple and Fast Method by Near-Infrared Spectroscopy for the Determination of the Sensory Attributes Elicited in Professional Coffee Cupping. J. Food Sci. 2019, 84, 1247–1255. [Google Scholar] [CrossRef]
- Yin, H.; Hu, X.; Huang, X.; Zou, X.; Xu, Y.; Shi, J.; Yang, M. Rapid Discrimination of Beer Flavors Using Ion-Selective Electrode Array System Combined with Chemometrics. Food Anal. Methods 2021, 14, 1836–1842. [Google Scholar] [CrossRef]
- Ouyang, Q.; Wang, L.; Park, B.; Kang, R.; Wang, Z.; Chen, Q.; Guo, Z. Assessment of matcha sensory quality using hyperspectral microscope imaging technology. LWT Food Sci. Technol. 2020, 125, 109254. [Google Scholar] [CrossRef]
- Liberto, E.; Bressanello, D.; Strocchi, G.; Cordero, C.; Ruosi, M.; Pellegrino, G.; Bicchi, C.; Sgorbini, B. HS-SPME-MS-Enose Coupled with Chemometrics as an Analytical Decision Maker to Predict In-Cup Coffee Sensory Quality in Routine Controls: Possibilities and Limits. Molecules 2019, 24, 4515. [Google Scholar] [CrossRef]
- Dowling, G.M.; Aliani, M. Editorial: Novel technologies applied to flavoromics and sensory evaluation of foods. Front. Nutr. 2025, 11, 1544709. [Google Scholar] [CrossRef]
- Belchior, V.; Botelho, B.G.; Oliveira, L.S.; Franca, A.S. Attenuated Total Reflectance Fourier Transform Spectroscopy (ATR-FTIR) and chemometrics for discrimination of espresso coffees with different sensory characteristics. Food Chem. 2019, 273, 178–185. [Google Scholar] [CrossRef]
- Gerhardt, N.; Schwolow, S.; Rohn, S.; Pérez-Cacho, P.; Galán-Soldevilla, H.; Arce, L.; Weller, P. Quality assessment of olive oils based on temperature-ramped HS-GC-IMS and sensory evaluation: Comparison of different processing approaches by LDA, kNN, and SVM. Food Chem. 2019, 286, 307–308. [Google Scholar] [CrossRef]
- Bressanello, D.; Marengo, A.; Cordero, C.; Strocchi, G.; Rubiolo, P.; Pellegrino, G.; Ruosi, M.; Bicchi, C.; Liberto, E. Chromatographic Fingerprinting Strategy to Delineate Chemical Patterns Correlated to Coffee Odor and Taste Attributes. J. Agric. Food Chem. 2021, 69, 4550–4560. [Google Scholar] [CrossRef] [PubMed]
- Basalekou, M.; Tataridis, P.; Georgakis, K.; Tsintonis, C. Measuring Wine Quality and Typicity. Beverages 2023, 9, 41. [Google Scholar] [CrossRef]
- Koljančić, N.; Gomes, A.A.; Špánik, I. A non-target geographical origin screening of botrytized wines through comprehensive two-dimensional gas chromatography coupled with high-resolution mass spectrometry. J. Sep. Sci. 2023, 46, 2300249. [Google Scholar] [CrossRef] [PubMed]
- Sáenz-Navajas, M.P.; Ballester, J.; Pêcher, C.; Peyron, D.; Valentin, D. Sensory drivers of intrinsic quality of red wines. Effect of culture and level of expertise. Food Res Int. 2013, 54, 1506–1518. [Google Scholar] [CrossRef]
- Villamor, R.; Ross, C. Wine matrix compounds affect perception of wine aromas. Annu. Rev. Food Sci. Technol. 2013, 4, 1–20. [Google Scholar] [CrossRef]
- Atanasova, B.; Thomas-Danguin, T.; Chabanet, C.; Langlois, D.; Nicklaus, S.; Etiévant, P. Perceptual interactions in odour mixtures: Odour quality in binary mixtures of woody and fruity wine odorants. Chem. Senses 2005, 30, 209–217. [Google Scholar] [CrossRef]
- Presa-Owens, C.; Noble, A.C. Descriptive analysis of three white wine varieties from Penedes. Am. J. Enol. Vitic. 1995, 46, 5–9. [Google Scholar] [CrossRef]
- Varela, P.; Ares, G. Sensory profiling, the blurred line between sensory and consumer science. A review of novel methods for product characterization. Food Res. Int. 2012, 48, 893–908. [Google Scholar] [CrossRef]
- Fariña, L.; Villar, V.; Ares, G.; Carrau, F.; Dellacassa, E.; Boido, E. Volatile composition and aroma profile of Uruguayan Tannat wines. Food Res. Int. 2015, 69, 244–255. [Google Scholar] [CrossRef]
- Dias, L.G.; Meirinho, S.G.; Veloso, A.C.A.; Rodrigues, L.R.; Peres, A.M. Electronic tongues and aptasensors. In Bioinspired Materials for Medical Applications; Rodrigues, L., Mota, M., Eds.; Woodhead Publishing: Cambridge, UK, 2017; pp. 371–402. [Google Scholar]
- Lozano, J.; Arroyo, T.; Santos, J.P.; Cabellos, J.M.; Horrillo, M.C. Electronic nose for wine ageing detection. Sens. Actuators B Chem. 2008, 133, 180–186. [Google Scholar] [CrossRef]
- Lozano, J.; Santos, J.P.; Horrillo, M.C. Enrichment sampling methods for wine discrimination with gas sensors. J. Food Compos. Anal. 2008, 21, 716–723. [Google Scholar] [CrossRef]
- Prieto, N.; Rodriguez-Méndez, M.L.; Leardi, R.; Oliveri, P.; Hernando-Esquisabel, D.; Iñiguez-Crespo, M.; de Saja, J.A. Application of multiway analysis to UV–visible spectroscopy, gas chromatography and electronic nose data for wine ageing evaluation. Anal. Chim. Acta 2012, 719, 43–51. [Google Scholar] [CrossRef]
- Berna, A.Z.; Trowell, S.; Clifford, D.; Cynkar, W.; Cozzolino, D. Geographical origin of Sauvignon Blanc wines predicted by mass spectrometry and metal oxide based electronic nose. Anal. Chim. Acta 2009, 648, 146–152. [Google Scholar] [CrossRef]
- Gehlken, J.; Pour Nikfardjam, M.; Zörb, C. Prediction of sensory attributes in winemaking grapes by on-line near-infrared spectroscopy based on selected volatile aroma compounds. Anal. Bioanal. Chem. 2023, 415, 1515–1527. [Google Scholar] [CrossRef]
- Muñoz-Castells, R.; Modesti, M.; Moreno-García, J.; Catini, A.; Capuano, R.; Di Natale, C.; Bellincontro, A.; Moreno, J. Application of an Electronic Nose to the Prediction of Odorant Series in Wines Obtained with Saccharomyces or Non-Saccharomyces Yeast Strains. Molecules 2025, 30, 1584. [Google Scholar] [CrossRef]
- Armstrong, C.; Niimi, J.; Boss, P.; Pagay, V.; Jeffery, D. Use of Machine Learning with Fused Spectral Data for Prediction of Product Sensory Characteristics: The Case of Grape to Wine. Foods 2023, 12, 757. [Google Scholar] [CrossRef]
- Rizwana, S.; Priya, P.; Suvarshitha, K.; Gayathri, M.; Ramakrishna, E.; Sireesha, M. Enhancing Wine Quality Prediction Through Machine Learning Techniques. In Proceedings of the 2025 IEEE International Conference on Interdisciplinary Approaches in Technology and Management for Social Innovation (IATMSI), Gwalior, India, 6–8 March 2025; pp. 1–6. [Google Scholar] [CrossRef]
- Vazaram, B.; Sankar, D.; Lokesh, M.; Mallikarjuna, M. Red Wine Quality Prediction using Machine Learning. Int. J. Innov. Sci. Res. Technol. 2024, 9, 2731–2734. [Google Scholar] [CrossRef]
- Dahal, K.; Dahal, J.; Banjade, H.; Gaire, S. Prediction of Wine Quality Using Machine Learning Algorithms. Open J. Stat. 2021, 11, 278–289. [Google Scholar] [CrossRef]
- Jain, K.; Kaushik, K.; Gupta, S.; Mahajan, S.; Kadry, S. Machine learning-based predictive modelling for the enhancement of wine quality. Sci. Rep. 2023, 13, 17042. [Google Scholar] [CrossRef]
- Kumar, S.; Agrawal, K.; Mandan, N. Red Wine Quality Prediction Using Machine Learning Techniques. In Proceedings of the 2020 International Conference on Computer Communication and Informatics (ICCCI), Coimbatore, India, 22–24 January 2020; pp. 1–6. [Google Scholar] [CrossRef]
- Zhou, M.; Yu, W.; Jiang, K. Wine Quality Detection Based on Improved Stacking Ensemble Learning. In Proceedings of the 2023 8th International Conference on Information Systems Engineering (ICISE), Dalian, China, 23–25 June 2023; pp. 226–229. [Google Scholar] [CrossRef]
- Lu, N.; Tansuchat, R.; Yuizono, T.; Huynh, V. Incorporating Active Learning into Machine Learning Techniques for Sensory Evaluation of Food. Int. J. Comput. Intell. Syst. 2020, 13, 655–662. [Google Scholar] [CrossRef]
- Sáenz-Navajas, M.; Ferrero-Del-Teso, S.; Romero, M.; Pascual, D.; Díaz, D.; Ferreira, V.; Fernández-Zurbano, P. Modelling wine astringency from its chemical composition using machine learning algorithms. Special Macrowine 2018 (Sarragosse). OENO One 2019, 53, 499–509. [Google Scholar] [CrossRef]
- Gupta, Y. Selection of important features and predicting wine quality using machine learning techniques. Procedia Comput. Sci. 2018, 125, 305–312. [Google Scholar] [CrossRef]
- Yavas, C.; Kim, J.; Chen, L. Mastering Precision in Pivotal Variables Defining Wine Quality via Incremental Analysis of Baseline Accuracy. IEEE Access 2024, 12, 105429–105459. [Google Scholar] [CrossRef]
- Ji, H.; Pu, D.; Yan, W.; Zhang, Q.; Zuo, M.; Zhang, Y. Recent advances and application of machine learning in food flavor prediction and regulation. T. Foods Sci. Technol. 2023, 138, 738–751. [Google Scholar] [CrossRef]
- Feng, T.; Hu, Z.; Chen, L.; Chen, D.; Wang, X.; Yao, L.; Sun, M.; Song, S.; Wang, H. Quantitative structure-activity relationships (QSAR) of aroma compounds in different aged Huangjiu. J. Food Sci. 2020, 85, 3273–3281. [Google Scholar] [CrossRef] [PubMed]
- Fuentes, S.; Torrico, D.D.; Tongson, E.; Gonzalez Viejo, C. Machine Learning Modeling of Wine Sensory Profiles and Color of Vertical Vintages of Pinot Noir Based on Chemical Fingerprinting, Weather and Management Data. Sensors 2020, 20, 3618. [Google Scholar] [CrossRef]
- De, P.; Kar, S.; Ambure, P.; Roy, K. Prediction reliability of QSAR models: An overview of various validation tools. Arch. Toxicol. 2022, 96, 1279–1295. [Google Scholar] [CrossRef]
- Konovalov, D.A.; Llewellyn, L.E.; Vander Heyden, Y.; Coomans, D. Robust cross-validation of linear regression QSAR models. J. Chem. Inf. Model. 2008, 48, 2081–2294. [Google Scholar] [CrossRef]
- Roy, K.; Mitra, I.; Kar, S.; Ojha, P.K.; Das, R.N.; Kabir, H. Comparative studies on some metrics for external validation of QSPR models. J. Chem. Inf. Model. 2012, 52, 396–408. [Google Scholar] [CrossRef]
- Ojha, P.K.; Mitra, I.; Das, R.N.; Roy, K. Further exploring rm2 metrics for validation of QSPR models. Chemom. Intell. Lab. Syst. 2011, 107, 194–205. [Google Scholar] [CrossRef]
- Roy, K.; Das, R.N.; Ambure, P.; Aher, R.B. Be aware of error measures. Further studies on validation of predictive QSAR models. Chemom. Intell. Lab. Syst. 2016, 152, 18–33. [Google Scholar] [CrossRef]
- Goodarzi, M.; Dejaegher, B.; Heyden, Y.V. Feature selection methods in QSAR studies. J. AOAC Int. 2012, 95, 636–651. [Google Scholar] [CrossRef] [PubMed]
- Palczewska, A.; Neagu, D.; Ridley, M. Using Pareto points for model identification in predictive toxicology. J. Cheminform 2013, 5, 16. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martínez, M.J.; Ponzoni, I.; Díaz, M.F.; E Vazquez, G.; Soto, A.J. Visual analytics in cheminformatics: User-supervised descriptor selection for QSAR methods. J. Cheminform 2015, 7, 39. [Google Scholar] [CrossRef] [PubMed]
- Shahlaei, M. Descriptor selection methods in quantitative structure-activity relationship studies: A review study. Chem. Rev. 2013, 113, 8093–8103. [Google Scholar] [CrossRef]
- Mamy, L.; Patureau, D.; Barriuso, E.; Bedos, C.; Bessac, F.; Louchart, X.; Benoit, P. Prediction of the Fate of Organic Compounds in the Environment From Their Molecular Properties: A Review. Crit. Rev. Environ. Sci. Technol. 2015, 45, 1277–1377. [Google Scholar] [CrossRef]
- Danishuddin; Khan, A.U. Descriptors and their selection methods in QSAR analysis: Paradigm for drug design. Drug Discov. Today 2016, 21, 1291–1302. [Google Scholar] [CrossRef]
- Ojha, P.K.; Roy, K. Chemometric modeling of odor threshold property of diverse aroma components of wine. RSC Adv. 2018, 8, 4750–4760. [Google Scholar] [CrossRef]
- Li, N.; Li, G.; Guan, X.; Li, A.; Tao, Y. Volatile aroma compound-based decoding and prediction of sweet berry aromas in dry red wine. Food Chem. 2025, 463, 141248. [Google Scholar] [CrossRef]
- Harris, N.; Gonzalez Viejo, C.; Barnes, C.; Fuentes, S. Non-Invasive Digital Technologies to Assess Wine Quality Traits and Provenance through the Bottle. Fermentation 2023, 9, 10. [Google Scholar] [CrossRef]
- Macías, M.M.; Manso, A.G.; Orellana, C.J.G.; Velasco, H.M.G.; Caballero, R.G.; Chamizo, J.C.P. Acetic acid detection threshold in synthetic wine samples of a portable electronic nose. Sensors 2013, 13, 208–220. [Google Scholar] [CrossRef] [PubMed]
- Santos, J.P.; Lozano, J.; Aleixandre, M.; Arroyo, T.; Cabellos, J.M.; Gil, M.; Horrillo, M.C. Threshold detection of aromatic compounds in wine with an electronic nose and a human sensory panel. Talanta 2010, 80, 1899–1906. [Google Scholar] [CrossRef] [PubMed]
- Diako, C.; Vixie, B.; Weller, K.M.; Dycus, D.A.; Ross, C.F. Determination of 4-ethylcatechol in a Merlot wine using sensory evaluation and the electronic tongue. Int. J. Food Sci. Technol. 2017, 52, 2489–2496. [Google Scholar] [CrossRef]
- Paup, V.D.; Cook-Barton, T.; Diako, C.; Edwards, C.G.; Ross, C.F. Detection of red wine faults over time with flash profiling and the electronic tongue. Beverages 2021, 7, 52. [Google Scholar] [CrossRef]
- Verrelli, G.; Francioso, L.; Paolesse, R.; Siciliano, P.; Di Natale, C.; D’Amico, A.; Logrieco, A. Development of silicon-based potentiometric sensors: Towards a miniaturized electronic tongue. Sens. Actuators B Chem. 2007, 123, 191–197. [Google Scholar] [CrossRef]
- Louw, L.; Oelofse, S.; Naes, T.; Lambrechts, M.; van Rensburg, P.; Nieuwoudt, H. Trained sensory panellists’ response to product alcohol content in the projective mapping task: Observations on alcohol content, product complexity and prior knowledge. Food Qual. Prefer. 2014, 34, 37–44. [Google Scholar] [CrossRef]
- Ragazzo-Sánchez, J.A.; Chalier, P.; Ghommidh, C. Coupling gas chromatography and electronic nose for dehydration and desalcoholization of alcoholized beverages: Application to off-flavour detection in wine. Sens. Actuators B Chem. 2005, 106, 253–257. [Google Scholar] [CrossRef]
- Sánchez, R.; Lozano, J.; Arroyo, P.; Meléndez, F. Detection of 2,4,6-Trichloroanisole in Sparkling Wines Using a Portable E-Nose and Chemometric Tools. Chemosensors 2025, 13, 178. [Google Scholar] [CrossRef]
- Loureiro, V.; Malfeito-Ferreira, M. Spoilage yeasts in the wine industry. Int. J. Food Microbiol. 2003, 86, 23–50. [Google Scholar] [CrossRef] [PubMed]
- Suárez, R.; Suárez-Lepe, J.A.; Morata, A.; Calderón, F. The production of ethylphenols in wine by yeasts of the genera Brettanomyces and Dekkera: A review. Food Chem. 2007, 102, 10–21. [Google Scholar] [CrossRef]
- Borisova, B.; Villalonga, M.L.; Arévalo-Villena, M.; Boujakhrout, A.; Sánchez, A.; Parrado, C.; Pingarrón, J.M.; Briones-Pérez, A.; Villalonga, R. Disposable electrochemical immunosensor for Brettanomyces bruxellensis based on nanogold-reduced graphene oxide hybrid nanomaterial. Anal. Bioanal. Chem. 2017, 409, 5667–5674. [Google Scholar] [CrossRef] [PubMed]
- Villalonga, M.L.; Borisova, B.; Arenas, C.B.; Villalonga, A.; Arévalo-Villena, M.; Sánchez, A.; Pingarrón, J.M.; Briones-Pérez, A.; Villalonga, R. Disposable electrochemical biosensors for Brettanomyces bruxellensis and total yeast content in wine based on core-shell magnetic nanoparticles. Sens. Actuators B Chem. 2019, 279, 15–21. [Google Scholar] [CrossRef]
- Portugal-Gómez, P.; Asunción Alonso-Lomillo, M.; Domínguez-Renedo, O. 4-ethyphenol detection in wine by fullerene modified screen-printed carbon electrodes. Microchem. J. 2022, 180, 107599. [Google Scholar] [CrossRef]
- Berna, A.Z.; Trowell, S.; Cynkar, W.; Cozzolino, D. Comparison of metal oxide-based electronic nose and mass spectrometry-based electronic nose for the prediction of red wine spoilage. J. Agric. Food Chem. 2008, 56, 3238–3244. [Google Scholar] [CrossRef]
- Cynkar, W.; Cozzolino, D.; Dambergs, B.; Janik, L.; Gishen, M. Feasibility study on the use of a head space mass spectrometry electronic nose (MS e_nose) to monitor red wine spoilage induced by Brettanomyces yeast. Sens. Actuators B Chem. 2007, 124, 167–171. [Google Scholar] [CrossRef]
- González-Calabuig, A.; del Valle, M. Voltammetric electronic tongue to identify Brett character in wines. On-site quantification of its ethylphenol metabolites. Talanta 2018, 179, 70–74. [Google Scholar] [CrossRef]
- Gonzalez Viejo, C.; Fuentes, S. Digital Assessment and Classification of Wine Faults Using a Low-Cost Electronic Nose, Near-Infrared Spectroscopy and Machine Learning Modelling. Sensors 2022, 22, 2303. [Google Scholar] [CrossRef]
- Gamboa, J.C.R.; Albarracin, E.S.A.; da Silva, A.; Ferreira, T.A.E. Electronic nose dataset for detection of wine spoilage thresholds. Data Brief 2019, 25, 104202. [Google Scholar] [CrossRef]
- Gamboa, J.C.R.; da Silva, A.J.; Araujo, I.C.; Albarracin, E.E.S.; Duran, A.C.M. Validation of the rapid detection approach for enhancing the electronic nose systems performance, using different deep learning models and support vector machines. Sens. Actuators B Chem. 2021, 327, 128921. [Google Scholar] [CrossRef]
- Meléndez, F.; Arroyo, P.; Herrero, J.L.; Fernández, J.A.; Carmona, P.; Rodríguez, S.; Lozano, J. Fast Detection of TCA in Cork Stoppers by Means of Electronic Noses. In Proceedings of the 2020 IEEE International Symposium on Circuits and Systems (ISCAS), Seville, Spain, 10–21 October 2020; pp. 1–4. [Google Scholar]
- Santos, J.P.; Sayago, I.; Sanjurjo, J.L.; Perez-Coello, M.S.; Díaz-Maroto, M.C. Rapid and Non-Destructive Analysis of Corky Off-Flavors in Natural Cork Stoppers by a Wireless and Portable Electronic Nose. Sensors 2022, 22, 4687. [Google Scholar] [CrossRef]
- Franceschi, D.; Cavalet, E.; Boatto, V.; Conte, G.; Bravi, M. Artificial diagnosis of sensory taints due to Brettanomyces spp. contamination in Valpolicella wines. Chem. Eng. Trans. 2016, 54, 343–348. [Google Scholar] [CrossRef]
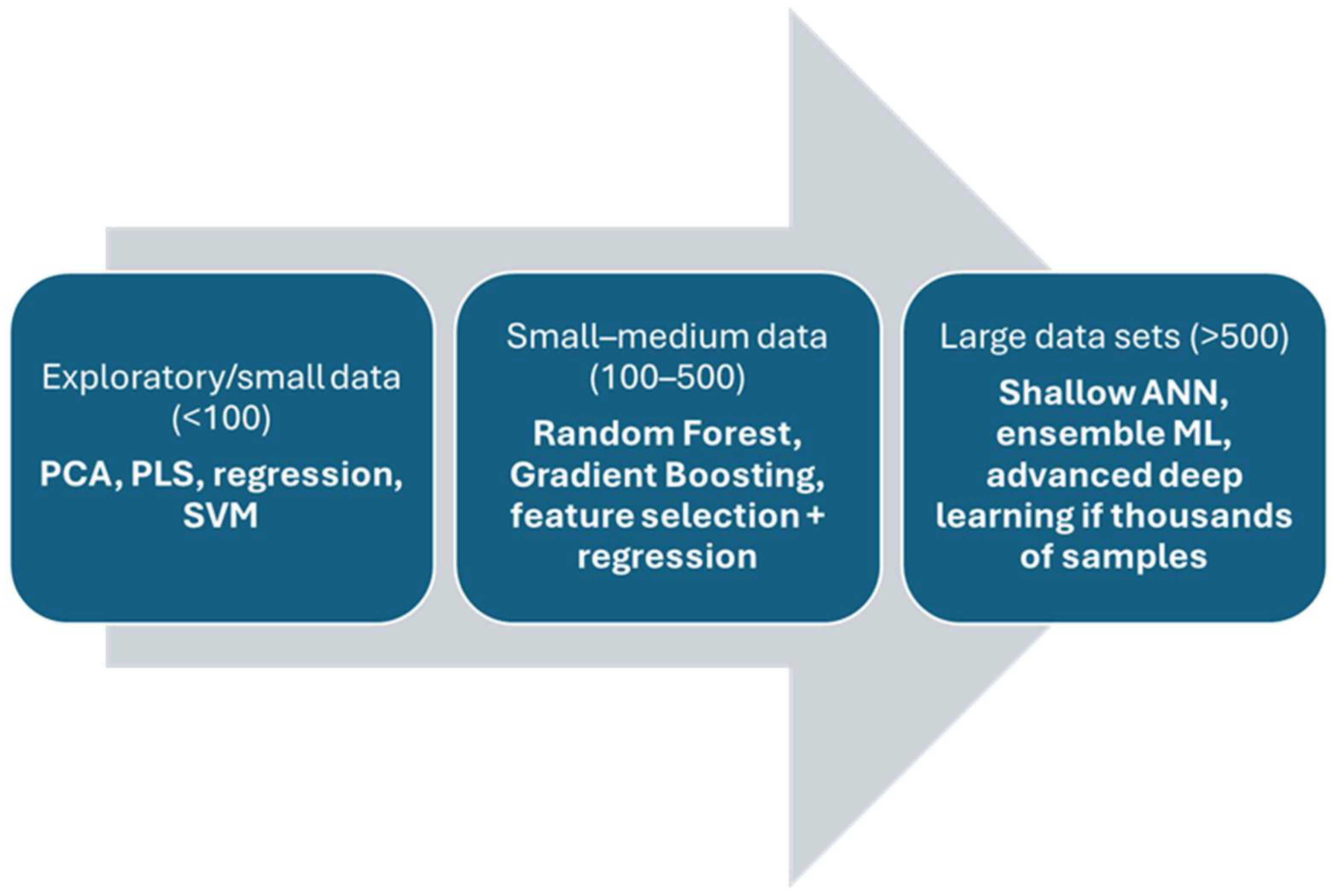
| Year/Period | Technology | Contribution/Development | Notes | References |
|---|---|---|---|---|
| Early 1900s | E-nose (concept) | Demonstrated detection of small amounts of aromatic compounds by non-biological means | Early concept of artificial olfaction | [17] |
| 1954 | E-nose | First odor measurement tool: microelectrode (platinum wire) and millivoltmeter | First instrumental approach | [18] |
| Late 1950s | E-nose | Mechanical olfactory system discriminating between simple and complex aromas | Early mechanical sensing | [19,20] |
| 1979 | E-nose | Introduction of acoustic wave chemical vapor sensors | Bulk Acoustic Wave and Surface Acoustic Wave sensors, operating at 1–500 MHz | [21] |
| 1982 | E-nose | First intelligent artificial nose with three metal oxide sensors, identifying up to 20 odorants | Milestone in E-nose development. Defined E-nose as sensor array and pattern recognition | [22] |
| Mid-1980s | E-nose | Integrated sensor with 6 metal oxide semiconductors | Quantified and identified scents s | [23] |
| 1985 | E-tongue | First liquid analysis system using a sensor array | Foundation of E-tongue technology | [24] |
| 1988 | E-nose | Coined the term “electronic nose” | Standard terminology established | [25] |
| 1990 | E-tongue | First system with partially selective sensors for qualitative liquid analysis | Enabled classification of liquids | [26] |
| 1990 | E-tongue | Pioneering concept of taste sensors | Marked the start of modern E-tongue development | [27] |
| Early 1990s | E-tongue | “E-tongue” using potentiometric electrodes with non-specific and cross-sensitive sensors | Key E-tongue breakthrough | [28] |
| 1990s | E-tongue | Active global research on liquid sensor arrays | Enabled commercialization around 2000 | [29] |
| Early 1990s | E-nose | First commercial electronic noses | Market introduction | [30,31] |
| 1997 | E-tongue | Voltammetric E-tongue with multiple metal electrodes reference, and auxiliary electrodes | Expanded sensing materials. Added reference and auxiliary electrodes | [32,33] |
| 1990s–Present | E-tongue | Development of multiple operational modes: electrochemical, enzymatic, optical, and mass-based | Tailored sensor arrays for target samples | [34] |
| 1990s–Present | E-tongue | Widespread use of potentiometric E-tongues (ion-selective electrodes) | Cost-effective, flexible, high selectivity; limited by temperature and adsorption effects | [35] |
| 1990s–Present | E-tongue | Development of voltammetric sensors for redox-active constituents | High selectivity, low detection limits; limited by temperature fluctuations and surface degradation | [36] |
| 2000s | E-nose | Portable E-nose developed | Enabled on-site analysis | [37] |
| Early 2000s | E-tongue | Introduction of impedimetric E-tongues | No reference electrode required; chemosensitive electrodes | [38] |
| 2010–2020 | E-nose | Nanoparticle-based sensors with higher sensitivity/selectivity | Significant performance improvements | [21] |
| 2010s | E-nose and E-tongue | Fusion of E-nose and E-tongue | Better classification accuracy | [39,40] |
| 2000s–present | E-nose | Bioelectronic noses and tongues integrating biosensors into sensor arrays | Use same chemometric tools as conventional systems | [41,42,43] |
| Recent years | E-nose | Integration with gas chromatography (including ultrafast and miniaturized headspace GC) | Improved identification capabilities | [44,45,46,47,48] |
| Species | Chemical Composition | Resulting Wine Properties | References |
|---|---|---|---|
| Traditional Woods Used in Cooperage | |||
| American oak (Q. alba) East USA | Contribution to whiskey-lactones | Slight risk of green taste, low tannin content, sugary character, fast wood intake | [117] |
| French oak (Q. petraea or Q. robur) North France | Higher content in phenols and flavonoids | Green taste with too short drying, high tannin content, limited aromatic contribution, slow wood intake | [117] |
| Non-traditional Woods in Cooperage from Oak Species | |||
| Q. pyrenaica Western Atlantic–Mediterranean regions | Ellagitannins, low-weight compounds, and aromatic compounds | Higher aromatic intensity and complexity. Woody, balsamic and cocoa notes. High levels of eugenol, guaiacol, cis-β-methyl-γ-octalactone, and other volatile phenols | [118,119,120,121] |
| Q. faginea Iberian Peninsula and North Africa | Castalagin and vescalagin are the main ellagitannins | Wines related to trans-resveratrol, p-hydroxybenzaldehyde, syringic acid, ellagic acid, and 5–HMF | [122,123] |
| Q. frainetto Balkan Peninsula, South Italy, and Northwest Turkey | High content in ellagitannins | High bitterness and particular and indefinable aromas. The natural drying and toasting of the wood can cushion both attributes. | [124] |
| Q. humboldtii Colombia | Phenolic acids, aldehydes, and ellagitannins | Balanced syringaldehyde/vanillin relationship. Higher concentrations of 5-methylfurfural, guaiacol, isoeugenol, trans-isoeugenol, and syringol. Lower furfural, 5–HMF, trans-β-methyl-γ-octalactone, and cis-β-methyl-γ-octalactone content | [115,125] |
| Q. oocarpa South America | Monomers of ellagitannins | Regarding the gustatory aspect, it is similar to Q. petraea | [124] |
| Untraditional Woods in Cooperage: Different from Oak Species | |||
| Castanea sativa Southern Europe and Asia | Low content of oxidizable polyphenols (less suitable for prolonged aging) | Higher content of total phenolic compounds and low molecular weight compounds. Higher antioxidant activities. Vanilla notes | [126,127] |
| Robinia pseudoacacia USA, Europe | Rich in mono and di-methoxyphenols, acetosyringone and ethyl vanillate. High content of simple volatile phenolic compounds | Red wines with higher smoky, spicy, and fruity notes | [128,129] |
| Fraxinus spp. Europe, Asia Minor, and North Africa | High content of 3-ethyl and 3,5-dimethylcyclotene, o-cresol, α-methylcrotonalactone, and vanillin. Low content of furanic derivatives | Less vanilla notes than oak | [128] |
| Morus spp. Asia, Africa, Europe, and North, Central, and South America | Decrease in fruity-note ethyl esters and ethyl-guaiacol and the high concentration of ethyl-phenol (a horsey-odor defect) | Hardly suitable for wine aging | [126,130] |
| P. avium and P. cerasus Europe and western Asia | Aromadendrin, naringenin, taxifolin, isosakuranetin, eriodictyol, and prunin | Greater oxygen penetration through their staves | [126,131,132] |
| Feature | Chemometric Approaches | Human Sensory Panels | References |
|---|---|---|---|
| Speed | Rapid, high-throughput | Slow, labor-intensive | [193,194] |
| Reproducibility | High, protocol-driven | Variable, subjective | [193,194,195] |
| Objectivity | Data-driven, unbiased | Prone to human bias | [193,194] |
| Scalability | Easily automated | Limited by panel size | [193,194] |
| Food Matrix | Approach Used | Prediction/Classification Accuracy | References |
|---|---|---|---|
| Wine | SERS (Surface-Enhanced Raman Scattering) + chemometrics + machine learning | Perfect accuracy in flavor quantification | [196] |
| Soybean Paste | E-nose/E-tongue + chemometrics + data fusion | Rp up to 0.96 for overall flavor prediction | [182,183] |
| Lamb Shashliks | GC-MS, E-nose, E-tongue + deep learning | R2 above 0.96 for VOCs and brand ID | [185] |
| Cocoa | UHPLC-HRMS + sensomics + chemometrics | Strong correlation with sensory descriptors | [169] |
| Dairy Products | Sensomics + chemometrics | Identification of key flavor compounds for reconstitution | [165] |
| Technique | Application Area | Notable Outcomes | References |
|---|---|---|---|
| XGBoost | Sensory attribute prediction | High R2 for multiple attributes | [177,221,224,225] |
| Random Forest | Quality classification | Highest accuracy in many studies | [222,223,224,225,226] |
| SVM | Quality and astringency modeling | Strong regression/classification | [223,224,226,229,230] |
| Ensemble/Stacking | Quality prediction | Enhanced accuracy/robustness | [223,225,227] |
| Active Learning | Sensory evaluation | Reduced labeling effort, high accuracy | [228] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cosme, F.; Vilela, A.; Oliveira, I.; Aires, A.; Pinto, T.; Gonçalves, B. From Volatile Profiling to Sensory Prediction: Recent Advances in Wine Aroma Modeling Using Chemometrics and Sensor Technologies. Chemosensors 2025, 13, 337. https://doi.org/10.3390/chemosensors13090337
Cosme F, Vilela A, Oliveira I, Aires A, Pinto T, Gonçalves B. From Volatile Profiling to Sensory Prediction: Recent Advances in Wine Aroma Modeling Using Chemometrics and Sensor Technologies. Chemosensors. 2025; 13(9):337. https://doi.org/10.3390/chemosensors13090337
Chicago/Turabian StyleCosme, Fernanda, Alice Vilela, Ivo Oliveira, Alfredo Aires, Teresa Pinto, and Berta Gonçalves. 2025. "From Volatile Profiling to Sensory Prediction: Recent Advances in Wine Aroma Modeling Using Chemometrics and Sensor Technologies" Chemosensors 13, no. 9: 337. https://doi.org/10.3390/chemosensors13090337
APA StyleCosme, F., Vilela, A., Oliveira, I., Aires, A., Pinto, T., & Gonçalves, B. (2025). From Volatile Profiling to Sensory Prediction: Recent Advances in Wine Aroma Modeling Using Chemometrics and Sensor Technologies. Chemosensors, 13(9), 337. https://doi.org/10.3390/chemosensors13090337









