Abstract
Hydrolates are aromatic aqueous solutions saturated with volatile water-soluble compounds of essential oil. Despite their potential, hydrolates remain less explored than essential oils. In this work, the hydrolate of Pelargonium odoratissimum (L.) L’Hér. has been analyzed by multiple analytical techniques in order to describe its chemical composition. Headspace (HS-) and Direct Immersion-Solid Phase Microextraction-Gas Chromatography/Mass spectrometry (DI-SPME-GC/MS) and Proton Transfer Reaction Time-of-Flight Mass Spectrometry (PTR-ToF-MS) were employed to reveal the VOC emission from the hydrolate. Further, a direct injection of the pure hydrolate and of the hydrolate after extraction with hexane was performed by Large-Volume Injection Gas Chromatography/Mass Spectrometry (LVI-GC/MS) and GC/MS. The results obtained by HS- and DI-SPME-GC/MS highlighted a nearly overlapping chemical profile with linalool, isomenthone, and α-terpineol as the main volatiles. On the other hand, analysis of the hydrolate by GC/MS after solvent extraction revealed a lower overall number of compounds but allowed the detection of thujone and cis-linalool oxide. In comparison, LVI-GC/MS was the technique that allowed the identification of a higher number of volatiles with citronellol, linalool, and α-terpineol as the principal compounds. Finally, PTR-ToF-MS was a fundamental approach to quantify and evaluate total terpene emissions from this complex matrix starting from low-molecular-weight compounds such as acetylene, methanol, acetaldehyde, acetone, and ethanol, which were the most abundant. Among the detected compounds, dimethyl sulfide and small amounts of dimethyl-furan and 2-butylfuran were also identified. Overall, the findings showed that the hydrolate was rich in monoterpene compounds while sesquiterpene compounds were missing. A very low intensity relating to sesquiterpenes was recorded only by PTR-ToF-MS technique.
1. Introduction
Hydrolate, also known as hydrosol, is a distilled aromatic water that remains after the process of hydrodistillation or steam distillation of plant material to obtain essential oil (EO) (ISO 9235:2013) [1]. In detail, hydrolates are heterogeneous mixtures consisting of essential oil droplets and water-soluble components. Hydrolates are easy and cheap to obtain, but since the main distillation product is the EO, they are often discarded because they are considered a by-product [2,3]. Moreover, being more delicate in their properties, because they are more diluted than the corresponding essential oils, they can be widely used [4,5]. For example, due to their antioxidant properties, hydrolates are widely applied in cosmetology as well as in the agri-food and pharmaceutical sectors [6,7]. Hydrolates, thanks to the fact that they contain a small percentage of essential oil, are safer to use, so much so that they can find application in the dermatological field or used in the food sector as they have a pleasant and not too aggressive odor [4]. Over the years, with a view to sustainability and adding value to this essential oil byproduct, interest in hydrolates has increased significantly, thanks also to scientific studies conducted to highlight their bioactive compound content and verify their biological properties [4,8,9].
Hydrolates are suspensions of essential oil droplets and other water-soluble substances, so oxygen-containing compounds are usually dominant among the volatiles detected. Most studies on the composition of hydrolates have focused on their volatile constituents. Frequently, hydrolates have been extracted with solvents before chemical analysis [10,11,12]. Headspace solid-phase microextraction (HS-SPME) is a solvent-free method used to describe the volatile chemical profile, which allows the extraction step to be avoided [13].
Despite the efforts made so far, information on how to maximize the potential of hydrolates, which are long-undervalued natural products, is still limited, especially regarding knowledge of their phytochemical profile.
Pelargonium odoratissimum (L.) L’Hér., originally from South Africa and Eswatini and belonging to the Geraniaceae family, is a particularly aromatic plant with very scented leaves. It produces small thin flowers of a pale pink, almost white, and grouped in small umbels. It is a plant particularly rich in bioactive metabolites, widely used in traditional medicine for the treatment of many diseases (to relieve the pain of hemorrhoids, dysentery and inflammation), and is less studied than other species of Pelargonium [14]. The chemical composition of P. odoratissimum essential oil has been extensively investigated, demonstrating how the prevalence of some compounds over others can vary especially depending on the growth region and the distillation process [15,16,17,18]. On the other hand, to the best of our knowledge, the hydrolate has never been chemically investigated until now. In our previous work, P. odoratissimum essential oil was investigated in terms of both its chemical composition and biological activity [18]. Chemical analysis revealed a volatile profile with significant qualitative and quantitative differences compared to the hydrolate.
The aim of this work was to describe a detailed characterization of the chemical composition of Pelargonium odoratissimum hydrolate through a metabolomic approach based on different analytical investigation techniques. In detail, the analysis performed by Headspace- and Direct Immersion Solid-Phase Microextraction-Gas Chromatography/Mass Spectrometry ((HS- and DI-) SPME-GC/MS), GC/MS, Large Volume Injection- Gas Chromatography/Mass Spectrometry (LVI-GC/MS) and Proton Transfer Reaction-Time of Flight-Mass Spectrometry (PTR-ToF-MS) techniques provided relevant information on the volatile and semi-volatile compositional profile of this unexplored matrix.
2. Materials and Methods
2.1. Plant Material
The hydrolate of P. odoratissimum obtained by steam distillation of the aerial parts (flowers and leaves) for 2 h at a pressure of 0.25 Atm, growing in Tuscany, Italy, was provided directly by “èssenziale” Azienda Agricola, San Donato in Poggio (FI), Italy.
2.2. Headspace Solid-Phase Microextraction (HS-SPME) Sampling
To characterize the chemical volatile profile of P. odoratissimum hydrolate, a SPME fiber was used for the extraction of the analytes. Approximately, 2.0 mL of the sample was placed into a 15 mL glass vial with PTFE-coated silicone septum. A thermostatic bath with constant magnetic stirring was utilized to reach thermal equilibrium. For the adsorbtion of the components, a SPME device from Supelco (Bellefonte, PA, USA) with 1 cm fiber coated with 50/30 μm DVB/CAR/PDMS (divinylbenzene/carboxen/polydimethylsiloxane) was used. Before use, the fiber was conditioned at 270 °C for 30 min. Subsequently, the fiber was inserted into the vial and exposed to the headspace for 10 min at 60 °C to collect the volatiles. Finally, the compounds were desorbed from the fiber by insertion into the GC injector held at 250 °C (splitless mode).
2.3. Direct Immersion Solid-Phase Microextraction (DI-SPME) Sampling
For DI-SPME, the fiber was immersed directly in the aqueous solution of P. odoratissimum for 10 min, thermally conditioned at 60 °C to ensure the extraction of both volatile and semi-volatile compounds. The analyte extraction process was performed as described in the previous section (Section 2.2).
2.4. Extraction Procedure of the P. odoratissimum Hydrolate
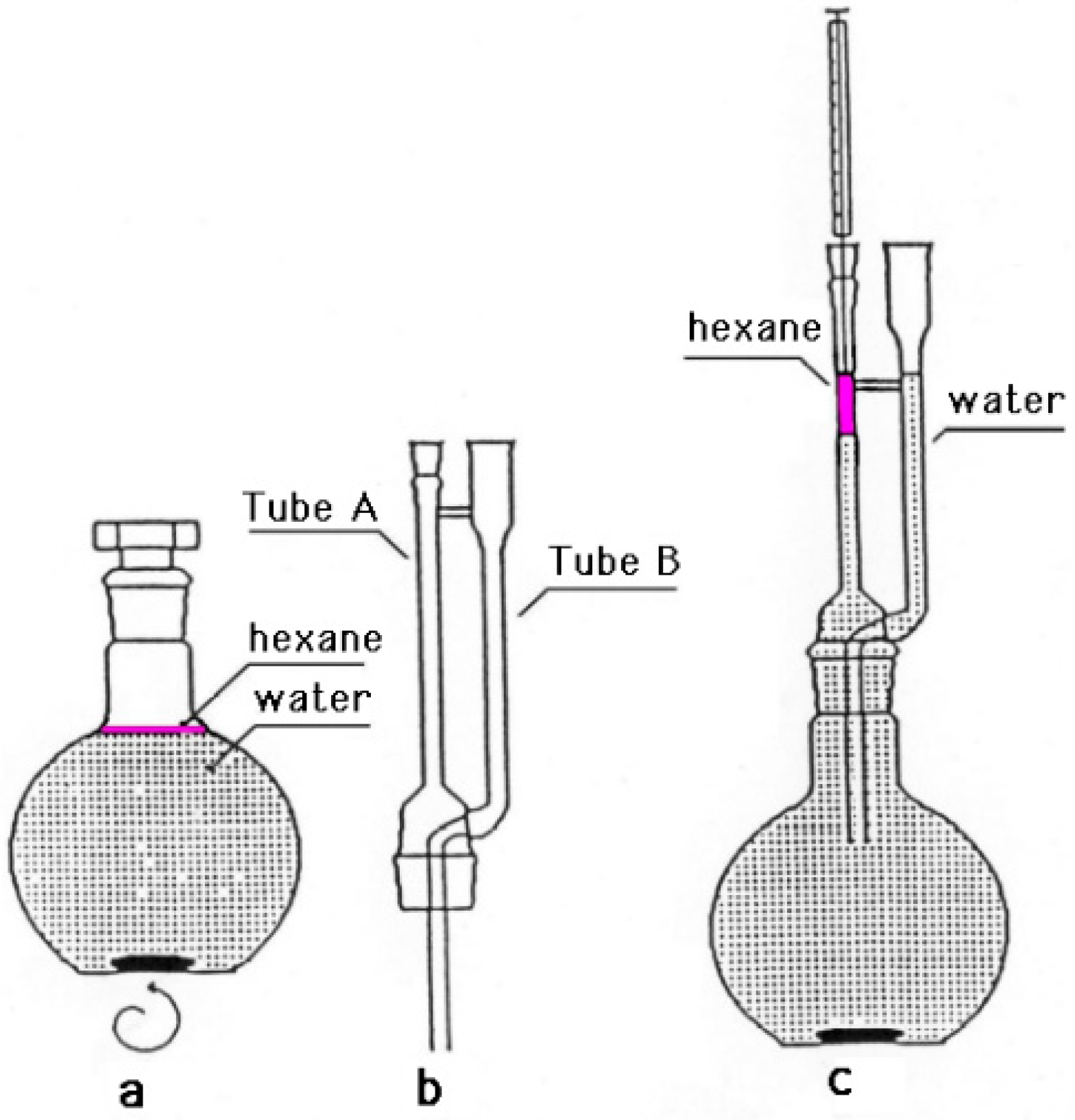
P. odoratissimum hydrolate (15 mL) was extracted using the method described by Zoccolillo et al. (1996). Briefly, 0.5 mL of hexane was added to the sample in a round-bottom flask. The resulting solution was mixed using a magnetic stirrer for 15 min (Figure 1a). After another 15 min of rest, the upper phase was recovered using a microextractor device (Figure 1b) by introducing a sufficient amount of water in tube B to allow the hexane layer to rise to the top of tube A (Figure 1c). Without reconcentrating the hexane phase, it is possible to take the sample and inject it into the GC-MS system.
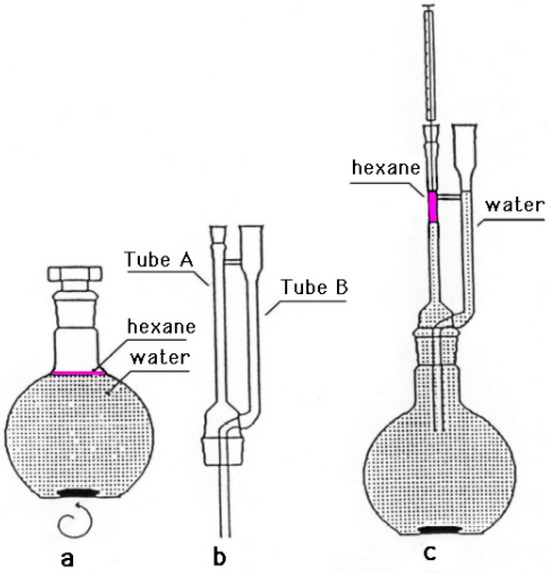
Figure 1.
Microextractor used for organic component of P. odoratissimum hydrolate.
2.5. Gas Chromatography/Mass Spectrometry (GC-MS) Analysis of P. odoratissimum Hydrolate
To perform GC/MS analysis, a Clarus 500 model Perkin Elmer (Waltham, MA, USA) gas chromatograph equipped with a FID (flame ionization detector) and coupled with a mass spectrometer was used. The chromatographic capillary column was a Varian Factor Four VF-5 (60 m, 0.25 mm, 1.00 µm, 7-inch cage) and helium was used as gas carrier at a flow rate of 1.0 mL min−1. The programmed temperature applied started at 40 °C then increased to 220 °C at 6 °C/min, and held for 10 min. The injector was set at 250 °C for SPME-GC/MS analysis and at 270 °C for injection of the hexane extract. The mass spectra were obtained in the electron ionization (EI) at 70 eV in scan mode in the range 35–400 m/z. The identification of the detected compounds was performed by the matching their mass spectra with those reported in the Wiley 2.2 and Nist 11 mass spectra libraries. In addition, linear retention indices (LRIs) were calculated using a homologous series of n-alkanes (C8–C30), injected in the column at the same operating conditions, and compared with the available literature values. Relative amounts of each component were expressed as percentage of its peak area in the total ion chromatogram without the use of an internal standard and any factor correction. The analyses were carried out in triplicate to ensure reproducibility.
2.6. LVI-GC-MS Analysis of P. odoratissimum Hydrolate
P. odoratissimum hydrolate was injected into the GC/MS (Konik, Sant Cugat del Vallés, Barcelona, Spain) using a TOTAD (Through Oven Transfer Adsorption Desorption) interface following Aragón et al., [19] with slight modifications. The adsorption temperature was set at 60 °C for 7.67 min under a nitrogen gas flow of 150 mL/min to eliminate the solvent (water) present in the hydrosol sample (20 µL), which was transferred to the interface with a methanol flow of 0.1 mL/min for 1 min. Adsorption of the compounds occurred at 260 °C for 3 min.
The GC/MS apparatus (Konik 5000C Sant Cugat del Vallés, Barcelona, Spain) consisted of a gas chromatograph at 5000 °C coupled to a Q12 MS mass spectrometer. The capillary column was a Merck SLB-5ms fused silica column (30 m × 0.25 mm, 1.0 µm) operating with helium carrier gas at 2.0 mL/min. During the adsorption phase (7.67 min), the oven was held at 38 °C, then raised to 280 °C at a rate of 5 °C and held for 5 min. The mass spectrometer interface was maintained at 260 °C, while the ion source (electron ionization mode at 70 eV) was set to 180 °C. The quadrupole operated in full scan mode from 41 to 450 m/z.
2.7. PTR -ToF- MS Analysis of P. odoratissimum Hydrolate
VOC acquisition was performed using a PTR-ToF 8000 from IONICON Analytik GmbH (Innsbruck, Austria). PTR-ToF-MS is widely used in many fields thanks to many advantages such as short response time, low limit of detection (LOD), direct sampling, and speed of analysis [20]. The advantages, limitations, and specific operational details of this tool are extensively discussed in [21,22]. In this study, the tool was used to investigate the chemical composition of P. odoratissimum hydrolate which was analyzed in triplicate. Raw data were recorded using TofDaq software (Tofwerk AG, Switzerland). To calibrate the instrument, we used a commercial gas mixture containing known compounds, such as acetone (m/z 59), limonene (m/z 137), and trichlorobenzene (m/z 181), at known concentrations, with the aim of generating a transmission curve (calibration curve) that allows for the quantification of each measured compound [23]. VOCs spontaneously emitted by hydrolate were acquired and measured using the same protocol reported by Caparrotta et al., 2019 [24]. In detail, 0.1 mL of hydrolate was put in a sealed glass vessel (2/3 L glass jar) and incubated for 100 sec at room temperature (22 °C). The drift tube operating parameters were set as follows: FC inlet 50 sccm, pressure 2.3 mbar, inlet and drift temperature 60 °C, voltage 600 V, extraction voltage (Udx) 35V which corresponded to an E/N value of 140 Td, optimizing the balance between water cluster formation and product ion fragmentation [25]. Each run involved a 180-s measurement to allow the acquisition of 180 spectra (acquisition of 1 spectra per sec) and was conducted in a conditioned room. To ensure data accuracy and prevent sample cross-contamination, the tool apparatus was cleaned using a zero-air generator, and a new, clean glass vessel was used for each replicate. Once each replicate was measured, the instrument was calibrated off-line using reference peaks detected at m/z 21.022 (H318O+), 59.049 (C3H7O+), and 137.132 (C10H17+). At the end of the analysis, we converted the signal intensities of each VOC from counts per second (cps) to parts per billion by volume (ppbv) using the primary ion signal (H318O+). Subsequently, the dataset was reduced to remove peaks associated with water chemistry and filtered out signals with average concentrations below 1 ppbv. So, the resulting cleaned dataset was used for statistical analysis. Finally, by comparison with the GC-MS literature for hydrolate and PTR-MS studies of fragmentation patterns, we tentatively identified each VOC detected.
3. Results and Discussion
3.1. P. odoratissimum Hydrolate Chemical Composition
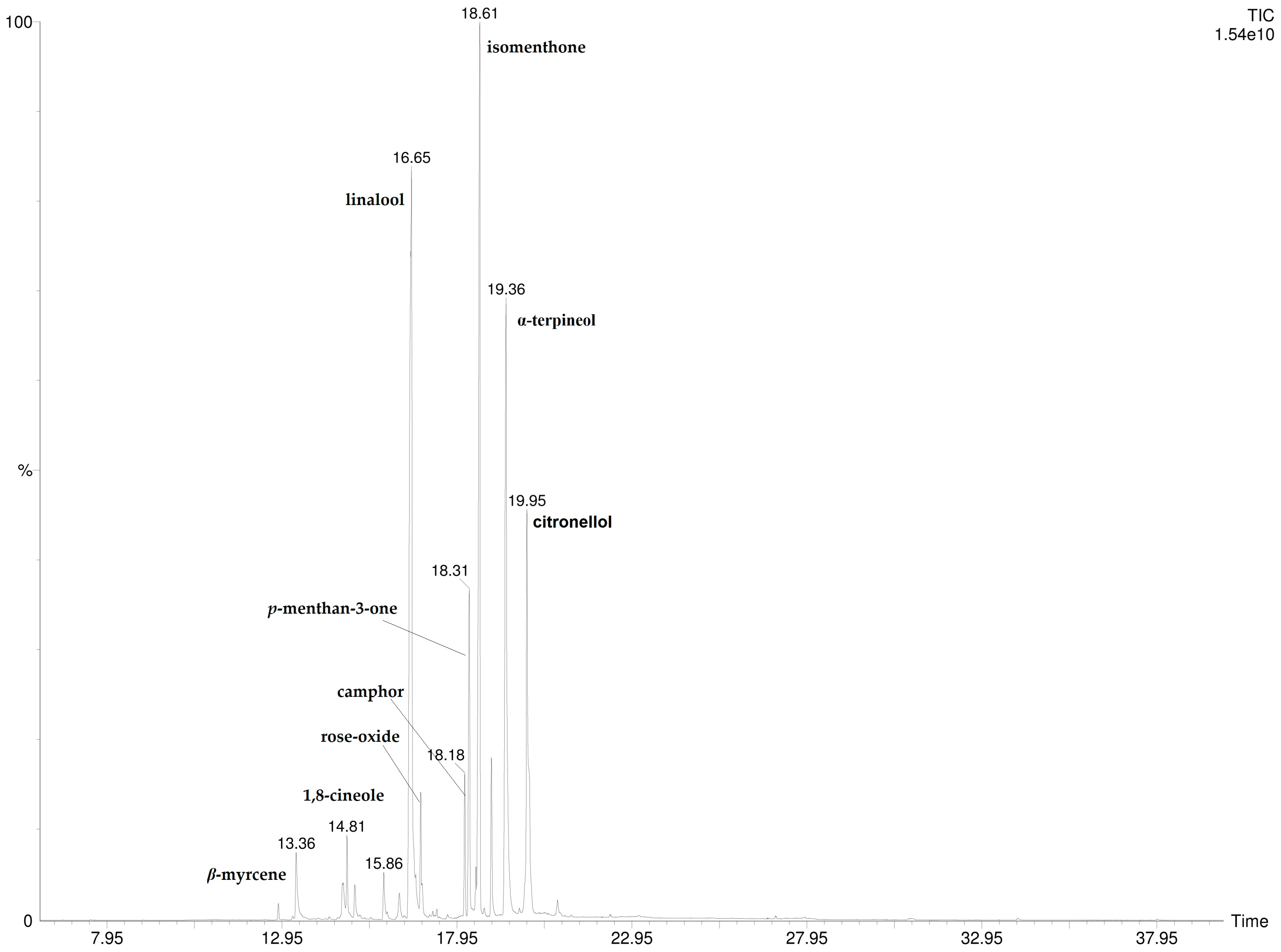
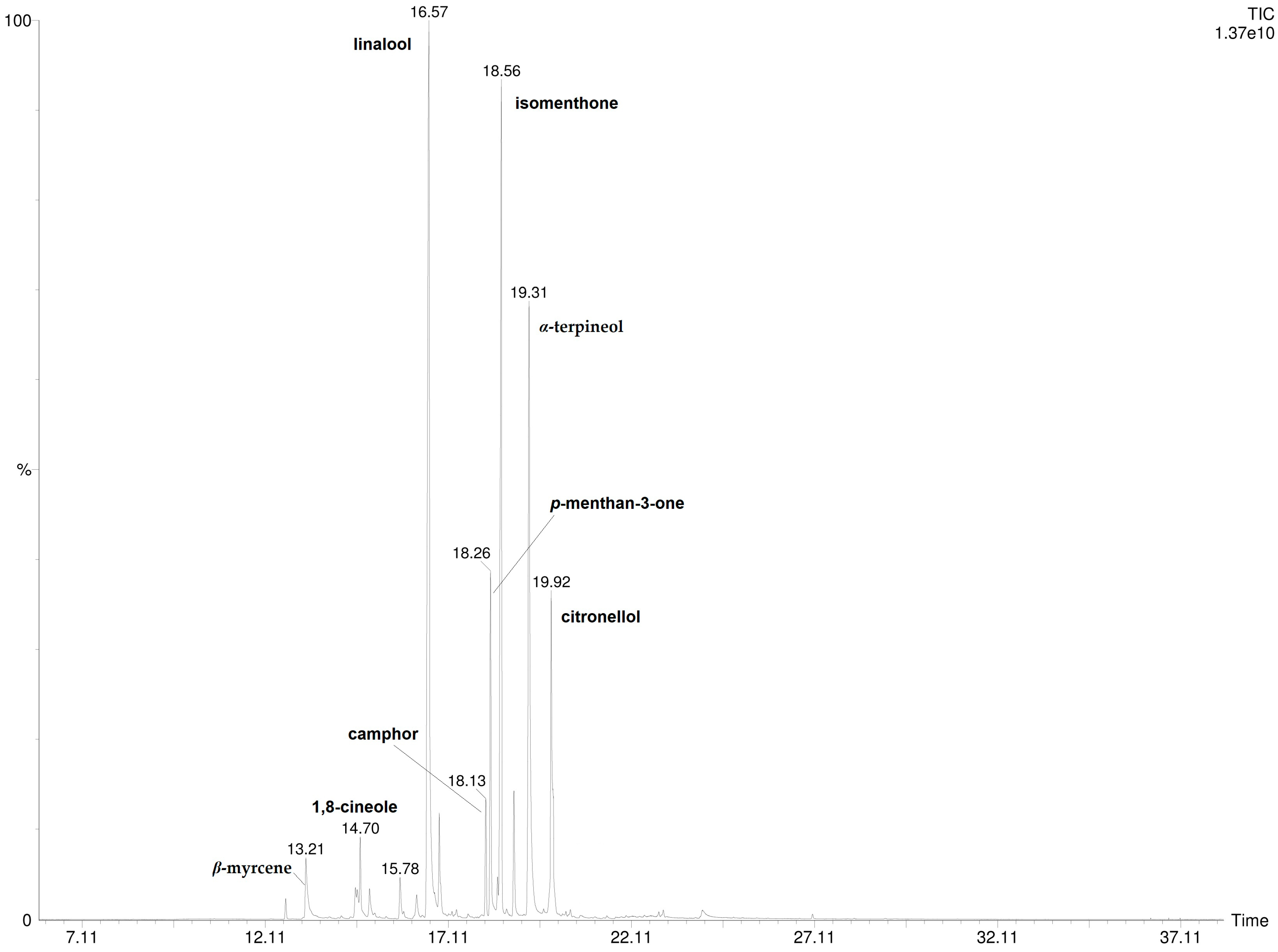
A total of 16 compounds were detected and identified by HS-SPME/GC-MS and by DI-SPME/GC-MS analysis (Table 1). The results show that P. odoratissimum hydrolate (Hy) is characterized by the presence of monoterpene compounds. No sesquiterpenes were found. Linalool (29.6%; 30.7%), isomenthone (21.1%; 19.9%), α-terpineol (18.9%; 18.7%), and p-menthan-3-one (6.6%; 6.9%) were the principal components detected by HS-SPME and DI-SPME, respectively. The volatile chemical profile obtained by applying the two extraction techniques was almost superimposable as there were no significant qualitative–quantitative differences. The DI- and HS-SPME-GC-MS chromatograms are reported in Figure 2 and Figure 3.

Table 1.
Chemical composition (percentage mean value ± standard deviation) of P. odoratissimum Hy as determined by HS-SPME-GC/MS and DI-SPME-GC/MS.
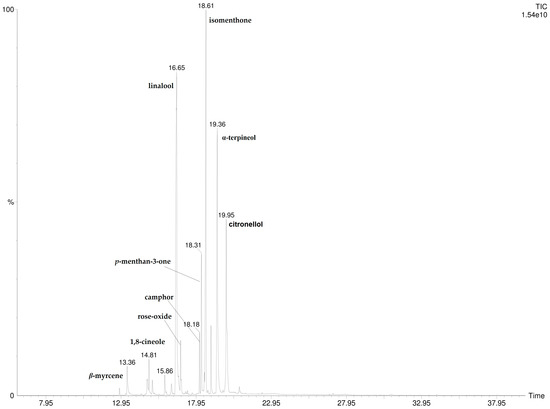
Figure 2.
HS-SPME-GC-MS chromatogram of P. odoratissimum hydrolate.
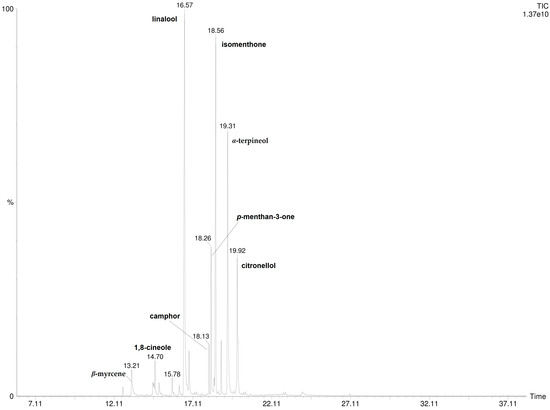
Figure 3.
DI-SPME-GC-MS chromatogram of P. odoratissimum hydrolate.
Generally, the volatile content of hydrolates is lower, both qualitatively and quantitatively, than the corresponding essential oils. In fact, monoterpene and sesquiterpene hydrocarbons, more hydrophobic, are present in reduced percentages in the hydrolate. On the other hand, the content of some oxygenated monoterpenes is higher in the hydrolate [26,27,28]. Our previous study conducted on P. odoratissimum essential oil showed that it was rich in oxygenated monoterpenes (81.2%), while sesquiterpenes accounted for only 11% [14]. The main compounds were citronellol (40.0%), nerol (15.3%), and citronellyl formate (12.6%) followed by (-)-aristolene (9.8%), isomenthone (7.4%), linalool (4.8%), and geranyl isobutyrate (2.6%). Among these major components, citronellol, isomenthone, and linalool were also detected in the hydrolate. On the contrary, α-terpineol, the third more abundant volatile in the hydrolate, was absent in the essential oil [18].
There are no previous works in the literature on the chemical composition of P. odoratissimum hydrolate or different Pelargonium species; therefore, comparative evaluations are not feasible. Only Rao et al. [2] discuss the chemical composition of P. graveolens hydrolate but only after extraction with hexane and subsequent distillation to produce a secondary essential oil in order to recover the oil dispersed in the hydrolate.
3.2. P. odoratissimum Hydrolate Hexane Extract Chemical Composition
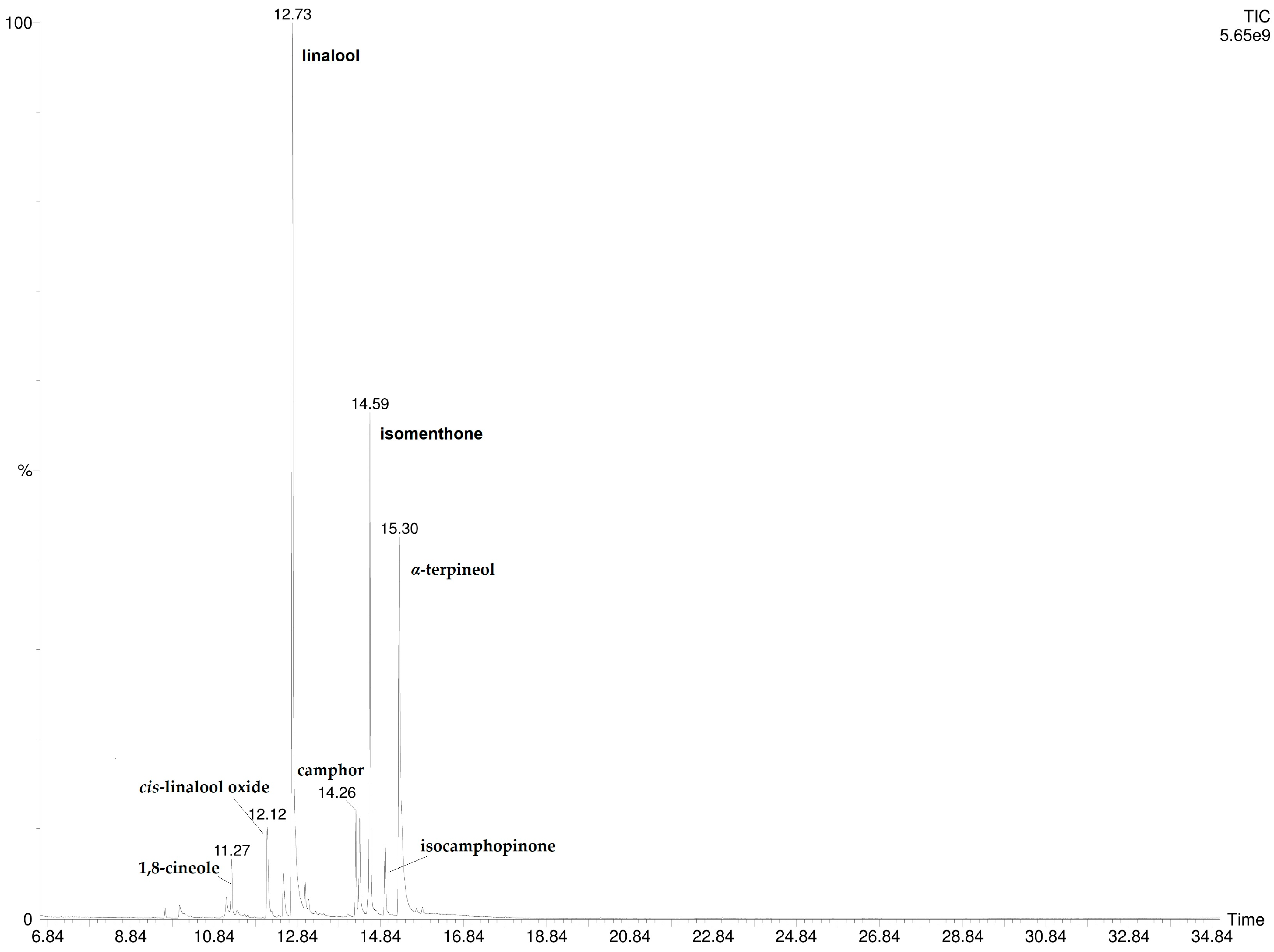
The injection into GC/MS of the hexane extract of P. odoratissimum hydrolate revealed the presence of 13 compounds, listed in Table 2. Among them, linalool was the most abundant (33.6%) followed by α-terpineol (20.9%), trans-linalool oxide (15.8%), and isomenthone (15.1%). Two compounds, thujone (0.9%) and cis-linalool oxide (3.5%), were not detected by SPME-GC/MS (both HS- and DI-) analysis. These components were also missing in the correspondent essential oil [18]. The GC-MS chromatogram is reported in Figure 4.

Table 2.
Chemical composition (percentage mean value ± standard deviation) of hexane extract of P. odoratissimum Hy as determined by GC/MS.
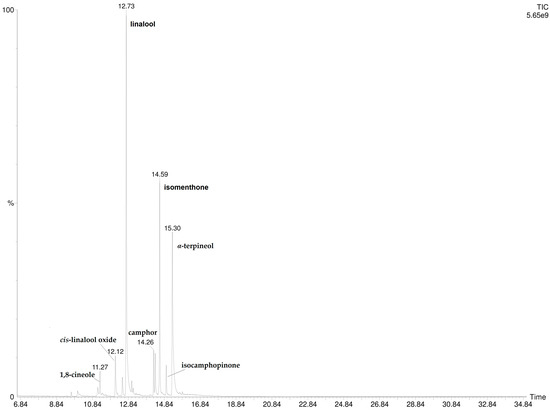
Figure 4.
GC-MS chromatogram of P. odoratissimum hydrolate hexane extract.
3.3. LVI-GC/MS Chemical Composition
P. odoratissimum hydrolate chemical composition obtained applying the LVI-GC-MS method is reported in Table 3. Monoterpene compounds covered 79% of the total composition and no sesquiterpenes were detected.

Table 3.
Chemical composition (percentage mean value ± standard deviation) of P. odoratissimum Hy as determined by LVI-GC/MS.
The results show important qualitative and quantitative differences with respect to SPME-GC/MS analysis.
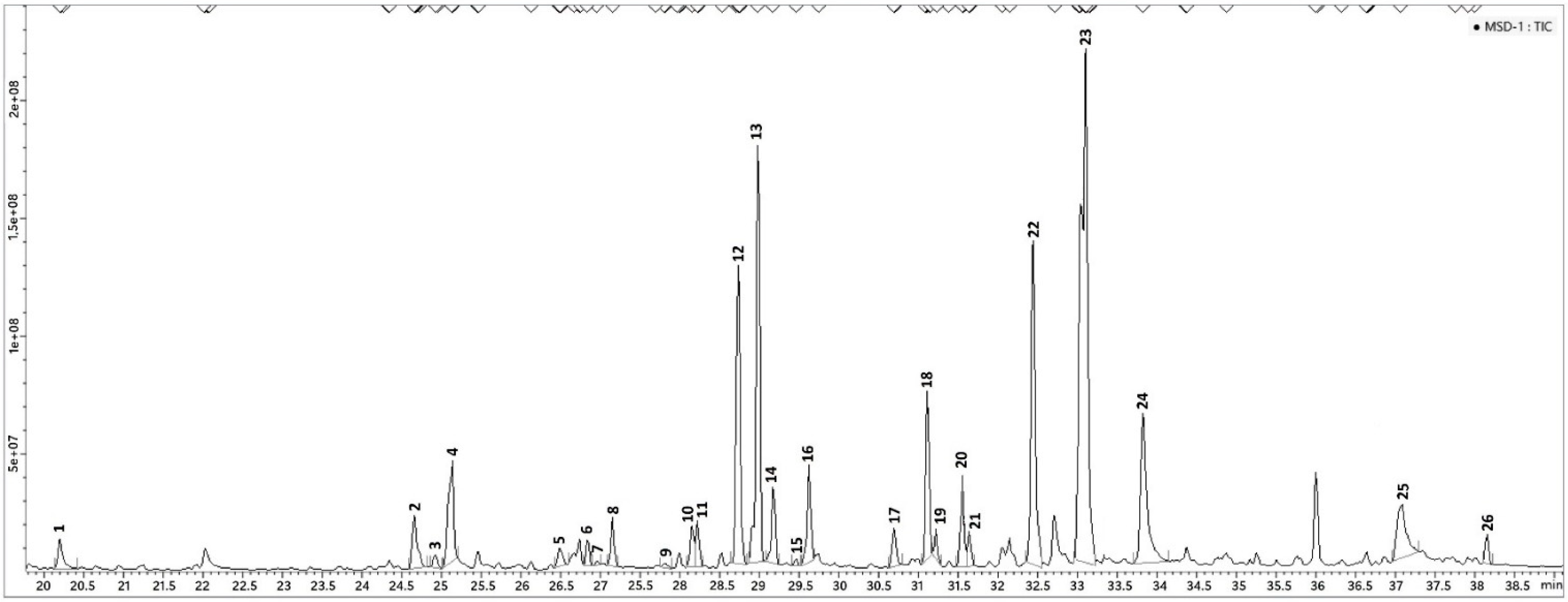
In total, 26 components were detected and identified, 10 more than those identified by HS-SPME/GC–MS and by DI-SPME/GC-MS analysis (Table 1), and 13 more than those identified in hexane extract (Table 2). Among these, the monoterpenes linalool (12.8%), α-terpineol (11.1%), and citronellol (25.8%) were the main detected compounds also relieved in both SPME analyses (HS- and DI-) but with different relative amounts. Particularly significant were the differences for linalool (29.6% in HS-, 30.7% in DI-) and for citronellol (8.8% in HS-, 8.3% in DI-). The relative amount of isomenthone (2.5%) also showed a remarkable difference compared to the SPME analysis, where rather higher quantities (21.1% in HS-, 19.9% in DI-) were found. Among compounds present in LVI analysis and absent in SPME analysis, the most abundant were benzenemethanol, α,α-dimethyl- (9.5%), geraniol (6.6%), myrcenol 2.8%), and hydroxy citronellol (4.0%). All other compounds (note 4) detected by SPME and LVI analysis, did not show significant variation. The LVI-GC-MS chromatogram was reported in Figure 5.
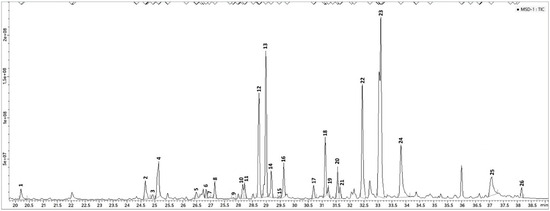
Figure 5.
LVI-GC-MS chromatogram of P. odoratissimum hydrolate. For peaks assignation, see Table 2.
3.4. PTR-ToF-MS
PTR-ToF-MS analysis of P. odoratissimum hydrolate identified 46 distinct peak signals with emission intensities exceeding 1 ppbv. Table 4 provides a comprehensive list of these compounds, including aldehydes, ketones, esters, sulfur-containing compounds, terpenes, and other unidentified compounds, detected within the mass range of m/z 20 to 220. PTR-ToF-MS is a powerful tool for high-frequency detection and quantification of gas-phase volatile organic compounds (VOCs). However, its soft ionization technique makes it difficult to distinguish structural isomers, limiting the ability to specifically identify individual terpene species [29]. Despite this limitation, total terpene concentrations expressed as the sum of isomers can still be estimated using characteristic terpene fragmentation patterns described in previous studies [29,30,31,32]. These studies highlight that sesquiterpenes, monoterpenes, and oxygenated terpenes can be assessed by targeting key m/z channels corresponding to their known fragmentation pathways. This approach provides viable means to quantify and evaluate total terpene emissions from complex natural matrices such as hydrolates.

Table 4.
VOC profile of P. odoratissimum hydrolate as determined by PTR-ToF-MS.
Analysis of the P. odoratissimum hydrolate revealed that the most abundant volatile compounds were detected at m/z 27 (fragments), 33 (methanol), 45 (acetaldehyde), and 59 (acetone), collectively accounting for over 60% of the total emissions (Table 4). These results suggest that water-soluble alcohols, aldehydes, and other polar compounds are effectively transferred into the aqueous phase during steam distillation. Moreover, a sulfur-containing compound, although present at a low concentration, was in line with the findings of Amel et al. [33]. This compound detected at m/z 63 was identified as dimethyl sulfide, known for its distinctive odor and potential contribution to the hydrolate’s sensory profile. Despite its low-level presence (approximately 2% of the total emissions), dimethyl sulfide may play an important role in defining the characteristic scent and potential bioactivity of the hydrolate. In contrast, nitrogen-containing compounds, often considered important contributors to the aromatic profile of geranium essential oils, were not detected in this study. In addition to the presence of dimethyl sulfide, which may contribute unique sensory nuances, another key class of volatiles identified in the P. odoratissimum hydrolate was terpenes.
Despite exhibiting low emission intensities, terpenes, known for their low perception thresholds, are characteristic of many plant hydrolates [34]. Indeed, the aroma profile of the P. odoratissimum hydrolate appeared to be strongly influenced by the presence of terpene compounds, as evidenced by the detection of characteristic mass-to-charge ratios (m/z) such as 135, 137, 153, and 155, along with their corresponding fragment ions (m/z 67, 69, 79, 81, 93, 95, 109). The detection of signals at m/z 135 and 137 is indicative of the presence of monoterpenes, while signals at m/z 153 and 155 are characteristic of oxygenated monoterpenes. However, no peaks corresponding to sesquiterpene compounds were detected with an intensity exceeding 1 ppbv. Consistent with the findings of Tavares et al. (2022) [34], hydrolates obtained from various plant species exhibit a diverse array of primary and secondary metabolites, making them valuable sources of biologically active compounds. Despite typically possessing a strong aroma, hydrolates usually contain low concentrations of essential oil and often exhibit a chemical resemblance to the corresponding essential oil composition, albeit with quantitative differences [34].
4. Conclusions
In this study, the chemical composition of P. odoratissimum hydrolate was explored by different techniques to describe an in-depth compositional framework. The findings highlighted the presence of a high number of monoterpenes. The comparison of the data obtained from the analyses demonstrates how the approach based on the different applied analytical techniques was useful for the detection of the individual secondary metabolites including volatiles and semi-volatiles.
SPME, a solvent-free technique, has proven to be a highly sensitive method for collecting analytes spontaneously emitted from the hydrolate. The monoterpene fraction dominated the volatile profile of P. odoratissimum hydrolate, with linalool, isomenthone, and α-terpineol being the most abundant. However, no significant differences were observed between the HS- and DI-SPME techniques. In contrast, the analysis of the hydrolate hexane extract by GC/MS showed a lower number of compounds but allowed the detection of thujone and cis-linalool oxide.
PTR-ToF-MS, with its exceptional sensitivity and selectivity, ensured comprehensive chemical characterization across a wide range of molecular weights, including compounds belonging to different chemical classes such as aldehydes, alcohols, terpenes, a sulfur containing compound, and traces of furan derivatives. On the other side, TOTAD interface allowed for the injection of large quantities (up to a few hundred microliters) of the hydrolate (predominantly aqueous sample) directly into the GC capillary column, thus ensuring a significant increase in sensitivity. Indeed, it was possible to detect other compounds such as benzenemethanol, α,α-dimethyl-, geraniol, myrcenol, and hydroxy-citronellol.
Overall, the applied analytical techniques proved invaluable for in-depth study of the chemical profile of the hydrolate, allowing for the precise identification and accurate quantification of VOCs.
In conclusion, the findings of this research provide important compositional information for potential use of P. odoratissimum hydrolate as a natural source of bioactives.
Author Contributions
Conceptualization, S.G.; methodology, C.T., V.V. and S.G. investigation, C.T., V.V., M.M.G. and S.G.; formal analysis, C.T., V.V., E.M., M.M.G. and S.G.; data curation, C.T., E.M., V.V. and S.G.; resources, S.G.; writing—original draft preparation, C.T., V.V. and S.G.; writing—review and editing, C.T., V.V. and S.G.; supervision, E.M., and S.G.; Project administration: S.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All generated data are included in this article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- ISO 9235:2013; Aromatic Natural Raw Materials—Vocabulary. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:9235:ed-3:v1:en (accessed on 14 July 2025).
- Rao, B.R.R.; Kaul, P.N.; Syamasundar, K.V.; Ramesh, S. Water soluble ractions of rose-scented geranium (Pelargonium species) essential oil. Bioresour. Technol. 2002, 84, 243–246. [Google Scholar]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant activity of some Moroccan hydrosols. J. Med. Plants Res. 2011, 5, 6688–6696. [Google Scholar]
- D’Amato, S.; Serio, A.; Lopez, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Traka, C.K.; Petrakis, E.A.; Kimbaris, A.C.; Polissiou, M.G.; Perdikis, D.C. Effects of Ocimum basilicum and Ruta chalepensis hydrosols on Aphis gossypii and Tetranychus urticae. J. Appl. Entomol. 2018, 142, 413–420. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Tuchowska, A.; Janda-Milczarek, K. Plant hydrolates—Antioxidant properties, chemical composition and potential applications. Biomed. Pharmacother. 2021, 142, 12033. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Tešević, V.V.; Smiljanić, K.T.; Cvetković, M.T.; Stanković, J.M.; Kiprovski, B.M.; Sikora, V.S. Hydrolates: By-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Catty, S. Hydrosols: The Next Aromatherapy; Healing Arts Press: Rochester, VT, USA, 2001. [Google Scholar]
- Price, L.; Price, S. Understanding Hydrolates: The Specific Hydrosols for Aromatherapy: A Guide for Health Professionals, 1st ed.; Churchill Livingstone: London, UK; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 9780443073168. [Google Scholar]
- Silva, H.N.P.; Sousa, E.M.O.; Maia, J.L.S.; Pinheiro, M.T.L.; Lameirão, S.V.O.C.; Mourão, R.H.V.; Maia, J.G.S.; Baldisserotto, B.; Silva, L.V.F. Lippia alba (Verbenaceae) hydrolate as sedative of tambaqui (Colossoma macropomum) juveniles in simulated transport conditions. Aquac. Res. 2018, 49, 128–134. [Google Scholar] [CrossRef]
- Djabou, N.; Dib, M.E.-A.; Tabti, B.; Costa, J.; Muselli, A. Chemical composition and antioxidant activity of hydrosol extracts obtained by liquid-liquid extraction (LLE) of Daucus muricatus L. J. Essent. Oil Res. 2014, 26, 393–399. [Google Scholar] [CrossRef]
- Zatla, A.T.; Dib, M.E.A.; Djabou, N.; Ilias, F.; Cost, J.; Muselli, A. Antifungal activities of essential oils and hydrosol extracts of Daucus carota subsp. sativus for the control of fungal pathogens, in particular gray rot of strawberry during storage. J. Essent. Oil Res. 2017, 29, 391–399. [Google Scholar] [CrossRef]
- Lancioni, C.; Castells, C.; Candal, R.; Tascon, M. Headspace Solid-Phase Microextraction: Fundamentals and Recent Advances. Adv. Sample Prep. 2022, 3, 100035. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Chahdoura, H.; Generalić Mekinić, I.; Maisto, M.; Kukula-Koch, W.; Ćavar Zeljković, S.; Koch, W.; Ben Akacha, B.; Taieb Bouteraa, M.; Ben Belgacem, A.; et al. A comprehensive review on traditional uses, chemical composition, pharmacological effects and applications in the food industry of Pelargonium odoratissimum (L.) L’Hér. in comparison to other Pelargonium spp. S. Afr. J. Bot. 2024, 174, 456–467. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Roth, G. Composition of the essential oils of Pelargonium odoratissimum, P. exstipulatum, and P. × fragrans (Geraniaceae) and their bioactivity. Flavour Fragr. J. 2020, 15, 391–394. [Google Scholar] [CrossRef]
- Andrade, A.M.; Cardoso, M.G.; Batista, L.R.; Freire, J.M.; Nelson, D.L. Antimicrobial activity and chemical composition of essential oil of Pelargonium odoratissimum. Rev. Bras. Farmacogn. 2011, 21, 47–52. [Google Scholar] [CrossRef]
- Szutt, A.; Dołhańczuk-Śródka, A.; Sporek, M. Evaluation of chemical composition of essential oils derived from different pelargonium species leaves. Ecol. Chem. Eng. S 2019, 26, 807–816. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Akacha, B.; Generalić Mekinić, I.; Čmiková, N.; Ben Belgacem, A.; Bouteraa, M.T.; Ben Saad, R.; Mnif, W.; Kluz, M.I.; Kačániová, M.; et al. Insight into Pelargonium odoratissimum Essential Oil Preservative Properties Effect on Ground Beef. Foods 2024, 13, 3181. [Google Scholar] [CrossRef] [PubMed]
- Aragón, A.; Toledano, R.M.; Vázquez, A.; Villén, J.; Cortés, J.M. Analysis of polycyclic aromatic hydrocarbons in aqueous samples by large volume injection gas chromatography-mass spectrometry using the through oven transfer adsorption desorption interface. Talanta 2015, 139, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, Q.; Zhou, W.; Zou, X.; Wang, H.; Huang, C.; Chu, Y. Detection of ketones by a novel technology: Dipolar proton transfer reaction mass spectrometry (DP-PTR-MS). J. Am. Soc. Mass Spectrom. 2017, 28, 873–879. [Google Scholar] [CrossRef]
- Blake, R.S.; Monks, P.S.; Ellis, A.M. Proton-transfer reaction mass spectrometry. Chem. Rev. 2009, 109, 861–896. [Google Scholar] [CrossRef]
- Taiti, C.; Costa, C.; Guidi Nissim, W.; Bibbiani, S.; Azzarello, E.; Masi, E.; Pandolfi, C.; Pallottino, F.; Menesatti, P.; Mancuso, S. Assessing VOC emission by different wood cores using the PTR-ToF-MS technology. Wood Sci. Technol. 2017, 51, 273–295. [Google Scholar] [CrossRef]
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Mark, L.; Seehauser, H.; Mark, T.D. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int. J. Mass Spectrom. 2009, 286, 122–128. [Google Scholar] [CrossRef]
- Caparrotta, S.; Comparini, D.; Marone, E.; Kimmenfield, R.; Luzzietti, L.; Taiti, C.; Mancuso, S. Correlation between VOC fingerprinting and antimicrobial activity of several essential oils extracted by plant resins against A. tumefaciens and P. savastanoi. Flavour Fragr. J. 2019, 34, 377–387. [Google Scholar] [CrossRef]
- Pang, X. Biogenic volatile organic compound analyses by PTR-TOF-MS: Calibration, humidity effect and reduced electric field dependency. J. Environ. Sci. 2015, 32, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, V.; Chahboun, J.; Russel, D.; Casabianca, H. Origanum compactum Bentham: Composition of the hydrolat aromatic fraction, comparison with the essential oil and its interest in arometherapy. Int. J. Aromather. 2003, 13, 90–94. [Google Scholar] [CrossRef]
- Śmigielski, K.B.; Prusinowska, R.; Krosowiak, K.; Sikora, M. Comparison of qualitative and quantitative chemical composition of hydrolate and essential oils of lavender (Lavandula angustifolia). J. Essent. Oil Res. 2013, 25, 291–299. [Google Scholar] [CrossRef]
- Chizzola, R.; Billiani, F.; Singer, S.; Novak, J. Diversity of essential oils and the respective hydrolates obtained from three Pinus cembra populations in the Austrian Alps. Appl. Sci. 2021, 11, 5686. [Google Scholar] [CrossRef]
- Misztal, P.K.; Heal, M.R.; Nemitz, E.; Cape, J.N. Development of PTR-MS selectivity for structural isomers: Monoterpenes as a case study. Int. J. Mass Spectrom. 2012, 310, 10–19. [Google Scholar] [CrossRef]
- Maleknia, S.D.; Bell, T.L.; Adams, M.A. PTR-MS analysis of reference and plant-emitted volatile organic compounds. Int. J. Mass Spectrom. 2007, 262, 203–210. [Google Scholar] [CrossRef]
- Demarcke, M.; Amelynck, C.; Schoon, N.; Dewulf, J.; Van Langenhove, H. Investigations on the influence of drift field and humidity on sesquiterpene product ion distributions in a PTR-MS instrument and implications for sesquiterpene detection sensitivity. In Proceedings of the 4th International PTR-MS Conference, Obergurgl, Austria, 16–21 February 2009; pp. 170–173. Available online: https://orfeo.belnet.be/handle/internal/3240 (accessed on 14 July 2025).
- Tani, A. Fragmentation and reaction rate constants of terpenoids determined by proton transfer reaction-mass spectrometry. Environ. Control Biol. 2013, 51, 23–29. [Google Scholar] [CrossRef]
- Amel, H.A.; Kamel, H.; Meriem, F.; Abdelkader, K. Traditional Uses, Botany, Phytochemistry, and Pharmacology of Pelargonium graveolens: A Comprehensive Review. Trop. J. Nat. Prod. Res. 2002, 6, 1547–1569. [Google Scholar]
- Tavares, C.S.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Hydrolates: A review on their volatiles composition, biological properties and potential uses. Phytochem. Rev. 2022, 21, 1661–1737. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).