In-Depth Investigation of the Chemical Profile of Pelargonium odoratissimum (L.) L’Hér. Hydrolate by SPME-GC/MS, GC/MS, LVI-GC/MS and PTR-Tof-MS Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Headspace Solid-Phase Microextraction (HS-SPME) Sampling
2.3. Direct Immersion Solid-Phase Microextraction (DI-SPME) Sampling
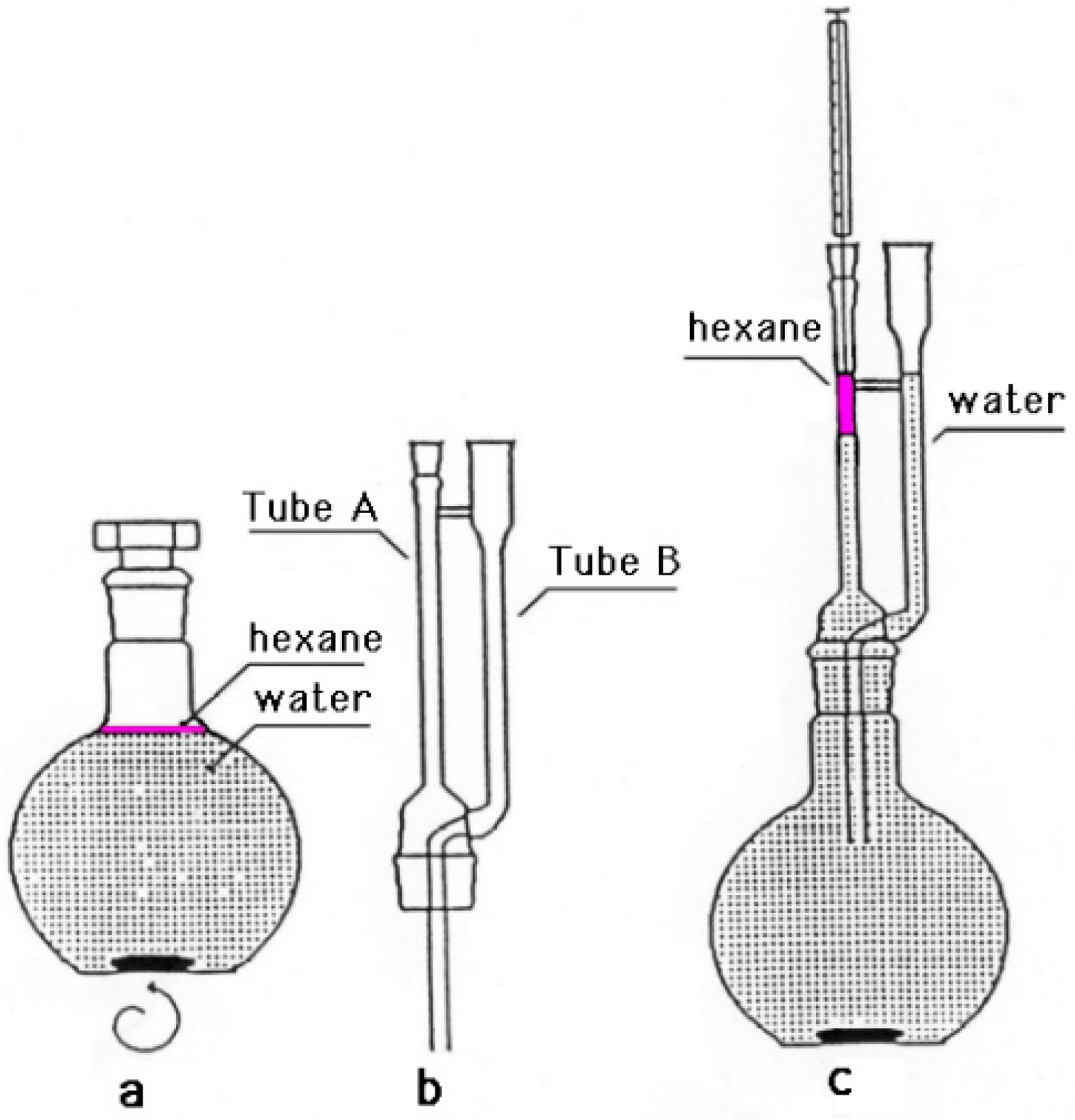
2.4. Extraction Procedure of the P. odoratissimum Hydrolate
2.5. Gas Chromatography/Mass Spectrometry (GC-MS) Analysis of P. odoratissimum Hydrolate
2.6. LVI-GC-MS Analysis of P. odoratissimum Hydrolate
2.7. PTR -ToF- MS Analysis of P. odoratissimum Hydrolate
3. Results and Discussion
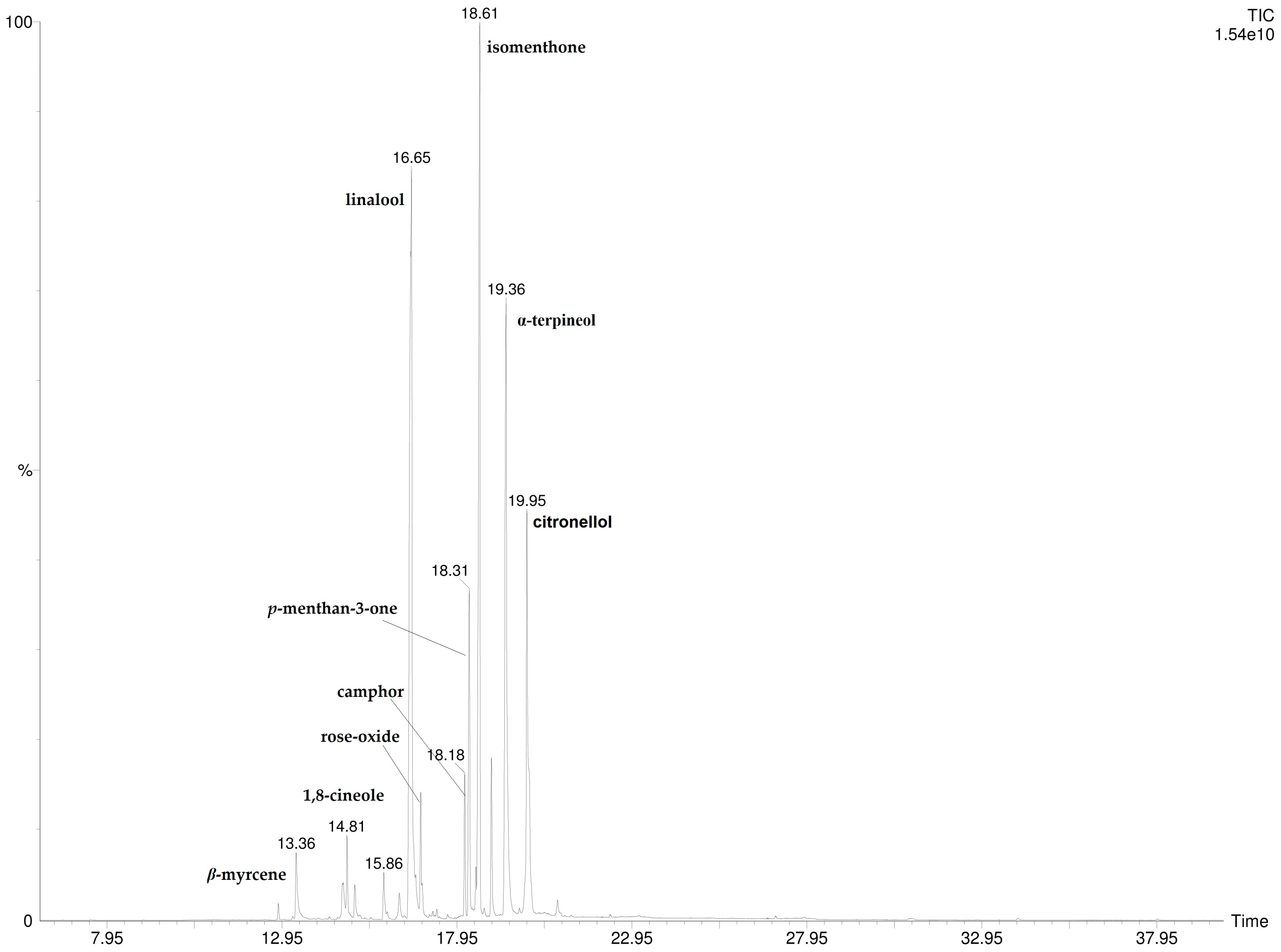
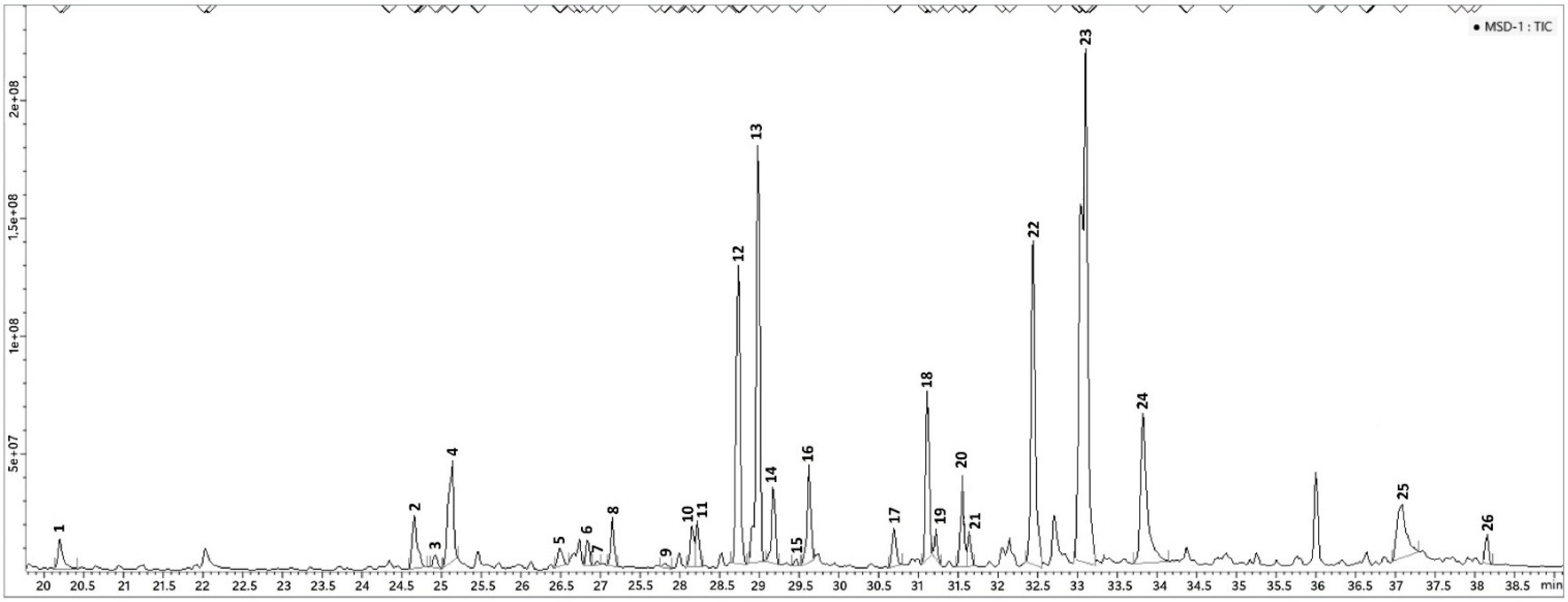
3.1. P. odoratissimum Hydrolate Chemical Composition
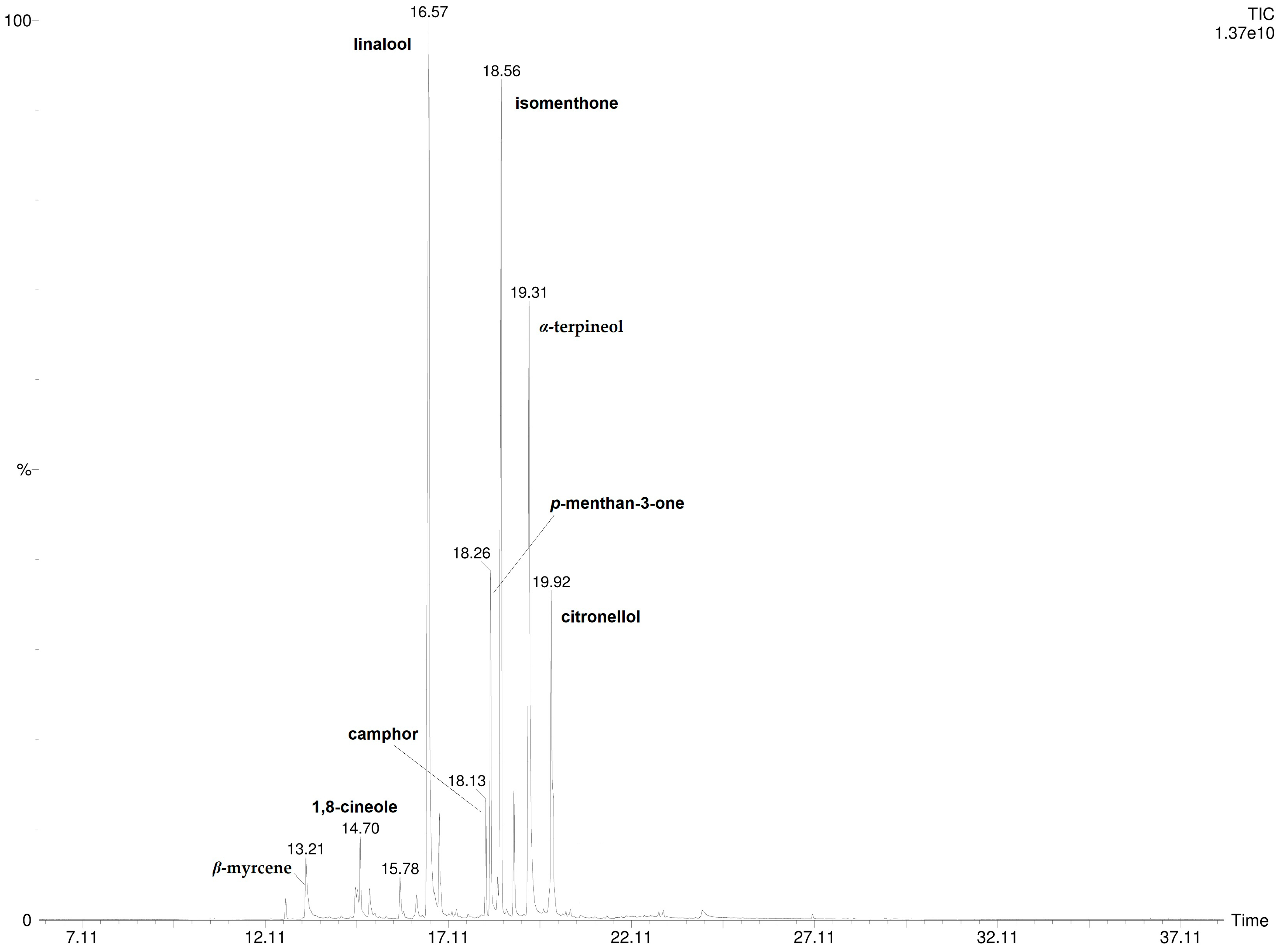
3.2. P. odoratissimum Hydrolate Hexane Extract Chemical Composition
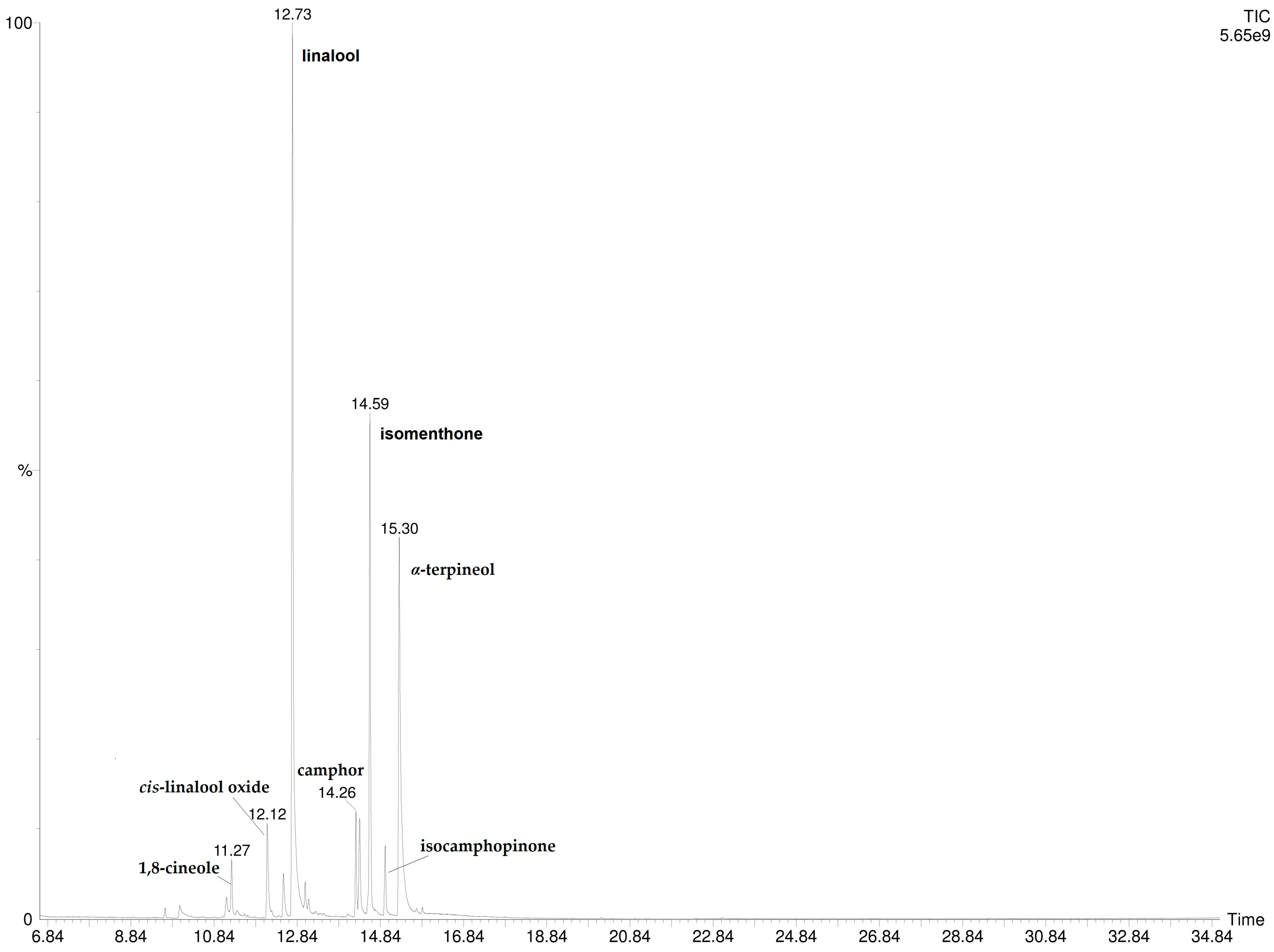
3.3. LVI-GC/MS Chemical Composition
3.4. PTR-ToF-MS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ISO 9235:2013; Aromatic Natural Raw Materials—Vocabulary. ISO: Geneva, Switzerland, 2021. Available online: https://www.iso.org/obp/ui/en/#iso:std:iso:9235:ed-3:v1:en (accessed on 14 July 2025).
- Rao, B.R.R.; Kaul, P.N.; Syamasundar, K.V.; Ramesh, S. Water soluble ractions of rose-scented geranium (Pelargonium species) essential oil. Bioresour. Technol. 2002, 84, 243–246. [Google Scholar]
- Aazza, S.; Lyoussi, B.; Miguel, M.G. Antioxidant activity of some Moroccan hydrosols. J. Med. Plants Res. 2011, 5, 6688–6696. [Google Scholar]
- D’Amato, S.; Serio, A.; Lopez, C.C.; Paparella, A. Hydrosols: Biological activity and potential as antimicrobials for food applications. Food Control 2018, 86, 126–137. [Google Scholar] [CrossRef]
- Traka, C.K.; Petrakis, E.A.; Kimbaris, A.C.; Polissiou, M.G.; Perdikis, D.C. Effects of Ocimum basilicum and Ruta chalepensis hydrosols on Aphis gossypii and Tetranychus urticae. J. Appl. Entomol. 2018, 142, 413–420. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Tuchowska, A.; Janda-Milczarek, K. Plant hydrolates—Antioxidant properties, chemical composition and potential applications. Biomed. Pharmacother. 2021, 142, 12033. [Google Scholar] [CrossRef]
- Aćimović, M.G.; Tešević, V.V.; Smiljanić, K.T.; Cvetković, M.T.; Stanković, J.M.; Kiprovski, B.M.; Sikora, V.S. Hydrolates: By-products of essential oil distillation: Chemical composition, biological activity and potential uses. Adv. Technol. 2020, 9, 54–70. [Google Scholar] [CrossRef]
- Catty, S. Hydrosols: The Next Aromatherapy; Healing Arts Press: Rochester, VT, USA, 2001. [Google Scholar]
- Price, L.; Price, S. Understanding Hydrolates: The Specific Hydrosols for Aromatherapy: A Guide for Health Professionals, 1st ed.; Churchill Livingstone: London, UK; Elsevier: Amsterdam, The Netherlands, 2004; ISBN 9780443073168. [Google Scholar]
- Silva, H.N.P.; Sousa, E.M.O.; Maia, J.L.S.; Pinheiro, M.T.L.; Lameirão, S.V.O.C.; Mourão, R.H.V.; Maia, J.G.S.; Baldisserotto, B.; Silva, L.V.F. Lippia alba (Verbenaceae) hydrolate as sedative of tambaqui (Colossoma macropomum) juveniles in simulated transport conditions. Aquac. Res. 2018, 49, 128–134. [Google Scholar] [CrossRef]
- Djabou, N.; Dib, M.E.-A.; Tabti, B.; Costa, J.; Muselli, A. Chemical composition and antioxidant activity of hydrosol extracts obtained by liquid-liquid extraction (LLE) of Daucus muricatus L. J. Essent. Oil Res. 2014, 26, 393–399. [Google Scholar] [CrossRef]
- Zatla, A.T.; Dib, M.E.A.; Djabou, N.; Ilias, F.; Cost, J.; Muselli, A. Antifungal activities of essential oils and hydrosol extracts of Daucus carota subsp. sativus for the control of fungal pathogens, in particular gray rot of strawberry during storage. J. Essent. Oil Res. 2017, 29, 391–399. [Google Scholar] [CrossRef]
- Lancioni, C.; Castells, C.; Candal, R.; Tascon, M. Headspace Solid-Phase Microextraction: Fundamentals and Recent Advances. Adv. Sample Prep. 2022, 3, 100035. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Chahdoura, H.; Generalić Mekinić, I.; Maisto, M.; Kukula-Koch, W.; Ćavar Zeljković, S.; Koch, W.; Ben Akacha, B.; Taieb Bouteraa, M.; Ben Belgacem, A.; et al. A comprehensive review on traditional uses, chemical composition, pharmacological effects and applications in the food industry of Pelargonium odoratissimum (L.) L’Hér. in comparison to other Pelargonium spp. S. Afr. J. Bot. 2024, 174, 456–467. [Google Scholar] [CrossRef]
- Lis-Balchin, M.; Roth, G. Composition of the essential oils of Pelargonium odoratissimum, P. exstipulatum, and P. × fragrans (Geraniaceae) and their bioactivity. Flavour Fragr. J. 2020, 15, 391–394. [Google Scholar] [CrossRef]
- Andrade, A.M.; Cardoso, M.G.; Batista, L.R.; Freire, J.M.; Nelson, D.L. Antimicrobial activity and chemical composition of essential oil of Pelargonium odoratissimum. Rev. Bras. Farmacogn. 2011, 21, 47–52. [Google Scholar] [CrossRef]
- Szutt, A.; Dołhańczuk-Śródka, A.; Sporek, M. Evaluation of chemical composition of essential oils derived from different pelargonium species leaves. Ecol. Chem. Eng. S 2019, 26, 807–816. [Google Scholar] [CrossRef]
- Ben Hsouna, A.; Ben Akacha, B.; Generalić Mekinić, I.; Čmiková, N.; Ben Belgacem, A.; Bouteraa, M.T.; Ben Saad, R.; Mnif, W.; Kluz, M.I.; Kačániová, M.; et al. Insight into Pelargonium odoratissimum Essential Oil Preservative Properties Effect on Ground Beef. Foods 2024, 13, 3181. [Google Scholar] [CrossRef] [PubMed]
- Aragón, A.; Toledano, R.M.; Vázquez, A.; Villén, J.; Cortés, J.M. Analysis of polycyclic aromatic hydrocarbons in aqueous samples by large volume injection gas chromatography-mass spectrometry using the through oven transfer adsorption desorption interface. Talanta 2015, 139, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhang, Q.; Zhou, W.; Zou, X.; Wang, H.; Huang, C.; Chu, Y. Detection of ketones by a novel technology: Dipolar proton transfer reaction mass spectrometry (DP-PTR-MS). J. Am. Soc. Mass Spectrom. 2017, 28, 873–879. [Google Scholar] [CrossRef]
- Blake, R.S.; Monks, P.S.; Ellis, A.M. Proton-transfer reaction mass spectrometry. Chem. Rev. 2009, 109, 861–896. [Google Scholar] [CrossRef]
- Taiti, C.; Costa, C.; Guidi Nissim, W.; Bibbiani, S.; Azzarello, E.; Masi, E.; Pandolfi, C.; Pallottino, F.; Menesatti, P.; Mancuso, S. Assessing VOC emission by different wood cores using the PTR-ToF-MS technology. Wood Sci. Technol. 2017, 51, 273–295. [Google Scholar] [CrossRef]
- Jordan, A.; Haidacher, S.; Hanel, G.; Hartungen, E.; Mark, L.; Seehauser, H.; Mark, T.D. A high resolution and high sensitivity proton-transfer-reaction time-of-flight mass spectrometer (PTR-TOF-MS). Int. J. Mass Spectrom. 2009, 286, 122–128. [Google Scholar] [CrossRef]
- Caparrotta, S.; Comparini, D.; Marone, E.; Kimmenfield, R.; Luzzietti, L.; Taiti, C.; Mancuso, S. Correlation between VOC fingerprinting and antimicrobial activity of several essential oils extracted by plant resins against A. tumefaciens and P. savastanoi. Flavour Fragr. J. 2019, 34, 377–387. [Google Scholar] [CrossRef]
- Pang, X. Biogenic volatile organic compound analyses by PTR-TOF-MS: Calibration, humidity effect and reduced electric field dependency. J. Environ. Sci. 2015, 32, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Jeannot, V.; Chahboun, J.; Russel, D.; Casabianca, H. Origanum compactum Bentham: Composition of the hydrolat aromatic fraction, comparison with the essential oil and its interest in arometherapy. Int. J. Aromather. 2003, 13, 90–94. [Google Scholar] [CrossRef]
- Śmigielski, K.B.; Prusinowska, R.; Krosowiak, K.; Sikora, M. Comparison of qualitative and quantitative chemical composition of hydrolate and essential oils of lavender (Lavandula angustifolia). J. Essent. Oil Res. 2013, 25, 291–299. [Google Scholar] [CrossRef]
- Chizzola, R.; Billiani, F.; Singer, S.; Novak, J. Diversity of essential oils and the respective hydrolates obtained from three Pinus cembra populations in the Austrian Alps. Appl. Sci. 2021, 11, 5686. [Google Scholar] [CrossRef]
- Misztal, P.K.; Heal, M.R.; Nemitz, E.; Cape, J.N. Development of PTR-MS selectivity for structural isomers: Monoterpenes as a case study. Int. J. Mass Spectrom. 2012, 310, 10–19. [Google Scholar] [CrossRef]
- Maleknia, S.D.; Bell, T.L.; Adams, M.A. PTR-MS analysis of reference and plant-emitted volatile organic compounds. Int. J. Mass Spectrom. 2007, 262, 203–210. [Google Scholar] [CrossRef]
- Demarcke, M.; Amelynck, C.; Schoon, N.; Dewulf, J.; Van Langenhove, H. Investigations on the influence of drift field and humidity on sesquiterpene product ion distributions in a PTR-MS instrument and implications for sesquiterpene detection sensitivity. In Proceedings of the 4th International PTR-MS Conference, Obergurgl, Austria, 16–21 February 2009; pp. 170–173. Available online: https://orfeo.belnet.be/handle/internal/3240 (accessed on 14 July 2025).
- Tani, A. Fragmentation and reaction rate constants of terpenoids determined by proton transfer reaction-mass spectrometry. Environ. Control Biol. 2013, 51, 23–29. [Google Scholar] [CrossRef]
- Amel, H.A.; Kamel, H.; Meriem, F.; Abdelkader, K. Traditional Uses, Botany, Phytochemistry, and Pharmacology of Pelargonium graveolens: A Comprehensive Review. Trop. J. Nat. Prod. Res. 2002, 6, 1547–1569. [Google Scholar]
- Tavares, C.S.; Gameiro, J.A.; Roseiro, L.B.; Figueiredo, A.C. Hydrolates: A review on their volatiles composition, biological properties and potential uses. Phytochem. Rev. 2022, 21, 1661–1737. [Google Scholar] [CrossRef]
| N° | Component 1 | LRI 2 | LRI 3 | Hy 4 (%) | Hy 5 (%) |
|---|---|---|---|---|---|
| 1 | 2H-piran, 2-ethenyl tetrahydro-2,6,6-trimethyl- | 958 | 960 | 0.29 ± 0.02 | 0.41 ± 0.02 |
| 2 | β-myrcene | 990 | 987 | 2.2 ± 0.06 | 3.0 ± 0.06 |
| 3 | limonene | 1025 | 1022 | 1.2 ± 0.04 | 0.69 ± 0.02 |
| 4 | 1,8-cineole | 1029 | 1027 | 1.4 ± 0.05 | 1.6 ± 0.05 |
| 5 | cis-β-ocimene | 1040 | 1037 | 0.81 ± 0.04 | 0.91 ± 0.03 |
| 6 | trans-linaool oxide | 1085 | 1082 | 1.0 ± 0.05 | 1.1 ± 0.04 |
| 7 | linalool | 1090 | 1089 | 29.6 ± 2.10 | 30.7 ± 2.64 |
| 8 | rose-oxide | 1110 | 1112 | 2.0 ± 0.06 | 2.1 ± 0.04 |
| 9 | camphor | 1132 | 1139 | 2.3 ± 0.05 | 2.2 ± 0.05 |
| 10 | p-menthan-3-one | 1139 | 1142 | 6.6 ± 0.09 | 6.9 ± 0.11 |
| 11 | trans-pinocamphone | 1144 | 1148 | 0.59 ± 0.02 | 0.59 ± 0.03 |
| 12 | isomenthone | 1155 | 1159 | 21.1 ± 1.52 | 19.9 ± 0.15 |
| 13 | isocamphopinone | 1164 | 1162 | 2.8 ± 0.04 | 2.7 ± 0.06 |
| 14 | α-terpineol | 1195 | 1198 | 18.9 ± 0.15 | 18.7 ± 0.11 |
| 15 | citronellol | 1209 | 1212 | 8.8 ± 0.10 | 8.3 ± 0.09 |
| 16 | methyleugenol | 1355 | 1360 | 0.41 ± 0.02 | 0.30 ± 0.02 |
| SUM | 100.0 | 100.0 | |||
| Monoterpenes | 95.0 | 95.0 | |||
| Sesquiterpenes | - | - | |||
| Others | 5.0 | 5.0 |
| N° | Component 1 | LRI 2 | LRI 3 | Hy (%) |
|---|---|---|---|---|
| 1 | β-myrcene | 985 | 987 | 1.1 ± 0.04 |
| 3 | 1,8-cineole | 1031 | 1027 | 2.0 ± 0.06 |
| 4 | cis-β-ocimene | 1042 | 1037 | 0.70 ± 0.03 |
| 5 | cis-linalool oxide | 1058 | 1062 | 3.5 ± 0.07 |
| 6 | trans-linaool oxide | 1085 | 1082 | 15.8 ± 1.03 |
| 7 | linalool | 1091 | 1089 | 33.6 ± 4.05 |
| 8 | rose oxide | 1115 | 1112 | 1.3 ± 0.07 |
| 9 | thujone | 1092 | 1099 | 0.90 ± 0.04 |
| 10 | camphor | 1142 | 1139 | 2.7 ± 0.05 |
| 11 | isomenthone | 1155 | 1159 | 15.1 ± 1.06 |
| 12 | isocamphopinome | 1160 | 1162 | 2.3 ± 0.06 |
| 13 | α-terpineol | 1189 | 1198 | 20.9 ± 3.22 |
| SUM | 99.9 | |||
| Monoterpenes | 98.6 | |||
| Sesquiterpenes | - | |||
| Others | 1.3 |
| N° | Component 1 | LRI 2 | LRI 3 | Hy (%) |
|---|---|---|---|---|
| 1 | hexenol (3z) | 857 | 857 | 1.1 ± 0.05 |
| 2 | 2h-pyran, 2-ethenyltetrahydro-2,6,6-trimethyl- | 978 | 971 | 2.1 ± 0.07 |
| 3 | sulcatone | 986 | 986 | 0.40 ± 0.03 |
| 4 | β-myrcene 4 | 992 | 989 | 4.3 ± 0.09 |
| 5 | 1-hexanol, 2-ethyl | 1030 | 1031 | 0.80 ± 0.03 |
| 6 | limonene4 | 1040 | 1039 | 0.69 ± 0.04 |
| 7 | β-phellandrene | 1044 | 1041 | 0.11 ± 0.01 |
| 8 | β-ocimene 4 | 1050 | 1044 | 1.3 ± 0.06 |
| 9 | seudenone | 1068 | 1046 | 0.20 ± 0.01 |
| 10 | acetophenone | 1078 | 1073 | 1.2 ± 0.05 |
| 11 | trans-linalool oxide 4 | 1081 | 1082 | 1.4 ± 0.06 |
| 12 | benzenemethanol, α,α-dimethyl- | 1095 | 1090 | 9.5 ± 0.12 |
| 13 | linalool 4 | 1102 | 1099 | 12.8 ± 0.13 |
| 14 | 6-methyl-3,5-heptadiene-2-one | 1109 | 1107 | 2.3 ± 0.06 |
| 15 | rose-oxide 4 | 1118 | 1113 | 0.20 ± 0.01 |
| 16 | myrcenol | 1122 | 1118 | 2.8 ± 0.06 |
| 17 | cis-ocimenol | 1156 | 1157 | 1.2 ± 0.04 |
| 18 | unknown | 1169 | / | 4.9 ± 0.07 |
| 19 | menthone | 1172 | 1163 | 1.1 ± 0.05 |
| 20 | Isomenthone 4 | 1182 | 1182 | 2.5 ± 0.06 |
| 21 | p-mentha-1,5-dien-8-ol | 1185 | 1185 | 1.0 ± 0.04 |
| 22 | α-terpineol 4 | 1211 | 1210 | 11.1 ± 0.10 |
| 23 | citronellol 4 | 1230 | 1228 | 25.8 ± 1.19 |
| 24 | geraniol | 1253 | 1255 | 6.6 ± 0.12 |
| 25 | hydroxy citronellol | 1368 | 1362 | 4.0 ± 0.11 |
| 26 | methyl eugenol 4 | 1406 | 1401,8 | 0.60 ± 0.05 |
| SUM | 100.0 | |||
| Monoterpenes | 79.0 | |||
| Sesquiterpenes | - | |||
| Others | 21.0 |
| Number of Detected Signals | m/z | Protonated Chemical Formula | Tentative Identifications | Hy | SD | % on the Total |
|---|---|---|---|---|---|---|
| 1 | 27.022 | C2H3+ | Acetylene | 401 | 119 | 10.2 |
| 2 | 30.046 | C2H6+ | Ethylene (isotope) | 42.8 | 6.06 | 1.09 |
| 3 | 31.018 | CH3O+ | Formaldehyde | 116 | 22.4 | 2.96 |
| 4 | 33.033 | CH5O+ | Methanol | 605 | 217 | 15.4 |
| 5 | 41.038 | C3H5+ | Alkyl fragments | 87.9 | 35.1 | 2.23 |
| 6 | 43.018 | C2H3O+ | Alkyl fragments (ethenone/incensole acetate) | 229 | 88.1 | 5.84 |
| 7 | 43.050 | C3H7+ | Alkyl fragments (propene) | 59.7 | 29.6 | 1.52 |
| 8 | 45.033 | C2H5O+ | Acetaldehyde | 684 | 415 | 17.4 |
| 9 | 47.049 | C2H7O+ | Ethanol | 409 | 123 | 10.4 |
| 10 | 55.055 | C4H7+ | C4 aldehydes fragment (Butanal) | 18.6 | 10.0 | 0.47 |
| 11 | 57.033 | C3H5O+ | Alkyl fragments (hexanal/1-butanol/1-octanol) | 5.63 | 0.67 | 0.14 |
| 12 | 57.069 | C4H9+ | Alkyl fragments (hexanol/valeric acid) | 12.8 | 5.84 | 0.33 |
| 13 | 59.049 | C3H7O+ | Propanal, acetone | 701 | 131 | 17.8 |
| 14 | 61.027 | C2H5O2+ | Acetates | 130 | 66.1 | 3.31 |
| 15 | 63.027 | C2H7S+ | Dimethyl sulfide | 78.5 | 15.8 | 2.00 |
| 16 | 65.038 | C5H5+ | Alkyl fragments | 4.73 | 2.07 | 0.12 |
| 17 | 67.054 | C5H7+ | Terpene fragments (e.g., myrcene) | 3.89 | 1.54 | 0.10 |
| 18 | 69.069 | C5H9+ | Isoprene/terpene fragments | 8.92 | 4.64 | 0.23 |
| 19 | 71.049 | C4H7O+ | Methyl vinyl ketone | 10.5 | 7.05 | 0.27 |
| 20 | 73.065 | C4H9O+ | Methyl ethyl ketone | 26.3 | 6.12 | 0.67 |
| 21 | 75.044 | C3H7O2+ | Methyl acetate | 3.57 | 2.32 | 0.09 |
| 22 | 79.054 | C6H7+ | Terpene fragments | 11.9 | 1.65 | 0.30 |
| 23 | 81.069 | C6H9+ | Terpene fragments/C6 fragments | 116 | 21.2 | 2.96 |
| 24 | 83.049 | C5H7O+ | 3-methyl furan | 3.43 | 1.90 | 0.09 |
| 25 | 83.086 | C6H11+ | C6 compounds/hexenol fragment | 6.18 | 3.29 | 0.16 |
| 26 | 85.065 | C5H9O+ | Methyl-butenal/ pentenone | 2.98 | 1.87 | 0.08 |
| 27 | 87.044 | C4H7O2+ | 2,3-Butandione/diacetyl | 13.7 | 3.91 | 0.35 |
| 28 | 87.080 | C5H11O+ | Pentanal/methylbutanal | 11.5 | 5.05 | 0.29 |
| 29 | 89.059 | C4H9O2+ | Ethyl acetate/methyl-propanoate | 2.32 | 1.48 | 0.06 |
| 30 | 93.069 | C7H9+ | Terpenes fragments | 25.6 | 3.54 | 0.65 |
| 31 | 95.086 | C7H11+ | Terpene fragments | 19.9 | 4.01 | 0.51 |
| 32 | 97.064 | C6H9O+ | Dimethyl-furan | 2.43 | 0.90 | 0.06 |
| 33 | 99.080 | C6H11O+ | Hexenals /methyl-pentenone | 8.75 | 1.64 | 0.22 |
| 34 | 101.059 | C6H13O+ | Hexenol | 3.25 | 1.39 | 0.08 |
| 35 | 107.086 | C8H11+ | Terpenes fragment | 1.51 | 0.85 | 0.04 |
| 36 | 109.085 | C8H11+ | Terpenes fragments | 3.63 | 2.54 | 0.09 |
| 37 | 111.080 | C7H11O+ | Dimethyl-2-cyclopenten-1-one | 1.98 | 0.64 | 0.05 |
| 38 | 113.096 | C7H13O+ | Methylcyclohexanone | 2.51 | 0.61 | 0.06 |
| 39 | 115.054 | C9H7+ | C9- aromatics | 2.28 | 0.67 | 0.06 |
| 40 | 123.101 | C9H15+ | Terpenes fragments | 2.66 | 0.39 | 0.07 |
| 41 | 125.096 | C8H13O+ | 2-Butylfuran | 1.94 | 0.59 | 0.05 |
| 42 | 135.117 | C10H15+ | p-cymene | 4.91 | 0.51 | 0.12 |
| 43 | 137.132 | C10H17+ | Monoterpenes (e.g., limonene) | 19.6 | 2.41 | 0.50 |
| 44 | 143.145 | C8H15O2+ | Nonanal | 2.94 | 0.51 | 0.07 |
| 45 | 153.127 | C10H17O+ | Terpenoid-like compound (e.g., camphor) | 14.6 | 5.10 | 0.37 |
| 46 | 155.143 | C10H19O+ | Terpenoid-like compound (e.g., 1,8-cineole) | 6.67 | 3.47 | 0.17 |
| Total Terpene Emission | 326.6 | 86.1 | 8.30 | |||
| Total VOC Emission | 3933.7 | 1379 | 100 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taiti, C.; Vinciguerra, V.; Graziano, M.M.; Masi, E.; Garzoli, S. In-Depth Investigation of the Chemical Profile of Pelargonium odoratissimum (L.) L’Hér. Hydrolate by SPME-GC/MS, GC/MS, LVI-GC/MS and PTR-Tof-MS Techniques. Chemosensors 2025, 13, 325. https://doi.org/10.3390/chemosensors13090325
Taiti C, Vinciguerra V, Graziano MM, Masi E, Garzoli S. In-Depth Investigation of the Chemical Profile of Pelargonium odoratissimum (L.) L’Hér. Hydrolate by SPME-GC/MS, GC/MS, LVI-GC/MS and PTR-Tof-MS Techniques. Chemosensors. 2025; 13(9):325. https://doi.org/10.3390/chemosensors13090325
Chicago/Turabian StyleTaiti, Cosimo, Vittorio Vinciguerra, Monica Mollica Graziano, Elisa Masi, and Stefania Garzoli. 2025. "In-Depth Investigation of the Chemical Profile of Pelargonium odoratissimum (L.) L’Hér. Hydrolate by SPME-GC/MS, GC/MS, LVI-GC/MS and PTR-Tof-MS Techniques" Chemosensors 13, no. 9: 325. https://doi.org/10.3390/chemosensors13090325
APA StyleTaiti, C., Vinciguerra, V., Graziano, M. M., Masi, E., & Garzoli, S. (2025). In-Depth Investigation of the Chemical Profile of Pelargonium odoratissimum (L.) L’Hér. Hydrolate by SPME-GC/MS, GC/MS, LVI-GC/MS and PTR-Tof-MS Techniques. Chemosensors, 13(9), 325. https://doi.org/10.3390/chemosensors13090325







