What’s New with the Old Ones: Updates on Analytical Methods for Fossil Research
Abstract
1. Fossils in Heritage Sciences: Current Status
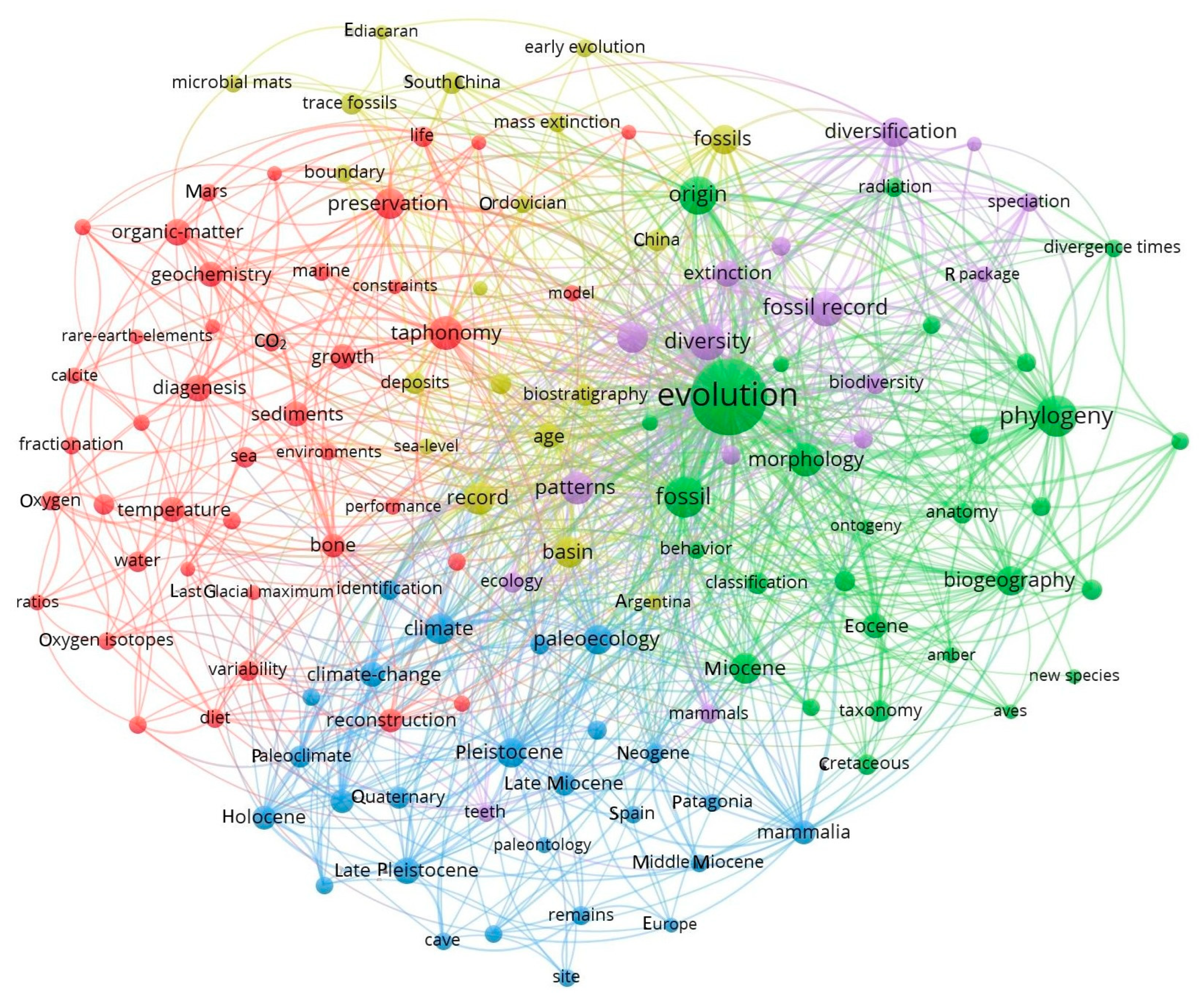
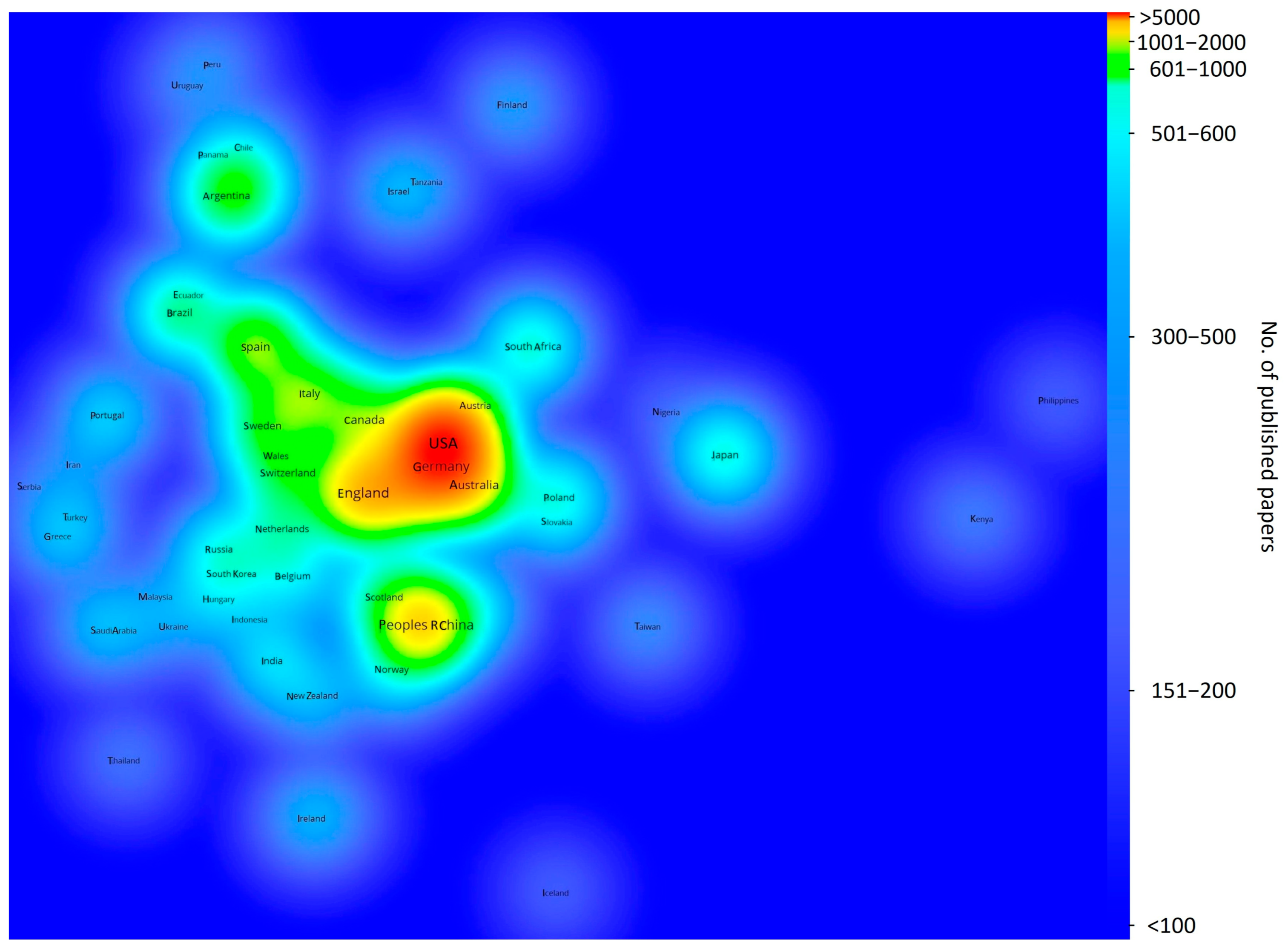
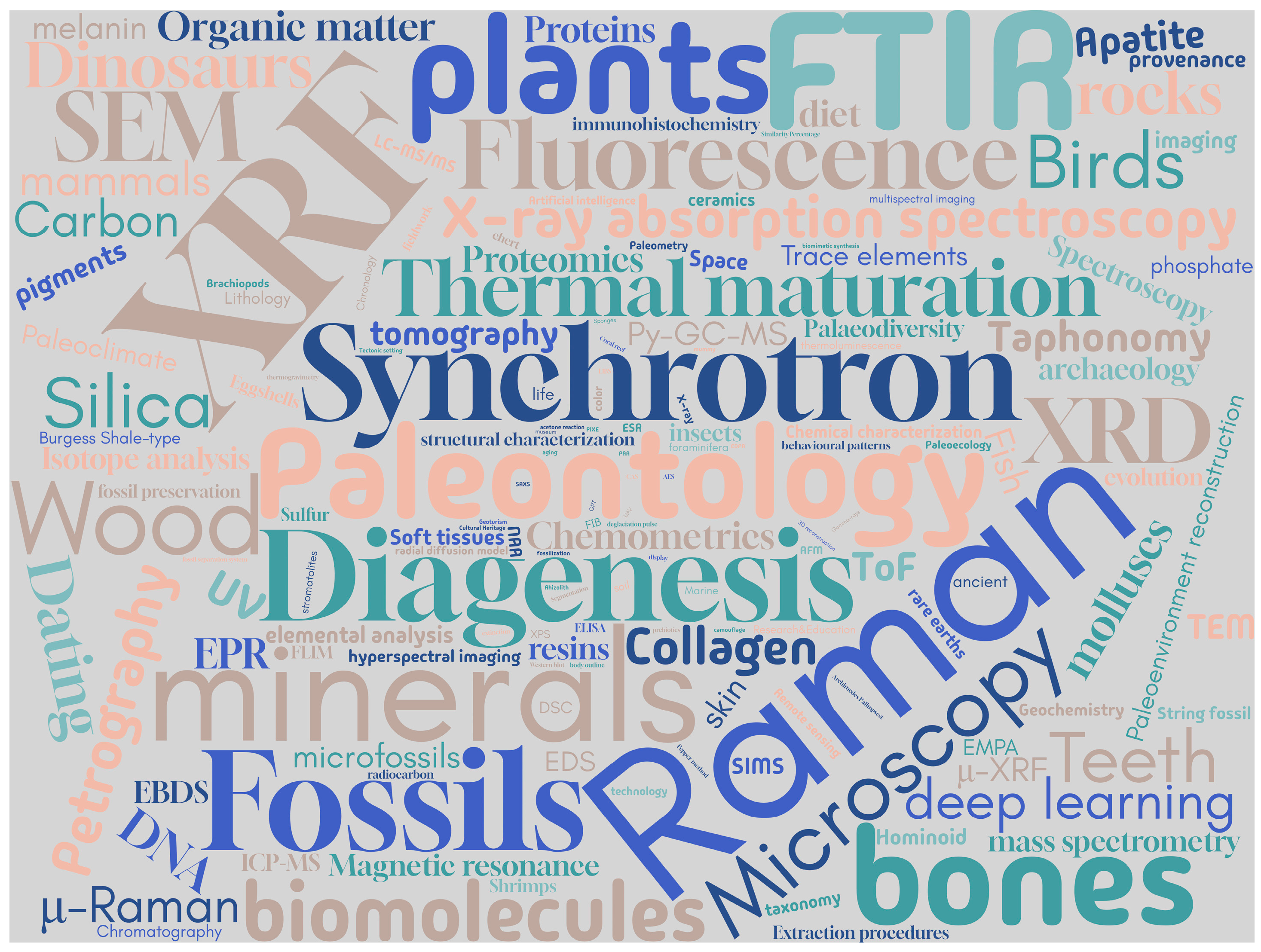
2. Bibliographic Study
3. Emerging Analytical Chemistry Principles for Fossil Research
| Type | Analytical Method | Aim | Pros (↑) and Cons (↓) | Type of Fossil | Selective References |
|---|---|---|---|---|---|
| NON-INVASIVE, NON-DESTRUCTIVE | (µ-)XRF | Elemental analysis | ↑ Suitable for elemental mapping of fossils ↑ Minimal or no sample preparation ↑ Highly portable ↑ Easily accessible ↑ High sensitivity for heavy elements, great for detection of REEs ↓ Limited to the detection of inorganic elements ↓ Can be affected by matrix effects | Bones Teeth Shells Fish Crustaceans Feathers Wood | [23,24,25,26,27,28] |
| (µ-)XAS, SAXS | ↑ Suitable for elemental mapping of fossils ↑ Can detect trace elements ↓ Micro-techniques require synchrotron-radiation | Bones Teeth Soft tissues Amber Kerogens Microfossils | [29,30,31] | ||
| (µ-)/(n-)CT, XRM | ↑ Suitable for visualization of dense fossils ↑ Allows 3D morphology ↓ Limited accuracy for low-contrast samples | Bones Shells Embryos Amber | [23,32,33,34] | ||
| Fluorescence, UV-Vis spectroscopy | Fossilization processes Morphological differentiation Preliminary screening of fossils | ↑ Suitable for screening of fossils ↑ Useful for understanding fossilization processes ↑ Suitable for differentiating organic and mineral phases ↑ Preliminary determination of morphological structure ↑Identification of the oxidation state ↑ Reconstruction of fossil paravian body ↑ Identification of unseen structures in fossil feathers ↑ Understanding nesting behavior ↓ Hard to discern overlapping signals ↓ May be affected by spectral interference | Amber Soft tissues Proteins | [35,36,37,38,39,40,41,42,43,44] | |
| Multi- or hyperspectral imaging | Surface mapping | ↑ Highly suitable for highlighting diagenetic transformations in fossils ↑ Fossil detection and screening ↑ Mapping surface textures and compositional variations ↑ Evaluation of collagen level in fossil bones ↓ Limited resolution | Wood Bones Amber Shells Soft tissues | [7,45,46,47,48,49] | |
| Raman spectroscopy | Molecular composition Taxonomic identification | ↑ Useful for the identification of compositional patterns ↑ Suitable for evaluating biogenicity ↑ Identification of biologically synthesized compounds ↑ Elucidating fossil preservation mechanisms ↑ Useful in palaeothermometry ↑ Characterization of diverse biosignatures ↑ Best suited for carbonaceous materials ↓ May be affected by fluorescence signal | Proteins Lipids Minerals | [36,50,51,52,53,54,55,56,57,58,59] | |
| INVASIVE, NON-DESTRUCTIVE | PIXE | Elemental analysis | ↑ High sensitivity to elements in trace amounts ↓ Requires thin sections | Bones Teeth Soft tissues | [35] |
| SEM(-EDS) | Surface analysis | ↑ Provides high-resolution images ↑Can offer information about the state of fossils ↑ Useful for the identification of melanosomes ↓ Can require sample coating for conductivity | Bone Teeth Scales | [59,60,61,62] | |
| EBSD | Crystalline structure | ↑ Suitable for examining fine-grain structures ↑ Evaluation of sedimentary processes ↑ Evaluation of diagenetic processes on foraminiferous fossils ↑ Allows the quantitative analysis of crystalline microstructures ↓ Requires sample preparation | Minerals | [54,63,64,65] | |
| (µ-)FTIR | Molecular composition | ↑ Suitable for analysis of functional groups ↑ Information about the molecular composition ↑ Taxonomic identification ↑ Evaluating biogenicity ↑ Identification of biologically synthesized compounds ↑ Identifying the effects of diagenetic processes ↑ Elucidating fossil preservation mechanisms ↓ May require crushing of the sample | Bones Amber | [1,47,50,66,67,68,69,70] | |
| EPR | Chemical composition | ↑ Information about chemical composition ↑ Molecular interactions ↑ Useful for detecting paramagnetic centers ↑ Effective for dating ↓ Requires specific sample preparation | Teeth Shell | [66,71,72,73] | |
| NAA | Chemical composition | ↑ Effective for dating ↑ Suitable for trace element analysis ↑ Determining chemical elements ↑ Useful in studies concerning the preservation of bone tissues ↓ Requires access to nuclear reactor or generator ↓ Limited elemental detection ↓ High operational costs | Bones Teeth Coprolites Shells Sediments | [72,74] | |
| (µ-)XRD | Mineralogical characterization | ↑ Current standard for identifying the crystalline structure of fossils ↓ Requires specific sample preparation | Minerals | [48] | |
| INVASIVE, DESTRUCTIVE | Thermoluminescence | Dating | ↑ Effective for dating ↓ Results may be affected by exposure of luminous centers to light after unearthing | Bones Minerals | [72,73] |
| Dendrochronology | Dating Species identification | ↑ Effective for dating ↑ Useful for the identification of wood species ↓ Requires core drilling or sawing | Wood | [73,75] | |
| TGA | Chemical fingerprinting | ↑ Suitable for determination of resin fossils’ origins ↑ Effective for dating ↑ Useful for understanding diagenetic processes ↓ Irreversible damage to the sample | Bones Coprolites | [70] | |
| FIB-SEM-EDS | Chemical and structural composition | ↑ Great for nano-scale chemical imaging ↓ Damages sample surface | Embryos Soft tissues Microbialmats Biofilms Amber | [32,53,61,76] | |
| TEM | Crystallographic structure Morphology | ↑ Can offer information about the size and shape of bioapatite crystals ↓ Requires thin sections | Proteins Biominerals | [53,60,69,71,77] | |
| Isotope analysis | Nutritional ecology Feeding and migration patterns | ↑ Useful for evaluating the ecological impact of certain species ↑ Effective for dating ↑ Useful in paleoenvironmental studies ↓ Frequently affected by external sample contamination | Teeth Bones Shells | [71,78,79,80,81,82] | |
| DNA analysis | Evolutionary studies Species identification | ↑ Highly suitable for elucidating evolutionary links ↑ Identifying extinct species ↓ Requires well-preserved organic material | Bones Teeth Hair | [1,5,83] | |
| Py-GC-MS | Organic composition | ↑ Information about chemical composition ↑ Identification of fossilized organic pigments ↑ Identification and classification of fossilized resins ↓ Sample destruction is unavoidable | Coprolites Resins Soft tissues | [38,66,84,85] | |
| (Tof-)/(NANO)SIMS | Molecular, elemental, and isotopic composition | ↑ suitable for isotopic mapping ↑ High spatial resolution ↑ Depth profiling ↑ Minimal sample preparation ↓ Expensive equipment ↓ Long acquisition time | Tissues Organelles | [33,86,87,88,89,90] | |
| DSC | Chemical fingerprinting | ↑ Investigating resin age ↓ Requires sample heating, altering its original properties | Collagen Resins | [70] | |
| LIBS | Surface and stratigraphical elemental analysis | ↑ Useful for fossil identification ↑ Allows controlled in-depth analysis ↑ Individual fingerprinting and fossil sampling ↓ Micro-destructive | Bones Teeth Sediments | [91,92] |
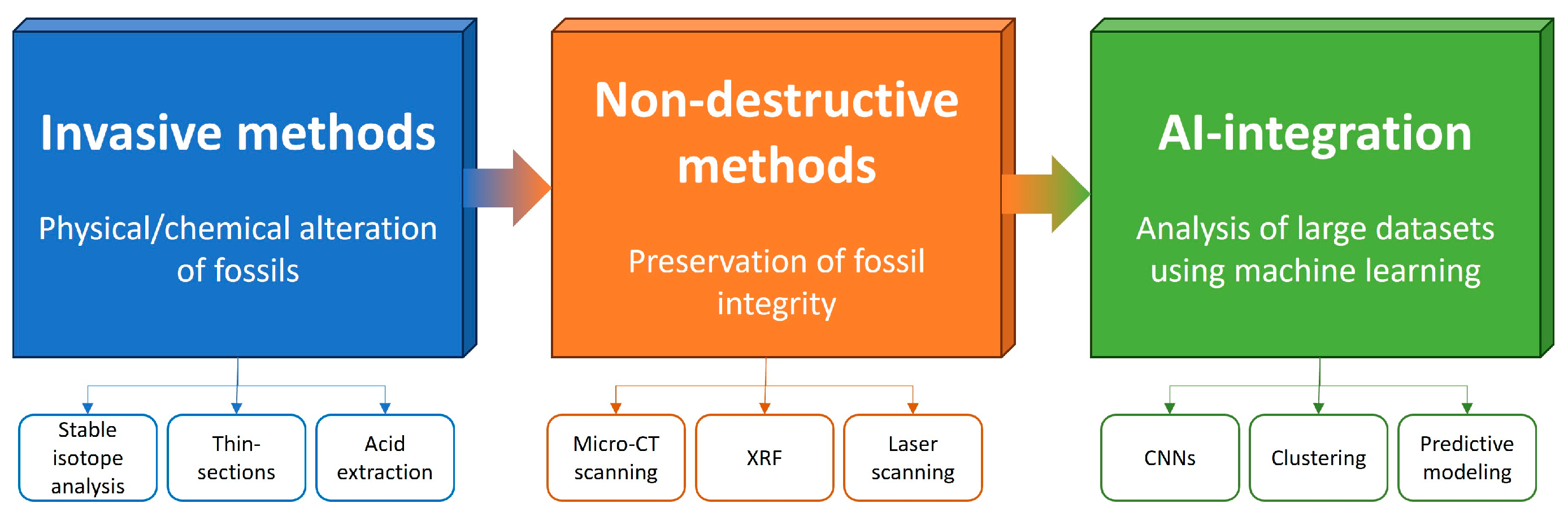
3.1. Non-Destructiveness
3.2. Operability
3.3. Downscaling
3.4. AI Integration
4. Conclusions and Future Perspectives
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| CT | computed tomography |
| DNA | deoxyribonucleic acid |
| DSC | differential scanning calorimetry |
| EBSD | electron backscatter diffraction |
| EPR | electron paramagnetic resonance |
| FIB-SEM-EDS | focused ion beam-scanning electron microscopy coupled with electron backscatter spectroscopy |
| FTIR | Fourier transform infrared spectroscopy |
| LA-ICP-MS | laser ablation-inductively coupled plasma-mass spectrometry |
| NAA | neutron activation analysis |
| NANO-SIMS | nanoscale secondary ion mass spectrometry |
| nCT | nano-computed tomography |
| PCCT | phase-contrast computed tomography (photon-counting computed tomography) |
| PIXE | particle-induced X-ray emission |
| Py-GC-MS | pyrolysis-gas chromatography-mass spectrometry |
| REE | rare-earth element |
| RS | Raman spectroscopy |
| SAXS | small-angle X-ray scattering |
| SEM | scanning electron microscopy |
| SEM-EDS | scanning electron microscopy coupled with electron backscatter spectroscopy |
| SIMS | secondary ion mass spectrometry |
| SR-FTIR | synchrotron radiation-based Fourier transform infrared spectroscopy |
| TEM | transmission electron microscopy |
| TGA | thermogravimetry |
| TOF-SIMS | time-of-flight secondary ion mass spectrometry |
| XANES | X-ray absorption near edge structure |
| XAS | X-ray absorption spectroscopy |
| XEOL | X-ray excited optical luminescence |
| XRD | X-ray diffraction spectroscopy |
| XRF | X-ray fluorescence spectroscopy |
| XRF-μCT | X-ray fluorescence spectroscopy-micro-computed tomography |
| μCT | micro-computed tomography |
| (μ-)XRF | micro-X-ray fluorescence spectroscopy |
| XRS | X-ray-induced Raman spectroscopy |
References
- Kontopoulos, I.; Penkman, K.; Mullin, V.E.; Winkelbach, L.; Unterländer, M.; Scheu, A.; Kreutzer, S.; Hansen, H.B.; Margaryan, A.; Teasdale, M.D.; et al. Screening archaeological bone for palaeogenetic and palaeoproteomic studies. PLoS ONE 2020, 15, e0235146. [Google Scholar] [CrossRef] [PubMed]
- van Eck, N.J.; Waltman, L. “VOSviewer 1.6.20,” Copyright (c) 2009–2023 Nees Jan van Eck and Ludo Waltman. Available online: https://www.vosviewer.com/ (accessed on 22 May 2025).
- Leach, J.D.; Gibson, G.R.; Van Loo, J. Human Evolution, Nutritional Ecology and Prebiotics in Ancient Diet. Biosci. Microflora 2006, 25, 1–8. [Google Scholar] [CrossRef]
- Cooper, R.B.; Flannery-Sutherland, J.T.; Silvestro, D. DeepDive: Estimating global biodiversity patterns through time using deep learning. Nat. Commun. 2024, 15, 4199. [Google Scholar] [CrossRef]
- Haouchar, D.; Haile, J.; McDowell, M.C.; Murray, D.C.; White, N.E.; Allcock, R.J.N.; Pjillips, M.J.; Prideaux, G.J.; Bunce, M. Thorough assessment of DNA preservation from fossil bone and sediments excavated from a late Pleistocene-Holocene cave deposit on Kangaroo Island, South Australia. Quat. Sci. Rev. 2014, 84, 56–64. [Google Scholar] [CrossRef]
- Yu, C.; Qin, F.; Watanabe, A.; Yao, W.; Li, Y.; Qin, Z.; Liu, Y.; Wang, H.; Jiangzuo, Q.; Hsiang, A.Y.; et al. Artificial intelligence in paleontology. Earth-Sci. Rev. 2024, 252, 104765. [Google Scholar] [CrossRef]
- Ghezzo, E.; Massironi, M.; Davis, E.B. Multispectral satellite imaging improves detection of large individual fossils. Geol. Mag. 2023, 160, 535–544. [Google Scholar] [CrossRef]
- Xiao, S.; Muscente, A.D.; Chen, L.; Zhou, C.; Schiffbauer, J.D.; Wood, A.D.; Polys, N.F.; Yuan, X. The Weng’an biota and the Ediacaran radiation of multicellular eukaryotes. Natl. Sci. Rev. 2014, 1, 498–520. [Google Scholar] [CrossRef]
- Rahman, I.A.; Darroch, S.A.F.; Racicot, R.A.; Laflamme, M. Organismal Biology: Suspension feeding in the enigmatic Ediacaran organism Tribrachidium demonstrates complexity of Neoproterozoic ecosystems. Sci. Adv. 2015, 1, e1500800. [Google Scholar] [CrossRef]
- Yang, C.; Li, Y.; Selby, D.; Wan, B.; Guan, C.; Zhou, C.; Li, X.-H. Implications for Ediacaran biological evolution from the ca. 602 Ma Lantian biota in China. Geology 2022, 50, 562–566. [Google Scholar] [CrossRef]
- Liu, H.; Dong, L.; Qin, S.; Liu, W.; Li, C. Restudy of string fossils from the Ediacaran-Cambrian Liuchapo Formation in Guizhou Province, South China. Precambrian Res. 2022, 376, 106693. [Google Scholar] [CrossRef]
- Callow, R.H.T.; Brasier, M.D. Remarkable preservation of microbial mats in Neoproterozoic siliciclastic settings: Implications for Ediacaran taphonomic models. Earth-Sci. Rev. 2009, 96, 207–219. [Google Scholar] [CrossRef]
- Zhang, X.; Shu, D. Current understanding on the Cambrian Explosion: Questions and answers. PalZ 2021, 95, 641–660. [Google Scholar] [CrossRef]
- Levinton, J.S. The Cambrian explosion: How do we use the evidence? BioScience 2008, 58, 855–864. [Google Scholar] [CrossRef]
- Cohen, D.R.; Cohen, E.J.; Graham, I.T.; Soares, G.G.; Hand, S.J.; Archer, M. Geochemical exploration for vertebrate fossils using field portable XRF. J. Geochem. Explor. 2017, 181, 1–9. [Google Scholar] [CrossRef]
- Saleh, F.; Pittet, B.; Sansjofre, P.; Guériau, P.; Lalonde, S.; Perrillat, J.-P.; Vidal, M.; Lucas, V.; El Hariri, K.; Kouraiss, K.; et al. Taphonomic pathway of exceptionally preserved fossils in the Lower Ordovician of Morocco. Geobios 2020, 60, 99–115. [Google Scholar] [CrossRef]
- Goldman, D.; Leslie, S.A.; Liang, Y.; Bergström, S.M. Ordovician biostratigraphy: Index fossils, biozones and correlation. Geol. Soc. Spec. Publ. 2023, 532, 31–62. [Google Scholar] [CrossRef]
- Young, S.A.; Edwards, C.T.; Ainsaar, L.; Lindskog, A.; Saltzman, M.R. Seawater signatures of Ordovician climate and environment. Geol. Soc. Spec. Publ. 2023, 532, 137–156. [Google Scholar] [CrossRef]
- Marinova, E.; Kirleis, W.; Bittmann, F. Human landscapes and climate change during the Holocene. Veg. Hist. Archaeobotany 2012, 21, 245–248. [Google Scholar] [CrossRef][Green Version]
- Araujo, A.G.M.; Correa, L.C.; Perez, G.C.; Di Gregorio, E.D.; Okumura, M. Human-environment interaction during the Holocene in Eastern South America: Rapid climate changes and population dynamics. PLoS ONE 2025, 20, e0315747. [Google Scholar] [CrossRef]
- Eurostat. R&D Expenditure. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=R%26D_expenditure (accessed on 22 July 2025).
- Word Cloud. Available online: https://www.wordclouds.com (accessed on 22 May 2025).
- Stelzner, J.; Million, S.; Stelzner, I.; Martinez-Garcia, J.; Gwerder, D.; Nelle, O.; Schuetz, P. Non-destructive dendrochronology with X-ray computed tomography: The influence of different conservation methods for waterlogged archaeological wood. Dendrochronologia 2023, 78, 126065. [Google Scholar] [CrossRef]
- Tintner, J.; Spangl, B.; Reiter, F.; Smidt, E.; Grabner, M. Infrared spectral characterization of the molecular wood decay in terms of age. Wood Sci. Technol. 2020, 54, 313–327. [Google Scholar] [CrossRef]
- Choi, S.; Park, Y.; Kweon, J.J.; Kim, S.; Jung, H.; Lee, S.K.; Lee, Y.-N. Fossil eggshells of amniotes as a paleothermometry tool. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2020, 571, 110376. [Google Scholar] [CrossRef]
- Kuczumow, A.; Cukrowska, E.; Stachniuk, A.; Gawęda, R.; Mroczka, R.; PAskowicz, W.; Skrzypiec, K.; Falkenberg, R.; Backwell, L. Investigation of chemical changes in bone material from South African fossil hominid deposits. J. Archaeol. Sci. 2010, 37, 107–115. [Google Scholar] [CrossRef]
- Herrera, E.Z.; Rios, S.D.; Aquino Ayala, C.; Colman, C.; Souberlich, R.; Idoyaga, M.L.; Sánchez, C.J.; Casanova Andrello, A.; Mello, A. Characterization of dental fragments of Toxodon sp. (Mammalia, Notoungulata) by multiple physical and chemical techniques. Vib. Spectrosc. 2021, 114, 103248. [Google Scholar] [CrossRef]
- Sousa Filho, F.E.; da Silva, J.H.; Saraiva, G.D.; Abagaro, B.T.O.; Barros, O.A.; Saraiva, A.A.F.; Viana, B.C.; Freire, P.T.C. Spectroscopic studies of the fish fossils (Cladocyclus gardneri and Vinctifer comptoni) from the Ipubi Formation of the Cretaceous Period. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2016, 157, 124–128. [Google Scholar] [CrossRef]
- Gueriau, P.; Réguer, S.; Leclercq, N.; Cupello, C.; Brito, P.M.; Jauvion, C.; Morel, S.; Charbonnier, S.; Thiaudière, D.; Mocuta, C. Visualizing mineralization processes and fossil anatomy using synchronous synchrotron X-ray fluorescence and X-ray diffraction mapping. J. R. Soc. Interface 2020, 17, 20200216. [Google Scholar] [CrossRef] [PubMed]
- Al-Nashar, A.M.; Hafez, N.A.A.; El-Moghny, M.W.A.; Awad, A.; Farouk, S.; Ayyad, H.M. Integrating facies, mineralogy, and paleomagnetism to constrain the age and provenance of Paleozoic siliciclastic sedimentary rocks along the northern Gondwana margin: Insights from the Araba and Naqus formations in western Gulf of Suez, Egypt. Int. J. Earth Sci. 2024, 113, 923–950. [Google Scholar] [CrossRef]
- Bergmann, U.; Bertrand, L.; Edwards, N.P.; Manning, P.L.; Wogelius, R.A. Chemical Mapping of Ancient Artifacts and Fossils with X-Ray Spectroscopy. In Synchrotron Light Sources and Free-Electron Lasers; Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Kitanaka, R.; Tsuboi, M.; Ozaki, Y. Biogenic apatite in carbonate concretions with and without fossils investigated in situ by micro-Raman spectroscopy. Sci. Rep. 2023, 13, 9714. [Google Scholar] [CrossRef] [PubMed]
- de Queiroz, L.C.; Carlisbino, T.; Agressott, E.V.H.; Paschoal, A.R.; de Tarso, C.; Freire, P.; Viana Neto, B.C.; da Silva, J.H. Paleoenvironmental interpretations of Irati and Mangrullo Formations (Permian of Paraná Basin) based on rocks and fossil bones through spectroscopy techniques. Vib. Spectrosc. 2020, 110, 103110. [Google Scholar] [CrossRef]
- Gueriau, P.; Mocuta, C.; Bertrand, L. Cerium Anomaly at Microscale in Fossils. Anal. Chem. 2015, 87, 8827–8836. [Google Scholar] [CrossRef]
- Moisan, P. The study of cuticular and epidermal features in fossil plant impressions using silicone replicas for scanning electron microscopy. Palaeontol. Electron. 2012, 15.2.23A. [Google Scholar] [CrossRef]
- Jiménez, V.C.; Monferran, M.D.; Sperança, M.A.; Castro, J.P.; Catelani, T.A.; Pellerano, R.G.; Perino, E.; Pereira–Filho, E.R.; Cabaleri, N.G.; Gallego, O.F. Combination of analytic techniques to chemical characterization and preservation of Jurassic clam shrimp carapaces from La Matilde formation, Patagonia. J. South Am. Earth Sci. 2021, 109, 103269. [Google Scholar] [CrossRef]
- Cáceres, J.O.; de los Terreros, J.Y.S. A real-world approach to identifying animal bones and Lower Pleistocene fossils by laser induced breakdown spectroscopy. Talanta 2021, 235, 122780. [Google Scholar] [CrossRef]
- Roberts, D.E.; Plessis, A.D.; Steyn, J.; Botha, L.R.; Pityana, S.; Berger, L.R. An investigation of Laser Induced Breakdown Spectroscopy for use as a control in the laser removal of rock from fossils found at the Malapa hominin site, South Africa. Spectrochim. Acta-Part B At. Spectrosc. 2012, 73, 48–54. [Google Scholar] [CrossRef]
- Schilling, R.; Jastram, B.; Wings, O.; Schwarz-Wings, D.; Issever, A.S. Reviving the Dinosaur: Virtual Reconstruction and Three-dimensional Printing of a Dinosaur Vertebra. Radiology 2014, 270, 864–871. [Google Scholar] [CrossRef]
- Kaye, T.G.; Falk, A.R.; Pittman, M.; Sereno, P.C.; Martin, L.D.; Burnham, D.A.; Gong, E.; Xu, X.; Wang, Y. Laser-stimulated fluorescence in paleontology. PLoS ONE 2015, 10, e0125923. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Avci, R.; Collier, T.; Goodwin, M.B. Microscopic, chemical and molecular methods for examining fossil preservation. Comptes Rendus-Palevol 2008, 7, 159–184. [Google Scholar] [CrossRef]
- Sasso, G.D.; Angelini, I.; Maritan, L.; Artioli, G. Raman hyperspectral imaging as an effective and highly informative tool to study the diagenetic alteration of fossil bones. Talanta 2018, 179, 167–176. [Google Scholar] [CrossRef]
- Demarchi, B.; Hall, S.; Roncal-Herrero, T.; Freeman, C.L.; Woolley, J.; Crisp, M.K.; Wilson, J.; Fotakis, A.; Fischer, R.; Kessler, B.M. Protein sequences bound to mineral surfaces persist into deep time. Elife 2016, 5, e17092. [Google Scholar] [CrossRef]
- Tiunstra, L.; Thomas, B.; Robinson, S.; Pawlak, K.; Elezi, G.; Faull, K.F.; Taylor, S. Evidence for Endogenous Collagen in Edmontosaurus Fossil Bone. Anal. Chem. 2025, 97, 2618–2628. [Google Scholar] [CrossRef] [PubMed]
- Lindgren, J.; Uvdal, P.; Engdahl, A.; Lee, A.H.; Alwmark, C.; Bergquist, K.-E.; Nilsson, E.; Ekström, P.; Rasmussen, M.; Douglas, D.A.; et al. Microspectroscopic evidence of cretaceous bone proteins. PLoS ONE 2011, 6, e19445. [Google Scholar] [CrossRef] [PubMed]
- Pawlicki, R.; Korbel, A.; Kubiak, H. Cells, collagen fibrils and vessels in dinosaur bone. Nature 1966, 211, 655–657. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Suo, Z.; Avci, R.; Asara, J.M.; Allen, M.A.; Teran Arce, F.; Horner, J.R. Analyses of soft tissue from Tyrannosaurus rex suggest the presence of protein. Science 2007, 316, 277–280. [Google Scholar] [CrossRef] [PubMed]
- Asara, J.M.; Schweitzer, M.H.; Freimark, L.M.; Phillips, M.; Cantley, L.C. Protein sequences from mastodon and Tyrannosaurus rex revealed by mass spectrometry. Science 2007, 316, 280–285. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Zheng, W.; Organ, C.L.; Avci, R.; Suo, Z.; Freimark, L.M.; Lebleu, V.S.; Duncan, M.B.; Vander Heiden, M.G.; Neveu, J.M.; et al. Biomolecular characterization and protein sequences of the Campanian hadrosaur B. canadensis. Science 2009, 324, 626–631. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Pasteris, J.D. Chemistry of bone mineral, based on the hypermineralized rostrum of the beaked whale Mesoplodon densirostris. Am. Mineral. 2014, 99, 645–653. [Google Scholar] [CrossRef][Green Version]
- Siddiqi, A.; Azhar, U. Carbonate substituted hydroxyapatite. In Handbook of Ionic Substituted Hydroxyapatites; Khan, A.S., Chaundry, A.A., Eds.; Woodhead Publishing: London, UK, 2020; pp. 149–173. [Google Scholar] [CrossRef]
- Bobroff, V.; Chen, H.H.; Javerzat, S.; Petibois, C. What can infrared spectroscopy do for characterizing organic remnant in fossils? TrAC-Trends Anal. Chem. 2016, 82, 443–456. [Google Scholar] [CrossRef]
- Kim, T.; Lee, Y.; Lee, Y.N. Fluorapatite diagenetic differences between Cretaceous skeletal fossils of Mongolia and Korea. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2018, 490, 579–589. [Google Scholar] [CrossRef]
- Mustoe, G.E. Mineralization of Fossil Wood with Macrocrystalline Quartz: A Microscopic Investigation. Minerals 2025, 15, 225. [Google Scholar] [CrossRef]
- Müller, K.J. Silicification of fossils. The Encyclopedia of Paleontology. Encyclopedia of Earth Sciences 1979, 7, 751–753. [Google Scholar] [CrossRef]
- Piga, G.; Baró, M.D.; Golvano Escobal, I.; Gonçalves, D.; Makhoul, C.; Amarante, A.; Malgosa, A.; Enzo, S.; Garroni, S. A structural approach in the study of bones: Fossil and burnt bones at nanosize scale. Appl. Phys. A Mater. Sci. Process. 2016, 122, 1031. [Google Scholar] [CrossRef]
- Spizzirri, P.G.; Cochrane, N.J.; Prawer, S.; Reynolds, E.C. A comparative study of carbonate determination in human teeth using Raman spectroscopy. Caries Res. 2012, 46, 353–360. [Google Scholar] [CrossRef]
- Mottram, H.R.; Evershed, R.P. Practical considerations in the gas chromatography/combustion/isotope ratio monitoring mass spectrometry of 13C-enriched compounds: Detection limits and carryover effects. Rapid Commun. Mass Spectrom. 2003, 17, 2669–2674. [Google Scholar] [CrossRef]
- Vonhof, H.B.; de Graaf, S.; Spero, H.J.; Schiebel, R.; Verdegaal, S.J.A.; Metcalfe, B.; Haug, G.H. High-precision stable isotope analysis of <5 μg CaCO3 samples by continuous-flow mass spectrometry. Rapid Commun. Mass Spectrom. 2020, 34, e8878. [Google Scholar] [CrossRef]
- Clementz, M.T. New insight from old bones: Stable isotope analysis of fossil mammals. J. Mammal. 2012, 93, 368–380. [Google Scholar] [CrossRef]
- Larsen, T.; Fernandes, R.; Wang, Y.V.; Roberts, P. Reconstructing Hominin Diets with Stable Isotope Analysis of Amino Acids: New Perspectives and Future Directions. BioScience 2022, 72, 618–637. [Google Scholar] [CrossRef]
- Naito, Y.I.; Meleg, I.N.; Robu, M.; Vlaicu, M.; Druckner, D.G.; Wißing, C.; Hofreiter, M.; Barlow, A.; Bocherens, H. Heavy reliance on plants for Romanian cave bears evidenced by amino acid nitrogen isotope analysis. Sci. Rep. 2020, 10, 6612. [Google Scholar] [CrossRef] [PubMed]
- Chadefaux, C.; Le Hô, A.-S.; Bellot-Gurlet, L.; Reiche, I. Curve-fitting micro-ATR-FTIR studies of the amide I and II bands of type I collagen in archaeological bone materials. e-PRESERVATION Sci. 2009, 6, 129–137. [Google Scholar]
- Long, D.A. The Raman Effect: A Unified Treatment of the Theory of Raman Scattering by Molecules; John Wiley & sons, LTD.: London, UK, 2002. [Google Scholar]
- Gauglitz, G.; Vo-Dinh, T. (Eds.) Handbook of Spectroscopy; WILEY-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2003. [Google Scholar]
- Edwards, H. Introduction to modern vibrational spectroscopy. Spectrochim. Acta Part A Mol. Spectrosc. 1994, 50, 2397–2398. [Google Scholar] [CrossRef]
- Derrick, M.R.; Stulik, D.; Landry, J. Infrared Spectroscopy in Conservation Science; Getty Conservation Institute: Los Angeles, CA, USA, 1999. [Google Scholar]
- Marshall, A.O.; Marshall, C.P. Vibrational spectroscopy of fossils. Palaeontology 2015, 58, 201–211. [Google Scholar] [CrossRef]
- Alleon, J.; Montagnac, G.; Reynard, B.; Brulé, T.; Thoury, M.; Gueriau, P. Pushing Raman spectroscopy over the edge: Purported signatures of organic molecules in fossil animals are instrumental artefacts. BioEssays 2021, 43, 2000295. [Google Scholar] [CrossRef] [PubMed]
- Herschel, W. Experiments on the Refrangibility of the Invisible Rays of the Sun By William Herschel, L.L.D.F.R. S. Philos. Trans. R. Soc. London 1800. [CrossRef]
- Coblentz, W.W. Supplementary Investigations of Infra-Red Spectra; The Carnegie Institution of Washington: Washington, DC, USA, 1908; Available online: https://archive.org/details/investigationsof03coblrich/investigationsof03coblrich/page/2/mode/2up (accessed on 26 June 2025).
- Griffiths, P.R. The early days of commercial FT-IR spectrometry: A personal perspective. Appl. Spectrosc. 2017, 71, 329–340. [Google Scholar] [CrossRef]
- Scientific and Analytical Instruments. Raman Laser Microprobe or MOLE (1977). Available online: https://www.horiba.com/int/scientific/resources/jobin-yvon-history-200-years-of-optical-innovation/instrumentation-1972-1989/raman-laser-microprobe-or-mole-1977/ (accessed on 26 June 2025).
- Edwards, H.G.M.; Vandenabeele, P.; Colomban, P. Historical Overview of Raman Spectroscopy. In Raman Spectroscopy in Cultural Heritage Preservation; Springer: Cham, Switzerland, 2023. [Google Scholar] [CrossRef]
- Tahoun, M.; Engeser, M.; Namasivayam, V.; Sander, P.M.; Müller, C.E. Chemistry and Analysis of Organic Compounds in Dinosaurs. Biology 2022, 11, 670. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.; Zhang, S.; Kim, N.-H.; Kweon, J.J.; Tanaka, K.; Kubota, K.; Lee, Y.-N.; Xie, J.; Paik, I.S.; Lee, S.K. Thermal maturity and colors of Cretaceous East Asian fossil eggs. Sediment. Geol. 2025, 481, 106855. [Google Scholar] [CrossRef]
- Rossi, V.; Unitt, R.; McNamara, M. A new non-destructive method to decipher the origin of organic matter in fossils using Raman spectroscopy. RSC Adv. 2024, 14, 26747–26759. [Google Scholar] [CrossRef]
- Del Sol Fernández, S.; García-Salcedo, R.; Guzmán Mendoza, J.; Sánchez-Guzmán, D.; Ramírez Rodríguez, G.; Gaona, E.; Rivera Montalvo, T. Thermoluminescent characteristics of LiF: MG, Cu, P and CaSO4: Dy for low dose measurement. Appl. Radiat. Isot. 2016, 111, 50–55. [Google Scholar] [CrossRef]
- Vichaidid, T.; Saeingjaew, P. Thermoluminescence and electron spin resonance dating of freshwater fossil shells from Pa Toh Roh Shelter archaeological site in southern Thailand. Heliyon 2022, 8, e10555. [Google Scholar] [CrossRef]
- Li, Y.; Feng, Y.; Du, J.; Wang, Y.; Shi, G.; Liang, Y. Spectral and chemical characterization of amber from Xixia, Henan Province, China via FTIR, three-dimensional fluorescence spectra and Py (HMDS)-GC-MS. Helyion 2024, 10, e35066. [Google Scholar] [CrossRef] [PubMed]
- Georgiou, R.; Gueriau, P.; Sahle, C.J.; Bernard, S.; Mirone, A.; Garrouste, R.; Bergmann, U.; Rueff, J.-P.; Bertrand, L. Carbon speciation in organic fossils using 2D to 3D x-ray Raman multispectral imaging. Sci. Adv. 2019, 5, eaaw5019. [Google Scholar] [CrossRef]
- de Winter, D.A.M.; Meirer, F.; Weckhuysen, B.M. FIB-SEM Tomography Probes the Mesoscale Pore Space of an Individual Catalytic Cracking Particle. ACS Catal. 2016, 6, 3158–3167. [Google Scholar] [CrossRef]
- Goldstein, J.I.; Newbury, D.E.; Michael, J.R.; Ritchie, N.W.M.; Scott, J.H.J.; Joy, D.C. Scanning Electron Microscopy and X-Ray Microanalysis; Springer Science+Business Media LLC: New York, NY, USA, 2017. [Google Scholar] [CrossRef]
- Natkaniec-Nowak, L.; Drzewicz, P.; Stach, P.; Mroczkowska-Szerszeń, M.; Żukowska, G. The overview of analytical methods for studying of fossil natural resins. Crit. Rev. Anal. Chem. 2023, 54, 2754–2776. [Google Scholar] [CrossRef] [PubMed]
- Wacey, D.; Battison, L.; Garwood, R.J.; Hickman-Lewis, K.; Brasier, M.D. Advanced analytical techniques for studying the morphology and chemistry of Proterozoic microfossils. Geol. Soc. Spec. Publ. 2016, 448, 81–104. [Google Scholar] [CrossRef]
- Prado, G.; Arthuzzi, J.C.L.; Osés, G.L.; Callefo, F.; Maldanis, L.; Sucerquia, P.; Becker-Kerber, B.; Romero, G.R.; Quiroz-Valle, F.R.; Galante, D. Synchrotron radiation in palaeontological investigations: Examples from Brazilian fossils and its potential to South American palaeontology. J. South Am. Earth Sci. 2021, 108, 102973. [Google Scholar] [CrossRef]
- Bergmann, U.; Manning, P.L.; Wogelius, R.A. Chemical mapping of paleontological and archeological artifacts with synchrotron X-Rays. Annu. Rev. Anal. Chem. 2012, 5, 361–389. [Google Scholar] [CrossRef]
- Zougrou, I.M.; Katsikini, M.; Pinakidou, F.; Paloura, E.C.; Papadopoulou, L.; Tsoukala, E. Study of fossil bones by synchrotron radiation micro-spectroscopic techniques and scanning electron microscopy. J. Synchrotron Radiat. 2014, 21, 149–160. [Google Scholar] [CrossRef]
- Imai, T.; Hattori, S.; Uesugi, K.; Hoshino, M. High-energy synchrotron-radiation-based X-ray micro-tomography enables non-destructive and micro-scale palaeohistological assessment of macro-scale fossil dinosaur bones. J. Synchrotron Radiat. 2023, 30, 627–633. [Google Scholar] [CrossRef]
- Gueriau, P.; Mocuta, C.; Dutheil, D.B.; Cohen, S.X.; Thiaudière, D.; The OT1 Consortium; Charbonnier, S.; Clément, G.; Bertrand, L. Trace elemental imaging of rare earth elements discriminates tissues at microscale in flat fossils. PLoS ONE 2014, 9, e86946. [Google Scholar] [CrossRef] [PubMed]
- Gueriau, P.; Jauvion, C.; Mocuta, C. Show me your yttrium, and I will tell you who you are: Implications for fossil imaging. Palaeontology 2018, 61, 981–990. [Google Scholar] [CrossRef]
- Lee, Y.C.; Chiang, C.-C.; Huang, P.-Y.; Chung, C.-Y.; Huang, T.D.; Wang, C.-C.; Chen, C.-I.; Chang, R.-S.; Liao, C.-H.; Reisz, R.R. Evidence of preserved collagen in an Early Jurassic sauropodomorph dinosaur revealed by synchrotron FTIR microspectroscopy. Nat. Commun. 2017, 8, 14220. [Google Scholar] [CrossRef]
- Wogelius, R.A.; Manning, P.L.; Barden, H.E.; Edwards, N.P.; Webb, S.M.; Sellers, W.I.; Taylor, K.G.; Larson, P.L.; Dodson, P.; You, K.; et al. Trace metals as biomarkers for eumelanin pigment in the fossil record. Science 2011, 333, 1622–1626. [Google Scholar] [CrossRef] [PubMed]
- Perrichot, V.; Marion, L.; Néraudeau, D.; Vullo, R.; Tafforeau, P. The early evolution of feathers: Fossil evidence from Cretaceous amber of France. Proc. R. Soc. B Biol. Sci. 2008, 275, 1197–1202. [Google Scholar] [CrossRef]
- Bergmann, U.; Morton, R.W.; Manning, P.L.; Sellers, W.I.; Farrar, S.; Huntley, K.G.; Wogelius, R.A.; Larson, P. Archaeopteryx feathers and bone chemistry fully revealed via synchrotron imaging. Proc. Natl. Acad. Sci. USA 2010, 107, 9060–9065. [Google Scholar] [CrossRef] [PubMed]
- Edwards, N.P.; Barden, H.E.; van Dongen, B.E.; Manning, P.L.; Larson, P.L.; Bergmann, U.; Sellers, W.I.; Wogelius, R.A. Infrared mapping resolves soft tissue preservation in 50 million year-old reptile skin. Proc. R. Soc. B Biol. Sci. 2011, 278, 3209–3218. [Google Scholar] [CrossRef]
- Boatman, E.M.; Goodwin, M.B.; Holman, H.-Y.N.; Fakra, S.; Zheng, W.; Gronsky, R.; Schweitzer, M.H. Mechanisms of soft tissue and protein preservation in Tyrannosaurus rex. Sci. Rep. 2019, 9, 15678. [Google Scholar] [CrossRef]
- Algamili, A.S.; Khir, M.H.M.; Dennis, J.O.; Ahmed, A.Y.; Alabsi, S.S.; Hashwan, S.S.B.; Junaid, M.M. A Review of Actuation and Sensing Mechanisms in MEMS-Based Sensor Devices. Nanoscale Res. Lett. 2021, 16, 16. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, X.; Shi, Q.; He, T.; Sun, Z.; Guo, X.; Liu, W.; Sulaiman, O.B.; Dong, B.; Lee, C. Development trends and perspectives of future sensors and MEMS/NEMS. Micromachines 2020, 11, 7. [Google Scholar] [CrossRef]
- Rodriguez-Saona, L.; Aykas, D.P.; Borba, K.R.; Urtubia, A. Miniaturization of optical sensors and their potential for high-throughput screening of foods. Curr. Opin. Food Sci. 2020, 31, 136–150. [Google Scholar] [CrossRef]
- Dahlin, A.B. Size matters: Problems and advantages associated with highly miniaturized sensors. Sensors 2012, 12, 3018–3036. [Google Scholar] [CrossRef]
- Santiago, J.B.; Sevilla, F.B. Headspace measurement of dimethyl sulfide in beer through paper-based smartphone-colorimetry. Food Chem. 2025, 485, 144391. [Google Scholar] [CrossRef] [PubMed]
- Fan, Y.; Li, J.; Guo, Y.; Xie, L.; Zhang, G. Digital image colorimetry on smartphone for chemical analysis: A review. Meas. J. Int. Meas. Confed. 2021, 171, 108829. [Google Scholar] [CrossRef]
- Hoffmeister, M.; Seum, T.; Ludwig, L.; Brenner, H. Performance of a smartphone-based stool test for use in colorectal cancer screening: Population-based study. Clin. Gastroenterol. Hepatol. 2025, in press. [Google Scholar] [CrossRef] [PubMed]
- Song, B.; Liang, R. Integrating artificial intelligence with smartphone-based imaging for cancer detection in vivo. Biosens. Bioelectron. 2025, 271, 116982. [Google Scholar] [CrossRef]
- Zadorozhnyy, O.; Kustryn, T.; Nasinnyk, I.; Nevska, A.; Guzun, O.; Korol, A.; Pasyechnikova, N. Application of smartphone-based infrared thermography devices for ocular surface thermal imaging. Med. Eng. Phys. 2024, 130, 104212. [Google Scholar] [CrossRef]
- Roy, B.; Maikap, A.; Das, S.; Chakraborty, S. Simultaneous detection of trace protein biomarkers from a single drop of blood using AI-enhanced smartphone-based digital microscopy. Biosens. Bioelectron. 2025, 276, 117259. [Google Scholar] [CrossRef]
- Wang, L.; Zhang, W.; Wu, L.; Song, X.; Li, F.; Fu, Y.; Bai, M. Fluorescence thermography based on smartphone and its application in defect inspection. Sens. Actuators A Phys. 2024, 368, 115171. [Google Scholar] [CrossRef]
- Tao, Z.; Zhang, Q.; Cao, Y.; Duan, X.; Wu, Y.; Fan, L.; Cao, C.; Liu, W. A Highly Portable Smartphone-Based Capillary Electrophoresis with Capacitively Coupled Contactless Conductivity Detection. Sensors 2025, 25, 2303. [Google Scholar] [CrossRef]
- Stigall, A.L.; Bauer, J.E.; Brame, H.M.R. The digital Atlas of Ordovician life: Digitizing and mobilizing data for paleontologists and the public. Est. J. Earth Sci. 2014, 63, 312–316. [Google Scholar] [CrossRef]
- Hendricks, J.R.; Stigall, A.L.; Lieberman, B.S. The digital atlas of ancient life: Delivering information on paleontology and biogeography via the web. Palaeontol. Electron. 2015, 18.2.3E. [Google Scholar] [CrossRef]
- Turner, M.G.; Wei, D.; Prentice, I.C.; Harrison, S.P. The impact of methodological decisions on climate reconstructions using WA-PLS. Quat. Res. 2021, 99, 341–356. [Google Scholar] [CrossRef]
- Juggins, S.; Birks, H.J.B. Quantitative Environmental Reconstructions from Biological Data. In Tracking Environmental Change Using Lake Sediments; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2012. [Google Scholar] [CrossRef]
- Marramà, G.; Kriwet, J. Principal component and discriminant analyses as powerful tools to support taxonomic identification and their use for functional and phylogenetic signal detection of isolated fossil shark teeth. PLoS ONE 2017, 12, e0188806. [Google Scholar] [CrossRef]
- Joshi, P.B. Navigating with chemometrics and machine learning in chemistry. Artif. Intell. Rev. 2023, 56, 9089–9114. [Google Scholar] [CrossRef]
- Lecun, Y.; Bengio, Y.; Hinton, G. Deep learning. Nature 2015, 521, 436–444. [Google Scholar] [CrossRef]
- Rial, R.C. AI in analytical chemistry: Advancements, challenges, and future directions. Talanta 2024, 274, 125949. [Google Scholar] [CrossRef] [PubMed]
- Houhou, R.; Bocklitz, T. Trends in artificial intelligence, machine learning, and chemometrics applied to chemical data. Anal. Sci. Adv. 2021, 2, 128–141. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Beresford, R. Basic concepts of artificial neural network (ANN) modeling and its application in pharmaceutical research. J. Pharm. Biomed. Anal. 2000, 22, 717–727. [Google Scholar] [CrossRef]
- Shao, F.; Shen, Z. How can artificial neural networks approximate the brain? Front. Psychol. 2023, 13, 970214. [Google Scholar] [CrossRef] [PubMed]
- Xue, X.; Sun, H.; Yang, M.; Liu, X.; Hu, H.-Y.; Deng, Y.; Wang, X. Advances in the Application of Artificial Intelligence-Based Spectral Data Interpretation: A Perspective. Anal. Chem. 2023, 95, 13733–13745. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; An, H.; Cai, W.; Shao, X. Deep learning in spectral analysis: Modeling and imaging. TrAC-Trends Anal. Chem. 2024, 172, 117612. [Google Scholar] [CrossRef]
- Open AI; Achiam, J.; Adler, S.; Agarwal, S.; Ahmad, L.; Akkaya, I.; Leoni Aleman, F.; Almeida, D.; Altenschmidt, J.; Altman, S.; et al. OpenAI (2023). arXiv 2024, arXiv:2412.06370. [Google Scholar] [CrossRef]
- GeminiTeam; Google. Gemini: A Family of Highly Capable Multimodal Models. arXiv 2023, arXiv:2312.11805. [Google Scholar] [CrossRef]
- OpenAI. DALL-E-3. Available online: https://openai.com/index/dall-e-3/ (accessed on 28 May 2025).
- Esser, P.; Kulal, S.; Blattmann, A.; Entezari, R.; Müller, J.; Saini, H.; Levi, Y.; Lorenz, D.; Sauer, A.; Boesel, F.; et al. Scaling Rectified Flow Transformers for High-Resolution Image Synthesis. arXiv 2024, arXiv:2403.03206. [Google Scholar] [CrossRef]
- Nayar, V. The Ethics of AI Generated Music. A Case Study of Suno AI. GRACE Glob. Rev. AI Community Ethics 2025, 3. [Google Scholar]
- Germanidis, A. Gen-2: Generate Novel Videos with Text, Images or Video Clips. Available online: https://runwayml.com/research/gen-2 (accessed on 28 May 2025).
- Baucon, A.; de Carvalho, C.N. Can AI Get a Degree in Geoscience? Performance Analysis of a GPT-Based Artificial Intelligence System Trained for Earth Science (GeologyOracle). Geoheritage 2024, 16, 121. [Google Scholar] [CrossRef]
- Schopf, J.W.; Kudryavtsev, A.B.; Agresti, D.G.; Czaja, A.D.; Wdowiak, T.J. Raman imagery: A new approach to assess the geochemical maturity and biogenicity of permineralized Precambrian fossils. Astrobiology 2005, 5, 333–371. [Google Scholar] [CrossRef]
- Beierlein, L.; Nehrke, G.; Brey, T. Confocal Raman microscopy in sclerochronology: A powerful tool to visualize environmental information in recent and fossil biogenic archives. Geochem. Geophys. Geosystems 2015, 16, 325–335. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, W.; Wu, S.; Yin, Z. Structural and Chemical Characterization of the Ediacaran Embryo-Like Fossils via the Combination of 3D-XRM and FIB-SEM Approaches. J. Earth Sci. 2024, 35, 1204–1214. [Google Scholar] [CrossRef]
- Pan, Y.; Hu, L.; Zhao, T. Applications of chemical imaging techniques in paleontology. Natl. Sci. Rev. 2019, 6, 1040–1053. [Google Scholar] [CrossRef] [PubMed]
- McMahon, S.; Bosak, T.; Grotzinger, J.P.; Milliken, R.E.; Summons, R.E.; Daye, M.; Newman, S.A.; Fraeman, A.; Williford, K.H.; Briggs, D.E.G. A Field Guide to Finding Fossils on Mars. J. Geophys. Res. Planets 2018, 123, 1012–1040. [Google Scholar] [CrossRef]
- McKay, D.S.; Gibson, E.K., Jr.; Thomas-Keprta, K.L.; Vali, H.; Romanek, C.S.; Clematt, S.J.; Chillier, X.D.F.; Maechling, C.R.; Zare, R.N. Search for past life on Mars: Possible relic biogenic activity in Martian meteorite ALH84001. Science 1996, 273, 924–930. [Google Scholar] [CrossRef]
- Steele, A.; Goddard, D.; Beech, I.B.; Tapper, R.C.; Stapleton, D.; Smith, J.R. Atomic force microscopy imaging of fragments from the martian meteorite ALH84001. J. Microsc. 1998, 189, 2–6. [Google Scholar] [CrossRef]
- Flynn, G.J.; McKay, D.S. An assessment of the meteoritic contribution to the Martian soil. J. Geophys. Res. 1990, 95, 14497–14509. [Google Scholar] [CrossRef]
- NASA. NASA’s Perseverance Rover Scientists Find Intriguing Mars Rock. Available online: https://www.nasa.gov/solar-system/nasas-perseverance-rover-scientists-find-intriguing-mars-rock/ (accessed on 3 April 2025).
- Freissinet, C.; Glavin, D.P.; Mahaffy, P.R.; Miller, K.E.; Eigenbrode, J.L.; Summons, R.E.; Brunner, A.E.; Buch, A.; Szopa, C.; Archer, P.D., Jr.; et al. Organic molecules in the Sheepbed Mudstone, Gale Crater, Mars. J. Geophys. Res. Planets 2015, 120, 495–514. [Google Scholar] [CrossRef]
- Li, J.; Pei, R.; Teng, F.; Qiu, H.; Tagle, R.; Yan, Q.; Wang, Q.; Chu, X.; Xu, X. Micro-XRF Study of the Troodontid Dinosaur Jianianhualong tengi Reveals New Biological and Taphonomical Signals. At. Spectrosc. 2021, 42, 1–11. [Google Scholar] [CrossRef]
- Harbowo, D.G.; Aswan; Zaim, Y.; Chaerun, S.K.; Chaerun, R.I.; Astuti, W.; Sato, T. Microanalytical approaches on the silicification process of wood fossil from Jasinga, West Java, Indonesia. Sci. Rep. 2024, 14, 19101. [Google Scholar] [CrossRef]
- Hiller, J.C.; Wess, T.J. The use of small-angle X-ray scattering to study archaeological and experimentally altered bone. J. Archaeol. Sci. 2006, 33, 560–572. [Google Scholar] [CrossRef]
- McNamara, M.E.; Saranathan, V.; Locatelli, E.R.; Noh, H.; Briggs, D.E.G.; Orr, P.J.; Cao, H. Cryptic iridescence in a fossil weevil generated by single diamond photonic crystals. J. R. Soc. Interface 2014, 11, 20140736. [Google Scholar] [CrossRef]
- Ober, M.F.; Baptist, A.; Wassermann, L.; Heuer-Jungemann, A.; Nickel, B. In situ small-angle X-ray scattering reveals strong condensation of DNA origami during silicification. Nat. Commun. 2022, 13, 5668. [Google Scholar] [CrossRef]
- Riquelme, F.; Sil, J.L.R.; Ortega, J.A. Palaeometry: Non-destructive analysis of fossil materials. Boletín la Soc. Geológica Mex. 2009, 61, 177–183. [Google Scholar] [CrossRef]
- Bunkin, A.F.; Pershin, S.M.; Artemova, D.G.; Gudkov, S.V.; Gomankov, A.V.; Sdvizhenskii, P.A.; Grishin, M.Y.; Lednev, V.N. Fossil Plant Remains Diagnostics by Laser-Induced Fluorescence and Raman Spectroscopies. Photonics 2023, 10, 15. [Google Scholar] [CrossRef]
- Boderau, M.; Jouault, C.; Aracheloff, C.; Ngô-Muller, V.; Engel, M.S.; Berthier, S.; Schöllhorn, B.; Huang, D.; Nel, A.; Garrouste, R. Morphological and palaeoecological aspects of fossil insects unveiled by UV-A light. MethodsX 2024, 13, 102794. [Google Scholar] [CrossRef]
- Pittman, M.; Kaye, T.G.; Graham, E.; Thorold, D. Laser-stimulated fluorescence in archaeology: Non-destructive fluorescence imaging for museum and field settings. J. Archaeol. Sci. Rep. 2022, 44, 103475. [Google Scholar] [CrossRef]
- Barlow, L.A.; Pittman, M.; Butcher, A.; Martill, D.M.; Kaye, T.G. Laser-stimulated fluorescence reveals unseen details in fossils from the Upper Jurassic Solnhofen Limestones. R. Soc. Open Sci. 2021, 8, 211601. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Pittman, M.; Zheng, X.; Kaye, T.G.; Falk, A.R.; Hartman, S.A.; Xu, X. Basal paravian functional anatomy illuminated by high-detail body outline. Nat. Commun. 2017, 8, 14576. [Google Scholar] [CrossRef]
- Kaye, T.G.; Pittman, M.; Mayr, G.; Schwarz, D.; Xu, X. Detection of lost calamus challenges identity of isolated Archaeopteryx feather. Sci. Rep. 2019, 9, 1182. [Google Scholar] [CrossRef]
- Kaye, T.G.; Pittman, M.; Marugán-Lobón, J.; Martín-Abad, H.; Sanz, J.L.; Buscalioni, A.D. Fully fledged enantiornithine hatchling revealed by Laser-Stimulated Fluorescence supports precocial nesting behavior. Sci. Rep. 2019, 9, 5006. [Google Scholar] [CrossRef]
- Delpueyo, X.; Vilaseca, M.; Furió, M.; Burgos-Fernández, F.J.; Pujol, J. Multispectral and colour imaging systems for the detection of small vertebrate fossils: A preliminary study. Palaeontol. Electron. 2016, 19. [Google Scholar] [CrossRef]
- Murphy, R.J.; Webster, J.M.; Nothdurft, L.; Dechnik, B.; McGregor, H.V.; Patterson, M.A.; Sanborn, K.L.; Webb, G.E.; Kearney, L.I.; Rintoul, L. High-resolution hyperspectral imaging of diagenesis and clays in fossil coral reef material: A nondestructive tool for improving environmental and climate reconstructions. Geochem. Geophys. Geosystems 2017, 18, 3209–3230. [Google Scholar] [CrossRef]
- Murphy, R.J.; Van Kranendonk, M.J.; Kelloway, S.J.; Wainwright, I.E. Complex patterns in fossilized stromatolites revealed by hyperspectral imaging (400-2496 nm). Geobiology 2016, 14, 419–439. [Google Scholar] [CrossRef] [PubMed]
- Damien, V.; Damien, E.; Antonio, F.P.J.; Vincent, B.; Bernard, B.; Pierre, D. Sorting of crop residues and fossil bones from soil by NIR Hyperspectral Imaging. In Proceedings of the 2014 6th Workshop on Hyperspectral Image and Signal Processing: Evolution in Remote Sensing (WHISPERS), Lausanne, Switzerland, 24–27 June 2014. [Google Scholar] [CrossRef]
- Marshall, A.O.; Wehrbein, R.L.; Lieberman, B.S.; Marshall, C.P. Raman spectroscopic investigations of burgess shaletype preservation: A new way forward. Palaios 2012, 27, 288–292. [Google Scholar] [CrossRef]
- Schweitzer, M.H.; Marshall, M.; Carron, K.; Bohle, D.S.; Busse, S.C.; Arnold, E.V.; Barnard, D.; Horner, J.R.; Starkey, J.R. Heme compounds in dinosaur trabecular bone. Proc. Natl. Acad. Sci. USA 1997, 94, 6291–6296. [Google Scholar] [CrossRef] [PubMed]
- Wiemann, J.; Briggs, D.E.G. Raman spectroscopy is a powerful tool in molecular paleobiology: An analytical response to Alleon et al. (https://doi.org/10.1002/bies.202000295). BioEssays 2022, 44, e2100070. [Google Scholar] [CrossRef] [PubMed]
- Bernard, S.; Benzerara, K.; Beyssac, O.; Menguy, N.; Guyot, F.; Brown, G.E., Jr.; Goffé, B. Exceptional preservation of fossil plant spores in high-pressure metamorphic rocks. Earth Planet. Sci. Lett. 2007, 262, 257–272. [Google Scholar] [CrossRef]
- da Silva, J.H.; Saraiva, G.D.; Memória Campelo, S.C.; Cisneros Martínez, J.C.; Viana, B.C.; Bezerra, F.I.; Abagaro, B.T.O.; Cavalcante Freire, P.T. Raman and infrared spectroscopy investigation of the root fossil (rhizoliths) from the Carboniferous period, Piauí Formation, Parnaíba Sedimentary Basin, Northeast Brazil. Vib. Spectrosc. 2019, 100, 117–122. [Google Scholar] [CrossRef]
- de Sousa, D.V.; Maia, P.V.S.; Eltink, E.; de Moura Guimaraes, L. Biomolecules in Pleistocene fossils from tropical cave indicate fossil biofilm. Sci. Rep. 2024, 14, 21071. [Google Scholar] [CrossRef] [PubMed]
- Cosmidis, J.; Benzerara, K.; Menguy, N.; Arning, E. Microscopy evidence of bacterial microfossils in phosphorite crusts of the Peruvian shelf: Implications for phosphogenesis mechanisms. Chem. Geol. 2013, 359, 10–22. [Google Scholar] [CrossRef]
- Saminpanya, S.; Sutherland, F.L. Silica phase-transformations during diagenesis within petrified woods found in fluvial deposits from Thailand-Myanmar. Sediment. Geol. 2013, 290, 15–26. [Google Scholar] [CrossRef]
- Roda, M.S.; Kim, D.; Brasier, A.T.; Griesshaber, E.; Lee, J.-H. Exploring electron backscatter diffraction analysis as a tool for understanding stromatolite: Quantitative description of Cretaceous lacustrine stromatolite reveals formative processes and high-resolution climatic cycles. Sedimentology 2024, 71, 2448–2469. [Google Scholar] [CrossRef]
- Procter, F.A.; Piazolo, S.; John, E.H.; Walshaw, R.; Pearson, P.N.; Lear, C.H.; Aze, T. Electron backscatter diffraction analysis unveils foraminiferal calcite microstructure and processes of diagenetic alteration. Biogeosciences 2024, 21, 1213–1233. [Google Scholar] [CrossRef]
- Choi, S.; Lee, S.K.; Kim, N.H.; Kim, S.; Lee, Y.N. Raman Spectroscopy Detects Amorphous Carbon in an Enigmatic Egg From the Upper Cretaceous Wido Volcanics of South Korea. Front. Earth Sci. 2020, 7, 349. [Google Scholar] [CrossRef]
- Kocheva, L.; Karmanov, A.; Telnova, O.; Marshall, J.E.A.; Lutoev, V.; Pokryshkin, S. Structural and chemical features of seed fossils from Paleozoic and Mesozoic sedimentary strata. Org. Geochem. 2022, 164, 104370. [Google Scholar] [CrossRef]
- Gosav, S.; Ene, A.; Aflori, M. Characterization and discrimination of plant fossils by ATR-FTIR, XRD and chemometric methods. Rom. J. Phys. 2019, 64, 806. [Google Scholar]
- Pagacz, J.; Naglik, B.; Stach, P.; Drzewicz, P.; Natkaniec-Nowak, L. Maturation process of natural resins recorded in their thermal properties. J. Mater. Sci. 2020, 55, 4504–4523. [Google Scholar] [CrossRef]
- Duval, M. Dating fossil teeth by electron paramagnetic resonance: How is that possible? Spectrosc. Eur. 2014, 26, 6–13. [Google Scholar]
- Cid, A.S.; Anjos, R.M.; Zamboni, C.B.; Cardoso, R.; Muniz, M.; Corona, A.; Valladares, D.L.; Kovacs, L.; Macario, K.; Perea, D.; et al. Na, K, Ca, Mg, and U-series in fossil bone and the proposal of a radial diffusion-adsorption model of uranium uptake. J. Environ. Radioact. 2014, 136, 131–139. [Google Scholar] [CrossRef] [PubMed]
- Brown, P.M.; Nash, S.E.; Kline, D. Identification and dendrochronology of wood found at the Ziegler Reservoir fossil site, Colorado, USA. Quat. Res. 2014, 82, 575–579. [Google Scholar] [CrossRef]
- Korbovyak, E.V.; Tishin, P.A.; Afonin, I.V.; Bether, O.V.; Konovalova, V.A. Mineralogical and geochemical characteristics of terrigenous sediments Vanavar formation (Siberian craton). In International Multidisciplinary Scientific GeoConference Surveying Geology and Mining Ecology Management; International Multidisciplinary Scientific GeoConferences SGEM: Sofia, Bulgaria, 2017. [Google Scholar] [CrossRef]
- Zheng, W.; Schweitzer, M.H. Chemical analyses of fossil bone. Methods Mol. Biol. 2012, 915, 153–172. [Google Scholar] [CrossRef]
- Feranec, R.S. Stable carbon isotope values reveal evidence of resource partitioning among ungulates from modern C3-dominated ecosystems in North America. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2007, 252, 575–585. [Google Scholar] [CrossRef]
- Hardy, F.C.; Rowland, S.M. Stable isotopic analysis of fossil Bison tooth enamel indicates flexible dietary ecology across Pleistocene North America. Quat. Sci. Rev. 2024, 334, 108741. [Google Scholar] [CrossRef]
- Xu, Z.; Pei, S.; Hu, Y.; de la Torre, I.; Ma, D. Stable Isotope Analysis of Mammalian Enamel From the Early Pleistocene Site of Madigou, Nihewan Basin: Implications for Reconstructing Hominin Paleoenvironmental Adaptations in North China. Front. Earth Sci. 2021, 9. [Google Scholar] [CrossRef]
- Ota, K.; Yokoyama, Y.; Miyairi, Y.; Obrochta, S.P.; Yamamoto, S.; Hubert-Ferrari, A.; Heyvaert, V.M.A.; De Batist, M.; Fujiwara, O. the QuakeRecNankai Team. Development of an automated extraction and radiocarbon dating method for fossil pollen deposited in lake Motosu, Japan. Quat. Sci. Adv. 2024, 15, 100207. [Google Scholar] [CrossRef]
- Oswald, J.A.; Allen, J.M.; Witt, K.E.; Folk, R.A.; Albury, N.A.; Steadman, D.W.; Guralnick, R.P. Ancient DNA from a 2,500-year-old Caribbean fossil places an extinct bird (Caracara creightoni) in a phylogenetic context. Mol. Phylogenet. Evol. 2019, 140, 106576. [Google Scholar] [CrossRef] [PubMed]
- Vinther, J. A guide to the field of palaeo colour. BioEssays 2015, 37, 643–656. [Google Scholar] [CrossRef]
- Nang, L.N.; Hieu, P.T.; Phat, L.V.; Tien, P.M.; Man, H.N.T.; Hang, H.T. Characteristics of Newly Discovered Amber from Phu Quoc, Vietnam. Gems Gemol. 2022, 58, 184–194. [Google Scholar] [CrossRef]
- Akse, S.P.; Das, G.; Agustí, S.; Pichevin, L.; Polerecky, L.; Middelburg, J.J. Imaging of organic signals in individual fossil diatom frustules with nanoSIMS and Raman spectroscopy. Mar. Chem. 2021, 228, 103906. [Google Scholar] [CrossRef]
- Kilburn, M.R.; Wacey, D. NanoSIMS analysis of Archean fossils and biomarkers. Appl. Surf. Sci. 2008, 255, 1465–1467. [Google Scholar] [CrossRef]
- Cisneros-Lazaro, D.; Adams, A.; Guo, J.; Bernard, S.; Baumgartner, L.P.; Daval, D.; Baronnet, A.; Grauby, O.; Vennemann, T.; Stolarski, J.; et al. Fast and pervasive diagenetic isotope exchange in foraminifera tests is species-dependent. Nat. Commun. 2022, 13, 113. [Google Scholar] [CrossRef] [PubMed]
- Heingård, M.; Sjövall, P.; Schultz, B.P.; Sylvestersen, R.L.; Lindgren, J. Preservation and Taphonomy of Fossil Insects from the Earliest Eocene of Denmark. Biology 2022, 11, 395. [Google Scholar] [CrossRef]
- Cleland, T.P.; Schroeter, E.R. A Comparison of Common Mass Spectrometry Approaches for Paleoproteomics. J. Proteome Res. 2018, 17, 936–945. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ghervase, L.; Dinu, M. What’s New with the Old Ones: Updates on Analytical Methods for Fossil Research. Chemosensors 2025, 13, 328. https://doi.org/10.3390/chemosensors13090328
Ghervase L, Dinu M. What’s New with the Old Ones: Updates on Analytical Methods for Fossil Research. Chemosensors. 2025; 13(9):328. https://doi.org/10.3390/chemosensors13090328
Chicago/Turabian StyleGhervase, Luminița, and Monica Dinu. 2025. "What’s New with the Old Ones: Updates on Analytical Methods for Fossil Research" Chemosensors 13, no. 9: 328. https://doi.org/10.3390/chemosensors13090328
APA StyleGhervase, L., & Dinu, M. (2025). What’s New with the Old Ones: Updates on Analytical Methods for Fossil Research. Chemosensors, 13(9), 328. https://doi.org/10.3390/chemosensors13090328






