Synthesis, Characterization, and Evaluation of Photocatalytic and Gas Sensing Properties of ZnSb2O6 Pellets
Abstract
1. Introduction
2. Experimental
2.1. Synthesis of ZnSb2O6 Particles
2.2. Physical Characterization
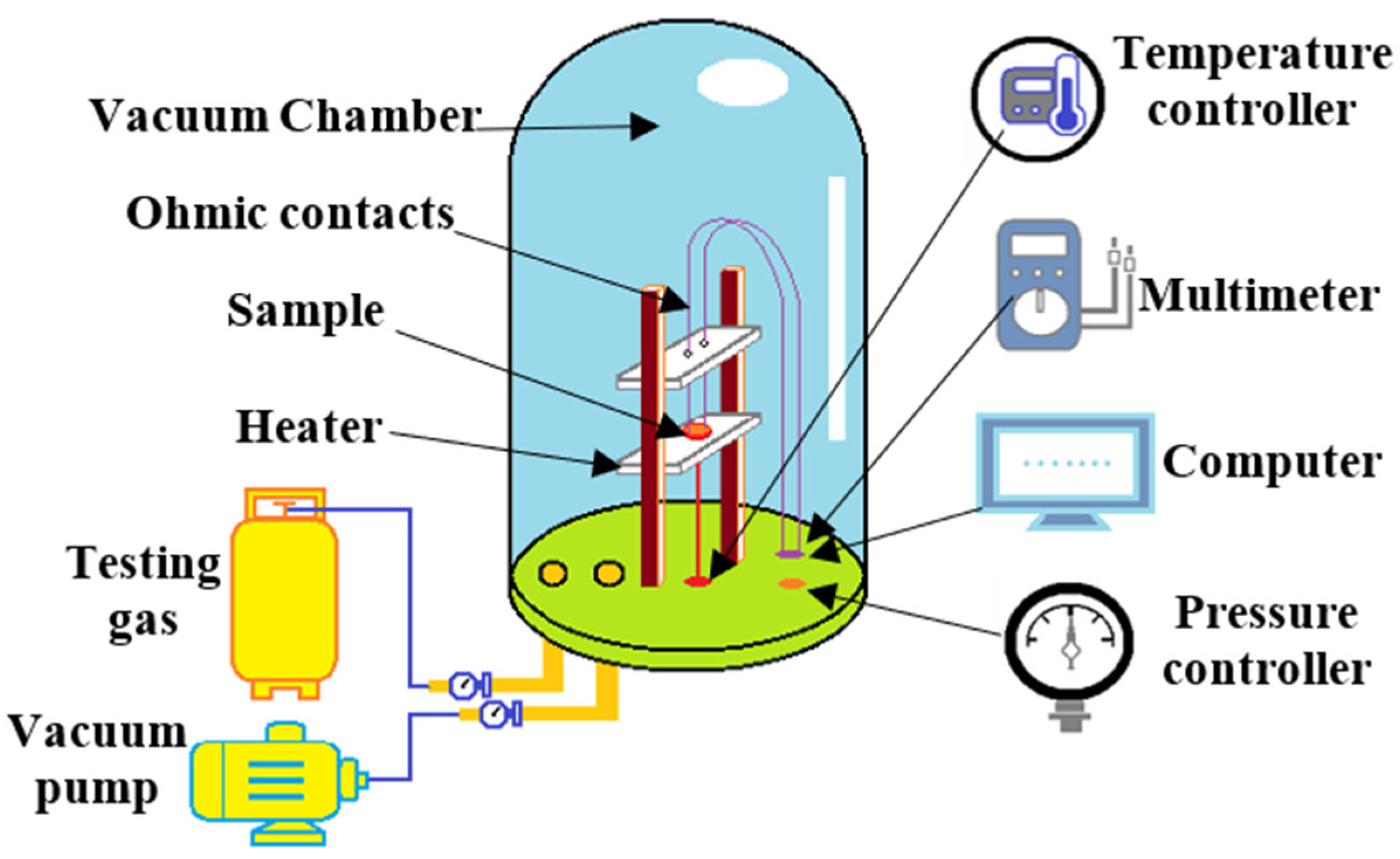
2.3. Gas Sensing Measurements
2.4. Photocatalytic Activity Test
3. Results
3.1. XRD Analysis
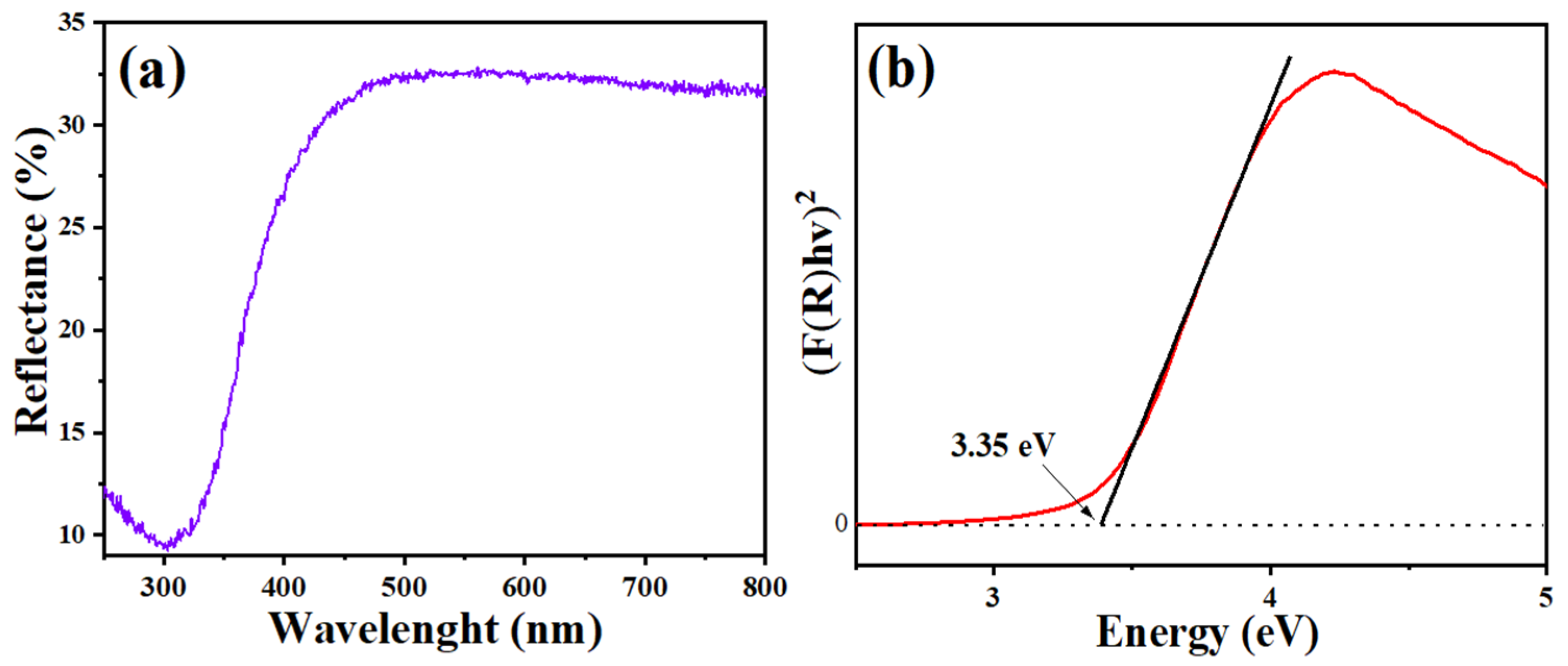
3.2. UV Analysis
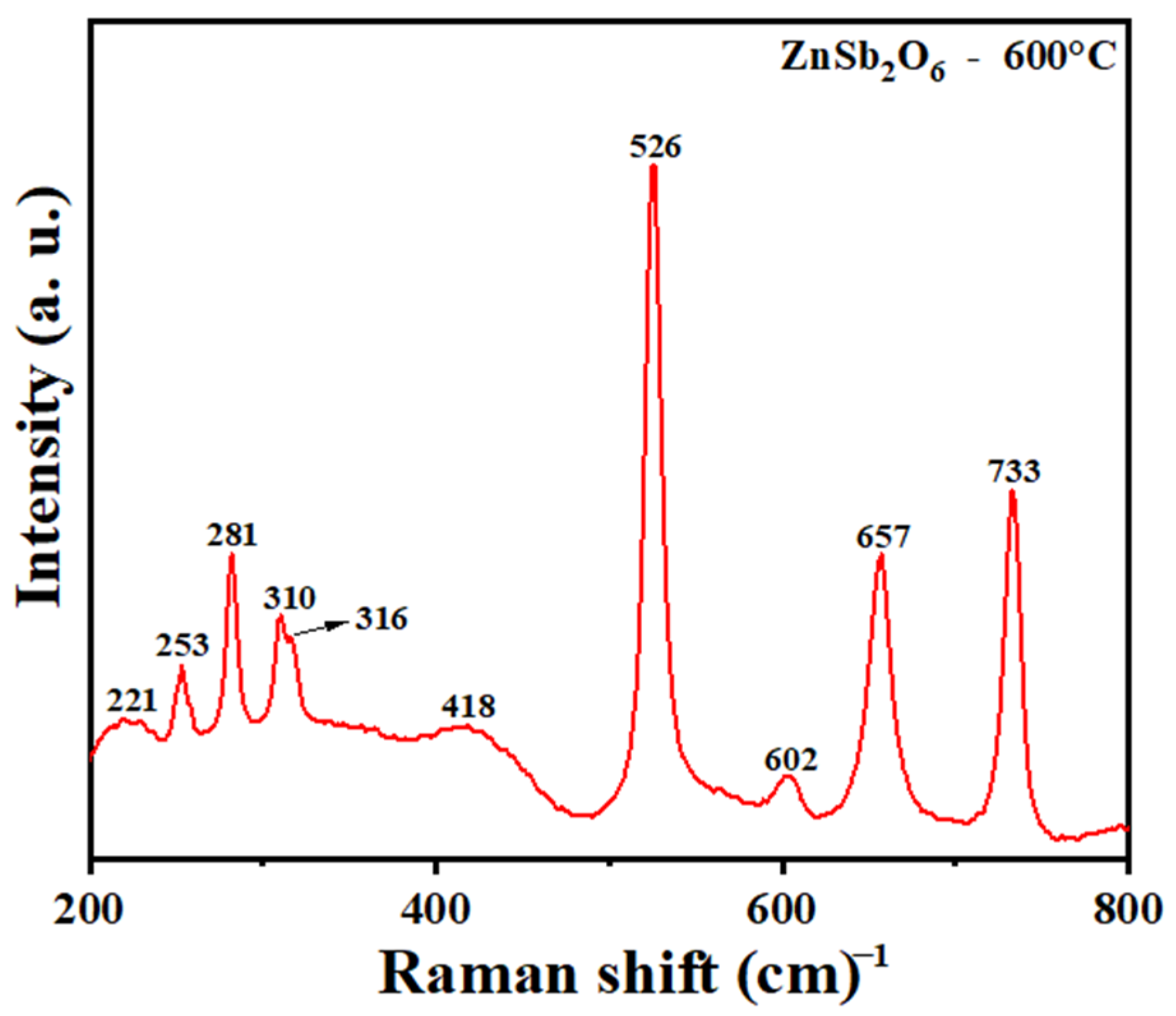
3.3. Raman Analysis
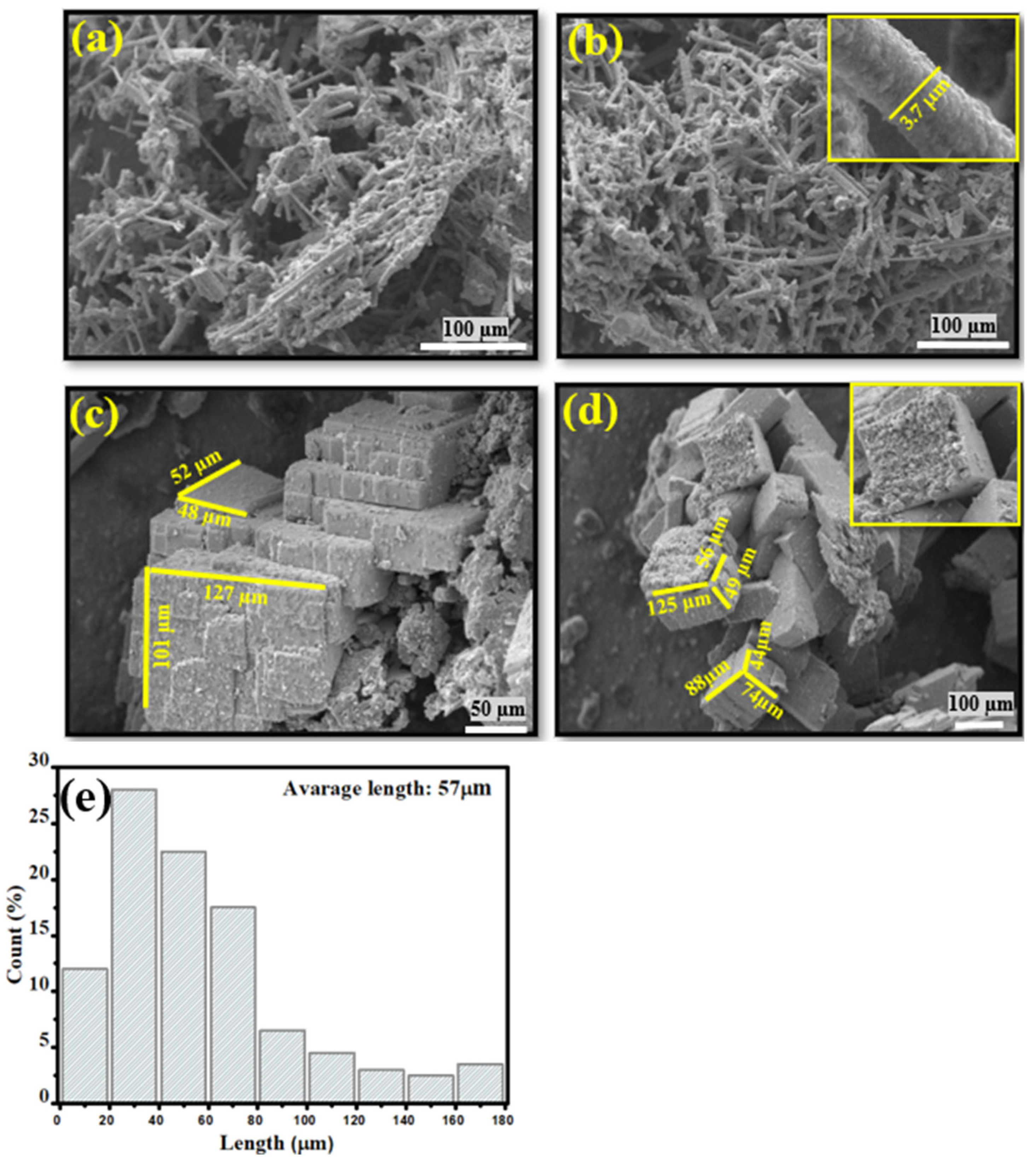
3.4. SEM Analysis
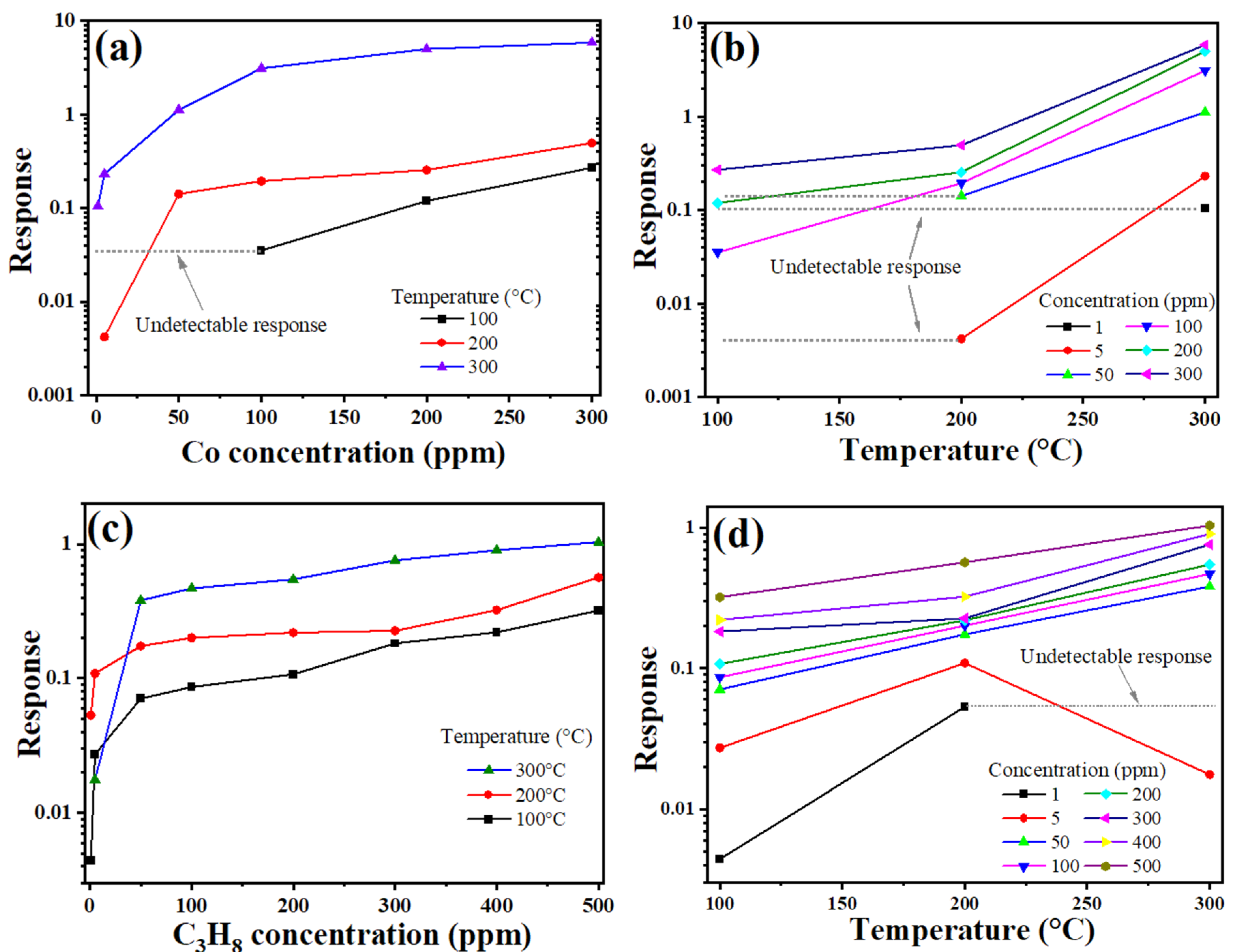
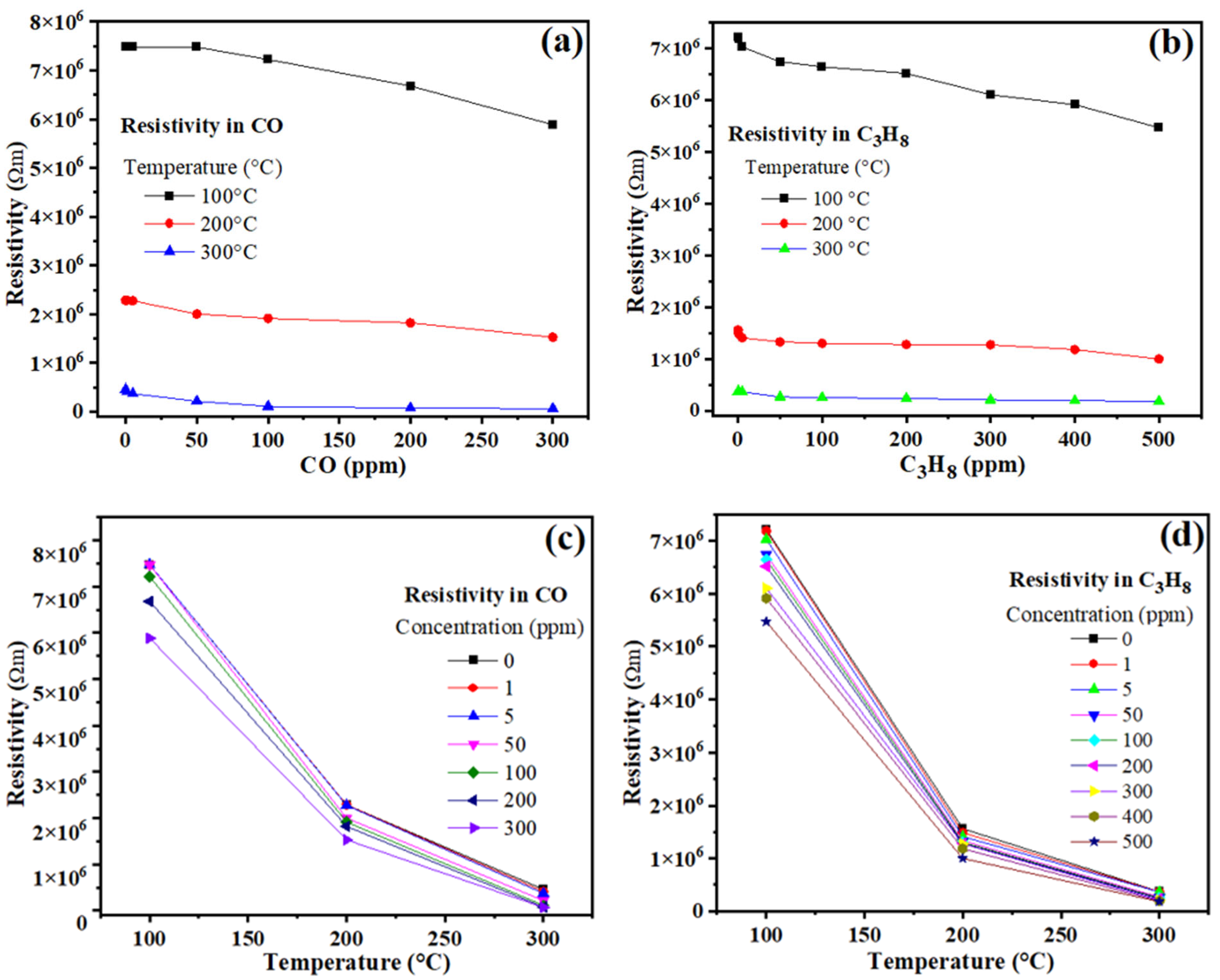
3.5. Gas Sensing Tests
3.6. Reaction Mechanism
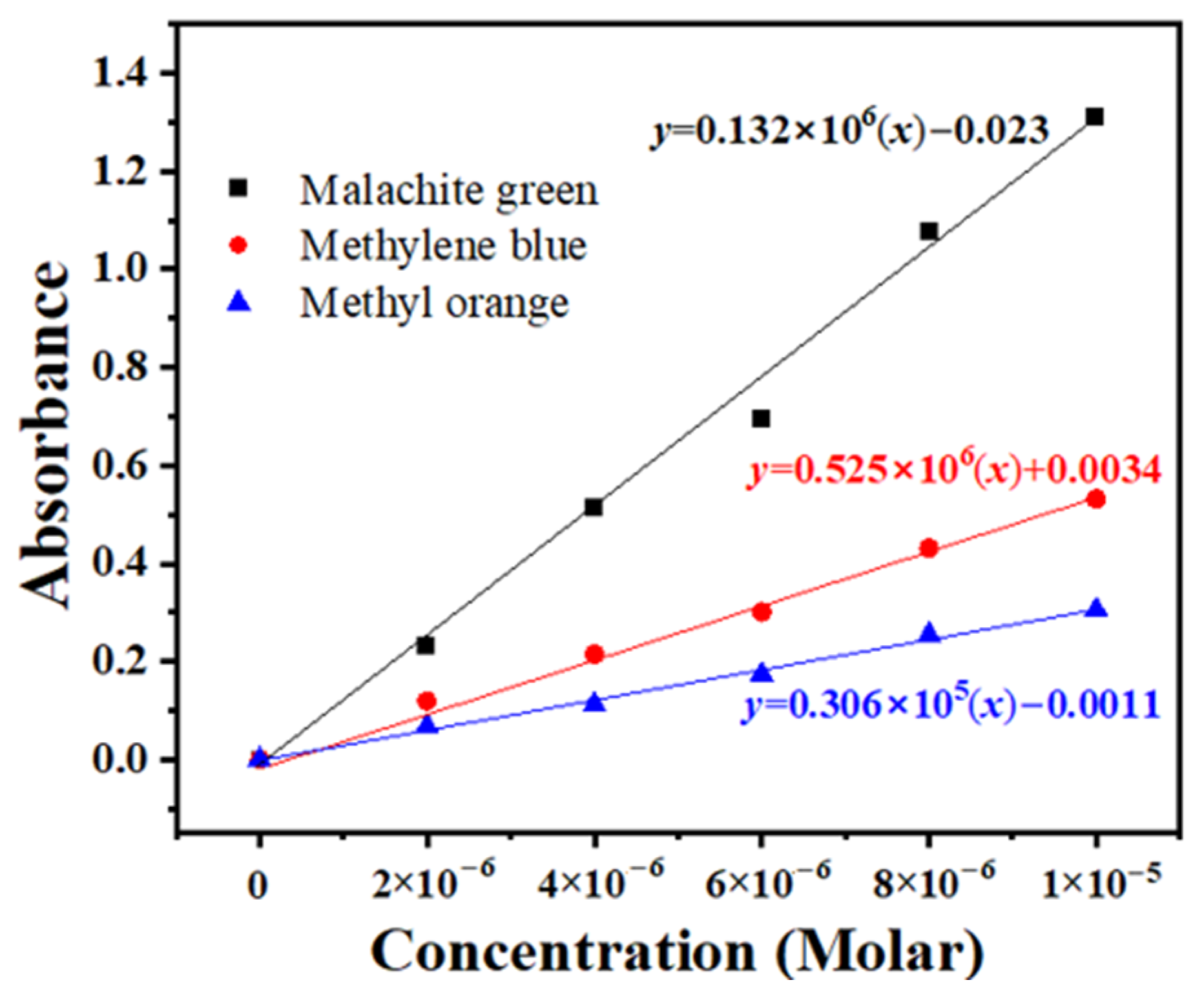
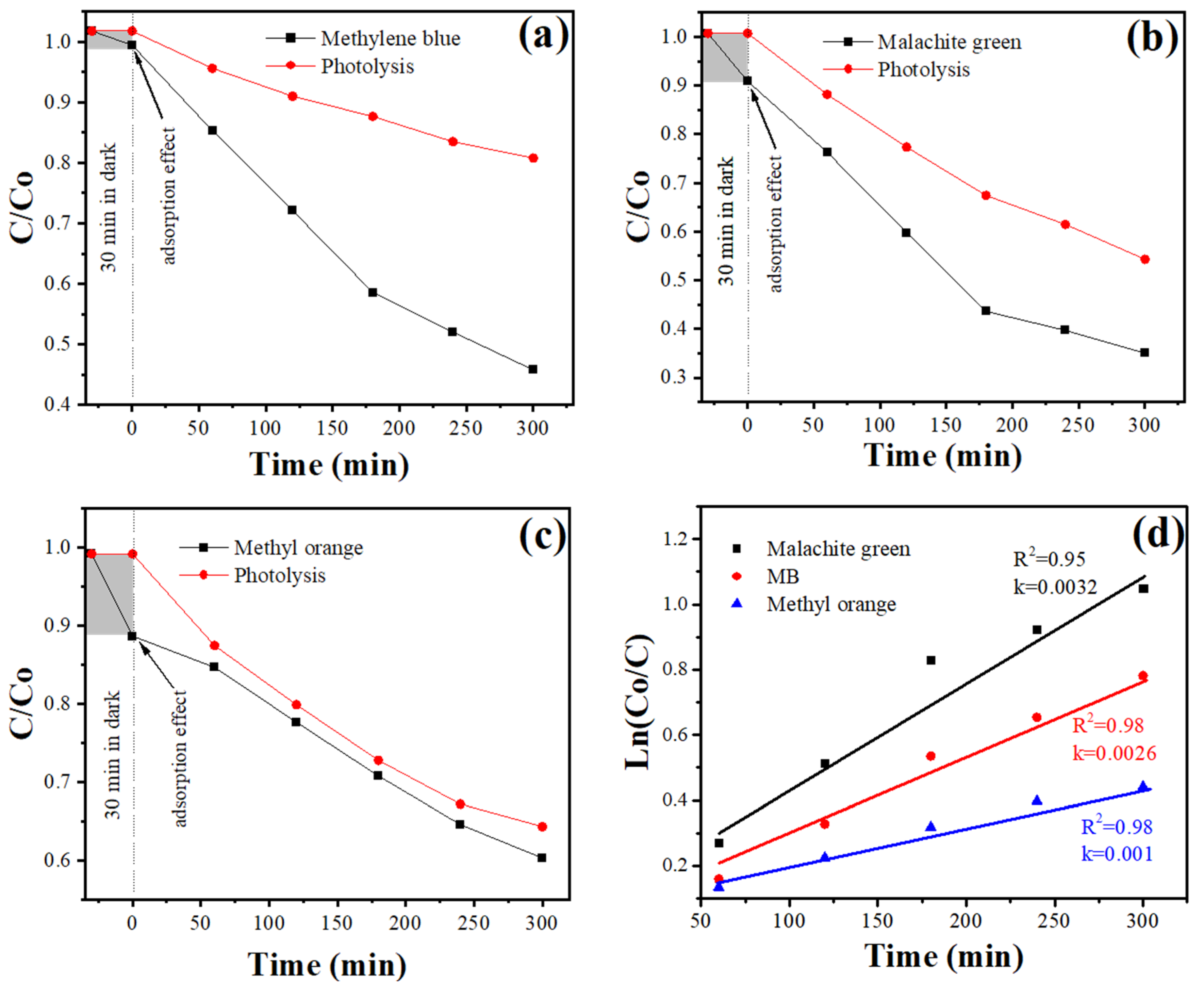
3.7. Photocatalytic Tests
4. Discussion and Limitations
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Correction Statement
References
- Roper, A.; Leverett, P.; Murphy, T.; Williams, P. Stabilities of byströmite, MgSb2O6, ordoñezite, ZnSb2O6 and rosiaite, PbSb2O6, and their possible roles in limiting antimony mobility in the supergene zone. Mineral. Mag. 2015, 79, 537–544. [Google Scholar] [CrossRef]
- Webmineral. Ordonezite. Available online: https://www.webmineral.com/data/Ordonezite.shtml (accessed on 21 May 2025).
- Jackson, A.J.; Parrett, B.J.; Willis, J.; Ganose, A.M.; Leung, W.W.; Liu, Y.; Williamson, B.A.D.; Kim, T.K.; Hoesch, M.; Veiga, L.S.I.; et al. Computational Prediction and Experimental Realization of Earth-Abundant Transparent Conducting Oxide Ga-Doped ZnSb2O6. ACS Energy Lett. 2022, 7, 3807–3816. [Google Scholar] [CrossRef]
- Dąbrowska, G. Reactivity of ZnSb1O6 with ZnTa2O6 and some properties of new limited ZnSb2-xTaxO6 solid solution with tri-rutile structure. Thermochim. Acta 2015, 614, 62–67. [Google Scholar] [CrossRef]
- Hussoyn, E.; Repelin, Y.; Brussrt, H.; Cerez, A. Spectres de vibration et calcul du champ de force des antimoniates et des tantalates de structure trirutile. Spectrochim. Acta 1979, 35A, 1177–1187. [Google Scholar] [CrossRef]
- Haeuseler, H. Infrared and Raman spectra and normal coordinate calculations on trirutile-type compounds. Spectrochim. Acta Part A 1981, 37, 487–495. [Google Scholar] [CrossRef]
- Balasubramaniam, M.; Balakumar, S. Nanostructuring of a one-dimensional zinc antimonate electrode material through a precipitation strategy for use in supercapacitors. New J. Chem. 2018, 42, 6613–6616. [Google Scholar] [CrossRef]
- Li, J.; Du, K.; Lai, Y.; Chen, Y.; Zhang, Z. ZnSb2O6: An advanced anode material for Li-ion batteries. J. Mater. Chem. A 2017, 5, 10843–10848. [Google Scholar] [CrossRef]
- Nagarajan, A.; Naraginti, S. Facile synthesis of N-MgSb2O6 trirutile antimonate and its enhanced photocatalytic performance. Int. J. Environ. Anal. Chem. 2020, 102, 7938–7952. [Google Scholar] [CrossRef]
- Nagarajan, A.; Vijayaraghavan, R. Enhanced photocatalytic activity of nanocrystalline N-doped ZnSb2O6: Role of N doping, cation ordering, particle size and crystallinity. RSC Adv. 2014, 4, 65223–65231. [Google Scholar]
- Dutta, D.P.; Ballal, A.; Singh, A.; Fulekar, M.H.; Tyagi, A.K. Multifunctionality of rare earth doped nano ZnSb2O6, CdSb2O6 and BaSb2O6: Photocatalytic properties and white light emission. Dalton Trans. 2013, 42, 16887–16897. [Google Scholar] [CrossRef] [PubMed]
- Nagarajan, A. Band gap engineering and photocatalytic activity of new trirutile structure Zn1-xMgxSb2O6 (0 < x < 1) solid solution. Indian J. Chem. 2021, 60A, 220–227. [Google Scholar]
- Michel, C.R.; Lopez Contreras, N.L.; Lopez-Alvarez, M.A.; Martinez-Preciado, A.H. Gas selectivity of nanostructured ZnSb2O6 synthesized by a colloidal method. Sens. Actuators B Chem. 2012, 171–172, 686–690. [Google Scholar] [CrossRef]
- Mizoguchi, H.; Woodward, P.M. Electronic structure studies of main group oxides possessing edge-sharing octahedra: Implications for the design of transparent conducting oxides. Chem. Mater. 2004, 16, 5233–5248. [Google Scholar] [CrossRef]
- Jiang, L.; Dong, C.; Lai, Y.; Chen, Y.; Zhang, Z.; Lv, S.; Wang, J.; Pan, S.; Wang, C.; Sun, P.; et al. Mixed potential type methanol gas sensor based on Gd2Zr2O7 solid state electrolyte and ZnSb2O6 sensing electrode. Sens. Actuators B Chem. 2023, 397, 134630. [Google Scholar] [CrossRef]
- Guillen-Bonilla, H.; Rodríguez-Betancourtt, V.-M.; Guillén-Bonilla, J.-T.; Reyes-Gómez, J.; Gildo-Ortiz, L.; Flores-Martínez, M.; Olvera-Amador, M.L. CO and C3H8 Sensitivity Behavior of Zinc Antimonate Prepared by a Microwave-Assisted Solution Method. J. Nanomater. 2015, 2015, 979543. [Google Scholar] [CrossRef]
- Manasa, S.; Gundeboina, R.; Perala, V.; Kammara, V.; Vithal, M. Carbon nanospheres supported visible-light-driven ZnSb2O6: Synthesis, characterization and photocatalytic degradation studies. SN Appl. Sci. 2019, 1, 1046. [Google Scholar]
- Balasubramaniam, M.; Balakumar, S. Contribution of Tin in Electrochemical Properties of Zinc Antimonate Nanostructures: An Electrode Material for Supercapacitors. AIP Conf. Proc. 2018, 1942, 140013. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Singh, A.; Tandon, P. A stable and highly sensitive room-temperature liquefied petroleum gas sensor based on nanocubes/cuboids of zinc antimonate. RSC Adv. 2020, 10, 20349–20357. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Wang, X.; Yang, C.; Wang, P.; Tian, W.; Zhao, K.; Zhang, K. Machine Learning-Motivated Trace Triethylamine Identification by Bismuth Vanadate/Tungsten Oxide Heterostructures. J. Colloid Interface Sci. 2025, 682, 1140–1150. [Google Scholar] [CrossRef]
- Teymourian, H.; Salimi, A.; Hallah, R. An Electrochemical Sensor Based on Gold Nanoparticle Decorated Carbon Nanotubes for Simultaneous Determination of Hydrazine and Hydroquinone. Int. J. Electrochem. Sci. 2019, 14, 6267–6275. [Google Scholar]
- Liu, K.; Xu, Y.; Tian, X.; Zhou, W.; Chen, H.; Zhang, X.; Li, Y.; Wang, M.; Yang, L.; Liu, F. Stacking Growth of Ionically Conductive MOF on Biofabrics Enables Reliable NH3 Sensor for Hepatic Encephalopathy Diagnosis. NPJ Flex. Electron. 2025, 9, 67. [Google Scholar] [CrossRef]
- Balasubramaniam, M.; Balakumar, S. A review on multifunctional attributes of zinc antimonate nanostructures towards energy and environmental applications. Chem. Pap. 2020, 74, 55–75. [Google Scholar] [CrossRef]
- Tamaki, J.; Yamada, Y.; Yamamoto, Y.; Matsuoka, M.; Ota, I. Sensing properties to dilute hydrogen sulfide of ZnSb2O6 thick-film prepared by dip-coating method. Sens. Actuators B Chem. 2000, 66, 70–73. [Google Scholar] [CrossRef]
- Biswas, A.; Bayer, I.S.; Biris, A.S.; Wang, T.; Dervishi, E.; Faupel, F. Advances in top–down and bottom–up surface nanofabrication: Techniques, applications & future prospects. Adv. Colloid Interface Sci. 2012, 170, 2–27. [Google Scholar] [CrossRef]
- Dhand, C.; Dwivedi, N.; Loh, X.J.; Jie Ying, A.N.; Verma, N.K.; Beuerman, R.W.; Lakshminarayanan, R.; Ramakrishna, S. Methods and strategies for the synthesis of diverse nanoparticles and their applications: A comprehensive overview. RSC Adv. 2015, 5, 105003–105037. [Google Scholar] [CrossRef]
- Anbuvannan, M.; Ramesh, M.; Viruthagiri, G.; Shanmugam, N.; Kannadasan, N. Synthesis, characterization and photocatalytic activity of ZnO nanoparticles prepared by biological method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 143, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Sing, S.W. Characterization of Porous Solids: An Introductory Survey. Stud. Surf. Sci. Catal. 1991, 62, 1–9. [Google Scholar]
- Lin, T.; Lv, X.; Li, S.; Wang, Q. The Morphologies of the Semiconductor Oxides and Their Gas-Sensing Properties. Sensors 2017, 17, 2779. [Google Scholar] [CrossRef]
- Qi, X.; Zhu, X.; Wu, J.; Wu, Q.; Li, X.; Gu, M. Controlled synthesis of BiVO4 with multiple morphologies via an ethylenediamine-assisted hydrothermal method. Mater. Res. Bull. 2014, 59, 435–441. [Google Scholar] [CrossRef]
- Mo, M.-S.; Wang, D.; Du, X.; Ma, J.; Qian, X.; Chen, D.; Qian, Y. Engineering of nanotips in ZnO submicrorods and patterned arrays. Cryst. Growth Des. 2009, 9, 2. [Google Scholar] [CrossRef]
- Deng, Z.-X.; Wang, C.; Sun, X.-M.; Li, Y.-D. Structure-directing coordination template effect of ethylenediamine in formations of ZnS and ZnSe nanocrystallites via solvothermal route. Inorg. Chem. 2002, 41, 869−873. [Google Scholar] [CrossRef]
- Gao, X.; Li, X.; Yu, W. Flowerlike ZnO nanostructures via hexamethylenetetramine-assisted thermolysis of zinc-ethylenediamine complex. J. Phys. Chem. B 2005, 109, 1155–1161. [Google Scholar] [CrossRef]
- Shen, X.; Liu, Q.; Ji, Z.; Zhu, G.; Zhou, H.; Chen, K. Controlled synthesis and gas sensing properties of porous Fe2O3/NiO hierarchical nanostructures. CrystEngComm 2015, 17, 5522–5529. [Google Scholar] [CrossRef]
- Jamil, S.; Jing, X.; Wang, J.; Li, S.; Liu, J.; Zhang, M. The synthesis of porous Co3O4 micro cuboid structures by solvothermal approach and investigation of its gas sensing properties and catalytic activity. Mater. Res. Bull. 2013, 48, 4513–4520. [Google Scholar] [CrossRef]
- Prabhakar Rai, H.; Song, H.-M.; Kim, Y.-S.; Song, M.-K.; Oh, P.-R.; Yoon, J.-M.; Yu, Y.-T. The synthesis and characterization of layered double hydroxides with enhanced thermal stability. Mater. Lett. 2012, 68, 90–93. [Google Scholar]
- Juárez-Amador, L.I.; Guillén-Bonilla, H.; Guillén-Bonilla, A.; Guillén-Bonilla, J.T.; Morales-Bautista, J.; Casillas-Zamora, A.; Rodríguez-Betancourtt, V.M.; Olvera-Amador, M.L. Photocatalytic and sensing properties in propane atmospheres of MgSb2O6 nanoparticles synthesized by a chemical method. J. Mater. Sci. Mater. Electron. 2024, 35, 1857. [Google Scholar] [CrossRef]
- Carbajal-Franco, G.; Tiburcio-Silver, A.; Domínguez, J.M.; Sánchez-Júarez, A. Thin film tin oxide-based propane gas sensors. Thin Solid Films 2000, 373, 141–144. [Google Scholar] [CrossRef]
- Gómez-Pozos, H.; Karthik, T.V.; Olvera, M.d.l.L.; Barrientos, A.G.; Pérez Cortés, O.; Vega-Pérez, J.; Maldonado, A.; Pérez-Hernández, R.; Rodríguez-Lugo, V. ZnO thin films as propane sensors: Band structure models to explicate the dependence between the structural and morphological properties on gas sensitivity. J. Phys. Chem. Solids 2017, 106, 16–28. [Google Scholar] [CrossRef]
- Karthik, T.V.K.; Olvera-Amador, M.L.; Maldonado, A.; Hernandez, A.G.; Gómez-Pozos, H. Propane gas-sensing properties of pure and Pd-doped tin oxide nanostructures. J. Mater. Sci. Mater. Electron. 2023, 34, 228. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Singh, A.; Rathore, S.; Yadav, B.C.; Tandon, P. Nanostructured cobalt antimonate: A fast responsive and highly stable sensing material for liquefied petroleum gas detection at room temperature. RSC Adv. 2020, 10, 33770–33781. [Google Scholar] [CrossRef]
- Meena, D.; Jain, M.; Bhatnagar, M.C. Resistive gas sensors based on nanostructured ternary metal oxide: A review. J. Mater. Sci. 2024, 59, 12177–12218. [Google Scholar] [CrossRef]
- Hsiao, C.-C.; Luo, L.-S. A Rapid Process for Fabricating Gas Sensors. Sensors 2014, 14, 12219–12232. [Google Scholar] [CrossRef]
- Baraton, M.-I.; Merhari, L. Electrical behavior of semiconducting nanopowders versus environment. Adv. Mater. Sci. 2003, 4, 15–24. [Google Scholar]
- Gao, X.; Zhang, T. An overview: Facet-dependent metal oxide semiconductor gas sensors. Sens. Actuators B Chem. 2018, 277, 604–633. [Google Scholar] [CrossRef]
- Gildo-Ortiz, L.; Rodríguez-Betancourtt, V.-M.; Ramírez Ortega, J.A.; Blanco-Alonso, O. An Alternative Approach for the Synthesis of Zinc Aluminate Nanoparticles for CO and Propane Sensing Applications. Chemosensors 2023, 11, 105. [Google Scholar] [CrossRef]
- Guillen Bonilla, J.T.; Jiménez Rodríguez, M.; Bonilla, H.G.; Bonilla, A.G.; Padilla, E.H.; Morales, M.E.S.; Flores Jiménez, A.B.; Gutiérrez, J.C.E. The Design, Simulation, and Construction of an O2, C3H8, and CO2 Gas Detection System Based on the Electrical Response of MgSb2O6 Oxide. Technologies 2025, 13, 79. [Google Scholar] [CrossRef]
- Guillén-Bonilla, A.; Guillén-Bonilla, J.T.; Guillén-Bonilla, H.; Huízar-Padilla, E.; Zamora, A.C.; Olvera Amador, M.d.L.L.; Rodríguez-Betancourtt, V.M. A theoretical and experimental study on the dynamic response in propane atmospheres of sensors made from trirutile magnesium antimonate powders. J. Mater. Sci. Mater. Electron. 2024, 35, 1849. [Google Scholar] [CrossRef]
- Yu, S.; Jia, X.; Zhang, J.; Yang, W.; Song, H. Recent advances in different materials for moisture resistance of metal oxide-based gas sensors: A review. Chem. Eng. J. 2025, 505, 159639. [Google Scholar] [CrossRef]
- Sheng, H.; Li, H.; Huang, Y.; Zhang, B.; Liang, J.; Zhou, X.; Tian, Y.; Li, Q. The Sensing Selectivity of Gas Sensors Based on Different Sn-Doped Indium Oxide Films. Chemosensors 2025, 13, 169. [Google Scholar] [CrossRef]
- Kita, J.; Engelbrecht, A.; Schubert, F.; Groß, A.; Rettig, F.; Moos, R. Some practical points to consider with respect to thermal conductivity and electrical resistivity of ceramic substrates for high-temperature gas sensors. Sens. Actuators B Chem. 2015, 213, 541–546. [Google Scholar] [CrossRef]
- Zhang, R.; Wang, Y.; Zhang, Z.; Cao, J. Highly Sensitive Acetone Gas Sensor Based on g-C3N4 Decorated MgFe2O4 Porous Microspheres Composites. Sensors 2018, 18, 2211. [Google Scholar] [CrossRef]
- Michel, G.; Martínez, A.H.; Jiménez, S. Gas sensing response of nanostructured trirutile-type CoSb2O6 synthesized by solution-polymerization method. Sens. Actuators B 2008, 132, 45–51. [Google Scholar] [CrossRef]
- Wang, C.; Yin, L.; Zhang, L.; Xiang, D.; Gao, R. Metal Oxide Gas Sensors: Sensitivity and Influencing Factors. Sensors 2010, 10, 2088–2106. [Google Scholar] [CrossRef]
- Rama Murthy, S.A.; Prabhu, E.; Clinsha, P.C.; Ravindranath Nair, A.; Gnanasekar, K.I.; Jayaraman, V. Role of oxygen species towards chemical sensing by semiconducting oxides. Appl. Surf. Sci. 2019, 487, 362–368. [Google Scholar]
- Srivastava, J.K.; Pandey, P.; Mishra, V.N.; Dwivedi, R. Sensing mechanism of Pd-doped SnO2 sensor for LPG detection. Solid State Sci. 2009, 11, 1602–1605. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, S.; Yu, Q.; Zhao, L.; Sun, P.; Wang, T.; Liu, F.; Yan, X.; Gao, Y.; Liang, X.; et al. One step synthesis of branched SnO2/ZnO heterostructures and their enhanced gas-sensing properties. Sens. Actuators B 2019, 281, 415–423. [Google Scholar] [CrossRef]
- Gildo-Ortiz, L.; Reyes-Gómez, J.; Flores-Álvarez, J.M.; Guillén-Bonilla, H.; Olvera, M.; Rodríguez Betancourtt, V.M.; Verde-Gómez, Y.; Guillén-Cervantes, A.; Santoyo-Salazar, J. Synthesis, characterization and sensitivity tests of perovskite-type LaFeO3 nanoparticles in CO and propane atmospheres. Ceram. Int. 2016, 42, 18821–18827. [Google Scholar] [CrossRef]
- Kapse, V.D. Preparation of Nanocrystalline Spinel-type oxide Materials for Gas sensing Applications. Res. J. Chem. Sci. 2015, 5, 7–12. [Google Scholar]
- Wu, S.; Li, G.; Zhang, Y.; Zhang, W. Surface photoelectric and visible light driven photocatalytic properties of zinc antimonate-based photocatalysts. Mater. Res. Bull. 2013, 48, 1117–1121. [Google Scholar] [CrossRef]
- Liu, W.; Lin, P.; Jin, H.; Xue, H.; Zhang, Y.; Li, Z. Nanocrystalline ZnSb2O6: Hydrothermal synthesis, electronic structure and photocatalytic activity. J. Mol. Catal. A Chem. 2011, 349, 80–85. [Google Scholar] [CrossRef]
- Gnanaprakasam, A.; Sivakumar, V.M.; Thirumarimurugan, M. Influencing parameters in the photocatalytic degradation of organic effluent via nanometal oxide catalyst: A review. Indian J. Mater. 2015, 2015, 601827. [Google Scholar] [CrossRef]
- Jabbar, Z.H.; Graimed, B.H. Recent developments in industrial organic degradation via semiconductor heterojunctions and the parameters affecting the photocatalytic process: A review study. J. Water Process Eng. 2022, 47, 102671. [Google Scholar] [CrossRef]
- Charif, R.; Makhloufi, R.; Chaba Mouna, S.; Chadli, A.; Barkat, A.; Nouiri, M. Structural, electronic, optical, mechanical, and phononic bulk properties of tetragonal trirutile antimonate MSb2O6 (M = Fe, Co, Ni, or Zn): A first-principles prediction for optoelectronic applications. Phys. Scr. 2024, 99, 125909. [Google Scholar] [CrossRef]
- Ham, K.; Hong, S.; Kang, S.; Cho, K.; Lee, J. Extensive active-site formation in trirutile CoSb2O6 by oxygen vacancy for oxygen evolution reaction in anion exchange membrane water splitting. ACS Energy Lett. 2021, 6, 364–370. [Google Scholar] [CrossRef]
- Tran, H.D.; Nguyen, D.Q.; Do, P.T.; Tran, U.N.P. Kinetics of photocatalytic degradation of organic compounds: A mini-review and new approach. RSC Adv. 2023, 13, 16915. [Google Scholar] [CrossRef]
- Shaikh, B.; Ali Bhatti, M.; Ahmed Shah, A.; Tahira, A.; Karim Shah, A.; Usto, A.; Aftab, U.; Bukhari, S.I.; Alshehri, S.; Shah Bukhari, S.D.U.; et al. Mn3O4@ZnO Hybrid Material: An Excellent Photocatalyst for the Degradation of Synthetic Dyes Including Methylene Blue, Methyl Orange and Malachite Green. Nanomaterials 2022, 12, 3754. [Google Scholar] [CrossRef]
- Kaur, H.; Hippargi, G.; Pophali, G.R.; Bansiwal, A.K. Treatment methods for removal of pharmaceuticals and personal care products from domestic wastewater. In Pharmaceuticals and Personal Care Products: Waste Management and Treatment Technology; Elsevier: Amsterdam, The Netherlands, 2019; pp. 129–150. [Google Scholar]
- Gouvea, C.A.K.; Wypych, F.; Moraes, S.G.; Duran, N.; Nagata, N.; Peralta-Zamora, P. Semiconductor-assisted photocatalytic degradation of reactive dyes in aqueous solution. Chemosphere 2000, 40, 433–440. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, L.; Li, X.; Xu, Y.; Wu, D.; Sakthivel, T.; Guo, Z.; Zhao, X.; Dai, Z. Asymmetric tacticity navigates the localized metal spin state for sustainable alkaline/sea water oxidation. Sci. Adv. 2025, 11, eads0861. [Google Scholar] [CrossRef] [PubMed]

| CO (300 ppm) | C3H8 (500 ppm) | ||||||
|---|---|---|---|---|---|---|---|
| Temp. (°C) | Resistance (MΩ) | Resistivity (Ω·m) | Sensor Response | Temp. (°C) | Resistance (MΩ) | Resistivity (Ω·m) | Sensor Response |
| 23 | 250 | 0 | 23 | 250 | 0 | ||
| 100 | 185 | 0.27 | 100 | 172 | 0.32 | ||
| 200 | 48.1 | 0.5 | 200 | 31.4 | 0.56 | ||
| 300 | 2.1 | 5.86 | 300 | 5.7 | 1.04 | ||
| Light Source | Analyte | Doping | Degradation Percentage (%) | Degradation Time (min) | Reference |
|---|---|---|---|---|---|
| 6 UV lamps | Rhodamine b | - | ~95 | 180 | [10] |
| 6 UV lamps | Rhodamine b | N | ~97 | 80 | |
| 4 UV lamps | Rhodamine b | - | ~95 | 95 | [11] |
| 4 UV lamps | Rhodamine b | Tb/Eu | ~95 | 95 | |
| Tungsten lamp | Methyl violet | - | ~40 | 180 | [17] |
| Tungsten lamp | Methyl violet | Carbon | ~92 | 180 | |
| Xe lamp | Rhodamine b | - | ~10 | 120 | [60] |
| Xe lamp | Rhodamine b | N | ~70 | 120 | |
| Full arc Xe lamp | Rhodamine b | - | ~10 | 120 | |
| Full arc Xe lamp | Rhodamine b | N | ~95 | 120 | |
| 4 UV lamps | Methyl orange | - | ~80 | 180 | [61] |
| 4 UV lamps | Rhodamine b | - | ~99 | 60 | |
| UV lamp | Methylene blue | - | ~60 | 300 | This work |
| UV lamp | Malachite green | - | ~50 | 300 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales-Bautista, J.; Guillén-Bonilla, H.; Juárez-Amador, L.I.; Guillén-Bonilla, A.; Rodríguez-Betancourtt, V.-M.; Ramírez-Ortega, J.A.; Guillén-Bonilla, J.T.; Olvera-Amador, M.d.l.L. Synthesis, Characterization, and Evaluation of Photocatalytic and Gas Sensing Properties of ZnSb2O6 Pellets. Chemosensors 2025, 13, 329. https://doi.org/10.3390/chemosensors13090329
Morales-Bautista J, Guillén-Bonilla H, Juárez-Amador LI, Guillén-Bonilla A, Rodríguez-Betancourtt V-M, Ramírez-Ortega JA, Guillén-Bonilla JT, Olvera-Amador MdlL. Synthesis, Characterization, and Evaluation of Photocatalytic and Gas Sensing Properties of ZnSb2O6 Pellets. Chemosensors. 2025; 13(9):329. https://doi.org/10.3390/chemosensors13090329
Chicago/Turabian StyleMorales-Bautista, Jacob, Héctor Guillén-Bonilla, Lucia Ivonne Juárez-Amador, Alex Guillén-Bonilla, Verónica-María Rodríguez-Betancourtt, Jorge Alberto Ramírez-Ortega, José Trinidad Guillén-Bonilla, and María de la Luz Olvera-Amador. 2025. "Synthesis, Characterization, and Evaluation of Photocatalytic and Gas Sensing Properties of ZnSb2O6 Pellets" Chemosensors 13, no. 9: 329. https://doi.org/10.3390/chemosensors13090329
APA StyleMorales-Bautista, J., Guillén-Bonilla, H., Juárez-Amador, L. I., Guillén-Bonilla, A., Rodríguez-Betancourtt, V.-M., Ramírez-Ortega, J. A., Guillén-Bonilla, J. T., & Olvera-Amador, M. d. l. L. (2025). Synthesis, Characterization, and Evaluation of Photocatalytic and Gas Sensing Properties of ZnSb2O6 Pellets. Chemosensors, 13(9), 329. https://doi.org/10.3390/chemosensors13090329







