Oxygen Vacancy-Engineered Cu2O@CuS p–p Heterojunction Gas Sensor for Highly Sensitive n-Butanol Detection
Abstract
1. Introduction
2. Experimental Section
2.1. Apparatus
2.2. Preparation of Materials
2.3. Construction and Testing of Gas Sensor
3. Results and Discussion
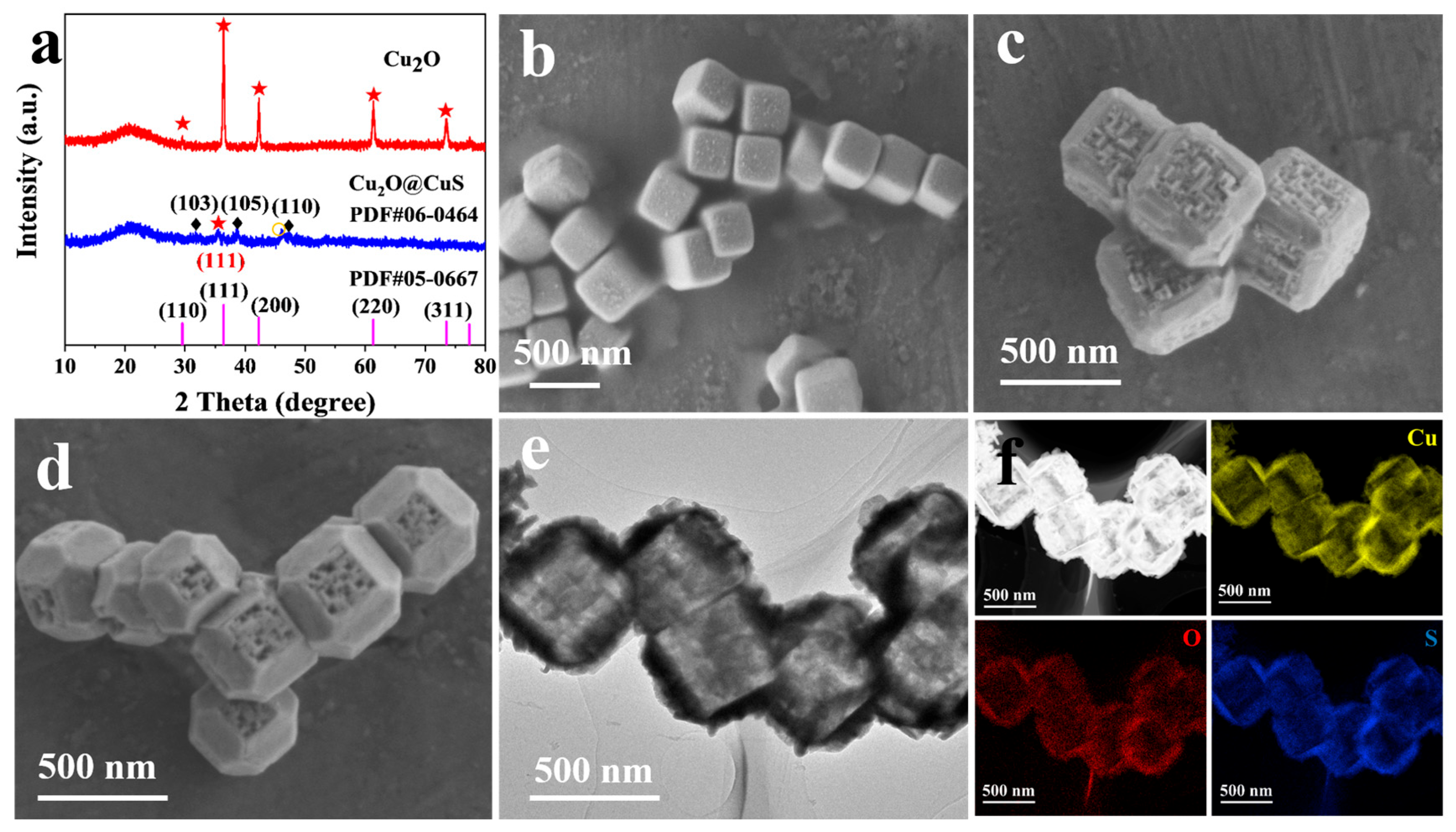
3.1. Characterization of Cu2O@CuS
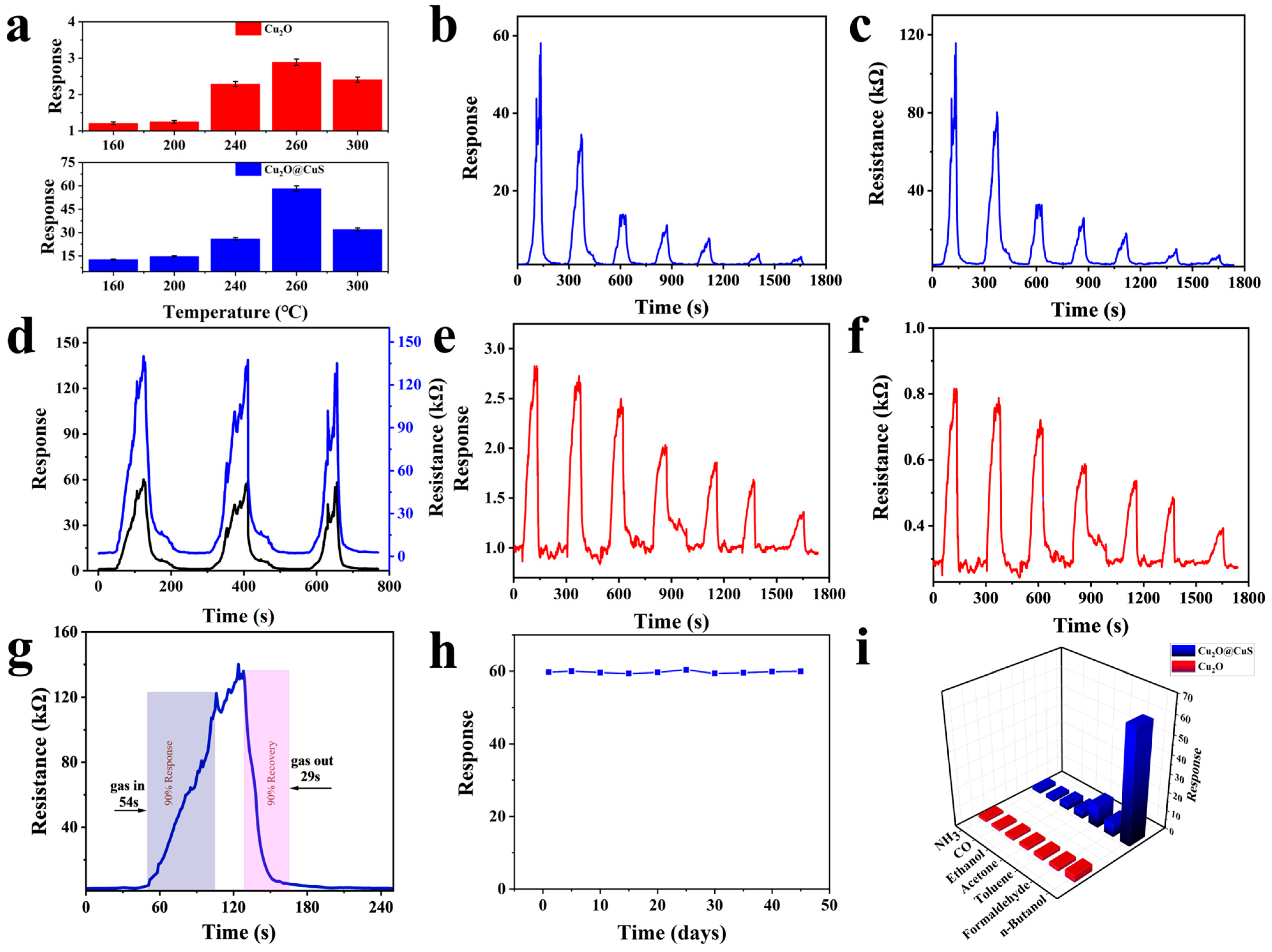
3.2. Gas-Sensing Properties
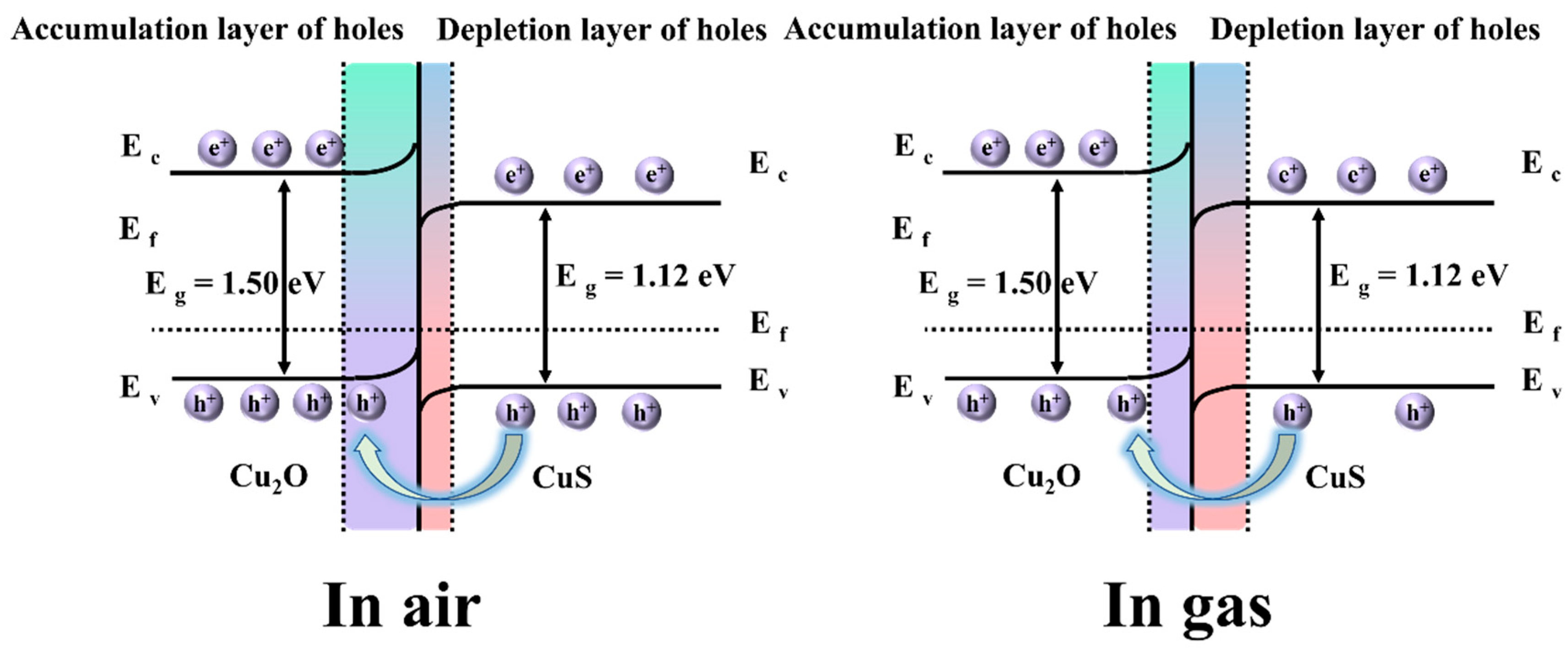
3.3. Plausible Gas-Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shang, Z.; Yu, X.; Ren, L.; Li, Z.; Wang, H.; Li, Y.; Wang, Y. Synergic effect of hydrogen and n-butanol on combustion and emission characteristics of a hydrogen direct injection SI engine fueled with n-butanol/gasoline. Int. J. Hydrogen Energy 2024, 49, 945–956. [Google Scholar] [CrossRef]
- Zhong, W.; Dai, L.; He, Z.; Wang, Q. Numerical study on the combustion characteristics of premixed ammonia flames with methanol/ethanol/n-butanol addition. Fuel 2025, 381, 133527. [Google Scholar] [CrossRef]
- Zhao, Z.; Su, Z.; Wu, L. Enhanced n-butanol gas sensors based on ZnO/CuCo2O4 heterojunction: Materials synthesis, sensing performance, and mechanism study. J. Alloys Compd. 2025, 1030, 180962. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, Q.; Wan, Y.; Gao, S.; Wang, Y.; Feng, C. High-performance and rapid-response n-butanol sensor based on ZnO/SnO2 heterojunction. J. Taiwan Inst. Chem. Eng. 2025, 170, 106027. [Google Scholar] [CrossRef]
- Cheng, L.; Yuan, Z.; Wu, R.; Li, Z.; Zheng, J.; Song, J. A leapfrog improvement in N-butanol detection achieved through single atom catalysts Co-N-C. Chem. Eng. J. 2025, 519, 164855. [Google Scholar] [CrossRef]
- Hu, X.; Zhang, K.; Li, R.; Cao, Y.; Tian, D.; Zhu, Z. Synergistic CdS/Ti3C2Tx MXene heterojunction for ultrasensitive and selective n-butanol detection prepared by facile hydrothermal strategy and improved by machine learning. Chem. Eng. J. 2025, 519, 165401. [Google Scholar] [CrossRef]
- Chu, J.; Deng, Z.; Pan, J.; Yang, A.; Wang, Q.; Yuan, H.; Yuan, H.; Xin, F.; Rong, M.; Wang, X. Parameter Optimization of Semiconductor Gas Sensor under AC Impedance Measurement. ACS Sens. 2024, 9, 5561–5569. [Google Scholar] [CrossRef] [PubMed]
- Wu, Z.; Wang, C.; Liu, X.; Tan, K.; Fu, Z.; Teat, S.J.; Li, Z.W.; Hei, X.; Huang, X.; Xu, G.; et al. Confinement of 1D Chain and 2D Layered CuI Modules in K-INA-R Frameworks via Coordination Assembly: Structure Regulation and Semiconductivity Tuning. J. Am. Chem. Soc. 2023, 145, 19293–19302. [Google Scholar] [CrossRef]
- Wang, H.; Zhao, Y.; Yuan, Z.; Ji, H.; Meng, F. Multiscale Bidirectional Temporal Convolutional Framework for Industrial Ketone Organic Solvent Detection Based on Semiconductor Gas Sensor. IEEE Trans. Ind. Electron. 2024, 71, 16772–16782. [Google Scholar] [CrossRef]
- Humayun, M.; Bououdina, M.; Usman, M.; Khan, A.; Luo, W.; Wang, C. Designing State-of-the-Art Gas Sensors: From Fundamentals to Applications. Chem. Rec. 2024, 24, e202300350. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Mao, J.; Yin, D.; Zhang, T.; Liu, C.; Hao, W.; Wang, Y.; Hao, J. Synergy of S-vacancy and heterostructure in BiOCl/Bi2S3−x boosting room-temperature NO2 sensing. J. Hazard. Mater. 2023, 455, 131591. [Google Scholar] [CrossRef]
- Zhang, W.; Yuan, Z.; Zhu, H.; Zhang, S.; Meng, F. Kinetic Modeling in Temperature-Modulated Semiconductor Gas Sensor Utilizing Eley–Rideal Mechanism and Its Application in Discriminative Detection of VOCs. ACS Sens. 2024, 9, 6460–6470. [Google Scholar] [CrossRef]
- Kou, Y.; Hua, L.; Chen, W.; Xu, X.; Song, L.; Yu, S.; Lu, Z. Material design and application progress of flexible chemiresistive gas sensors. J. Mater. Chem. A 2024, 12, 21583–21604. [Google Scholar] [CrossRef]
- Hu, C.; Feng, Y.; Huo, J.; Zhang, B.; Cui, H.; Wang, S.; Song, Q.; Cao, J.; Dong, X. Regulating microscopic surfaces and structures to boost n-butanol sensing performances in NiCo2O4/NiO composites. Sens. Actuators B Chem. 2025, 429, 137341. [Google Scholar] [CrossRef]
- Jiang, B.; Cao, J.; Zhang, K.; Chen, Q.; Ma, P. Modulation of ZnFe2O4 material growth using NiO nanoparticles for highly sensitive response to n-butanol. Sens. Actuators B Chem. 2025, 430, 137321. [Google Scholar] [CrossRef]
- Zhou, P.; Tsang, J.; Blackman, C.; Shen, Y.; Liang, J.; Covington, J.A.; Saffell, J.; Danesh, E. A Novel Mechanism Based on Oxygen Vacancies to Describe Isobutylene and Ammonia Sensing of p-Type Cr2O3 and Ti-Doped Cr2O3 Thin Films. Chemosensors 2024, 12, 218. [Google Scholar] [CrossRef]
- Wang, G.; Chen, T.; Guo, L.; Wang, H.; Wang, X.; Zeng, H.; Feng, Y.; Zhao, W.; Wang, Y.; Liu, X.; et al. Chemiresistive n-butanol gas sensors based on Co3O4@ZnO hollow-sphere-array thin films prepared by template-assisted magnetron sputtering. Sens. Actuators B Chem. 2024, 413, 135862. [Google Scholar] [CrossRef]
- Zheng, Z.; Liu, K.; Zhou, Y.; Zhang, Z.; Su, H.; Nie, X.; Debliquy, M.; Yu, Z.; Zhang, C. Spinel type MCo2O4 (M = Mn, Mg, Ni, Cu, Fe and Zn) for chemoresistance gas sensors. Mater. Today Chem. 2024, 36, 101928. [Google Scholar] [CrossRef]
- Zang, D.; Wei, X.; Liu, Q.; Li, Y.; You, R. In-situ growth of ZnO@ZnWO4 heterojunction with flower-like structure by chemical vapor deposition for H2S gas sensor. Appl. Surf. Sci. 2025, 679, 161149. [Google Scholar] [CrossRef]
- Chen, X.; Wang, T.; Shi, J.; Lv, W.; Han, Y.; Zeng, M.; Yang, J.; Hu, N.; Su, Y.; Wei, H.; et al. A Novel Artificial Neuron-Like Gas Sensor Constructed from CuS Quantum Dots/Bi2S3 Nanosheets. Nano-Micro Lett. 2021, 14, 8. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Mirzaei, A.; Zheng, Y.; Lee, J.; Kim, J.; Kim, H.W.; Kim, S.S. Enhancement of H2S sensing performance of p-CuO nanofibers by loading p-reduced graphene oxide nanosheets. Sens. Actuators B Chem. 2019, 281, 453–461. [Google Scholar] [CrossRef]
- Huang, C.; He, X.; Dai, T. Realization of a self-powered Cu2O ozone gas sensor through the lateral photovoltaic effect. J. Mater. Chem. C 2022, 10, 16517–16523. [Google Scholar] [CrossRef]
- Ren, W.; Luan, J.; Yin, L.; Xu, R.; Wang, C.; Zhang, P.; Cui, G.; Yang, K. Ultrasensitive room-temperature NO2 gas sensor based on graphene-modified Cu2O nanocomposites: A combined experimental and first-principles study. Measurement 2025, 245, 116647. [Google Scholar] [CrossRef]
- Tan, C.; Hsu, S.; Ke, W.; Chen, L.; Huang, M.H. Facet-Dependent Electrical Conductivity Properties of Cu2O Crystals. Nano Lett. 2015, 15, 2155–2160. [Google Scholar] [CrossRef] [PubMed]
- Azmoodeh, Z.; Nasirian, S.; Milani Moghaddam, H. Improving H2 gas sensing with ZnMn2O4/Polypyrrole Nanocomposite. Int. J. Hydrogen Energy 2024, 85, 854–864. [Google Scholar] [CrossRef]
- Cho, D.; Lee, G.; Lee, Y.; Cho, A.; Lee, S.; Kim, G.H.; Park, G.; Jang, S.; Choi, M.; Shim, Y.; et al. Ultrathin Copper Monosulfide Films for an Optically Semitransparent, Highly Selective Ammonia Chemosensor. ACS Appl. Mater. Interfaces 2024, 16, 60530–60540. [Google Scholar] [CrossRef]
- Li, Z.; Liu, X.; Li, S.; Feng, E.; Wu, Z.; Yang, Z.; Ma, X.; Han, X. Shape-controlled hollow Cu2O@CuS nanocubes with enhanced photocatalytic activities towards degradation of tetracycline. Environ. Technol. 2023, 44, 2702–2712. [Google Scholar] [CrossRef]
- Pan, L.; Dai, L.; Burton, O.J.; Chen, L.; Andrei, V.; Zhang, Y.; Ren, D.; Cheng, J.; Wu, L.; Frohna, K.; et al. High carrier mobility along the [111] orientation in Cu2O photoelectrodes. Nature 2024, 628, 765–770. [Google Scholar] [CrossRef]
- Frankcombe, T.J.; Liu, Y. Interpretation of Oxygen 1s X-ray Photoelectron Spectroscopy of ZnO. Chem. Mater. 2023, 35, 5468–5474. [Google Scholar] [CrossRef]
- Idriss, H. On the wrong assignment of the XPS O1s signal at 531–532 eV attributed to oxygen vacancies in photo- and electro-catalysts for water splitting and other materials applications. Surf. Sci. 2021, 712, 121894. [Google Scholar] [CrossRef]
- Dai, W.; Wan, K.; Pang, K.; Guo, J.; Liu, S.; Wu, K.; Tang, C.; Sun, Y.; Shi, X.; Tang, Z.; et al. In-depth understanding and precise modulation of surface reconstruction during heterogeneous electrocatalysis: From model to practical catalyst. Chem 2025, 11, 102345. [Google Scholar] [CrossRef]
- Yu, Z.; Li, F.; Di, H.; Pan, Y.; Lv, L.; Ma, Y.; Chen, Q. A facile one-pot method for preparation of the rGO–CuS/Cu2S with enhanced photocatalytic activity under visible light irradiation. J. Mater. Sci. Mater. Electron. 2016, 27, 5136–5144. [Google Scholar] [CrossRef]
- Hung, C.M.; Le, D.T.T.; Van Hieu, N. On-chip growth of semiconductor metal oxide nanowires for gas sensors: A review. J. Sci. Adv. Mater. Devices 2017, 2, 263–285. [Google Scholar] [CrossRef]
- Teterycz, H.; Halek, P.; Wiśniewski, K.; Halek, G.; Koźlecki, T.; Polowczyk, I. Oxidation of Hydrocarbons on the Surface of Tin Dioxide Chemical Sensors. Sensors 2011, 11, 4425–4437. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lefferts, M.J.; Wang, Y.; Lister, A.M.; Forssberg, A.; Chen, R.; Wang, L.; Castell, M.R. Chemiresistive Polymer Percolation Network Gas Sensor Created with a Nanosphere Template. Adv. Mater. Interfaces 2023, 10, 2202042. [Google Scholar] [CrossRef]
- Mallik, M.; Monia, S.; Gupta, M.; Ghosh, A.; Toppo, M.P.; Roy, H. Synthesis and characterization of Cu2O nanoparticles. J. Alloys Compd. 2020, 829, 154623. [Google Scholar] [CrossRef]
- Wu, H.; Zhou, Y.; Guo, J.; Zhao, L.; Wang, T.; Yan, X.; Wang, C.; Liu, F.; Sun, P.; Lu, G. Highly sensitive and selective xylene sensor based on p-p heterojunctions composites derived from off-stoichiometric cobalt tungstate. Sens. Actuators B Chem. 2022, 351, 130973. [Google Scholar] [CrossRef]
- Yamazoe, N.; Fuchigami, J.; Kishikawa, M.; Seiyama, T. Interactions of tin oxide surface with O2, H2O and H2. Surf. Sci. 1979, 86, 335–344. [Google Scholar] [CrossRef]
- Zhu, Y.; Meng, X.; Wang, X.; Gao, W. Low detection based on Pd Pt /In2O3 nanospheres for rapid hydrogen detection. Sens. Actuators B Chem. 2024, 410, 135654. [Google Scholar] [CrossRef]
- An, D.; Liu, N.; Zhang, H.; Sun, Q.; Li, C.; Li, Y.; Zhang, Q.; Lu, Y. Enhanced n-butanol sensing performance of SnO2-based gas sensors by doping In2O3 via co-precipitation method. Sens. Actuators B Chem. 2021, 340, 129944. [Google Scholar] [CrossRef]
- Guo, W.; Shuai, Y.; Liu, X.; Zhang, J.; Wang, J.; Mahmoud, K.H.; El-Bahy, Z.M.; Mubarak, N.M. A n-butanol gas sensor with enhanced gas sensing performance based on Co-doped BiVO4 polyhedrons. Sens. Actuators B Chem. 2022, 354, 131221. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, B.; Zhao, Z.; Zhang, Z.; Zhang, S.; Bala, H.; Zhang, Z. Enhanced n-butanol sensing properties of Au modified TiO2 nanorod arrays: A combined experimental and first-principle study. Appl. Surf. Sci. 2023, 641, 158458. [Google Scholar] [CrossRef]
- Wang, B.; Wang, Z.; Wan, Y.; Wang, Y.; Gao, S.; Chen, Y.; Luo, Q.; Feng, C. Novel Bi-doped ZnFe2O4 nanofibers based gas sensor for enhanced n-butanol sensing. J. Taiwan Inst. Chem. Eng. 2024, 157, 105395. [Google Scholar] [CrossRef]
- Pan, H.; Li, Z.; Lou, C.; Lei, G.; Xie, J.; Zheng, W.; Liu, X.; Zhang, J. Anchoring Fe2O3 nanosheets on NiO nanoprisms to regulate the electronic properties for improved n-butanol detection. Sens. Actuators B Chem. 2022, 354, 131223. [Google Scholar] [CrossRef]
- Qian, X.; Chen, Y.; Tao, Y.; Zhang, J.; Zhang, G.; Xu, H. Facile synthesis of NiFe2O4-based nanoblocks for low-temperature detection of trace n-butanol. RSC Adv. 2024, 14, 2214–2225. [Google Scholar] [CrossRef]
- Lv, L.; Cheng, P.; Wang, Y.; Xu, L.; Zhang, B.; Lv, C.; Ma, J.; Zhang, Y. Sb-doped three-dimensional ZnFe2O4 macroporous spheres for N-butanol chemiresistive gas sensors. Sens. Actuators B Chem. 2020, 320, 128384. [Google Scholar] [CrossRef]


| CH3CH2OH | NH3 | HCHO | C4H10O | C3H6O | C7H8 | CO | |
|---|---|---|---|---|---|---|---|
| Source | liquid | liquid | liquid | liquid | liquid | liquid | gas cylinder |
| Purity | AR | 25% | 37% | AR | AR | AR | 99.9% |
| Materials | Lattice Oxygen | Vacant Oxygen | Adsorbed Oxygen | Cu (I) | Cu (II) |
|---|---|---|---|---|---|
| Cu2O | 59.34 | 31.3 | 9.36 | 47.24 | 42.31 |
| Cu2O@CuS | 35.86 | 43.48 | 20.66 | 56.29 | 33.73 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, D.; Qu, Z.; Li, C.; Wang, H.; Zhang, Y.; Ren, X.; Xu, R. Oxygen Vacancy-Engineered Cu2O@CuS p–p Heterojunction Gas Sensor for Highly Sensitive n-Butanol Detection. Chemosensors 2025, 13, 324. https://doi.org/10.3390/chemosensors13090324
Zhang D, Qu Z, Li C, Wang H, Zhang Y, Ren X, Xu R. Oxygen Vacancy-Engineered Cu2O@CuS p–p Heterojunction Gas Sensor for Highly Sensitive n-Butanol Detection. Chemosensors. 2025; 13(9):324. https://doi.org/10.3390/chemosensors13090324
Chicago/Turabian StyleZhang, Di, Zhengfang Qu, Chenchen Li, Huan Wang, Yong Zhang, Xiang Ren, and Rui Xu. 2025. "Oxygen Vacancy-Engineered Cu2O@CuS p–p Heterojunction Gas Sensor for Highly Sensitive n-Butanol Detection" Chemosensors 13, no. 9: 324. https://doi.org/10.3390/chemosensors13090324
APA StyleZhang, D., Qu, Z., Li, C., Wang, H., Zhang, Y., Ren, X., & Xu, R. (2025). Oxygen Vacancy-Engineered Cu2O@CuS p–p Heterojunction Gas Sensor for Highly Sensitive n-Butanol Detection. Chemosensors, 13(9), 324. https://doi.org/10.3390/chemosensors13090324






