Resistive-Based Nanostructured CeO2 Gas Sensors: A Review
Abstract
1. Introduction
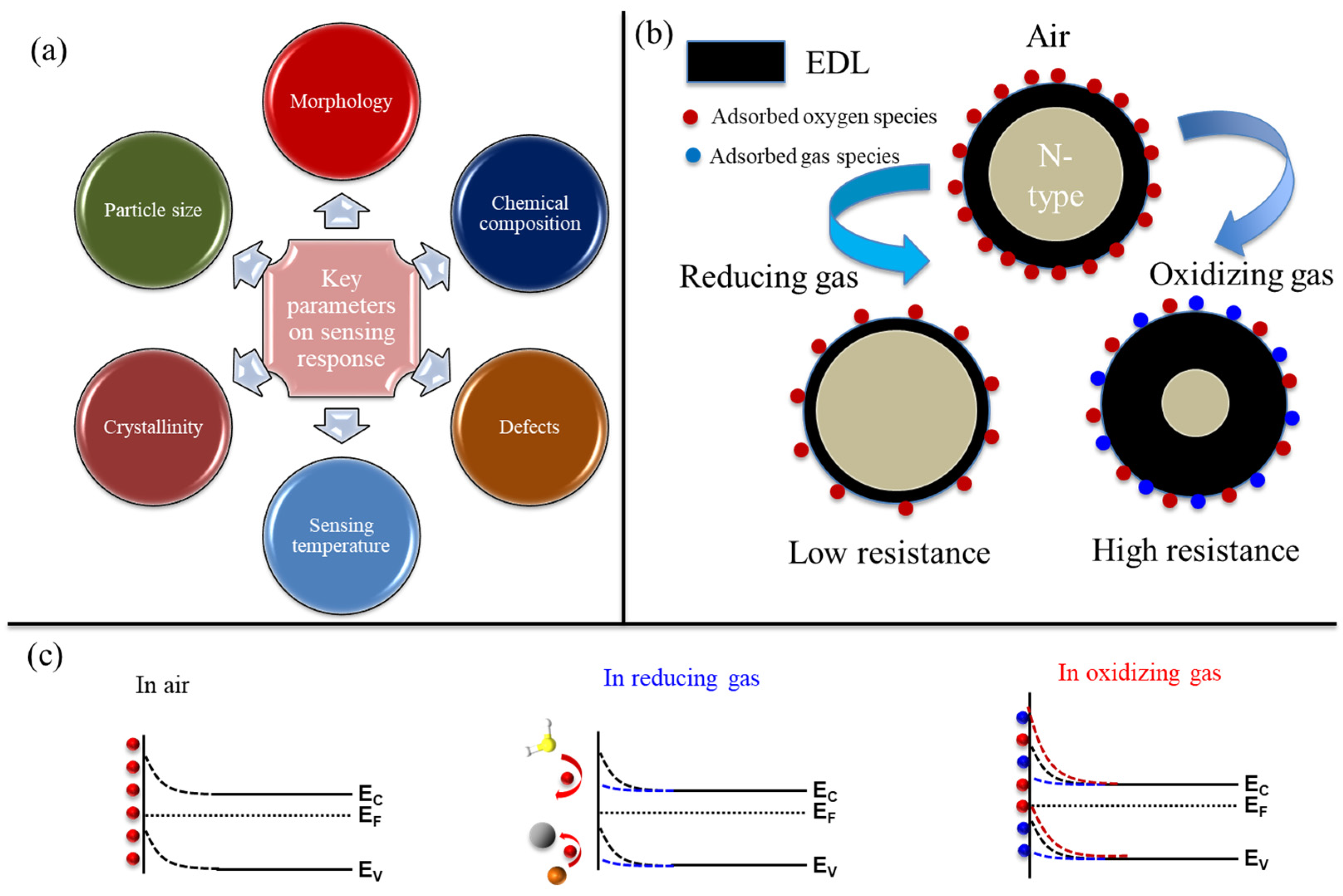
2. General Gas Sensing Mechanism of Resistive Gas Sensors
3. CeO2-Based Resistive Gas Sensors
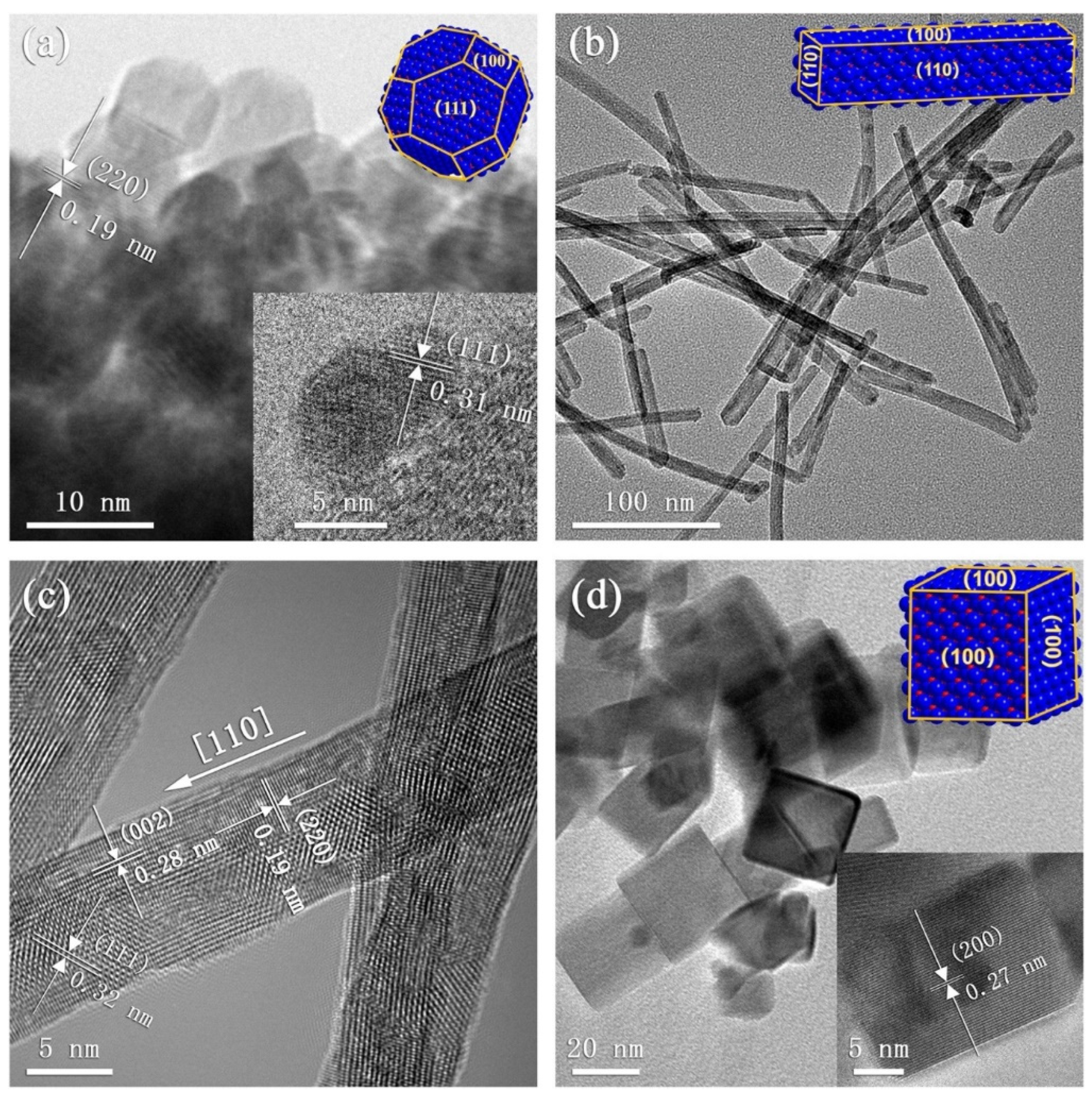
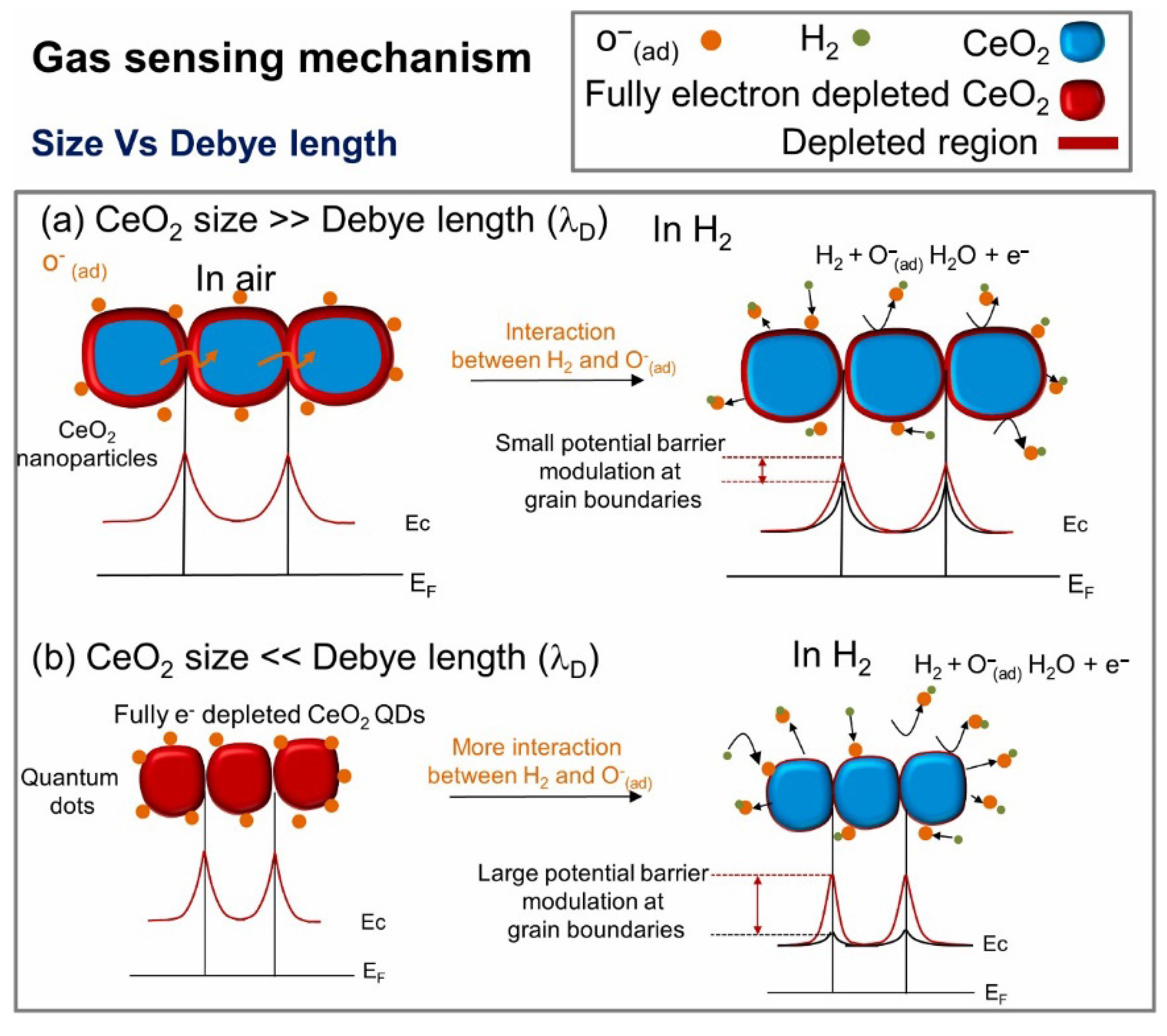
3.1. Pristine CeO2 Gas Sensors
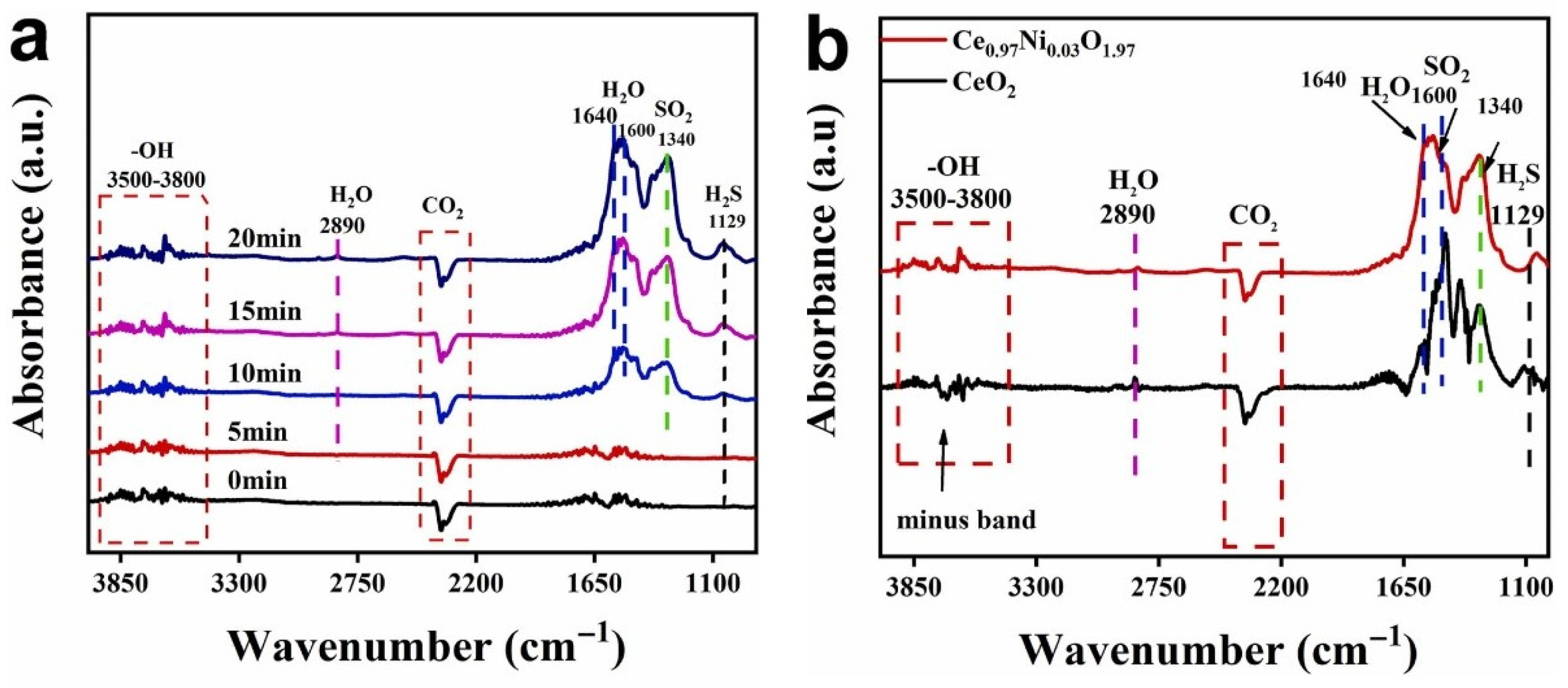
3.2. Doped CeO2 Gas Sensors
3.3. Noble Metal Decorated CeO2 Gas Sensors
3.4. Composite CeO2 Gas Sensors
4. Conclusions and Outlooks
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Momeni, M.M.; Mohammadinejad, F.; Ghasemipur, F.; Lee, B.-K. Asymmetric Photo-Assisted Supercapacitor and Symmetric Flexible Supercapacitors Based on CeO2-MnO2 Supported on Carbon Cloth. J. Alloys Compd. 2025, 1029, 180761. [Google Scholar] [CrossRef]
- Tran, D.P.H.; Pham, M.-T.; Bui, X.-T.; Wang, Y.-F.; You, S.-J. CeO2 as a Photocatalytic Material for CO2 Conversion: A Review. Sol. Energy 2022, 240, 443–466. [Google Scholar] [CrossRef]
- Montini, T.; Melchionna, M.; Monai, M.; Fornasiero, P. Fundamentals and Catalytic Applications of CeO2-Based Materials. Chem. Rev. 2016, 116, 5987–6041. [Google Scholar] [CrossRef]
- Kumar, R.; Gupta, V.K.; Khosya, M.; Singh, S.; Kumar, U. Comparative computational and experimental insights into the structural, electrical, and biological properties of CeO2 fluorite ceramics. Sci. Rep. 2025, 15, 19269. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Lu, Y.; Zhang, L.; Kong, Z.; Yang, T.; Tao, L.; Zou, Y.; Wang, S. Defect Engineering on CeO2-Based Catalysts for Heterogeneous Catalytic Applications. Small Struct. 2021, 2, 2100058. [Google Scholar] [CrossRef]
- Ma, R.; Zhang, S.; Wen, T.; Gu, P.; Li, L.; Zhao, G.; Niu, F.; Huang, Q.; Tang, Z.; Wang, X. A Critical Review on Visible-Light-Response CeO2-Based Photocatalysts with Enhanced Photooxidation of Organic Pollutants. Catal. Today 2019, 335, 20–30. [Google Scholar] [CrossRef]
- Beie, H.-J.; Gnörich, A. Oxygen Gas Sensors Based on CeO2 Thick and Thin Films. Sens. Actuators B Chem. 1991, 4, 393–399. [Google Scholar] [CrossRef]
- Gangopadhyay, S.; Frolov, D.D.; Masunov, A.E.; Seal, S. Structure and Properties of Cerium Oxides in Bulk and Nanoparticulate Forms. J. Alloys Compd. 2014, 584, 199–208. [Google Scholar] [CrossRef]
- Ackland, K.; Coey, J.M.D. Room Temperature Magnetism in CeO2—A Review. Phys. Rep. 2018, 746, 1–39. [Google Scholar] [CrossRef]
- Li, Q.; Song, L.; Liang, Z.; Sun, M.; Wu, T.; Huang, B.; Luo, F.; Du, Y.; Yan, C.-H. A Review on CeO2-Based Electrocatalyst and Photocatalyst in Energy Conversion. Adv. Energy Sustain. Res. 2021, 2, 2000063. [Google Scholar] [CrossRef]
- Salaev, M.A.; Salaeva, A.A.; Kharlamova, T.S.; Mamontov, G.V. Pt–CeO2-Based Composites in Environmental Catalysis: A Review. Appl. Catal. B Environ. 2021, 295, 120286. [Google Scholar] [CrossRef]
- Wang, W.; Wang, L.; Zheng, Z.; Zhou, Z.; Li, S.; Wang, J.; Chen, Y. Effect of Pd Chemical States on Three-Way Catalytic Reaction and C3H8 Total Combustion Reaction in Pd/CeO2-ZrO2-Al2O3 Catalysts. Fuel 2024, 372, 132052. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, S.; Feng, J.; Song, S.; Shi, W.; Wang, D.; Zhang, H. Unraveling the Physical Chemistry and Materials Science of CeO2-Based Nanostructures. Chem 2021, 7, 2022–2059. [Google Scholar] [CrossRef]
- Gu, W.; Wang, Z.; Kondamareddy, K.K.; Xie, L.; Yang, J.; Ho, W.; Khosla, A.; Lu, D. Assembling Lamellar G-C3N4 on a Hydrophobic PVDF Film Induced Spatial Electric Field to Construct a Piezotronic Effect-Enhanced CeO2/g-C3N4/PVDF Hybrid Flexible Film Photocatalyst. Appl. Mater. Today 2025, 44, 102744. [Google Scholar] [CrossRef]
- Bao, B.; Sun, Y.; Li, X.; Li, L.; Yu, Y. Tuning the UV Absorbing Ability of CeO2 Nanoparticles with F− Doping. FlatChem 2023, 39, 100494. [Google Scholar] [CrossRef]
- Rajendran, P.; Muthuraj, A.; Rajagounder, N.E. Review on CeO2-Based Corrosion Coatings. Trans. Indian Ceram. Soc. 2022, 81, 158–174. [Google Scholar] [CrossRef]
- Li, H.; Xia, P.; Pan, S.; Qi, Z.; Fu, C.; Yu, Z.; Kong, W.; Chang, Y.; Wang, K.; Wu, D.; et al. The Advances of Ceria Nanoparticles for Biomedical Applications in Orthopaedics. Int. J. Nanomed. 2020, 15, 7199–7214. [Google Scholar] [CrossRef]
- Godavarthi, S.; Kushvaha, S.S.; Saha, D.; Altaf, M.; Nallabala, N.K.R.; Yuvaraj, C.; Reddy, M.R.; Kesarla, M.K.; Bakash, K.R.; Krishna, G.G.; et al. Realization of CO2 Gas Sensors and Broadband Photodetectors Using Metal/High-k CeO2/p-Si Heterojunction. Ceram. Int. 2024, 50, 31845–31858. [Google Scholar] [CrossRef]
- Bhandari, S.; Valsalakumar, S.; Ali, M.S.; Mallick, T.K.; Hinshelwood, J.; Sundaram, S. Influence of Adjustable CeO2 Morphology on the Performance of Ambient Hole Transport Layer-Free Carbon-Based Perovskite Solar Cells. Energy Fuels 2025, 39, 9566–9575. [Google Scholar] [CrossRef]
- Dan, X.; Li, W.; Ning, F.; Wen, Q.; He, C.; Chai, Z.; Zhu, X.; Huang, W.; Zhou, X. Rare Earth Doped CeO2 Uniformly Enwraps Graphite Felt as High Current Density Electrode for All Vanadium Redox Flow Battery. J. Power Sources 2025, 628, 235921. [Google Scholar] [CrossRef]
- Bobade, R.G.; Pandit, B.; Khedulkar, A.P.; Raykar, V.S.; Sarawade, P.B.; Shaikh, S.F.; Huang, C.; Ambare, R.C. Electrochemical Investigation of Crinum Asiaticum-like BaO-CeO2 Nanostructure for High-Performance Asymmetric Supercapacitor. Inorg. Chem. Commun. 2025, 177, 114370. [Google Scholar] [CrossRef]
- Ueda, T.; Oide, N.; Kamada, K.; Hyodo, T.; Shimizu, Y. Improved Toluene Response of Mixed-Potential Type YSZ-Based Gas Sensors Using CeO2-Added Au Electrodes. ECS Sens. Plus 2022, 1, 013604. [Google Scholar] [CrossRef]
- Li, X.; Li, Z.; Chen, Y.; Zhao, K.; Jiang, Y.; Di, Y.; Liu, Y.; Zhou, C. High-Sensitivity CO Electrochemical Gas Sensor Based on a Superconductive C-Loaded CuO-CeO2 Nanocomposite Sensing Material. Mater. Sci. Eng. B 2021, 271, 115272. [Google Scholar] [CrossRef]
- Renganathan, B.; Rao, S.K.; Ganesan, A.R.; Deepak, A. Investigating the Gas Sensing Potential in CeO2 Fiber Optic Sensor via Trivalent Gadolinium Ion Substitution at Room Temperature. Mater. Lett. 2022, 325, 132766. [Google Scholar] [CrossRef]
- Joy, N.A.; Nandasiri, M.I.; Rogers, P.H.; Jiang, W.; Varga, T.; Kuchibhatla, S.V.N.T.; Thevuthasan, S.; Carpenter, M.A. Selective Plasmonic Gas Sensing: H2, NO2, and CO Spectral Discrimination by a Single Au-CeO2 Nanocomposite Film. Anal. Chem. 2012, 84, 5025–5034. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Huang, H.; Wang, L.; Cai, D.; Liu, B.; Wang, D.; Li, Q.; Wang, T. Electrospun CeO2 Nanoparticles/PVP Nanofibers Based High-Frequency Surface Acoustic Wave Humidity Sensor. Sens. Actuators B Chem. 2016, 223, 730–737. [Google Scholar] [CrossRef]
- Liu, Y.; Lei, Y. Pt-CeO2 Nanofibers Based High-Frequency Impedancemetric Gas Sensor for Selective CO and C3H8 Detection in High-Temperature Harsh Environment. Sens. Actuators B Chem. 2013, 188, 1141–1147. [Google Scholar] [CrossRef]
- Yu, S.; Jia, X.; Yang, J.; Wang, S.; Li, Y.; Song, H. Highly Sensitive and Low Detection Limit of Ethanol Gas Sensor Based on CeO2 Nanodot-Decorated ZnSnO3 Hollow Microspheres. Ceram. Int. 2022, 48, 14865–14875. [Google Scholar] [CrossRef]
- Pourfayaz, F.; Khodadadi, A.; Mortazavi, Y.; Mohajerzadeh, S.S. CeO2 Doped SnO2 Sensor Selective to Ethanol in Presence of CO, LPG and CH4. Sens. Actuators B Chem. 2005, 108, 172–176. [Google Scholar] [CrossRef]
- Singh, A.; Singh, S.; Yadav, B.C. Gigantic Enhancement in Response of Heterostructured CeO2/CdS Nanospheres Based Self-Powered CO2 Gas Sensor: A Comparative Study. Sens. Actuators B Chem. 2023, 377, 133085. [Google Scholar] [CrossRef]
- Xu, L.; Song, H.; Hu, J.; Lv, Y.; Xu, K. A Cataluminescence Gas Sensor for Triethylamine Based on Nanosized LaF3–CeO2. Sens. Actuators B Chem. 2012, 169, 261–266. [Google Scholar] [CrossRef]
- Steinhauer, S. Gas Sensors Based on Copper Oxide Nanomaterials: A Review. Chemosensors 2021, 9, 51. [Google Scholar] [CrossRef]
- Almaev, A.V.; Karipbayev, Z.T.; Kakimov, A.B.; Yakovlev, N.N.; Kukenov, O.I.; Korchemagin, A.O.; Akmetova-Abdik, G.A.; Kumarbekov, K.K.; Zhunusbekov, A.M.; Mochalov, L.A.; et al. High-Temperature Methane Sensors Based on ZnGa2O4:Er Ceramics for Combustion Monitoring. Technologies 2025, 13, 286. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor Metal Oxide Gas Sensors: A Review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Mirzaei, A.; Bharath, S.P.; Kim, J.-Y.; Pawar, K.K.; Kim, H.W.; Kim, S.S. N-Doped Graphene and Its Derivatives as Resistive Gas Sensors: An Overview. Chemosensors 2023, 11, 334. [Google Scholar] [CrossRef]
- Yan, Z.; Zhang, Y.; Kang, W.; Deng, N.; Pan, Y.; Sun, W.; Ni, J.; Kang, X. TiO2 Gas Sensors Combining Experimental and DFT Calculations: A Review. Nanomaterials 2022, 12, 3611. [Google Scholar] [CrossRef]
- Spencer, M.J.S. Gas Sensing Applications of 1D-Nanostructured Zinc Oxide: Insights from Density Functional Theory Calculations. Prog. Mater. Sci. 2012, 57, 437–486. [Google Scholar] [CrossRef]
- Cruz-Martínez, H.; Rojas-Chávez, H.; Montejo-Alvaro, F.; Peña-Castañeda, Y.A.; Matadamas-Ortiz, P.T.; Medina, D.I. Recent Developments in Graphene-Based Toxic Gas Sensors: A Theoretical Overview. Sensors 2021, 21, 1992. [Google Scholar] [CrossRef]
- Fu, M.; Ma, Z.; Gao, C.; Ye, Y.; Li, W.; Hou, D.; Cao, Y. A Multi-Functional VOC Sensor Based on Cascaded Quartz Crystal Resonators. IEEE Electron Device Lett. 2025, 46, 476–479. [Google Scholar] [CrossRef]
- Navale, S.; Shahbaz, M.; Mirzaei, A.; Kim, S.S.; Kim, H.W. Effect of Ag Addition on the Gas-Sensing Properties of Nanostructured Resistive-Based Gas Sensors: An Overview. Sensors 2021, 21, 6454. [Google Scholar] [CrossRef]
- Ansari, H.R.; Mirzaei, A.; Shokrollahi, H.; Kumar, R.; Kim, J.-Y.; Kim, H.W.; Kumar, M.; Kim, S.S. Flexible/Wearable Resistive Gas Sensors Based on 2D Materials. J. Mater. Chem. C 2023, 11, 6528–6549. [Google Scholar] [CrossRef]
- Sonawane, L.D.; Mandawade, A.S.; Bhoye, L.N.; Ahemad, H.I.; Tayade, S.S.; Aher, Y.B.; Gite, A.B.; Nikam, L.K.; Shinde, S.D.; Jain, G.H.; et al. Sol-Gel and Hydrothermal Synthesis of CeO2 NPs: Their Physiochemical Properties and Applications for Gas Sensor with Photocatalytic Activities. Inorg. Chem. Commun. 2024, 164, 112313. [Google Scholar] [CrossRef]
- Izu, N.; Shin, W.; Murayama, N.; Kanzaki, S. Resistive Oxygen Gas Sensors Based on CeO2 Fine Powder Prepared Using Mist Pyrolysis. Sens. Actuators B Chem. 2002, 87, 95–98. [Google Scholar] [CrossRef]
- Kapuscik, P.; Wojcieszak, D.; Domaradzki, J.; Weichbrodt, W.; Mazur, M.; Chodasewicz, P.; Kosto, Y.; Morales, C.; Flege, I. Analysis of Gas Sensing Properties of Thin Film Coating Based on Cerium Oxides. In Proceedings of the 17 th International Conference on Optical and Electronic Sensors COE 2024, Wrocław, Poland, 24–26 June 2024. [Google Scholar]
- Li, P.; Wang, B.; Qin, C.; Han, C.; Sun, L.; Wang, Y. Band-Gap-Tunable CeO2 Nanoparticles for Room-Temperature NH3 Gas Sensors. Ceram. Int. 2020, 46, 19232–19240. [Google Scholar] [CrossRef]
- Li, H.; Qu, Y.; Zhang, X. The Gas Sensor Utilizing CeO2 Nanorods for the Low Temperature Detection of Hydrogen. Inorg. Chem. Commun. 2021, 130, 108692. [Google Scholar] [CrossRef]
- Hussain, S.; Aslam, N.; Yang, X.Y.; Javed, M.S.; Xu, Z.; Wang, M.; Liu, G.; Qiao, G. Unique Polyhedron CeO2 Nanostructures for Superior Formaldehyde Gas-Sensing Performances. Ceram. Int. 2018, 44, 19624–19630. [Google Scholar] [CrossRef]
- Bi, H.; Zhang, L.-X.; Xing, Y.; Zhang, P.; Chen, J.-J.; Yin, J.; Bie, L.-J. Morphology-Controlled Synthesis of CeO2 Nanocrystals and Their Facet-Dependent Gas Sensing Properties. Sens. Actuators B Chem. 2021, 330, 129374. [Google Scholar] [CrossRef]
- Lyu, L.; Xie, Q.; Yang, Y.; Wang, R.; Cen, W.; Luo, S.; Yang, W.; Gao, Y.; Xiao, Q.; Zou, P.; et al. A Novel CeO2 Hollow-Shell Sensor Constructed for High Sensitivity of Acetone Gas Detection. Appl. Surf. Sci. 2022, 571, 151337. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kordrostami, Z.; Shahbaz, M.; Kim, J.-Y.; Kim, H.W.; Kim, S.S. Resistive-Based Gas Sensors Using Quantum Dots: A Review. Sensors 2022, 22, 4369. [Google Scholar] [CrossRef]
- Rohit; Kaur, S.; Hussain, S.; Park, J.Y.; Katoch, V.; Parkash, B.; Katoch, A.; Jamwal, D. Size Dependent Dual Functionality of CeO2 Quantum Dots: A Correlation among Parameters for Hydrogen Gas Sensor and Pollutant Remediation. Chemosphere 2024, 364, 142959. [Google Scholar] [CrossRef]
- Matussin, S.N.; Harunsani, M.H.; Khan, M.M. CeO2 and CeO2-Based Nanomaterials for Photocatalytic, Antioxidant and Antimicrobial Activities. J. Rare Earths 2023, 41, 167–181. [Google Scholar] [CrossRef]
- Dong, Z.; Hu, Q.; Liu, H.; Wu, Y.; Ma, Z.; Fan, Y.; Li, R.; Xu, J.; Wang, X. 3D Flower-like Ni Doped CeO2 Based Gas Sensor for H2S Detection and Its Sensitive Mechanism. Sens. Actuators B Chem. 2022, 357, 131227. [Google Scholar] [CrossRef]
- Zheng, L.; Regenstein, J.M.; Teng, F.; Li, Y. Tofu products: A review of their raw materials, processing conditions, and packaging. Compr. Rev. Food Sci. Food Saf. 2020, 19, 3683–3714. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Zhang, H.; Chen, C. Preparation and Freshness Detection of Tofu Based on In-Doped CeO2 Ammonia Gas Sensor. Appl. Surf. Sci. 2024, 669, 160506. [Google Scholar] [CrossRef]
- Wu, K.; Debliquy, M.; Zhang, C. Room Temperature Gas Sensors Based on Ce Doped TiO2 Nanocrystals for Highly Sensitive NH3 Detection. Chem. Eng. J. 2022, 444, 136449. [Google Scholar] [CrossRef]
- Kumar, D.K.; Bharathi, P.; Govind, A.; Archana, J.; Navaneethan, M.; Harish, S. Detection of NO2 at Ppm-Level Using Al-Doped CeO2 Based Gas Sensor with High Sensitivity and Selectivity at Room Temperature. J. Environ. Chem. Eng. 2024, 12, 112253. [Google Scholar] [CrossRef]
- Duan, Q.; Zhang, W.; Li, L.; Ying, B.; Li, T.; Chen, B.; Li, H.-Y.; Liu, H. W-CeO2 Nanospheres Gas Sensor Array for Accurate and Selective H2S Detection in Exhaled Breath. Chem. Eng. J. 2024, 479, 147748. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. Resistive-Based Gas Sensors for Detection of Benzene, Toluene and Xylene (BTX) Gases: A Review. J. Mater. Chem. C 2018, 6, 4342–4370. [Google Scholar] [CrossRef]
- Qin, R.; Yuan, Q.; Yu, J.; Hu, J.; Zhang, W.; Wang, Y.; Cao, Y.; Ma, Q.; Li, S.; Li, G.; et al. WO3/Ru@CeO2 Bilayer Gas Sensor for Ppb-Level Xylene Detection Based on a Catalytic-Sensitive Synergistic Mechanism. ACS Appl. Mater. Interfaces 2025, 17, 16920–16931. [Google Scholar] [CrossRef]
- Venkatraman, M.; Kadian, A.; Ganesan, A.; Dong, C.-L.; Singh, A.; Dev, K.; Selvaraj, M.; Subramanian, A.; Marappan, S. Ru-Doped CeO2 Nanoparticles as Sensing Materials for the Detection of Formaldehyde. ACS Appl. Nano Mater. 2025, 8, 4680–4693. [Google Scholar] [CrossRef]
- Jin, C.; Kim, S.; Kim, D.E.; Mirzaei, A.; Roh, J.W.; Choi, S.-W.; Choi, M.S. Gadolinium-Doped CeO2 Gas Sensor for H2S Sensing. Korean J. Met. Mater. 2023, 61, 414–421. [Google Scholar] [CrossRef]
- Baier, D.; Priamushko, T.; Weinberger, C.; Kleitz, F.; Tiemann, M. Selective Discrimination between CO and H2 with Copper–Ceria-Resistive Gas Sensors. ACS Sens. 2023, 8, 1616–1623. [Google Scholar] [CrossRef] [PubMed]
- Zhou, F.; Xin, Q.; Fu, Y.; Hua, Z.; Dong, Y.; Ran, M.; Song, H.; Liu, S.; Qu, R.; Yang, Y.; et al. Efficient Catalytic Oxidation of Chlorinated Volatile Organic Compounds over RuO2-WOx/Sn0.2Ti0.8O2 Catalysts: Insight into the Cl Poisoning Mechanism of Acid Sites. Chem. Eng. J. 2023, 464, 142471. [Google Scholar] [CrossRef]
- Wang, C.; Zhang, L.; He, B.; Zhou, Q.; Zhang, S.-H.; Kong, X.-L.; Chen, Z.; Pan, G.-B. Three-Dimensional CeO2@carbon-Quantum-Dots Scaffold Modified with Au Nanoparticles on Flexible Substrates for High Performance Gas Sensing at Room Temperature. Rare Met. 2023, 42, 1946–1958. [Google Scholar] [CrossRef]
- Hashtroudi, H.; Yu, A.; Juodkazis, S.; Shafiei, M. Ultra-Sensitive Photo-Induced Hydrogen Gas Sensor Based on Two-Dimensional CeO2-Pd-PDA/rGO Heterojunction Nanocomposite. Nanomaterials 2022, 12, 1628. [Google Scholar] [CrossRef]
- Zakaria, S.A.; Amini, M.H.; Ahmadi, S.H. CeO2/Ni–Al Layered Double Hydroxide Composite Decorated with Ag Nanoparticles as a Gas Sensor. Mater. Sci. Semicond. Process. 2024, 178, 108391. [Google Scholar] [CrossRef]
- Liao, L.; Mai, H.X.; Yuan, Q.; Lu, H.B.; Li, J.C.; Liu, C.; Yan, C.H.; Shen, Z.X.; Yu, T. Single CeO2 Nanowire Gas Sensor Supported with Pt Nanocrystals: Gas Sensitivity, Surface Bond States, and Chemical Mechanism. J. Phys. Chem. C 2008, 112, 9061–9065. [Google Scholar] [CrossRef]
- Drobek, M.; Kim, J.-H.; Bechelany, M.; Vallicari, C.; Julbe, A.; Kim, S.S. MOF-Based Membrane Encapsulated ZnO Nanowires for Enhanced Gas Sensor Selectivity. ACS Appl. Mater. Interfaces 2016, 8, 8323–8328. [Google Scholar] [CrossRef]
- Me’nil, F.; Lucat, C.; Debe’da, H. The Thick-Film Route to Selective Gas Sensors. Sens. Actuators B Chem. 1995, 25, 415–420. [Google Scholar] [CrossRef]
- Li, H.; Wu, R.; Tian, X.; Han, L.; Chen, T.; Yang, B.; Zhi, Z.; Hua, Z.; Fan, S. A Catalytic Filter Based on Pt-CeO2 for Selective Methane Detection with SnO2 Sensors. Sens. Actuators B Chem. 2023, 389, 133872. [Google Scholar] [CrossRef]
- Kim, J.-H.; Mirzaei, A.; Osada, M.; Kim, H.W.; Kim, S.S. Hydrogen Sensing Characteristics of Pd-Decorated Ultrathin ZnO Nanosheets. Sens. Actuators B Chem. 2021, 329, 129222. [Google Scholar] [CrossRef]
- Mirzaei, A.; Yousefi, H.R.; Falsafi, F.; Bonyani, M.; Lee, J.-H.; Kim, J.-H.; Kim, H.W.; Kim, S.S. An Overview on How Pd on Resistive-Based Nanomaterial Gas Sensors Can Enhance Response toward Hydrogen Gas. Int. J. Hydrogen Energy 2019, 44, 20552–20571. [Google Scholar] [CrossRef]
- Wang, Y.; Yu, M.; Fan, Y.; Sun, X.; Lei, R.; Guo, L.; Cao, J.; Qin, C. Visible Light-Assisted Room-Temperature Hydrogen Sensors Using Pd/CeO2/ZnO Nanocomposites. Int. J. Hydrogen Energy 2025, 149, 150121. [Google Scholar] [CrossRef]
- Liu, J.; Dai, M.; Wang, T.; Sun, P.; Liang, X.; Lu, G.; Shimanoe, K.; Yamazoe, N. Enhanced Gas Sensing Properties of SnO2 Hollow Spheres Decorated with CeO2 Nanoparticles Heterostructure Composite Materials. ACS Appl. Mater. Interfaces 2016, 8, 6669–6677. [Google Scholar] [CrossRef]
- Hui, G.; Zhu, M.; Yang, X.; Liu, J.; Pan, G.; Wang, Z. Highly Sensitive Ethanol Gas Sensor Based on CeO2/ZnO Binary Heterojunction Composite. Mater. Lett. 2020, 278, 128453. [Google Scholar] [CrossRef]
- Al-Kuhaili, M.F.; Durrani, S.M.A.; Bakhtiari, I.A. Carbon Monoxide Gas-Sensing Properties of CeO2–ZnO Thin Films. Appl. Surf. Sci. 2008, 255, 3033–3039. [Google Scholar] [CrossRef]
- Kabure, A.A.; Shirke, B.S.; Mane, S.R.; Garadkar, K.M. Microwave-Assisted Sol-Gel Synthesis of CeO2–NiO Nanocomposite Based NO2 Gas Sensor for Selective Detection at Lower Operating Temperature. J. Indian Chem. Soc. 2022, 99, 100369. [Google Scholar] [CrossRef]
- Takte, M.A.; Ingle, N.N.; Dole, B.N.; Tsai, M.-L.; Hianik, T.; Shirsat, M.D. A Stable and Highly-Sensitive Flexible Gas Sensor Based on Ceria (CeO2) Nano-Cube Decorated rGO Nanosheets for Selective Detection of NO2 at Room Temperature. Synth. Met. 2023, 297, 117411. [Google Scholar] [CrossRef]
- Qin, W.; Xu, L.; Song, J.; Xing, R.; Song, H. Highly Enhanced Gas Sensing Properties of Porous SnO2–CeO2 Composite Nanofibers Prepared by Electrospinning. Sens. Actuators B Chem. 2013, 185, 231–237. [Google Scholar] [CrossRef]
- Yan, S.; Liang, X.; Song, H.; Ma, S.; Lu, Y. Synthesis of Porous CeO2-SnO2 Nanosheets Gas Sensors with Enhanced Sensitivity. Ceram. Int. 2018, 44, 358–363. [Google Scholar] [CrossRef]
- Amoresi, R.A.C.; de Oliveira, R.C.; Cichetto, L.; Desimone, P.M.; Aldao, C.M.; Ponce, M.A.; Gracia, L.; Sambrano, J.R.; Longo, E.; Andres, J.; et al. Pure and Ni2O3-Decorated CeO2 Nanoparticles Applied as CO Gas Sensor: Experimental and Theoretical Insights. Ceram. Int. 2022, 48, 14014–14025. [Google Scholar] [CrossRef]
- Kabure, A.A.; Shirke, B.S.; Mane, S.R.; Garadkar, K.M.; Sargar, B.M.; Pakhare, K.S. LPG Gas Sensor Activities of CeO2-Fe2O3 Nanocomposite Thin Film at Optimum Temperature. Appl. Phys. A 2021, 127, 711. [Google Scholar] [CrossRef]
- Naganaboina, V.R.; Singh, S.G. Graphene-CeO2 Based Flexible Gas Sensor: Monitoring of Low Ppm CO Gas with High Selectivity at Room Temperature. Appl. Surf. Sci. 2021, 563, 150272. [Google Scholar] [CrossRef]
- Zhang, L.; Fang, Q.; Huang, Y.; Xu, K.; Chu, P.K.; Ma, F. Oxygen Vacancy Enhanced Gas-Sensing Performance of CeO2/Graphene Heterostructure at Room Temperature. Anal. Chem. 2018, 90, 9821–9829. [Google Scholar] [CrossRef]
- Farea, M.A.; Mohammed, H.Y.; Shirsat, S.M.; Sayyad, P.W.; Ingle, N.N.; Al-Gahouari, T.; Mahadik, M.M.; Bodkhe, G.A.; Shirsat, M.D. Hazardous Gases Sensors Based on Conducting Polymer Composites: Review. Chem. Phys. Lett. 2021, 776, 138703. [Google Scholar] [CrossRef]
- Liu, X.; Zheng, W.; Kumar, R.; Kumar, M.; Zhang, J. Conducting Polymer-Based Nanostructures for Gas Sensors. Coord. Chem. Rev. 2022, 462, 214517. [Google Scholar] [CrossRef]
- Wen, J.; Wang, S.; Feng, J.; Ma, J.; Zhang, H.; Wu, P.; Li, G.; Wu, Z.; Meng, F.; Li, L.; et al. Recent Progress in Polyaniline-Based Chemiresistive Flexible Gas Sensors: Design, Nanostructures, and Composite Materials. J. Mater. Chem. A 2024, 12, 6190–6210. [Google Scholar] [CrossRef]
- Esmaeili, C.; Ashtiani, S.; Regmi, C.; Laposa, A.; Voves, J.; Kroutil, J.; Friess, K.; Povolny, V.; Lotfian, S. Preparation and Characterisation of NH3 Gas Sensor Based on PANI/Fe-Doped CeO2 Nanocomposite. Heliyon 2024, 10, e34801. [Google Scholar] [CrossRef]
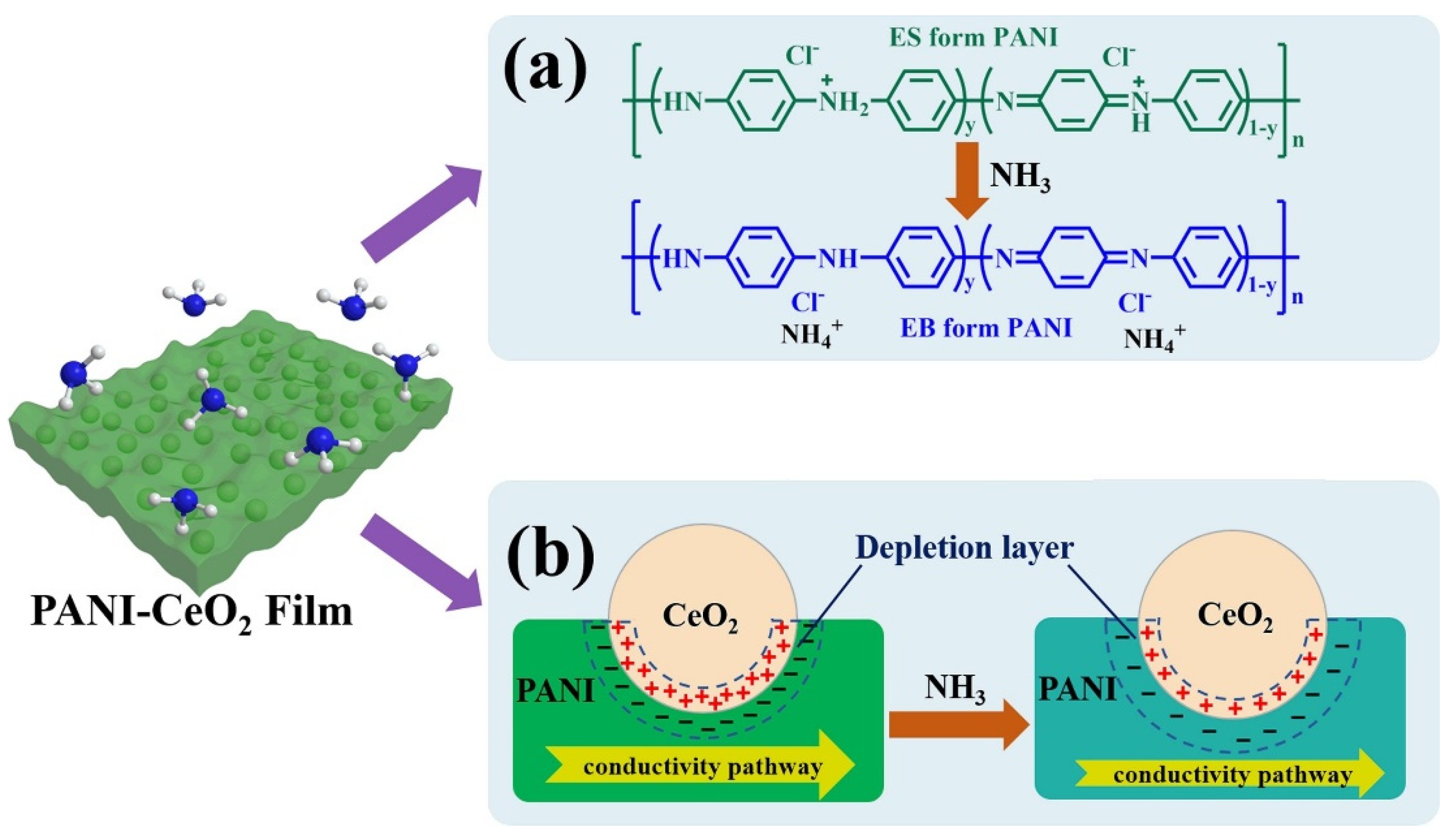
- Liu, C.; Tai, H.; Zhang, P.; Yuan, Z.; Du, X.; Xie, G.; Jiang, Y. A High-Performance Flexible Gas Sensor Based on Self-Assembled PANI-CeO2 Nanocomposite Thin Film for Trace-Level NH3 Detection at Room Temperature. Sens. Actuators B Chem. 2018, 261, 587–597. [Google Scholar] [CrossRef]
- Ema, T.; Choi, P.G.; Takami, S.; Masuda, Y. Facet-Controlled Synthesis of CeO2 Nanoparticles for High-Performance CeO2 Nanoparticle/SnO2 Nanosheet Hybrid Gas Sensors. ACS Appl. Mater. Interfaces 2022, 14, 56998–57007. [Google Scholar] [CrossRef] [PubMed]
- Motaung, D.E.; Mhlongo, G.H.; Makgwane, P.R.; Dhonge, B.P.; Cummings, F.R.; Swart, H.C.; Ray, S.S. Ultra-High Sensitive and Selective H2 Gas Sensor Manifested by Interface of n–n Heterostructure of CeO2-SnO2 Nanoparticles. Sens. Actuators B Chem. 2018, 254, 984–995. [Google Scholar] [CrossRef]
- Oosthuizen, D.N.; Motaung, D.E.; Swart, H.C. Gas Sensors Based on CeO2 Nanoparticles Prepared by Chemical Precipitation Method and Their Temperature-Dependent Selectivity towards H2S and NO2 Gases. Appl. Surf. Sci. 2020, 505, 144356. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Bang, J.H.; Kim, H.W.; Kim, S.S. Selective H2S sensing without external heat by a synergy effect in self-heated CuO-functionalized SnO2-ZnO core-shell nanowires. Sens. Actuators B Chem. 2019, 300, 126981. [Google Scholar] [CrossRef]
- Shin, K.Y.; Nguyen, L.H.T.; Nguyen, H.L.; Mirzaei, A.; Tran, V.N.H.; Mai, N.X.D.; Tran, N.Q.; Oum, W.; Kim, E.B.; Kim, H.M.; et al. Titanium-Based Metal-Organic-Framework-Coated SnO2 Nanowires with Enhanced NO2 Gas Sensing Capability in Humid Environment. Sens. Actuators B Chem. 2023, 394, 134425. [Google Scholar] [CrossRef]
- Bang, J.H.; Kwon, Y.J.; Lee, J.-H.; Mirzaei, A.; Lee, H.Y.; Choi, H.; Kim, S.S.; Jeong, Y.K.; Kim, H.W. Proton-Beam Engineered Surface-Point Defects for Highly Sensitive and Reliable NO2 Sensing under Humid Environments. J. Hazard. Mater. 2021, 416, 125841. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Chang, X.; Tang, S.; Chen, X.; Gao, W.; Niu, S.; Li, J.; Jiang, Y.; Sun, S. Humidity-Tolerant Chemiresistive Gas Sensors Based on Hydrophobic CeO2/SnO2 Heterostructure Films. ACS Appl. Mater. Interfaces 2022, 14, 25680–25692. [Google Scholar] [CrossRef]
- Ng, W.C.; Yaw, C.S.; Shaffee, S.N.A.; Samad, N.A.A.; Koi, Z.K.; Chong, M.N. Elevating the Prospects of Green Hydrogen (H2) Production through Solar-Powered Water Splitting Devices: A Systematic Review. Sustain. Mater. Technol. 2024, 40, e00972. [Google Scholar] [CrossRef]
- Alamri, M. A Review of Chemoresistive Hydrogen Gas Sensors Based on Low-Dimensional Materials. Arab. J. Sci. Eng. 2025, 50, 6195–6219. [Google Scholar] [CrossRef]
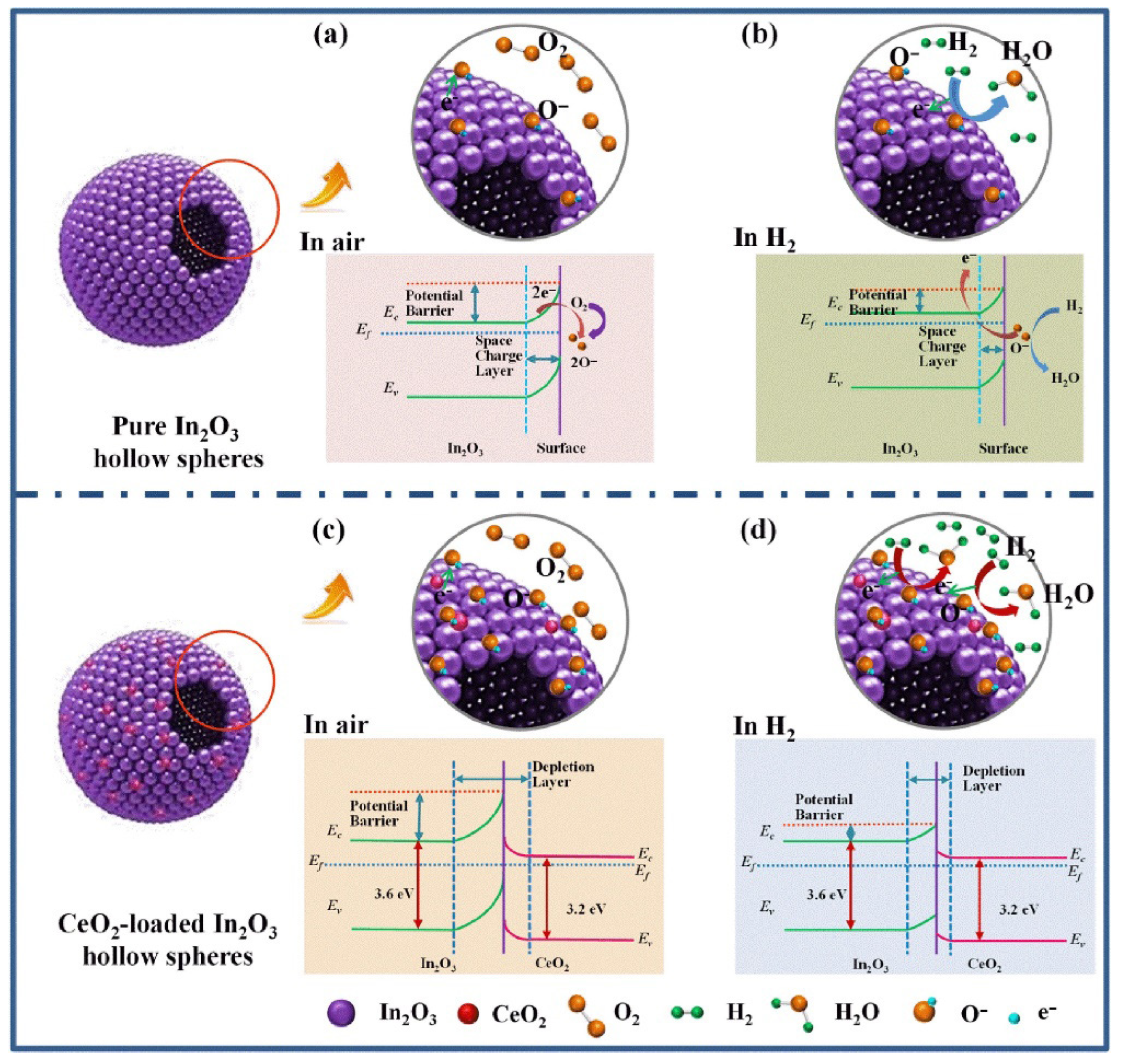
- Hu, J.; Sun, Y.; Xue, Y.; Zhang, M.; Li, P.; Lian, K.; Zhuiykov, S.; Zhang, W.; Chen, Y. Highly Sensitive and Ultra-Fast Gas Sensor Based on CeO2-Loaded In2O3 Hollow Spheres for Ppb-Level Hydrogen Detection. Sens. Actuators B Chem. 2018, 257, 124–135. [Google Scholar] [CrossRef]
- Yuan, K.; Wang, C.-Y.; Zhu, L.-Y.; Cao, Q.; Yang, J.-H.; Li, X.-X.; Huang, W.; Wang, Y.-Y.; Lu, H.-L.; Zhang, D.W. Fabrication of a Micro-Electromechanical System-Based Acetone Gas Sensor Using CeO2 Nanodot-Decorated WO3 Nanowires. ACS Appl. Mater. Interfaces 2020, 12, 14095–14104. [Google Scholar] [CrossRef]
- Neri, G.; Bonavita, A.; Rizzo, G.; Galvagno, S.; Capone, S.; Siciliano, P. Methanol Gas-Sensing Properties of CeO2–Fe2O3 Thin Films. Sens. Actuators B Chem. 2006, 114, 687–695. [Google Scholar] [CrossRef]
- Li, M.; Ren, W.; Wu, R.; Zhang, M. CeO2 Enhanced Ethanol Sensing Performance in a CdS Gas Sensor. Sensors 2017, 17, 1577. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, Z.; Zhang, S.; Yan, S.; Cao, B.; Wang, Z.; Fu, Y. Enhanced NH3 Gas-Sensing Performance of Silica Modified CeO2 Nanostructure Based Sensors. Sens. Actuators B Chem. 2018, 255, 862–870. [Google Scholar] [CrossRef]
- Hjiri, M.; Benmansour, N.; Alonizan, N.H.; Neri, G. Ppt Level Gas Sensing by Resistive Gas Sensors: A Review. Microchem. J. 2025, 213, 113670. [Google Scholar] [CrossRef]
- Fang, H.; Shang, E.; Wang, D.; Ma, X.; Zhao, B.; Han, C.; Zheng, C. A Chemiresistive Ppt Level NO2 Gas Sensor Based on CeO2 Nanoparticles Modified CuO Nanosheets Operated at 100 °C. Sens. Actuators B Chem. 2023, 393, 134277. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Han, L.; Li, X.; Xu, Y. Highly Sensitive and Selective Triethylamine Gas Sensor Based on Hierarchical Radial CeO2/ZnO n-n Heterojunction. Sens. Actuators B Chem. 2022, 367, 132031. [Google Scholar] [CrossRef]
- El-Muraikhi, M.D.; Ayesh, A.I.; Mirzaei, A. Review of Nanostructured Bi2O3, Bi2WO6, and BiVO4 as Resistive Gas Sensors. Surf. Interfaces 2025, 60, 106003. [Google Scholar] [CrossRef]
- Meng, X.; Kang, S.; Zhao, Z.; Jin, G.; Shao, Z.; Wu, L. Core-Shell Bi2O3/CeO2 Heterojunction for Enhanced Formaldehyde Gas Sensor. Ceram. Int. 2025, 51, 6067–6077. [Google Scholar] [CrossRef]
- Singh, S.; Shin, K.Y.; Moon, S.; Kim, S.S.; Kim, H.W. Phase-Engineered MoSe2/CeO2 Composites for Room-Temperature Gas Sensing with a Drastic Discrimination of NH3 and TEA Gases. ACS Sens. 2024, 9, 3994–4006. [Google Scholar] [CrossRef]
- Zhou, Q.; Geng, Z.; Yang, L.; Shen, B.; Kan, Z.; Qi, Y.; Hu, S.; Dong, B.; Bai, X.; Xu, L.; et al. A Wearable Healthcare Platform Integrated with Biomimetical Ions Conducted Metal–Organic Framework Composites for Gas and Strain Sensing in Non-Overlapping Mode. Adv. Sci. 2023, 10, 2207663. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Fu, G.; Xu, P.; Fan, C.; Shen, L.; Yang, G.; Wen, C.; Liu, W. Ultrasensitive Detection of Dimethylamine Gas for Early Diagnosis of Parkinson’s Disease Using CeO2-Coated Ti3C2Tx MXene/Carbon Nanofibers. ACS Sens. 2024, 9, 6400–6410. [Google Scholar] [CrossRef]
- Shin, K.Y.; Kim, Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Bimetal-Decorated Resistive Gas Sensors: A Review. J. Mater. Chem. C 2025, 13, 9930–9950. [Google Scholar] [CrossRef]
- Park, H.; Kim, J.-H.; Vivod, D.; Kim, S.; Mirzaei, A.; Zahn, D.; Park, C.; Kim, S.S.; Halik, M. Chemical-Recognition-Driven Selectivity of SnO2-Nanowire-Based Gas Sensors. Nano Today 2021, 40, 101265. [Google Scholar] [CrossRef]
- Niu, G.; Wang, F. A Review of MEMS-Based Metal Oxide Semiconductors Gas Sensor in Mainland China. J. Micromech. Microeng. 2022, 32, 054003. [Google Scholar] [CrossRef]
- Bhattacharyya, P. Technological Journey Towards Reliable Microheater Development for MEMS Gas Sensors: A Review. IEEE Trans. Device Mater. Reliab. 2014, 14, 589–599. [Google Scholar] [CrossRef]
| Amount of Oxygen Vacancies | Stability | Adsorption Capacity |
|---|---|---|
| {100} > {110} > {111} | {111} > {110} > {100} | {100} > {110} > {111} |
| Sensing Material | Gas | Conc (ppm) | T(°C) | Response (Ra/Rg) or (Rg/Ra) or [(ΔR)/Ra) × 100] | Ref |
|---|---|---|---|---|---|
| CeO2 NPs | NH3 | 500 | RT | 23 | [45] |
| CeO2 NRs | H2 | 1000 | 100 | 6 | [46] |
| CeO2 nanostructures | TEA | 100 | 100 | 150 | [47] |
| CeO2 nanopolyhedra | DMA | 100 | 300 | 150 | [48] |
| hollow CeO2 microspheres | Acetone | 100 | 260 | 270% | [49] |
| CeO2 QDs | H2 | 10 | 220 | 4.2 | [51] |
| Ni-doped CeO2 | H2S | 0.5 | 200 | 3.2 | [53] |
| In-doped CeO2 nanostructures | NH3 | 1000 | RT | 5412.93 | [55] |
| Ce-doped TiO2 nanostructures | NH3 | 20 | RT | 25 | [56] |
| W6+-doped CeO2 NPs | H2S | 10 | 225 | 18.6 | [58] |
| Ru-doped CeO2 NSs | Xylene | 5 | 160 | 37 | [60] |
| Pt loaded CeO2 -porous SnO2 | CH4 | 1000 | 350 | 12.4 | [71] |
| Pd-decorated CeO2-ZnO NRs composite | H2 | 1000 | RT | 300 | [74] |
| graphene-CeO2 nanocomposite | CO | 10 | RT | 1.5% | [84] |
| CeO2-graphene nanocomposite | NO2 | 5 | RT | 10.6% | [85] |
| PANI-CeO2 nanocomposite | NH3 | 10 | RT | 82% | [90] |
| {100} CeO2 NCs/SnO2 NS | Acetone | 20 | 550 | 14.8 | [91] |
| SnO2-CeO2 nanocomposite | H2 | 60 | 300 | 1323 | [92] |
| CeO2-SnO2 nanocomposite | TEA | 20 | 200 | 22.5 | [97] |
| CeO2-In2O3 hollow composite spheres | H2 | 50 | 160 | 20.7 | [100] |
| CeO2 nanodot-decorated WO3 NWs | Acetone | 0.5 | 1.30 | [101] | |
| CeO2-Fe2O3 nanocomposite | Methanol | 100 | 400 | 4 | [102] |
| CeO2-CdS composite NWs | Acetone | 100 | 160 | 52 | [103] |
| SiO2 modified CeO2 NPs | NH3 | 80 | RT | 3245% | [104] |
| CeO2-decorated CuO NSs | NO2 | 20 | 100 | 6.96 | [106] |
| CeO2-ZnO composites | TEA | 100 | 92 | 92 | [107] |
| Bi2O3-CeO2 core-shell nanocomposites | HCHO | 100 | RT | 137 | [109] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torkamani Cheriani, M.; Mirzaei, A.; Kim, J.-H. Resistive-Based Nanostructured CeO2 Gas Sensors: A Review. Chemosensors 2025, 13, 298. https://doi.org/10.3390/chemosensors13080298
Torkamani Cheriani M, Mirzaei A, Kim J-H. Resistive-Based Nanostructured CeO2 Gas Sensors: A Review. Chemosensors. 2025; 13(8):298. https://doi.org/10.3390/chemosensors13080298
Chicago/Turabian StyleTorkamani Cheriani, Mahmoud, Ali Mirzaei, and Jae-Hun Kim. 2025. "Resistive-Based Nanostructured CeO2 Gas Sensors: A Review" Chemosensors 13, no. 8: 298. https://doi.org/10.3390/chemosensors13080298
APA StyleTorkamani Cheriani, M., Mirzaei, A., & Kim, J.-H. (2025). Resistive-Based Nanostructured CeO2 Gas Sensors: A Review. Chemosensors, 13(8), 298. https://doi.org/10.3390/chemosensors13080298







