Microfluidic Paper-Based Sensors and Their Applications for Glucose Sensing
Abstract
1. Introduction
2. Recent Development of the Paper-Based Glucose Sensor and Their Application in Glucose Sensing
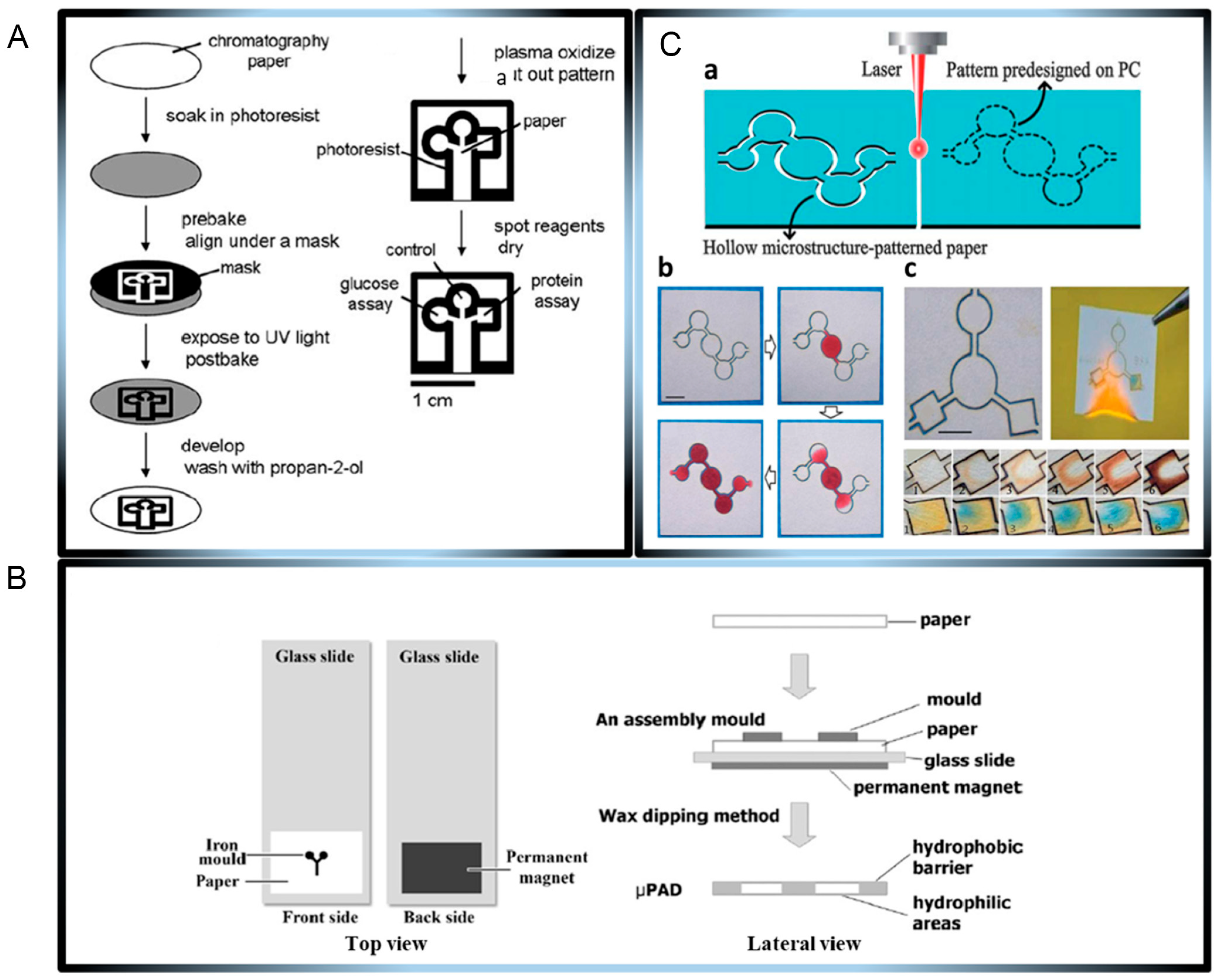
2.1. Overview of Microfluidic Paper-Based Sensors
2.2. Overview of Microfluidic Paper-Based Glucose Sensors
2.3. Signal Analysis and Technological Relevance
2.3.1. Paper-Based Colorimetric Sensor
2.3.2. Paper-Based Electrochemical Sensor
2.3.3. Paper-Based Mass Spectrometry
3. Study of the Paper-Based Glucose Sensor Advancement Through Typical Examples
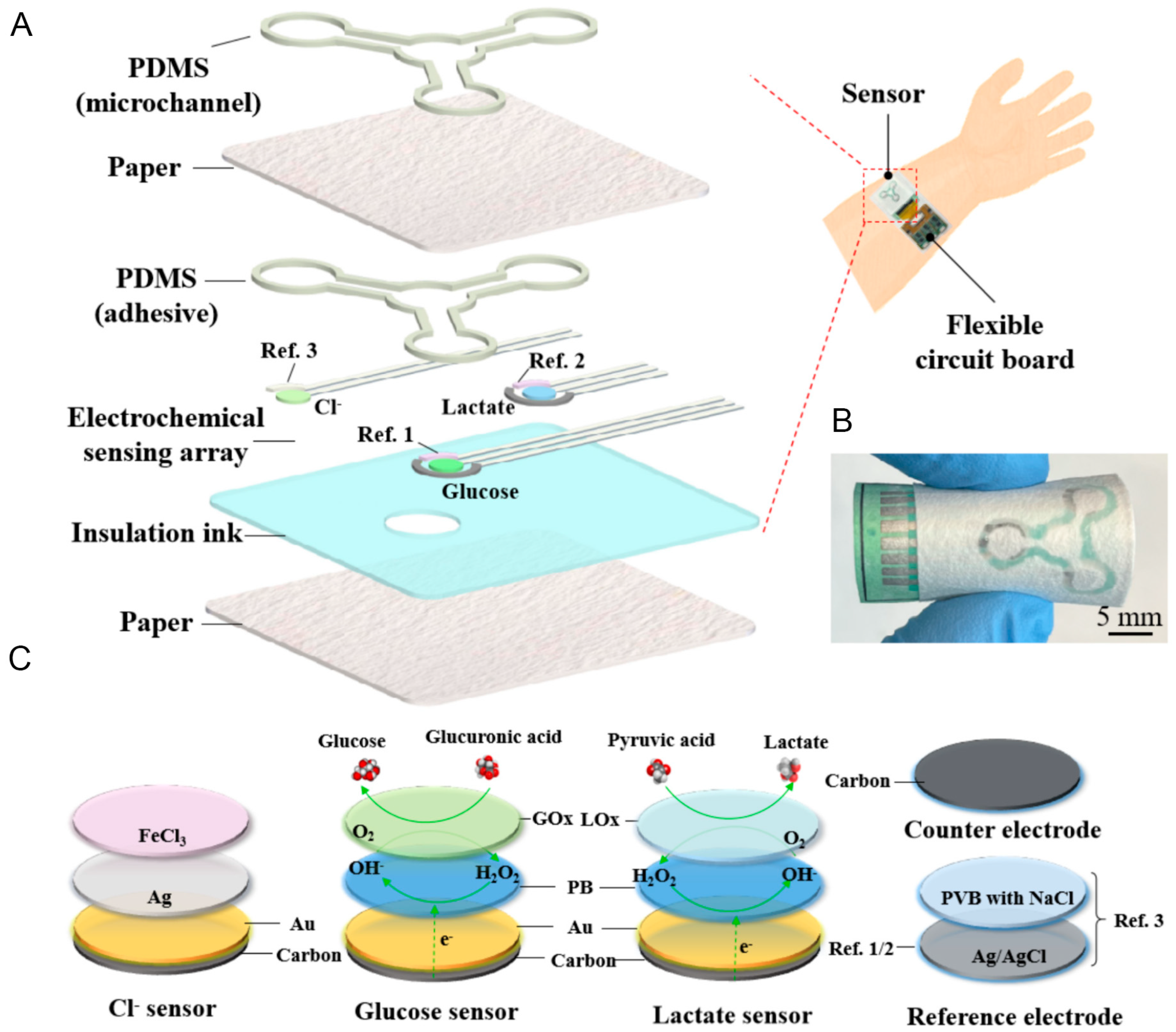
3.1. Recent Development of Enzymatic Microfluidic Paper-Based Glucose Sensor
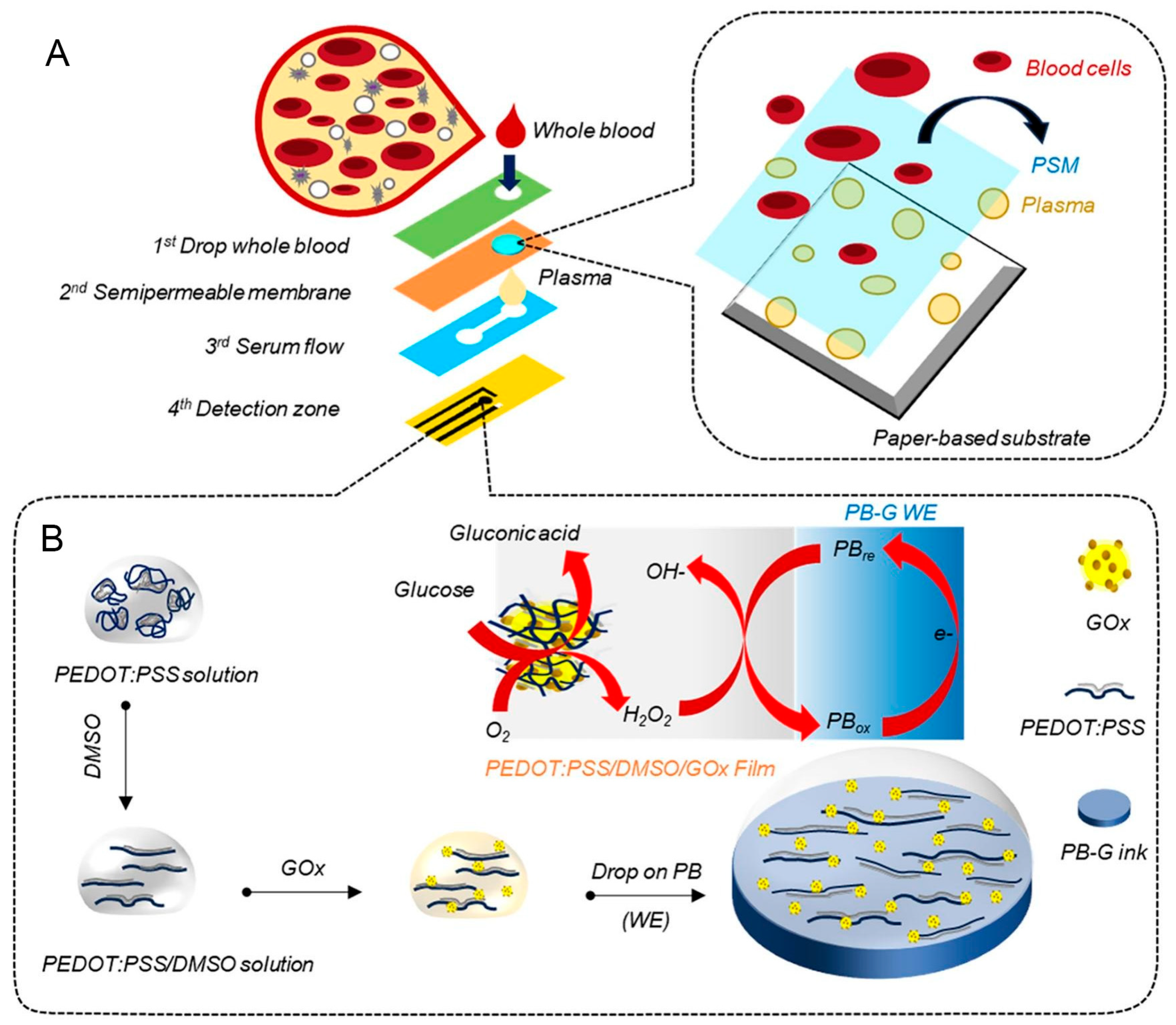
3.2. Recent Development of Hybrid Microfluidic Paper-Based Glucose Sensor
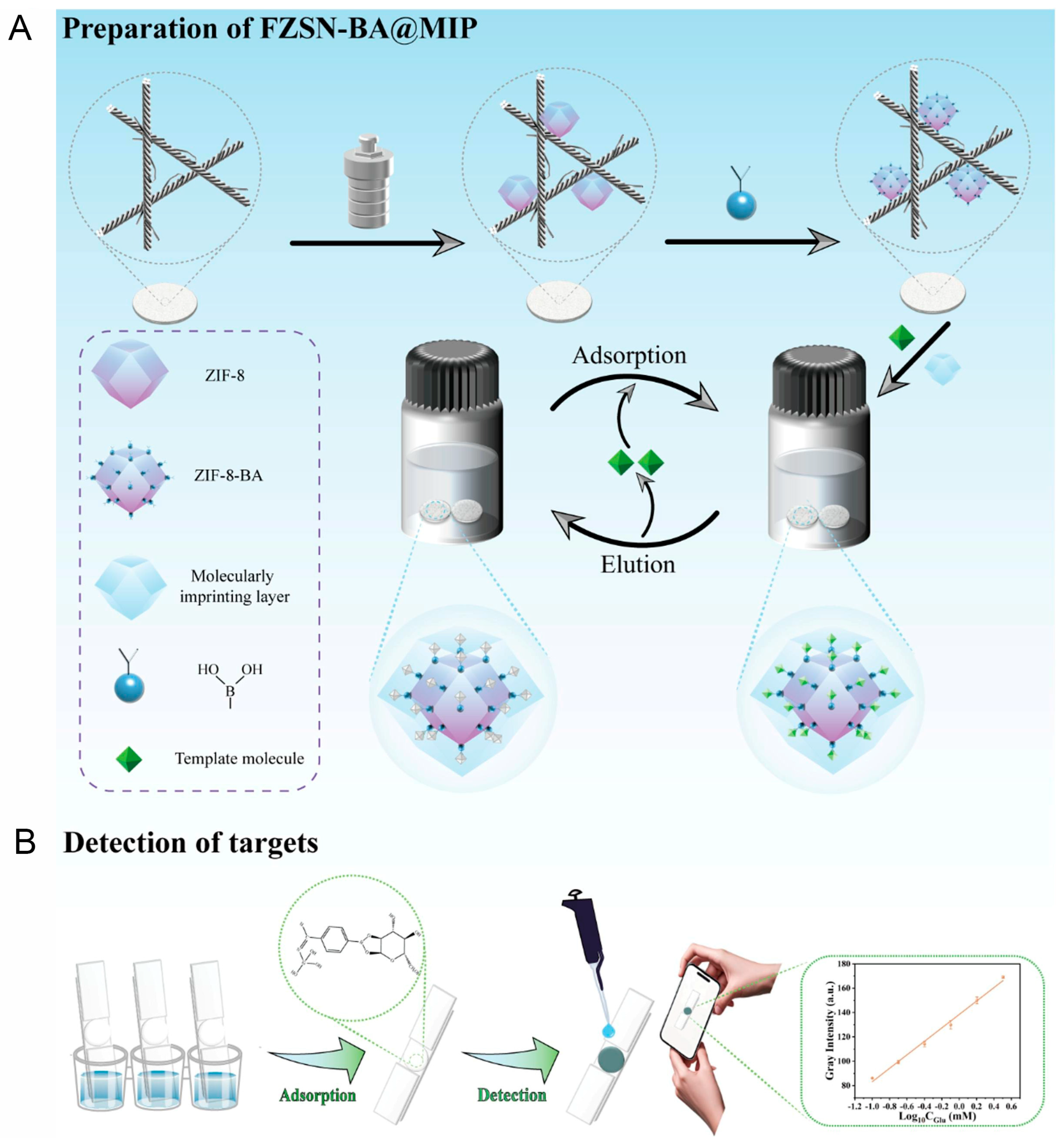
3.3. Recent Development of Non-Enzymatic Microfluidic Paper-Based Glucose Sensor
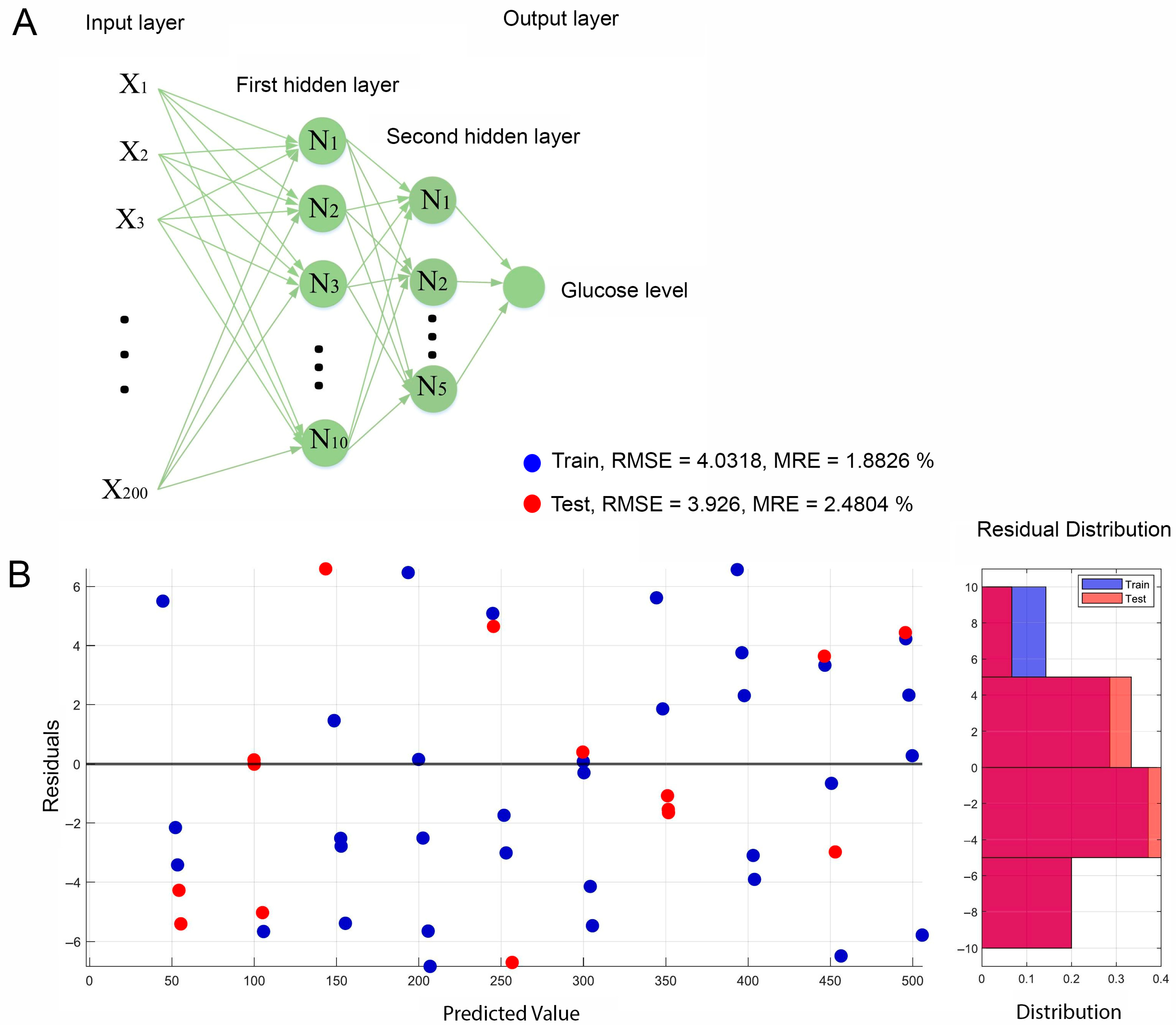
3.4. Assistance of AI and Machine Learning for Microfluidic Paper-Based Glucose Sensor Processing
4. Conclusions and Future Perspectives
- The development of novel nanomaterials integrated within paper substrates enhances the sensor response speed and sensitivity.
- Surface engineering of paper-based biosensors for improving target specificity.
- The transformation of conventional paper-based biosensors into wearable formats to broaden their applicability and user convenience.
- Commercial products incorporating AI and ML are required
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Correction Statement
Abbreviations
| µPAD | Microfluidic paper analytic devices |
| AI | Artificial intelligence |
| HRP | Horseradish peroxidase |
| GOx | Glucose oxidase |
| MIP | Molecularly imprinted polymer |
| ML | Machine learning |
| PDMS | Polydimethylsiloxane |
| POCT | Point-of-care testing |
References
- Lu, X.; Xie, Q.; Pan, X.; Zhang, R.; Zhang, X.; Peng, G.; Zhang, Y.; Shen, S.; Tong, N. Type 2 Diabetes Mellitus in Adults: Pathogenesis, Prevention and Therapy. Signal Transduct. Target Ther. 2024, 9, 262. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Sharaky, M.; Khattab, M.; Ashour, N.A.; Zaid, R.T.; Roh, E.J.; Elkamhawy, A.; Al-Karmalawy, A.A. Diabetes Mellitus: Classification, Mediators, and Complications; A Gate to Identify Potential Targets for the Development of New Effective Treatments. Biomed. Pharmacother. 2023, 168, 115734. [Google Scholar] [CrossRef] [PubMed]
- Ojo, O.A.; Ibrahim, H.S.; Rotimi, D.E.; Ogunlakin, A.D.; Ojo, A.B. Diabetes Mellitus: From Molecular Mechanism to Pathophysiology and Pharmacology. Med. Nov. Technol. Devices 2023, 19, 100247. [Google Scholar] [CrossRef]
- Lee, H.; Hong, Y.J.; Baik, S.; Hyeon, T.; Kim, D.-H. Enzyme-Based Glucose Sensor: From Invasive to Wearable Device. Adv. Healthc. Mater. 2018, 7, 1701150. [Google Scholar] [CrossRef]
- Govindaraj, M.; Srivastava, A.; Muthukumaran, M.K.; Tsai, P.-C.; Lin, Y.-C.; Raja, B.K.; Rajendran, J.; Ponnusamy, V.K.; Arockia Selvi, J. Current Advancements and Prospects of Enzymatic and Non-enzymatic Electrochemical Glucose Sensors. Int. J. Biol. Macromol. 2023, 253, 126680. [Google Scholar] [CrossRef]
- Ghosh, R.; Li, X.; Yates, M.Z. Non-enzymatic Glucose Sensor Using Bimetallic Catalysts. ACS Appl. Mater. Interfaces 2024, 16, 17–29. [Google Scholar] [CrossRef]
- Somsiri, S.; Kaewjangwad, C.; Malarat, N.; Wangchuk, S.; Saichanapan, J.; Samoson, K.; Promsuwan, K.; Saisahas, K.; Soleh, A.; Phairatana, T.; et al. Portable NFC Potentiostat Integrated with a 3D Paper-based Microfluidic Electrochemical Device for Glucose Detection in Whole Blood using PEDOT:PSS/DMSO/GOx Sensitive Film. Microchem. J. 2025, 213, 113623. [Google Scholar] [CrossRef]
- Tong, L.; Wu, L.; Zai, Y.; Zhang, Y.; Su, E.; Gu, N. Paper-based Colorimetric Glucose Sensor using Prussian Blue Nanoparticles as Mimic Peroxidase. Biosens. Bioelectron. 2023, 219, 114787. [Google Scholar] [CrossRef]
- Nguyen, Q.H.; Lee, D.H.; Nguyen, P.T.; Le, P.G.; Kim, M.I. Foldable Paper Microfluidic Device Based on Single Iron Site-containing Hydrogel Nanozyme for Efficient Glucose Biosensing. Chem. Eng. J. 2023, 454, 140541. [Google Scholar] [CrossRef]
- Le, P.G.; Le, X.A.; Duong, H.S.; Jung, S.H.; Kim, T.; Kim, M.I. Ultrahigh Peroxidase-like Catalytic Performance of Cu–N4 and Cu–N4S Active Sites-containing Reduced Graphene Oxide for Sensitive Electrochemical Biosensing. Biosens. Bioelectron. 2024, 255, 116259. [Google Scholar] [CrossRef]
- Le, P.G.; Wu, Q.; Kong, D.Y.; Ge, J.; Kim, M.I. Tailoring Nanostructured Supports to Achieve High Performance in Enzymatic Biofuel Cells. ACS Appl. Energy Mater. 2022, 5, 13113–13127. [Google Scholar] [CrossRef]
- Le, P.G.; Cho, S. Recent Developments in MXene-Based Enzyme-Free Electrochemical Glucose Sensing. BioChip J. 2024, 18, 521–534. [Google Scholar] [CrossRef]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef]
- Kanchan, M.; Tambe, P.K.; Bharati, S.; Powar, O.S. Convolutional Neural Network for Colorimetric Glucose Detection using a Smartphone and Novel multilayer polyvinyl film microfluidic device. Sci. Rep. 2024, 14, 28377. [Google Scholar] [CrossRef] [PubMed]
- Xu, B.; Xiang, X.; Ding, Z.; Luo, Z.; Huang, J. A Colorimetric Assay for Detection of Glucose by Enzymatic Etching of Triangular Gold Nanosheets. Mater. Chem. Phys. 2023, 301, 127619. [Google Scholar] [CrossRef]
- Lan, N.T.; Phan, L.G.; Hoang, L.H.; Huan, B.D.; Van Hong, L.; Anh, T.X.; Chinh, H.D. Hydrothermal Synthesis, Structure and Photocatalytic Properties of La/Bi Co-Doped NaTaO3. Mater. Trans. 2016, 57, 1–4. [Google Scholar] [CrossRef]
- Colvin, L.; Tu, D.; Dunlap, D.; Rios, A.; Coté, G. A Polarity-Sensitive Far-Red Fluorescent Probe for Glucose Sensing through Skin. Biosensors 2023, 13, 788. [Google Scholar] [CrossRef]
- Sawayama, J.; Okitsu, T.; Nakamata, A.; Kawahara, Y.; Takeuchi, S. Hydrogel Glucose Sensor with In Vivo Stable Fluorescence Intensity Relying on Antioxidant Enzymes for Continuous Glucose Monitoring. iScience 2020, 23, 101243. [Google Scholar] [CrossRef]
- Li, D.; Zhang, Y.; Sun, F.; Felidj, N.; Gagey-Eilstein, N.; Lamouri, A.; Hémadi, M.; Nizard, P.; Luo, Y.; Mangeney, C. Dual-Probe SERS Nanosensor: A Promising Approach for Sensitive and Ratiometric Detection of Glucose in Clinical Settings. ACS Appl. Bio Mater. 2024, 7, 2254–2263. [Google Scholar] [CrossRef]
- Sun, X. Glucose Detection through Surface-enhanced Raman Spectroscopy: A Review. Anal. Chim. Acta 2022, 1206, 339226. [Google Scholar] [CrossRef]
- Naikoo, G.A.; Bano, M.; Arshad, F.; Hassan, I.U.; BaOmar, F.; Alfagih, I.M.; Tambuwala, M.M. Non-enzymatic glucose sensors composed of trimetallic CuO/Ag/NiO based composite materials. Sci. Rep. 2023, 13, 6210. [Google Scholar] [CrossRef]
- Guesmi, S.; Moulaee, K.; Bressi, V.; Kahri, H.; Khaskhoussi, A.; Espro, C.; Barhoumi, H.; Neri, G. Non-Enzymatic Amperometric Glucose Sensing by Novel Cu-MOF Synthesized at Room Temperature. Mater. Adv. 2024, 5, 1160–1170. [Google Scholar] [CrossRef]
- Dung, N.Q.; Duong, T.T.T.; Lam, T.D.; Dung, D.D.; Huy, N.N.; Van Thanh, D. A Simple Route for Electrochemical Glucose Sensing using Background Current Subtraction of Cyclic Voltammetry Technique. J. Electroanal. Chem. 2019, 848, 113323. [Google Scholar] [CrossRef]
- Phasuksom, K.; Sirivat, A. Chronoampermetric Detection of Enzymatic Glucose Sensor Based on Doped Polyindole/MWCNT Composites Modified onto Screen-printed Carbon Electrode as Portable Sensing Device for Diabetes. RSC Adv. 2022, 12, 28505–28518. [Google Scholar] [CrossRef] [PubMed]
- Scandurra, A.; Iacono, V.; Boscarino, S.; Scalese, S.; Grimaldi, M.G.; Ruffino, F. Model of Chronoamperometric Response towards Glucose Sensing by Arrays of Gold Nanostructures Obtained by Laser, Thermal and Wet Processes. Nanomaterials 2023, 13, 1163. [Google Scholar] [CrossRef] [PubMed]
- Patil, V.B.; Sawkar, R.R.; Ilager, D.; Shetti, N.P.; Tuwar, S.M.; Aminabhavi, T.M. Glucose-based Carbon Electrode for Trace-level Detection of Acetaminophen. Electrochem. Sci. Adv. 2022, 2, e202100117. [Google Scholar] [CrossRef]
- Patras, M.A.; Davalos, J.Z.; Kuhnert, N. Understanding the Fragmentation of Glucose in Mass Spectrometry. J. Mass Spectrom. 2023, 58, e4972. [Google Scholar] [CrossRef]
- Taylor, V.F.; March, R.E.; Longerich, H.P.; Stadey, C.J. A Mass Spectrometric Study of Glucose, Sucrose, and Fructose using an Inductively Coupled Plasma and Electrospray Ionization. Int. J. Mass Spectrom. 2005, 243, 71–84. [Google Scholar] [CrossRef]
- Omer, A.E.; Shaker, G.; Safavi-Naeini, S.; Kokabi, H.; Alquié, G.; Deshours, F.; Shubair, R.M. Low-cost Portable Microwave Sensor for Non-invasive Monitoring of Blood Glucose Level: Novel Design Utilizing a Four-cell CSRR Hexagonal Configuration. Sci. Rep. 2020, 10, 15200. [Google Scholar] [CrossRef]
- Rattan, P.; Houngkamhang, N.; Orankitanun, T.; Phasukkit, P. Metamaterial Microwave Sensor for Glucose Level Measurement Based on Strip Line with Complementary Split Ring Resonator. Phys. Status Solidi (A) 2025, 222, 2400180. [Google Scholar] [CrossRef]
- Brazaca, L.C.; Imamura, A.H.; Blasques, R.V.; Camargo, J.R.; Janegitz, B.C.; Carrilho, E. The Use of Biological Fluids in Microfluidic Paper-based Analytical Devices (μPADs): Recent Advances, Challenges and Future Perspectives. Biosens. Bioelectron. 2024, 246, 115846. [Google Scholar] [CrossRef]
- Le, P.G.; Jung, S.-C.; Han, J.-H.; Cho, S. Nanozyme-integrated Paper Chip Based on High Peroxidase-like Activity of Zn-doped Reduced Graphene Oxide for Glucose Sensing. Microchem. J. 2025, 213, 113895. [Google Scholar] [CrossRef]
- Yeon, S.Y.; Seo, M.; Kim, Y.; Hong, H.; Chung, T.D. Paper-based Electrochromic Glucose Sensor with Polyaniline on Indium Tin Oxide Nanoparticle Layer as the Optical Readout. Biosens. Bioelectron. 2022, 203, 114002. [Google Scholar] [CrossRef]
- Sung, W.-H.; Hung, J.-T.; Lu, Y.-J.; Cheng, C.-M. Paper-Based Detection Device for Alzheimer’s Disease—Detecting β-amyloid Peptides (1–42) in Human Plasma. Diagnostics 2020, 10, 272. [Google Scholar] [CrossRef] [PubMed]
- Duan, S.; Cai, T.; Liu, F.; Li, Y.; Yuan, H.; Yuan, W.; Huang, K.; Hoettges, K.; Chen, M.; Lim, E.G.; et al. Automatic Offline-Capable Smartphone Paper-based Microfluidic Device for Efficient Biomarker Detection of Alzheimer's Disease. Anal. Chim. Acta 2024, 1308, 342575. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Raucci, A.; Cimmino, W.; Cinti, S. Paper-Based Analytical Devices for Cancer Liquid Biopsy. Anal. Chem. 2024, 96, 3698–3706. [Google Scholar] [CrossRef] [PubMed]
- Shalaby, A.A.; Tsao, C.-W.; Ishida, A.; Maeki, M.; Tokeshi, M. Microfluidic Paper-based Analytical Devices for Cancer Diagnosis. Sens. Actuators B Chem. 2023, 379, 133243. [Google Scholar] [CrossRef]
- Fan, H.; Sasaki, Y.; Zhou, Q.; Tang, W.; Nishina, Y.; Minami, T. Non-enzymatic Detection of Glucose Levels in Human Blood Plasma by a Graphene Oxide-modified Organic Transistor Sensor. Chem. Commun. 2023, 59, 2425–2428. [Google Scholar] [CrossRef]
- Psotta, C.; Cirovic, S.; Gudmundsson, P.; Falk, M.; Mandal, T.; Reichhart, T.; Leech, D.; Ludwig, R.; Kittel, R.; Schuhmann, W.; et al. Continuous Ex Vivo Glucose Sensing in Human Physiological Fluids using an Enzymatic Sensor in a Vein Replica. Bioelectrochemistry 2023, 152, 108441. [Google Scholar] [CrossRef]
- Lee, H.-A.; Lin, P.-Y.; Solomatina, A.I.; Koshevoy, I.O.; Tunik, S.P.; Lin, H.-W.; Pan, S.-W.; Ho, M.-L. Glucose Sensing in Human Whole Blood Based on Near-Infrared Phosphors and Outlier Treatment with the Programming Language “R”. ACS Omega 2022, 7, 198–206. [Google Scholar] [CrossRef]
- Math, C.; Income, K.; Khachornsakkul, K.; Duenchay, P.; Dungchai, W. A Sensitive and Facile Electrochemical Paper-based Sensor for Glucose Detection in Whole Blood using the Pd/CB-Ni@rGO Modified Electrode. Analyst 2023, 148, 4753–4761. [Google Scholar] [CrossRef] [PubMed]
- Akiiga, N.S.; El-Bab, A.M.R.F.; Yoshihisa, M.; El-Moneim, A.A. Enzyme-Free Glucose Detection in Sweat using 2D inkjet-printed Cobalt Sulfide Anchored on Graphene in a Paper-based Microfluidic Device. J. Colloid Interface Sci. 2025, 688, 490–504. [Google Scholar] [CrossRef]
- Moradi, S.; Hosseini, M.; Firoozbakhtian, A.; Barshan Tashnizi, M.; Tashnizi, M.B. Electrochemical Detection of Glucose in Sweat using a Paper-based Origami Biosensor Based on PEDOT:PSS/Ti3C2/MWCNT Composite. J. Electroanal. Chem. 2024, 973, 118675. [Google Scholar] [CrossRef]
- Li, Y.; Kong, Y.; Hu, Y.; Li, Y.; Asrosa, R.; Zhang, W.; Boruah, B.D.; Yetisen, A.K.; Davenport, A.; Lee, T.-C.; et al. A Paper-based Dual Functional Biosensor for Safe and User-friendly Point-of-care Urine Analysis. Lab Chip 2024, 24, 2454–2467. [Google Scholar] [CrossRef]
- Lee, T.; Kim, I.; Cheong, D.Y.; Roh, S.; Jung, H.G.; Lee, S.W.; Kim, H.S.; Yoon, D.S.; Hong, Y.; Lee, G. Selective Colorimetric Urine Glucose Detection by Paper Sensor Functionalized with Polyaniline Nanoparticles and Cell Membrane. Anal. Chim. Acta 2021, 1158, 338387. [Google Scholar] [CrossRef]
- Ruiz, R.A.; Gonzalez, J.L.; Vazquez-Alvarado, M.; Martinez, N.W.; Martinez, A.W. Beyond Wax Printing: Fabrication of Paper-Based Microfluidic Devices Using a Thermal Transfer Printer. Anal. Chem. 2022, 94, 8833–8837. [Google Scholar] [CrossRef]
- Malik, S.; Singh, J.; Saini, K.; Chaudhary, V.; Umar, A.; Ibrahim, A.A.; Akbar, S.; Baskoutas, S. Paper-based Sensors: Affordable, Versatile, and Emerging Analyte Detection Platforms. Anal. Methods 2024, 16, 2777–2809. [Google Scholar] [CrossRef]
- Costa-Rama, E.; Fernández-Abedul, M.T. Paper-Based Screen-Printed Electrodes: A New Generation of Low-Cost Electroanalytical Platforms. Biosensors 2021, 11, 51. [Google Scholar] [CrossRef]
- Cao, Q.; Liang, B.; Tu, T.; Wei, J.; Fang, L.; Ye, X. Three-dimensional Paper-based Microfluidic Electrochemical Integrated Devices (3D-PMED) for Wearable Electrochemical Glucose Detection. RSC Adv. 2019, 9, 5674–5681. [Google Scholar] [CrossRef]
- Lee, S.; Park, J.S.; Woo, H.; Yoo, Y.K.; Lee, D.; Chung, S.; Yoon, D.S.; Lee, K.-B.; Lee, J.H. Rapid Deep Learning-assisted Predictive Diagnostics for Point-of-care Testing. Nat. Commun. 2024, 15, 1695. [Google Scholar] [CrossRef] [PubMed]
- Han, G.-R.; Goncharov, A.; Eryilmaz, M.; Ye, S.; Palanisamy, B.; Ghosh, R.; Lisi, F.; Rogers, E.; Guzman, D.; Yigci, D.; et al. Machine Learning in Point-of-care Testing: Innovations, Challenges, and Opportunities. Nat. Commun. 2025, 16, 3165. [Google Scholar] [CrossRef] [PubMed]
- Gao, Q.; Li, S. Intelligent Point of Care Testing for Medicine Diagnosis. Interdiscip. Med. 2024, 2, e20230031. [Google Scholar] [CrossRef]
- Wang, K.; Meng, X.; Yan, X.; Fan, K. Nanozyme-based Point-of-Care testing: Revolutionizing Environmental Pollutant Detection with High Efficiency and Low Cost. Nano Today 2024, 54, 102145. [Google Scholar] [CrossRef]
- Kuswandi, B.; Hidayat, M.A.; Noviana, E. Paper-based Sensors for Rapid Important Biomarkers Detection. Biosens. Bioelectron. X 2022, 12, 100246. [Google Scholar] [CrossRef]
- Bilgen, E.; Suvacı, Z.; Çetinkol, Ö.P.; Forough, M. 26—Disposable Paper-based Sensors. In Fundamentals of Sensor Technology; Barhoum, A., Altintas, Z., Eds.; Woodhead Publishing: Cambridge, UK, 2023; pp. 803–860. [Google Scholar]
- Tong, X.; Ga, L.; Zhao, R.; Ai, J. Research Progress on the Applications of Paper Chips. RSC Adv. 2021, 11, 8793–8820. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y.; Ji, Y.; Zheng, S.; Wang, F.; Li, C. Cheap and Portable Paper Chip with Terrific Oxidase-like Activity and SERS Enhancement Performance for SERS-colorimetric Bimodal Detection of Intracellular Glutathione. Biosens. Bioelectron. 2024, 244, 115817. [Google Scholar] [CrossRef]
- Noviana, E.; Ozer, T.; Carrell, C.S.; Link, J.S.; McMahon, C.; Jang, I.; Henry, C.S. Microfluidic Paper-Based Analytical Devices: From Design to Applications. Chem. Rev. 2021, 121, 11835–11885. [Google Scholar] [CrossRef]
- Chen, J.L.; Njoku, D.I.; Tang, C.; Gao, Y.; Chen, J.; Peng, Y.-K.; Sun, H.; Mao, G.; Pan, M.; Tam, N.F.-Y. Advances in Microfluidic Paper-Based Analytical Devices (µPADs): Design, Fabrication, and Applications. Small Methods 2024, 8, 2400155. [Google Scholar] [CrossRef]
- Yu, L.; Shi, Z.Z. Microfluidic Paper-based Analytical Devices Fabricated by Low-cost Photolithography and Embossing of Parafilm®. Lab Chip 2015, 15, 1642–1645. [Google Scholar] [CrossRef]
- García-Casas, X.; Aparicio, F.J.; Budagosky, J.; Ghaffarinejad, A.; Orozco-Corrales, N.; Ostrikov, K.; Sánchez-Valencia, J.R.; Barranco, Á.; Borrás, A. Paper-based ZnO Self-powered Sensors and Nanogenerators by Plasma Technology. Nano Energy 2023, 114, 108686. [Google Scholar] [CrossRef]
- Mukhopadhyay, M.; Subramanian, S.G.; Durga, K.V.; Sarkar, D.; DasGupta, S. Laser Printing Based Colorimetric Paper Sensors for Glucose and Ketone Detection: Design, Fabrication, and Theoretical Analysis. Sens. Actuators B Chem. 2022, 371, 132599. [Google Scholar] [CrossRef]
- Aghababaie, M.; Foroushani, E.S.; Changani, Z.; Gounani, Z.; Mobarakeh, M.S.; Hadady, H.; Khedri, M.; Maleki, R.; Asadnia, M.; Razmjou, A. Recent Advances In the Development of Enzymatic Paper-based Microfluidic Biosensors. Biosens. Bioelectron. 2023, 226, 115131. [Google Scholar] [CrossRef] [PubMed]
- Verma, D.; Dubey, N.; Yadav, A.K.; Saraya, A.; Sharma, R.; Solanki, P.R. Disposable Paper-based Screen-printed Electrochemical Immunoplatform for Dual Detection of Esophageal Cancer Biomarkers in Patients’ Serum Samples. Mater. Adv. 2024, 5, 2153–2168. [Google Scholar] [CrossRef]
- Juang, Y.-J.; Chen, P.-S.; Wang, Y. Rapid Fabrication of Microfluidic Paper-based Analytical Devices by Microembossing. Sens. Actuators B Chem. 2019, 283, 87–92. [Google Scholar] [CrossRef]
- Mei, X.; Chen, Z.; Wen, A.; Zhang, J.; Wei, X.; Wang, F.; Zhou, L.; Wang, B.; Wu, Y. Wearable Three-dimensional Paper-based Microfluidic Electrochemical Sensors for Real-time Sweat Monitoring. Chem. Eng. J. 2025, 515, 163786. [Google Scholar] [CrossRef]
- Ilyasov, I.R.; Beloborodov, V.L.; Selivanova, I.A.; Terekhov, R.P. ABTS/PP Decolorization Assay of Antioxidant Capacity Reaction Pathways. Int. J. Mol. Sci. 2020, 21, 1131. [Google Scholar] [CrossRef]
- Liu, D.-M.; Dong, C.; Ma, R.-T. A Colorimetric Method for Screening α-glucosidase Inhibitors from Flavonoids using 3,3′,5,5′-tetramethylbenzidine as a Chromogenic Probe. Colloids Surf. B Biointerfaces 2021, 197, 111400. [Google Scholar] [CrossRef]
- Singh, K.R.B.; Nayak, V.; Sarkar, T.; Singh, R.P. Cerium Oxide Nanoparticles: Properties, Biosynthesis and Biomedical Application. RSC Adv. 2020, 10, 27194–27214. [Google Scholar] [CrossRef]
- Monisha; Shrivas, K.; Kant, T.; Patel, S.; Devi, R.; Dahariya, N.S.; Pervez, S.; Deb, M.K.; Rai, M.K.; Rai, J. Inkjet-printed Paper-based Colorimetric Sensor Coupled with Smartphone for Determination of Mercury (Hg2+). J. Hazard. Mater. 2021, 414, 125440. [Google Scholar] [CrossRef]
- Uhlikova, N.; Almeida, M.I.G.S.; McKelvie, I.; Morrison, R.; Kolev, S.D. Use of Scanners for Colorimetric Analysis of Microfluidic Paper-based Analytical Devices (µPADs): A Practical Guide. Microchem. J. 2023, 191, 108879. [Google Scholar] [CrossRef]
- Nota, G.; Cimmino, W.; Singh, S.; Darwish, I.A.; La Rocca, C.; Carbone, F.; Matarese, G.; Cinti, S. A Portable and Ecological Paper-based Device for Glucose Monitoring in Peripheral Blood Mononuclear Cell Lysates. Anal. Methods 2025, 17, 2529–2535. [Google Scholar] [CrossRef]
- Zheng, Y.; Huang, Y.; Zuo, Q.; Zhang, Y.; Wu, Y.; Zhang, Z. On-Demand Portable Paper-Based Electrospray Ionization Mass Spectrometry for High-Sensitivity Analysis of Complex Samples. Anal. Chem. 2023, 95, 6163–6171. [Google Scholar] [CrossRef]
- Pumbua, R.; Sricharoen, N.; Wongravee, K.; Praneenararat, T. Paper Spray Mass Spectrometry as an Effective Tool for Differentiating Coffees Based on their Geographical Origins. Food Chem. X 2023, 18, 100624. [Google Scholar] [CrossRef]
- Amor-Gutiérrez, O.; Costa Rama, E.; Costa-García, A.; Fernández-Abedul, M.T. Paper-based Maskless Enzymatic Sensor for Glucose Determination Combining Ink and Wire Electrodes. Biosens. Bioelectron. 2017, 93, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Chun, H.J.; Park, Y.M.; Han, Y.D.; Jang, Y.H.; Yoon, H.C. Paper-based Glucose Biosensing System Utilizing a Smartphone as a Signal Reader. BioChip J. 2014, 8, 218–226. [Google Scholar] [CrossRef]
- Li, N.; Zhou, Y.; Sun, H.; Wang, N.; Yang, N.; Ren, P.; Fu, L.; Zhang, Y.; Liu, W.; Li, Y.; et al. Rapid Analysis of Salivary Glucose Content using MOF/MIPs Biomimetic Microfluidic Paper Chips. Chem. Eng. J. 2025, 504, 159023. [Google Scholar] [CrossRef]
- Lian, M.; Shi, F.; Cao, Q.; Wang, C.; Li, N.; Li, X.; Zhang, X.; Chen, D. Paper-based Colorimetric Sensor using Bimetallic Nickel-Cobalt Selenides Nanozyme with Artificial Neural Network-assisted for Detection of H2O2 on Smartphone. Spectrophim. Acta A Mol. Biomol. Spectrosc. 2024, 311, 124038. [Google Scholar] [CrossRef]
- Yan, L.; Zhang, B.; Zhou, W.; Hao, J.; Shi, H.; Wang, S.; Shuang, S.; Shi, L. An Artificial Intelligence Handheld Sensor for Direct Reading of Nickel Ion and Ethylenediaminetetraacetic Acid in Food Samples using Ratiometric Fluorescence Cellulose Paper Microfluidic Chip. Int. J. Biol. Macromol. 2024, 279, 135083. [Google Scholar] [CrossRef]
- Coskun, M. Intrinsic Graph Topological Correlation for Graph Convolutional Network Propagation. Comput. Stand. Interfaces 2023, 83, 103655. [Google Scholar] [CrossRef]
- Kumbure, M.M.; Luukka, P. Generalizing Fuzzy K-Nearest Neighbor Classifier using an OWA Operator with a RIM Quantifier. Expert Syst. Appl. 2025, 282, 127795. [Google Scholar] [CrossRef]
- Vásquez, J.C.G.; Kumral, M. Artificial Neural Networks and Support Vector Machines for More Accurate Cost Estimation in Underground Mining: A Contractor’s Viewpoint. Mach. Learn. Appl. 2025, 21, 100689. [Google Scholar] [CrossRef]
- Hamdi, T.; Ben Ali, J.; Di Costanzo, V.; Fnaiech, F.; Moreau, E.; Ginoux, J.-M. Accurate Prediction of Continuous Blood Glucose Based on Support Vector Regression and Differential Evolution Algorithm. Biocybern. Biomed. Eng. 2018, 38, 362–372. [Google Scholar] [CrossRef]
- Pantic, I.V.; Paunovic Pantic, J.; Valjarevic, S.; Corridon, P.R.; Topalovic, N. Artificial Intelligence—Based Approaches Based on Random Forest Algorithm for Signal Analysis: Potential Applications in Detection of Chemico—Biological Interactions. Chem. Biol. Interact. 2025, 418, 111624. [Google Scholar] [CrossRef] [PubMed]
- Karim, R.A.H.; Vassányi, I.; Kósa, I. After-meal Blood Glucose Level Prediction using an Absorption Model for Neural Network Training. Comput. Biol. Med. 2020, 125, 103956. [Google Scholar] [CrossRef]
- Agrawal, H.; Jain, P.; Joshi, A.M. Machine Learning Models for Non-invasive Glucose Measurement: Towards Diabetes Management in Smart Healthcare. Health Technol. 2022, 12, 955–970. [Google Scholar] [CrossRef]
- Abreu, A.; dos Santos Oliveira, D.; Vinagre, I.; Cavouras, D.; Alves, J.A.; Pereira, A.I.; Lima, J.; Moreira, F.T.C. A Machine Learning Approach for Enhanced Glucose Prediction in Biosensors. Chemosensors 2025, 13, 52. [Google Scholar] [CrossRef]
- Shalini, J.; Kumar, A. An Explainable Artificial Intelligence Driven Fall System for Sensor Data Analysis Enhanced by Butterworth Filtering. Eng. Appl. Artif. Intell. 2025, 158, 111364. [Google Scholar] [CrossRef]
- Medanki, S.; Dommati, N.; Bodapati, H.H.; Katru, V.; Moses, G.; Komaraju, A.; Donepudi, N.S.; Yalamanchili, D.; Sateesh, J.; Turimerla, P. Artificial Intelligence Powered Glucose Monitoring and Controlling System: Pumping Module. World J. Exp. Med. 2024, 14, 87916. [Google Scholar] [CrossRef]
- Zhang, G.; Zhang, Q.; Wang, C.; Ji, X.; Weng, Z.; Mayet, A.M.; Miao, X. A Microstrip-based Sensor for Glucose Monitoring: Towards Non-invasive Blood Glucose Detection. Measurement 2025, 256, 118116. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Le, P.G.; Cho, S. Microfluidic Paper-Based Sensors and Their Applications for Glucose Sensing. Chemosensors 2025, 13, 293. https://doi.org/10.3390/chemosensors13080293
Le PG, Cho S. Microfluidic Paper-Based Sensors and Their Applications for Glucose Sensing. Chemosensors. 2025; 13(8):293. https://doi.org/10.3390/chemosensors13080293
Chicago/Turabian StyleLe, Phan Gia, and Sungbo Cho. 2025. "Microfluidic Paper-Based Sensors and Their Applications for Glucose Sensing" Chemosensors 13, no. 8: 293. https://doi.org/10.3390/chemosensors13080293
APA StyleLe, P. G., & Cho, S. (2025). Microfluidic Paper-Based Sensors and Their Applications for Glucose Sensing. Chemosensors, 13(8), 293. https://doi.org/10.3390/chemosensors13080293





