Illuminating Pollutants: The Role of Carbon Dots in Environmental Sensing
Abstract
1. Introduction
2. Importance of Pollutant Sensing in the Present Scenario
3. Existing Sensors in Pollutant Sensing and Their Limitations
3.1. Metal-Based Sensors
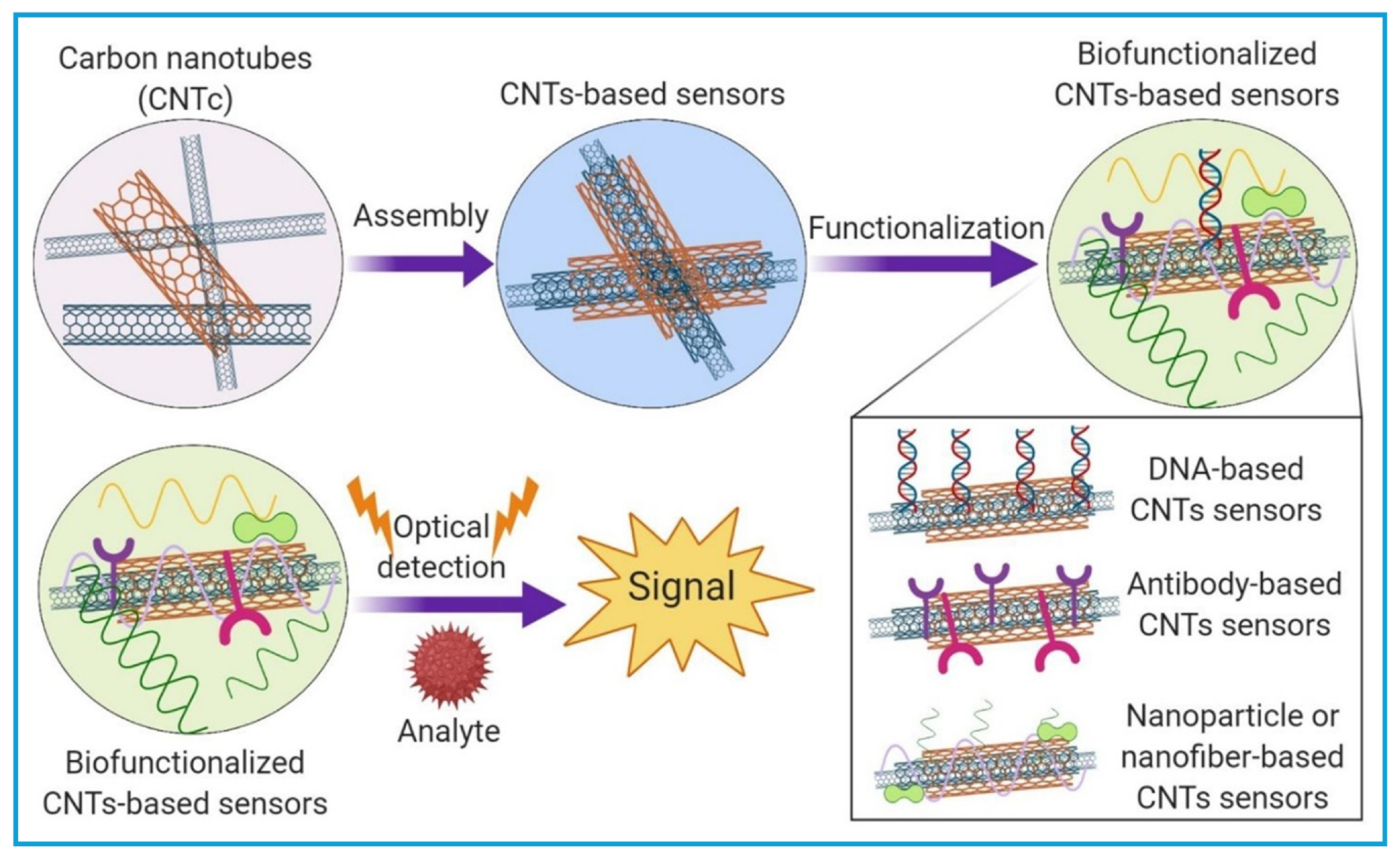
3.2. Carbon Nanotube (CNT)-Based Sensors
3.3. Chemical Sensors: Advancements
3.4. Electrochemical-Based Sensors
3.5. Voltammetric Sensors
3.6. Amperometric Sensors
3.7. Potentiometric Sensors
3.8. Optical Sensors
3.9. Fluorescence Sensors
3.10. Surface Plasmon Resonance (SPR) Sensors
3.11. CD-Based Antigen–Antibody Biosensors
4. Modern Nanotechnologies for Sensing Pollutants
4.1. Nanotechnology for Clean Water
4.2. Nanoscale Zerovalent Iron (nZVI)
4.3. Bioactive Nanoparticles for Water Disinfection
4.4. Bimetallic Nanoparticles (BNPs) for Remediation
4.5. Semiconductor Nanoparticles for Remediation
4.6. Nanoadsorbents
4.7. Nanomembranes
4.8. Nanocatalyst
4.9. Nanotechnology for the Adsorption of Toxic Gases
4.10. Adsorption of Dioxin
4.11. Adsorption of Nitrogen Oxides
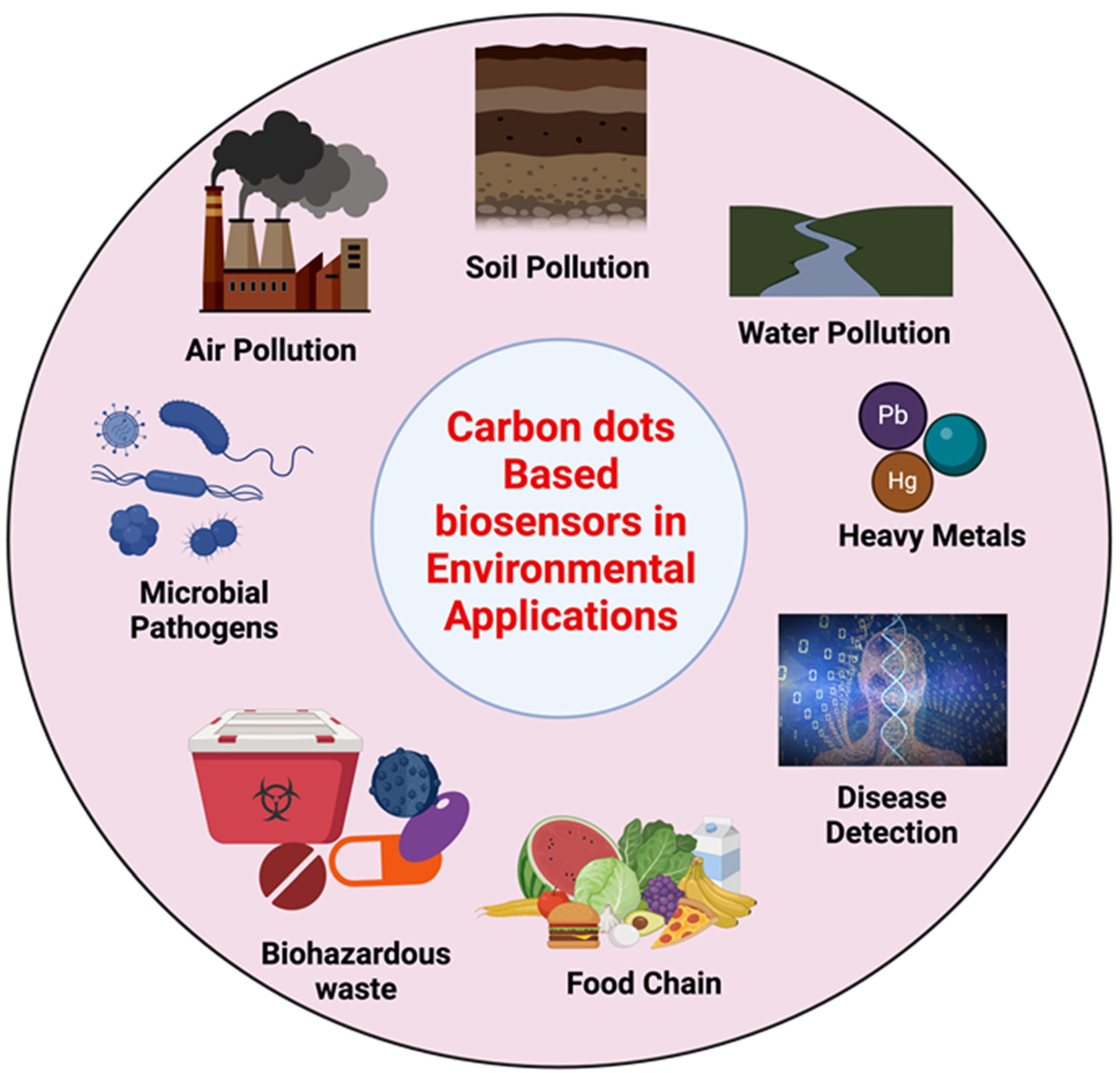
5. Importance of CDs in Pollutant Sensing
5.1. Unique Optical and Physicochemical Properties of Carbon Dots (CDs) Relevant to Pollutant Sensing
5.2. Detection of Pathogens Using CDs
5.3. CDs for Pollutant Degradation
6. Recent Trends in Developing Pollutant-Sensing CDs
6.1. Transition-Metal-Doped CDs
6.2. Nanohybrid-Based CDs
7. Limitations and Future Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saxena, V. Water Quality, Air Pollution, and Climate Change: Investigating the Environmental Impacts of Industrialization and Urbanization. Water Air Soil Pollut. 2025, 236, 1–40. [Google Scholar] [CrossRef]
- Awewomom, J.; Dzeble, F.; Takyi, Y.D.; Ashie, W.B.; Ettey, E.N.Y.O.; Afua, P.E.; Akoto, O. Addressing global environmental pollution using environmental control techniques: A focus on environmental policy and preventive environmental management. Discov. Environ. 2024, 2, 8. [Google Scholar] [CrossRef]
- Diez-Pascual, A.M.; Rahdar, A. Functional nanomaterials in biomedicine: Current uses and potential applications. ChemMedChem 2022, 17, e202200142. [Google Scholar] [CrossRef] [PubMed]
- Dutta, V.; Verma, R.; Gopalkrishnan, C.; Yuan, M.H.; Batoo, K.M.; Jayavel, R.; Chauhan, A.; Lin, K.Y.A.; Balasubramani, R.; Ghotekar, S. Bio-inspired synthesis of carbon-based nanomaterials and their potential environmental applications: A state-of-the-art review. Inorganics 2022, 10, 169. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Kim, Y.J. Recent developments in the design and fabrication of electrochemical biosensors using functional materials and molecules. Biosensors 2023, 13, 424. [Google Scholar] [CrossRef]
- Rezaei, M.; Mehdinia, A. A Review on the Applications of Quantum Dots in Sample Preparation. J. Sep. Sci. 2025, 48, e70061. [Google Scholar] [CrossRef]
- Tejwan, N.; Saini, A.K.; Sharma, A.; Singh, T.A.; Kumar, N.; Das, J. Metal-doped and hybrid carbon dots: A comprehensive review on their synthesis and biomedical applications. J. Control. Release 2021, 330, 132–150. [Google Scholar] [CrossRef]
- Liu, H.; Ding, J.; Zhang, K.; Ding, L. Construction of biomass carbon dots-based fluorescence sensors and their applications in chemical and biological analysis. TrAC Trends Anal. Chem. 2019, 118, 315–337. [Google Scholar] [CrossRef]
- Long, C.; Jiang, Z.; Shangguan, J.; Qing, T.; Zhang, P.; Feng, B. Applications of carbon dots in environmental pollution control: A review. Chem. Eng. J. 2020, 406, 126848. [Google Scholar] [CrossRef]
- Meng, X.; Shen, F.; Wang, D.; Zhang, S.; Hou, J.; Ding, L.; Sun, J. Carbon dots-based hybrid materials: Synthesis, properties and applications in environmental pollution control. Chem. Eng. J. 2024, 503, 158278. [Google Scholar] [CrossRef]
- Keçili, R.; Hussain, C.G.; Hussain, C.M. Fluorescent nanosensors based on green carbon dots (CDs) and molecularly imprinted polymers (MIPs) for environmental pollutants: Emerging trends and future prospects. Trends Environ. Anal. Chem. 2023, 40, e00213. [Google Scholar] [CrossRef]
- Nieuwenhuijsen, M.; de Nazelle, A.; Garcia-Aymerich, J.; Khreis, H.; Hoffmann, B. Shaping urban environments to improve respiratory health: Recommendations for research, planning, and policy. Lancet Respir. Med. 2024, 12, 247–254. [Google Scholar] [CrossRef] [PubMed]
- Edo, G.I.; Samuel, P.O.; Oloni, G.O.; Ezekiel, G.O.; Ikpekoro, V.O.; Obasohan, P.; Agbo, J.J. Environmental persistence, bioaccumulation, and ecotoxicology of heavy metals. Chem. Ecol. 2024, 40, 322–349. [Google Scholar] [CrossRef]
- Mathew, N.; Somanathan, A.; Tirpude, A.; Arfin, T. The impact of short-lived climate pollutants on the human health. Environ. Pollut. Manag. 2024, 1, 1–14. [Google Scholar] [CrossRef]
- Ramaiyan, K.; Tsui, L.K.; Brosha, E.L.; Kreller, C.; Stetter, J.; Russ, T.; Du, W.; Peaslee, D.; Hunter, G.W.; Xu, J.; et al. Recent Developments in Sensor Technologies for Enabling the Hydrogen Economy. ECS Sens. Plus 2023, 2, 045601. [Google Scholar] [CrossRef]
- Wami-Amadi, C.F. The Impact of Air Borne Toxins from Gas Flaring on Cardiopulmonary and Other Systemic Functions. Sch. Int. J. Anat. Physiol. 2025, 8, 12–28. [Google Scholar] [CrossRef]
- Ding, L.; Wang, S.; Liu, Y.; Cao, J.; Fang, Y. Bispyrene/surfactant assemblies as fluorescent sensor platform: Detection and identification of Cu2+ and Co2+ in aqueous solution. J. Mater. Chem. A 2013, 1, 8866–8875. [Google Scholar] [CrossRef]
- Olugbemi, S.A.; Amoniyan, O.A.; Salami, A.A. Assessment of the Adsorption Potential of Synthesized Chitosan-Pyrrole-2-Carboxaldehyde Schiff Base for Cr2+ and Pb2+ Ions from Dumpsite Leachate. Eur. J. Adv. Chem. Res. 2023, 4, 30–39. [Google Scholar] [CrossRef]
- Kasonga, T.K.; Coetzee, M.A.; Kamika, I.; Ngole-Jeme, V.M.; Momba, M.N.B. Endocrine-disruptive chemicals as contaminants of emerging concern in wastewater and surface water: A review. J. Environ. Manag. 2021, 277, 111485. [Google Scholar] [CrossRef]
- Al Naggar, Y.; Khalil, M.S.; Ghorab, M.A. Environmental pollution by heavy metals in the aquatic ecosystems of Egypt. Open Acc. J. Toxicol. 2018, 3, 555603. [Google Scholar]
- Penticoff, H.B.; Fortin, J.S. Toxic metabolic diseases of the nervous system. In Neurobiology of Brain Disorders; Academic Press: Cambridge, MA, USA, 2023; pp. 379–401. [Google Scholar]
- Amirian, H.; Dalvand, K.; Ghiasvand, A. Seamless integration of Internet of Things, miniaturization, and environmental chemical surveillance. Environ. Monit. Assess. 2024, 196, 582. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, D.; Wang, H.; Huang, W.; Hu, L.; Tang, Y.; Guo, Z.; Ouyang, Z.; Zhang, H. Recent advances in two-dimensional-material-based sensing technology toward health and environmental monitoring applications. Nanoscale 2020, 12, 3535–3559. [Google Scholar] [CrossRef]
- Elmi, I.; Zampolli, S.; Cozzani, E.; Mancarella, F.; Cardinali, G.C. Development of ultra-low-power consumption MOX sensors with ppb-level VOC detection capabilities for emerging applications. Sens. Actuators B Chem. 2008, 135, 342–351. [Google Scholar] [CrossRef]
- Pal, S.; Yu, S.S.; Kung, C.W. Group 4 metal-based metal—Organic frameworks for chemical sensors. Chemosensors 2021, 9, 306. [Google Scholar] [CrossRef]
- Mittelsteadt, C.; Norman, T.; Rich, M.; Willey, J. PEM Electrolyzers and PEM regenerative fuel cells industrial view. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Elsevier: Amsterdam, The Netherlands, 2015; pp. 159–181. [Google Scholar]
- Kanan, S.; Obeideen, K.; Moyet, M.; Abed, H.; Khan, D.; Shabnam, A.; El-Sayed, Y.; Arooj, M.; Mohamed, A.A. Recent advances on metal oxide-based sensors for environmental gas pollutants detection. Crit. Rev. Anal. Chem. 2024, 1–34. [Google Scholar] [CrossRef]
- Tyagi, S.; Chaudhary, M.; Ambedkar, A.K.; Sharma, K.; Gautam, Y.K.; Singh, B.P. Metal oxide nanomaterial-based sensors for monitoring environmental NO 2 and its impact on the plant ecosystem: A review. Sens. Diagn. 2022, 1, 106–129. [Google Scholar] [CrossRef]
- Shinde, R.S.; Khairnar, S.D.; Patil, M.R.; Adole, V.A.; Koli, P.B.; Deshmane, V.V.; Halwar, D.K.; Shinde, R.A.; Pawar, T.B.; Jagdale, B.S.; et al. Synthesis and characterization of ZnO/CuO nanocomposites as an effective photocatalyst and gas sensor for environmental remediation. J. Inorg. Organomet. Polym. Mater. 2022, 32, 1045–1066. [Google Scholar] [CrossRef]
- Lupan, C.; Mishra, A.K.; Wolff, N.; Drewes, J.; Krüger, H.; Vahl, A.; Lupan, O.; Pauporté, T.; Viana, B.; Kienle, L.; et al. Nanosensors based on a single ZnO: Eu nanowire for hydrogen gas sensing. ACS Appl. Mater. Interfaces. 2022, 14, 41196–41207. [Google Scholar] [CrossRef]
- Luo, K.; Peng, H.; Zhang, B.; Chen, L.; Zhang, P.; Peng, Z.; Fu, X. Advances in carbon nanotube-based gas sensors: Exploring the path to the future. Coord. Chem. Rev. 2024, 518, 216049. [Google Scholar] [CrossRef]
- Kim, S.; Han, J.; Choi, J.M.; Nam, J.S.; Lee, I.H.; Lee, Y.; Novikov, I.V.; Kauppinen, E.I.; Lee, K.; Jeon, I. Aerosol-Synthesized Surfactant-Free Single-Walled Carbon Nanotube-Based NO2 Sensors: Unprecedentedly High Sensitivity and Fast Recovery. Adv. Mater. 2024, 36, 2313830. [Google Scholar] [CrossRef]
- Singh, S.; Oum, W.; Kim, S.S.; Kim, H.W. Functionalized multiwalled carbon nanotubes for highly stable room temperature and humidity-tolerant triethylamine sensing. ACS Sens. 2023, 8, 4664–4675. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Sidhu, H.K.; Paul, A.K.; Bhardwaj, N.; Thakur, N.S.; Deep, A. Bioengineered multi-walled carbon nanotube (MWCNT) based biosensors and applications thereof. Sens. Diagn. 2023, 2, 1390–1413. [Google Scholar] [CrossRef]
- Hamayun, U.; Marwat, M.A.; Abdullah, S.M.; Ullah, R.; Humayun, M.; Bououdina, M.; Karim, M.R.; Khan, M.Z.; Hanif, M.B. Synergistic integration of Ag@Fe0.67Cu0.22Co0.11S core-shell nanostructures and SWCNTs for improved supercapacitor performance. J. Alloys Compd. 2025, 1012, 178422. [Google Scholar] [CrossRef]
- Mahmoudi, T.; de la Guardia, M.; Baradaran, B. Lateral flow assays towards point-of-care cancer detection: A review of current progress and future trends. TrAC Trends Anal. Chem. 2020, 125, 115842. [Google Scholar] [CrossRef]
- Ding, W.T.; Jiao, X.Y.; Zhao, Y.M.; Sun, X.Y.; Chen, C.; Wu, A.P.; Ding, Y.T.; Hou, P.X.; Liu, C. Enhancing the electrical conductivity and strength of PET by single-wall carbon nanotube film coating. ACS Appl. Mater. Interfaces 2023, 15, 37802–37809. [Google Scholar] [CrossRef]
- Rasheed, T.; Nabeel, F.; Adeel, M.; Rizwan, K.; Bilal, M.; Iqbal, H.M. Carbon nanotubes-based cues: A pathway to future sensing and detection of hazardous pollutants. J. Mol. Liq. 2019, 292, 111425. [Google Scholar] [CrossRef]
- Meng, Z.; Stolz, R.M.; Mendecki, L.; Mirica, K.A. Electrically transduced chemical sensors based on two-dimensional nanomaterials. Chem. Rev. 2019, 119, 478–598. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Sruthi, V.P.; Satija, J.; Senthilkumar, S.; Kim, Y.J. Materials and design strategies for the electrochemical detection of antineoplastic drugs: Progress and perspectives. Mater. Sci. Eng. R Rep. 2024, 161, 100840. [Google Scholar] [CrossRef]
- El-Fatah, G.A.; Magar, H.S.; Hassan, R.Y.; Mahmoud, R.; Farghali, A.A.; Hassouna, M.E. A novel gallium oxide nanoparticles-based sensor for the simultaneous electrochemical detection of Pb2+, Cd2+ and Hg2+ ions in real water samples. Sci. Rep. 2022, 12, 20181. [Google Scholar] [CrossRef]
- Hoyos-Arbeláez, J.; Vázquez, M.; Contreras-Calderón, J. Electrochemical methods as a tool for determining the antioxidant capacity of food and beverages: A review. Food Chem. 2017, 221, 1371–1381. [Google Scholar] [CrossRef]
- Kaur, H.; Siwal, S.S.; Chauhan, G.; Saini, A.K.; Kumari, A.; Thakur, V.K. Recent advances in electrochemical-based sensors amplified with carbon-based nanomaterials (CNMs) for sensing pharmaceutical and food pollutants. Chemosphere 2022, 304, 135182. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.; Nisar, A.; Khan, K.; Nisar, J.; Niaz, A.; Ashiq, M.N.; Akhter, M.S. Amino acid functionalized glassy carbon electrode for the simultaneous detection of thallium and mercuric ions. Electrochim. Acta 2019, 321, 34658. [Google Scholar] [CrossRef]
- Bi, Z.; Kong, Q.; Cao, Y.; Sun, G.; Su, F.; Wei, X.; Li, X.; Ahmad, A.; Xie, L.; Chen, C.-M. Biomass-derived porous carbon materials with different dimensions for supercapacitor electrodes: A review. J. Mater. Chem. A 2019, 7, 16028–16045. [Google Scholar] [CrossRef]
- Keerthana, P.; George, A.; Bharath, M.; Ghosh, M.; Varghese, A. Dual ion specific electrochemical sensor using aminothiazole-engineered carbon quantum dots. Chem. Eng. J. 2024, 480, 148018. [Google Scholar] [CrossRef]
- Beitollahi, H.; Khalilzadeh, M.A.; Tajik, S.; Safaei, M.; Zhang, K.; Jang, H.W.; Shokouhimehr, M. Recent advances in applications of voltammetric sensors modified with ferrocene and its derivatives. ACS Omega 2020, 5, 2049–2059. [Google Scholar] [CrossRef]
- Ahmed, H.E.H.; SOYLAK, M. Exploring the potential of carbon quantum dots (CQDs) as an advanced nanomaterial for effective sensing and extraction of toxic pollutants. TrAC Trends Anal. Chem. 2024, 180, 117939. [Google Scholar] [CrossRef]
- Rojas, L.M.G.; Huerta-Aguilar, C.A.; Tecuapa-Flores, E.D.; Huerta-José, D.S.; Thangarasu, P.; Sidhu, J.S.; Singh, N.; Téllez, M.D.L.L.C. Why ionic liquids coated ZnO nanocomposites emerging as environmental remediates: Enhanced photo-oxidation of 4-nitroaniline and encouraged antibacterial behavior. J. Mol. Liq. 2020, 319, 114107. [Google Scholar] [CrossRef]
- Tajik, S.; Beitollahi, H.; Nejad, F.G.; Safaei, M.; Zhang, K.; Van Le, Q.; Varma, R.S.; Jang, H.W.; Shokouhimehr, M. Developments and applications of nanomaterial-based carbon paste electrodes. RSC Adv. 2020, 10, 21561–21581. [Google Scholar] [CrossRef]
- Ronkainen, N.J.; Halsall, H.B.; Heineman, W.R. Electrochemical biosensors. Chem. Soc. Rev. 2010, 39, 1747–1763. [Google Scholar] [CrossRef]
- Theyagarajan, K.; Lakshmi, B.A.; Kim, C.; Kim, Y.J. A nanohybrid-based smartphone-compatible high performance electrochemical glucose sensor. Mater. Today Chem. 2024, 41, 102282. [Google Scholar] [CrossRef]
- Hernandez-Vargas, G.; Sosa-Hernández, J.E.; Saldarriaga-Hernandez, S.; Villalba-Rodríguez, A.M.; Parra-Saldivar, R.; Iqbal, H.M. Electrochemical biosensors: A solution to pollution detection with reference to environmental contaminants. Biosensors 2018, 8, 29. [Google Scholar] [CrossRef] [PubMed]
- Altintas, Z.; Akgun, M.; Kokturk, G.; Uludag, Y. A fully automated microfluidic-based electrochemical sensor for real-time bacteria detection. Biosens. Bioelectron. 2018, 100, 541–548. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Hu, Y.; Singh, S.; Mizaikoff, B. Emerging biosensor platforms for the assessment of water-borne pathogens. Analyst 2018, 143, 359–373. [Google Scholar] [CrossRef]
- Pingarrón, J.M.; Labuda, J.; Barek, J.; Brett, C.M.; Camoes, M.F.; Fojta, M.; Hibbert, D.B. Terminology of electrochemical methods of analysis (IUPAC Recommendations 2019). Pure Appl. Chem. 2020, 92, 641–694. [Google Scholar] [CrossRef]
- Yuan, Z.; Hou, L.; Bariya, M.; Nyein, H.Y.Y.; Tai, L.C.; Ji, W.; Li, L.; Javey, A. A multi-modal sweat sensing patch for cross-verification of sweat rate, total ionic charge, and Na+ concentration. Lab. Chip. 2019, 19, 3179–3189. [Google Scholar] [CrossRef]
- Eivazzadeh-Keihan, R.; Pashazadeh, P.; Hejazi, M.; de la Guardia, M.; Mokhtarzadeh, A. Recent advances in nanomaterial-mediated bio and immune sensors for detection of aflatoxin in food products. TrAC Trends Anal. Chem. 2017, 87, 112–128. [Google Scholar] [CrossRef]
- Ding, J.; Qin, W. Recent advances in potentiometric biosensors. TrAC Trends Anal. Chem. 2020, 124, 115803. [Google Scholar] [CrossRef]
- Fauzi, A.S.A.; Hamidah, N.L.; Sato, S.; Shintani, M.; Putri, G.K.; Kitamura, S.; Hatakeyama, K.; Quitain, A.T.; Kida, T. Carbon-based potentiometric hydrogen sensor using a proton conducting graphene oxide membrane coupled with a WO3 sensing electrode. Sens. Actuators B Chem. 2020, 323, 128678. [Google Scholar] [CrossRef]
- Liu, J.; Jalali, M.; Mahshid, S.; Wachsmann-Hogiu, S. Are plasmonic optical biosensors ready for use in point-of-need applications? Analyst 2020, 145, 364–384. [Google Scholar] [CrossRef]
- Zhang, M.; Liao, C.; Mak, C.H.; You, P.; Mak, C.L.; Yan, F. Highly sensitive glucose sensors based on enzyme-modified whole-graphene solution-gated transistors. Sci. Rep. 2015, 5, 8311. [Google Scholar] [CrossRef]
- Kong, Y.; Liu, D.; Guo, X.; Chen, X. Fluorescence detection of three types of pollutants based on fluorescence resonance energy transfer and its comparison with colorimetric detection. RSC Adv. 2023, 13, 22043–22053. [Google Scholar] [CrossRef] [PubMed]
- Tabasi, O.; Falamaki, C. Recent advancements in the methodologies applied for the sensitivity enhancement of surface plasmon resonance sensors. Anal. Methods 2018, 10, 3906–3925. [Google Scholar] [CrossRef]
- Çaktü, K.; Özgür, E.; Bereli, N.; Denizli, A. Diclofenac Imprinted Surface Plasmon Resonance (SPR) Based Sensor. ChemistrySelect 2022, 7, e202200436. [Google Scholar] [CrossRef]
- Çaktü Güler, K.; Göktürk, I.; Yılmaz, F.; Araz, A.; Denizli, A. Plasmonic nanosensors for environmental pollutants sensing: Recent advances and perspectives. Essential Chem. 2024, 1, 1–19. [Google Scholar] [CrossRef]
- Achadu, O.J.; Abe, F.; Hossain, F.; Nasrin, F.; Yamazaki, M.; Suzuki, T.; Park, E.Y. Sulfur-doped carbon dots@polydopamine-functionalized magnetic silver nanocubes for dual-modality detection of norovirus. Biosens. Bioelectron. 2021, 193, 113540. [Google Scholar] [CrossRef]
- Xue, Y.; Liu, C.; Andrews, G.; Wang, J.; Ge, Y. Recent advances in carbon quantum dots for virus detection, as well as inhibition and treatment of viral infection. Nano Converg. 2022, 9, 15. [Google Scholar] [CrossRef]
- Pourmadadi, M.; Nouralishahi, A.; Shalbaf, M.; Shabani Shayeh, J.; Nouralishahi, A. An electrochemical aptasensor for detection of prostate-specific antigen-based on carbon quantum dots-gold nanoparticles. Biotechnol. Appl. Biochem. 2023, 70, 175–183. [Google Scholar] [CrossRef]
- Alonso-Fradejas, A. Leaving no one unscathed in sustainability transitions: The life purging agro-extractivism of corporate renewables. J. Rural Stud. 2021, 81, 127–138. [Google Scholar] [CrossRef]
- Neill, A.J.; Tetzlaff, D.; Strachan, N.J.C.; Hough, R.L.; Avery, L.M.; Watson, H.; Soulsby, C. Using spatial-stream-network models and long-term data to understand and predict dynamics of fecal contamination in a mixed land-use catchment. Sci. Total Environ. 2018, 612, 840–852. [Google Scholar] [CrossRef]
- Sachs, J.D.; Schmidt-Traub, G.; Mazzucato, M.; Messner, D.; Nakicenovic, N.; Rockström, J. Six transformations to achieve the sustainable development goals. Nat. Sustain. 2019, 2, 805–814. [Google Scholar] [CrossRef]
- Yasmeen, R.; Khan, F.S.; Nisa, W.U.; Saleem, A.R.; Awais, M.; Jameel, M.; Dara, R.N. Enhanced water purification by using graphene oxide nano-membranes: A novel approach for mitigating industrial pollutant. Carbon. Trends 2025, 19, 100486. [Google Scholar] [CrossRef]
- Baddianaah, I.; Dongzagla, A.; Salifu, S.N. Navigating access to safe water by rural households in sub-Saharan Africa: Insights from north-western Ghana. Sustain. Environ. 2024, 10, 2303803. [Google Scholar] [CrossRef]
- Dinar, A. Challenges to water resource management: The role of economic and modeling approaches. Water 2024, 16, 610. [Google Scholar] [CrossRef]
- Chaturvedi, S.; Dave, P.N. Water purification using nanotechnology an emerging opportunity. Chem. Methodol. 2019, 3, 115–144. [Google Scholar]
- Bolisetty, S.; Peydayesh, M.; Mezzenga, R. Sustainable technologies for water purification from heavy metals: Review and analysis. Chem. Soc. Rev. 2019, 48, 463–487. [Google Scholar] [CrossRef]
- Nizamuddin, S.; Siddiqui, M.T.H.; Mubarak, N.M.; Baloch, H.A.; Abdullah, E.C.; Mazari, S.A.; Tanksale, A. Iron oxide nanomaterials for the removal of heavy metals and dyes from wastewater. In Nanoscale Materials in Water Purification; Elsevier: Amsterdam, The Netherlands, 2019; pp. 447–472. [Google Scholar]
- Mukhopadhyay, R.; Sarkar, B.; Khan, E.; Alessi, D.S.; Biswas, J.K.; Manjaiah, K.M.; Eguchi, M.; Wu, K.C.W.; Yamauchi, Y.; Ok, Y.S. Nanomaterials for sustainable remediation of chemical contaminants in water and soil. Crit. Rev. Environ. Sci. Technol. 2022, 52, 2611–2660. [Google Scholar] [CrossRef]
- Fiorentini, E.F.; Valdés Rodríguez, E.M.; Bonilla-Petriciolet, A.; Escudero, L.B. Carbon dots in organic pollutant removal. In Carbon Dots: Recent Developments and Future Perspectives; American Chemical Society: Washington, DC, USA, 2024; pp. 259–275. [Google Scholar]
- Aboelfetoh, E.F.; Elabedien, M.E.Z.; Ebeid, E.-Z.M. Effective treatment of industrial wastewater applying SBA-15 mesoporous silica modified with graphene oxide and hematite nanoparticles. J. Environ. Chem. Eng. 2021, 9, 104817. [Google Scholar] [CrossRef]
- Kristanti, R.A.; Hadibarata, T.; Syafrudin, M.; Yılmaz, M.; Abdullah, S. Microbiological contaminants in drinking water: Current status and challenges. Water Air Soil Pollut. 2022, 233, 299. [Google Scholar] [CrossRef]
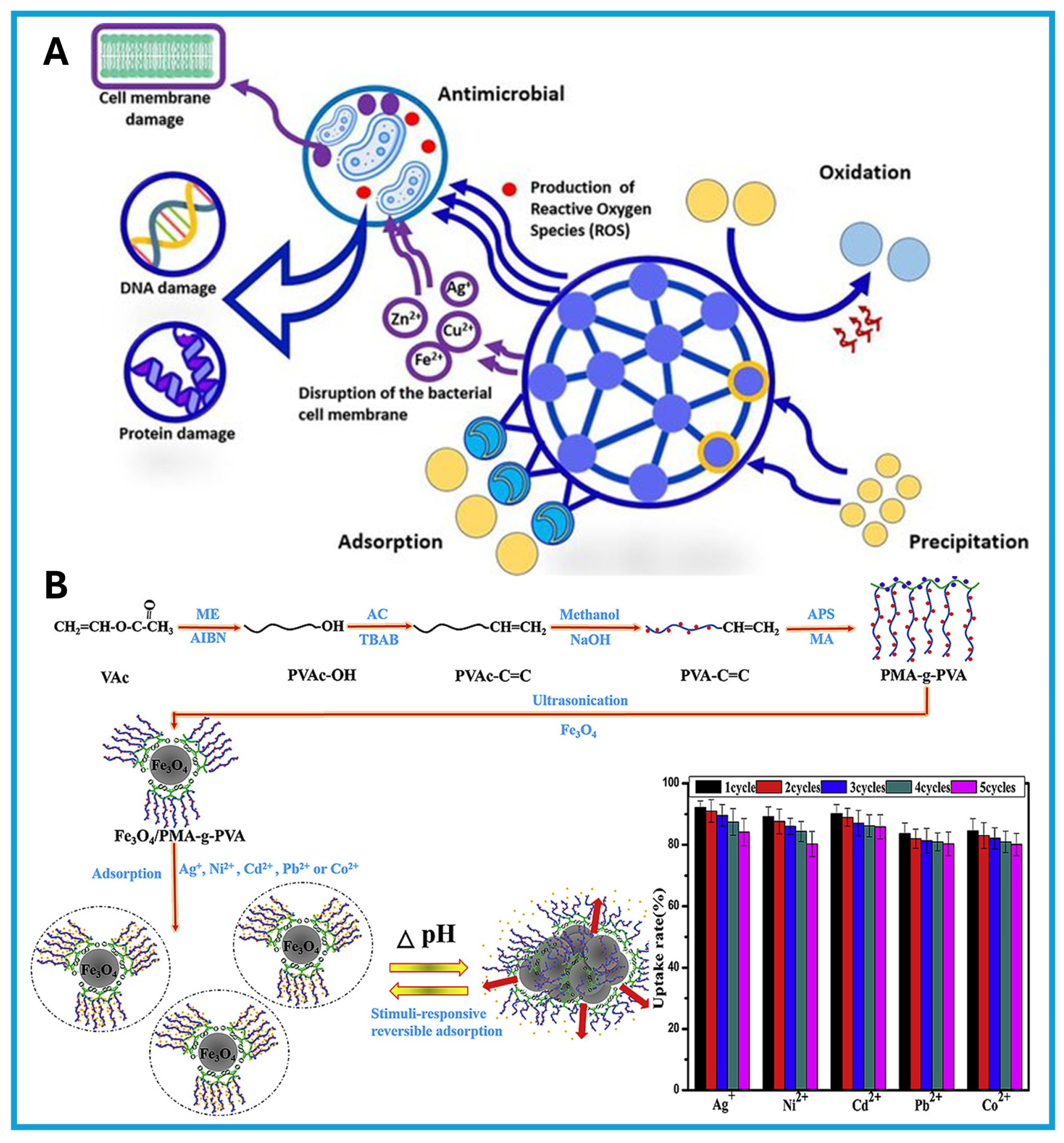
- Liu, X.; Guan, J.; Lai, G.; Xu, Q.; Bai, X.; Wang, Z.; Cui, S. Stimuli-responsive adsorption behavior toward heavy metal ions based on comb polymer functionalized magnetic nanoparticles. J. Clean. Prod. 2020, 253, 119915. [Google Scholar] [CrossRef]
- Elhenawy, S.; Khraisheh, M.; AlMomani, F.; Al-Ghouti, M.; Selvaraj, R.; Al-Muhtaseb, A.A. Emerging nanomaterials for drinking water purification: A new era of water treatment technology. Nanomaterials 2024, 14, 1707. [Google Scholar] [CrossRef]
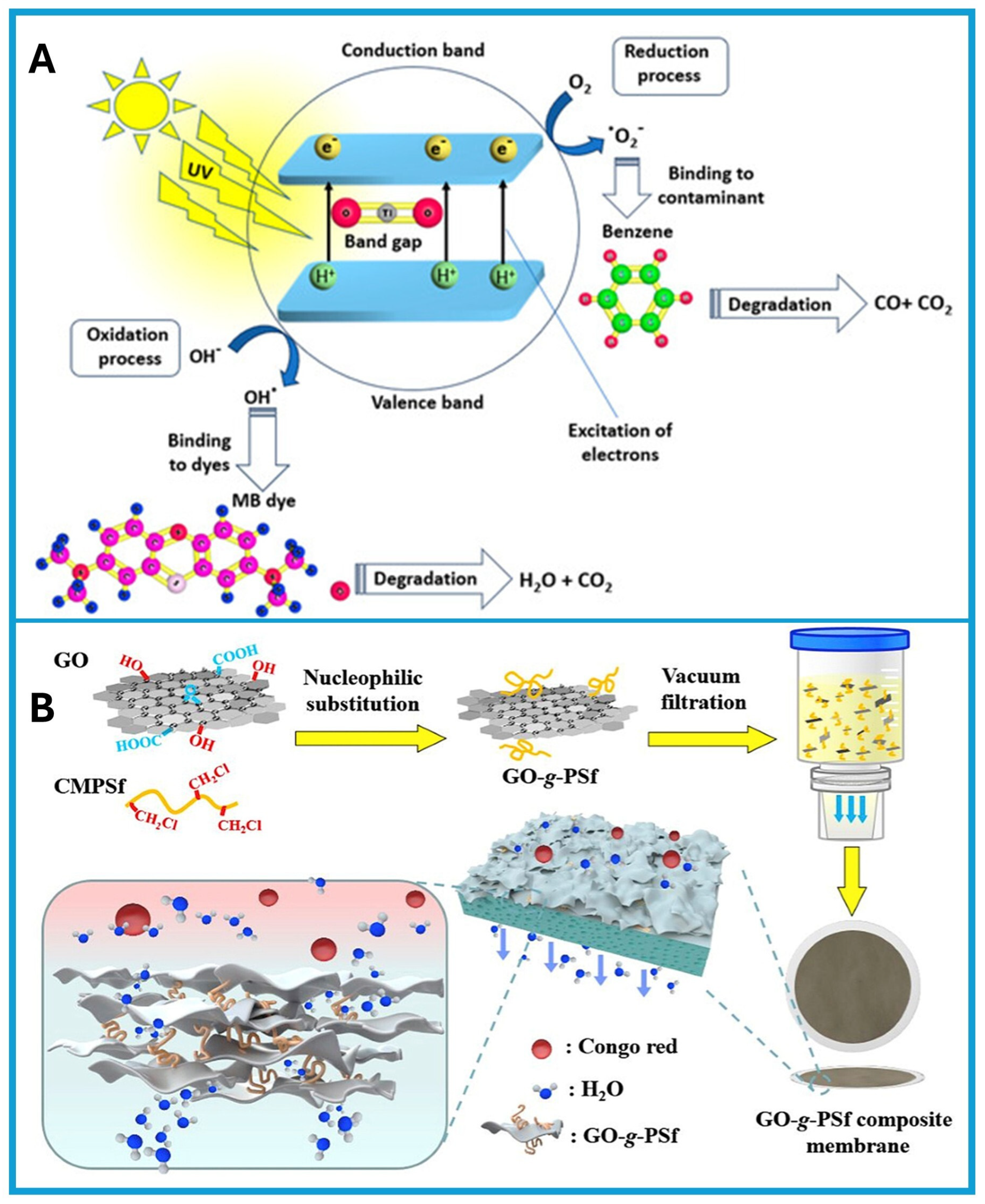
- Li, J.; Hu, M.; Pei, H.; Ma, X.; Yan, F.; Dlamini, D.S.; Cui, Z.; He, B.; Li, J.; Matsuyama, H. Improved water permeability and structural stability in a polysulfone-grafted graphene oxide composite membrane used for dye separation. J. Membr. Sci. 2020, 595, 117547. [Google Scholar] [CrossRef]
- Jiang, D.; Zeng, G.; Huang, D.; Chen, M.; Zhang, C.; Huang, C.; Wan, J. Remediation of contaminated soils by enhanced nanoscale zero-valent iron. Environ. Res. 2018, 163, 217–227. [Google Scholar] [CrossRef] [PubMed]
- Kunduru, K.R.; Nazarkovsky, M.; Farah, S.; Pawar, R.P.; Basu, A.; Domb, A.J. Nanotechnology for water purification: Applications of nanotechnology methods in wastewater treatment. In Water Purification; Academic press: Cambridge, MA, USA, 2017; pp. 33–74. [Google Scholar]
- Mu, Y.; Jia, F.; Ai, Z.; Zhang, L. Iron oxide shell mediated environmental remediation properties of nano zero-valent iron. Environ. Sci. Nano 2017, 4, 27–45. [Google Scholar] [CrossRef]
- Karim, S.; Bae, S.; Greenwood, D.; Hanna, K.; Singhal, N. Degradation of 17α-ethinylestradiol by nano zero-valent iron under different pH and dissolved oxygen levels. Water. Res. 2017, 125, 32–41. [Google Scholar] [CrossRef]
- Pathakoti, K.; Manubolu, M.; Hwang, H.M. Nanotechnology applications for environmental industry. In Handbook of Nanomaterials for Industrial Applications; Elsevier: Amsterdam, The Netherlands, 2018; pp. 894–907. [Google Scholar]
- Chi, Z.; Lei, J.; Ding, L.; Dong, J. Mechanism on emulsified vegetable oil stimulating nitrobenzene degradation coupled with dissimilatory iron reduction in aquifer media. Bioresour. Technol. 2018, 260, 38–43. [Google Scholar] [CrossRef]
- Primo, J.d.O.; Horsth, D.F.; Correa, J.d.S.; Das, A.; Bittencourt, C.; Umek, P.; Buzanich, A.G.; Radtke, M.; Yusenko, K.V.; Zanette, C.; et al. Synthesis and characterization of Ag/ZnO nanoparticles for bacteria disinfection in water. Nanomaterials 2022, 12, 1764. [Google Scholar] [CrossRef]
- Pan, Y.; Liu, X.; Zhang, W.; Liu, Z.; Zeng, G.; Shao, B.; Chen, M. Advances in photocatalysis based on fullerene C60 and its derivatives: Properties, mechanism, synthesis, and applications. Appl. Catal. B 2020, 265, 118579. [Google Scholar] [CrossRef]
- Gopinath, K.P.; Madhav, N.V.; Krishnan, A.; Malolan, R.; Rangarajan, G. Present applications of titanium dioxide for the photocatalytic removal of pollutants from water: A review. J. Environ. Manag. 2020, 270, 110906. [Google Scholar] [CrossRef]
- Nanditha, T.K.; Vishwashreelakshmi, P.S.; Bhat, S.; Waiker, M.R.; Sonkawade, R.G.; Ballal, M.; Gurumurthy, S.C. Eco-friendly synthesis and multifaceted environmental applications of AgCu bimetallic nanoparticles. Mater. Res. Bull. 2025, 184, 113233. [Google Scholar] [CrossRef]
- Khin, M.M.; Nair, A.S.; Babu, V.J.; Murugan, R.; Ramakrishna, S. A review on nanomaterials for environmental remediation. Energy Environ. Sci. 2012, 5, 8075–8109. [Google Scholar] [CrossRef]
- Hong, J.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Nanomaterials in agricultural production: Benefits and possible threats? In Sustainable Nanotechnology and the Environment: Advances and Achievements; ASC Publications: Washington, DC, USA, 2013; pp. 73–90. [Google Scholar]
- Das, P.; Maity, P.P.; Ganguly, S.; Ghosh, S.; Baral, J.; Bose, M.; Choudhary, S.; Gangopadhyay, S.; Dhara, S.; Das, A.K. Biocompatible carbon dots derived from κ-carrageenan and phenylboronic acid for dual-modality sensing platform of sugar and its anti-diabetic drug release behavior. Int. J. Biol. Macromol. 2019, 132, 316–329. [Google Scholar] [CrossRef] [PubMed]
- Wadhawan, S.; Jain, A.; Nayyar, J.; Mehta, S.K. Role of nanomaterials as adsorbents in heavy metal ion removal from waste water: A review. J. Water Process Eng. 2020, 33, 101038. [Google Scholar] [CrossRef]
- Xiao, J.; Lv, W.; Xie, Z.; Song, Y.; Zheng, Q. L-cysteine-reduced graphene oxide/poly (vinyl alcohol) ultralight aerogel as a broad-spectrum adsorbent for anionic and cationic dyes. J. Mater. Sci. 2017, 52, 5807–5821. [Google Scholar] [CrossRef]
- Chatzimarkou, A.; Stalikas, C. Adsorptive Removal of Estriol from Water Using Graphene-Based Materials and Their Magnetite Composites: Heterogeneous Fenton-Like Non-toxic Degradation on Magnetite/Graphene Oxide. Int. J. Environ. Res. 2020, 14, 269–287. [Google Scholar] [CrossRef]
- Singh, N.B.; Nagpal, G.; Agrawal, S. Water purification by using adsorbents: A review. Environ. Technol. Innov. 2018, 11, 187–240. [Google Scholar] [CrossRef]
- Baruah, J.; Chaliha, C.; Nath, B.K.; Kalita, E. Enhancing arsenic sequestration on ameliorated waste molasses nanoadsorbents using response surface methodology and machine-learning frameworks. Environ. Sci. Pollut. Res. 2021, 28, 11369–11383. [Google Scholar] [CrossRef]
- Osman, A.I.; Chen, Z.; Elgarahy, A.M.; Farghali, M.; Mohamed, I.M.; Priya, A.K.; Hawash, H.B.; Yap, P.S. Membrane technology for energy saving: Principles, techniques, applications, challenges, and prospects. Adv. Energy Sustain. Res. 2024, 5, 2400011. [Google Scholar] [CrossRef]
- Linklater, D.P.; Baulin, V.A.; Le Guével, X.; Fleury, J.B.; Hanssen, E.; Nguyen, T.H.P.; Juodkazis, S.; Bryant, G.; Crawford, R.J.; Stoodley, P.; et al. Antibacterial action of nanoparticles by lethal stretching of bacterial cell membranes. Adv. Mater. 2020, 32, 2005679. [Google Scholar] [CrossRef]
- Jadhav, S.A.; Khedkar, M.V.; Somvanshi, S.B.; Jadhav, K.M. Magnetically retrievable nanoscale nickel ferrites: An active photocatalyst for toxic dye removal applications. Ceram. Int. 2021, 47, 28623–28633. [Google Scholar] [CrossRef]
- Haque, F.; Blanchard, A.; Laipply, B.; Dong, X. Visible-light-activated TiO2-based photocatalysts for the inactivation of pathogenic bacteria. Catalysts 2024, 14, 855. [Google Scholar] [CrossRef]
- Kamali, M.; Persson, K.M.; Costa, M.E.; Capela, I. Sustainability criteria for assessing nanotechnology applicability in industrial wastewater treatment: Current status and future outlook. Environ. Int. 2019, 125, 261–276. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Lin, Q.; Chang, H.T. Recent advances and sensing applications of carbon dots. Small Methods 2020, 4, 1900387. [Google Scholar] [CrossRef]
- Das, S.; Chakraborty, J.; Chatterjee, S.; Kumar, H. Prospects of biosynthesized nanomaterials for the remediation of organic and inorganic environmental contaminants. Environ. Sci. Nano. 2018, 5, 2784–2808. [Google Scholar] [CrossRef]
- Zhang, W.J.; Liu, S.G.; Han, L.; Luo, H.Q.; Li, N.B. A ratiometric fluorescent and colorimetric dual-signal sensing platform based on N-doped carbon dots for selective and sensitive detection of copper (II) and pyrophosphate ion. Sens. Actuators B Chem. 2019, 283, 215–221. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Niu, Q.; Gu, X.; Yang, N.; Zhao, G. Recent progress on carbon nanomaterials for the electrochemical detection and removal of environmental pollutants. Nanoscale 2019, 11, 11992–12014. [Google Scholar] [CrossRef]
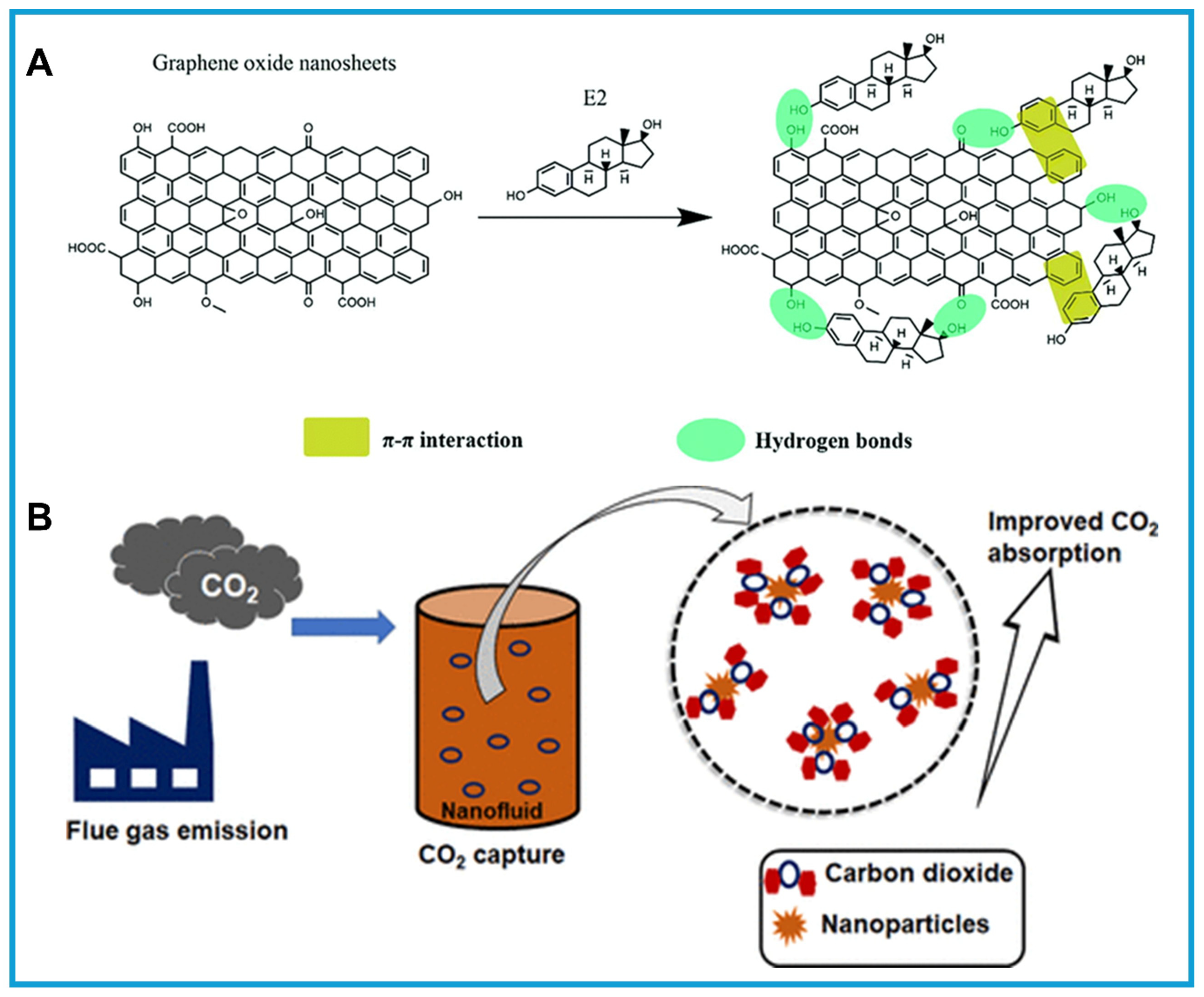
- Khumalo, N.P.; Mahlangu, O.T.; Mamba, B.B.; Motsa, M.M. Graphene Oxide Enhanced Monoethanolamine and Ethylenediamine Nanofluids for Efficient Carbon Dioxide Uptake from Flue Gas. ACS Omega 2024, 9, 25625–25637. [Google Scholar] [CrossRef]
- Jiang, L.H.; Liu, Y.G.; Zeng, G.M.; Xiao, F.Y.; Hu, X.J.; Hu, X.; Wang, H.; Li, T.-T.; Zhou, L.; Tan, X.F. Removal of 17β-estradiol by few-layered graphene oxide nanosheets from aqueous solutions: External influence and adsorption mechanism. Chem. Eng. J. 2016, 284, 93–102. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, Y.; Kang, S.; Wang, Z.; Wu, C. Microplastics in soil: A review on methods, occurrence, sources, and potential risk. Sci. Total Environ. 2021, 780, 146546. [Google Scholar] [CrossRef]
- Stephens, V.R.; Horner, K.B.; Avila, W.M.; Spicer, S.K.; Chinni, R.; Bernabe, E.B.; Gaddy, J.A. The impact of persistent organic pollutants on fertility: Exposure to the environmental toxicant 2, 3, 7, 8-tetrachlorodibenzo-p-dioxin alters reproductive tract immune responses. Front. Immunol. 2024, 15, 1497405. [Google Scholar] [CrossRef]
- Rai, P.K. Novel adsorbents in remediation of hazardous environmental pollutants: Progress, selectivity, and sustainability prospects. Clean. Mater. 2022, 3, 100054. [Google Scholar] [CrossRef]
- Singh, D.V.; Bhat, R.A.; Dervash, M.A.; Qadri, H.; Mehmood, M.A.; Dar, G.H.; Hameed, M.; Rashid, N. Wonders of nanotechnology for remediation of polluted aquatic environs. In Fresh Water Pollution Dynamics and Remediation; Springer: Cham, Switzerland, 2020; pp. 319–339. [Google Scholar]
- Wang, Y.; Wang, T.; Gu, Q.; Shang, J. Adsorption Removal of NO2 Under Low-Temperature and Low-Concentration Conditions: A Review of Adsorbents and Adsorption Mechanisms. Adv. Mater. 2025, 37, 2401623. [Google Scholar] [CrossRef] [PubMed]
- Reddy, B.M. Two-Dimensional Nanomaterials for Antibacterial and Antimicrobial Activity for Water Contaminants Disinfection. In Advanced Two-Dimensional Nanomaterials for Environmental and Sensing Applications; CRC Press: Boca Raton, FL, USA, 2024; pp. 234–280. [Google Scholar]
- Kumar, A.; Jayeoye, T.J.; Mohite, P.; Singh, S.; Rajput, T.; Munde, S.; Parihar, A. Sustainable and consumer-centric nanotechnology-based materials: An update on the multifaceted applications, risks and tremendous opportunities. Nano-Struct. Nano-Objects 2024, 38, 101148. [Google Scholar] [CrossRef]
- Huang, J.; Liu, J.; Wang, J. Optical properties of biomass-derived nanomaterials for sensing, catalytic, biomedical and environmental applications. TrAC Trends Anal. Chem. 2020, 124, 115800. [Google Scholar] [CrossRef]
- Seevakan, K.; Manikandan, A.; Devendran, P.; Baykal, A.; Alagesan, T. Electrochemical and magneto-optical properties of cobalt molybdate nano-catalyst as high-performance supercapacitor. Ceram. Int. 2018, 44, 17735–17742. [Google Scholar] [CrossRef]
- Mashangva, T.T.; Goel, A.; Bagri, U.; Prasher, S.; Sharma, A.; Kumar, M.; Singh, P.K. Frontiers in MXene research: Pioneering synthesis, unveiled properties, and emerging applications in VOC detection. Appl. Mater. Today 2024, 38, 102163. [Google Scholar] [CrossRef]
- Sheikh, R.A.; Kingsley, D. Carbon Dots in Cancer Therapeutics: Advancements in Drug Delivery and Imaging Technologies. Res. J. Biotechnol. 2024, 20, 225–240. [Google Scholar] [CrossRef]
- Abbas, K.; Jiang, L.; Li, Y.; Li, Z.; Bi, H. Carbon Dots. In Elemental Carbon; Royal Society of Chemistry: London, UK, 2024; pp. 238–300. [Google Scholar]
- Muniandy, S.; The, S.J.; Thong, K.L.; Thiha, A.; Dinshaw, I.J.; Lai, C.W.; Ibrahim, F.; Leo, B. F Carbon nanomaterial-based electrochemical biosensors for foodborne bacterial detection. Crit. Rev. Anal. Chem. 2019, 49, 510–533. [Google Scholar] [CrossRef]
- Castelo, R.M.; de Albuquerque Oliveira, M.; Furtado, R.F.; de Oliveira, B.P.; Martoni, L.V.L.; Machado, T.F.; Muniz, C.R.; Abreu, F.O.M.d.S.; Machado, S.A.S.; Melo, A.M.A.; et al. Carbon-dot pequi-nut in the development of immunosensor to detect pathogenic bacteria. Braz. J. Microbiol. 2025, 56, 275–284. [Google Scholar] [CrossRef]
- Kumari, M.; Chaudhary, G.R.; Chaudhary, S.; Umar, A.; Baskoutas, S. Fluorescent Carbon Dots from Disposable Masks: Pathogen Sensing and UV-Blocking Film Integration. J. Fluoresc. 2025. ahead of print. [Google Scholar] [CrossRef]
- Xiao, M.; Mei, L.; Qi, J.; Zhu, L.; Wang, F. Functionalized carbon quantum dots fluorescent sensor array assisted by a machine learning algorithm for rapid foodborne pathogens identification. Microchem. J. 2024, 201, 110701. [Google Scholar] [CrossRef]
- Sharma, S.; Dutta, V.; Singh, P.; Raizada, P.; Rahmani-Sani, A.; Hosseini-Bandegharaei, A.; Thakur, V.K. Carbon quantum dot supported semiconductor photocatalysts for efficient degradation of organic pollutants in water: A review. J. Clean. Prod. 2019, 228, 755–769. [Google Scholar] [CrossRef]
- Dhanush, C.; Ismayati, M.; Sethuraman, M.G. Photocatalysis of an organic pollutant using ZnOsemiconductor nanoparticles embedded biogenic nitrogen doped carbon dots. Mater. Sci. Eng. B 2025, 316, 118090. [Google Scholar] [CrossRef]
- Wang, Q.; Wen, G.; Yang, Z.; Guo, Q.; Zhang, B.; Nie, Y.; Wang, D. Preparation and study of CDs-WO3 composites with enhanced photocatalytic antimicrobial properties and degradation of dyes. Biochem. Eng. J. 2025, 217, 109670. [Google Scholar] [CrossRef]
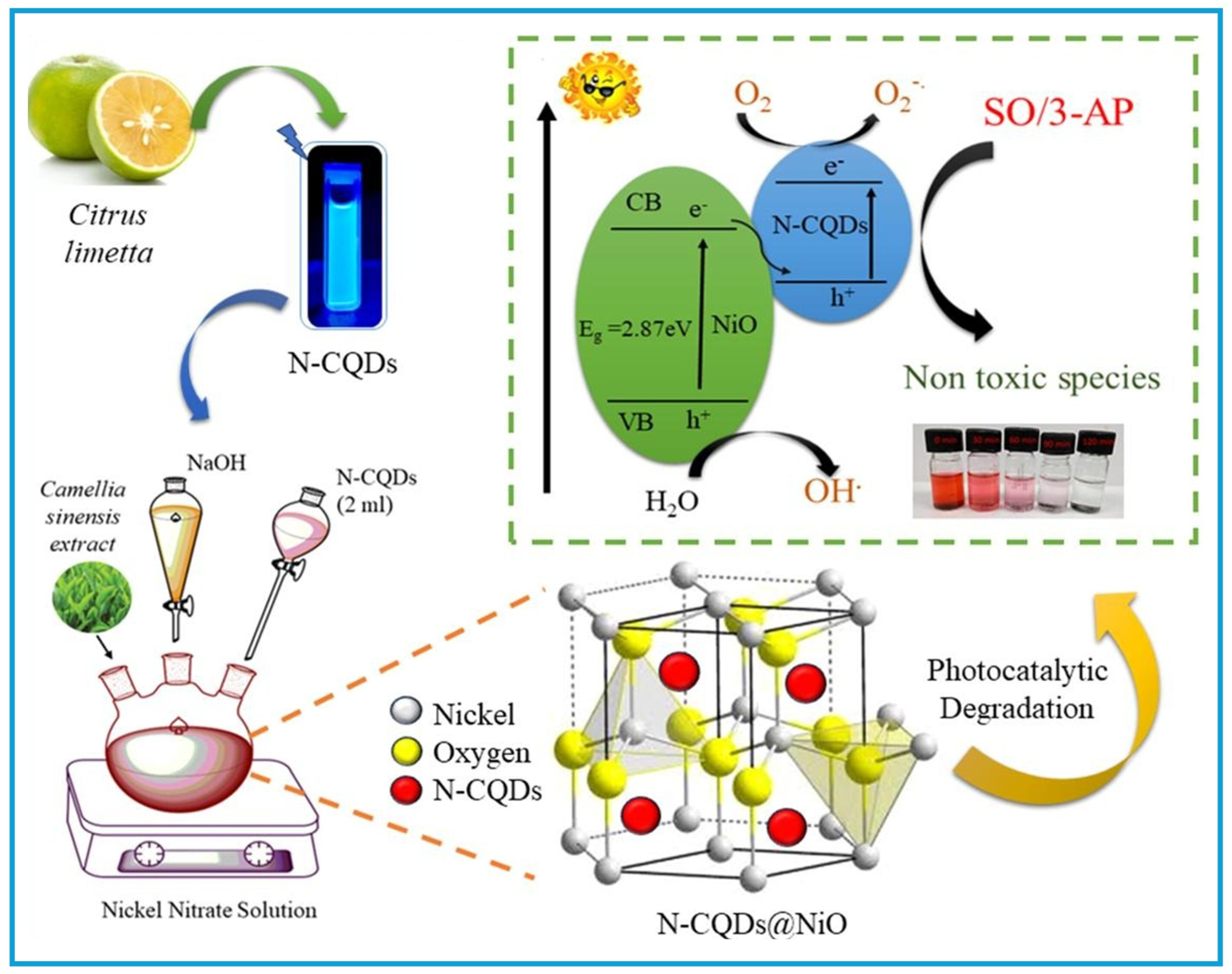
- Rani, M.; Kaur, D.; Shanker, U.; Sillanpää, M. Green synthesized N-CQDs@NiO nanocomposites for efficient photocatalytic degradation of organic pollutants from water. Inorg. Chem. Commun. 2024, 170, 113326. [Google Scholar] [CrossRef]
- Tahir, M.B.; Rafique, M.; Rafique, M.S.; Nawaz, T.; Rizwan, M.; Tanveer, M. Photocatalytic nanomaterials for degradation of organic pollutants and heavy metals. In Nanotechnology and Photocatalysis for Environmental Applications; Elsevier: Amsterdam, The Netherlands, 2020; pp. 119–138. [Google Scholar]
- Iraqui, S.; Dubey, A.; Savarimuthu, I.; Shankar, A.; Jaiswal, A.; Bahadur, I.; Uddin, I.; Mohammad, F. Bi-functional aqueous starch capped CdS quantum dots synthesis and their application as sensor of heavy metal-ions as well as photocatalytic dye degradation. J. Mol. Liq. 2023, 388, 122794. [Google Scholar] [CrossRef]
- Jarque, S.; Bittner, M.; Blaha, L.; Hilscherova, K. Yeast biosensors for detection of environmental pollutants: Current state and limitations. Trends Biotechnol. 2016, 34, 408–419. [Google Scholar] [CrossRef]
- Mezzaroba, L.; Alfieri, D.F.; Simão, A.N.C.; Reiche, E.M.V. The role of zinc, copper, manganese and iron in neurodegenerative diseases. Neurotoxicology 2019, 74, 230–241. [Google Scholar] [CrossRef]
- Beaumier, E.P.; Pearce, A.J.; See, X.Y.; Tonks, I.A. Modern applications of low-valent early transition metals in synthesis and catalysis. Nat. Rev. Chem. 2019, 3, 15–34. [Google Scholar] [CrossRef]
- Nodzewska, A.; Wadolowska, A.; Watkinson, M. Recent advances in the catalytic oxidation of alkene and alkane substrates using immobilized manganese complexes with nitrogen containing ligands. Coord. Chem. Rev. 2019, 382, 181–216. [Google Scholar] [CrossRef]
- Báez-Santos, Y.M.; John, S.E.S.; Mesecar, A.D. The SARS-coronavirus papain-like protease: Structure, function and inhibition by designed antiviral compounds. Antiviral Res. 2015, 115, 21–38. [Google Scholar] [CrossRef]
- Kumar, P.; Kim, K.H.; Bansal, V.; Lazarides, T.; Kumar, N. Progress in the sensing techniques for heavy metal ions using nanomaterials. J. Ind. Eng. Chem. 2017, 54, 30–43. [Google Scholar] [CrossRef]
- Molaei, M.J. Principles, mechanisms, and application of carbon quantum dots in sensors: A review. Anal. Methods 2020, 12, 1266–1287. [Google Scholar] [CrossRef]
- Wolfbeis, O.S. An overview of nanoparticles commonly used in fluorescent bioimaging. Chem. Soc. Rev. 2015, 44, 4743–4768. [Google Scholar] [CrossRef]
- Mishra, V.; Patil, A.; Thakur, S.; Kesharwani, P. Carbon dots: Emerging theranostic nanoarchitectures. Drug Discov. Today 2018, 23, 1219–1232. [Google Scholar] [CrossRef]
- Dhenadhayalan, N.; Lin, K.C.; Saleh, T.A. Recent advances in functionalized carbon dots toward the design of efficient materials for sensing and catalysis applications. Small 2020, 16, 1905767. [Google Scholar] [CrossRef]
- Liu, S.; Cui, J.; Huang, J.; Tian, B.; Jia, F.; Wang, Z. Facile one-pot synthesis of highly fluorescent nitrogen-doped carbon dots by mild hydrothermal method and their applications in detection of Cr(VI) ions. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 206, 65–71. [Google Scholar] [CrossRef]
- Feng, H.; Qian, Z. Functional carbon quantum dots: A versatile platform for chemosensing and biosensing. Chem. Rec. 2018, 18, 491–505. [Google Scholar] [CrossRef]
- Xu, J.; Wei, D.; Wang, F.; Bai, C.; Du, Y. Bioassay: A useful tool for evaluating reclaimed water safety. J. Environ. Sci. 2020, 88, 165–176. [Google Scholar] [CrossRef]
- Praneerad, J.; Thongsai, N.; Supchocksoonthorn, P.; Kladsomboon, S.; Paoprasert, P. Multipurpose sensing applications of biocompatible radish-derived carbon dots as Cu2+ and acetic acid vapor sensors. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2019, 211, 59–70. [Google Scholar] [CrossRef]
- Wang, C.; Shi, H.; Yang, M.; Yan, Y.; Liu, E.; Ji, Z.; Fan, J. Facile synthesis of novel carbon quantum dots from biomass waste for highly sensitive detection of iron ions. Mater. Res. Bull. 2020, 124, 110730. [Google Scholar] [CrossRef]
- Devi, B.S.; Laxmi, B.; Raju, C.L.; Rosaiah, G.; Reddy, A.S. Colorimetric detection of silver ions in different pH conditions using Pongamia pinnata leaf extract derived carbon dots. Mater. Lett. 2023, 336, 133872. [Google Scholar] [CrossRef]
- Sahoo, N.K.; Jana, G.C.; Aktara, M.N.; Das, S.; Nayim, S.; Patra, A.; Bhattacharjee, P.; Bhadra, K.; Hossain, M. Carbon dots derived from lychee waste: Application for Fe3+ ions sensing in real water and multicolor cell imaging of skin melanoma cells. Mater. Sci. Eng. C 2020, 108, 110429. [Google Scholar] [CrossRef] [PubMed]
- Bandi, R.; Dadigala, R.; Gangapuram, B.R.; Guttena, V. Green synthesis of highly fluorescent nitrogen–doped carbon dots from Lantana camara berries for effective detection of lead (II) and bioimaging. J. Photochem. Photobiol. B 2018, 178, 330–338. [Google Scholar] [CrossRef]
- Pourreza, N.; Ghomi, M. Green synthesized carbon quantum dots from Prosopis juliflora leaves as a dual off-on fluorescence probe for sensing mercury (II) and chemet drug. Mater. Sci. Eng. C 2019, 98, 887–896. [Google Scholar] [CrossRef]
- Komalavalli, L.; Amutha, P.; Monisha, S. A facile approach for the synthesis of carbon dots from Hibiscus sabdariffa & its application as bio-imaging agent and Cr (VI) sensor. Mater. Today Proc. 2020, 33, 2279–2285. [Google Scholar]
- Arumugham, T.; Alagumuthu, M.; Amimodu, R.G.; Munusamy, S.; Iyer, S.K. A sustainable synthesis of green carbon quantum dot (CQD) from Catharanthus roseus (white flowering plant) leaves and investigation of its dual fluorescence responsive behavior in multi-ion detection and biological applications. Sustain. Mater. Technol. 2020, 23, 00138. [Google Scholar] [CrossRef]
- Qi, H.; Teng, M.; Liu, M.; Liu, S.; Li, J.; Yu, H.; Teng, C.; Huang, Z.; Liu, H.; Shao, Q.; et al. Biomass-derived nitrogen-doped carbon quantum dots: Highly selective fluorescent probe for detecting Fe3+ ions and tetracyclines. J. Colloid Interface Sci. 2019, 539, 332–341. [Google Scholar] [CrossRef]
- Bhatt, S.; Bhatt, M.; Kumar, A.; Vyas, G.; Gajaria, T.; Paul, P. Green route for synthesis of multifunctional fluorescent carbon dots from Tulsi leaves and its application as Cr(VI) sensors, bio-imaging and patterning agents. Colloids Surf. B 2018, 167, 126–133. [Google Scholar] [CrossRef]
- Huang, Y.; Chen, S.; Huang, W.; Zhuang, X.; Zeng, J.; Rong, M.; Niu, L. Visualized test of environmental water pollution and meat freshness: Design of Au NCs-CDs-test paper/PVA film for ratiometric fluorescent sensing of sulfide. Food Chem. 2024, 432, 137292. [Google Scholar] [CrossRef]
- Yang, M.; Yan, Y.; He, F.; Wang, Z.; Liu, E.; Fan, J. Green synthesis of fluorescent carbon dots from discarded cigarette butts as an effective fluorescence probe for sensing iron ions. Mater. Lett. 2024, 357, 135613. [Google Scholar] [CrossRef]
- Shinohara, H.; Tiwari, A. Graphene: An Introduction to the Fundamentals and Industrial Applications; John Wiley & Sons: Hoboken, NJ, USA, 2015. [Google Scholar]
- Issa, M.A.; Abidin, Z.Z.; Sobri, S.; Rashid, S.A.; Mahdi, M.A.; Ibrahim, N.A. Fluorescent recognition of Fe3+ in the acidic environment by enhanced-quantum yield N-doped carbon dots: Optimization of variables using central composite design. Sci. Rep. 2020, 10, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Trpkov, D.; Sredojević, D.; Pajović, J.; Tošić, D.; Božanić, D.K.; Djoković, V. Hybrid nanostructures of nitrogen-doped carbon dots and aromatic amino acids: Synthesis, interactions at interfaces and optical properties. Colloids Surf. B Biointerfaces 2024, 238, 113878. [Google Scholar] [CrossRef] [PubMed]
- Sendao, R.; de Yuso, M.D.V.M.; Algarra, M.; da Silva, J.C.E.; da Silva, L.P. Comparative life cycle assessment of bottom-up synthesis routes for carbon dots derived from citric acid and urea. J. Clean. Prod. 2020, 254, 120080. [Google Scholar] [CrossRef]
- Emam, A.N.; Loutfy, S.A.; Mostafa, A.A.; Awad, H.; Mohamed, M.B. Cytotoxicity, biocompatibility, and cellular response of carbon dots–plasmonic-based nano-hybrids for bioimaging. RSC Adv. 2017, 7, 23502–23514. [Google Scholar] [CrossRef]
- Munusamy, S.; Mandlimath, T.R.; Swetha, P.; Al-Sehemi, A.G.; Pannipara, M.; Koppala, S.; Shanmugam, P.; Boonyuen, S.; Pothu, R.; Boddula, R. Nitrogen-doped carbon dots: Recent developments in its fluorescent sensor applications. Environ. Res. 2023, 231, 116046. [Google Scholar] [CrossRef]
- Dhariwal, J.; Rao, G.K.; Vaya, D. Recent advancements towards the green synthesis of carbon quantum dots as an innovative and eco-friendly solution for metal ion sensing and monitoring. RSC Sustain. 2024, 2, 11–36. [Google Scholar] [CrossRef]
- Liu, Y.; Duan, W.; Song, W.; Liu, J.; Ren, C.; Wu, J.; Chen, H. Red emission B, N, S-co-doped carbon dots for colorimetric and fluorescent dual-mode detection of Fe3+ ions in complex biological fluids and living cells. ACS Appl. Mater. Interfaces 2017, 9, 12663–12672. [Google Scholar] [CrossRef]
- Wu, H.; Jiang, J.; Gu, X.; Tong, C. Nitrogen and sulfur co-doped carbon quantum dots for highly selective and sensitive fluorescent detection of Fe(III) ions and L-cysteine. Microchim. Acta 2017, 184, 2291–2298. [Google Scholar] [CrossRef]
- Wang, J.; Wang, J.; Han, L.; Liao, C.; Cai, W.; Kan, Y.; Hu, Y. Fabrication of an anode composed of an N, S co-doped carbon nanotube hollow architecture with CoS 2 confined within: Toward Li and Na storage. Nanoscale 2019, 11, 20996–21007. [Google Scholar] [CrossRef]
- Chandra, S.; Bano, D.; Pradhan, P.; Singh, V.K.; Yadav, P.K.; Sinha, D.; Hasan, S.H. Nitrogen/sulfur-co-doped carbon quantum dots: A biocompatible material for the selective detection of picric acid in aqueous solution and living cells. Anal. Bioanal. Chem. 2020, 412, 3753–3763. [Google Scholar] [CrossRef]
- Shangguan, J.; Huang, J.; He, D.; He, X.; Wang, K.; Ye, R.; Yang, X.; Qing, T.; Tang, J. Highly Fe3+-selective fluorescent nanoprobe based on ultrabright N/P codoped carbon dots and its application in biological samples. Anal. Chem. 2017, 89, 7477–7484. [Google Scholar] [CrossRef] [PubMed]
- Tall, A.; Cunha, F.A.; Kaboré, B.; do ES Barbosa, C.D.A.; Rocha, U.; Sales, T.O.; Santos, J.C.C. Green emitting N, P-doped carbon dots as efficient fluorescent nanoprobes for determination of Cr(VI) in water and soil samples. Microchem. J. 2021, 166, 106219. [Google Scholar] [CrossRef]
- Tu, Y.; Wang, S.; Yuan, X.; Song, P.; Wei, Y.; Qin, K.; Ji, X. Hydrothermal synthesis of Auricularia auricula derived nitrogen, phosphorus-doped carbon dots and application in Ag(I) and 4-nitrophenol detection and bioimaging. Anal. Methods 2020, 12, 2237–2243. [Google Scholar] [CrossRef]
- Kalaiyarasan, G.; Joseph, J.; Kumar, P. Phosphorus-doped carbon quantum dots as fluorometric probes for iron detection. ACS Omega 2020, 5, 22278–22288. [Google Scholar] [CrossRef]
- Gallareta-Olivares, G.; Rivas-Sanchez, A.; Cruz-Cruz, A.; Hussain, S.M.; González-González, R.B.; Cárdenas-Alcaide, M.F.; Iqbal, H.M.; Parra-Saldívar, R. Metal-doped carbon dots as robust nanomaterials for the monitoring and degradation of water pollutants. Chemosphere 2023, 312, 137190. [Google Scholar] [CrossRef]
- Lu, W.; Guo, Y.; Zhang, J.; Yue, Y.; Fan, L.; Li, F.; Shuang, S. A high catalytic activity nanozyme based on cobalt-doped carbon dots for biosensor and anticancer cell effect. ACS Appl. Mater. Interfaces 2022, 14, 57206–57214. [Google Scholar] [CrossRef]
- Swayamprabha, S.S.; Nagar, M.R.; Yadav, R.A.K.; Gull, S.; Dubey, D.K.; Jou, J.H. Hole-transporting materials for organic light-emitting diodes: An overview. J. Mater. Chem. C 2019, 7, 7144–7158. [Google Scholar]
- Liu, Q.; Wang, H.; Ji, J.; Zhang, W.; Wang, A.; Zhao, B.; Chen, Z. Cu-doped graphitic carbon nitride composite functionalized sensor for sensitive Cd2+ detection. Comput. Electron. Agric. 2023, 215, 108383. [Google Scholar] [CrossRef]
- Chen, D.; Miao, Q.; Liao, H.; Xiao, S.; Xiao, B.; Zhang, X. Transition Metal (Rh, Pd, and Pt) Doped SnS2 Monolayer as, Promising Work Function Gas Sensors for H2Se Detection: A DFT Study. IEEE Sens. J. 2024, 24, 10740597. [Google Scholar] [CrossRef]
- Michenzi, C.; Proietti, A.; Rossi, M.; Espro, C.; Bressi, V.; Vetica, F.; Simonis, B.; Chiarotto, I. Carbon nanodots from orange peel waste as fluorescent probes for detecting nitrobenzene. RSC Sustain. 2024, 2, 933–942. [Google Scholar] [CrossRef]
- Umarani, P.; Sivakumar, K.; Sathiya, S.; Lims, S.C.; Aswathappa, S.; Kumar, R.S.; Almansour, A.I.; Dhas, S.S.J. Tuning of optical and vapour sensing properties of manganese-doped cadmium oxide thin films for sensor applications. Opt. Mater. 2024, 149, 115126. [Google Scholar] [CrossRef]
- Ansi, V.A.; Vijisha, K.R.; Muraleedharan, K.; Renuka, N.K. Fluorescent carbon nanodots as efficient nitro aromatic sensor-analysis based on computational perspectives. Sens. Actuators A Phys. 2020, 302, 111817. [Google Scholar] [CrossRef]
- Massad-Ivanir, N.; Bhunia, S.K.; Raz, N.; Segal, E.; Jelinek, R. Synthesis and characterization of a nanostructured porous silicon/carbon dot-hybrid for orthogonal molecular detection. NPG Asia Mater. 2018, 10, e463. [Google Scholar] [CrossRef]
- Liu, M.; Ren, X.; Meng, X.; Li, H. Metal-organic frameworks-based fluorescent nanocomposites for bioimaging in living cells and in vivo. Chin. J. Chem. 2021, 39, 473–487. [Google Scholar] [CrossRef]
- Bloch, K.; Chakraborty, S.; Mishra, R.; Ghosh, S. Nanohybrids for the detection and control of infectious diseases. In Medical Nanobiotechnology; Woodhead Publishing: Cambridge, UK, 2025; pp. 609–633. [Google Scholar]
- Niu, Y.; Tan, H.; Li, X.; Zhao, L.; Xie, Z.; Zhang, Y.; Qu, X. Protein–carbon dot nanohybrid-based early blood–brain barrier damage theranostics. ACS Appl. Mater. Interfaces. 2020, 12, 3445–3452. [Google Scholar] [CrossRef]
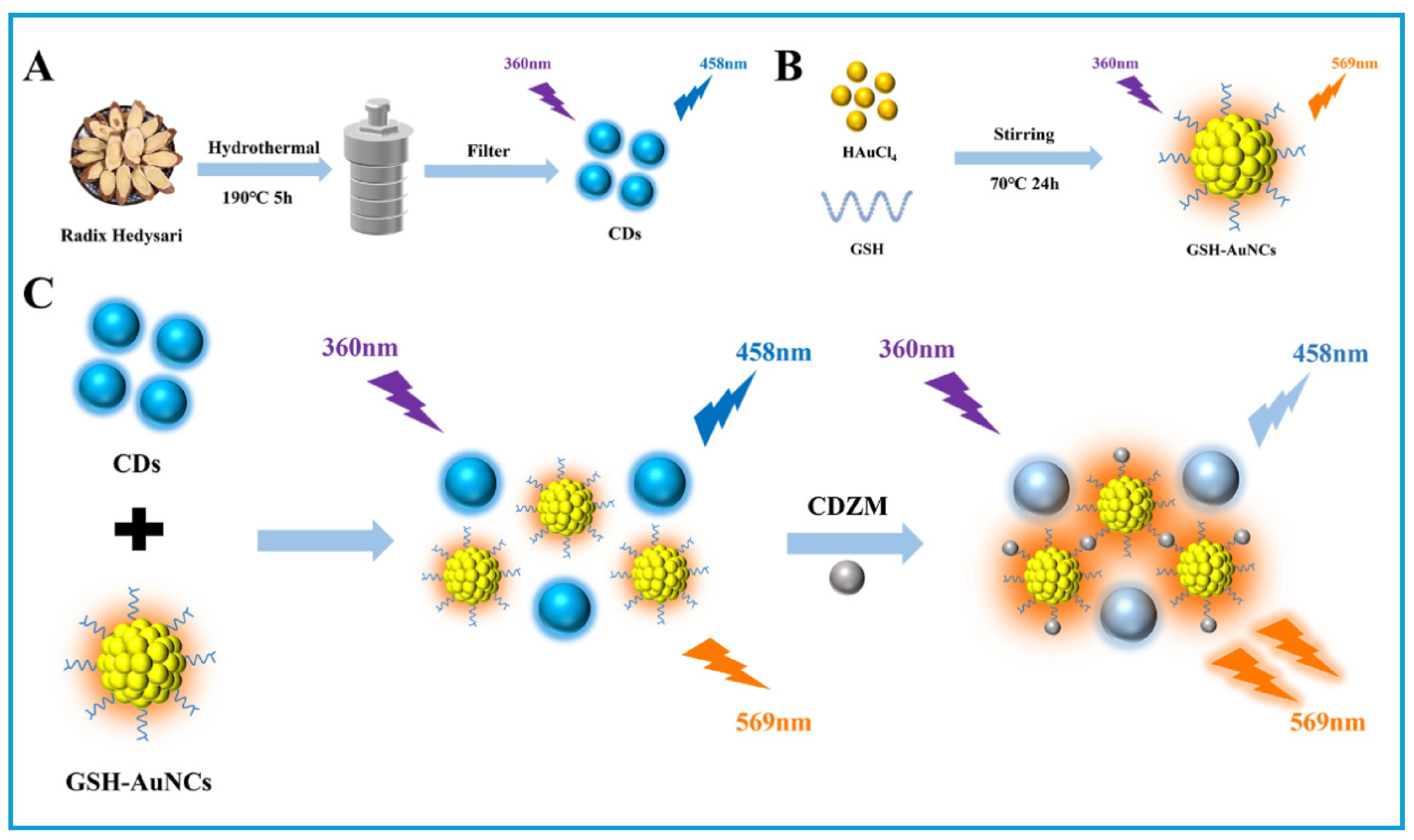
- Guo, Y.X.; Guo, X.R.; Chen, X.Y. Design of Ratio-Fluorescence Nanohybrid Based on Radix Hedysari Green-Synthesized CDs and GSH-AuNCs for Sensitive Detection of Cefodizime Sodium in Urine Sample. Int. J. Mol. Sci. 2024, 25, 5971. [Google Scholar] [CrossRef]
- Kayani, K.F.; Mohammed, S.J.; Mohammad, N.N.; Rahim, M.K.; Mustafa, M.S.; Ahmed, H.R.; Karim, W.O.; Sidiq, M.K.; Aziz, S.B. Exploring green practices: A review of carbon dot-based sustainable sensing approaches. J. Fluoresc. 2025. [Google Scholar] [CrossRef]
- Nguyen, D.H.; Muthu, A.; Elsakhawy, T.; Sheta, M.H.; Abdalla, N.; El-Ramady, H.; Prokisch, J. Carbon Nanodots-Based Sensors: A Promising Tool for Detecting and Monitoring Toxic Compounds. Nanomaterials 2025, 15, 725. [Google Scholar] [CrossRef]
- Koç, Ö.K.; Üzer, A.; Apak, R. Molecular aggregation-dependent “turn-on” fluorescence strategy: Carbon quantum dots as sensing material for dual-mode detection of intact TATP at nanomolar level. Appl. Mater. Today 2025, 44, 102760. [Google Scholar] [CrossRef]
- Yang, L.; Hou, H.; Li, J. Frontiers in fluorescence imaging: Tools for the in situ sensing of disease biomarkers. J. Mater. Chem. B 2025, 13, 1133–1158. [Google Scholar] [CrossRef]
- Bravo-Yagüe, J.C.; Paniagua-González, G.; Garcinuño, R.M.; García-Mayor, A.; Fernández-Hernando, P. Colorimetric molecularly imprinted polymer-based sensors for rapid detection of organic compounds: A review. Chemosensors 2025, 13, 163. [Google Scholar] [CrossRef]
- Singh, B.; Adcock, A.F.; Dumra, S.; Collins, J.; Yang, L.; Bunker, C.E.; Sun, Y.P. Microwave-Assisted Carbonization Processing for Carbon Dot-like Nanomaterials with Antimicrobial Properties. Micro 2025, 5, 14. [Google Scholar] [CrossRef]
- Wibowo, A.; Jahir Khan, M.; Sansanaphongpricha, K.; Khemthong, P.; Laosiripojana, N.; Yu, Y.S.; Wu, K.C.; Sakdaronnarong, C. Carbon Dots in Photodynamic Therapy: The Role of Dopant and Solvent on Optical and Photo-Responsive Properties. Chem.-Eur. J. 2024, 30, e202400885. [Google Scholar] [CrossRef] [PubMed]
- Sekar, R.; Basavegowda, N.; Jena, S.; Jayakodi, S.; Elumalai, P.; Chaitanyakumar, A.; Somu, P.; Baek, K.H. Recent developments in heteroatom/metal-doped carbon dot-based image-guided photodynamic therapy for cancer. Pharmaceutics 2022, 14, 1869. [Google Scholar] [CrossRef]
- Farid, M.A.A.; Lease, J.; Yoshito, A. Lignocellulosic biomass-derived carbon quantum dots (CQDs): A novel approach utilizing organosolv lignin from Moso bamboo waste. J. Clean. Prod. 2024, 467, 142852. [Google Scholar] [CrossRef]
- George, E.; Mustafa, M.N.; Walvekar, R.; Mathkor, D.M.; Haque, S.; Numan, A.; Khalid, M. Carbon Dot-Based Biosensors for Continuous Glucose Monitoring in Point-of-Care Devices: Advancements, Challenges, and Future Prospects. J. Electrochem. Soc. 2025, 172, 047508. [Google Scholar] [CrossRef]

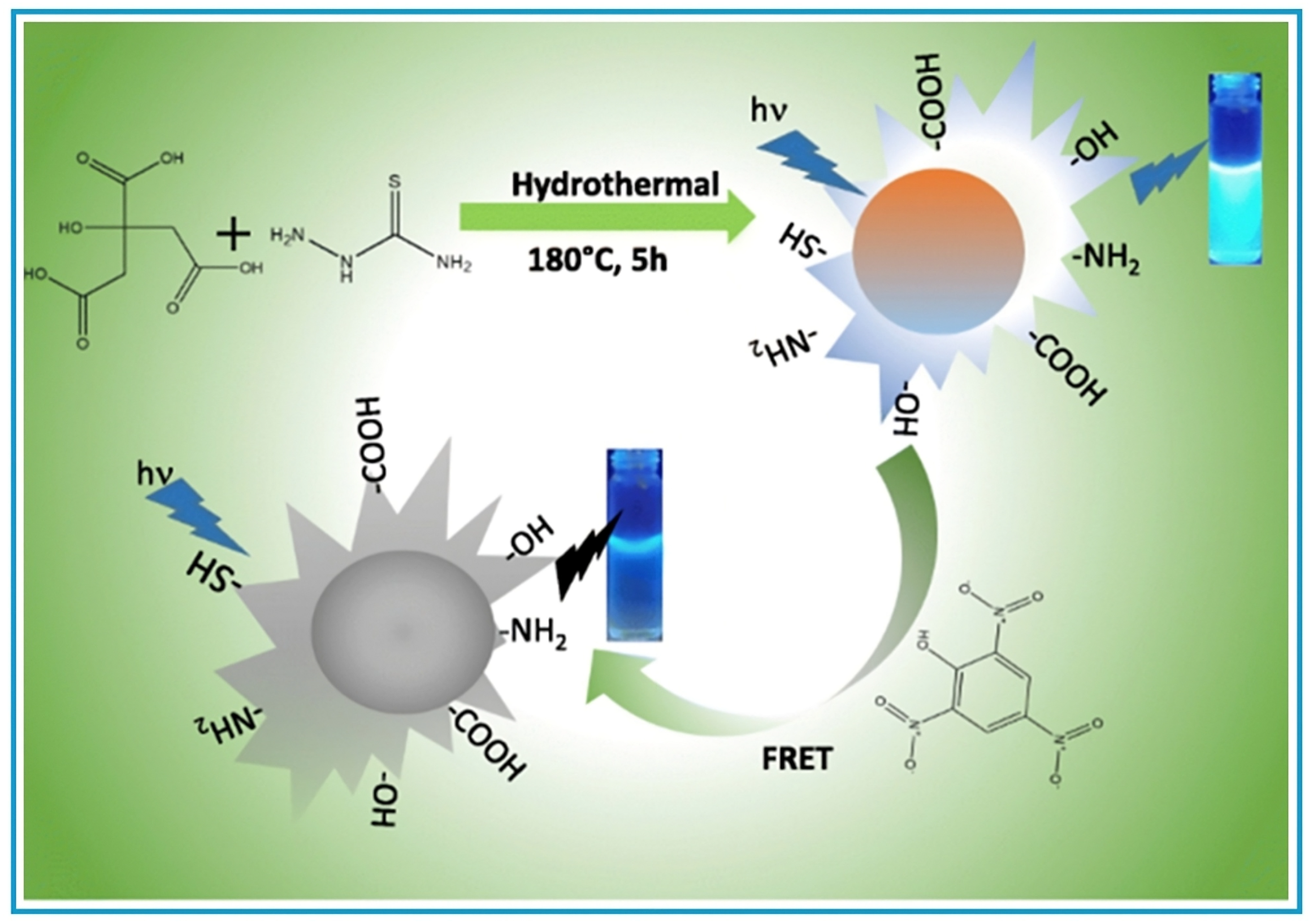
| S. No. | Compounds | Source | Sensing Application | LOD | References |
|---|---|---|---|---|---|
| 1 | CDs | Radish | Cu2+ and acetic acid vapors in water samples | 0.16 μM and 6.8 μM | [150] |
| 2 | CDs | Rice residue | Fe3+ and Tetracyclines | 0.073 μM | [151] |
| 3 | CDs | Pongamia pinnata | Ag2+ | 0.758 μM and 0.340 μM | [152] |
| 4 | CDs | Lychee Waste | Fe3+ | 2.36 nM | [153] |
| 5 | Nitrogen-doped CDs | Lantana camara berries | Pb2+ | 9.64 nM | [154] |
| 6 | CDs | Prosopis juliflora | Hg2+ | 1.26 ng mL−1 | [155] |
| 7 | CDs | Hibiscus sabdariffa | Cr6+ | - | [156] |
| 8 | CDs | Catharanthus roseus leaves | Al3+ and Fe3+ | 0.5 and 0.3 μM | [157] |
| 9 | Nitrogen-doped CDs | Rice residue | Fe3+ and Tetracycline | 0.7462 μM | [158] |
| 10 | CDs | Tulasi leaves | Cr6+ | 229 nM | [159] |
| 11 | Au NCs-CDs | Nanocomposite test paper and polyvinyl alcohol film | H2S | 4.20 nM | [160] |
| 12 | Fluorescent CDs | Discarded cigarette butts | Fe3+ | 0.5–800 μM | [161] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Thanjavur, N.; Kim, Y.-J. Illuminating Pollutants: The Role of Carbon Dots in Environmental Sensing. Chemosensors 2025, 13, 241. https://doi.org/10.3390/chemosensors13070241
Thanjavur N, Kim Y-J. Illuminating Pollutants: The Role of Carbon Dots in Environmental Sensing. Chemosensors. 2025; 13(7):241. https://doi.org/10.3390/chemosensors13070241
Chicago/Turabian StyleThanjavur, Naveen, and Young-Joon Kim. 2025. "Illuminating Pollutants: The Role of Carbon Dots in Environmental Sensing" Chemosensors 13, no. 7: 241. https://doi.org/10.3390/chemosensors13070241
APA StyleThanjavur, N., & Kim, Y.-J. (2025). Illuminating Pollutants: The Role of Carbon Dots in Environmental Sensing. Chemosensors, 13(7), 241. https://doi.org/10.3390/chemosensors13070241







