Abstract
MicroRNAs (miRNAs) are small, non-coding RNAs that regulate gene expression and have emerged as critical biomarkers in various diseases, including cancer. Their stability in bodily fluids and role as oncogenes or tumor suppressors make them attractive targets for non-invasive diagnostics. However, conventional detection methods, such as Northern blotting, RT-PCR, and microarrays, are limited by low sensitivity, lengthy protocols, and limited specificity. Electrochemical biosensors offer a promising alternative, providing high sensitivity, rapid response times, portability, and cost-effectiveness. These biosensors translate miRNA hybridization events into quantifiable electrochemical signals, often leveraging redox-active labels, mediators, or intercalators. Recent advancements in nanomaterials and signal amplification strategies have further enhanced detection capabilities, enabling sensitive, label-free miRNA quantification. This review provides a comprehensive overview of the recent advances in electrochemical biosensing of miRNAs, emphasizing innovative redox-based detection strategies, probe immobilization techniques, and hybridization modalities. The critical challenges and future perspectives in advancing electrochemical miRNA biosensors toward clinical translation and point-of-care diagnostics are discussed.
1. Introduction
MicroRNAs (miRNAs) are a class of small, non-coding RNA molecules, typically comprising 19–25 nucleotides, that play crucial roles in the post-transcriptional regulation of gene expression. Through sequence-specific binding to target messenger RNAs (mRNAs), miRNAs induce mRNA degradation or translational repression, thereby orchestrating key biological processes such as cell proliferation, differentiation, apoptosis, and tumorigenesis [1]. Dysregulation of miRNA expression has been extensively implicated in the onset and progression of numerous pathological conditions, including cardiovascular diseases, viral infections, renal and neurological disorders, and various cancers [2]. In oncology, miRNAs can function either as oncogenes or tumor suppressors, and abnormal miRNA profiles have been correlated with tumor initiation, progression, and metastasis across a broad spectrum of cancer types [3]. Consequently, miRNAs have garnered considerable interest as minimally invasive biomarkers for cancer diagnosis, prognosis, and therapeutic monitoring [4].
The characterization of miRNA expression signatures in tissues and bodily fluids has opened new avenues for early disease detection; however, the accurate and sensitive detection of miRNAs remains technically challenging. Conventional miRNA detection methods, including Northern blotting, reverse transcription–polymerase chain reaction (RT-PCR), and microarray analysis, each suffer from critical limitations [5,6,7]. Northern blotting, regarded as the traditional gold standard, is labor-intensive, time-consuming, and insufficiently sensitive for detecting low-abundance targets. RT-PCR offers improved sensitivity but requires complex sample processing and expensive instrumentation and is prone to false positives due to the short primer sequences used for amplification. Meanwhile, microarrays enable high-throughput analysis but exhibit limited sensitivity and specificity, rendering them suboptimal for clinical translation.
To overcome these obstacles, electrochemical biosensors have emerged as a powerful alternative for miRNA detection [8]. These platforms translate the hybridization of miRNAs with complementary probes immobilized on electrode surfaces into measurable electrochemical signals. By monitoring changes in electrical properties such as current, voltage, or impedance, miRNA concentrations can be quantified rapidly and with high sensitivity. Moreover, electrochemical biosensors offer several inherent advantages over traditional techniques, including low-cost fabrication, portability, minimal sample preparation, and potential for real-time analysis [9]. Their adaptability to various electrode substrates and detection chemistries allows for customizable designs that can be tailored to specific diagnostic needs [10]. Furthermore, the integration of nanomaterials (e.g., nanoparticles, graphene derivatives, and conductive polymers) and the implementation of advanced signal amplification strategies have dramatically enhanced the analytical performance of electrochemical miRNA sensors, enabling detection at femtomolar to attomolar levels [11].
Building on these technological advances, there is growing interest in refining redox-based detection strategies and hybridization modes to further enhance the sensitivity, specificity, and clinical applicability of miRNA biosensors. This review provides a comprehensive overview of the recent advancements in electrochemical biosensors for miRNA detection, with a focus on redox-based strategies and innovative hybridization modes. Spanning the period from 2013 to 2025, it critically examines the underlying principles of sensor design, highlights key technological breakthroughs, and discusses current limitations. Finally, this review outlines future directions for translating these biosensing platforms into clinically viable and point-of-care (POC) diagnostic tools.
2. Electrochemical miRNA Biosensors
Among various biosensing modalities, electrochemical biosensors have emerged as particularly well-suited for POC applications due to their inherent advantages [12]. In contrast, optical biosensors often require bulky and costly external equipment [13]; mechanical biosensors are challenging to miniaturize and exhibit slower response times [14]; photothermal biosensors necessitate strict temperature control, limiting their sensitivity [15], and magnetic biosensors are expensive, require external magnetic fields, and are less amenable to portable formats [16]. Electrochemical biosensors, by contrast, offer facile miniaturization, low fabrication costs, high sensitivity and selectivity, rapid analysis, low background noise, and compatibility with user-friendly platforms [17], making them attractive candidates for next-generation POC diagnostics.
2.1. Fundamental Designs of Electrochemical Biosensors for MicroRNA Quantification
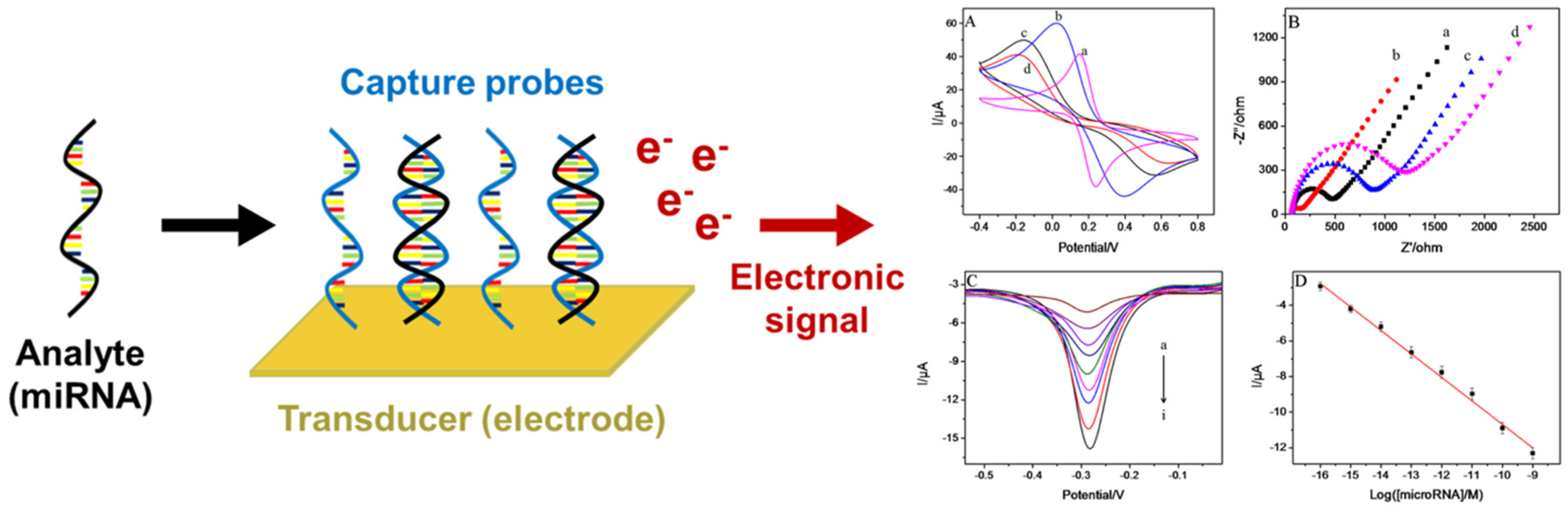
A wide array of electrochemical biosensors has been developed for miRNA detection, reflecting ongoing innovation in sensor design and functionality. Fundamentally, an electrochemical biosensor for miRNA detection is a bioanalytical device that transduces the hybridization event between a target miRNA and complementary nucleic acid probes into a quantifiable electrical signal, as shown in Figure 1. These biosensors traditionally consist of three essential components: an electrode, immobilized nucleic acid probes, and electrochemical reporters. The electrode surface serves as the transduction interface, where target miRNAs hybridize with single-stranded DNA (ssDNA), RNA (ssRNA), or peptide nucleic acid (ssPNA) probes anchored to the electrode. This configuration, referred to as the direct or linear hybridization structure, represents a primary strategy for miRNA quantification [18]. Upon hybridization, the formation of DNA/RNA/PNA-miRNA duplexes induces detectable changes in the electrochemical response. These variations can be measured using techniques such as voltammetry, chronocoulometry, chronoamperometry, or electrochemical impedance spectroscopy (EIS), often facilitated by the presence of redox-active labels, intercalators, or mediators [19,20,21,22,23]. The resulting signals are then processed and correlated with miRNA concentration, enabling sensitive and specific detection, which is critical for clinical diagnostics.
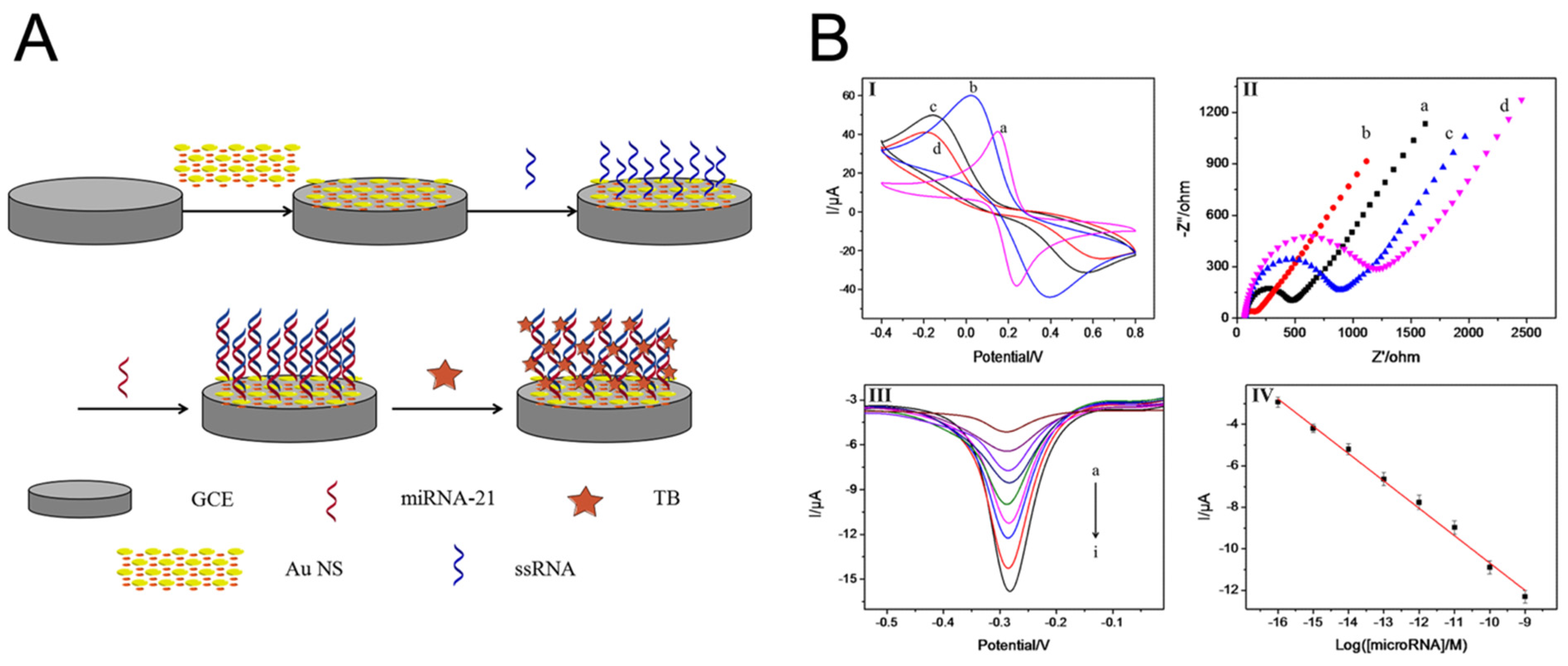
Figure 1.
Schematic illustration of the miRNA electrochemical biosensor components and sample electrochemical techniques: CVs (A) and EIS (B) GCE (a), AuNS/GCE (b), SS-RNA probe/AuNS/GCE (c), and mi-croRNA/SS-RNA probe/AuNS/GCE (d). (C) Differential pulse voltammetry (DPV) in the presence of different concentrations of miR-21 from (a) to (i): 0 M to 1 nM. (D) Calibration curve [23].
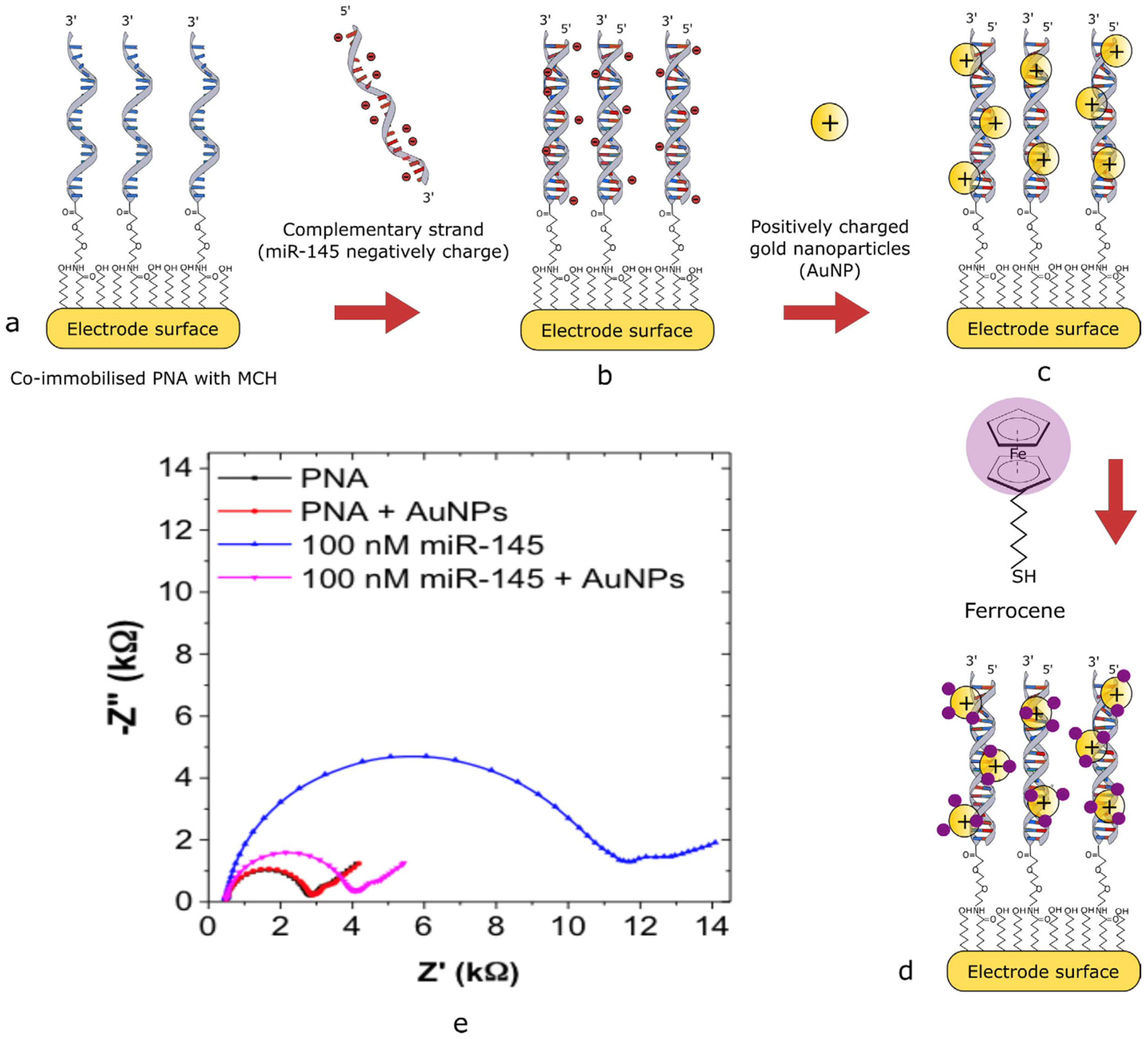
Jolly et al. [19] reported a highly sensitive voltammetric biosensor for miR-145 detection, demonstrating a multi-step electrode modification strategy that exemplifies the integration of nanomaterials and redox reporters to enhance sensitivity. The design utilized co-immobilization of a thiolated ssPNA probe complementary to miR-145 and 6-mercapto-1-hexanol (MCH) on a gold electrode. The use of MCH served to passivate nonspecific binding sites and maintain the structural integrity of the hybridization interface. Upon successful hybridization of miR-145 with the ssPNA probe, positively charged gold nanoparticles (AuNPs) were introduced. These nanoparticles are electrostatically associated with the negatively charged phosphate backbone of the miRNA, amplifying the detection signal. Subsequent conjugation of thiolated ferrocene to the AuNPs enabled redox-based signal generation, which was quantified using square wave voltammetry (SWV). This stepwise amplification approach yielded a remarkable limit of detection (LOD) of 0.37 fM and a wide dynamic range spanning from 1 fM to 100 nM, highlighting the biosensor’s potential for early-stage disease diagnostics. Figure 2 illustrates the biosensor assembly and the calibration curve derived from SWV measurements.
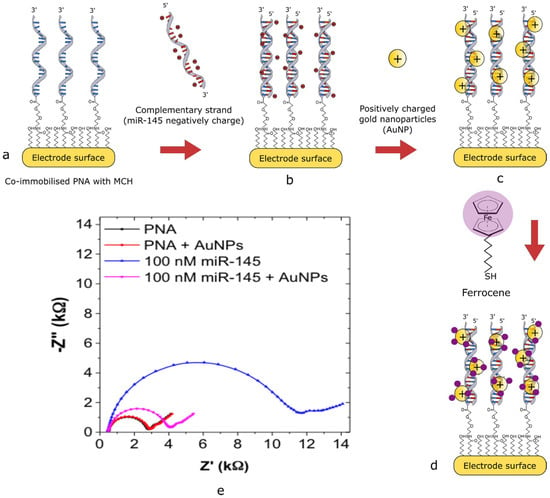
Figure 2.
Schematic of AuNP/thiolated ferrocene detection strategy (a–d) and the Nyquist plot of the modified electrode surface (e) [19].
In contrast, Rafiee-Pour et al. [21] introduced an intercalation-based electrochemical biosensor for miR-21 detection, offering a simpler yet effective route for hybridization detection without requiring complex nanostructures or covalent modifications. Their approach utilized a glassy carbon electrode (GCE) modified with carboxylated multi-walled carbon nanotubes (MWCNT-COOH), which facilitated strong adsorption of a ssDNA-21 probe via π–π interactions. Following hybridization with miR-21, the electrode was immersed in methylene blue (MB), a redox-active dye known to preferentially intercalate into double-stranded nucleic acid structures. Differential pulse voltammetry (DPV) was used to interrogate the intercalated MB, where the magnitude of the oxidation peak current correlated directly with the extent of hybridization. This intercalation-based detection method yielded an LOD of 84.3 pM and a dynamic range of 0.1 to 500 pM, making it well-suited for mid-femtomolar to low-nanomolar detection in complex sample matrices.
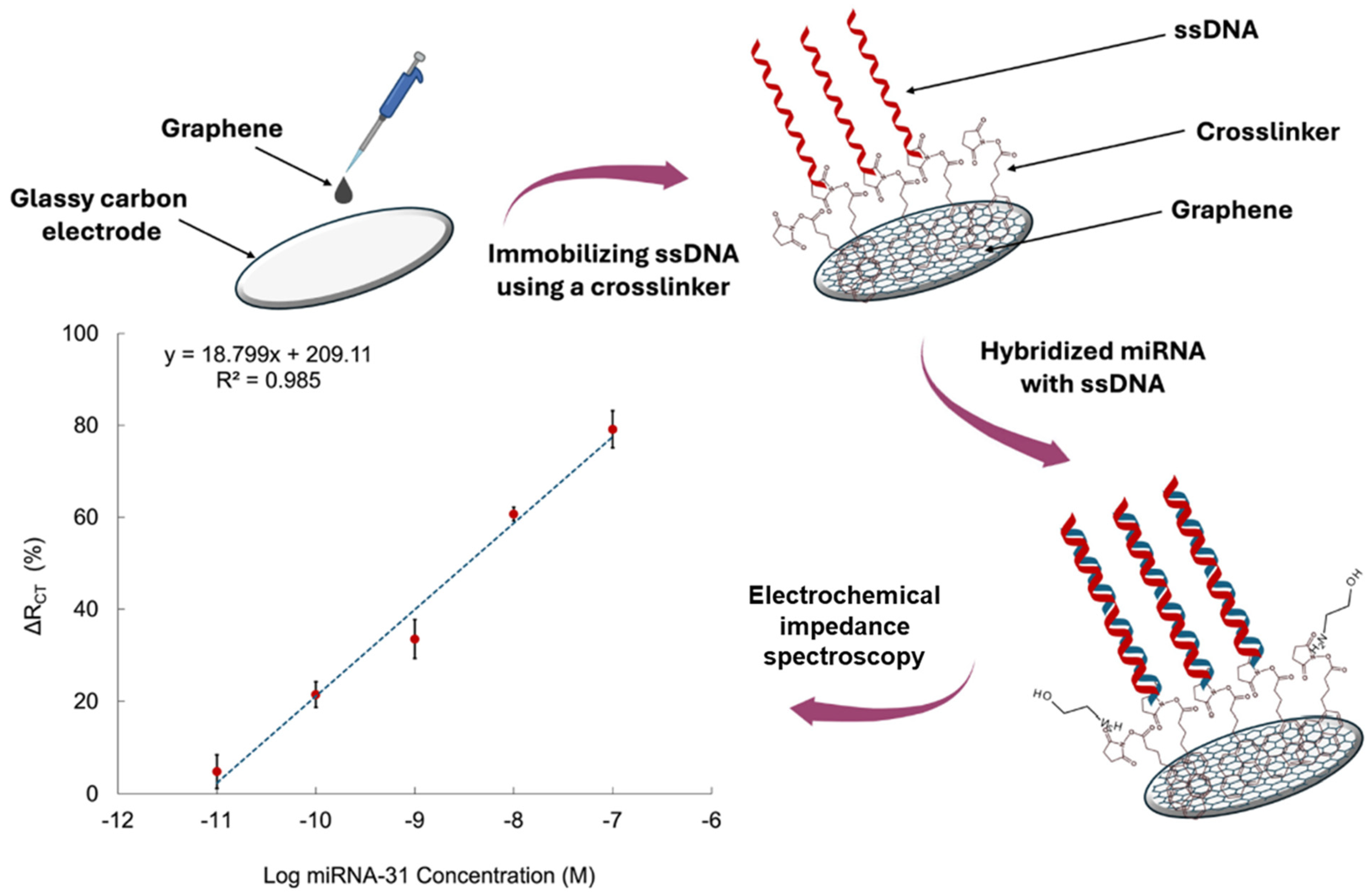
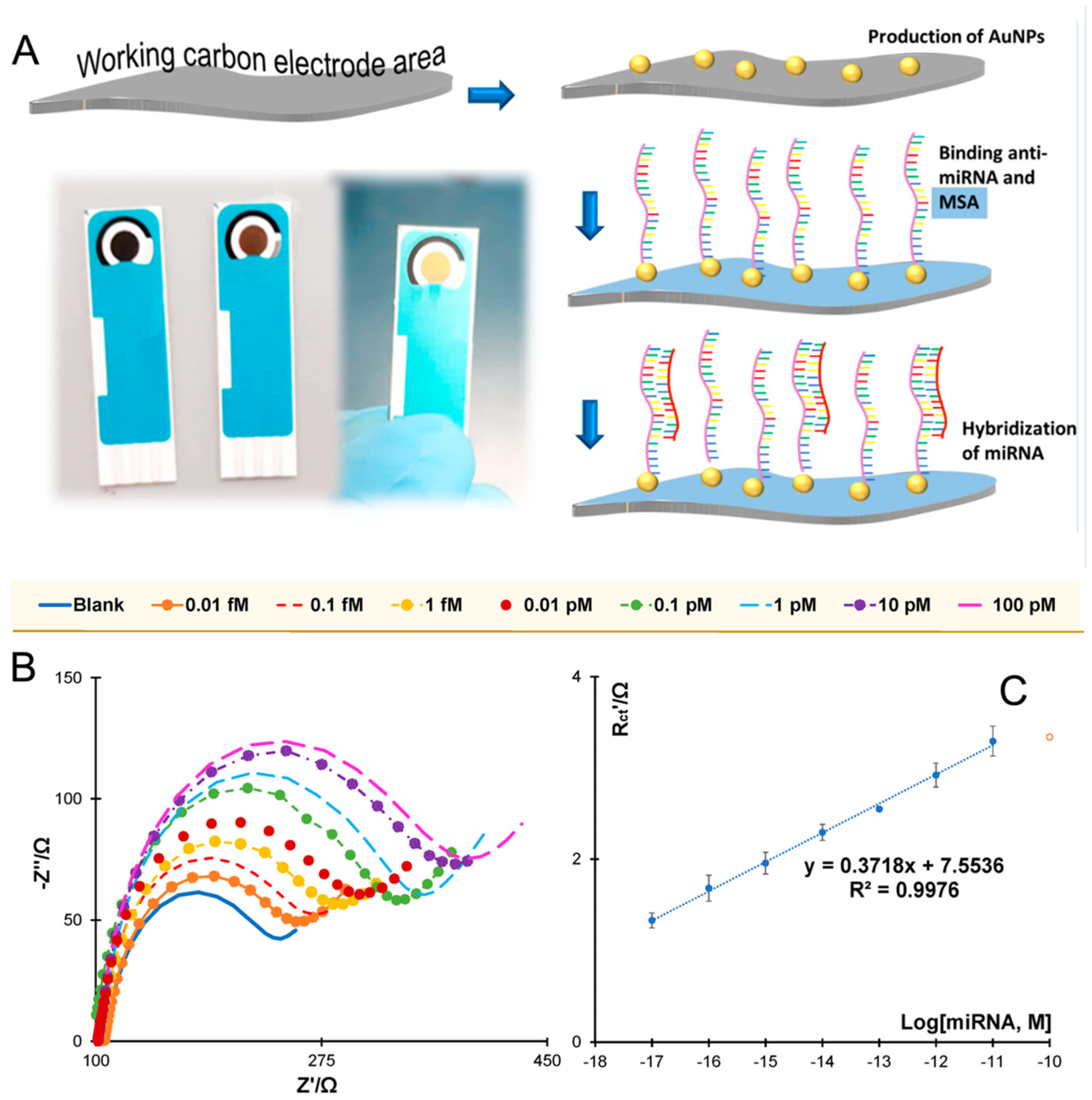
Impedimetric sensing offers another label-free alternative, leveraging changes in charge transfer resistance (Rct) at the electrode interface upon target hybridization. This technique is highly sensitive and preserves biomolecular structure, yet it is often limited by poor specificity in complex matrices and susceptibility to non-Faradaic background noise [22]. It is also difficult to differentiate between small variations in impedance without precise control over surface chemistry. Nevertheless, Nagdeve et al. [24] developed an impedimetric biosensor for miR-31 detection in a recent report. A GCE was modified with graphene to enhance electron transfer and provide a high surface area platform. Figure 3 provides a schematic representation of the sensor’s architecture and the corresponding calibration curve obtained from EIS analysis. 1-pyrenebutanoic acid succinimidyl ester was employed as a bifunctional crosslinker to anchor ssDNA-31 probes onto the graphene-modified surface. Upon hybridization with the target miR-31 in diluted human serum, the resulting nucleic acid duplex caused an increase in Rct, indicating successful target capture. EIS was conducted using [Fe(CN)6]3−/4− as the redox probe, enabling sensitive detection without the need for enzymatic or nanoparticle-based signal amplification. The sensor achieved a LOD of 100 pM and a linear response range of 100 pM to 10 nM.
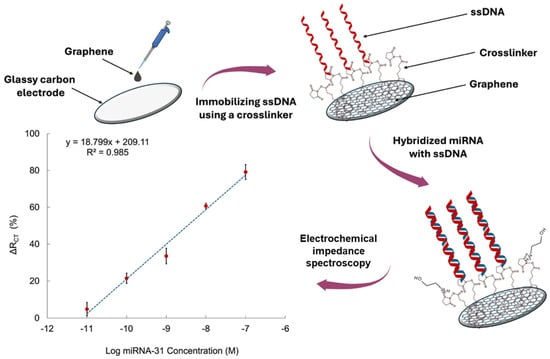
Figure 3.
Schematic of the glassy carbon electrode (GCE)/graphene-based biosensor design and the sensitivity calibration curve based on the relationship between ΔRCT (%) and the logarithmic concentration of miRNA-31 in diluted serum [24].
2.2. Emerging Architectures and Amplification Techniques for Electrochemical miRNA Detection
Beyond direct hybridization strategies, several advanced assay architectures have been developed to enhance the sensitivity, specificity, and dynamic range of electrochemical miRNA biosensors. These include sandwich-type hybridization structures, signal-on/off strategies, and catalytic amplification schemes. Enzyme-assisted amplification strategies, including horseradish peroxidase (HRP), alkaline phosphatase (ALP), and DNAzyme-based reporters, significantly boost detection limits by catalyzing redox reactions in situ [25,26,27,28]. These strategies are especially effective when paired with nanomaterials for increased signal output. However, they introduce issues of enzymatic instability, temperature sensitivity, and batch variability, which may hinder scalability and field deployment. In sandwich-type designs, a secondary probe labeled with a redox reporter or nanomaterial hybridizes to a different region of the target miRNA, enabling dual-site recognition and signal enhancement. Mandli et al. [25] developed a sandwich hybridization assay on an AuNP-modified pencil graphite electrode for detecting miR-21, integrating enzymatic amplification to enhance sensitivity. In this system, the target miRNA bridges a thiolated capture probe (SH-P1) and a biotinylated detection probe, forming a ternary complex. The subsequent introduction of streptavidin-conjugated alkaline phosphatase enables enzymatic conversion of the electrochemically inactive substrate α-naphthyl phosphate into the electroactive product α-naphthol. Detection via DPV yielded a LOD of 100 pM and a broad dynamic range from 200 pM to 388 nM. This work demonstrates the effectiveness of enzyme-based signal amplification in sandwich assay formats, especially for mid-nanomolar to low-picomolar detection in complex sample matrices.
Signal-on strategies generate an increase in the electrochemical response upon target binding, while signal-off formats produce a decrease, both offering distinct advantages depending on the application requirements. Interestingly, Rezaei et al. [26] proposed an innovative ratiometric biosensor for detecting miR-18a that integrates both signal-on and signal-off modalities in a dual-amplification architecture. Their platform employs a DNA hairpin probe immobilized on the electrode surface, which forms RNA/DNA duplexes upon hybridization with the target miR-18a. These duplexes serve as substrates for duplex-specific nuclease-assisted target recycling, which selectively cleaves the DNA strand, releasing MB tags and resulting in a signal-off response. Notably, the target miRNA is not consumed but rather recycled to catalyze multiple cleavage cycles, enhancing the signal suppression. Simultaneously, the remaining DNA fragments are hybridized with azide-modified signal DNA strands, which undergo a click reaction with 3-butynyl-2-bromoisobutyrate to initiate electrochemical atom transfer radical polymerization of ferrocenylmethyl methacrylate (FMMA). This reaction generates a ferrocene-rich surface, producing a strong signal-on response. By evaluating the signal ratio between the ferrocene and MB (IFMMA/IMB), the platform achieves ratiometric quantification, effectively mitigating background noise and signal drift. This dual-response biosensor exhibited an ultralow LOD of 2.5 aM and a linear range spanning from 100 aM to 50 pM. Such integration of opposing signal mechanisms exemplifies a new generation of ultrasensitive and noise-resilient biosensors suitable for early disease diagnostics.
The integration of enzymatic or nanomaterial-based amplification, such as rolling circle amplification (RCA), hybridization chain reaction (HCR), or DNA walkers, has significantly pushed the limits of detection to the femtomolar and even attomolar range. RCA, an isothermal amplification technique, involves a circular DNA template and primer that, upon target binding and ligation, initiate polymerase-driven synthesis of long ssDNA with tandem repeats. These amplified sequences can hybridize with redox-labeled probes (e.g., MB, ferrocene), allowing for robust electrochemical signal generation. This strategy not only enhances sensitivity into the femtomolar and attomolar ranges but also maintains isothermal conditions favorable for POC applications. The modular nature of RCA enables integration with various detection platforms and supports multiplexing potential, which is critical for comprehensive biomarker profiling in clinical settings. When functioning solely as electron-conductivity enhancers, nanomaterials such as graphene oxide, reduced graphene oxide (rGO), and carbon nanotubes (CNTs) significantly increase the electroactive surface area and improve the rate of charge transfer [29]. Their high conductivity allows for a more efficient transduction of the hybridization signal. However, in these passive roles, their contribution to selectivity is minimal, necessitating careful design of the biorecognition interface to avoid nonspecific interactions. In more active roles, metallic nanoparticles such as gold (AuNPs), silver (AgNPs), and platinum (PtNPs) contribute to both electron mediation and signal amplification by participating in redox cycling or catalysis [30,31]. For instance, AuNPs can serve as catalytic platforms for HRP [32] or ALP [33] in enzymatic amplification cascades, and their surface chemistry enables strong thiol–gold immobilization of capture probes, effectively functioning as both anchoring sites and amplification agents. Furthermore, many nanomaterials serve as immobilization scaffolds due to their high surface-area-to-volume ratio and tunable functionalization. For example, magnetic nanoparticles (MNPs) not only facilitate easy separation but also provide multiple attachment points for capture probes, increasing the local concentration of recognition elements and enhancing hybridization efficiency [34]. Similarly, hybrid nanocomposites, such as metal–organic frameworks (MOFs) and quantum dot-nanoparticle conjugates, have been engineered to integrate all three functions (i.e., signal enhancement, electron mediation, and molecular capture) within a single platform [35].
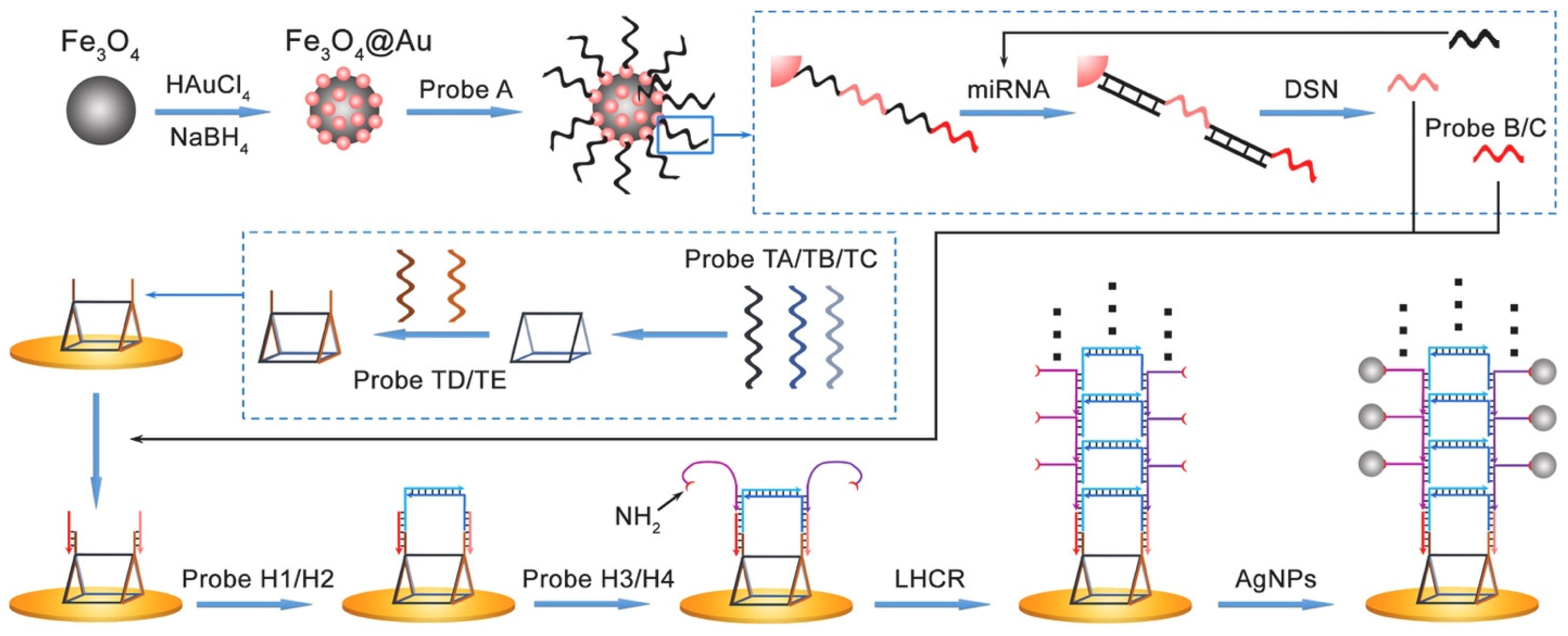
Isothermal, enzyme-free nucleic acid amplification strategies have gained considerable attention in recent years for their simplicity, versatility, and compatibility with POC biosensors. Among these, the HCR has emerged as a particularly effective tool for electrochemical detection of miRNAs, especially when integrated with electrode-bound capture probes. HCR is initiated when a target miRNA (the initiator strand) binds to a complementary probe, typically immobilized on the electrode surface, triggering a cascade of strand displacement events between metastable DNA hairpins (e.g., H1 and H2). This process forms long, nicked double-helix DNA polymers, which can be labeled with electroactive molecules to generate amplified current signals proportional to miRNA concentration. In an adaptation of this mechanism, Chai et al. [36] introduced a ladder hybridization chain reaction (LHCR) strategy to detect miR-141 derived from prostate cancer cells. Their platform, illustrated in Figure 4, incorporates Fe3O4/Au nanoparticles functionalized with thiol-modified DNA probes (Probe A) for magnetic separation and target preconcentration. Upon miRNA binding, duplex-specific nuclease-assisted target recycling is employed to release single-stranded Probes B and C, while preserving and reusing the target miRNA to amplify signal input. These released probes initiate a hierarchical LHCR process at the electrode interface, facilitated by a uniquely engineered triangular prism DNA nanostructure that improves hybridization kinetics and probe accessibility. The resulting DNA assembly, featuring sequential hybridization of H1/H2 followed by H3/H4, yields a three-dimensional, ladder-like nanostructure densely functionalized with silver nanoparticles (AgNPs), tethered via amino group modification for electrochemical signal generation. This system achieved an ultralow LOD of 5 aM and a dynamic range spanning 10 aM to 1 pM, highlighting the diagnostic potential of LHCR for early-stage cancer biomarker detection. The integration of duplex-specific nuclease (DSN)-based recycling and spatially structured DNA scaffolds demonstrates how structural innovation can synergistically improve hybridization efficiency and signal strength in HCR-based systems.
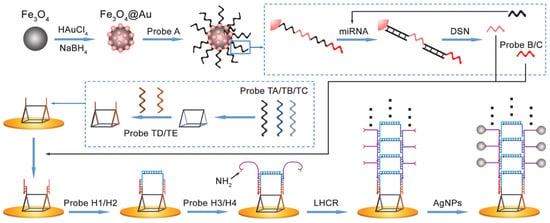
Figure 4.
Schematic illustration of Fe3O4@AuNPs-assisted DSN cleavage and LHCR-driven signal amplification for electrochemical biosensing [36].
DNA walkers represent a paradigm shift in dynamic, target-responsive biosensing. These synthetic molecular machines are designed to “walk” stepwise along a DNA track through enzyme-catalyzed cleavage or toehold-mediated strand displacement, amplifying signal output with each step. Upon recognition of a specific miRNA, the DNA walker is activated and initiates a sequential, directional motion that either deposits or releases electroactive species on the electrode surface, producing quantifiable changes in current. Hou et al. [37] developed a multiregion linear DNA walker (MLDW) optimized for fast, ultrasensitive detection of miR-21. This single-legged walker was engineered with small steric hindrance and multiple functional domains to enhance mobility and reaction efficiency. Upon binding to miR-21, the hairpin probe H1 opens, exposing a toehold that mediates duplex formation and releases both the miRNA and a primer strand. This primer, in turn, initiates RCA with the assistance of a padlock probe, T4 DNA ligase, Phi29 polymerase, and dNTPs, generating long DNA tracks enriched with binding domains for the walker. Once immobilized via a polyA–Au linkage to a second hairpin probe (H2) on the electrode, the MLDW progresses along the surface, driven by an embedded DNAzyme domain that cleaves RNA linkages in the presence of Mg2+, resulting in the release of ferrocene and a corresponding electrochemical signal. This walker-mediated RCA cascade achieved a LOD of 36 aM in under 30 min, representing at least a fourfold reduction in assay time compared to conventional DNA walkers. These innovations collectively contribute to the growing array of amplification strategies (enzymatic, nanomaterial-based, RCA, HCR, DNA walkers) to improve sensitivity, specificity, and dynamic range, all of which are central to the development of electrochemical biosensors for miRNA diagnostics. Table 1 provides a comparative overview of key amplification strategies used in electrochemical miRNA detection.

Table 1.
Comparison of Amplification Strategies for Electrochemical miRNA Detection.
2.3. Challenges and Advances in Nanomaterial and Amplification Strategy Integration
A central criticism in the application of nanomaterials for biosensing is their tendency to promote nonspecific adsorption. Notably, carbon-based materials such as CNTs, graphene oxide (GO), and rGO, possess high surface areas and hydrophobic or π-rich surfaces that can interact indiscriminately with proteins, lipids, and other biomolecules found in complex biological samples like serum or plasma [38]. This aspecific adsorption can severely compromise the sensor’s performance by introducing false-positive signals, increasing background noise, and reducing hybridization efficiency with the target miRNA. Carbon materials, although advantageous for electron transfer and mechanical strength, have an inherent propensity to bind non-target molecules via van der Waals forces and hydrophobic interactions, leading to sensor fouling and signal drift [39].
To mitigate these issues, several surface passivation strategies have been developed. One approach involves the use of polyethylene glycol (PEG), a hydrophilic polymer that forms steric barriers on the nanomaterial surface, thereby preventing protein adhesion and enhancing biocompatibility [40]. Alternatively, self-assembled monolayers (SAMs) comprising alkanethiols, silanes, or zwitterionic compounds can be used to tune surface properties and create antifouling layers [41]. Albumin or bovine serum albumin (BSA) blocking is also frequently employed to reduce nonspecific binding, particularly in point-of-care formats [42]. Furthermore, the functionalization of nanomaterials with chemically active groups such as carboxyl, hydroxyl, or amine moieties enhances their compatibility with capture probes and improves selectivity by reducing unintended interactions [43]. These functionalizations not only enable covalent attachment of biomolecules but can also be engineered to modulate surface charge and hydrophilicity, which are key determinants of fouling resistance.
Another major challenge is the batch-to-batch variability in nanoparticle synthesis, which affects critical parameters such as particle size, shape, surface charge, and catalytic properties [44]. Inconsistent synthesis can result in significant variation in biosensor performance, hampering reproducibility and standardization, critical barriers for regulatory approval and clinical deployment. For example, the electrochemical properties of gold or silver nanoparticles are highly sensitive to even minor deviations in size or aggregation state [45]. Recent advances in microfluidic synthesis platforms [46] and automated nanoparticle fabrication systems [47] have begun to address this issue. These technologies offer better control over reaction conditions such as temperature, flow rate, and precursor concentration, allowing for the generation of more uniform and reproducible nanomaterial batches. Additionally, in situ characterization techniques such as dynamic light scattering (DLS), zeta potential analysis, and electron microscopy are increasingly used to qualify nanomaterials before biosensor assembly, ensuring quality control and standardization.
Amplification strategies also face important limitations. Many of the most sensitive detection strategies rely on enzymatic amplification processes, such as RCA, HCR, and CHA, that introduce vulnerabilities in terms of thermal and chemical stability. Enzymes like Phi29 DNA polymerase or DSN may be denatured by storage conditions, compromised during shipping, or inhibited by endogenous substances present in clinical samples [48,49]. This can lead to loss of amplification efficiency or inconsistent results across different testing environments. Furthermore, amplification reactions are often time-consuming and require precise temperature control, which limits their suitability for rapid diagnostics or field use. Amplification bias, where certain sequences are preferentially amplified over others, may also distort quantification in multiplexed or complex sample settings [50].
To overcome these amplification challenges, researchers have developed thermostable enzyme variants and explored enzyme-free amplification mechanisms [51,52,53]. DNAzyme mimetics and entropy-driven strand displacement cascades have shown promise in maintaining high signal amplification without reliance on fragile enzymatic components [54,55]. In particular, strategies that integrate nanomaterials to localize and concentrate reactants, such as magnetic nanoparticles [56,57], DNA-functionalized gold nanostructures [58], or DNA origami scaffolds [59], have been highly effective in enhancing amplification kinetics. These nanomaterial-assisted amplification systems can significantly reduce reaction time while achieving attomolar detection limits by increasing the local effective concentration of target and probes.
Several advances have significantly expanded the capabilities of nanomaterial-integrated miRNA biosensors. For instance, the development of hybrid nano-biointerfaces, combining MOFs with conductive polymers or metal nanoparticles, has yielded multifunctional sensing platforms that simultaneously offer catalytic activity, electrical conductivity, and high biomolecule loading capacity [60,61,62]. Similarly, the implementation of multiplexed sensor arrays with spatially resolved immobilization zones and orthogonal redox labels allows for simultaneous detection of multiple miRNAs, which is critical for disease signature profiling [63,64]. Additionally, the rise of smartphone-integrated readout platforms and portable potentiostats has facilitated the deployment of nanomaterial-enhanced biosensors in resource-limited and POC settings [65]. These platforms leverage wireless data transmission, cloud-based analytics, and user-friendly interfaces to enable rapid, decentralized diagnostics. Table 2 summarizes the roles, advantages, limitations, and performance metrics of various common nanomaterials used to enhance electrochemical miRNA detection.

Table 2.
Comparison of Nanomaterial-Based Enhancements in Electrochemical miRNA Detection.
3. Redox-Reporting Methods in Electrochemical miRNA Biosensors
Various redox-reporting strategies have been developed to enhance the sensitivity, specificity, and reproducibility of electrochemical miRNA biosensors. These can be broadly categorized into electroactive species-labeled probes, inorganic and organic redox mediators, and intercalating molecules [16,66,67,68]. Electroactive species-labeled probes represent a direct and efficient approach, where redox-active molecules are covalently linked to nucleic acid probes. Upon hybridization with the target miRNA, measurable changes in electron transfer can be monitored by voltammetry, amperometry, or EIS. Both inorganic and organic redox labels have been utilized. Inorganic reporters include nanoparticles such as gold, silver, or platinum, which exhibit strong electrochemical signatures and enable signal amplification through catalytic or conductivity-enhancing effects [69]. Organic molecules, such as ferrocene and MB, are more commonly employed due to their well-defined redox potentials, chemical stability, and ease of functionalization [70,71]. However, they are diffusional in nature, which can cause nonspecific background currents and reduced spatial resolution. Additionally, signal readouts depend heavily on probe–analyte proximity and electrode cleanliness, which complicates reproducibility. In contrast, electroactive species-labeled capture probes offer more controlled signal generation. These probes typically involve covalently bound redox molecules that report hybridization events through signal modulation. While this method enhances spatial specificity and allows for miniaturized and multiplexed formats, it requires careful probe design and costly synthesis. Moreover, the stability and orientation of the redox tags on the electrode surface can affect signal intensity.
3.1. Redox-Labeled Hybridization Modes
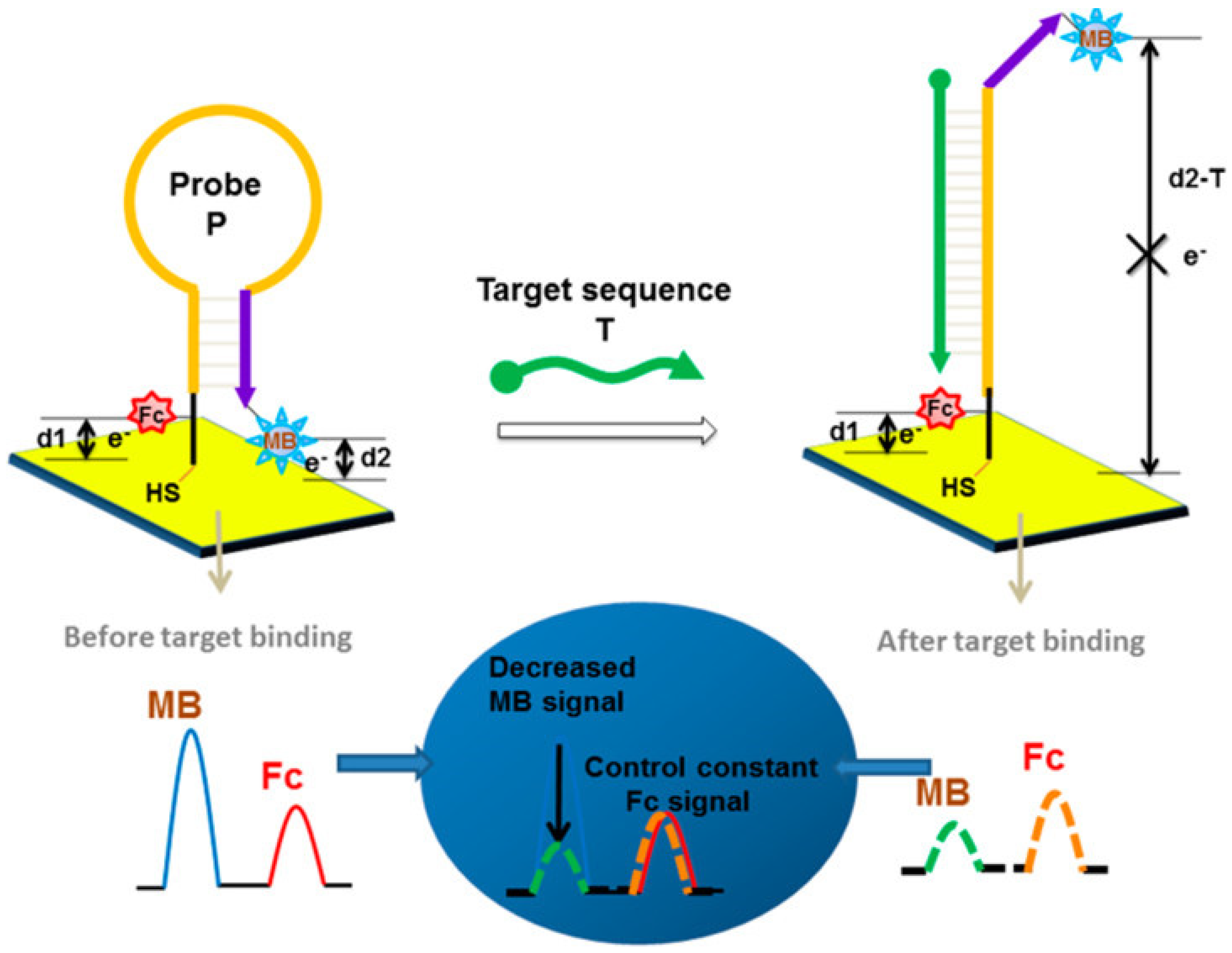
Several hybridization modes leverage these labeled probes to enhance signal output. Besides the linear (direct hybridization) format, hairpin (stem–loop) structures and sandwich assays are widely employed, while more complex approaches such as toehold-mediated strand displacement and three-dimensional DNA nanostructures have also been explored, albeit less frequently due to their fabrication complexity and cost [72]. In hairpin-based designs, a single-stranded nucleic acid probe folds into a stem–loop structure, positioning the redox label in proximity to the electrode surface [73]. In the closed-loop state, efficient electron transfer results in a strong electrochemical signal. Upon hybridization with the target miRNA, the stem opens, altering the distance and orientation of the redox reporter relative to the electrode, thereby modulating the signal. Du et al. [74] developed a ratiometric detection strategy using two redox probes: MB for dynamic signal reporting and ferrocene as an internal control. In their system, target hybridization induced the opening of the stem–loop, altering the distance between the MB probe and the electrode, thereby selectively affecting the MB signal while leaving the ferrocene reference unchanged. This design mechanism, shown in Figure 5, provided enhanced precision and robustness against environmental fluctuations, achieving LOD of 25.1 pM.
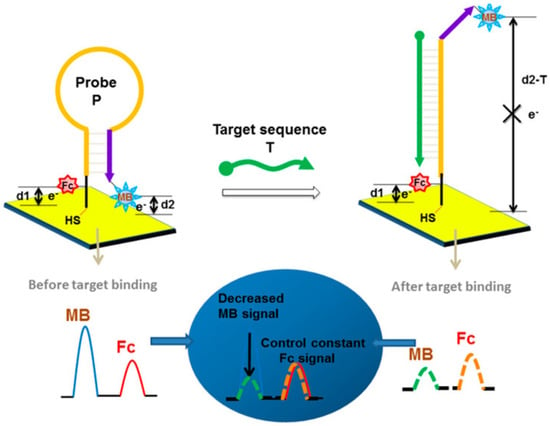
Figure 5.
Schematic illustrations of nucleic acid electrochemical biosensor using hairpin probe [75].
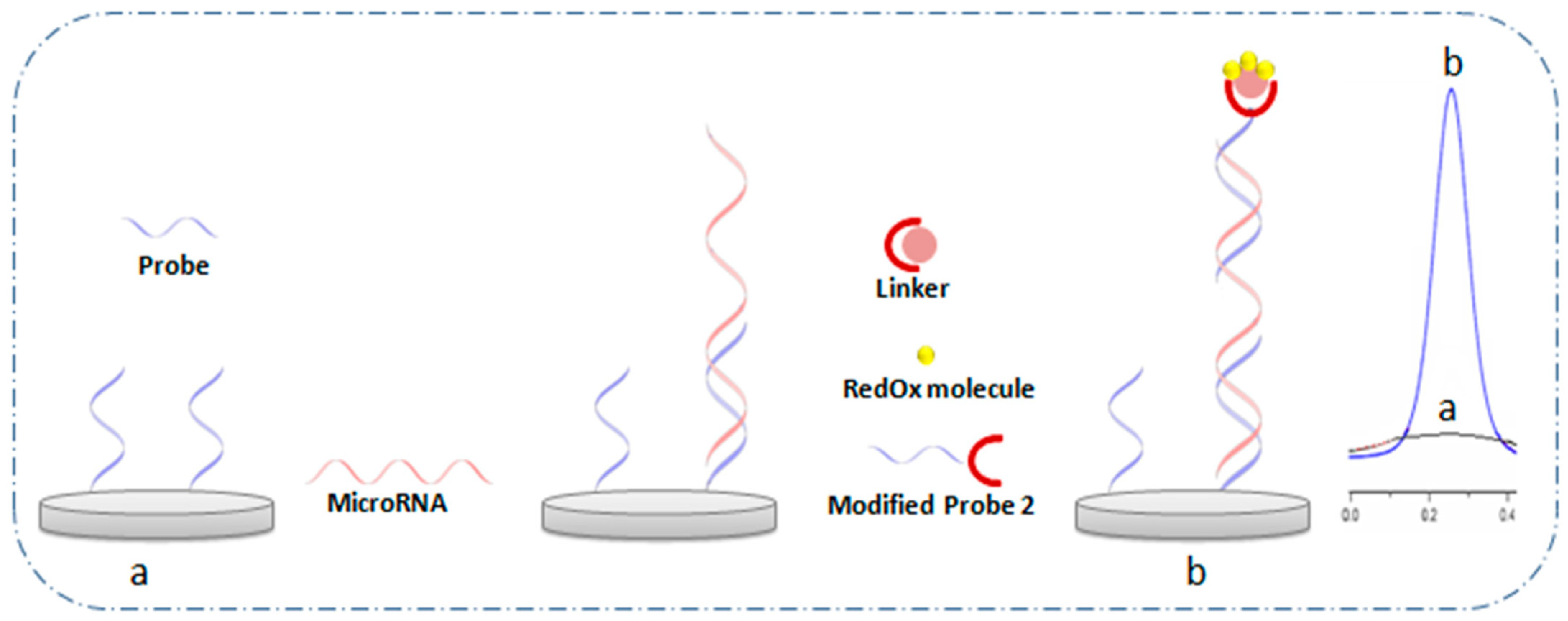
Alternatively, sandwich-type hybridization assays employ a three-component structure involving a capture probe, the target miRNA, and a detection probe labeled with a signal reporter [75]. The capture probe (e.g., ssDNA) first hybridizes to a segment of the miRNA, leaving an overhang region available for the labeled detection probe (e.g., redox probe, enzyme, or electroactive nanomaterial). Upon binding, a “sandwich” structure forms, bringing the redox reporter into proximity with the electrode and generating a quantifiable signal. This strategy offers improved selectivity and amplification capabilities compared to direct hybridization, especially for low-abundance targets. A general schematic of the sandwich assay architecture is illustrated in Figure 6 [16].
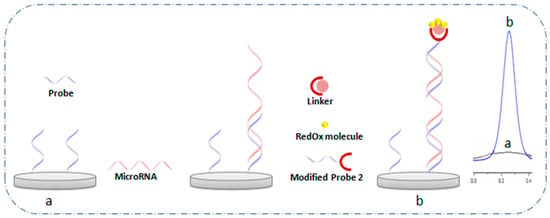
Figure 6.
General schematic of the sandwich structure method (a) before and (b) after hybridization [16].
Each redox-reporting approach presents unique advantages and challenges. Labeled-probe strategies provide simplicity and rapid response but may suffer from steric hindrance or incomplete hybridization efficiency. Sandwich assays offer higher sensitivity but require careful optimization of probe sequences to avoid nonspecific binding. Advanced designs, such as ratiometric measurements or dual-probe systems, are increasingly employed to address these limitations and ensure accurate miRNA quantification in complex biological matrices.
3.2. Redox Mediators in Label-Free Electrochemical miRNA Biosensing
Redox mediators are small molecules or metal complexes that facilitate electron transfer between the electrode surface and the biomolecular recognition event, enabling the indirect detection of target miRNA hybridization without the need for covalent labeling [76]. In label-free miRNA biosensors, redox mediators play a crucial role in signal generation by modulating the electrode’s electrochemical response upon hybridization events [77]. Based on their chemical composition and redox behavior, redox mediators can be broadly categorized into inorganic and organic classes. Inorganic redox mediators, such as transition metal complexes, exhibit reversible oxidation-reduction reactions at specific electrode potentials [78]. A widely employed example is the potassium ferri/ferrocyanide system (K3[Fe(CN)6]/K4[Fe(CN)6]), which operates as an indirect charge–transfer probe [79]. Unlike direct labeling strategies, ferri/ferrocyanide detects hybridization-induced alterations to the electrode interface. Specifically, nucleic acid backbones possess negatively charged phosphate groups that form an electrostatic barrier, impeding the access of the anionic [Fe(CN)6]3−/4− species to the electrode surface. Upon target hybridization, the increased density of double-stranded structures exacerbates this barrier, leading to a measurable decrease in the electrochemical current, which can be quantified to determine miRNA concentration.
Serrano et al. [80] fabricated an impedimetric biosensor for miR-21-5p detection by electrodepositing gold nanoparticles (AuNPs) onto a carbon screen-printed electrode (SPE), enabling the immobilization of thiol-modified complementary ssRNA probes (Figure 7A). EIS was performed to monitor the miR-21-5p/ssDNA-21 hybridization using the [Fe(CN)6]3−/4− redox probe. The direct linear relationship between miRNA concentration and impedance was observed, as shown in Figure 7B,C, thereby achieving a detection range from 10 aM to 10 pM and a LOD of 4.31 aM.
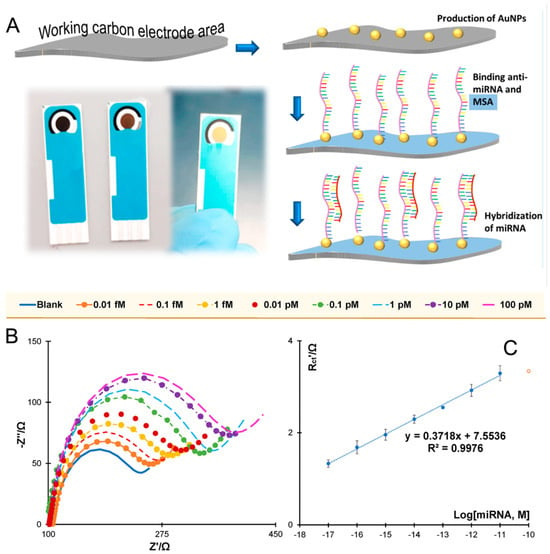
Figure 7.
(A) Schematic representation of the assembly of the miR-21-5p on carbon electrode modified with gold nanoparticles (AuNPs). (B) Nyquist plots. (C) Corresponding calibration curve in 5.0 × 10−3 M [Fe(CN)6]3−/4−, in standard solutions prepared in a 1000-fold diluted human serum and using relative charge transfer resistance (Rct) [78] data.
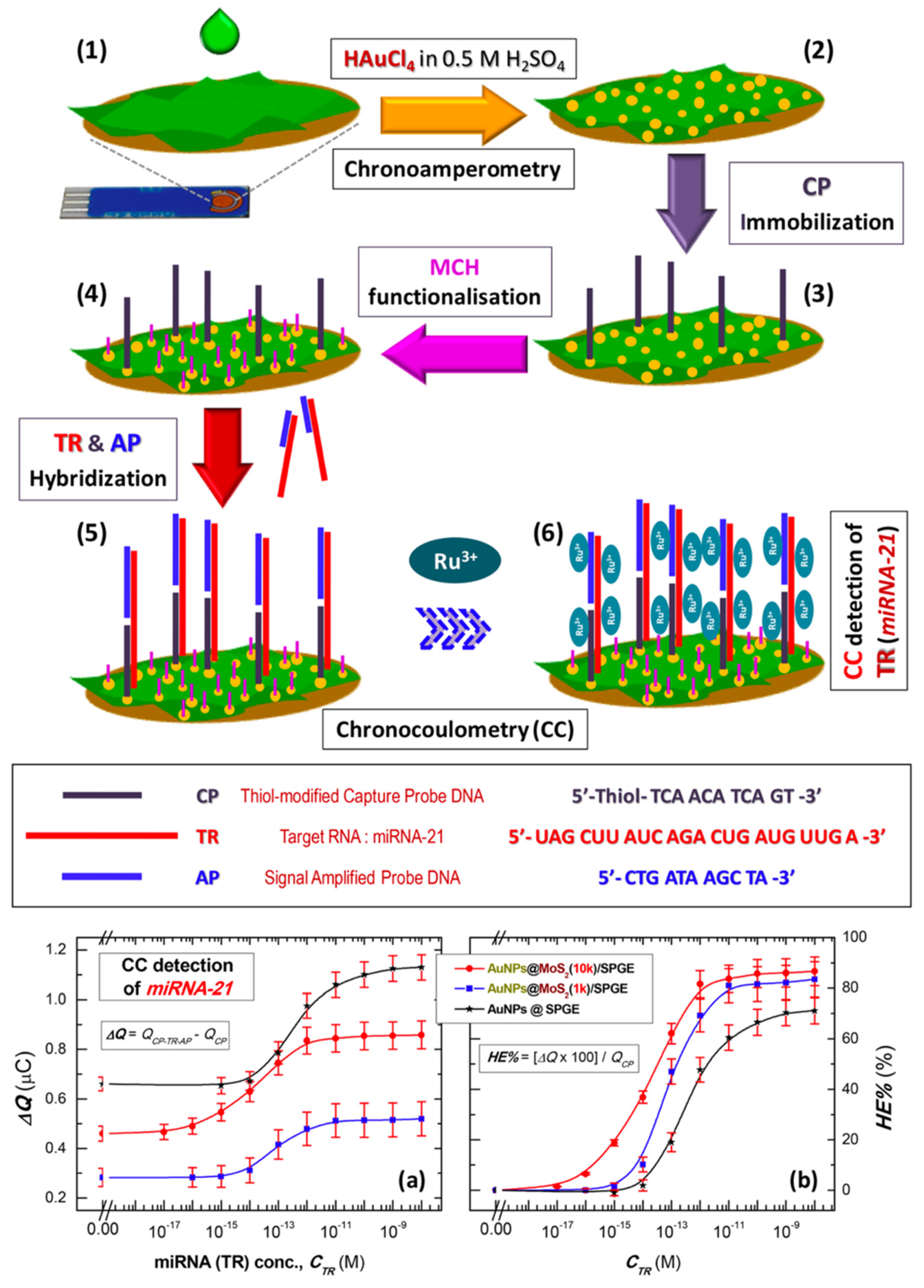
Expanding on label-free sensing platforms, Torul et al. [81] reported a novel approach for the detection of miR-21 and miR-155 using nitrocellulose paper-based electrodes. The electrodes were fabricated by screen printing conductive carbon and Ag/AgCl inks onto nitrocellulose substrates, followed by wax deposition to stabilize electrode configuration and prevent reagent diffusion. To enhance sensitivity, two nanomaterial modifications, AuNP-reduced graphene oxide (AuNP/RGO) and AuNP–molybdenum disulfide (AuNP/MoS2), were explored as illustrated by the voltammograms in Figure 8A,B. Using DPV with ferri/ferrocyanide as the redox mediator, a linear correlation between miRNA concentration and current decrease was observed, as depicted in Figure 8C,D. The AuNP/RGO-modified electrode exhibited good sensitivity, with a LOD of 51.6 nM, and the detection assay was completed within 35 min at room temperature using only 5.0 µL of sample. This study demonstrates the potential for low-cost, rapid, and user-friendly miRNA diagnostics without the need for complex surface chemistries. In contrast to negatively charged mediators, positively charged redox mediators, such as hexaammineruthenium(II)/(III) ([Ru(NH3)6]2+/3+), offer an alternative detection mechanism. Owing to their strong electrostatic attraction to the negatively charged nucleic acid backbones, [Ru(NH3)6]2+/3+, preferentially binds to nucleic acid duplexes formed upon hybridization. In this system, the amount of mediator accumulated correlates directly with the density of hybridized structures. Subsequent electrochemical analysis by voltammetry (oxidation of Ru2+ to Ru3+) or coulometry (measuring total charge, Q) enables sensitive quantification of miRNA targets. Ganguly et al. [22] demonstrated this principle by electrodepositing AuNP-decorated MoS2 nanosheets onto a gold SPE to develop a chronocoulometric biosensor for miR-21 detection (Figure 9a). Thiolated ssDNA probes were immobilized onto the AuNPs to capture target miRNA, and a secondary complementary DNA sequence was hybridized to the overhang of miR-21, thereby amplifying the charge signal. Chronocoulometric analysis of [Ru(NH3)6]3+ binding exhibited a linear relationship between miR-21 concentration and ΔQ (charge difference), as shown in Figure 9b demonstrating the effectiveness of positive redox mediators for highly sensitive label-free detection.
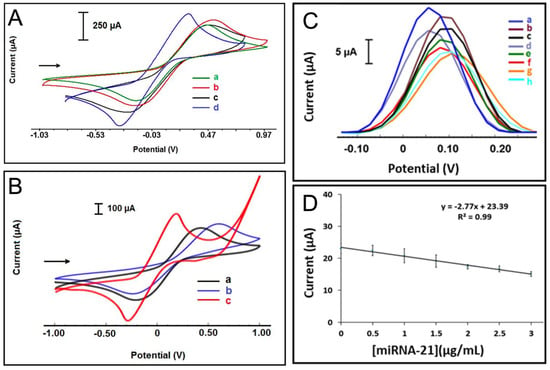
Figure 8.
Cyclic voltammograms (CVs) performed in 50 mM [Fe(CN)6]3−/4− containing 0.1 M KCl of (A) (a) unmodified paper electrode, (b) RGO-modified paper electrode, (c) activated RGO-modified paper electrode using covalent agents, and (d) AuNPs electrodeposited activated RGO-modified electrode. (B) (a) Unmodified paper electrode, (b) MoS2-modified paper electrode, (c) AuNPs electrodeposited MoS2-modified paper electrodes. (C) Oltammograms of (a) Probe-2 immobilized AuNP/MoS2-paper electrode in the absence of miR-21 target, after hybridization of Probe-2 with miR-21 target at concentrations of (b–h) from 0.5 to 5 µg/mL. (D) Corresponding calibration plot [81].
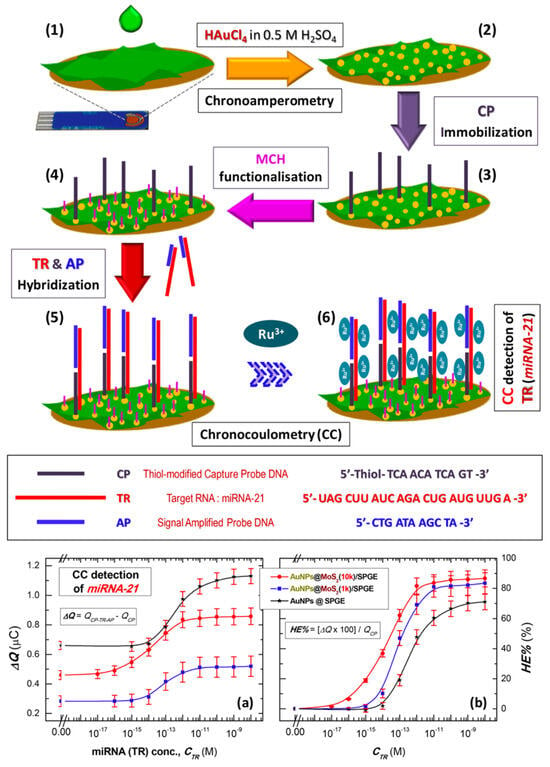
Figure 9.
Fabrication and detection strategy of miR-21 sensor. Coating of MoS2 nanosheets (MoS2 NSs) on commercial screen-printed gold electrodes (SPGEs) (1); decoration of MoS2 NSs with AuNPs (2); assembly of ssDNA capture probe: anti-miR-21 (CP), miR-21 target (TR) and signal amplification probe (AP) on AuNPs@MoS2/SPGE sensor (3–5); chronocoulometric (CC) detection of miRNA (TR) by monitoring [Ru(NH3)6]3+ (RuHex) electrostatically bound to phosphate backbones of oligonucleotides (6); CC detection of miR-21. Logarithmic plot for (a) CC signal (ΔQ) and (b) corresponding hybridization efficiency (HE%) versus target miRNA (TR) concentration (CTR) for the CP-immobilized on AuNPs@SPGE and AuNPs@MoS2 modified SPGEs employing MoS2 nanosheets produced at centrifugation speeds of 1 k rpm (AuNPs@MoS2(1k)/SPGE) and 10 k rpm (AuNPs@MoS2(10k)/SPGE) [22].
Each mediator-based sensing strategy presents inherent advantages and trade-offs. Ferri/ferrocyanide-based systems are cost-effective and simple but may suffer from nonspecific interactions in complex matrices. In contrast, positively charged mediators like [Ru(NH3)6]3+ provide enhanced specificity through electrostatic interactions but require careful control of ionic strength and background signal. Continued innovation in mediator chemistry, electrode modification, and assay design is essential for advancing label-free electrochemical detection of miRNAs toward clinical and POC applications.
3.3. Intercalation-Based Redox Probes for Label-Free miRNA Detection
Intercalation is a molecular process where planar aromatic molecules insert themselves between the stacked base pairs of double-stranded nucleic acid helices, stabilized primarily through π–π stacking and van der Waals interactions. In label-free miRNA biosensing, electroactive intercalators selectively bind to hybridized miRNA-capture probe duplexes and undergo redox reactions upon electrochemical stimulation, producing a measurable current proportional to the amount of target miRNA [82]. The degree of intercalation, and, hence, the magnitude of the resulting electrochemical signal, is directly correlated with the extent of nucleic acid hybridization, offering a quantitative approach for miRNA detection without requiring covalent labeling or target modification. This strategy circumvents the need for covalent labeling, thereby streamlining sensor fabrication and preserving target integrity.
The efficacy of intercalation-based sensing hinges on several key parameters: (i) the binding affinity of the intercalator for duplex versus single-stranded nucleic acids, (ii) the number of accessible binding sites, (iii) the redox reversibility and stability of the intercalator under operational conditions, and (iv) the absence of nonspecific adsorption on the electrode surface, which can confound signal interpretation. A variety of redox-active intercalators have been explored for this purpose. Organic molecules such as MB [81], toluidine blue (TB) [23], and anthraquinone derivatives like Oracet Blue (OB) [83] are among the most widely used due to their well-defined redox behaviors and strong affinity for nucleic acid duplexes. Beyond organic dyes, metallic nanoparticles (e.g., palladium nanoparticles) [84], organometallic complexes (e.g., cobalt-phenanthroline) [85], and bioorganic molecules such as hemin [86] have also been employed to facilitate or enhance intercalative electrochemical sensing, offering diversified approaches to increase sensitivity, stability, and selectivity in miRNA biosensors.
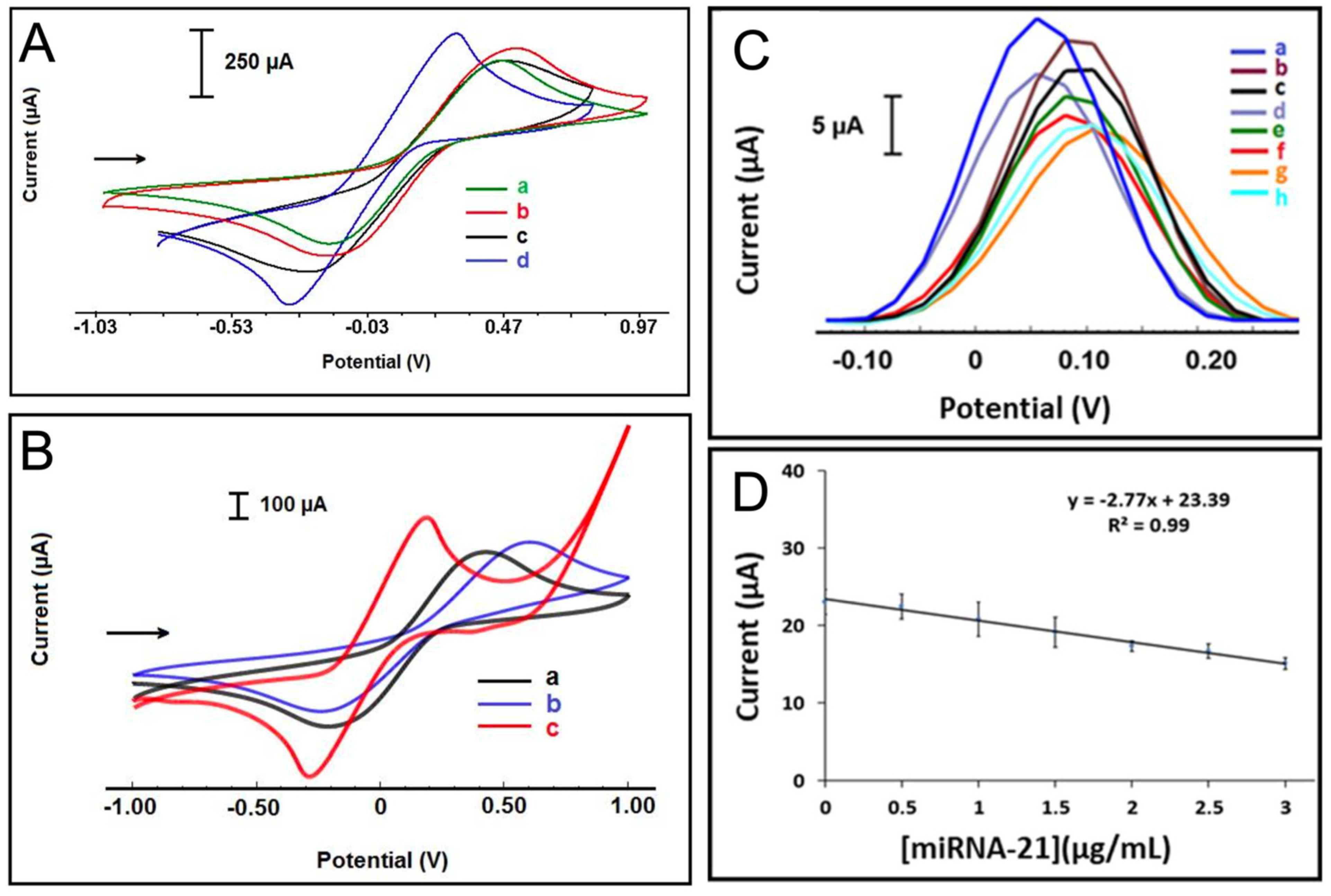
Tian et al. [23] demonstrated an application of this strategy by engineering an ultra-sensitive electrochemical biosensor for the detection of miR-21 and miR-141 using TB as the electroactive intercalator. Their biosensor construction involved the deposition of a polypyrrole (PPy)-coated gold nanoparticle superlattice (AuNS) onto a GCE, illustrated in Figure 10A. The highly ordered and densely packed AuNP superlattice architecture provided a large electroactive surface area and enhanced electron transfer kinetics, facilitating the immobilization of a high density of ssRNA capture probes. This high probe-loading capacity translated into improved hybridization efficiency with the target miRNA sequences. Following hybridization, TB was introduced as the intercalative redox probe, leveraging its strong π–π stacking interactions with the nucleobases of the RNA duplexes. Upon hybridization of the target miRNA with the capture probes, TB molecules intercalated into the formed duplexes and underwent electrochemical redox reactions, thereby generating a signal that scaled linearly with miRNA concentration (Figure 10B). Importantly, the PPy-coated AuNS scaffold further contributed to signal enhancement by offering electrocatalytic sites that lowered the overpotential required for TB redox cycling, minimizing background noise, and improving the limit of detection. The synergistic combination of the conductive AuNS scaffold and the efficient electron transfer properties of TB significantly amplified the electrochemical response, achieving a low detection limit of 78 aM and a wide dynamic range of 100 aM–1 nM, suitable for detecting miRNAs in complex biological matrices.
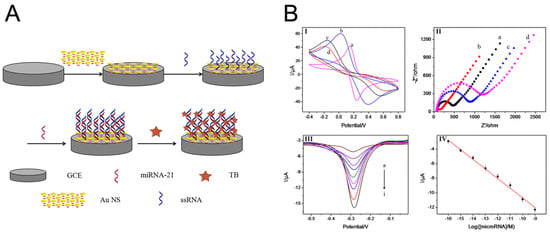
Figure 10.
(A) Schematic representation of the miRNA biosensor and detection principle. (B) CVs (I) and EIS (II) of bare GCE (a), AuNS/GCE (b), SS-RNA probe/AuNS/GCE (c), and microRNA/SS-RNA probe/AuNS/GCE (d) in 5.0 mM Fe(CN)64−/3− containing 0.1 M KCl at 100 mV/s. (III) Differential pulse voltammetry (DPV) responses to SS-RNA probe/AuNS/GCE biosensor in the presence of different concentrations of miR-21 from (a) to (i): 0 M, 100 aM, 1 fM, 10 fM, 100 fM, 1 pM, 10 pM, 100 pM, and 1 nM. (IV) Calibration curve of peak current vs. logarithmic microRNA-21 concentration [23].
As summarized in Table 3, the representative intercalative redox mediators have demonstrated promise for label-free electrochemical detection of miRNAs, owing to their ability to intercalate within nucleic acid duplexes and transduce hybridization events into measurable electrical signals. The design strategy demonstrates the synergistic benefits of combining nanostructured electrode platforms with efficient intercalative redox mediators to overcome the intrinsic challenges of label-free miRNA detection, such as low target abundance and high matrix complexity in clinical samples. However, many of these redox mediators suffer from chemical and electrochemical instability, particularly under physiological or ambient conditions, leading to signal drift or compromised reproducibility over time. This instability underscores the need for next-generation mediators with enhanced stability or immobilization strategies that prevent leaching and preserve electrochemical integrity over extended periods.

Table 3.
Representative Intercalative Redox Mediators for Label-Free miRNA Detection.
4. Challenges and Perspectives
Despite remarkable advancements in the electrochemical detection of miRNAs, several technical and translational hurdles remain to be addressed to enable reliable clinical deployment. One of the most pressing challenges is nonspecific adsorption, which often leads to elevated background signals and diminished signal-to-noise ratios, particularly in complex biological fluids such as serum, saliva, and urine. The positively charged nature of many intercalative redox mediators (e.g., methylene blue, hexaammineruthenium(III)) exacerbates this issue by promoting electrostatic interactions with non-target molecules, thereby undermining detection specificity. To mitigate such effects, future electrochemical miRNA biosensor platforms must incorporate robust antifouling strategies, such as the development of zwitterionic polymer coatings, nanostructured antifouling interfaces, and selective permselective membranes, all of which can reduce biofouling while preserving access to the immobilized probes.
Equally limiting is the poor multiplexing capability of current electrochemical miRNA biosensors. While increasing evidence supports the clinical value of miRNA signatures, panels of multiple miRNAs, in disease diagnosis and prognosis, simultaneous detection remains rare due to overlapping redox potentials, probe cross-reactivity, and electronic crosstalk among sensing channels. Overcoming these barriers will require the integration of orthogonal redox mediators, electrically addressable electrode arrays, and spatially resolved immobilization strategies, which, together, can support high-throughput and interference-free detection. Another significant bottleneck is the suboptimal hybridization kinetics between target miRNAs and surface-tethered capture probes, especially at low target concentrations or when secondary RNA structures impede binding. This can result in delayed response times and reduced assay sensitivity. Optimizing probe design using locked nucleic acids, stem–loop structures, or thermodynamically favorable conformations, combined with assisted hybridization techniques such as localized heating, electrokinetic preconcentration, or nanopore confinement, may significantly improve hybridization efficiency.
Although many platforms report promising sensitivity and selectivity in idealized conditions (e.g., buffered solutions), their translation to unprocessed clinical samples remains limited. Biological fluids introduce a host of variables, such as pH variation, ionic strength, proteolytic enzymes, and nuclease activity, that can destabilize both the redox mediator and the capture probe. To ensure robustness in real-world diagnostics, sensor development must focus on protective probe chemistries, enzymatic shielding, and microfluidic systems capable of on-chip sample conditioning and matrix isolation. Moving forward, the path to POC diagnostics lies in the convergence of material innovation, system integration, and validation in real-world samples. Biosensors that are selective, stable, multiplex-capable, and amenable to POC deployment will be critical to unlocking the full diagnostic potential of miRNA-based electrochemical detection.
5. Conclusions and Future Outlook
MicroRNAs have emerged as powerful biomarkers due to their unique expression profiles across diverse pathological conditions and their stable presence in accessible biological fluids such as urine, blood, and saliva. Despite their clinical potential, the practical translation of miRNA-based diagnostics has been constrained by the intrinsic limitations of conventional detection methods, including labor-intensive workflows, insufficient sensitivity at low target concentrations, and limited adaptability for POC applications. Electrochemical biosensors have rapidly advanced as a promising technological solution to these challenges. By transducing biomolecular recognition events directly into electrical signals, these platforms offer rapid, sensitive, and often label-free detection formats suitable for decentralized testing. The integration of diverse redox-based reporting mechanisms, including covalently attached electroactive probes, solution-phase redox mediators, and intercalative redox agents, has enabled the development of biosensors capable of detecting miRNAs at attomolar to femtomolar concentrations. These strategies not only allow for quantitative signal generation but also afford considerable flexibility in assay design, supporting multiplexed analysis, signal amplification, and integration into miniaturized devices.
The recent innovations in nanomaterials engineering, particularly the incorporation of metal nanoparticles, conductive polymers, and nanostructured composites, have significantly amplified electrochemical responses while facilitating sensor miniaturization. Moreover, the adoption of advanced nucleic acid architectures such as hairpin probes, toehold-mediated strand displacement systems, and sandwich-type hybridization structures has markedly enhanced target specificity and reduced background noise, further elevating the reliability of miRNA detection in complex biological samples. Despite these advances, critical challenges remain to be addressed before the widespread clinical implementation of electrochemical miRNA biosensors. These include the need for standardized, scalable sensor fabrication protocols; the development of universal platforms capable of robust multi-analyte detection; and the demonstration of long-term stability, reproducibility, and performance consistency under physiologically relevant conditions. Additionally, regulatory approval pathways will require comprehensive clinical validation studies, rigorous quality control measures, and integration into established diagnostic frameworks.
Nonetheless, the inherent advantages of electrochemical biosensing, namely, high sensitivity, excellent specificity, portability, low cost, and operational simplicity, position these devices as strong candidates for next-generation molecular diagnostics. With continued interdisciplinary efforts across bioengineering, materials science, clinical medicine, and regulatory science, electrochemical biosensors are poised to play a transformative role in the future of personalized healthcare, early disease detection, and decentralized diagnostic testing.
Author Contributions
Conceptualization, A.H. and G.S.; Methodology, G.S.; Formal analysis, A.H. and G.S.; Investigation, A.H.; Resources, G.S.; Data curation, A.H.; Writing—original draft, A.H.; Writing—review & editing, G.S.; Supervision, G.S.; Project administration, G.S.; Funding acquisition, G.S. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Ranganathan, K.; Sivasankar, V. MicroRNAs—Biology and clinical applications. J. Oral Maxillofac. Pathol. 2014, 18, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Ha, T.Y. MicroRNAs in Human Diseases: From Cancer to Cardiovascular Disease. Immune Netw. 2011, 11, 135–154. [Google Scholar] [CrossRef] [PubMed]
- Chakrabortty, A.; Patton, D.J.; Smith, B.F.; Agarwal, P. miRNAs: Potential as Biomarkers and Therapeutic Targets for Cancer. Genes 2023, 14, 1375. [Google Scholar] [CrossRef] [PubMed]
- Singh, V.; Sen, A.; Saini, S.; Dwivedi, S.; Agrawal, R.; Bansal, A.; Shekhar, S. MicroRNA Significance in Cancer: An Updated Review on Diagnostic, Prognostic, and Therapeutic Perspectives. Ejifcc 2024, 35, 265–284. [Google Scholar]
- Lu, D.P.; Read, R.L.; Humphreys, D.T.; Battah, F.M.; Martin, D.I.; Rasko, J.E. PCR-based expression analysis and identification of microRNA. J. RNAi Gene Silenc. Int. J. RNA Gene Target. Res. 2005, 1, 44–49. [Google Scholar]
- Zippelius, A.; Kufer, P.; Honold, G.; Köllermann, M.W.; Oberneder, R.; Schlimok, G.; Riethmüller, G.; Pantel, K. Limitations of reverse-transcriptase polymerase chain reaction analyses for detection of micrometastatic epithelial cancer cells in bone marrow. J. Clin. Oncol. 1997, 15, 2701–2708. [Google Scholar] [CrossRef]
- Jaksik, R.; Iwanaszko, M.; Rzeszowska-Wolny, J.; Kimmel, M. Microarray experiments and factors which affect their reliability. Biol. Direct 2015, 10, 46. [Google Scholar] [CrossRef]
- Hunt, A.; Slaughter, G. Electrochemical Detection of Prostate Cancer—Associated miRNA-141 Using a Low-Cost Disposable Biosensor. Biosensors 2025, 15, 364. [Google Scholar] [CrossRef]
- Yáñez-Sedeño, P.; Agüí, L.; Campuzano, S.; Pingarrón, J.M. What Electrochemical Biosensors Can Do for Forensic Science? Unique Features and Applications. Biosensors 2019, 9, 127. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, W.; Lv, C.; Liu, X.; Yang, M.; Guo, M.; Fu, Q. Electrochemical biosensors represent promising detection tools in medical field. Adv. Sens. Energy Mater. 2023, 2, 100081. [Google Scholar] [CrossRef]
- Liyanage, T.; Alharbi, B.; Quan, L.; Esquela-Kerscher, A.; Slaughter, G. Plasmonic-based biosensor for the early diagnosis of prostate cancer. ACS Omega 2022, 7, 2411–2418. [Google Scholar] [CrossRef]
- Bharti, A.; Mittal, S.; Rana, S.; Dahiya, D.; Agnihotri, N.; Prabhakar, N. Electrochemical biosensor for miRNA-21 based on gold-platinum bimetallic nanoparticles coated 3-aminopropyltriethoxy silane. Anal. Biochem. 2020, 609, 113908. [Google Scholar] [CrossRef]
- Liyanage, T.; Lai, M.; Slaughter, G. Label-free tapered optical fiber plasmonic biosensor. Anal. Chim. Acta 2021, 1169, 338629. [Google Scholar] [CrossRef] [PubMed]
- Arlett, J.L.; Myers, E.B.; Roukes, M.L. Comparative advantages of mechanical biosensors. Nat. Nanotechnol. 2011, 6, 203–215. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Pu, H.; Sun, D.-W. Photothermal detection of food hazards using temperature as an indicator: Principles, sensor developments and applications. Trends Food Sci. Technol. 2024, 146, 104393. [Google Scholar] [CrossRef]
- Sin, M.L.; Mach, K.E.; Wong, P.K.; Liao, J.C. Advances and challenges in biosensor-based diagnosis of infectious diseases. Expert Rev. Mol. Diagn. 2014, 14, 225–244. [Google Scholar] [CrossRef] [PubMed]
- Campuzano, S.; Pedrero, M.; Gamella, M.; Serafín, V.; Yáñez-Sedeño, P.; Pingarrón, J.M. Beyond Sensitive and Selective Electrochemical Biosensors: Towards Continuous, Real-Time, Antibiofouling and Calibration-Free Devices. Sensors 2020, 20, 3376. [Google Scholar] [CrossRef]
- El Aamri, M.; Yammouri, G.; Mohammadi, H.; Amine, A.; Korri-Youssoufi, H. Electrochemical Biosensors for Detection of MicroRNA as a Cancer Biomarker: Pros and Cons. Biosensors 2020, 10, 186. [Google Scholar] [CrossRef]
- Jolly, P.; Batistuti, M.R.; Miodek, A.; Zhurauski, P.; Mulato, M.; Lindsay, M.A.; Estrela, P. Highly sensitive dual mode electrochemical platform for microRNA detection. Sci. Rep. 2016, 6, 36719. [Google Scholar] [CrossRef]
- Miglione, A.; Raucci, A.; Amato, J.; Marzano, S.; Pagano, B.; Raia, T.; Lucarelli, M.; Fuso, A.; Cinti, S. Printed Electrochemical Strip for the Detection of miRNA-29a: A Possible Biomarker Related to Alzheimer’s Disease. Anal. Chem. 2022, 94, 15558–15563. [Google Scholar] [CrossRef]
- Rafiee-Pour, H.-A.; Behpour, M.; Keshavarz, M. A novel label-free electrochemical miRNA biosensor using methylene blue as redox indicator: Application to breast cancer biomarker miRNA-21. Biosens. Bioelectron. 2016, 77, 202–207. [Google Scholar] [CrossRef] [PubMed]
- Ganguly, A.; Benson, J.; Papakonstantinou, P. Sensitive chronocoulometric detection of miRNA at screen-printed electrodes modified by gold-decorated MoS2 nanosheets. ACS Appl. Bio Mater. 2018, 1, 1184–1194. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Qian, K.; Qi, J.; Liu, Q.; Yao, C.; Song, W.; Wang, Y. Gold nanoparticles superlattices assembly for electrochemical biosensor detection of microRNA-21. Biosens. Bioelectron. 2018, 99, 564–570. [Google Scholar] [CrossRef]
- Nagdeve, S.N.; Suganthan, B.; Ramasamy, R.P. An electrochemical biosensor for the detection of microRNA-31 as a potential oral cancer biomarker. J. Biol. Eng. 2025, 19, 24. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chang, Y.; Lou, J.; Zhang, S.; Yi, X. Overview on the Development of Alkaline-Phosphatase-Linked Optical Immunoassays. Molecules 2023, 28, 6565. [Google Scholar] [CrossRef]
- Shao, Y.; Zhou, H.; Wu, Q.; Xiong, Y.; Wang, J.; Ding, Y. Recent advances in enzyme-enhanced immunosensors. Biotechnol. Adv. 2021, 53, 107867. [Google Scholar] [CrossRef]
- Peng, H.; Newbigging, A.M.; Wang, Z.; Tao, J.; Deng, W.; Le, X.C.; Zhang, H. DNAzyme-Mediated Assays for Amplified Detection of Nucleic Acids and Proteins. Anal. Chem. 2018, 90, 190–207. [Google Scholar] [CrossRef]
- Xianyu, Y.; Chen, Y.; Jiang, X.; Peroxidase-Mediated, H. Iodide-Catalyzed Cascade Reaction for Plasmonic Immunoassays. Anal. Chem. 2015, 87, 10688–10692. [Google Scholar] [CrossRef]
- Liyanage, T.; Qamar, A.Z.; Slaughter, G. Application of nanomaterials for chemical and biological sensors: A review. IEEE Sens. J. 2020, 21, 12407–12425. [Google Scholar] [CrossRef]
- Malekzad, H.; Zangabad, P.S.; Mirshekari, H.; Karimi, M.; Hamblin, M.R. Noble metal nanoparticles in biosensors: Recent studies and applications. Nanotechnol. Rev. 2017, 6, 301–329. [Google Scholar] [CrossRef]
- Hunt, A.; Torati, S.R.; Slaughter, S.R.G. Based DNA Biosensor for Rapid and Selective Detection of miR-21. Biosensors 2024, 14, 485. [Google Scholar] [CrossRef] [PubMed]
- Talapphet, N.; Huh, C.S. The optimization of gold nanoparticles–horseradish peroxidase as peroxidase mimic using central composite design for the detection of hydrogen peroxide. Nano Express 2024, 5, 015012. [Google Scholar] [CrossRef]
- Liu, L.; Xia, N.; Liu, H.; Kang, X.; Liu, X.; Xue, C.; He, X. Highly sensitive and label-free electrochemical detection of microRNAs based on triple signal amplification of multifunctional gold nanoparticles, enzymes and redox-cycling reaction. Biosens. Bioelectron. 2014, 53, 399–405. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; He, Z.; Liu, H.; Xu, Y.; Huang, H.; Yang, G.; Xiao, Z.; Li, S.; Liu, H.; Deng, Y.; et al. Application of magnetic nanoparticles in nucleic acid detection. J. Nanobiotechnol. 2020, 18, 62. [Google Scholar] [CrossRef]
- Chandra, D.K.; Kumar, A.; Mahapatra, C. Smart nano-hybrid metal-organic frameworks: Revolutionizing advancements; applications, and challenges in biomedical therapeutics and diagnostics. Hybrid Adv. 2025, 9, 100406. [Google Scholar] [CrossRef]
- Chai, H.; Zhu, J.; Guo, Z.; Tang, Y.; Miao, P. Ultrasensitive miRNA biosensor amplified by ladder hybridization chain reaction on triangular prism structured DNA. Biosens. Bioelectron. 2023, 220, 114900. [Google Scholar] [CrossRef]
- Hou, T.-L.; Zhu, L.; Zhang, X.-L.; Chai, Y.-Q.; Yuan, R. Multiregion Linear DNA Walker-Mediated Ultrasensitive Electrochemical Biosensor for miRNA Detection. Anal. Chem. 2022, 94, 10524–10530. [Google Scholar] [CrossRef]
- Bhattacharya, K.; Mukherjee, S.P.; Gallud, A.; Burkert, S.C.; Bistarelli, S.; Bellucci, S.; Bottini, M.; Star, A.; Fadeel, B. Biological interactions of carbon-based nanomaterials: From coronation to degradation. Nanomed. Nanotechnol. Biol. Med. 2016, 12, 333–351. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Unlocking the Future: Carbon Nanotubes as Pioneers in Sensing Technologies. Chemosensors 2025, 13, 225. [Google Scholar] [CrossRef]
- Yue, Z.; Molino, P.J.; Liu, X.; Wallace, G.G. PEGylation of platinum bio-electrodes. Electrochem. Commun. 2013, 27, 54–58. [Google Scholar] [CrossRef]
- Choi, Y.; Tran, H.-V.; Lee, T.R. Self-assembled monolayer coatings on gold and silica surfaces for antifouling applications: A review. Coatings 2022, 12, 1462. [Google Scholar] [CrossRef]
- Contreras-Naranjo, J.E.; Aguilar, O. Suppressing Non-Specific Binding of Proteins onto Electrode Surfaces in the Development of Electrochemical Immunosensors. Biosensors 2019, 9, 15. [Google Scholar] [CrossRef]
- Aydin, D.; Gübbük, İ.H.; Ersöz, M. Recent advances and applications of nanostructured membranes in water purification. Turk. J. Chem. 2024, 48, 1–20. [Google Scholar] [CrossRef] [PubMed]
- Mülhopt, S.; Diabaté, S.; Dilger, M.; Adelhelm, C.; Anderlohr, C.; Bergfeldt, T.J. Gómez de la Torre, Y.; Jiang, E. Valsami-Jones, D.; et al. Characterization of Nanoparticle Batch-To-Batch Variability. Nanomaterials 2018, 8, 311. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle classification, physicochemical properties, characterization, and applications: A comprehensive review for biologists. J. Nanobiotechnol. 2022, 20, 262. [Google Scholar] [CrossRef]
- Gimondi, S.; Ferreira, H.; Reis, R.L.; Neves, N.M. Microfluidic Devices: A Tool for Nanoparticle Synthesis and Performance Evaluation. ACS Nano 2023, 17, 14205–14228. [Google Scholar] [CrossRef]
- Keal, M.E.; Rees, N.V. Recent advances in nanomaterial fabrication and electrocatalysis applications of single-entity nano-impact electrochemistry. Curr. Opin. Electrochem. 2024, 45, 101525. [Google Scholar] [CrossRef]
- Song, T.; Guo, X.; Li, X.; Zhang, S. Label-free electrochemical detection of RNA based on “Y” junction structure and restriction endonuclease-aided target recycling strategy. J. Electroanal. Chem. 2016, 781, 251–256. [Google Scholar] [CrossRef]
- Zhou, C.; Huang, R.; Zhou, X.; Xing, D. Sensitive and specific microRNA detection by RNA dependent DNA ligation and rolling circle optical signal amplification. Talanta 2020, 216, 120954. [Google Scholar] [CrossRef]
- Chugh, P.; Dittmer, D.P. Potential pitfalls in microRNA profiling. Wiley Interdiscip. Rev. RNA 2012, 3, 601–616. [Google Scholar] [CrossRef]
- Islam, M.M.; Koirala, D. Toward a next-generation diagnostic tool: A review on emerging isothermal nucleic acid amplification techniques for the detection of SARS-CoV-2 and other infectious viruses. Anal. Chim. Acta 2022, 1209, 339338. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, B.B.; Veigas, B.; Baptista, P.V. Isothermal amplification of nucleic acids: The race for the next “gold standard”. Front. Sens. 2021, 2, 752600. [Google Scholar] [CrossRef]
- Wang, M.; Liu, H.; Ren, J.; Huang, Y.; Deng, Y.; Liu, Y.; Chen, Z.; Chow, F.W.-N.; Leung, P.H.-M.; Li, S. Enzyme-Assisted Nucleic Acid Amplification in Molecular Diagnosis: A Review. Biosensors 2023, 13, 160. [Google Scholar] [CrossRef]
- Brown, C.W., III; Lakin, M.R.; Horwitz, E.K.; Fanning, M.L.; West, H.E.; Stefanovic, D.; Graves, S.W. Signal propagation in multi-layer DNAzyme cascades using structured chimeric substrates. Angew. Chem. Int. Ed. 2014, 53, 7183–7187. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.; Kang, S.; Kim, S.; Park, N. Advances and Trends in miRNA Analysis Using DNAzyme-Based Biosensors. Biosensors 2023, 13, 856. [Google Scholar] [CrossRef]
- Xue, Z.; Wang, L.; Pan, S.; Yan, J.; You, M.; Yao, C. The nucleic acid reactions on the nanomaterials surface for biomedicine. J. Nanobiotechnology 2025, 23, 308. [Google Scholar] [CrossRef]
- Comanescu, C. Recent Advances in Surface Functionalization of Magnetic Nanoparticles. Coatings 2023, 13, 1772. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Interface-Driven Hybrid Materials Based on DNA-Functionalized Gold Nanoparticles. Matter 2019, 1, 825–847. [Google Scholar] [CrossRef]
- Zhan, P.; Peil, A.; Jiang, Q.; Wang, D.; Mousavi, S.; Xiong, Q.; Shen, Q.; Shang, Y.; Ding, B.; Lin, C.; et al. Recent Advances in DNA Origami-Engineered Nanomaterials and Applications. Chem. Rev. 2023, 123, 3976–4050. [Google Scholar] [CrossRef]
- Sohrabi, H.; Ghasemzadeh, S.; Shakib, S.; Majidi, M.R.; Razmjou, A.; Yoon, Y.; Khataee, A. Metal–Organic Framework-Based Biosensing Platforms for the Sensitive Determination of Trace Elements and Heavy Metals: A Comprehensive Review. Ind. Eng. Chem. Res. 2023, 62, 4611–4627. [Google Scholar] [CrossRef]
- Mohanty, B.; Kumari, S.; Yadav, P.; Kanoo, P.; Chakraborty, A. Metal-organic frameworks (MOFs) and MOF composites based biosensors. Coord. Chem. Rev. 2024, 519, 216102. [Google Scholar] [CrossRef]
- Sha, M.; Xu, W.; Fang, Q.; Wu, Y.; Gu, W.; Zhu, C.; Guo, S. Metal-organic-framework-involved nanobiocatalysis for biomedical applications. Chem Catal. 2022, 2, 2552–2589. [Google Scholar] [CrossRef]
- Sen, D.; Lazenby, R.A. Electrochemical biosensor arrays for multiple analyte detection. Anal. Sens. 2024, 4, e202300047. [Google Scholar] [CrossRef]
- Jet, T.; Gines, G.; Rondelez, Y.; Taly, V. Advances in multiplexed techniques for the detection and quantification of microRNAs. Chem. Soc. Rev. 2021, 50, 4141–4161. [Google Scholar] [CrossRef] [PubMed]
- Xing, E.; Chen, H.; Xin, X.; Cui, H.; Dou, Y.; Song, S. Recent Advances in Smart Phone-Based Biosensors for Various Applications. Chemosensors 2025, 13, 221. [Google Scholar] [CrossRef]
- Shanbhag, M.M.; Manasa, G.; Mascarenhas, R.J.; Mondal, K.; Shetti, N.P. Fundamentals of bio-electrochemical sensing. Chem. Eng. J. Adv. 2023, 16, 100516. [Google Scholar] [CrossRef]
- Hickey, D.P.; Milton, R.D.; Rasmussen, M.; Abdellaoui, S.; Nguyen, K.; Minteer, S.D. Fundamentals and applications of bioelectrocatalysis. Electrochemistry 2015, 13, 97–132. [Google Scholar]
- Rashid, J.I.A.; Yusof, N.A. The strategies of DNA immobilization and hybridization detection mechanism in the construction of electrochemical DNA sensor: A review. Sens. Bio Sens. Res. 2017, 16, 19–31. [Google Scholar] [CrossRef]
- Anushka; Bandopadhyay, A.; Das, P.K. Paper based microfluidic devices: A review of fabrication techniques and applications. Eur. Phys. J. Spec. Top. 2023, 232, 781–815. [Google Scholar] [CrossRef]
- Cimmino, W.; Raucci, A.; Grosso, S.P.; Normanno, N.; Cinti, S. Enhancing sensitivity towards electrochemical miRNA detection using an affordable paper-based strategy. Anal. Bioanal. Chem. 2024, 416, 4227–4236. [Google Scholar] [CrossRef]
- Liu, S.; Su, W.; Li, Z.; Ding, X. Electrochemical detection of lung cancer specific microRNAs using 3D DNA origami nanostructures. Biosens. Bioelectron. 2015, 71, 57–61. [Google Scholar] [CrossRef] [PubMed]
- Walbrun, A.; Wang, T.; Matthies, M.; Šulc, P.; Simmel, F.C.; Rief, M. Single-molecule force spectroscopy of toehold-mediated strand displacement. Nat. Commun. 2024, 15, 7564. [Google Scholar] [CrossRef]
- Farahani, N.; Behmanesh, M.; Ranjbar, B. Evaluation of Rationally Designed Label-free Stem-loop DNA Probe Opening in the Presence of miR-21 by Circular Dichroism and Fluorescence Techniques. Sci. Rep. 2020, 10, 4018. [Google Scholar] [CrossRef] [PubMed]
- Du, Y.; Lim, B.J.; Li, B.; Jiang, Y.S.; Sessler, J.L.; Ellington, A.D. Reagentless, ratiometric electrochemical DNA sensors with improved robustness and reproducibility. Anal. Chem. 2014, 86, 8010–8016. [Google Scholar] [CrossRef]
- Gong, P.; Antrim, A.K.; Bickman, S.R.; Cooley, E.G.; Chung, S.H. Sandwich Hybridization Assay for In Situ Real-Time Cyanobacterial Detection and Monitoring: A Review. Biosensors 2022, 12, 640. [Google Scholar] [CrossRef] [PubMed]
- Silveira, C.M.; Almeida, M.G. Small electron-transfer proteins as mediators in enzymatic electrochemical biosensors. Anal. Bioanal. Chem. 2013, 405, 3619–3635. [Google Scholar] [CrossRef]
- Keshavarz, M.; Behpour, M.; Rafiee-pour, H.-A. Recent trends in electrochemical microRNA biosensors for early detection of cancer. RSC Adv. 2015, 5, 35651–35660. [Google Scholar] [CrossRef]
- Fu, Y.; Liu, T.; Wang, H.; Wang, Z.; Hou, L.; Jiang, J.; Xu, T. Applications of nanomaterial technology in biosensing. J. Sci. Adv. Mater. Devices 2024, 9, 100694. [Google Scholar] [CrossRef]
- Koç, Y.; Morali, U.; Erol, S.; Avci, H. Investigation of electrochemical behavior of potassium ferricyanide/ferrocyanide redox probes on screen printed carbon electrode through cyclic voltammetry and electrochemical impedance spectroscopy. Turk. J. Chem. 2021, 45, 1895–1915. [Google Scholar]
- Serrano, V.M.; Silva, I.S.P.; Cardoso, A.R.; Sales, M.G.F. Carbon electrodes with gold nanoparticles for the electrochemical detection of miRNA 21-5p. Chemosensors 2022, 10, 189. [Google Scholar] [CrossRef]
- Torul, H.; Yarali, E.; Eksin, E.; Ganguly, A.; Benson, J.; Tamer, U.; Papakonstantinou, P.; Erdem, A. Based electrochemical biosensors for voltammetric detection of miRNA biomarkers using reduced graphene oxide or MoS2 nanosheets decorated with gold nanoparticle electrodes. Biosensors 2021, 11, 236. [Google Scholar] [CrossRef] [PubMed]
- Su, S.; Hao, Q.; Yan, Z.; Dong, R.; Yang, R.; Zhu, D.; Chao, J.; Zhou, Y.; Wang, L. A molybdenum disulfide@Methylene Blue nanohybrid for electrochemical determination of microRNA-21, dopamine and uric acid. Microchim. Acta 2019, 186, 607. [Google Scholar] [CrossRef] [PubMed]
- Azimzadeh, M.; Rahaie, M.; Nasirizadeh, N.; Naderi-Manesh, H. Application of Oracet Blue in a novel and sensitive electrochemical biosensor for the detection of microRNA. Anal. Methods 2015, 7, 9495–9503. [Google Scholar] [CrossRef]
- Zhang, C.; Li, D.; Li, D.; Wen, K.; Yang, X.; Zhu, Y. Rolling circle amplification-mediated in situ synthesis of palladium nanoparticles for the ultrasensitive electrochemical detection of microRNA. Analyst 2019, 144, 3817–3825. [Google Scholar] [CrossRef] [PubMed]
- Erdem, A.; Eksin, E.; Kadikoylu, G.; Yildiz, E. Voltammetric detection of miRNA hybridization based on electroactive indicator-cobalt phenanthroline. Int. J. Biol. Macromol. 2020, 158, 819–825. [Google Scholar] [CrossRef]
- Yi, X.; Lu, Z.; Kong, Y.; Chen, Z. Label-Free Electrochemical Detection of MicroRNAs via Intercalation of Hemin into the DNA/RNA Hybridization. Int. J. Electrochem. Sci. 2017, 12, 2813–2821. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).