Abstract
The demand of biosensors for field testing is increasing in various application scenarios such as food testing, environmental analysis, and medical diagnostics. However, conventional non-intelligent biosensors have been largely restricted in such applications. With the popularity of smart phones, biosensors combined with smart phones have successfully solved these problems. Acting as both analytical instruments and data platforms, smart phones have greatly improved optical and electrical biosensors for field testing. Importantly, coupled with cloud computing and artificial intelligence algorithms, smart phone-based biosensors enable real-time and dynamic testing and monitoring. This review focuses on how smart phones are combined with biosensors and what their combination methods are. At the same time, it introduces how their combination is applied in medical diagnostics, food testing, environmental analysis, and other aspects. Smart phones will enhance their crucial role in field testing, while simultaneously promoting technological innovation and interdisciplinary integration. With breakthroughs in other technologies, smart phone-based biosensors are poised to play an important character role in the future.
1. Introduction
A biosensor is an analytical and detection device consisting of a sensitive element formed by the integration of biologically active materials with molecular recognition capabilities [1]. These materials are combined with transducers processed through electronic technology to record electrical signals and optical signals, etc. Biosensors are developed based on chemical sensors and represent an interdisciplinary product arising from the convergence of modern biotechnology, chemistry, and electronic physics [2]. The sensing principle of biosensors can be described as follows: The analyte diffuses into the immobilized biosensitive film layer, where it undergoes molecular recognition and subsequent biochemical reactions. The resulting signal is then transformed into a quantifiable and processable electrical signal by an appropriate chemical or physical transducer. Following amplification and output by the secondary instrument, the concentration of the analyte can be determined, thereby reflecting the performance characteristics of the biosensor.
In 1962, the American scientist Leland Clark Jr. first introduced the concept of an enzyme electrode and utilized it to measure glucose concentration [3], which is considered the inaugural prototype of a biosensor. Over the subsequent decades, biosensors progressively diversified into various types [4,5], achieving significant advancements across multiple critical domains. Meanwhile, the research and application of disease diagnostic biomarkers have significantly advanced the development of precision medicine. Biomarkers, encompassing diverse molecular categories such as proteins, nucleic acids, metabolites, cells, and microbial components, serve as core tools for early screening, rapid diagnosis, and therapeutic monitoring by specifically reflecting disease states. In the domain of medical diagnosis, both the depth and breadth of its applications have been steadily expanding. This kind of biosensor based on analyte detection was initially limited to straightforward tasks such as blood glucose monitoring; it has now progressed to encompass long-term management of chronic conditions like diabetes and hypertension, as well as early screening and precise diagnosis of complex diseases such as cancer [6,7,8]. For instance, biosensors leveraging enzyme electrode principles are capable of rapidly and accurately detecting blood glucose levels, thereby offering significant convenience for the daily blood glucose monitoring of diabetic patients [9]. In the realm of cancer diagnosis, immunosensors have progressively assumed a critical role in enhancing the early detection rate of cancer through the identification of specific tumor markers, thereby providing patients with valuable treatment opportunities [10]. Furthermore, the integration of multi-marker panels and artificial intelligence has enabled multidimensional diagnostic models for complex diseases such as cancer and neurodegenerative disorders. In the field of food safety testing, traditional biosensors have emerged as a robust tool for ensuring the safety of food supplies. Research on pesticide residues and veterinary drug residues as key targets in food safety testing, along with advancements in rapid detection technologies, has significantly enhanced global food risk prevention and control. PR detection focuses on organophosphorus insecticides, herbicides, and metabolites with higher toxicity, while VDR monitoring prioritizes antibiotics, banned additives, and complex co-contaminants such as heavy metals. The rapid detection technologies based on biosensing of smart phones have achieved remarkable improvements in sensitivity and efficiency, enabling on-site screening from laboratories to fields and markets, thereby effectively intercepting non-compliant food products before they reach consumers. These biosensors are capable of rapidly detecting pathogenic bacteria, pesticide residues, veterinary drug residues, and other harmful substances in food products [11,12,13]. This capability effectively prevents unsafe food from entering the market, thereby protecting public health. Biosensors also play a crucial role in environmental monitoring. In environmental analysis, the identification of biological and chemical pollutants as critical targets has become essential for ecological and public health protection. Biological pollutants encompass pathogenic microorganisms, biotoxins, and allergens, while chemical pollutants include heavy metals, persistent organic pollutants, and emerging contaminants. Rapid detection technologies have revolutionized environmental monitoring by enabling on-site, real-time analysis with ultra-high sensitivity and multi-pollutant profiling capabilities. By utilizing specific biometric recognition elements such as enzymes, antibodies, microorganisms, or DNA, these biosensors bind to target contaminants and convert biochemical reactions into quantifiable electrical or optical signals. This enables highly sensitive and real-time detection of environmental conditions. For example, in water quality monitoring, biosensors can rapidly detect heavy metals (e.g., mercury and lead), organic pollutants (e.g., pesticides and benzene compounds), and eutrophication indicators in water bodies (e.g., nitrates and phosphates) [14,15]. In atmospheric monitoring, they can identify harmful gases such as sulfur dioxide and nitrogen oxides [16]. Compared to traditional methods, biosensors offer advantages including rapid response time, high portability, and low cost. They are especially suited for on-site rapid screening and long-term dynamic monitoring, thereby providing efficient technical support for environmental pollution early warning and governance. In various application scenarios such as medical diagnosis, food inspection, and environmental analysis, the demand for on-site detection of biosensors is increasing day by day. Due to their advantages of rapidity, accuracy, and portability, they are gradually replacing traditional laboratory detection methods and are widely used in real-time monitoring and immediate diagnosis fields.
In diverse application scenarios such as food inspection, environmental analysis, and medical diagnosis, the demand for on-site real-time detection by biosensors is experiencing explosive growth. The traditional non-intelligent biosensors face many bottlenecks in their application in these fields: complex operation processes, the need for professional personnel to operate, limited data analysis capabilities, and untimely result output, which seriously restrict their practical application effects. Especially in remote areas with limited resources or in emergency situations, these limitations are more prominent. It is important to highlight that, with the rapid advancement of mobile communication technology and the widespread adoption of smartphones, an innovative solution has emerged: the integrated biosensor system for smartphones. This intelligent detection platform successfully merges the high specificity of biosensors with the advanced functionalities of smartphones, marking a revolutionary advancement in the detection process. Smartphones not only offer reliable power supply, efficient data processing capabilities, and user-friendly human-machine interaction interfaces for biosensors but also enable real-time analysis of detection results, geolocation tagging, and remote sharing via their built-in cameras, GPS positioning systems, wireless transmission functionalities, and other advanced features [17,18]. This integrated design significantly simplifies and enhances the efficiency of on-site detection compared to previous methods. After undergoing brief training, even non-professionals can operate the system with proficiency. Moreover, the detection results can be transmitted to the cloud in real time via mobile networks for further analysis or expert consultation. More significantly, the widespread adoption of smartphones has substantially decreased the overall system costs, facilitating the broad application of high-performance detection technologies in primary healthcare facilities, food safety surveillance locations, and environmental pollution monitoring stations. This truly realizes the democratization and widespread accessibility of detection technologies, thereby providing robust technical support for enhancing public health standards, safeguarding environmental safety, and improving access to medical services.
For instance, the biosensors combined with smart phones can achieve portable and real-time health monitoring in the field of medical diagnosis, significantly improving the efficiency of early disease screening and management [19]. The electrocardiogram (ECG) function of the Apple Watch detects abnormal heart rate through built-in sensors, helping users identify heart problems such as atrial fibrillation; In addition, blood glucose monitoring devices such as Glucometer can be connected via mobile phones to record the blood glucose levels of diabetic patients in real time and generate trend reports. This type of technology lowers the threshold for professional medical equipment and makes personal health management more convenient. In the field of food safety, biosensors based on smart phones can quickly detect pathogens and chemical contaminants to revolutionize food safety supervision [18]. In the field of disease diagnosis, the development of biosensors on smartphones has also brought unprecedented new opportunities for real-time detection [20]. For different individuals, real-time detection (POC) may have different interpretations, but they all follow the same fundamental principle: quickly collecting diagnostic results to enable patients to initiate appropriate treatment in a timely manner. It is obvious that ubiquitous POC devices can significantly enhance the workflow of patient management, clinical care delivery, and healthcare providers, especially in the context of the current pandemic challenges. In the past decade, there has been a sharp increase in biosensors based on smart phones. These sensors enable artificial systems to sense environmental stimuli, many of which are now compatible with mainstream smartphone platforms [21]. This has further promoted the rapid development of smart phones in the POC field. Meanwhile, biosensors combined with artificial intelligence and smart phones can effectively monitor food safety, for example, the bacterial levels in food. Foodborne bacterial pathogens pose a significant threat to global food safety. Foodborne diseases caused by pathogens are among the most serious risks to human health, resulting in a large number of hospitalizations and deaths every year. Unlike chemical contaminants, pathogenic bacteria are living organisms capable of rapid proliferation in food matrices over time. Notably, individual foodborne pathogens often comprise multiple strains, yet only specific virulent strains threaten human health. This biological characteristic necessitates stringent screening throughout production chains, as even trace amounts of lethal pathogenic strains are strictly prohibited in food products. The substantial public health risks associated with foodborne pathogens underscore the urgent need for developing rapid and accurate detection methodologies. Point-of-care testing (POCT) has emerged as a promising approach for real-time pathogen detection in field settings. The ubiquitous presence of foodborne microorganisms across production, packaging, and transportation processes highlights the critical importance of their identification in ensuring food safety, monitoring sanitary processing efficacy, and verifying product quality and shelf-life stability. Specificity remains the most important consideration in food safety detection methods, fundamentally depending on the selectivity of the identified elements. Bacteriophages–viruses specifically infecting host bacteria–have attracted considerable interest as alternative biorecognition elements in biosensor development. Their exceptional affinity and specificity for target bacteria, extending even to strain-level discrimination, make them particularly valuable [22]. However, designing appropriate signal amplification strategies that maintain high sensitivity while ensuring operational simplicity remains a critical challenge in bacteriophage-based biosensing systems. In the field of environmental monitoring, biosensing devices integrated with smartphones are employed for rapid on-site water quality analysis to assess water pollution [23]. These devices can quantitatively detect heavy metals, such as lead and mercury, in groundwater within 10 min, achieving an accuracy comparable to that of laboratory-based inductively coupled plasma mass spectrometry (ICP-MS). The above-mentioned research indicates that biosensors based on the smartphone platform have successfully broken through the boundaries of traditional medical detection through deep integration with microfluidic technology, nanomaterials, and the Internet of Things (IoT), providing efficient and portable innovative tools for addressing global challenges such as food safety monitoring, environmental pollution assessment and agricultural health management.
Microfluidic chip technology has demonstrated exceptional potential for high-throughput identification of complex sample components in food safety, clinical diagnostics, and environmental monitoring. A crucial factor enabling high-throughput microfluidic analysis lies in the development of cost-effective, compact, and reliable detection systems. Smartphones have garnered significant attention for on-site detection due to their ability to integrate with microfluidic systems for direct, rapid, and accurate quantification. Advanced smartphone applications enable real-time analysis of captured video or image data through dedicated algorithms. The integration of microfluidics with miniaturized instrumentation has evolved into a promising POCT strategy with broad application prospects [24,25].
Miniaturized automated microfluidic devices represent a transformative approach for pathogen detection and containment across clinical, industrial, and agricultural sectors. Precise pathogen identification is essential for understanding microbial physiology, investigating food safety incidents, and ensuring clinical accuracy in therapeutic interventions. Recent advancements in automated systems integrating microfluidic chips with computerized control architectures demonstrate remarkable capabilities, including live bacteria detection in real samples. These innovative platforms address the limitations of conventional methods characterized by low efficiency and cumbersome procedures, achieving high-precision automated detection through streamlined operational workflows [26]. Smartphones have played a revolutionary role in the development of biosensing technology. The global popularity of smart phones (with over 6 billion global users by 2023) and their highly integrated hardware modules provide a unique technical platform for the innovation of biosensing technology. As a bridge between biosensing technology and end users, smartphones not only solve the problem of complex operation of traditional biosensors, but also upgrade instantaneous detection from a “single diagnostic tool” to an “intelligent health management system” through digital and networked capabilities. This integration marks the entry of intelligent biosensors into the era of “intelligent health management systems” and a new stage of “universal accessibility and data interconnection” [27,28].
Smartphones are the supporting platforms for multimodal biosensing. The core hardware of smart phones (such as optical cameras, environmental sensors, audio interfaces, etc.) can be directly used as the core module for biological signal collection and analysis after adaptation, significantly reducing the hardware cost of biosensing detection equipment. For different signal output modes, its advantages are reflected in three aspects: (1) Optical sensing: the high resolution (up to 20 million pixels) and programmable flash of the mobile phone camera enable it to capture weak signals such as colorimetry, fluorescence, and chemiluminescence. (2) Electrochemical sensing: the audio interface of the smart phone (3.5 mm or USB-C) can output a stable low-frequency signal to drive the miniaturized electrochemical sensor. (3) Environmental sensing: the temperature, humidity and air pressure sensors built into the mobile phone can correct the detection of environmental interference in real time, thereby improving the reliability of the detection [29]. This multi-mode integration capability makes smartphones an “integrated” biosensing carrier. In addition, the deep integration of sensing technology with smartphones is driving the development of biosensing technology toward a more “mobile-friendly” orientation. Specifically, the following advancements have been made: (1) Nano-signal amplification: nano-materials such as gold nanoparticles and quantum dots, leveraging their superior optical properties, are extensively utilized to enhance the signal-capturing capabilities of smartphone cameras. (2) Miniaturization of microfluidic chips: given the size constraints of smartphones, the thickness of the new-generation microfluidic chips has been reduced to less than 5 mm, and a folding design has been adopted to align with the camera field of view of smartphones. (3) Passive sensing technology: to eliminate reliance on external power sources, near-field communication (NFC) and radio-frequency identification (RFID) technologies have been integrated into biosensors. These innovations have enabled smartphone-based biosensing devices to achieve sensitivity, throughput, and portability comparable to professional laboratory equipment.
It is anticipated that the rapid advancement of artificial intelligence will compensate for deficiencies in signal analysis and enable swift decision-making. The integration of smartphone computing power (CPU + GPU) with artificial intelligence algorithms has resolved two key challenges faced by traditional biosensors: subjective interpretation errors and multi-parameter correlation analysis. Artificial intelligence is expected to offer a more robust data processing paradigm for the sensing system of smart mobile devices [30]: (1) Automation of Image Recognition: algorithms leveraging convolutional neural networks (CNNs) can autonomously detect and analyze features, such as the T/C line intensity and cell morphology in test strips. (2) Dynamic Signal Analysis: for time-dependent detection (e.g., CRISPR fluorescence curves), recurrent neural networks (RNNs) enable real-time tracking of signal variations, thereby reducing the overall detection time. (3) Multi-modal Data Integration: smartphones facilitate the integration of biosensing data with additional patient information, including medical history, geographical location, and other relevant parameters, to generate personalized diagnostic recommendations. Moreover, smartphones contribute to the transformation of the global healthcare infrastructure by enabling real-time data transmission through wireless communication technologies (4G/5G, Bluetooth). This capability supports two critical advancements: telemedicine and hierarchical diagnostics, alongside real-time epidemiological surveillance.
The rapid advancement of biosensing technology and its cross-sector integration with smartphones are anticipated to establish a novel paradigm for on-site detection. Firstly, the miniaturization and multifunctionality of biosensing devices tailored for smartphones address the pressing demands of real-time detection. Secondly, as a versatile platform, smartphones have substantially enhanced the detection efficiency and data analysis capabilities of biosensors through hardware augmentation and algorithmic empowerment. The synergistic innovation between these two technologies not only facilitates the realization of application scenarios such as medical diagnostics, food safety monitoring, and environmental analysis but also offers cost-effective and high-performance detection solutions for resource-constrained regions. Looking ahead, with the continued evolution of cutting-edge technologies, the deep integration of biosensors and smartphones will expedite the transition towards “decentralized” and “intelligent” detection paradigms. Nevertheless, challenges related to standardization, security, and interdisciplinary collaboration persist and require further exploration and resolution. Despite the rapid progress of smartphone-integrated biosensors, the following limitations remain: (1) It is difficult to achieve the best balance between detection throughput and multi-parameter collaborative sensing; (2) Lack of the robust quality control and standardization framework; (3) Lack of cost-effective and effective control methods in low-income areas [31]. In the future, the convergence of flexible electronics, synthetic biology, and artificial intelligence may propel the development of next-generation biosensors integrated with advanced smartphones.
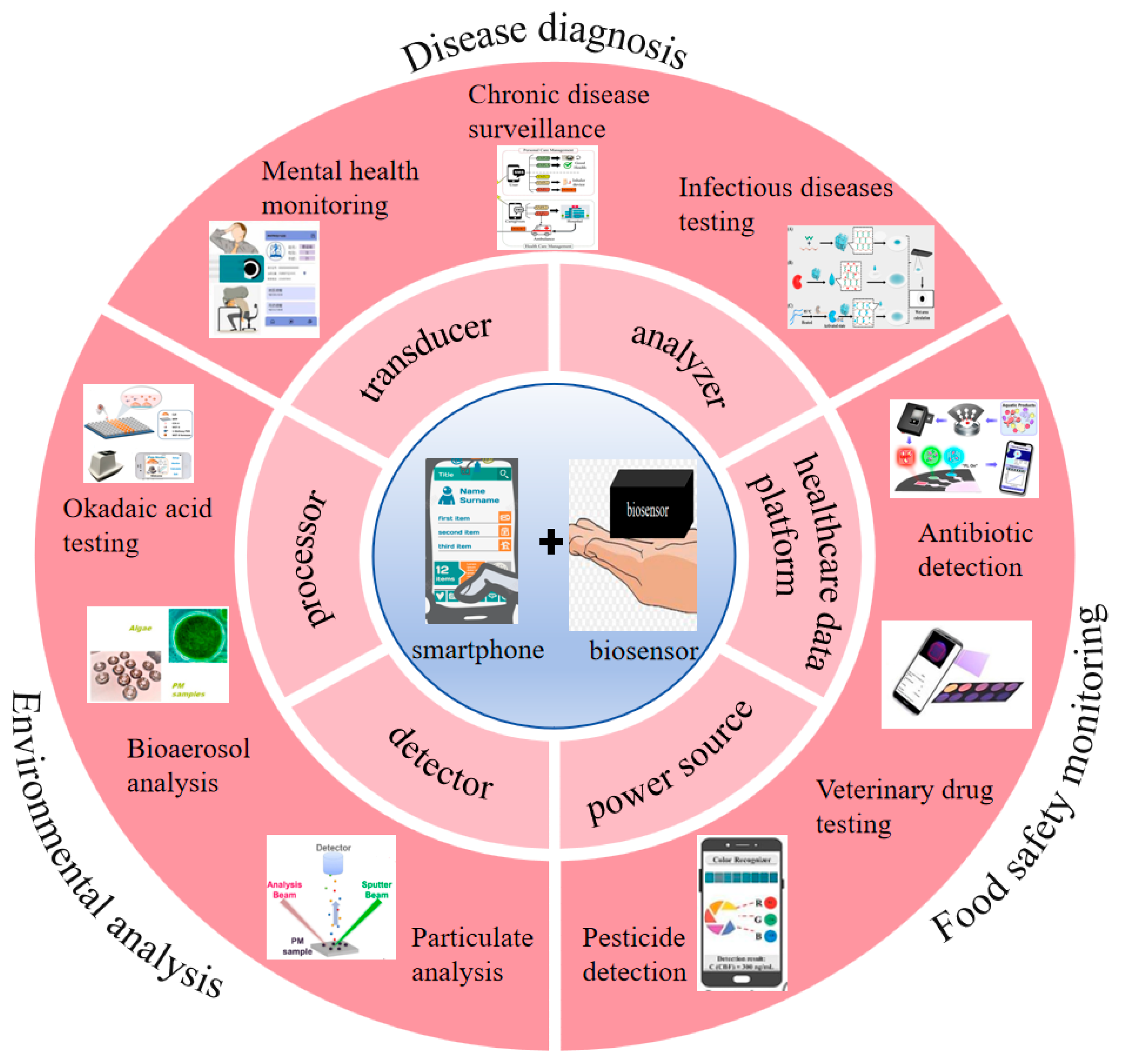
Based on this, many researchers have introduced and analyzed the current research status in the field of biosensing for smart phones. Shiyu Qian et al. reviewed the principles, characteristics and advantages of colorimetric biosensors based on smart phones, and focused on introducing the latest application progress in fields such as biomedical analysis, medical diagnosis, environmental monitoring and food safety [20]. Gaowa Xing et al. summarized different detection methods of microfluidic sensors based on smart phones and reviewed their applications in fields such as disease diagnosis, environmental monitoring and food safety detection in recent years [24]. Irina Ganeevad et al. introduced the research progress of adoptive cell immunotherapy bioreactors [32]. Mirkomil Sharipovd et al. introduced the latest progress of biochemical and environmental analysis sensors based on Arduino and smart phones [33]. These reviews only discuss the specific detection principles of biosensors based on smart phones and their applications in specific fields, but do not cover the different functions of smart phones in this sensing field and their broader applications. However, the development of biosensors for smart phones is rapid and they have wide applicability. They will continue to be used in more and more fields. Researchers need to have a deeper and broader understanding of biosensors in smart phones. Therefore, this paper aims to review the different functions, performance advantages, system analysis, technical principles, cutting-edge applications and transformation potential of smart phones in the field of biosensing, thereby providing theoretical guidance (Figure 1). We discussed the latest trends, major achievements, and remaining challenges of smartphone biosensors.
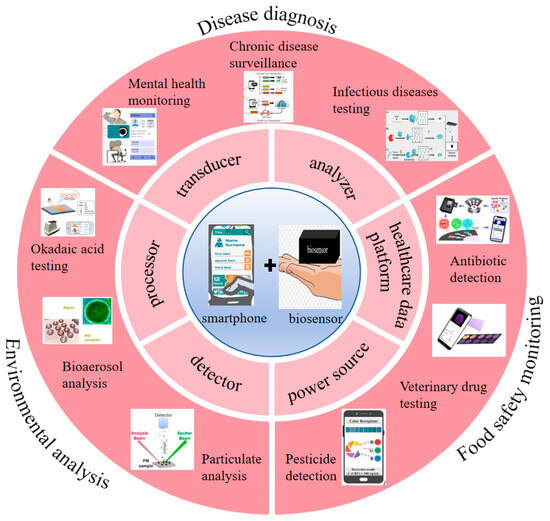
Figure 1.
The application of smartphones in biosensors [34,35,36,37,38,39,40,41,42]. Copyright 2017, Elsevier. Copyright 2019, Elsevier. Copyright 2025, Elsevier. Copyright 2025, Elsevier. Copyright 2023, Elsevier. Copyright 2025, Elsevier. Copyright 2022, Elsevier. Copyright 2022, American Chemical Society. Copyright 2017, Elsevier.
2. Different Functions of Smartphones in Biosensing
In the field of biosensing, amperometric biosensors based on electrical signal output are extensively utilized. When a defined voltage is applied to the working electrode, electroactive substances undergo redox reactions during enzyme-catalyzed biochemical processes, generating current signals that are proportional to the concentration of the target biochemical molecules. In addition, bioanalytical sensing technologies based on electrical signal output also include chronoamperometry (CA), cyclic voltammetry (CV), differential pulse voltammetry (DPV), and square wave voltammetry, among others [43,44]. The reported miniaturized electrical biosensing system, which integrates electrical signal output-type biosensors with smartphones, comprises a disposable microfluidic chip featuring biosensor electrodes, a weak-signal processing circuit, and a detection system [44,45,46]. The current measurement module of this biosensor is designed to be highly compact and cost-effective, thereby rendering it particularly suitable for on-site detection. A miniature universal serial bus (USB) port on a smartphone can provide an electronic interface between the miniaturized current measurement system and the smartphone for medical data transfer and upload. In addition, Deng et al. demonstrated a smartphone-based electrochemical test strip for POC gender validation, which is a key factor in forensic analysis [47]. The miniaturized system was developed using an ampere meter characterization module and a smartphone application.
In addition, optical biosensors transduce biomolecular binding events into detectable optical signals through mechanisms such as fluorescence emission, surface plasmon resonance (SPR), or colorimetric reactions [48]. When target molecules, such as proteins and nucleic acids, specifically interact with probes immobilized on sensor surfaces, measurable changes in light intensity, wavelength, or polarization are induced. These changes can be quantified via spectral analysis, image processing, or interferometry. Smartphone-based optical detection systems, leveraging high-resolution cameras, portability, and computational power, have emerged as ideal platforms for point-of-care testing (POCT). These advantages render the system highly suitable for disease diagnosis in resource-limited settings. The multiplexing capability of optical sensing based on smart phones is particularly advantageous. The flash of a smart phone provides uniform illumination, while open-source image processing algorithms (such as the ImageJ 1.54f plugin) can conduct quantitative analysis of the target object within minutes. In the development process of biosensors based on electrical and optical signal output, smartphones demonstrate different functions. Next, we discuss in detail later the different functions of smart phones in biochemical sensing detection.
2.1. Analyzer Application
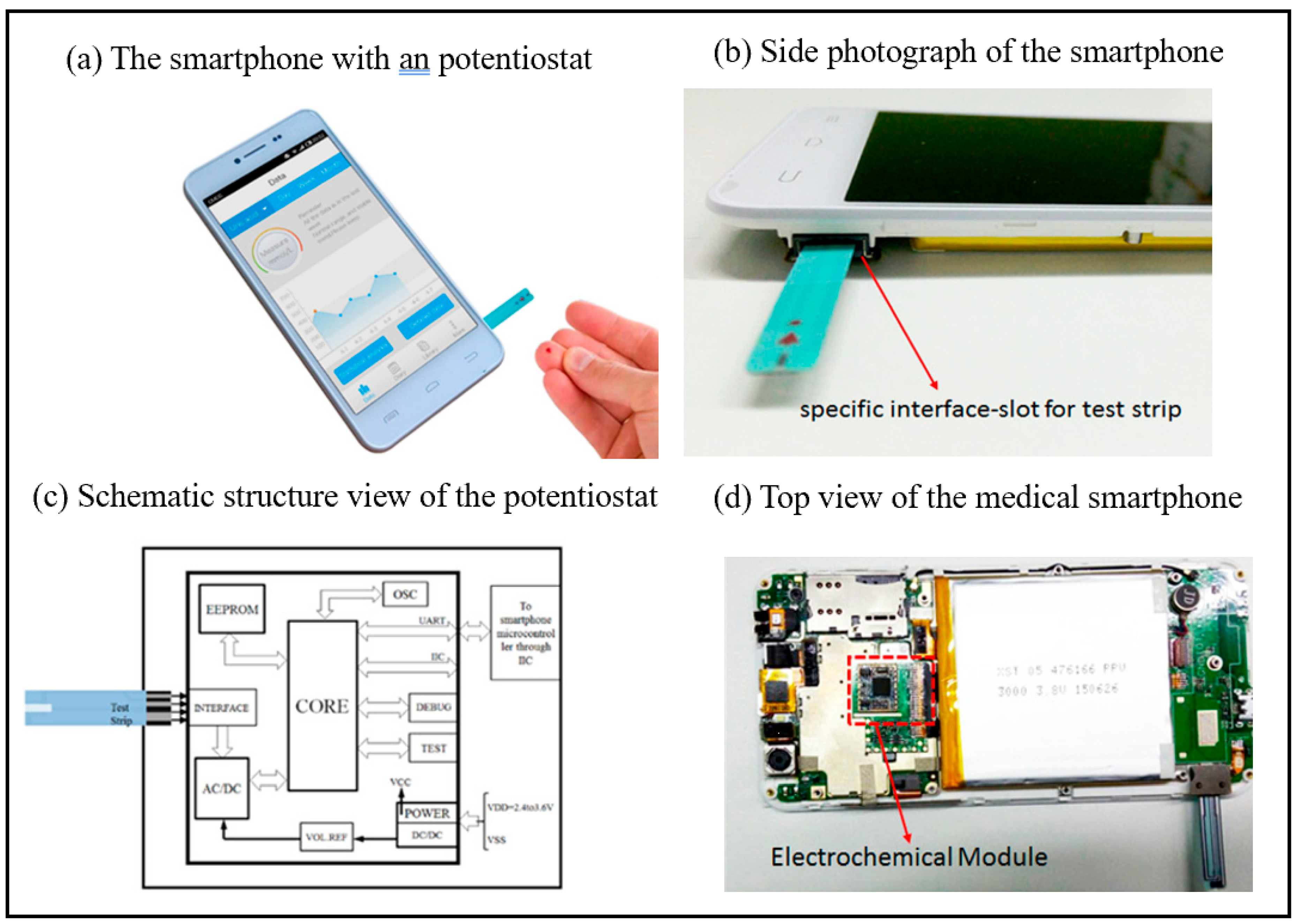
Smartphones can serve as portable analyzers for biosensing applications. By incorporating optical, electrochemical, and other sensor modules, they enable real-time detection of biomarkers, thereby substantially improving the convenience of on-site analysis. Additionally, their integrated algorithms and networking capabilities facilitate rapid data processing and remote sharing, offering cost-effective and efficient solutions for medical diagnostics, food safety monitoring, and environmental analysis [49,50]. Guo et al. successfully demonstrated a medical-grade smartphone as a miniaturized electrochemical analyzer station for measuring uric acid in finger-pricked whole blood through collaboration with POC electrochemical test strips [43]. The medical smartphone has the advantage of being low-cost, costing less than $60. The photograph of the medical smartphone is as shown in Figure 2. The disposable enzyme uric acid (UA) test strip is inserted into the built-in ammeter module of a smart phone through a specially designed interface for uric acid detection. This medical smart phone is regarded as a promising solution that can meet the rigid demands of mobile health management. Guo et al. provided another typical example of a smartphone-driven medical dongle as a compact current analyzer used in conjunction with enzymatic β-hydroxybutyric acid test strips for the precise measurement of blood ketones in finger-pricked whole blood in POC [44]. The proposed system can provide a solution for the characterization and treatment of diabetic ketoacidosis (DK) and diabetic ketoacidosis (DKA).
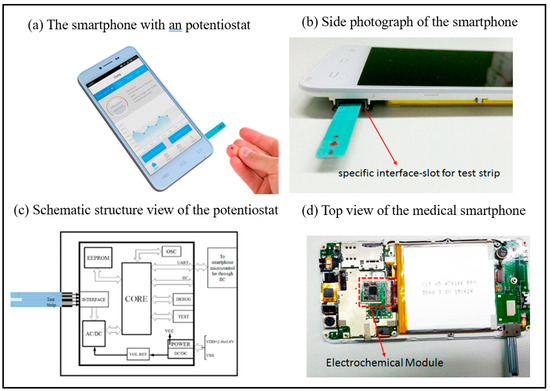
Figure 2.
(a) Photograph of the proposed medical smartphone in which an electrometer has been preburied. (b) Side photograph of the proposed medical smartphone: slot is the interface for insertion of UA test strip. (c) Schematic structure view of the electrometer; a micro controller for resolving the electrochemical current formed in the test strip. (d) Top view of the layout of the medical smartphone: the electrochemical module is integrated with the main print circuit board of the smartphone [43]. Copyright 2016, American Chemical Society.
Smartphones, as portable biosensing analyzers, also have some limitations. The sensor sensitivity and accuracy are usually lower than those of professional laboratory equipment, which may affect the detection reliability of low-concentration biomarkers. It is greatly disturbed by external factors such as ambient light and temperature, and the hardware differences among different models can lead to inconsistent test results. In addition, the processing capacity of biological samples is limited, and complex samples usually need to be preprocessed before they can be adapted to the mobile phone detection module.
2.2. Healthcare Data Platform Applications
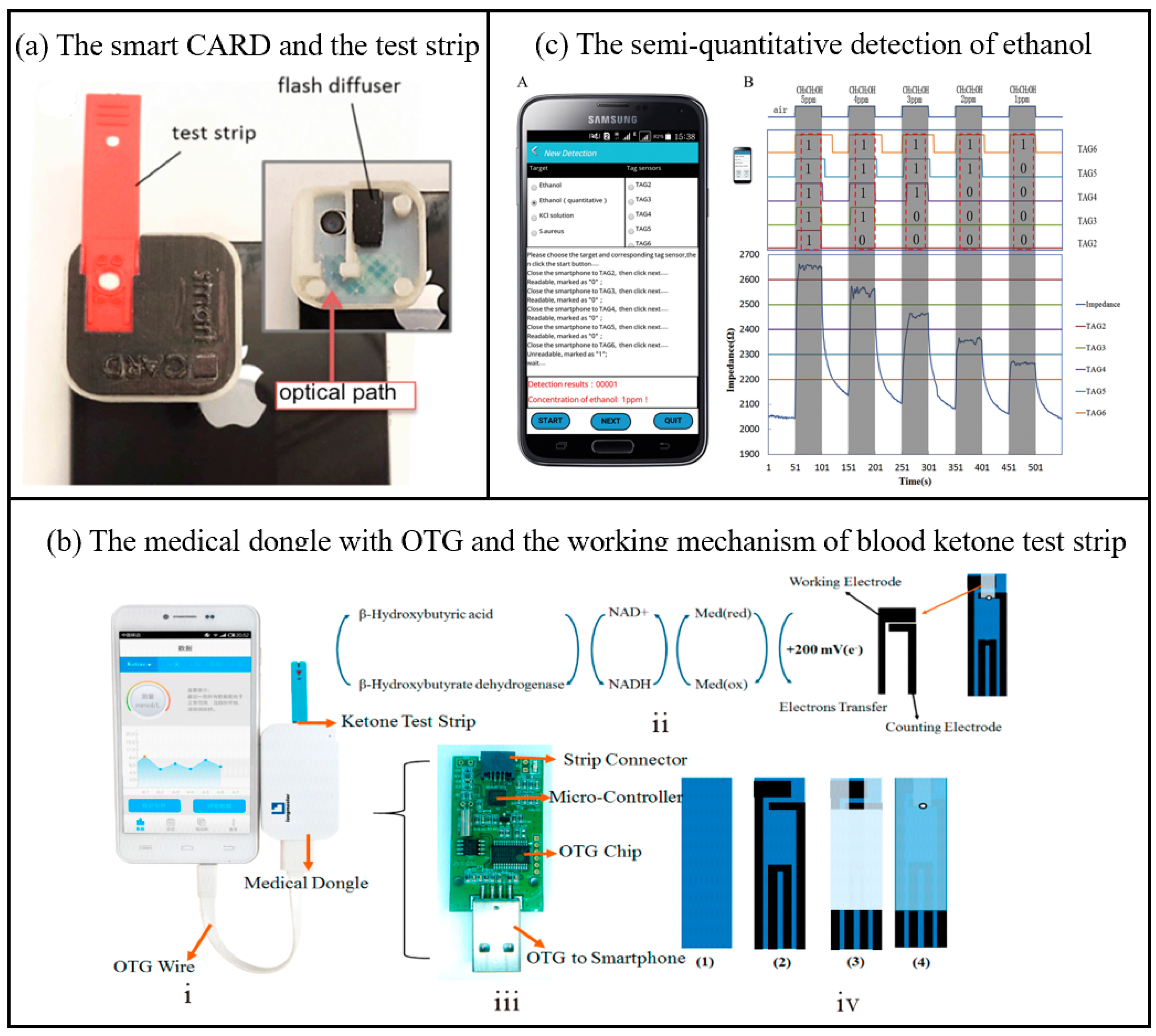
As a health data platform for biosensing, smartphones can collect users’ physiological indicators (such as heart rate, blood oxygen, sleep quality, etc.) in real time through built-in or external sensors, and integrate the data into health applications for analysis and visualization. Its portability and networking capabilities enable users and medical professionals to remotely monitor health conditions, achieving personalized health management and early disease warnings. As shown in Figure 3a, Erickson et al. developed a smartphone-based cholesterol rapid diagnostic system application that quantifies cholesterol levels through colorimetric analysis of the enzymatic reaction occurring on test strips [51]. The system comprises a smartphone accessory for capturing images of test strips and an application that serves as a medical data platform. This platform is utilized for analyzing parameters in the test region and directly measuring cholesterol levels on the smartphone. The measurement accuracy was maintained within 1.8% across the relevant physiologic range (140 mg/dL to 400 mg/dL). In all cases, the maximum difference between the predicted value of the user test and the measured value using the CardioChek PA system was less than 5.5%. The system recognizes erroneous readings and is reproducible. In addition, the disposable electrochemical blood ketone strips are associated with a medical adapter (Figure 3b) that is powered by a smartphone via OTG (On-the-Go: a device communication standard) [44]. Blood ketone levels were assessed by measuring electrochemical currents. Depending on the signal-to-noise ratio (SNR) level, the limit of detection (LOD) of the proposed medical dongle can be close to 0.001 mmol/L. The linear detection range of blood ketones lies between 0.001 and 6.100 mmol/L. This β-ketone test strip demonstrates significant advantages in detection accuracy, cost, and time efficiency. Its results show strong agreement with laboratory biochemical analyzers (the gold standard), achieving a correlation coefficient of 0.987 and a detection limit as low as 0.001 mmol/L. The per-test cost remains under $10 (excluding the smartphone), with a rapid detection time of approximately 10 s. In contrast, laboratory biochemical analyzers, while offering the highest accuracy, require expensive equipment and hours of sample processing. Traditional urine ketone test strips are highly cost-effective ($0.5–2 per test) but suffer from poor specificity and delayed results. Existing portable blood ketone meters exhibit comparable detection times (30 s to 2 min) and moderate-to-high accuracy, yet rely on dedicated devices, resulting in higher overall costs. By integrating smartphone technology, this test strip enables low-cost, highly sensitive, and real-time detection, making it particularly suitable for primary healthcare and home-based health management.
Another example is a smartphone-based passive and wireless near-field communication (NFC) biochemical sensor, which integrates a smartphone data reader with a passive NFC tag transponder [52]. As shown in Figure 3c, the smartphone can wirelessly power the sensor, receive detection results via inductive coupling, and transmit data through signal modulation [53]. By incorporating varistors into the resonant circuit of the sensor, the system enables precise semi-quantitative detection of various analytes. This sensor demonstrates versatile detection performance across different applications: For ethanol gas detection, it achieves a limit of detection (LOD) of 1 ppm with an average accuracy of 97.6% (based on 50 repeated tests). In ion detection for KCl solutions, the LOD is 0.009 M with 99% accuracy. For Staphylococcus aureus detection, the LOD reaches 105 CFU/mL, and while its sensitivity is relatively low (slope = 7.03 × 10−5 Ω/(CFU/mL)), the sensor array effectively distinguishes concentration ranges using a calibration curve (R² = 0.8620). The core reason for the limited performance of bacterial detection in this experiment is that the conductivity change caused by microorganisms is weak, and no specific biometric recognition elements are used, resulting in low signal strength and high noise. The author emphasizes that this experiment is only a proof-of-concept and needs to be combined with the optimization of biologically modified electrodes in the future. This tag sensor is capable of differentiating between analytes such as ions and bacteria at different concentrations in solution, unfolding a new dimension of biochemical sensing at the point of care using smartphones. The medical data platform of biosensors for smart phones has been widely studied, but it may have deficiencies such as insufficient data accuracy, privacy leakage risks, lack of clinical verification and standardized supervision, which still deserve attention.
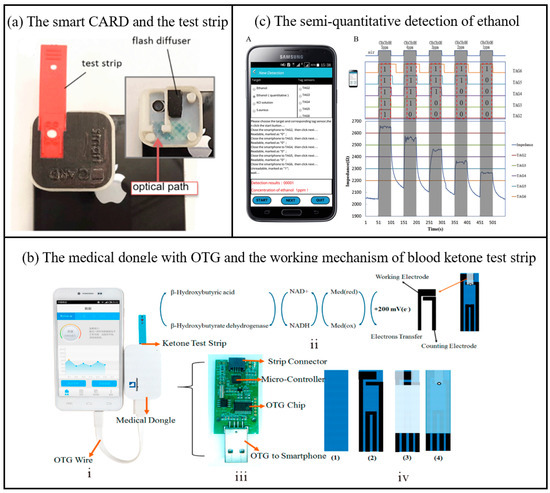
Figure 3.
(a) Picture of the smart CARD (smartphone Cholesterol Application for Rapid Diagnostics) accessory and the test strip; the inset shows the inside of the accessory with the diffuser and the optical path of the flash used to illuminate the strip [51]. Copyright 2014, Royal Society of Chemistry. (b) Photograph of the medical dongle with OTG connecting to smartphone; (b) the working mechanism of blood ketone test strip; the printed circuit board (PCB) and electronic elements in medical dongle; the fabrication procedure of the blood ketone test strip [44]. OTG (On-The-Go: a kind of device communication standard). Copyright 2017, American Chemical Society. (c) The semi-quantitative detection of ethanol from 1 ppm to 5 ppm by the sensor array and smartphone. With the different readabilities of the five sensors in the detections, different concentrations were successfully discriminated A—the interface of our NFC detection application program which displayed the detection results of ethanol in 1 ppm. B—Rt values of the sensor array were shown as different colored lines [53]. Copyright 2017, Elsevier.
Figure 3.
(a) Picture of the smart CARD (smartphone Cholesterol Application for Rapid Diagnostics) accessory and the test strip; the inset shows the inside of the accessory with the diffuser and the optical path of the flash used to illuminate the strip [51]. Copyright 2014, Royal Society of Chemistry. (b) Photograph of the medical dongle with OTG connecting to smartphone; (b) the working mechanism of blood ketone test strip; the printed circuit board (PCB) and electronic elements in medical dongle; the fabrication procedure of the blood ketone test strip [44]. OTG (On-The-Go: a kind of device communication standard). Copyright 2017, American Chemical Society. (c) The semi-quantitative detection of ethanol from 1 ppm to 5 ppm by the sensor array and smartphone. With the different readabilities of the five sensors in the detections, different concentrations were successfully discriminated A—the interface of our NFC detection application program which displayed the detection results of ethanol in 1 ppm. B—Rt values of the sensor array were shown as different colored lines [53]. Copyright 2017, Elsevier.
2.3. Power Source Applications
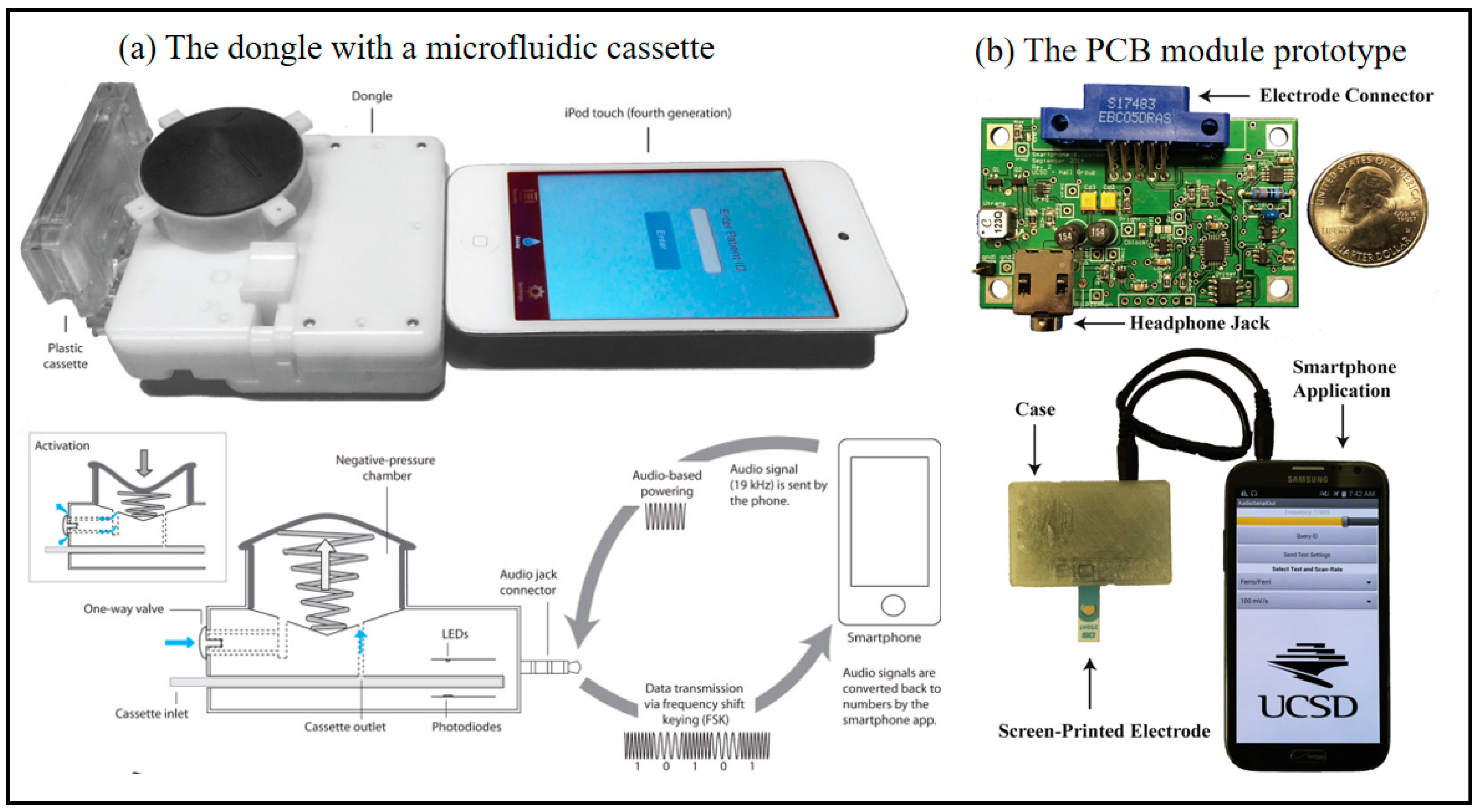
Smart phones can function as portable power sources in biosensing detection, supplying power to small sensors or detection modules while enabling real-time data collection and analysis. Their built-in batteries, combined with rechargeable capabilities, ensure the continuous operation of detection equipment. Additionally, the USB or wireless charging functions significantly enhance the convenience of power management. Laksanasopin et al. investigated a complete laboratory-quality immunoassay that can be performed on a small diagnostic accessory made of a dongle attached to a smartphone that provides all necessary power (Figure 4a) [54]. Notably, in this design, the smartphone is used not only as a passive interface to receive sensing information but also as an active interface to control the test program, and the dongle performs the POC enzyme-linked immunosorbent assay (ELISA) quality test. Compared with laboratory-based diagnostic devices, this device gives patients the preference for rapid and accurate HIV and syphilis diagnostic results with only a single finger prick, and can be made by any population with a smartphone. Hall et al. developed a smartphone-based electrochemical biosensor system to detect the concentration of secreted leukocytes protease inhibitors (SLPLs), which is used for POC diagnosis and health tracking [55]. This system communicates and even supplies power through the audio port on the smartphone. As shown in Figure 4b, the system consists of a low-cost electronic module including a low-power potentiostat, which efficiently collects power from a variety of smartphones via the audio port. In this study, the collection efficiency is as high as 79% due to active impedance matching, and the module consumes a peak power of 6.9 mW, in addition to losses due to power conditioning and rectification, and can measure bi-directional currents of < 1 nA. The prototype can be executed within the available power budget of a smartphone and produces data that matches that of expensive laboratory instruments. However, as the power source of biosensors, smartphones have limited battery capacity and unstable continuous power supply, making it difficult to meet the long-term demands of high-power consumption sensors. Meanwhile, the internal circuit noise and voltage fluctuations of mobile phones may interfere with the precise collection of biological signals.
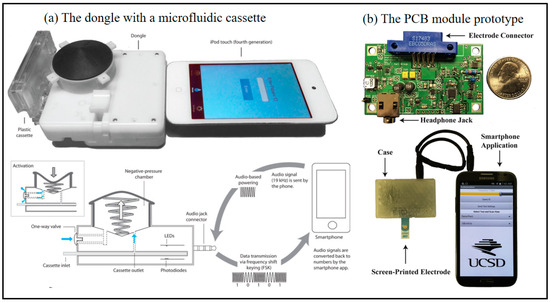
Figure 4.
Smartphone-based immune biosensor at POC. (a) Images of the dongle with a microfluidic cassette connected to an iPod touch and schematic diagram of dongle highlighting a power-free vacuum generator using the audio jack connector for audio based powering and frequency shift keying (FSK) data transmission to a smart-enabled device [54]. Copyright 2015, The American Association for the Advancement of Science. (b) Photograph of the PCB module prototype and the module being powered by and communicating with a Samsung Note 2 (Seoul, South Korea, Samsung Electronics), including electrode connector with headphone Jack (Independently developed by the team), smartphone application, Screen-printed electrode [55]. Copyright 2016, Elsevier.
2.4. Detector Applications
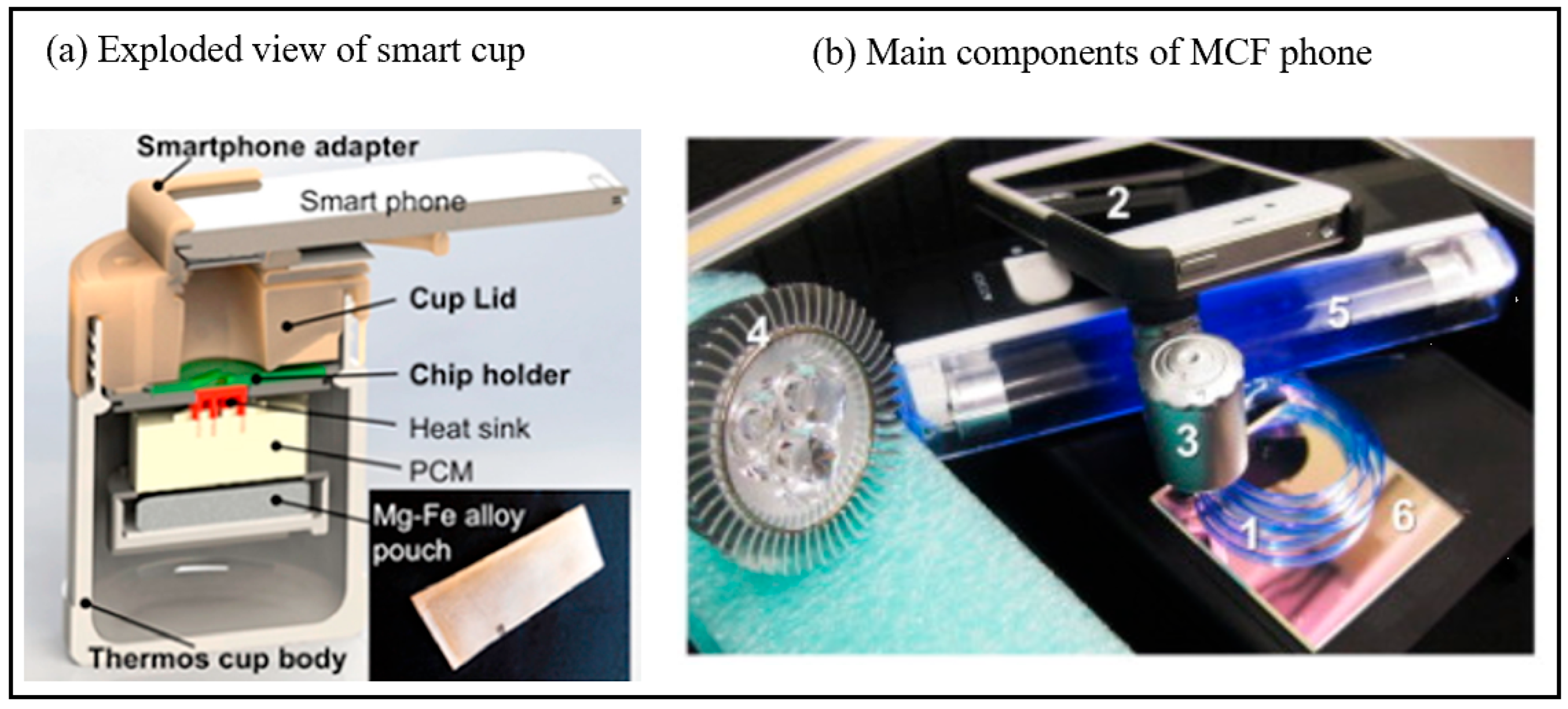
Smartphones can serve as portable detection platforms in biosensing detection. By integrating optical, electrochemical or biosensor modules, they can analyze target substances (such as glucose, proteins or pathogens) in biological samples in real time. Its high-performance cameras, data processing capabilities and wireless transmission functions significantly reduce the detection cost and enhance the accessibility of on-site detection [56,57]. Seo et al. achieved the first example of pathogen monitoring via the Internet of Things (IoT) for smartphone-based healthcare [58]. In their study, a CMOS image sensor (CIS) was used to detect signals generated by a smartphone-based immunosensor system to address the difficulties of healthcare monitoring via IoT. The system was developed in a pocket-sized dimension and then used to measure foodborne pathogens in real samples. The smartphone in this work was used to control and monitor the analysis process, which was also used to upload the results to an internet server to share the data with the public. However, the obstacle to its application is the relatively expensive cost, including sample preparation, specific laboratory equipment, and training for specific researchers. To address these challenges, Song et al. developed a highly sensitive reverse transcription loop-mediated isothermal amplification (RT-LAMP) assay for rapid Zika virus detection, integrated into a simple, user-friendly, and cost-effective disposable point-of-care (POC) cartridge capable of performing all unit operations from sample introduction to detection [59]. For thermal control of the cartridge, a chemically heated cup was employed (Figure 5a), eliminating the need for electrical power. Amplification products were visually detected using leuco crystal violet (LCV) dye without requiring instrumentation. As an inexpensive and optional disposable heating unit, a simple thermally insulated portable cup was designed for chip-based isothermal amplification, utilizing exothermic reactions for heating. Briefly, the cup system comprises three components: an insulated cup body, a microfluidic chip holder, and a 3D-printed cup lid. The body of the insulated cup can be made of polystyrene foam plastic, which further saves costs. A Mg-Fe alloy serves as the heat source, housed within a dedicated drawer in the cup lid. Water is introduced through a port on the lid to activate the Mg-Fe alloy, triggering an exothermic reaction. To thermally isolate the amplification reactor from environmental fluctuations, phase change material (PCM) with a melting point of 68 °C was incorporated for temperature regulation. An aluminum heat sink embedded within the PCM enhances thermal transfer from the PCM to the cartridge. After approximately 40 min, results can be directly visualized by naked-eye observation or documented using smartphone imaging. This integrated system demonstrates a practical approach for resource-limited settings by combining low-cost materials with simplified thermal management and detection methodologies. Another recent example is the device integrated with a custom-designed holder designed by Giavazzi et al. [60]. The device is fitted with a disposable sensing cassette, a miniature magnetic stirrer and some passive optics. In this work, a reflector-mode interface based on the measured light intensity was reflected by the surface of an amorphous fluoropolymer substrate that immobilized the antibodies on the same chip. A flash LED installed on a smartphone camera can be used as an illumination source. In addition, Barbosa et al. designed a portable detection system [61], the microcapillary film (MCF) phone, forcolorimetric and fluorescent quantitative sandwich immunoassays for prostate-specific antigen (PSA). The MCF phone (Figure 5b) integrates a magnifying glass, a simple light source, and a miniaturized immunoassay platform, the MCF. The MCF has excellent properties in terms of fluoropolymer transparency and planar geometry. The MCF handpiece has been demonstrated to provide fluorescence detection with high sensitivity, colorimetric volume, and good recoveries, further demonstrating a major breakthrough in the integration of low-cost and portable microfluidic devices, commercially available immunoassay reagents, and smartphone technology. However, these portable detection platforms based on smart phones are limited by sensor accuracy, environmental interference and non-professional hardware, and the accuracy and reliability of the detection results are often not as good as those of dedicated equipment.
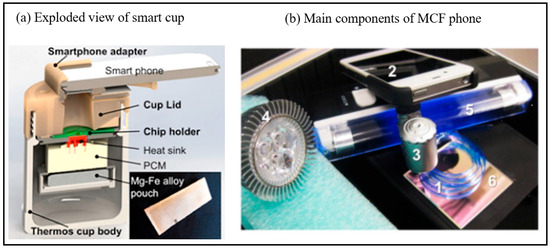
Figure 5.
(a) Exploded view of the chemically heated cup. The cup consists of a thermos cup body, a 3D-printed cup lid, a chip holder, PCM material, heat sink and single-use Mg–Fe alloy pack heat source [59]. Copyright 2016, American Chemical Society. (b) Main components of MCF phone (1) Microcapillary Film (MCF) (2) Smartphone (3) Magnifying lens (4) Blue LED (5) UV black light for fluorescence detection, light source for chromogenic detection (6) dichroic filter [61]. Copyright 2015, Elsevier.
2.5. Processor Applications
As portable data processing devices, smartphones are capable of receiving, analyzing, and visualizing physiological or biochemical data collected by biosensors in real time. This significantly enhances the efficiency and convenience of detection processes. Equipped with built-in algorithms and wireless transmission capabilities, smartphones further facilitate remote diagnosis and health monitoring, thereby offering critical technical support for personalized medicine and point-of-care diagnostics. To achieve fast immunosensing for POC testing, Dou et al. improved a smartphone-based electrochemical biosensor with an electric field-driven acceleration strategy [62]. They developed an immunosensor (Figure 6a) made of smartphone embedded circuitry for signal processing and a screen-printed carbon electrode (SPCE) modified with multi-walled carbon nanotubes (MWNTs) and a goat anti-mouse immunoglobulin G (IgG) sensing layer (MWNTs-I layer) for a fast and sensitive immunosensing assay for clenbuterol hydrochloride (CLB In their work, the competitive immunoassay was completed in 6 min by electric field-driven acceleration with a limit of detection of 0.076 ng/mL by horseradish peroxidase-coupled CLB (CLB-HRP). Potential as a POC platform for rapid and sensitive detection of all food safety relevant species. Wang’s group then devised another improved and efficient biochemical analysis method for the detection of marine toxins using a portable smartphone-based system, the Bionic e-Eye [63]. The Bionic e-Eye offers the advantages of rapid on-site measurement and real-time on-line analysis with comparable accuracy, dynamic range, detection limits and sensitivity compared to traditional marine toxin detection methods such as the Microtitre Plate Reader (MTPR). The Bionic e-Eye is powered by software called iPlate( iOS application developed using Swift 1.0 and Objective-C in Xcode 7 on macOS) which integrates image capture and more in-depth data processing. In the Bionic e-Eye, two color models, HSV and RGB, are used to evaluate the bioanalytical performance of the Stonehouse clam toxin (STX) assay and Okadaic acid (OA), which are the standard representations of Paralytic Shellfish Poison (PSP) and Diarrhoeal Shellfish Poison (DSP), respectively. The biomimetic e-Eye has great potential to become a new assay with an on-site test platform rather than the traditional off-site assay. Smartphones, as portable data processing devices, are widely used in the field of biosensing. However, these devices also have certain drawbacks. Their limited battery life and heat dissipation capacity make it difficult to maintain high-performance computing for a long time, and they are prone to lag or frequency reduction in multi-tasking or complex computing scenarios.
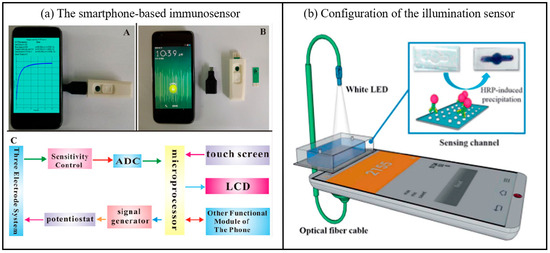
Figure 6.
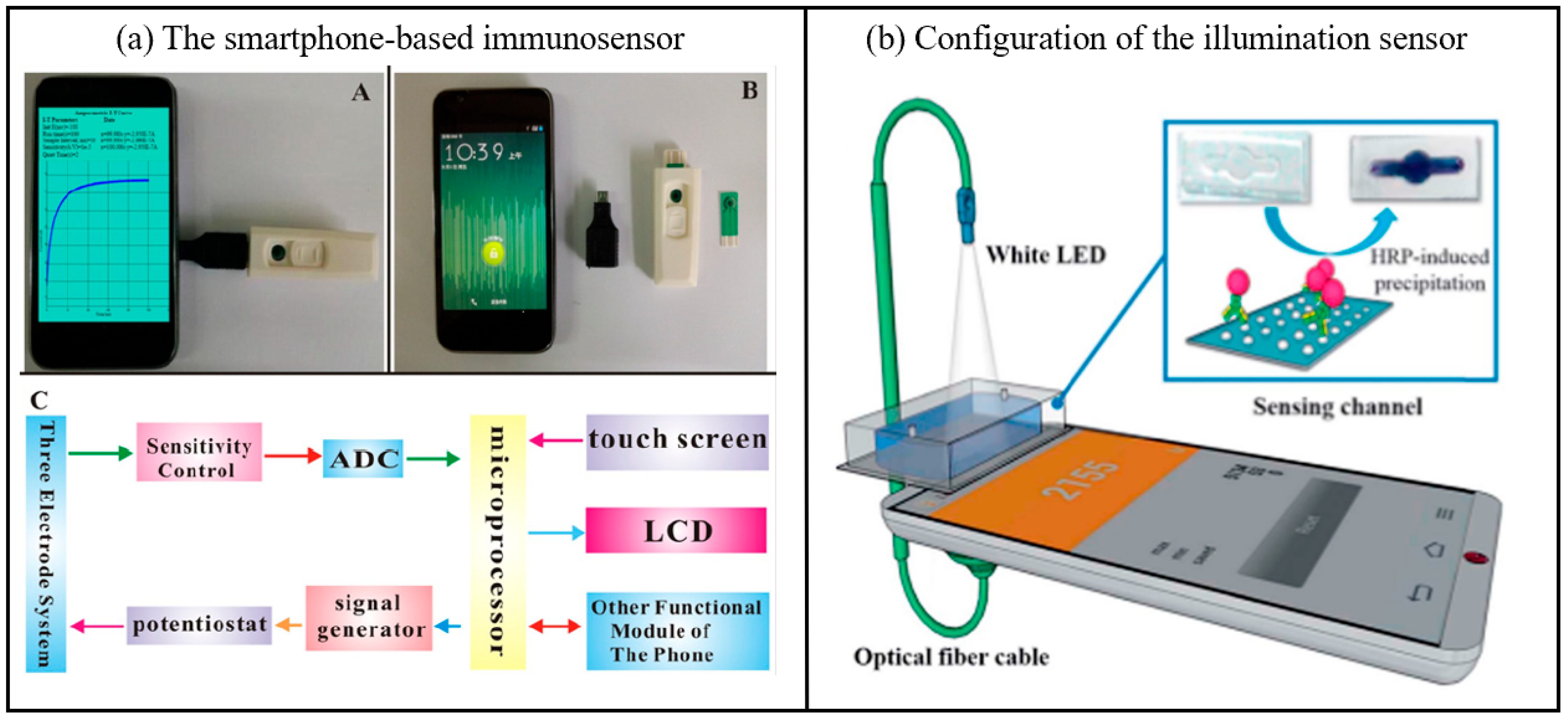
(a) A photograph of the smartphone-based immunosensor, a picture of the smartphone, USB port, chip box and screen-printed carbon electrode, An electrical circuit frame diagram of the mobile electrochemical device (ADC: analog to-digital converter. LCD: liquid crystal display.) [62]. Copyright 2016, Elsevier. (b) Configuration of the illumination sensor, including white LED, sensing channel, optical fiber cable [48]. Copyright 2018, Elsevier.
2.6. Transducer Applications
In biosensing detection, smartphones function as transducers to convert biological signals (e.g., light, electricity, and chemical reactions) into quantifiable digital signals, thereby facilitating portable and real-time data analysis. The integration of high-precision sensors and advanced algorithms further enhances the sensitivity and specificity of detection, offering efficient solutions for on-site analysis and remote monitoring [64]. In addition, Yoon’s group developed a novel smartphone-based immunosensor fitted with an immunoblotting assay and a built-in illumination sensor (Figure 6b) [65]. The optical biosensing system can be easily and efficiently constructed thanks to the use of smartphone embedded components, including white LEDs and illumination sensors, which are designed as a light source and a light receiver, respectively. Compared to conventional optical sensors, illumination sensors respond to changes in external light intensity over a wider wavelength range, making them more sensitive. In the illumination sensor, the smartphone is used as a signal sensor in the optical system. In addition, the new system introduces immunoblotting technology to uniformly change the intensity of light [66]. The specific mechanism of immunosensor conduction is that horseradish peroxidase-induced insoluble precipitates interfere with the penetration of incident light, facilitating changes in the intensity of the applied light. The light then passes through the biosensing channel and is analyzed by an illuminometer in a mobile application. Indeed, illumination is a promising candidate for quantitative diagnostics and POC testing.
The main disadvantage of the above system is that the smartphone has to be integrated with other devices such as dongles, circuits, etc., which implies additional consumption and a more complex system. More importantly, the excessive number of accessories attached to the smartphone can lead to results and analyses that are susceptible to their conditions.
3. Smartphone Applications in Biosensing
3.1. Disease Diagnosis and Monitoring
With the rapid development of mobile health (mHealth) technology, the combination of smartphones and biosensors provides a revolutionary solution for disease monitoring. By integrating optical, electrochemical, microfluidic, and artificial intelligence (AI) technologies, smartphone platforms have enabled the detection and analysis of multimodal biomarkers. Each perspective will be presented later in the article [32,67,68,69].
3.1.1. Chronic Disease Surveillance and Management
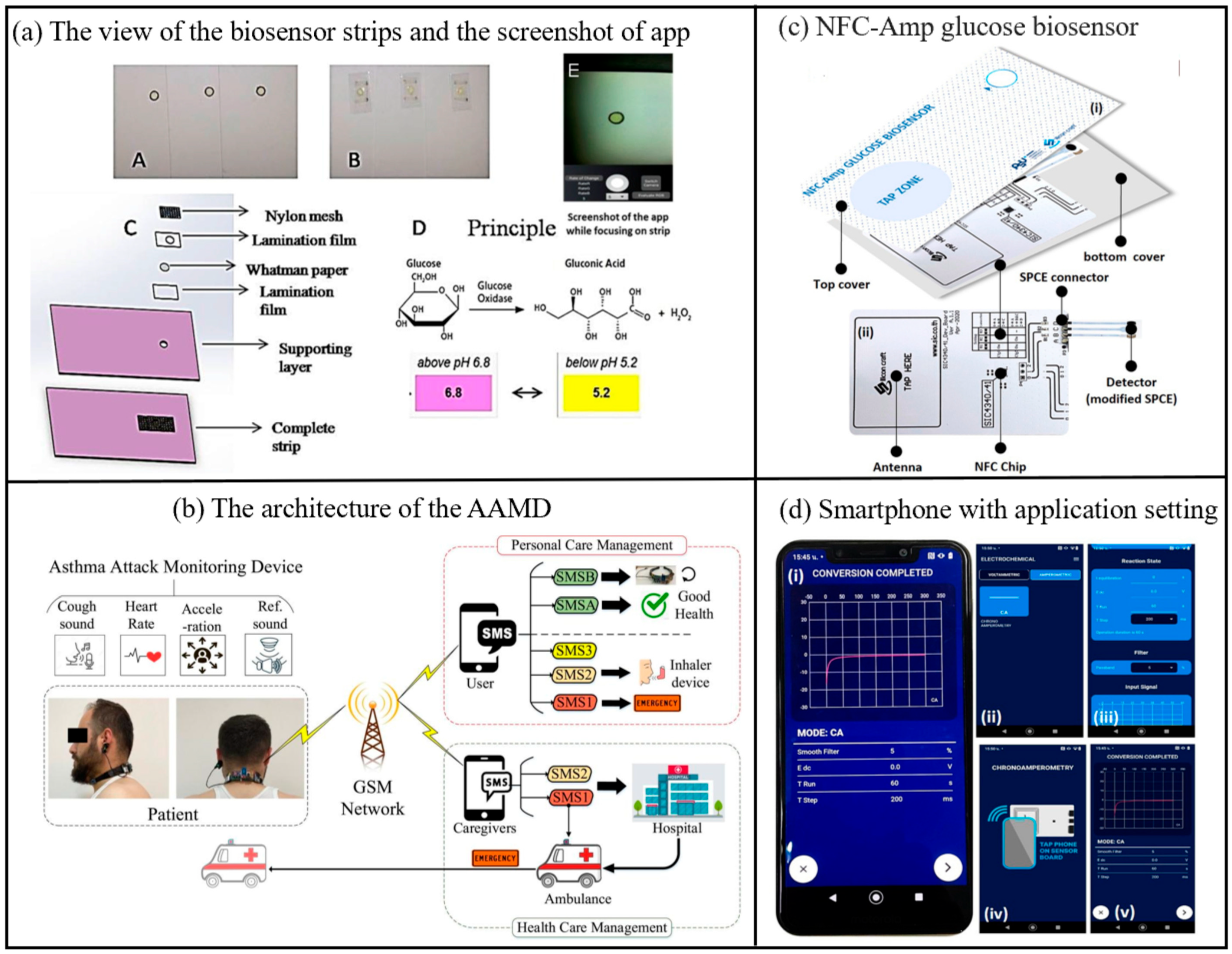
Smartphone-based biosensors enable convenient and continuous tracking of daily health data for patients with chronic conditions through real-time monitoring of physiological parameters such as heart rate, blood glucose levels, and blood oxygen saturation, and acute kidney injury biomarkers in urine [70,71]. This facilitates the early detection of abnormal trends. Furthermore, by incorporating advanced AI algorithms and cloud-based data analytics, these biosensors can provide personalized health management recommendations, thereby supporting physicians in refining treatment strategies and empowering patients to enhance their self-management capabilities. Take diabetes as an example, the most common laboratory tests used to diagnose diabetes include the Fasting Glucose Test (FPG), Oral Glucose Tolerance Test (OGTT), Random Glucose Test, and Hemoglobin A1c Test (HbA1c test). In recent years, self-monitoring of blood glucose (SMBG) has become a better option. Anuradha Soni et al. developed a non-invasive glucose biosensor based smart phone for the diagnosis of diabetes mellitus using saliva samples (Figure 7) [72]. It creates a biosensor by immobilizing glucose oxidase and pH indicator on a strip of paper. An in-house developed android application was used to estimate the color change of the pH indicator upon reaction with glucose. Clinical validation of the biosensor was performed on healthy and diabetic subjects to correlate blood and saliva glucose levels. Blood glucose levels can be measured in the field using a smartphone without the involvement of any specialized instruments. Ferris et al. report a smartphone-based analyte sensing platform that utilizes the built-in magnetometer for signal transduction via analyte-responsive magnetic hydrogel composites [73]. As these hydrogels swell in response to target stimuli, they displace the attached magnetic material relative to the phone’s magnetometer. Employing a bilayer hydrogel geometry to amplify this displacement enables sensitive, optics-free, quantitative liquid-phase analyte measurements. This approach requires no additional electronics or power sources beyond those integrated within the smartphone itself. The concept was demonstrated using both glucose-specific and pH-responsive hydrogels, achieving glucose detection down to single-digit micromolar concentrations with potential extension to nanomolar sensitivity. This versatile platform is adaptable to diverse analytes, opening a pathway for portable, low-cost sensing of multiple analytes or biomarkers of interest. Saif Saad Fakhrulddin et al. have proposed a novel Asthma Attack Monitoring Device (AAMD) [36]. Featuring a unique U-shaped design and lightweight construction, this device allows users to carry out daily activities without hindrance. The AAMD integrates an innovative asthma attack prediction algorithm with advanced biosensors, employing a novel approach for predicting asthma episodes by monitoring coughing through sound and accelerometer sensors while simultaneously collecting heart rate data. It incorporates groundbreaking enhancements such as improved noise reduction, optimized sensor placement, and smartphone integration for user alerts and health updates. By addressing limitations in prior asthma monitoring technologies through refined design, enhanced accuracy, and increased user engagement, the device represents a substantial technological leap. Additionally, it includes a GSM module to alert both patients and healthcare providers during critical events. The AAMD marks a significant advancement in patient-friendly asthma management, combining wearability comfort, diagnostic precision, and real-time alert systems to enable effective monitoring and timely intervention. Abnormal catecholamine (CA) levels are critical biomarkers for potential health disorders including Alzheimer’s disease, Parkinson’s disease, cardiomyopathy, hypertension, and obesity. To address the need for rapid and reliable CA detection, Zhang et al. engineered an adaptive carbon-based fluorescent probe that synthesizes multicolor fluorescent carbon dots (CDs) in real time, enabling both visual discrimination and quantitative analysis of multiple CAs in a portable, point-of-care device [74]. To enhance accuracy, the system integrates a fluorescence-stable reference material to mitigate environmental interference. Quantitative detection is achieved through dual methodologies: fluorescence spectrophotometry, which calculates CA concentrations via fluorescence emission intensity ratios, and a smartphone-compatible platform that analyzes RGB channel intensity ratios from captured images, both achieving nanomolar (nM)-level sensitivity. The technology’s clinical utility was demonstrated through successful CA biosensing in human serum samples and the development of a paper-based assay integrated with smartphone imaging for real-time monitoring. The biosensor demonstrates a limit of detection (LOD) of 24.6 mg/dL, calculated based on the sensor noise level and equivalent glucose concentration. The sensor exhibits a sensitivity of 0.0012 pixels·sec−¹/mg·dL−¹ within the linear detection range of 50–540 mg/dL. This breakthrough not only advances early disease diagnosis and precision management of CA-related pathologies but also provides a robust tool for elucidating disease mechanisms. By enabling simultaneous multi-CA detection, the approach supports comprehensive biomarker profiling, offering transformative potential for understanding and managing neurodegenerative and metabolic diseases.
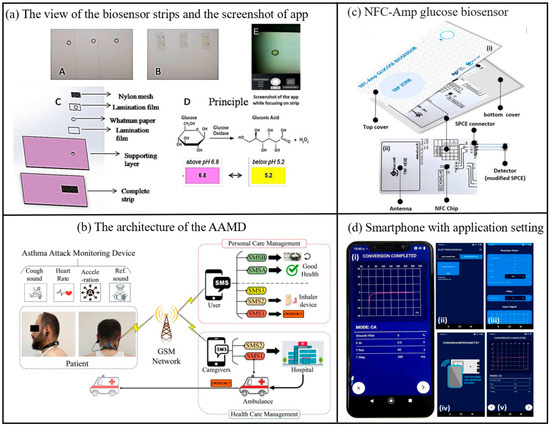
Figure 7.
(a) (A and B) Front and back view of the biosensor strips, (C) Layered structure of the strip (D) Principle involved in color change of biosensor strip due to Glucose oxidase reaction, (E) Screenshot of developed android app focusing on detection zone of test strip [72]. Copyright 2017, Elsevier. (b) Proposed architecture of the AAMD, including asthma attack monitoring device, GSM network [36]. Copyright 2025, Elsevier. (c) The components of the developed NFC glucose biosensing device (d) Screenshots of the Chemist application, (i) real chronoamperogram, (ii) selecting chronoamperometry mode, (iii) saving chronoamperometric measurement, (iv) tapping smartphone to initiate glucose biosensing, (v) electrochemical measuring step [75]. Copyright 2023, Elsevier.
Electrochemical glucose biosensors are now an acceptable option for measuring serum glucose. They provide high sensitivity and tunable selectivity through enzymes that specifically catalyze glucose. Most electrochemical glucose biosensors utilize glucose oxidase (GOx) immobilized at the electrode interface. The catalytic reaction between glucose and GOx produces hydrogen peroxide (H2O2), which is detected electrochemically by oxidation of H2O2 at high applied potentials [76]. However, serum contains reducing compounds such as uric acid (UA), dopamine (DA), and ascorbic acid (AA), which are readily oxidized at the same potentials and may produce interfering currents [77,78,79]. A glucose biosensor controlled by a battery-free NFC-enabled smartphone has been developed for diagnosing diabetes by Kiattisak et al. (Figure 7) [75]. The biosensor utilizes Prussian blue to mediate the redox reaction catalyzed by glucose oxidase. The biosensor quantifies glucose in human serum using a biosensor controlled by a smartphone in chronoamperometric detection mode. Near-field communication transmits glucose data at the point of care. It can be analyzed for a variety of properties including linearity, limit of detection (LOD), sensitivity, reproducibility, reproducibility, as stability, and selectivity, with good linearity and selectivity and a low limit of detection. This versatile smartphone-based NFC sensing device can be part of the next generation of portable electrochemical sensors.
3.1.2. Rapid Detection of Infectious Diseases
The biosensor, when integrated with smartphones and leveraging highly sensitive biometric technology, is capable of rapidly detecting infectious disease pathogens, thereby enabling immediate diagnosis and early warning. Its portability, coupled with its data-sharing capabilities, has substantially enhanced detection efficiency in remote regions and during public health emergencies, offering an innovative approach to the prevention and control of infectious diseases. Researchers have conducted extensive studies on infectious diseases such as HPV, 5-generation dengue, and infectious pneumonia using biosensors based on smart phones [80,81,82]. Take the detection of the novel coronavirus that spread worldwide in 2020 as an example, the highly contagious and rapidly spreading nature of SARS-CoV-2 poses a great challenge to the prevention and control of standing outbreaks. All these require easy and accurate identification of pathogens in a timely manner. Long Ma et al. developed a CRISPR-Cas12a-driven visual biosensor with a smartphone readout for the ultrasensitive and selective detection of SARS-CoV-2 [83]. It produces a color change that can be easily distinguished by the naked eye as well as by a smartphone with a Color Picker app. The biosensor detects the SARS-CoV-2 gene in synthetic vectors, transcribed RNA, and SARS-CoV-2 pseudoviruses. It achieves “single-copy separation” with a detection limit of 1 copy/μL of pseudovirus and no cross-reactivity. The developed biosensor provided 100% concordance (positive and negative) with qPCR results when the developed biosensor was attacked with SARS-CoV-2 clinical biological samples. The sample-to-result time was approximately 90 min. this biosensor provides a novel and powerful technique for the ultrasensitive detection of SARS-CoV-2 for clinical use. Cristina Adrover-Jaume et al. organized previous findings into a new design for decentralized COVID-19 diagnosis [84]. It consists of a device made entirely of paper and consists of two parts: a paper cube containing a nanoparticle reservoir and a paper strip for target capture (Figure 8). The mobile biosensor was used to detect cytokine storm biomarkers. The immunosensor was made only of cellulose modified with antibody-modified nanoparticles. This collective, amplified signal was then quantified where needed using a previously developed smartphone app for optical densitometry. The turnaround time was less than 10 min.
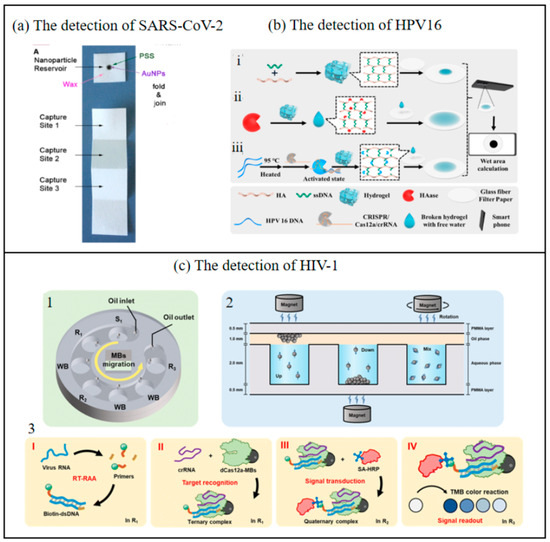
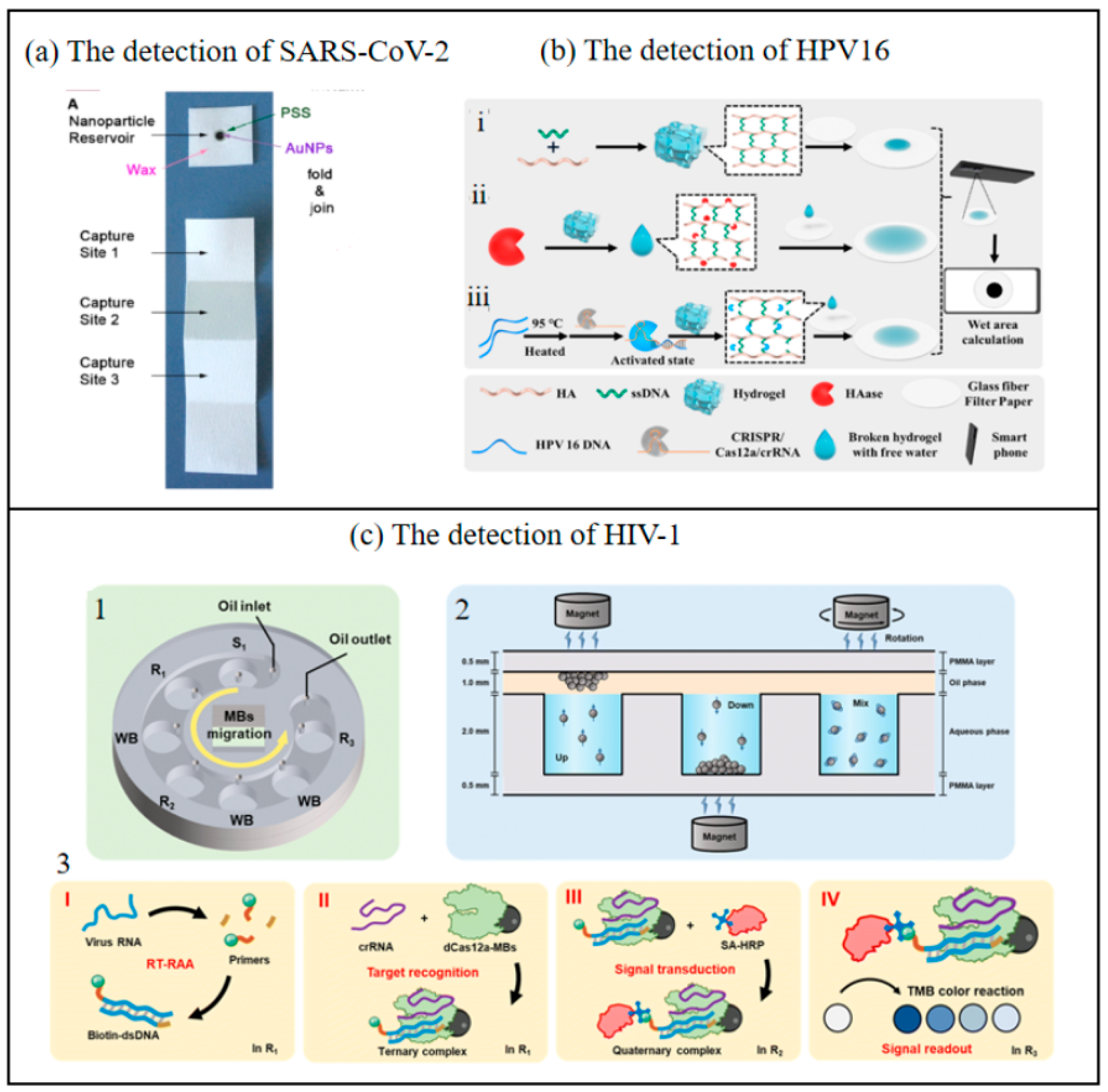
Figure 8.
(a) The chip of SARS-CoV-2 detection [84]. Copyright 2021, Elsevier. (b) Working principle of the up and down chip for HIV-1 detection. i—Preparation of hydrogel; ii—HAase detection process; iii—HPV 16 DNA detection process. (HA-Hyaluronic Acid, HAase- Hyaluronidase) [37]. Copyright 2025, Elsevier. (c) Paper-based biosensor for the points of care testing HAase and HPV 16 DNA using wet area as readout. 1—Schematic diagram of the chip structure. 2—MB migration and mixing mode. 3—Principle of nucleic acid detection based on dCas12a in the up and down chip. I—Reverse transcription and exponential amplification of target RNA through RT-RAA reaction. II—Target recognition by dCas12a-modified beads. III—Signal transduction through the capture of HRP-SA. IV—Signal readout based on the color change reaction. (SA-HRP—Streptavidin Conjugated with Horseradish Peroxidase) [85]. Copyright 2023, Royal Society of Chemistry.
Huang et al. developed an integrated system combining a CRISPR-Cas12a-based biosensor with microfluidic technology, significantly enhancing its practicality for point-of-care (POC) nucleic acid detection [85]. This system consolidates multiple processes—including nucleic acid amplification, target capture, affinity binding, magnetic bead (MB) washing, and chromogenic reactions—into distinct chambers within a microfluidic chip. These chambers are sealed with a unified oil-phase compartment to prevent cross-interference. Furthermore, a smartphone-compatible color recognition application (app) was developed to convert the shade intensity in the color reaction chamber into distinct RGB values, which are then processed using an empirical formula to calculate corresponding luminance values. This approach enables advanced signal transduction, improving detection sensitivity and allowing semi-quantitative/qualitative analysis of target nucleic acids without requiring specialized equipment. The limit of detection (LOD) reaches 1 copy/μL for pseudoviruses, highlighting its ultrahigh sensitivity, even at single-copy resolution. The colorimetric readout, analyzed via a smartphone app (Color Picker), exhibited strong linear correlations between lightness values/absorbance and viral concentrations (R² = 0.990–0.998), enabling semi-quantitative analysis. The method demonstrated robust reproducibility and repeatability, with relative standard deviation (RSD) values below 6%, and maintained consistent performance across different smartphones and lighting conditions. When applied to HIV-1 detection and compared with existing systems, the results demonstrated that the detection outcomes could be visually interpreted or digitally read via smartphone, achieving high sensitivity and specificity without reliance on bulky instruments. The entire detection process is integrated into a single chip, ensuring operational safety and substantial clinical potential. This advancement holds practical significance for evaluating viral loads to improve patient survival rates, particularly in resource-limited settings. Li et al. developed an innovative biosensing strategy integrating target-regulated water-absorbing hydrogels with glass fiber filter paper featuring a large microporous structure and excellent modifiability (optimized for diffusion wet zone generation) to detect hyaluronidase (HAase) activity and HPV 16 DNA [37]. This method involves depositing fragmented hydrogels and free water droplets released from the hydrogel onto the modified glass fiber filter paper, where they form visible diffusion wet zones. The images of these wet zones on the filter paper are captured using a smartphone and subsequently analyzed through computational processing to quantitatively determine HAase activity and HPV 16 DNA concentration. This approach effectively eliminates risks associated with signal probe leakage and minimizes potential human error inherent in conventional methods. By synergizing the unique physicochemical properties of hydrogels with modern technological tools, this strategy advances the development of portable biosensors utilizing area-based signal readouts. Furthermore, the creation of a simple yet versatile dual-functional biosensor for simultaneous detection of HPV DNA and HAase establishes a promising diagnostic platform for innovative applications. This breakthrough holds significant clinical relevance for monitoring HPV status and enabling early cervical cancer screening in HPV-positive patients. The proposed system demonstrates substantial potential for practical implementation in HPV surveillance and early-stage cervical cancer detection, particularly in resource-limited settings requiring cost-effective and user-friendly diagnostic solutions.
3.1.3. Mental Health Monitoring
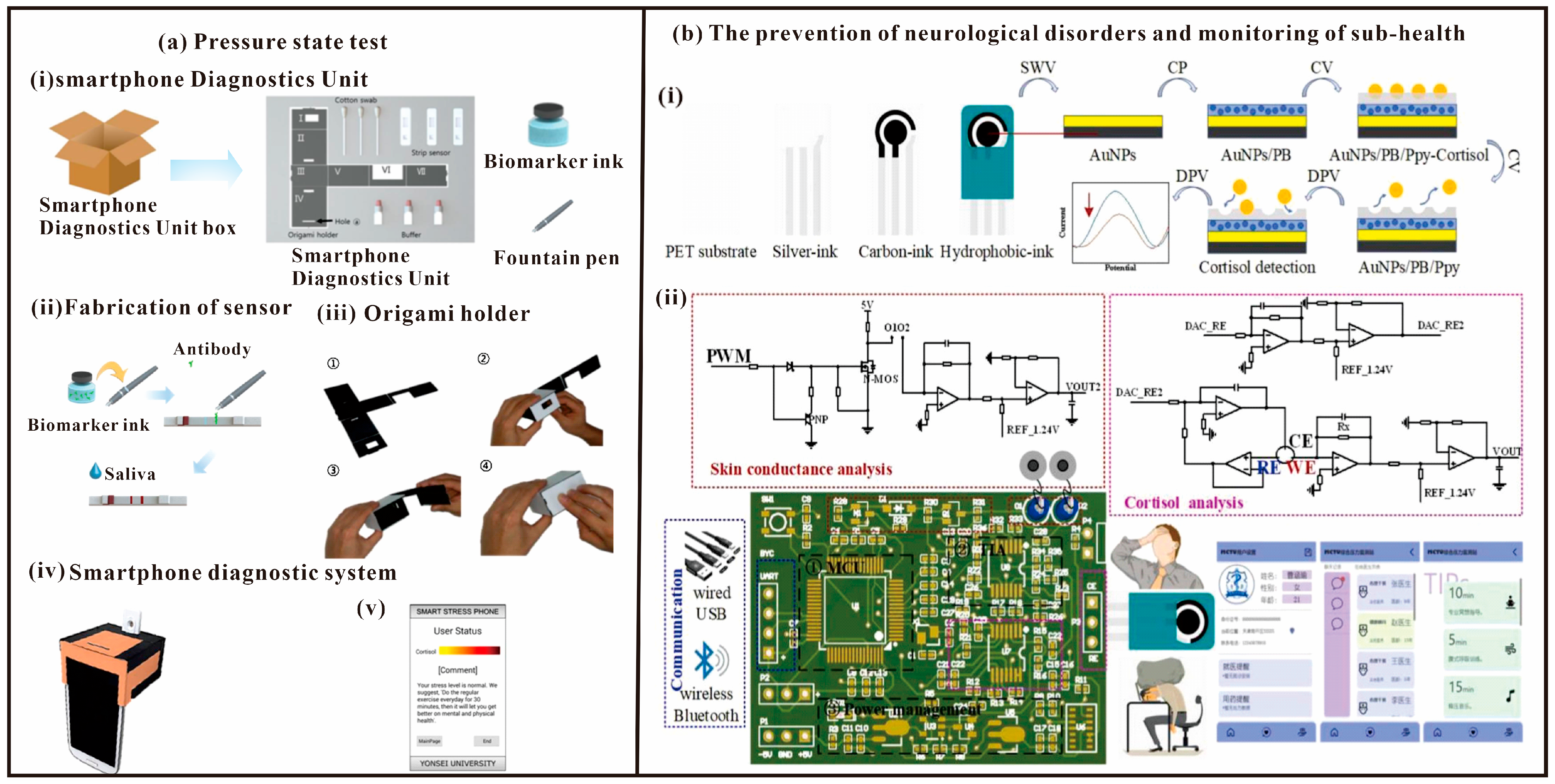
Over the past two decades, invisible disorders related to human emotions, such as psychological stress, fear, and depression, have been the subject of increasing attention as political, social, educational, and medical issues in human societies [86]. Moreover, since stress is one of the key factors contributing to various disorders in a wide range of human emotions, many research teams have investigated the psychological responses to stress [86]. Studies have shown that smartphones can be used in systems for detecting clinical diseases using saliva, sweat, and blood [27,87,88,89] instead of traditional methods. In addition, colorimetric analysis has recently been performed using smartphones and an implementation of lateral chromatography assay (LFA) has been developed [90]. Jung-Sik Yang et al. designed a method to select cortisol and C-reactive protein (CRP) levels as indicators of psychological stress, and measured the stress status of subjects through a smartphone application and LFA (Figure 9a) [91]. Finally, they proposed an economical and lightweight Smartphone Diagnostic Unit (SDU), which measured stress levels similar to those of the 3D stent, and finally successfully measured salivary cortisol and C-reactive protein (CRP) levels. It will help to assess psychological stress levels more easily.
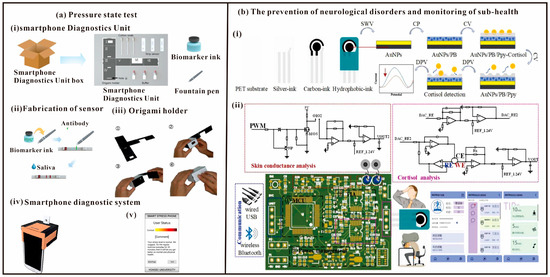
Figure 9.
(a) The method to select cortisol and C-reactive protein (CRP) levels as indicators of psychological stress, and measured the stress status of subjects through a smartphone application and LFA. (i)—Components of the smartphone diagnostics unit: origami holder, cotton swab, strip biosensor, and buffer solution. (ii)—Fabrication of the LFA with biomarker ink and fountain pen, which makes it easy to draw lines on the strip biosensor. (iii)—Assembly of the origami holder: Paper holder plate after removal from box. The bulged bottom layer is folded and inserted into the slit in the upper side. The box is enclosed with the vertical piece. (iv)—A complete SDU unit with strip biosensor, origami holder, and smartphone. (v)—A screen from the smartphone application. [91]. Copyright 2017, Elsevier. (b) Integrated biosensing system for cortisol and skin conductance analysis on smartphone, including (i)—Cortisol sensing electrode and (ii)—Integrated system [38]. Copyright 2023, Elsevier.
With modern social changes and the renewal of culture and living habits in the post-COVID-19 period, mental stress caused by closed spaces, high-intensity work, low-density social interactions, and fast-paced life has become an important factor that directly affects human health [92,93,94]. In particular, during COVID-19 pandemic, surveys demonstrated that medical and quarantine personnel were subjected to significantly elevated levels of stress, which affected their health and daily life [95,96]. High levels of stress are associated with negative physiological and psychological consequences, including alterations in cognition processes and behavior, the onset of anxiety and depression, and the development of physiological skin, endocrine, and cardiovascular disorders, which greatly affect personal health and social stability [97,98,99]. Most wearable devices allow crude monitoring of stress levels by detecting skin conductance [100,101]. In addition, cortisol detection, as an important biomarker of psychological and physiological stress, is important for the prevention of neurological disorders and monitoring of sub-health. Bai et al. proposed an electrochemical and electrophysiological integrated biosensing system for the analysis of cortisol and skin conductance on a smartphone (Figure 9b) [38]. Then a portable integrated physiological and biochemical sensing system was developed, which contains a microcontroller module, a transimpedance amplifier (TIA) module, a power management module, a signaling module, and a cortisol and skin conductance detection module. The system is characterized by low noise, low input bias current, cell gain stability, precision voltage control and weak current signal conditioning. In addition, the prepared multimodal sensing system shows good performance in combined physiological and biochemical sensing, which proves its promising application in precise complementarity monitoring.
The past decade has witnessed an explosive proliferation of smartphone-based biosensors. As comprehensively and innovatively analyzed by Rossana E. Madrid et al. in “Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users,” the global ubiquity of smartphones has unlocked novel opportunities to leverage their computational power, network connectivity, battery efficiency, and imaging capabilities [102]. These features enable the deployment of portable POC devices near patients for real-time healthcare monitoring. Such advancements empower patients and clinicians to address health concerns more rapidly, efficiently, and reliably, whether at home or outside traditional healthcare facilities. Furthermore, the extensive connectivity options offered by contemporary wireless communication infrastructure position smartphones as a ubiquitous platform ideally suited for developing biosensing and diagnostic systems, particularly for point-of-care and telemedicine applications. This integration not only enhances accessibility but also redefines healthcare delivery by bridging gaps between clinical settings and decentralized care environments.
In conclusion, the biosensors combined with smart phones can monitor physiological indicators such as heart rate and skin electrical activity in real time, dynamically track changes in psychological states such as stress and anxiety, and provide early warnings. Its portable and non-invasive features are integrated into daily life, lowering the usage threshold and enhancing user compliance. By integrating AI algorithms to analyze multi-dimensional data, it can also provide personalized feedback, assist in precise intervention, and make up for the lag and subjective limitations of traditional scale assessment. Table 1 shows the advantages and disadvantages of biosensors for disease diagnosis compared with traditional methods.

Table 1.
The advantages and disadvantages of using smartphone biosensors for the diagnosis of different types of diseases compared with traditional methods.
3.2. Food Safety Testing
In recent years, smartphones have become an important carrier of biosensing technology for food testing by virtue of their portability, high-performance cameras, data processing capabilities and wireless transmission functions. By integrating miniaturized sensors, image analysis algorithms and cloud-based data processing platforms, smartphones have demonstrated the advantages of high sensitivity, fast response and low cost in food safety detection. In the following, the progress of its application is analyzed with examples, categorized by different detectives.
3.2.1. Pesticide Residue Detection
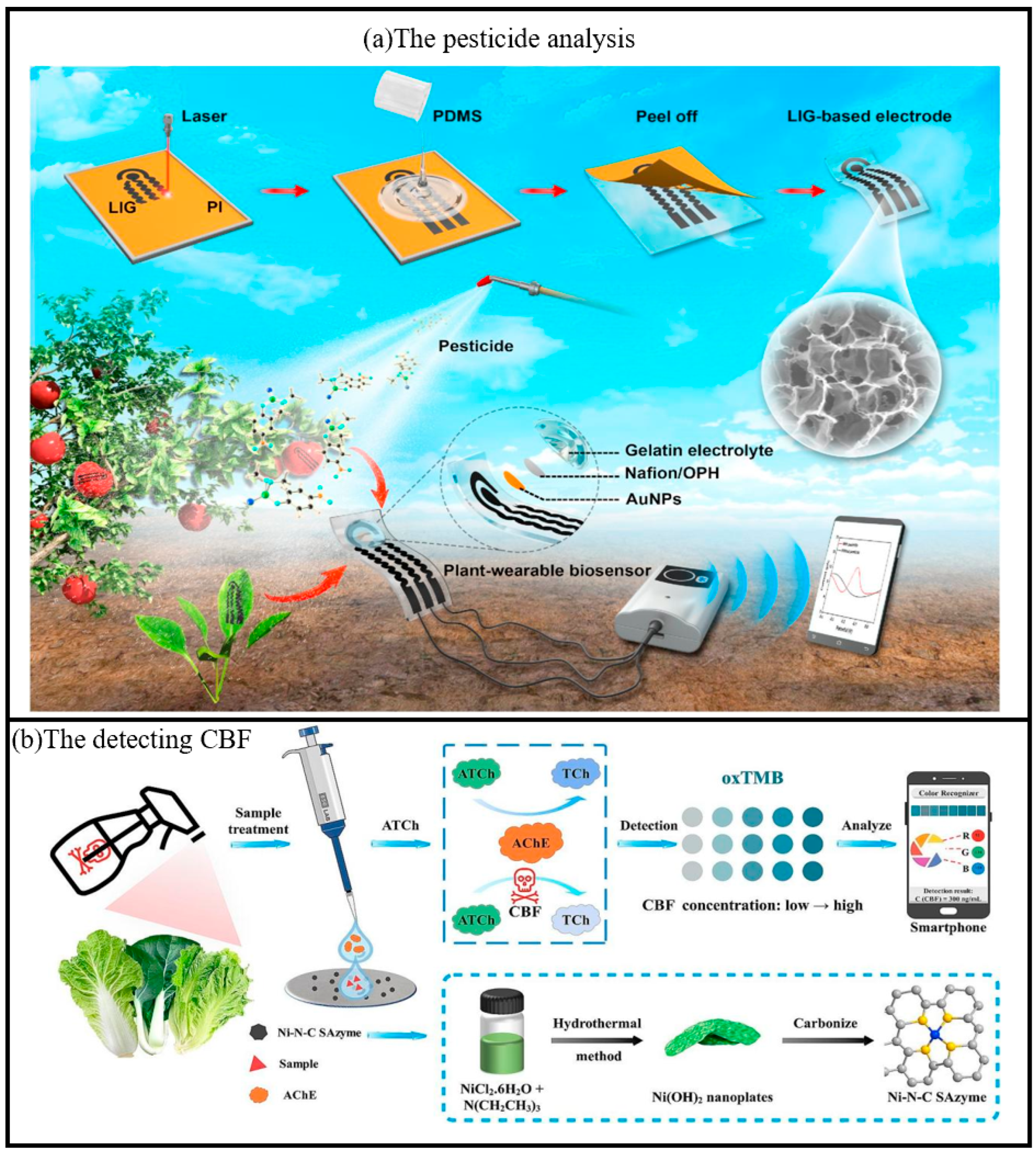
Biosensors integrated with smartphones have served as portable and efficient tools for the detection of pesticide residues. By enabling real-time data collection and analysis, these biosensors have substantially enhanced both the efficiency and accuracy of detection processes. The incorporation of an intelligent user interface and cloud-based data sharing capabilities facilitates on-site rapid screening and large-scale monitoring, thereby providing robust support for food safety supervision. Zhao et al. proposed a plant wearable electrochemical biosensor, which can be mounted on the surface of a crop for in situ analysis of organophosphorus pesticides (OPs) (Figure 10) [110]. It was fabricated with a flexible serpentine-shaped three-electrode system using laser-induced technology. Modified with organophosphorus hydrolase (OPH) and equipped with biocompatible semi-solid electrolytes, our plant wearable biosensor can selectively capture and recognize methyl parathion on the crop surface. With a Bluetooth-equipped handheld electrochemical workstation, real-time information on pesticide residues can be wirelessly received on a smartphone. This plant wearable analysis strategy eliminates the need for complex sample pre-treatment and allows for rapid, non-destructive and in situ identification of pesticides on crop surfaces. Real-time pesticide residue information can also be received wirelessly on smartphones.
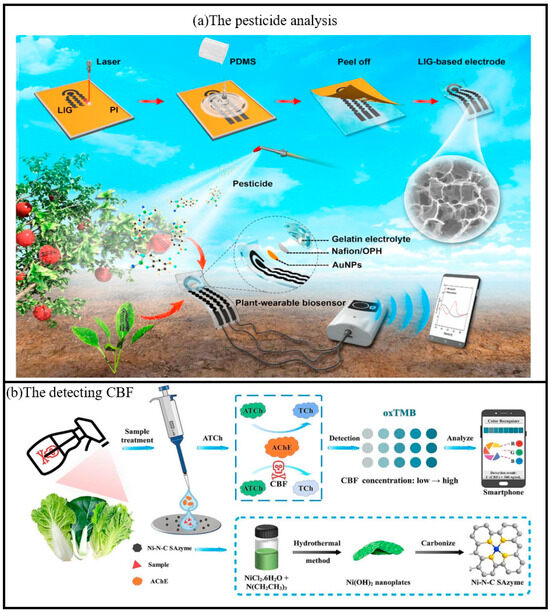
Figure 10.
(a) Schematic diagram of the fabrication process of the plant-wearable biosensor based on 3D porous LIG and the application of in situ pesticide analysis for agricultural products [110]. Copyright 2020, Elsevier. (b) Schematic diagram of detecting CBF by colorimetric paper chip sensor based on smartphone using Ni-N-C SAzyme [39]. Copyright 2025, Elsevier.
Pesticides are widely used in agriculture and forestry due to their significant impact on crop disease control and pest management [111]. Carbofuran (CBF) is a carbamate insecticide that is used for agricultural pest control due to its broad spectrum, high efficiency and low toxicity [112,113]. However, irrational or excessive application of CBF poses a major threat to human health. There is an urgent need to develop a simple, rapid and sensitive method for CBF detection. In recent years, the application of test paper sensors in handheld sensing has become a hot research topic, and its advantages include light weight, flexibility, low cost, disposable after use, and simple process [114,115,116]. Zhang et al. investigated a portable smartphone-assisted colorimetric paper-chip sensor based on Ni-N-C SAzyme, which can detect CBF by using nickel hydroxide nanoplatelets (Ni(OH)2) as the CBF precursor [39]. AChE plays an important role in the biological nervous system as a hydrolase that catalyzes the conversion of acetylcholine (ATCh) to the strongly reducing choline (TCh) [117]. The sensor demonstrated a limit of detection (LOD) of 0.42 ng/mL with a linear range of 1–500 ng/mL for carbofuran (CBF) using UV-vis absorption spectroscopy. For the smartphone-assisted paper chip colorimetric sensor, the LOD was 8.79 ng/mL with a linear range of 10–500 ng/mL. In real-sample testing (Chinese cabbage, cabbage, and lettuce), the spiked recoveries ranged from 81.09% to 125.27%, with relative standard deviations (RSD) below 10.81%, indicating satisfactory accuracy and precision. This enzymatic process reduces the POD activity of Ni-N-C, leading to a decrease in oxTMB production. Upon addition of CBF molecules, the enzymatic activity of AChE was inhibited, leading to a decrease in TCh content and a significant blue color recovery catalyzed by Ni-N-C (Figure 10). In addition, the prepared colorimetric paper sheet sensor had good selectivity and reliability, and was successfully applied to the detection of three vegetable samples, including cabbage, cabbage and lettuce, with satisfactory results.
3.2.2. Veterinary Drug Residue Testing
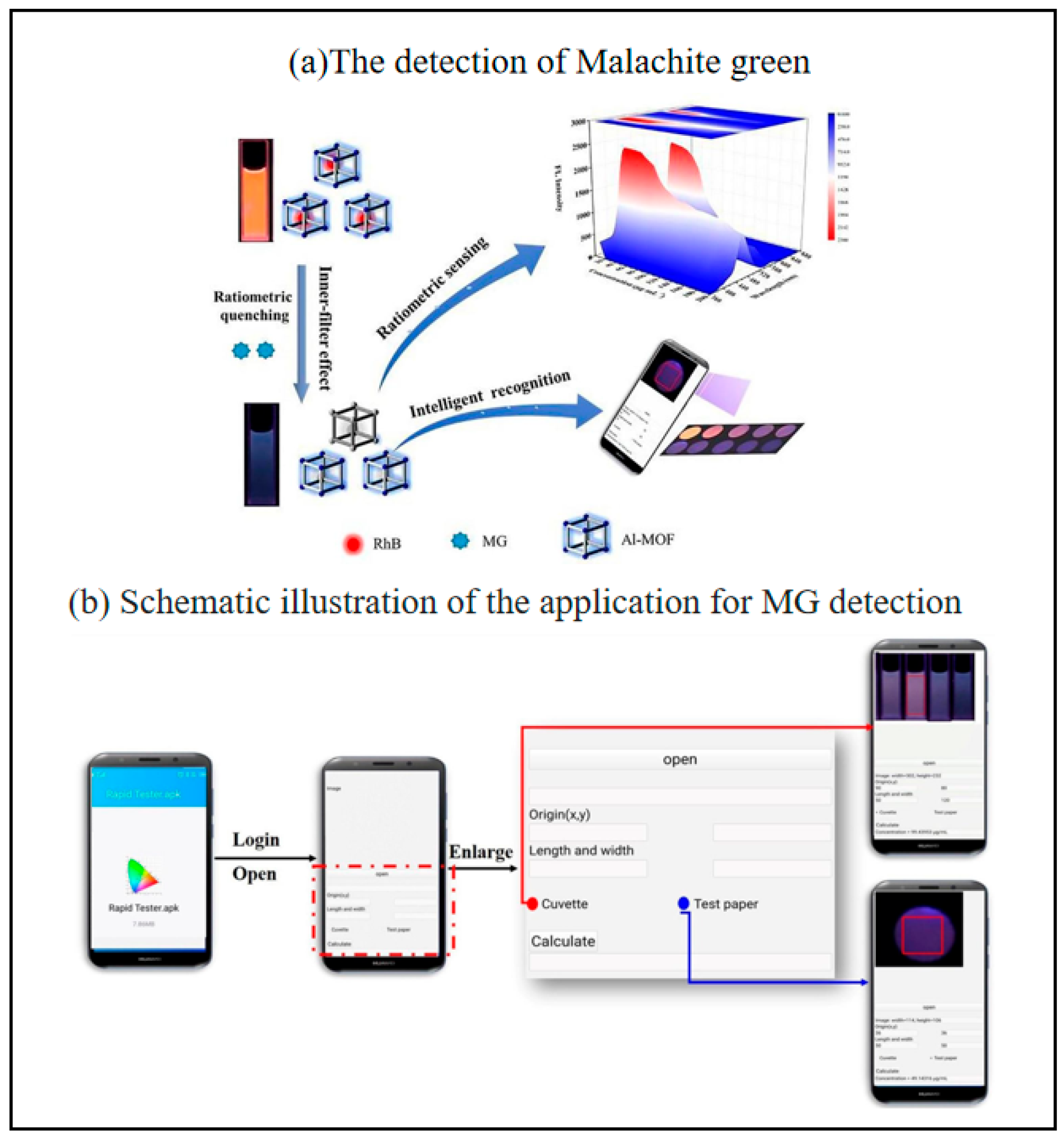
The development of intelligent, sensitive, and rapid veterinary residue assays is a practical need to ensure food quality and safety. Malachite green (MG) is considered one of the most toxic veterinary residues because of its potential carcinogenicity and teratogenicity in animals and humans [118,119]. Although its use in aquaculture and food production is banned in many countries, it is used illegally because of its broad antimicrobial spectrum, low cost and high availability [120,121]. Therefore, there is a need for a rapid, sensitive and reliable method to monitor MG residues to ensure food safety. Yue et al. prepared a sensitive ratiometric fluorescent sensor using Al-MOF/RhB as a fluorescent probe [40]. The fluorescence color of their designed probe showed a wide range of color changes from orange to blue. Meanwhile, a smartphone-assisted Al-MOF/RhB-based fluorescent paper was used as a portable platform for MG intelligence and visual inspection (Figure 11). The portable smartphone-assisted ratiometric fluorescence sensor developed in this study has a limit of detection (LOD) of 1.6 μg/mL and a limit of quantification (LOQ) of 5.3 μg/mL for malachite green (MG). In spiked fish tissue samples, the results obtained by fluorescence spectrophotometry and smartphone detection are in good agreement with those of high-performance liquid chromatography (HPLC), with recoveries ranging from 81.90% to 108.00% and relative standard deviations (RSD) of 1.00–6.55%, indicating that the sensor has high accuracy and reliability. It is a paper-based analyzing device integrate with a smartphone.
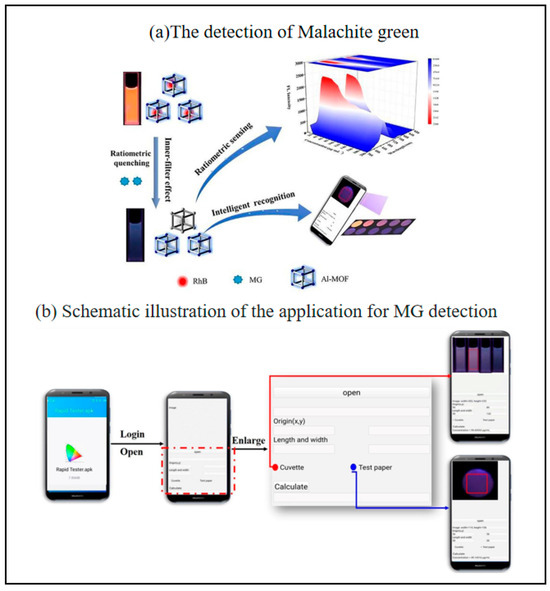
Figure 11.
Schematic illustration of the smartphone-assisted colorimetric fluorescent probe for MG detection (a)the detection of malachite green, (b) schematic illustration of the application [40]. Copyright 2022, Elsevier.
Metronidazole (MNZ) belongs to the class of nitroimidazole (NIIM) antibiotics and has been widely used in humans and animals due to its excellent performance in the treatment of parasitic and anaerobic infections [122,123,124]. Metronidazole is also recommended and approve by FDA for Humans [123]. However, studies have shown that MNZ can cause neurotoxicity, carcinogenicity and mutagenicity [125]. Conventional methods used to detect MNZ include HPLC [126], LC-MS [127], and capillary electrophoresis [128,129]. Despite the advantages of high sensitivity and accurate quantification, these methods have complex, time-consuming detection procedures and require expensive, large-scale instrumentation, which limits their application for rapid, on-site and batch low-cost analysis. Therefore, it is necessary and challenging to explore efficient methods and portable devices for the rapid determination and differentiation of MNZ in food samples. Wang et al. developed a fluorometric method for the rapid detection of multicolored CDs based on MNZ residues using a one-step hydrothermal method for the preparation of multicolored carbon dots using a fluorescent sensor array that was used to differentiate between different nitroimidazole antibiotics. Fluorescence quantification of MNZ based on fluorescent images (R/G values) can be achieved in minutes by building a simple device and introducing a smartphone. Fluorescence quantification of MNZ in food samples (milk and honey) based on a smartphone-integrated platform showed good recoveries and accuracy of the proposed method. The smartphone integrated platform is more convenient for on-site testing, therefore, this analytical strategy and smartphone integrated platform provide a practical tool for quantification and differentiation of drug residues in food samples [130]. Concentration can be measured by the designed app by calculating RGB values of different images. The signals read from the designed app provide a viable method of quantitative detection. Intelligent visualization and analysis methods can show satisfactory detection results [40].
3.2.3. Detection of Antibiotics
Antibiotics are widely abused as the most effective class of drugs for the prevention and treatment of infectious diseases. Many antibiotics can be metabolized into livestock products such as milk through accumulation in the food chain and overdosed in the human body, inevitably causing poisons and side effects in humans [131]. On-site detection of antibiotics is of vital importance for food safety monitoring. The biosensing device for smart phones, by integrating highly sensitive biosensors and portable detection modules, can detect antibiotic residues in real time and rapidly, and is suitable for on-site screening. By integrating image analysis algorithms and cloud data platforms, it can also achieve visual interpretation and remote sharing of test results, providing a convenient solution for food safety and medical monitoring. Liquid chromatography combined with mass spectrometry to quantify the concentration of analytes is a standard technique for antibiotic residue detection. However, inherent limitations such as time-consuming, the need for expensive equipment and complex workflows hinder the application of on-site testing. It is urgent to accelerate the research on highly sensitive and rapid simultaneous detection methods for multiple antibiotics in milk. A smartphone-based pH-responsive 3-channel colorimetric biosensor for non-enzymatic antibiotic residues was proposed by Zhou et al. [132]. The system also introduced a smartphone to replace the traditional optical signal capture and detection result analysis instruments for accurate quantification of multiple target antibiotic residues in milk. Based on the above, a pH-responsive 3-channel rapid antibiotic residue detection system and accompanying kit were developed. It can identify multiple antibiotics simultaneously and accurately within 40 min, and has the advantages of high sensitivity, strong anti-interference, simple operation, short time-consumption, and easy to store, which provides a broad application prospect in the on-site analysis of antibiotics, and effectively protects food safety. The sensor exhibits detection limits (LODs) of 0.085, 0.168, and 0.307 pg/mL for oxytetracycline (OTC), kanamycin (KAN), and streptomycin (STR), respectively, which are far below the maximum residue limits (MRLs) set by the European Union for antibiotics in milk, demonstrating exceptional sensitivity. In terms of accuracy, the spiked recoveries in milk samples ranged from 96.2% to 105.7%, with relative standard deviations (RSDs) of 0.7–5.3%. Specifically, recoveries for OTC, KAN, and STR were 96.8–101.8% (RSD 1.4–5.3%), 96.2–97.4% (RSD 0.7–1.4%), and 97.4–105.7% (RSD 0.8–2.0%), respectively, confirming high accuracy and reproducibility in real-sample analysis.
Antibiotics are commonly used in aquaculture to control various bacterial diseases, improve feed utilization and reduce the need for certain nutrients [133,134]. At the same time, there are increasing concerns about the safety of aquaculture products, especially after the widespread rejection of aquaculture products in importing countries due to the presence of banned antibiotic residues. Tong et al. designed a laser-printed paper-based microfluidic chip based on a multicolor fluorescent CD aptasensor (mCD-μPAD aptasensor) for the simultaneous in situ [41]. In addition, a 3D-printed portable test kit was designed to visually analyze sulfadimethoxine (SMZ), OTC, and CAP via a smartphone, and a third-party smartphone color-recognition application recognizes the RGB value of the fluorescent paper image in the presence of the target, enabling simultaneous and visual detection of antibiotics in aquatic products (Figure 12). Under optimal conditions, the limits of detection (LOD) of this sensor for sulfamethazine (SMZ), oxytetracycline (OTC), and chloramphenicol (CAP) are 0.47, 0.48, and 0.34 ng/mL, respectively. In the detection of actual shrimp samples, the recoveries of the three antibiotics are 95.2–101.2%, 96.4–105%, and 96.7–106.1%, with relative standard deviations (RSD) all below 6%. Under optimal conditions, this μPAD allows for a fast response of 15 min for SMZ, OTC, and CAP, and this paper-based sensor opens up avenues for in situ, high-throughput, and rapid detection methods that can be widely used for POCT in food safety.
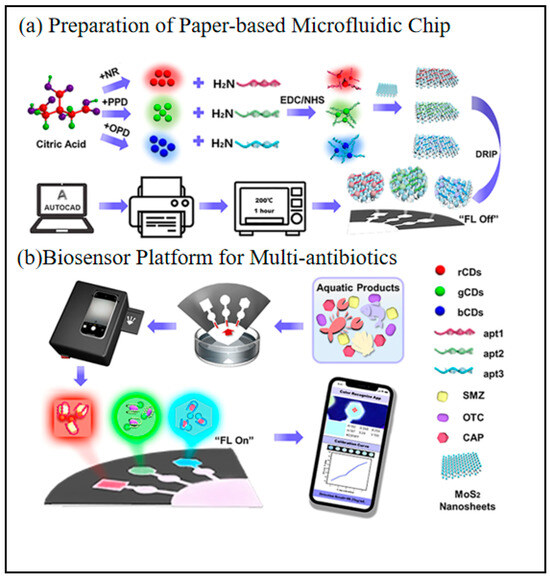
Figure 12.
Simultaneous visual detection of multiple antibiotics via the mCD-μPAD aptamer sensor and a portable detection device with a smartphone, (a) preparation of paper-based microfluidic chip, (b) biosensor platform for multi-antibiotics [41]. Copyright 2022, American chemical Society.
Ensuring food safety through simple and rapid contaminant screening is critical for protecting human health and preventing foodborne incidents. As elaborated by Qin et al. in their review “Advances in Smartphone-Based Biosensors for Food Testing,” traditional electrochemical sensors primarily rely on bulky laboratory-based amplifiers, rendering them unsuitable for rapid on-site detection [135]. In contrast, the integration of biosensors with smartphones simplifies and intellectualizes sensing systems. Given the global ubiquity of mobile internet access, the combination of smartphones with disposable sensors holds transformative potential to democratize sensing methodologies, enabling universal accessibility and real-time participation by users anywhere, anytime. With minimal additional hardware, smartphones can collect and analyze results independently, serving as all-in-one platforms for data processing and user interaction in food quality assessment. The practicality of this approach is undeniable, particularly in reducing costs and improving accessibility in developing countries. Advances in microelectronics have further driven the miniaturization and enhanced functionality of smartphones, significantly boosting their portability and broadening their applicability in resource-limited settings.
To summarize, smartphone-based biosensors currently applied in the field of food detection provide great convenience for this field, but still face technical challenges as well as complex future trends. It needs to address such aspects as sensitivity enhancement: development of novel nanomaterials to enhance the signal; multi-target detection: integration of microfluidic chips with array sensors; data analysis: deep learning algorithms to optimize signal parsing; and standardization: establishment of cross-platform detection protocols. It is believed that with the development of various technologies, these problems can be solved in the future. Table 2 shows the advantages and disadvantages of biosensors for food safety testing compared with traditional methods.

Table 2.
The advantages and disadvantages of using smartphone biosensors for food safety testing compared with traditional methods.
3.3. Environmental Analysis
As environmental pollution problems intensify, the combination of smartphone and biosensor technologies offers innovative solutions for real-time, portable environmental monitoring. By integrating optical sensing, electrochemical detection, microbial response modules, and Internet of Things (IoT) technologies, smartphone platforms have enabled rapid analysis of air, water, soil, and biological pollutants [33,142].
3.3.1. Biological Contaminants
Okadaic acid (OA) is a diarrheal shellfish poisoning toxin produced by some unicellular algae from planktonic and benthic microalgae and accumulates in the digestive glands of shellfish [143]. It is prone to cause pollution of environmental water bodies and is likely to cause illness in humans after consumption. Su et al. developed a novel portable system called the CVBS system based on the combination of image analysis and smartphone-based applications (Figure 13) [42]. This biosensing system was used in conjunction with a cytotoxicity-free CCK-8 kit for label-free, non-invasive and long-term monitoring of cell viability. The detection system for okadaic acid (OA) exhibits a detection limit of 33.95 µg/L and a linear range of 10–800 µg/L. Validation via spiked recovery experiments demonstrated recovery rates ranging from 93.54% to 104.03%, with an average recovery of 99.06%, indicating accuracy comparable to the mouse bioassay (MBA, average recovery: 100.16%). Compared to the traditional MBA method (detection limit: 220 µg/kg), this sensor significantly improves sensitivity and complies with the European Commission (EC) regulatory standard for OA (maximum permitted concentration: 160 µg/kg). By integrating image analysis with portable technology, the system achieves low-cost, high-throughput, and wide-range detection capabilities, making it highly suitable for practical applications in food safety and environmental monitoring. In addition, the HepG2 cell line was selected as the sensing element for the specific detection of OA. this smartphone-based system exhibits low cost, high throughput, wide detection range, and portability for OA detection. The cell culture process used in this system is more common than other cell-based biosensors, which could facilitate the popularization of this system in the field of shellfish toxin measurement. The primary objective of this study by Kim et al. was to develop the on-site deployable microalgae monitoring platform using only a smartphone and inexpensive acrylic color filters [144]. The platform aims to assess the morphological status of microalgae, including stress levels, cell concentration, and the ecologically dominant species. It employs the smartphone’s white LED flash and camera to detect both fluorescence and reflectance intensities from microalgal samples under various combinations of excitation and emission colors. This approach provides non-experts with accessible field-based microalgae monitoring and has the potential for application in monitoring harmful algal blooms.
Figure 13.
(a) The action principle of CCK-8 kit. (b) Construction of CVBS, (c) the picture of portable smartphone-based system and (d) the main interface of the homemade software iPlate Monitor [42]. Copyright 2017, Elsevier.
3.3.2. Chemical Contaminants
Heavy metal contamination in aquatic environments poses a significant threat to both ecosystems and human health. It can lead to the mortality or genetic mutation of aquatic or ganisms, thereby disrupting the stability of the food chain. Heavy metals, such as lead and mercury, when ingested via drinking water or the food chain, may accumulate in vital organs and result in chronic conditions, including cancer and neurological disorders. Furthermore, heavy metal pollution is characterized by its persistence and irreversibility, presenting a substantial obstacle to the sustainable management of water resources. Smartphone-based biosensors offer substantial advantages for the detection of heavy metal ions in aquatic environments. Their portability facilitates rapid on-site analysis without reliance on elaborate instrumentation, thereby enhancing monitoring efficiency significantly. The high sensitivity and specificity of these biosensors enable precise identification of low-concentration heavy metal ions, such as lead and mercury, minimizing the likelihood of false positives. Furthermore, leveraging the data processing and cloud-sharing capabilities of smartphones allows for real-time analysis and remote transmission of test results, providing timely decision-making support for environmental oversight. Ramar et al. employed a strategy combining spectrophotometry with a smartphone-based detection platform [145]. Utilizing surface plasmon resonance (SPR)-assisted colorimetry, they achieved rapid, visually quantifiable on-site detection of Cr(III) ions. Furthermore, they developed a spectrophotometric technique that utilizes smartphone RGB color analysis for the field analysis of Cr(III) ions in environmental water samples. This approach highlights the potential of their chemical sensing system for application in portable quantitative detection devices. Additionally, the catalytic performance of the MMT-supported gold nanoparticles (MMT-AuNPs) was demonstrated through the reduction of p-nitroaniline in the presence of sodium borohydride.
In summary, smartphone environmental sensors have some advantages in terms of portability and cost. But despite this, we still face many challenges, such as: sensitivity and selectivity; standardization and certification; and long-term stability. Of course, this technology will certainly continue to evolve in the future. Development directions include, for example: multimodal sensing fusion; AI-driven data analysis; self-powered system development; and citizen science network construction. Table 3 shows the advantages and disadvantages of biosensors for environmental analysis compared with traditional methods.

Table 3.
The advantages and disadvantages of using smartphone biosensors for environmental analysis compared with traditional methods.
4. Conclusions and Outlook
The deep integration of on-site testing based on biosensing technology and smartphones is reshaping the paradigm of medical diagnosis, food safety monitoring and environmental analysis. Through the integration of microfluidics, nanomaterials, and artificial intelligence (AI) technologies, smartphones have not only become portable, low-cost testing terminals, but also driven biosensing technology from a single testing tool to an intelligent health management system through multimodal hardware adaptation and data interconnection capabilities. In the medical field, the smartphone-biosensing system has realized dynamic monitoring of chronic diseases, rapid screening of infectious diseases and mental illnesses, significantly improving the accessibility of diagnosis and treatment in resource-poor areas; in the field of food safety, the innovation of multiple detection methods combined with AI algorithms has realized the high-sensitivity, multi-targeted co-testing of pollutants; and in environmental monitoring, the smartphone platform has also provided real-time, portable in environmental monitoring, smartphone platforms also provide real-time, portable solutions.
However, the current technology still faces multiple challenges: detection accuracy is limited by environmental interference and lack of equipment standardization; the complexity of multi-parameter co-testing is not yet fully balanced with the demand for high throughput; and data privacy and long-term stability issues constrain large-scale diffusion. In the future, through the integration of flexible electronics and wearable devices, POCT systems will develop towards passivation and miniaturization; federated learning and edge computing technologies are expected to optimize the generalization ability of AI models under the premise of privacy protection; and the combination of the Internet of Things (IoT) and Citizen Science Networks (CSNs) will promote real-time sharing of testing data and dynamic tracking of epidemiology. In addition, the further application of nanomaterials (e.g., MXene, quantum dots) and CRISPR technology may break through the existing detection limit and realize single-molecule-level sensitivity.
Looking ahead, the intelligent and decentralized trend of smartphone-biosensing will accelerate the equitable distribution of healthcare resources and provide sustainable technological support for global health monitoring. Interdisciplinary collaboration, development of standardized protocols, and improvement of ethical regulations will be key to realizing this vision. With the penetration of cutting-edge technologies such as 6G communication, organ chips and synthetic biology, traditional biosensors are expected to evolve from “on-site detection” to integrated full-cycle health management, and ultimately reshape the future of humanity in terms of disease, food safety, and a friendly environment.
Author Contributions
Conceptualization, E.X. and S.S.; methodology, E.X. and X.X.; investigation, E.X. and H.C. (Haoran Cui); writing—original draft preparation, E.X. and Y.D.; writing—review and editing, S.S. and Y.D.; supervision, S.S. and H.C. (Hongfei Chen). All authors have read and agreed to the published version of the manuscript.
Funding
This research was supported by the National Key Research and Development Program of China (2023YFB3208200) and National Natural Science Foundation of China (21974147).
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Wongkaew, N.; Simsek, M.; Griesche, C.; Baeumner, A.J. Functional Nanomaterials and Nanostructures Enhancing Electrochemical Biosensors and Lab-on-a-Chip Performances: Recent Progress, Applications, and Future Perspective. Chem. Rev. 2019, 119, 120–194. [Google Scholar] [CrossRef]
- Comstock, M.J. Biosensor Design and Application, Copyright, 1992 Advisory Board, Foreword. In Biosensor Design and Application; ACS Symposium Series; American Chemical Society: Washington, DC, USA, 1992; Volume 511, pp. i–vi. [Google Scholar]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef] [PubMed]
- Chikkaveeraiah, B.V.; Bhirde, A.A.; Morgan, N.Y.; Eden, H.S.; Chen, X. Electrochemical Immunosensors for Detection of Cancer Protein Biomarkers. ACS Nano 2012, 6, 6546–6561. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Li, Q.; Wang, L.; Zhang, G.-J.; Fan, C. Framework-Nucleic-Acid-Enabled Biosensor Development. ACS Sensors 2018, 3, 903–919. [Google Scholar] [CrossRef]
- Gerdan, Z.; Saylan, Y.; Denizli, A. Biosensing Platforms for Cardiac Biomarker Detection. ACS Omega 2024, 9, 9946–9960. [Google Scholar] [CrossRef] [PubMed]
- Ki, H.; Jang, H.; Oh, J.; Han, G.-R.; Lee, H.; Kim, S.; Kim, M.-G. Simultaneous Detection of Serum Glucose and Glycated Albumin on a Paper-Based Sensor for Acute Hyperglycemia and Diabetes Mellitus. Anal. Chem. 2020, 92, 11530–11534. [Google Scholar] [CrossRef]
- Shi, Y.F.; Jiang, Y.P.; Wang, X.Z.; Sun, P.P.; Zhu, N.J.; Wang, K.; Zhang, Z.Q.; Liu, Y.Y.; Huo, J.; Wang, X.R.; et al. Chiral Luminescent Sensor Eu-BTB@d-Carnitine Applied in the Highly Effective Ratiometric Sensing of Curing Drugs and Biomarkers for Diabetes and Hypertension. Inorg. Chem. 2022, 61, 15921–15935. [Google Scholar] [CrossRef]
- Wang, F.; Feng, X.; Gao, Y.; Ding, X.; Wang, W.; Zhang, J. Green Synthesis of PtPdNiFeCu High-Entropy Alloy Nanoparticles for Glucose Detection. ACS Omega 2023, 8, 47773–47780. [Google Scholar] [CrossRef]
- Sun, S.; Chen, J. Recent Advances in Hydrogel-Based Biosensors for Cancer Detection. ACS Appl. Mater. Interfaces 2024, 16, 46988–47002. [Google Scholar] [CrossRef]
- Hernández, B.; Sanchez-Cortes, S.; Houzé, P.; Ghomi, M. Nonenzymatic Hydrolysis of Acetylthiocholine by Silver Nanoparticles. J. Phys. Chem. C 2019, 123, 2378–2385. [Google Scholar] [CrossRef]
- Liu, G.; Chen, H.; Peng, H.; Song, S.; Gao, J.; Lu, J.; Ding, M.; Li, L.; Ren, S.; Zou, Z.; et al. A carbon nanotube-based high-sensitivity electrochemical immunosensor for rapid and portable detection of clenbuterol. Biosens Bioelectron 2011, 28, 308–313. [Google Scholar] [CrossRef]
- Wei, S.; Dou, Y.; Song, S.; Li, T. Functionalized-Graphene Field Effect Transistor-Based Biosensor for Ultrasensitive and Label-Free Detection of β-Galactosidase Produced by Escherichia coli. Biosensors 2023, 13, 925. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Khondakar, K.R.; Mazumdar, H.; Kaushik, A.; Mishra, Y.K. AI and IoT: Supported Sixth Generation Sensing for Water Quality Assessment to Empower Sustainable Ecosystems. ACS ES&T Water 2025, 5, 490–510. [Google Scholar] [CrossRef]
- Gao, W.; Wang, J. The Environmental Impact of Micro/Nanomachines: A Review. ACS Nano 2014, 8, 3170–3180. [Google Scholar] [CrossRef] [PubMed]
- Forbes, P. Atmospheric Chemistry Analysis: A Review. Anal. Chem. 2020, 92, 455–472. [Google Scholar] [CrossRef]
- Padha, B.; Yadav, I.; Dutta, S.; Arya, S. Recent Developments in Wearable NEMS/MEMS-Based Smart Infrared Sensors for Healthcare Applications. ACS Appl. Electron. Mater. 2023, 5, 5386–5411. [Google Scholar] [CrossRef]
- Yousefi, H.; Su, H.-M.; Imani, S.M.; Alkhaldi, K.; Filipe, M.C.D.; Didar, T.F. Intelligent Food Packaging: A Review of Smart Sensing Technologies for Monitoring Food Quality. ACS Sensors 2019, 4, 808–821. [Google Scholar] [CrossRef]
- Min, J.; Tu, J.; Xu, C.; Lukas, H.; Shin, S.; Yang, Y.; Solomon, S.A.; Mukasa, D.; Gao, W. Skin-Interfaced Wearable Sweat Sensors for Precision Medicine. Chem. Rev. 2023, 123, 5049–5138. [Google Scholar] [CrossRef]
- Qian, S.; Cui, Y.; Cai, Z.; Li, L. Applications of smartphone-based colorimetric biosensors. Biosens. Bioelectron. X 2022, 11, 100173. [Google Scholar] [CrossRef]
- Upadhyay, S.; Kumar, A.; Srivastava, M.; Srivastava, A.; Dwivedi, A.; Singh, R.K.; Srivastava, S.K. Recent advancements of smartphone-based sensing technology for diagnosis, food safety analysis, and environmental monitoring. Talanta 2024, 275, 126080. [Google Scholar] [CrossRef]
- You, H.; Wang, M.; Wang, S.; Xu, J.; Hu, S.; Li, T.; Yu, Z.; Tang, D.; Gan, N. Ultrasensitive and Specific Phage@DNAzyme Probe-Triggered Fluorescent Click Chemistry for On-Site Detection of Foodborne Pathogens Using a Smartphone. Anal. Chem. 2023, 95, 11211–11218. [Google Scholar] [CrossRef] [PubMed]
- Patabadige, D.E.; Jia, S.; Sibbitts, J.; Sadeghi, J.; Sellens, K.; Culbertson, C.T. Micro Total Analysis Systems: Fundamental Advances and Applications. Anal. Chem. 2016, 88, 320–338. [Google Scholar] [CrossRef]
- Xing, G.; Li, N.; Lin, H.; Shang, Y.; Pu, Q.; Lin, J.-M. Microfluidic biosensor for one-step detection of multiplex foodborne bacteria ssDNA simultaneously by smartphone. Talanta 2023, 253, 123980. [Google Scholar] [CrossRef]
- Xing, G.; Ai, J.; Wang, N.; Pu, Q. Recent progress of smartphone-assisted microfluidic sensors for point of care testing. TrAC Trends Anal. Chem. 2022, 157, 116792. [Google Scholar] [CrossRef]
- Yin, B.; Zhu, H.; Zeng, S.; Sohan, A.S.M.M.F.; Wan, X.; Liu, J.; Zhang, P.; Lin, X. Chip-based automated equipment for dual-mode point-of-care testing foodborne pathogens. Biosens. Bioelectron. 2024, 257, 116338. [Google Scholar] [CrossRef] [PubMed]
- Roda, A.; Michelini, E.; Zangheri, M.; Di Fusco, M.; Calabria, D.; Simoni, P. Smartphone-based biosensors: A critical review and perspectives. TrAC Trends Anal. Chem. 2016, 79, 317–325. [Google Scholar] [CrossRef]
- Bui, T.H.; Thangavel, B.; Sharipov, M.; Chen, K.; Shin, J.H. Smartphone-Based Portable Bio-Chemical Sensors: Exploring Recent Advancements. Chemosensors 2023, 11, 468. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, 1806739. [Google Scholar] [CrossRef]
- Yang, Y.; Xu, F.; Chen, J.; Tao, C.; Li, Y.; Chen, Q.; Tang, S.; Lee, H.K.; Shen, W. Artificial intelligence-assisted smartphone-based sensing for bioanalytical applications: A review. Biosens. Bioelectron. 2023, 229, 115233. [Google Scholar] [CrossRef]
- Esch, E.W.; Bahinski, A.; Huh, D. Organs-on-chips at the frontiers of drug discovery. Nat. Rev. Drug Discov. 2015, 14, 248–260. [Google Scholar] [CrossRef]
- Ganeeva, I.; Zmievskaya, E.; Valiullina, A.; Kudriaeva, A.; Miftakhova, R.; Rybalov, A.; Bulatov, E. Recent Advances in the Development of Bioreactors for Manufacturing of Adoptive Cell Immunotherapies. Bioengineering 2022, 9, 808. [Google Scholar] [CrossRef] [PubMed]
- Sharipov, M.; Uzokboev, S.; Nghia, N.N.; Azizov, S.; Ryu, W.; Tawfik, S.M.; Lee, Y.-I. Recent progress in Arduino- and smartphone-based sensors for biochemical and environmental analysis. TrAC Trends Anal. Chem. 2025, 183, 118103. [Google Scholar] [CrossRef]
- Huang, D.; Hua, X.; Xiu, G.-L.; Zheng, Y.-J.; Yu, X.-Y.; Long, Y.-T. Secondary ion mass spectrometry: The application in the analysis of atmospheric particulate matter. Anal. Chim. Acta 2017, 989, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Buiarelli, F.; Sonego, E.; Uccelletti, D.; Bruni, E.; Di Filippo, P.; Pomata, D.; Riccardi, C.; Perrino, C.; Marcovecchio, F.; Simonetti, G. Determination of the main bioaerosol components using chemical markers by liquid chromatography–tandem mass spectrometry. Microchem. J. 2019, 149, 103974. [Google Scholar] [CrossRef]
- Fakhrulddin, S.S.; Bhatt, V.; Gharghan, S.K. A novel predictive algorithm for integrating asthma attack monitor device with smartphone. Measurement 2025, 248, 116949. [Google Scholar] [CrossRef]
- Li, P.; Xu, S.; Dong, Z.; Liu, H.; Huang, J.; Deng, X.; Tao, Y.; Liu, H.; Lin, Z.; Li, Z. Dual-function paper-based biosensor for sensitive detection of hyaluronidase and human papillomavirus DNA using diffusion wet area as readout. Biosens. Bioelectron. 2025, 271, 117087. [Google Scholar] [CrossRef]
- Bai, Y.; Fu, J.; Qin, Z.; Gao, Q.; Li, S. Integrated biosensing system of electrochemistry and electrophysiology for cortisol and skin conductance analysis on smartphone. Sens. Actuators B Chem. 2023, 394, 134368. [Google Scholar] [CrossRef]
- Zhang, L.; Lang, Z.; Lu, B.; Yang, T.; Zhang, X.; Wang, M.; Zhang, X.; Cao, H.; Ye, D. Smartphone-based colorimetric paper chip sensor using single-atom nanozyme for the detection of carbofuran pesticide residues in vegetables. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2025, 327, 125415. [Google Scholar] [CrossRef]
- Yue, X.; Li, Y.; Xu, S.; Li, J.; Li, M.; Jiang, L.; Jie, M.; Bai, Y. A portable smartphone-assisted ratiometric fluorescence sensor for intelligent and visual detection of malachite green. Food Chem. 2022, 371, 131164. [Google Scholar] [CrossRef]
- Tong, X.; Lin, X.; Duan, N.; Wang, Z.; Wu, S. Laser-Printed Paper-Based Microfluidic Chip Based on a Multicolor Fluorescence Carbon Dot Biosensor for Visual Determination of Multiantibiotics in Aquatic Products. ACS Sensors 2022, 7, 3947–3955. [Google Scholar] [CrossRef]
- Su, K.; Pan, Y.; Wan, Z.; Zhong, L.; Fang, J.; Zou, Q.; Li, H.; Wang, P. Smartphone-based portable biosensing system using cell viability biosensor for okadaic acid detection. Sens. Actuators B Chem. 2017, 251, 134–143. [Google Scholar] [CrossRef]
- Guo, J. Uric Acid Monitoring with a Smartphone as the Electrochemical Analyzer. Anal. Chem. 2016, 88, 11986–11989. [Google Scholar] [CrossRef]
- Guo, J. Smartphone-Powered Electrochemical Dongle for Point-of-Care Monitoring of Blood β-Ketone. Anal. Chem. 2017, 89, 8609–8613. [Google Scholar] [CrossRef]
- Guo, J.; Ma, X. Simultaneous monitoring of glucose and uric acid on a single test strip with dual channels. Biosens. Bioelectron. 2017, 94, 415–419. [Google Scholar] [CrossRef] [PubMed]
- White, B.J.; James Harmon, H. Novel optical solid-state glucose sensor using immobilized glucose oxidase. Biochem. Biophys. Res. Commun. 2002, 296, 1069–1071. [Google Scholar] [CrossRef]
- Deng, W.; Dou, Y.; Song, P.; Xu, H.; Aldalbahi, A.; Chen, N.; El-Sayed, N.N.; Gao, J.; Lu, J.; Song, S.; et al. Lab on smartphone with interfaced electrochemical chips for on-site gender verification. J. Electroanal. Chem. 2016, 777, 117–122. [Google Scholar] [CrossRef]
- Xu, D.; Huang, X.; Guo, J.; Ma, X. Automatic smartphone-based microfluidic biosensor system at the point of care. Biosens. Bioelectron. 2018, 110, 78–88. [Google Scholar] [CrossRef]
- Daffara, C.; Ambrosini, D. Smartphone-based diagnostics with coherent and infrared imaging for cultural heritage. J. Phys. Photonics 2024, 6, 045006. [Google Scholar] [CrossRef]
- Li, H.; Zheng, S.; Tan, Q.-G.; Zhan, L.; Martz, T.R.; Ma, J. Toward Citizen Science-Based Ocean Acidification Observations Using Smartphone Devices. Anal. Chem. 2023, 95, 15409–15417. [Google Scholar] [CrossRef]
- Oncescu, V.; Mancuso, M.; Erickson, D. Cholesterol testing on a smartphone. Lab A Chip 2014, 14, 759–763. [Google Scholar] [CrossRef]
- Beck, J.J.; Alenicheva, V.; Rahn, K.L.; Russo, M.J.; Baldo, T.A.; Henry, C.S. Evaluating the performance of an inexpensive, commercially available, NFC-powered and smartphone controlled potentiostat for electrochemical sensing. Electroanalysis 2023, 35, e202200552. [Google Scholar] [CrossRef]
- Xu, G.; Zhang, Q.; Lu, Y.; Liu, L.; Ji, D.; Li, S.; Liu, Q. Passive and wireless near field communication tag sensors for biochemical sensing with smartphone. Sens. Actuators B Chem. 2017, 246, 748–755. [Google Scholar] [CrossRef]
- Laksanasopin, T.; Guo, T.W.; Nayak, S.; Sridhara, A.A.; Xie, S.; Olowookere, O.O.; Cadinu, P.; Meng, F.; Chee, N.H.; Kim, J.; et al. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci. Transl. Med. 2015, 7, 273re1. [Google Scholar] [CrossRef]
- Sun, A.C.; Yao, C.; Hall, D.A. An efficient power harvesting mobile phone-based electrochemical biosensor for point-of-care health monitoring. Sens. Actuators B Chem. 2016, 235, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Mesas Gómez, M.; Julián, E.; Armengou, L.; Pividori, M.I. Evaluating smartphone-based optical readouts for immunoassays in human and veterinary healthcare: A comparative study. Talanta 2024, 275, 126106. [Google Scholar] [CrossRef]
- Yang, C.; Wang, Z.; Xiao, K.; Ushakov, N.; Kumar, S.; Li, X.; Min, R. Portable optical fiber biosensors integrated with smartphone: Technologies, applications, and challenges [Invited]. Biomed. Opt. Express 2024, 15, 1630–1650. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-M.; Kim, S.-W.; Jeon, J.-W.; Kim, J.-H.; Kim, H.-S.; Cho, J.-H.; Lee, W.-H.; Paek, S.-H. Food contamination monitoring via internet of things, exemplified by using pocket-sized immunosensor as terminal unit. Sens. Actuators B Chem. 2016, 233, 148–156. [Google Scholar] [CrossRef]
- Song, J.; Mauk, M.G.; Hackett, B.A.; Cherry, S.; Bau, H.H.; Liu, C. Instrument-Free Point-of-Care Molecular Detection of Zika Virus. Anal. Chem. 2016, 88, 7289–7294. [Google Scholar] [CrossRef]
- Giavazzi, F.; Salina, M.; Ceccarello, E.; Ilacqua, A.; Damin, F.; Sola, L.; Chiari, M.; Chini, B.; Cerbino, R.; Bellini, T.; et al. A fast and simple label-free immunoassay based on a smartphone. Biosens. Bioelectron. 2014, 58, 395–402. [Google Scholar] [CrossRef]
- Barbosa, A.I.; Gehlot, P.; Sidapra, K.; Edwards, A.D.; Reis, N.M. Portable smartphone quantitation of prostate specific antigen (PSA) in a fluoropolymer microfluidic device. Biosens. Bioelectron. 2015, 70, 5–14. [Google Scholar] [CrossRef]
- Dou, Y.; Jiang, Z.; Deng, W.; Su, J.; Chen, S.; Song, H.; Aldalbahi, A.; Zuo, X.; Song, S.; Shi, J.; et al. Portable detection of clenbuterol using a smartphone-based electrochemical biosensor with electric field-driven acceleration. J. Electroanal. Chem. 2016, 781, 339–344. [Google Scholar] [CrossRef]
- Su, K.; Qiu, X.; Fang, J.; Zou, Q.; Wang, P. An improved efficient biochemical detection method to marine toxins with a smartphone-based portable system—Bionic e-Eye. Sens. Actuators B Chem. 2017, 238, 1165–1172. [Google Scholar] [CrossRef]
- Sami, M.A.; Tayyab, M.; Hassan, U. Excitation modalities for enhanced micro and nanoparticle imaging in a smartphone coupled 3D printed fluorescent microscope. Lab A Chip 2022, 22, 3755–3769. [Google Scholar] [CrossRef]
- Park, Y.M.; Han, Y.D.; Chun, H.J.; Yoon, H.C. Ambient light-based optical biosensing platform with smartphone-embedded illumination sensor. Biosens. Bioelectron. 2017, 93, 205–211. [Google Scholar] [CrossRef]
- Benjamin, E.M. Self-Monitoring of Blood Glucose: The Basics. Clin. Diabetes 2002, 20, 45–47. [Google Scholar] [CrossRef]
- Aliyeva, N.; Saylan, Y.; Denizli, A. Chapter 26—Smartphone-based point-of-care device. In Advanced Sensors for Smart Healthcare; Nguyen, T.A., Ed.; Elsevier: Amsterdam, The Netherlands, 2025; pp. 437–454. [Google Scholar]
- Zahra, Q.u.a.; Mohsan, S.A.H.; Shahzad, F.; Qamar, M.; Qiu, B.; Luo, Z.; Zaidi, S.A. Progress in smartphone-enabled aptasensors. Biosens. Bioelectron. 2022, 215, 114509. [Google Scholar] [CrossRef]
- Beduk, T.; Beduk, D.; Hasan, M.R.; Guler Celik, E.; Kosel, J.; Narang, J.; Salama, K.N.; Timur, S. Smartphone-Based Multiplexed Biosensing Tools for Health Monitoring. Biosensors 2022, 12, 583. [Google Scholar] [CrossRef]
- Zhang, Q.; Li, Y.; Hu, Q.; Xie, R.; Zhou, W.; Liu, X.; Wang, Y. Smartphone surface plasmon resonance imaging for the simultaneous and sensitive detection of acute kidney injury biomarkers with noninvasive urinalysis. Lab A Chip 2022, 22, 4941–4949. [Google Scholar] [CrossRef]
- Sreenan, B.; Gulbag, A.; Kafil, V.; Kharal, G.; Lee, B.; Zhu, X. Smartphone-based Devices for Biomarkers. In Sensing Materials and Devices for Biomarkers; Mutreja, V., Kathuria, D., Sareen, S., Park, J., Eds.; Royal Society of Chemistry: London, UK, 2024; Volume 28, pp. 191–227. [Google Scholar]
- Soni, A.; Jha, S.K. Smartphone based non-invasive salivary glucose biosensor. Anal. Chim. Acta 2017, 996, 54–63. [Google Scholar] [CrossRef]
- Ferris, M.; Zabow, G. Quantitative, high-sensitivity measurement of liquid analytes using a smartphone compass. Nat. Commun. 2024, 15, 2801. [Google Scholar] [CrossRef]
- Zhang, J.; An, J.; Han, Y.; Fang, J.; Liu, Y. Spermine induced in-situ synthesis of polychromatic carbon nanodots towards smartphone-readable ratiometric fluorescence sensing of multiple catecholamines. Talanta 2025, 285, 127292. [Google Scholar] [CrossRef]
- Promsuwan, K.; Soleh, A.; Samoson, K.; Saisahas, K.; Wangchuk, S.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Limbut, W. Novel biosensor platform for glucose monitoring via smartphone based on battery-less NFC potentiostat. Talanta 2023, 256, 124266. [Google Scholar] [CrossRef] [PubMed]
- Garjonyte, R.; Malinauskas, A. Glucose biosensor based on glucose oxidase immobilized in electropolymerized polypyrrole and poly(o-phenylenediamine) films on a Prussian Blue-modified electrode. Sens. Actuators B Chem. 2000, 63, 122–128. [Google Scholar] [CrossRef]
- Pananon, P.; Sriprachuabwong, C.; Wisitsoraat, A.; Chuysinuan, P.; Tuantranont, A.; Saparpakorn, P.; Dechtrirat, D. A facile one-pot green synthesis of gold nanoparticle-graphene-PEDOT:PSS nanocomposite for selective electrochemical detection of dopamine. RSC Adv. 2018, 8, 12724–12732. [Google Scholar] [CrossRef]
- Samoson, K.; Soleh, A.; Saisahas, K.; Promsuwan, K.; Saichanapan, J.; Kanatharana, P.; Thavarungkul, P.; Chang, K.H.; Lim Abdullah, A.F.; Tayayuth, K.; et al. Facile fabrication of a flexible laser induced gold nanoparticle/chitosan/ porous graphene electrode for uric acid detection. Talanta 2022, 243, 123319. [Google Scholar] [CrossRef]
- Uzunçar, S.; Kaç, H.; Ak, M. Electro-templating of prussian blue nanoparticles in PEDOT:PSS and soluble silkworm protein for hydrogen peroxide sensing. Talanta 2023, 252, 123841. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Yang, J.; Li, Y.; Lu, C.; Hao, Y.; He, G.; Zhang, Y.; Song, Q.; Long, J.; et al. Smartphone-based urine colourimetric assay for home self-screening of HPV infection. Spectrochim. Acta. A. Mol. Biomol. Spectrosc. 2025, 334, 125923. [Google Scholar] [CrossRef] [PubMed]
- Ghaderinia, M.; Abadijoo, H.; Mahdavian, A.; Kousha, E.; Shakibi, R.; Taheri, S.M.-R.; Simaee, H.; Khatibi, A.; Moosavi-Movahedi, A.A.; Khayamian, M.A. Smartphone-based device for point-of-care diagnostics of pulmonary inflammation using convolutional neural networks (CNNs). Sci. Rep. 2024, 14, 6912. [Google Scholar] [CrossRef]
- Hasan, M.R.; Sharma, P.; Singh, S.; Narang, J. Smartphone-Integrated Wireless Portable Potentiostat to Develop 5th-Generation Dengue Pocket Aptasensor toward Portronicx-Approach. ACS Appl. Bio Mater. 2024, 7, 2299–2308. [Google Scholar] [CrossRef]
- Ma, L.; Yin, L.; Li, X.; Chen, S.; Peng, L.; Liu, G.; Ye, S.; Zhang, W.; Man, S. A smartphone-based visual biosensor for CRISPR-Cas powered SARS-CoV-2 diagnostics. Biosens. Bioelectron. 2022, 195, 113646. [Google Scholar] [CrossRef] [PubMed]
- Adrover-Jaume, C.; Alba-Patiño, A.; Clemente, A.; Santopolo, G.; Vaquer, A.; Russell, S.M.; Barón, E.; González del Campo, M.d.M.; Ferrer, J.M.; Berman-Riu, M.; et al. Paper biosensors for detecting elevated IL-6 levels in blood and respiratory samples from COVID-19 patients. Sens. Actuators B Chem. 2021, 330, 129333. [Google Scholar] [CrossRef] [PubMed]
- Huang, D.; Zhao, Y.; Fang, M.; Shen, P.; Xu, H.; He, Y.; Chen, S.; Si, Z.; Xu, Z. Magnetofluid-integrated biosensors based on DNase-dead Cas12a for visual point-of-care testing of HIV-1 by an up and down chip. Lab A Chip 2023, 23, 4265–4275. [Google Scholar] [CrossRef]
- Lee, J.-H.; Hwang, Y.; Cheon, K.-A.; Jung, H.-I. Emotion-on-a-chip (EOC): Evolution of biochip technology to measure human emotion using body fluids. Med. Hypotheses 2012, 79, 827–832. [Google Scholar] [CrossRef]
- Erickson, D.; O’Dell, D.; Jiang, L.; Oncescu, V.; Gumus, A.; Lee, S.; Mancuso, M.; Mehta, S. Smartphone technology can be transformative to the deployment of lab-on-chip diagnostics. Lab A Chip 2014, 14, 3159–3164. [Google Scholar] [CrossRef]
- Torous, J.; Powell, A.C. Current research and trends in the use of smartphone applications for mood disorders. Internet Interv. 2015, 2, 169–173. [Google Scholar] [CrossRef]
- Vashist, S.K.; van Oordt, T.; Schneider, E.M.; Zengerle, R.; von Stetten, F.; Luong, J.H.T. A smartphone-based colorimetric reader for bioanalytical applications using the screen-based bottom illumination provided by gadgets. Biosens. Bioelectron. 2015, 67, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Tang, R.; Yang, H.; Choi, J.R.; Gong, Y.; Hu, J.; Feng, S.; Pingguan-Murphy, B.; Mei, Q.; Xu, F. Improved sensitivity of lateral flow assay using paper-based sample concentration technique. Talanta 2016, 152, 269–276. [Google Scholar] [CrossRef]
- Yang, J.-S.; Shin, J.; Choi, S.; Jung, H.-I. Smartphone Diagnostics Unit (SDU) for the assessment of human stress and inflammation level assisted by biomarker ink, fountain pen, and origami holder for strip biosensor. Sens. Actuators B Chem. 2017, 241, 80–84. [Google Scholar] [CrossRef]
- Farina, M.P.; Zhang, Z.; Donnelly, R. Anticipatory stress, state policy contexts, and mental health during the COVID-19 pandemic. SSM-Popul. Health 2023, 23, 101415. [Google Scholar] [CrossRef]
- Nuryana, Z.; Xu, W.; Kurniawan, L.; Sutanti, N.; Makruf, S.A.; Nurcahyati, I. Student stress and mental health during online learning: Potential for post-COVID-19 school curriculum development. Compr. Psychoneuroendocrinology 2023, 14, 100184. [Google Scholar] [CrossRef]
- Wu, T.; Jia, X.; Shi, H.; Niu, J.; Yin, X.; Xie, J.; Wang, X. Prevalence of mental health problems during the COVID-19 pandemic: A systematic review and meta-analysis. J. Affect. Disord. 2021, 281, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Grover, S.; Dua, D.; Sahoo, S.; Mehra, A.; Nehra, R.; Chakrabarti, S. Why all COVID-19 hospitals should have mental health professionals: The importance of mental health in a worldwide crisis! Asian J Psychiatr 2020, 51, 102147. [Google Scholar] [CrossRef] [PubMed]
- Qiu, Y.; Wu, Q.; Chen, R.; Guan, C. Research on psychological stress and mental health of medical staff in COVID-19 prevention and control. Int. J. Disaster Risk Reduct. 2021, 65, 102524. [Google Scholar] [CrossRef] [PubMed]
- Esler, M. Mental stress and human cardiovascular disease. Neurosci Biobehav Rev 2017, 74, 269–276. [Google Scholar] [CrossRef]
- Sloover, M.; van Est, L.A.C.; Janssen, P.G.J.; Hilbink, M.; van Ee, E. A meta-analysis of mentalizing in anxiety disorders, obsessive-compulsive and related disorders, and trauma and stressor related disorders. J. Anxiety Disord. 2022, 92, 102641. [Google Scholar] [CrossRef]
- Yang, K.; Zhang, T.; Ananiadou, S. A mental state Knowledge–aware and Contrastive Network for early stress and depression detection on social media. Inf. Process. Manag. 2022, 59, 102961. [Google Scholar] [CrossRef]
- Billeci, L.; Brunori, E.; Crifaci, G.; Tartarisco, G.; Scardigli, S.; Pioggia, G.; Maestro, G.; Morales, M.A. Skin conductance (SC) monitoring during relaxation in anorexia nervosa adolescents by wearable sensors combined with wireless technologies. Int. J. Psychophysiol. 2012, 85, 373–374. [Google Scholar] [CrossRef]
- Chugh, V.; Basu, A.; Kaushik, A.; Basu, A.K. E-skin–Based advanced wearable technology for Health Management. Curr. Res. Biotechnol. 2023, 5, 100129. [Google Scholar] [CrossRef]
- Madrid, R.E.; Ashur Ramallo, F.; Barraza, D.E.; Chaile, R.E. Smartphone-Based Biosensor Devices for Healthcare: Technologies, Trends, and Adoption by End-Users. Bioengineering 2022, 9, 101. [Google Scholar] [CrossRef]
- Manzocchi, S.; Furman, E.; Freeman, K. Comparison of 3 options for choosing control limits in biochemistry testing. Vet. Clin. Pathol. 2017, 46, 120–125. [Google Scholar] [CrossRef]
- Sacks, D.A.; Chen, W.; Wolde-Tsadik, G.; Buchanan, T.A. Fasting Plasma Glucose Test at the First Prenatal Visit as a Screen for Gestational Diabetes. Obstet. Gynecol. 2003, 101. [Google Scholar]
- Warren, B.; Lee, A.K.; Ballantyne, C.; Hoogeveen, R.; Pankow, J.S.; Kottgen, A.; Selvin, E. Abstract MP018: Performance of 1,5-anhydroglucitol Compared to the Oral Glucose Tolerance Test and Fasting Glucose for Identification of Diabetes in the Community. Circulation 2017, 135, AMP018. [Google Scholar] [CrossRef]
- Joh, D.Y.; Hucknall, A.M.; Wei, Q.; Mason, K.A.; Lund, M.L.; Fontes, C.M.; Hill, R.T.; Blair, R.; Zimmers, Z.; Achar, R.K.; et al. Inkjet-printed point-of-care immunoassay on a nanoscale polymer brush enables subpicomolar detection of analytes in blood. Proc. Natl. Acad. Sci. USA 2017, 114, E7054–E7062. [Google Scholar] [CrossRef] [PubMed]
- Mulberry, G.; White, K.A.; Vaidya, M.; Sugaya, K.; Kim, B.N. 3D printing and milling a real-time PCR device for infectious disease diagnostics. PLoS ONE 2017, 12, e0179133. [Google Scholar] [CrossRef] [PubMed]
- Cocker, C.; Minnis, H.; Sweeting, H. Potential value of the current mental health monitoring of children in state care in England. BJPsych Open 2018, 4, 486–491. [Google Scholar] [CrossRef]
- Doom, J.R.; Peckins, M.K.; Hein, T.C.; Dotterer, H.L.; Mitchell, C.; Lopez-Duran, N.L.; Brooks-Gunn, J.; McLanahan, S.; Hyde, L.W.; Abelson, J.L.; et al. Differential associations of parental harshness and parental disengagement with overall cortisol output at 15 years: Implications for adolescent mental health. Dev. Psychopathol. 2022, 34, 129–146. [Google Scholar] [CrossRef]
- Zhao, F.; He, J.; Li, X.; Bai, Y.; Ying, Y.; Ping, J. Smart plant-wearable biosensor for in-situ pesticide analysis. Biosens. Bioelectron. 2020, 170, 112636. [Google Scholar] [CrossRef]
- Zhang, C.; Guanming, S.; Shen, J.; Hu, R.-f. Productivity effect and overuse of pesticide in crop production in China. J. Integr. Agric. 2015, 14, 1903–1910. [Google Scholar] [CrossRef]
- Li, S.; Wu, X.; Liu, C.; Yin, G.; Luo, J.; Xu, Z. Application of DNA aptamers as sensing layers for detection of carbofuran by electrogenerated chemiluminescence energy transfer. Anal. Chim. Acta 2016, 941, 94–100. [Google Scholar] [CrossRef]
- Oliveira, T.M.B.F.; Ribeiro, F.W.P.; Sousa, C.P.; Salazar-Banda, G.R.; de Lima-Neto, P.; Correia, A.N.; Morais, S. Current overview and perspectives on carbon-based (bio)sensors for carbamate pesticides electroanalysis. TrAC Trends Anal. Chem. 2020, 124, 115779. [Google Scholar] [CrossRef]
- Agarwal, T.; Borrelli, M.R.; Makvandi, P.; Ashrafizadeh, M.; Maiti, T.K. Paper-Based Cell Culture: Paving the Pathway for Liver Tissue Model Development on a Cellulose Paper Chip. ACS Appl. Bio Mater. 2020, 3, 3956–3974. [Google Scholar] [CrossRef]
- Khan, S.M.; Nassar, J.M.; Hussain, M.M. Paper as a Substrate and an Active Material in Paper Electronics. ACS Appl. Electron. Mater. 2021, 3, 30–52. [Google Scholar] [CrossRef]
- Li, D.; Duan, H.; Ma, Y.; Deng, W. Headspace-Sampling Paper-Based Analytical Device for Colorimetric/Surface-Enhanced Raman Scattering Dual Sensing of Sulfur Dioxide in Wine. Anal. Chem. 2018, 90, 5719–5727. [Google Scholar] [CrossRef]
- Cao, J.; Wang, M.; She, Y.; Abd El-Aty, A.M.; Hacımüftüoğlu, A.; Wang, J.; Yan, M.; Hong, S.; Lao, S.; Wang, Y. Rapid colorimetric determination of the pesticides carbofuran and dichlorvos by exploiting their inhibitory effect on the aggregation of peroxidase-mimicking platinum nanoparticles. Mikrochim. Acta 2019, 186, 390. [Google Scholar] [CrossRef]
- Hu, Y.; Gao, Z.; Luo, J. Fluorescence detection of malachite green in fish tissue using red emissive Se,N,Cl-doped carbon dots. Food Chem. 2021, 335, 127677. [Google Scholar] [CrossRef]
- Luo, S.; Yan, G.; Sun, X. Molecular imprinting based on phosphorescent resonance energy transfer for malachite green detection in fishes and water. New J. Chem. 2018, 42, 9510–9516. [Google Scholar] [CrossRef]
- Abbasi, A.; Hanif, S.; Shakir, M. Gum acacia-based silver nanoparticles as a highly selective and sensitive dual nanosensor for Hg(ii) and fluorescence turn-off sensor for S2− and malachite green detection. RSC Advances 2020, 10, 3137–3144. [Google Scholar] [CrossRef] [PubMed]
- Ju, Y.J.; Li, N.; Liu, S.G.; Han, L.; Xiao, N.; Luo, H.Q.; Li, N.B. Ratiometric fluorescence method for malachite green detection based on dual-emission BSA-protected gold nanoclusters. Sens. Actuators B Chem. 2018, 275, 244–250. [Google Scholar] [CrossRef]
- Niu, X.; Yang, J.; Ma, J.-F. Ni/MoN nanoparticles embedded with mesoporous carbon as a high-efficiency electrocatalyst for detection of nitroimidazole antibiotics. Sens. Actuators B Chem. 2023, 387, 133819. [Google Scholar] [CrossRef]
- Qi, H.; Qiu, L.; Zhang, X.; Yi, T.; Jing, J.; Sami, R.; Alanazi, S.F.; Alqahtani, Z.; Aljabri, M.D.; Rahman, M.M. Novel N-doped carbon dots derived from citric acid and urea: Fluorescent sensing for determination of metronidazole and cytotoxicity studies. RSC Adv. 2023, 13, 2663–2671. [Google Scholar] [CrossRef]
- Simsir, E.A.; Erdemir, S.; Tabakci, M.; Tabakci, B. Nano-scale selective and sensitive optical sensor for metronidazole based on fluorescence quenching: 1H-Phenanthro[9,10-d]imidazolyl-calix[4]arene fluorescent probe. Anal. Chim. Acta 2021, 1162, 338494. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Glennon, L.; Fenelon, O.; Breslin, C.B. Electrodeposition of bismuth at a graphene modified carbon electrode and its application as an easily regenerated sensor for the electrochemical determination of the antimicrobial drug metronidazole. Talanta 2023, 251, 123758. [Google Scholar] [CrossRef] [PubMed]
- Sun, Q.; Fu, L.; Yin, C.; Wu, M.; Liu, H.; Niu, N.; Chen, L. Construction of biomass carbon dots@molecularly imprinted polymer fluorescent sensor array for accurate identification of 5-nitroimidazole antibiotics. Sens. Actuators B Chem. 2022, 373, 132716. [Google Scholar] [CrossRef]
- Xu, X.; Zhao, W.; Ji, B.; Han, Y.; Xu, G.; Jie, M.; Wu, N.; Wu, Y.; Li, J.; Li, K.; et al. Application of silanized melamine sponges in matrix purification for rapid multi-residue analysis of veterinary drugs in eggs by UPLC-MS/MS. Food Chem. 2022, 369, 130894. [Google Scholar] [CrossRef]
- Lin, Y.; Su, Y.; Liao, X.; Yang, N.; Yang, X.; Choi, M.M. Determination of five nitroimidazole residues in artificial porcine muscle tissue samples by capillary electrophoresis. Talanta 2012, 88, 646–652. [Google Scholar] [CrossRef]
- Moreno-González, D.; Lara, F.J.; Jurgovská, N.; Gámiz-Gracia, L.; García-Campaña, A.M. Determination of aminoglycosides in honey by capillary electrophoresis tandem mass spectrometry and extraction with molecularly imprinted polymers. Anal. Chim. Acta 2015, 891, 321–328. [Google Scholar] [CrossRef]
- Wang, S.; Wang, Y.; Ning, Y.; Wang, W.; Liu, Q. Multicolor emissive carbon dot-based fluorometric analysis platform for rapid quantification and discrimination of nitroimidazole antibiotic residues. Talanta 2024, 271, 125679. [Google Scholar] [CrossRef]
- Naik, L.; Sharma, R.; Mann, B.; Lata, K.; Rajput, Y.S.; Surendra Nath, B. Rapid screening test for detection of oxytetracycline residues in milk using lateral flow assay. Food Chem. 2017, 219, 85–92. [Google Scholar] [CrossRef]
- Zhou, X.; Fu, S.; Li, J.; Yi, Y.; Hu, Y.; Lu, J.; Yang, C.; Miao, J.; Xu, Y. Smartphone-based pH responsive 3-channel colorimetric biosensor for non-enzymatic multi-antibiotic residues. Food Chem. 2023, 429, 136953. [Google Scholar] [CrossRef]
- Chen, J.; Sun, R.; Pan, C.; Sun, Y.; Mai, B.; Li, Q.X. Antibiotics and Food Safety in Aquaculture. J. Agric. Food Chem. 2020, 68, 11908–11919. [Google Scholar] [CrossRef]
- Shah, B.R.; Mraz, J. Advances in nanotechnology for sustainable aquaculture and fisheries. Rev. Aquac. 2020, 12, 925–942. [Google Scholar] [CrossRef]
- Qin, S.; Sun, X.; Zhao, X. Advances in smartphone-based biosensors for food testing. Curr. Opin. Food Sci. 2025, 61, 101236. [Google Scholar] [CrossRef]
- Dzantiev, B.B.; V, Z.A.; G, R.O.; Sapegova, L.A. Development and Comparative Study of Different Immunoenzyme Techniques for Pesticides Detection. Int. J. Environ. Anal. Chem. 1996, 65, 95–111. [Google Scholar] [CrossRef]
- Zhang, H.-X.; Wei, R.-B.; Chen, C.-Z.; Tuo, X.-L.; Wang, X.-G. A novel fluorescent epoxy resin for organophosphate pesticide detection. Chin. Chem. Lett. 2015, 26, 39–42. [Google Scholar] [CrossRef]
- Balizs, G.; Hewitt, A. Determination of veterinary drug residues by liquid chromatography and tandem mass spectrometry. Anal. Chim. Acta 2003, 492, 105–131. [Google Scholar] [CrossRef]
- Wittenberg, J.B.; Simon, K.A.; Wong, J.W. Targeted Multiresidue Analysis of Veterinary Drugs in Milk-Based Powders Using Liquid Chromatography–Tandem Mass Spectrometry (LC-MS/MS). J. Agric. Food Chem. 2017, 65, 7288–7293. [Google Scholar] [CrossRef]
- Majdinasab, M.; Mishra, R.K.; Tang, X.; Marty, J.L. Detection of antibiotics in food: New achievements in the development of biosensors. TrAC Trends Anal. Chem. 2020, 127, 115883. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Liang, L. Recent development of antibiotic detection in food and environment: The combination of sensors and nanomaterials. Microchim. Acta 2021, 188, 21. [Google Scholar] [CrossRef]
- Li, H.; Fang, T.; Tan, Q.-G.; Ma, J. Development of a versatile smartphone-based environmental analyzer (vSEA) and its application in on-site nutrient detection. Sci. Total Environ. 2022, 838, 156197. [Google Scholar] [CrossRef]
- Sassolas, A.; Hayat, A.; Catanante, G.; Marty, J.-L. Detection of the marine toxin okadaic acid: Assessing seafood safety. Talanta 2013, 105, 306–316. [Google Scholar] [CrossRef]
- Kim, S.; Sosnowski, K.; Hwang, D.S.; Yoon, J.-Y. Smartphone-Based Microalgae Monitoring Platform Using Machine Learning. ACS EST Eng. 2024, 4, 186–195. [Google Scholar] [CrossRef]
- Rajamanikandan, R.; Ilanchelian, M.; Ju, H. Smartphone-enabled colorimetric visual quantification of highly hazardous trivalent chromium ions in environmental waters and catalytic reduction of p-nitroaniline by thiol-functionalized gold nanoparticles. Chemosphere 2023, 340, 139838. [Google Scholar] [CrossRef]
- Buttner Mark, P.; Cruz-Perez, P.; Stetzenbach Linda, D. Enhanced Detection of Surface-Associated Bacteria in Indoor Environments by Quantitative PCR. Appl. Environ. Microbiol. 2001, 67, 2564–2570. [Google Scholar] [CrossRef] [PubMed]
- Jolly, C.; Katsiadaki, I.; Le Belle, N.; Mayer, I.; Dufour, S. Development of a stickleback kidney cell culture assay for the screening of androgenic and anti-androgenic endocrine disrupters. Aquat. Toxicol. 2006, 79, 158–166. [Google Scholar] [CrossRef] [PubMed]
- Ollson, C.J.; Smith, E.; Herde, P.; Juhasz, A.L. Influence of co-contaminant exposure on the absorption of arsenic, cadmium and lead. Chemosphere 2017, 168, 658–666. [Google Scholar] [CrossRef] [PubMed]
- Abd-Elhakim, Y.M.; Sharkawy, N.I.E.; Moustafa, G.G. An investigation of selected chemical contaminants in commercial pet foods in Egypt. J. Vet. Diagn. Investig. 2016, 28, 70–75. [Google Scholar] [CrossRef]
- Li, G.; Xu, L.; Wu, W.; Wang, D.; Jiang, J.; Chen, X.; Zhang, W.; Poapolathep, S.; Poapolathep, A.; Zhang, Z.; et al. On-Site Ultrasensitive Detection Paper for Multiclass Chemical Contaminants via Universal Bridge-Antibody Labeling: Mycotoxin and Illegal Additives in Milk as an Example. Anal. Chem. 2019, 91, 1968–1973. [Google Scholar] [CrossRef]
- Villa, C.A.; Finlayson, S.; Limpus, C.; Gaus, C. A multi-element screening method to identify metal targets for blood biomonitoring in green sea turtles (Chelonia mydas). Sci. Total Environ. 2015, 512–513, 613–621. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).