Trimethylamine Gas Sensor Based on Electrospun In2O3 Nanowires with Different Grain Sizes for Fish Freshness Monitoring
Abstract
1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis of In2O3 Nanowires
2.3. Characterization
2.4. Gas Sensor Fabrication and Test
3. Results and Discussion
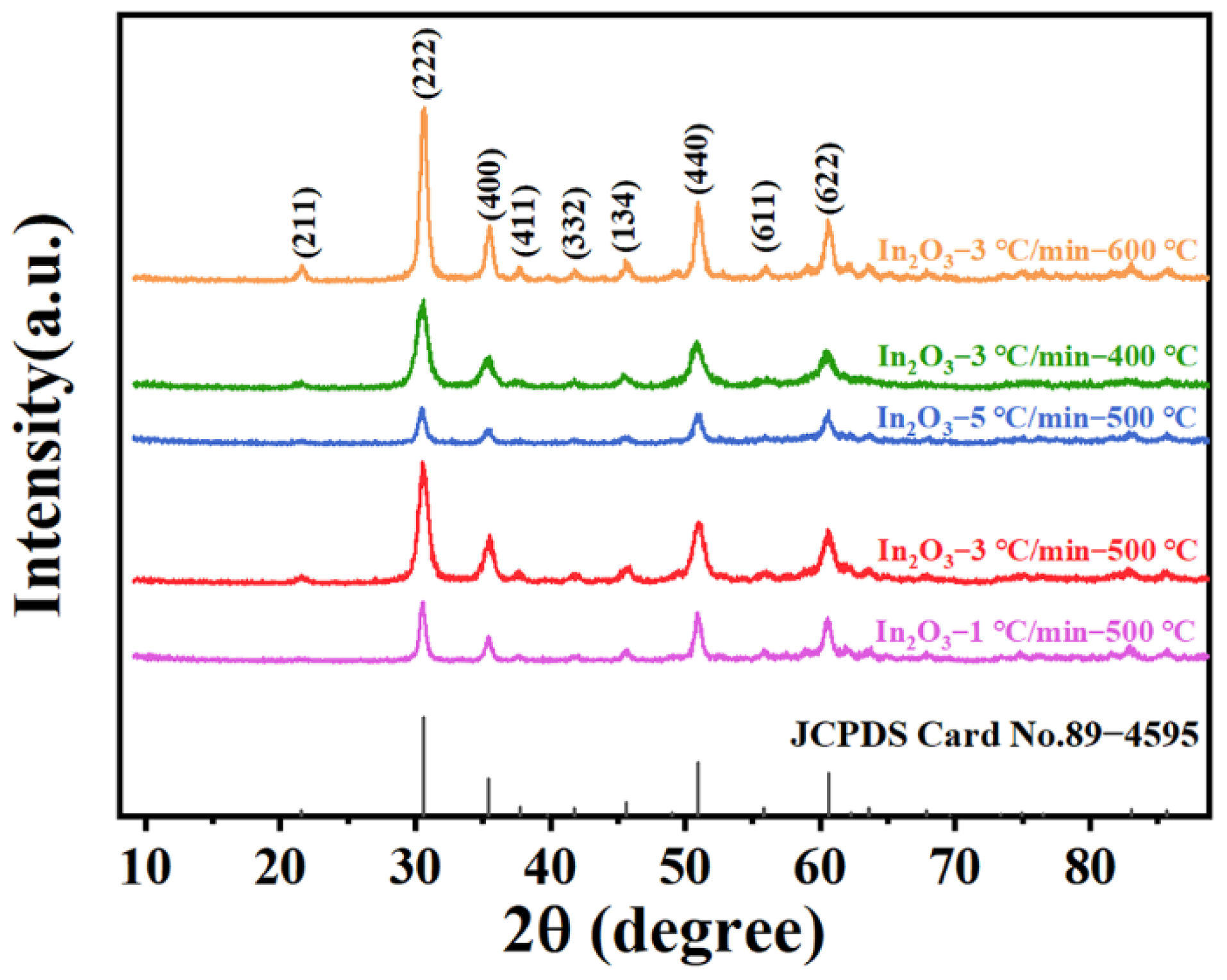
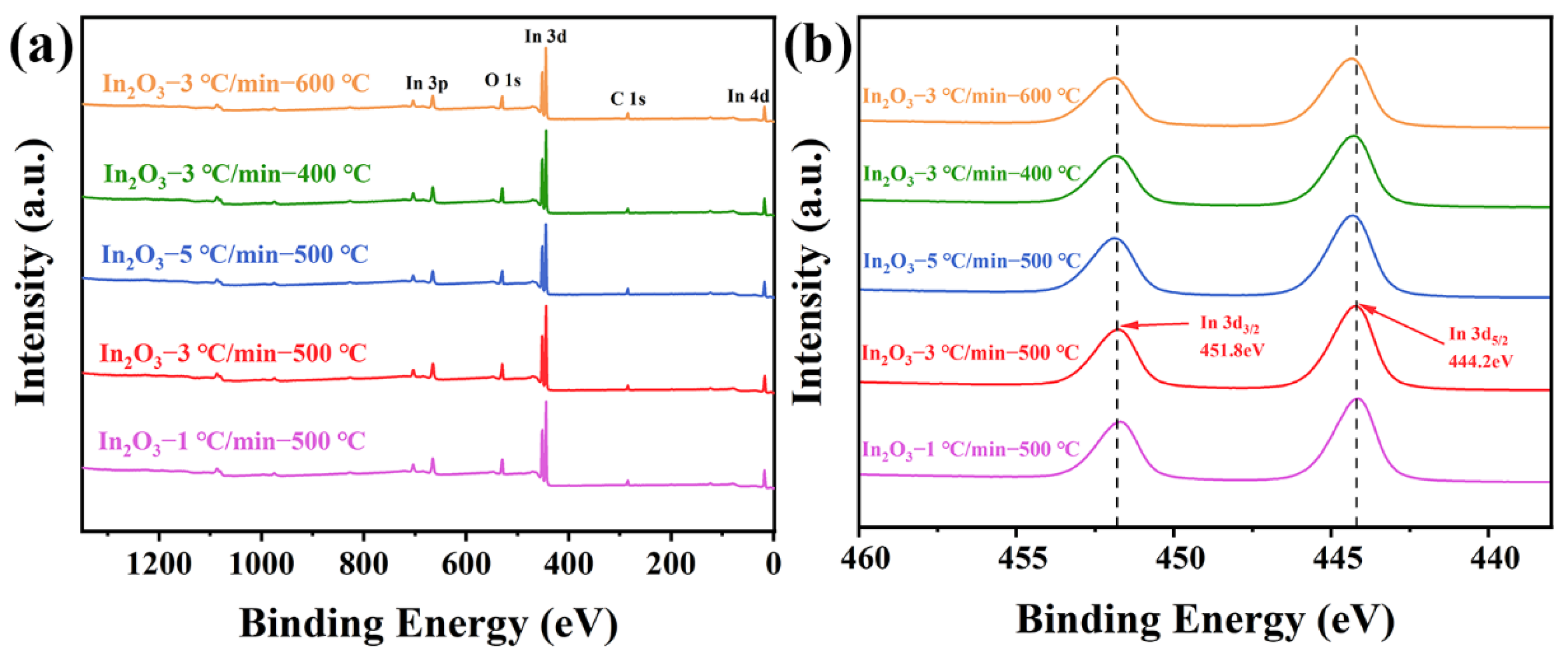
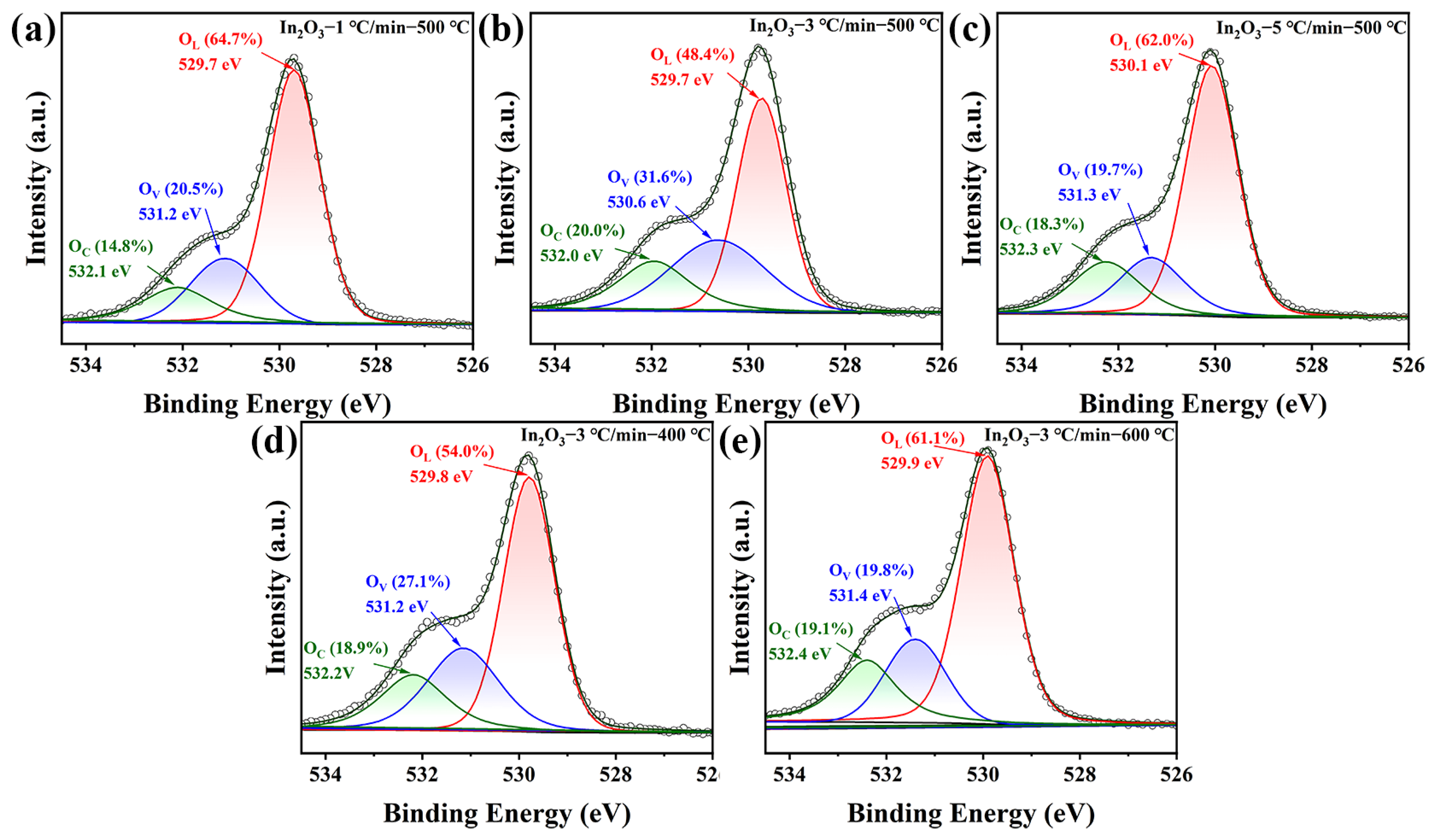
3.1. Structural and Morphological Features
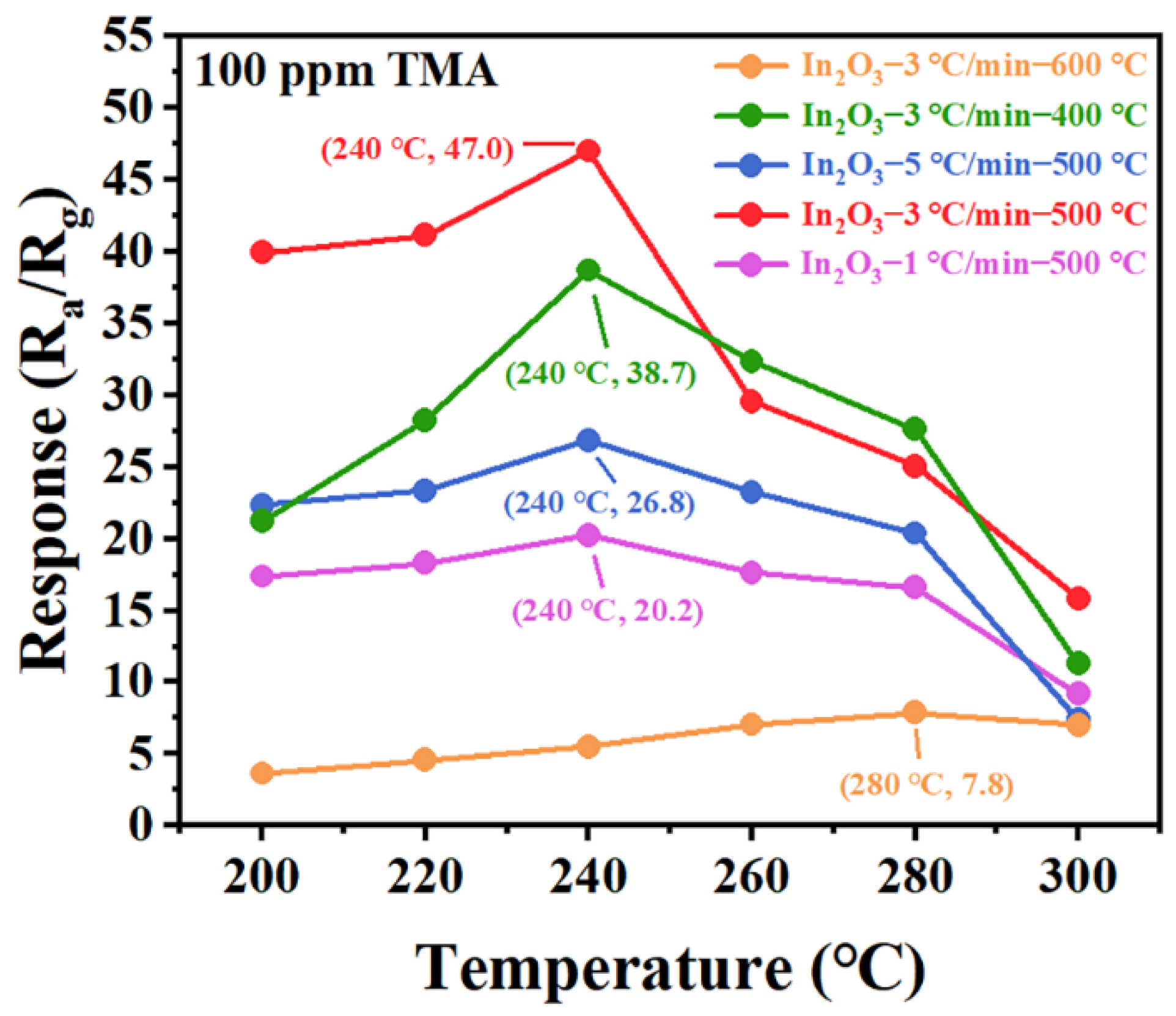
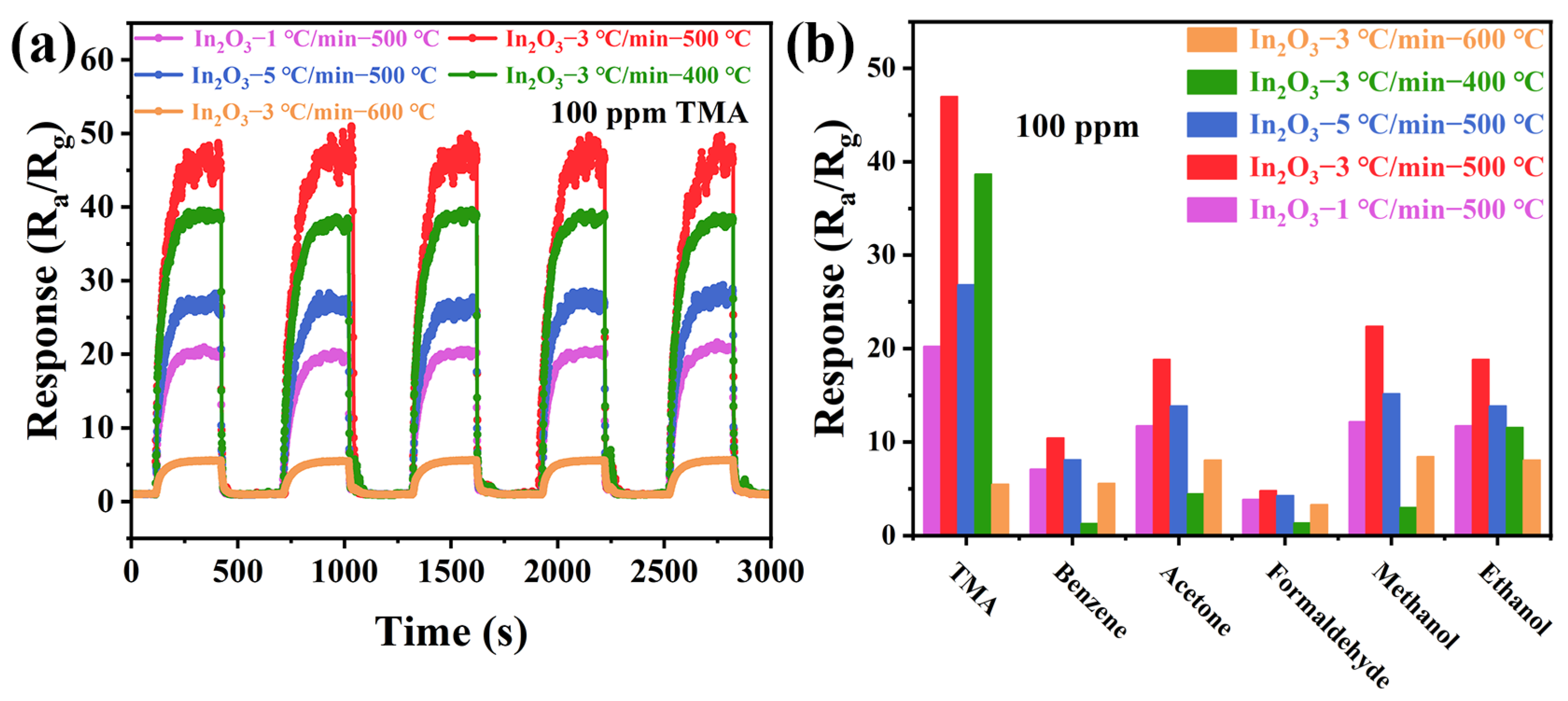
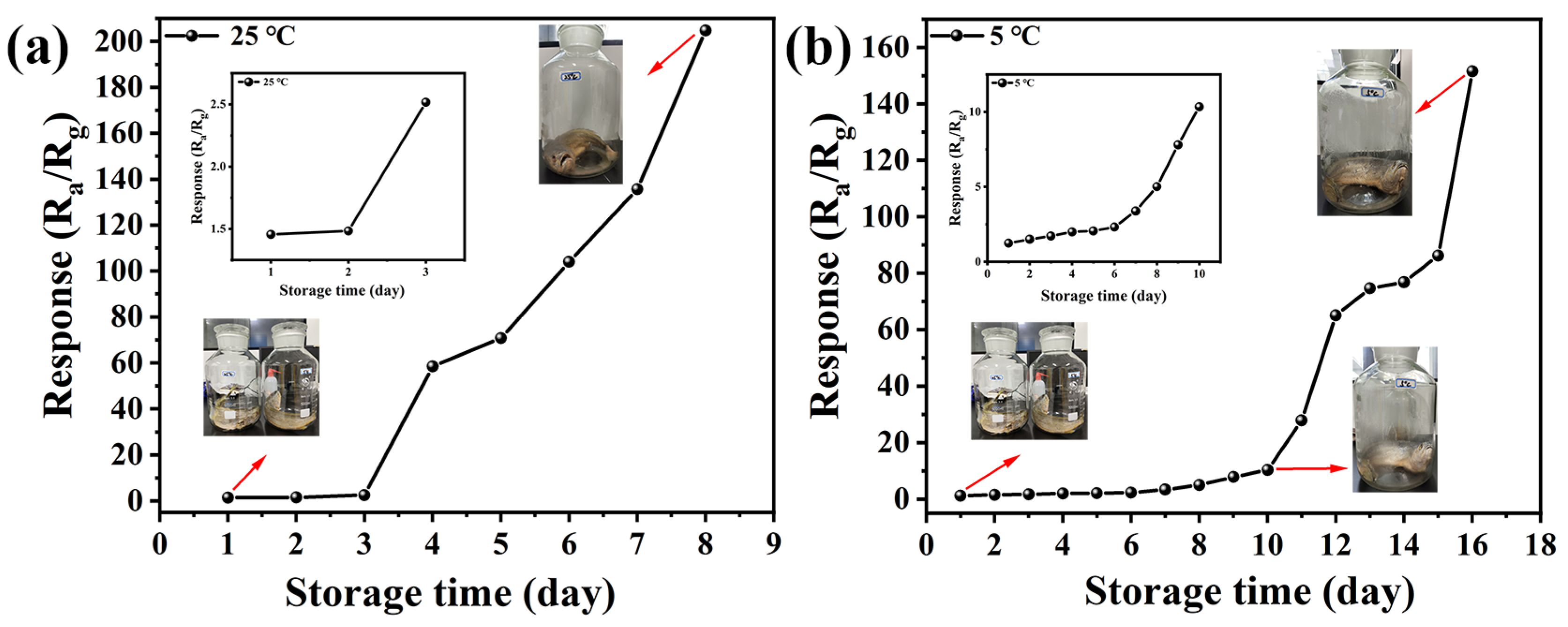
3.2. Gas-Sensing Properties
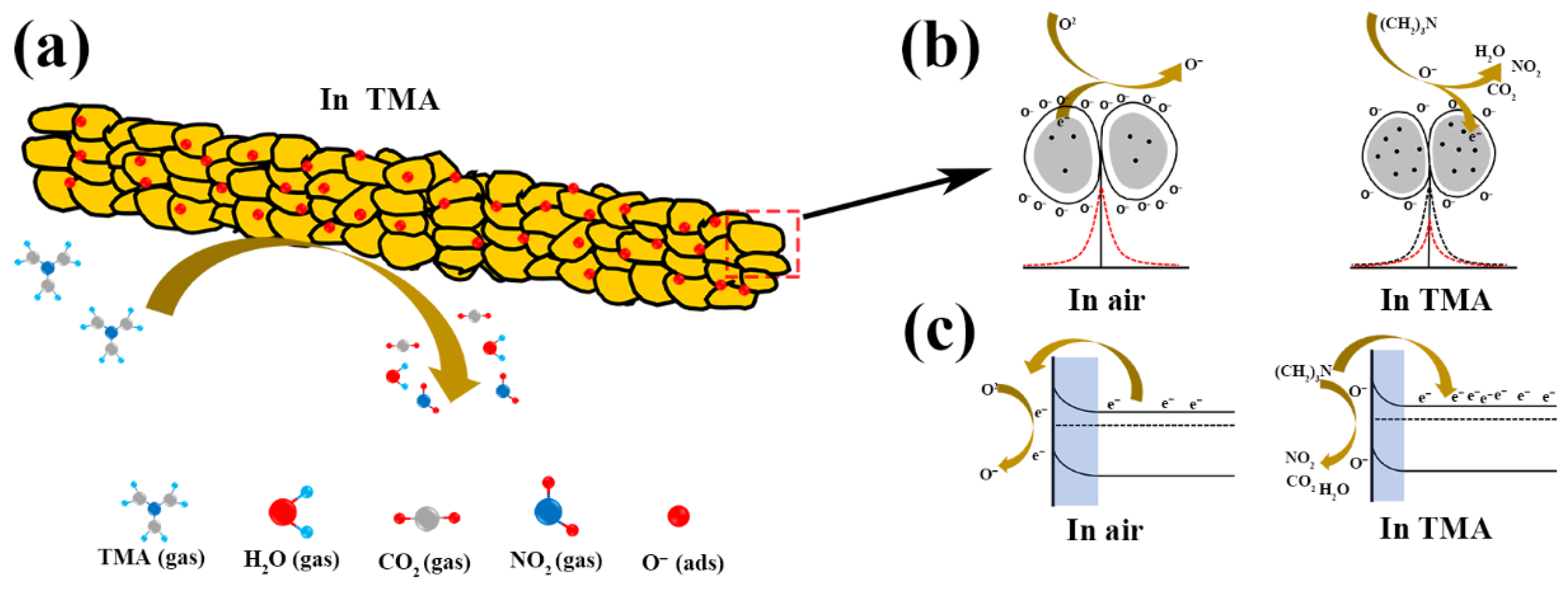
3.3. Gas-Sensing Mechanism
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Weichselbaum, E.; Coe, S.; Buttriss, J.; Stanner, S. Fish in the diet: A review. Nutr. Bull. 2013, 38, 128–177. [Google Scholar] [CrossRef]
- Ashie, I.N.A.; Smith, J.P.; Simpson, B.K.; Haard, N.F. Spoilage and shelf-life extension of fresh fish and shellfish. Crit. Rev. Food Sci. Nutr. 2009, 36, 87–121. [Google Scholar] [CrossRef]
- Shen, J.; Xu, S.; Zhao, C.; Qiao, X.; Liu, H.; Zhao, Y.; Wei, J.; Zhu, Y. Bimetallic Au@Pt nanocrystal sensitization mesoporous α-Fe2O3 hollow nanocubes for highly sensitive and rapid detection of fish freshness at low temperature. ACS Appl. Mater. Interfaces 2021, 13, 57597–57608. [Google Scholar] [CrossRef] [PubMed]
- Prabhakar, P.K.; Vatsa, S.; Srivastav, P.P.; Pathak, S.S. A comprehensive review on freshness of fish and assessment: Analytical methods and recent innovations. Food Res. Int. 2020, 133, 109157. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Debliquy, M.; Zhang, C. Metal–oxide–semiconductor resistive gas sensors for fish freshness detection. Compr. Rev. Food Sci. Food Saf. 2022, 22, 913–945. [Google Scholar] [CrossRef]
- Bernardo, Y.A.A.; Rosario, D.K.A.; Delgado, I.F.; Conte-Junior, C.A. Fish quality index method: Principles, weaknesses, validation, and alternatives—A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 2657–2676. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.H.; Sun, D.W.; Zeng, X.A.; Liu, D. Recent advances in methods and techniques for freshness quality determination and evaluation of fish and fish fillets: A review. Crit. Rev. Food Sci. Nutr. 2015, 55, 1012–1225. [Google Scholar] [CrossRef]
- Hassoun, A.; Karoui, R. Quality evaluation of fish and other seafood by traditional and nondestructive instrumental methods: Advantages and limitations. Crit. Rev. Food Sci. Nutr. 2017, 57, 1976–1998. [Google Scholar] [CrossRef]
- Amari, A.; El Barbri, N.; Llobet, E.; El Bari, N.; Correig, X.; Bouchikhi, B. Monitoring the freshness of moroccan sardines with a neural–network based electronic nose. Sensors 2006, 6, 1209–1223. [Google Scholar] [CrossRef]
- Wu, D.; Zhang, M.; Chen, H.; Bhandari, B. Freshness monitoring technology of fish products in intelligent packaging. Crit. Rev. Food Sci. Nutr. 2020, 61, 1279–1292. [Google Scholar] [CrossRef]
- Freitas, J.; Vaz-Pires, P.; Câmara, J.S. Quality Index Method for fish quality control: Understanding the applications, the appointed limits and the upcoming trends. Trends Food Sci. 2021, 111, 333–345. [Google Scholar] [CrossRef]
- Ritter, D.O.; Lanzarin, M.; Novaes, S.F.; Monteiro, M.L.G.; Almeida, E.S.; Mársico, E.T.; Franco, R.M.; Conte, C.A.; Freitas, M.Q. Quality Index Method (QIM) for gutted ice–stored hybrid tambatinga (Colossoma macropomum x Piaractus brachypomum) and study of shelf life. LWT—Food Sci. Technol. 2016, 67, 55–61. [Google Scholar] [CrossRef]
- Khodabux, K.; Lomelette, M.; Jhaumeerlaulloo, S.; Ramasami, P.; Rondeau, P. Chemical and near-infrared determination of moisture, fat and protein in tuna fishes. Food Chem. 2007, 102, 669–675. [Google Scholar] [CrossRef]
- ElMasry, G.; Wold, J.P. High-speed assessment of fat and water content distribution in fish fillets using online imaging spectroscopy. J. Agric. Food Chem. 2008, 56, 7672–7677. [Google Scholar] [CrossRef]
- Ozogul, Y.; Ozyurt, G.; Ozogul, F.; Kuley, E.; Polat, A. Freshness assessment of European eel (Anguilla anguilla) by sensory, chemical and microbiological methods. Food Chem. 2005, 92, 745–751. [Google Scholar] [CrossRef]
- Heising, J.K.; Dekker, M.; Bartels, P.V.; van Boekel, M.A.J.S. A non-destructive ammonium detection method as indicator for freshness for packed fish: Application on cod. J. Food Eng. 2012, 110, 254–261. [Google Scholar] [CrossRef]
- Sivertsen, A.H.; Kimiya, T.; Heia, K. Automatic freshness assessment of cod (Gadus morhua) fillets by Vis/Nir spectroscopy. J. Food Eng. 2011, 103, 317–323. [Google Scholar] [CrossRef]
- Godiksen, H.; Morzel, M.; Hyldig, G.; Jessen, F. Contribution of cathepsins B, L and D to muscle protein profiles correlated with texture in rainbow trout (Oncorhynchus mykiss). Food Chem. 2009, 113, 889–896. [Google Scholar] [CrossRef]
- Herrero, A.M. Raman spectroscopy a promising technique for quality assessment of meat and fish: A review. Food Chem. 2008, 107, 1642–1651. [Google Scholar] [CrossRef]
- Folkestad, A.; Wold, J.P.; Rørvik, K.-A.; Tschudi, J.; Haugholt, K.H.; Kolstad, K.; Mørkøre, T. Rapid and non-invasive measurements of fat and pigment concentrations in live and slaughtered atlantic salmon (Salmo salar L.). Aquaculture 2008, 280, 129–135. [Google Scholar] [CrossRef]
- Shibata, M.; ElMasry, G.; Moriya, K.; Rahman, M.M.; Miyamoto, Y.; Ito, K.; Nakazawa, N.; Nakauchi, S.; Okazaki, E. Smart technique for accurate monitoring of ATP content in frozen fish fillets using fluorescence fingerprint. LWT 2018, 92, 258–264. [Google Scholar] [CrossRef]
- Møretrø, T.; Normann, M.A.; Sæbø, H.R.; Langsrud, S. Evaluation of ATP bioluminescence-based methods for hygienic assessment in fish industry. J. Appl. Microbiol. 2019, 127, 186–195. [Google Scholar] [CrossRef]
- Wu, L.; Pu, H.; Sun, D.-W. Novel techniques for evaluating freshness quality attributes of fish: A review of recent developments. Trends Food Sci. Technol. 2019, 83, 259–273. [Google Scholar] [CrossRef]
- Summers, G.; Wibisono, R.D.; Hedderley, D.I.; Fletcher, G.C. Trimethylamine oxide content and spoilage potential of New Zealand commercial fish species. N. Z. J. Mar. Freshwater Res. 2016, 51, 393–405. [Google Scholar] [CrossRef]
- Zhu, K.; Zhu, Z.; Xu, S.; Zhao, C.; Ni, T. Controlled synthesis of α-Fe2O3 nanocubes for gas-sensing applications: Feasibility of assessing crucian carp (Carassius auratus) freshness via trimethylamine levels. Food Chem. 2024, 441, 138361. [Google Scholar] [CrossRef] [PubMed]
- Tewari, D.; Sah, A.N.; Tripathi, Y.C. Chemical composition of angelica glauca roots volatile oil from indian himalayan region by GC-MS. J. Essent. Oil Bear. Plants 2019, 21, 1636–1641. [Google Scholar] [CrossRef]
- Cheng, S.; Li, H.; Jiang, D.; Chen, C.; Zhang, T.; Li, Y.; Wang, H.; Zhou, Q.; Li, H.; Tan, M. Sensitive detection of trimethylamine based on dopant–assisted positive photoionization ion mobility spectrometry. Talanta 2017, 162, 398–402. [Google Scholar] [CrossRef]
- Yan, W.; Xu, H.; Ling, M.; Zhou, S.; Qiu, T.; Deng, Y.; Zhao, Z.; Zhang, E. MOF-derived porous hollow Co3O4@ZnO cages for high-performance mems trimethylamine sensors. ACS Sens. 2021, 6, 2613–2621. [Google Scholar] [CrossRef]
- Li, F.; Gao, X.; Wang, R.; Zhang, T.; Lu, G.; Barsan, N. Design of core–shell heterostructure nanofibers with different work function and their sensing properties to trimethylamine. ACS Appl. Mater. Interfaces 2016, 8, 19799–19806. [Google Scholar] [CrossRef]
- Hu, S.; Xiao, Y.; Zhang, A.; Lin, Z.; Li, F.; Zhang, B.; Wang, G.; Qu, F.; Yao, D.; Zhang, H. Ultrasensitive xylene sensor based on RuO2-modified BiVO4 nanosheets. Sens. Actuators B 2025, 422, 136623. [Google Scholar] [CrossRef]
- Kadam, S.A.; Kadam, K.P.; Maria, C.C.S.; Ma, Y.R.; Molane, A.C.; Lolage, S.A.; Patil, V.B.; More, P.D. Microporous α-Fe2O3@NiO heterostructures synthesized for enhanced supercapacitor performance. Chemistryselect 2024, 9, e202302376. [Google Scholar] [CrossRef]
- Shen, S.; Zhang, X.; Cheng, X.; Xu, Y.; Gao, S.; Zhao, H.; Zhou, X.; Huo, L. Oxygen–vacancy–enriched porous α-MoO3 nanosheets for trimethylamine sensing. ACS Appl. Nano Mater. 2019, 2, 8016–8026. [Google Scholar] [CrossRef]
- Liu, L.; Fu, S.; Lv, X.; Yue, L.; Fan, L.; Yu, H.; Gao, X.; Zhu, W.; Zhang, W.; Li, X.; et al. A gas sensor with Fe2O3 nanospheres based on trimethylamine detection for the rapid assessment of spoilage degree in fish. Front. Bioeng. Biotechnol. 2020, 8, 567584. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, S.; Huang, C.; Qu, F.; Yao, D.; Guo, H.; Xu, H.; Jiang, C.; Yang, M. Mesoporous WO3 modified by Au nanoparticles for enhanced trimethylamine gas sensing properties. Dalton Trans. 2021, 50, 970–978. [Google Scholar] [CrossRef]
- Shanmugasundaram, A.; Ramireddy, B.; Basak, P.; Manorama, S.V.; Srinath, S. Hierarchical In(OH)3 as a precursor to mesoporous In2O3 nanocubes: A facile synthesis route, mechanism of self-assembly, and enhanced sensing response toward hydrogen. J. Phys. Chem. C 2014, 118, 6909–6921. [Google Scholar] [CrossRef]
- Nomani, M.W.K.; Kersey, D.; James, J.; Diwan, D.; Vogt, T.; Webb, R.A.; Koley, G. Highly sensitive and multidimensional detection of NO2 using In2O3 thin films. Sens. Actuators B 2011, 160, 251–259. [Google Scholar] [CrossRef]
- Huang, F.; Yang, W.; He, F.; Liu, S. Controlled synthesis of flower-like In2O3 microrods and their highly improved selectivity toward ethanol. Sens. Actuators B 2016, 235, 86–93. [Google Scholar] [CrossRef]
- Lu, X.; Yu, Q.; Wang, K.; Shi, L.; Liu, X.; Qiu, A.; Wang, L.; Cui, D. Synthesis, characterization and gas sensing properties of flowerlike In2O3 composed of microrods. Cryst. Res. Technol. 2010, 45, 557–561. [Google Scholar] [CrossRef]
- Li, Y.; Liu, J.; Zhang, J.; Liang, X.; Zhang, X.; Qi, Q. Deposition of In2O3 nanofibers on polyimide substrates to construct high-performance and flexible trimethylamine sensor. Chin. Chem. Lett. 2020, 31, 2142–2144. [Google Scholar] [CrossRef]
- Zheng, W.; Lu, X.; Wang, W.; Li, Z.; Zhang, H.; Wang, Y.; Wang, Z.; Wang, C. A highly sensitive and fast-responding sensor based on electrospun In2O3 nanofibers. Sens. Actuators B 2009, 142, 61–65. [Google Scholar] [CrossRef]
- Cantalini, C.; Wlodarski, W.; Sun, H.T.; Atashbar, M.Z.; Passacantando, M.; Santucci, S. NO2 response of In2O3 thin film gas sensors prepared by sol-gel and vacuum thermal evaporation techniques. Sens. Actuators B 2000, 65, 101–104. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cerneavschi, A.; Brinzari, V.; Vasiliev, A.; Ivanov, M.; Cornet, A.; Morante, J.; Cabot, A.; Arbiol, J. In2O3 films deposited by spray pyrolysis as a material for ozone gas sensors. Sens. Actuators B 2004, 99, 297–303. [Google Scholar] [CrossRef]
- Zhang, B.; Wang, J.; Yu, P.; Guo, L.; Xu, Y.; Dong, Y.; Ni, Y.; Ao, J.; Wei, Q.; Xia, Y. In/Fe cospinning nanowires for triethylamine gas sensing. ACS Appl. Nano Mater. 2022, 5, 9554–9566. [Google Scholar] [CrossRef]
- Li, W.; Zhang, B.; Dong, X.; Lu, Q.; Shen, H.; Ni, Y.; Liu, Y.; Song, H. Electrospun WO3/TiO2 core–shell nanowires for triethylamine gas sensing. Chemosensors 2025, 13, 45. [Google Scholar] [CrossRef]
- Li, Z.; Guo, L.; Feng, Z.; Gao, S.; Zhang, H.; Yang, X.; Liu, H.; Shao, J.; Sun, C.; Cheng, Y.; et al. Metal–organic framework–derived ZnO decorated with CuO for ultra–high response and selectivity H2S gas sensor. Sens. Actuators B 2022, 366, 131995. [Google Scholar] [CrossRef]
- Wang, L.; Cao, J.; Qian, X.; Zhang, H. Facile synthesis and enhanced gas sensing properties of grain size–adjustable In2O3 micro/nanotubes. Mater. Lett. 2016, 171, 30–33. [Google Scholar] [CrossRef]
- Van Hoang, N.; Hung, C.M.; Hoa, N.D.; Van Duy, N.; Van Hieu, N. Facile on-chip electrospinning of ZnFe2O4 nanofiber sensors with excellent sensing performance to H2S down ppb level. J. Hazard. Mater. 2018, 360, 6–16. [Google Scholar] [CrossRef]
- He, K.; Chen, N.; Wang, C.; Wei, L.; Chen, J. Method for determining crystal grain size by x-ray diffraction. Cryst. Res. Technol. 2018, 53, 1700157. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, L.; Tang, X.; Sun, D.; Tian, C.; Yang, Y. Nanoflake assembled hierarchical porous flower-like α-Fe2O3 with large specific surface area for enhanced acetone sensing. Func. Mater. Lett. 2020, 13, 2051038. [Google Scholar] [CrossRef]
- Wang, Y.; Sun, X.; Cao, J. Enhanced methane sensing performance of Ag modified In2O3 microspheres. J. Alloys Compd. 2022, 895, 162557. [Google Scholar] [CrossRef]
- Liu, W.; Gu, D.; Li, X. Detection of Ppb–level NO2 using mesoporous ZnSe/SnO2 core–shell microspheres based chemical sensors. Sens. Actuators B 2020, 320, 128365. [Google Scholar] [CrossRef]
- Wu, J.; Feng, S.; Li, Z.; Tao, K.; Chu, J.; Miao, J.; Norford, L.K. Boosted sensitivity of graphene gas sensor via nanoporous thin film structures. Sens. Actuators B 2018, 255, 1805–1813. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, N.; Xu, J.; Jin, Q.; San, X.; Wang, X. Co3O4/In2O3 p–n heterostructures based gas sensor for efficient structure–driven trimethylamine detection. Ceram Int. 2023, 49, 17354–17362. [Google Scholar] [CrossRef]
- Meng, D.; Qiao, T.; Ji, Y.; Wang, R.; Zhang, Y.; San, X.; Jin, Q.; Wang, X. Hydrangea-like In2O3@In2S3 n–n heterostructures for high-efficiency TMA measurement. IEEE Trans. Instrum. Meas. 2023, 72, 1–9. [Google Scholar] [CrossRef]
- Wang, W.; Zhang, L.; Liu, Z.; Kang, Y.; Chen, Q.; Wang, W.; Liu, M.; Ye, B.-C.; Yu, F.; Li, Y. Visible–light–activated TiO2–NiFe2O4 heterojunction for detecting sub–ppm trimethylamine. J. Alloys Compd. 2022, 898, 162990. [Google Scholar] [CrossRef]
- Mahata, B.; Giri, S.; Banerji, P.; Guha, P.K. Cactus-like NiO nanostructure–based ppb level trimethylamine gas sensor for monitoring saltwater fish freshness. ACS Food Sci. Technol. 2024, 5, 284–291. [Google Scholar] [CrossRef]
- Meng, D.; He, C.; Zhang, Y.; San, X.; Zhang, L.; Jin, Q.; Qi, J. Heterostructures constructed by NiMoO4 functionalized 2D MoO3 nanosheets for ultrasensitive trimethylamine detection with ppb level detection. Sens. Actuators B 2024, 421, 136442. [Google Scholar] [CrossRef]
- Chen, Y.; Li, Y.; Feng, B.; Wu, Y.; Zhu, Y.; Wei, J. Self-templated synthesis of mesoporous Au–ZnO nanospheres for seafood freshness detection. Sens. Actuators B 2022, 360, 131662. [Google Scholar] [CrossRef]
- Meng, D.; Qiao, T.; Wang, G.; Shen, Y.; San, X.; Pan, Y.; Meng, F. NiO-functionalized In2O3 flower like structures with enhanced trimethylamine gas sensing performance. Appl. Surf. Sci. 2022, 577, 151877. [Google Scholar] [CrossRef]
- Wang, L.; Gao, L.; Liu, H.; Zhang, Q.; Fu, H.; Liu, Z. Constructing of Bi2O3-In2O3 nanofibers for sensitive detection of freshness gas markers in aquatic products. J. Alloys Compd. 2025, 1017, 179019. [Google Scholar] [CrossRef]
- Zhang, K.; Shen, Y.; Lin, Z.; Dong, Z. Ultra-high ethanol sensitivity sensor based on porous In2O3 decorated with gold nanoparticles. Mater. Sci. Semicond. Process. 2021, 135, 106124. [Google Scholar] [CrossRef]
- Sun, Z.; Yan, X.; Huang, L.; Zhang, Y.; Hu, Z.; Sun, C.; Yang, X.; Pan, G.; Cheng, Y. AuPd bimetallic functionalized monodisperse In2O3 porous spheres for ultrasensitive trimethylamine detection. Sens. Actuators B 2023, 381, 133355. [Google Scholar] [CrossRef]
- He, J.; Meng, H.; Wang, X.; Xu, Y.; Feng, L. S–doped SnO2 nanoparticles with a smaller grain size for highly efficient detection of greenhouse gas hexafluoroethane. Sens. Actuators B 2024, 418, 136335. [Google Scholar] [CrossRef]
- Kong, D.; Wang, Y.; Han, J.; Gao, Y.; Liu, F.; Zhou, W.; Gao, Y.; Lu, G. Tailoring the triethylamine vapor sensing on Co–Fe bimetallic oxide nanosheets assemblies by crystal structure and doping sites. Sens. Actuators B 2024, 407, 135447. [Google Scholar] [CrossRef]
- Katoch, A.; Sun, G.-J.; Choi, S.-W.; Byun, J.-H.; Kim, S.S. Competitive influence of grain size and crystallinity on gas sensing performances of ZnO nanofibers. Sens. Actuators B 2013, 185, 411–416. [Google Scholar] [CrossRef]



| Sample | Grain Size (nm) a |
|---|---|
| In2O3–1 °C/min– 500 °C | 15.30 |
| In2O3–3 °C/min– 500 °C | 9.06 |
| In2O3–5 °C/min– 500 °C | 12.67 |
| In2O3–3 °C/min– 400 °C | 7.66 |
| In2O3–3 °C/min– 600 °C | 12.80 |
| Sample | OL (%) a | OV (%) a | OC (%) a |
|---|---|---|---|
| In2O3–1 °C/min–500 °C | 64.7 | 20.5 | 14.8 |
| In2O3–3 °C/min–500 °C | 48.4 | 31.6 | 20.0 |
| In2O3–5 °C/min–500 °C | 62.0 | 19.7 | 18.3 |
| In2O3–3 °C/min–400 °C | 54.0 | 27.1 | 18.9 |
| In2O3–3 °C/min–600 °C | 61.1 | 19.8 | 19.1 |
| Mater. | Temp. (°C) | Conc. (ppm) | Res. | τres./τrec. (s) | Ref. |
|---|---|---|---|---|---|
| Co2O3/In2O3 | 200 | 10 | 11.67 | 25/68 | [53] |
| In2O3@In2S3 | 100 | 10 | 5.5 | 28/48 | [54] |
| α-Fe2O3 | 250 | 20 | 7.1 | 100/200 | [33] * |
| Au–WO3 | 268 | 100 | 42.56 | 1/323 | [34] |
| TiO2–NiFe2O4 | 307 | 10 | 11.2 | 50/45 | [55] |
| NiO | 260 | 100 | 13.51 | 24/39 | [56] * |
| NiMoO4/MoO3 | 200 | 10 | 62.79 | 13/78 | [57] * |
| Au/ZnO | 250 | 10 | 52.6 | 12/127 | [58] * |
| In2O3–NiO | 200 | 10 | 20.51 | 39/43 | [59] |
| 3 %Bi2O3–In2O3 NFs | 250 | 100 | 48.6 | 320/58 | [60] |
| In2O3 | 240 | 100 | 47.0 | 6/37 | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dong, X.; Zhang, B.; Shen, M.; Lu, Q.; Shen, H.; Ni, Y.; Liu, Y.; Song, H. Trimethylamine Gas Sensor Based on Electrospun In2O3 Nanowires with Different Grain Sizes for Fish Freshness Monitoring. Chemosensors 2025, 13, 218. https://doi.org/10.3390/chemosensors13060218
Dong X, Zhang B, Shen M, Lu Q, Shen H, Ni Y, Liu Y, Song H. Trimethylamine Gas Sensor Based on Electrospun In2O3 Nanowires with Different Grain Sizes for Fish Freshness Monitoring. Chemosensors. 2025; 13(6):218. https://doi.org/10.3390/chemosensors13060218
Chicago/Turabian StyleDong, Xiangrui, Bo Zhang, Mengyao Shen, Qi Lu, Hao Shen, Yi Ni, Yuechen Liu, and Haitao Song. 2025. "Trimethylamine Gas Sensor Based on Electrospun In2O3 Nanowires with Different Grain Sizes for Fish Freshness Monitoring" Chemosensors 13, no. 6: 218. https://doi.org/10.3390/chemosensors13060218
APA StyleDong, X., Zhang, B., Shen, M., Lu, Q., Shen, H., Ni, Y., Liu, Y., & Song, H. (2025). Trimethylamine Gas Sensor Based on Electrospun In2O3 Nanowires with Different Grain Sizes for Fish Freshness Monitoring. Chemosensors, 13(6), 218. https://doi.org/10.3390/chemosensors13060218






