Electrochemical Sensors for the Detection of TiO2 Nanoparticles Genotoxicity at Different pH Values Simulating the Gastrointestinal Tract
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Apparatus
2.3. Preparation of the Biosensor
2.4. Incubation of TiO2 NPs in an Experimental Environment
2.5. Methods
2.6. Data Treatment
3. Results and Discussion
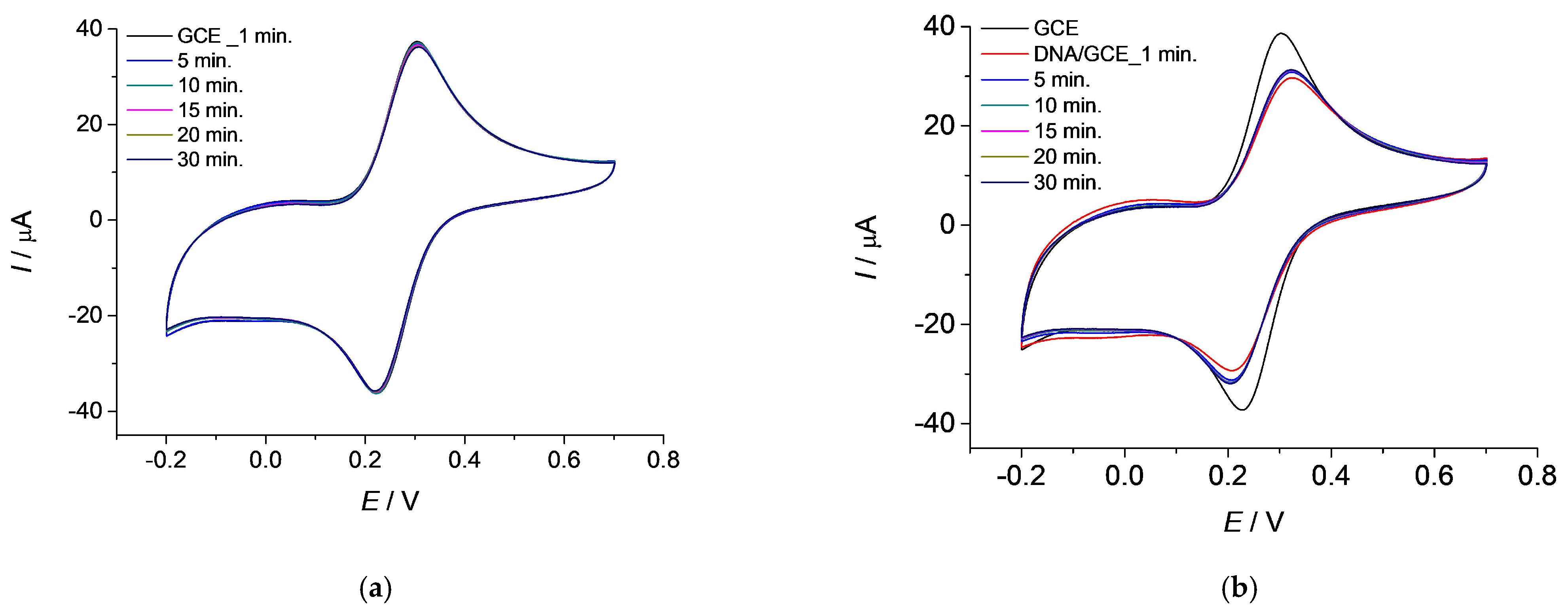
3.1. Monitoring the Stability of the GCE Sensor and the DNA/GCE Biosensor
3.2. Effect of TiO2 Nanoparticles on the Surface of the GCE Sensor and the DNA/GCE Biosensor
3.2.1. Effect of TiO2 Concentration on the GCE Biosensor
3.2.2. Effect of TiO2 Concentration on the DNA/GCE Biosensor
3.3. Influence of pH on TiO2 NPs and Their Effect on the DNA/GCE Biosensor
3.3.1. Influence of pH on the DNA/GCE Biosensor
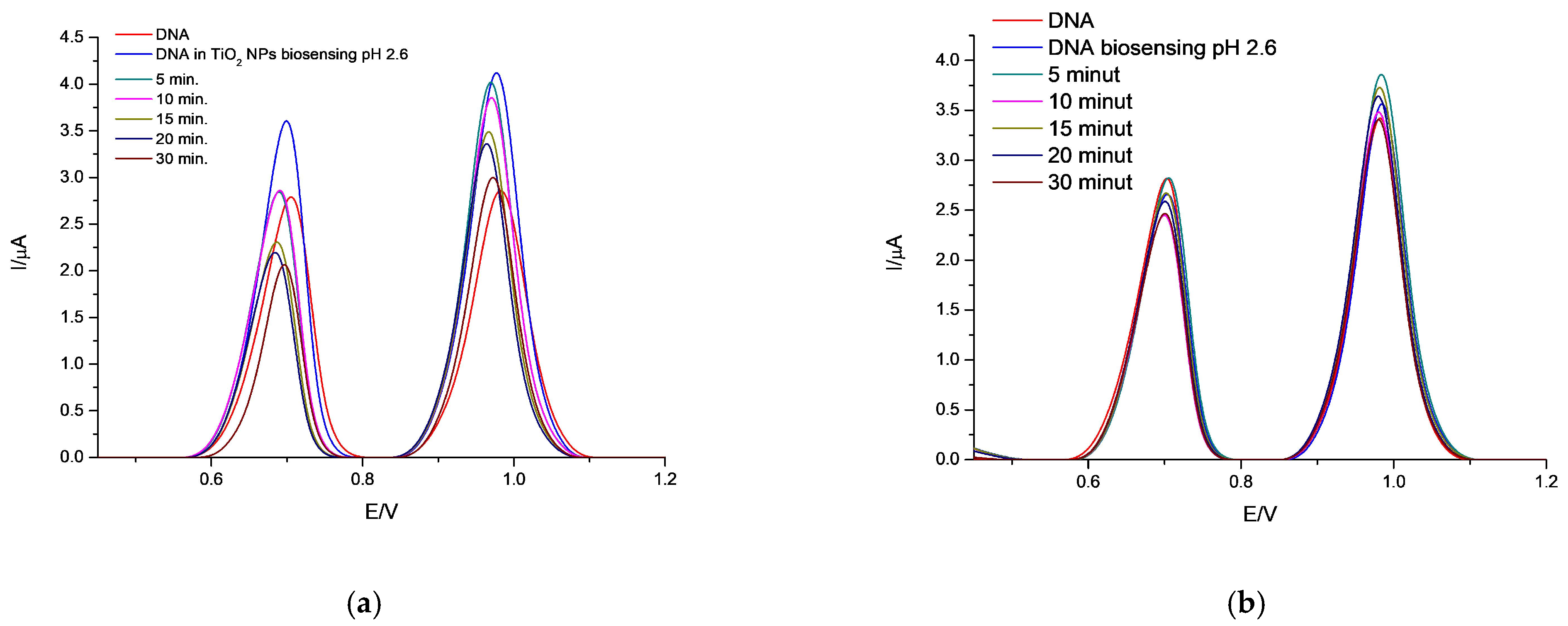
3.3.2. Influence of TiO2 Exposed to pH Values Simulating the GIT Environment on the DNA/GCE Biosensor
3.4. Monitoring the Genotoxicity of TiO2 NPs on DNA Structure by Biosensing
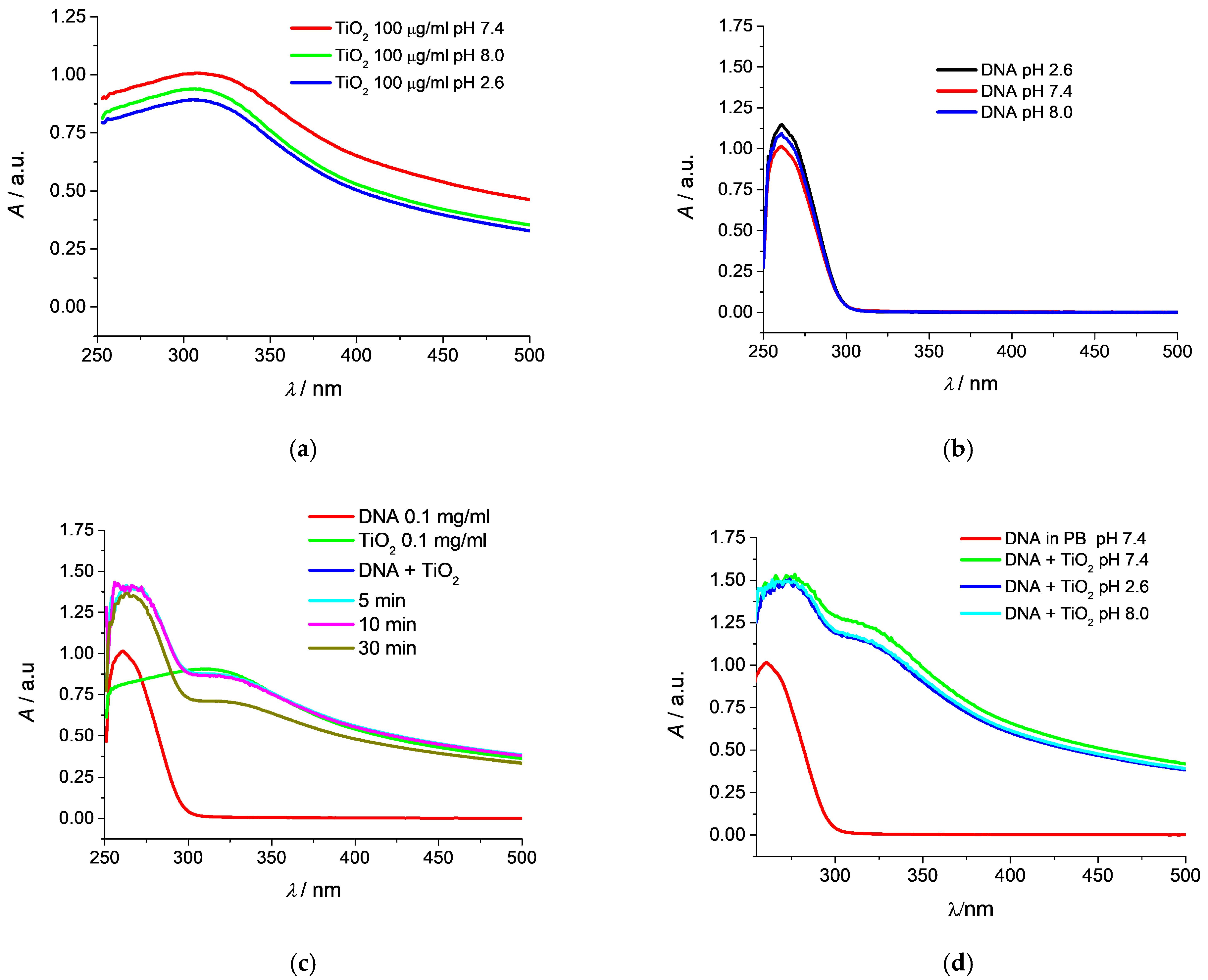
3.5. Monitoring the Genotoxicity of TiO2 NPs Toward DNA by UV–Vis Spectrophotometry
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CV | Cyclic voltammetry |
| DPV | Differential pulse voltammetry |
| EFSA | European Food Safety Authority |
| FDA | US Food and Drug Administration |
| GCE | Glassy carbon electrode |
| GIT | Gastrointestinal tract |
| IARC | International Agency for Research on Cancer |
| ROS | Reactive oxygen species |
| TiO2 | Titanium dioxide |
| TiO2 NPs | Titanium dioxide nanoparticles |
References
- Mao, X.X.; Hao, C.L. Recent advances in the use of composite titanium dioxide nanomaterials in the food industry. J. Food Sci. 2024, 89, 1310–1323. [Google Scholar] [CrossRef] [PubMed]
- Heckman, M.M.; Albright, M.C.; Poulsen, K.M.; Tighe, R.M.; Payne, C.K. Cellular and In Vivo Response to Industrial, Food Grade, and Photocatalytic TiO2 Nanoparticles. J. Phys. Chem B 2024, 128, 8878–8885. [Google Scholar] [CrossRef]
- Inagaki, Y.; Ishitani, Y.; Tame, A.; Uematsu, K.; Tomioka, N.; Ushikubo, T.; Ujiié, Y. Foraminiferal detoxification breakdown induced by fatal levels of TiO2 nanoparticles. Front. Mar. Sci. 2024, 11, 1381247. [Google Scholar] [CrossRef]
- Bastardo-Fernández, I.; Chekri, R.; Oster, C.; Thoury, V.; Fisicaro, P.; Jitaru, P.; Noireaux, J. Assessment of TiO2 (nano)particles migration from food packaging materials to food simulants by single particle ICP-MS/MS using a high efficiency sample introduction system. Nanoimpact 2024, 34, 100503. [Google Scholar] [CrossRef]
- Singha, S.K.; Hoque, S.M.; Das, H.; Alim, M.A. Evaluation of chitosan-Ag/TiO2 nanocomposite for the enhancement of shelf life of chili and banana fruits. Heliyon 2023, 9, e21752. [Google Scholar] [CrossRef]
- Abdulagatov, I.M.; Maksumova, A.M.; Magomedov, M.Z.; Tsakhaeva, R.O.; Khidirova, S.M.; Salikhov, A.M. Antibacterial Food Packaging Nanomaterial Based on Atomic Layer Deposition for Long-Term Food Storage. J. Food Sci. Technol.-Mysore 2024, 61, 596–606. [Google Scholar] [CrossRef]
- Li, J.R.; Zhang, D.Q.; Hou, C.L. Application of Nano-Titanium Dioxide in Food Antibacterial Packaging Materials. Bioengineering 2025, 12, 19. [Google Scholar] [CrossRef]
- Phuinthiang, P.; Channei, D.; Ratananikom, K.; Nakaruk, A.; Khanitchaidecha, W. Antibacterial properties of TiO2 nano coating on food packaging surfaces against Escherichia coli and Salmonella typhimurium. Surf. Eng. 2023, 39, 433–444. [Google Scholar] [CrossRef]
- Raymond, P.; St-Germain, F.; Paul, S.; Chabot, D.; Deschênes, L. Impact of Nanoparticle-Based TiO2 Surfaces on Norovirus Capsids and Genome Integrity. Foods 2024, 13, 1527. [Google Scholar] [CrossRef]
- Gea, M.; Bonetta, S.; Iannarelli, L.; Giovannozz, A.M.; Maurino, V.; Bonetta, S.; Hodoroaba, V.-D.; Armato, C.; Rossi, A.M.; Schilirò, T. Shape-engineered titanium dioxide nanoparticles (TiO2 NP): Cytotoxicity and genotoxicity in bronchial epithelial cells. Food Chem. Toxicol. 2019, 127, 89–100. [Google Scholar] [CrossRef]
- Al Mutairi, M.A.; BinSaeedan, N.M.; Alnabati, K.K.; Alotaibi, A.; Al-Mayouf, A.M.; Ali, R.; Alowaifeer, A.M. Characterisation of engineered titanium dioxide nanoparticles in selected food. Food Addit. Contam. Part B-Surveill. 2023, 16, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Marucco, A.; Prono, M.; Beal, D.; Alasonati, E.; Fisicaro, P.; Bergamaschi, E.; Carriere, M.; Fenoglio, I. Biotransformation of food-grade and nanometric TiO2 in the oral-gastro-intestial tract: Driving forces and Effect on the toxicity toward intestinal epithelial cells. Nanomaterials 2020, 10, 2132. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Wójcik, E.; Rymuszka, A.; Sieroslawska, A.; Szwajgier, D. Effect of Food Additive E171 and Titanium Dioxide Nanoparticles (TiO2-NPs) on Caco-2 Colon Cancer Cells. Appl. Sci. 2024, 14, 9387. [Google Scholar] [CrossRef]
- Baranowska-Wójcik, E.; Welc-Stanowska, R.; Acek, D.W.; Nawrocka, A.; Szwajgier, D. The interactions of pectin with TiO2 nanoparticles measured by FT-IR are confirmed in a model of the gastrointestinal tract. Food Res. Int. 2024, 189, 114509. [Google Scholar] [CrossRef]
- Ryabtseva, M.S.; Umanskaya, S.F.; Shevchenko, M.A.; Krivobok, V.S.; Kolobov, A.V.; Nastulyavichus, A.A.; Chentsov, S.I.; Sibirtsev, V.D. Transformation of Nano-Size Titanium Dioxide Particles in the Gastrointestinal Tract and Its Role in the Transfer of Nanoparticles through the Intestinal Barrier. Int. J. Mol. Sci. 2023, 24, 14911. [Google Scholar] [CrossRef]
- Tsymbalyuk, O.V.; Davydovska, T.L.; Naumenko, A.M.; Voiteshenko, I.S.; Veselsky, S.P.; Nyporko, A.Y.; Pidhaietska, A.Y.; Kozolup, M.S.; Skryshevsky, V.A. Mechanisms of regulation of motility of the gastrointestinal tract and the hepatobiliary system under the chronic action of nanocolloids. Sci. Rep. 2023, 13, 3823. [Google Scholar] [CrossRef]
- Buowmeester, H.; Van Der Zande, M.; Jepson, M.A. Effects of food-borne nanomaterials on gastrointestinal tissues and microbiota. WIREs Nanomed. Nanobiotechnol. 2018, 10, 1481. [Google Scholar] [CrossRef]
- Bucher, G.; El Hadri, H.; Asensio, O.; Auger, F.; Barrero, J.; Rosec, J.P. Large-scale screening of E171 food additive (TiO2) on the French market from 2018 to 2022: Occurrence and particle size distribution in various food categories. Food Control 2024, 155, 110102. [Google Scholar] [CrossRef]
- Espada-Bernabé, E.; Moreno-Martín, G.; Gómez-Gómez, B.; Madrid, Y. Assesing the behaviour of particulate/nanoparticulate form of E171 (TiO2) food additive in colored chocolate candies before and after in vitro oral ingestion by spICP-MS, TEM and cellular in vitro models. Food Chem. 2024, 432, 137201. [Google Scholar] [CrossRef]
- Warheit, D.B. Safety of titanium dioxide (E171) as a food additive for humans. Front. Toxicol. 2024, 6, 1333746. [Google Scholar] [CrossRef]
- Chen, T.; Yan, J.; Li, Y. Genotoxicity of titanium dioxide nanoparticles. J. Food Drug Anal. 2014, 22, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Andreoli, C.; Leter, G.; De Berardis, B.; Degan, P.; De Angelis, I.; Pacchierotti, F.; Crebelli, R.; Barone, F.; Zijno, A. Critical issues in genotoxicity assessment of TiO2 nanoparticles by human peripheral blood mononuclear cells. J. Appl. Toxicol. 2018, 38, 1471–1482. [Google Scholar] [CrossRef]
- Ma, T.; Wang, M.; Gong, S.; Tian, B. Impact of Sediment Organic Matter Content and pH on Ecotoxicity of Coexposure of TiO2 Nanoparticles and Cadmium to Freshwater Snails Bellamya aeruginosa. Arch. Environ. Contam. Toxicol. 2017, 72, 153–165. [Google Scholar] [CrossRef]
- Tice, R.; Agurell, E.; Anderson, D.; Burlinson, B.; Hartmann, A.; Kobayashi, H.; Miyamae, Y.; Rojas, E.; Ryu, J.-C.; Sasaki, Y.F. Single cell gel/comet assay: Guidelines for in vitro and in vivo genetic toxicology testing. Environ. Mol. Mutagen. 2000, 35, 206–221. [Google Scholar] [CrossRef]
- Rejman, J.; Oberle, V.; Zuhorn, I.S.; Heokstra, D. Size-dependent internalization of particles via pathways of clathrin- and caveolae-mediated endocytosis. Biochem. J. 2004, 377, 159–169. [Google Scholar] [CrossRef]
- Chen, M.; Von Mikecz, A. Formation of nucleoplasmic protein aggregates impairs nuclear function in ressponse to SiO2 nanoparticles. Exp. Cell Res. 2005, 305, 51–62. [Google Scholar] [CrossRef]
- Li, S.; Zhu, H.; Zhu, R.; Sun, X.; Yao, S.; Wang, S. Impact and mechanism of TiO2 nanoparticles on DNA synthesis in vitro. Sci. China Ser. B Chem. 2008, 51, 367–372. [Google Scholar] [CrossRef]
- Zhu, R.R.; Wang, S.L.; Zhang, R.; Sun, X.Y.; Yao, S.D. A Novel Toxicological Evaluation of TiO2 Nanoparticles on DNA structure. Chin. J. Chem. 2007, 25, 958–961. [Google Scholar] [CrossRef]
- Federici, G.; Shaw, B.J.; Handy, R.D. Toxicity of titanium dioxide nanoparticles to rainbow trout (Oncorhynchus mykiss): Gill injury, oxidative stress, and other physiological effects. Aquat. Toxicol. 2007, 84, 415–430. [Google Scholar] [CrossRef]
- Veranth, J.M.; Kaser, E.G.; Veranth, M.M.; Koch, M.; Yost, G.S. Cytokine responses of human lung cells (BEAS-2B) treated with micron-sized and nanoparticles of metal oxides compared to soil dusts. Part. Fibre Toxicol. 2007, 4, 2. [Google Scholar] [CrossRef]
- Dunford, R.; Salinaro, A.; Cai, L.; Serpone, N.; Horikoshi, S.; Hidaka, H.; Knowland, J. Chemical oxidation and DNA damage catalyzed by inorganic sunscreen ingredients. FEBS Lett. 1997, 418, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Kashige, N.; Kakita, Y.; Nakashima, Y.; Miake, F.; Watanabe, K. Mechanism of the photocatalytic inactivation of Lactobacillus casei PL-1 by titania thin film. Curr. Microbiol. 2001, 42, 184–189. [Google Scholar] [CrossRef]
- Alfhili, M.A.; Hindawi, M.-H.L. Review Article Triclosan: An Update on Biochemical and Molecular Mechanisms. Oxid. Med. Cell. Longev. 2019, 2019, 1607304. [Google Scholar] [CrossRef]
- Blaskovicova, J.; Augustin, M.; Vyskocil, V.; Purdesova, A. Ethanol and NaCl-induced gold nanoparticle aggregation toxicity toward DNA investigated with DNA/GCE biosensor. Sensors 2023, 23, 3425. [Google Scholar] [CrossRef]
- Svitkova, V.; Blaskovicova, J.; Tekelova, M.; Kallai, B.M.; Ignat, T.; Horackova, V.; Skladal, P.; Kopel, P.; Adam, V.; Farkasova, D.; et al. Assessment of CdS quantum dots effect on UV damage to DNA using a DNA/quantum dots structured electrochemical biosensor and DNA biosensing in solution. Sens. Acutators BChem. 2017, 243, 435–444. [Google Scholar] [CrossRef]
- Blaskovicova, J.; Labuda, J. Effect of Triclosan and Silver Nanoparticles on DNA Damage Investigated with DNA-Based Biosensor. Sensors 2022, 22, 4332. [Google Scholar] [CrossRef]
- Blaskovicova, J.; Purdesova, A. Simultaneous detection of purine metabolites by membrane modified electrochemical sensors. Acta Chim. Slov. 2022, 15, 54–60. [Google Scholar] [CrossRef]
- Blaskovicova, J.; Sochr, J.; Koutsogiannis, A.; Diamantidou, D.; Kopel, P.; Adam, V.; Labuda, J. Detection of ROS Generated by UV-C Irradiation of CdS Quantum Dots and their Effect on Damage to Chromosomal and Plasmid DNA. Electroanalysis 2018, 30, 698–704. [Google Scholar] [CrossRef]
- Hlavata, L.; Striesova, I.; Ignat, T.; Blaskovicova, J.; Ruttkay-Nedecky, B.; Kopel, P.; Adam, V.; Kizek, R.; Labuda, J. An electrochemical DNA-based biosensor to study the effects of CdTe quantum dots on UV-induced damage of DNA. Mikrochim. Acta 2015, 182, 1715–1722. [Google Scholar] [CrossRef]
- Svitkova, V.; Steffelova, L.; Blaškovičová, J.; Labuda, J. DNA-based biosensors with polyvinyl alcohol external membrane as a tool for the evaluation of antioxidant activity of white wines. Acta Chim. Slov. 2015, 8, 197–202. [Google Scholar] [CrossRef]
- Labuda, J.; Brett, A.M.O.; Evtugyn, G.; Fojta, M.; Mascini, M.; Ozsoz, M.; Palchetti, I.; Paleček, E.; Wang, J. Electrochemical nucleic acid-based biosensors: Concepts, terms and methodology. Pure Appl. Chem. 2010, 82, 1161–1187. [Google Scholar] [CrossRef]
- Labuda, J.; Vyskocil, V. DNA/Electrode Interface Detection of Damage to DNA Using DNA Modified Electrodes. In Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; pp. 346–350. [Google Scholar]
- Wang, J. Towards Genoelectronics: Electrochemical Biosensing of DNA Hybridization. Chem. Eur. J. 1999, 5, 1681–1685. [Google Scholar] [CrossRef]
- Sadecka, J.; Netriova, J.; Majek, P. Analyticke Metody v Klinickej Chemii; STU: Bratislava, Slovakia, 2008; pp. 16–17. ISBN 8022728218. [Google Scholar]
- Ophus, E.M.; Rode, L.; Gylseth, B.; Nicholson, D.G.; Saeed, K. Analysis of titanium pigments in human lung tissue. J. Work. Environ. Health 1979, 5, 290–296. [Google Scholar] [CrossRef]
- Patel, S.; Patel, P.; Bakshi, S.R. Titanium dioxide nanoparticles: An in vitro study of DNA binding, chromosome aberration assay and comet assay. Cytotechnology 2017, 69, 245–263. [Google Scholar] [CrossRef]
- Trouiller, B.; Reliene, R.; Westbrook, A.; Solaimani, P.; Schiestl, R.H. Titanium dioxide nanoparticles induce DNA damage and genetic instability in vivo in mice. Cancer Res. 2009, 22, 69–84. [Google Scholar] [CrossRef]
- El-Said, K.S.; Ali, E.M.; Kanehira, K.; Taniguchi, A. Molecular mechanism of DNA damage induced by titanium dioxide nanoparticles in toll-like receptor 3 or 4 expressing human hepatocarcinoma cell lines. J. Nanotechnol. 2014, 48, 12–22. [Google Scholar] [CrossRef]
- Dobrucka, R. Synthesis of Titanium Dioxide Nanoparticles Using Echinacea purpurea Herba. J. Pharm. Res. 2017, 16, 756–762. [Google Scholar]
- Patel, S.; Patel, P.; Undre, S.B.; Pandya, S.R.; Singh, M.; Bakshi, S. DNA binding and dispersion activities of titanium dioxide nanoparticles with UV/vis spectrophotometry, fluorescence spectroscopy and physicochemical analysis at physiological temperature. J. Mol. Liq. 2016, 213, 304–311. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Blaškovičová, J.; Bartánusová, D. Electrochemical Sensors for the Detection of TiO2 Nanoparticles Genotoxicity at Different pH Values Simulating the Gastrointestinal Tract. Chemosensors 2025, 13, 194. https://doi.org/10.3390/chemosensors13060194
Blaškovičová J, Bartánusová D. Electrochemical Sensors for the Detection of TiO2 Nanoparticles Genotoxicity at Different pH Values Simulating the Gastrointestinal Tract. Chemosensors. 2025; 13(6):194. https://doi.org/10.3390/chemosensors13060194
Chicago/Turabian StyleBlaškovičová, Jana, and Dominika Bartánusová. 2025. "Electrochemical Sensors for the Detection of TiO2 Nanoparticles Genotoxicity at Different pH Values Simulating the Gastrointestinal Tract" Chemosensors 13, no. 6: 194. https://doi.org/10.3390/chemosensors13060194
APA StyleBlaškovičová, J., & Bartánusová, D. (2025). Electrochemical Sensors for the Detection of TiO2 Nanoparticles Genotoxicity at Different pH Values Simulating the Gastrointestinal Tract. Chemosensors, 13(6), 194. https://doi.org/10.3390/chemosensors13060194




