Abstract
Many popular alcoholic beverages, such as Brazilian sugar cane spirit (cachaça), are aged in wood casks to achieve a smoother and more pleasant taste. The type of wood plays an important role in improving the quality of the spirit, with oak being the most widely used. Due to its elevated price and poor local availability, oak has been gradually replaced in Brazil by other woods, such as Amburana cearensis (Amburana), Cariniana legalis (Jequitibá), Hymenaea courbaril (Jatobá), and Ocotea odorifera (Cinnamon sassafras). For general purposes in beverage quality control and wood identification, and using ethanol/water extracts (cachaça 47% v/v) as a model, this article describes the construction of a low-cost electronic nose that quickly identifies the wood species that was used for aging a cachaça sample. The nose is made of an array of four chemoresistive conductive polymer gas sensors. Principal component and leave-one-out analyses showed perfect classification of all tested samples.
1. Introduction
Cachaça is a typical Brazilian distilled alcoholic beverage, with an alcohol content ranging from 38% to 48% ABV at 20 °C, produced by the fermentation of sugar cane juice. It can be used in cocktails prepared from freshly squeezed fruits such as lime (Caipirinha) or appreciated in its pristine form. In the latter case, the freshly distilled beverage may present sensory characteristics that are not very appreciated, a fact that can be overcome by aging it for several months in wooden barrels before being marketed. During the storage, the internal walls of the barrel undergo ethanolysis, and the compounds liberated by the wood, extracted by the “aquo-ethanol” solvent, are incorporated into the beverage, leading to changes in its chemical and sensory properties as a function of the wood characteristics [1,2,3]. Although oak is currently the most commonly used wood for barrel construction, other species of wood are being employed as an alternative to oak due to its high price and low availability [2,4]. Native Brazilian woods such as Amendoim (Pterogyne nitens), Amburana (Amburana Cearensis), and Cabreúva (Myroxylon peruiferum) can offer peculiar and attractive flavor characteristics due to the extraction of specific compounds, making them advantageous and viable options for cachaça producers [5]. This requires an appropriate control methodology that can identify the type of wood that was used in the aging process in order to detect and avoid eventual fraud and adulteration [2].
Wood identification is very important for an extensive range of professional fields. It may assist in forensic science and law, impart invaluable support to customs and to antique dealers, and contribute to biology, pharmaceutics, and other sciences [6]. The anatomy of wood species has been widely used as a tool for their identification, and this requires imaging tools for the evaluation of tissues and cells, including hand lenses, light or electron microscopes, and 2D and 3D scans, among other techniques [7]. Corroborating these practices, the International Association of Wood Anatomists (IAWA) has developed a list that standardizes the microscopic diagnostic characteristics that provide the basis for identifying hardwood species, taking into account anatomical patterns such as vessels, rays, parenchyma, and fibers [8]. Although these methodologies are standardized and highly efficient, they are not applicable in situations requiring rapid and accurate assessment. Therefore, fast, accessible, portable, and reliable methods would be highly beneficial for controlling wood transportation, general carpentry, and the cooperage industry [9,10].
In the case of aged alcoholic beverages, direct access to the barrel in which it was aged is no longer possible in most situations, as the beverage is bottled and displayed on store shelves. Therefore, the usual wood identification techniques that use samples of the wood itself are not useful. Instead, methods capable of detecting “chemical signatures” left by the woods in the aged beverages would be required. In that sense, using UV-Vis spectroscopy combined with multivariate data analysis, a methodology was developed that allows 10 different types of ethanolic wood extracts to be distinguished [11]. The identification of woods used in the maturation of sugarcane spirits has also paved the way for exploring alternative techniques that combine spectral data with supervised classification models, as reported in the literature [12,13,14,15]. The use of e-noses has been reported by Silvello and Alcarde [16], who employed MOS-type sensors and experimental design for optimizing sampling, successfully differentiating the studied wood types. Further exploring the application of e-noses, direct coupling to headspace and mass spectrometry has been used to recognize the aging time of spirits stored in oak barrels [17]. Although traditional techniques such as gas chromatography coupled with mass spectrometry (GC-MS) and Fourier transform infrared spectroscopy (FTIR) provide valuable insights into the chemical characterization of the headspace fraction, they require significant investment and must be conducted by a qualified analyst. In contrast, e-noses offer a portable, compact, cost-effective, and user-friendly alternative. Advances in the development of electronic noses using conductive and semiconductive materials have opened up a vast field of exploration. While specific sensors are designed to detect a single odorant, non-specific sensor arrays offer the advantage of identifying odor patterns, making them particularly useful for characterizations that do not require high specificity. Furthermore, the use of classification algorithms and machine learning techniques has enhanced the interpretation of data sets generated by these arrays, improving the accuracy and applicability of electronic noses across various fields [18]. The sensor array in electronic nose systems is the critical component that requires proper selection in the e-nose construction design, with possibilities such as conductive polymer sensors, piezoelectric mass sensors, optical sensors, or MOSFET sensors. The use of chemiresistive sensors has a wide field of application and is highly applicable to electronic noses due to their simple electrical properties and reading interface circuit. These can be divided into two types: metal oxide and conductive polymer, and these two types of sensors exhibit a variety of resistance values when exposed to odors. Both metal oxide and conductive polymer sensors have unique advantages [19,20], such as fast response and recovery times (MOS), flexibility, and lightweight (conductive polymers), and disadvantages, such as high operating temperatures (MOS) and environmental sensitivity (conductive polymers).
In parallel studies, conductive polymers have been successfully used as sensing materials in gas sensors for the detection of methanol in Brazilian sugarcane spirit and in electronic noses for the identification of several types of timber, using low-cost equipment and straightforward data processing [20]. Notably, these sensors have demonstrated insensitivity to water, acetic acid, and ethanol vapors, which together account for more than 98% of the total chemical composition of sugarcane spirits. Building on these properties and leveraging the ability of the water–ethanol mixture to extract volatile organic compounds from wood, this study describes the application of an electronic nose for distinguishing different ethanolic wood extracts by identifying the woods used. Since this method is based on detecting volatile components, it is far more straightforward and less susceptible to interference from the background sample composition compared to the UV-Vis spectroscopy approach mentioned before [9].
2. Materials and Methods
2.1. General Methods
Proton Nuclear Magnetic Resonance (1H NMR) Fourier Transform (FT) spectra were obtained using a Bruker (Karlsruhe, Germany) AC-200 spectrometer operating at 200 MHz. Deuterated chloroform (CDCl3) with tetramethylsilane (TMS) from Merck (Brazil) served as the solvent and internal reference. Fourier transform infrared (FTIR) spectra were acquired either as potassium bromide (KBr) discs or in chloroform (CHCl3) solution, employing a Perkin-Elmer (Shelton, CT, USA) 1750 series grating spectrophotometer. Only the prominent or significant absorption bands are reported. UV-Vis spectra were measured in chloroform (CHCl3) solution using a Hitachi (Tokyo, Japan) U-2000 spectrophotometer. Cyclic voltammetry (CV) of the polymeric precursors and the preparative controlled potential electrolyses were carried out using an Autolab (Metrohm, Switzerland) PGSTAT100 potentiostat/galvanostat. Elemental composition was determined using a Perkin Elmer (Shelton, CT, USA) 2400 CHN Elemental Analyzer. Molecular weight analysis was performed via SEC in tetrahydrofuran (THF) at a flow rate of 1 mL min−1. The SEC system consisted of a Shimadzu (Kyoto, Japan) Class-LC10 HPLC equipped with three Supelco Progel columns (G5000, G4000, and G3000). Molecular weights are reported relative to polystyrene standards of narrow dispersity (2500, 5000, 17,500, 30,000, 50,000, 95,800, and 184,200 gmol−1).
N,N-dimethylformamide (DMF) (Merck GPR), used for cyclic voltammetry (CV) and preparative electrolyses, was dried over anhydrous copper(II) sulfate (CuSO4) for 48 h, followed by distillation at 44–45 °C (25 mmHg) through a 40 cm Vigreux column. The purified DMF was stored over activated 4 Å molecular sieves. Tetraethylammonium bromide, used as the supporting electrolyte, was dried at 150 °C overnight. Carbon tetrachloride (CCl4) was purified by refluxing over phosphorus pentoxide (P2O5) for 10 h and subsequent distillation, then stored over activated 4 Å molecular sieves. All other commercially available materials were used as received.
2.2. Conductive Polymers Size Exclusion Chromatography
Poly(2,5-biphenyleneethylene) (1) and poly(4′-hexyloxy-2,5-biphenylene ethylene) (2) were synthesized according to literature procedures [21,22], respectively.
1,4-Bis-bromomethyl-9,9-dioctyl-fluorene (5): 1,4-dimethyl-9,9-dioctyl-fluorene (7) (1.11 g; 2.65 mmol), N-bromosuccinimide (NBS) (0.995 g; 5.59 mmol), and dibenzoyl peroxide (3.0 mg) were combined in 8 mL of dry carbon tetrachloride. The mixture was heated to reflux for 4 h under illumination from a 500 W halogen lamp, then allowed to cool to room temperature. The resulting succinimide precipitate was removed by filtration and washed with hot chloroform. The combined filtrate was washed sequentially with aqueous sodium chloride solution and water. After drying the organic layer over anhydrous magnesium sulfate and evaporating the solvent, a viscous brown oil was obtained (1.36 g; 2.36 mmol; 89%). 1H NMR δ: 0.786 (t, J = 7.0 Hz, 6H), 0.905–1.30 (m, 24H), 1.97–2.33 (m, 4H), 4.73 (s, 2H), 4.88 (s, 2H), 7.22–7.43 (m, 4H), 7.67–8.10 (m, 2H). FTIR (CHCl3), cm−1: 3045, 3019 (CH aromatic), 2927, 2854 (CH aliphatic), 1600 (C=C aromatic), 1462, 1375 (CH aliphatic), 828 (CH aromatic), 652 (CBr). Anal. Calc. for C31H44Br2: C, 64.59; H, 7.69. Found: C, 64.93; H, 7.94. CV: −1.3 V vs. Ag/AgBr (−1.6 V vs. SCE).
Poly(9,9-dioctyl-1,4-fluorenylene-1,2-ethylene) (3): 1,4-bis-bromomethyl-9,9-dioctyl-fluorene (5) (0.906 g, 1.57 mmol) was polymerized via electrolysis at a mercury pool cathode in a divided cell. The electrolyte solution consisted of 0.1 M tetraethylammonium bromide (Et4NBr) in N,N-dimethylformamide (DMF) (50 mL). Electrolysis was performed at −1.3 V versus Ag/AgBr, using a graphite rod as the anode. The cathode compartment was continuously purged with dry nitrogen. A yellow precipitate formed during the reaction. Upon passage of approximately 2.1 Fmol−1, the cell current decreased to background levels. The precipitate was collected by filtration, washed extensively with water to remove DMF and Et4NBr, and dried under vacuum. The filtrate was then treated with water to precipitate an additional polymer fraction (DMF-soluble), which was also washed and dried. The insoluble fraction yielded 0.119 g (0.286 mmol, 18%), and the DMF-soluble fraction yielded 0.332 g (0.798 mmol, 51%). Total yield: 69 %. 1H NMR δ: 0.69–0.98 (m, 6H), 1.03–1.38 (m, 24H), 2.17–2.50 (m, 4H), 3.25–3.58 (m, 4H), 7.30–7.37 (m, 4H), 7.82–7.97 (m, 2H). FTIR (CHCl3), cm−1: 3041, 3014 (CH, aromatic), 2927, 2854 (CH aliphatic), 1597 (C=C, aromatic), 1461, 1374 (CH aliphatic), and 819 (CH aromatic). UV-Vis (CHCl3): bandgap = 3.53 eV. SEC: Mw = 2.8 × 104 g mol−1, Mn = 1.3 × 104 g mol−1, and Mw/Mn = 2.1.
4,4′-Bis-bromomethyl-2-decyloxy-biphenyl (6): 2-decyloxy-4,4′-dimethyl-biphenyl (8) (1.80 g; 5.32 mmol), NBS (1.98 g; 11.1 mmol), and dibenzoyl peroxide (10 mg) were treated as described above for compound 5. A viscous yellow oil was obtained, which crystallized after the addition of some drops of hexane. The solid was recrystallized from hexane/chloroform to give 1.51 g (3.04 mmol; 57%) of a white solid. MP. 46–51 °C. 1H NMR δ: 0.886 (t, J = 6.0 Hz, 3H), 1.27 (m, 14H), 1.72 (m, 2H), 3.97 (t, J = 6.6 Hz, 2H), 4.52 (s, 2H), 4.54 (s, 2H), 6.99 (s, 1H), 7.03 (d, J = 8.0 Hz, 1H), 7.28 (d, J = 8.0 Hz, 1H), 7.41 (d, J = 8.0 Hz, 2H), and 7.51 (d, J = 8.0 Hz, 2H). FTIR (KBr), cm−1: 3029 (CH aromatic), 2957, 2919, 2853 (CH aliphatic), 1607, 1474 (C=C aromatic), 1457, 1396 (CH aliphatic), 1279, 1019 (COC), and 849, 815 (CH aromatic). Anal. Calc. for C24H32Br2O: C, 58.1; H, 6.49. Found: C, 57.6; H, 6.29. CV: −1.8 V vs. Ag/AgBr (−2.1 V vs. SCE).
Poly(2-decyloxy-4,4′-biphenylene-1,2-ethylene) (4): 4,4′-bis-bromomethyl-2-decyloxy-biphenyl (6) (1.33 g, 2.68 mmol) was electrolyzed at −1.8 V (vs. Ag/AgBr) as described above for polymer 3. Yield: 75 mg (0.22 mmol; 8 %) of insoluble fraction and 573 mg (1.71 mmol; 64%) of DMF-soluble fraction. Total yield: 72%. 1H NMR δ: 0.886 (m, 3H), 1.26 (m, 14H), 1.70 (m, 2H), 2.99 (m, 4H), 3.93 (m, 2H), 6.78 (m, 1H), 6.82 (m, 1H), 7.17–7.22 (m, 3H), 7.42–7.46 (m, 2H). FTIR (KBr), cm−1: 3024 (CH, aromatic), 2922, 2852 (CH aliphatic), 1604, 1494 (C=C, aromatic), 1466, 1399 (CH aliphatic), 1272, 1032 (COC), and 845, 814 (CH aromatic). UV-Vis (CHCl3): bandgap = 3.02 eV. SEC: Mw = 1.3 × 105 g mol−1, Mn = 4.3 × 104 g mol−1, Mw/Mn = 3.0.
2.3. Preparation of the Sensors
The sensors were prepared by spin coating (300 rpm, 30 s, 25 °C) 40 µL of a polymer solution onto an interdigitated electrode, forming an adherent thin film of ~10 µm thickness, measured with a Dektak 3 profilometer (Veeco Instruments). The polymer solution was prepared by dissolving 2.5 mg of polymer 1, 2, 3, or 4 and 0.7 mg of dodecylbenzenesulfonic acid (DBSA) (dopant) in 0.7 mL of chloroform. The interdigitated electrodes (1 cm2, 24 µm gap between digits) were prepared by conventional lithography.
2.4. Cachaça and Wood Samples
A certified sample of non-aged cachaça, without added sugar, was kindly provided by Indústrias Müller de Bebidas (Pirassununga, São Paulo, Brazil) and was used as a solvent for the extraction procedure, without dilution and maintaining alcohol content ≈ 47% v/v for all samples prepared. Certified air-dried wood samples of the following species were purchased from the Laboratório de Madeiras e Estrutura da Universidade de São Paulo (São Carlos, São Paulo, Brazil): jatobá (Hymenaea courbaril), canela-sassafras (Ocotea odorifera), jequitibá (Cariniana legalis), and amburana (Amburana cearensis). The certified air-dried or kiln-dried oak samples were provided by Prof. John Piggot (Department of Biosciences, University of Strathclyde, Glasgow, Scotland). The extracts were prepared following a procedure established to mimic an accelerated aging process, previously described [11], where dry and ground sawdust (100 ± 20 mesh) of the certified wood species was used and subjected to extraction with unaged cachaça (47% v/v) for 26 days on an automatic vibrating table at room temperature (25 ± 2 °C).
2.5. E-Nose Measurements
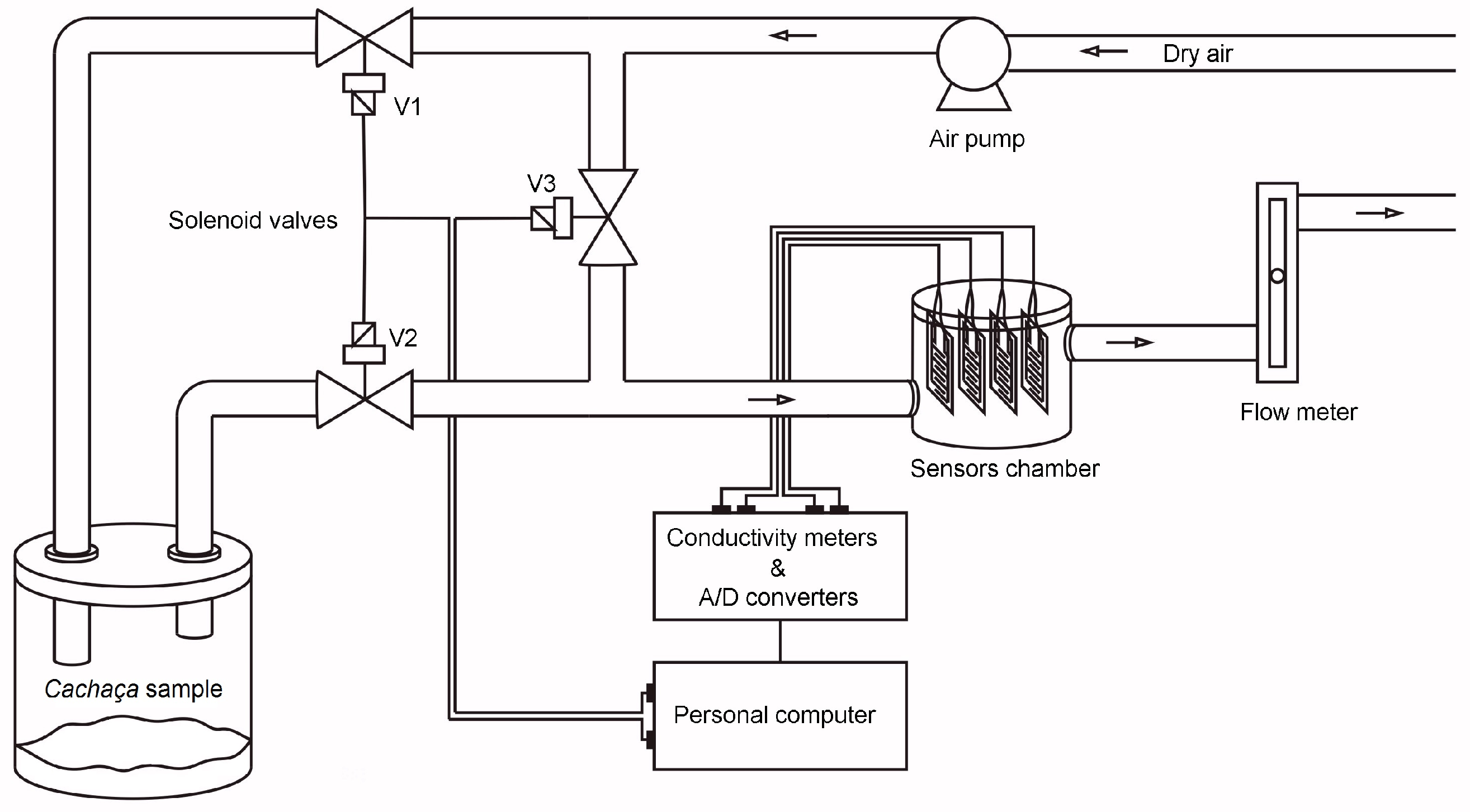
These were carried out with the aid of a home-made pneumatic assembly suitable for dynamic sampling (Figure 1), which is comprised of an air pump (0.5 L min−1), three solenoid valves, a sample chamber, a sensor chamber, and a flow meter. Ten exposure (5 s)/recovery (40 s) cycles were performed per cachaça sample. Sensor electrical conductance was measured every 50 milliseconds using four precise conductivity meters [23]. These meters utilized an 80 mV peak-to-peak, 2 kHz triangle wave AC voltage and were interfaced with a computer via a 10-bit analog-to-digital converter for real-time data capture.
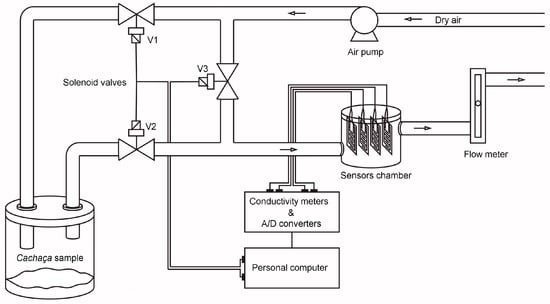
Figure 1.
Setup of the measuring system. The arrows show the direction of the air flow.
3. Results and Discussion
3.1. Syntheses
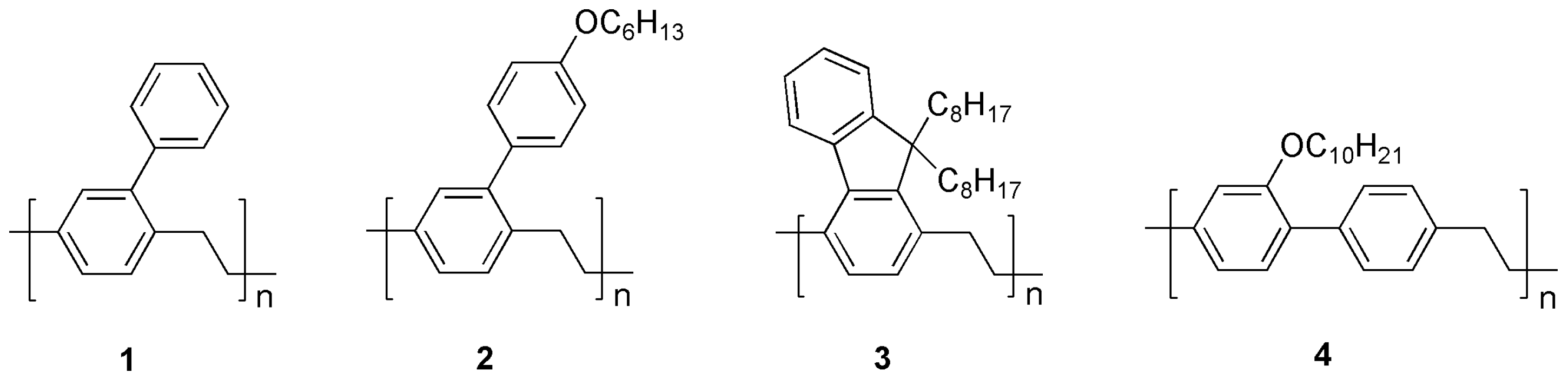
The polymers used (Figure 2) for assembling the chemoresistive layers of the sensors are derivatives of poly-p-xylylene (PPX), which is a class of high-performance materials with excellent dielectric and insulating properties. Nevertheless, the introduction of conjugated side groups, as in 1, 2, and 3, or of a substituted biphenyl system (4), lowers the energy gap of the polymers, which become conductive upon doping with Lewis acids, e.g., DBSA, without significantly affecting their high chemical stability. This is particularly important in gas sensors, since the devices cannot be encapsulated, exposing the polymer films to air and volatile analytes.
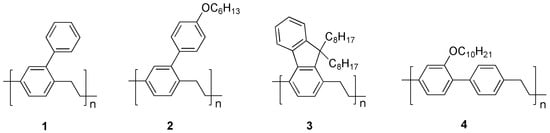
Figure 2.
Structural formulae of the polymers used for assembling the sensors.
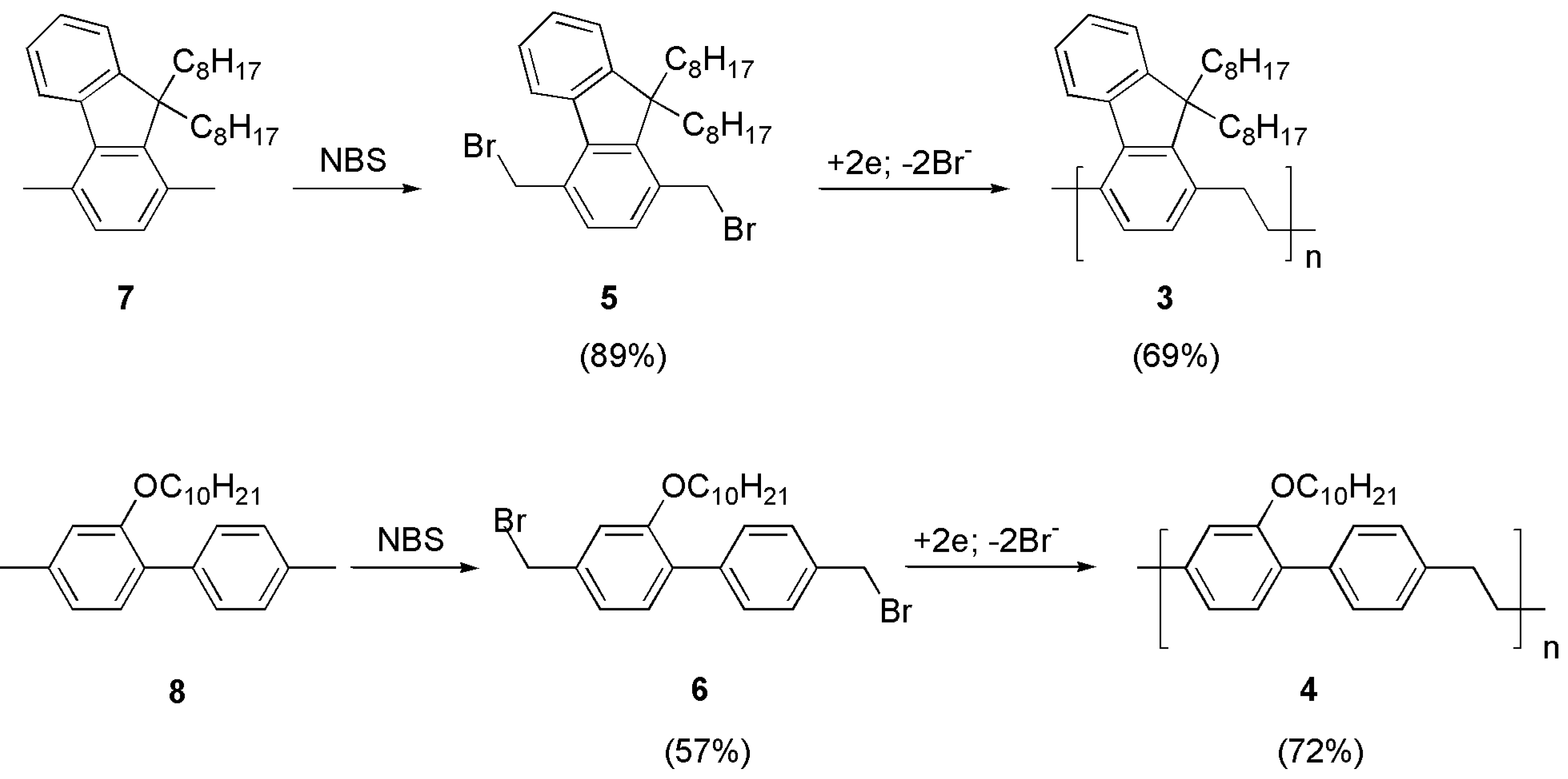
The syntheses of polymers 1 and 2 have been described before [21,22]. Polymers 3 and 4 are new materials and were prepared by the potentiostatic cathodic reduction in their corresponding bis-bromomethylated precursors (5, 6), which were obtained by benzylic bromination of compounds 7 and 8, respectively (Figure 3).
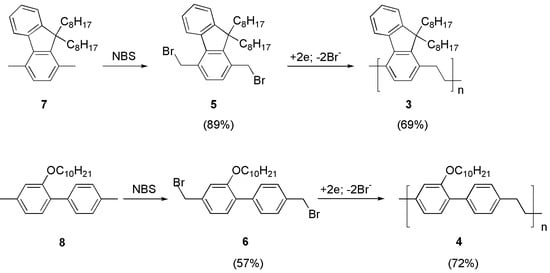
Figure 3.
Synthetic routes to polymers 3 and 4. The yield of each step is indicated in parentheses.
3.2. E-Nose
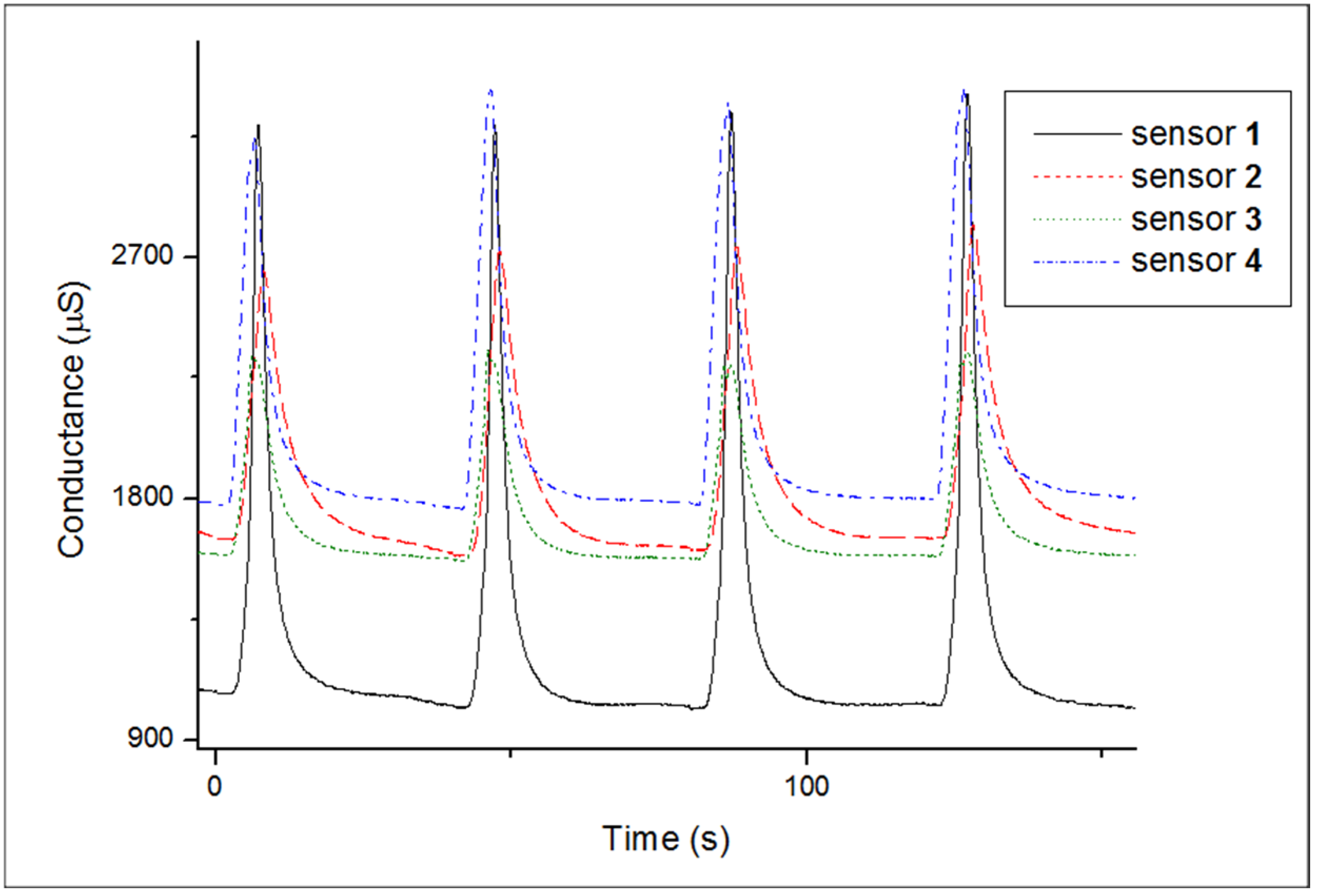
The electrical conductance of an array of four sensors was monitored during exposure to the headspace of cachaça samples followed by dry air. A typical response pattern is shown in Figure 4 only for the first four exposure/recovery cycles for one cachaça sample aged in an oak barrel. The graph reveals: (i) different patterns for each sensor, (ii) high relative responses (up to 210%), (iii) the reversible nature of the sensors, and (iv) fast response and recovery times.
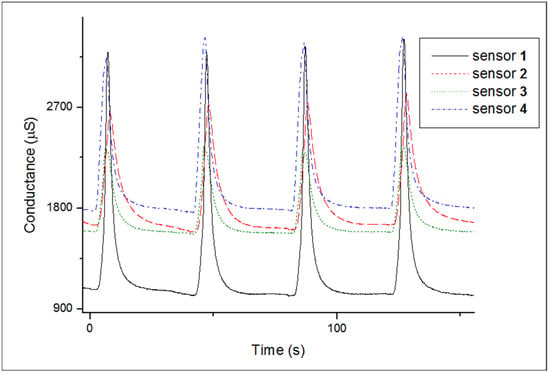
Figure 4.
Response of four sensors (coated with polymer 1, 2, 3, and 4, respectively) to a sequence of four exposures/recoveries to the headspace of a cachaça sample aged in an oak barrel/air at 30 °C. The sensor array is exposed to the headspace of the sample under analysis for 5 s, then exposed to air for 40 s.
The relative responses (Ra) for all the exposure/recovery cycles for all the tested samples were calculated according to Equation (1).
where Gf is the conductance at the end of the exposure period and Go is the initial conductance.
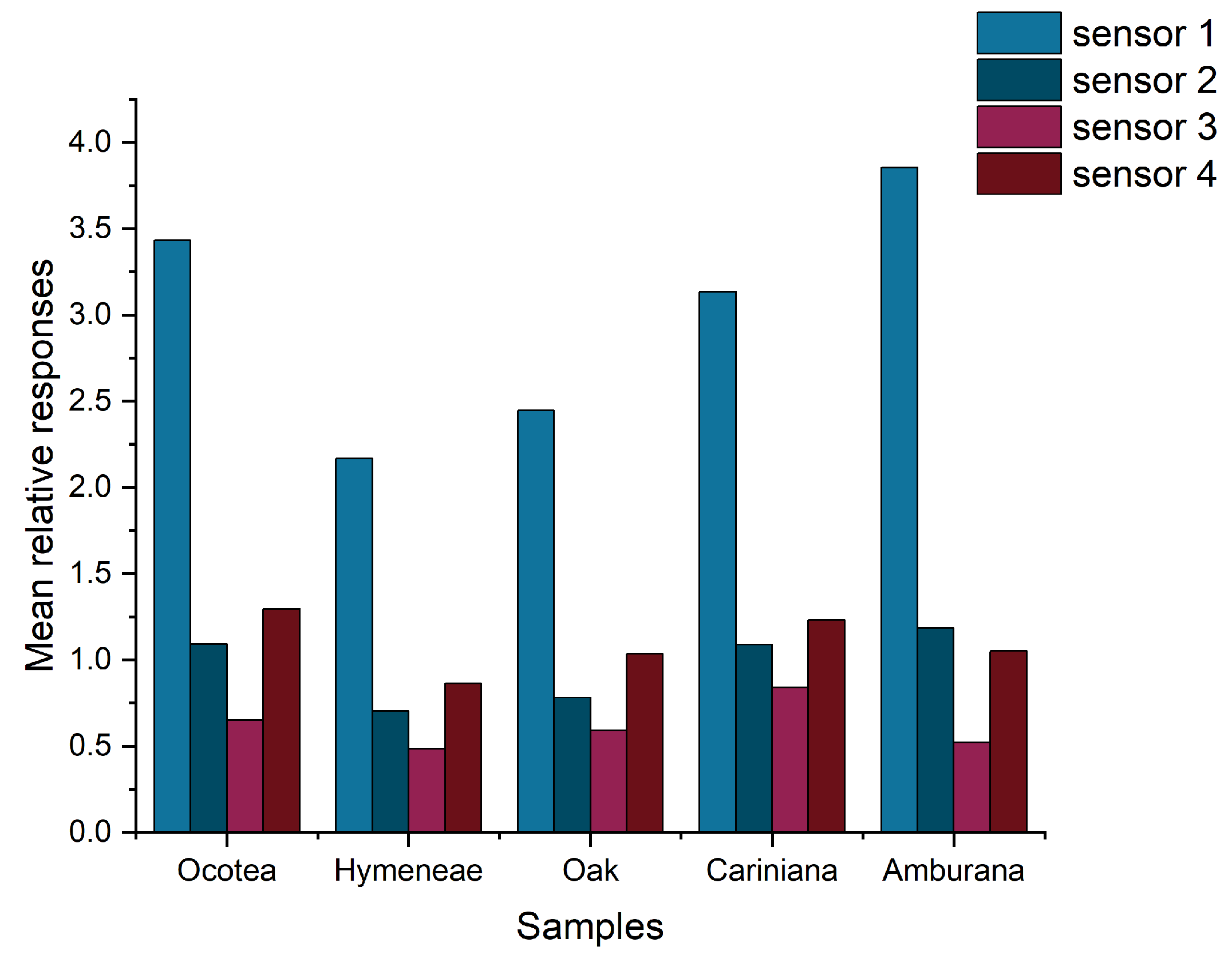
The set of Ra values obtained by the four sensors for all the samples was normalized by Z-score normalization. A bar graph of the mean Ra values is shown in Figure 5.
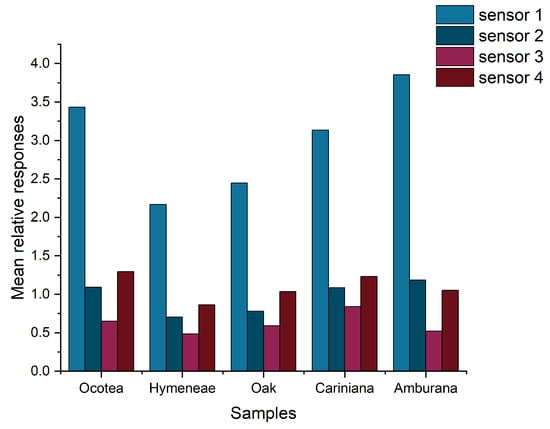
Figure 5.
Bar graph of the mean normalized Ra values of the sensors exposed to cachaça samples aged in 5 different woods.
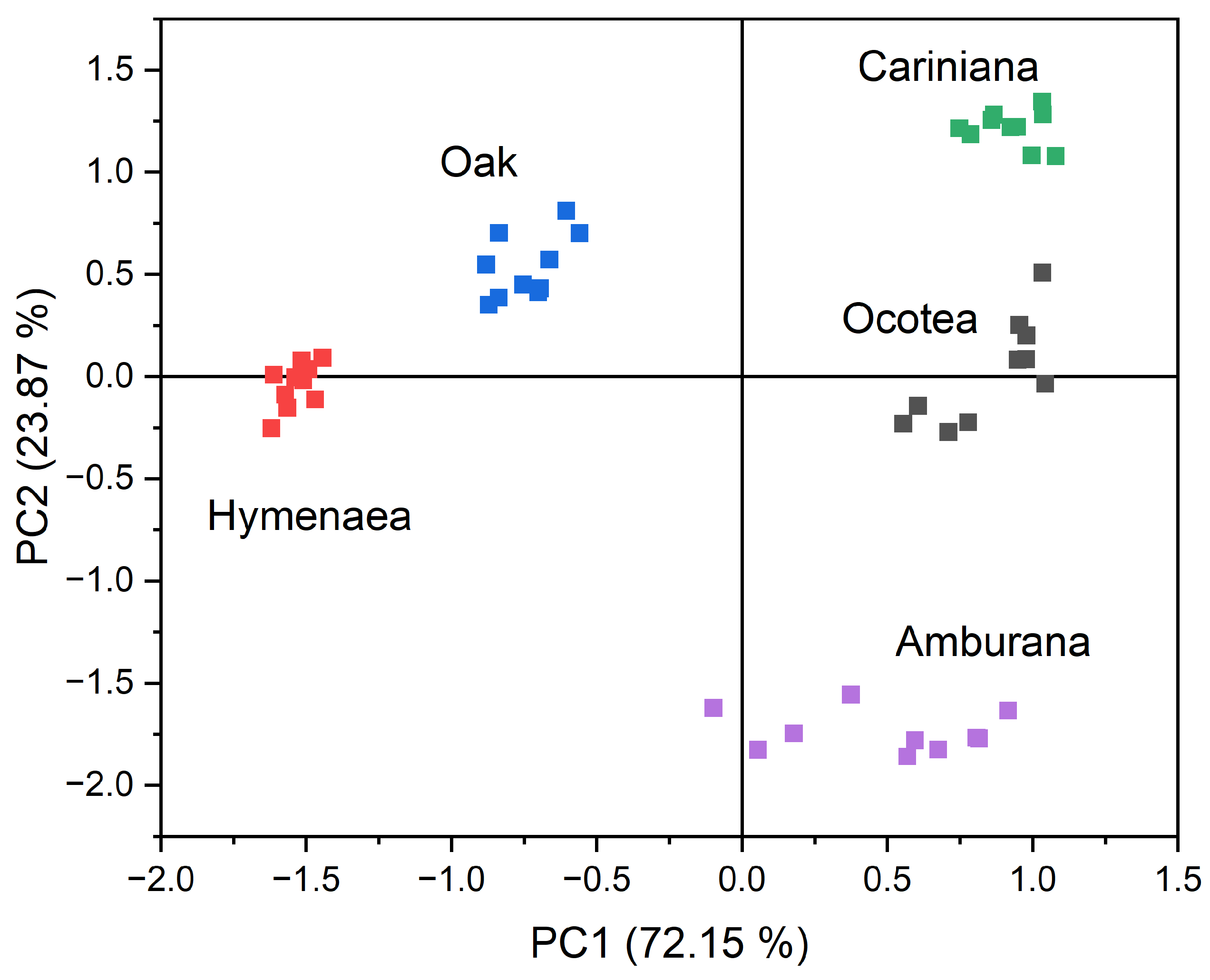
This set of Ra values was used as input data for principal component analysis (PCA) to evaluate the potential separation and classification power of the different aqueous-ethanolic wood extracts studied. A cluster plot with the first two principal components (PCs) (Figure 6) demonstrated the system’s ability to classify the samples into distinct clusters, presenting a grouping according to the wood species of the corresponding aged cachaça samples. Each cluster has 10 points corresponding to the 10 repetitions (exposure/recovery cycles). The sum of the first two PCs (PC1: 72.15% and PC2: 23.87%) accounted for 96.02% of the total variance in the original data. While PC1 is important for separating oak and Hymenaea from the other woods, PC2 is crucial in differentiating the Cariniana and Amburana clusters. To validate the predictive capability of the method, the leave-one-out cross-validation technique [24,25] was applied, achieving a 100% accuracy rate for the test samples. This result highlights the method’s potential as a reliable tool for distinguishing different wood species in water–ethanol solutions. The portability of a system of this scale, combined with the rapid response of the measurements, can be highly valuable for in situ analyses. Additionally, pre-programmed statistical software can be used to process analytical data in real time during collection. In case of replacement, each sensor—including the interdigitated electrode and polymer film—costs only USD 5.00, making it a cost-effective solution.
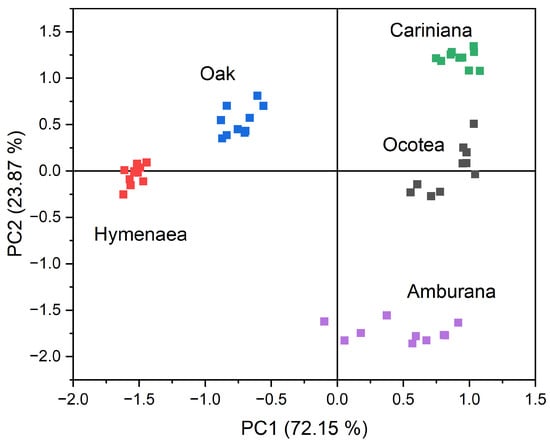
Figure 6.
Bidimensional principal component analysis plot for the five wood species in which the cachaça samples were aged. Altogether 200 relative responses (Ra) (10 cycles x 4 sensors x 5 types of wood) were used as the input data for the PCA analysis, which gave 5 separated clusters, one for each type of wood. Each cluster has 10 points corresponding to the 10 repetitions (exposure/recovery cycles).
Finally, it is worth mentioning that although the use of techniques such as HPLC, fluorescence, and GC-MS have proven to be powerful methods with significant identification power to evaluate potential specific descriptors for the wood samples to be analyzed [26], these tend to be time-consuming, expensive, and require laboratory infrastructure and a qualified operator, often not available at the site where the analysis is to be performed. In studies using the same sample preparation method as ours and spectrophotometric analysis [11], it appears to be a much cheaper and faster alternative method and yielded good results for oak (Quercus petraea), jatobá (Hymenaea courbaril), jequitibá (Cariniana legalis), and amburana (Amburana cearensis), but failed to group data for cinnamon sassafras (Ocotea odorifera). The easy assembly of the complete system, as reported for analysis, requiring minimal space for operation, makes it an important tool for use in remote locations with little laboratory infrastructure. Therefore, methods using an electronic nose setup as presented in this article represent progress and offer an alternative for this type of analysis due to their inherent ease of operation, low cost, portability, and efficiency.
4. Conclusions
The electronic nose described, based on four sensors formed by different poly-p-xylylene derivatives, can recognize five common woods in which Brazilian sugar cane spirit (cachaça) is usually aged. The equipment is low-cost (about USD 200), portable, and can be operated by rechargeable batteries. Data can be processed on small computers (e.g., notebooks, tablets), and the analysis time is only 5 min. This method can be extended to other alcoholic beverages and other wood species. These features make this e-nose viable for quality control in the industrial sectors, as well as to aid tampering detection by consumers or by legal authorities.
Author Contributions
Conceptualization, J.G. and A.A.S.; methodology, J.G., A.A.S., B.R.V., E.Y.Y., and R.W.C.L.; software, A.A.S. and E.Y.Y.; validation, J.G. and A.A.S.; formal analysis, J.G. and A.A.S.; investigation, B.R.V., E.Y.Y., and R.W.C.L.; resources, J.G.; data curation, J.G. and A.A.S.; writing—original draft preparation, J.G. and A.A.S.; writing—review and editing, J.G.; visualization, J.G. and A.A.S.; supervision, J.G.; project administration, J.G.; funding acquisition, J.G. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), grant number 310048/2022-2 to J.G.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
This paper is dedicated to Douglas W. Franco (in memoriam). The authors would like to thank Marcelo N. P. Carreño and Gustavo P. Rehder (EP-USP) for assembling the interdigitated electrodes, and Flavio Makoto Shimizu for his help with the principal component analysis. Thanks are due to Elina W. Gruber for the language revision.
Conflicts of Interest
The authors declare no conflicts of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| AC | Alternating current |
| CV | Cyclic voltammetry |
| DBSA | Dodecylbenzenesulfonic acid |
| DMF | N,N-dimethylformamide |
| FT | Fourier-transform |
| FTIR | Fourier-transform infrared |
| HPLC/DAD | High-performance liquid chromatography with diode-array detection |
| IAWA | International Association of Wood Anatomists |
| NBS | N-bromosuccinimide |
| NMR | Nuclear magnetic resonance |
| PC | Principal component |
| PCA | Principal component analysis |
| PPX | Poly-p-xylylene |
| SCE | Saturated calomel electrode |
| SEC | Size exclusion chromatography |
| THF | Tetrahydrofuran |
| TMS | Tetramethylsilane |
| UV-Vis | Ultraviolet–visible |
References
- Tarko, T.; Krankowski, F.; Duda-Chodak, A. The Impact of Compounds Extracted from Wood on the Quality of Alcoholic Beverages. Molecules 2023, 28, 620. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Gil, A.; del Alamo-Sanza, M.; Sánchez-Gómez, R.; Nevares, I. Different Woods in Cooperage for Oenology: A Review. Beverages 2018, 4, 94. [Google Scholar] [CrossRef]
- Reazin, G.H. Chemical analysis of whisky maturation. In Flavour of Distilled Beverages: Origin and Development; Piggott, J.R., Ed.; Ellis Horwood: Chichester, UK, 1983; pp. 225–240. [Google Scholar]
- García-Moreno, M.V.; Sánchez-Guillén, M.M.; de Mier, M.R.; Delgado-González, M.J.; Rodríguez-Dodero, M.C.; García-Barroso, C.; Guillén-Sánchez, D.A. Use of Alternative Wood for the Ageing of Brandy de Jerez. Foods 2020, 9, 250. [Google Scholar] [CrossRef] [PubMed]
- Lima, C.M.G.; Benoso, P.; Pierezan, M.D.; Santana, R.F.; Hassemer, G.S.; Rocha, R.A.; Nora, F.M.D.; Verruck, S.; Caetano, D.; Simal-Gandara, J. A state-of-the-art review of the chemical composition of sugarcane spirits and current advances in quality control. J. Food Compos. Anal. 2022, 106, 104338. [Google Scholar] [CrossRef]
- Machado, J.S.; Pereira, F.; Quilhó, T. Assessment of old timber members: Importance of wood species identification and direct tensile test information. Constr. Build. Mater. 2019, 207, 651–660. [Google Scholar] [CrossRef]
- Ruffinatto, F.; Crivellaro, A.; Wiedenhoeft, A.C. Review of Macroscopic Features for Hardwood and Softwood Identification and a Proposal for a New Character List. IAWA J. 2015, 36, 208–241. [Google Scholar] [CrossRef]
- Wheeler, E.A.; Baas, P.; Gasson, P.E. (Eds.) IAWA List of Microscopic Features for Hardwood Identification; International Association of Wood Anatomists: Leiden, the Netherlands, 1989; Volume 10, pp. 219–332. [Google Scholar]
- Zielinski, K.M.; Scabini, L.; Ribas, L.C.; da Silva, N.R.; Beeckman, H.; Verwaeren, J.; Bruno, O.M.; De Baets, B. Advanced wood species identification based on multiple anatomical sections and using deep feature transfer and fusion. Comput. Electron. Agr. 2025, 231, 109867. [Google Scholar] [CrossRef]
- Dierickx, S.; Genbrugge, S.; Beeckman, H.; Hubau, W.; Kibleur, P.; Van den Bulcke, J. Non-destructive wood identification using X-ray μCT scanning: Which resolution do we need? Plant Methods 2024, 20, 1–14. [Google Scholar] [CrossRef]
- Da Silva, A.A.; De Keukeleire, D.; Cardoso, D.R.; Franco, D.W. Multivariate analyses of UV-Vis absorption spectral data from cachaça wood extracts: A model to classify aged Brazilian cachaças according to the wood species used. Anal. Methods 2012, 4, 642–646. [Google Scholar] [CrossRef]
- de Sousa Fernandes, D.D.; de Almeida, V.E.; Fontes, M.M.; de Araújo, M.C.U.; Véras, G.; Diniz, P.H.G.D. Simultaneous identification of the wood types in aged cachaças and their adulterations with wood extracts using digital images and SPA-LDA. Food Chem. 2019, 273, 77–84. [Google Scholar] [CrossRef]
- Rodrigues, B.; Costa, R.M.; Salvini, R.; Soares, A.S.; Silva, F.; Caliari, M.; Cardoso, K.R.; Ribeiro, T. Cachaça classification using chemical features and computer vision. Procedia Comput. Sci. 2014, 29, 2024–2033. [Google Scholar] [CrossRef]
- Silveira, A.L.; Barbeira, P.J.S. A fast and low-cost approach for the discrimination of commercial aged cachaças using synchronous fluorescence spectroscopy and multivariate classification. J. Sci. Food Agric. 2022, 102, 4918–4926. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, S.C.; Oldoni, T.L.C.; Veras, G.; Sousa, E.S.; Fernandes, D.D.S. Non-destructive authentication of Cachaças from Breno Paraibano based on MIR spectroscopy. Food Chem. 2025, 477, 143554. [Google Scholar] [CrossRef] [PubMed]
- Sivello, G.C.; Alcarde, A.R. Experimental design and chemometric techniques applied in electronic nose analysis of wood-aged sugar cane spirit (cachaça). J. Agr. Food Res. 2020, 2, 100037. [Google Scholar]
- Matrí, M.P.; Pino, J.; Boqué, R.; Busto, O.; Giasch, J. Determination of ageing time of spirits in oak barrels using a headspace–mass spectrometry (HS-MS) electronic nose system and multivariate calibration. Anal. Bioanal. Chem. 2005, 382, 440–443. [Google Scholar]
- Chiu, S.-W.; Tang, K.-T. Towards a Chemiresistive Sensor-Integrated Electronic Nose: A Review. Sensors 2013, 13, 14214–14247. [Google Scholar] [CrossRef]
- Niinomi, T.; Nakao, A.; Hanai, Y.; Ushio, H.; Hayashi, T.; Nakatani, M. A Compact 16-Channel Input Thermally Adsorption-/Desorption-Controlled Intelligent Odor Sensing System. IEEE Sens. J. 2024, 24, 9334–9340. [Google Scholar] [CrossRef]
- Esteves, H.A.; Gonçalves, W.B.; Teixeira, W.S.R.; Pádua, A.C.C.S.; Gruber, J. Conductive polymer-based sensors. In Organic and Inorganic Materials Based Sensors, 1st ed.; Das, S., Thomas, S., Das, P., Eds.; Wiley: Weinheim, Germany, 2024; Volume 2, pp. 559–591. [Google Scholar]
- Li, R.W.C.; Carvalho, L.R.F.; Ventura, L.; Gruber, J. low cost selective sensor for carbonyl compounds in air based on a novel conductive poly(p-xylylene) derivative. Mater. Sci. Eng. C 2009, 29, 426–429. [Google Scholar] [CrossRef]
- Li, R.W.C.; Ventura, L.; Gruber, J.; Kawano, Y.; Carvalho, L.R.F. A selective conductive polymer-based sensor for volatile halogenated organic compounds (VHOC). Sens. Actuators B Chem. 2008, 131, 646–651. [Google Scholar] [CrossRef]
- Rocha, R.T.; Gutz, I.G.R.; Lago, C.L. A Low-Cost and High-Performance Conductivity Meter. J. Chem. Educ. 1997, 74, 572–574. [Google Scholar] [CrossRef]
- Lopes, F.M.; Martins, D.C.; Cesar, R.M. Feature selection environment for genomic applications. BMC Bioinform. 2008, 9, 451. [Google Scholar] [CrossRef] [PubMed]
- Pomerantsev, A.L.; Rodionova, O.Y. Procrustes Cross-Validation of short datasets in PCA context. Talanta 2021, 226, 122104. [Google Scholar] [CrossRef] [PubMed]
- Flamini, R.; Vedova, A.D.; Cancian, D.; Panighel, A.; De Rosso, M. GC/MS-positive ion chemical ionization and MS/MS study of volatile benzene compounds in five different woods used in barrel making. J. Mass Spectrom. 2007, 42, 641–646. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).