Functionalized Three–Dimensional Graphene Containing Chitosan and Bovine Serum Albumin for Recognizing Chiral Drug Intermediates
Abstract
1. Introduction
2. Experimental Section
2.1. Reagents and Apparatus
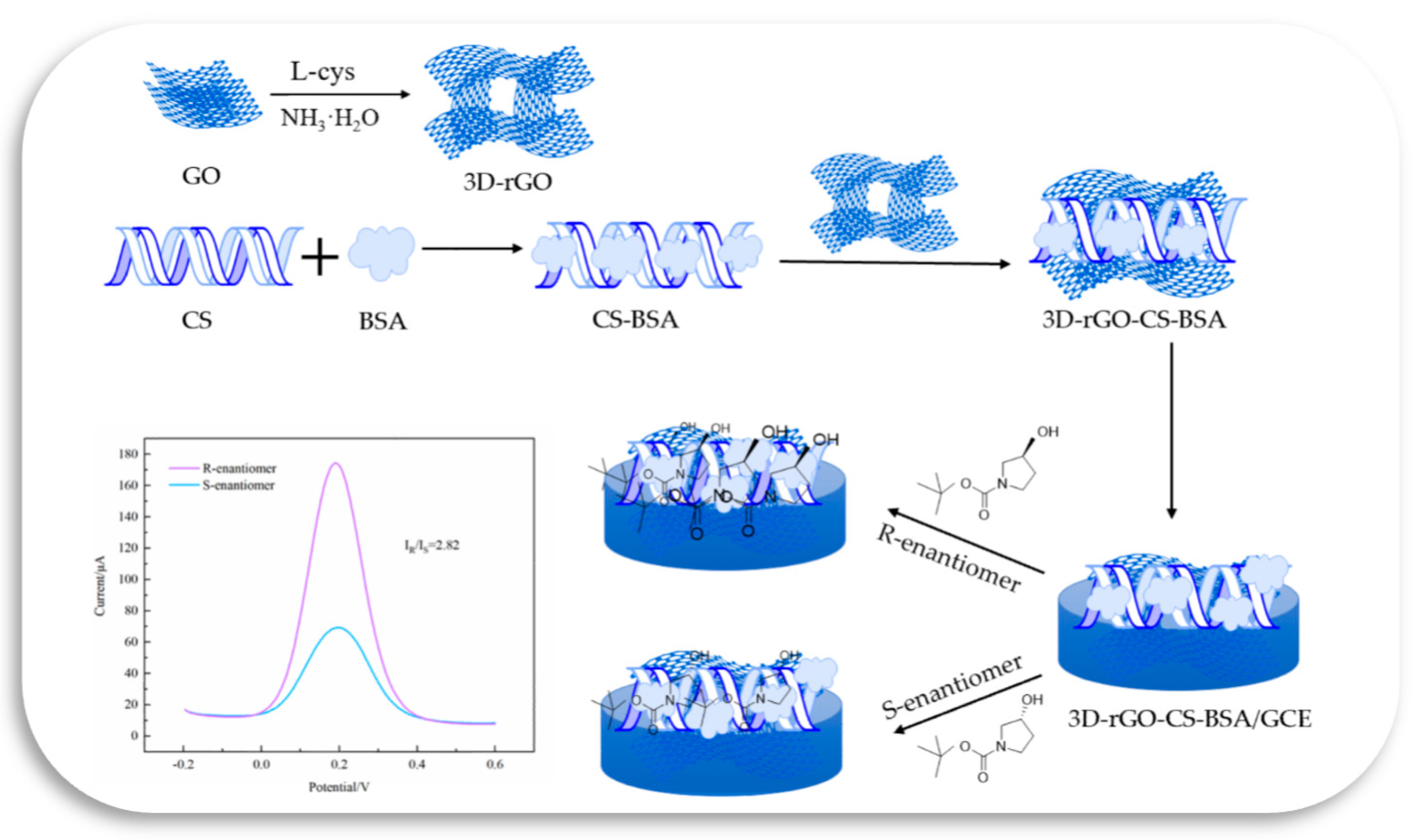
2.2. Preparation of 3D–rGO–CS–BSA/GCE
2.3. Electrochemical Chiral Discrimination of 1–Boc–3–Hydroxypyrrolidine Enantiomers
2.4. Preparation of Samples for Characterization
3. Results and Discussion
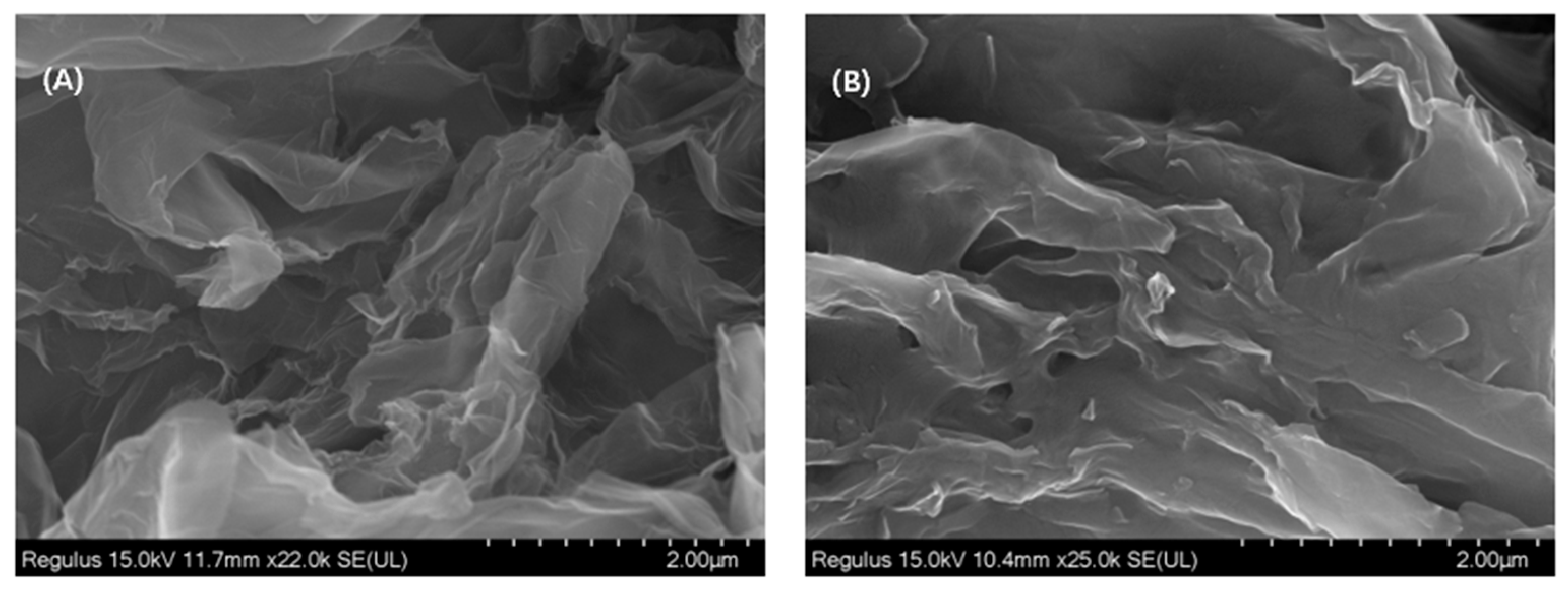
3.1. SEM Images of 3D–rGO and 3D–rGO–CS–BSA
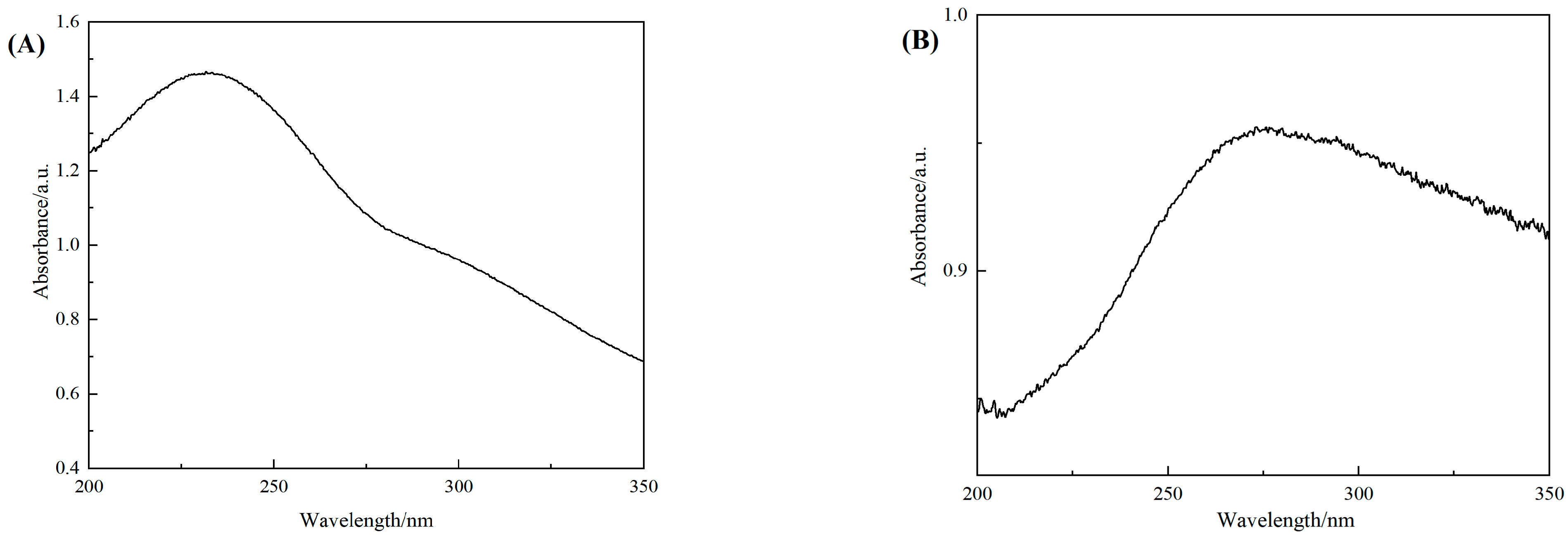
3.2. UV Spectra of GO and 3D–rGO
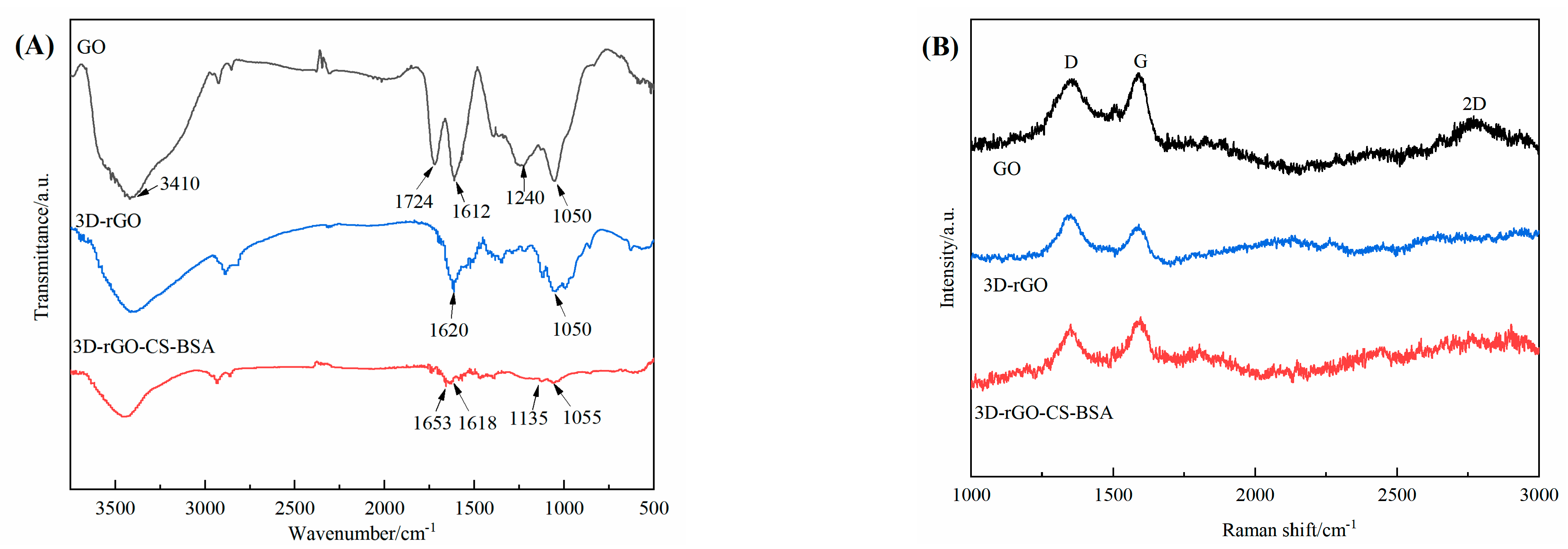
3.3. FT–IR and Raman Spectra of GO, 3D–rGO, and 3D–rGO–CS–BSA
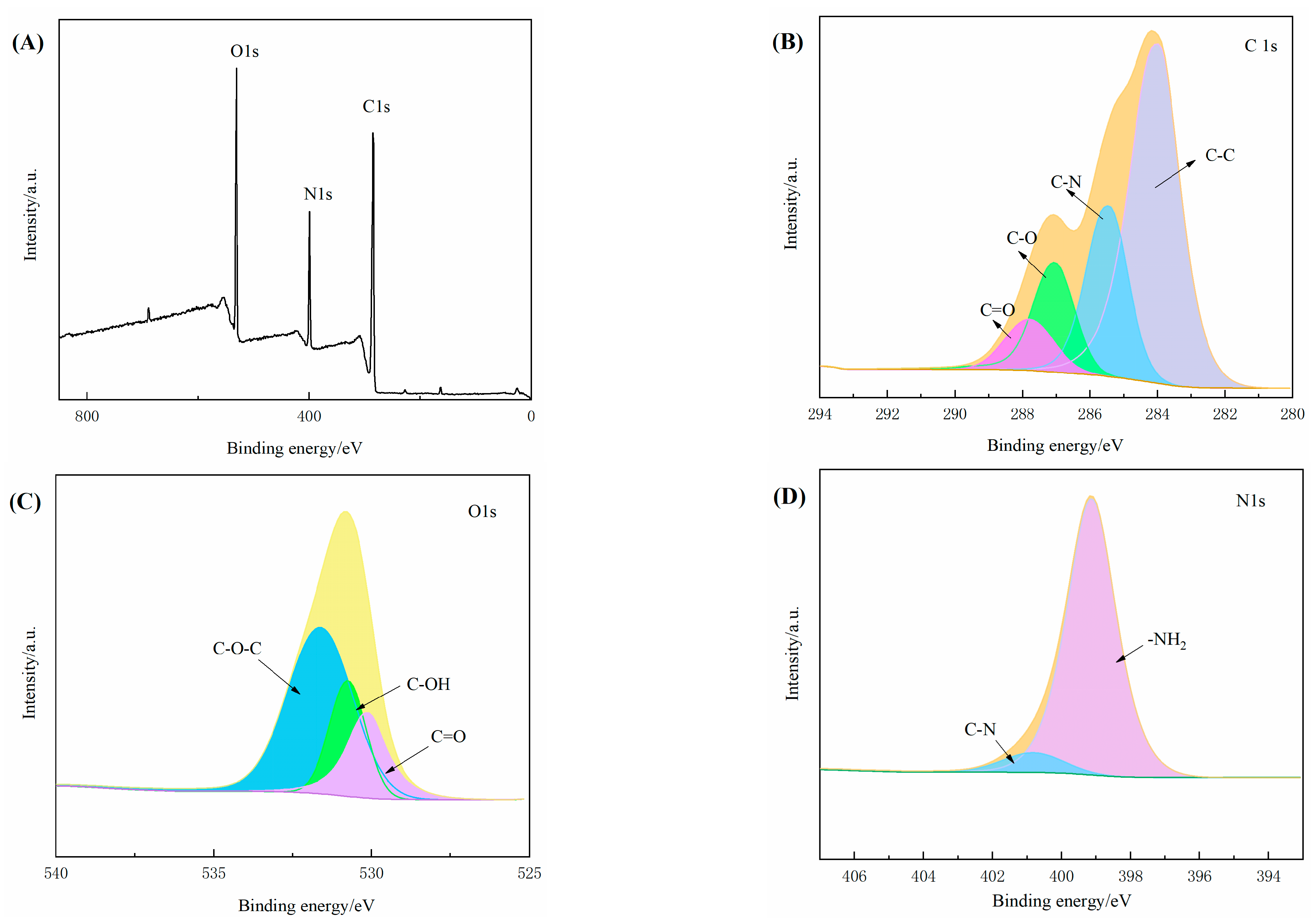
3.4. XPS of 3D–rGO–CS–BSA
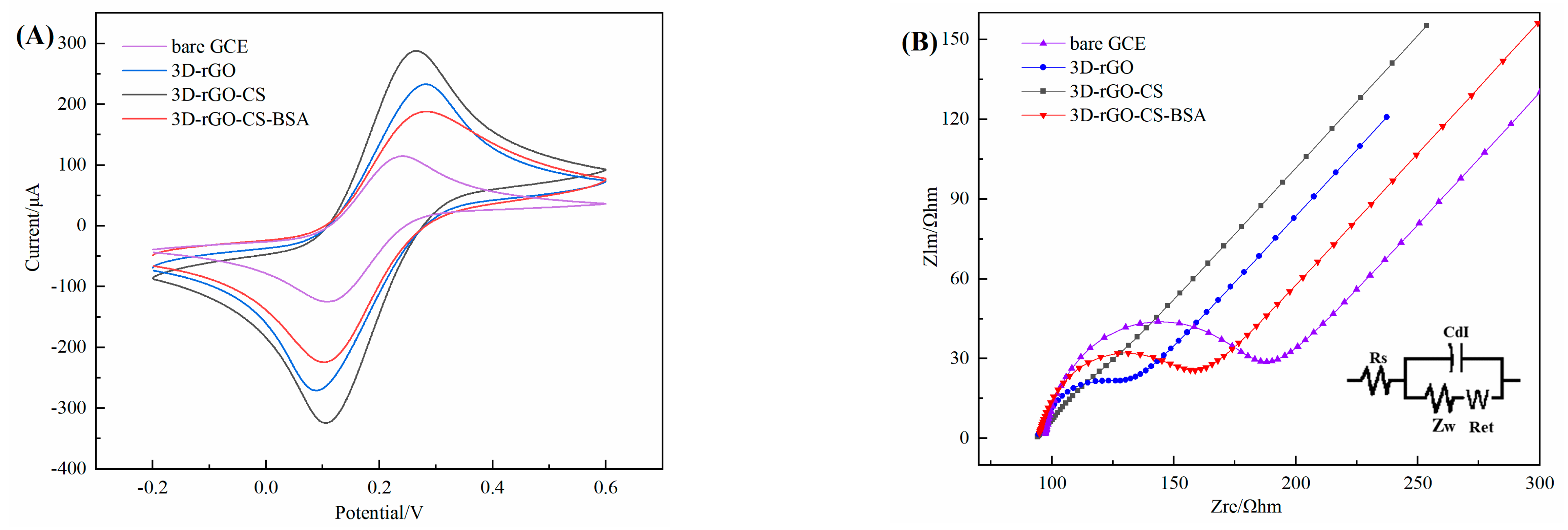
3.5. Electrochemical Characterization
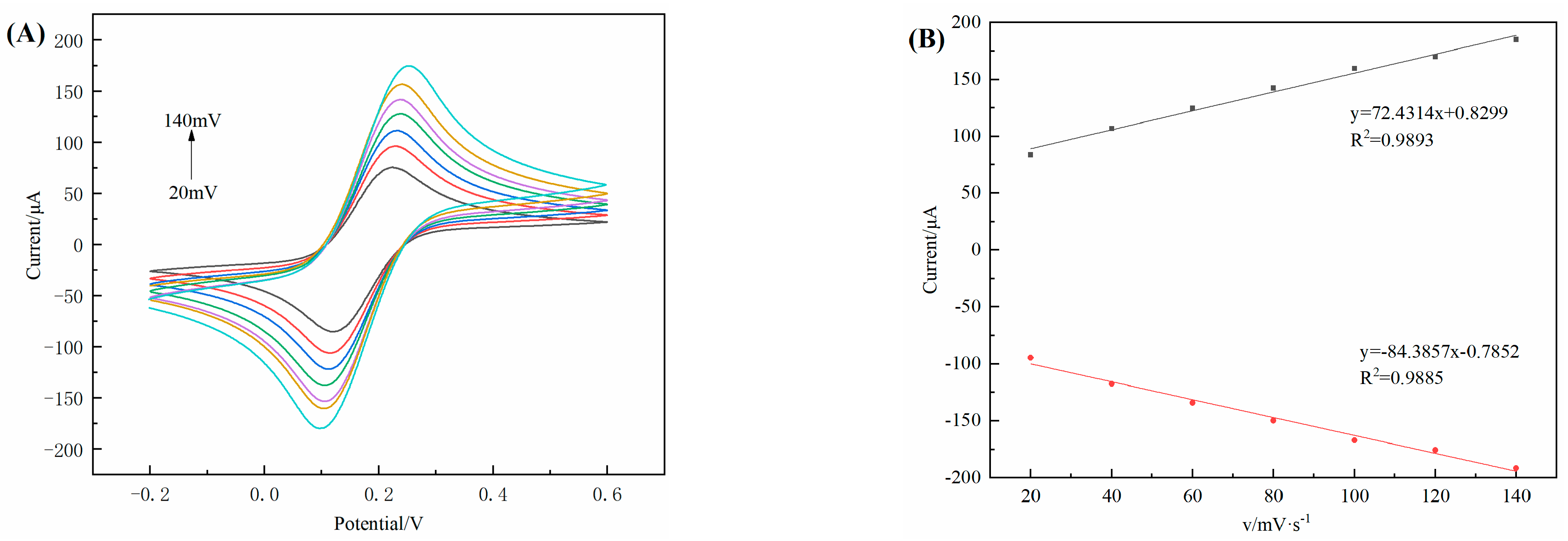
3.6. Electrochemical Kinetics of 3D–rGO–CS–BSA/GCE
3.7. Electrochemical Chiral Discrimination of Enantiomers
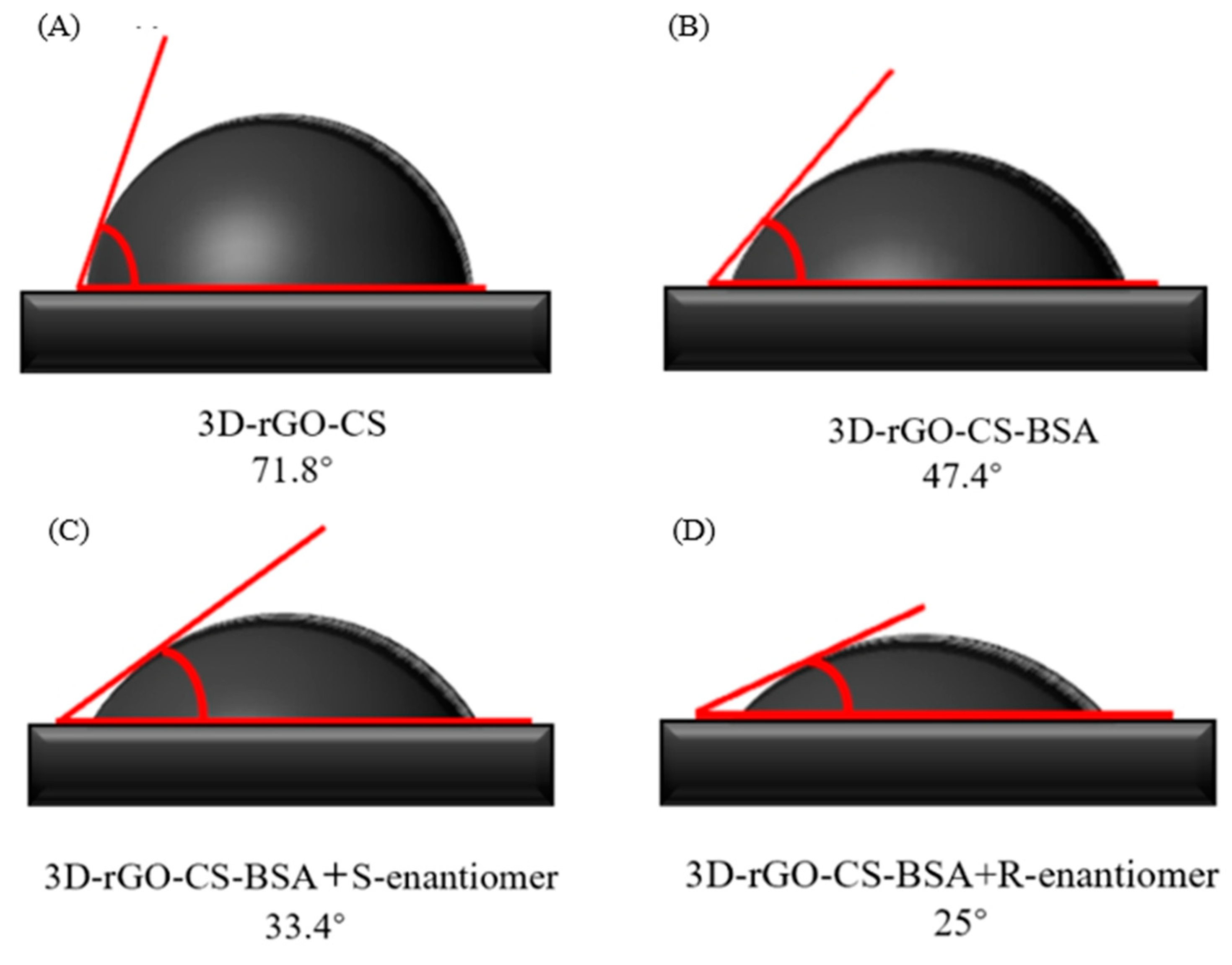
3.8. Water Contact Angle on Different Samples
3.9. The Calculation of the Binding Constant
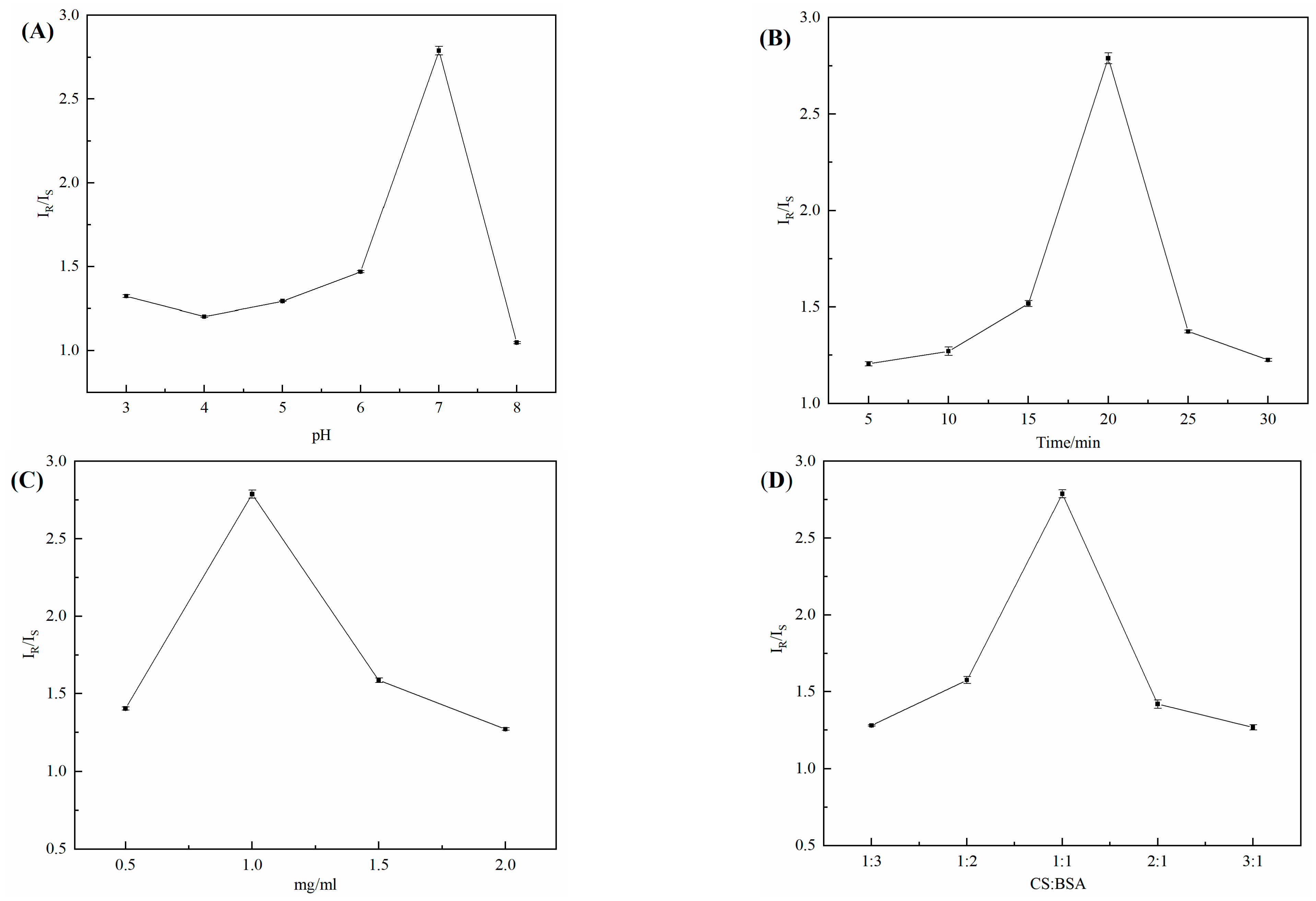
3.10. Chiral Condition Optimization
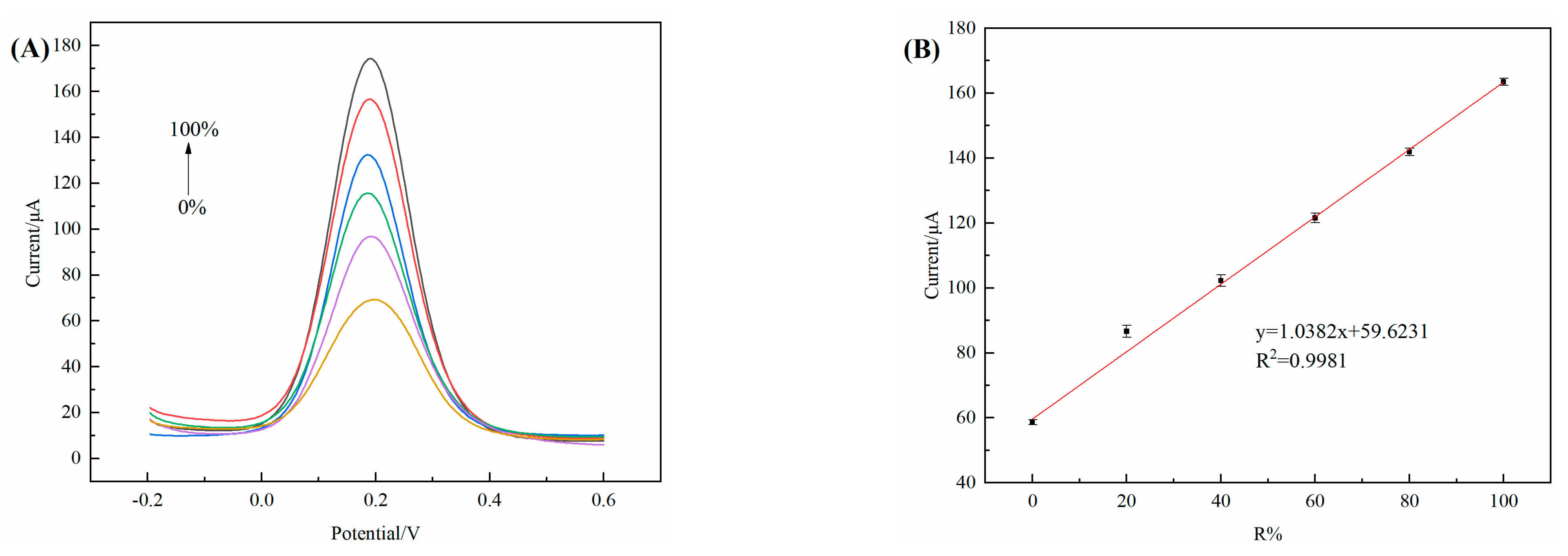
3.11. Detection Line of 3D–rGO–CS–BSA/GCE
3.12. Stability and Reproducibility
3.13. Chiral Recognition of R–Enantiomers in Mixture Solutions
3.14. Specificity of 3D–rGO–CS–BSA/GCE
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.; Liu, G.; Yao, X.; Gao, S.; Xie, J.; Xu, H.; Lin, N. Electrochemical chiral sensor based on cellulose nanocrystals and multiwall carbon nanotubes for discrimination of tryptophan enantiomers. Cellulose 2018, 25, 3861–3871. [Google Scholar] [CrossRef]
- Rutkowska, M.; Plotka-Wasylka, J.; Morrison, C.; Wieczorek, P.P.; Namieanik, J.; Marc, M. Application of molecularly imprinted polymers in analytical chiral separations and analysis. TrAC Trends Anal. Chem. 2018, 102, 91–102. [Google Scholar] [CrossRef]
- Huang, X.-Y.; Quan, K.-J.; Pei, D.; Liu, J.-F.; Di, D.-L. The development of biphasic chiral recognition in chiral separation. Chirality 2018, 30, 974–981. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Kingsford, O.J.; Yi, Y.; Wong, K.-Y. Review—Recent Advances in Electrochemical Chiral Recognition. J. Electrochem. Soc. 2019, 166, H205–H217. [Google Scholar] [CrossRef]
- Chen, Q.; Zhou, J.; Han, Q.; Wang, Y.; Fu, Y. A new chiral electrochemical sensor for the enantioselective recognition of penicillamine enantiomers. J. Solid State Electrochem. 2012, 16, 2481–2485. [Google Scholar] [CrossRef]
- Lu, Q.; Chen, L.; Meng, Q.; Jiang, Y.; Xie, L. A biomolecule chiral interface base on BSA for electrochemical recognition of amine enantiomers. Chirality 2021, 33, 385–396. [Google Scholar] [CrossRef]
- Yang, S.; Wang, Y.; Jiang, Y.; Li, S.; Liu, W. Molecularly Imprinted Polymers for the Identification and Separation of Chiral Drugs and Biomolecules. Polymers 2016, 8, 216. [Google Scholar] [CrossRef]
- Harada, N. HPLC Separation of Diastereomers: Chiral Molecular Tools Useful for the Preparation of Enantiopure Compounds and Simultaneous Determination of Their Absolute Configurations. Molecules 2016, 21, 1328. [Google Scholar] [CrossRef]
- Wang, J.; Huang, S.H.; Chen, W.; Bai, Z.W. Eluent Tolerance and Enantioseparation Recovery of Chiral Packing Materials Based on Chitosan Bis(Phenylcarbamate)-(n-Octyl Urea)s for High Performance Liquid Chromatography. Molecules 2016, 21, 1528. [Google Scholar] [CrossRef]
- Behrendt, R.; White, P.; Offer, J. Advances in Fmoc solid-phase peptide synthesis. J. Pept. Sci. 2016, 22, 4–27. [Google Scholar] [CrossRef]
- Monteagudo, E.; Virgili, A.; Parella, T.; Pérez-Trujillo, M. Chiral Recognition by Dissolution DNP NMR Spectroscopy of 13C-Labeled DL-Methionine. Anal. Chem. 2017, 89, 4939–4944. [Google Scholar] [CrossRef]
- Li, T.; Wang, Y.; Kan, X. Electrochemical chiral recognition of tryptophan enantiomers based on copper-modified β-cyclodextrin. J. Electroanal. Chem. 2021, 902, 115817. [Google Scholar] [CrossRef]
- Jing, P.; Yin, Z.-Z.; Cai, W.; Li, J.; Wu, D.; Kong, Y. The hybrids of perylene tetracarboxylic acid functionalized multi-walled carbon nanotubes and chitosan for electrochemical chiral sensing of tryptophan enantiomers. Bioelectrochemistry 2022, 146, 108110. [Google Scholar] [CrossRef]
- Dong, L.; Zhang, Y.; Duan, X.; Zhu, X.; Sun, H.; Xu, J. Chiral PEDOT-Based Enantioselective Electrode Modification Material for Chiral Electrochemical Sensing: Mechanism and Model of Chiral Recognition. Anal. Chem. 2017, 89, 9695–9702. [Google Scholar] [CrossRef]
- Caroff, E.; Hubler, F.; Meyer, E.; Renneberg, D.; Gnerre, C.; Treiber, A.; Rey, M.; Hess, P.; Steiner, B.; Hilpert, K.; et al. 4-((R)-2-{[6-((S)-3-Methoxypyrrolidin-1-yl)-2-phenylpyrimidine-4-carbonyl]amino}-3-phosphonopropionyl)piperazine-1-carboxylic Acid Butyl Ester (ACT-246475) and Its Prodrug (ACT-281959), a Novel P2Y12 Receptor Antagonist with a Wider Therapeutic Window in the Rat Than Clopidogrel. J. Med. Chem. 2015, 58, 9133–9153. [Google Scholar] [CrossRef]
- El-ahmad, Y.; Tabart, M.; Halley, F.; Certal, V.; Thompson, F.; Filcoche-romme, B.; Gruss-leleu, F.; Muller, C.; Brollo, M.; Fabien, L.; et al. Discovery of 6-(2,4-Dichlorophenyl)-5-[4-[(3S)-1-(3-fluoropropyl)pyrrolidin-3-yl]oxyphenyl]-8,9-dihydro-7H-benzo[7]annulene-2-carboxylic acid (SAR439859), a Potent and Selective Estrogen Receptor Degrader (SERD) for the Treatment of Estrogen-Receptor-Positive Breast Cancer. J. Med. Chem. 2020, 63, 512–528. [Google Scholar] [CrossRef]
- Ge, Z.; Yang, L.; Xiao, F.; Wu, Y.; Yu, T.; Chen, J.; Lin, J.; Zhang, Y. Graphene Family Nanomaterials: Properties and Potential Applications in Dentistry. Int. J. Biomater. 2018, 2018, 1539678. [Google Scholar] [CrossRef]
- Saleem, H.; Haneef, M.; Abbasi, H.Y. Synthesis route of reduced graphene oxide via thermal reduction of chemically exfoliated graphene oxide. Mater. Chem. Phys. 2018, 204, 1–7. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Three-dimensional graphene architectures. Nanoscale 2012, 4, 5549–5563. [Google Scholar] [CrossRef]
- Tarcan, R.; Todor-Boer, O.; Petrovai, I.; Leordean, C.; Astilean, S.; Botiz, I. Reduced graphene oxide today. J. Mater. Chem. C 2020, 8, 1198–1224. [Google Scholar] [CrossRef]
- Wang, H.D.; Chu, L.Y.; Song, H.; Yang, J.P.; Xie, R.; Yang, M. Preparation and enantiomer separation characteristics of chitosan/β-cyclodextrin composite membranes. J. Membr. Sci. 2007, 297, 262–270. [Google Scholar] [CrossRef]
- Huq, T.; Khan, A.; Brown, D.; Dhanyagude, N.; He, Z.; Ni, Y. Sources, production and commercial applications of fungal chitosan: A review. J. Bioresour. Bioprod. 2022, 7, 85–98. [Google Scholar] [CrossRef]
- Madni, A.; Kousar, R.; Naeem, N.; Wahid, F. Recent advancements in applications of chitosan-based biomaterials for skin tissue engineering. J. Bioresour. Bioprod. 2021, 6, 11–25. [Google Scholar] [CrossRef]
- Grant, J.J.; Pillai, S.C.; Perova, T.S.; Hehir, S.; Hinder, S.J.; McAfee, M.; Breen, A. Electrospun Fibres of Chitosan/PVP for the Effective Chemotherapeutic Drug Delivery of 5-Fluorouracil. Chemosensors 2021, 9, 70. [Google Scholar] [CrossRef]
- Ardean, C.; Davidescu, C.M.; Nemes, N.S.; Negrea, A.; Ciopec, M.; Duteanu, N.; Negrea, P.; Duda-seiman, D.; Musta, V. Factors Influencing the Antibacterial Activity of Chitosan and Chitosan Modified by Functionalization. Int. J. Mol. Sci. 2021, 22, 7449. [Google Scholar] [CrossRef]
- Mabrouk, M.; Hammad, S.F.; Abdella, A.A.; Mansour, F.R. Enantioselective chitosan-based racemic ketoprofen imprinted polymer: Chiral recognition and resolution study. Int. J. Biol. Macromol. 2022, 200, 327–334. [Google Scholar] [CrossRef]
- Cai, W.R.; Zhu, W.K.; Yang, B.Z.; Wu, D.T.; Li, J.Y.; Yin, Z.Z.; Kong, Y. Porphyrin-Based Metal–Organic Frameworks for Efficient Electrochemiluminescent Chiral Recognition of Tyrosine Enantiomers. Chemosensors 2022, 10, 519. [Google Scholar] [CrossRef]
- Petrucci, R.; Pasquali, M.; Scaramuzzo, F.A.; Curulli, A. Recent Advances in Electrochemical Chitosan-Based Chemosensors and Biosensors: Applications in Food Safety. Chemosensors 2021, 9, 254. [Google Scholar] [CrossRef]
- Mashuni, M.; Ritonga, H.; Jahiding, M.; Rubak, B.; Hamid, F.H. Highly Sensitive Detection of Carbaryl Pesticides Using Potentiometric Biosensor with Nanocomposite Ag/r-Graphene Oxide/Chitosan Immobilized Acetylcholinesterase Enzyme. Chemosensors 2022, 10, 138. [Google Scholar] [CrossRef]
- Gu, Y.; Gong, G.; Jiang, Y.; Qin, J.; Mei, Y.; Han, J. Electrochemical Immunosensor Modified with Nitrogen-Doped Reduced Graphene Oxide@Carboxylated Multi-Walled Carbon Nanotubes/Chitosan@Gold Nanoparticles for CA125 Detection. Chemosensors 2022, 10, 272. [Google Scholar] [CrossRef]
- Pei, H.; Chen, F.; Niu, X.; Jia, Q.; Guo, R.; Liu, N.; Mo, Z. Self-assembled chitosan-sodium alginate composite material for electrochemical recognition of tyrosine isomers. J. Electroanal. Chem. 2021, 895, 115525. [Google Scholar] [CrossRef]
- Wei, X.; Chen, Y.; He, S.; Lian, H.; Cao, X.; Liu, B. Bovine serum albumin-coated titanium dioxide modified electrochemical interface for enantioselective discrimination of D/L-aspartic acid. Chirality 2021, 33, 731–744. [Google Scholar] [CrossRef]
- Huang, Y.; Deng, S.; Luo, X.; Liu, Y.; Xu, W.; Pan, J.; Wang, M.; Xia, Z. Evaluation of Intestinal Absorption Mechanism and Pharmacokinetics of Curcumin-Loaded Galactosylated Albumin Nanoparticles. Int. J. Nanomed. 2019, 14, 9721–9730. [Google Scholar] [CrossRef]
- Yao, W.; Li, S.; Xie, L.; Jiang, Y. Chiral recognition of tryptophan enantiomer based on the electrode modified by polyaniline adsorption bovine serum albumin complex. Chirality 2023, 35, 129–144. [Google Scholar] [CrossRef]
- Singh, R.; Ullah, S.; Rao, N.; Singh, M.; Patra, I.; Darko, D.A.; Issac, C.P.J.; Esmaeilzadeh-Salestani, K.; Kanaoujiya, R.; Vijayan, V. Synthesis of Three-Dimensional Reduced-Graphene Oxide from Graphene Oxide. J. Nanomater. 2022, 2022, 8731429. [Google Scholar] [CrossRef]
- Chen, Z.; Jin, L.; Hao, W.; Ren, W.; Cheng, H.-M. Synthesis and applications of three-dimensional graphene network structures. Mater. Today Nano 2019, 5, 100027. [Google Scholar] [CrossRef]
- Liang, W.; Rong, Y.; Fan, L.; Zhang, C.; Dong, W.; Li, J.; Niu, J.; Yang, C.; Shuang, S.; Dong, C.; et al. Simultaneous electrochemical sensing of serotonin, dopamine and ascorbic acid by using a nanocomposite prepared from reduced graphene oxide, Fe3O4 and hydroxypropyl-β-cyclodextrin. Microchim. Acta 2019, 186, 751. [Google Scholar] [CrossRef]
- Zagitova, L.; Yarkaeva, Y.; Zagitov, V.; Nazyrov, M.; Gainanova, S.; Maistrenko, V. Voltammetric chiral recognition of naproxen enantiomers by N-tosylproline functionalized chitosan and reduced graphene oxide based sensor. J. Electroanal. Chem. 2022, 922, 116744. [Google Scholar] [CrossRef]
- Niu, Q.; Jin, P.; Huang, Y.; Fan, L.; Zhang, C.; Yang, C.; Dong, C.; Liang, W.; Shuang, S. A selective electrochemical chiral interface based on a carboxymethyl-β-cyclodextrin/Pd@Au nanoparticles/3D reduced graphene oxide nanocomposite for tyrosine enantiomer recognition. Analyst 2022, 147, 880–888. [Google Scholar] [CrossRef]
- Chen, F.; Niu, X.; Yang, X.; Pei, H.; Guo, R.; Liu, N.; Mo, Z. Self-assembled reduced graphene oxide/polyaniline/sodium carboxymethyl cellulose nanocomposite for voltammetric recognition of tryptophan enantiomers. J. Mater. Sci. Mater. Electron. 2021, 32, 11791–11804. [Google Scholar] [CrossRef]
- Yang, J.; Wu, D.; Fan, G.-C.; Ma, L.; Tao, Y.; Qin, Y.; Kong, Y. A chiral helical self-assembly for electrochemical recognition of tryptophan enantiomers. Electrochem. Commun. 2019, 104, 106478. [Google Scholar] [CrossRef]
- Gou, H.; He, J.; Mo, Z.; Wei, X.; Hu, R.; Wang, Y.; Guo, R. A Highly Effective Electrochemical Chiral Sensor of Tryptophan Enantiomers Based on Covalently Functionalize Reduced Graphene Oxide with L-Lysine. J. Electrochem. Soc. 2016, 163, B272–B279. [Google Scholar] [CrossRef]
- Li, H.; Wang, L.; Yan, S.; Chen, J.; Zhang, M.; Zhao, R.; Niu, X.; Wang, K. Fusiform-like metal-organic framework for enantioselective discrimination of tryptophan enantiomers. Electrochim. Acta 2022, 419, 140409. [Google Scholar] [CrossRef]
- Yao, W.; Li, S.; Kong, Y.; Xie, L.; Jiang, Y. Chiral Recognition of Phenylglycinamide Enantiomer Based on Electrode Modified by Silver-Ammonia Ion-Functionalized Carbon Nanotubes Complex. Chemosensors 2023, 11, 86. [Google Scholar] [CrossRef]
- Pei, H.; Wang, J.; Jin, X.; Zhang, X.; Liu, W.; Guo, R.; Liu, N.; Mo, Z. An electrochemical chiral sensor based on glutamic acid functionalized graphene-gold nanocomposites for chiral recognition of tryptophan enantiomers. J. Electroanal. Chem. 2022, 913, 116283. [Google Scholar] [CrossRef]



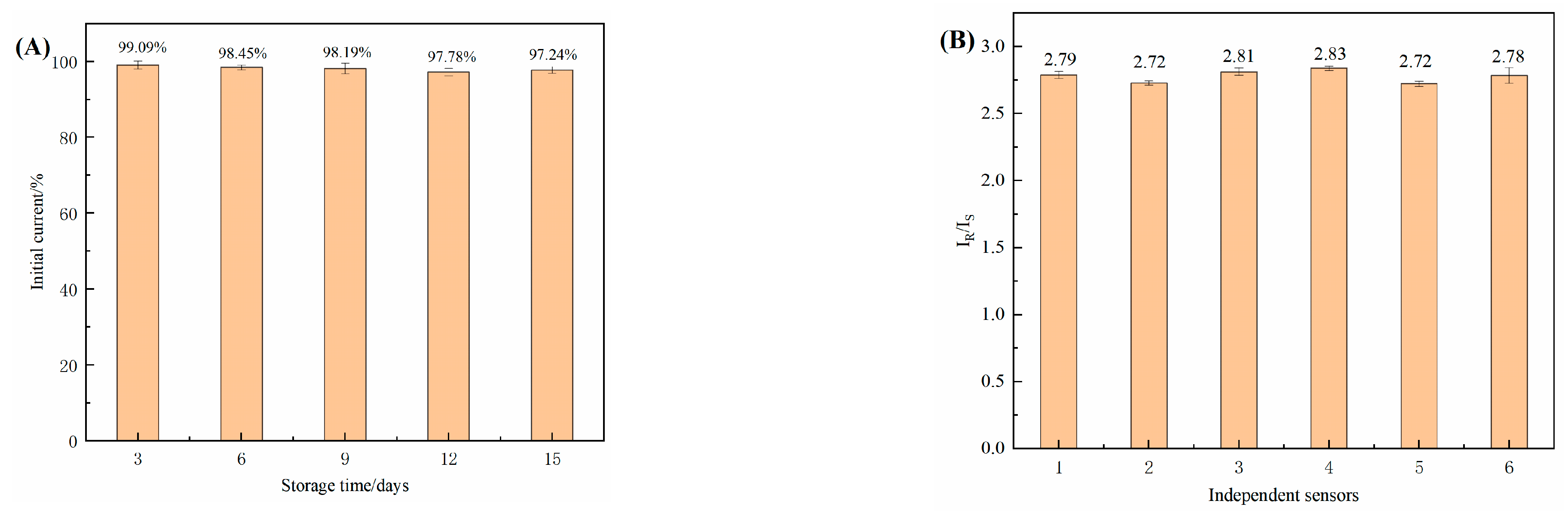
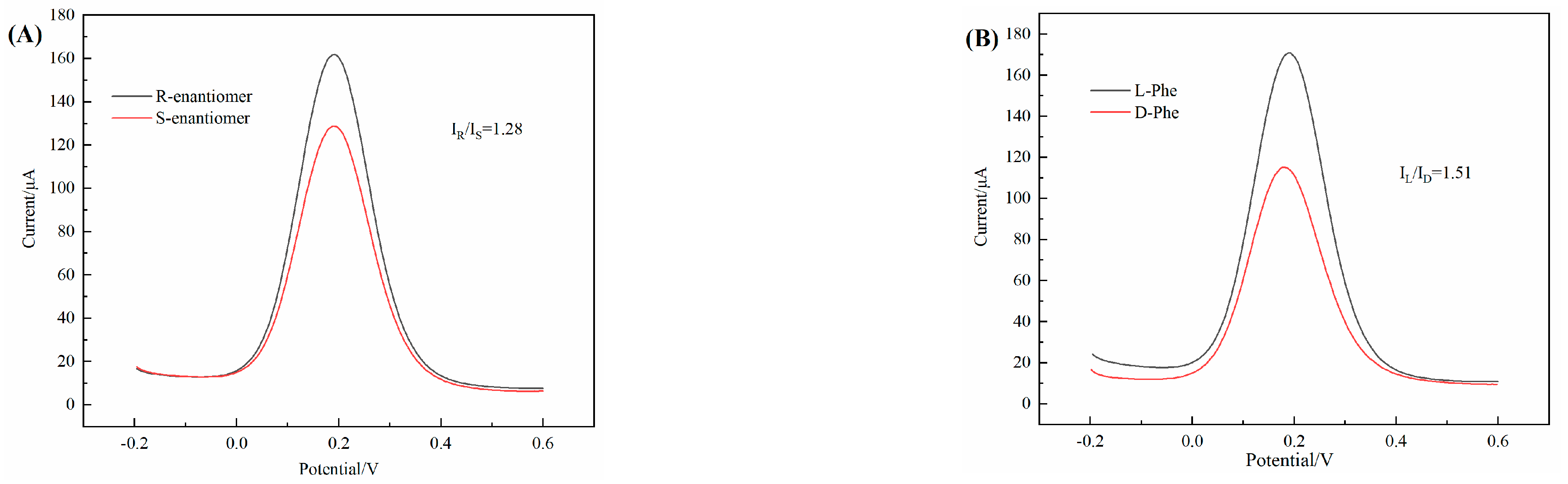
| Electrode | Detection Object | LOD | References | |
|---|---|---|---|---|
| APS–DPANI–BSA | L–/D–Tryptophan | 0.071 mM (L) | 0.0478 mM (D) | [33] |
| PAA–MWCNTs–Ag–SCS | L–/D–Phenylglycinamide | 0.015 mM (L) | 0.036 mM (D) | [44] |
| RGO–Au/L–Glu | L–/D–Tryptophan | 0.28 mM (L) | 0.86 mM (D) | [45] |
| Pt–CLMOF | L–/D–Methionine | 0.33 μM (L) | 0.60 μM (D) | [41] |
| 3D–rGO/Pd@Au/CM–β–CD | L–/D–Tryptophan | 52 nM (L) | 96 nM (D) | [38] |
| 3D–rGO–CS–BSA | R–/S–1–Boc–3–hydroxypyrrolidine | 4.85 nM (R) | 11.76 nM (S) | This work |
| Number | Added (mM) | Peak Current (μA) | Actual (mM) | Recovery (%) | RSD |
|---|---|---|---|---|---|
| 1 | 6.68 | 73.27 | 6.57 | 98.35 | 1.04% |
| 2 | 14.43 | 88.61 | 13.96 | 96.74 | |
| 3 | 21.39 | 103.7 | 21.22 | 99.20 | |
| 4 | 36.90 | 135.3 | 36.44 | 98.75 | |
| 5 | 41.71 | 145.6 | 41.40 | 99.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, S.; Yao, W.; Xie, L.; Jiang, Y. Functionalized Three–Dimensional Graphene Containing Chitosan and Bovine Serum Albumin for Recognizing Chiral Drug Intermediates. Chemosensors 2023, 11, 243. https://doi.org/10.3390/chemosensors11040243
Li S, Yao W, Xie L, Jiang Y. Functionalized Three–Dimensional Graphene Containing Chitosan and Bovine Serum Albumin for Recognizing Chiral Drug Intermediates. Chemosensors. 2023; 11(4):243. https://doi.org/10.3390/chemosensors11040243
Chicago/Turabian StyleLi, Sha, Wenyan Yao, Licheng Xie, and Yan Jiang. 2023. "Functionalized Three–Dimensional Graphene Containing Chitosan and Bovine Serum Albumin for Recognizing Chiral Drug Intermediates" Chemosensors 11, no. 4: 243. https://doi.org/10.3390/chemosensors11040243
APA StyleLi, S., Yao, W., Xie, L., & Jiang, Y. (2023). Functionalized Three–Dimensional Graphene Containing Chitosan and Bovine Serum Albumin for Recognizing Chiral Drug Intermediates. Chemosensors, 11(4), 243. https://doi.org/10.3390/chemosensors11040243






