Determination of the Critical Micelle Concentration of Gelatin, ι-Carrageenan, Pectin, Gellan Gum and Xanthan Gum by Mid Infrared Spectroscopy Among Other Techniques
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
- Gelatin (Bloom 210, 20 Mesh, Type A S4814, Nactis, Furdenheim, France)
- Gellan gum (Modulose LA800, Kalys, Saint Nolff, France)
- Iota-carrageenan (ι-Carrageenan) (Genuvisco carrageenan type J, Alliances Gums et Industries, Cormeilles En Parisis, France)
- Pectin (GRINSED Pectin XSS 100, PD 234149-4.5EN, Danisco, Neuilly-Sur-Seine, France)
- Xanthan gum (Ref. GE060, E415, Millbaker, Torcy Le Petit, France)
2.2. Preparation of Hydrocolloid Solutions
2.3. Mid-Infrared Analysis
2.4. Conductivity Analysis
2.5. Viscosity Analysis
2.6. Surface Tension Analysis
2.7. Statistical Analysis
3. Results and Discussion
3.1. Mid-Infrared Measurements
3.2. Conductivity Measurements
3.3. Viscosity Measurements
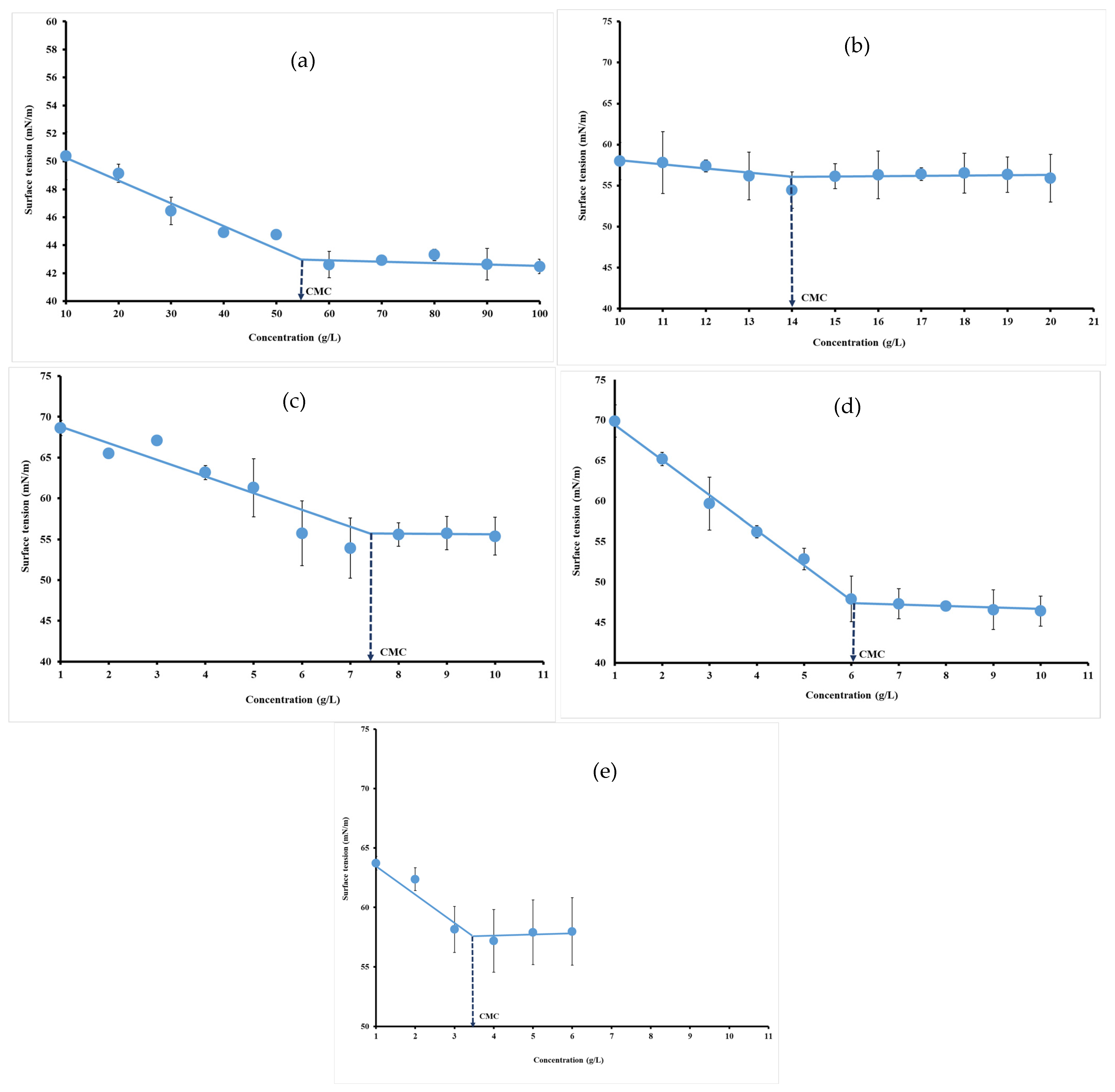
3.4. Surface Tension Measurements
3.5. CMC Comparison of Hydrocolloids According to the Applied Techniques
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phillips, G.O.; Williams, P.A. Introduction to Food Hydrocolloids, 2nd ed.; Glyndwr University: Wrexham, UK, 2000; pp. 1–22. [Google Scholar]
- Olorunsola, E.O.; Adedokun, M.O. Surface activity as basis for pharmaceutical applications of hydrocolloids: A review. J. Appl. Pharm. Sci. 2014, 4, 110–116. [Google Scholar] [CrossRef]
- Munoz, J.; Rincon, F.; Alfaro, M.C.; Zapata, I.; Fuente, J.; Beltran, O.; De Pinto, G.L. Rheological properties and surface tension of Acacia tortuosa. Carbohydr. Polym. 2007, 70, 198–205. [Google Scholar] [CrossRef]
- Huang, X.; Kakuda, Y.; Cui, W. Hydrocolloids in emulsions: Particle size distribution and interfacial activity. Food Hydrocoll. 2001, 15, 533–542. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids at interfaces and the influence on the properties of dispersed systems. Food Hydrocoll. 2003, 17, 25–39. [Google Scholar] [CrossRef]
- Dickinson, E. Hydrocolloids as emulsifiers and emulsion stabilizers. Food Hydrocoll. 2009, 23, 1473–1482. [Google Scholar] [CrossRef]
- Osano, J.P.; Hosseini-Parvar, S.H.; Matia-Merino, L.; Golding, M. Emulsifying properties of a novel polysaccharide extracted from basil seed (Ocimum bacilicum L.): Effect of polysaccharide and protein content. Food Hydrocoll. 2014, 37, 40–48. [Google Scholar] [CrossRef]
- Obi, C.; Adebayo, I. Determination of critical micelle concentration and thermodynamic evaluations of micellization of GMS. Mod. Chem. Appl. 2018, 6, 251. [Google Scholar]
- Perinelli, D.R.; Cespi, M.; Lorusso, N.; Palmieri, G.F.; Bonacucina, G.; Blasi, P. Surfactant self-assembling and critical micelle concentration: One approach fits all? Langmuir 2020, 36, 5745–5753. [Google Scholar] [CrossRef]
- Behari, M.; Das, D.; Mohanty, A.M. Influence of surfactant for stabilization and pipeline transportation of iron ore water slurry: A Review. ACS Omega 2022, 7, 28708–28722. [Google Scholar] [CrossRef]
- Smith, O.E.P.; Waters, L.J.; Small, W.; Mellor, S. CMC determination using isothermal titration calorimetry for five industrially significant non-ionic surfactants. Colloids Surf. B Biointerfaces 2022, 211, 112320. [Google Scholar] [CrossRef]
- Wu, C.; Li, N.J.; Chen, K.C.; Hsu, H.F. Determination of critical micelle concentrations of ionic and nonionic surfactants based on relative viscosity measurements by capillary electrophoresis. Res. Chem. Intermed. 2014, 40, 2371–2379. [Google Scholar] [CrossRef]
- Krstonošić, V.; Dokić, L.; Milanović, J. Micellar properties of OSA starch and interaction with xanthan gum in aqueous solution. Food Hydrocoll. 2011, 25, 361–367. [Google Scholar] [CrossRef]
- Seo, S.; Kwon, S.B.; Park, Y. MOF (CuBDC)-Microcantilever IR Spectroscopy for methane sensing with high sensitivity and selectivity. Chemosensors 2025, 13, 8. [Google Scholar] [CrossRef]
- Al-Saidi, S.M.K.; Al-Kharousi, Z.S.N.; Rahman, M.S.; Sivakumar, N.; Suleria, H.A.R.; Ashokkumar, M.; Hussain, M.; Al-Habsi, N. Thermal and structural characteristics of date-pits as digested by Trichoderma reesei. Heliyon 2024, 10, e28313. [Google Scholar] [CrossRef]
- Karoui, R.; Hammami, M.; Rouissi, H.; Blecker, C. Mid infrared and fluorescence spectroscopies coupled with factorial discriminant analysis technique to identify sheep milk from different feeding systems. Food Chem. 2011, 127, 743–748. [Google Scholar] [CrossRef]
- Karoui, R.; Mouazen, A.M.; Dufour, E.; Pillonel, L.; Picque, D.; De Baerdemaeker, J.; Bosset, J. Application of the MIR for the determination of some chemical parameters in European Emmental cheeses produced during summer. Eur. Food Res. Technol. 2006, 222, 165–170. [Google Scholar] [CrossRef]
- Karoui, R.; Mazerolles, G.; Bosset, J.; De Baerdemaeker, J.; Dufour, E. Utilisation of mid-infrared spectroscopy for determination of the geographic origin of Gruyère PDO and L’Etivaz PDO Swiss cheeses. Food Chem. 2007, 105, 847–854. [Google Scholar] [CrossRef]
- Ibrahima, M.N.M.; Ngaha, W.S.W.; Norliyanaa, M.S.; Daudb, W.R.W.; Rafatullahb, M.; Sulaimanb, O.; Hashim, R. A novel agricultural waste adsorbent for the removal of lead (II) ions from aqueous solutions. J. Hazard. Mater. 2010, 182, 377–385. [Google Scholar] [CrossRef]
- Almeida, P.; Lannes, S.; Calarge, F.; Farias, T.M.B.; Santana, J. FTIR characterization of gelatin from chicken feet. J. Chem. Eng. 2012, 6, 1029–1032. [Google Scholar]
- Muyonga, J.H.; Cole, C.G.B.; Duodu, K.G. Fourier transform infrared (FTIR) spectroscopic study of acid soluble collagen and gelatin from skins and bones. Food Chem. 2004, 86, 325–332. [Google Scholar] [CrossRef]
- Li, F.; Wei, Y.; Liang, L.; Huang, L.; Yu, G.; Li, Q. A novel low-molecular-mass pumpkin polysaccharide: Structural characterization, antioxidant activity, and hypoglycemic potential. Carbohydr. Polym. 2021, 251, 117090. [Google Scholar] [CrossRef] [PubMed]
- Misra, N.N.; Hae, I.Y.; Rohit, P.; Cheorun, J. Atmospheric pressure cold plasma improves viscosifying and emulsion stabilizing properties of xanthan gum. Food Hydrocoll. 2018, 82, 29–33. [Google Scholar] [CrossRef]
- Faria, S.; Petkowicz, C.L.D.O.; De Morais, S.A.L.; Terrones, M.G.H.; De Resende, M.M.; De França, F.P.; Cardoso, V.L. Characterization of xanthan gum produced from sugar cane broth. Carbohydr. Polym. 2011, 86, 469–476. [Google Scholar] [CrossRef]
- Synytsya, A.; Čopíková, A.; Matějka, P.; Machovič, V. Fourier transform Raman and infrared spectroscopy of pectins. Carbohydr. Polym. 2003, 54, 97–106. [Google Scholar] [CrossRef]
- Yang, Z.; Wang, D.; Hu, K.; Xiao, J.; Wu, L. Efficient extraction of pectin from sisal waste by combined enzymatic and ultrasonic process. Food Hydrocoll. 2018, 79, 189–196. [Google Scholar] [CrossRef]
- Maiti, S.; Chakravorty, A.; Chowdhury, M. Gellan co-polysaccharide micellar solution of budesonide for allergicanti-rhinitis: An in vitro appraisal. Int. J. Biol. Macromol. 2014, 68, 241–246. [Google Scholar] [CrossRef]
- Xu, L.; Qiua, Z.; Gonga, H.; Zhua, C.; Lia, Z.; Lia, Y.; Dong, M. Rheological behaviors of microbial polysaccharides with different substituents in aqueous solutions: Effects of concentration, temperature, inorganic salt and surfactant. Carbohydr. Polym. 2019, 219, 162–171. [Google Scholar] [CrossRef]
- Guo, C.; Liu, H.-Z.; Chen, J.-Y.A. Fourier transform infrared study on water-induced reverse micelle formation of block copoly(oxyethylene–oxypropylene–oxyethylene) in organic solvent. Colloids Surf. A Physicochem. Eng. Asp. 2000, 175, 193–202. [Google Scholar] [CrossRef]
- Scheuing, D.R.; Weers, J.G.A. Fourier transform infrared spectroscopic study of dodecyltrimethylammonium chloride/sodium dodecyl sulfate surfactant mixtures. Langmuir 1990, 6, 665–671. [Google Scholar] [CrossRef]
- Becherová, L.; Prokopec, V.; Čejková, J. Vibrational spectroscopic analysis of critical micelle concentration in sodium decanoate solutions. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2021, 250, 119387. [Google Scholar] [CrossRef]
- Bouyer, E.; Mekhloufi, G.; Rosilio, V.; Grossiord, J.L.; Agnely, F. Proteins, polysaccharides, and their complexes used as stabilizers for emulsions: Alternatives to synthetic surfactants in the pharmaceutical field? Int. J. Pharm. 2012, 436, 359–378. [Google Scholar] [CrossRef] [PubMed]
- Bai, L.; Huan, S.; Li, Z.; McClements, D.J. Comparison of emulsifying properties of food-grade polysaccharides in oil-in-water emulsions: Gum arabic, beet pectin, and corn fiber gum. Food Hydrocoll. 2017, 66, 144–153. [Google Scholar] [CrossRef]
- Higiro, J.; Herald, T.J.; Alavi, S.; Bean, S. Rheological study of xanthan and locust bean gum interaction in dilute solution: Effect of salt. Food Res. Int. 2007, 40, 435–447. [Google Scholar] [CrossRef]
- Banipal, T.S.; Kaur, H.; Kaur, A.; Banipal, P.K. Effect of tartarate and citrate based food additives on the micellar properties of sodium dodecylsulfate for prospective use as food emulsifier. Food Chem. 2016, 190, 599–606. [Google Scholar] [CrossRef]
- Cancella, M.J.; Cerqueira, A.F.L.W.; Teodoro, L.C.; Pereira, J.R.; Ludwig, Z.M.C.; Anjos, V.C.; Denadai, A.M.L.; Hungaro, H.M.; Rodar, M.P. Xanthan gum produced from milk permeate and deproteinized cheese whey: A comparative analysis with commercial xanthan gums. Biocatal. Agric. Biotechnol. 2024, 56, 103053. [Google Scholar] [CrossRef]
- Haghighi, H.; Biard, S.; Bigi, F.; De Leo, R.; Bedin, E.; Pfeifer, F.; Siesler, H.W.; Licciardello, F.; Pulvirenti, A. Comprehensive characterization of active chitosan-gelatin blend films enriched with different essential oils. Food Hydrocoll. 2019, 95, 33–42. [Google Scholar] [CrossRef]
- Ibrahim, M.I.; Mahmoud, A.A.; Osman, O.; El-Aal, M.A.; Eid, M. Molecular spectroscopic analyses of gelatin. Spectrochim. Acta A 2011, 81, 724–729. [Google Scholar] [CrossRef]
- Hentati, F.; Tounsi, L.; Pierre, G.; Barkallah, M.; Ursu, A.V.; Hlima, B.H.; Desbrières, J.; Le Cerf, D.; Fendri, I.; Michaud, P.; et al. Structural characterization and rheological and antioxidant properties of novel polysaccharide from calcareous red seaweed. Mar. Drugs 2022, 20, 546. [Google Scholar] [CrossRef]
- Zhang, H.; Zhao, Y. Preparation, characterization and evaluation of tea polyphenole Zn complex loaded b-chitosan nanoparticles. Food Hydrocoll. 2015, 48, 260–273. [Google Scholar] [CrossRef]
- Pan, X.; Zhao, W.; Wang, Y.; Xu, Y.; Zhang, W.; Lao, F.; Liao, X.; Wu, J. Physicochemical and structural properties of three pectin fractions from muskmelon (Cucumis melo) and their correlation with juice cloud stability. Food Hydrocoll. 2022, 124, 107–313. [Google Scholar] [CrossRef]
- Da Silva, J.A.; Cardoso, L.G.; Assis, D.D.J.; Gomes, G.V.P.; Oliveira, M.B.P.P.; De Souza, C.O.; Druzian, J.I. Xanthan gum production by xanthomonas campestris pv. campestris ibsbf 1866 and 1867 from lignocellulosic agroindustrial wastes. Appl. Biochem. Biotechnol. 2018, 186, 750–763. [Google Scholar] [CrossRef]
- Niknezhad, S.V.; Asadollahi, M.A.; Zamanin, A.; Biria, D.; Doostmohammadi, M. Optimization of xanthan gum production using cheese whey and response surface methodology. Food Sci. Biotechnol. 2015, 24, 453–460. [Google Scholar] [CrossRef]
- Dominguez, A.; Fernandez, A.; Gonzalez, N.; Iglesias, E.; Montenegro, L. Determination of Critical Micelle Concentration of Some Surfactants by Three Techniques. J. Chem. Educ. 1997, 74, 1227. [Google Scholar] [CrossRef]
- Chen, C.; Jiang, C.; Tripp, C.P. Molecular dynamics of the interaction of anionic surfactants with liposomes. Colloids Surf. B Biointerfaces 2013, 105, 173–179. [Google Scholar] [CrossRef]
- Abram, T.; Chfaira, R. Etude de la solubilisation micellaire ionique d’un polluant organique, cas du phénol (Study of micelle solubilization ionic of an organic pollutant case of phenol). J. Mater. Environ. Sci. 2015, 6, 491–498. [Google Scholar]
- Togbe, F.C.A.; Yete, P.; Azandegbe, E.C.; Wotto, D.V. Évaluation du comportement de quelques savons traditionnels en solution aqueuse: Détermination de la concentration micellaire critique et de la température de Krafft. J. Appl. Biosci. 2014, 83, 7493–7498. [Google Scholar] [CrossRef]
- Ofori-Kwakye, K.; Asantewaa, Y.; Kipo, S.L. Physicochemical and binding properties of cashew tree gum in metronidazole tablet formulation. Int. J. Pharm. Pharm. Sci. 2010, 2, 105–109. [Google Scholar]
- Cookey, G.A.; Nwokobia, F.U. Conductivity studies of binary mixtures of ionic and non-ionic surfactants at different temperatures and concentrations. Int. J. Appl. Environ. Sci. 2014, 18, 530–534. [Google Scholar]
- Rodd, A.B.; Dunstan, D.E.; Boger, D.V. Characterization of xanthan gum solutions using dynamic light scattering and rheology. Carbohydr. Polym. 2000, 42, 159–174. [Google Scholar] [CrossRef]
- Egermayer, M.; Karlberg, M.; Piculell, L. Gels of hydrophobically modified ethyl (hydroxyethyl) cellulose cross-linked by amylase: Effect of hydrophobe architecture. Langmuir 2004, 20, 2208–2214. [Google Scholar] [CrossRef]
- Wang, F.; Sun, Z.; Wang, Y.J. Study of xanthan gum/waty corn starch interaction in solution by viscosity. Food hydrocoll. 2001, 15, 575–581. [Google Scholar] [CrossRef]
- Kalkandelen, C.; Ozbek, B.; Ergul, N.M.; Akyol, S.; Moukbil, Y.; Oktar, F.N.; Ekren, N.; Kılıc, O.; Kılıc, B.; Gunduz, O. Effect of temperature, viscosity and surface tension on gelatine structures produced by modified 3D printer. IOP Conf. Ser. Mater. Sci. Eng. 2017, 293, 012001. [Google Scholar] [CrossRef]
- Mahfoudhi, N.; Sessa, M.; Chouaibi, M.; Ferrari, G.; Donsì, F.; Hamdi, S. Assessment of emulsifying ability of almond gum in comparison with gum arabic using response surface methodology. Food Hydrocoll. 2014, 37, 49–59. [Google Scholar] [CrossRef]
- Petri, D.F.S. Xanthan gum: A versatile biopolymer for biomedical and technological applications. J. Appl. Polym. Sci. 2015, 132, 42035. [Google Scholar] [CrossRef]
- Abbott, S. Surfactant Science: Principles and Practice, 1st ed.; University of Leeds: Leeds, UK, 2015; pp. 1–260. [Google Scholar]
- Beverung, C.J.; Radke, C.J.; Blanch, H.W. Protein adsorption at the oil water interface: Characterization of adsorption kinetics by dynamic interfacial tension measurements. Biophys. Chem. 1999, 81, 59–80. [Google Scholar] [CrossRef]
- Ganzevles, R.A. Protein/Polysaccharide Complexes at Air/Water Interfaces. Ph.D. Thesis, Wageningen University, Wageningen, The Netherlands, 2007. [Google Scholar]
- Saxena, N.; Nilanjan, P.; Keka, O.; Swapan, D.; Ajay, M. Synthesis, characterization, physical and thermodynamic properties of a novel anionic surfactant derived from sapindus laurifolius. RSC Adv. 2018, 8, 24485–24499. [Google Scholar] [CrossRef]
- Benichou, A.; Aserin, A.; Lutz, R.; Garti, N. Formation and characteriztion of amphiphilic, conjugates of whey protein isolate (WPI)/xanthan to improve surface activity. Food Hydrocoll. 2007, 21, 379–391. [Google Scholar] [CrossRef]
- Liu, J.; Tan, J.; Hua, X.; Jiang, Z.; Wang, M.; Yang, R.; Cao, Y. Interfacial properties of ultrahigh methoxylated pectin. Int. J. Biol. Macromol. 2020, 152, 403–410. [Google Scholar] [CrossRef]
- Castellani, O.; Guibert, D.; Al-Assaf, S.; Axelos, M.; Phillips, G.O.; Anton, M. Hydrocolloids with emulsifying capacity. Part 1: Emulsifying properties and interfacial characteristics of conventional (Acacia senegal (L.) Willd. var. senegal) and matured (Acacia (sen) Super gum) Acacia Senegal. Food Hydrocoll. 2010, 24, 193–199. [Google Scholar] [CrossRef]
- Castellani, O.; Gaillard, C.; Vié, V.; Al-Assaf, S.; Axelos, M.; Phillips, G.O.; Anton, M. Hydrocolloids with emulsifying capacity. Part 3: Adsorption and structural properties at the air-water surface. Food Hydrocoll. 2010, 24, 131–141. [Google Scholar] [CrossRef]
- Du, F.; Qi, Y.; Huang, H.; Wang, P.; Xu, X.; Yang, Z. Stabilization of O/W emulsions via interfacial protein concentrating induced by thermodynamic incompatibility between sarcoplasmic proteins and xanthan gum. Food Hydrocoll. 2022, 124, 107–242. [Google Scholar] [CrossRef]
- Cai, Y.; Deng, X.; Liu, T.; Zhao, M.; Zhao, Q.; Chen, S. Effect of xanthan gum on walnut protein/xanthan gum mixtures, interfacial adsorption, and emulsion properties. Food Hydrocoll. 2018, 79, 391–398. [Google Scholar] [CrossRef]
- Zaho, Q.; Long, Z.; Kong, J.; Liu, T.; Sun-Waterhouse, D.; Zhao, M. Sodium caseinate/flaxseed gum interactions at oil-water interface: Effect on protein adsorption and functions in oil-in-water emulsion. Food Hydrocoll. 2015, 43, 137–145. [Google Scholar] [CrossRef]
- Prosser, A.J.; Franses, E.I. Adsorption and surface tension of ionic surfactants at the air–water interface: Review and evaluation of equilibrium models. Colloids Surf. A Physicochem. Eng. Asp. 2001, 178, 1–40. [Google Scholar] [CrossRef]
- Mukherjee, I.; Moulik, S.P.; Rakshit, A.K. Tensiometric determination of Gibbs surface excess and micelle point: A critical revisit. J. Colloid Interface Sci. 2013, 394, 329–336. [Google Scholar] [CrossRef]
- Leroux, J.; Langendorff, V.; Schick, G.; Vaishnav, V.; Mazoyer, J. Emulsion stabilizing properties of pectin. Food Hydrocoll. 2003, 17, 455–462. [Google Scholar] [CrossRef]
- Ampatzidis, C.D.; Varka, E.M.A.; Karapantsios, T.D. Adsorption behavior of non-conventional eco-friendly tyrosine glycerol ether surfactants. Colloids Surf. A Physicochem. Eng. Asp 2013, 438, 104–111. [Google Scholar] [CrossRef]
- Shirzad, S.; Rahmat, S. Micellization properties and related thermodynamic parameters of aqueous sodium dodecyl sulfate and sodium dodecyl sulfonate solutions in the presence of 1-propanol. Fluid Phase Equilib. 2014, 377, 1–8. [Google Scholar] [CrossRef]
- Sanlıer, H.; Yasa, S.M.; Cihnioglu, A.O.; Abdulhayoglu, M.; Yılmaz, H.; Güliz, A. Development of gemcitabine-adsorbed magnetic gelatin nanoparticles for targeted drug delivery in lung cancer. Artif. Cells Nanomed. Biotechnol. 2016, 44, 943–949. [Google Scholar]
- Yoshimura, T.; Sakato, A.; Tsuchiya, K.; Ohkubo, T.; Sakai, H.; Abe, M.; Esumi, K. Adsorption and aggregation properties of amino acid-based n-alkyl cysteine monomeric and dialkyl cystine gemini surfactants. J. Colloid Interface Sci. 2007, 308, 466–473. [Google Scholar] [CrossRef]
- Dauvergne, J. Synthèse et Etude Physico-Chimique de Nouveaux Tensioactifs Utilisables pour la Cristallisation 2D sur Film Lipidique et l’étude des Protéines Membranaires. Ph.D. Thèse, Université d’Avignon et des Pays de Vaucluse, Avignon, France, 2010. [Google Scholar]
- Zdziennicka, A.; Katarzyna, S.; Joanna, K.; Bronisław, J. Critical micelle concentration of some surfactants and thermodynamic parameters of their micellization. Fluid. Phase Equilib. 2012, 322–323, 126–134. [Google Scholar] [CrossRef]
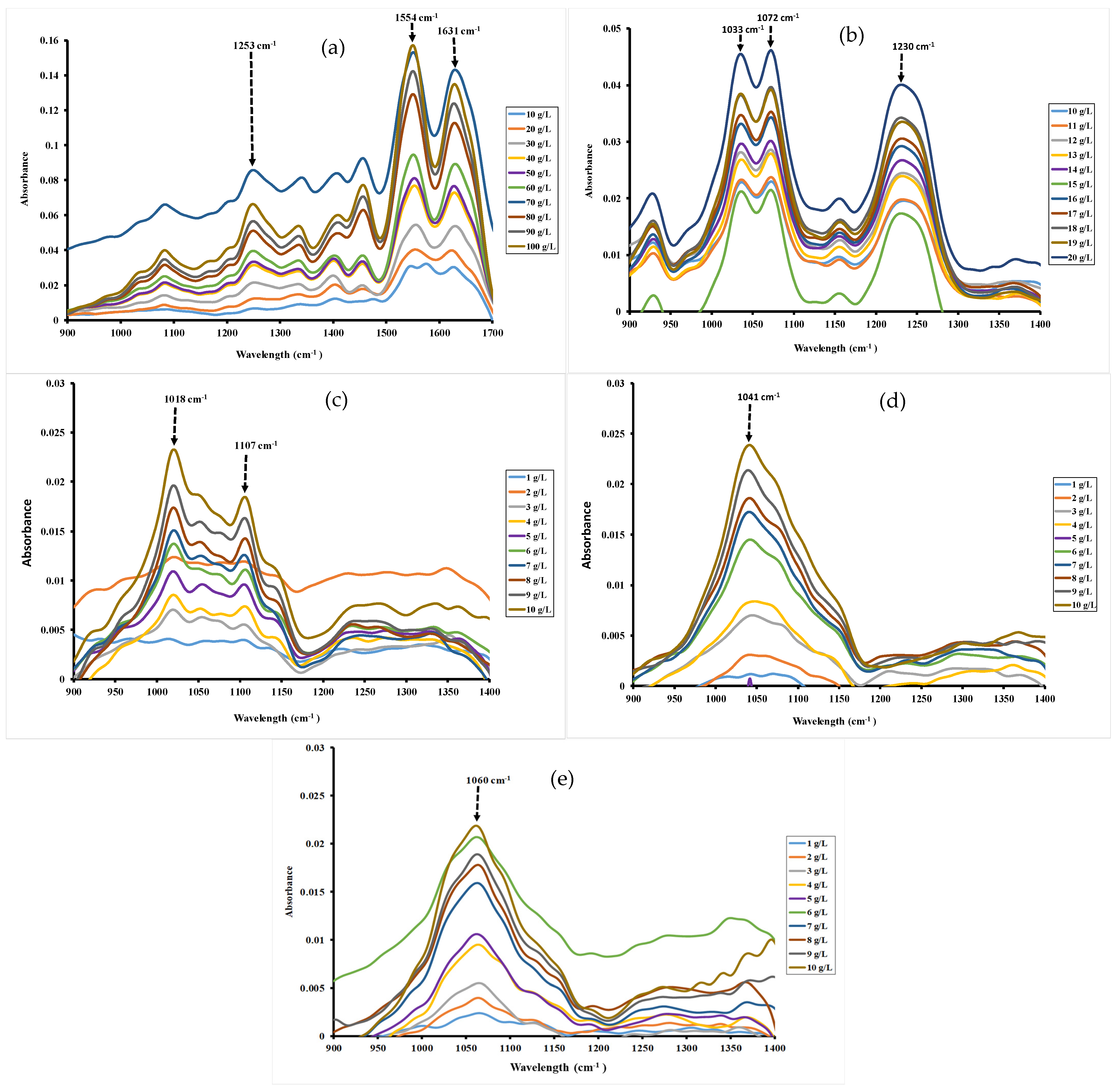

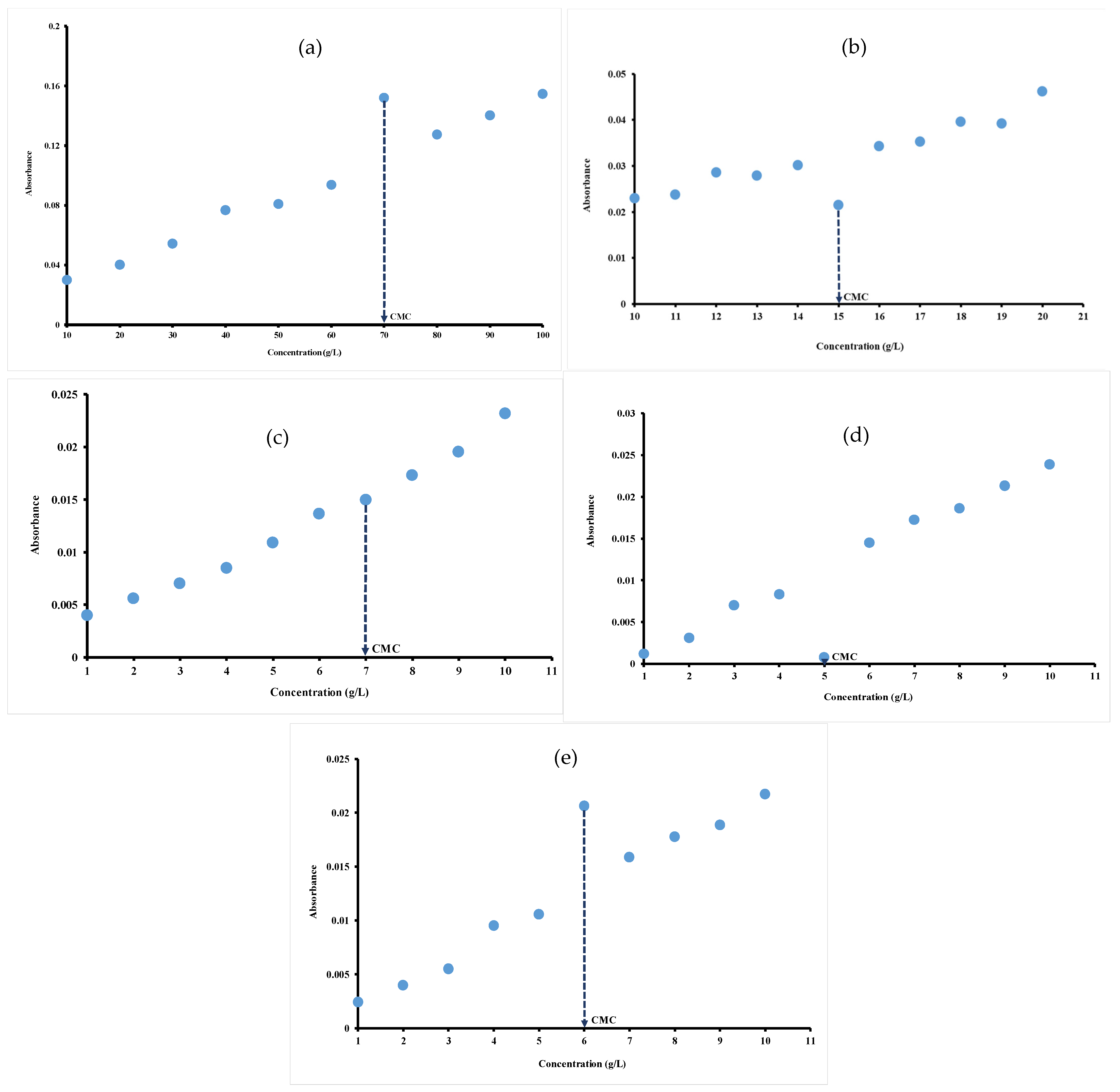
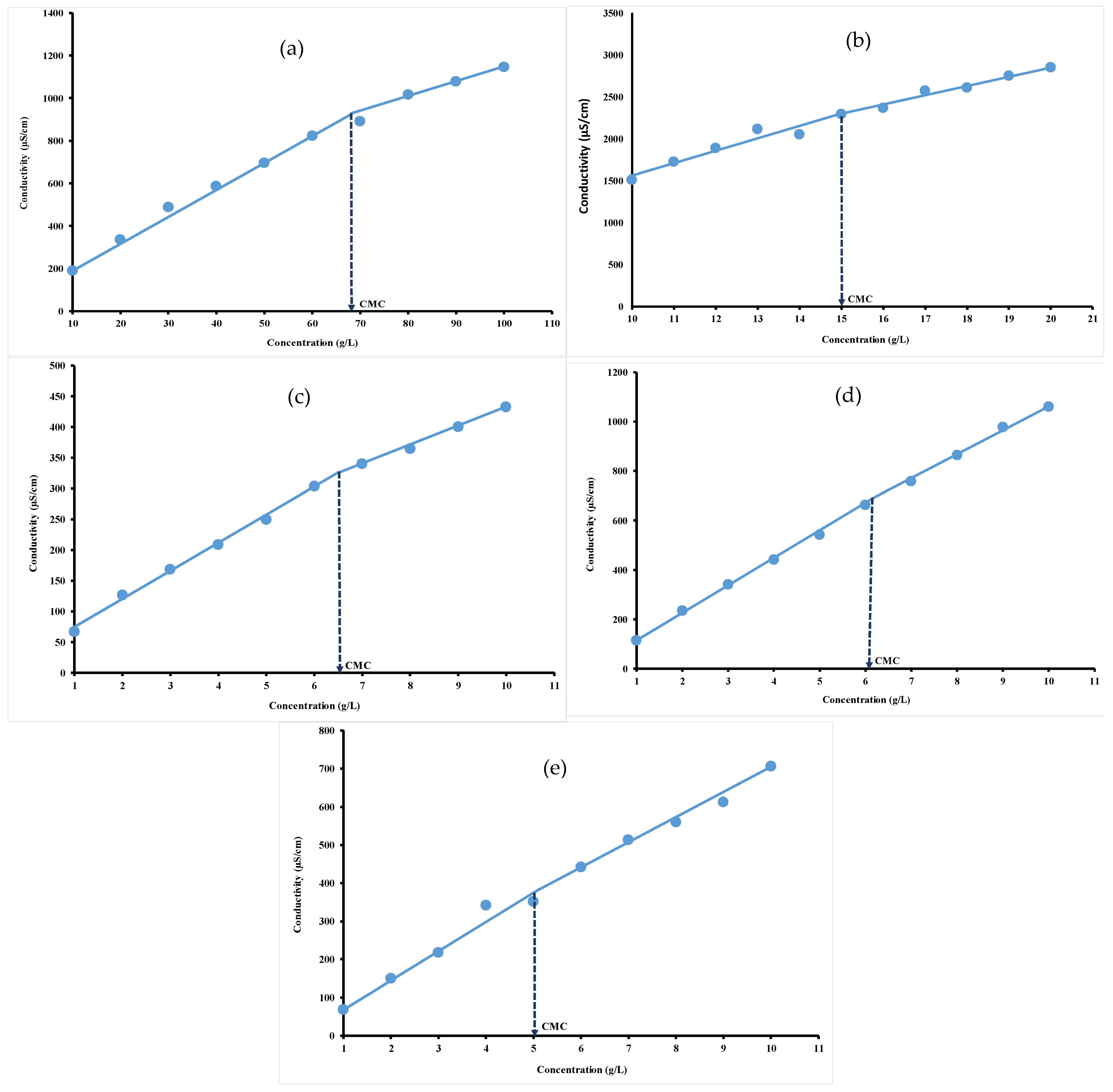
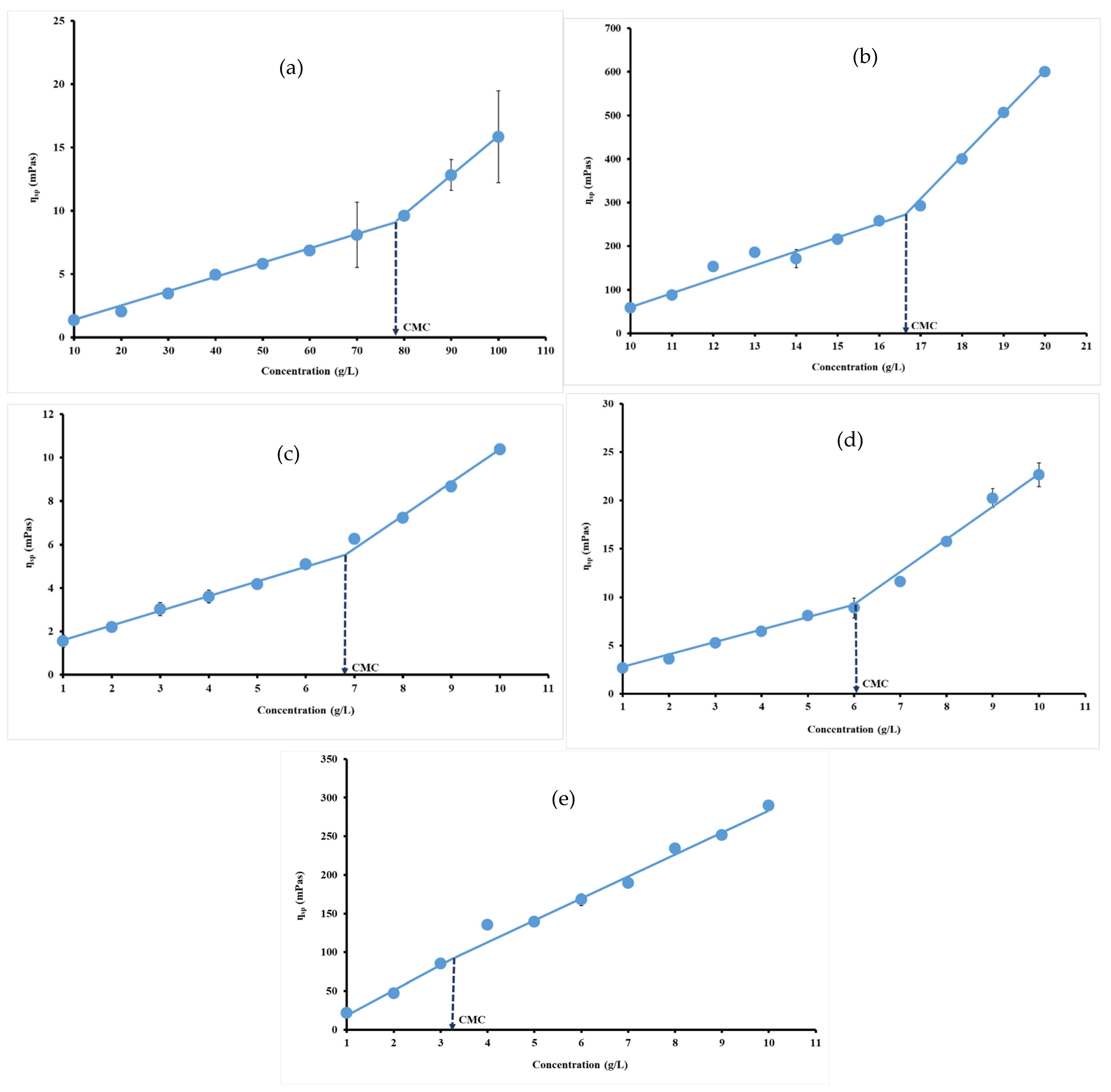
| Hydrocolloids | CMC · 10−5 (mol/L) | CMC (mN/m) | ∏CMC (mNm) | (nmol/m2) | (nm2) | −7 (kJ/mol) | (kJ/mol) |
|---|---|---|---|---|---|---|---|
| Gelatin | 55.16 ± 1.07 | 43.72 | 28.28 | 63.6 | 0.036 | −1.95 | −44,592.42 |
| ι-Carrageenan | 7 ± 0.57 | 54.46 | 17.54 | 100 | 0.023 | −1.24 | −175,203.05 |
| Pectin | 7.46 ± 1.98 | 53.76 | 18.24 | 34.86 | 0.066 | −2.47 | −523,193.6 |
| Gellan gum | 1.2 ± 0.56 | 46.02 | 25.975 | 11.09 | 0.21 | −58.96 | −341,098.59 |
| Xanthan gum | 0.17 ± 0.62 | 56.93 | 15.07 | 2.32 | 1 | −17.26 | −6,493,369.73 |
| Hydrocolloids | CMC (g/L) | |||
|---|---|---|---|---|
| Conductivity | Viscosity | Surface Tension | MIR | |
| Gelatin | 68.59 ± 4.35 a | 79.15 ± 6.45 b | 55.16 ± 1.07 c | 70 ± 0 a |
| ι-carrageenan | 15 ± 1 a | 16.64 ± 1.25 b | 14 ± 0.57 a | 15 ± 0 a |
| Pectin | 6.53 ± 0.43 a | 6.91 ± 0.31 a | 7.46 ± 1.98 a | 7 ± 0 a |
| Gellan gum | 6.16 ± 0.51 a | 6.03 ± 0.56 a | 6.04 ± 0.56 a | 5 ± 0 a |
| Xanthan gum | 5.08 ± 0.08 a | 3.29 ± 0.89 b | 3.48 ± 0.62 b | 6 ± 0 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Koko, J.H.K.K.; Tia, V.E.; Karamoko, G.; Karoui, R. Determination of the Critical Micelle Concentration of Gelatin, ι-Carrageenan, Pectin, Gellan Gum and Xanthan Gum by Mid Infrared Spectroscopy Among Other Techniques. Chemosensors 2025, 13, 99. https://doi.org/10.3390/chemosensors13030099
Koko JHKK, Tia VE, Karamoko G, Karoui R. Determination of the Critical Micelle Concentration of Gelatin, ι-Carrageenan, Pectin, Gellan Gum and Xanthan Gum by Mid Infrared Spectroscopy Among Other Techniques. Chemosensors. 2025; 13(3):99. https://doi.org/10.3390/chemosensors13030099
Chicago/Turabian StyleKoko, Joel Henri Konan Kouakou, Vama Etienne Tia, Gaoussou Karamoko, and Romdhane Karoui. 2025. "Determination of the Critical Micelle Concentration of Gelatin, ι-Carrageenan, Pectin, Gellan Gum and Xanthan Gum by Mid Infrared Spectroscopy Among Other Techniques" Chemosensors 13, no. 3: 99. https://doi.org/10.3390/chemosensors13030099
APA StyleKoko, J. H. K. K., Tia, V. E., Karamoko, G., & Karoui, R. (2025). Determination of the Critical Micelle Concentration of Gelatin, ι-Carrageenan, Pectin, Gellan Gum and Xanthan Gum by Mid Infrared Spectroscopy Among Other Techniques. Chemosensors, 13(3), 99. https://doi.org/10.3390/chemosensors13030099





