Abstract
In the past few decades, metal–organic frameworks (MOFs) have been widely employed for a variety of applications such as sensors, adsorption, and catalysis. MOFs have excellent gas sensing properties and a large specific surface area which makes them a suitable candidate for the determination of toxic and hazardous gases. Some reports have also shown that integration of MOFs with other materials such as graphene, metal oxides, or conducting polymers may further improve their sensing performance. MOF-derived materials have also demonstrated excellent gas sensing properties. In this review article, we have compiled the recent progress in MOFs, MOF-based composites, and MOF-derived materials for gas sensing applications. We believe that the present review article may benefit readers who are planning or working on the development of MOF-based gas sensors.
1. Introduction
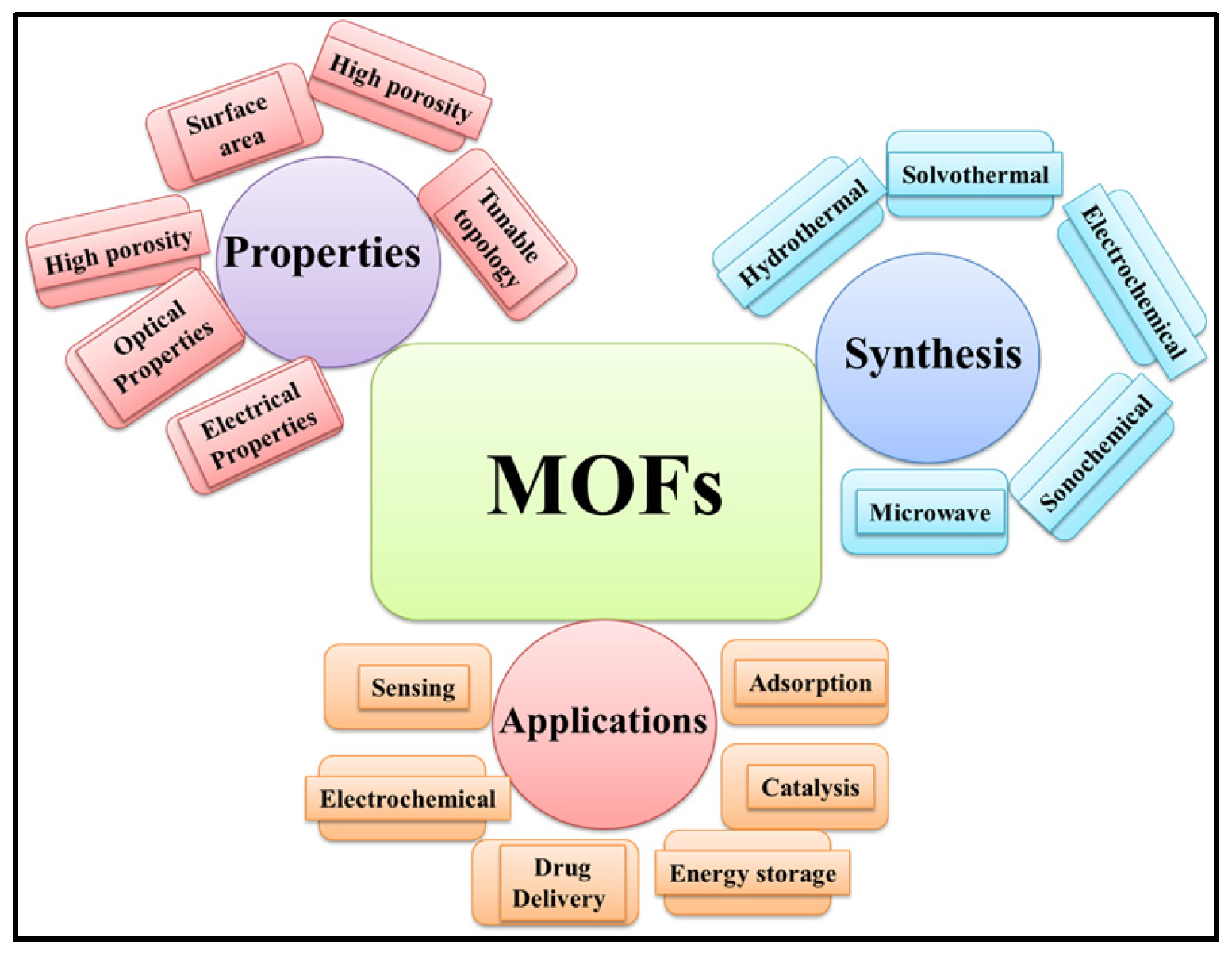
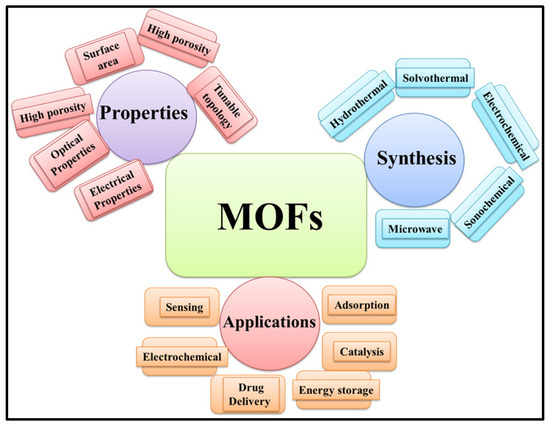
Metal–organic frameworks (MOFs) are crystalline porous materials which consist of inorganic metal ions and organic ligands [1]. MOF materials possess excellent porosity, a large surface area, tunable microporosity, and adsorption capacity [2]. MOF-based materials have received significant attention due to their excellent surface properties, acceptable conductivity, decent thermal stability, and crystalline nature [3]. MOF-based materials have been extensively used in a variety of applications, including gas adsorption [4], chemical sensing [5], energy storage [6], water treatment [7], drug delivery [8], catalysis/electro-catalysis, etc. [9,10]. MOFs can be synthesized by various approaches, including hydrothermal, solvothermal, microwave, electrochemical, and sonochemical methods (Scheme 1).
Scheme 1.
Schematic representation of the properties, synthesis methods, and applications of MOFs.
The development of high-performance gas sensors is of great significance for monitoring volatile organic compounds (VOCs) and hazardous and toxic gases in the environment [11,12]. VOCs and other toxic gases are released from various industries, which may cause environmental pollution [13]. In the past few years, various metal oxide [14], reduced graphene oxide (rGO) [15], graphitic-carbon nitride (g-C3N4) [16], polymer [17], metal sulfide [18], and MXene-based [19] materials have been employed in gas sensing applications. Due to their excellent adsorption properties and excellent surface properties, MOFs and MOF-based materials have been considered promising gas sensing materials.
MOFs have excellent surface properties and it has been found that MOF-derived materials may also retain high surface properties [20]. The thermal decomposition of the highly porous framework of MOF materials transforms into metal oxides which possess abundant active sites, interconnected porosity, and a large surface area [21,22]. These features may enhance gas adsorption and facilitate fast charge transport which is crucial for gas sensing applications. Moreover, MOF-derived metal oxides demonstrate better stability and robustness. Therefore, various reports have demonstrated the fabrication of gas sensors using MOF-derived metal oxides as gas sensing materials.
In this review report, we have summarized the progress in the preparation of MOFs and MOF-derived materials for gas sensing applications. This review article would be beneficial for readers who are working on the synthesis of MOFs, MOF-based composites, and MOF-derived metal oxides for gas sensing applications.
2. Synthetic Process for MOFs
MOFs have been widely synthesized by utilizing various methods such as hydrothermal, solvothermal, microwave, and electrochemical methods. In this section, we have briefly summarized the four most widely used methods for the synthesis of MOFs.
2.1. Microwave Method
In a previous study, Klinowski et al. [23] stated that the microwave synthesis method was discovered by P.L. Spencer in 1946 at Raytheon Corporation. In the past few decades, it has received extensive interest from researchers due to its rapid heating, short reaction time, and energy efficiency [24]. The microwave synthesis method generates direct heating within the reaction medium via dielectric heating by harnessing the interaction of electromagnetic waves with mobile electric charges, such as electrons or ions in solids and polar solvent molecules or ions in solutions [25]. The microwave-assisted synthesis method may generate thermal and kinetic effects that reduce reaction times, improve purity, enhance product yields, and boost overall efficiency [26]. The microwave-assisted synthesis method may also enable high yields with significant phase selectivity, rapid crystallization, and precise control over crystal size and shape, from microcrystals to nanoparticles [27,28,29]. Yahia et al. [30] reported the microwave synthesis of the MOFs UiO-66 and MOF-808 for application in CO2/CH4 separation. The synthetic procedure is described in Figure 1a. Skoda et al. [31] also fabricated a Co-MOF using the microwave approach and used it as anode electrode material for sodium-ion battery applications.
2.2. Sonochemical Method
The literature has suggested that the sonochemical synthesis method has various advantages, including simplicity, ease of use, and eco/environmentally friendly nature [32]. Thus, the sonochemical synthesis method has been utilized for the preparation of MOFs to achieve phase selectivity, control crystallite size, and speed up crystallization [33]. Vaitsis et al. [34] reported the sonochemical synthesis of a Zn-based MOF for electrochemical reduction of CO2 applications. In another study, Abaszadeh et al. [35] synthesized a Cu-MOF as shown in Figure 1b and evaluated its antibacterial properties. Moradi et al. [36] also examined antibacterial properties of a sonochemically synthesized Zn-based MOF. The synthesized MOF also exhibited good catalytic properties for the removal of mercury. Due to fast reaction rates, large surface area, simplicity, and excellent energy efficiency, the sonochemical method has been considered a more advanced synthesis technique compared to conventional synthesis methods [37].
2.3. Hydrothermal Method
The hydrothermal method is one of the most widely used approaches for the synthesis of MOFs and metal oxides. Hydrothermal synthesis uses PTFE liners inside tightly sealed stainless steel autoclaves to synthesize crystalline materials from aqueous solutions at autogenous pressure and high temperatures (60–220 °C). The hydrothermal method generates single crystals by utilizing the material’s solubility in hot water under high vapor pressure while maintaining a temperature difference across the reactor [38]. In some cases, solvents are used. This is referred to as the solvothermal method and it is particularly effective for materials with vapor pressures near their melting points and yields high-quality crystals [39]. Wu et al. [40] reported hydrothermal synthesis of Ni(II)- or Co(II)-based MOFs as illustrated in Figure 1c. The authors investigated the electro-catalytic properties of the synthesized MOFs and explored them as electrode materials for hydrogen evolution applications. Despite various advantages, a major drawback of this approach is the difficulty in monitoring crystal growth, as the process can take three to four days. Thus, it is still a challenge to develop a highly efficient synthetic method for the preparation of MOFs with high scalability and efficiency with controlled crystal growth.
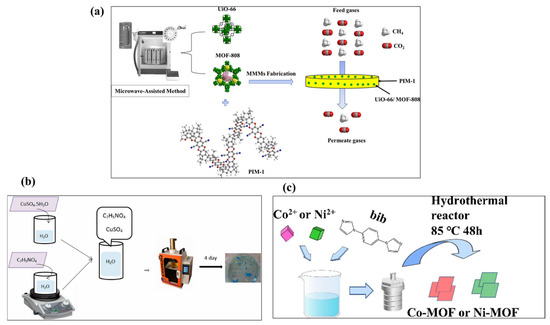
Figure 1.
Schematic graph for the synthesis of MOFs using microwave (a), sonochemical, (b) and hydrothermal (c) methods. Reprinted with permission [30,35,40].
Figure 1.
Schematic graph for the synthesis of MOFs using microwave (a), sonochemical, (b) and hydrothermal (c) methods. Reprinted with permission [30,35,40].
2.4. Electrochemical Method
The electrochemical method may be considered as an environmental friendly approach for the synthesis of MOFs and other materials. The electrochemical method demonstrates several unique advantages, including precise control over various reaction parameters, such as current, voltage, and reaction time, which may enable the customization of the growth and morphology of the MOFs. Moreover, the electrochemical method operates under mild conditions, typically at room temperature (RT) and atmospheric pressure, leading to reduced energy consumption and increased environmental sustainability. Additionally, the electrochemical method may facilitate faster synthesis of MOFs compared to traditional methods. The electrochemical method also eliminates the need for harsh chemicals, resulting in a cleaner and more sustainable process. In addition, this method can directly deposit MOFs onto conductive substrates such as fluorine-doped tin oxide (FTO), making it particularly advantageous for applications in energy storage and electrochemical sensors. It is seen that the electrochemical synthetic process can be classified in two ways: anodic dissolution and the cathodic synthesis method. In the anodic approach, the electrodes are electrochemically oxidized to generate the metal ions which can combine with organic ligands and form MOFs. In the cathodic method, a solution contacting metal ions and organic ligands produced protons at the cathodic surface. Electrochemical synthesis offers several advantages over other conventional methods, including minimal reaction conditions (ambient temperature and pressure), reduced crystal growth time, and greater ease of scaling up the process. However, a major drawback of the electrochemical method for the synthesis of MOFs is the risk of producing non-uniform MOF materials. The electrochemical process often depends on applied potentials which can create localized reaction conditions, leading to inconsistencies in crystal size, shape, and phase purity. Additionally, the electrochemical method demands precise control over electrochemical parameters such as current density and voltage which makes it more complex and less tolerant to variations. It is expected that this may affect reproducibility and scalability, especially for large-scale production. In addition, some unwanted side reactions or by-products arising from the electrochemical environment can complicate the synthesis further, negatively affecting the quality and functionality of the resulting MOF product.
3. MOF-Based Materials in Sensing Applications
3.1. MOF and MOFs/Composite
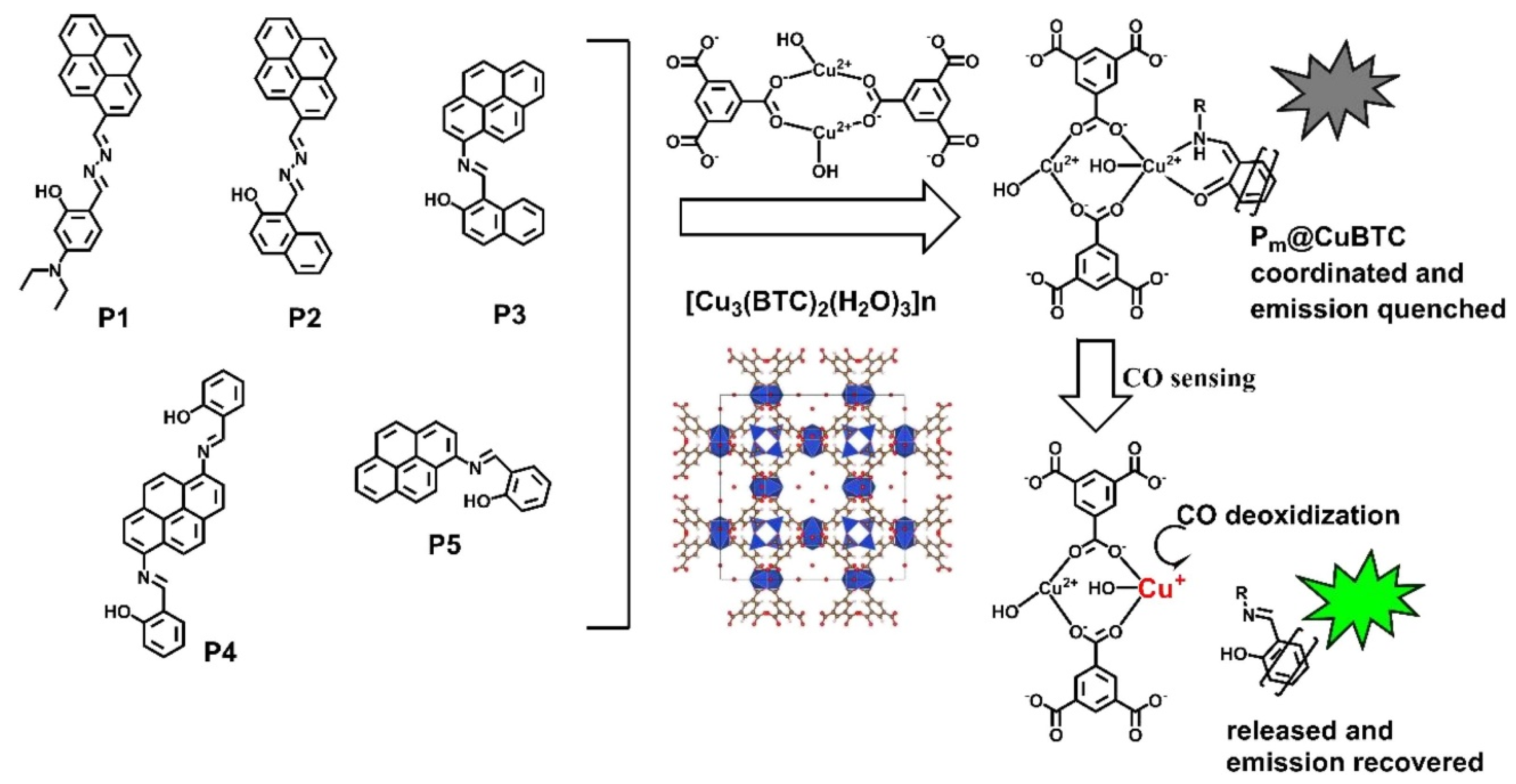
In previous decades, MOFs have been extensively used in a variety of applications because of their extraordinary and unique physicochemical features. MOFs have been integrated with various nanostructured and conductive materials to boost their gas sensing performance. In this context, Zheng et al. [41] reported the preparation of a copper-MOF (Cu-MOF) integrated with pyrene-cored probes and explored it as sensitive and efficient indicator for carbon monoxide (CO) in coal mine gas. Figure 2 shows the molecular structure of pyrene-cored probes (P1–P5) and composites with [Cu3(BTC)2(H2O)3]n. The formation of the Cu-MOF ([Cu3(BTC)2(H2O)3]n (H3BTC is 1,3,5-benzenetricarboxylic acid)) was authenticated by the NMR technique. The proposed sensing strategies demonstrated that a Cu-MOF attached to a pyrene-cored probe has a limit of detection (LOD) of 0.005% vs. N2, 50 ppm, for the determination of CO.
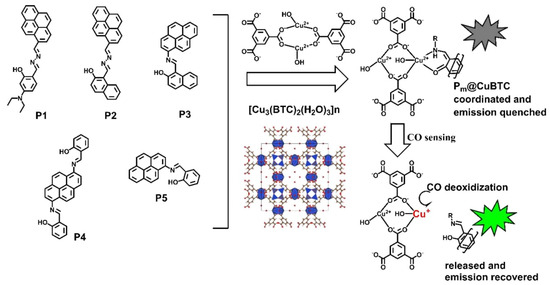
Figure 2.
Molecular structure of pyrene-cored probes (P1–P5) and [Cu3(BTC)2(H2O)3]n. (Pm@CuBTC; m = 1, 2). Reproduced with permission [41].
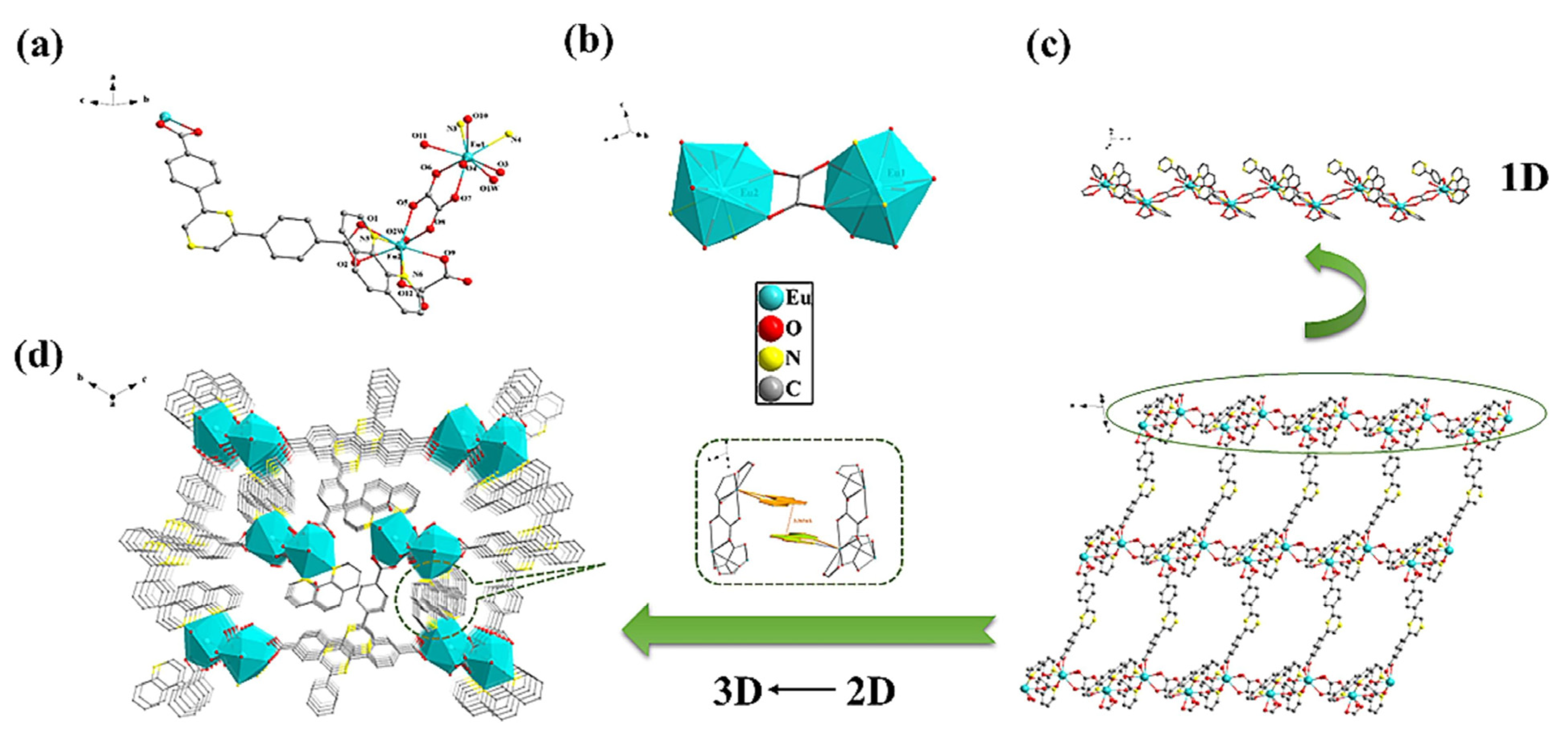
In a recent report, Fan et al. [42] reported a novel Eu-based MOF ({[Eu2(L)(phen)2(ox)2(H2O)2]⋅10H2O⋅phen}n (H2L = 2,6-bis(4-carboxyphenyl) pyrazine, phen = 1,10-phenanthroline)) using the hydrothermal method. The coordination environment and one-, two-, and three-dimensional (1D, 2D, and 3D) structural representations of the Eu-MOF are demonstrated in Figure 3a–d. The authors constructed convenient test strips using the Eu-MOF and applied it as a sensor for the detection of liquid/gas benzaldehyde, Cr2O72−/CrO42−, and mercury (Hg2+). The major advantage of the abovementioned study was the determination of benzaldehyde in gas and liquid phases. The authors also successfully detected Hg2+ in tap water, river water, and tea samples which demonstrated excellent recoveries in the range of 99.84 5 to 102.34%. The authors also found that the proposed sensor has decent repeatability with an RSD value of less than 2.01%.
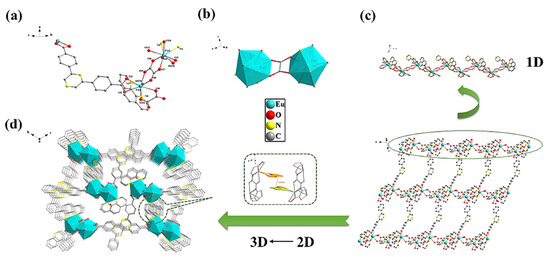
Figure 3.
(a) Coordination environment, (b) asymmetric unit, (c) 2D layer construction demonstration by 1D chain and (d) 3D network. Reproduced with permission [42].
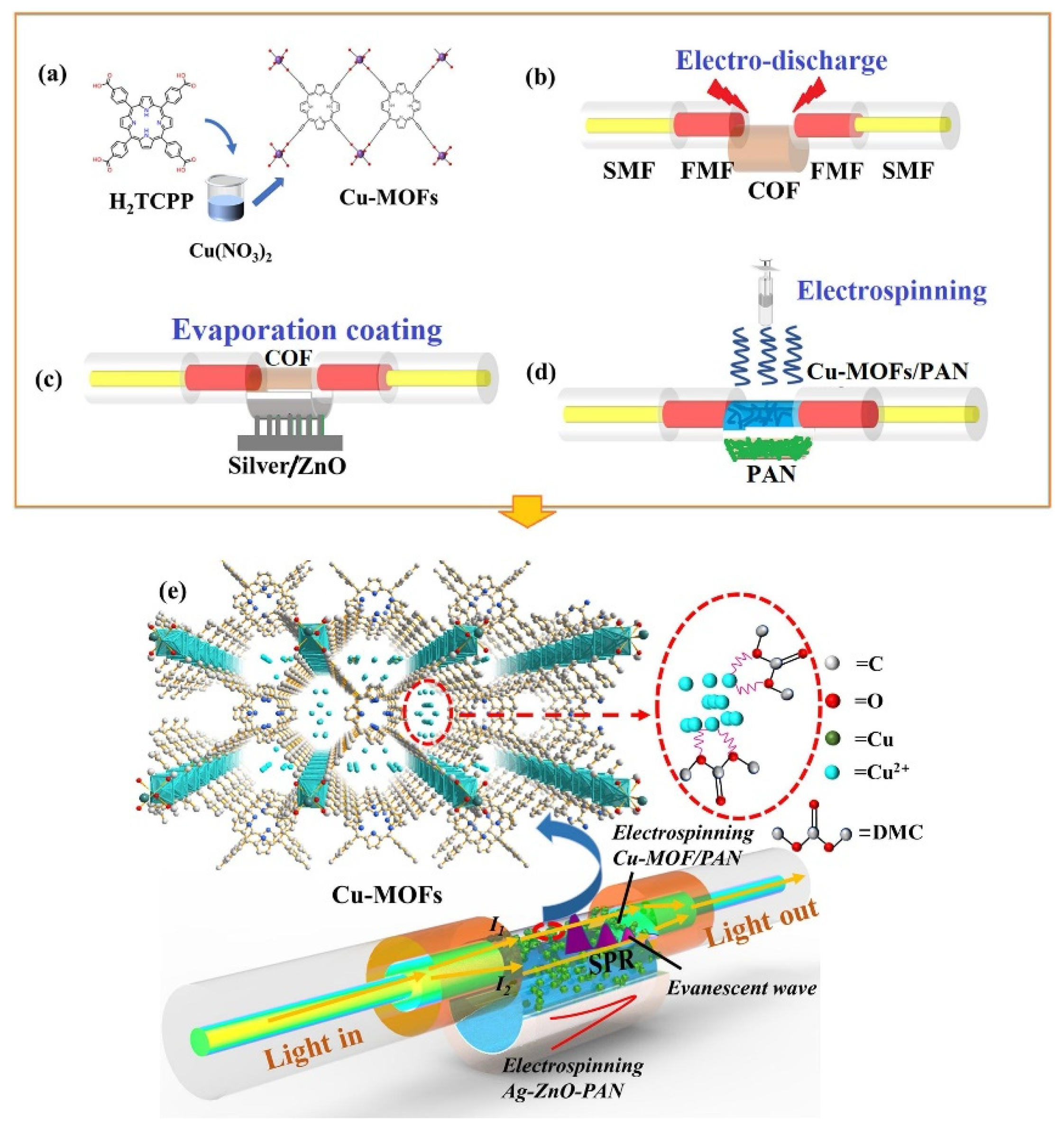
Previously, Liang et al. [43] designed and synthesized two indium-based MOFs (In-MOF1 and In-MOF2) by using simple and novel strategies. The authors used X-ray photoelectron spectroscopy (XPS) to characterize the proposed In-MOF. The authors also reported that In-MOF1 contains [In3(μ3–O) (CO2)6N2O] units whereas In-MOF2 has [In3(μ3–O)(CO2)6N3] units. The proposed In-based MOF has excellent sensing properties for the detection of Fe3+. In-MOF1 and In-MOF2 exhibited LODs of 2.89 × 10−4 M and 6.79 × 10−4 M, respectively. Recently, Kong et al. [44] proposed simple strategies and approaches for the sensing of dimethyl carbonate (DMC). The authors designed and synthesized Cu-MOF integrated polyacrylonitrile (PAN) material for the sensing of DMC. The synthesis procedure is described in Figure 4a. The fabrication of the sensor (Cu-MOF/PAN) is presented in Figure 4b–d. The sensing activity of the developed sensor is shown in Figure 4e. The proposed sensor exhibited an LOD of 53 ppb for the sensing of DMC.
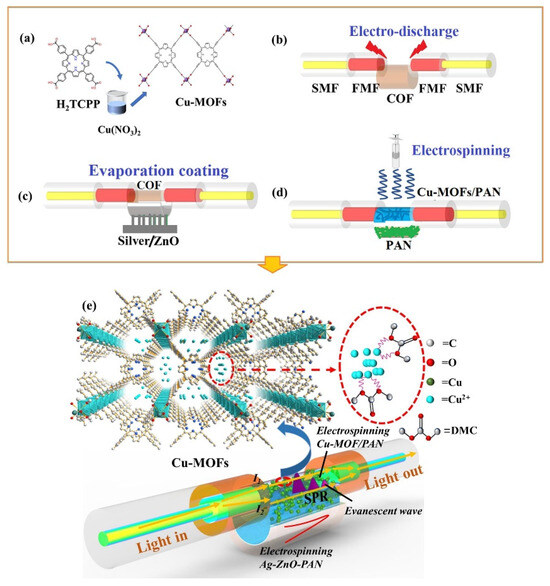
Figure 4.
(a) Schematic picture showing the preparation of Cu-MOF and construction of sensor (b–d). (e) Schematic illustration for the sensing of DMC. Reproduced with permission [44].
It has been observed from the reported literature that Cu-MOFs possess excellent gas sensing properties and are widely used in gas sensing and adsorption applications. In this context, Cai et al. [45] reported the preparation of a 2D conductive Cu-MOF (Cu3(HITP)2) for the sensing of ammonia. The schematic illustration of the synthesized Cu-MOF (Cu3(HITP)2) is shown in Figure 5a. The authors characterized Cu3(HITP)2 by using scanning electron microscopy (SEM) and found that it consists of irregular particles. X-ray diffraction (XRD) studies also suggested that Cu3(HITP)2 has a decent crystalline nature. The authors also adopted the XPS technique to further verify the valence states of the Cu2p, N1s, and C1s elements in the prepared Cu-MOF. The manufacturing procedure for the proposed sensor is described in Figure 5b. The authors adopted four synthesis systems which yielded 20 mg samples. The authors also found that samples were uniformly dispersed in DI water. In further steps, 2 µL of each solution was drop casted on alumina-ceramic-based substrate featuring Au interdigitated electrodes. Finally, the fabricated sensors electrodes were dried at 60 °C. The synthesized Cu-MOF was explored as sensing material for the determination of ammonia gas as shown in Figure 5c. The Cu-MOF exhibited an excellent LOD of 0.015 ppm for the detection of ammonia in dry air and RT conditions.
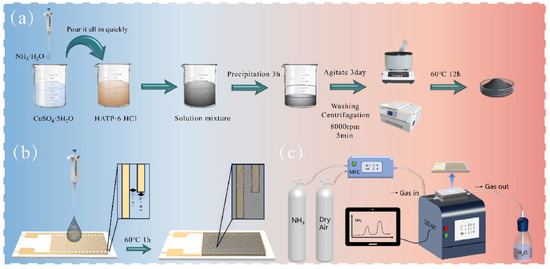
Figure 5.
(a) Schematic illustration for the synthesis of Cu3(HITP)2. (b) Schematic graph showing the construction of sensor and (c) gas sensing performance testing equipment. Reproduced with permission [45].
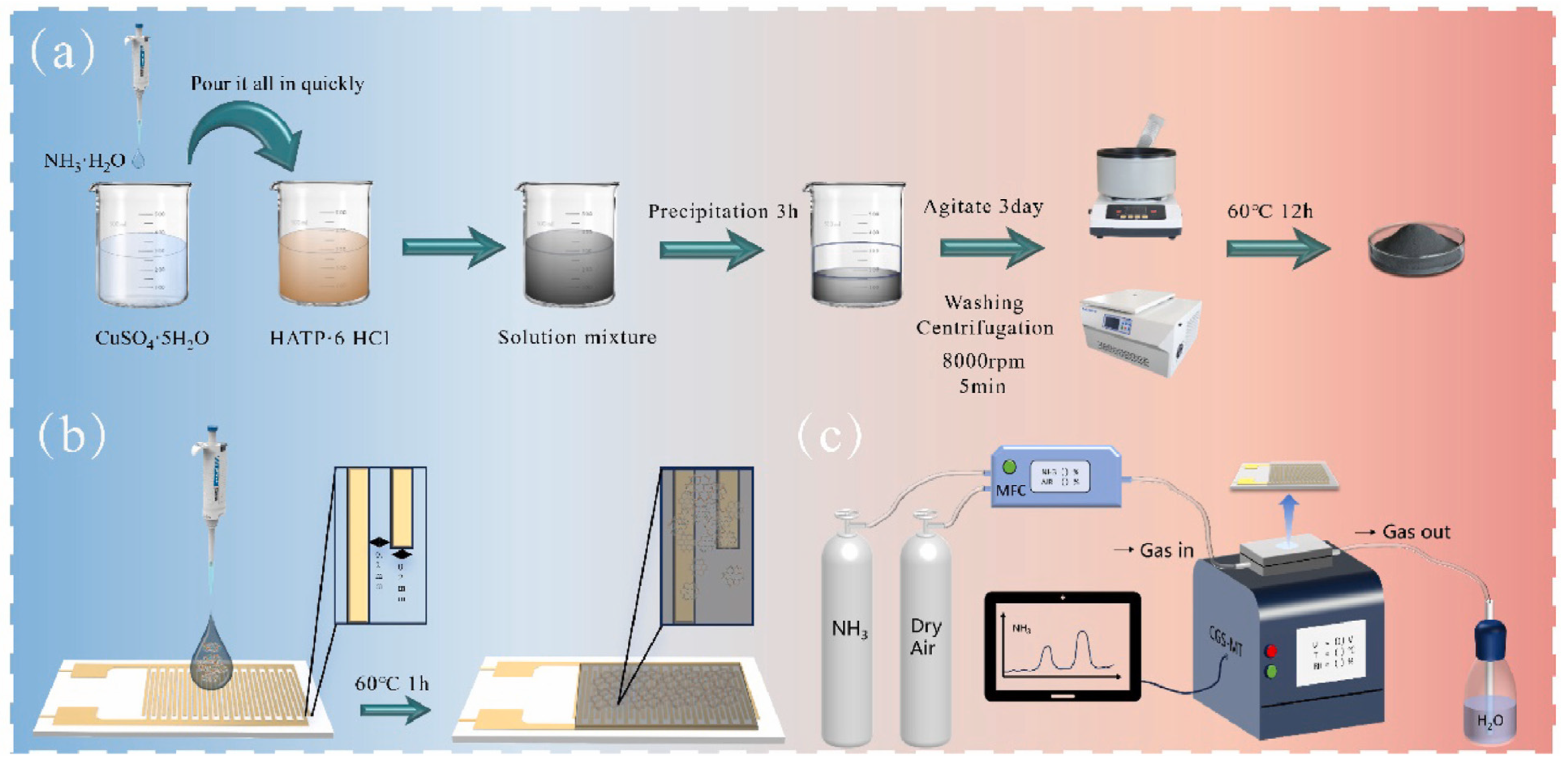
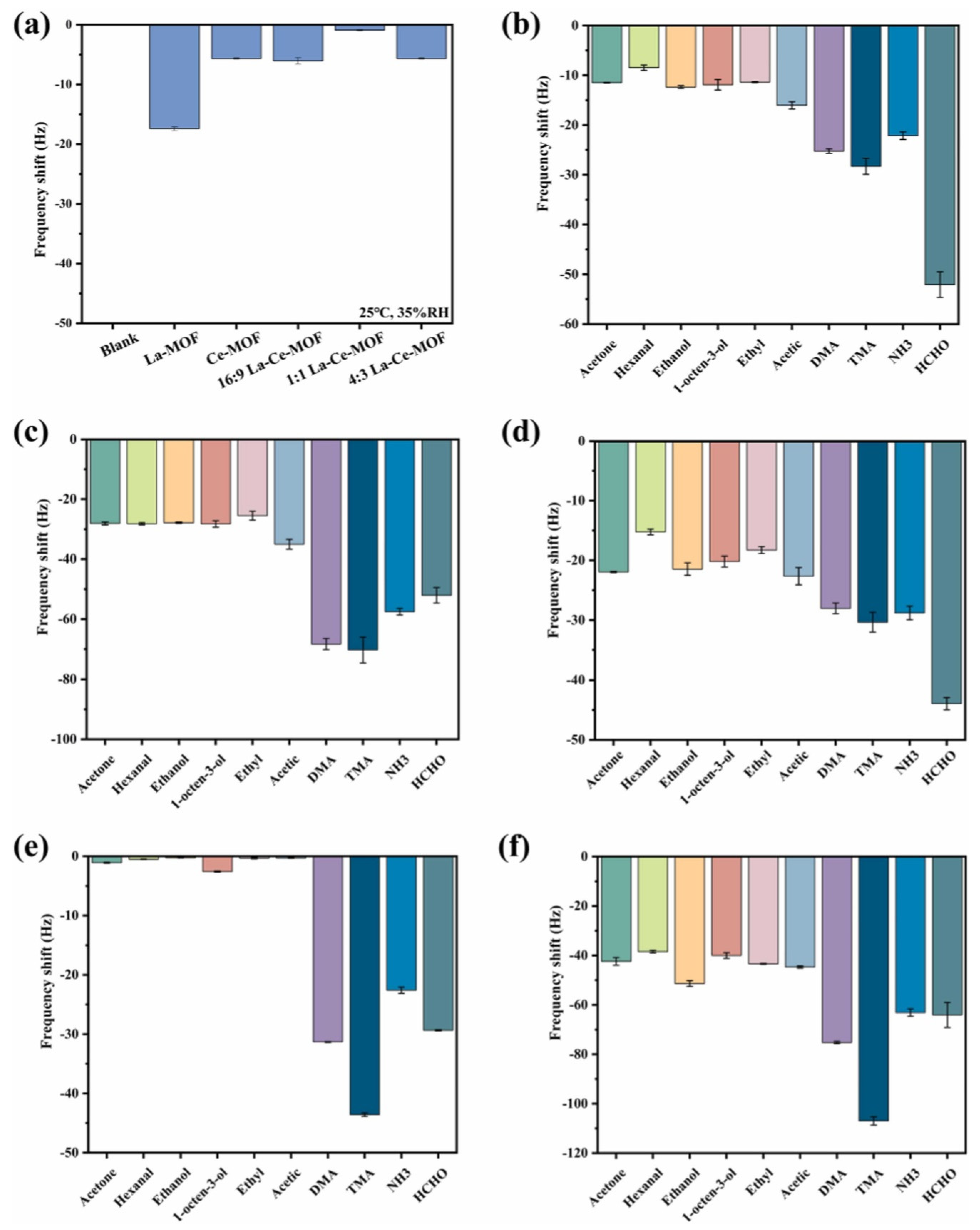
Chen et al. [46] reported the synthesis of a lanthanum- and cerium-based MOF (La-Ce-MOF). The synthesized MOF was used as gas sensing material for the detection of trimethylamine (TMA), ammonia (NH3), dimethylamine (DMA), and formaldehyde (HCHO). These gases are volatile in nature and may cause negative impacts on human health and the environment. Thus, it is important to monitor the level of these gases. The La-Ce-MOF was prepared using the hydrothermal method and characterized by SEM, XRD, and XPS. Sensor selectivity is an essential parameter. The target gases selected for analysis were key indicators commonly produced during the spoilage of salmon meat. Although interfering gases were mostly insignificant in the context of salmon spoilage, they could serve as signature indicators for spoilage in other materials. The concentration of all the substances was 20 μmol/L and obtained findings are presented in Figure 6a. To determine the sensor’s functionality under atmospheric conditions, a quartz crystal microbalance (QCM) gas sensor was modified with various sensing materials and tested in air. It was reported that response to the 1:1 La-Ce-MOF was almost negligible and is it suitable for the detection of volatile gases under atmospheric conditions. In further investigations, the authors used a La-MOF, Ce-MOF, and different proportions of La and Ce to optimize the sensing performance (Figure 6b–f). The obtained results suggested that a 16:9 La-Ce-MOF demonstrates relatively better selectivity for HCHO. The 1:1 La-Ce-MOF shows a significant sensing response to the target gases with negligible responses to the interfering gases. On the other side, the Ce-MOF and 4:3 La-Ce-MOF demonstrated remarkable selectivity for TMA but minimal responses to other target gases. In conclusion, the La-Ce-MOF nanocomposite demonstrated improved sensitivity to the target gases over interfering ones, highlighting its potential for the simultaneous detection of four target gases and its applicability in evaluating the freshness of salmon meat. LODs of 0.16 µmol/L, 0.35 µmol/L, 0.94 µmol/L, and 1.42 µmol/L were obtained for the sensing of TMA, DMA, NH3, and HCHO, respectively.
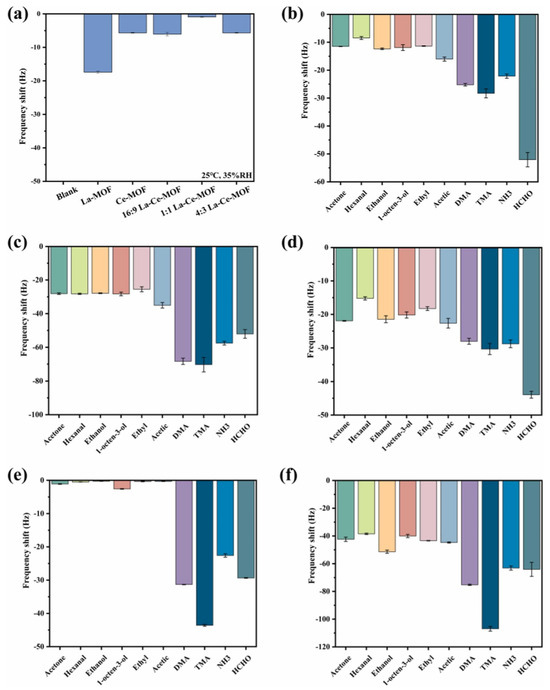
Figure 6.
(a) Sensing data for blank, La-MOF, Ce-MOF, and different La-Ce-MOFs. Sensing results of various analytes with 20 µmol·L−1 concentration for La-MOF (b), Ce-MOF (c), 16:9 La-Ce-MOF (d), 1:1 La-Ce-MOF (e), and 4:3 La-Ce-MOF (f). Reproduced with permission [46].
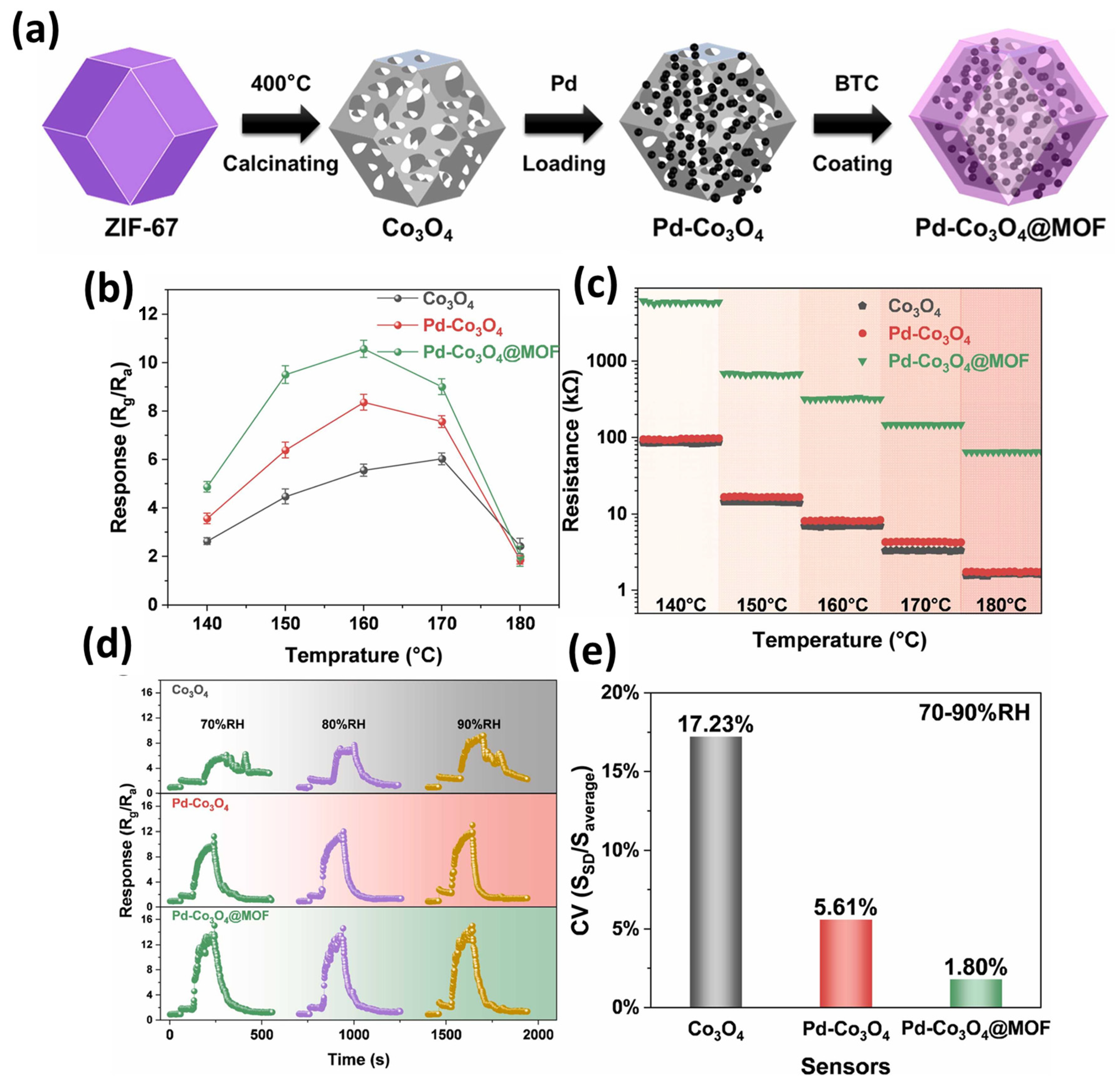
Previously, Jin et al. [47] proposed the synthesis of functionalized cobalt oxide (Co3O4) porous cages/palladium (Pd)/Co-MOFs. The synthesis of the Pd-Co3O4@Co-MOF is described in Figure 7a. The authors found that the ZIF-67-derived Pd-Co3O4@Co-MOF has excellent gas sensing properties. The physicochemical properties of the Pd-Co3O4@Co-MOF were examined by SEM, XRD, XPS, and energy dispersive X-ray spectroscopic (EDX) studies. The authors also found that the synthesized porous cage composite has humidity-independent and sensitive properties for the detection of acetone. Figure 7b shows the response of the Co3O4, Pd-Co3O4, and Pd-Co3O4@MOF to 50 ppm acetone at temperatures from 140–180 °C. The Pd-Co3O4@MOF sensor exhibits the highest response compared to Co3O4 or Pd-Co3O4. The authors found that sensor response increases with increasing temperature but it started to decrease after 160 °C for the Pd-Co3O4@MOF. The resistances of the Co3O4, Pd-Co3O4, and Pd-Co3O4@MOF in air under different temperature conditions are shown in Figure 7c. The initial resistance decreases with increasing temperature as shown in Figure 7c. The dynamic response–recovery transient curves of the Co3O4, Pd-Co3O4, and Pd-Co3O4@MOF for 50 ppm acetone in different humidity conditions are shown in Figure 7d. The response value increases using dry air as background gas under relative humidity. The performance of the sensor is positive under relative humidity conditions (Figure 7d). The CV values of the Co3O4, Pd-Co3O4, and Pd-Co3O4@MOF for 50 ppm acetone under different humidity conditions at 160 °C are presented in Figure 7e. The CV values of the Co3O4, Pd-Co3O4, and Pd-Co3O4@MOF for 50 ppm acetone were found to be 17.23%, 5.61%, and 1.80%, respectively. The authors also stated that dual functionalization of Pd and the Co-MOF may enhance the humidity resistance of the Co3O4 sensor. This exceptional moisture resistance may be attributed to the oxygen adsorption regulation provided by Pd functionalization and the hydrophobic substituent linkers within the Co-MOF.
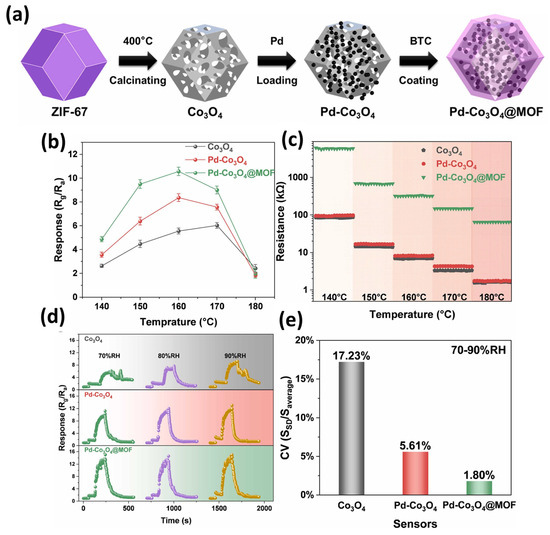
Figure 7.
(a) Schematic graph showing the synthesis of Pd-Co3O4@Co-MOF. (b) Response of Co3O4, Pd-Co3O4, and Pd-Co3O4@MOF to 50 ppm acetone at different temperatures. (c) Resistances of Co3O4, Pd-Co3O4, and Pd-Co3O4@MOF in air under different temperature conditions. (d) Response and (e) CV values of Co3O4, Pd-Co3O4, and Pd-Co3O4@MOF for 50 ppm acetone under different humidity conditions at 160 °C. Reproduced with permission [47].
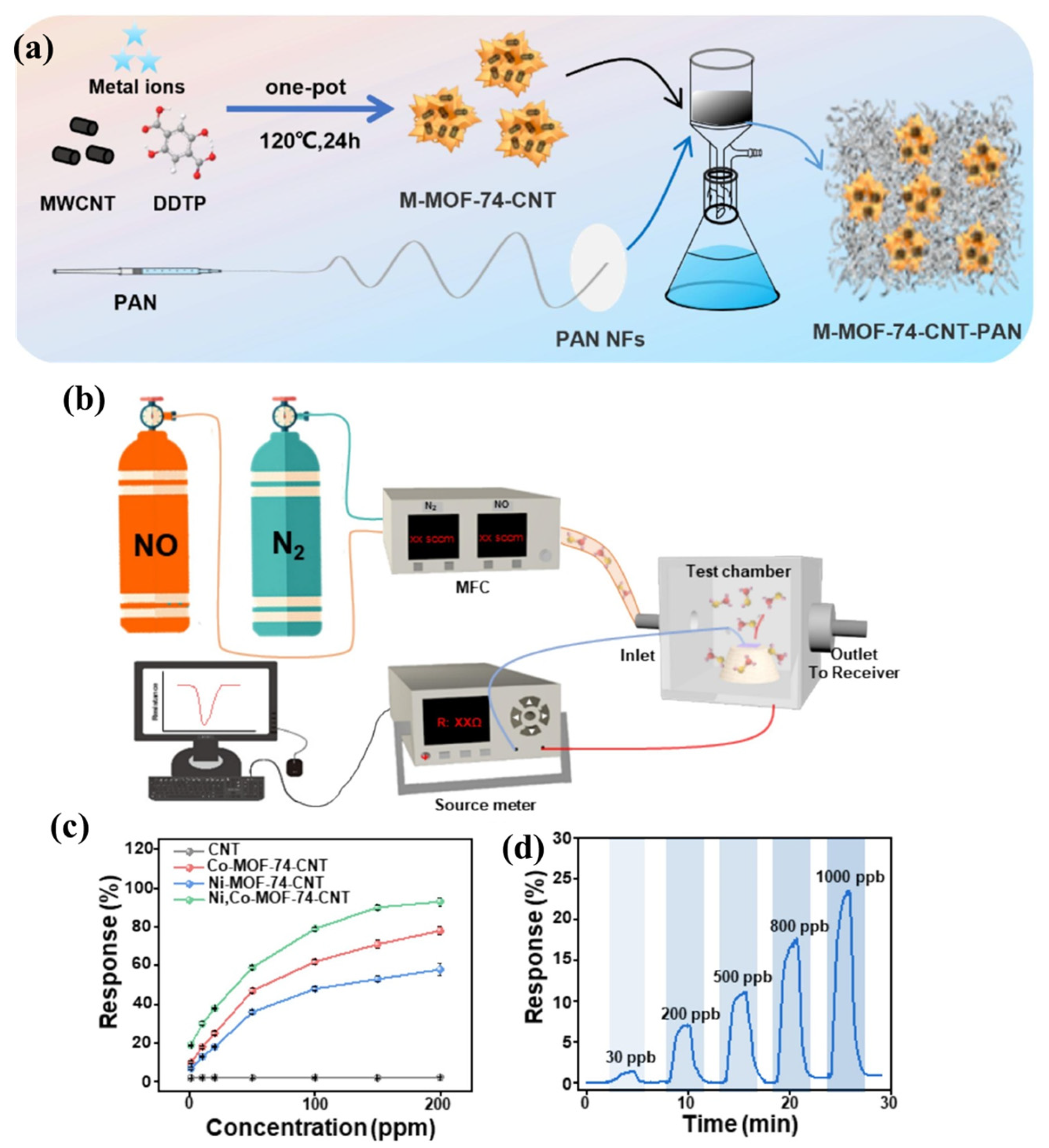
Figure 8a shows the schematic illustration for the synthesis of M-MOF-74-CNT (where M = Ni or Co). The surface morphological characteristics of the synthesized Ni-MOF-74, Co-MOF-74, Ni, Co-MOF-74 and Ni, Co-MOF-74-CNT were determined by using the SEM method. The sensing system for the determination of NO is represented in Figure 8b. The response versus concentration of the different sensing materials is presented in Figure 8c. It can be clearly understood that Ni, Co-MOF-74-CNT has excellent sensing behavior compared to the other materials. The real-time responses of the Ni, Co-MOF-74-CNT for different concentrations of NO are presented in Figure 8d, which demonstrates that Ni, Co-MOF-74-CNT possesses excellent sensing properties for the determination of NO. This sensor exhibits an excellent LOD of 18.6 ppb in the detection of NO [48].
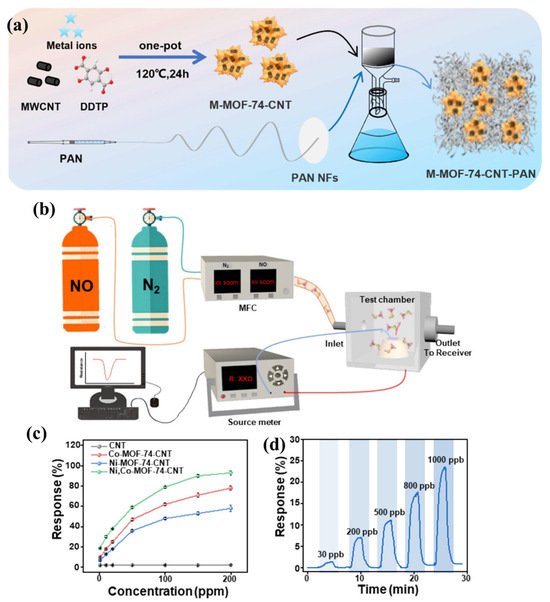
Figure 8.
(a) Synthetic steps for the preparation of M-MOF-74-CNT. (b) Schematic picture for NO detection system. (c) Response versus concentration curves for CNT, Co-MOF-74-CNT, Ni-MOF-74-CNT, and Ni, Co-MOF-74-CNT. (d) Real-time responses of Ni, Co-MOF-74-CNT with different NO concentrations. Reproduced with permission [48].
In another study, a vanadium (V)-based MOF was synthesized by employing the hydrothermal method [49]. The hydrothermally prepared V-MOF(PTA) and V-MOF(PMA) were characterized by XRD, SEM, and XPS methods. The authors pyrolyzed the organic ligand whereas vanadium ions were oxidized to form the V-MOF120(PTA), V-MOF150(PTA), V-MOF120(PMA), and V-MOF150(PMA). Furthermore, the authors explored the synthesized materials (V-MOF120(PTA), V-MOF150(PTA), V-MOF120(PMA), and V-MOF150(PMA)) for NO2 gas sensing applications. The V-MOF120(PTA) exhibited the highest response with an average of 800.8% at 100 ppm NO2. The proposed sensing material shows the decent LOD of 1 ppm with acceptable selectivity for the determination of NO2 compared to ethanol, acetone, methanol, and ammonia. Yu et al. [50] successfully prepared a (Cu–S)n MOF-PANI nanocomposite by using an in situ polymerization process. The authors found that (Cu–S)n MOF particles are well-distributed in the fiber-like matrix of polyaniline (PANI). Furthermore, a 3wt% MOF-PANI-coated IDE was constructed for hydrogen sulfide (H2S) gas sensing applications. The proposed sensing material demonstrated an excellent LOD, selectivity, and repeatability and acceptable stability. Carbon dioxide (CO2) is the well-known greenhouse gas which is present in the atmosphere and its amount is increasing due to deforestation and the extensive use of fossil fuels. Although a low level of CO2 does not harm human beings, a higher concentration may affect the human respiratory system. Thus, sensing of CO2 gas is of great significance and Wang et al. [51] reported the synthesis of M-MOF-74 (M = Mg, Ni, Zn, and Co) for the sensing of CO2 gas.
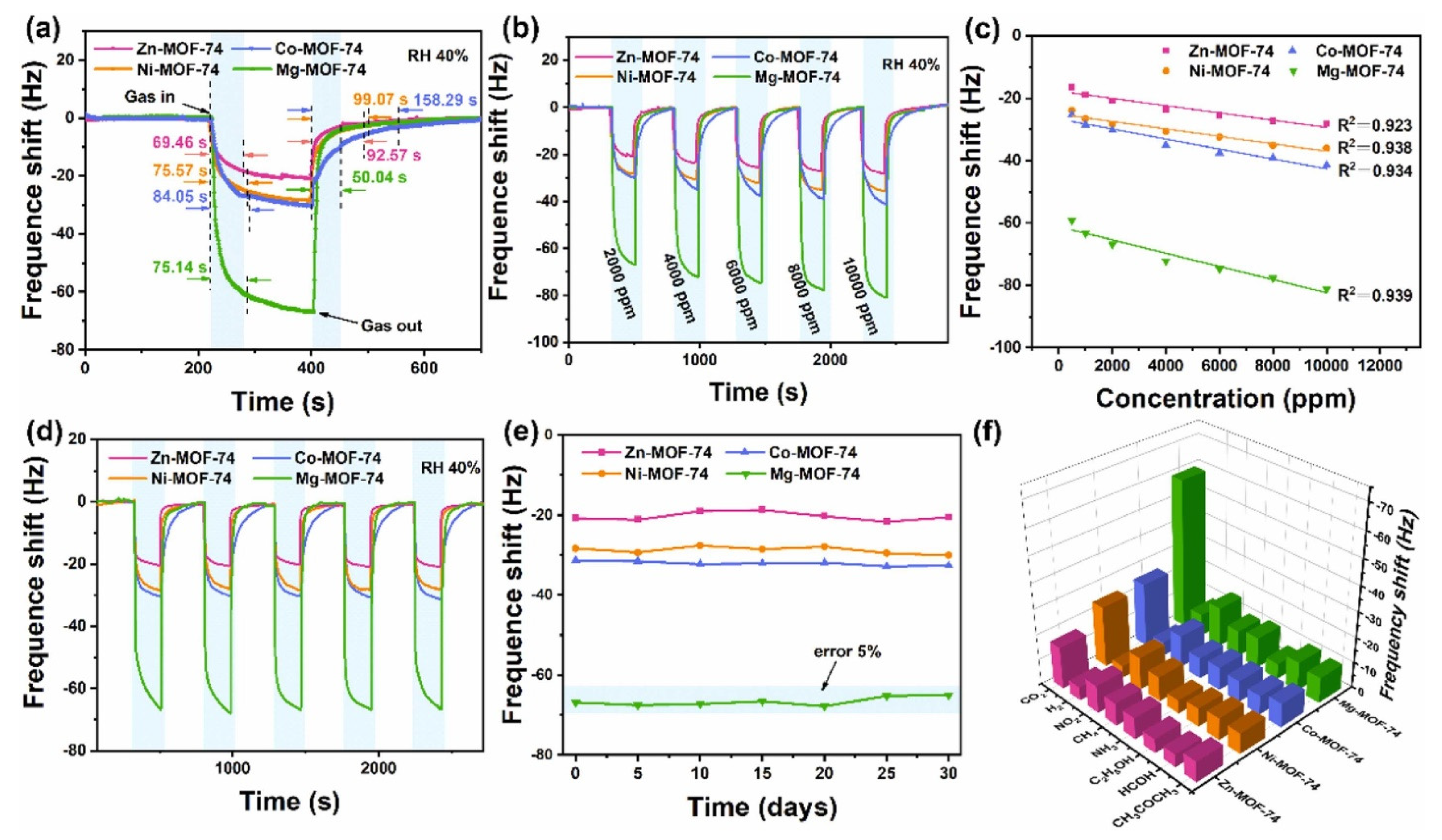
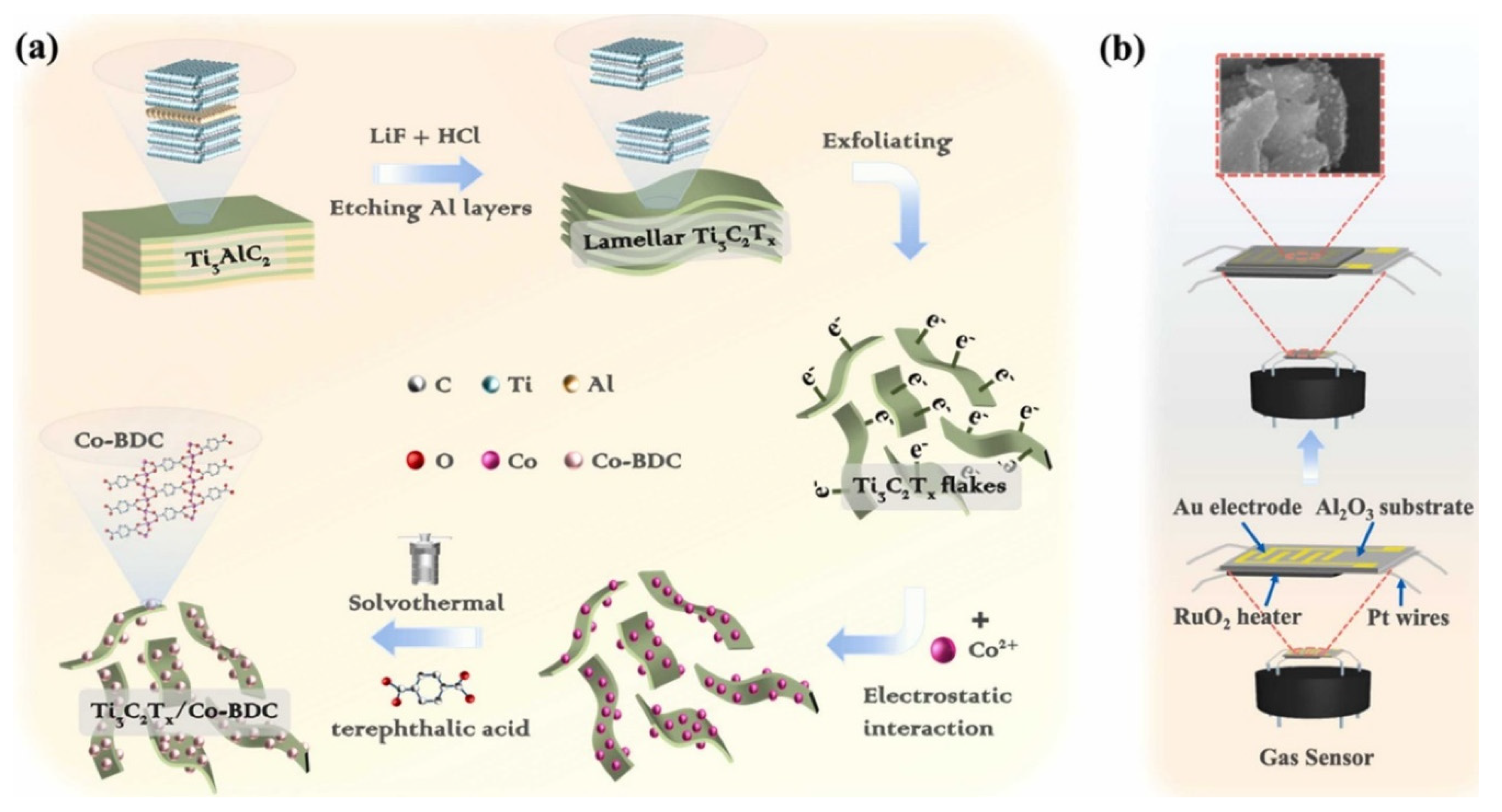
Figure 9a shows the responses of the various sensing materials for the sensing of CO2 gas. The concentration of CO2 gas was 2000 ppm and observations suggested that Mg-MOF-74 is most efficient sensing material compared to the other materials. The dynamic response of the sensors was also checked under 2000 to 10,000 ppm CO2 gas at RT (Figure 9b). The frequency responses of the M-MOF-74 (M = Mg, Ni, Zn, and Co) increase with increasing CO2 concentration as shown in Figure 9b. The obtained results shows that Mg-MOF-74 demonstrated a significantly good response in the range of 500 to 1000 ppm CO2 and suggested its potential for practical applications. Figure 9c shows the fitted curves exhibiting the relationship between the frequency shift and CO2 gas concentration for M-MOF-74 (M = Mg, Ni, Zn, and Co). Correlation coefficients (R2) of 0.923, 0.938, 0.9834, and 0.939 were found for Zn-MOF-74, Ni-MOF-74, Co-MOF-74, and Mg-MOF-74, respectively (Figure 9c). The Mg-MOF-74 exhibited the best linearity for CO2 gas sensing compared to the other sensors. Since repeatability is the major concern for these sensors, reproducibility of the sensor was evaluated for 2000 ppm CO2 and obtained results are shown in Figure 9d. The sensing studies exhibit acceptable repeatability of the proposed sensor. The responses of the sensors for repetitive tests of more than one month for 2000 ppm CO2 are summarized in Figure 9e. The obtained results demonstrated acceptable results in terms of fluctuations with a less than 5% deviation. The proposed sensor also exhibited excellent selectivity as shown in Figure 9f. Wei et al. [52] reported the preparation of Mg-doped MOF-ZnO for the determination of n-butanol gas at lower temperatures. The authors adopted a co-precipitation approach for the synthesis of the Mg-doped MOF-ZnO composite. The gas sensing features of the pristine MOF-ZnO and Mg-doped MOF-ZnO samples with different doping percentages of 1%, 3%, 5%, and 7% (wt%) were evaluated by a gas-sensitive system. The investigations showed that the 3% Mg:MOF-ZnO sensor has a large surface area and the highest oxygen vacancy and demonstrated excellent gas sensing activity compared to the other sensors. Maji et al. [53] proposed the synthesis of a fully flexible Fe/Co hydrogel-based sensitive sensor for the detection of acetone gas at RT. The Fe/Co MOF HG sensor exhibited a lower LOD value of 103 ppb with acceptable selectivity and sensitivity. The gas sensing performance of this sensor was evaluated by examining the adsorption of a large number of acetone molecules onto the hydrogel surface and their interaction with the abundant oxygen-containing groups of PVA in the hydrogel. Reliability tests of mechanical strength and humidity resistance suggested that the proposed sensor is stable over time and it its performance is unaffected by humidity. Cai et al. [54] observed that a quasi-Zn-MOF may be a suitable sensing material for the detection of n-butanol. The Zn-MOF-210 exhibited a good response of 173 for 30 ppm n-butanol at 200 °C. The authors proposed and emphasized the importance of the quasi-MOF-based materials for gas sensing applications. Liu et al. [55] have proposed the synthesis of La2O3 modified MOF-SnO2 composite using a one-step hydrothermal method. In other work, Nguyen et al. [56] prepared a series of porphyrinic-Al-based MOFs using a benign solvothermal procedure. The authors prepared porphyrinic pristine and Co2+ and Cu2+ metalated Al-based MOFs. The synthesized material was denoted as AlPMOF(M) (M = Cu, Co). The reported MOF-based material was applied for a CO sensing application and it was found that AlPMOF(Cu) and AlPMOF(Co) materials exhibited better performance compared to the pristine sensor. The AlMOF (Cu) and AlMOF (Co) exhibited a good response for 10 ppm CO gas at RT and 200 °C. Fang et al. [57] reported the synthesis of a CuNi-MOF derivative with TiO2 quantum dots for the sensing of H2S gas. The proposed sensor demonstrated an LOD of 100 ppb with excellent repeatability and selectivity. It has been observed that MXene materials may boost the sensing performance of MOF-based materials. It would be worth combining MOFs and MXene materials for gas sensing applications. In this context, Ding et al. [58] designed and prepared a 0D-2D heterostructure of a Co-MOF integrated MXene composite for H2S gas sensing applications. It is a well-understood fact that Co-MOFs have a large surface area, the ability to capture more gas molecules, and improved host–guest interactions which can subsequently boost the sensing performance in terms of selectivity and sensitivity. The Co-MOF@MXene exhibited an acceptable LOD value of 50 ppb for H2S sensing with high selectivity for H2S determination against interfering species. It is well-known that triethylamine (TEA) is a volatile organic environmental pollutant which may have significant negative impacts on the environment. Thus, Liu et al. [59] reported simple and efficient strategies for the sensing of TEA using MOF integrated MXene as sensing material. The authors synthesized a Ti3C2Tx/Co-BDC composite and characterized it with transmission electron microscopy (TEM), XRD, and XPS. The formation of Ti3C2Tx/Co-BDC composite is described in Figure 10a. The synthesized Ti3C2Tx/Co-BDC composite was further applied as a sensor for the determination of TEA as shown in Figure 10b. The proposed novel sensor demonstrated an LOD of 0.085 ppm for the detection of TEA with decent selectivity. This shows that MXene-based MOF materials may be suitable sensing materials for various gas sensing applications. More in-depth study is required on the development of MOFs/MXene-based materials for gas sensing applications.
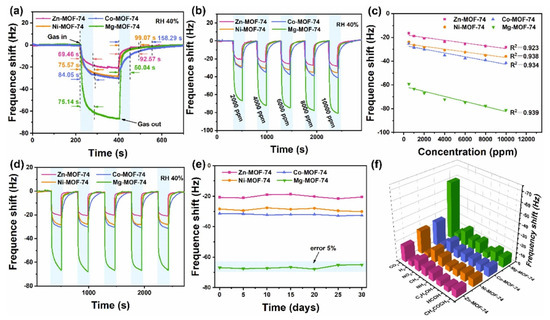
Figure 9.
(a) Response curves of the M-MOF-74-based QCM sensors for 2000 ppm CO2 gas at RT. (b) Dynamic responses of the M-MOF-74-based QCM sensors exposed to CO2. (c) Fitting curves of sensors with respect to different CO2 concentrations. (d) Repeatability test and (e) stability and (f) selectivity study. Reproduced with permission [51].
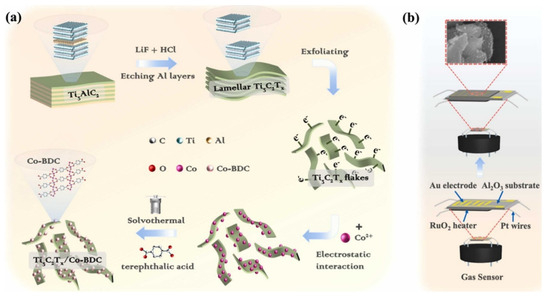
Figure 10.
Schematic illustration of the preparation of (a) Ti3C2Tx/Co-BDC and gas sensing procedure (b). Reproduced with permission [59].
Zhang et al. [60] used a derivative of tin (Sn)-MOF for NO2 sensing applications. The authors prepared the SnO2-M-Ov-T using thermal treatment of the Sn-MOF which was further applied as NO2 sensing material. The SnO2-M-Ov-300 exhibited a better response and sensing performance for the monitoring of NO2. Yan et al. [61] reported the fabrication of a chemiresistive ethylene gas sensor by employing single-walled carbon nanotube (SWCNT) modified Pd NPs/Cu-MOF-74 as sensing material. The SWCNTs/PdNPs/Cu-MOF-74 composite was attached to a flexible PET substrate and the developed sensor showed acceptable sensing performance. The sensor exhibited an LOD of 31 ppb with excellent selectivity and sensitivity. Yang et al. [62] reported the fabrication of accordion-like ZIF-8/MoO3 composite via the hydrothermal method. The prepared material was explored for H2S gas sensing applications and demonstrated decent performance. The above observations indicated that MOF-based hybrid composite materials are promising gas sensing materials and further studies and investigations are required to explore them for practical applications. The gas sensing performance data of the MOF-based materials are compiled in Table 1.

Table 1.
Summary of the gas sensing performance of the previously reported gas sensors using MOF-based materials.
3.2. MOF-Derived Materials
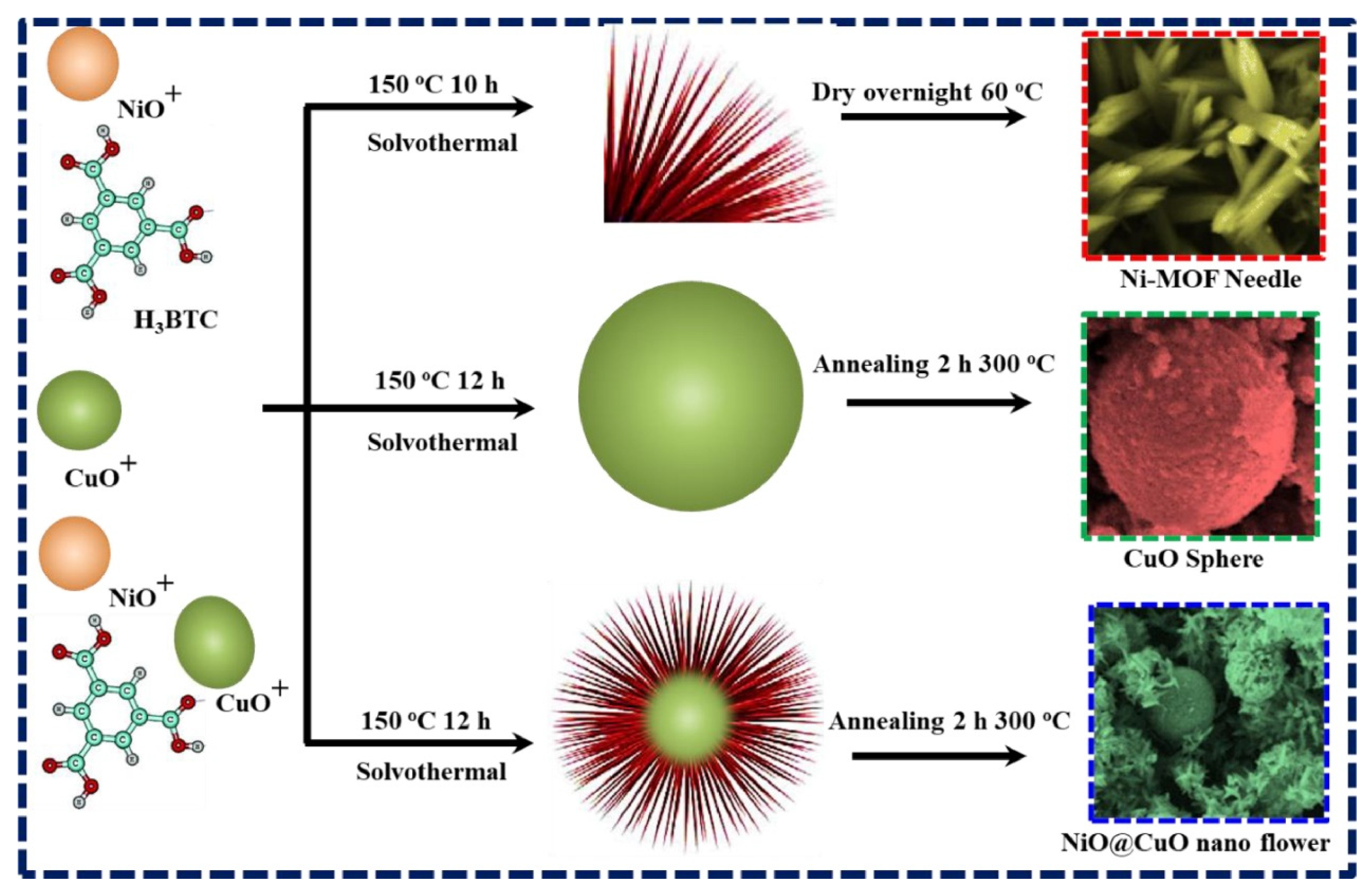
It is reported that MOF-derived nanostructured materials may offer significant advantages for gas sensing applications due to their exceptional structural and functional versatility. The large specific surface area and porosity inherited from their parent MOFs provide abundant active sites for gas adsorption which may increase sensitivity. The tailored morphologies of the MOF-derived nanostructured materials may promote efficient gas diffusion/interactions with the sensing material. In addition, MOF-derived materials may often demonstrate enhanced electrical conductivity. In particular, those doped with carbon or metallic phases may enable faster and more reliable signal transduction. MOF-derived materials are thermally and chemically stable which make them suitable candidates for sensing operations in harsh environments. It is also understood that flexibility in design and composition may further allow the fabrication of materials with desirable properties which may include selective gas sensing and/or increased response speed. Thus, the unique features of MOF-derived materials make them efficient sensing materials for the development of advanced gas sensing technologies. In this regard, Wang et al. [63] adopted an electrospinning method for the preparation of bimetallic MOF-derived tungsten oxide/zinc tungstate/cobalt tungstate (WO3/ZnWO4/CoWO4) composite. The authors used MOF material as a precursor and subsequently transformed the MOF into WO3/ZnWO4/CoWO4 composite. The obtained WO3/ZnWO4/CoWO4 composite was used for the sensing of n-butanol. The MOF-derived WO3/ZnWO4/CoWO4 composite exhibited decent gas sensing performance. Nickel oxide (NiO) an efficient gas sensing material for the detection of hydrogen (H2) gas due to its high sensitivity and low resistance. However, low electron–hole transport may result in a low gas response value. Thus, it is worth combing it with other metal oxides such as copper oxide (CuO) to form a heterostructure. It is also of great significance to use MOF-based structures as precursors to obtain gas sensing materials with desirable and improved properties. Thus, Shah et al. [64] prepared unique NiO@CuO nanoflowers (NFs) and morphological studies showed that the synthesized material has a porous sharp-tip and nanospherical structure. The synthesis of the gas sensing material is described in Figure 11. A Ni-MOF was prepared using the solvothermal method and a morphological study demonstrated that the synthesized Ni-MOF has a needle-like surface structure. CuO was also prepared using the solvothermal method and it was observed that CuO has a spherical structure. The authors observed that 2 wt% NiO@CuO NFs have excellent selectivity towards the detection of H2 gas and demonstrated high sensitivity with an LOD of 300 ppb. The improved gas sensing performance of the 2 wt% NiO@CuO NFs may be ascribed to the presence of synergistic interactions between the NiO and CuO nanoflowers.
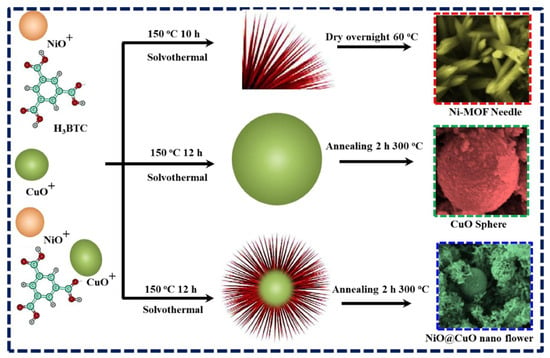
Figure 11.
Schematic description for the preparation of NiO@CuO NFs. Reproduced with permission [64].
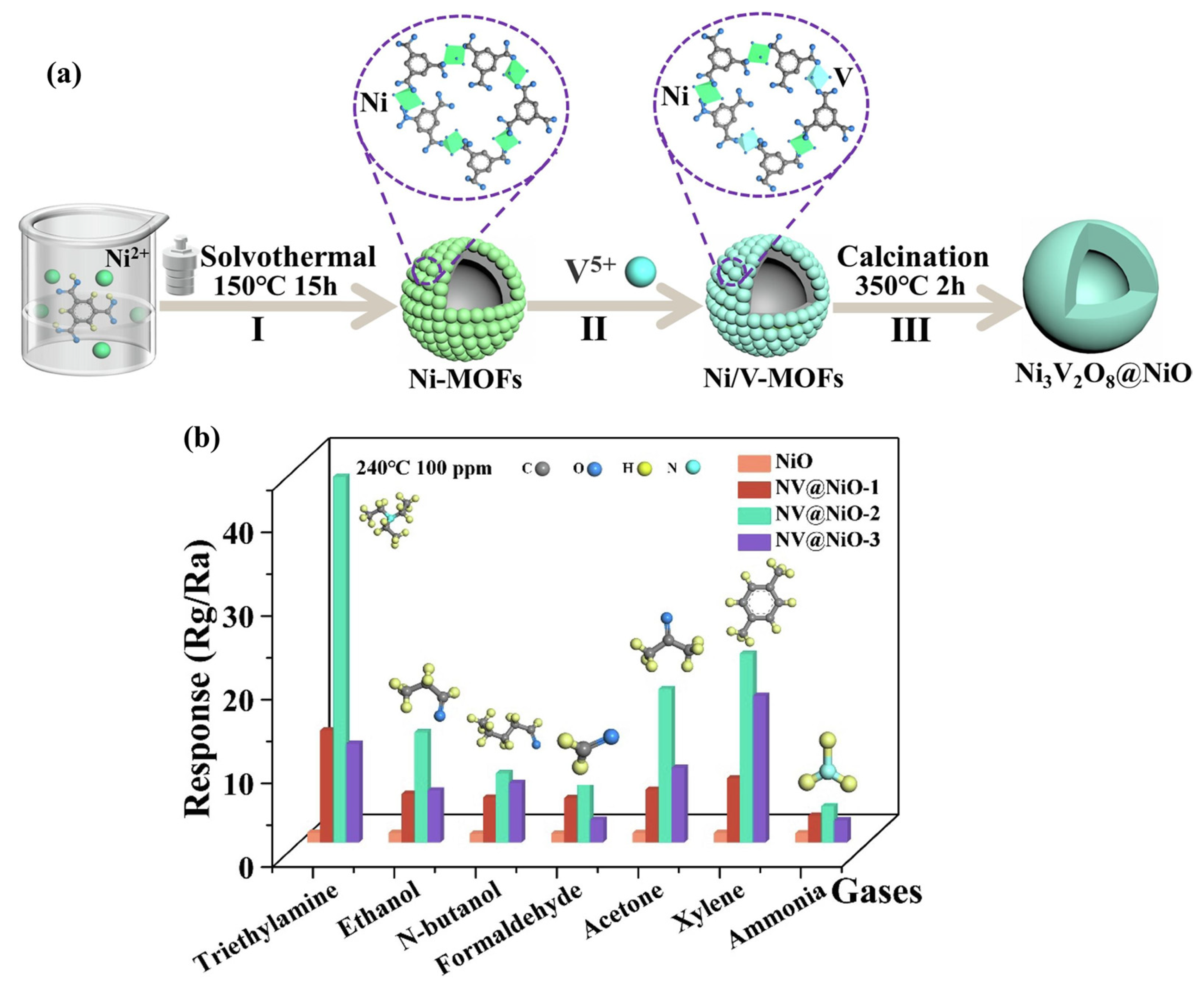
Zhang et al. [65] have adopted different contents of 1, 3, 5-benzenetricarboxylic acid (BTC) and stannous sulfate (molar ratio 0.5:1, 1.5:1, and 2:1) to design and obtain Sn-based MOF structures by employing the solvothermal method. The calcination of the prepared Sn-MOF yielded SnO2 particles. The SnO2-1.5 exhibited better gas sensing properties compared to the other prepared materials. To further improve the gas sensing properties of the SnO2-1.5, Ce was used as dopant and different mole percentages (0.25%, 0.75%, and 1.25%) of Ce were used to prepare the MOF-derived Ce-doped SnO2. The SnO2-Ce-0.75 further demonstrated enhanced gas sensing response and better selectivity for the detection of ethylene glycol. Hussain et al. [66] reported the simple and straightforward calcination process for the preparation of Mo-doped Co3O4 using MOFs as precursors. The authors used the ion-assisted solvothermal method for obtain Co3O4 and Mo-Co3O4 nanostructures with yeast-like morphology. The 2 mol % Mo-Co3O4 showed better CO sensing performance compared to the pristine Co3O4. Geng et al. [67] proposed the fabrication of Ni3V2O8@NiO composite for the sensing of TEA. The authors adopted a three-step solvothermal method, cation-exchange method, and annealing process approach for the preparation of Ni3V2O8@NiO composite. In the first step, the authors prepared a Ni-MOF structure using the solvothermal method as shown in Figure 12a. In the second step, a Ni/V-MOF was prepared which was further converted to Ni3V2O8@NiO composite by the annealing method. The gas sensing activity (response) of the different materials for 100 ppm of different gases at 240 °C is shown in Figure 12b. It can be observed that the NV@NiO-2 sensor exhibited a better response for the sensing of TEA compared to the other materials. The obtained results also demonstrated acceptable selectivity for the detection of TEA.
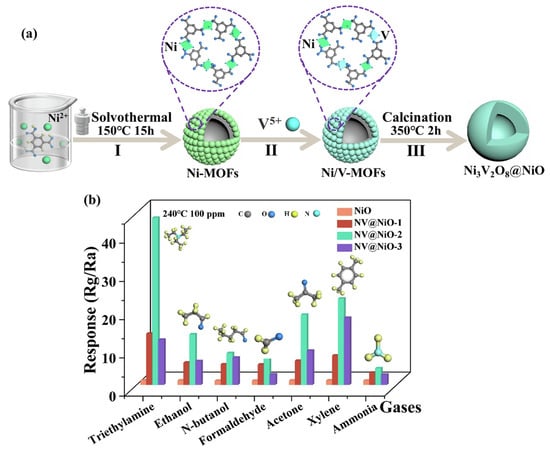
Figure 12.
(a) Schematic representation of the synthesis of Ni3V2O8@NiO. (b) Response of the different sensors for 100 ppm of different gases at 240 °C. Reproduced with permission [67].
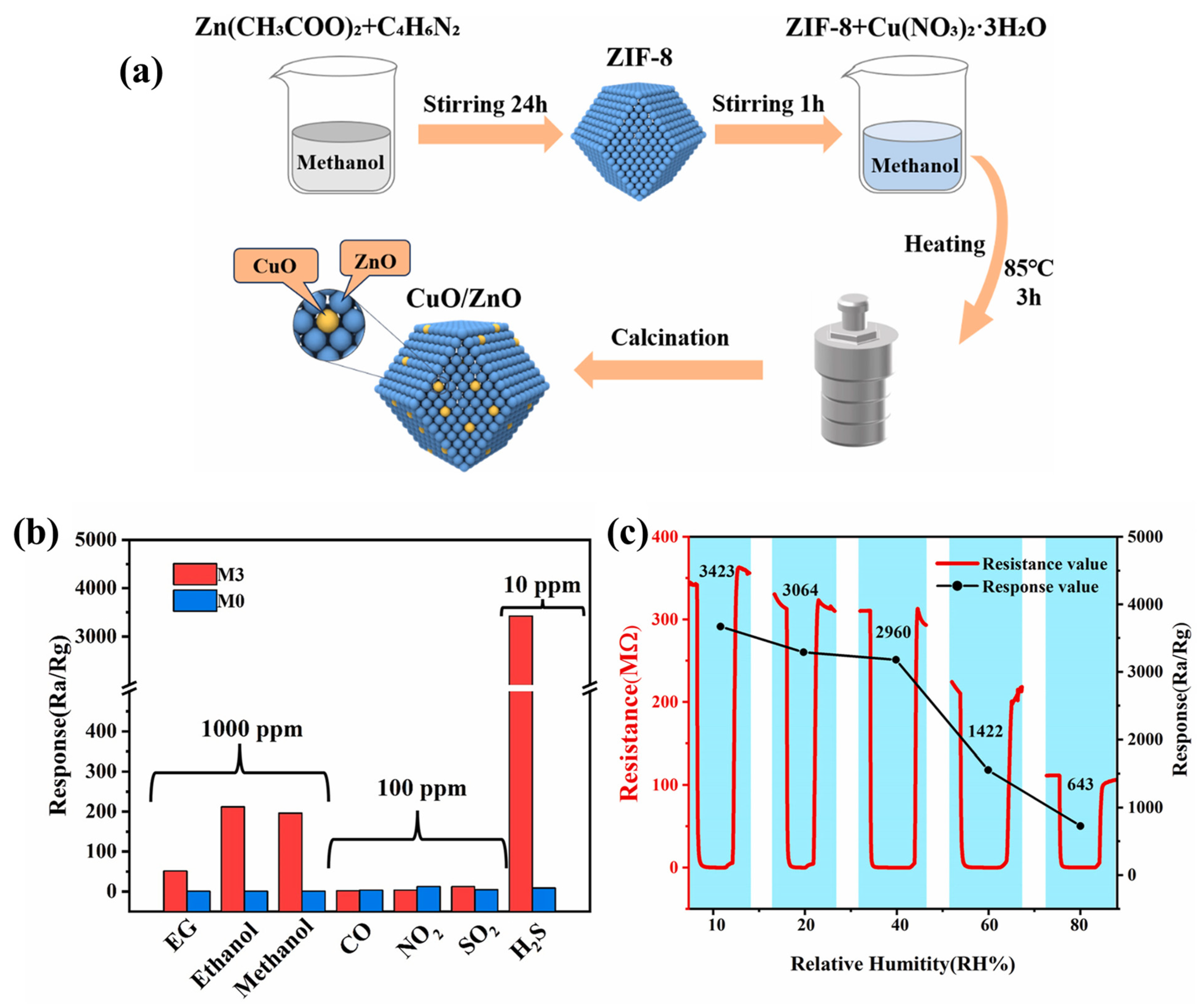
Xiao et al. [68] reported the synthesis of ZIF-8-derived CuO/ZnO composite for the sensing of H2S gas. The synthetic protocols are described in Figure 13a. Various percentages of CuO were used to optimize the gas sensing performance of the CuO/ZnO composite. The authors found that the M3 sample has better performance compared to the other prepared materials. The selectivities of M3 and M0 were also checked as shown in Figure 13b. It is clear that M3 has better selectivity compared to the pristine M0 sample. The resistances of the M3 sample under different humidity conditions are summarized in Figure 13c. It was found that the M3 sample has excellent gas sensing activity up to 40% RH. However, M3 also demonstrated acceptable performance at 80% RH. The CuO/ZnO composite demonstrated excellent gas sensing behavior for 10 ppm H2S gas. Lu et al. [69] have explored novel strategies to synthesize different valent metal cation (Zn2+, In3+, and Zr4+) doped Co3O4 materials using ZIF-67 as a precursor. The authors investigated the influence of different doping and valence contents of metal cations on the surface structure properties of the prepared doped Co3O4 for toluene sensing. The authors reported that valence Zr doping significantly enhanced the toluene gas sensing properties of the prepared Co2.717Zr0.189O4 sample. The Co2.717Zr0.189O4 sample exhibited the highest response value of 94.58 to 100 ppm toluene gas which was better than that of pristine Co3O4.
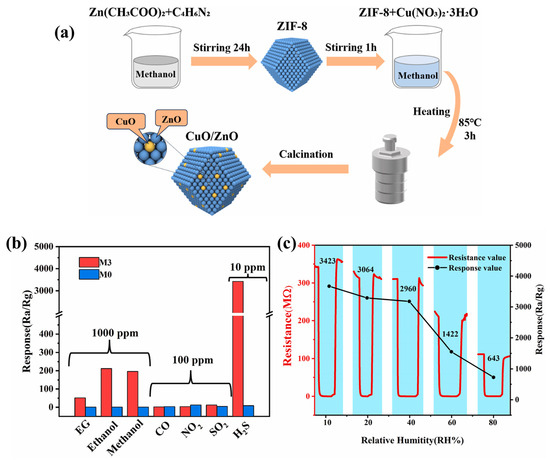
Figure 13.
(a) Schematic graph for the synthesis of CuO/ZnO composite. (b) Selectivity response test of M3 and M0 for multiple gases. (c) Response of M3 for 10 ppm H2S under different humidity conditions. Reproduced with permission [68].
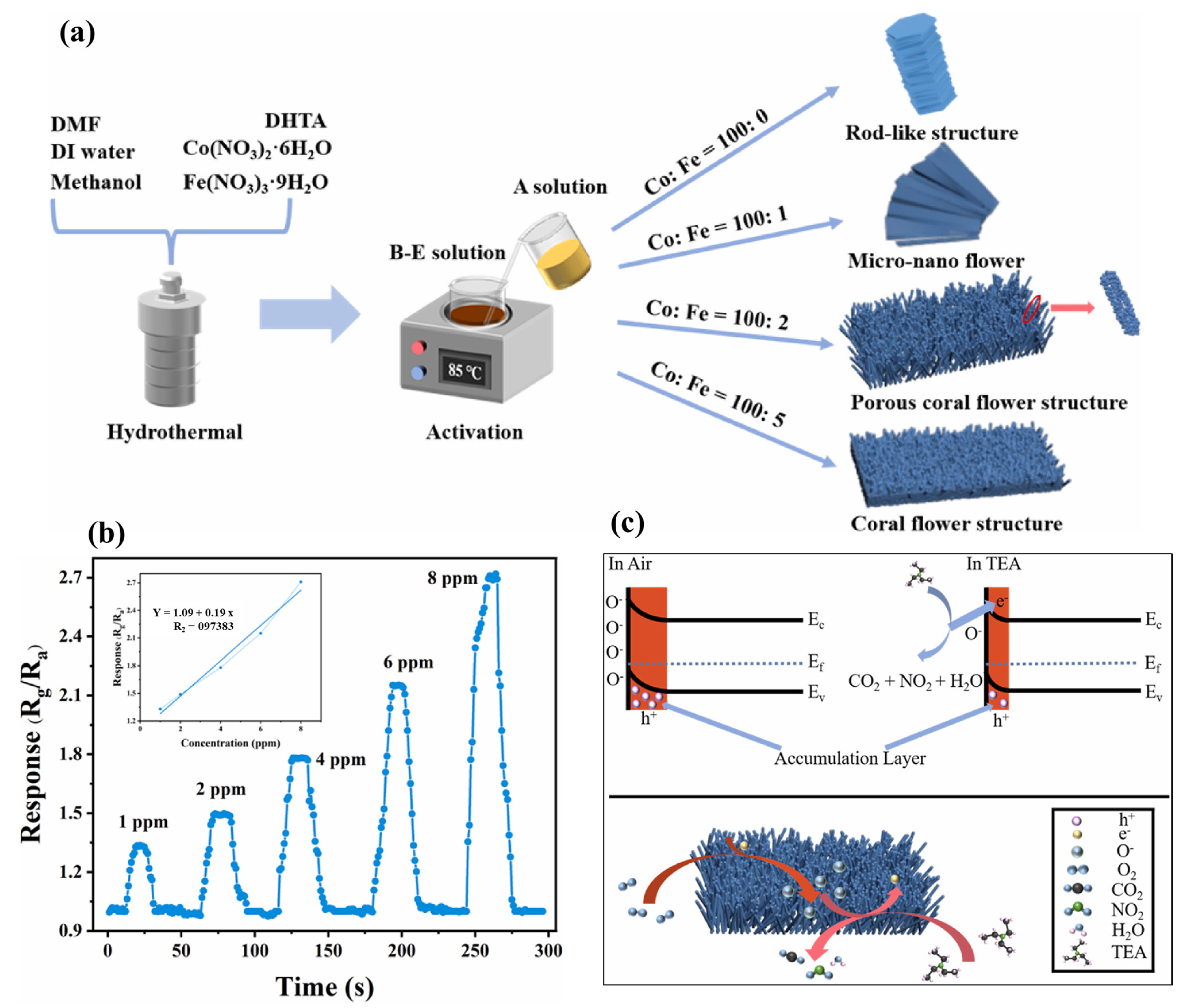
Ding et al. [70] have reported the synthesis of coral-flower-like Fe-doped Co3O4 material using a sacrificial template method as shown in Figure 14a. The authors observed that Fe3+ doping may lead to the presence of stable Co2+ within the prepared sample and form p-n heterojunctions. In addition, lattice distortion enhanced oxygen vacancies and improved the redox reaction for the detection of the target gas. The authors reported that the FC-2 sample has a better response of 21.2 and fast recovery time of 13 s/15 s for 100 ppm TEA. The gas sensing responses of FC-2 for different concentrations of TEA are shown in Figure 14b whereas the gas sensing mechanism is illustrated in Figure 14c. FC-2 demonstrated an acceptable LOD of 1 ppm with a good linear relationship. The proposed sensor also showed good stability and selectivity under acceptable humidity conditions.
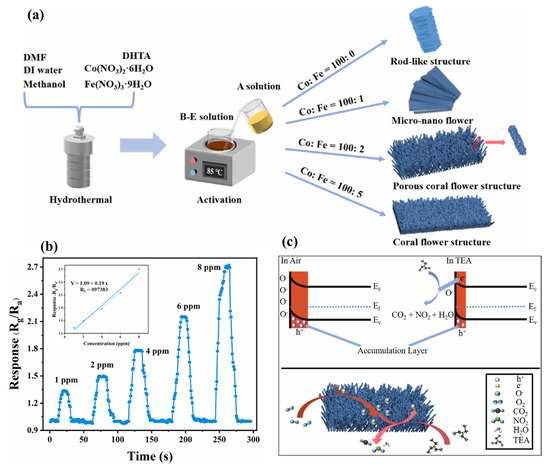
Figure 14.
(a) Schematic graph for the synthesis of gas sensing materials. (b) Gas sensing performance (response) of FC-2 with 1 to 8 ppm TEA. (c) Schematic for gas sensing mechanism of FC-X composite. Reproduced with permission [70].
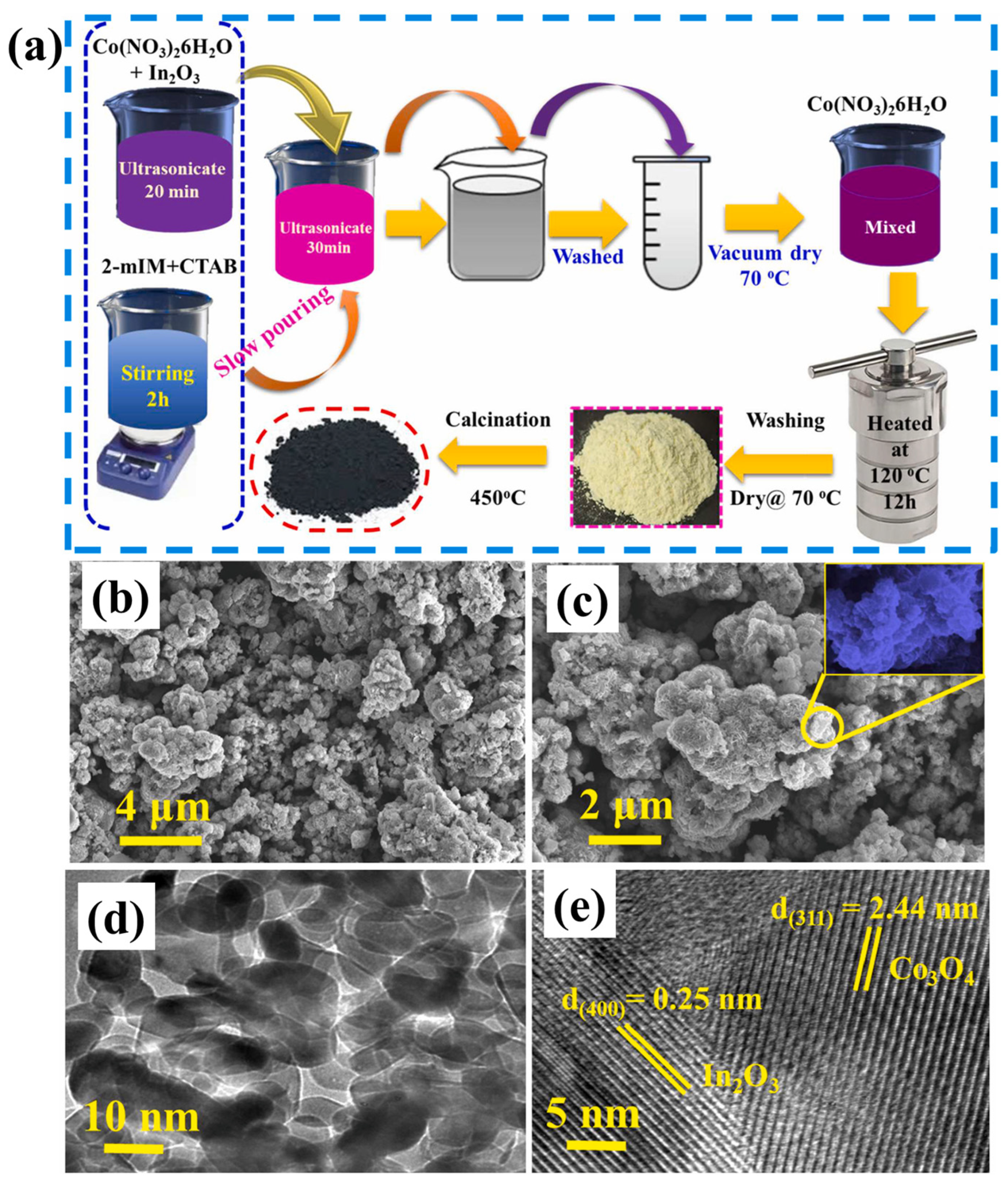
Begi et al. [71] synthesized indium oxide decorated Co3O4 flower-like composite using a two-step hydrothermal and heat treatment method. The synthesis of In2O3/Co3O4 composite is described in Figure 15a. It can be seen that the authors used MOF-derived strategies for the preparation of In2O3/Co3O4 composite. Furthermore, the authors used SEM and transmission electron microscopy (TEM) methods to study the morphological characteristics of the prepared In2O3/Co3O4 composite. SEM images of the Co3O4 and In2O3/Co3O4 composite are shown in Figure 15b and 15c, respectively. It can be observed that Co3O4 consists of nanoflower-like architecture. In another case, In2O3/Co3O4 composite shows the combination of In2O3 particles along with the Co3O4 nanoflowers (Figure 15c). The TEM results of the In2O3/Co3O4 composite also suggested the successful formation of In2O3/Co3O4 composite (Figure 15d). The high-resolution TEM image of the In2O3/Co3O4 composite indicated the presence of In2O3 and Co3O4 composite (Figure 15e). The synthesized In2O3/Co3O4 composite was further explored for NH3 sensing and obtained results demonstrated a decent LOD value of 500 ppb for NH3 at 250 °C.
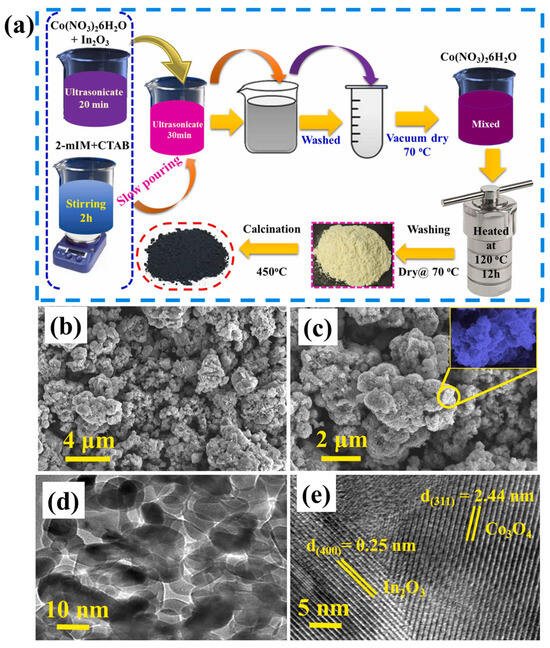
Figure 15.
(a) Schematic diagram for the synthesis of In2O3/Co3O4 composite. SEM image of (b) Co3O4 and (c) In2O3/Co3O4 composite. (d) TEM image and (e) HRTEM image of In2O3/Co3O4 composite. Reproduced with permission [71].
Hussain et al. [72] reported the synthesis of In-doped Co3O4 nanoflowers using a solvent heating-assisted calcination method via an MOF self-sacrificial template. Characterization such as XPS confirmed the presence of In ions in the prepared In-doped Co3O4 sample. The authors observed that 5% In-doped Co3O4 has excellent responses for the detection of 50 ppm H2S gas and demonstrated an LOD of 1.8 ppm. Zhang et al. [73] demonstrated the role of H3BTC and H2ABDC ligand-based Zn-MOF-derived ZnO combined with Tb for gas sensing applications. The authors synthesized Tb@ZnO-1 and Tb@ZnO-2 and examined their sensing ability for the monitoring of acetic acid. Interestingly, Tb@ZnO-2 exhibited better performance compared to Tb@ZnO-1. Begi et al. [74] also prepared MOF-derived Co3O4 by employing a precipitation approach for the monitoring of H2S gas. The prepared sample was characterized by various sophisticated techniques and explored for the determination of H2S gas. The obtained results exhibited excellent selectivity, stability, and response towards H2S with an LOD of 500 ppb. According to Peng et al. [75], it was observed that MXene may enhance the physicochemical properties of CuO NPs. The authors fabricated CuO NPs/Ti3C2Tx MXene composite using MOFs as precursor sources for CuO NPs. The obtained results showed excellent selectivity, stability, and response time for the sensing of NO2 using CuO NPs/Ti3C2Tx MXene composite at RT. The authors found that the proposed sensor has an excellent LOD of 0.03 ppm in the determination of NO2. The authors found that the presence of oxygen vacancies and a large specific surface area were responsible for the enhanced sensing activity of the prepared material. In addition, the presence of MXene may further boost electron transport ability and improved performance was observed for the sensing of NO2. Geng et al. [76] reported the synthesis of bimetallic Ni/Zr-MOF microspheres using the solvothermal method. This prepared MOF was transformed into NiO/ZrO2 composite using the annealing method. Gas sensing studies showed that NiO/ZrO2-2 has a relatively better response (32.3 to 100 ppm), a decent LOD of 7.2 ppb, and excellent repeatability for the detection of TEA. In addition, the proposed sensor demonstrated a wide specific range of 1 to 200 ppm and a strong linear relationship (R2) of 0.9588. Geng et al. [77] proposed the preparation of MOF-derived In2O3/ZnCo2O4 composites by employing a two-step solvothermal method. Morphological studies showed that In2O3/ZnCo2O4 composite comprised hollow microtubes and microflowers. It was also observed that In2O3/ZnCo2O4-2 composite has better gas sensing properties compared to other prepared control samples. A decent LOD of 4.2 ppb with a response value of 208.7 was reported for the determination of n-butanol. The proposed sensor also has excellent selectivity and repeatability which may open doors for practical applications.
Hu et al. [78] also adopted the hydrothermal method for the preparation of MOF-derived Sn/W co-doped NiO hollow flower-like spherical structures. The synthesized material was further explored for the sensing of TEA and demonstrated a decent LOD of 0.1 ppm with good sensing response. Wang et al. [79] adopted a one-step hydrothermal method for the preparation of MOF-derived Au functionalized CeO2/Co3O4 composite. The MOF-derived CeO2/Co3O4 composite was further used as toluene gas sensing material which demonstrated a response to 100 ppm toluene and an excellent LOD of 0.1 ppm was obtained with selectivity and stability. Duan et al. [80] proposed synthetic strategies for the fabrication of MOF-derived porous xPd-NPs@ZnO. The authors also observed that the prepared sample has a large surface area of 471.08 m2/g which may significantly enhance the sensing ability of the prepared sample. A wide range of 0.2 to 4000 ppm was observed for the monitoring of H2 gas. The authors reported an excellent LOD of less than 10−3 ppm for the detection of H2 gas using 5.0Pd-NPs@ZnO material. Xin et al. [81] designed and synthesized an Al/Co metal–organic framework (MOF) by adopting the hydrothermal method. Furthermore, the authors prepared MOF-derived Al3+-doped Co3O4 (Al3+-Co3O4) material for the sensing of n-butanol. The observations revealed that 10% Al3+-Co3O4 nanocomposite has improved gas sensing properties and excellent selectivity, stability, and repeatability were observed for the sensing of n-butanol. The authors stated that doping may have significantly improved the gas sensing performance of the proposed materials due to the insertion of Al3+ into the Co3O4 which created oxygen vacancies. Majhi et al. [82] reported the role of hydrothermally prepared Sn-MOF-derived SnO2 NPs in NO2 sensing studies. It was also mentioned that SnO2 NPs were prepared with a size of 20 to 30 nm with a large surface area of 185.31 m2/g. The proposed Sn-MOF-derived SnO2 NPs showed decent and acceptable gas sensing activity towards NO2 gas. Liu et al. [83] synthesized MOF-derived Ru-doped SnO2 for the sensing of TEA. The authors reported that 0.4 mol % Ru-SnMOF@SnO2 has better sensitivity, a fast response, and decent selectivity with good long-term stability. In another study, Xie et al. [84] prepared MOF-derived Cr2O3 modified reduced graphene oxide composite to form p-p junctions by using the hydrothermal method. The prepared Cr2O3/rGO p-p heterojunction was explored for n-butanol sensing applications. The authors observed that rGO not only prevented the agglomeration/stacking of Cr2O3 NPs but also facilitated the formation of Cr2O3/rGO p-p heterojunctions. The theoretical LOD was determined to be 8.6 ppb. Montoro et al. [85] synthesized M-MOFs (M = Cu, Ni, and Zn) and converted them into metal oxides by a calcination process. The MOF-derived metal oxides (CuO, NiO, and ZnO) demonstrated improved selectivity for the determination of H2S, CO, and H2 gases, respectively. Liu et al. [86] prepared MoO3/TiO2-X (MMT-X) composites using a condensation reflux and annealing process. The authors prepared exfoliated-Ti3C2Tx-derived TiO2 and assembled it on rod-shaped Mo-MOF-derived MoO3. The prepared MMT-X exhibited good sensitivity towards 100 ppm TEA. It is well-understood that MOF-derived materials may offer a large interfacial surface area for prepared hybrid composite materials and facilitate swift adsorption and charge transfer while ensuring crucial structural stability. Lu et al. [87] synthesized a carbon-derived dodecahedral MOF (ZIF-8-C) and subsequently prepared 3D hollow dodecahedral ZIF-derived carbon-PANI composite (ZCPx; x = mass fraction of ZIF-derived carbon). The 3D hollow ZCP50 demonstrated excellent repeatability, good long-term stability, and sensitivity for the detection of NH3. Ding et al. [88] reported the preparation of bimetallic Ni/Sn-BTC MOFs (BTC: benzene-1,3,5-tricarboxylic)-derived NiO-SnO2 composite by utilizing solvothermal and calcination methods. The authors found that the prepared MOF-derived NiO-SnO2 composite has a large surface area of 74.73 m2/g and porosity of 0.343 m3/g. This may maximize the number of active sites for sufficient pathways for NO2 and improve the capacity to capture NO2. A decent LOD of 25 ppb was achieved for the detection of NO2 with acceptable long-term stability. Qiao et al. [89] synthesized MOF-derived CdS@In2O3 composite for the determination of NO2. The prepared materials showed good sensitivity and response for the monitoring of NO2. The improved performance may be ascribed to the presence of synergistic interactions and quantum dot size of the CdS. Gao et al. [90] adopted a ZIF-67 derivative for the preparation of Co3O4. The prepared sensing material showed good sensing performance for the determination of TEA. Qin et al. [91] proposed a benign approach for the preparation of MOF-derived Mn-doped In2O3 hollow nanotubes using solvothermal and annealing methods. The 3 mol % Mn-In2O3 showed the highest sensing performance compared to the other prepared control materials. The 3 mol % Mn-In2O3 demonstrated a decent LOD of 25 ppb with high selectivity and response. Li et al. [92] prepared MOF-derived Cu2O/CuO-C composite using thermal decomposition of Cu-MOF-based precursors. The Cu-MOF-74-derived Cu2O/CuO-C-250 exhibited sensitivity of 1.03 to 30 ppb for the detection of NO2 with acceptable selectivity. Maji et al. [93] prepared MOF-derived ZnCo2O4 using the solvothermal method. The prepared material was explored for the detection of CH4 gas. The MOF-derived ZnCo2O4 exhibited good response for 100 ppm CH4 gas. The proposed material also demonstrated excellent stability for 45 days. The performances of the MOF-derived materials for gas sensing applications are summarized in Table 2.

Table 2.
Summary of the gas sensing performance of the previously reported gas sensors using MOF-derived materials.
4. Conclusions and Future Perspectives
MOFs, MOF-based composites, and MOF-derived materials have been reviewed in terms of gas sensing applications in the monitoring of CO, DMC, TMA, NH3, NO, NO2, TEA, H2S, butanol, methane, H2, and toluene. It was observed from the reported literature that MOF-based hybrid materials demonstrated reasonably good responses and detection limits. Despite numerous efforts, MOFs suffer from poor conductivity and limited mass transfer. In this regard, MOFs have been incorporated with various materials such as polymers, MXenes, and metal oxides. The hybrid MOF-based composites showed improved performance. The hybrid composite of MOFs/polymer and MOFs/MXene showed decent performance in terms of detection limit. In addition, MOF-derived materials also demonstrated excellent performance for the sensing of various toxic and hazardous gases. The MOF-derived materials retain excellent surface properties and porosity compared to metal oxides synthesized by conventional methods. The large surface area of the MOFs provides a better pathway for the sensing of analytes with low concentrations. Although MOF-derived materials show promising gas sensing performance, they still have many challenges, including high working temperature, limited choices of precursors/methods, and limited variety of detected gases. The synergistic interactions between the hybrid composite still need to be studied in depth due to their unclear mechanism. We believe that understanding the mechanism for the synergistic interactions among ternary or binary MOF-based composites may further improve the sensing performance of MOF-based gas sensors. The development of new cost-effective and environmentally friendly synthesis methods would be of great significance towards the development of low-cost gas sensors. For practical applications, MOFs and MOF-derived materials should have robust stability, low cost, and durability. In this regard, rational design and cost-effective synthetic approaches are required to fabricate highly stable MOFs for gas sensing applications. The structural stability of the MOFs can be improved by coating with protective layers. To improve the selectivity of the MOFs, it is required to design and prepare special-group-based functionalized MOFs for the determination of a selective gas. MOF-derived material showed decent stability but suffers from poor electrical conductivity. Thus, it is required to design and construct hybrid composite materials with MXenes and layered double hydroxide (LDH) materials to further enhance the electrical conductivity of the MOFs and MOF-derived materials. Future research may focus on the preparation of functionalized MOFs/MOF-derived materials with MXene and LDH to obtain conductive and stable hybrid composites for gas sensing applications.
Author Contributions
Conceptualization: K.A.; writing—original draft preparation: K.A.; supervision: T.H.O.; writing—review and editing: T.H.O. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Luo, F.; Che, Y.-X.; Zheng, J.M. Construction of microporous metal–organic frameworks (MOFs) by Mn–O–C rod-like secondary building units (SBUs): Solvothermal synthesis, structure, thermostability, and magnetic properties. Inorg. Chem. Commun. 2008, 11, 358–362. [Google Scholar] [CrossRef]
- Samanidou, V.F.; Deliyanni, E.A. Metal Organic Frameworks: Synthesis and Application. Molecules 2020, 25, 960. [Google Scholar] [CrossRef] [PubMed]
- Thiam, A.; Lopez-Ruiz, J.A.; Barpaga, D.; Garcia-Segura, S. The Surge of Metal–Organic-Framework (MOFs)-Based Electrodes as Key Elements in Electrochemically Driven Processes for the Environment. Molecules 2021, 26, 5713. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Wang, Y.; Zhang, J.; Cheng, S.; Sun, Y. A Short Review of Advances in MOF Glass Membranes for Gas Adsorption and Separation. Membranes 2024, 14, 99. [Google Scholar] [CrossRef]
- Gomez, G.E.; dos Santos Afonso, M.; Baldoni, H.A.; Roncaroli, F.; Soler-Illia, G.J.A.A. Luminescent Lanthanide Metal Organic Frameworks as Chemosensing Platforms towards Agrochemicals and Cations. Sensors 2019, 19, 1260. [Google Scholar] [CrossRef]
- Pettinari, C.; Tombesi, A. MOFs for Electrochemical Energy Conversion and Storage. Inorganics 2023, 11, 65. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, T.; Wang, B.; Wang, W. The Application of Metal–Organic Frameworks in Water Treatment and Their Large-Scale Preparation: A Review. Materials 2024, 17, 1972. [Google Scholar] [CrossRef]
- Saeb, M.R.; Rabiee, N.; Mozafari, M.; Mostafavi, E. Metal-Organic Frameworks (MOFs)-Based Nanomaterials for Drug Delivery. Materials 2021, 14, 3652. [Google Scholar] [CrossRef]
- Králik, M.; Koóš, P.; Markovič, M.; Lopatka, P. Organic and Metal–Organic Polymer-Based Catalysts—Enfant Terrible Companions or Good Assistants? Molecules 2024, 29, 4623. [Google Scholar] [CrossRef]
- Xie, W.-J.; Mulina, O.M.; Terent’ev, A.O.; He, L.-N. Metal–Organic Frameworks for Electrocatalytic CO2 Reduction into Formic Acid. Catalysts 2023, 13, 1109. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, H.; Yang, K.; Shen, Y.; Qin, K.; Yuan, P.; Li, E. Highly Sensitive and Selective Defect WS2 Chemical Sensor for Detecting HCHO Toxic Gases. Sensors 2024, 24, 762. [Google Scholar] [CrossRef]
- Nalimova, S.S.; Shomakhov, Z.V.; Kozodaev, D.A.; Rybina, A.A.; Buzovkin, S.S.; Bui, C.D.; Novikov, I.A.; Moshnikov, V.A. VOC Gas Sensors Based on Zinc Stannate Nanoparticles Decorated with Silver. Nanomaterials 2024, 14, 1993. [Google Scholar] [CrossRef]
- David, E.; Niculescu, V.-C. Volatile Organic Compounds (VOCs) as Environmental Pollutants: Occurrence and Mitigation Using Nanomaterials. Int. J. Environ. Res. Public Health 2021, 18, 13147. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiao, M. A Mini-Review on Metal Oxide Semiconductor Gas Sensors for Carbon Monoxide Detection at Room Temperature. Chemosensors 2024, 12, 55. [Google Scholar] [CrossRef]
- Chakraborthy, A.; Nuthalapati, S.; Nag, A.; Afsarimanesh, N.; Alahi, M.E.E.; Altinsoy, M.E. A Critical Review of the Use of Graphene-Based Gas Sensors. Chemosensors 2022, 10, 355. [Google Scholar] [CrossRef]
- Kotbi, A.; Benyoussef, M.; Ressami, E.M.; Lejeune, M.; Lakssir, B.; Jouiad, M. Gas Sensors Based on Exfoliated g-C3N4 for CO2 Detection. Chemosensors 2022, 10, 470. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Long, X.; Cao, J.; Xin, X.; Guan, X.; Peng, J.; Zheng, X. Gas Sensors Based on Mechanically Exfoliated MoS2 Nanosheets for Room-Temperature NO2 Detection. Sensors 2019, 19, 2123. [Google Scholar] [CrossRef]
- Vaishag, P.V.; Noh, J.-S. A Comparative Review of Graphene and MXene-Based Composites towards Gas Sensing. Molecules 2024, 29, 4558. [Google Scholar] [CrossRef]
- Ren, X.; Xu, Z.; Zhang, Z.; Tang, Z. Enhanced NO2 Sensing Performance of ZnO-SnO2 Heterojunction Derived from Metal-Organic Frameworks. Nanomaterials 2022, 12, 3726. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, C.; Wang, Q.; Kong, Q.; Chen, G.; Guan, H.; Dong, C. MOFs-Derived Porous NiFe2O4 Nano-Octahedrons with Hollow Interiors for an Excellent Toluene Gas Sensor. Nanomaterials 2019, 9, 1059. [Google Scholar] [CrossRef] [PubMed]
- Qi, B.; Wang, X.; Wang, X.; Cheng, J.; Shang, Y. Synthesis and H2S-Sensing Properties of MOF-Derived Cu-Doped ZnO Nanocages. Nanomaterials 2022, 12, 2579. [Google Scholar] [CrossRef] [PubMed]
- Klinowski, J.; Paz, F.A.A.; Silva, P.; Rocha, J. Microwave-Assisted Synthesis of Metal–Organic Frameworks. Dalton Trans. 2011, 40, 321–330. [Google Scholar] [CrossRef]
- Zhao, Z.; Li, H.; Zhao, K.; Wang, L.; Gao, X. Microwave-assisted synthesis of MOFs: Rational design via numerical simulation. Chem. Eng. J. 2022, 428, 131006. [Google Scholar] [CrossRef]
- Phan, P.T.; Hong, J.; Tran, N.; Le, T.H. The Properties of Microwave-Assisted Synthesis of Metal–Organic Frameworks and Their Applications. Nanomaterials 2023, 13, 352. [Google Scholar] [CrossRef] [PubMed]
- Tanan, W.; Panpinit, S.; Saengsuwan, S. Comparison of microwave-assisted and thermal-heated synthesis of P(HEMA-co-AM)/PVA interpenetrating polymer network (IPN) hydrogels for Pb(II) removal from aqueous solution: Characterization, adsorption and kinetic study. Eur. Polym. J. 2021, 143, 110193. [Google Scholar] [CrossRef]
- Taylor-Pashow, K.M.L.; Della Rocca, J.; Xie, Z.; Tran, S.; Lin, W. Postsynthetic Modifications of Iron-Carboxylate Nanoscale Metal-Organic Frameworks for Imaging and Drug Delivery. J. Am. Chem. Soc. 2009, 131, 14261–14263. [Google Scholar] [CrossRef]
- Khan, N.A.; Kang, I.J.; Seok, H.Y.; Jhung, S.H. Facile synthesis of nano-sized metal- organic frameworks, chromium-benzenedicarboxylate, MIL-101. Chem. Eng. J. 2011, 166, 1152–1157. [Google Scholar] [CrossRef]
- Zhu, Y.-J.; Chen, F. Microwave-Assisted Preparation of Inorganic Nanostructures in Liquid Phase. Chem. Rev. 2014, 114, 6462–6555. [Google Scholar]
- Yahia, M.; Lozano, L.A.; Zamaro, J.M.; Téllez, C.; Coronas, J. Microwave-assisted synthesis of metal–organic frameworks UiO-66 and MOF-808 for enhanced CO2/CH4 separation in PIM-1 mixed matrix membranes. Sep. Purif. Technol. 2024, 330, 125558. [Google Scholar] [CrossRef]
- Skoda, D.; Kazda, T.; Munster, L.; Hanulikova, B.; Styskalik, A.; Eloy, P.; Debecker, D.P.; Vilcakova, J.; Cech, O.; Simonikova, L.; et al. Microwave-assisted synthesis of platelet-like cobalt metal-organic framework, its transformation to porous layered cobalt-carbon nanocomposite discs and their utilization as anode materials in sodium-ion batteries. J. Energy Storage 2020, 27, 101113. [Google Scholar] [CrossRef]
- Dehane, A.; Merouani, S.; Chibani, A.; Hamdaoui, O.; Ashokkumar, M. Sonochemical and sono-assisted reduction of carbon dioxide: A critical review. Chem. Eng. Process. Process Intensif. 2022, 179, 109075. [Google Scholar] [CrossRef]
- Vaitsis, C.; Sourkouni, G.; Argirusis, C. Metal Organic Frameworks (MOFs) and ultrasound: A review. Ultrason. Sonochem. 2019, 52, 106–119. [Google Scholar] [CrossRef]
- Vaitsis, C.; Kanellou, E.; Pandis, P.K.; Papamichael, I.; Sourkouni, G.; Zorpas, A.A.; Argirusis, C. Sonochemical synthesis of zinc adipate Metal-Organic Framework (MOF) for the electrochemical reduction of CO2: MOF and circular economy potential. Sustain. Chem. Pharm. 2022, 29, 100786. [Google Scholar] [CrossRef]
- Abaszadeh, N.; Afzali, D.; Sargazi, G.; Golpayegani, A. Sonochemical-assisted method for efficient synthesis of Cu-MOF and evaluating its antibacterial properties. Heliyon 2024, 10, e31024. [Google Scholar] [CrossRef]
- Moradi, E.; Rahimi, R.; Safarifard, V.; Azari, S. A Sonochemically-Synthesized Microporous Metal-Organic Framework for the Rapid and Efficient Ultrasonic-Assisted Removal of Mercury (II) Ions in a Water Solution and a Study of the Antibacterial Activity. Proceedings 2019, 41, 31. [Google Scholar] [CrossRef]
- Pokhrel, N.; Vabbina, P.K.; Pala, N. Sonochemistry: Science and Engineering. Ultrason. Sonochem 2016, 29, 104–128. [Google Scholar] [CrossRef]
- Wang, X.; Li, Q.; Yang, N.; Yang, Y.; He, F.; Chu, J.; Gong, M.; Wu, B.; Zhang, R.; Xiong, S. Hydrothermal synthesis of NiCo-based bimetal-organic frameworks as electrode materials for supercapacitors. J. Solid State Chem. 2019, 270, 370–378. [Google Scholar] [CrossRef]
- Qi, Q.; Liu, Z.; Chen, X.; Yu, J.; Li, X.; Wang, R.; Liu, Y.; Chen, J. Promoted electrochemical performance by MOF on MOF composite catalyst of microbial fuel cell: CuCo-MOF@ZIF-8 and the comparison between two-step hydrothermal method and dual-solution method. Biosens. Bioelectron. 2024, 264, 116693. [Google Scholar] [CrossRef]
- Wu, L.-Z.; Zhou, X.-Y.; Zeng, P.-C.; Huang, J.-Y.; Zhang, M.-D.; Qin, L. Hydrothermal synthesis of Ni(II) or Co(II)-based MOF for electrocatalytic hydrogen evolution. Polyhedron 2022, 225, 116035. [Google Scholar] [CrossRef]
- Zheng, X.; Chu, X.; Liang, H. Cu-MOF attached with pyrene-cored probes as a highly sensitive indicator for carbon monoxide in coal mine gas: Synthesis and performance. Microchem. J. 2024, 199, 109983. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, J.; Zhao, Y.; Sun, C.; Li, W.; Chang, Z. A robust Eu-MOF as a multi-functional fluorescence sensor for detection of benzaldehyde, Hg2+, and Cr2O72−/CrO42−. Microchem. J. 2024, 196, 109712. [Google Scholar] [CrossRef]
- Liang, F.; Feng, Y.; Huang, S.; Duan, B.; Gao, D.; Cheng, Z.; Kong, L.; Guo, Y.; Liu, C.; Ding, T. Two In-MOFs containing pyridine dicarboxylic acids with different functional sites: Sensing of Fe3+ ion in water and selective gas capture. J. Mol. Struct. 2024, 1316, 139018. [Google Scholar] [CrossRef]
- Kong, L.-X.; Sun, J.-Z.; Shi, F.-F.; Li, Y.; Li, X.-Y. Temperature self-compensating fiber-optic misalignment sensor based on Cu-MOFs/PAN film cavity for detecting dimethyl carbonate gas. Opt. Laser Technol. 2025, 181, 111926. [Google Scholar] [CrossRef]
- Cai, S.; Huang, X.; Luo, M.; Xiong, D.; Pang, W.; Wang, M.; Wang, L.; Li, S.; Luo, P.; Gao, Z. High-performance ammonia sensor at room temperature based on 2D conductive MOF Cu3(HITP)2. Talanta 2024, 285, 127226. [Google Scholar] [CrossRef]
- Chen, S.; Duan, X.; Liu, C.; Liu, S.; Li, P.; Su, D.; Sun, X.; Guo, Y.; Chen, W.; Wang, Z. La-Ce-MOF nanocomposite coated quartz crystal microbalance gas sensor for the detection of amine gases and formaldehyde. J. Hazard. Mater. 2024, 467, 133672. [Google Scholar] [CrossRef]
- Jin, W.; Zhang, N.; Jia, M.; Wang, J.; Yang, S.; Liu, Y.; Chen, W. Dual functionalized Co3O4 porous cages with Pd and Co-MOF for acetone gas sensing under high humidity. Mater. Today Commun. 2024, 40, 109582. [Google Scholar] [CrossRef]
- Zhao, J.; Li, P.; Zhang, Q.; Ye, Z.; Wang, Y.; Tian, J.; Xu, Z.; Peng, N.; Ren, H.; Zhang, X. MOF nanoflowers-based flexible portable NO sensors for human airway inflammation detection. Chem. Eng. J. 2024, 499, 156184. [Google Scholar] [CrossRef]
- Yang, C.-R.; Huang, J.-G.; Huang, M.-J.; Shen, H.-Y.; Tseng, S.-F. High-performance NO2 gas sensors based on vanadium metal organic frameworks (V-MOFs) on flexible graphene electrodes. J. Alloys Compd. 2024, 1008, 176675. [Google Scholar] [CrossRef]
- Yu, Y.-H.; Lin, X.-Y.; Teng, K.-L.; Hu, C.-C.; Wang, W.-Y.; Hung, Y.-H.; Tseng, H.-Y.; Luo, K.-H.; Yeh, J.-M.; Lu, K.-L.; et al. Semiconductive (Cu–S)n Metal–Organic Frameworks Hybrid Polyaniline Nanocomposites as Hydrogen Sulfide Gas Sensor. Surf. Interfaces 2024, 44, 103698. [Google Scholar] [CrossRef]
- Wang, X.; Xu, X.; Zhou, T.; Zhang, T. Nanoscale MOF-74-based QCM gas sensor for CO2 detection at room temperature. Sens. Actuators B Chem. 2024, 413, 135874. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.; Guo, Y.; Liu, Y.; Wang, L.; Wang, Q.; Cheng, L.; Jiao, Z. UV-enhanced Mg:MOF-ZnO sensor for n-butanol gas detection at a lower operating temperature. Appl. Surf. Sci. 2024, 678, 161138. [Google Scholar] [CrossRef]
- Maji, B.; Singh, P.; Badhulika, S. A highly sensitive and fully flexible Fe-Co metal-organic framework hydrogel based gas sensor for ppb level detection of acetone. Appl. Surf. Sci. 2024, 678, 161047. [Google Scholar] [CrossRef]
- Cai, H.; Luo, H.; Hu, F.; Wang, J.; Zhou, J.; An, D. High-response n-butanol gas sensor based on quasi-Zn-MOFs with tunable surface oxygen vacancies. J. Alloys Compd. 2025, 1010, 177274. [Google Scholar] [CrossRef]
- Liu, Z.; Kang, M.; Zhang, Z.; Yue, C.; Mu, Y.; Yang, Z.; Wang, F.; Dastan, D.; Yin, X.-T.; Ma, X. One-step preparation of La2O3-modified MOF-SnO2 gas sensor for ethanol detection. Appl. Surf. Sci. 2025, 681, 161594. [Google Scholar] [CrossRef]
- Van Nguyen, M.; Kim, T.-U.; Nguyen, L.H.T.; Mirzaei, A.; Pham, A.T.T.; Tran, T.Q.; Mai, N.X.D.; Tran, N.Q.; Kim, Y.; Phan, T.B.; et al. Efficient low-temperature detection of CO gas by various metalated porphyrinic-Al-based MOF (Cu and Co) materials. Sens. Actuators B Chem. 2025, 424, 13691. [Google Scholar] [CrossRef]
- Fang, H.; Ma, X.; Li, S.; Tan, R.; Chen, H.; Zhang, J.; Yuan, K.; Wang, D. H2S gas sensing based on CuNi MOFs derivative with TiO2 quantum dot decoration applied for meat freshness detection. Sens. Actuators B Chem. 2025, 422, 136612. [Google Scholar] [CrossRef]
- Ding, J.; Wang, Q.; Liu, X.; Li, S.; Li, H. Ultrasensitive detection of hazardous gas at room temperature enabled by MOF@MXene 0D-2D heterostructure. J. Hazard. Mater. 2024, 480, 136261. [Google Scholar] [CrossRef]
- Liu, H.; Chen, Q.; Xu, T.; Liu, H.; Miao, L.; Liu, W.; Cheng, J.; Yin, S.; Wang, C.; Zhao, J. Chemiresistive triethylamine detection based on the novel Ti3C2Tx/Co-BDC gas sensor. Sens. Actuators B Chem. 2025, 423, 136738. [Google Scholar] [CrossRef]
- Zhang, S.; Zhao, Z.; Jia, L.; Guo, X.; Yang, R.; Deng, Q.; Zhang, D. Ultrahigh sensitive and selectivity NO2 gas sensors based on Sn-MOF derivates at low temperature. Sens. Actuators B Chem. 2024, 417, 136073. [Google Scholar] [CrossRef]
- Yan, H.; Wang, J.; Shi, N.; Han, Y.; Zhang, S.; Zhao, G. A flexible and wearable chemiresistive ethylene gas sensor modified with PdNPs-SWCNTs@Cu-MOF-74 nanocomposite: A targeted strategy for the dynamic monitoring of fruit freshness. Chem. Eng. J. 2024, 488, 151142. [Google Scholar] [CrossRef]
- Yang, Y.; Yue, J.; Zhang, X.; Ren, B.; Fu, S.; Sun, Y.; Luo, Z. Accordion-like ZIF-8/MoO3 composite gas sensor for highly selective and sensitive H2S detection. Ceram. Int. 2024, 50, 38253–38262. [Google Scholar] [CrossRef]
- Wang, X.; Ma, Q.; Wang, Y.; Li, L.; Zhao, D.; Zhang, H.; Li, B. Bimetallic MOFs-derived hierarchical WO3/ZnWO4/CoWO4 heterostructures for enhanced n-butanol gas-sensing performance. Chem. Phys. Lett. 2024, 844, 141260. [Google Scholar] [CrossRef]
- Shah, S.; Hussain, S.; Khan, L.A.; Yusuf, K.; Manavalan, R.K.; Tianyan, Y.; Zhang, X.; Liu, G.; Qiao, G. ppb-level H2 gas-sensor based on porous Ni-MOF derived NiO@CuO nanoflowers for superior sensing performance. Mater. Res. Bull. 2024, 180, 113021. [Google Scholar] [CrossRef]
- Zhang, S.; Pu, Y.; Du, X.; Cao, S.; Zhu, D. Effect of Ce doping and MOF-derived structure on gas sensing performance of SnO2 to ethylene glycol. Mater. Sci. Eng. B 2024, 302, 117247. [Google Scholar] [CrossRef]
- Hussain, S.; Begi, A.N.; Amu-Darko, J.N.O.; Yusuf, K.; Manavalan, R.K.; Iqbal, A.; Zhang, X.; Qiao, G.; Liu, G. MOF-derived Mo-doped Co3O4: A hierarchical yeast-like structure for superior carbon monoxide sensing. Sens. Actuators B Chem. 2024, 420, 136489. [Google Scholar] [CrossRef]
- Geng, W.; Song, P.; Cao, X.; Duan, L. Bimetallic Ni/V-MOFs-derived Ni3V2O8@NiO hollow microspheres for ultrasensitive detection of triethylamine. Appl. Surf. Sci. 2024, 670, 160637. [Google Scholar] [CrossRef]
- Xiao, D.; Wang, Y.; Zhang, D.; Liu, Y.; Wang, H.; Li, Y.; Wei, H.; Wang, S.; Sun, M.; Sun, M. CuO/ZnO hollow nanocages derived from metal−organic frameworks for ultra-high and rapid response H2S gas sensor. Ceram. Int. 2024, 50, 15767–15779. [Google Scholar] [CrossRef]
- Lu, Z.J.; Yue, J.H.; Xu, J.C.; Hong, B.; Li, J.; Zeng, Y.X.; Peng, X.L.; Chen, H.W.; Wang, X.Q. Highly-enhanced toluene gas-sensing behavior of high-valent metal-cations doped Co3O4 nanostructures derived from ZIF-67 MOF. Chem. Phys. 2024, 581, 112266. [Google Scholar] [CrossRef]
- Ding, Q.; Li, H.; Liu, W.; Huang, D.; Tan, X.; Zhao, M.; Cheng, Q.; Yi, M.; Ren, Y. Fast response triethylamine sensor based on MOF-derived coral flower-like Fe-doped Co3O4. Mater. Sci. Semicond. Process. 2024, 180, 108557. [Google Scholar] [CrossRef]
- Begi, A.N.; Hussain, S.; Liaqat, M.J.; Alsaiari, N.S.; Ouladsmane, M.; Qiao, G.; Liu, G. Unlocking low-concentration NH3 gas sensing: An innovative MOF-derived In2O3/Co3O4 nanocomposite approach. Mater. Sci. Semicond. Process. 2024, 181, 108641. [Google Scholar] [CrossRef]
- Hussain, S.; Peng, L.; Amu-Darko, J.N.O.; Shahid, A.; Yusuf, K.; Wang, S.; Liaqat, M.J.; Manavalan, R.K.; Zhang, X.; Qiao, G. ZIF-67 MOF derived in-doped Co3O4 nanoflowers for H2S gas-sensing performances. Mater. Sci. Semicond. Process. 2024, 184, 108840. [Google Scholar] [CrossRef]
- Zhang, S.; Ling, W.; Zhao, T.; Pu, Y.; Cao, S.; Zhu, D. High response ZnO gas sensor derived from Tb@Zn-MOFs to acetic acid under UV excitation. Sens. Actuators A Phys. 2024, 365, 114862. [Google Scholar] [CrossRef]
- Begi, A.N.; Hussain, S.; Amu-Darko, J.N.O.; Shah, S.; Junhao, W.; Zhang, X.; Yusuf, K.; Manavalan, R.K.; Qiao, G.; Liu, G. Low-concentration H2S gas sensors based on MOF-derived Co3O4 nanomaterials. Sens. Actuators A Phys. 2024, 378, 115776. [Google Scholar] [CrossRef]
- Peng, H.; Yang, J.; Lin, C.; Qi, L.; Li, L.; Shi, K. Gas-sensitive performance of metal-organic framework-derived CuO NPs/Ti3C2TX MXene heterostructures for efficient NO2 detection at room temperature. J. Alloys Compd. 2024, 980, 173657. [Google Scholar] [CrossRef]
- Geng, W.; Song, P.; Cao, X.; Duan, L. NiO/ZrO2 hollow microspheres derived from bimetallic Ni/Zr-MOFs for fast and sensitive detection of triethylamine. J. Alloys Compd. 2024, 1006, 176277. [Google Scholar] [CrossRef]
- Geng, W.; Song, P.; Duan, L.; Luan, T. MOFs-derived In2O3 hollow microtubes/ZnCo2O4 microflowers for fast and sensitive detection of n-butanol. Sens. Actuators B Chem. 2025, 423, 136803. [Google Scholar] [CrossRef]
- Hu, Z.; Yang, X.; Zhang, Y.; Sun, Z.; Liu, W.; Pan, G.; Wang, H.; Sun, M. High-performance trimethylamine gas sensor based on Sn-W co-doped MOF-derived hollow flower-like nickel oxide. J. Alloys Compd. 2025, 1010, 177110. [Google Scholar] [CrossRef]
- Wang, S.; Liu, Q.; Shao, J.; Pan, G.; Qi, Y.; Qiu, M. MOF-derived Au functional CeO2/Co3O4 heterostructure gas sensor with moisture resistance for low-temperature and efficient toluene detection. J. Alloys Compd. 2025, 1010, 177358. [Google Scholar] [CrossRef]
- Duan, P.; Wang, H.; Zhou, H.; Zhang, S.; Meng, X.; Duan, Q.; Jin, K.; Sun, J. MOF-derived xPd-NPs@ZnO porous nanocomposites for ultrasensitive ppb-level gas detection with photoexcitation: Design, diverse-scenario characterization, and mechanism. J. Colloid Interface Sci. 2024, 660, 974–988. [Google Scholar] [CrossRef] [PubMed]
- Xin, J.; Wang, W.; Xie, L.; Li, X.; Yao, Y.; Zhao, X.; Zhu, Z. MOF-derived Al3+-doped Co3O4 nanocomposites for highly n-butanol gas sensing performance at low operating temperature. J. Alloys Compd. 2024, 978, 173341. [Google Scholar] [CrossRef]
- Majhi, S.M.; Kim, J.-Y.; Mirzaei, A.; Surya, S.G.; Kim, H.W.; Kim, S.S. MOF-derived SnO2 nanoparticles for realization of ultrasensitive and highly selective NO2 gas sensing. Sens. Actuators B Chem. 2024, 419, 136369. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Y.; Li, Y.; Sui, C.; Liu, Y.; Liu, Y.; Zhao, Y.; Liang, X.; Liu, F.; Lu, G. Bimetallic MOF derived mesoporous structure of Ru doped SnO2 enable high-sensitivity gas sensors for triethylamine in high humidity. Sens. Actuators B Chem. 2024, 405, 135275. [Google Scholar] [CrossRef]
- Xie, T.; Li, F.; Song, P.; Fang, M.; Duan, L.; Zhang, Q.; Geng, W. High responsive n-butanol gas sensor based on MOFs-derived Cr2O3/RGO p-p heterojunctions materials. J. Alloys Compounds 2024, 1002, 175271. [Google Scholar] [CrossRef]
- Montoro, C.; Kim, J.-Y.; Mirzaei, A.; Lee, J.-H.; Sayegh, S.; Makhoul, E.; Iatsunskyi, I.; Coy, E.; Bechelany, M.; Kim, H.W.; et al. MOF-derived metal oxide (Cu, Ni, Zn) gas sensors with excellent selectivity towards H2S, CO and H2 gases. Compos. Part B Eng. 2024, 283, 111637. [Google Scholar] [CrossRef]
- Liu, W.; Li, H.; Huang, D.; Tan, X.; Zhao, M.; Cheng, Q.; Yi, M.; Ding, Q.; Ren, Y.; Liu, G. Engineering α-MoO3/TiO2 heterostructures derived from MOFs/MXene hybrids for high-performance triethylamine sensor. Chem. Eng. J. 2024, 483, 149340. [Google Scholar] [CrossRef]
- Lu, X.; Qu, Y.; Zhang, F.; Ding, Z.; Zheng, H.; Lei, Y.; Liu, S.; Li, S. A sensitive NH3 chemiresistive sensor with wide detection range: Employing a MOF-derived mesoporous carbon composite with polyaniline. Sens. Actuators B Chem. 2024, 416, 135938. [Google Scholar] [CrossRef]
- Ding, Y.; Du, B.; Guo, X.; Dong, Y.; Zhang, M.; Jin, W.; Gao, C.; Peng, D.; He, Y. An ultrasensitive NO2 gas sensor based on a NiO-SnO2 composite with a sub-ppb detection limit at room temperature. Sens. Actuators B Chem. 2024, 414, 135916. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhang, J.; Liu, J.; Liu, Y.; Zhang, X.; Yang, Z.; Yin, X.; Gao, J.; Wang, C.; Lu, H. MOF-derived porous In2O3 nanospheres sensitized by CdS quantum dots: A ppb-level NO2 sensor with ultra-high response at room-temperature. J. Alloys Compd. 2024, 997, 174889. [Google Scholar] [CrossRef]
- Gao, S.; Li, X.; Wang, C.; Yuan, R.; Zhang, Q. High-performance triethylamine gas sensor with low working temperature based on amorphous derivative from ZIF-67. J. Alloys Compd. 2024, 998, 174952. [Google Scholar] [CrossRef]
- Qin, C.; Wei, Z.; Zhao, X.; Cao, J.; Wang, Y. High-temperature hydrogen sensor based on MOFs-derived Mn-doped In2O3 hollow nanotubes. Int. J. Hydrog. Energy 2024, 78, 1024–1033. [Google Scholar] [CrossRef]
- Li, N.; Hu, J.; Li, J.; Cheng, M.; Wei, T.; Liu, Q.; Wang, R.; Li, W.; Ling, Y.; Zhang, Y.; et al. Cu-MOF-74-derived Cu2O/CuO-C nanocomposite as an effective chemiresistive sensor for detection of NO2 at room temperature. J. Alloys Compd. 2024, 976, 173074. [Google Scholar] [CrossRef]
- Maji, B.; Dash, P. Investigation into the enhanced gas sensing performance for CH4: Comparative study of MOF-derived and traditionally synthesized ZnCo2O4 flower based composite. Sens. Actuators B Chem. 2024, 403, 135182. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).