Abstract
Carbon nanotubes (CNTs) have opened new routes in the field of chemical sensing due to their unparalleled electrical conductivity, high surface area, and versatile functionalization capabilities. This review systematically examined the latest advancements in CNT-based chemical sensors, with a focus on their sensing mechanism, functionalization strategies, and applications. A spotlight was cast on the wide-ranging applications of CNT-based chemical sensors, spanning environmental analysis, drug detection, healthcare, food quality control, gases detection, strain sensing, etc. Finally, through a comprehensive SWOT analysis, the strengths, weaknesses, opportunities, and existing threats, along with emerging trends of CNTs in the sensing field, were elucidated. This review systematically summarized the applications of CNTs across six major fields, highlighting more than 60 CNT-based sensing materials. We aim to provide a forward-looking perspective on how CNTs will continue to shape the future of chemical sensing.
1. Introduction
The advancement of chemical sensor technologies has become indispensable in addressing global challenges across environmental, industrial, healthcare, and food-control domains. With the growing need for sensitive, selective, and real-time chemical detection, the exploration of novel nanomaterials (such as carbon nanotubes, metal–organic frameworks, graphene, quantum dots, metallic nanoparticles, etc.) has become a cornerstone of modern sensor design [1]. Among these, CNTs have been a rising star due to their remarkable electrical, mechanical, and chemical properties, which make them uniquely suited for use in chemical sensing platforms [2,3].
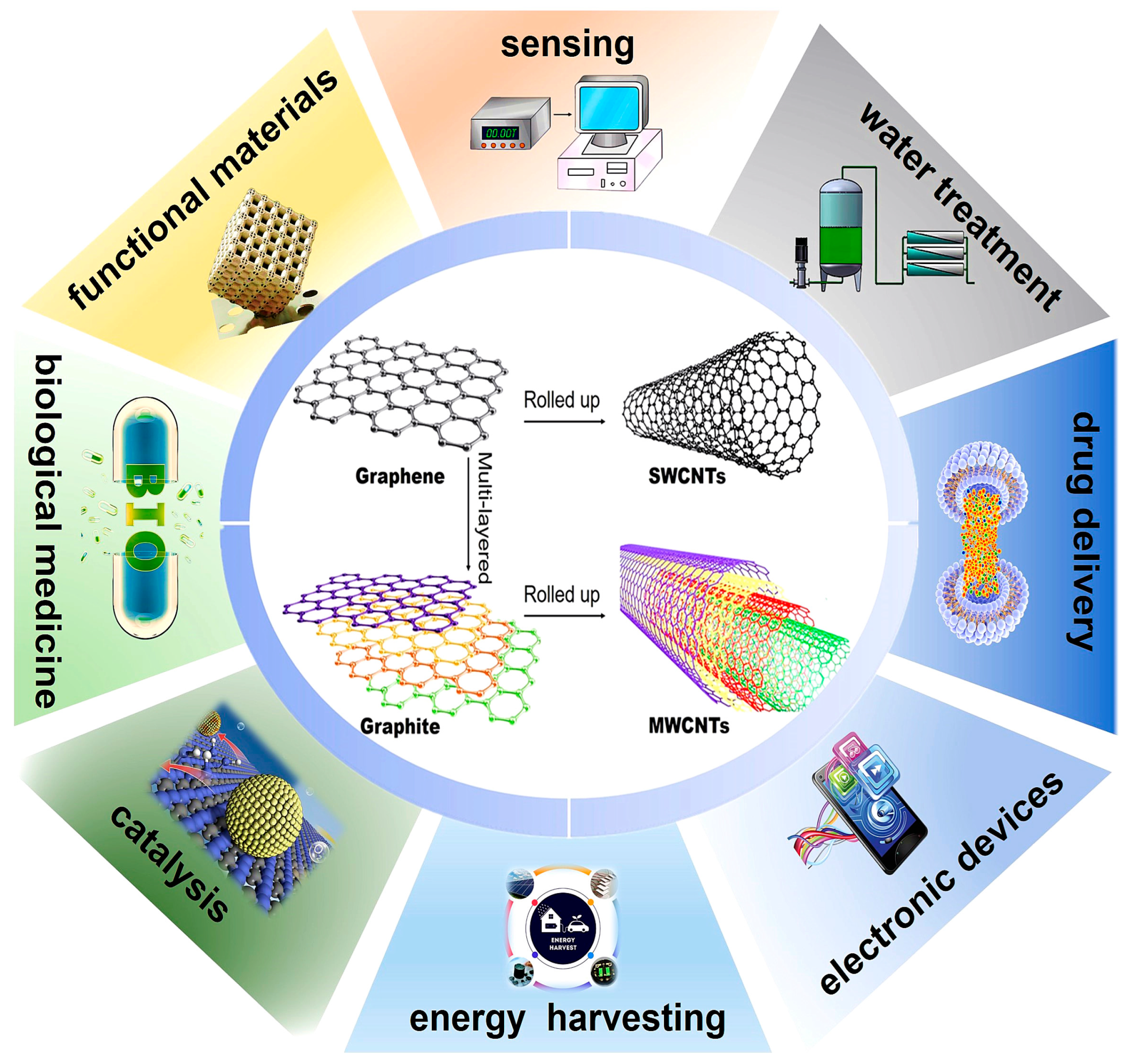
CNTs were first observed by Radushkevich and Lukyanovich in 1952 [4], but they attracted worldwide attention after Iijima reported their detailed structure in 1991 [5,6], which is generally considered as the starting point of CNT research. The diameters of CNTs range from a few tenths of a nanometer to several tens of nanometers, and their lengths can reach up to several centimeters. Essentially, carbon nanotubes are rolled-up graphene sheets in single-walled (SWCNTs) or multi-walled (MWCNTs) forms (Figure 1), which can be discriminated into “armchair (θ = 30°)”, “chiral (0° < θ < 30°)” and “zigzag (θ = 0°)” forms based on different chiral angles “θ” and curvatures [7,8]. CNTs exhibit ballistic charge transport [9], high aspect ratios [10], large surface-to-volume ratios [11], and exceptional chemical stability [12]. Due to these features, CNTs are widely applied in sensing areas [13,14,15], functional materials [16], biological medicine [17], catalysis [18], energy harvesting and conversion devices [19], electronic devices [20], drug delivery [21], and water treatment [22].
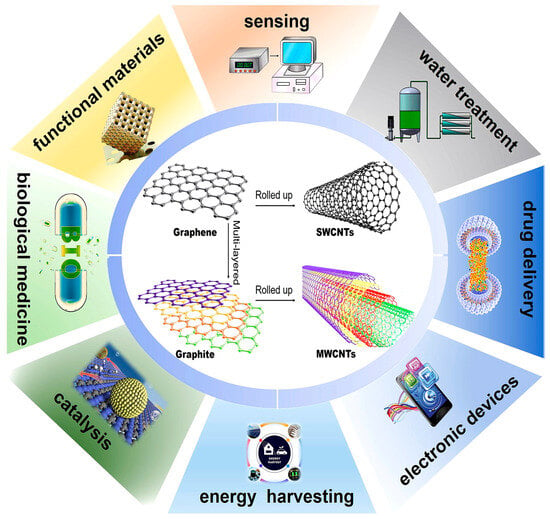
Figure 1.
Schematic diagram of SWCNTs and MWCNTs together with their applications.
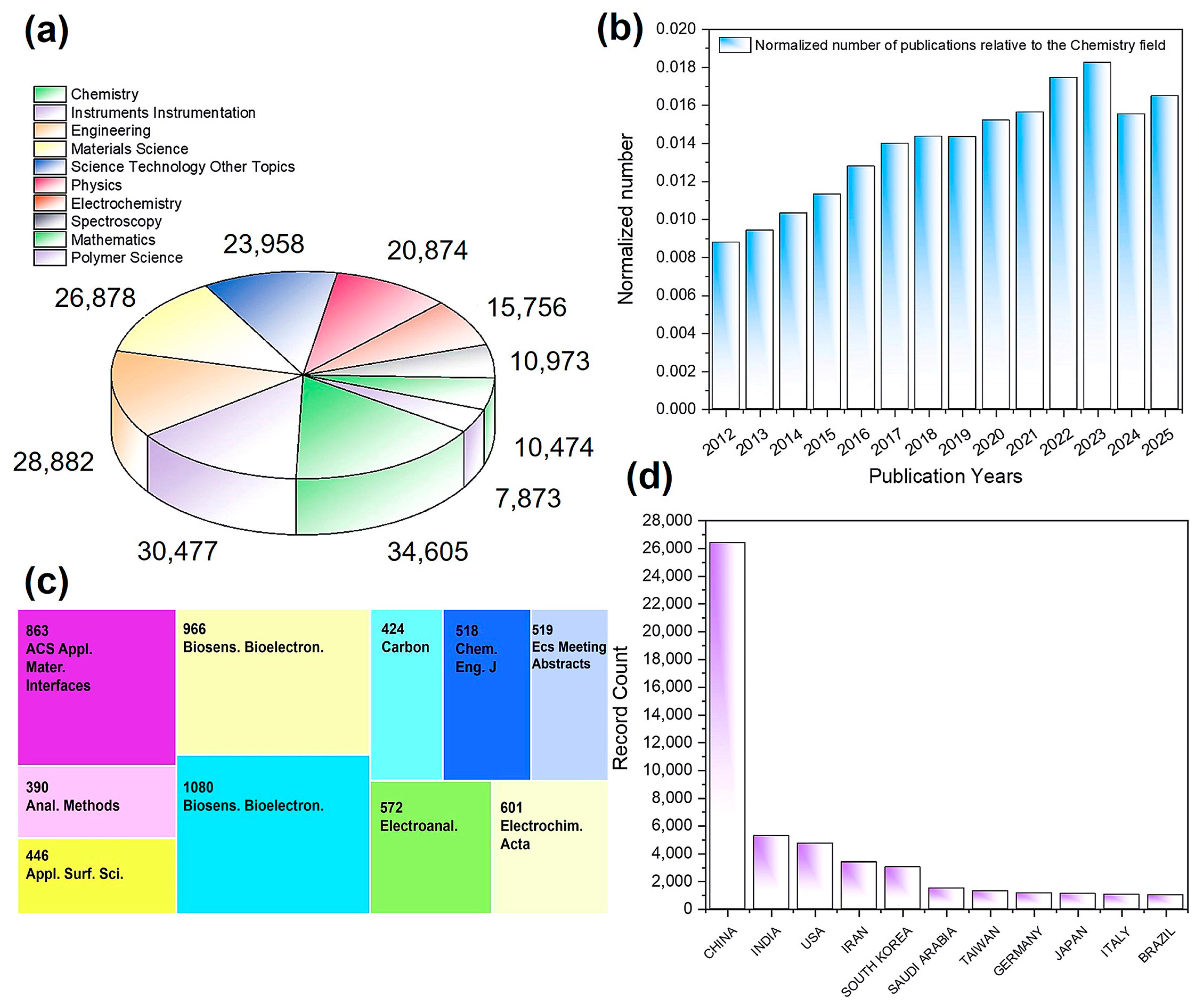
In the field of sensing, a literature search on the Web of ScienceTM using the keyword “carbon nanotube + sensor” from 2012 to the present yielded 47,913 publications. This demonstrated that CNT-based sensors have already extended into multiple disciplines, including chemistry, instrumentation, engineering, materials science, physics, and electrochemistry (Figure 2a). The annual publication output of carbon nanotube sensors was normalized to the total annual publication output in the field of chemistry, yielding the normalized number of publications. The results indicated that this field has been in a state of continuous yearly growth (Figure 2b). In mainstream journals related to sensing, materials, electrochemistry, and analytical sciences, the yearly output often reaches several hundred papers (Figure 2c), underscoring the broad academic attention this topic has attracted. Overall, CNT-based sensing is currently experiencing an explosive growth phase, and it has become a research hotspot favored by scholars from many countries and regions worldwide (Figure 2d).
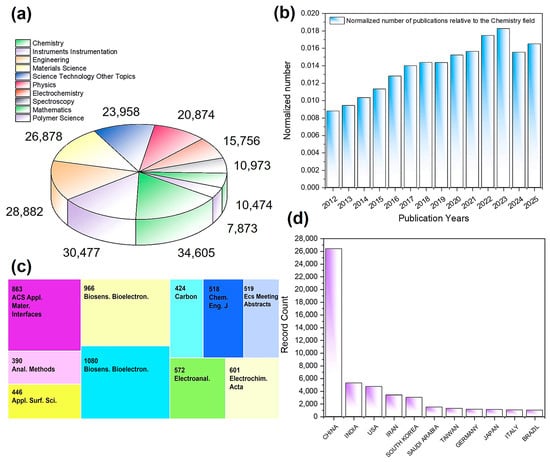
Figure 2.
Analysis of 47,913 publications searched with keywords “carbon nanotube + sensor” on Web of ScienceTM: (a) research areas, (b) years, (c) publications, and (d) countries/regions.
Despite their unparalleled advantages, pristine CNTs are often limited in practical applications due to poor dispersibility (CNTs tend to aggregate in solvents or matrices) [23], inadequate selectivity and sensitivity (pristine CNTs may exhibit non-specific responses to multiple analytes and show relatively low signal changes) [24], surface inertness [25], potential cytotoxicity and environmental concerns [26], and reproducibility issues [13]. Therefore, functionalization and modification of CNTs have become the mainstream approach [27], allowing for the design of target-specific chemical sensors with enhanced performance metrics such as sensitivity, selectivity, response time, and operational stability. Functionalization methods can broadly be categorized into covalent and non-covalent strategies [28,29]. Covalent modifications involve the chemical bonding of functional groups onto the CNT sidewalls or ends, often improving solubility and stability at the cost of partial degradation of the π-conjugated network. In contrast, non-covalent interactions preserve the intrinsic electronic properties of CNTs and rely on π-π stacking, van der Waals forces, or electrostatic interactions to immobilize recognition elements (details are given in Section 3). The choice of functionalization approach directly influences the sensing mechanism, signal transduction mode, and device architecture. Over the past two decades, considerable research efforts have been devoted to functionalizing or hybridizing CNTs with various molecular and polymeric agents [30,31], (metal) nanoparticles [32], metal oxides [33,34], biomolecules [35], graphene [36,37], and metal–organic frameworks (MOFs) [38,39] to tailor their surface properties and enhance their interaction with analytes. Hybridization with these functional materials has created synergistic effects that boost the sensor performance beyond the capabilities of pristine CNTs; as a result, modified CNTs can act as highly responsive transducers capable of detecting even trace levels of chemical species.
In terms of application, as shown in Figure 2a, CNT-based chemical sensors have been successfully applied in various areas. Specifically, CNTs have shown exceptional performance in environmental analysis, such as the detection of organic pollutants and heavy metal ions. Their high sensitivity and fast response have also been leveraged in drug monitoring. In addition, recent studies have demonstrated the use of functionalized CNTs in healthcare, food safety control, and gases detection, underlining their versatility and commercial viability.
This review presents a comprehensive and up-to-date overview of the sensing mechanisms, surface functionalization strategies, and applications of CNT-based chemical sensors. We also critically discuss cutting-edge application cases, highlighting how CNT sensors are deployed in real-world environments. Lastly, through Strengths-Weaknesses-Opportunities-Threats (SWOT) analysis, we further identify the emerging trends and future research directions. By providing both a technical- and application-oriented perspective, we aim to support the next generation of researchers in harnessing the full potential of CNTs for chemical sensing innovation.
2. Sensing Mechanism
Sensors based on CNTs can take various forms, mainly including electrical, optical, mechanical/piezoresistive, thermal, and mass-sensitive types [40,41]. Among these, electrical sensors are the most widely used and can be further subdivided into several categories, such as resistive sensors, field-effect transistor (FET) sensors, electrochemical sensors, and impedance/capacitance-based sensors. Optical sensors, on the other hand, are particularly suited for high-sensitivity detection, while mechanical and piezoresistive sensors are rapidly advancing in the field of wearable devices. In each type of sensor, CNTs perform distinct roles, leveraging their unique properties and participating in different sensing mechanisms to achieve precise and efficient detection. Apart from the differences in sensing mechanisms arising from the forms of application, the mechanisms also vary depending on the physical state of the analyte. For example, gas-phase sensing primarily relies on adsorption, charge transfer, and electrostatic gating effects, where analyte molecules directly interact with the CNT surface [42]. In contrast, liquid-phase sensing is more complex, as it involves additional factors such as solvation effects, ionic strength, double-layer screening, and diffusion dynamics in the medium [43]. These differences highlight the need for tailored sensing mechanisms in CNT-based sensors to effectively detect and differentiate between gas- and liquid-phase analytes.
2.1. Electrical Sensors
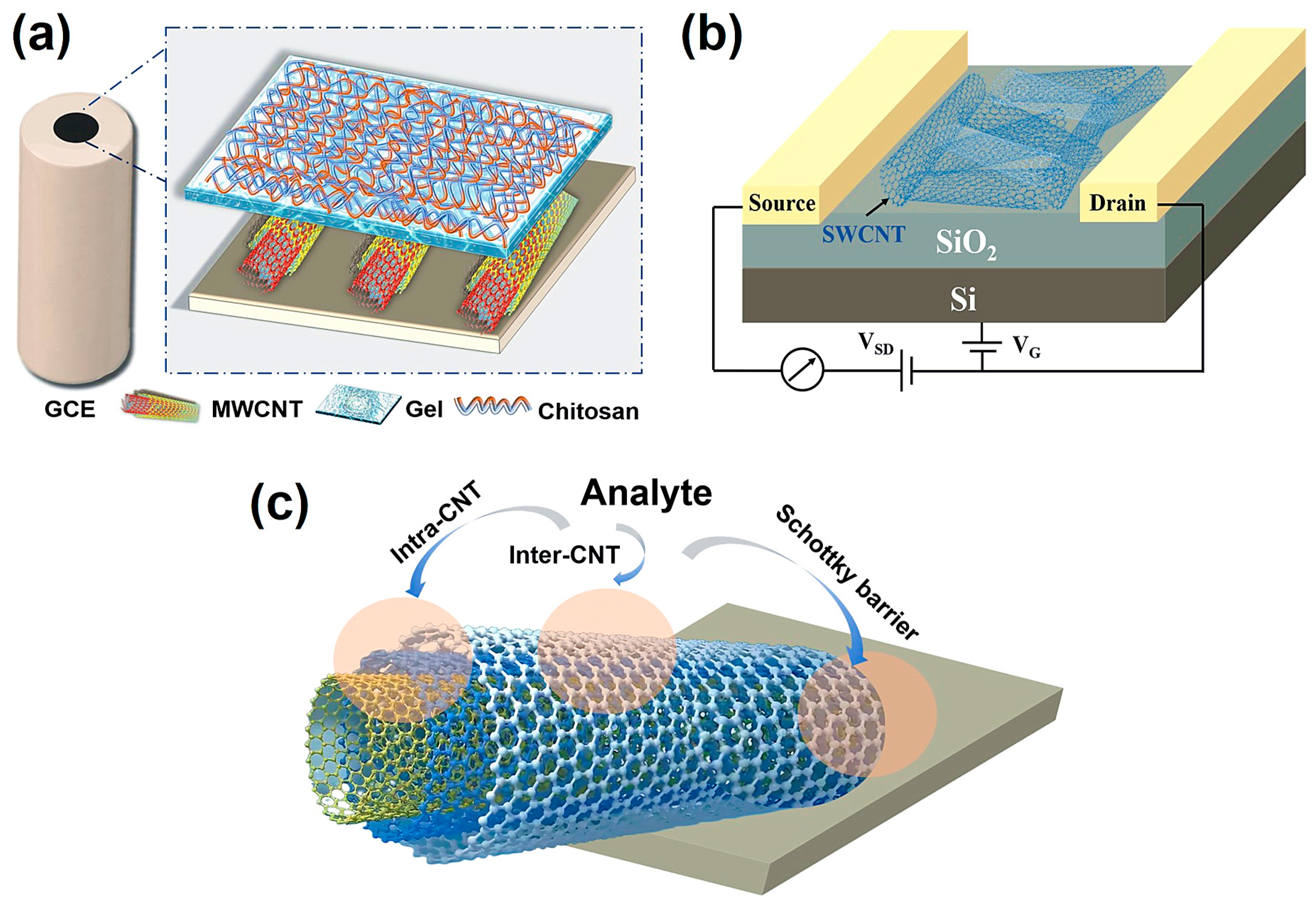
CNTs exhibit excellent conductivity and high carrier mobility, making them highly suitable for electrical sensing. The detection mechanism mainly relies on changes in electrical conductance. When target molecules adsorb onto the CNT surface, charge–transfer or electrostatic interactions occur, shifting the Fermi level and modulating conductivity. In resistive sensors, analytes act as electron donors or acceptors, leading to either an increase or decrease in conductance. In resistive sensors, analytes act as electron donors or acceptors, leading to either an increase or decrease in conductance. To further enhance the sensing performance and control the selectivity of CNT-based resistive sensors, dispersing CNTs into a polymer matrix to form polymer nanocomposites is an alternative. In such composites, the polymer serves a dual role: it stabilizes the conducting CNT network and allows tuning of the CNT-CNT junction distances, which directly modulates quantum tunneling conduction within the network. This mechanism is particularly relevant for chemoresistive sensors, where changes in tunneling pathways due to analyte adsorption lead to variations in conductivity [44]. In electrochemical sensing, CNTs can be used to modify the electrode (Figure 3a), providing a large surface area and efficient electron transport channels, thereby accelerating electrode reaction kinetics and amplifying detection signals. Recently, FET sensors have been widely used for the analysis and detection of various substances due to their high sensitivity and rapid response [45]. A typical FET generally consists of a source, a drain, a channel, a gate, and a dielectric layer [46]. Generally, since SWCNTs are single graphene layers rolled into tubes and possess stronger semiconducting properties, they offer the advantages of a single conduction channel, well-defined semiconductor behavior, and high-sensitivity functionalization. Therefore, SWCNTs are more widely employed in FET applications than MWCNTs [47]. In a general CNT-FET device, CNTs serve as the semiconducting channel bridging the source and drain electrodes, with the current modulated by the gate voltage (Figure 3b). When target gas molecules or chemical species adsorb onto the CNT surface, they induce charge transfer or electrostatic gating effects, which alter the carrier density and thereby modulate the channel conductance [48]. In particular, CNT-FET gas sensors can be integrated into a logic inverter configuration, where conductance or threshold changes induced by gas molecules are converted into digital voltage transitions. This converts weak analog sensing signals into distinct logic-level changes, facilitating digital readout and system-level integration. Such an approach enhances signal readability, improves noise immunity, and enables device miniaturization and low-power operation [49]. In more detail, the sensing mechanisms of CNTs can be roughly summarized into the following three points (Figure 3c): (1) Intra-CNT (occurs on the sidewall or along the length of CNTs). The major sensing mechanism comes from direct charge transfer between the analyte and CNTs. Sometimes the degradation of the CNT sidewalls caused by analytes can increase the number of defect sites of the SWCNT, thus changing its sensing properties; (2) Inter-CNT (occurs on the CNT-CNT interface). This is mainly affected by the distance between two CNTs; (3) Schottky barrier modulation. The Schottky barrier refers to the junction of metal electrode and CNT, though there remain some inconsistencies; the sensing mechanism may be strongly controlled by the bottlenecks in the conduction pathways [50]. A brief comparison of different types of CNT-based electrical sensors is given in Table 1.
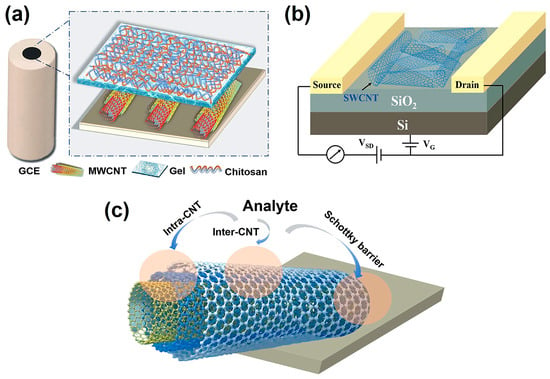
Figure 3.
(a) CNT-modified GCE, excerpted from our recently published work, (b) schematic representation of a general CNT-FET, (c) schematic of sensing mechanisms in CNT-based sensors.

Table 1.
Comparison of different types of CNT-based electrical sensors (quantitative data can be found in Section 4).
2.2. Optical Sensors
The unique electronic band structure and strong optical response of CNTs allow their integration into optical sensing platforms, such as fluorescence [51], surface plasmon resonance (SPR) [52], and surface-enhanced Raman scattering (SERS) [53]. The detection mechanism is based on variations in optical signal intensity or energy states. In fluorescence sensors, analytes induce energy transfer or quenching effects, altering the photoluminescence intensity of CNTs. In SPR or localized surface plasmon resonance (LSPR) sensors, CNTs combined with metallic nanostructures enhance the local electromagnetic field, thereby amplifying the signal of adsorbed molecules. In SERS-based sensing, CNTs act as Raman-active substrates where molecular adsorption causes charge–transfer interactions, leading to enhanced Raman peaks of the analyte.
2.3. Mechanical/Mass Sensors
With their high Young’s modulus and ultra-low mass density, CNTs are ideal candidates for nanoelectromechanical systems (NEMS) [54,55]. Their sensing mechanism relies on changes in mechanical properties or resonant frequency. Adsorption of molecules or nanoparticles on the CNT surface increases the effective mass, resulting in measurable shifts in resonance frequency, which enables ultra-high-sensitivity mass detection. Additionally, CNTs can act as strain sensors [56,57]: external pressure or deformation alters their band structure and electrical resistance, thus transducing mechanical stimuli into electrical signals.
2.4. Biological Sensors
This type of sensor will focus more on dynamically monitoring key molecular changes in physiological and pathological processes, providing real-time information for precision medicine. The surface of a CNT can be chemically modified (such as covalent bonding and noncovalent modification) to connect various biological recognition elements (such as antibodies, aptamers, enzymes), polymers, or functional molecules, thereby achieving specific detection of different target substances. SWCNTs especially have fluorescence emission in the near-infrared region II (NIR-II), strong tissue penetration ability, and low spontaneous fluorescence background, making them very suitable for sensing and imaging in complex biological environments. In addition, a CNT has good mechanical strength and flexibility, making it very suitable for building flexible electronic devices and wearable sensors. Multifunctional integration and wearability are important development trends at present, such as simultaneous detection of multiple biomarkers, pH, and temperature; it can be combined with flexible electronic technology to develop more comfortable, portable, and intelligent wearable devices for continuous health monitoring.
3. Functionalization, Modification, and Hybridization Strategies
Functionalization and surface modification of CNTs are often necessary to enhance their performance in sensing fields. Generally, pristine CNTs lack inherent selectivity and may show poor dispersion in solution due to strong van der Waals interactions and hydrophobicity; compared to individual CNTs, these bundles and aggregates can lead to a deterioration in the mechanical and electrical properties of the resulting composites [58]. Meanwhile, functionalization introduces specific chemical groups or recognition elements onto the CNT surface, which can significantly improve the selectivity toward target analytes through mechanisms such as hydrogen bonding, electrostatic interactions, π-π stacking, etc. Additionally, surface modification enhances the sensitivity of CNT-based sensors by facilitating stronger and more specific interactions with analytes, even at trace levels. It also improves the solubility and processability of CNTs, enabling the fabrication of more uniform and reproducible sensor devices. Importantly, appropriate functionalization further enhances biocompatibility and reduces cytotoxicity of the CNT, making it more suitable for biological applications. Therefore, functionalization is a crucial strategy for tailoring the physicochemical properties of CNTs to meet the specific requirements of chemical or biosensing platforms, which can be divided into covalent and noncovalent strategies. Comprehensive reviews on the functionalization and modification of CNTs have been published in earlier years [59,60,61,62,63]. To avoid redundancy, this review focuses primarily on the methods and advancements in CNTs’ functionalization reported in recent years, especially in the sensing area.
Covalent functionalization involves the chemical modification of CNTs’ graphitic skeleton through the formation of covalent bonds with functional groups (attached to the ends or sidewalls of CNTs). A widely used method is oxidation reaction, which often begins with oxidative treatments using strong acids (e.g., HNO3 or H2SO4) or non-acidic oxidants (e.g., H2O2 or KMnO4) to introduce carboxyl (–COOH), hydroxyl (–OH), or carbonyl (–C=O) groups on the CNT surface or open ends. On one hand, hydrophilic groups improve the solution dispersibility of CNTs. On the other hand, these oxygen-containing groups serve as reactive sites for further chemical derivatization, such as amide or ester formation via coupling with amines or alcohols.
3.1. Covalent Functionalization
3.1.1. Oxidation Reaction
Oxidation is a classic chemical modification method for CNTs. One of the most commonly used approaches involves treatment with strong acids, such as ultrasonic treatment of pristine MWCNTs at 40 °C for 3 h/at 70 °C for 7 h [64,65] with a mixture of concentrated H2SO4:HNO3 acids 3:1 (v/v) to introduce carboxyl functional groups on the surface of MWCNTs, or by refluxing in a 3:1 (v/v) mixture of concentrated HNO3 (65%) and H2SO4 (98%) at 70 °C for 4 h [66]. In addition, the reflux method can also be used to obtain oxidized CNTs. For example, preparation of COOH–MWCNTs by refluxing MWCNTs and a 3M HNO3 + H2SO4 mixing solution (1:3, v/v) at 80 °C for 5 h [67]. Sometimes simple stirring is also an alternative approach; for instance, stirring MWCNTs in a mixed solution of HNO3 and H2SO4 (3:1, v/v) at room temperature for 12 h [68].
3.1.2. Esterification Reaction
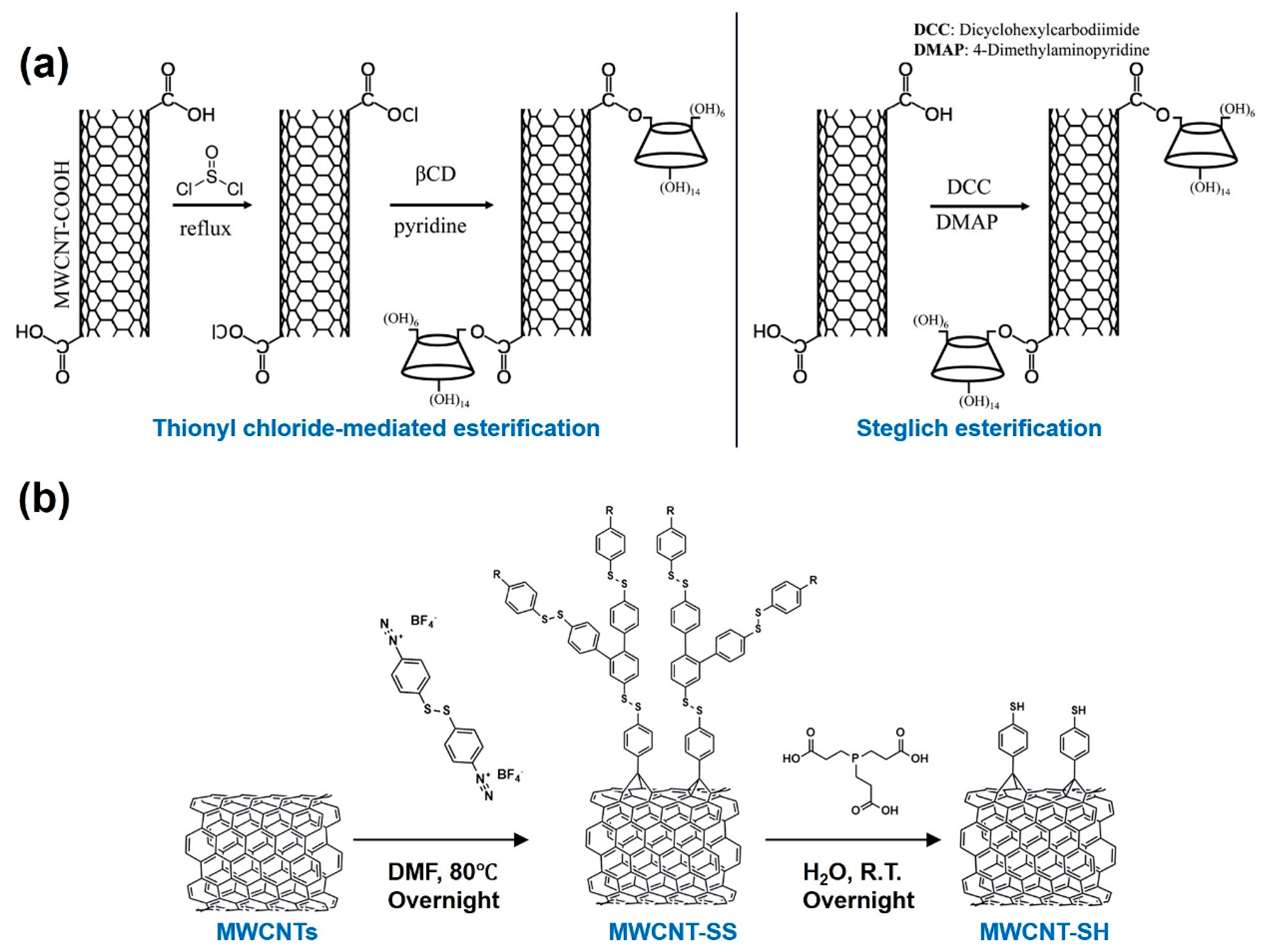
Alam’s group prepared β-cyclodextrin (β-CD)-functionalized MWCNTs using two different approaches: thionyl chloride (TC) esterification and Steglich esterification (SE). Briefly, in the former, MWCNT–COOH was reacted with SOCl2 to yield MWCNT–COCl, which then reacted with β-CD to produce MWCNT–β-CD. In the SE method, 4-(dimethylamino) pyridine (DMAP), β-CD, and dicyclohexylcarbodiimide (DCC) were added to a DMF suspension of MWCNT–COOH, and the product was obtained via a one-pot reaction (Figure 4a). Compared to TC esterification, the SE method requires a shorter reaction time and lower temperature and yields MWCNT–β-CD with a moderate β-CD loading capacity [69]. Thus, they continued to employ Steglich esterification to prepare MWCNT–β-CD in subsequent studies [70]. This functionalization approach is based on an esterification reaction between the carboxyl groups on the sidewalls of oxidized CNTs and the hydroxyl groups of β-CD, enabling covalent grafting of β-CD onto the CNT surface.
Yola et al. [68] reported a method for synthesizing thiol-functionalized MWCNTs via a self-assembled esterification reaction. First, a carboxylated MWCNT ethanol solution was reacted with N-hydroxysuccinimide and 1-ethyl-3-(3-dimethylaminopropyl) carbodiimide for 12 h to activate the carboxyl groups of the MWCNTs. Subsequently, the activated MWCNTs were added to a 4-aminothiophenol solution and reacted for 2 h to obtain the final product.
3.1.3. Radical Reaction
Radical reaction is a common approach for the covalent functionalization of CNTs, typically achieved by introducing radical intermediates onto the CNT surface, which then form chemical bonds with the C=C bonds or defect sites of the nanotube, thereby enabling functionalization. A research team functionalized MWCNTs with 4,4′-dithiodibenzenediazonium tetrafluoroborate (DTDBBF4) at 80 °C [71]. Under heating conditions, DTDBBF4 decomposes to generate aryl radicals, which covalently attach to the CNT surface through the formation of stable C–C bonds. This process introduced aryl substituents containing disulfide linkages, yielding disulfide-functionalized MWCNTs (MWCNT–SS). The disulfide groups can be further reduced to thiol (–SH) functionalities, thereby converting MWCNT–SS into thiol-functionalized MWCNTs (MWCNT–SH), as displayed in Figure 4b.
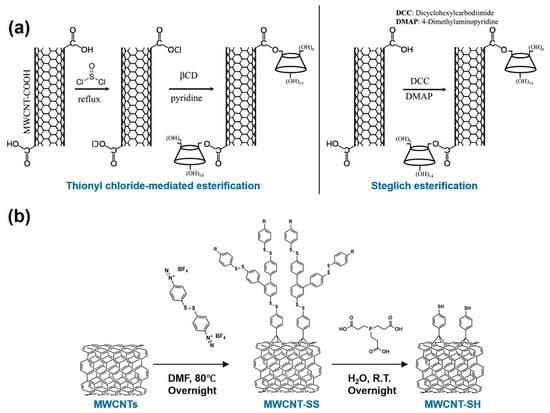
Figure 4.
(a) Synthesis of MWCNT–β-CD through thionyl chloride-mediated esterification (left) and Steglich esterification (right). Copyright 2018 American Chemical Society. (b) Synthesis of disulphide-functionalized MWCNTs by a radical reaction. Copyright 2024 Elsevier.
Figure 4.
(a) Synthesis of MWCNT–β-CD through thionyl chloride-mediated esterification (left) and Steglich esterification (right). Copyright 2018 American Chemical Society. (b) Synthesis of disulphide-functionalized MWCNTs by a radical reaction. Copyright 2024 Elsevier.
3.1.4. Other Reactions
Cycloaddition/Substitution Reaction and Nucleophilic/Electrophilic Additions
In addition to commonly used oxidation, acylation (or esterification), and radical reactions, some classical reactions, such as cycloaddition [72], substitution reactions [59], nucleophilic additions [73], and electrophilic additions [74], are also frequently employed for the covalent functionalization of carbon nanotubes.
Click Chemistry
The concept of click chemistry was first proposed by Sharpless and co-workers in 2001 [75]. Its main characteristics include high stereoselectivity, efficiency, mild reaction conditions, and high yields [76]. Click chemistry offers a mild, efficient, and controllable strategy for the functionalization of CNTs [77], enabling their effective coupling with various functional materials. For example, a click chemistry-mediated MWCNT–βCD conjugate was synthesized by reacting alkyne-functionalized MWCNTs with azide-modified β-CD in the presence of copper(I) iodide and 1,8-diazabicyclo[5.4.0]undec-7-ene under argon bubbling at 70 °C for 48 h [69].
Covalent functionalization enables high stability and tunability, improves compatibility with other materials, and allows for the introduction of specific recognition elements. It should be noticed that, as shown in Figure 1, MWCNTs consist of multiple concentric graphene layers, providing higher structural stability. While covalent grafting may alter the outer layers, electrons can still circulate through the inner walls with minimal disruption. But SWCNTs, which are composed of a single graphene layer, are relatively fragile. Covalent strategy may change the length of CNTs or disrupt their π-conjugated structure, thereby altering their intrinsic properties such as optical characteristics, mechanical strength, and electrical conductivity. Therefore, this approach is typically favored when chemical stability and robust molecular anchoring are prioritized, or when the introduction of functional groups is required for subsequent functionalization steps.
3.2. Noncovalent Functionalization and Hybridization
Noncovalent functionalization represents a milder and structurally conservative approach to modify CNTs without altering their intrinsic sp2 hybridized carbon framework. This method leverages weak physical interactions such as π-π stacking, van der Waals forces, electrostatic interactions, and hydrophobic effects to adsorb molecules onto the CNT surface. Typical functionalization agents include carbon nanosheets (CNS), ionic liquids (ILs), graphene oxide (GO), and polymers [78]. For instance, surfactants such as sodium dodecyl sulfate (SDS) or cetyltrimethylammonium bromide (CTAB) can effectively solubilize CNTs, while π-conjugated molecules such as pyrene derivatives provide targeted non-covalent anchoring through π-π interactions. Non-covalent functionalization preserves the electrical and optical properties of CNTs, making them particularly attractive for applications requiring high conductivity, such as chemical and biosensors.
In contrast, noncovalent hybridization involves combining CNTs with other nanomaterials (e.g., metal or metal oxide nanoparticles, MOFs, or 2D materials) to form composite architectures. Unlike functionalization, hybridization primarily modifies the conducting or structural framework of the transducer rather than the chemical selectivity of CNTs. While it does not introduce specific recognition sites, hybridization can enhance overall device performance by improving conductivity, stability, or catalytic activity.
Although less chemically robust than covalent methods, noncovalent approaches (both functionalization and hybridization) offer greater reversibility and processability and are often preferred when structural integrity and electronic performance must be maintained.
3.2.1. Thermal Method
Zhao et al. [79] reported a ball-milling and thermal decomposition strategy for the synthesis of 3D interconnected porous carbon nanotubes/carbon nanosheets (3D IPCNT/CNS). In this method, sodium citrate was first ball-milled for 8 h and then mixed with CNTs, followed by additional ball-milling for 2 h. The resulting mixture was carbonized at 850 °C for 1 h under an argon atmosphere. The obtained black powder was sequentially washed with HCl and deionized water and then dried to obtain the 3D IPCNT/CNS composite. Later, Liu and coworkers [80] introduced a slight modification to Zhao’s method. After mixing the ball-milled sodium citrate with CNTs, the mixture was ball-milled for 2 h, followed by high-temperature activation at 850 °C for 3 h, yielding a carbon nanosheets@carbon nanotubes (CNS@CNT) composite.
Besides thermal decomposition, hydrothermal methods are also widely employed for the functionalization of CNTs. Early studies have demonstrated that 3D graphene–carbon nanotube (GN–CNTs) composites exhibit outstanding electrical conductivity and catalytic activity. However, its poor dispersibility and stability in solvents limited their practical applications. To address this issue, subsequent research introduced ionic liquids to modify GN–CNTs. ILs not only promote the dispersion of GN–CNTs but also serve as linkers to assemble them into hybrid nanostructures. For instance, Yang et al. [81] synthesized a GN–CNTs–IL composite via a hydrothermal approach. Specifically, a suspension of MWCNTs, [APMIM]Br, and GO was thoroughly mixed and ultrasonicated for 2 h, followed by hydrothermal treatment at 180 °C for 12 h. The resulting product was collected by centrifugation, washed, and dried to obtain the GN–CNTs–IL composite. Building upon this, the same group further developed an AuPd/GN–CNTs–IL nanocomposite in order to combine the conductivity and catalytic activity of metal nanoparticles [82]. In this work, HAuCl4 and Na2PdCl4 were added to a suspension containing [HOEMIM][NTf2], GO, and CNTs. The mixture was subjected to a hydrothermal reaction at 180 °C for 12 h, yielding the target nanocomposite, which possessed high conductivity and electro-catalytic property.
Zhang’s group [83] reported a hydrothermal method for preparing GO/MWCNT composites. In this approach, GO and MWCNTs were dispersed in deionized water to form a suspension, followed by the addition of 35% hydrazine solution and 28 wt% ammonia solution. The mixture was then reacted at 80 °C for 30 min and subsequently at 90 °C for 1 h. After centrifugation, filtration, and drying, GO/MWCNT powder was obtained.
A hydrothermal reaction was applied to the preparation of CNT/MoS2 nanocomposites. Ammonium molybdate and thiourea were added to an aqueous solution of CNTs containing cetyltrimethylammonium bromide (CTAB), and the mixture was subjected to hydrothermal treatment at 220 °C for 24 h. The resulting product was collected by centrifugation, washed, and dried to obtain black CNT/MoS2 composite [84]. Moreover, MOF-functionalized CNT composites can be synthesized via a hydrothermal method. For example, dispersing ZrCl4, 2-aminoterephthalic acid, glacial acetic acid, and MWCNTs in DMF followed by heating at 120 °C for 48 h can yield UiO-66-NH2@MWCNTs composites [85].
3.2.2. Ultrasound-Assisted Method
Ultrasound-assisted treatment is another widely used method for non-covalent functionalization of CNTs. Through this way, materials such as MOFs, metal (or metallic oxide) nanoparticles, cyclodextrins, and amino acids can be combined with CNTs to form functionalized composite materials. This strategy effectively integrates the advantages of individual components, enhancing the overall performance of the resulting hybrid system.
MOF
Ultrasonication of MWCNTs with Cu-functionalized metal–organic frameworks (UiO-bpydc-Cu) for 30 min can yield a composite material with enhanced electrical conductivity and sensing performance. The improved stability of the resulting hybrid was attributed to electrostatic attraction between the positively charged MOF and the negatively charged MWCNTs, as well as π-π stacking interactions between the conjugated structures [86]. Han et al. [87] prepared SCN@UIO-66 nanocomposites by ultrasonically treating single-walled carbon nanotubes (SCN) with an equal mass of MOF UIO-66 nanoparticles for 60 min, followed by magnetic stirring at 80 °C for 2 h.
Metal/Metallic Oxide Nanoparticles
CNT-modified metal oxides have attracted considerable attention due to their excellent properties and broad application prospects. These hybrid materials can be readily prepared through a simple ultrasonication process. According to the study by Kunene et al. [88], a uniform paste was obtained by dispersing 2.0 mg of MWCNTs and 2.5 mg of silver-doped zinc oxide nanoparticles (Ag-ZnONPs) in 2 mL of a DMF:H2O (1:1) mixture, followed by ultrasonication for 3 h.
Cyclodextrins
CDs are a family of cyclic oligosaccharides composed of 6–8 D-glucopyranose units linked by α-1,4-glycosidic bonds, characterized by a hydrophilic outer surface and a hydrophobic inner cavity. Based on the number of glucose units, CDs are mainly classified into three types: α-cyclodextrin (α-CD, 6 units), β-cyclodextrin (β-CD, 7 units), and γ-cyclodextrin (γ-CD, 8 units). Owing to their hydrophobic cavity, CDs are capable of forming stable host–guest inclusion complexes with various small molecules, making them highly valuable in applications such as drug delivery, food formulation, environmental sensing, and chemical sensors. The integration of CDs with CNTs significantly enhances sensor performance. Specifically, the molecular recognition ability of CDs can synergize with the excellent electrical conductivity of CNTs to amplify response signals, particularly in electrochemical sensors. This combination improves both the sensitivity and selectivity of detection. Moreover, the hydrophilic outer shell of CDs markedly improves the dispersibility of CNTs in aqueous or polar solvents, thereby reducing aggregation and facilitating uniform distribution. In addition, the multiple hydroxyl groups on cyclodextrin molecules allow for versatile chemical modifications. Functionalization strategies such as thiolation, carboxylation, and amination can introduce groups like thiol (-SH), carboxyl (-COOH), and amino (-NH2) onto the CD structure. These modifications significantly enhance the binding affinity between the CD-based composite materials and target analytes. CD-modified CNTs have attracted much attention in sensing area, and one of the main methods to prepare them is ultrasound treatment. For instance, carboxylic acid-functionalized SWCNTs–β-CD suspensions can be prepared by doping β-CD powder with SWCNT suspensions, followed by continuous ultrasound [89].
Amino Acid
It was reported that after ultrasonication of CNTs with L-cysteine for 2 h followed by 1.5 h of magnetic stirring, amine-functionalized carbon nanotubes (NH2-CNTs) can be obtained. This is achieved through adsorption, electrostatic interactions, or other non-covalent forces that enable the attachment of L-cysteine to the CNT surface [90].
Sometimes, a combination of thermal and ultrasound methods is also a good choice. For example, in the synthesis of a MoS2/MW-CNTs heterostructure. Firstly, a suspension of MWCNTs in deionized water was ultrasonically treated for 30 min with a mixed solution of ammonium molybdate, thiourea, and deionized water. The mixture was then subjected to a hydrothermal reaction at 160 °C for 27 h to obtain the final product [91].
3.2.3. Physical Absorption
In the adsorption approach, the surface of CNTs serves as a supporting matrix, onto which functional materials are noncovalently coated through electrostatic interactions, van der Waals forces, hydrogen bonding, and π–π stacking, while preserving the intrinsic structural integrity of the CNTs. For example, HAuCl4·3H2O and MWCNT-COOH were first stirred for 20 min, followed by the addition of sodium formate as a reducing agent and further reaction for 60 min at room temperature, leading to the in situ generation of AuNPs in the presence of MWCNT-COOH. The resulting AuNPs were subsequently adsorbed onto the surface of MWCNT-COOH through noncovalent interactions [92].
3.2.4. Other Methods
Melt blending is a common method for preparing carbon nanotube–polymer composites [93], typically involving the mixing of thermoplastic polymers with bundles of CNTs at elevated temperatures. This process is fast, cost-effective, solvent-free, and suitable for insoluble polymers; however, the high shear forces and elevated temperatures involved may damage both the CNTs and the polymer chains [94]. Meanwhile, melt blending makes dispersion more difficult; it is generally much more difficult to obtain an aggregate free transducer and a film less than 100 µm [67,95].
3.3. Combination of Covalent and Noncovalent Functionalization
Chio and coworkers [96] reported a dual covalent and noncovalent SWCNT functionalization method. Firstly, they used cyanuric chloride to produce triazine-functionalized SWCNTs (Trz-SWCNTs) by a re-aromatization reaction, then the chlorine of Trz-SWCNTs with high-labeling densities (Trz-H-SWCNTs) was replaced by a primary amine through nucleophilic substitution. In this way, they established a library of surface-functionalized SWCNTs, facilitating the introduction of functional groups such as thiol, PEG2-Biotin, and glycine. Following covalent functionalization, researchers employed non-covalent strategies based on π-π aromatic stabilization and hydrophobic packing to further modify the SWCNT surface with amphiphilic polymers, phospholipids, and surfactants. This approach involved a mild covalent modification protocol that preserved the intrinsic optical properties of SWCNTs, ensuring that their fluorescence characteristics remained intact even after subsequent non-covalent coating. The results showed that all SWCNT complexes were still capable of acting as optical nanosensors after noncovalent association of amphiphilic polymers or phospholipids. This study highlighted the reliability and potential of combined covalent and non-covalent functionalization techniques for developing robust and versatile SWCNT-based sensing platforms.
4. Applications of CNTs in Chemical Sensing
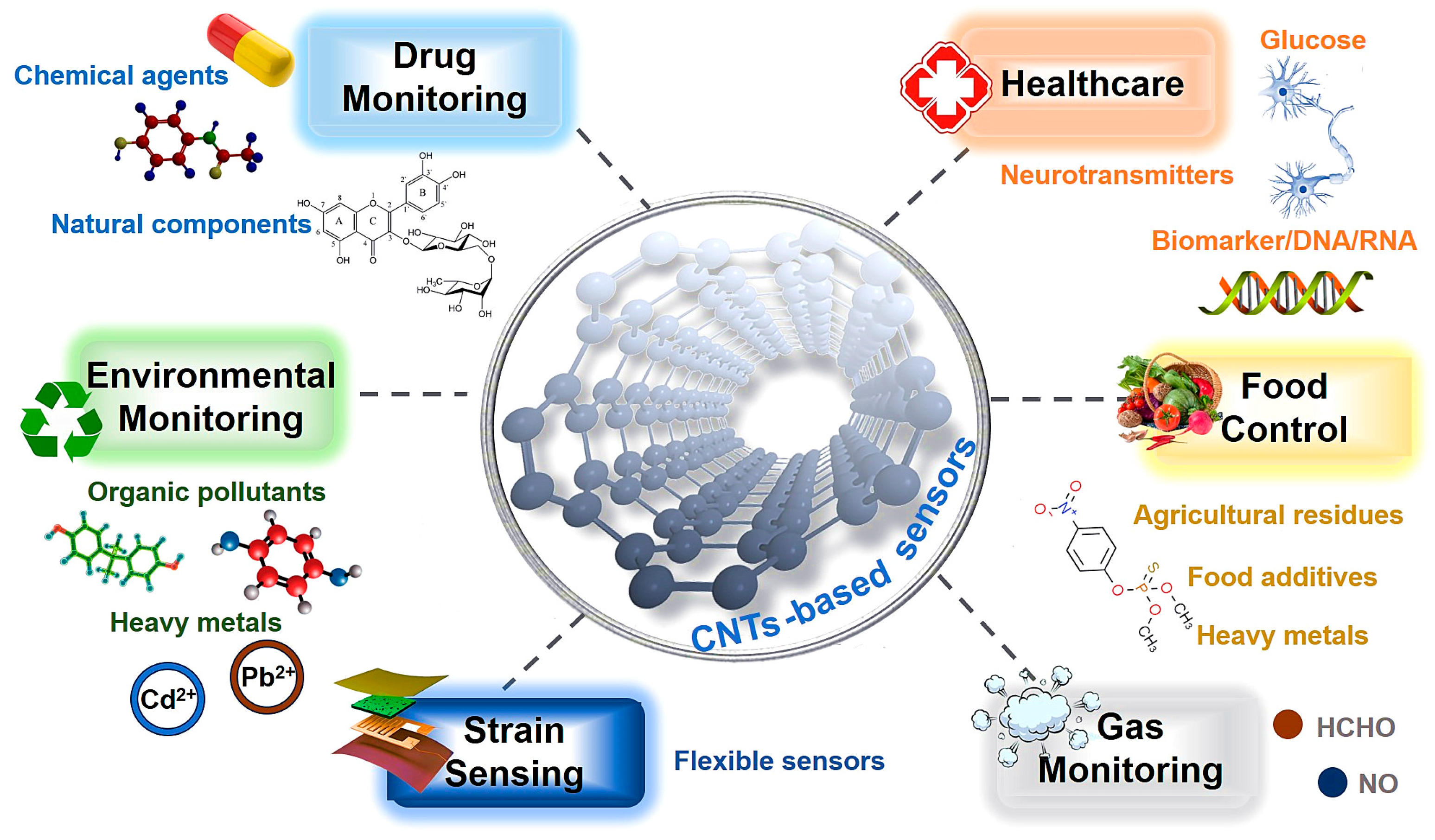
Currently, various CNT-based sensors have been successfully applied across multiple industries (Figure 5). For example, in the environmental field, they are employed to monitor organic pollutants and heavy metals [97]; in the pharmaceutical sector, they can be used to detect chemical formulations or natural products; in healthcare, CNT-based sensors have been explored for monitoring blood glucose, neurotransmitters, tumor markers, and other biomarkers. When applied in food quality control, they are capable of detecting active ingredients, additive residues, and potential contaminants. In the area of gas sensing, CNT-based devices not only detect hazardous gases in the environment but also analyze specific biomarkers in human breath, providing valuable information for disease diagnosis. Moreover, some next-generation flexible CNT-based sensors have recently been developed, opening new opportunities for wearable and portable sensing platforms.
Figure 5.
Applications of CNT-based sensors in various fields.
4.1. Environmental Monitoring: Detecting Hazardous Molecules and Heavy Metals
Bisphenol A (BPA) is an industrial chemical widely used in many plastic products encountered in daily life. It can easily leach into food and source water cycles. As BPA is known to disrupt the human endocrine system, its monitoring in the environment is of significant importance. Some researchers utilized β-CD-modified MWCNTs to modify a screen-printed carbon electrode (SPCE) for the detection of BPA. The hydrophilic nature of β-CD and the large surface area of MWCNTs enhanced the sensor’s performance; the sensor MWCNTs-βCD/SPCE achieved a detection limit of 13.76 nM. When applied to detecting BPA in lake water and tap water, the recovery rates reached as high as 96.05% to 108.70% [98]. Meanwhile, the same group [70] developed a sensor for BPA detection in water using GO and β-CD-functionalized MWCNTs. The β-CD was covalently grafted onto CNTs via a one-step Steglich esterification reaction, yielding MWCNT-βCD. The MWCNT-βCD suspension was then mixed with GO suspension to form the GO-MWCNT-βCD suspension, which was subsequently drop-casted onto a screen-printed electrode (SPE) to fabricate GO-MWCNT-βCD/SPEs. This sensor offers a simple fabrication process and takes advantage of the high surface area of GO and CNTs, along with the excellent host–guest interaction capability of β-CD. The resulting sensor exhibited a LOD of 6 nM for BPA, showing reproducible and stable signals, strong anti-interference performance, and outstanding recovery in real water samples. Some researchers developed an electrochemical sensor for the detection of BPA by modifying a carbon screen-printed electrode with WMCNTs-functionalized silver-doped zinc oxide nanoparticles (Ag-ZnONPs), followed by the immobilization of laccase (Lac). The resulting sensor, denoted as Lac/Ag–ZnO/MWCNTs/C-SPE, exhibited excellent performance for BPA detection, with a LOD as low as 6.0 nM, enabling accurate quantification of BPA in plastic bottle samples [88].
CNTs have also demonstrated satisfactory performance in the detection of hazardous phenolic compounds in the environment. For example, a research group developed a biosensor for phenol detection by immobilizing aminated Coriolus hirsuta laccase (ChLa) onto carboxyl-functionalized multi-walled carbon nanotubes (COOH-MWCNTs) and modified screen-printed carbon electrodes via electrostatic adsorption, resulting in a ChLa/COOH–MWCNT/SPCE biosensor. In this system, COOH-MWCNTs not only provide excellent sensitivity and electrical conductivity, but the carboxyl groups also serve as effective functional sites for enzyme immobilization. Using catechol as the substrate, the biosensor exhibited rapid response, good repeatability, and retained its activity even after 20 days of storage, indicating strong operational stability [99]. Zhang et al. [86] designed an electrochemical sensor based on UiO-bpydc-Cu and MWCNTs-modified GCE, denoted as UiO-bpydc-Cu/MWCNTs/GCE, for the simultaneous detection of environmental pollutants hydroquinone (HQ) and catechol (CT). Owing to the synergistic effects between the MOF material (UiO-bpydc-Cu) and MWCNTs, the sensor exhibited rapid and sensitive responses toward both dihydroxybenzene compounds, along with advantages such as a wide linear range, low detection limits, and excellent stability. The sensor also delivered satisfactory results when applied to the determination of HQ and CT in tap water and river water samples.
In detecting heavy metals that pose threats to both the environment and human health, CNTs also play an important role. On one hand, CNTs possess a high specific surface area, excellent electrical conductivity, and outstanding mechanical properties, making them excellent electrode materials. On the other hand, functionalized CNTs (e.g., COOH–MWCNTs) exhibit strong metal-chelating abilities, making them particularly suitable for heavy metal ion detection. For example, a GCE modified with a triple-layer assembly film (MWCNTs–COOH/UiO-66-NH2/MWCNTs–COOH/GCE) enabled simultaneous quantitative determination of Cd2+ and Pb2+ [67].
4.2. Drug Monitoring: Chemical Agents and Natural Bioactive Components
Currently, CNTs find successful applications in the detection of drugs [100], including chemical agents and natural medicines. For example, some researchers developed a method for the determination of the nonsteroidal anti-inflammatory drug diclofenac using a gold electrode modified with a nanocomposite film of functionalized MWCNTs and GO combined with gold nanoparticles (AuNPs). This sensor exhibited excellent catalytic activity toward diclofenac molecules, achieving a detection limit of 0.09 μM [36]. Pour and colleagues [39] developed a GCE modified with a nanocomposite of MWCNTs and a copper metal–organic framework (CuMOF), denoted as CuMOFs-MWCNTs/GCE. This sensor could be used for the quantitative detection of the anticancer drug imatinib in human serum, urine, and pharmaceutical formulations, exhibiting linear ranges of 0.01–20 µM and 20–220 µM, with a LOD of 4.1 nM. A research team functionalized MWCNTs with β-CD to modify a GCE for monitoring trace amounts of acetaminophen and estrogen in drinking water. The detection limits for the two analytes were 3.3 nM and 2.5 nM, respectively [69]. Yang’s group [82] developed an electrochemical sensor for the detection of paracetamol (PCM) by modifying a GCE with a gold–palladium nanoparticles–ionic liquid-functionalized graphene–carbon nanotube nanocomposite (AuPd/GN-CNTs-IL). The 3D GN-CNTs composite provided excellent electrical conductivity and catalytic activity, facilitating rapid mass transport. The resulting electrochemical sensor exhibited high selectivity and sensitivity, enabling accurate monitoring of PCM concentrations in the urine samples of patients with fever and cold symptoms. Later, Kokab et al. [101] developed an electrochemical sensor, fCNTs/ZnO/fCNTs/GCE, capable of the simultaneous detection of acetaminophen, diclofenac, and ofloxacin. This sensor can achieve femtomolar-level detection limits for the three drugs, specifically 46.8 fM, 78 fM, and 60 fM, respectively.
Meanwhile, CNT-based composites have been widely employed as electrode modification materials for the electrochemical detection and analysis of various natural bioactive substances, achieving remarkable performance in a broad range of applications. In our recent study, a three-layer composite was fabricated via a layer-by-layer assembly method using long-chain amino acid-type imidazolium ionic liquids ([Cₙmim][AA]), MWCNTs, and chitosan. This composite was employed to modify a glassy carbon electrode (GCE) for the electrochemical detection of the flavonoid compound rutin. The [Cₙmim][AA] ionic liquid served as a molecular recognition element, providing selective interactions with rutin, while the MWCNTs offered excellent charge transport capabilities. The modified electrode exhibited outstanding anti-interference properties and achieved a detection limit as low as 0.05 μM for rutin, surpassing even some metal-modified electrodes in sensitivity [102].
Hu and colleagues [103] developed a zeolitic imidazolate framework (ZIF)-derived Co3O4@N-CNTs composite with excellent electrochemical activity via a pyrolysis method. Based on this, they constructed a ZIF-derived cobalt trioxide@nitrogen doped carbon nanotube/amino-functionalized graphene quantum dots composite-modified GCE, denoted as Co3O4@N-CNTs/NH2-GQDs/GCE, for the electrochemical detection of luteolin. The modified electrode exhibited ultra-sensitive detection performance toward the flavonoid compound luteolin, with a remarkable LOD of 0.1 nM. In real sample analysis, the sensor also demonstrated excellent sensitivity, stability, and reproducibility. Some researchers [79] have developed an electrochemical sensor for the detection of gallic acid (GA) by modifying a GCE with a 3D IPCNT/CNS hybrid composite. In this hybrid carbon material, CNTs and carbon nanosheets were integrated into a 3D interconnected hierarchical porous structure featuring a high specific surface area and abundant surface functional groups. During the electrochemical detection of GA, the composite provided a sufficient electrolyte–electrode interface, facilitated electron transfer, and enhanced surface affinity between the modified electrode and GA at the interface. Experimental results demonstrated that the sensor exhibited a low detection limit, as well as excellent reproducibility, stability, and selectivity. Moreover, the sensor achieved satisfactory results in the analysis of real tea samples, including green tea and black tea. Araujo et al. [64] employed acid-functionalized MWCNTs to modify SPEs. The oxidized MWCNTs were ultrasonically dispersed in a mixed solution of zein, 70% ethanol, and water, followed by drop-casting onto the SPE surface to fabricate the modified electrode. This electrode was then applied for the determination of caffeic acid, an antioxidant compound in tea samples. It was shown that the acid-functionalized MWCNTs were found to enhance the electrochemical behavior of the SPE, significantly improving the sensor’s analytical performance.
4.3. Healthcare: Glucose, Neurotransmitters, Biomarker, and DNA/RNA Detection
In the field of medical health and disease diagnosis, CNTs have been widely applied in the detection of glucose, neurotransmitters, tumor markers, gases from human exhalation, and other biomolecules.
4.3.1. Glucose
The prevalence of diabetes has continued to rise, making accurate determination of blood glucose levels critically important for clinical diagnosis and treatment. Electrochemical sensing has emerged as a popular approach due to its high sensitivity, rapid response, and operational simplicity. Against this backdrop, numerous CNT-based glucose sensors have been developed, demonstrating excellent performance and significant application potential [104]. It was reported that a GCE modified with CNTs, molybdenum disulfide (MoS2), and nickel nanoparticles (NiNPs) (i.e., GCE/CNT/MoS2/NiNPs) exhibited excellent electrochemical performance for glucose detection, with a detection limit as low as 0.197 μM and a rapid response time of only 3 s [84]. In one of our previous studies, a nanocomposite of magnetic nanoparticles (Fe3O4-CS-CD) and MWCNTs, namely Fe3O4-CS-CD/MWCNTs, was employed as a functional material. Subsequently, glucose oxidase (GOx) was immobilized on the modified electrode to construct a novel glucose biosensing platform. This sensor exhibited a relatively wide linear range (40 µM to 1.04 mM), a high sensitivity of 23.59 μA mM−1 cm−2, and a low detection limit of 19.30 µM. Moreover, satisfactory performance was achieved in practical application tests with human serum samples [105]. A study reported the development of an electrochemical sensor based on palladium–nickel nanoparticles-decorated functionalized MWCNTs (Pd–Ni@f-MWCNTs/GCE), which exhibited excellent reproducibility and stability. The sensor demonstrated outstanding sensitivity for glucose detection, with a wide linear range from 0.01 to 1.4 mM and a remarkably low limit of detection of 0.026 μM [106].
In addition to electrochemical detection, glucose can also be detected using optical fiber sensors. CNTs have been shown to significantly enhance the performance of such sensors due to their large surface area and strong adsorption capacity. Li et al. [107] developed a localized surface plasmon resonance (LSPR) optical fiber sensor with a serial quadruple tapered fiber structure. The fiber was functionalized with AuNPs and further modified with GO and MWCNTs to increase the number of binding sites for GOx. The resulting sensor exhibited excellent sensing performance and good stability in the detection of glucose.
4.3.2. Neurotransmitters
Neurotransmitters are endogenous chemical substances that are essential for the efficiency of both the central and peripheral nervous systems, and they play a key role in regulating vital activities such as heartbeat, respiration, mood, and memory. CNT-based sensors have been successfully applied to the analysis and detection of various neurotransmitters [108], including dopamine, serotonin, and adrenaline, norepinephrine, acetylcholine [109], glutathione [110], glutamate, etc.
Dopamine (DA), as a crucial neurotransmitter, plays a vital role in regulating human motor functions, cognitive processes, and behavioral control. Numerous CNT-based sensors have been developed for DA detection. Elugoke et al. [111] have reviewed the CNT-based sensors for dopamine detection that emerged between 2010 and 2019, such as β-CD-MWCNTs/Plu-AuNPs/GCE [112], Nafion/Ni(OH)2-CNT-modified GCE [113], metal oxides NPs/MWCNT nanocomposite [114], Au@NAC-MWCNTs/GCE [115], a simple SWCNTs array immobilized on GCE [116], etc. Subsequently, a variety of materials have been developed, including acid-treated MWCNTs, MoS2/MWCNT/PPy, and MWCNT/AgNPs nanocomposites [66]. Recently, some researchers developed an electrochemical sensor based on a 3D sheet-like NiO/CoO@porous carbon nanosheets/carbon nanotubes/electrochemically reduced graphene oxide composite-modified GCE (NiO/CoO@PCNs/CNTs/erGO/GCE) for the detection of DA. This sensor exhibited a linear detection range of 0.10 μM–22.0 μM with a LOD of 0.045 μM [117].
In addition to electrochemical sensing, CNTs can also serve as fluorescent sensors for the detection of DA. This capability arises from the property that semiconducting SWCNTs exhibit near-infrared (NIR) fluorescence, where the fluorescence intensity is sensitive to the local environment while the emission wavelength is determined by the chirality of the SWCNTs. These features make SWCNTs highly attractive as multifunctional NIR labels and sensing building blocks. Based on this principle, a group of researchers employed chirality-pure (6,5)-SWCNTs, which emit fluorescence at approximately 990 nm, for the detection of DA. The sensor demonstrated a strong fluorescence response to DA and exhibited long-term stability exceeding 14 days [118].
Serotonin (5-hydroxytryptamine, 5-HT) is an important neurotransmitter with multiple physiological regulatory functions [119], and its detection has attracted considerable attention in the field of sensor technology. Due to their unique advantages, CNTs have been successfully employed for the analysis and detection of 5-HT [120]. A research team employed 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride as a cross-linking agent to immobilize monoamine oxidase A (MAO-A) onto MWCNTs, forming an MWCNT/MAO-A composite, which was deposited on a glassy carbon plate as a conductive surface for 5-HT detection. Electrochemical measurements performed in simulated body fluid yielded a detection limit of 2 × 10−7 M [121]. Wu et al. [92] developed a nanocomposite of ferrocene covalently linked gold nanoparticles and MWCNTs (FeC-AuNPs-MWCNT), which was used to modify a SPCE to create a high-density catalyst-conjugated conductive network for electrochemical detection of 5-HT. Compared with the unmodified SPCE, the nanocomposite-modified electrode exhibited a 61-fold enhancement in electrocatalytic activity toward 5-HT and achieved a detection limit of 17 nM. Other researchers reported an electrochemical sensor capable of simultaneously detecting DA and 5-HT, in which oxidized carbon nanotubes (oCNTs) were first pre-modified onto a GCE, followed by electrochemical deposition of curcumin (CM) as a metal-free platform. Compared with the bare electrode, CM-oCNT exhibited a 4.42-fold increase in surface area and a 60-fold increase in anodic current [122]. In another study, a sensor was fabricated by simple electrodeposition of 2-amino-5-mercapto-1,3,4-thiadiazole (AMT) and gold nanoparticles (nAu) onto a functionalized CNT-coated GCE, yielding the nAu/pAMT/f-CNT/GCE configuration. This sensor achieved a detection limit as low as 7.8 nM for 5-HT and showed strong anti-interference capability in the presence of DA [123]. Recently, Misia et al. [71] designed a sensor based on MWCNTs decorated with ligand-free gold nanoparticles synthesized via a metal vapor technique. This sensor efficiently catalyzed the oxidation of 5-HT in plasma, achieving a sensitivity of 6.7 μA μmol L−1 cm−2 and a detection limit of 1 μmol L−1.
CNT-based sensors have played a crucial role in the detection of epinephrine (or adrenaline) and norepinephrine (or noradrenaline) [124]. Several research groups have investigated the electrochemical detection of adrenaline using carbon nanotube-based modified electrodes. A comparison between bare carbon nanotube paste electrodes (BCNTPE) and poly oxalic acid-modified carbon nanotube paste electrodes (POXMCNTPE) demonstrated that the latter exhibited superior detection capability, with a detection limit as low as 3.1 × 10−8 M, while also showing strong anti-interference ability against folic acid and acetaminophen [125]. Kumar et al. [91] synthesized molybdenum disulfide (MoS2) heterostructure-functionalized multi-walled carbon nanotubes (MoS2/MWCNTs) via a hydrothermal method and employed them for adrenaline sensing. The MoS2/MWCNT-modified GCE exhibited good stability, with a LOD of 3.0 μM and a linear response range of 9.9–137.9 μM. In another study, de-bundled single-walled carbon nanotubes (D-SWCNTs) were used to modify SPCE for adrenaline detection. The D-SWCNT/SPCE sensor demonstrated significantly enhanced sensitivity compared to either bare SPCE or bundled SWCNT-modified SPCE, achieving a detection limit of 62.0 nM [126]. Manjunatha and colleagues [127] modified carbon paste electrode (CPE) with MWCNTs, strontium ferrite nanoparticle (SrF/NP), and carbon paste to prepare the sensor SrF/MWCNT/MCPE, which achieved a LOD of 5.8 μM toward adrenaline. Liu et al. [128] designed rice-stick-like Ag/polyoxometalates/nitrogen-doped carbon nanotubes (Ag/POM/N-CNTs) to detect adrenaline; the composite exhibited excellent specificity and stability, achieving a broad linear range (0.40–700 μM) and a low detection limit of 0.3 μM. Some researchers proposed a sensor based on a poly(aspartic acid)-modified carbon nanotube mixed carbon paste electrode (PAGMCNTMCPE), which achieved a detection limit of 0.25 µM for adrenaline. Notably, the sensor maintained selectivity toward adrenaline even in the presence of acetaminophen and folic acid [129]. Zeng’s group developed a carbon fiber microelectrode array (CFMEA) sensor coated with a composite of MWCNTs, graphene, and alizarin polymer (p-AZ/MWCN-GR/CFMEA). This electrode enabled the simultaneous detection of norepinephrine and 5-HT. For norepinephrine, the sensor exhibited a linear response range of 0.08–8 μM, with a detection limit as low as 4.22 nM [130].
Additionally, numerous CNT-based sensors have been developed for the detection of other neurotransmitters. For the detection of acetylcholine, an electrochemical sensor fabricated from a doped polypyrrole/multi-walled carbon nanotube (dPIn-MWCNT) composite exhibited an impressive LOD of 1.27 nM [131], while another sensor based on a braided copper cobaltite/MWCNT hybrid achieved an even lower LOD of 0.8 nM, highlighting its remarkable sensitivity and selectivity [132]. In the case of glutathione detection, several CNT-based sensing platforms have also been reported. Examples include SWCNTs decorated with molybdenum disulfide nanosheets (SWCNTs@MoS2) [133], copper nanoparticles encapsulated within B and N co-doped carbon nanotubes (Cu@BCNNTs) [134], and laser-ablated MWCNT-Au heterostructures (LAMWCNT-Au) [135]. With regard to glutamate detection, copper oxide (CuO) nanostructures physically mixed with MWCNTs were integrated onto SPCE to construct an electrochemical sensor with high sensitivity toward glutamate [136]. In another study, nickel hydroxide nanosheets were combined with MWCNTs to form NH/MWCNT composites, which were subsequently employed to modify the electrode surface. This sensor exhibited not only excellent anti-interference capability but also a remarkably low detection limit, demonstrating great promise for reliable detection of glutamate in complex biological environments [137].
4.3.3. Biomarker
CNT-based sensors have also been widely applied in the detection of biomarkers and specific antigens. For example, in the detection of prostate-specific antigen (PSA), carboxylated carbon nanotubes (COOH-MWCNTs)/polyaniline (PANI)/gold nanoparticles (AuNPs)-modified GCE exhibited satisfied performance. In this work, carboxyl groups were first introduced onto the surface of MWCNTs via oxidation to obtain COOH-MWCNTs, which were then drop-cast onto a polished GCE. Subsequently, aniline was electropolymerized onto the GCE/COOH-MWCNTs surface, followed by electrodeposition of AuNPs onto the PANI layer using chronoamperometry, resulting in the final composite-modified electrode GCE/COOH-MWCNTs/PANI/AuNPs. This sensor integrated the general advantages of MWCNTs, while COOH-MWCNTs can act as a sensitive substrate, as the hydrogen bonding interactions between the amino group of aniline and the carboxylic acid group of MWCNTs facilitate the polymerization of aniline. Owing to the synergistic effects of functional materials, the sensor exhibited enhanced signal amplification, demonstrating good reproducibility, selectivity, and acceptable stability in the electrochemical detection of PSA [65]. Wang et al. [138] fabricated a multifunctional electrochemical sensor by twisting functionalized MWCNTs into helical fiber bundles that mimic the hierarchical structure of muscle. When implanted into mouse tumors, the sensor was capable of detecting H2O2, and it could also measure Ca2+ and glucose levels in the venous blood of cats. Yola’s group developed an electrochemical sensor for the detection of the pro-inflammatory cytokine TNF-α. The sensor featured a gold nanoparticle-encapsulated, thiol-functionalized WMCNTs (AuNPs/S-MWCNTs) platform constructed on a GCE, with bimetallic Ni/Cu MOFs employed as signal amplifiers. This sensor exhibited a linear detection range of 0.01–1.0 pg·mL−1 and a LOD as low as 2.00 fg·mL−1 [68].
A research team fabricated a FET biosensor based on semiconducting carbon nanotube thin films for the detection of β-amyloid (Aβ), a core blood biomarker of Alzheimer’s disease (AD). This sensor exhibited excellent performance, featuring a broad dynamic range and rapid response time, demonstrating its potential for early diagnosis and monitoring of neurodegenerative disorders [139].
4.3.4. Other Biomolecules
In the detection of DNA and RNA, CNT-based sensors have also been widely applied. For example, an electrochemical sensing platform based on a polypyrrole nanowire array functionalized with MWCNTs and Prussian blue (PPY/MWCNTs/PB) was developed to detect microRNA (miRNA). Owing to the excellent catalytic activity and inherent redox properties of the MWCNTs/PB composite, the platform exhibits superior electrocatalytic performance for hydrogen peroxide reduction, enabling sensitive detection of miRNA [140]. In another example, a nanocomposite of MWCNTs and amine-functionalized ionic liquid-reduced graphene oxide (NH2-IL-rGO)-modified GCE was employed for the electrochemical detection of human papillomavirus (HPV16) DNA in patients with HPV16-positive head and neck cancer [141].
A research group reported a CNT-based near-infrared sensor capable of rapidly detecting the SARS-CoV-2 spike protein. This sensor featured a noncovalent functionalization strategy in which the host protein ACE2, possessing high binding affinity for the SARS-CoV-2 spike protein, was passivated onto the surface of SWCNTs. Due to ACE2’s strong affinity for the SWCNT surface, a stable and robust adsorption can be achieved. In this surface-immobilized state, the nanosensor exhibited rapid responsiveness, reaching more than 70% fluorescence turn-on within seconds of exposure to SARS-CoV-2 virus-like particles [142].
4.4. Food Quality Control
In the field of food quality control, attention needs to be paid to the content of active ingredients in raw materials; meanwhile, the residues of certain agricultural residues, food additives, or heavy metals contaminants also need to be tested. CNTs play an important role in both aspects. For example, in our recently published work, we developed a novel glucose biosensor by integrating the advantages of tropine–amino acid-based ionic liquids (TABILs), glucose oxidase, silver nanoparticles (AgNPs), and MWCNTs. MWCNTs were first reacted with AgNO3 and NaBH4 to prepare Ag/MWCNTs nanocomposites. Ag/MWCNTs were then dispersed and drop-cast onto the surface of a GCE. Subsequently, TABILs and a chitosan solution containing GOx were successively drop-cast to fabricate the IL/Ag/MWCNTs/GOx/GCE glucose biosensor. The results demonstrated that the synthesized composite significantly reduced the impedance of bare GCE, facilitated efficient electron transfer, and preserved the bioactivity of GOx. The biosensor exhibited excellent anti-interference capability, reproducibility, repeatability, and stability during electrochemical glucose detection. Furthermore, it showed satisfactory performance in real honey sample analysis, suggesting promising potential for applications in the food industry [143].
Histamine is a potentially harmful biogenic amine found in fish and other aquatic products, its production and accumulation are closely associated with the spoilage process of fish. Therefore, it can serve as an important indicator for evaluating the safety and freshness of fish meat. Zhang et al. [83] developed an LSPR optical fiber sensor for histamine detection, in which AuNPs and GO/MWCNT nanocomposites were used to enhance probe sensitivity, while diamine oxidase enzyme was employed to improve the sensor’s specificity. The sensor exhibited rapid response, high sensitivity, a linear range of 0–1000 µM, and a detection limit of 59.45 µM.
Carbendazim (CBZ) is a broad-spectrum fungicide widely used in agriculture. However, its residues can cause hepatic diseases and chromosomal abnormalities, posing toxic effects to mammals. To detect CBZ residues in apple juice, researchers developed an electrochemical sensor based on β-CD-functionalized carbon nanosheets@carbon nanotubes (CNS@CNT). This sensor took advantage of the large specific surface area of CNS and the excellent electrical conductivity of CNTs, significantly enhancing its electrocatalytic performance. As a result, the sensor exhibited high sensitivity toward CBZ with a low detection limit of 9.4 nM, along with good stability, reproducibility, and practical applicability in real sample analysis [80].
At present, a variety of CNT-based sensors have been successively developed and widely applied in the detection of organophosphorus pesticide methyl parathion (MP), achieving satisfactory results. For example, a silver–zinc oxide (Ag–ZnO) composite-decorated semiconducting single-walled carbon nanotube (s-SWCNT)-based field-effect transistor exhibited a wide linear concentration range of 1 × 10−16 M to 1 × 10−4 M, with a LOD of 0.27 × 10−16 M [144]. A self-assembled monolayer of mono-6-thio-β-cyclodextrin (SH-β-CD) on gold nanoparticles/single-walled carbon nanotubes/glassy carbon electrode (SH-β-CD/AuNPs/SWCNTs/GCE) showed a linear range of 2–80 nM and a LOD of 0.1 nM [145]. A sensor based on carboxyl-functionalized single-walled carbon nanotubes-β-cyclodextrin-modified glassy carbon electrode (ƒ-SWCNT-β-CD/GCE) demonstrated two linear ranges (0.002–0.02 μg mL−1 and 0.02–17.5 μg mL−1) with a LOD of 0.4 ng mL−1 [89]. And a novel electrochemical sensor based on zirconium-based metal–organic framework (UIO-66) nanoparticles-modified single-walled carbon nanotube networks (SCN) achieved a linear concentration range of 0.01–10 μM, with a LOD of 8.05 nM [87].
Vanillin (VL) is an additive commonly found in various food products and daily necessities. Although it exhibits certain pharmacological effects, VL also has some side effects on organisms. Excessive intake of VL may cause damage to the human liver and kidneys, making it necessary to perform quantitative detection and analysis of vanillin in food. Early studies employed commercially available disposable multi-walled carbon nanotube screen-printed electrodes (CNT-SPEs) for the detection and determination of vanillin in natural vanilla [146]. However, this method exhibited low sensitivity and a relatively high LOD. To address these limitations, subsequent researchers developed an electrochemically polymerized glutamic acid (GA)-functionalized multi-walled carbon nanotube and graphite (GT) composite paste sensor (CPS), denoted as Poly (GA)/(MWCNTs-GT) CPS, for the determination of VL in food. This sensor combines the catalytic activity, conductivity, high active surface sites, together with the outstanding mechanical, chemical, electronic, and thermal stability of both MWCNTs and graphite. Additionally, due to π-π stacking, electronic effects, and hydrophobic interactions, the CPS demonstrates simple and rapid analytical characteristics. The results indicated that Poly (GA)/(MWCNTs-GT) CPS achieved satisfactory recovery rates and RSDs in various vanilla-containing food products [147].
A research team fabricated a SPCE modified with 3D CuO nanoflowers and NH2-CNTs for the detection of the food preservative tert-butylhydroquinone (TBHQ). The integration of CuO and CNTs significantly enhanced the overall electrical conductivity of the sensor, thereby improving its detection performance. The resulting sensor exhibited an ultra-low detection limit and exceptionally high sensitivity. When applied to real sample analysis, such as edible oils and water samples, the sensor demonstrated excellent reproducibility, with a maximum RSD of only 2.71% [90].
CNT-based sensors have also been successfully applied in detecting heavy metal contaminants in food. For instance, Wang et al. [85] developed an electrochemical sensor based on a composite of amine-functionalized Zr (IV) MOF (UiO-66-NH2) and MWCNTs, denoted as UiO-66-NH2@MWCNTs/GCE, for the detection of cadmium ions (Cd2+) in meat samples. In this design, UiO-66-NH2 offered a unique octahedral structure and amine groups for effective target interaction, while MWCNTs contributed excellent electron transport properties. The synergistic effect between the two materials significantly enhanced the electrode performance, enabling a linear detection range of 0.5–170 μg/L and a low LOD of 0.2 μg/L.
In addition to detecting harmful components in food, CNT-based sensors can also be employed to identify toxic substances in condiments. For instance, a composite material of carboxylated MWCNTs (cMWCNTs) and MOF-199 (cMWCNT/MOF-199) was synthesized and subsequently used to modify a GCE. The resulting electrochemical sensor demonstrated high sensitivity for the detection of chloropropanols, specifically 3-chloro-1,2-propanediol, in soy sauce, achieving a detection limit as low as 4.3 × 10−10 mol L−1 with recovery rates ranging from 96% to 108% [38].
4.5. Gases Detection
CNT-based sensors have achieved remarkable success in gas detection, encompassing applications in industrial process monitoring, environmental gas analysis, and the detection of specific biomarkers in human breath [148], with targets including chlorine (Cl2) [149], ammonia gas (NH3) [150], methanol (CH3OH) [151], carbon monoxide (CO) [152], nitric oxide (NO) [153], nitrogen dioxide (NO2) [154], ethanol (CH3CH2OH), acetone (C3H6O) [155], as well as the vapor of chemical warfare agents (such as methyl methylphosphonate and dichloromethane) [156].
Hydrogen is a highly reactive and explosive hazardous gas; therefore, its timely monitoring is of critical importance. Numerous CNT-based hydrogen sensors have been developed to date, such as acid-treated CNT yarns [157], NiN4S-doped SWCNTs [158], and Pd-functionalized CNTs. In particular, studies have shown that Pd-coated CNTs exhibit enhanced sensitivity to H2 compared with pristine CNTs [159,160]. Recently, Son et al. [161] reported a wearable hydrogen early-warning platform by employing a dual-coiled structure of palladium oxide nanoparticles (PdO NPs) and spinnable CNT buckypapers, yielding a yarn-type hydrogen sensing platform (HGSP) with an ultrafast response time of only 2 s and a sensitivity as high as 1198%. Similarly, the Girma group [162] fabricated single-layer semiconducting SWCNT (sc-SWNT) films using chemical cross-linkers, which were further modified with Pd nanoparticles to obtain an optimized sc-SWNT hydrogen sensor. This device not only demonstrated selectivity toward hydrogen but also exhibited rapid response and recovery times of 10 and 3 s, respectively. Furthermore, some researchers investigated CNT film/graphene heterostructure H2 sensors modified with catalytic platinum nanoparticles via atomic layer deposition (Pt-NPs/CNTs/Gr). By systematically comparing CNT films composed of SWCNTs, double-walled carbon nanotubes (DWCNTs), and MWCNTs of different diameters, they found that Pt-NPs/MWCNTs/Gr exhibited the best hydrogen sensing performance [163].
Recently, a research team developed an advanced CNT-based gas sensor by in situ polymerizing polypyrrole (PPy) onto the sidewalls of MWCNTs to obtain MCNT@PPy. Subsequently, cobalt tetrakis (β-trifluoromethylphenoxy) phthalocyanine (TfmpoPcCo) was adsorbed onto the MCNT@PPy surface, forming a MCNT@PPy/TfmpoPcCo composite sensor. This sensor exhibited a rapid response and an ultra-low detection limit of 11 ppb for NH3 at 20 °C, demonstrating its strong potential for environmental monitoring applications [30]. Liu et al. [164] employed semiconducting carbon nanotubes (s-CNTs) as the channel material and catalytic metals and as the gate electrodes in a FET-type gas sensor for formaldehyde (HCHO) detection. The CNT-based gas sensor achieved a detection limit as low as 20 ppb at room temperature, which could be further improved to 10 ppb upon heating. Moreover, the device exhibited excellent reproducibility, stability, and recovery performance. In addition, a research team employed CNT-FETs to monitor NO2. In this design, the channel, dielectric layer, and floating gate were composed of CNTs, Y2O3, and Pd/WS2, respectively, enabling the detection of NO2 at concentrations as low as 20 ppb [165].
Beyond environmental monitoring, CNT-based sensors also show great potential for detecting volatile organic compounds (VOCs) or biomarkers in exhaled human breath. Thousands of VOCs can be detected in human breath [166], many of which are closely associated with the onset of certain diseases, making breath analysis a promising non-invasive diagnostic tool. For example, detecting NO in exhaled breath plays an important role in the auxiliary diagnosis and evaluation of treatment responses in clinical conditions such as asthma, rhinitis, and gastrointestinal diseases. In this context, CNT-based gas sensors have been successfully employed for the detection of characteristic gases. Jeong et al. [167] developed a highly sensitive NO gas sensor based on SWNTs, achieving a detection limit at the parts-per-billion (ppb) level under ambient conditions. The sensor exhibited excellent selectivity toward NO, with negligible interference from CO and volatile organic compounds. Nag et al. [168] grafted buckminsterfullerene (C60) onto MWNTs and reduced graphene oxide (rGO) to construct a novel conductive architecture, in which the presence of nanoscale C60 at the junctions allows easy disruption of the network. This design significantly enhances the sensitivity and selectivity of chemoresistive vapor sensors toward seven biomarkers: ethanol, methanol, acetone, chloroform, benzene, toluene, cyclohexane, and water.
Currently, carbon nanotube (CNT)-based electronic noses (E-noses) have become a popular research area. An E-nose is an artificial device inspired by the olfactory system [169,170], which combines an array of chemical sensors with pattern recognition techniques, and can serve as a low-cost tool for detecting and identifying gases or volatile compounds. CNT-based E-noses have been successfully applied for various diseases, particularly as an aid in the diagnosis of respiratory and pulmonary disorders [171].
4.6. Strain Sensing and Novel Sensors
At present, CNTs have also attracted extensive attention in the field of strain sensing [172,173] and flexible wearable devices [174], owing to their outstanding mechanical and electrical properties. Their high aspect ratio, exceptional tensile strength, and excellent piezoresistive response enable CNT networks to convert mechanical deformation into detectable electrical signals with high sensitivity and reliability. When incorporated into flexible substrates, CNT-based strain sensors exhibit superior stretchability, lightweight characteristics, and conformability to complex surfaces, making them ideal for wearable electronics. Furthermore, their intrinsic flexibility and chemical stability provide long-term durability under repeated mechanical stress. These unique advantages allow CNT-based wearable strain sensors to monitor subtle human motions, physiological signals, and biomechanical activities, thereby offering great potential for applications in personalized healthcare, human–machine interfaces, and soft robotics. For instance, the resistive sensors are typically designed with a sandwich structure, consisting of an encapsulation layer, a conductive layer, and an electrode layer. Some research groups incorporate CNTs into the conductive layer (or sensing layer) to enhance sensing performance. Guo et al. [175] fabricated a flexible pressure sensor with a sandwich structure using a layer-by-layer self-assembly technique. The piezoresistive sensing layer was composed of a graphene oxide/hydroxyl-functionalized CNTs/bovine serum albumin nanocomposite, where GO and CNTs served as the primary conductive components. Additionally, in resistive sensors there are CNT-filled silicone rubber composites as conductive materials [176], interlayer-structured sensors fabricated by embedding SWNTs into 3D polydimethylsiloxane (PDMS) films [177], and porous microstructured piezoresistive materials made from MWCNTs and PDMS [178].
To conclude, applications of CNT-based sensors are summarized in Table 2 (where ED = electrical detection, OD = optical detection, CA = colorimetric analysis, MS = mechanical sensing).

Table 2.
Applications of CNTs in chemical sensing mentioned in this review.
5. SWOT Analysis and Emerging Trends
5.1. Strengths
CNTs have a huge specific surface area and excellent electrical properties and are extremely sensitive to changes in surface charge. They can achieve ultra-low detection limits for the analyte (even at the single-molecule level). Its electrical performance is very good, with high electron mobility and edge planar structure that can effectively promote electron transfer, enhance electrochemical response signals, and promote high signal-to-noise ratio. It is easy to functionalize and can flexibly modify various recognition elements such as antibodies, aptamers, enzymes, polymers, etc., on the surface through covalent or noncovalent means, achieving specific detection of different target substances. It has diverse transduction mechanisms; it can be detected based on both electrical signals (such as resistance, current, and field-effect transistor characteristics) and optical signals (such as fluorescence quenching/enhancement), with flexible applications. The nanoscale size makes it easy to construct high-density sensor arrays, which can be combined with microfluidic technology to develop miniaturized and portable lab-on-a-chip devices [179]. Its excellent mechanical performance makes it very suitable for developing flexible and stretchable wearable sensors that can be closely adhered to human skin for long-term monitoring [180,181].
5.2. Weaknesses
Despite the remarkable success of CNTs in chemical sensing, challenges hinder their large-scale deployment, and practical translation still cannot be ignored. One of the most significant issues is synthesis of CNTs. The diameter, length, and metallicity/semiconductor ratio of CNTs are difficult to precisely control, resulting in differences in sensor performance between different batches and difficulties in standardized production. Current synthetic techniques such as chemical vapor deposition, arc discharge, laser ablation, vapor-phase growth, etc. [182], still cannot guarantee the material heterogeneity. CNTs are often synthesized as mixtures of metallic and semiconducting tubes, with variations in size, chirality, defect density, and even purity, resulting in unstable sensor performance, poor reproducibility, and limited standardization across different devices. Moreover, most synthetic routes are not environmentally friendly or economical enough [183]. Finally, the controllable synthesis cost of high-purity, specific-scale CNTs is still high, and large-scale, low-cost standardized preparation is still a bottleneck for industrialization.
Another limitation is the dispersion, stability, and environmental sensitivity of CNTs, which can also affect their sensing performance. CNTs tend to aggregate due to strong van der Waals interactions [184], which reduces their accessible surface area and limits active sites for analyte interaction. Moreover, their high surface-to-volume ratio makes them highly susceptible to environmental factors such as humidity, temperature, and pH. While CNTs possess high surface area and strong adsorption capability, these same properties can cause nonspecific binding and sensor drift in complex matrices such as biological fluids or environmental samples, which compromise the long-term stability and reliability of CNT-based sensors. Therefore, the selectivity, long-term stability, and reliability of CNT-based sensors require attention, and the anti-pollution ability of sensors in real complex samples such as blood and sewage need to be improved. Besides that, the integration of CNTs into device architectures—whether in flexible substrates, wearable formats, or miniaturized platforms—still requires optimization to ensure mechanical robustness, reproducibility, and cost-effectiveness. Finally, the toxicity and environmental impact of CNTs, especially during sensor disposal, have raised concerns that must be addressed through sustainable design and lifecycle assessment. Specifically, CNTs as nanofillers may cause potential health risks due to their fibrous shape and persistence, which resemble asbestos-like effects in some studies [185]. Their possible cytotoxicity, inflammatory responses, and bioaccumulation raise safety concerns for large-scale use, especially in biomedical and wearable applications [186]. Thus, systematic toxicological evaluation, safe-by-design strategies, and the development of biocompatible or biodegradable CNT composites are necessary to mitigate these risks. Moreover, the lack of standardized protocols for CNT purification, dispersion, and functionalization limits the comparability and scalability of reported sensing systems.
5.3. Opportunities
Precision medicine and personalized health monitoring are currently hot topics. The market’s demand for real-time, continuous, and non-invasive health monitoring has surged, driving the rapid development of various CNT sensors. The demand for real-time detection is undoubtedly enormous, providing a broad market for CNT sensors in areas such as rapid disease diagnosis, home healthcare, on-site food safety screening, and environmental emergency monitoring, where there is a need for portable and fast devices. AI algorithms can be used to process complex sensor data and improve recognition accuracy and anti-interference ability [187]. The demand for high sensitivity and rapid detection of air pollutants, heavy metals in water quality, chemical warfare agents, explosives, etc., is an important application scenario for CNT sensors. Frontier interdisciplinary fields are rapidly developing; the deep integration of nanotechnology, biotechnology, information technology, and materials science provides unprecedented opportunities for the development of a new generation of multifunctional and intelligent sensing systems.
Moreover, the integration of CNTs with microelectromechanical systems (MEMS) provides a feasible approach for developing miniaturized sensors with small size, low power consumption, excellent performance, and scalable mass production [188]. MEMS technology can provide robust platforms with precise microfabrication, allowing CNTs to be patterned, aligned, or assembled directly on devices [189]. This integration not only enhances mechanical stability and reproducibility but also facilitates seamless connection with existing microelectronics, broadening the range of real-world applications [190].
5.4. Threats
Currently, there is fierce competition among emerging nanomaterials. Emerging nanomaterials such as graphene, transition metal sulfides, MXenes, and various organic frameworks are also rapidly developing, which may provide better performance or lower costs. Traditional detection methods (e.g., enzyme-linked immunosorbent assay, ELISA; polymerase chain reaction, PCR; etc.) and commercialized electrochemical sensor technologies are stable and cost-effective, forming a strong market inertia. Researchers need strong reasons to switch to using new sensors. As medical diagnostic sensors, they also face strict supervision from drug regulatory agencies in various countries, with lengthy clinical validation and approval processes, high costs, and extremely high commercialization barriers. Patent and intellectual property barriers are also important threats, as core material preparation, functionalization methods, and device design may be protected by a few companies’ or institutions’ patents, forming a technological monopoly. In addition, stricter regulations on the handling, manufacturing, and disposal of CNTs may emerge as more evidence of their potential health and environmental risks becomes available [191]. Compliance with occupational exposure limits, biosafety standards, and environmental regulations could increase production costs and slow down commercialization. Therefore, addressing the safety issues of CNT-based nanofillers is not only a scientific challenge but also a regulatory and industrial bottleneck for future deployment.
5.5. Emerging Trends
Looking forward, several emerging trends are reshaping the landscape of CNT-based sensors. First, hybrid nanomaterials are increasingly being explored, where CNTs are combined with metals, metal oxides, polymers, or 2D nanomaterials. These composites exploit synergistic effects to enhance sensitivity, selectivity, and catalytic activity. Second, advanced functionalization strategies, such as bio-orthogonal click chemistry and molecular imprinting, are opening avenues for highly specific recognition elements tailored to target analytes. Third, integration with microelectronics and wearable technologies is driving the development of portable, low-power, and wireless CNT sensor platforms, enabling real-time and on-site monitoring. Finally, the emergence of artificial intelligence and machine learning in synthesis of CNTs [192] and data analysis is expected to play a pivotal role. By addressing issues such as signal drift, cross-sensitivity, and complex background interference, AI-driven approaches can significantly improve the reliability and interpretability of CNT sensor outputs. Together, these advances suggest that while substantial challenges remain, CNT-based chemical sensors are poised to transition from laboratory demonstrations toward impactful applications in healthcare, environmental monitoring, and industrial safety.
To effectively exploit these opportunities, we provide here the following possible guidelines: 1. Alignment with MEMS platforms. Developing standardized protocols for CNT integration into MEMS architectures (e.g., microcantilevers, resonators, lab-on-chip) will allow scalable, high-density sensor arrays; 2. System-level integration. Coupling CNT sensors with low-power microelectronics and wireless communication modules is necessary for wearable and portable devices, enabling real-time data transmission; 3. AI-driven design and analytics. AI and machine learning should not only be used for post-processing sensor data, but also actively guide CNT synthesis optimization, device calibration, and self-compensation for environmental drift. Together, these guidelines provide a concrete direction to align CNT research with application-driven requirements, pushing the limits of performance and accelerating the translation of CNT-based chemical sensors into practical, scalable technologies.
6. Conclusions
Owing to their high specific surface area, excellent electrical conductivity, and chemical stability, carbon nanotubes have emerged as highly promising materials in the field of sensing. CNT-based sensors exhibit a wide range of application formats, including electrical, optical, mechanical, thermal, mass-sensitive types, etc., and have demonstrated unparalleled advantages in diverse fields such as environmental monitoring, drug analysis, healthcare, food quality control, gas detection, and strain sensing. Although pristine CNTs face inherent limitations, including poor dispersion, low selectivity, and moderate sensitivity, these challenges can be effectively addressed by covalent or noncovalent functionalization and hybridization with functional materials such as graphene, cyclodextrins, MOFs, and metal nanoparticles, leading to the development of more versatile and high-performance sensing platforms. Moreover, the integration of CNT-based sensors with microelectronics and wearable technologies enables rapid real-time detection, further expanding their practical applications in everyday life. Looking ahead, the combination of CNT sensors with frontier disciplines such as artificial intelligence, machine learning, biotechnology, and advanced nanomaterials is expected to usher in a new era of chemical sensing, unlocking unprecedented capabilities and applications.
Author Contributions
Conceptualization, J.T.; investigation, and writing—original draft, J.T.; software, R.L.; writing—editing and review, S.M.; visualization and data curation, J.L.; supervision, S.Y.; project administration, S.Y.; funding acquisition, S.Y. All authors have read and agreed to the published version of the manuscript.
Funding
This review was funded by the National Natural Science Foundation of China (82273814), Fundamental Research Funds for the Central Universities/Sichuan University-Luzhou Science and Technology Innovation Platform Construction Project (2022CDLZ-20) and supported by Sichuan Provincial Department of Science and Technology (No. 2025ZNSFSC1729) and Open Fund of Key Laboratories of Fine Chemicals and Surfactants in Sichuan Provincial Universities (No. 2024JXY10).
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
The following abbreviations are used in this manuscript:
| AD | Alzheimer’s disease |
| Ag–ZnO | Silver–zinc oxide |
| Ag-ZnONPs | Silver-doped zinc oxide nanoparticles |
| AuNPs | Gold nanoparticles |
| CBZ | Carbendazim |
| CNS | Carbon nanosheets |
| CPE | Carbon paste electrode |
| COOH–MWCNTs | Carboxyl-functionalized multi-walled carbon nanotubes |
| CTAB | Cetyltrimethylammonium bromide |
| ChLa | Coriolus hirsuta laccase |
| CDs | Cyclodextrins |
| DWCNTs | Double-walled carbon nanotubes |
| ELISA | Enzyme-linked immunosorbent assay |
| E-nose | Electronic noses |
| FET | Field-effect transistor |
| GA | Gallic acid |
| GCE | Glassy carbon electrode |
| GO | Graphene oxide |
| Lac | Laccase enzyme |
| LSPR | Localized surface plasmon resonance |
| MEMS | Microelectromechanical systems |
| MOFs | Metal–organic frameworks |
| MoS2 | Molybdenum disulfide |
| MWCNTs | Multi-walled carbon nanotubes |
| NEMS | Nanoelectromechanical systems |
| NH2-CNTs | Amine-functionalized carbon nanotubes |
| PCM | Paracetamol |
| PCR | Polymerase chain reaction |
| PDMS | Polydimethylsiloxane |
| SPCE | Screen-printed carbon electrode |
| SPE/SCPE | Screen-printed electrodes |
| SWCNTs | Single-walled carbon nanotube |
| s-SWCNT | Semiconducting single-walled carbon nanotube |
| SWOT | Strengths-weaknesses-opportunities-threats |
| TBHQ | Tert-butylhydroquinone |
| VL | Vanillin |
| VOC | Volatile organic compounds |
References
- Ullah, N.; Mansha, M.; Khan, I.; Qurashi, A. Nanomaterial-based optical chemical sensors for the detection of heavy metals in water: Recent advances and challenges. TrAC Trend. Anal. Chem. 2018, 100, 155–166. [Google Scholar] [CrossRef]
- Parvin, N.; Joo, S.W.; Jung, J.H.; Mandal, T.K. Unlocking the Future: Carbon Nanotubes as Pioneers in Sensing Technologies. Chemosensors 2025, 13, 225. [Google Scholar] [CrossRef]
- Yao, X.; Zhang, Y.; Jin, W.; Hu, Y.; Cui, Y. Carbon nanotube field-effect transistor-based chemical and biological sensors. Sensors 2021, 21, 995. [Google Scholar] [CrossRef]
- Radushkevich, L.V.; Lukyanovich, V.M. About the structure of carbon formed by thermal decomposition of carbon monoxide on iron substrate. J. Phys. Chem. 1952, 26, 88–95. [Google Scholar]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Rathinavel, S.; Priyadharshini, K.; Panda, D. A review on carbon nanotube: An overview of synthesis, properties, functionalization, characterization, and the application. Mater. Sci. Eng. B 2021, 268, 115095. [Google Scholar] [CrossRef]
- Hou, P.X.; Zhang, F.; Zhang, L.; Liu, C.; Cheng, H.M. Synthesis of carbon nanotubes by floating catalyst chemical vapor deposition and their applications. Adv. Funct. Mater. 2022, 32, 2108541. [Google Scholar] [CrossRef]
- Zahra, A.; Razzokov, J.; Kashif, M.; Khalilov, U.; Li, H.; Shahzad, A.; Ren, G.; Vakili-Nezhaad, G. Strained Mechanical and Fracture Analyses of Armchair-Chiral-Zigzag-Based Carbon Nanotubes Using Molecular Dynamics Simulations. ACS Omega 2024, 9, 48055–48069. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Oh, Y.; Shin, J.; Yang, M.; Shin, N.; Shekhar, S.; Hong, S. Nanoscale mapping of carrier mobilities in the ballistic transports of carbon nanotube networks. ACS Nano 2022, 16, 21626–21635. [Google Scholar] [CrossRef]
- Onyancha, R.B.; Aigbe, U.O.; Ukhurebor, K.E.; Muchiri, P.W. Facile synthesis and applications of carbon nanotubes in heavy-metal remediation and biomedical fields: A comprehensive review. J. Mol. Struct. 2021, 1238, 130462. [Google Scholar] [CrossRef]
- Saifuddin, N.; Raziah, A.Z.; Junizah, A.R. Carbon nanotubes: A review on structure and their interaction with proteins. J. Chem. 2013, 2013, 676815. [Google Scholar] [CrossRef]
- Chronopoulos, D.D.; Saini, H.; Tantis, I.; Zbořil, R.; Jayaramulu, K.; Otyepka, M. Carbon nanotube based metal–organic framework hybrids from fundamentals toward applications. Small 2022, 18, 2104628. [Google Scholar] [CrossRef]
- Luo, S.X.L.; Swager, T.M. Chemiresistive sensing with functionalized carbon nanotubes. Nat. Rev. Method Primers 2023, 3, 73. [Google Scholar] [CrossRef]
- Wang, R.; Sun, L.; Zhu, X.; Ge, W.; Li, H.; Li, Z.; Zhang, H.; Huang, Y.; Li, Z.; Zhang, Y.; et al. Carbon nanotube-based strain sensors: Structures, fabrication, and applications. Adv. Mater. Technol. 2023, 8, 2200855. [Google Scholar] [CrossRef]
- Ferrier, D.C.; Honeychurch, K.C. Carbon nanotube (CNT)-based biosensors. Biosensors 2021, 11, 486. [Google Scholar] [CrossRef]
- Wu, Y.; Zhao, X.; Shang, Y.; Chang, S.; Dai, L.; Cao, A. Application-driven carbon nanotube functional materials. ACS Nano 2021, 15, 7946–7974. [Google Scholar] [CrossRef]
- Murjani, B.O.; Kadu, P.S.; Bansod, M.; Vaidya, S.S.; Yadav, M.D. Carbon nanotubes in biomedical applications: Current status, promises, and challenges. Carbon Lett. 2022, 32, 1207–1226. [Google Scholar] [CrossRef] [PubMed]
- Javahershenas, R.; Soloshonok, V.A.; Klika, K.D.; Jervis, P.J. Carbon nanotubes as heterogeneous catalysts for the multicomponent reaction synthesis of heterocycles. Carbon Lett. 2025, 35, 75–105. [Google Scholar] [CrossRef]
- Hu, X.; Bao, X.; Zhang, M.; Fang, S.; Liu, K.; Wang, J.; Liu, R.; Kim, S.H.; Baughman, R.H.; Ding, J. Recent advances in carbon nanotube-based energy harvesting technologies. Adv. Mater. 2023, 35, 2303035. [Google Scholar] [CrossRef]
- Maheswaran, R.; Shanmugavel, B.P. A critical review of the role of carbon nanotubes in the progress of next-generation electronic applications. J. Electron. Mater. 2022, 51, 2786–2800. [Google Scholar] [CrossRef]
- Rahamathulla, M.; Bhosale, R.R.; Osmani, R.A.; Mahima, K.C.; Johnson, A.P.; Hani, U.; Ghazwani, M.; Begum, M.Y.; Alshehri, S.; Ghoneim, M.M. Carbon nanotubes: Current perspectives on diverse applications in targeted drug delivery and therapies. Materials 2021, 14, 6707. [Google Scholar] [CrossRef]
- Aslam, M.M.-A.; Kuo, H.-W.; Den, W.; Usman, M.; Sultan, M.; Ashraf, H. Functionalized carbon nanotubes (CNTs) for water and wastewater treatment: Preparation to application. Sustainability 2021, 13, 5717. [Google Scholar] [CrossRef]
- Salah, L.S.; Ouslimani, N.; Bousba, D.; Huynen, I.; Danlée, Y. Carbon nanotubes (CNTs) from synthesis to functionalized (CNTs) using conventional and new chemical approaches. J. Nanomater. 2021, 2021, 4972770. [Google Scholar] [CrossRef]
- Chatterjee, S.; Castro, M.; Feller, J.F. Tailoring selectivity of sprayed carbon nanotube sensors (CNT) towards volatile organic compounds (VOC) with surfactants. Sens. Actuators B Chem. 2015, 220, 840–849. [Google Scholar] [CrossRef]
- Chen, J.; Liu, B.; Gao, X.; Xu, D. A review of the interfacial characteristics of polymer nanocomposites containing carbon nanotubes. RSC Adv. 2018, 8, 28048–28085. [Google Scholar] [CrossRef] [PubMed]
- Kyriakidou, K.; Brasinika, D.; Trompeta, A.; Bergamaschi, E.; Karoussis, I.; Charitidis, C. In vitro cytotoxicity assessment of pristine and carboxyl-functionalized MWCNTs. Food Chem. Toxicol. 2020, 141, 111374. [Google Scholar] [CrossRef]
- Kharlamova, M.V.; Paukov, M.; Burdanova, M.G. Nanotube functionalization: Investigation, methods and demonstrated applications. Materials 2022, 15, 5386. [Google Scholar] [CrossRef]
- Liu, D.; Shi, L.; Dai, Q.; Lin, X.; Mehmood, R.; Gu, Z.; Dai, L. Functionalization of carbon nanotubes for multifunctional applications. Trends Chem. 2024, 6, 186–210. [Google Scholar] [CrossRef]
- Abreu, B.; Pires, A.S.; Guimarães, A.; Fernandes, R.M.F.; Oliveira, I.S.; Marques, E.F. Polymer/surfactant mixtures as dispersants and non-covalent functionalization agents of multiwalled carbon nanotubes: Synergism, morphological characterization and molecular picture. J. Mol. Liq. 2022, 347, 118338. [Google Scholar] [CrossRef]
- Gai, S.; Wang, B.; Wang, X.; Zhang, R.; Miao, S.; Wu, Y. Ultrafast NH3 gas sensor based on phthalocyanine-optimized non-covalent hybrid of carbon nanotubes with pyrrole. Sens. Actuators B Chem. 2022, 357, 131352. [Google Scholar] [CrossRef]
- Mostafa, M.H.; Ali, E.S.; Darwish, M.S.A. Recent developments of conductive polymers/carbon nanotubes nanocomposites for sensor applications. Polym.-Plast. Technol. Mater. 2022, 61, 1456–1480. [Google Scholar] [CrossRef]
- Fritea, L.; Banica, F.; Costea, T.O.; Moldovan, L.; Dobjanschi, L.; Muresan, M.; Cavalu, S. Metal nanoparticles and carbon-based nanomaterials for improved performances of electrochemical (Bio) sensors with biomedical applications. Materials 2021, 14, 6319. [Google Scholar] [CrossRef]
- Aykaç, A.; Gergeroglu, H.; Beşli, B.; Akkaş, E.; Yavaş, A.; Güler, S.; Güneş, F.; Erol, M. An overview on recent progress of metal oxide/graphene/CNTs-based nanobiosensors. Nanoscale Res. Lett. 2021, 16, 65. [Google Scholar] [CrossRef]
- Fazio, E.; Spadaro, S.; Corsaro, C.; Neri, G.; Leonardi, S.G.; Neri, F.; Lavanya, N.; Sekar, C.; Donato, N.; Neri, G. Metal-oxide based nanomaterials: Synthesis, characterization and their applications in electrical and electrochemical sensors. Sensors 2021, 21, 2494. [Google Scholar] [CrossRef] [PubMed]
- Kelich, P.; Jeong, S.; Navarro, N.; Adams, J.; Sun, X.; Zhao, H.; Landry, M.P.; Vuković, L. Discovery of DNA–carbon nanotube sensors for serotonin with machine learning and near-infrared fluorescence spectroscopy. ACS Nano 2021, 16, 736–745. [Google Scholar] [CrossRef]
- Nasiri, F.; Rounaghi, G.H.; Ashraf, N.; Deiminiat, B. A new electrochemical sensing platform for quantitative determination of diclofenac based on gold nanoparticles decorated multiwalled carbon nanotubes/graphene oxide nanocomposite film. Int. J. Environ. Anal. Chem. 2021, 101, 153–166. [Google Scholar] [CrossRef]
- Xu, Y.; Xie, X.; Huang, H.; Wang, Y.; Yu, J.; Hu, Z. Encapsulated core–sheath carbon nanotube–graphene/polyurethane composite fiber for highly stable, stretchable, and sensitive strain sensor. J. Mater. Sci. 2021, 56, 2296–2310. [Google Scholar] [CrossRef]
- Han, S.; Ding, Y.; Teng, F.; Yao, A.; Leng, Q. Determination of chloropropanol with an imprinted electrochemical sensor based on multi-walled carbon nanotubes/metal-organic framework composites. RSC Adv. 2021, 11, 18468–18475. [Google Scholar] [CrossRef] [PubMed]
- Pour, B.H.; Haghnazari, N.; Keshavarzi, F.; Ahmadi, E.; Zarif, B.R. High sensitive electrochemical sensor for imatinib based on metal-organic frameworks and multiwall carbon nanotubes nanocomposite. Microchem. J. 2021, 165, 106147. [Google Scholar] [CrossRef]
- Sinha, N.; Ma, J.; Yeow, J.T.W. Carbon nanotube-based sensors. J. Nanosci. Nano Technol. 2006, 6, 573–590. [Google Scholar] [CrossRef]
- Saino, H.V.; Remya, N.; Baiju, G.N.; Hanajiri, T.; Maekawa, T.; Yoshida, Y.; Sakthi Kumar, D. Sensors based on carbon nanotubes and their applications: A review. Curr. Nanosci. 2010, 6, 331–346. [Google Scholar] [CrossRef]
- Tian, T.; Yin, H.; Zhang, L.; Zhu, M.; Ma, D.; Shao, F.; Hu, N.; Yang, Z.; Zhang, Y.; Su, Y. Gas sensing performance and charge-transfer mechanism of semiconducting single-walled carbon nanotubes. Appl. Surf. Sci. 2023, 609, 155357. [Google Scholar] [CrossRef]
- Escobar-Teran, F.; Perrot, H.; Sel, O. Ion dynamics at the single wall carbon nanotube based composite electrode/electrolyte interface: Influence of the cation size and electrolyte pH. J. Phys. Chem. C 2019, 123, 4262–4273. [Google Scholar] [CrossRef]
- Feller, J.F.; Gatt, N.; Kumar, B.; Castro, M. Selectivity of chemoresistive sensors made of chemically functionalized carbon nanotube random networks for volatile organic compounds (VOC). Chemosensors 2014, 2, 26–40. [Google Scholar] [CrossRef]
- Dai, C.; Liu, Y.; Wei, D. Two-dimensional field-effect transistor sensors: The road toward commercialization. Chem. Rev. 2022, 122, 10319–10392. [Google Scholar] [CrossRef] [PubMed]
- Jing, X.; Illarionov, Y.; Yalon, E.; Zhou, P.; Grasser, T.; Shi, Y.; Lanza, M. Engineering field effect transistors with 2D semiconducting channels: Status and prospects. Adv. Funct. Mater. 2020, 30, 1901971. [Google Scholar] [CrossRef]
- Hughes, K.J.; Iyer, K.A.; Bird, R.E.; Ivanov, J.; Banerjee, S.; Georges, G.; Zhou, Q.A. Review of carbon nanotube research and development: Materials and emerging applications. ACS Appl. Nano Mater. 2024, 7, 18695–18713. [Google Scholar] [CrossRef]
- Sacco, L.; Forel, S.; Florea, I.; Cojocaru, C.-S. Ultra-sensitive NO2 gas sensors based on single-wall carbon nanotube field effect transistors: Monitoring from ppm to ppb level. Carbon 2020, 157, 631–639. [Google Scholar] [CrossRef]
- Forel, S.; Sacco, L.; Castan, A.; Florea, I.; Cojocaru, C.S. Simple and rapid gas sensing using a single-walled carbon nanotube field-effect transistor-based logic inverter. Nanoscale Adv. 2021, 3, 1582–1587. [Google Scholar] [CrossRef]
- Schroeder, V.; Savagatrup, S.; He, M.; Lin, S.; Swager, T.M. Carbon nanotube chemical sensors. Chem. Rev. 2018, 119, 599–663. [Google Scholar] [CrossRef]
- Ackermann, J.; Metternich, J.T.; Herbertz, S.; Kruss, S. Biosensing with fluorescent carbon nanotubes. Angew. Chem. Int. Edit. 2022, 61, e202112372. [Google Scholar] [CrossRef]
- Wang, Y.; Cui, Z.; Zhang, X.; Zhu, Y.; Chen, S.; Hu, H. Excitation of surface plasmon resonance on multiwalled carbon nanotube metasurfaces for pesticide sensors. ACS Appl. Mater. Interfaces 2020, 12, 52082–52088. [Google Scholar] [CrossRef] [PubMed]
- Fang, W.; Zhang, Z.; Miao, N.; Yang, R.; Xiao, Z.; Cao, Y.; Xin, W. Highly uniform fabrication of gold nanoparticles on carbon nanotube sheets for sensors based on surface-enhanced Raman spectroscopy with improved reproducibility. ACS Appl. Nano Mater. 2023, 6, 9949–9957. [Google Scholar] [CrossRef]
- Hierold, C.; Jungen, A.; Stampfer, C.; Helbling, T. Nano electromechanical sensors based on carbon nanotubes. Sens. Actuators A Phys. 2007, 136, 51–61. [Google Scholar] [CrossRef]
- Stampfer, C.; Jungen, A.; Linderman, R.; Obergfell, D.; Roth, S.; Hierold, C. Nano-electromechanical displacement sensing based on single-walled carbon nanotubes. Nano Lett. 2006, 6, 1449–1453. [Google Scholar] [CrossRef]
- Wang, Y.; Qin, W.; Hu, X.; Liu, Z.; Ren, Z.; Cao, H.; An, B.; Zhou, X.; Shafiq, M.; Yin, S.; et al. Hierarchically buckled Ti3C2Tx MXene/carbon nanotubes strain sensor with improved linearity, sensitivity, and strain range for soft robotics and epidermal monitoring. Sens. Actuators B Chem. 2022, 368, 132228. [Google Scholar] [CrossRef]
- He, Y.; Wu, D.; Zhou, M.; Zheng, Y.; Wang, T.; Lu, C.; Liu, H.; Liu, C. Wearable strain sensors based on a porous polydimethylsiloxane hybrid with carbon nanotubes and graphene. ACS Appl. Mater. Interfaces 2021, 13, 15572–15583. [Google Scholar] [CrossRef]
- Norizan, M.N.; Moklis, M.H.; Demon, S.Z.N.; Halim, N.A.; Samsuri, A.; Mohamad, I.S.; Knight, V.F.; Abdullah, N. Carbon nanotubes: Functionalisation and their application in chemical sensors. RSC Adv. 2020, 10, 43704–43732. [Google Scholar] [CrossRef]
- Karousis, N.; Tagmatarchis, N.; Tasis, D. Current progress on the chemical modification of carbon nanotubes. Chem. Rev. 2010, 110, 5366–5397. [Google Scholar] [CrossRef]
- Maiti, U.N.; Lee, W.J.; Lee, J.M.; Oh, Y.; Kim, J.Y.; Kim, J.E.; Shim, J.; Han, T.H.; Kim, S.O. 25th anniversary article: Chemically modified/doped carbon nanotubes & graphene for optimized nanostructures & nanodevices. Adv. Mater. 2014, 26, 40–67. [Google Scholar] [CrossRef]
- Mallakpour, S.; Soltanian, S. Surface functionalization of carbon nanotubes: Fabrication and applications. RSC Adv. 2016, 6, 109916–109935. [Google Scholar] [CrossRef]
- Balasubramanian, K.; Burghard, M. Chemically functionalized carbon nanotubes. Small 2005, 1, 180–192. [Google Scholar] [CrossRef]
- Hirsch, A. Functionalization of single-walled carbon nanotubes. Angew. Chem. Int. Edit. 2002, 41, 1853–1859. [Google Scholar] [CrossRef]
- Araujo, D.A.G.; Camargo, J.R.; Pradela-Filho, L.A.; Lima, A.P.; Muñoz, R.A.A.; Takeuchi, R.M.; Janegitz, B.C.C.; Santos, A.L.D. A lab-made screen-printed electrode as a platform to study the effect of the size and functionalization of carbon nanotubes on the voltammetric determination of caffeic acid. Microchem. J. 2020, 158, 105297. [Google Scholar] [CrossRef]
- Assari, P.; Rafati, A.A.; Feizollahi, A.; Joghani, R.A. Fabrication of a sensitive label free electrochemical immunosensor for detection of prostate specific antigen using functionalized multi-walled carbon nanotubes/polyaniline/AuNPs. Mater. Sci. Eng. C 2020, 115, 111066. [Google Scholar] [CrossRef]
- Anshori, I.; Nuraviana Rizalputri, L.; Rona Althof, R.; Sean Surjadi, S.; Harimurti, S.; Gumilar, G. Functionalized multi-walled carbon nanotube/silver nanoparticle (f-MWCNT/AgNP) nanocomposites as non-enzymatic electrochemical biosensors for dopamine detection. Nanocomposites 2021, 7, 97–108. [Google Scholar] [CrossRef]
- Lu, Z.; Zhao, W.; Wu, L.; He, J.; Dai, W.; Zhou, C.; Du, H.; Ye, J. Tunable electrochemical of electrosynthesized layer-by-layer multilayer films based on multi-walled carbon nanotubes and metal-organic framework as high-performance electrochemical sensor for simultaneous determination cadmium and lead. Sens. Actuators B Chem. 2021, 326, 128957. [Google Scholar] [CrossRef]
- Yola, M.L.; Atar, N. Novel voltammetric tumor necrosis factor-alpha (TNF-α) immunosensor based on gold nanoparticles involved in thiol-functionalized multi-walled carbon nanotubes and bimetallic Ni/Cu-MOFs. Anal. Bioanal. Chem. 2021, 413, 2481–2492. [Google Scholar] [CrossRef]
- Alam, A.U.; Qin, Y.; Catalano, M.; Wang, L.; Kim, M.J.; Howlader, M.M.R.; Hu, N.; Deen, M.J. Tailoring MWCNTs and β-cyclodextrin for sensitive detection of acetaminophen and estrogen. ACS Appl. Mater. Interfaces 2018, 10, 21411–21427. [Google Scholar] [CrossRef] [PubMed]
- Alam, A.U.; Deen, M.J. Bisphenol A electrochemical sensor using graphene oxide and β-cyclodextrin-functionalized multi-walled carbon nanotubes. Anal. Chem. 2020, 92, 5532–5539. [Google Scholar] [CrossRef]
- Misia, G.; Evangelisti, C.; Merino, J.P.; Pitzalis, E.; Abelairas, A.M.; Mosquera, J.; Criado, A.; Prato, M.; Silvestri, A. Design and optimization of an electrochemical sensor based on carbon nanotubes for the reliable voltammetric detection of serotonin in complex biological fluids. Carbon 2024, 229, 119550. [Google Scholar] [CrossRef]
- Stathouraki, M.M.; Pantazidis, C.; Mygiakis, E.; Avgeropoulos, A.; Sakellariou, G. Functionalization of single-walled carbon nanotubes with end-capped polystyrene via a single-step Diels–Alder cycloaddition. Polymers 2021, 13, 1169. [Google Scholar] [CrossRef] [PubMed]
- Wunderlich, D.; Hauke, F.; Hirsch, A. Preferred functionalization of metallic and small-diameter single-walled carbon nanotubes by nucleophilic addition of organolithium and-magnesium compounds followed by reoxidation. Chem. Eur. J. 2008, 14, 1607–1614. [Google Scholar] [CrossRef]
- Xu, Y.; Wang, X.; Tian, R.; Li, S.; Wan, L.; Li, M.; You, H.; Li, Q.; Wang, S. Microwave-induced electrophilic addition of single-walled carbon nanotubes with alkylhalides. Appl. Surf. Sci. 2008, 254, 2431–2435. [Google Scholar] [CrossRef]
- Palacin, T.; Khanh, H.L.; Jousselme, B.; Jegou, B.; Filoramo, A.; Ehli, C.; Guldi, D.M.; Campidelli, S. Efficient functionalization of carbon nanotubes with porphyrin dendrons via click chemistry. J. Am. Chem. Soc. 2009, 131, 15394–15402. [Google Scholar] [CrossRef]
- Devaraj, N.K.; Finn, M.G. Introduction: Click chemistry. Chem. Rev. 2021, 121, 6697–6698. [Google Scholar] [CrossRef] [PubMed]
- Clave, G.; Campidelli, S. Efficient covalent functionalisation of carbon nanotubes: The use of “click chemistry”. Chem. Sci. 2011, 2, 1887–1896. [Google Scholar] [CrossRef]
- Mohd Nurazzi, N.; Asyraf, M.R.M.; Khalina, A.; Abdullah, N.; Sabaruddin, F.A.; Kamarudin, S.H.; Ahmad, S.; Mahat, A.M.; Lee, C.L.; Aisyah, H.A.; et al. Fabrication, functionalization, and application of carbon nanotube-reinforced polymer composite: An overview. Polymers 2021, 13, 1047. [Google Scholar] [CrossRef]
- Zhao, H.; Ran, Q.; Li, Y.; Li, B.; Liu, B.; Ma, H.; Zhang, M.; Komarneni, S. Highly sensitive detection of gallic acid based on 3D interconnected porous carbon nanotubes/carbon nanosheets modified glassy carbon electrode. J. Mater. Res. Technol. 2020, 9, 9422–9433. [Google Scholar] [CrossRef]
- Liu, R.; Li, B.; Li, F.; Dubovyk, V.; Chang, Y.; Li, D.; Ding, K.; Ran, Q.; Wang, G.; Zhao, H. A novel electrochemical sensor based on β-cyclodextrin functionalized carbon nanosheets@ carbon nanotubes for sensitive detection of bactericide carbendazim in apple juice. Food Chem. 2022, 384, 132573. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Zhao, F.; Zeng, B. Electrochemical determination of eugenol using a three-dimensional molecularly imprinted poly (p-aminothiophenol-co-p-aminobenzoic acids) film modified electrode. Electrochim. Acta 2016, 210, 293–300. [Google Scholar] [CrossRef]
- Yang, L.; Zhang, B.; Xu, B.; Zhao, F.; Zeng, B. Ionic liquid functionalized 3D graphene-carbon nanotubes–AuPd nanoparticles–molecularly imprinted copolymer based paracetamol electrochemical sensor: Preparation, characterization and application. Talanta 2021, 224, 121845. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Singh, R.; Wang, Z.; Li, G.; Xie, Y.; Jha, R.; Marques, C.; Zhangm, B.; Kumar, S. Humanoid shaped optical fiber plasmon biosensor functionalized with graphene oxide/multi-walled carbon nanotubes for histamine detection. Opt. Express 2023, 31, 11788–11803. [Google Scholar] [CrossRef]
- Fall, B.; Sall, D.D.; Hémadi, M.; Fall, M.; Randriamahazaka, H.; Thomas, S. Highly efficient non-enzymatic electrochemical glucose sensor based on carbon nanotubes functionalized by molybdenum disulfide and decorated with nickel nanoparticles (GCE/CNT/MoS2/NiNPs). Sens. Actuators 2023, 5, 100136. [Google Scholar] [CrossRef]
- Wang, X.; Xu, Y.; Li, Y.; Li, Y.; Li, Z.; Zhang, W.; Zou, X.; Shi, J.; Huang, X.; Liu, C.; et al. Rapid detection of cadmium ions in meat by a multi-walled carbon nanotubes enhanced metal-organic framework modified electrochemical sensor. Food Chem. 2021, 357, 129762. [Google Scholar] [CrossRef]
- Zhang, H.; Zou, X.; Chen, W.; Sun, Q.; Gao, E. A Cu-functionalized MOF and multi-walled carbon nanotube composite modified electrode for the simultaneous determination of hydroquinone and catechol. Anal. Methods 2022, 14, 3961–3969. [Google Scholar] [CrossRef]
- Han, J.; Li, F.; Zhao, M.; Guo, M.; Liu, Y.; Guo, X.; Ran, Q.; Wang, Z.; Zhao, H. Ultrasensitive electrochemical sensing platform based on Zr-based MOF embellished single-wall carbon nanotube networks for trace analysis of methyl parathion. Microchem. J. 2024, 200, 110323. [Google Scholar] [CrossRef]
- Kunene, K.; Sabela, M.; Kanchi, S.; Bisetty, K. High performance electrochemical biosensor for bisphenol A using screen printed electrodes modified with multiwalled carbon nanotubes functionalized with silver-doped zinc oxide. Waste Biomass Valorization 2020, 11, 1085–1096. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, L.; Xu, J.; Wang, X.; Duan, X.; Wen, Y. Rapid and sensitive stripping voltammetric analysis of methyl parathion in vegetable samples at carboxylic acid-functionalized SWCNTs–β-cyclodextrin modified electrode. J. Electroanal. Chem. 2014, 713, 1–8. [Google Scholar] [CrossRef]
- Balram, D.; Lian, K.Y.; Sebastian, N.; Rasana, N. Ultrasensitive detection of cytotoxic food preservative tert-butylhydroquinone using 3D cupric oxide nanoflowers embedded functionalized carbon nanotubes. J. Hazard. Mater. 2021, 406, 124792. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Awasthi, A.; Sharma, M.D.; Singh, K.; Singh, D. Functionalized multiwall carbon nanotube-molybdenum disulphide nanocomposite based electrochemical ultrasensitive detection of neurotransmitter epinephrine. Mater. Chem. Phys. 2022, 290, 126656. [Google Scholar] [CrossRef]
- Wu, B.; Yeasmin, S.; Liu, Y.; Cheng, L. Sensitive and selective electrochemical sensor for serotonin detection based on ferrocene-gold nanoparticles decorated multiwall carbon nanotubes. Sens. Actuators B Chem. 2022, 354, 131216. [Google Scholar] [CrossRef]
- Venkataraman, A.; Amadi, E.V.; Chen, Y.; Papadopoulos, C. Carbon nanotube assembly and integration for applications. Nanoscale Res. Lett. 2019, 14, 220. [Google Scholar] [CrossRef]
- Papageorgiou, D.G.; Li, Z.; Liu, M.; Kinloch, I.A.; Young, R.J. Mechanisms of mechanical reinforcement by graphene and carbon nanotubes in polymer nanocomposites. Nanoscale 2020, 12, 2228–2267. [Google Scholar] [CrossRef] [PubMed]
- Kobashi, K.; Villmow, T.; Andres, T.; Pötschke, P. Liquid sensing of melt-processed poly (lactic acid)/multi-walled carbon nanotube composite films. Sens. Actuators B Chem. 2008, 134, 787–795. [Google Scholar] [CrossRef]
- Chio, L.; Pinals, R.L.; Murali, A.; Goh, N.S.; Landry, M.P. Covalent surface modification effects on single-walled carbon nanotubes for targeted sensing and optical imaging. Adv. Funct. Mater. 2020, 30, 1910556. [Google Scholar] [CrossRef]
- Cho, G.; Azzouzi, S.; Zucchi, G.; Lebental, B. Electrical and electrochemical sensors based on carbon nanotubes for the monitoring of chemicals in water—A review. Sensors 2021, 22, 218. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.Y.; Alam, A.U.; Howlader, M.M.R. Fabrication of highly sensitive Bisphenol A electrochemical sensor amplified with chemically modified multiwall carbon nanotubes and β-cyclodextrin. Sens. Actuators B Chem. 2020, 320, 128319. [Google Scholar] [CrossRef]
- Othman, A.M.; Wollenberger, U. Amperometric biosensor based on coupling aminated laccase to functionalized carbon nanotubes for phenolics detection. Int. J. Biol. Macromol. 2020, 153, 855–864. [Google Scholar] [CrossRef]
- Pushpanjali, P.A.; Manjunatha, J.G.; Hareesha, N. An overview of recent developments of carbon-based sensors for the analysis of drug molecules. J. Electrochem. Sci. Eng. 2021, 11, 161–177. [Google Scholar] [CrossRef]
- Kokab, T.; Shah, A.; Khan, M.A.; Arshad, M.; Nisar, J.; Ashiq, M.N.; Zia, M.A. Simultaneous femtomolar detection of paracetamol, diclofenac, and orphenadrine using a carbon nanotube/zinc oxide nanoparticle-based electrochemical sensor. ACS Appl. Nano Mater. 2021, 4, 4699–4712. [Google Scholar] [CrossRef]
- Tang, J.; Peng, L.; Ali, A.; Zhao, S.; Zeng, Z.; Yuan, K.; Yao, S. Electrochemical detection of rutin in black tartary buckwheat tea and related health-care pills with new ionic liquid-based supramolecular hydrogels. Food Control 2024, 155, 110045. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, L.; Zhao, P.; Wang, C.; Fei, J.; Xie, Y. Ultrasensitive luteolin electrochemical sensor based on zeolitic imidazolate frameworks-derived cobalt trioxide@ nitrogen doped carbon nanotube/amino-functionalized graphene quantum dots composites modified glass carbon electrode. Sens. Actuators B Chem. 2022, 351, 130938. [Google Scholar] [CrossRef]
- Yuwen, T.; Shu, D.; Zou, H.; Yang, X.; Zhang, S.; Liu, Q.; Wang, X.; Wang, G.; Zhang, Y.; Zang, G. Carbon nanotubes: A powerful bridge for conductivity and flexibility in electrochemical glucose sensors. J. Nanobiotechnol. 2023, 21, 320. [Google Scholar] [CrossRef]
- Peng, L.; Luo, Y.; Xiong, H.; Yao, S.; Zhu, M.; Song, H. A novel amperometric glucose biosensor based on Fe3O4–chitosan–β-cyclodextrin/MWCNTs nanobiocomposite. Electroanalysis 2021, 33, 723–732. [Google Scholar] [CrossRef]
- Karimi-Maleh, H.; Cellat, K.; Arıkan, K.; Savk, A.; Karimi, F.; Sen, F. Palladium–Nickel nanoparticles decorated on Functionalized-MWCNT for high precision non-enzymatic glucose sensing. Mater. Chem. Phys. 2020, 250, 123042. [Google Scholar] [CrossRef]
- Li, G.; Xu, Q.; Singh, R.; Zhang, W.; Marques, C.; Xie, Y. Graphene oxide/multiwalled carbon nanotubes assisted serial quadruple tapered structure-based LSPR sensor for glucose detection. IEEE Sens. J. 2022, 22, 16904–16911. [Google Scholar] [CrossRef]
- Song, H.; Liu, Y.; Fang, Y.; Zhang, D. Carbon-based electrochemical sensors for in vivo and in vitro neurotransmitter detection. Crit. Rev. Anal. Chem. 2023, 53, 955–974. [Google Scholar] [CrossRef]
- Shakil, S.; Yuan, D.; Li, M. Electrochemical Sensors for Acetylcholine Detection. J. Electrochem. Soc. 2024, 171, 067512. [Google Scholar] [CrossRef]
- Bagherpour, S.; Pérez-García, L. Recent advances on nanomaterial-based glutathione sensors. J. Mater. Chem. B 2024, 12, 8285–8309. [Google Scholar] [CrossRef]
- Elugoke, S.E.; Adekunle, A.S.; Fayemi, O.E.; Mamba, B.B.; Nkambule, T.T.I.; Sherif, E.M.; Ebenso, E.E. Progress in electrochemical detection of neurotransmitters using carbon nanotubes/nanocomposite based materials: A chronological review. Nano Select 2020, 1, 561–611. [Google Scholar] [CrossRef]
- Jia, D.; Dai, J.; Yuan, H.; Lei, L.; Xiao, D. Selective detection of dopamine in the presence of uric acid using a gold nanoparticles-poly (luminol) hybrid film and multi-walled carbon nanotubes with incorporated β-cyclodextrin modified glassy carbon electrode. Talanta 2011, 85, 2344–2351. [Google Scholar] [CrossRef]
- Babaei, A.; Taheri, A.R. Nafion/Ni (OH)2 nanoparticles-carbon nanotube composite modified glassy carbon electrode as a sensor for simultaneous determination of dopamine and serotonin in the presence of ascorbic acid. Sens. Actuators B Chem. 2013, 176, 543–551. [Google Scholar] [CrossRef]
- Fayemi, O.E.; Adekunle, A.S.; Ebenso, E.E. Metal oxide nanoparticles/multi-walled carbon nanotube nanocomposite modified electrode for the detection of dopamine: Comparative electrochemical study. J. Biosens. Bioelectron 2015, 6, 190. [Google Scholar] [CrossRef]
- Wang, Z.; Guo, H.; Gui, R.; Jin, H.; Xia, J.; Zhang, F. Simultaneous and selective measurement of dopamine and uric acid using glassy carbon electrodes modified with a complex of gold nanoparticles and multiwall carbon nanotubes. Sens. Actuators B Chem. 2018, 255, 2069–2077. [Google Scholar] [CrossRef]
- Yang, Y.; Li, M.; Zhu, Z. A novel electrochemical sensor based on carbon nanotubes array for selective detection of dopamine or uric acid. Talanta 2019, 201, 295–300. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, J.; Li, J.; Li, Y.; Yang, P.; Zhao, P.; Fei, J.; Xie, Y. A novel dopamine electrochemical sensor based on 3D flake nickel oxide/cobalt oxide@ porous carbon nanosheets/carbon nanotubes/electrochemical reduced of graphene oxide composites modified glassy carbon electrode. Colloid Surf. A 2023, 666, 131284. [Google Scholar] [CrossRef]
- Nißler, R.; Kurth, L.; Li, H.; Spreinat, A.; Kuhlemann, I.; Flavel, B.S.; Kruss, S. Sensing with chirality-pure near-infrared fluorescent carbon nanotubes. Anal. Chem. 2021, 93, 6446–6455. [Google Scholar] [CrossRef]
- Dăscălescu, D.; Apetrei, C. Nanomaterials based electrochemical sensors for serotonin detection: A review. Chemosensors 2021, 9, 14. [Google Scholar] [CrossRef]
- Ahmad, I.; Sead, F.F.; Kanjariya, P.; Kumar, A.; Rajivm, A.; Shankhyan, A.; Jaidka, S.; Kumar, H.; Aminov, Z. Nanomaterial sensors for enhanced detection of serotonin. Clin. Chim. Acta 2025, 569, 120160. [Google Scholar] [CrossRef] [PubMed]
- Becerra-Hernández, A.; Galindo-de-la-Rosa, J.; Martínez-Pimentel, Y.; Ledesma-García, J.; Álvarez-Contreras, L.; Guerra-Balcázar, M.; Aguilar-Elguezabal, A.; Chávez-Ramírez, A.U.; Vallejo-Becerra, V. Novel biomaterial based on monoamine oxidase-A and multi-walled carbon nanotubes for serotonin detection. Biochem. Eng. J. 2019, 149, 107240. [Google Scholar] [CrossRef]
- Nayak, S.P.; Prathyusha, V.; Kumar, J.K.K. Eco-friendly surface modification of oxidized carbon nanotubes with curcumin for simultaneous electrochemical detection of dopamine and serotonin. Mater. Chem. Phys. 2022, 287, 126293. [Google Scholar] [CrossRef]
- Shekher, K.; Sampath, K.; Vandini, S.; Satyanarayana, M.; Vengatajalabathy Gobi, K. Gold nanoparticle assimilated polymer layer on carbon nanotube matrices for sensitive detection of serotonin in presence of dopamine in-vitro. Inorg. Chim. Acta 2023, 549, 121399. [Google Scholar] [CrossRef]
- Yao, Y.; Shen, R.; Xu, J.; Feng, Z. Progress in electrochemical sensing of epinephrine using carbon nanomaterials: A review. Int. J. Electrochem. Sci. 2024, 19, 100750. [Google Scholar] [CrossRef]
- Charithra, M.M.; Manjunatha, J.G. Electrochemical sensing of adrenaline using surface modified carbon nanotube paste electrode. Mater. Chem. Phys. 2021, 262, 124293. [Google Scholar] [CrossRef]
- Rajarathinam, T.; Thirumalai, D.; Kwon, M.; Lee, S.; Jayaraman, S.; Paik, H.; Lee, J.; Chang, S. Screen-printed carbon electrode modified with de-bundled single-walled carbon nanotubes for voltammetric determination of norepinephrine in ex vivo rat tissue. Bioelectrochemistry 2022, 146, 108155. [Google Scholar] [CrossRef]
- Manjunatha, K.G.; Swamy, B.E.K.; Jayaprakash, G.K.; Madhuchandra, H.D.; Vishnumurthy, K.A. Electro-sensitive detection of adrenaline and paracetamol at SrF/MWCNT modified carbon paste electrode. Inorg. Chem. Commun. 2023, 158, 111504. [Google Scholar] [CrossRef]
- Liu, L.; Hao, L.; Shen, W.; Yang, X.; Zhang, Y. Synthesis of ternary heterostructured rice-stick-like Ag/polyoxometalates/nitrogen-doped carbon nanotube hybrids for the sensitive detection of adrenaline. Talanta 2025, 294, 128222. [Google Scholar] [CrossRef] [PubMed]
- Charithra, M.M.; Manjunatha, J.G.; Al-Kahtani, A.A.; Tighezza, A.M.; Ataollahi, N. Electroanalysis of epinephrine using polymerized carbon nanotube composite sensor. Top. Catal. 2025, 68, 1349–1359. [Google Scholar] [CrossRef]
- Zeng, C.; Li, Y.; Zhu, M.; Du, Z.; Liang, H.; Chen, Q.; Ye, H.; Li, R.; Liu, W. Simultaneous detection of norepinephrine and 5-hydroxytryptophan using poly-alizarin/multi-walled carbon nanotubes-graphene modified carbon fiber microelectrode array sensor. Talanta 2024, 270, 125565. [Google Scholar] [CrossRef]
- Phasuksom, K.; Thongwattana, N.; Ariyasajjamongkol, N.; Sirivat, A. Non-enzymatic sensor based on doped polyindole/multi-walled carbon nanotube for detecting neurotransmitter acetylcholine. J. Electroanal. Chem. 2024, 964, 118337. [Google Scholar] [CrossRef]
- Poolakkandy, R.R.; Ramalakshmi, N.A.; Padmalayam, K.A.; Krishnamurthy, R.G.; Menamparambath, M.M. Braided copper cobaltite/MWCNT composites enable acetylcholine detection at sub-nanomolar levels in vitro. Sens. Diagn. 2023, 2, 726–735. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, L.; Chu, S.; Zhang, S.; Chen, X.; Gong, Y.; Du, Z.; Mao, G.; Wang, H. One-pot fabrication of nanozyme with 2D/1D heterostructure by in-situ growing MoS2 nanosheets onto single-walled carbon nanotubes with enhanced catalysis for colorimetric detection of glutathione. Anal. Chim. Acta 2022, 1221, 340083. [Google Scholar] [CrossRef]
- Liu, Y.; Yan, X.; Li, L.; Xing, Y.; Zhao, P.; Liu, M.; Zhu, Y.; Liu, N.; Zhang, Z. Nanoreactor based on Cu nanoparticles confined in B, N co-doped porous carbon nanotubes for glutathione biosensing. Microchim. Acta 2023, 190, 325. [Google Scholar] [CrossRef]
- Kaimal, R.; Senthilkumar, P.; Aljafari, B.; Anandan, S. A nanosecond pulsed laser-ablated MWCNT-Au heterostructure: An innovative ultra-sensitive electrochemical sensing prototype for the identification of glutathione. Analyst 2022, 147, 3894–3907. [Google Scholar] [CrossRef]
- Ali, M.Y.; Knight, D.; Howlader, M.M.R. Nonenzymatic electrochemical glutamate sensor using copper oxide nanomaterials and multiwall carbon nanotubes. Biosensors 2023, 13, 237. [Google Scholar] [CrossRef]
- Poolakkandy, R.R.; AR, N.; Padmalayam, K.A. Nickel hydroxide nanoflake/carbon nanotube composites for the electrochemical detection of glutamic acid using In vitro stroke model. ACS Appl. Nano Mater. 2023, 6, 1347–1359. [Google Scholar] [CrossRef]
- Wang, L.; Xie, S.; Wang, Z. Functionalized helical fibre bundles of carbon nanotubes as electrochemical sensors for long-term in vivo monitoring of multiple disease biomarkers. Nat. Biomed. Eng. 2020, 4, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Xiao, M.; He, J.; Zhang, Y.; Liang, Y.; Liu, H.; Zhang, Z. Aptamer-functionalized carbon nanotube field-effect transistor biosensors for Alzheimer’s disease serum biomarker detection. ACS Sens. 2022, 7, 2075–2083. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Wang, J.; Lü, H.; Hui, N. Electrochemical sensor based on Prussian blue/multi-walled carbon nanotubes functionalized polypyrrole nanowire arrays for hydrogen peroxide and microRNA detection. Microchim. Acta 2021, 188, 25. [Google Scholar] [CrossRef]
- Farzin, L.; Sadjadi, S.; Shamsipur, M.; Sheibani, S. Electrochemical genosensor based on carbon nanotube/amine-ionic liquid functionalized reduced graphene oxide nanoplatform for detection of human papillomavirus (HPV16)-related head and neck cancer. J. Pharmaceut. Biomed. Anal. 2020, 179, 112989. [Google Scholar] [CrossRef]
- Pinals, R.L.; Ledesma, F.; Yang, D.; Navarro, N.; Jeong, S.; Pak, J.E.; Chuang, Y.; Cheng, Y.; Sun, H.; Landry, M.P. Rapid SARS-CoV-2 spike protein detection by carbon nanotube-based near-infrared nanosensors. Nano Lett. 2021, 21, 2272–2280. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Zhou, G.; Jiang, W.; Chen, Y.; Wu, T.; Yuan, K.; Ali, A.; Yao, S. A new Ionic Liquid-Modified Silver/Multi-Walled Carbon Nanotubes Biosensor for Detection of Glucose in Honey Samples. Food Bioprocess Technol. 2025, 18, 2962–2984. [Google Scholar] [CrossRef]
- Kumar, T.H.V.; Pillai, S.K.R.; Chan-Park, M.B.; Sundramoorthy, A.K. Highly selective detection of an organophosphorus pesticide, methyl parathion, using Ag–ZnO–SWCNT based field-effect transistors. J. Mater. Chem. C 2020, 8, 8864–8875. [Google Scholar] [CrossRef]
- Fu, X.C.; Zhang, J.; Tao, Y.Y.; Wu, J.; Xie, C.G.; Kong, L.T. Three-dimensional mono-6-thio-β-cyclodextrin covalently functionalized gold nanoparticle/single-wall carbon nanotube hybrids for highly sensitive and selective electrochemical determination of methyl parathion. Electrochim. Acta 2015, 153, 12–18. [Google Scholar] [CrossRef]
- Chen, L.; Chaisiwamongkhol, K.; Chen, Y.; Compton, R.G. Rapid electrochemical detection of vanillin in natural vanilla. Electroanalysis 2019, 31, 1067–1074. [Google Scholar] [CrossRef]
- Hareesha, N.; Manjunatha, J.G.; Amrutha, B.M.; Sreeharsha, N.; Basheeruddin Asdaq, S.M.; Anwer, M.K. A fast and selective electrochemical detection of vanillin in food samples on the surface of poly (glutamic acid) functionalized multiwalled carbon nanotubes and graphite composite paste sensor. Colloids Surf. A 2021, 626, 127042. [Google Scholar] [CrossRef]
- Guo, S.Y.; Hou, P.X.; Zhang, F.; Liu, C.; Cheng, H.M. Gas sensors based on single-wall carbon nanotubes. Molecules 2022, 27, 5381. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Kim, B.M.; Oh, S.H.; Byun, Y.T. Selective detection of chlorine at room temperature utilizing single-walled carbon nanotubes functionalized with platinum nanoparticles synthesized via ultraviolet irradiation. Sens. Actuators B Chem. 2017, 249, 414–422. [Google Scholar] [CrossRef]
- Tian, X.; Wang, Q.; Chen, X.; Yang, W.; Wu, Z.; Xu, X.; Jiang, M.; Zhou, Z. Enhanced performance of core-shell structured polyaniline at helical carbon nanotube hybrids for ammonia gas sensor. Appl. Phys. Lett. 2014, 105, 203109. [Google Scholar] [CrossRef]
- Tung, T.T.; Pham-Huu, C.; Janowska, I.; Kim, T.; Castro, M.; Feller, J.F. Hybrid films of graphene and carbon nanotubes for high performance chemical and temperature sensing applications. Small 2015, 11, 3485–3493. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.W.; Kim, J.; Lee, J.H.; Byun, Y.T. Remarkable improvement of CO-sensing performances in single-walled carbon nanotubes due to modification of the conducting channel by functionalization of Au nanoparticles. Sens. Actuators B Chem. 2016, 232, 625–632. [Google Scholar] [CrossRef]
- Bensifia, M.; Bouanis, F.; Léonard, C. Theoretical study of the non-covalent functionalization of carbon nanotubes for NO and CO detection. Comput. Condens. Matter 2025, 42, e00998. [Google Scholar] [CrossRef]
- Choi, S.W.; Kim, J.; Byun, Y.T. Highly sensitive and selective NO2 detection by Pt nanoparticles-decorated single-walled carbon nanotubes and the underlying sensing mechanism. Sens. Actuators B Chem. 2017, 238, 1032–1042. [Google Scholar] [CrossRef]
- Evans, G.P.; Buckley, D.J.; Skipper, N.T.; Parkin, I.P. Single-walled carbon nanotube composite inks for printed gas sensors: Enhanced detection of NO2, NH3, EtOH and acetone. RSC Adv. 2014, 4, 51395–51403. [Google Scholar] [CrossRef]
- Chang, C.P.; Yuan, C.L. The fabrication of a MWNTs–polymer composite chemoresistive sensor array to discriminate between chemical toxic agents. J. Mater. Sci. 2009, 44, 5485–5493. [Google Scholar] [CrossRef]
- Han, M.; Kim, J.K.; Lee, J.; An, H.K.; Yun, J.P.; Kang, S.-W.; Jung, D. Room-temperature hydrogen-gas sensor based on carbon nanotube yarn. J. Nanosci. Nanotechnol. 2020, 20, 4011–4014. [Google Scholar] [CrossRef] [PubMed]
- Imeni, S.; Rouhani, M.; Aliabad, J.M. NiN4S-doped single walled carbon nanotube as an ultrafast H2 gas sensor: A DFT simulation. Inorg. Chem. Commun. 2023, 148, 110334. [Google Scholar] [CrossRef]
- Kim, J.K.; Han, M.; Kim, Y.; An, H.K.; Lee, S.; Kong, S.H.; Jung, D. Pd-decorated multi-walled carbon nanotube sensor for hydrogen detection. J. Nanosci. Nanotechnol. 2021, 21, 3707–3710. [Google Scholar] [CrossRef]
- Han, M.; Kim, J.K.; Lee, J.; An, H.K.; Yun, J.P.; Kang, S.-W.; Jung, D. H2 gas sensor based on Pd-loaded carbon nanotube film. J. Nanosci. Nanotechnol. 2020, 20, 4470–4473. [Google Scholar] [CrossRef]
- Son, W.; Lee, D.W.; Kim, Y.K.; Chun, S.; Lee, J.M.; Choi, J.H.; Shim, W.S.; Suh, D.; Lim, S.K.; Choi, C. PdO-nanoparticle-embedded carbon nanotube yarns for wearable hydrogen gas sensing platforms with fast and sensitive responses. ACS Sens. 2023, 8, 94–102. [Google Scholar] [CrossRef] [PubMed]
- Girma, H.G.; Park, K.H.; Ji, D.; Kim, Y.; Lee, H.M.; Jeon, S.; Jung, S.H.; Kim, J.Y.; Noh, Y.Y.; Lim, B. Room-temperature hydrogen sensor with high sensitivity and selectivity using chemically immobilized monolayer single-walled carbon nanotubes. Adv. Funct. Mater. 2023, 33, 2213381. [Google Scholar] [CrossRef]
- Alamri, M.; Liu, B.; Berrie, C.L.; Walsh, M.; Wu, J.Z. Probing the role of CNTs in Pt nanoparticle/CNT/graphene nanohybrids H2 sensors. Nano Express 2022, 3, 035004. [Google Scholar] [CrossRef]
- Liu, C.; Hu, J.; Wu, G.; Cao, J.; Zhang, Z.; Zhang, Y. Carbon nanotube-based field-effect transistor-type sensor with a sensing gate for ppb-level formaldehyde detection. ACS Appl. Mater. Interfaces 2021, 13, 56309–56319. [Google Scholar] [CrossRef]
- Sun, Y.; Zhang, Y. Wafer-scale floating-gate field effect transistor sensor built on carbon nanotubes film for ppb-level NO2 detection. Chem. Eng. J. 2023, 473, 145480. [Google Scholar] [CrossRef]
- Velumani, M.; Prasanth, A.; Narasimman, S.; Chandrasekhar, A.; Sampson, A.; Meher, S.R.; Rajalingam, S.; Rufus, E.; Alex, Z.C. Nanomaterial-based sensors for exhaled breath analysis: A review. Coatings 2022, 12, 1989. [Google Scholar] [CrossRef]
- Jeong, D.W.; Kim, K.H.; Kim, B.S.; Byun, Y.T. Characteristics of highly sensitive and selective nitric oxide gas sensors using defect-functionalized single-walled carbon nanotubes at room temperature. Appl. Surf. Sci. 2021, 550, 149250. [Google Scholar] [CrossRef]
- Nag, S.; Castro, M.; Choudhary, V.; Feller, J.-F. Boosting selectivity and sensitivity to biomarkers of quantum resistive vapour sensors used for volatolomics with nanoarchitectured carbon nanotubes or graphene platelets connected by fullerene junctions. Chemosensors 2021, 9, 66. [Google Scholar] [CrossRef]
- Gelperin, A.; Johnson, A. Nanotube-based sensor arrays for clinical breath analysis. J. Breath Res. 2008, 2, 037015. [Google Scholar] [CrossRef]
- Hu, W.; Wan, L.; Jian, Y.; Ren, C.; Jin, K.; Su, X.; Bai, X.; Haick, H.; Yao, M.; Wu, W. Electronic noses: From advanced materials to sensors aided with data processing. Adv. Mater. Technol. 2019, 4, 1800488. [Google Scholar] [CrossRef]
- Freddi, S.; Sangaletti, L. Trends in the development of electronic noses based on carbon nanotubes chemiresistors for breathomics. Nanomaterials 2022, 12, 2992. [Google Scholar] [CrossRef]
- Dai, H.; Thostenson, E.T.; Schumacher, T. Processing and characterization of a novel distributed strain sensor using carbon nanotube-based nonwoven composites. Sensors 2015, 15, 17728–17747. [Google Scholar] [CrossRef]
- Bautista-Quijano, J.R.; Pötschke, P.; Brünig, H.; Heinrich, G. Strain sensing, electrical and mechanical properties of polycarbonate/multiwall carbon nanotube monofilament fibers fabricated by melt spinning. Polymer 2016, 82, 181–189. [Google Scholar] [CrossRef]
- Cheng, Y.; Wang, R.; Sun, J.; Gao, L. A stretchable and highly sensitive graphene–based fiber for sensing tensile strain, bending, and torsion. Adv. Mater. 2015, 27, 7365–7371. [Google Scholar] [CrossRef]
- Guo, X.; Hong, W.; Zhao, Y.; Zhu, T.; Li, H.; Zheng, G.; Wang, J.; Tang, G.; Cao, J.; Wang, Y.; et al. Bioinspired sandwich-structured pressure sensors based on graphene oxide/hydroxyl functionalized carbon nanotubes/bovine serum albumin nanocomposites for wearable textile electronics. Compos. Part A-Appl. S. 2022, 163, 107240. [Google Scholar] [CrossRef]
- Wang, L.; Lv, D.; Wang, F. Electrode-shared differential configuration for pressure sensor made of carbon nanotube-filled silicone rubber composites. IEEE Trans. Instrum. Meas. 2018, 67, 1417–1424. [Google Scholar] [CrossRef]
- Su, Y.; Zhang, W.; Chen, S.; Yao, D.; Zhang, X.; Chen, H. Piezoresistive electronic-skin sensors produced with self-channeling laser microstructured silicon molds. IEEE Trans. Electron Devices 2021, 68, 786–792. [Google Scholar] [CrossRef]
- Li, W.; Jin, X.; Han, X.; Wang, W.; Lin, T.; Zhu, Z. Synergy of porous structure and microstructure in piezoresistive material for high-performance and flexible pressure sensors. ACS Appl. Mater. Interfaces 2021, 13, 19211–19220. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Chen, X.; Ren, L.; Wang, M. Integrated Biomarker Separation and Detection with Carbon Nanotubes: Advances and Prospects. Small Methods 2025, 9, e00117. [Google Scholar] [CrossRef]
- Palumbo, A.; Li, Z.; Yang, E.H. Trends on carbon nanotube-based flexible and wearable sensors via electrochemical and mechanical stimuli: A review. IEEE Sens. J. 2022, 22, 20102–20125. [Google Scholar] [CrossRef]
- Rdest, M.; Janas, D. Carbon nanotube wearable sensors for health diagnostics. Sensors 2021, 21, 5847. [Google Scholar] [CrossRef]
- Shoukat, R.; Khan, M.I. Carbon nanotubes: A review on properties, synthesis methods and applications in micro and nanotechnology. Microsyst. Technol. 2021, 27, 4183–4192. [Google Scholar] [CrossRef]
- Gacem, A.; Modi, S.; Yadav, V.K.; Islam, S.; Patel, A.; Dawane, V.; Jameel, M.; Inwati, G.K.; Piplode, S.; Solanki, V.S.; et al. Recent advances in methods for synthesis of carbon nanotubes and carbon nanocomposite and their emerging applications: A descriptive review. J. Nanomater. 2022, 2022, 7238602. [Google Scholar] [CrossRef]
- Gao, C.; Guo, M.; Liu, Y.; Zhang, D.; Gao, F.; Sun, L.; Li, J.; Chen, X.; Wang, Y. Surface modification methods and mechanisms in carbon nanotubes dispersion. Carbon 2023, 212, 118133. [Google Scholar] [CrossRef]
- Kobayashi, N.; Izumi, H.; Morimoto, Y. Review of toxicity studies of carbon nanotubes. J. Occup. Health 2017, 59, 394–407. [Google Scholar] [CrossRef]
- Sousa, S.P.; Peixoto, T.; Santos, R.M.; Lopes, A.; Paiva, M.d.C.; Marques, A.T. Health and safety concerns related to CNT and graphene products, and related composites. J. Compos. Sci. 2020, 4, 106. [Google Scholar] [CrossRef]
- Aliyana, A.K.; Naveen Kumar, S.K.; Marimuthu, P.; Baburaj, A.; Adetunji, M.; Frederick, T.; Sekhar, P.; Fernandez, R.E. Machine learning-assisted ammonium detection using zinc oxide/multi-walled carbon nanotube composite based impedance sensors. Sci. Rep. 2021, 11, 24321. [Google Scholar] [CrossRef]
- Algamili, A.S.; Khir, M.H.M.; Dennis, J.O.; Ahmed, A.Y.; Alabsi, S.S.; Ba Hashwan, S.S.; Junaid, M.M. A review of actuation and sensing mechanisms in MEMS-based sensor devices. Nanoscale Res. Lett. 2021, 16, 16. [Google Scholar] [CrossRef]
- Lee, K.; Park, J.; Jung, S.I.; Hajra, S.; Kim, H.J. Direct integration of carbon nanotubes on a suspended Pt microheater for hydrogen gas sensing. J. Mater. Sci. Mater. Electron. 2021, 32, 19626–19634. [Google Scholar] [CrossRef]
- Tang, S.; Chen, W.; Jin, L.; Zhang, H.; Li, Y.; Zhou, Q.; Zen, W. SWCNTs-based MEMS gas sensor array and its pattern recognition based on deep belief networks of gases detection in oil-immersed transformers. Sens. Actuators B Chem. 2020, 312, 127998. [Google Scholar] [CrossRef]
- Kim, M.; Goerzen, D.; Jena, P.V.; Zeng, E.; Pasquali, M.; Meidl, R.A.; Heller, D.A. Human and environmental safety of carbon nanotubes across their life cycle. Nat. Rev. Mater. 2024, 9, 63–81. [Google Scholar] [CrossRef]
- Li, Y.; Wang, S.; Lv, Z.; Wang, Z.; Zhao, Y.; Xie, Y.; Xu, Y.; Qian, L.; Yang, Y.; Zhao, Z.; et al. Transforming the synthesis of carbon nanotubes with machine learning models and automation. Matter 2025, 8, 101913. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).