Fabrication and Characterization of a Portable and Electrochemical System for Field Determination of Nitrate in Coastal Seawater
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Apparatus
2.3. Construction of a Portable Electrochemical System
2.4. Electrochemical Analysis Procedure
2.5. Coastal Seawater Sample Collection
3. Results and Discussion
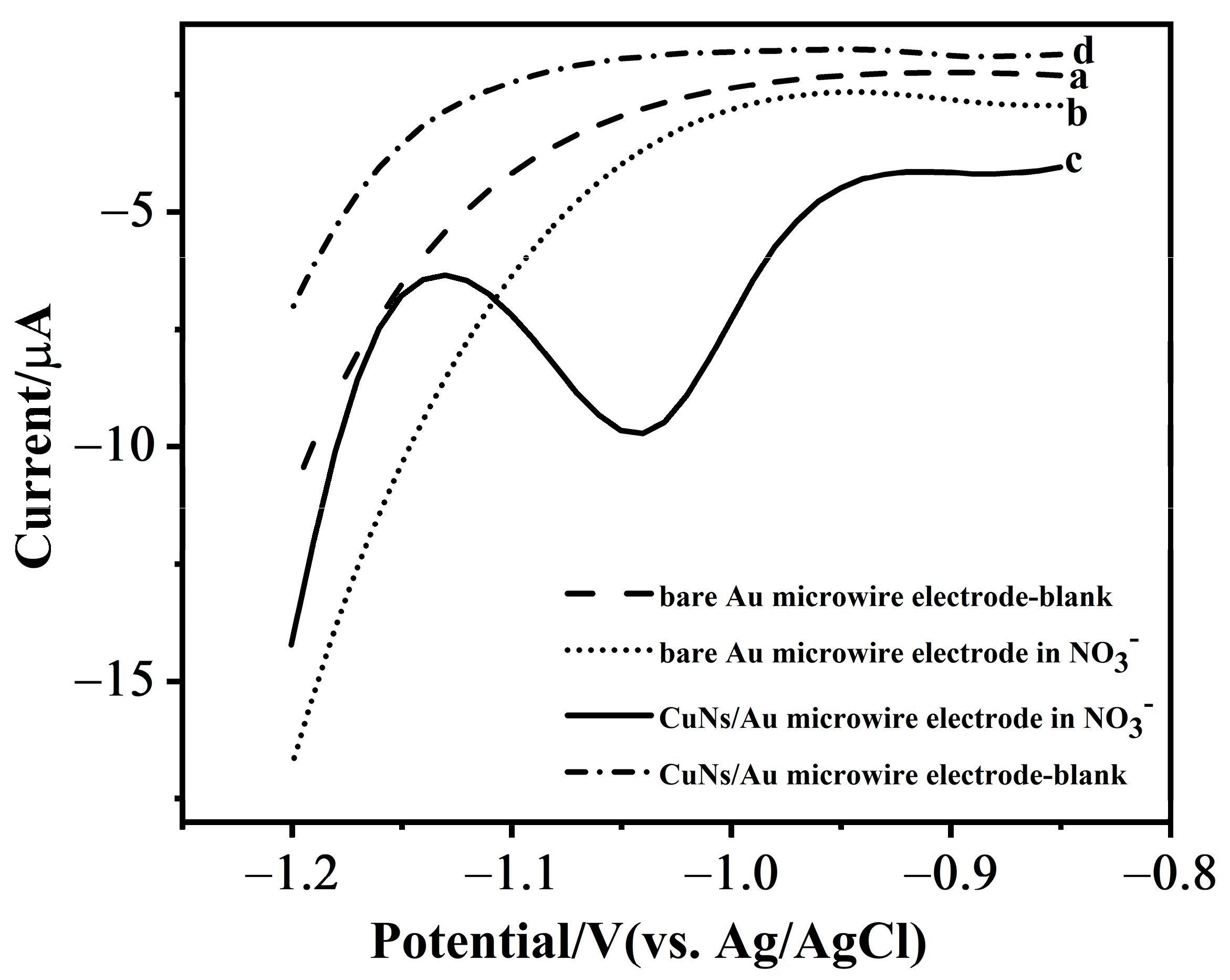
3.1. Detection Mechanism
3.2. Characterization of CuNs/Au Microwire Electrode
3.2.1. Scanning Electron Microscopy
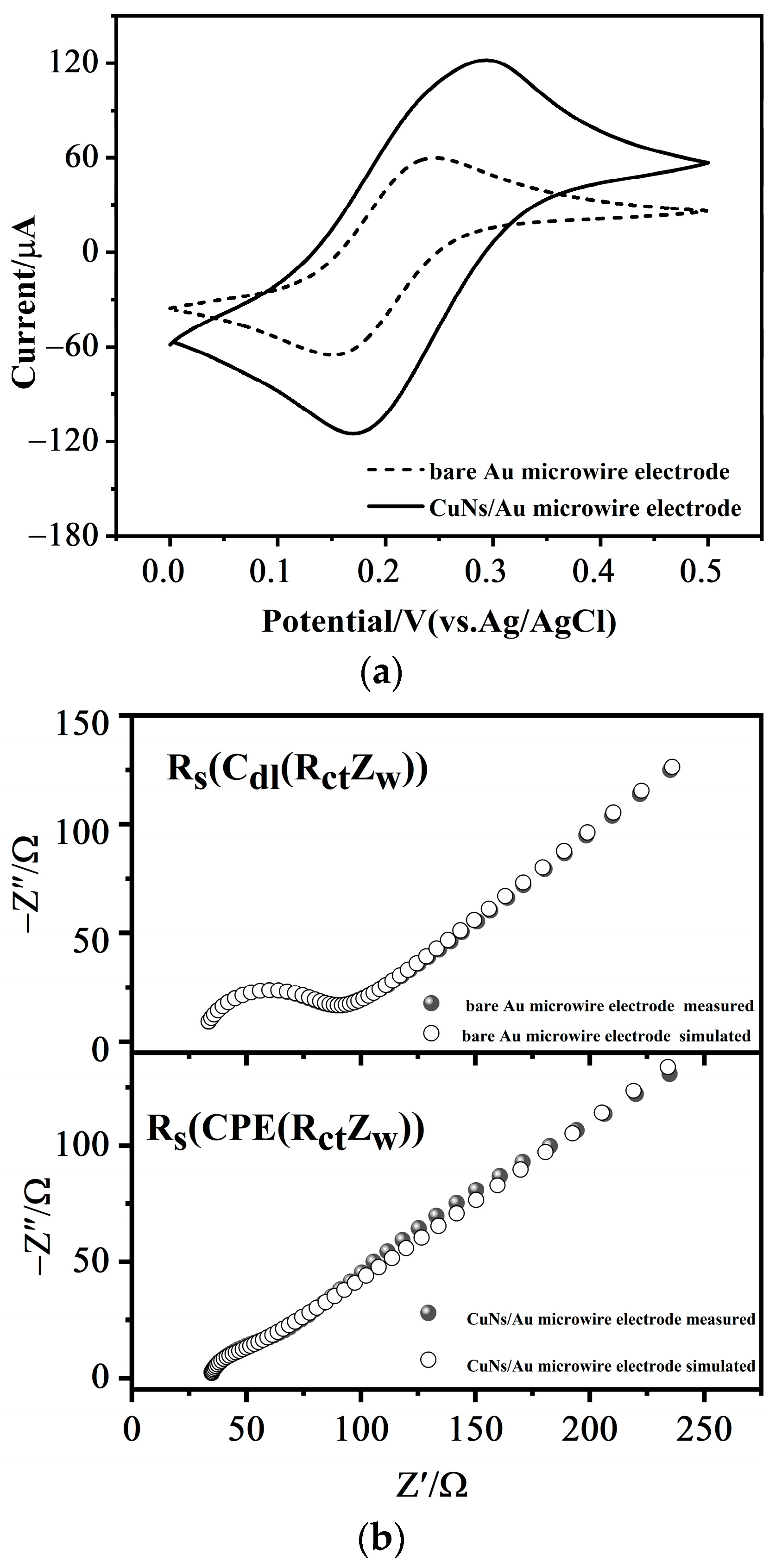
3.2.2. Cyclic Voltammetry
3.2.3. Electrochemical Impedance Spectroscopy
3.3. Voltammetric Response of Portable Electrochemical System in Artificial Seawater
3.3.1. Optimization Conditions
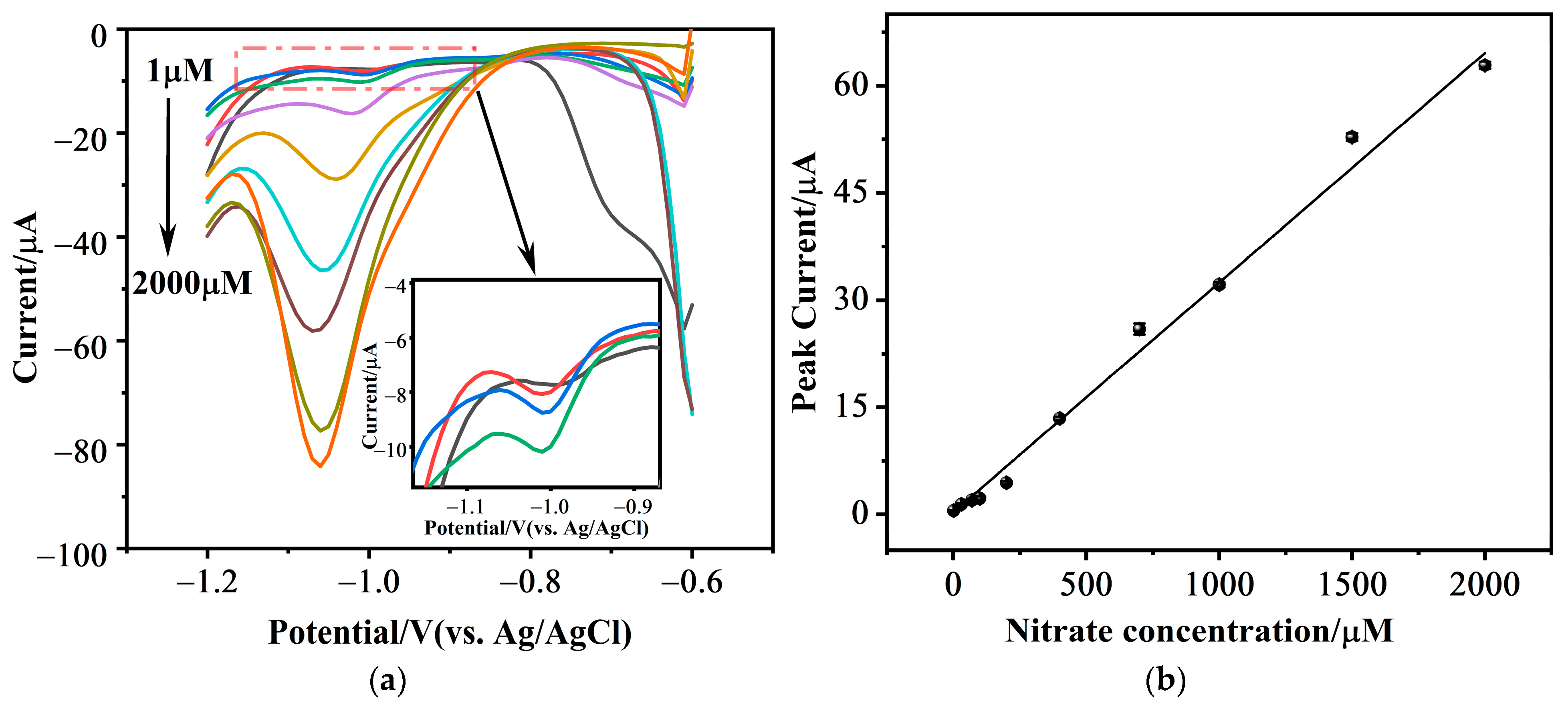
3.3.2. Typical Different Pulse Voltammetric Response
3.3.3. Linearity and Limit of Detection
3.3.4. Selectivity, Stability, and Reproducibility
3.4. Practical Application of Portable Electrochemical System in Coastal Seawater
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Casciotti, K.L. Nitrogen and oxygen isotopic studies of the marine nitrogen cycle. Annu. Rev. Mar. Sci. 2016, 8, 379–407. [Google Scholar] [CrossRef]
- Akçay, İ.; Özbay, Ö. Assessment of ecological and potential health risk caused by nitrate pollution of the berdan and göksu river basins, Turkey. J. Water Chem. Technol. 2024, 46, 645–651. [Google Scholar] [CrossRef]
- Smith, S.L.; Yamanaka, Y.; Pahlow, M.; Oschlies, A. Optimal uptake kinetics: Physiological acclimation explains the pattern of nitrate uptake by phytoplankton in the ocean. Mar. Ecol.-Prog. Ser. 2009, 384, 1–12. [Google Scholar] [CrossRef]
- Banerjee, P.; Garai, P.; Saha, N.C.; Saha, S.; Sharma, P.; Maiti, A.K. A critical review on the effect of nitrate pollution in aquatic invertebrates and fish. Water Air Soil Pollut. 2023, 234, 333. [Google Scholar] [CrossRef]
- Liefer, J.D.; White, A.E.; Finkel, Z.V.; Irwin, A.J.; Dugenne, M.; Inomura, K.; Ribalet, F.; Armbrust, E.V.; Karl, D.M.; Fyfe, M.H.; et al. Latitudinal patterns in ocean C:N:P reflect phytoplankton acclimation and macromolecular composition. Proc. Natl. Acad. Sci. USA 2024, 121, e2404460121. [Google Scholar] [CrossRef]
- Howarth, R.W.; Chan, F.; Swaney, D.P.; Marino, R.M.; Hayn, M. Role of external inputs of nutrients to aquatic ecosystems in determining prevalence of nitrogen vs. phosphorus limitation of net primary productivity. Biogeochemistry 2021, 154, 293–306. [Google Scholar] [CrossRef]
- Liang, W.; Wang, Y.; Mu, J.L.; Wu, N.; Wang, J.Y.; Liu, S.M. Nutrient changes in the Bohai Sea over the past two decades. Sci. Total Environ. 2023, 903, 166696. [Google Scholar] [CrossRef]
- Valiela, I.; Liu, D.; Lloret, J.; Chenoweth, K.; Hanacek, D. Stable isotopic evidence of nitrogen sources and C4 metabolism driving the world’s largest macroalgal green tides in the Yellow Sea. Sci. Rep. 2018, 8, 17437. [Google Scholar] [CrossRef]
- Levitus, S.; Conkright, M.E.; Reid, J.L.; Najjar, R.G.; Mantyla, A. Distribution of nitrate, phosphate and silicate in the world oceans. Prog. Oceanogr. 1993, 31, 245–273. [Google Scholar] [CrossRef]
- Wu, J.; Wang, Z.; Tian, J.; Li, N.; Wang, K.; Song, L.; Song, G.; Xu, X. Seasonal and long-term variations of nutrients in Liaodong Bay, China: Influencing factors and ecological effects. Mar. Environ. Res. 2024, 202, 106815. [Google Scholar] [CrossRef] [PubMed]
- Tuholske, C.; Halpern, B.S.; Blasco, G.; Villasenor, J.C.; Frazier, M.; Caylor, K. Mapping global inputs and impacts from of human sewage in coastal ecosystems. PLoS ONE 2021, 16, e0258898. [Google Scholar] [CrossRef]
- Xi, Y.; Wang, Q.; Zhu, J.; Yang, M.; Hao, T.; Chen, Y.; Zhang, Q.; He, N.; Yu, G. Atmospheric wet organic nitrogen deposition in China: Insights from the national observation network. Sci. Total Environ. 2023, 898, 165629. [Google Scholar] [CrossRef]
- Huesemann, M.H.; Skillman, A.D.; Crecelius, E.A. The inhibition of marine nitrification by ocean disposal of carbon dioxide. Mar. Pollut. Bull. 2002, 44, 142–148. [Google Scholar] [CrossRef]
- Arai, R.; Sakai, T.; Nishida, Y.; Inada, M. In situ temperature-compensated ultraviolet spectrophotometry to estimate nitrate and chloride concentrations in estuarine seawater with different salinity and composition. J. Environ. Manag. 2023, 344, 118689. [Google Scholar] [CrossRef] [PubMed]
- Niu, W.; Li, H.; Guo, X.; Chen, J.; Shi, X.; Zhu, Y. Field determination of nitrate in seawater using a novel on-line coppered cadmium column: A comparison study with the vanadium reduction method. Front. Mar. Sci. 2023, 10, 1138734. [Google Scholar] [CrossRef]
- Cheong, C.; Suzuki, T.; Miura, T.; Hioki, A. Comparison of continuous flow analysis and ion chromatography for determinations of nitrate, nitrite and phosphate ions in seawater and development of related seawater certified reference materials. Accredit. Qual. Assur. 2024, 29, 243–251. [Google Scholar] [CrossRef]
- Tahoun, I.F.; Rend, E.A.; Shehab, E. Optimization and validation of reversed phase ion-pair liquid chromatographic method for accurate determination of nitrate in high saline seawater. Egypt. J. Chem. 2024, 68, 135–139. [Google Scholar] [CrossRef]
- Lee, J.G.; Hong, J.; Lee, Y.; Lee, W.J.; Jeong, T.Y.; Oh, J.W. Point-of-care-testing NO3–N detection technology with selected transition-metal-based colorimetric sensor arrays. ACS Sens. 2025, 10, 986–994. [Google Scholar] [CrossRef]
- Tu, C.; Kee, L.H. Determination of nitrate in seawater by capillary zone electrophoresis with chloride-induced sample self-stacking. J. Chromatogr. A 2002, 966, 205–212. [Google Scholar] [CrossRef]
- Küster, T.; Bothun, G.D. In situ SERS detection of dissolved nitrate on hydrated gold substrates. Nanoscale Adv. 2021, 3, 4098–4105. [Google Scholar] [CrossRef]
- Chandrasekara, N.P.G.N.; Pashley, R.M. Study of a new process for the efficient regeneration of ion exchange resins. Desalination 2015, 357, 131–139. [Google Scholar] [CrossRef]
- Yuan, W.; Chen, X.; Yu, Z.; Wan, Y.; Lin, J.; Ye, W. Critical review of membrane fouling in reverse osmosis treatment: Characterizations, models, mechanisms, and controls. Sep. Purif. Technol. 2025, 363, 132119. [Google Scholar] [CrossRef]
- Wei, H.; Pan, D.; Han, H. Electrochemical monitoring of marine nutrients: From principle to application. Trac-Trends Anal. Chem. 2021, 138, 116242. [Google Scholar] [CrossRef]
- Paliwal, A.; Bandas, C.D.; Thornburg, E.S.; Haasch, R.T.; Gewirth, A.A. Enhanced nitrate reduction activity from Cu-alloy electrodes in an alkaline electrolyte. ACS Catal. 2023, 13, 6754–6762. [Google Scholar] [CrossRef]
- Li, D.; Xu, S.; Jin, H.; Wang, J.; Yan, F. Copper nanoparticles confined in a silica nanochannel film for the electrochemical detection of nitrate ions in water samples. Molecules 2023, 28, 7515. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, M.; Li, S.; Ren, Y.; Qin, G. Copper wires with seamless 1D nanostructures: Preparation and electrochemical sensing performance. Mater. Lett. 2018, 211, 247–249. [Google Scholar] [CrossRef]
- Brug, G.J.; van den Eeden, A.L.G.; Sluyters-Rehbach, M.; Sluyters, J.H. The Analysis of electrode impedances complicated by the presence of a constant phase element. J. Electroanal. Chem. 1984, 176, 275–295. [Google Scholar] [CrossRef]
- Randles, J.E.B. Kinetics of Rapid Electrode Reactions. Discuss. Faraday Soc. 1947, 1, 11–19. [Google Scholar] [CrossRef]
- Outaleb, H.; Kouzbour, S.; Audonnet, F.; Vial, C.; Gourich, B. Electrocatalytic nitrate reduction for brackish groundwater treatment: From engineering aspects to implementation. Appl. Sci. 2024, 14, 8986. [Google Scholar] [CrossRef]
- Pushpanjali, P.A.; Manjunatha, J.G.; Hareesha, N.; Souza, E.S.D.; Charithra, M.M.; Prinith, N.S. Voltammetric analysis of antihistamine drug cetirizine and paracetamol at poly(L-Leucine) layered carbon nanotube paste electrode. Surf. Interface 2021, 24, 101154. [Google Scholar] [CrossRef]
- Wu, K.; Sun, C.; Wang, Z.; Song, Q.; Bai, X.; Yu, X.; Li, Q.; Wang, Z.; Zhang, H.; Tong, X.; et al. Surface reconstruction on uniform Cu nanodisks boosted electrochemical nitrate reduction to ammonia. ACS Mater. Lett. 2022, 4, 650–656. [Google Scholar] [CrossRef]
- Amini, N.; Maleki, A.; Maleki, P. Electrochemical detection of nitrate ions via reduction of NO2− and oxidation of NO reactions based on Cu@TiO2 coreshell/nafion/polyalizarin immobilized electrode. Mater. Chem. Phys. 2021, 264, 124384. [Google Scholar] [CrossRef]
- Patella, B.; Russo, R.R.; O’Riordan, A.; Aiello, G.; Sunseri, C.; Inguanta, R. Copper nanowire array as highly selective electrochemical sensor of nitrate ions in water. Talanta 2021, 221, 121643. [Google Scholar] [CrossRef] [PubMed]
- Abed, S.; Gibilaro, M.; Chamelot, P.; David, A.; Barus, C.; Massot, L. The optimization of bimetallic electrodes’ sensitivity using copper nucleation on metallic substrates to detect nitrates in seawater. J. Electroanal. Chem. 2022, 918, 116497. [Google Scholar] [CrossRef]
- Abir, A.Y.; Uddin, S.M.N.; Hasan, M.; Aziz, M.A.; Shah, S.; Ahmed, J.; Hasnat, M.A. Cu-electrodeposited gold electrode for the sensitive electrokinetic investigations of nitrate reduction and detection of the nitrate ion in acidic medium. Results Chem. 2023, 5, 100702. [Google Scholar] [CrossRef]
- Talbi, M.; Anurag, A.; Tegenkamp, C.; Ali, M.B.; Kanoun, O. Enhanced electrochemical detection of nitrite and nitrate in water using Chitosan-Copper phthalocyanine nanocomposite. Measurement 2024, 238, 115395. [Google Scholar] [CrossRef]
- Wei, S.; Xiao, D.; Bian, C.; Li, Y. Phosphate and nitrate electrochemical sensor based on a bifunctional boron-doped diamond electrode. ACS Omega 2024, 9, 20293–20303. [Google Scholar] [CrossRef]
- Zhu, X.; Xu, J.; Zhang, N.; Wu, C. Nitrate measurement in seawater based on environmental correction algorithm. IEEE Sens. J. 2023, 23, 15803–15812. [Google Scholar] [CrossRef]
- Prabhu, K.; Malode, S.J.; Shetti, N.P. Highly sensitive electrochemical sensor for the detection and quantification of Linuron based on silica gel modified carbon paste electrode. Environ. Technol. Innov. 2021, 23, 101687. [Google Scholar] [CrossRef]
- Zheng, Y.L.; Hou, L.J.; Liu, M.; Liu, Z.F.; Li, X.F.; Lin, X.B.; Yin, G.Y.; Gao, J.; Yu, C.D.; Wang, R.; et al. Tidal pumping facilitates dissimilatory nitrate reduction in intertidal marshes. Sci. Rep. 2016, 6, 21338. [Google Scholar] [CrossRef]
- Wallace, C.D.; Sawyer, A.H.; Barnes, R.T.; Soltanian, M.R.; Gabor, R.S.; Wilkins, M.J.; Moore, M.T. A model analysis of the tidal engine that drives nitrogen cycling in coastal riparian aquifers. Water Resour. Res. 2020, 56, e2019WR025662. [Google Scholar] [CrossRef]
- Sendrowski, A.; Castañeda-Moya, E.; Twilley, R.; Passalacqua, P. Biogeochemical and hydrological variables synergistically influence nitrate variability in coastal deltaic wetlands. J. Geophys. Res. Biogeosciences 2021, 126, e2020JG005737. [Google Scholar] [CrossRef]
- Cuartero, M.; Crespo, G.; Cherubini, T.; Pankratova, N.; Confalonieri, F.; Massa, F.; Tercier-Waeber, M.L.; Abdou, M.; Schäfer, J.; Bakker, E. In situ detection of macronutrients and chloride in seawater by submersible electrochemical sensors. Anal. Chem. 2018, 90, 4702–4710. [Google Scholar] [CrossRef] [PubMed]




| Electrode | Methods | LOD (μM) | Linear Range (μM) | pH Value | Sample Matrix | Real Samples | Ref. |
|---|---|---|---|---|---|---|---|
| Cu@TiO2-Nf 1/PAR 2/GCE 3 | DPV | 2.1 | 5~7500 | 1.7 | phosphate buffer | River water; tap water | [32] |
| Cu-NWs 4/Poretics™-Au 5 | CV, LSV | 9 | 10~50 50~1500 | 2.5 | 0.1 M Na2SO4 | Rain water; river water; drinking water | [33] |
| Cu/Pt 6 | SWV | 0.5 | 0.5~15 | 8 | 0.56 M NaCl | Artificial seawater | [34] |
| CuNPs/NH2-VMSF 7/ITO 8 | DPV | 2.3 | 5.0~1000 | 3.0 | 0.1 M Na2SO4 | Tap water; pond water; rain water; seawater | [25] |
| Cu/Au | DPV | 0.52 | 20~800 | 1 | 0.1 M H2SO4 | Firm-land water; tap water; power plant water | [35] |
| Chit 9/Ts-CuPc 10/CSPE 11 | CV, SWV | 0.05 | 1~10 | 2.0 | 0.01 M phosphate buffer | Mineral water; olive canned water | [36] |
| Cu/BDD 12 | LSV | 4.6 | 5~214 214~7143 | 1.5 | 0.1 M Na2SO4 | Tap water; lake water | [37] |
| CuNs 13/Au microwire electrode | DPV | 0.3 | 1~2000 | 6 | 0.6 M NaCl | Coastal seawater | This work |
| Real Seawater Samples | UV–Visible Spectrophotometry (μM) | DPV Method (μM) |
|---|---|---|
| Seawater 1 | 3.23 ± 0.03 | 3.21 ± 0.20 |
| Seawater 2 | 8.34 ± 0.04 | 8.13 ± 0.91 |
| Seawater 3 | 5.06 ± 0.03 | 5.13 ± 0.06 |
| Seawater 4 | 6.26 ± 0.04 | 6.12 ± 0.16 |
| Seawater 5 | 20.33 ± 0.06 | 21.21 ± 0.95 |
| Seawater 6 | 11.86 ± 0.08 | 11.79 ± 0.36 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
He, X.; Wei, H.; Ouyang, T.; Xu, Z.; Liu, T.; Cheng, Y.; Ma, Z.; Tao, W.; Pan, D. Fabrication and Characterization of a Portable and Electrochemical System for Field Determination of Nitrate in Coastal Seawater. Chemosensors 2025, 13, 366. https://doi.org/10.3390/chemosensors13100366
He X, Wei H, Ouyang T, Xu Z, Liu T, Cheng Y, Ma Z, Tao W, Pan D. Fabrication and Characterization of a Portable and Electrochemical System for Field Determination of Nitrate in Coastal Seawater. Chemosensors. 2025; 13(10):366. https://doi.org/10.3390/chemosensors13100366
Chicago/Turabian StyleHe, Xiaoling, Hong Wei, Tian Ouyang, Ziwen Xu, Taoda Liu, Ying Cheng, Ziman Ma, Wenyan Tao, and Dawei Pan. 2025. "Fabrication and Characterization of a Portable and Electrochemical System for Field Determination of Nitrate in Coastal Seawater" Chemosensors 13, no. 10: 366. https://doi.org/10.3390/chemosensors13100366
APA StyleHe, X., Wei, H., Ouyang, T., Xu, Z., Liu, T., Cheng, Y., Ma, Z., Tao, W., & Pan, D. (2025). Fabrication and Characterization of a Portable and Electrochemical System for Field Determination of Nitrate in Coastal Seawater. Chemosensors, 13(10), 366. https://doi.org/10.3390/chemosensors13100366






