Indium Oxide Decorated WS2 Microflakes for Selective Ammonia Sensors at Room Temperature
Abstract
:1. Introduction
2. Experimental Method
2.1. Synthesis of In2O3/WS2 Nanocomposites
2.2. Characterization
2.3. Fabrication and Measurement of the Gas Sensors
3. Results and Discussion
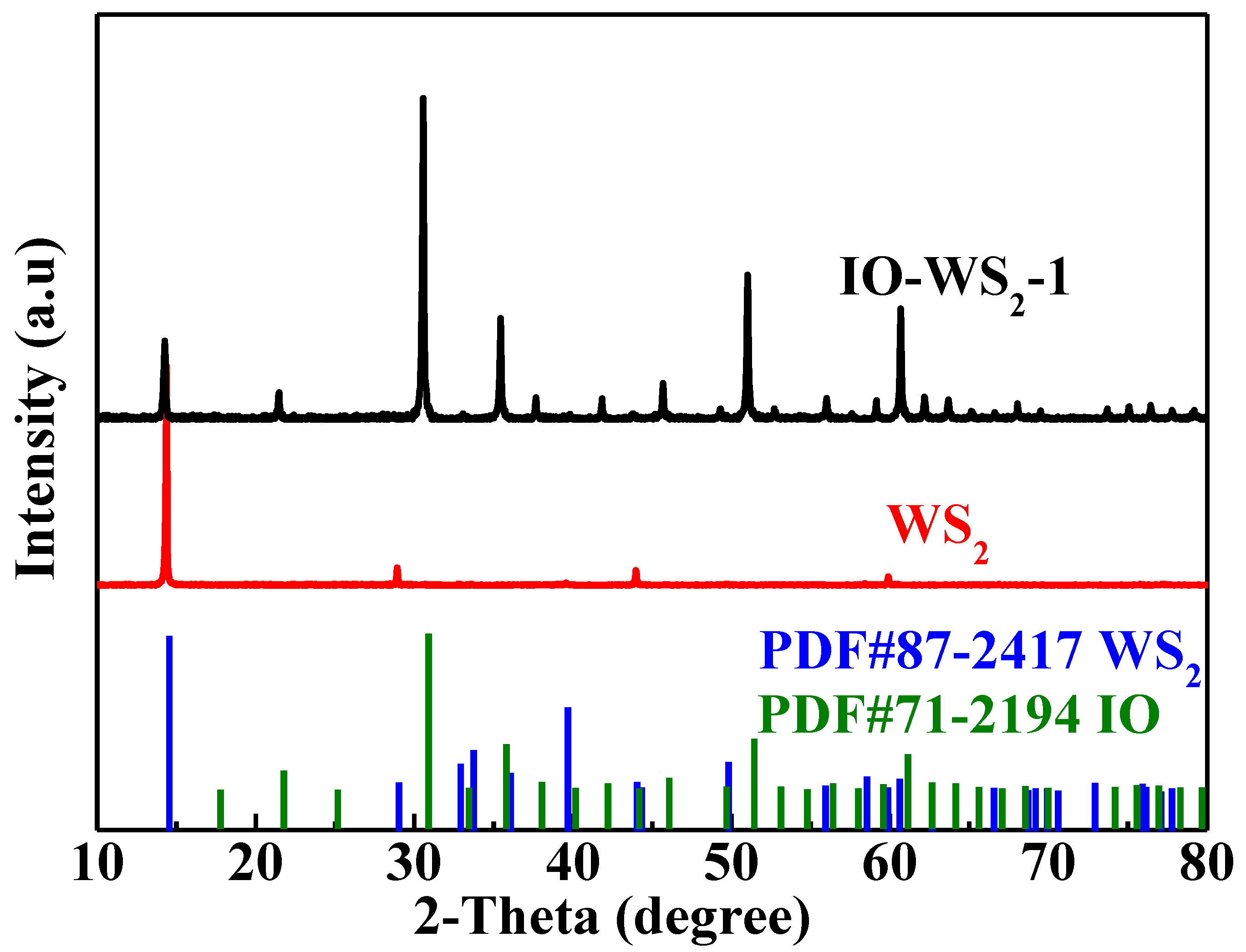
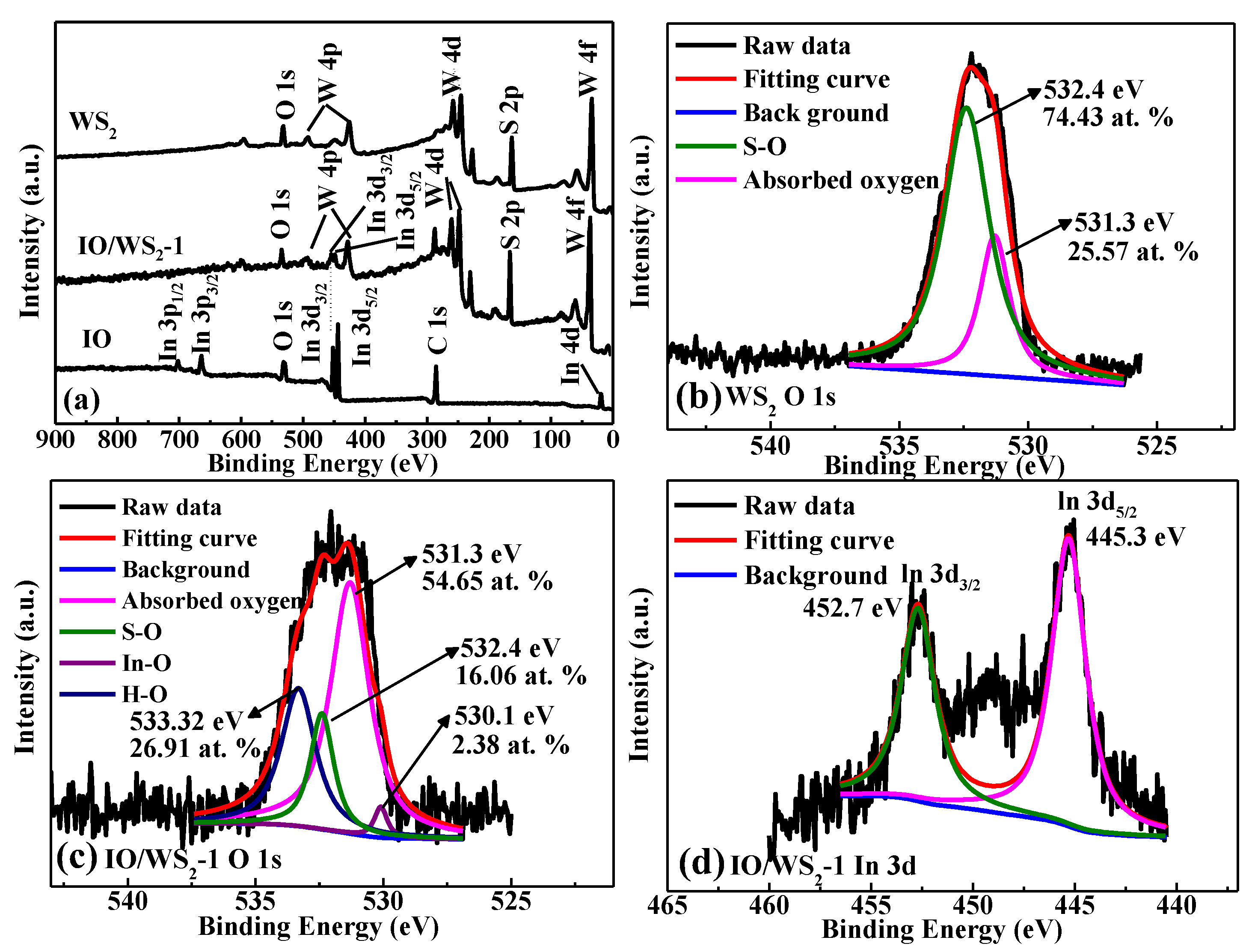
3.1. Microstructure Characterizations
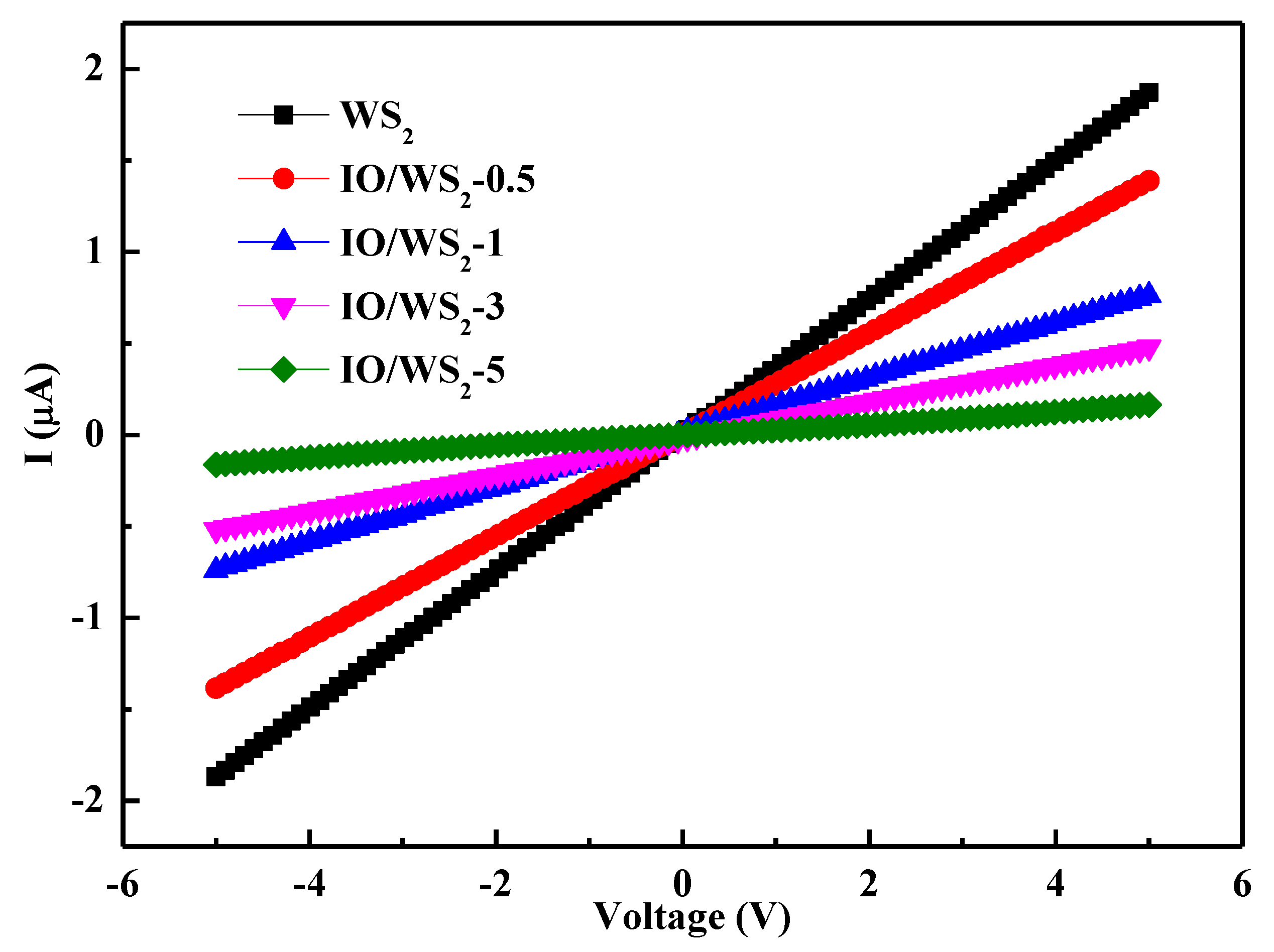
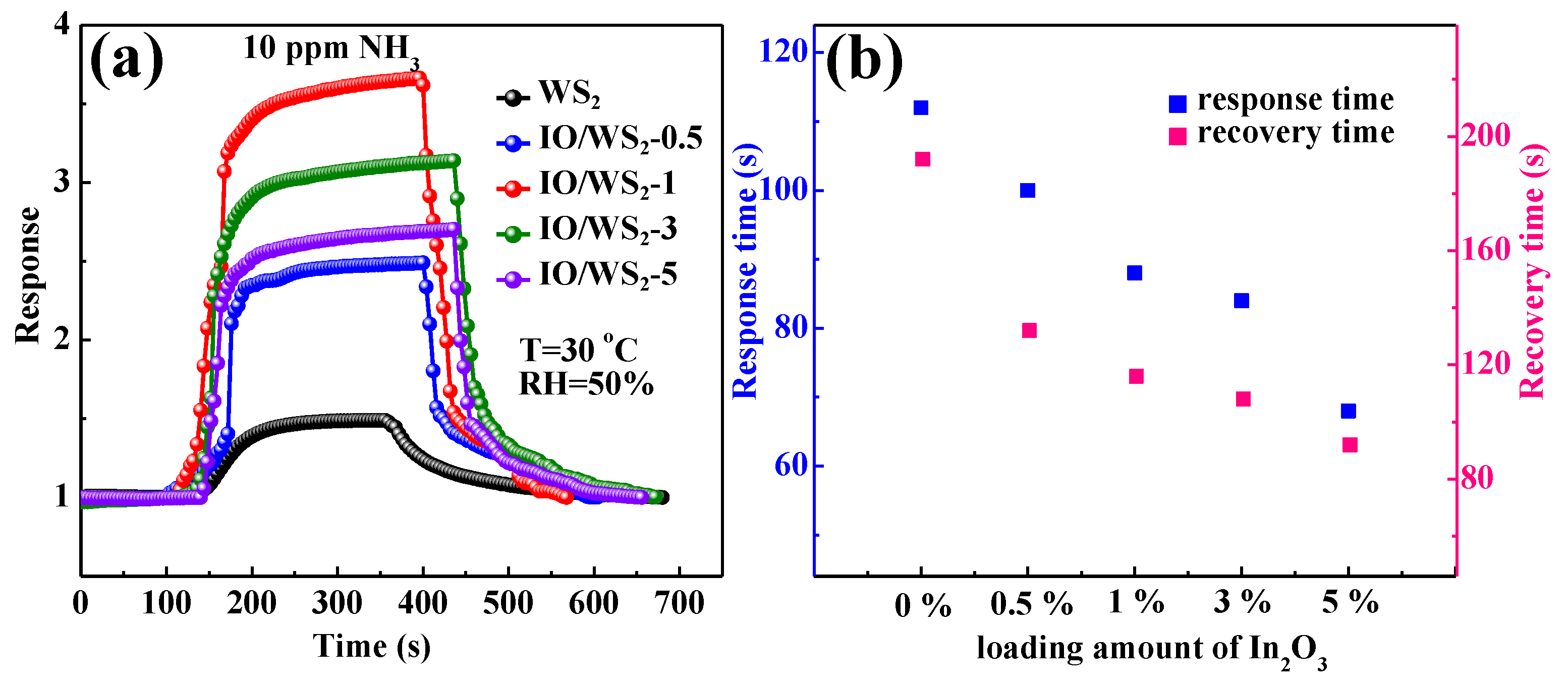
3.2. Electrical and Sensing Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Negwamba, T.; Dinka, M.O. Assessing the performance of Trichardt Wastewater Treatment Plant (South Africa). Phys. Chem. Earth 2019, 114, 102784. [Google Scholar] [CrossRef]
- Cecilia, C.; Federica, B.; Marcella, G. Ammonia concentration and recommended threshold values in pig farming: A review. In Proceedings of the IEEE International Workshop on Metrology for Agriculture and Forestry, Trento-Bolzano, Italy, 3–5 November 2021; pp. 162–166. [Google Scholar]
- Macedonio, F.; Frappa, M.; Bamaga, O. Application of a membrane condenser system for ammonia recovery from humid waste gaseous streams at a minimum energy consumption. Appl. Water Sci. 2022, 12, 90. [Google Scholar] [CrossRef]
- Berwal, P.; Kumar, S.; Khandelwal, B. A comprehensive review on synthesis, chemical kinetics, and practical application of ammonia as future fuel for combustion. J. Energy Inst. 2021, 99, 273–298. [Google Scholar] [CrossRef]
- Mallouppas, G.; Ioannou, C.; Yfantis, E.A. A Review of the Latest Trends in the Use of Green Ammonia as an Energy Carrier in Maritime Industry. Energies 2022, 15, 1453. [Google Scholar] [CrossRef]
- Gardner, R. Use of the reciprocal calculation procedure for setting workplace emergency action levels for hydrocarbon mixtures and their relationship to lower explosive limits. Ann. Occup. Hyg. 2011, 55, 326–339. [Google Scholar]
- Pedersen, J.M.; Feilberg, A.; Kamp, J.N.; Hafner, S.; Nyord, T. Ammonia emission measurement with an online wind tunnel system for evaluation of manure application techniques. Atmos. Environ. 2020, 230, 117562. [Google Scholar] [CrossRef]
- Orozco, J.L.; Caneghem, J.V.; Hens, L.; González, L.; Lugo, R.; Díaz, S.; Pedroso, I. Assessment of an ammonia incident in the industrial area of Matanzas. J. Clean. Prod. 2019, 222, 934–941. [Google Scholar] [CrossRef]
- Kwak, D.; Lei, Y.; Maric, R. Ammonia gas sensors: A comprehensive review. Talanta 2019, 204, 713–730. [Google Scholar] [CrossRef]
- Aarya, S.; Kumar, Y.; Chahota, R.K. Recent Advances in Materials, Parameters, Performance and Technology in Ammonia Sensors: A Review. J. Inorg. Organomet. Polym. 2020, 30, 269–290. [Google Scholar] [CrossRef]
- Tsymbalov, A.V.; Kalygina, V.M.; Maksimova, N.K.; Chernikov, E.V. Ammonia sensors based on resistive structures M–SnO2: Sb–M. J. Phys. Conf. Ser. 2020, 1499, 012037. [Google Scholar] [CrossRef]
- Kathwate, L.H.; Umadevi, G.; Kulal, P.M.; Nagaraju, P.; Dubal, D.P.; Nanjundan, A.K.; Mote, V.D. Ammonia gas sensing properties of Al doped ZnO thin films. Sens. Actuators A Phys. 2020, 313, 112193. [Google Scholar] [CrossRef]
- Yamazoe, N.; Tamaki, J.; Miura, N. Role of hetero-junctions in oxide semiconductor gas sensors. Mater. Sci. Eng. B 1996, 41, 178–181. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, J.; Xu, Z.; Shen, W.; Wu, Y.; Corriou, J.P. Computational assisted tuning of Co-doped TiO2 nanoparticles for ammonia detection at room temperatures. Appl. Surf. Sci. 2022, 601, 154214. [Google Scholar] [CrossRef]
- Büyükköse, S. Highly selective and sensitive WO3 nanoflakes based ammonia sensor. Mater. Sci. Semicond. Process. 2020, 110, 104969. [Google Scholar] [CrossRef]
- Bhardwaj, S.K.; Bhardwaj, N.; Kukkar, M.; Sharma, A.L.; Kim, K.H.; Deep, A. Formation of High-Purity Indium Oxide Nanoparticles and Their Application to Sensitive Detection of Ammonia. Sensors 2015, 15, 31930–31938. [Google Scholar] [CrossRef] [Green Version]
- Sharieh, J.K.; Rahim, G.; Maryam, S. Attached two folded graphene nanoribbons as sensitive gas sensor. Phys. B Condens. Matter 2022, 628, 413630. [Google Scholar]
- Tang, X.; Debliquy, M.; Lahem, D.; Yan, Y.; Raskin, J.P. A Review on Functionalized Graphene Sensors for Detection of Ammonia. Sensors 2021, 21, 1443. [Google Scholar] [CrossRef]
- Rani, S.; Sharma, M.; Verma, D.; Ghanghass, A.; Bhatia, R.; Sameera, I. Two-dimensional transition metal dichalcogenides and their heterostructures: Role of process parameters in top-down and bottom-up synthesis approaches. Mater. Sci. Semicond. Process. 2022, 139, 106313. [Google Scholar] [CrossRef]
- Sukhwinder, S.; Jyotirmoy, D.; Utpal, S.; Sandeep, S. MoS2/WO3Nanosheets for Detection of Ammonia. ACS Appl. Nano Mater. 2021, 4, 2594–2605. [Google Scholar]
- Xia, Y.; Xu, L.; He, S.; Zhou, L.; Wang, M.; Wang, J.; Komarneni, S. UV-activated WS2/SnO2 2D/0D heterostructures for fast and reversible NO2 gas sensing at room temperature. Sens. Actuators B Chem. 2022, 364, 131903. [Google Scholar] [CrossRef]
- Wang, X.; Huang, B.; Wu, X.; Gu, D.; Li, X. Enhanced ammonia sensing properties of rGO/WS2 heterojunction based chemiresistive sensor by marginal sulfonate decoration. Sens. Actuators B Chem. 2021, 337, 129776. [Google Scholar] [CrossRef]
- Kim, J.H.; Mirzaei, A.; Kim, J.Y.; Yang, D.H.; Kim, S.S.; Kim, H.W. Selective CO gas sensing by Au-decorated WS2-SnO2 core-shell nanosheets on flexible substrates in self-heating mode. Sens. Actuators B Chem. 2022, 353, 131197. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Li, Z.; Wang, J.; Zhang, J. WS2 nanoflakes based selective ammonia sensors at room temperature. Sens. Actuators B Chem. 2017, 240, 273–277. [Google Scholar] [CrossRef]
- Tian, J.; Chen, X.; Wang, T.; Pei, W.; Li, F.; Li, D.; Yang, Y.; Dong, X. Modification of indium oxide nanofibers by polyoxometalate electron acceptor doping for enhancement of gas sensing at room temperature. Sens. Actuators B Chem. 2021, 344, 130227. [Google Scholar]
- Yang, Z.; Zhang, D.; Chen, H. MOF-derived indium oxide hollow microtubes/MoS2 nanoparticles for NO2 gas sensing. Sens. Actuators B Chem. 2019, 300, 127037. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, J.; Cao, Y. Cobalt-doped indium oxide/molybdenum disulfide ternary nanocomposite toward carbon monoxide gas sensing. J. Alloy. Compd. 2019, 777, 443–453. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Zhang, J.; Yan, H.; Li, J.; Yang, Z.; Wang, Q. Highly sensitive detection of acetone using mesoporous In2O3 nanospheres decorated with Au nanoparticles. Sens. Actuators B 2017, 242, 983–993. [Google Scholar] [CrossRef]
- Zoleikha, H.; Reza, T.L.; Fereshteh, R.A. 3-Identification and analytical methods. In Heterogeneous Micro and Nanoscale Composites for the Catalysis of Organic Reactions; Elsevier: Amsterdam, The Netherlands, 2022; pp. 33–51. [Google Scholar]
- Guang, Q.; Huang, B.; Li, X. Au-Decorated WS2 Microflakes Based Sensors for Selective Ammonia Detection at Room Temperature. Chemosensors 2022, 10, 9. [Google Scholar] [CrossRef]
- Huang, B.; Wang, Y.; Hu, Q.; Mu, X.; Zhang, Y.; Bai, J.; Wang, Q.; Sheng, Y.; Zhang, Z.; Xie, E. A low temperature and highly sensitive ethanol sensor based on Au modified In2O3 nanofibers by coaxial electrospinning. J. Mater. Chem. C 2018, 6, 10935. [Google Scholar] [CrossRef]
- Liu, Z.; He, T.; Sun, H.; Huang, B.; Li, X. Layered MXene heterostructured with In2O3 nanoparticles for ammonia sensors at room temperature. Sens. Actuators B Chem. 2022, 365, 131918. [Google Scholar] [CrossRef]
- Liu, W.; Gu, D.; Li, X. Ultrasensitive NO2 Detection Utilizing Mesoporous ZnSe/ZnO Heterojunction-Based Chemiresistive-Type Sensors. ACS Appl. Mater. Interfaces 2019, 11, 29029–29040. [Google Scholar] [CrossRef] [PubMed]
- Silva, J.C.M.; Piasentin, R.M.; Spinace, E.V.; Neto, A.O.; Baranova, E.A. The effect of antimony-tin and indium-tin oxide supports on the catalytic activity of Pt nanoparticles for ammonia electro-oxidation. Mater. Chem. Phys. 2016, 180, 97–103. [Google Scholar] [CrossRef]
- Wang, X.Y.; Gu, D.; Li, X.; Lin, S.; Zhao, S.; Rumyantseva, M.N.; Gaskov, A.M. Reduced graphene oxide hybridized with WS2 nanoflakes based heterojunctions for selective ammonia sensors at room temperature. Sens. Actuators B Chem. 2019, 282, 290–299. [Google Scholar] [CrossRef]
- Zhao, C.; Wang, P.; Niu, G.; Luo, D.; Wang, Q.; Wang, F. Rapid and Efficient Detection of NH3 at Room Temperature Using CuO/WS2 Nanohybrids. IEEE Sens. J. 2022, 17, 3175827. [Google Scholar] [CrossRef]
- Perrozzi, F.; Emamjomeh, S.M.; Paolucci, V.; Taglieri, G.; Ottaviano, L.; Cantalini, C. Thermal stability of WS2 flakes and gas sensing properties of WS2/WO3 composite to H2, NH3 and NO2. Sens. Actuators B Chem. 2017, 243, 812–822. [Google Scholar] [CrossRef]

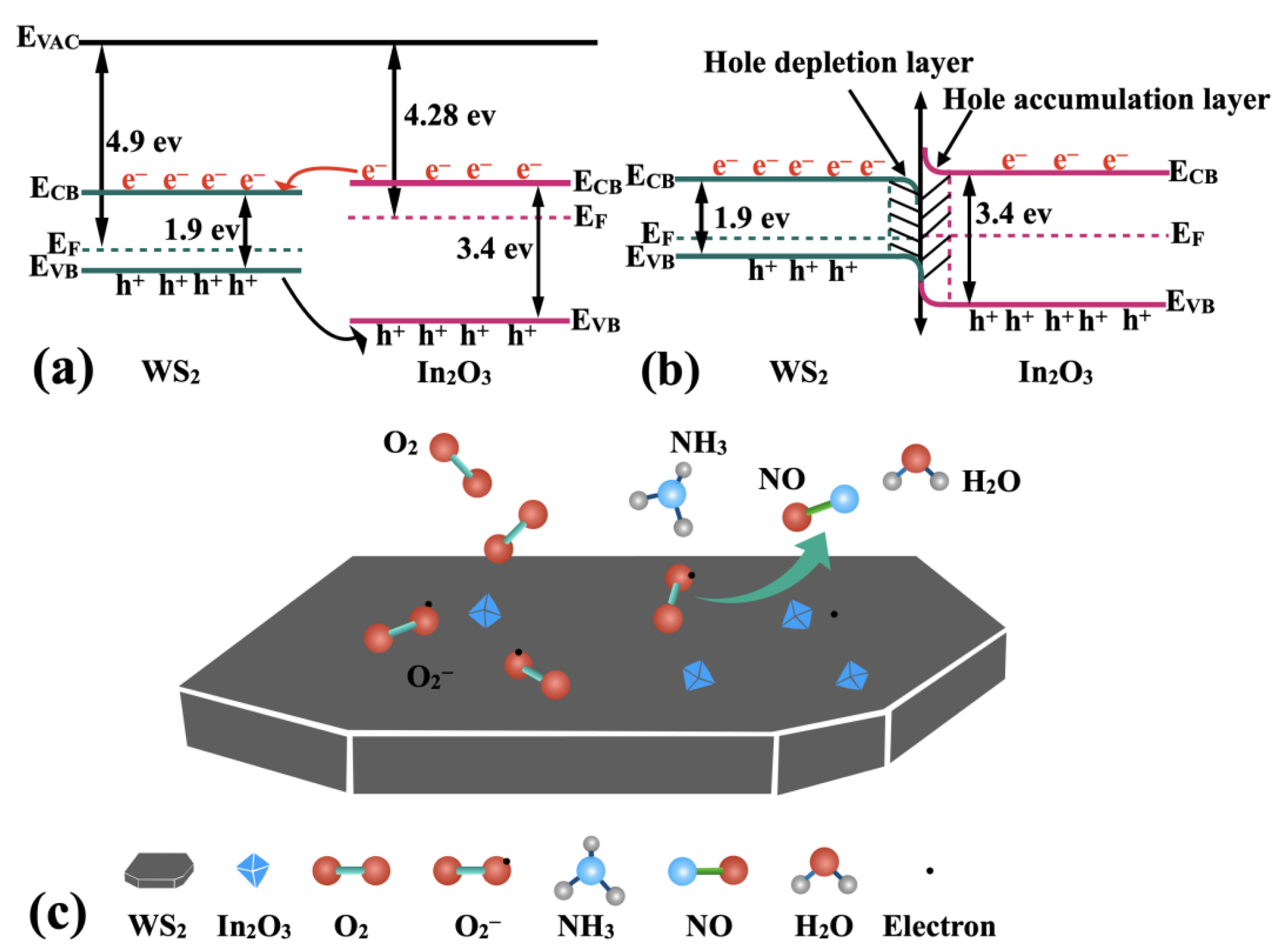
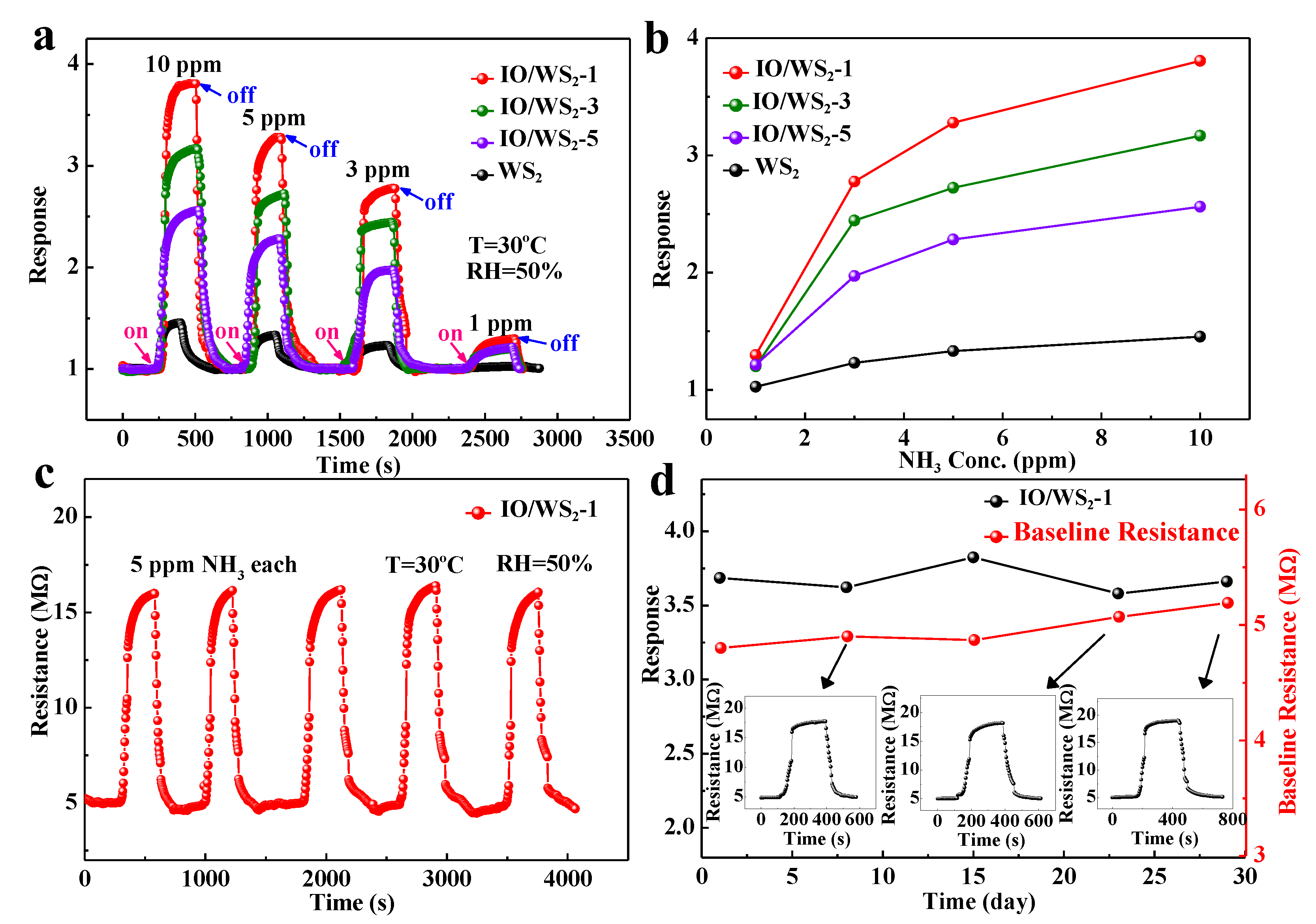
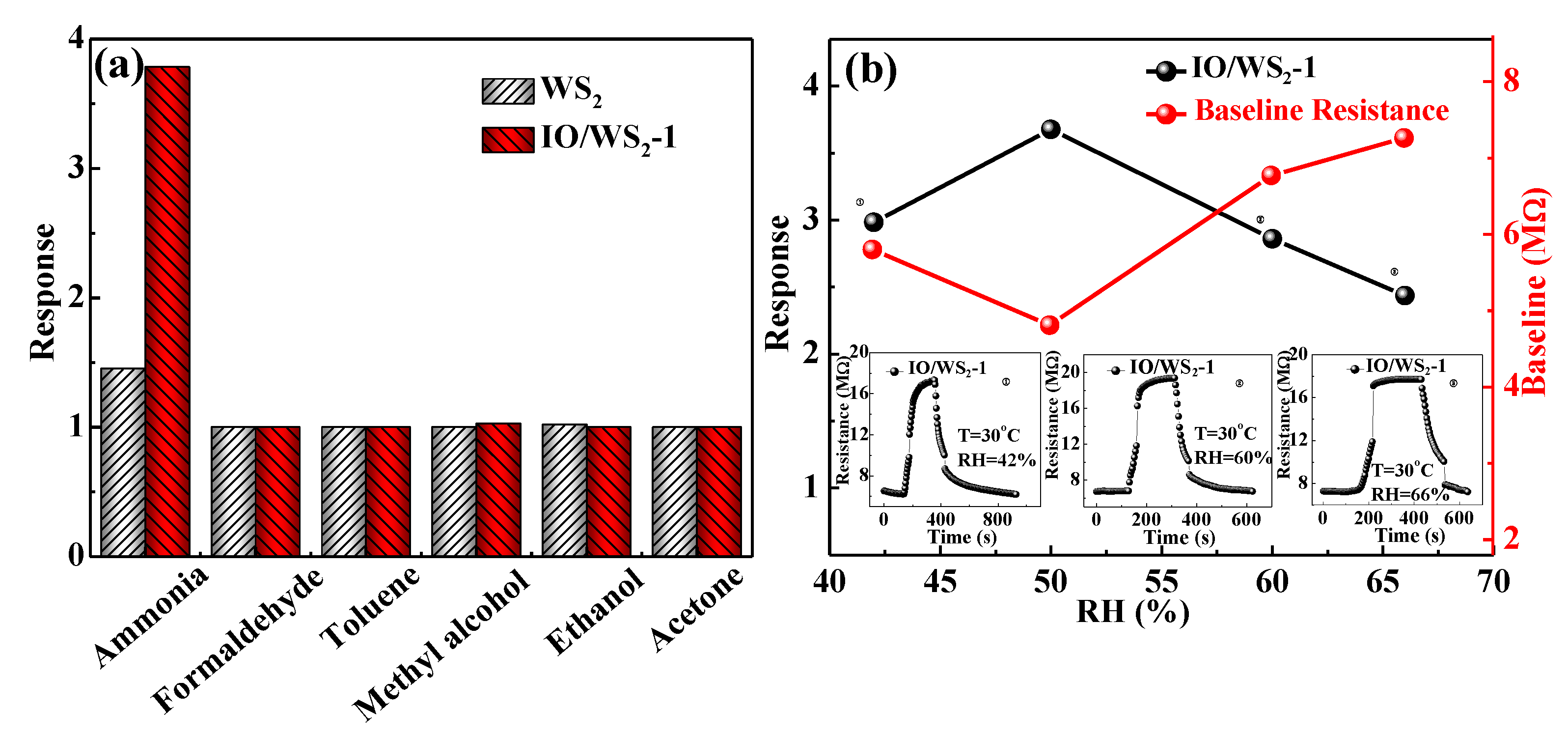
| Sensitive Material | NH3 Concentration | Temperature (°C) | Response (%) | Response/ Recovery (s/s) | Ref. |
|---|---|---|---|---|---|
| WS2 nanoflakes | 5 ppm | RT | 217 a | 120/150 | [24] |
| rGO/WS2 | 10 ppm | RT | 71 | 240/600 | [35] |
| 2:1 CuO/WS2 nanohybrids | 60 ppm | RT (30 °C) | 59.5 | 35/213 | [36] |
| WS2/WO3 Au/WS2 IO/WS2 ZnO/WS2 SnO2/WS2 | 10 ppm 1 ppm 1 ppm 1 ppm 1 ppm | 150 °C RT RT RT RT | 400 191.54 129.84 133.63 117 | ~150/~100 968/788 160/44 288/44 224/24 | [37] [30] This work This work This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guang, Q.; Huang, B.; Yu, J.; Zhang, J.; Li, X. Indium Oxide Decorated WS2 Microflakes for Selective Ammonia Sensors at Room Temperature. Chemosensors 2022, 10, 402. https://doi.org/10.3390/chemosensors10100402
Guang Q, Huang B, Yu J, Zhang J, Li X. Indium Oxide Decorated WS2 Microflakes for Selective Ammonia Sensors at Room Temperature. Chemosensors. 2022; 10(10):402. https://doi.org/10.3390/chemosensors10100402
Chicago/Turabian StyleGuang, Qiyilan, Baoyu Huang, Jun Yu, Jianwei Zhang, and Xiaogan Li. 2022. "Indium Oxide Decorated WS2 Microflakes for Selective Ammonia Sensors at Room Temperature" Chemosensors 10, no. 10: 402. https://doi.org/10.3390/chemosensors10100402
APA StyleGuang, Q., Huang, B., Yu, J., Zhang, J., & Li, X. (2022). Indium Oxide Decorated WS2 Microflakes for Selective Ammonia Sensors at Room Temperature. Chemosensors, 10(10), 402. https://doi.org/10.3390/chemosensors10100402






