High-Performance Sulfur Dioxide Gas Sensor Based on Graphite-Phase Carbon-Nitride-Functionalized Tin Diselenide Nanorods Composite
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
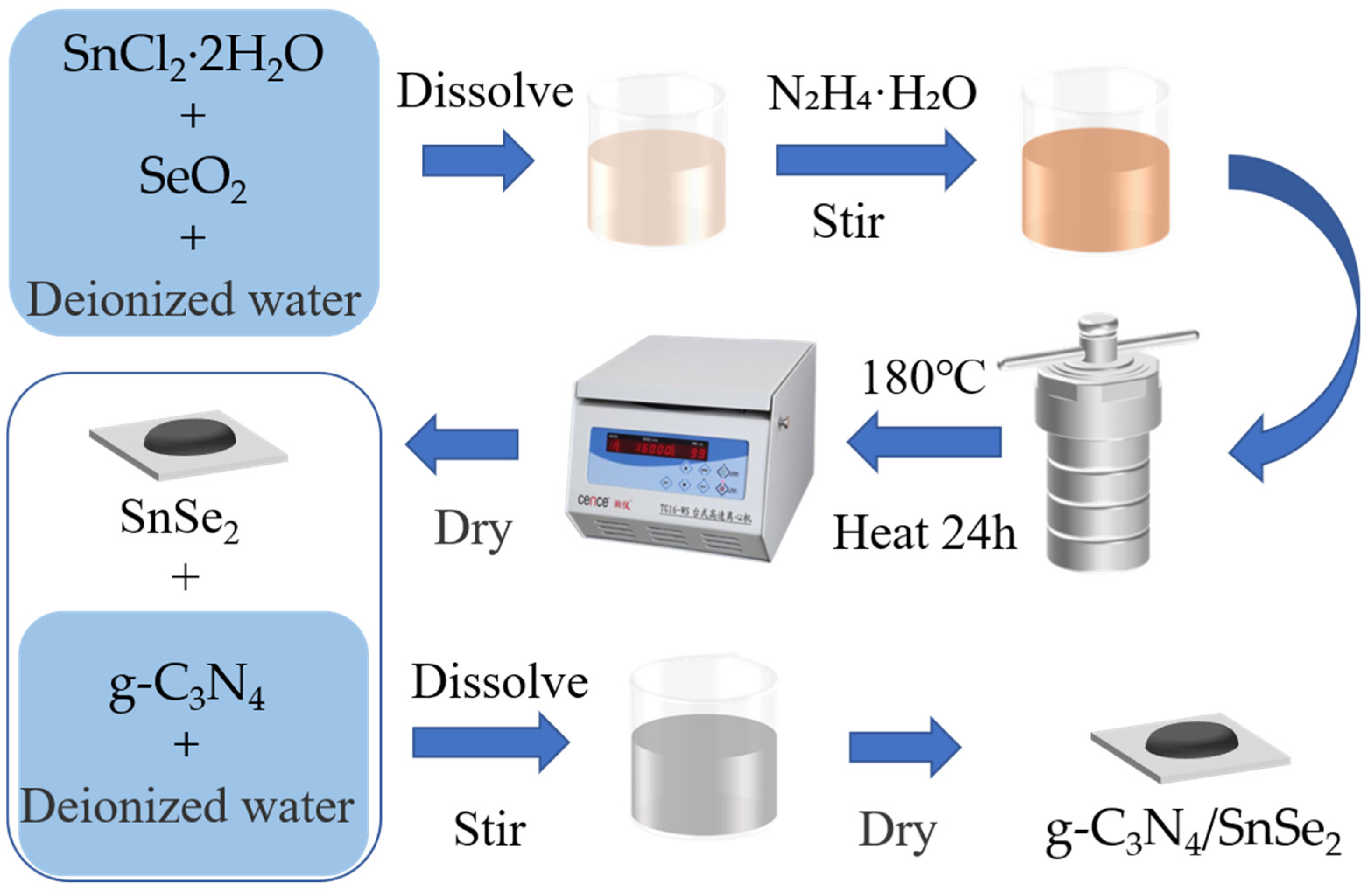
2.2. Material Synthesis and Sensor Fabrication
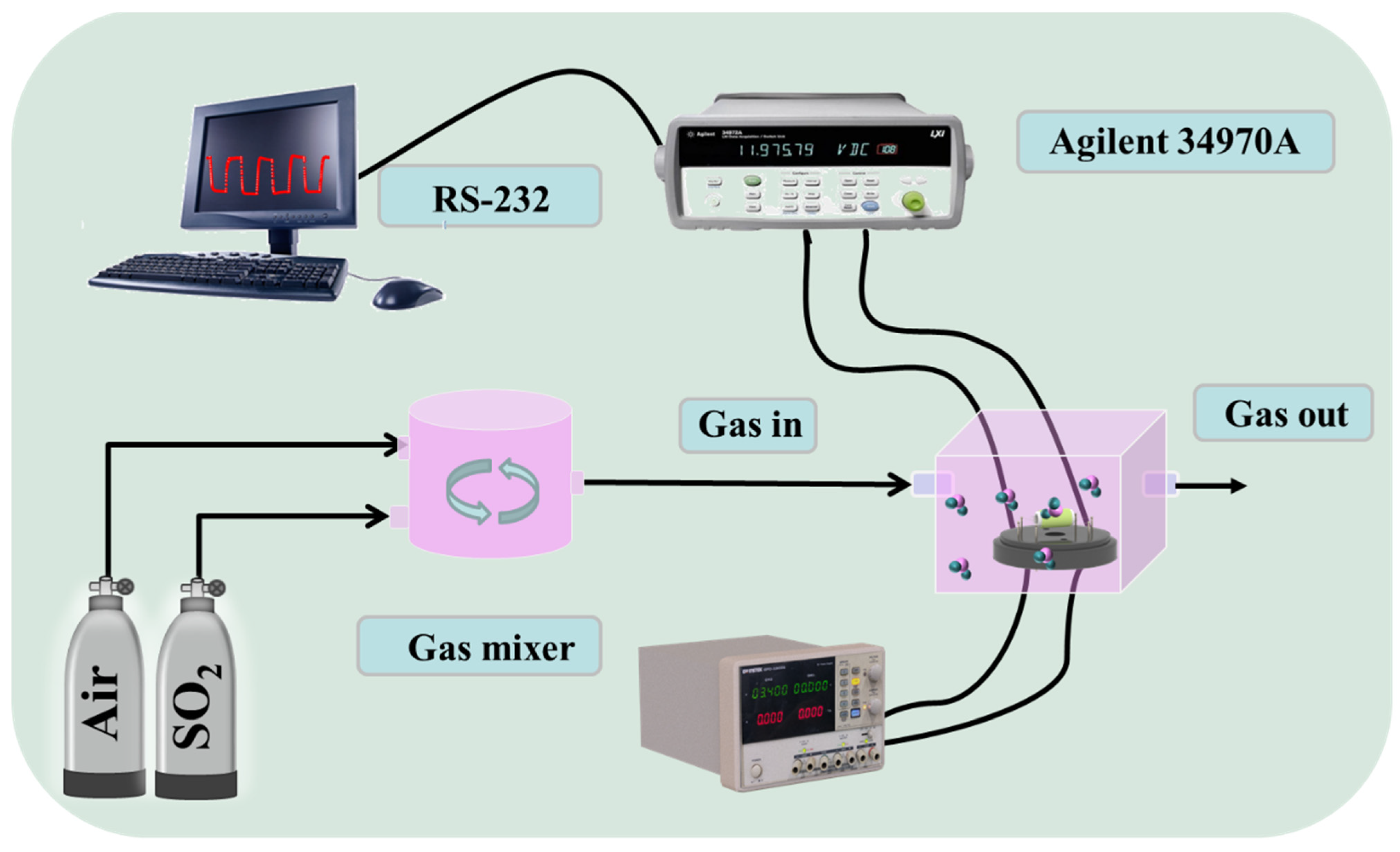
2.3. Gas Sensor Fabrication
3. Results and Discussion
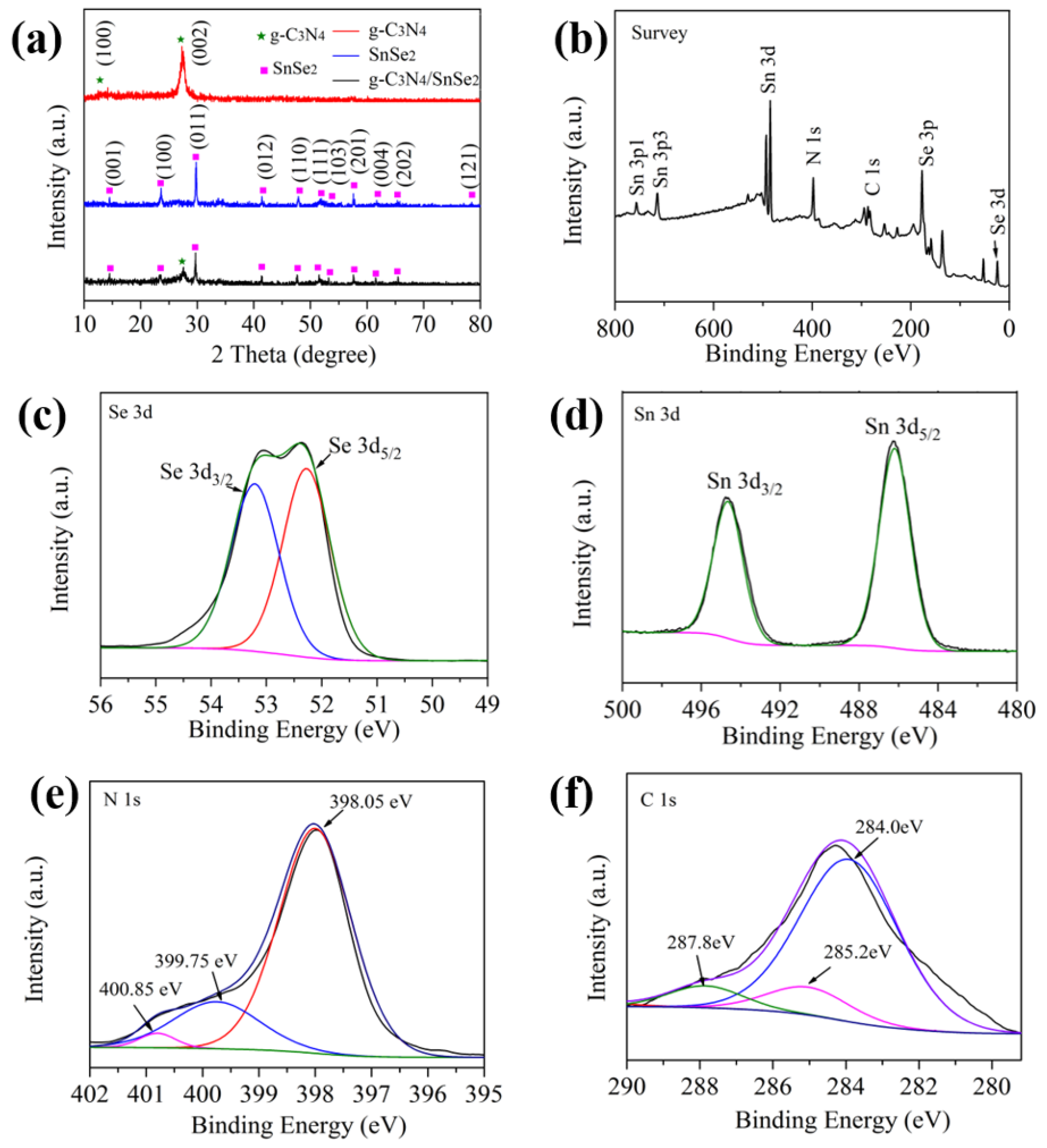
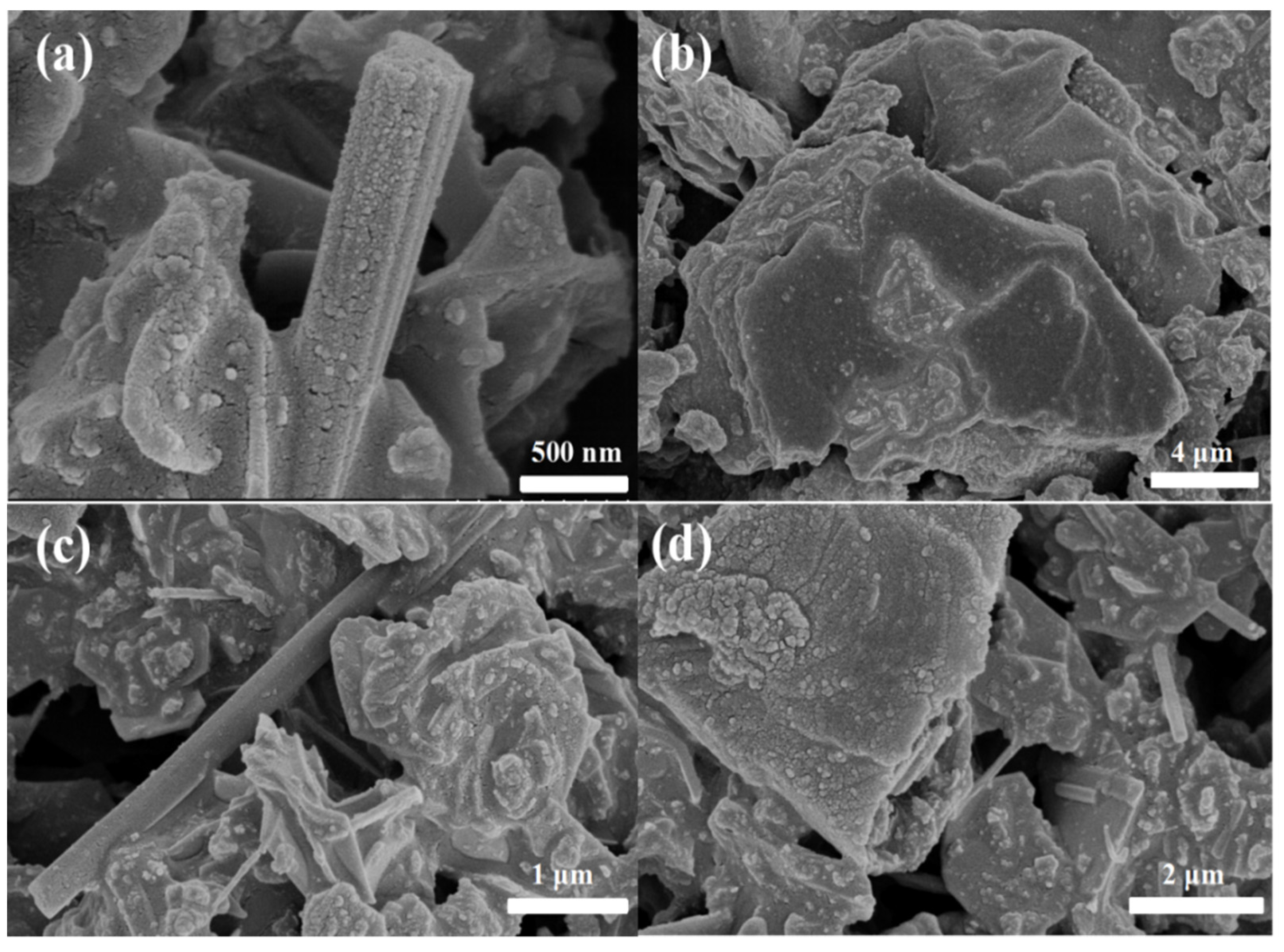
3.1. Structure Characterization
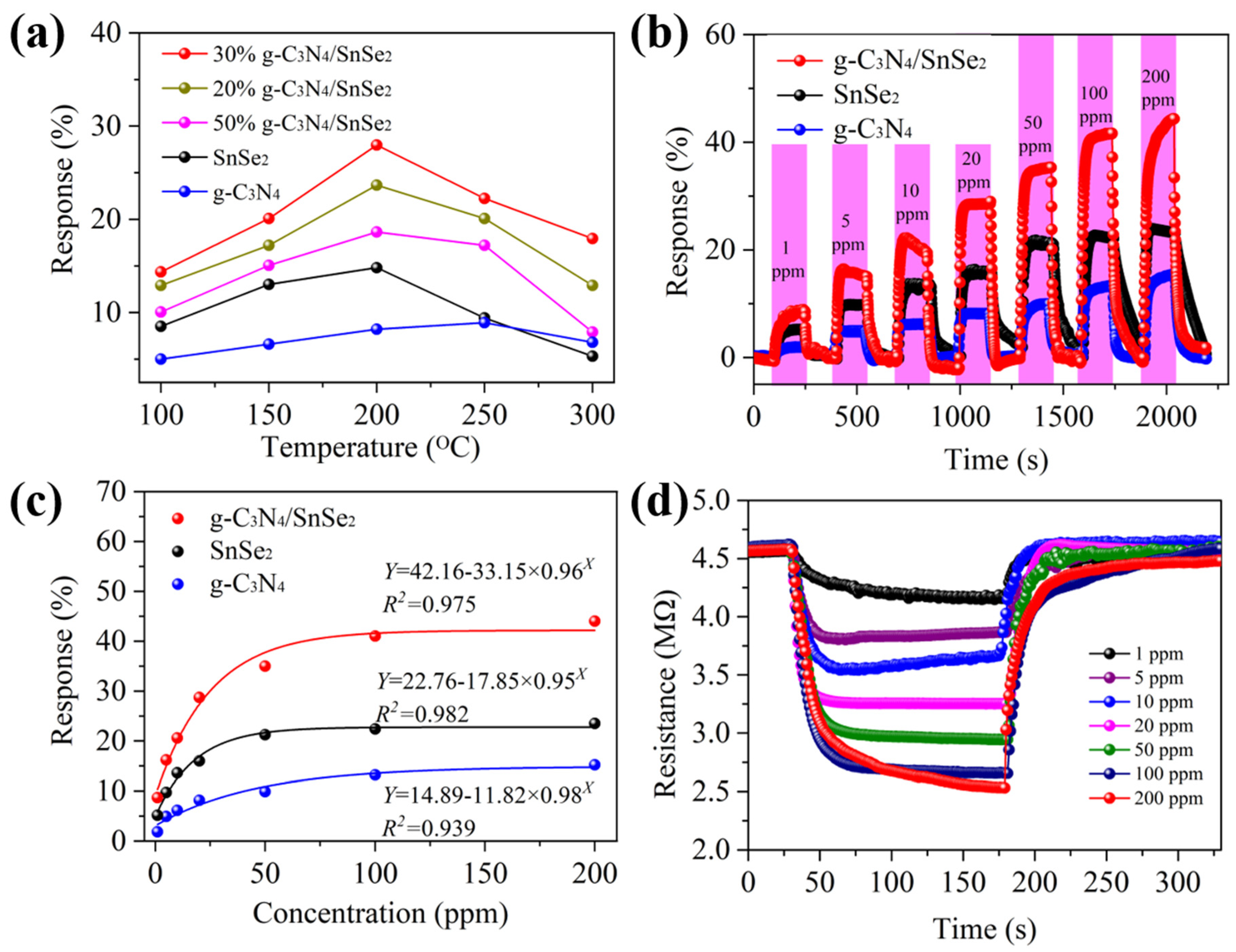
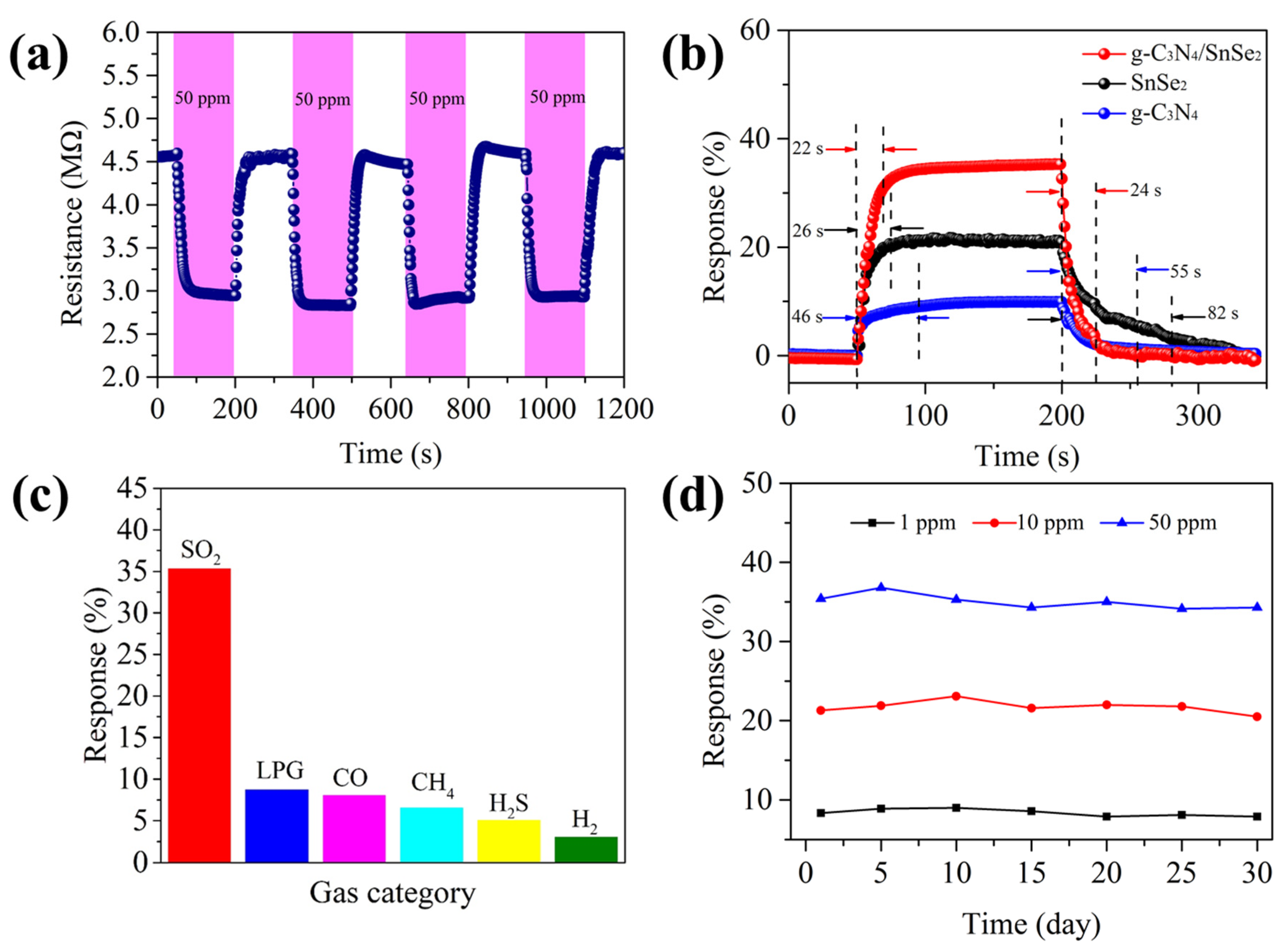
3.2. SO2-Sensing Properties
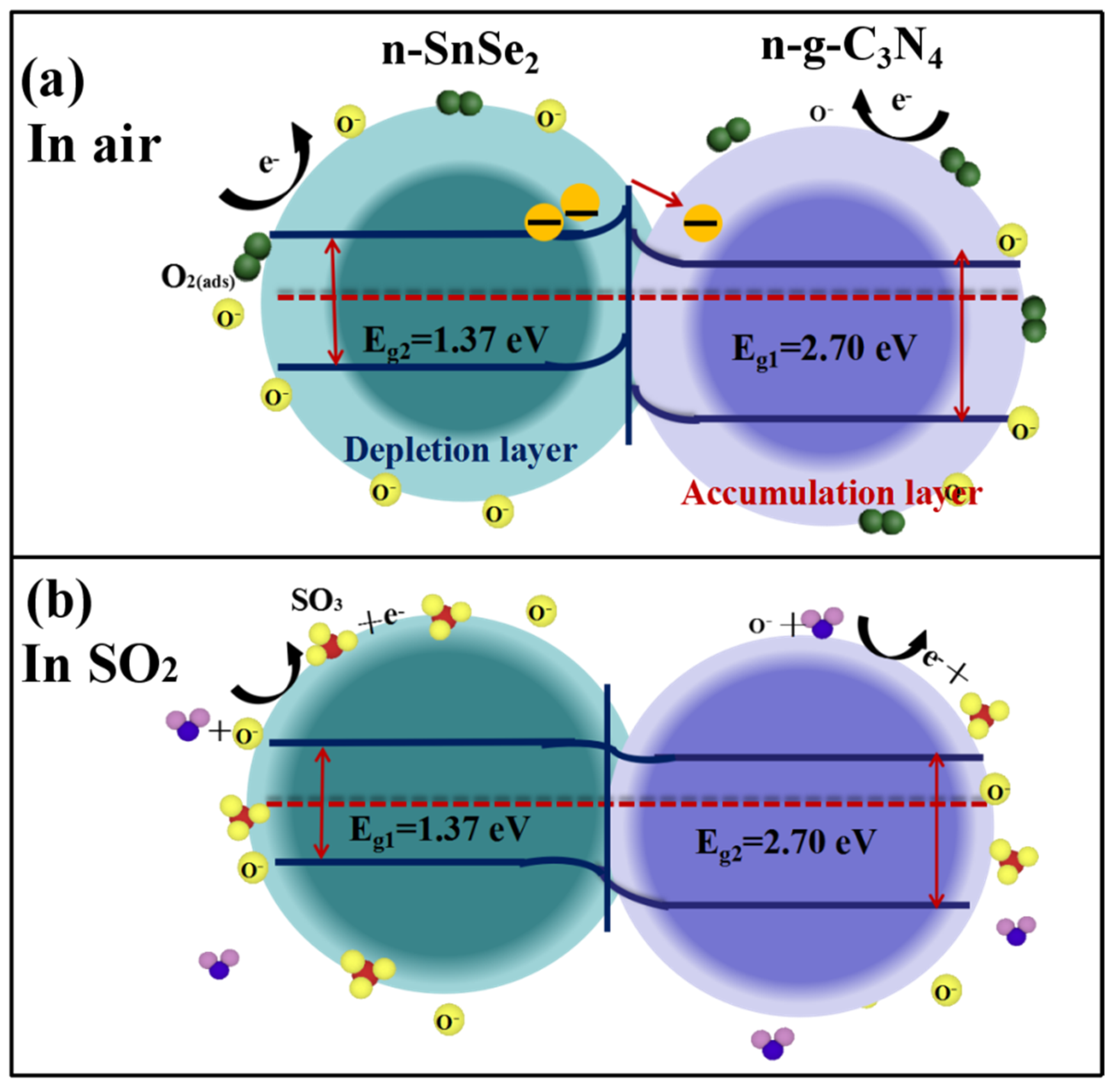
3.3. SO2 Gas-Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Das, S.; Rana, S.; Mursalin, S.; Rana, P.; Sen, A. Sonochemically prepared nanosized BiFeO3 as novel SO2 sensor. Sens. Actuators B 2015, 218, 122–127. [Google Scholar] [CrossRef]
- Jung, G.; Jeong, Y.; Hong, Y.; Wu, M.; Hong, S.; Shin, W.; Park, J.; Jang, D.; Lee, J. SO2 gas sensing characteristics of FET- and resistor-type gas sensors having WO3 as sensing material. Solid-State Electron. 2020, 165, 107747. [Google Scholar] [CrossRef]
- Ma, C.; Hao, X.; Yang, X.; Liang, X.; Liu, F.; Liu, T.; Yang, C.; Zhu, H.; Lu, G. Sub-ppb SO2 gas sensor based on NASICON and LaxSm1−xFeO3 sensing electrode. Sens. Actuators B Chem. 2018, 256, 648–655. [Google Scholar] [CrossRef]
- Liu, L.; Liu, S. Oxygen vacancies as an efficient strategy for promotion of low concentration SO2 gas sensing: The case of Au-modified SnO2. ACS Sustain. Chem. Eng. 2018, 6, 13427–13434. [Google Scholar] [CrossRef]
- Liu, F.; Wang, J.; Jiang, L.; You, R.; Wang, Q.; Wang, C.; Lin, Z.; Yang, Z.; He, J.; Liu, A.; et al. Compact and planar type rapid response ppb-level SO2 sensor based on stabilized zirconia and SrMoO4 sensing electrode. Sens. Actuators B Chem. 2020, 307, 127655. [Google Scholar] [CrossRef]
- Tong, P.; Hoa, N.; Nha, H.; Duy, N.; Hung, C.; Hieu, N. SO2 and H2S sensing properties of hydrothermally synthesized CuO nanoplates. J. Electron. Mater. 2018, 47, 7170–7178. [Google Scholar] [CrossRef]
- Goel, N.; Kumar, R.; Jain, S.; Rajamani, S.; Roul, B.; Gupta, G.; Kumar, M.; Krupanidhi, S. A high-performance hydrogen sensor based on a reverse-biased MoS2/GaN heterojunction. Nanotechnology 2019, 30, 314001. [Google Scholar] [CrossRef]
- Xu, T.; Han, Y.; Lin, L.; Xu, J.; Fu, Q.; He, H.; Song, B.; Gai, Q.; Wang, X. Self-power position-sensitive detector with fast optical relaxation time and large position sensitivity basing on the lateral photovoltaic effect in tin diselenide films. J. Alloys Compd. 2019, 790, 941–946. [Google Scholar] [CrossRef]
- Javed, Y.; Mirza, S.; Li, C.; Xu, X.; Rafiq, M. The role of biaxial strain and pressure on the thermoelectric performance of SnSe2: A first principles study. Semicond. Sci. Technol. 2019, 34, 055009. [Google Scholar] [CrossRef]
- Gu, D.; Wang, X.; Liu, W.; Li, X.; Lin, S.; Wang, J.; Rumyantseva, M.; Gaskovc, A.; Akbar, S. Visible-light activated room temperature NO2 sensing of SnS2 nanosheets based chemiresistive sensors. Sens. Actuators B Chem. 2020, 305, 127455. [Google Scholar] [CrossRef]
- Jaiswal, J.; Sanger, A.; Tiwari, P.; Chandra, R. MoS2 hybrid heterostructure thin film decorated with CdTe quantum dots for room temperature NO2 gas sensor. Sens. Actuators B Chem. 2020, 305, 127437. [Google Scholar] [CrossRef]
- Shen, J.; Yanga, Z.; Wang, Y.; Xu, L.; Liu, R.; Liu, X. The gas sensing performance of borophene/MoS2 heterostructure. Appl. Surf. Sci. 2020, 504, 144412. [Google Scholar] [CrossRef]
- Liu, D.; Tang, Z.; Zhang, Z. Comparative study on NO2 and H2S sensing mechanisms of gas sensors based on WS2 nanosheets. Sens. Actuators B Chem. 2020, 303, 127114. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhu, L.; Zheng, C.; Wang, J. Nanoporous Functionalized WS2/MWCNTs Nanocomposite for Trimethylamine Detection Based on Quartz Crystal Microbalance Gas Sensor. ACS Appl. Mater. Interfaces 2021, 13, 41339–41350. [Google Scholar] [CrossRef] [PubMed]
- Pawar, M.; Kadam, S.; Late, D. High-performance sensing behavior using electronic ink of 2D SnSe2 nanosheets. Chem. Sel. 2017, 2, 4068–4075. [Google Scholar] [CrossRef]
- Wang, X.; Liu, Y.; Dai, J.; Chen, Q.; Huang, X.; Huang, W. Solution-processed p-SnSe/n-SnSe2 hetero-structure layers for ultrasensitive NO2 detection. Chem. Eur. J. 2020, 26, 3870–3876. [Google Scholar] [CrossRef]
- Moreira, O.; Cheng, W.; Fuh, H.; Chien, W.; Yan, W.; Fei, H.; Xu, H.; Zhang, D.; Chen, Y.; Zhao, Y.; et al. High Selectivity Gas Sensing and Charge Transfer of SnSe2. ACS Sens. 2019, 4, 2546–2552. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Zhang, D.; Pan, Q.; Tang, M.; Yu, S. UV enhanced NO2 gas sensing at room temperature based on coral-like tin diselenide/MOFs-derived nanoflower-like tin dioxide heteronanostructures. Sens. Actuators B Chem. 2022, 355, 131049. [Google Scholar] [CrossRef]
- Wang, D.; Zhang, D.; Pan, Q.; Wang, T.; Chen, F. Gas sensing performance of carbon monoxide sensor based on rod-shaped tin diselenide/MOFs derived zinc oxide polyhedron at room temperature. Sens. Actuators B Chem. 2022, 371, 132481. [Google Scholar] [CrossRef]
- Pan, Q.; Li, T.; Zhang, D. Ammonia gas sensing properties and density functional theory investigation of coral-like Au-SnSe2 Schottky junction. Sens. Actuators B Chem. 2021, 332, 129440. [Google Scholar] [CrossRef]
- Sun, K.; Zhan, G.; Chen, H.; Lin, S. Low-Operating-Temperature NO2 Sensor Based on a CeO2/ZnO Heterojunction. Sensors 2022, 21, 8269. [Google Scholar] [CrossRef] [PubMed]
- Liu, K.; Liu, H.; Wang, J.; Feng, L. Synthesis and characterization of SnSe2 hexagonal nanoflakes. Mater. Lett. 2009, 63, 512–514. [Google Scholar] [CrossRef]
- David, S.P.S.; Veeralakshmi, S.; Priya, M.S.; Nehru, S.; Kalaiselvam, S. Room-temperature chemiresistive g-C3N4/Ag2ZrO3 nanocomposite gas sensor for ethanol detection. J. Mater. Sci.-Mater. El. 2022, 33, 11498–11510. [Google Scholar] [CrossRef]
- Pan, W.; Zhang, Y.; Yu, S.; Liu, X.; Zhang, D. Hydrogen sulfide gas sensing properties of metal organic framework-derived α-Fe2O3 hollow nanospheres decorated with MoSe2 nanoflowers. Sens. Actuators B Chem. 2021, 344, 130221. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, D.; Chen, H. MOF-derived indium oxide hollow microtubes/MoS2 nanoparticles for NO2 gas sensing. Sens. Actuators B Chem. 2019, 300, 127037. [Google Scholar] [CrossRef]
- Liu, C.; Huang, Z.; Wang, D.; Wang, X.; Miao, L.; Wang, X.; Wu, S.; Toyama, N.; Asaka, T.; Chen, J.; et al. Dynamic Ag+-intercalation with AgSnSe2 nanoprecipitates in Cl-doped polycrystalline SnSe2 toward ultra-high thermoelectric performance. J. Mater. Chem. A 2019, 7, 9761–9772. [Google Scholar] [CrossRef]
- Hang, N.; Zhang, S.; Yang, W. Efficient exfoliation of g-C3N4 and NO2 sensing behavior of graphene/g-C3N4 nanocomposite. Sens. Actuators B Chem. 2017, 248, 940–948. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Li, P.; Pang, M.; Xue, Q. Flexible self-powered high-performance ammonia sensor based on Au-decorated MoSe2 nanoflowers driven by single layer MoS2-flake piezoelectric nanogenerator. Nano Energy 2019, 65, 103974. [Google Scholar] [CrossRef]
- Yan, J.; Song, Z.; Xu, H.; Lee, L. Carbon-mediated electron transfer channel between SnO2 QDs and g-C3N4 for enhanced photocatalytic H2 production. Chem. Eng. J. 2021, 425, 131512. [Google Scholar] [CrossRef]
- Yan, J.; Wang, T.; Qiu, S.; Song, Z.; Zhu, W.; Liu, X.; Liang, J.; Sun, C.; Li, H. Insights into the efficient charge separation over Nb2O5/2D-C3N4 heterostructure for exceptional visible-light driven H2 evolution. J. Energy Chem. 2022, 65, 548–555. [Google Scholar] [CrossRef]
- Xiong, Y.; Lu, W.; Ding, D.; Zhu, L.; Li, X.; Ling, C.; Xue, Q. Enhanced room temperature oxygen sensing properties of LaOCl-SnO2 hollow spheres by UV light illumination. ACS Sens. 2017, 2, 679–686. [Google Scholar] [CrossRef] [PubMed]
- Zeng, B.; Zhang, L.; Wan, X. Fabrication of α-Fe2O3/g-C3N4 composites for cataluminescence sensing of H2S. Sens. Actuators B Chem. 2015, 211, 370–376. [Google Scholar] [CrossRef]
- Guo, J.; Zhang, J.; Zhu, M.; Ju, D.; Xu, H.; Cao, B. High-performance gas sensor based on ZnO nanowires functionalized by Au nanoparticles. Sens. Actuator B 2014, 199, 339–345. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, W.; Wang, C.; Hu, X.; Li, X.; Sun, P.; Shimanoe, K.; Yamazoe, N.; Lu, G. Porous ZnO/ZnCo2O4 hollow spheres: Synthesis, characterization, and applications in gas sensing. J. Mater. Chem. A 2014, 2, 17683–17690. [Google Scholar] [CrossRef]
- Bai, S.; Chen, C.; Luo, R.; Chen, A.; Li, D. Synthesis of MoO3/reduced graphene oxide hybrids and mechanism of enhancing H2S sensing performances. Sens. Actuator B 2015, 216, 113–120. [Google Scholar] [CrossRef]
- Zhang, D.; Cao, Y.; Wu, J.; Zhang, X. Tungsten trioxide nanoparticles decorated tungsten disulfide nanoheterojunction for highly sensitive ethanol gas sensing application. Appl. Surf. Sci. 2020, 503, 144063. [Google Scholar] [CrossRef]
- Zhang, D.; Yang, Z.; Yu, S.; Mi, Q.; Pan, Q. Diversiform metal oxide-based hybrid nanostructure for gas sensing with versatile prospects. Coord. Chem. Rev. 2020, 413, 213272. [Google Scholar] [CrossRef]
- Triet, N.M.; Duy, L.T.; Hwang, B.U.; Hanif, A.; Siddiqui, S.; Park, K.H.; Cho, C.Y.; Lee, N.E. High-performance Schottky diode gas sensor based on the heterojunction of three-dimensional nanohybrids of reduced graphene oxide-vertical ZnO vanorods on an AIGaN/GaN layer. ACS Appl. Mater. Interfaces 2017, 9, 30722–30732. [Google Scholar] [CrossRef]
- Li, W.; Guo, J.; Cai, L.; Qi, W.; Sun, Y.; Xu, J.; Sun, M.; Zhu, H.; Xiang, L.; Xie, D.; et al. UV light irradiation enhanced gas sensor selectivity of NO2 and SO2 using rGO functionalized with hollow SnO2 nanofibers. Sens. Actuators B Chem. 2019, 290, 443–452. [Google Scholar] [CrossRef]
- Chen, D.; Tang, J.; Zhang, X.; Fang, J.; Li, Y.; Zhuo, R. Detecting decompositions of sulfur hexafluoride using reduced graphene oxide decorated with Pt nanoparticles. J. Phys. D Appl. Phys. 2018, 51, 185304. [Google Scholar] [CrossRef]
- Chen, A.; Liu, R.; Peng, X.; Chen, Q.; Wu, J. 2D hybrid nanomaterials for selective detection of NO2 and SO2 using “light on and off” strategy. ACS Appl. Mater. Interfaces 2017, 9, 37191–37200. [Google Scholar] [CrossRef] [PubMed]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. A comparative study of RGO-SnO2 and MWCNT-SnO2 nanocomposites based SO2 gas sensors. Sens. Actuators B Chem. 2017, 248, 980–986. [Google Scholar] [CrossRef]
- Chaudhary, V.; Kaur, A. Enhanced room temperature sulfur dioxide sensing behaviour of in situ polymerized polyaniline–tungsten oxide nanocomposite possessing honeycomb morphology. RSC Adv. 2015, 5, 73535–73544. [Google Scholar] [CrossRef]
- Stankova, M.; Vilanova, X.; Calderer, J. Detection of SO2 and H2S in CO2 stream by means of WO3-based micro-hotplate sensors. Sens. Actuators B Chem. 2004, 2, 219–225. [Google Scholar] [CrossRef]
- Ghimbeu, M.; Lumbreras, M.; Schoonman, J. Electrosprayed metal oxide semiconductor films for sensitive and selective detection of hydrogen sulfide. Sensors 2009, 9, 9122–9132. [Google Scholar] [CrossRef] [PubMed]
- Shen, F.; Wang, D.; Liu, R. Edge-tailored graphene oxide nanosheet-based field effect transistors for fast and reversible electronic detection of sulfur dioxide. Nanoscale 2012, 5, 537–540. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.; Liu, J.; Jiang, C.; Li, P.; Sun, Y. High-performance sulfur dioxide sensing properties of layer-by-layer self-assembled titania-modified graphene hybrid nanocomposite. Sens. Actuators B Chem. 2017, 245, 560–567. [Google Scholar] [CrossRef]
- Tyagi, P.; Sharma, A.; Tomar, M.; Gupta, V. Metal oxide catalyst assisted SnO2 thin film based SO2 gas sensor. Sens. Actuators B Chem. 2016, 224, 282–289. [Google Scholar] [CrossRef]
- Zhang, X.; Tie, J.; Zhang, J. A Pt-doped TiO2 nanotube arrays sensor for detecting SF6 decomposition products. Sensors 2013, 13, 14764–14776. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, V.; Kaur, A. Solitary surfactant assisted morphology dependent chemiresistive polyanilne sensors for room temperature monitoring of low ppm sulphur dioxide. Polym. Int. 2015, 64, 1475–1481. [Google Scholar] [CrossRef]
- Lupan, O.; Chow, L.; Chai, G. A single ZnO tetrapod-based sensor. Sens. Actuators B Chem. 2009, 141, 511–517. [Google Scholar] [CrossRef]
- Zhang, D.; Wu, J.; Peng, L. Room-temperature SO2 gas-sensing properties based on a metal-doped MoS2 nanoflower: An experimental and density functional theory investigation. J. Mater. Chem. A 2017, 5, 20666–20677. [Google Scholar] [CrossRef]
- Das, S.; Chakraborty, S.; Parkash, O. Vanadium doped tin dioxide as a novel sulfur dioxide sensor. Talanta 2008, 75, 385–389. [Google Scholar] [CrossRef] [PubMed]
- Morris, D.; Egdell, R. Application of V-doped TiO2 as a sensor for detection of SO2. J. Mater. Chem. 2001, 11, 3207–3210. [Google Scholar] [CrossRef]
- Kumar, R.; Avasthi, D.; Kaur, A. Fabrication of chemiresistive gas sensors based on multistep reduced graphene oxide for low parts per million monitoring of sulfur dioxide at room temperature. Sens. Actuators B Chem. 2017, 242, 461–468. [Google Scholar] [CrossRef]
- Nisar, J.; Topalian, Z.; de Sarkar, A.; Osterlund, L.; Ahuja, R. TiO2 based gas sensor: A possible application to SO2. ACS Appl. Mater. Interfaces 2013, 5, 8516–8522. [Google Scholar] [CrossRef]
- Zhang, S.; Song, P.; Liu, L.; Yang, Z.; Wang, Q. In2O3 nanosheets array directly grown on Al2O3 ceramic tube and their gas sensing performance. Ceram. Int. 2017, 43, 7942–7947. [Google Scholar] [CrossRef]
- Si, W.; Du, W.; Wang, F.; Wu, L.; Liu, J.; Liu, W.; Cui, P.; Zhang, X. One-pot hydrothermal synthesis of nano-sheet assembled NiO/ZnO microspheres for efficient sulfur dioxide detection. Ceram. Int. 2020, 46, 7279–7287. [Google Scholar] [CrossRef]
- Zhuo, Q.; Zeng, W.; Chen, W.; Xu, L.; Kumar, R.; Umar, A. High sensitive and low-concentration sulfur dioxide (SO2) gas sensor application of heterostructure NiO-ZnO nanodisks. Sens. Actuators B Chem. 2019, 298, 126870. [Google Scholar] [CrossRef]
- Ye, H.; Liu, L.; Xu, Y.; Wang, L.; Chen, X.; Zhang, K.; Liu, Y.; Koh, S.; Zhang, G. SnSe monolayer: A promising candidate of SO2 sensor with high adsorption quantity. Appl. Surf. Sci. 2019, 484, 33–38. [Google Scholar] [CrossRef]
- Seif, A.; nikfarjam, A.; Ghassem, H. UV enhanced ammonia gas sensing properties of PANI/TiO2 core-shell nanofibers. Sens. Actuators B Chem. 2019, 298, 126906. [Google Scholar]
- Bai, J.; Zhao, C.; Gong, H.; Wang, Q.; Huang, B.; Sun, G.; Wang, Y.; Zhou, J.; Xie, E.; Wang, F. Debye-length controlled gas sensing performances in NiO@ZnO p-n junctional core-shell nanotubes. J. Phys. D Appl. Phys. 2019, 52, 285103. [Google Scholar] [CrossRef]
- Su, P.; Zheng, Y. Room-temperature ppb-level SO2 gas sensors based on RGO/WO3 and MWCNTs/WO3 nanocomposites. Anal. Methods 2021, 13, 782–788. [Google Scholar] [CrossRef] [PubMed]
- Veeralingam, S.; Sahatiya, P.; Badhulika, S. Low cost, flexible and disposable SnSe2 based photoresponsive ammonia sensor for detection of ammonia in urine samples. Sens. Actuators B Chem. 2019, 297, 126725. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, D.; Wang, D. Carbon monoxide gas sensing properties of metal-organic frameworks-derived tin dioxide nanoparticles/molybdenum diselenide nanoflowers. Sens. Actuators B Chem. 2020, 304, 127369. [Google Scholar] [CrossRef]
- Guo, J.; Li, Y.; Jiang, B.; Gao, H.; Wang, T.; Sun, P.; Liu, F.; Yan, X.; Liang, X.; Gao, Y.; et al. Xylene gas sensing properties of hydrothermal synthesized SnO2-Co3O4 microstructure. Sens. Actuators B Chem. 2020, 310, 127780. [Google Scholar] [CrossRef]
- Beniwai, A.; Sunny. Electrospun SnO2/PPy nanocomposite for ultra-low ammonia concentration detection at room temperature. Sens. Actuators B Chem. 2019, 296, 126660. [Google Scholar] [CrossRef]
- Xue, Q.; Chen, H.; Li, Q.; Yan, K.; Besenbacher, F.; Dong, M. Room-temperature high-sensitivity detection of ammonia gas using the capacitance of carbon/silicon heterojunctions. Energy Environ. Sci. 2010, 3, 288–291. [Google Scholar] [CrossRef]

| Sensing Material | Sensing Environment | Response | Concentration | Ref. |
|---|---|---|---|---|
| AlGaN/ZnO/rGO | RT | 2.5% | 120 ppb | [38] |
| SnO2/rGO | RT/UV | 1.7% | 5 ppm | [39] |
| Pt/rGO | 120 °C | 5% | 100 ppm | [40] |
| g-C3N4/rGO | RT | 3.2% | 100 ppm | [41] |
| SnO2/MWCNT | 60 °C | 6 | 500 ppm | [42] |
| V2O5/WO3/TiO2 | 400 °C | 5% | 20 ppm | [43] |
| WO3 | 350 °C | 5% | 1 ppm | [44] |
| Cu–SnO2 | 400 °C | 1.1% | 20 ppm | [45] |
| SnO2–PANI | RT | 3.1% | 4 ppm | [46] |
| TiO2/rGO | RT | 11.14% | 5 ppm | [47] |
| NiO–SnO2 | 180 °C | 8.3% | 50 ppm | [48] |
| TiO2 | 200 °C | 11% | 10 ppm | [49] |
| ZnO | RT | 0.2% | 100 ppm | [50] |
| WO3–PANI | RT | 4.3% | 5 ppm | [51] |
| Ni–MoS2 | RT | 7.4% | 5 ppm | [52] |
| V2O5/SnO2 | 350 °C | 45% | 5 ppm | [53] |
| V-doped TiO2 | 400 °C | 10% | 10 ppm | [54] |
| GO | RT | 6% | 5 ppm | [55] |
| PANI | RT | 4.2% | 10 ppm | [56] |
| g-C3N4/SnSe2 | 200 °C | 28.9% | 20 ppm | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, H.; Pan, Q.; Zhang, Y.; Zhang, Y.; Zhang, D. High-Performance Sulfur Dioxide Gas Sensor Based on Graphite-Phase Carbon-Nitride-Functionalized Tin Diselenide Nanorods Composite. Chemosensors 2022, 10, 401. https://doi.org/10.3390/chemosensors10100401
Zhang H, Pan Q, Zhang Y, Zhang Y, Zhang D. High-Performance Sulfur Dioxide Gas Sensor Based on Graphite-Phase Carbon-Nitride-Functionalized Tin Diselenide Nanorods Composite. Chemosensors. 2022; 10(10):401. https://doi.org/10.3390/chemosensors10100401
Chicago/Turabian StyleZhang, Hao, Qiannan Pan, Yating Zhang, Yanting Zhang, and Dongzhi Zhang. 2022. "High-Performance Sulfur Dioxide Gas Sensor Based on Graphite-Phase Carbon-Nitride-Functionalized Tin Diselenide Nanorods Composite" Chemosensors 10, no. 10: 401. https://doi.org/10.3390/chemosensors10100401
APA StyleZhang, H., Pan, Q., Zhang, Y., Zhang, Y., & Zhang, D. (2022). High-Performance Sulfur Dioxide Gas Sensor Based on Graphite-Phase Carbon-Nitride-Functionalized Tin Diselenide Nanorods Composite. Chemosensors, 10(10), 401. https://doi.org/10.3390/chemosensors10100401






