A Molecularly Imprinted Polymer-Disposable Pipette Tip Extraction-Capillary Electrophoresis (MISPE-DPX-CE) Method for the Preconcentration and Determination of Scopolamine in Synthetic Urine Samples
Abstract
:1. Introduction
2. Experimental
2.1. Reagents and Materials
2.2. Synthesis of Molecular Imprinted Polymers (MIPs) and Non-Imprinted Polymers (NIPs)
2.3. Procedure for Preparation, Conditioning, and Application of MISPE-DPX Cartridges for the Extraction and Preconcentration of Scopolamine
2.4. Synthetic Urine Samples
3. Results and Discussion
3.1. Evaluation of the Solvent for the Preconditioning of the MISPE-DPX Device
3.2. Optimization of the Extraction Conditions
3.3. Optimization of the Elution Conditions
3.4. Analytical Validation
3.5. Application in Synthetic Urine Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gamal, M. Analytical Review: Analytical Techniques for Hyoscine: N Butyl Bromide. Analyst 2020, 145, 2025–2037. [Google Scholar] [CrossRef] [PubMed]
- Martinez, S.T.; Almeida, M.R. Alucinógenos Naturais: Um Voo Da Europa Medieval Ao Brasil. Quím. Nova 2009, 32, 2501–2507. [Google Scholar] [CrossRef]
- Jalali, F.; Afshari, R.; Babaei, A. Smoking Crushed Hyoscine/Scopolamine Tablets as Drug Abuse. Subst. Use Misuse 2014, 49, 793–797. [Google Scholar] [CrossRef]
- Le Garff, E.; Delannoy, Y.; Mesli, V.; Hédouin, V.; Tournel, G. Forensic Features of a Fatal Datura Poisoning Case during a Robbery. Forensic Sci. Int. 2016, 261, e17–e21. [Google Scholar] [CrossRef] [PubMed]
- Ribeiro, M.M.A.C.; Barreto, D.N.; Petruci, J.F.D.S.; Richter, E.M. Simultaneous Determination of Scopolamine and Butylscopolamine in Pharmaceutical and Beverage Samples by Capillary Zone Electrophoresis. Microchem. J. 2022, 172, 106985. [Google Scholar] [CrossRef]
- Kummer, S.; Rickert, A.; Daldrup, T.; Mayatepek, E. Abuse of the Over-the-Counter Antispasmodic Butylscopolamine for the Home Synthesis of Psychoactive Scopolamine. Eur. J. Pediatr. 2016, 175, 1019–1021. [Google Scholar] [CrossRef]
- Lusthof, K.J.; Bosman, I.J.; Kubat, B.; Vincenten-van Maanen, M.J. Toxicological Results in a Fatal and Two Non-Fatal Cases of Scopolamine-Facilitated Robberies. Forensic Sci. Int. 2017, 274, 79–82. [Google Scholar] [CrossRef]
- Sáiz, J.; Mai, T.D.; López, M.L.; Bartolomé, C.; Hauser, P.C.; García-Ruiz, C. Rapid Determination of Scopolamine in Evidence of Recreational and Predatory Use. Sci. Justice 2013, 53, 409–414. [Google Scholar] [CrossRef]
- Dufayet, L.; Alcaraz, E.; Dorol, J.; Rey-Salmon, C.; Alvarez, J.C. Attempt of Scopolamine-Facilitated Robbery: An Original Case of Poisoning by Inhalation Confirmed by LC–MS/MS and Review of the Literature. Forensic Toxicol. 2020, 38, 264–268. [Google Scholar] [CrossRef]
- De Castro, A.; Lendoiro, E.; Quintela, Ó.; Concheiro, M.; López-Rivadulla, M.; Cruz, A. Hair Analysis Interpretation of an Unusual Case of Alleged Scopolamine-Facilitated Sexual Assault. Forensic Toxicol. 2012, 30, 193–198. [Google Scholar] [CrossRef]
- Corallo, C.E.; Whitfield, A.; Wu, A. Anticholinergic Syndrome Following an Unintentional Overdose of Scopolamine. Ther. Clin. Risk Manag. 2009, 5, 719. [Google Scholar] [CrossRef] [PubMed]
- Mulder, H.A.; Halquist, M.S. Growing Trends in the Efficient and Selective Extraction of Compounds in Complex Matrices Using Molecularly Imprinted Polymers and Their Relevance to Toxicological Analysis. J. Anal. Toxicol. 2021, 45, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Gouda, A.A.; El Shafey, Z.; Hossny, N.; El-Azzazy, R. Spectrophotometric Determination of Hyoscine Butylbromide and Famciclovir in Pure Form and in Pharmaceutical Formulations. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2008, 70, 785–792. [Google Scholar] [CrossRef]
- Dias, B.C.; Batista, A.D.; da Silveira Petruci, J.F. ΜOPTO: A Microfluidic Paper-Based Optoelectronic Tongue as Presumptive Tests for the Discrimination of Alkaloid Drugs for Forensic Purposes. Anal. Chim. Acta 2021, 1187, 339141. [Google Scholar] [CrossRef] [PubMed]
- Da Costa Oliveira, T.; Santana, M.H.P.; Banks, C.E.; Munoz, R.A.A.; Richter, E.M. Electrochemical Portable Method for on Site Screening of Scopolamine in Beverage and Urine Samples. Electroanalysis 2019, 31, 567–574. [Google Scholar] [CrossRef]
- Ferreira, J.B.; de Jesus Macrino, C.; Dinali, L.A.F.; Filho, J.F.A.; Silva, C.F.; Borges, K.B.; Romão, W. Molecularly Imprinted Polymers as a Selective Sorbent for Forensic Applications in Biological Samples—A Review. Anal. Bioanal. Chem. 2021, 413, 6013–6036. [Google Scholar] [CrossRef]
- Strano-Rossi, S.; Mestria, S.; Bolino, G.; Polacco, M.; Grassi, S.; Oliva, A. Scopolamine Fatal Outcome in an Inmate after Buscopan® Smoking. Int. J. Leg. Med. 2021, 135, 1455–1460. [Google Scholar] [CrossRef]
- Gadzikowska, M.; Petruczynik, A.; Waksmundzka-Hajnos, M.; Hawrył, M.; Jóźwiak, G. Two-Dimensional Planar Chromatography of Tropane Alkaloids from Datura Innoxia Mill. J. Planar Chromatogr.-Mod. TLC 2005, 18, 127–131. [Google Scholar] [CrossRef]
- Min, J.Y.; Jung, H.Y.; Kang, S.M.; Kim, Y.D.; Kang, Y.M.; Park, D.J.; Prasad, D.T.; Choi, M.S. Production of Tropane Alkaloids by Small-Scale Bubble Column Bioreactor Cultures of Scopolia Parviflora Adventitious Roots. Bioresour. Technol. 2007, 98, 1748–1753. [Google Scholar] [CrossRef]
- Brown, K.; Jacquet, C.; Biscay, J.; Allan, P.; Dennany, L. Tale of Two Alkaloids: PH-Controlled Electrochemiluminescence for Differentiation of Structurally Similar Compounds. Anal. Chem. 2020, 92, 2216–2223. [Google Scholar] [CrossRef] [Green Version]
- Luger, S.; Mayerhuber, L.; Weigelhofer, G.; Hein, T.; Holzer, B.; Hametner, C.; Fruhmann, P. Development of Ion-selective Electrodes for Tropane, Atropine, and Scopolamine—A Concept for the Analysis of Tropane Alkaloids. Electroanalysis 2022, 34, 1–9. [Google Scholar] [CrossRef]
- Boysen, R.I. Advances in the Development of Molecularly Imprinted Polymers for the Separation and Analysis of Proteins with Liquid Chromatography. J. Sep. Sci. 2019, 42, 51–71. [Google Scholar] [CrossRef] [PubMed]
- Zuo, J.; Zhang, X.; Li, X.; Li, Z.; Li, Z.; Li, H.; Zhang, W. Preparation of Monoethyl Fumarate-Based Molecularly Imprinted Polymers and Their Application as a Solid-Phase Extraction Sorbent for the Separation of Scopolamine from Tropane Alkaloids. RSC Adv. 2019, 9, 19712–19719. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, S.K.; Fisher, J.; Brogden, N.K.; Kandimalla, K.K. Development and Validation of a Sensitive LC-MS/MS Method for the Estimation of Scopolamine in Human Serum. J. Pharm. Biomed. Anal. 2019, 164, 41–46. [Google Scholar] [CrossRef]
- Belbruno, J.J. Molecularly Imprinted Polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef]
- Da Silva, A.T.M.; de Oliveira, H.L.; Silva, C.F.; Fonseca, M.C.; Pereira, T.F.D.; Nascimento, C.S.; de Figueiredo, E.C.; Borges, K.B. Efficient Molecularly Imprinted Polymer as a Pipette-Tip Solid-Phase Sorbent for Determination of Carvedilol Enantiomers in Human Urine. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2017, 1061–1062, 399–410. [Google Scholar] [CrossRef]
- Wan, Q.; Liu, H.; Deng, Z.; Bu, J.; Li, T.; Yang, Y.; Zhong, S. A Critical Review of Molecularly Imprinted Solid Phase Extraction Technology. J. Polym. Res. 2021, 28, 401. [Google Scholar] [CrossRef]
- Jayasinghe, G.D.T.M.; Moreda-piñeiro, A. Molecularly Imprinted Polymers for Dispersive (Micro) Solid Phase Extraction: A Review. Separations 2021, 8, 99. [Google Scholar] [CrossRef]
- Tiwari, A.; Uzun, L. (Eds.) Advanced Molecularly Imprinted Materials; Scrivener Publishing LLC Wiley: Hoboken, NJ, USA, 2017; ISBN 9781119336297. [Google Scholar]
- Leibl, N.; Haupt, K.; Gonzato, C.; Duma, L. Molecularly Imprinted Polymers for Chemical Sensing: A Tutorial Review. Chemosensors 2021, 9, 123. [Google Scholar] [CrossRef]
- Regina, M.; Figueira, E.C.; Del Pilar, M.; Sotomayor, T. Analytical Methods Electroanalytical Determination of Bumetanide Employing a Biomimetic Sensor for Detection of Doping in Sports †. Anal. Methods 2014, 6, 5792–5798. [Google Scholar] [CrossRef]
- Wang, B.; Hong, J.; Liu, C.; Zhu, L.; Jiang, L. An Electrochemical Molecularly Imprinted Polymer Sensor for Rapid β-Lactoglobulin Detection. Sensors 2021, 21, 8240. [Google Scholar] [CrossRef] [PubMed]
- Arabi, M.; Ghaedi, M.; Ostovan, A. Synthesis and Application of In-Situ Molecularly Imprinted Silica Monolithic in Pipette-Tip Solid-Phase Microextraction for the Separation and Determination of Gallic Acid in Orange Juice Samples. J. Chromatogr. B 2017, 1048, 102–110. [Google Scholar] [CrossRef] [PubMed]
- Vasconcelos, I.; da Silva, P.H.R.; Dias, D.R.D.; de Freitas Marques, M.B.; da Nova Mussel, W.; Pedrosa, T.A.; Ribeiro e Silva, M.E.S.; de Souza Freitas, R.F.; de Sousa, R.G.; Fernandes, C. Synthesis and Characterization of a Molecularly Imprinted Polymer (MIP) for Solid-Phase Extraction of the Antidiabetic Gliclazide from Human Plasma. Mater. Sci. Eng. C 2020, 116, 111191. [Google Scholar] [CrossRef] [PubMed]
- Turiel, E.; Martín-Esteban, A. Molecularly Imprinted Polymers-Based Microextraction Techniques. TrAC-Trends Anal. Chem. 2019, 118, 574–586. [Google Scholar] [CrossRef]
- Andrade, F.N.; Nazario, C.E.D.; Santos-Neto, Á.J.; Lanças, F.M. Development of On-Line Molecularly Imprinted Solid Phase Extraction-Liquid Chromatography-Mass Spectrometry for Triazine Analysis in Corn Samples. Anal. Methods 2016, 8, 1181–1186. [Google Scholar] [CrossRef]
- Sirumapea, L.; Zulfikar, M.A.; Amran, M.B.; Alni, A. Selective Solid-Phase Extraction of Meropenem from Human Blood Plasma Using a Molecularly Imprinted Polymer. Indones. J. Chem. 2021, 21, 1167–1179. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Green Analytical Chemistry Metrics: A Review. Talanta 2022, 238, 123046. [Google Scholar] [CrossRef]
- Płotka-wasylka, J.; Szczepan, N. Miniaturized Solid-Phase Extraction Techniques. Trends Anal. Chem. 2015, 73, 19–38. [Google Scholar] [CrossRef]
- Carasek, E.; Morés, L.; Huelsmann, R.D. Disposable Pipette Extraction: A Critical Review of Concepts, Applications, and Directions. Anal. Chim. Acta 2022, 1192, 339383. [Google Scholar] [CrossRef]
- Mozaner Bordin, D.C.; Alves, M.N.; Cabrices, O.G.; de Campos, E.G.; De Martinis, B.S. A Rapid Assay for the Simultaneous Determination of Nicotine, Cocaine and Metabolites in Meconium Using Disposable Pipette Extraction and Gas Chromatography—Mass Spectrometry (GC–MS). J. Anal. Toxicol. 2014, 38, 31–38. [Google Scholar] [CrossRef] [Green Version]
- Gomes, I.C.; Martins, R.O.; Machado, L.S.; Cardoso, A.T.; de Souza, P.S.; Coltro, W.K.T.; de Tarso Garcia, P.; Chaves, A.R. Molecularly Imprinted Polymer as Sorbent Phase for Disposable Pipette Extraction: A Potential Approach for Creatinine Analysis in Human Urine Samples. J. Pharm. Biomed. Anal. 2022, 211, 114625. [Google Scholar] [CrossRef] [PubMed]
- Trujillo-Rodríguez, M.J.; Pacheco-Fernández, I.; Taima-Mancera, I.; Díaz, J.H.A.; Pino, V. Evolution and Current Advances in Sorbent-Based Microextraction Configurations. J. Chromatogr. A 2020, 1634, 461670. [Google Scholar] [CrossRef]
- Silva, W.R.; Sote, W.O.; Comar Júnior, M.; Petruci, J.F.S. The Use of in Silico Models for the Rationalization of Molecularly Imprinted Polymer Synthesis. Eur. Polym. J. 2022, 166, 111024. [Google Scholar] [CrossRef]
- Zeng, S.; She, Y.; Jiao, B.; Liu, G.; Wang, J.; Su, X.; Ma, X.; Jin, M.; Jin, F.; Wang, S. Molecularly Imprinted Polymer for Selective Extraction and Simultaneous Determination of Four Tropane Alkaloids from Przewalskia Tangutica Maxim. Fruit Extracts Using LC-MS/MS. RSC Adv. 2015, 5, 94997–95006. [Google Scholar] [CrossRef]
- Nakamura, M.; Ono, M.; Nakajima, T.; Ito, Y.; Aketo, T.; Haginaka, J. Uniformly Sized Molecularly Imprinted Polymer for Atropine and Its Application to the Determination of Atropine and Scopolamine in Pharmaceutical Preparations Containing Scopolia Extract. J. Pharm. Biomed. Anal. 2005, 37, 231–237. [Google Scholar] [CrossRef] [PubMed]
- Theodoridis, G.; Kantifes, A.; Manesiotis, P.; Raikos, N.; Tsoukali-Papadopoulou, H. Preparation of a Molecularly Imprinted Polymer for the Solid-Phase Extraction of Scopolamine with Hyoscyamine as a Dummy Template Molecule. J. Chromatogr. A 2003, 987, 103–109. [Google Scholar] [CrossRef]
- Gao, Y.; Tian, Y.; Wang, E. Simultaneous Determination of Two Active Ingredients in Flos Daturae by Capillary Electrophoresis with Electrochemiluminescence Detection. Anal. Chim. Acta 2005, 545, 137–141. [Google Scholar] [CrossRef]
- Cunha, R.R.; Ribeiro, M.M.A.C.; Muñoz, R.A.A.; Richter, E.M. Fast Determination of Codeine, Orphenadrine, Promethazine, Scopolamine, Tramadol, and Paracetamol in Pharmaceutical Formulations by Capillary Electrophoresis. J. Sep. Sci. 2017, 40, 1815–1823. [Google Scholar] [CrossRef]
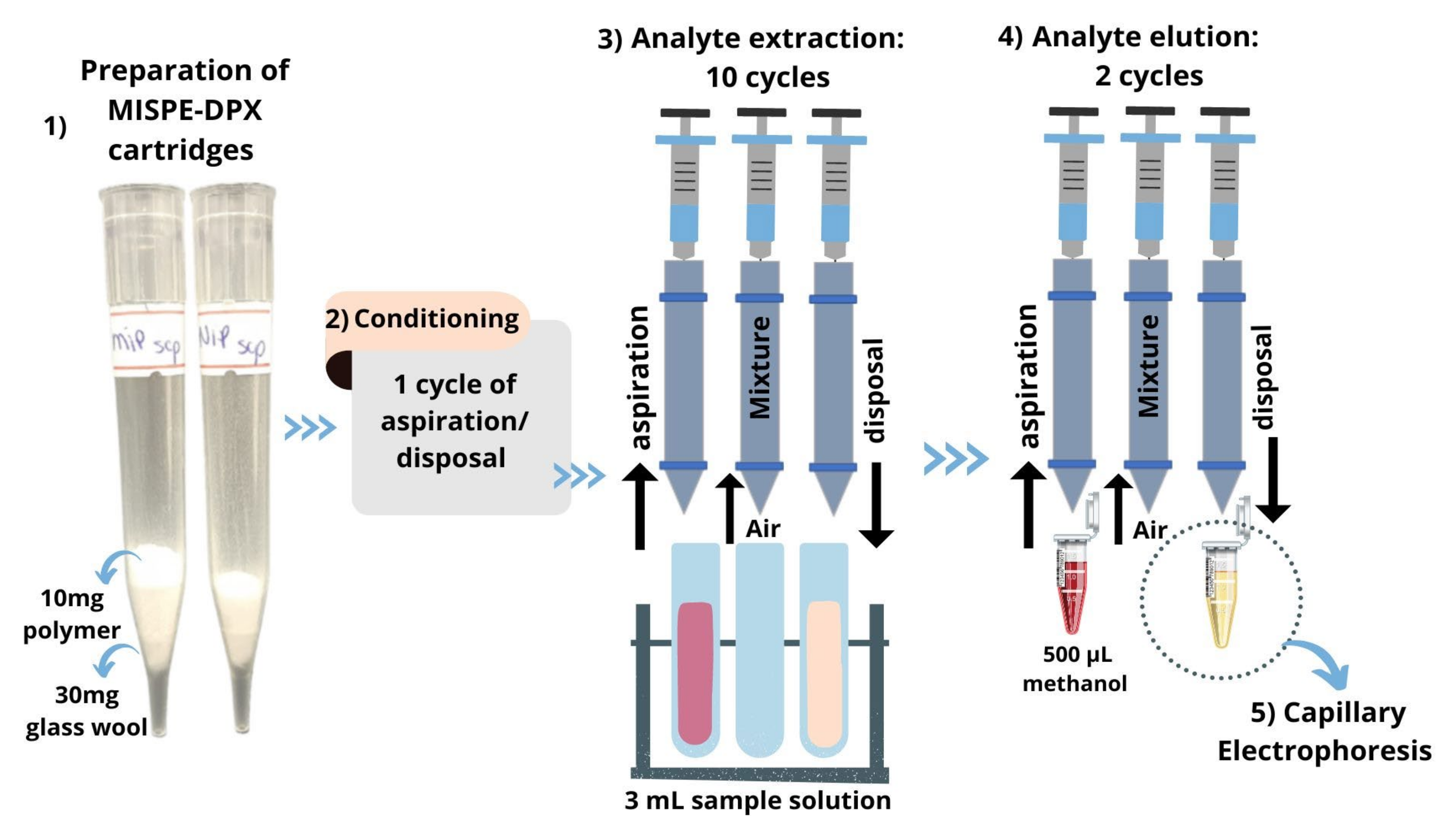
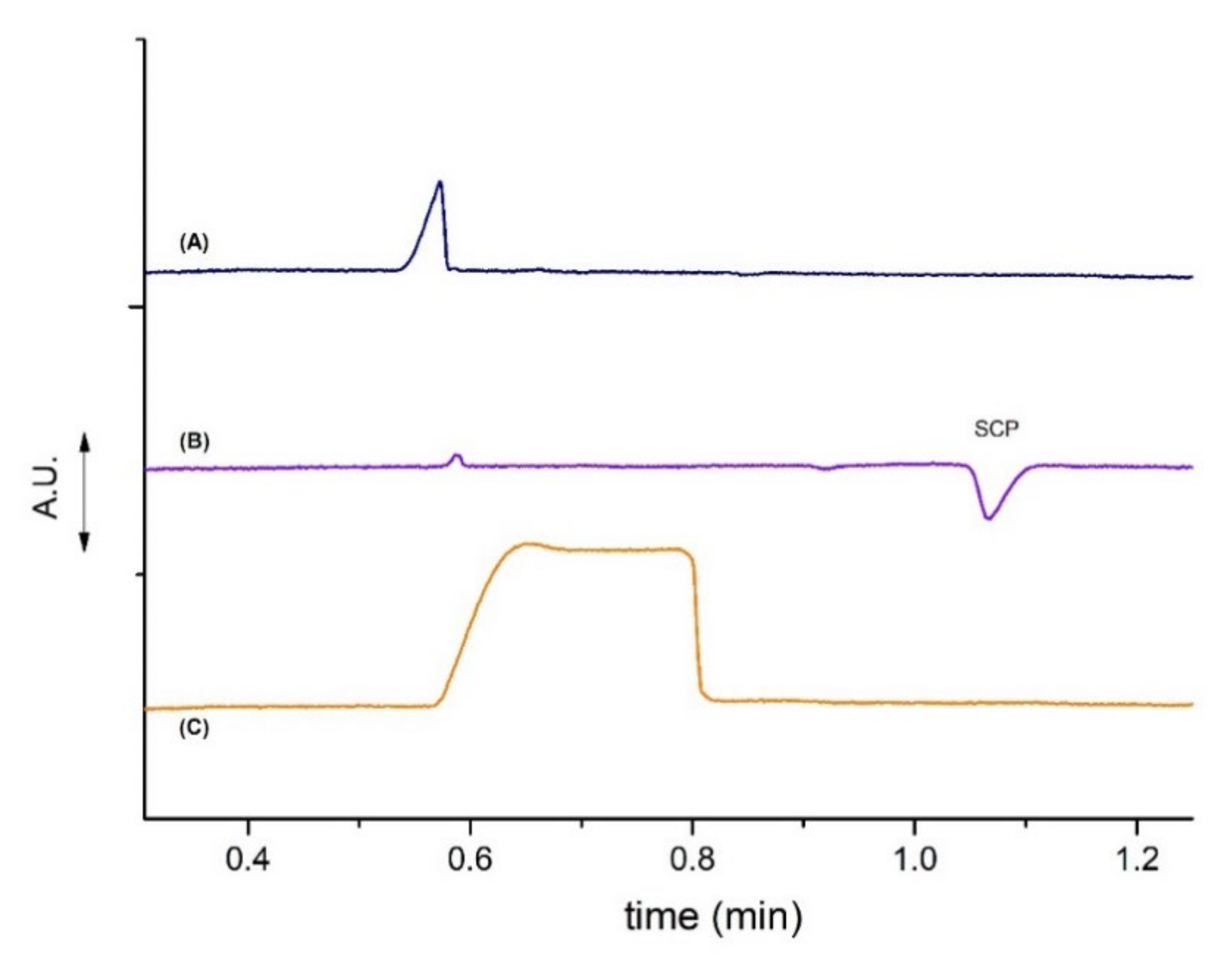
| Solvent | Polymer | Q (mg/g) | IF |
|---|---|---|---|
| Water | MIP | 1.60 ± 0.02 | 1.42 |
| NIP | 1.13 ± 0.01 | ||
| Methanol | MIP | 1.33 ± 0.01 | 1.17 |
| NIP | 1.14 ± 0.01 | ||
| Water:Methanol (50:50) | MIP | 1.50 ± 0.02 | 1.25 |
| NIP | 1.20 ± 0.02 | ||
| No conditioning | MIP | 1.42 ± 0.03 | 1.27 |
| NIP | 1.12 ± 0.01 |
| Experiment | Coded Values | Real Values | Q (mg/g) | Response | |||||
|---|---|---|---|---|---|---|---|---|---|
| Time (s) | Mass (mg) | Cycles of Extraction | Time (s) | Mass (mg) | Cycles of Extraction | QMIP | QNIP | IF | |
| 1 | + | + | + | 60 | 10.0 | 10 | 2.24 | 1.72 | 1.30 |
| 2 | − | + | + | 20 | 10.0 | 10 | 1.51 | 1.01 | 1.50 |
| 3 | + | − | + | 60 | 5.0 | 10 | 1.84 | 1.80 | 1.02 |
| 4 | − | − | + | 20 | 5.0 | 10 | 2.39 | 2.34 | 1.02 |
| 5 | + | + | − | 60 | 10.0 | 2 | 1.69 | 1.67 | 1.01 |
| 6 | − | + | − | 20 | 10.0 | 2 | 1.35 | 1.21 | 1.12 |
| 7 | + | − | − | 60 | 5.0 | 2 | 0.46 | 1.69 | 0.27 |
| 8 | − | − | − | 20 | 5.0 | 2 | 0.19 | 1.23 | 0.15 |
| cp1 | 0 | 0 | 0 | 40 | 7.5 | 6 | 1.97 | 0.99 | 1.99 |
| cp2 | 0 | 0 | 0 | 40 | 7.5 | 6 | 2.06 | 1.99 | 1.04 |
| cp3 | 0 | 0 | 0 | 40 | 7.5 | 6 | 2.00 | 2.89 | 0.69 |
| Experiment | Coded Values | Real Values | Concentration (mg L−1) | Response | |||||
|---|---|---|---|---|---|---|---|---|---|
| Volume (mL) | [Solvent] % | Cycles Elution | Volume (mL) | [Solvent] % | Cycles Elution | CMIP | CNIP | MIP/ NIP | |
| 1 | + | + | + | 2.0 | 100 | 10 | 9.52 | 5.06 | 1.88 |
| 2 | − | + | + | 0.50 | 100 | 10 | 15.26 | 16.85 | 0.91 |
| 3 | + | − | + | 2.0 | 50 | 10 | 6.60 | 5.55 | 1.19 |
| 4 | − | − | + | 0.50 | 50 | 10 | 14.57 | 14.35 | 1.02 |
| 5 | + | + | − | 2.0 | 100 | 2 | 5.14 | 4.27 | 1.20 |
| 6 | − | + | − | 0.50 | 100 | 2 | 21.76 | 15.25 | 1.43 |
| 7 | + | - | − | 2.0 | 50 | 2 | 7.48 | 6.37 | 1.18 |
| 8 | − | − | − | 0.50 | 50 | 2 | 10.79 | 10.90 | 0.99 |
| cp1 | 0 | 0 | 0 | 1.25 | 75 | 6 | 10.30 | 6.19 | 1.66 |
| cp2 | 0 | 0 | 0 | 1.25 | 75 | 6 | 8.00 | 7.92 | 1.01 |
| cp3 | 0 | 0 | 0 | 1.25 | 75 | 6 | 10.26 | 7.38 | 1.39 |
| Parameters | Values |
|---|---|
| Linear range | 0.50–6.00 µM |
| r | 0.9988 |
| R2 | 0.9972 |
| Intraday repeatability (DPR%, n = 7) | 6.43% |
| LD (µM) | 0.04 |
| LQ (µM) | 0.12 |
| Recovery 1 (2 µM) | 84% |
| Recovery 2 (6 µM) | 101% |
| Preconcentration factor | 20 |
| Migration time | 59.4 ± 1.1 s |
| Method | Solid-Phase | Linear Range | Sample | LD | Ref. |
|---|---|---|---|---|---|
| MISPE-DPX-CE | MIP | 0.5–6 µM | Synthetic urine | 0.04 µM | This work |
| MISPE–LC–MS/MS | MIP | 0.011–2.28 µM | Extracts of Przewalskia tangutica Maxim. fruits | 0.005 µM | [45] |
| MISPE–HPLC–UV | MIP | 91–82 µM | Plant samples | 0.005 µM | [23] |
| Column–switching HPLC–UV | MIP | 0.0068–0.205 µM | Pharmaceutical preparations | 0.002 µM | [46] |
| Reversed-phase HPLC–UV | MIP | 2.28–228 µM | Human urine | − | [47] |
| CE–ELC | − | 10–1000 µM | Chinese herb | 0.05 µM | [48] |
| CE–C4D | − | 100–350 µM | Pharmaceutical samples | 2.5 µM | [49] |
| CE–C4D | − | 10–1000 µM | Beverages and pharmaceutical formulations | 2.4 µM | [5] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Silva, W.R.; Ribeiro, M.M.A.C.; Richter, E.M.; Batista, A.D.; da Silveira Petruci, J.F. A Molecularly Imprinted Polymer-Disposable Pipette Tip Extraction-Capillary Electrophoresis (MISPE-DPX-CE) Method for the Preconcentration and Determination of Scopolamine in Synthetic Urine Samples. Chemosensors 2022, 10, 387. https://doi.org/10.3390/chemosensors10100387
Silva WR, Ribeiro MMAC, Richter EM, Batista AD, da Silveira Petruci JF. A Molecularly Imprinted Polymer-Disposable Pipette Tip Extraction-Capillary Electrophoresis (MISPE-DPX-CE) Method for the Preconcentration and Determination of Scopolamine in Synthetic Urine Samples. Chemosensors. 2022; 10(10):387. https://doi.org/10.3390/chemosensors10100387
Chicago/Turabian StyleSilva, Weida Rodrigues, Michelle M. A. C. Ribeiro, Eduardo Mathias Richter, Alex D. Batista, and João Flávio da Silveira Petruci. 2022. "A Molecularly Imprinted Polymer-Disposable Pipette Tip Extraction-Capillary Electrophoresis (MISPE-DPX-CE) Method for the Preconcentration and Determination of Scopolamine in Synthetic Urine Samples" Chemosensors 10, no. 10: 387. https://doi.org/10.3390/chemosensors10100387
APA StyleSilva, W. R., Ribeiro, M. M. A. C., Richter, E. M., Batista, A. D., & da Silveira Petruci, J. F. (2022). A Molecularly Imprinted Polymer-Disposable Pipette Tip Extraction-Capillary Electrophoresis (MISPE-DPX-CE) Method for the Preconcentration and Determination of Scopolamine in Synthetic Urine Samples. Chemosensors, 10(10), 387. https://doi.org/10.3390/chemosensors10100387









