Au-Decorated Polyaniline-ZnO Electrospun Composite Nanofiber Gas Sensors with Enhanced Response to NO2 Gas
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of ZnO Nanofibers
2.3. Au Decoration
2.4. Preparation of Composite Nanofibers
2.5. Characterization
2.6. Gas Sensing Measurements
3. Results and Discussion
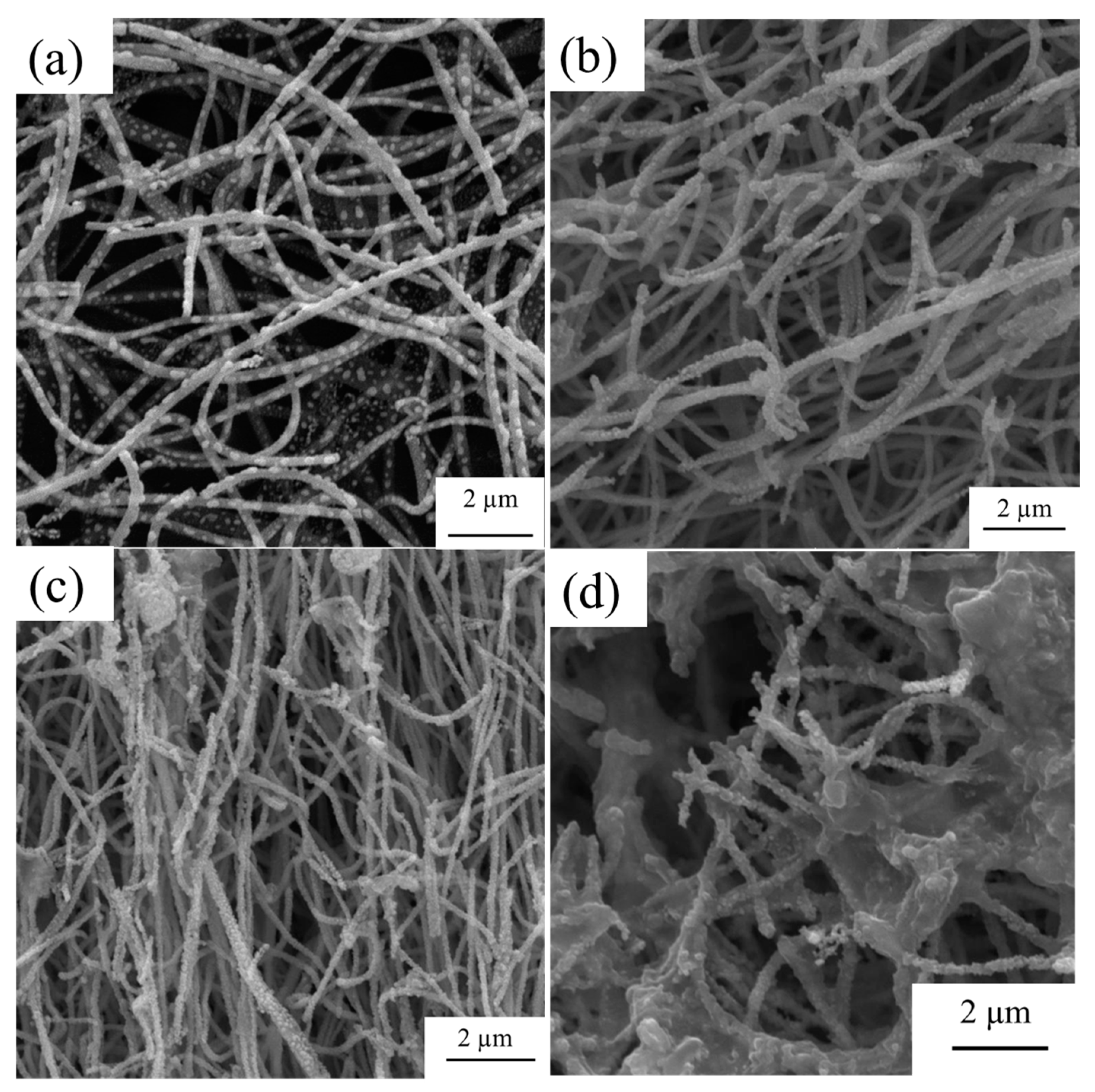
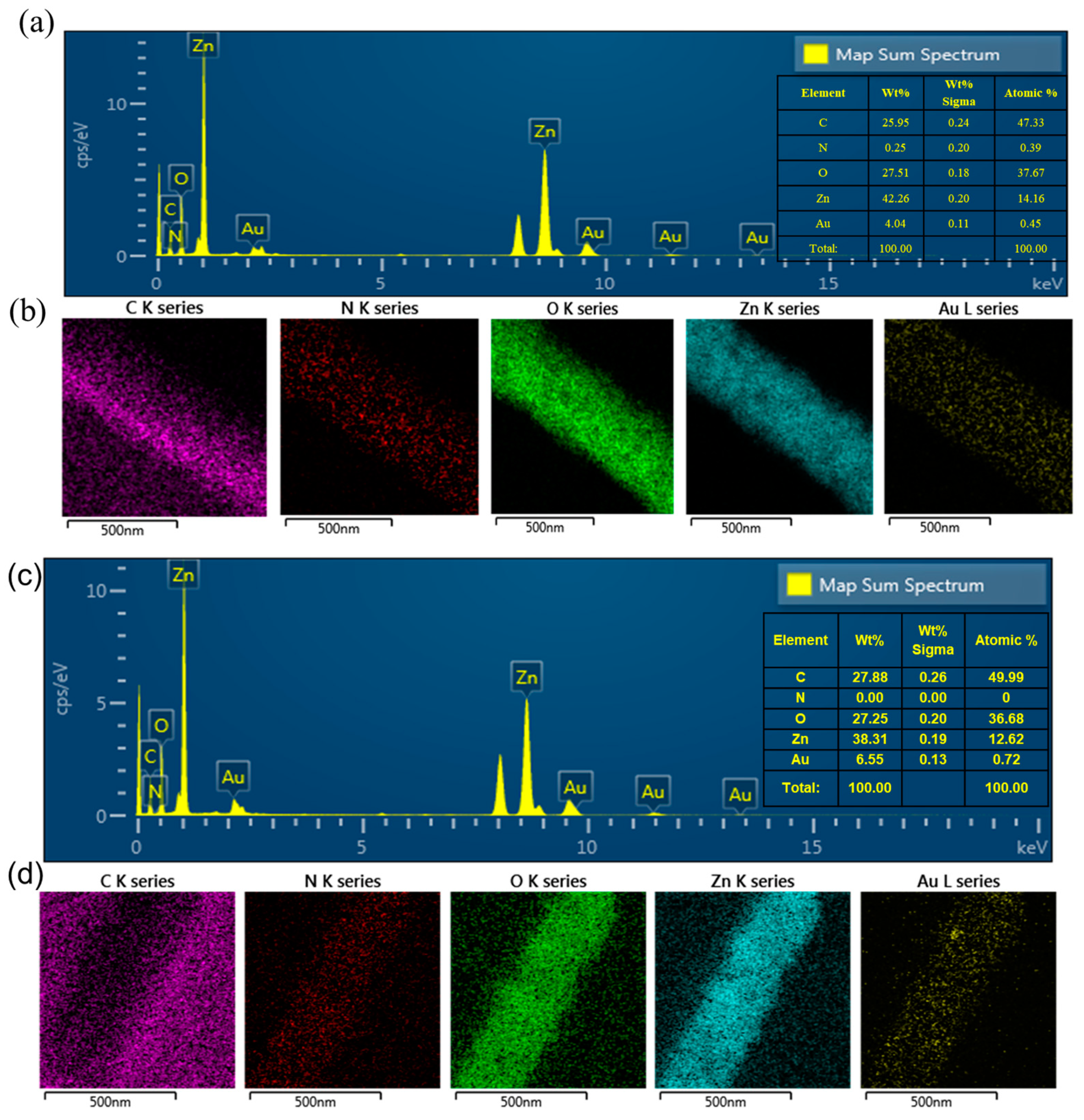
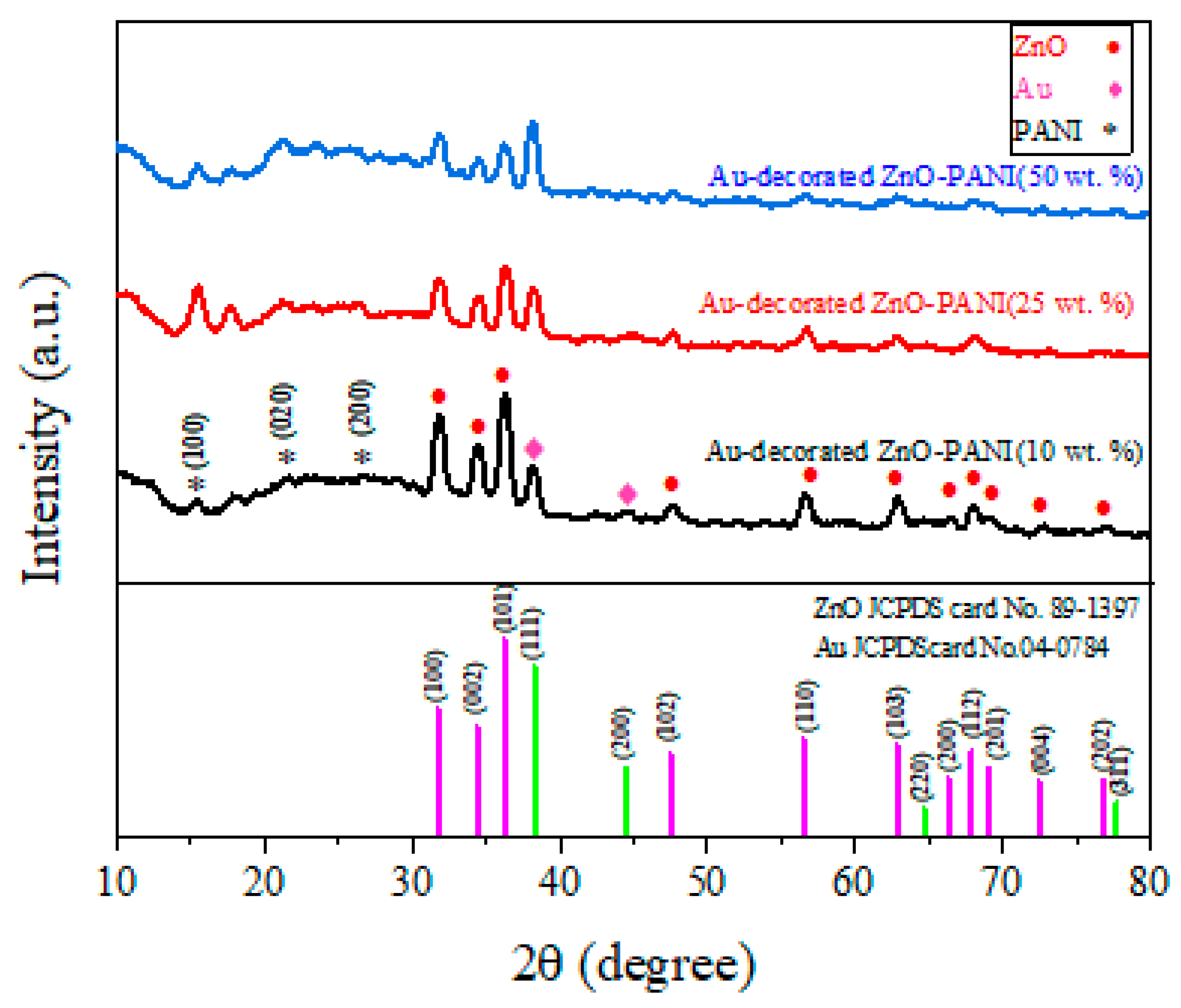
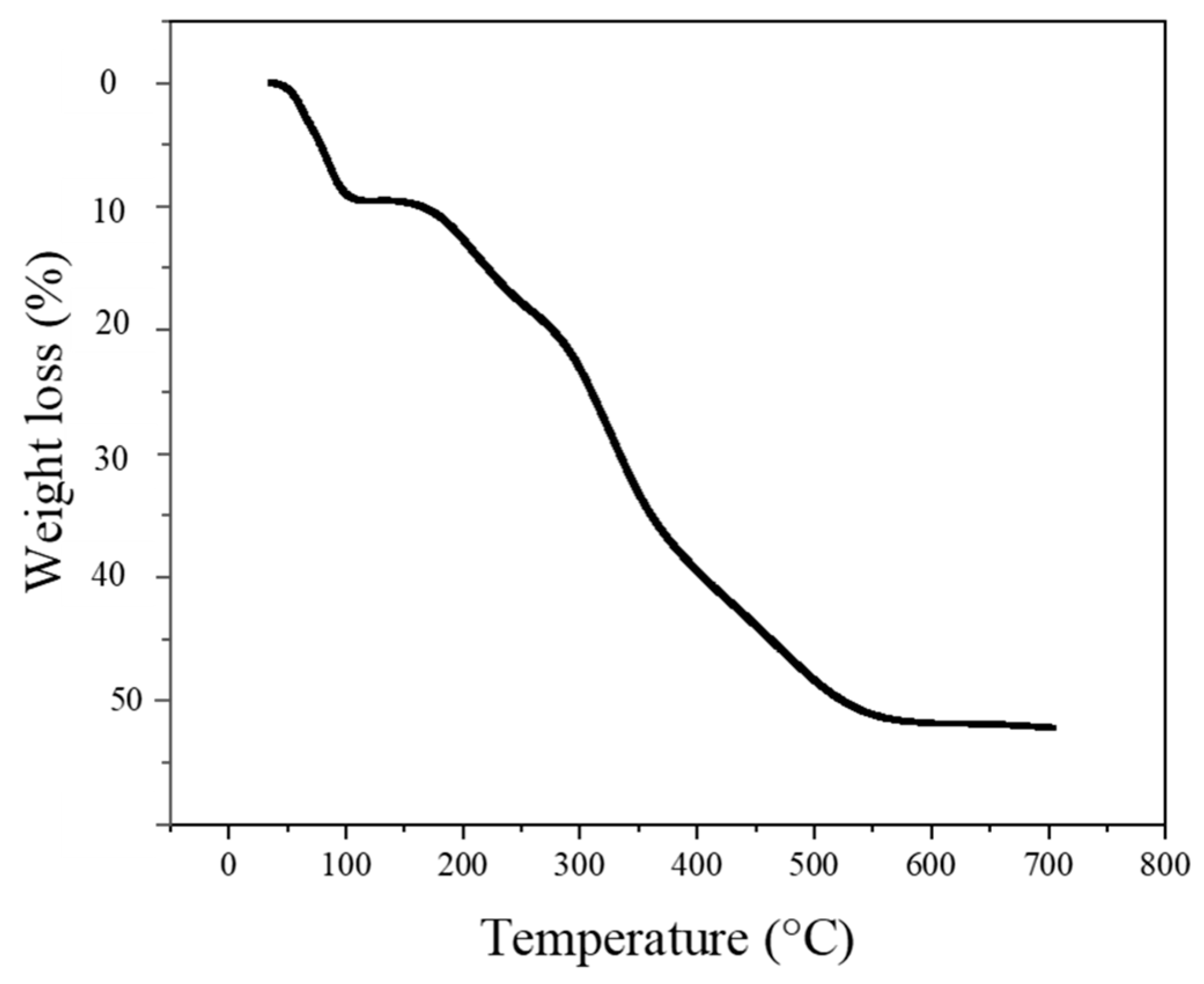
3.1. Morphological, Structural, and Thermal Studies
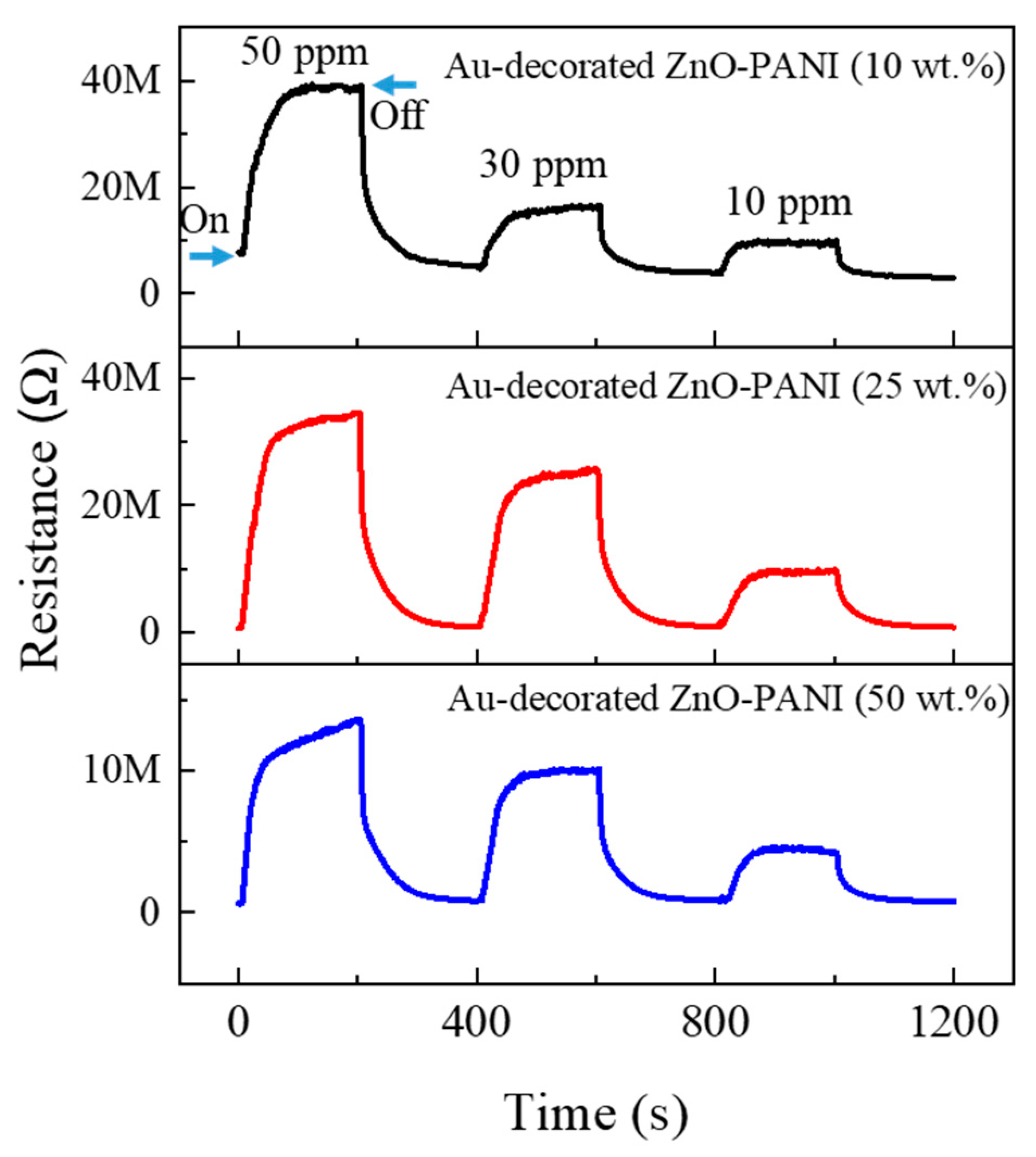
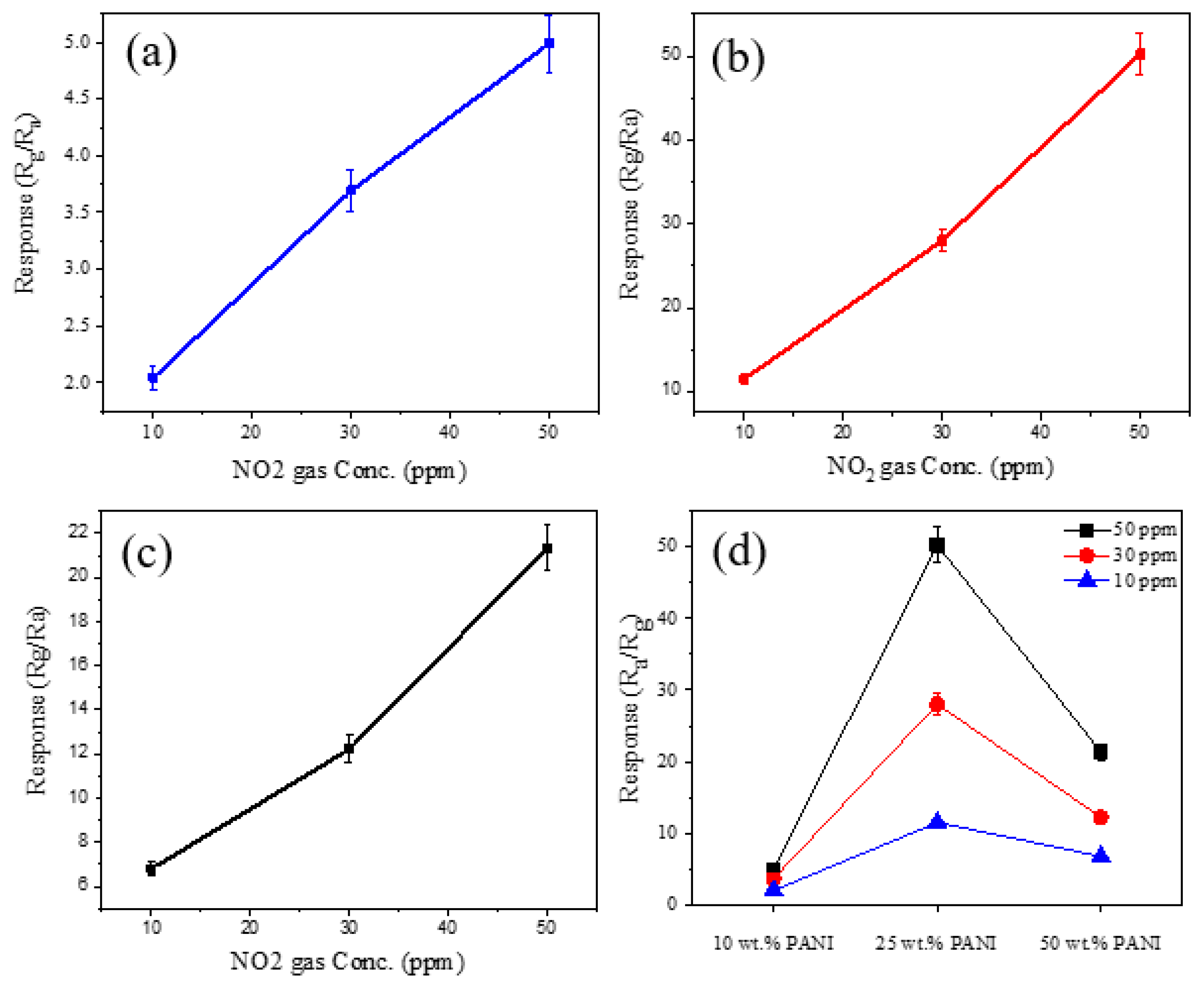
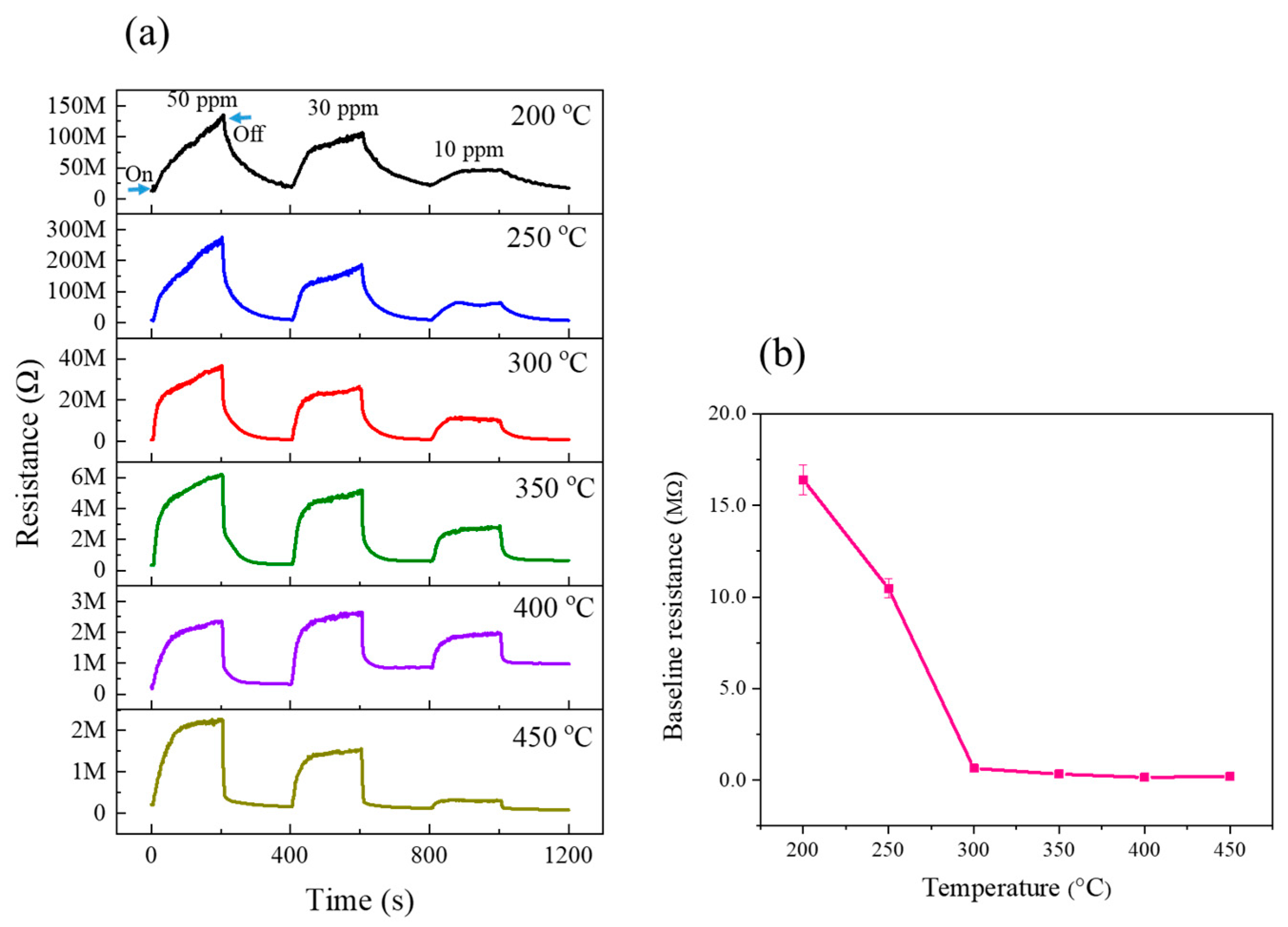
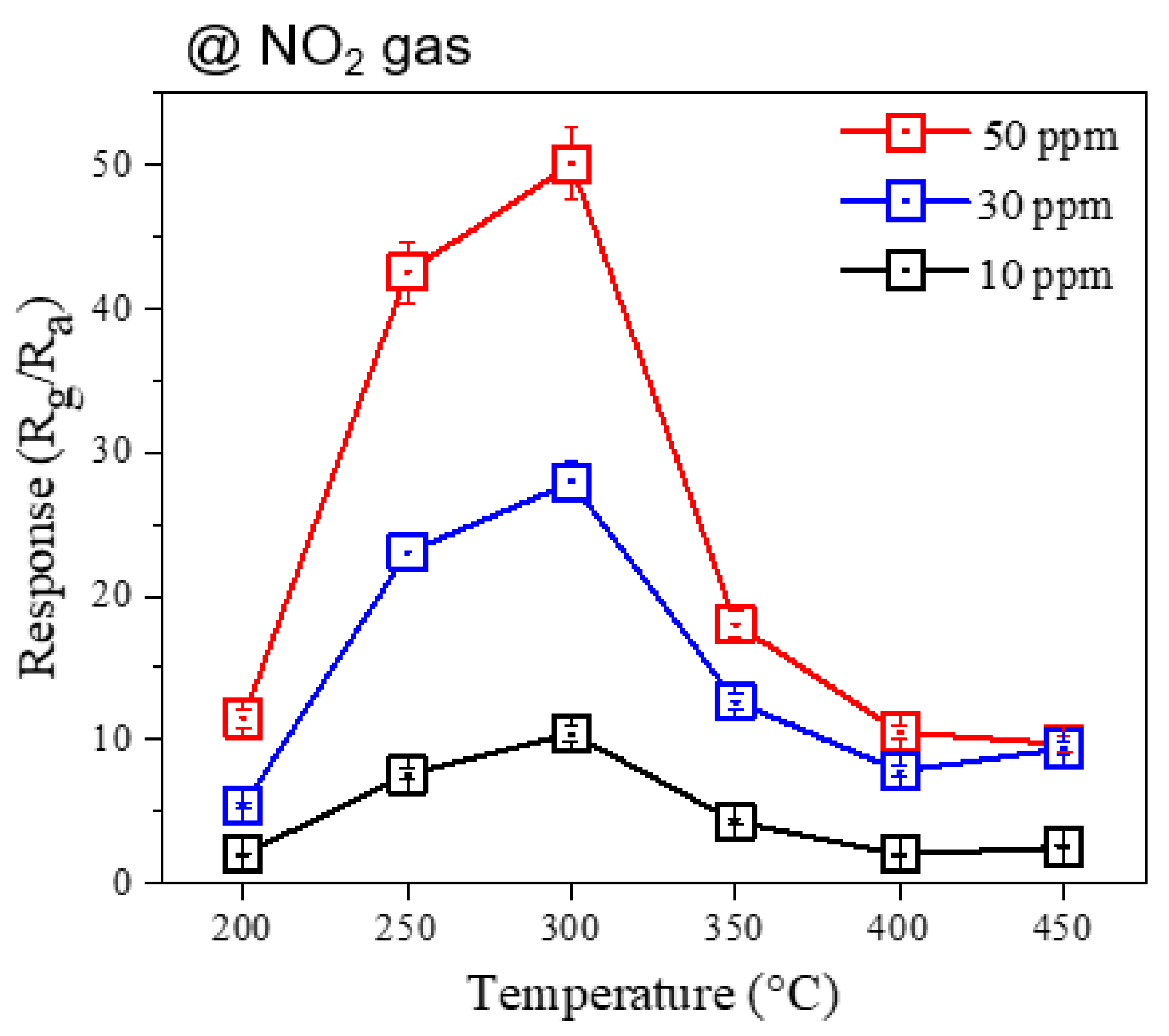
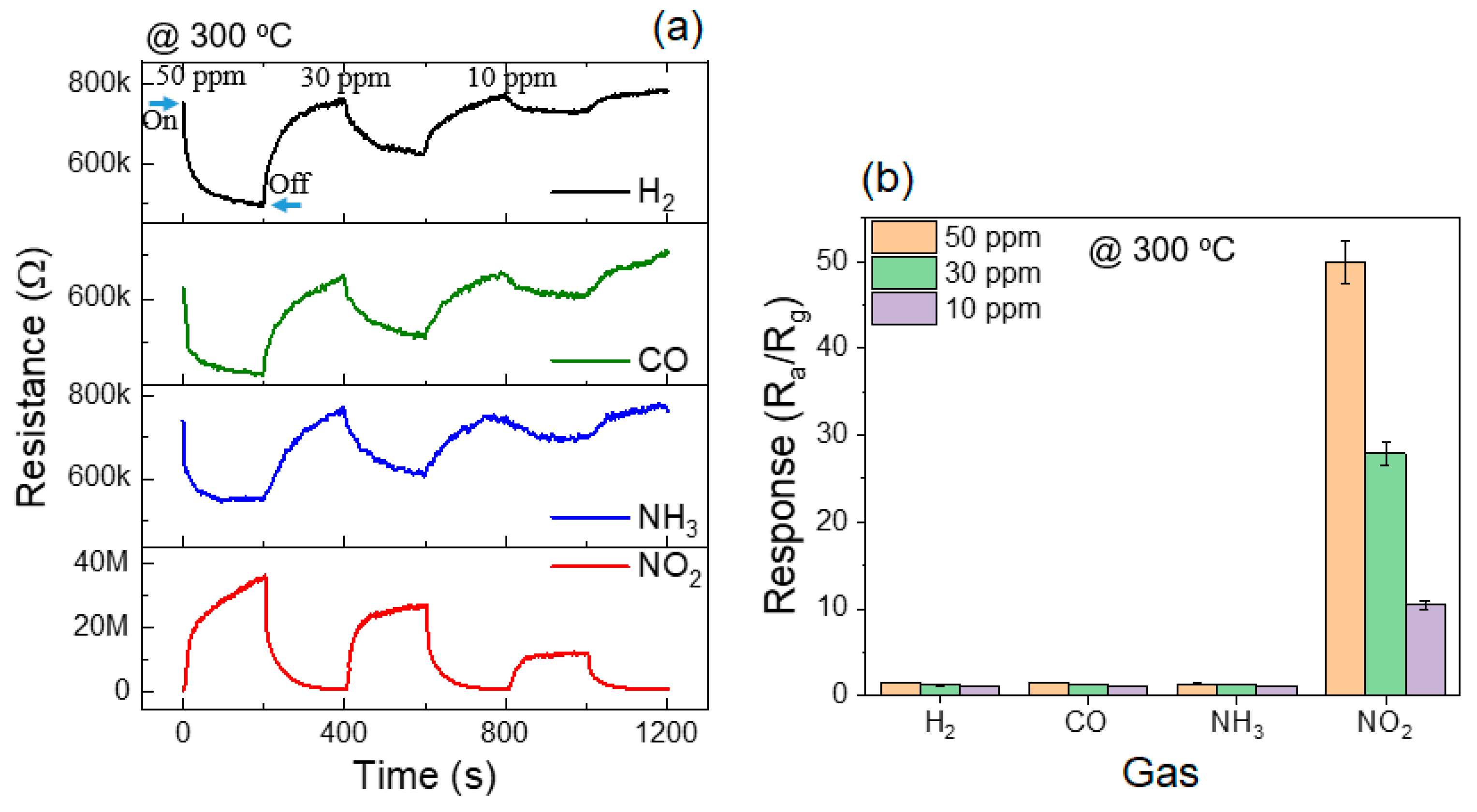
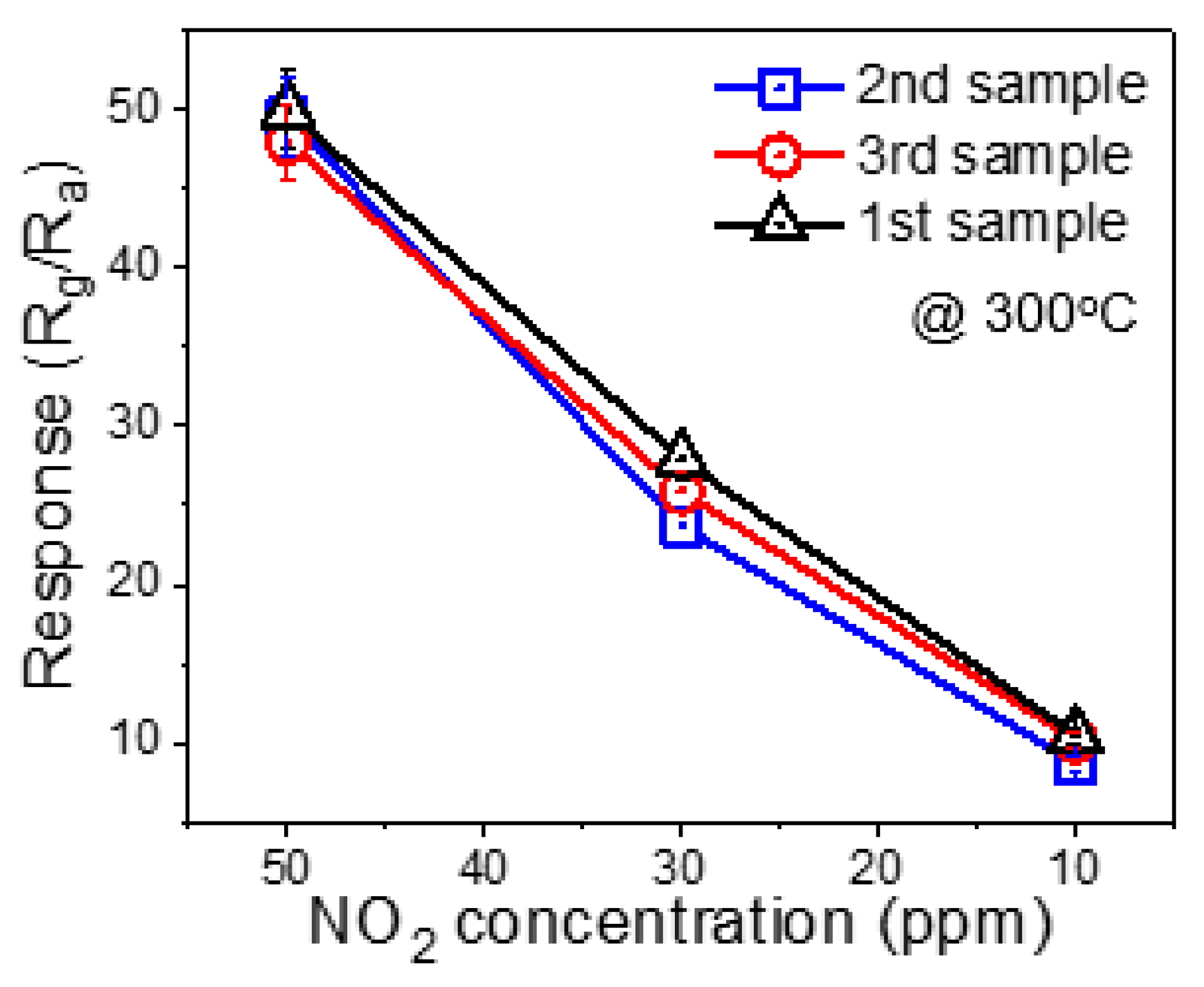
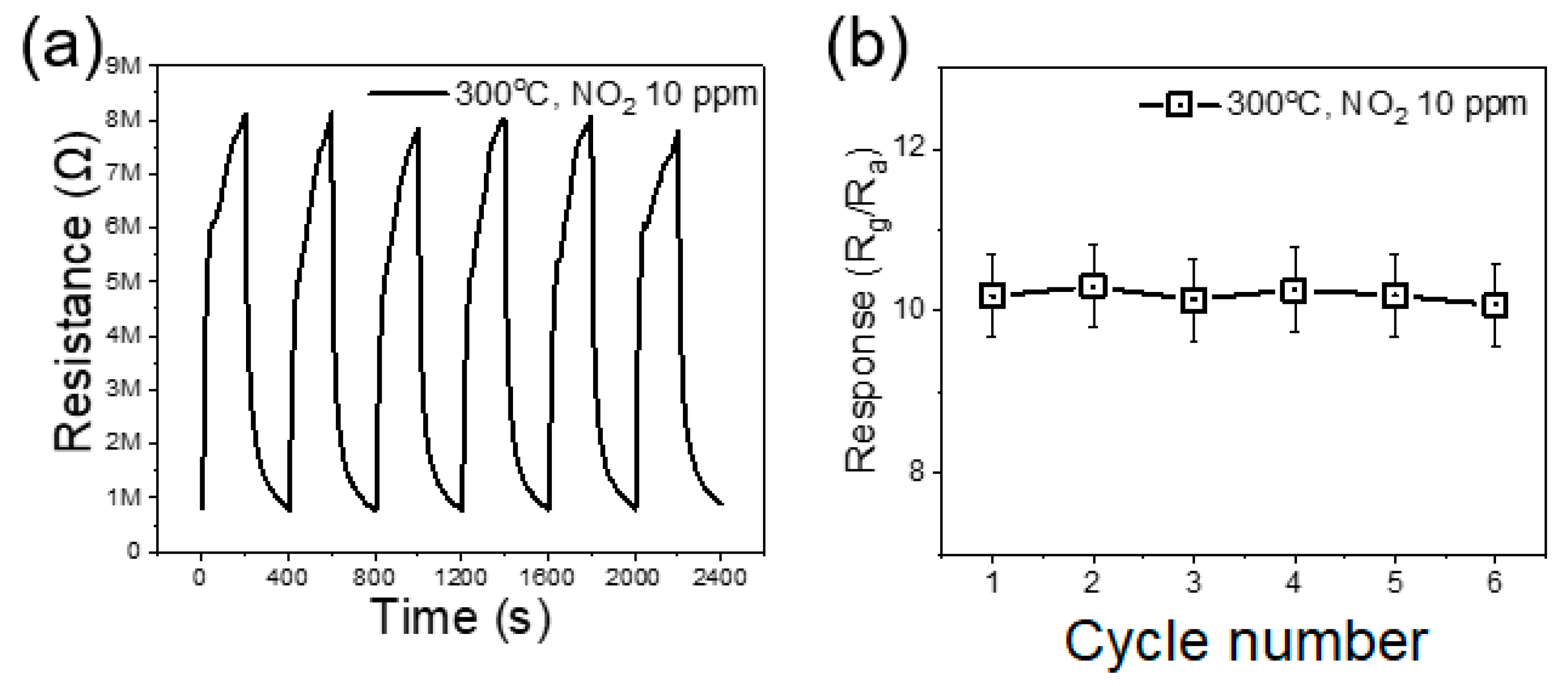
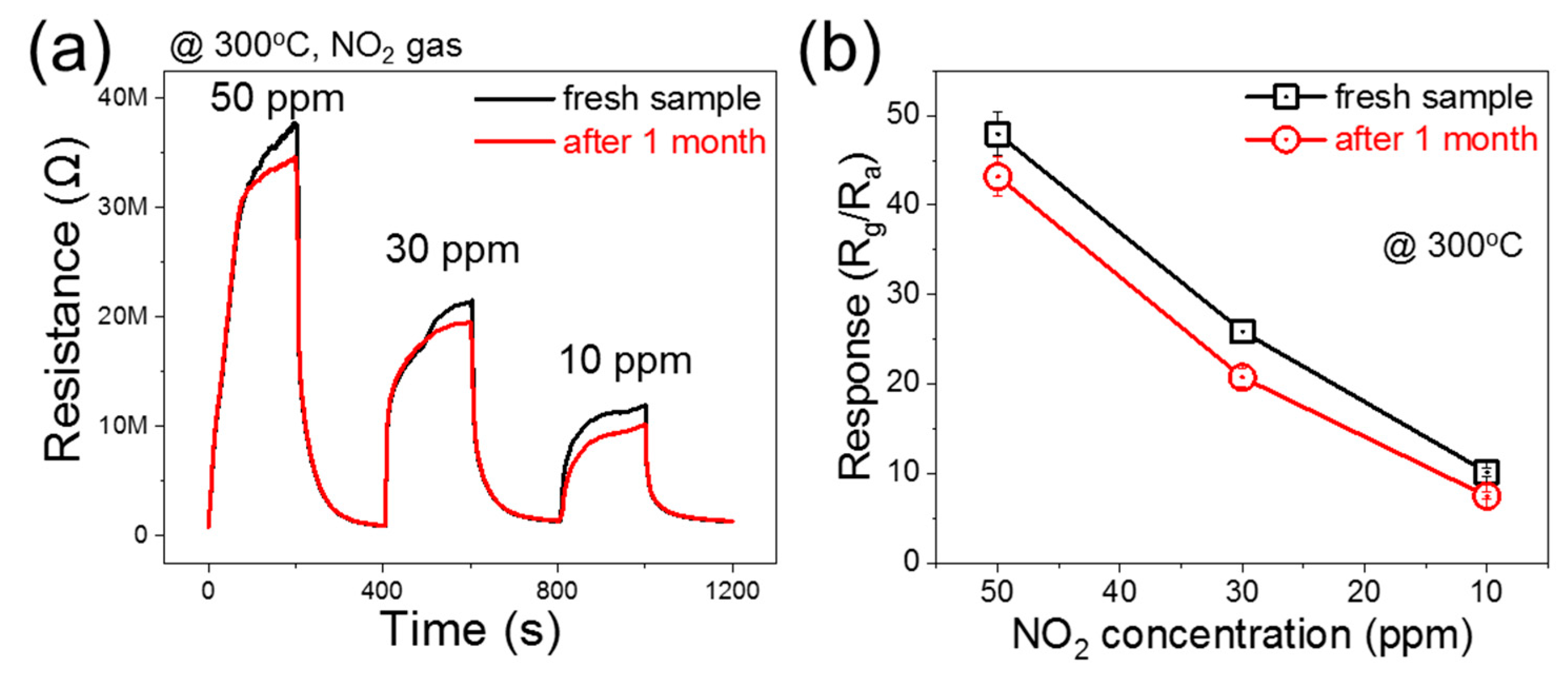
3.2. Gas Sensing Investigations
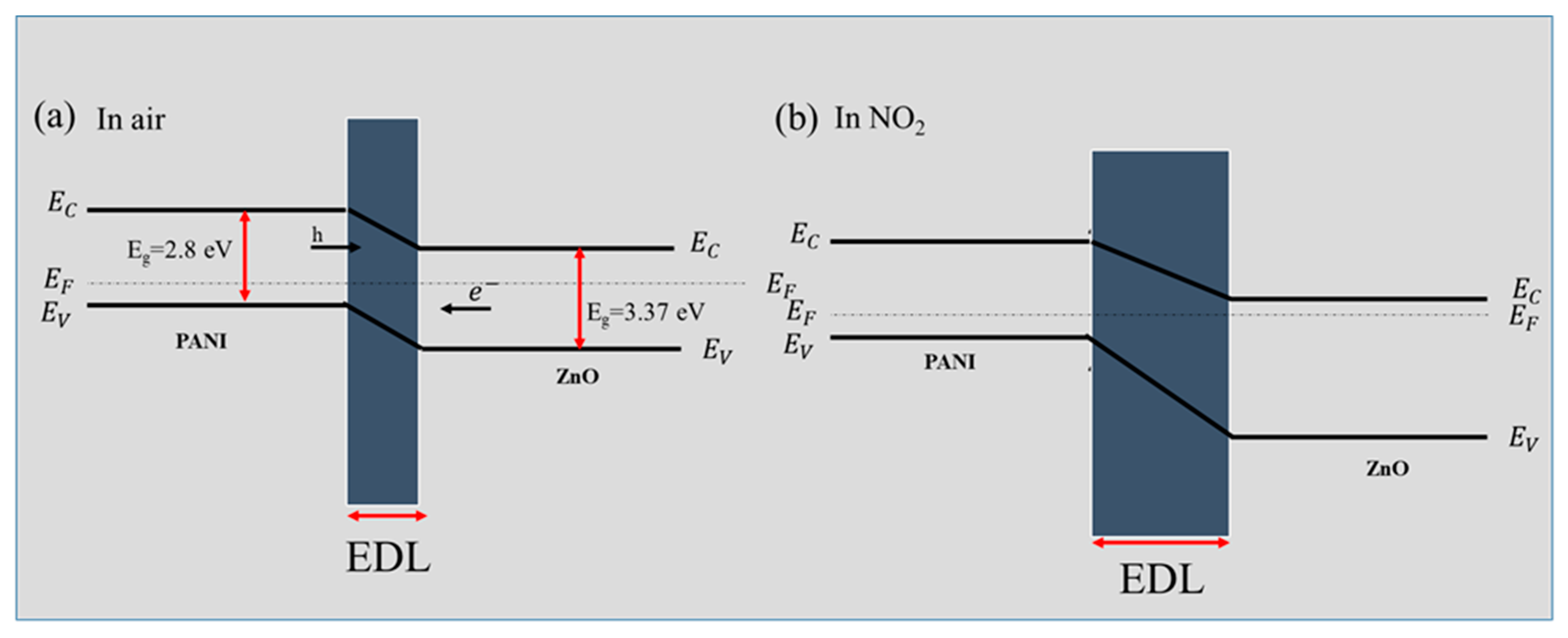
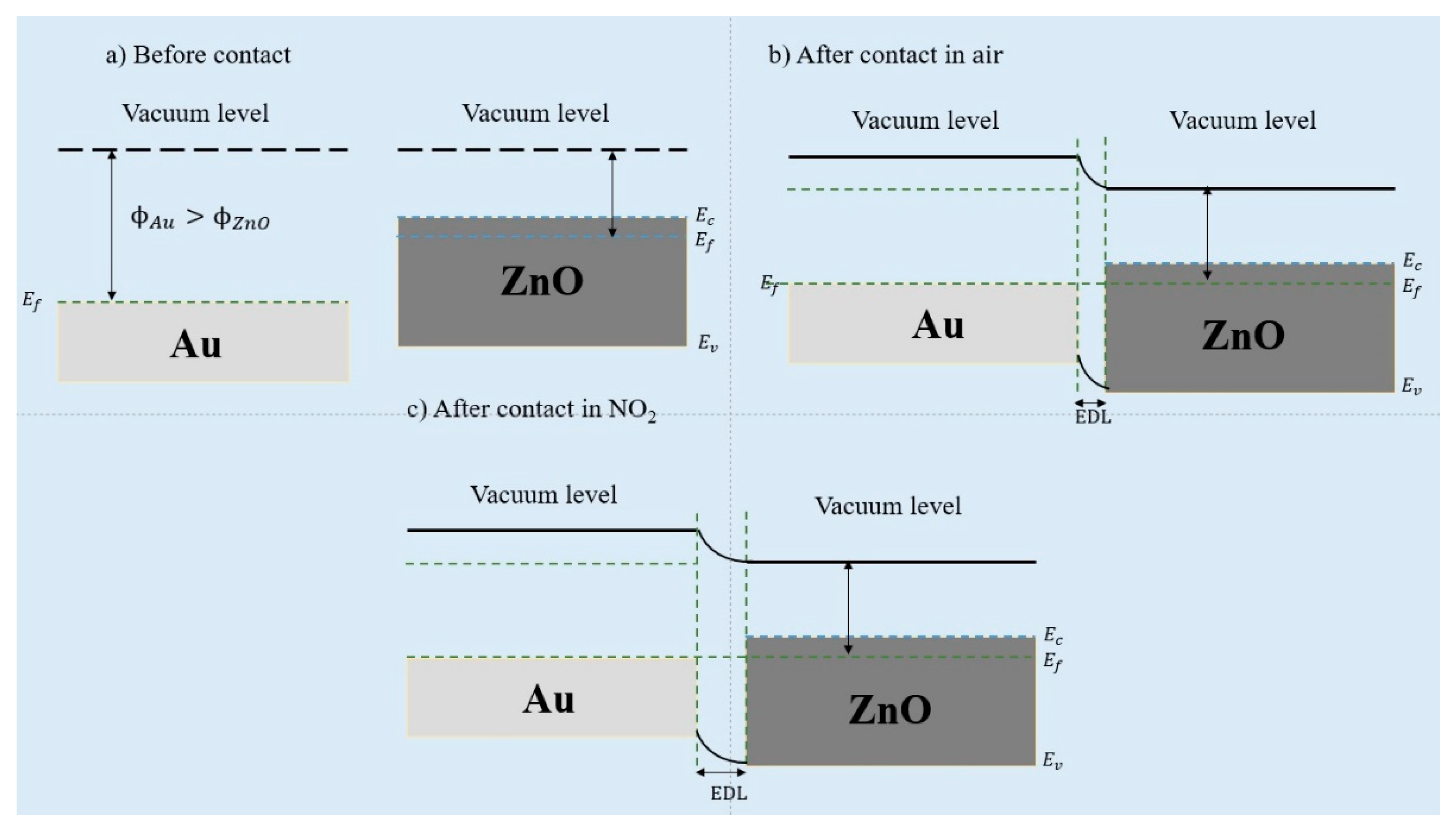
3.3. Gas Sensing Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sharma, N.; Kumar, S.; Gupta, A.; Dolmanan, S.B.; Patil, D.S.K.; Tan, S.T.; Tripathy, S.; Kumar, M. MoS2 Functionalized AlGaN/GaN Transistor based Room Temperature NO2 Gas Sensor. Sens. Actuator A Phys. 2022, 342, 113647. [Google Scholar] [CrossRef]
- Rani, S.; Kumar, M.; Sheoran, H.; Singh, R.; Singh, V.N. Rapidly Responding Room Temperature NO2 gas Sensor based on SnSe Nanostructured Film. Mater. Today Commun. 2022, 30, 103135. [Google Scholar] [CrossRef]
- Li, Q.; Zeng, W.; Li, Y. Metal oxide gas sensors for detecting NO2 in industrial exhaust gas: Recent developments. Sens. Actuators B Chem. 2022, 359, 131579. [Google Scholar] [CrossRef]
- Hong, H.S.; Ha, N.H.; Thinh, D.D.; Nam, N.H.; Huong, N.T.; Hue, N.T.; Hoang, T.V. Enhanced sensitivity of self-powered NO2 gas sensor to sub-ppb level using triboelectric effect based on surface-modified PDMS and 3D-graphene/CNT network. Nano Energy 2021, 87, 106165. [Google Scholar] [CrossRef]
- Kumar, R.R.; Murugesan, T.; Dash, A.; Hsu, C.-H.; Gupta, S.; Manikandan, A.; Kumar Anbalagan, A.; Lee, C.-H.; Tai, N.-H.; Chueh, Y.-L. Ultrasensitive and light-activated NO2 gas sensor based on networked MoS2/ZnO nanohybrid with adsorption/desorption kinetics study. Appl. Surf. Sci. 2021, 536, 147933. [Google Scholar] [CrossRef]
- Zhang, C.; Hong, X.; Jiang, C.; Luo, F.; Wan, B. Zheng, X. A special gas sensor based on mesoporous WO3 sensing electrode in medium low temperature for NO2 detection. Mater. Lett. 2022, 306, 130927. [Google Scholar] [CrossRef]
- Sun, J.; Xiong, W.; Zhang, J.; Zhang, Y.; Xie, B. SnS2 nanoparticle-based gas sensor with highly sensitive NO2 detection at room temperature. Mater. Lett. 2022, 308, 131214. [Google Scholar] [CrossRef]
- Su, P.-G.; Yu, J.-H. Enhanced NO2 gas-sensing properties of Au-Ag bimetal decorated MWCNTs/WO3 composite sensor under UV-LED irradiation. Sens. Actuator A Phys. 2020, 303, 111718. [Google Scholar] [CrossRef]
- Bharathi, P.; Harish, S.; Mathankumar, G.; Mohan, M.K.; Archana, J.; Kamalakannan, S.; Prakash, M.; Shimomura, M.; Navaneethan, M. Solution processed edge activated Ni-MoS2 nanosheets for highly sensitive room temperature NO2 gas sensor applications. Appl. Surf. Sci. 2022, 600, 154086. [Google Scholar] [CrossRef]
- Andrysiewicz, W.; Krzekinski, J.; Skarzynski, K.; Marszalek, K.; Sloma, M.; Rydosz, A. Flexible gas sensor printed on a polymer substrate for sub-ppm acetone detection. Electron. Mater. Lett. 2020, 16, 146–155. [Google Scholar] [CrossRef]
- Kim, S.; Bang, J.H.; Choi, M.S.; Oum, W.; Mirzaei, A.; Lee, N.; Kwon, H.C.; Lee, D.; Jeon, H.; Kim, S.S.; et al. Synthesis, characterization and gas-sensing properties of pristine and SnS2 functionalized TeO2, nanowires. Met. Mater. Int. 2019, 25, 805–813. [Google Scholar] [CrossRef]
- Park, S.; Kheel, H.; Sun, G.J.; Kim, H.W.; Ko, T.; Lee, C. Room-temperature hydrogen gas sensing properties of the networked Cr2O3-functionalized Nb2O5 nanostructured sensor. Met. Mater. Int. 2016, 22, 730–736. [Google Scholar] [CrossRef]
- Kim, S.S.; Na, H.G.; Kwon, Y.J.; Cho, H.Y.; Kim, H.W. Synthesis and room-temperature NO2 sensing properties of Sb2O5 nanowires. Met. Mater. Int. 2015, 21, 415–421. [Google Scholar] [CrossRef]
- Kim, J.H.; Zheng, Y.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Synthesis and selective sensing properties of rGO/metal-coloaded SnO2 nanofibers. J. Elec. Mater. 2017, 46, 3531–3541. [Google Scholar] [CrossRef]
- Li, T.; Yin, W.; Gao, S.; Sun, Y.; Xu, P.; Wu, S.; Kong, H.; Yang, G.; Wei, G. The combination of two-dimensional nanomaterials with metal oxide nanoparticles for gas sensors: A review. Nanomaterials. 2022, 12, 982. [Google Scholar] [CrossRef]
- Güntner, A.T.; Pineau, N.J.; Pratsinis, S.E. Flame-made chemoresistive gas sensors and devices. Prog. Energy Combust. Sci. 2022, 90, 100992. [Google Scholar] [CrossRef]
- Franco, M.A.; Conti, P.P.; Andre, R.S.; Correa, D.S. A review on chemiresistive ZnO gas sensors. Sens. Actuators Rep. 2022, 4, 100100. [Google Scholar] [CrossRef]
- Chen, Y.; Li, H.; Huang, D.; Wang, X.; Wang, Y.; Wang, W.; Yi, M.; Cheng, Q.; Song, Y.; Han, G. Highly sensitive and selective acetone gas sensors based on modified ZnO nanomaterials. Mater. Sci. Semi. Proc. 2022, 148, 106807. [Google Scholar] [CrossRef]
- Syue, Y.-K.; Hsu, K.-C.; Fang, T.-H.; Lee, C.-I.; Shih, C.-J. Characteristics and gas sensor applications of ZnO-Perovskite heterostructure. Ceram. Int. 2022, 48, 12585–12591. [Google Scholar] [CrossRef]
- Zhang, Z.; Wen, Z.; Ye, Z.; Zhu, L. Ultrasensitive ppb-level NO2 gas sensor based on WO3 hollow nanosphers doped with Fe. Appl. Surf. Sci. 2018, 434, 891–897. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc oxide nanostructures for NO2 gas–sensor applications: A review. Micro Nano Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Khudiar, S.S.; Nayef, U.M.; Mutlak, F.A.H.; Abdulridha, S.K. Characterization of NO2 gas sensing for ZnO nanostructure grown hydrothermally on porous silicon. Optik 2022, 249, 168300. [Google Scholar] [CrossRef]
- Wang, J.; Yang, Y.; Xia, Y. Mesoporous MXene/ZnO nanorod hybrids of high surface area for UV-activated NO2 gas sensing in ppb-level. Sens. Actuators B Chem. 2022, 353, 131087. [Google Scholar] [CrossRef]
- Lontio Fomekong, R.; Saruhan, B. Influence of humidity on NO2-sensing and selectivity of spray-CVD grown ZnO thin film above 400 °C. Chemosensors 2019, 7, 42. [Google Scholar] [CrossRef]
- Chang, C.-J.; Lin, C.-Y.; Chen, J.-K.; Hsu, M.-H. Ce-doped ZnO nanorods based low operation temperature NO2 gas sensors. Ceram. Int. 2014, 40, 10867–10875. [Google Scholar] [CrossRef]
- Wang, J.; Fan, S.; Xia, Y.; Yang, C.; Komarneni, S. Room-temperature gas sensors based on ZnO nanorod/Au hybrids: Visible-light-modulated dual selectivity to NO2 and NH3. J. Haz. Mater. 2020, 381, 120919. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.; Gupta, V.; Tomar, M. Fabrication and characterization of ZnO-TiO2-PANI (ZTP) micro/nanoballs for the detection of flammable and toxic gases. J. Haz. Mater. 2019, 370, 126–137. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, L.; Fan, J.; Zhu, B.; Yu, J. Triethylamine gas sensor based on Pt-functionalized hierarchical ZnO microspheres. Sens. Actuators B Chem. 2021, 331, 129425. [Google Scholar] [CrossRef]
- Sun, L.; Sun, J.K.; Zhang, X.; Sun, S.; Bai, Y.; Zhao, R.; Luo, D.; Li Chen, A. rGO functionalized α-Fe2O3/Co3O4 heterojunction for NO2 detection. Sens. Actuators B Chem. 2022, 354, 131194. [Google Scholar] [CrossRef]
- Zhu, L.; Zeng, W. Room-temperature gas sensing of ZnO-based gas sensor: A review. Sens. Actuator A Phys. 2017, 267, 242–261. [Google Scholar] [CrossRef]
- Namsheer, K.; Rout, C.S. Conducting polymers: A comprehensive review on recent advances in synthesis, properties and applications. RSC Adv. 2021, 11, 5659–5697. [Google Scholar]
- Wong, Y.C.; Ang, B.C.; Haseeb, A.S.M.A.; Baharuddin, A.A.; Wong, Y.H. Conducting polymers as chemiresistive gas sensing materials: A review. J. Electrochem. Soc. 2019, 167, 037503. [Google Scholar] [CrossRef]
- Ramanavicius, S.; Ramanavicius, A. Conducting polymers in the design of biosensors and biofuel cells. Polymers. 2020, 13, 49. [Google Scholar] [CrossRef]
- Kumar, V.; Mirzaei, A.; Bonyani, M.; Kim, K.-H.; Kim, H.W.; Kim, S.S. Advances in electrospun nanofiber fabrication for polyaniline (PANI)-based chemoresistive sensors for gaseous ammonia. Trends Analyt. Chem. 2020, 129, 115938. [Google Scholar] [CrossRef]
- Li, Z.; Gong, L. Research progress on applications of polyaniline (PANI) for electrochemical energy storage and conversion. Materials 2020, 13, 548. [Google Scholar] [CrossRef]
- Tiwari, A.; Kumar, R.; Prabaharan, M.; Pandey, R.R.; Kumari, P.; Chaturvedi, A.; Mishra, A.K. Nanofibrous polyaniline thin film prepared by plasma-induced polymerization technique for detection of NO2 gas. Polym. Adv. Technol. 2010, 21, 615–620. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.C.; Dzhardimalieva, G.I. Preparation and properties of nanostructured PANI thin film and its application as low temperature NO2 sensor. J. Inorg. Organomet. Polym. Mater. 2016, 26, 1428–1433. [Google Scholar] [CrossRef]
- Xu, H.; Chen, X.; Zhang, J.; Wang, J.; Cao, B.; Cui, D. NO2 gas sensing with SnO2–ZnO/PANI composite thick film fabricated from porous nanosolid. Sens. Actuators B Chem. 2013, 176, 166–173. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.C.; Sharma, A.; Tomar, M.; Gupta, V. Experimental investigations on NO2 sensing of pure ZnO and PANI–ZnO composite thin films. RSC Adv. 2016, 6, 56149–56158. [Google Scholar] [CrossRef]
- Jain, S.; Karmakar, N.; Shah, A.; Shimpi, N.G. Development of Ni doped ZnO/polyaniline nanocomposites as high response room temperature NO2 sensor. Mater. Sci. Eng. B 2019, 247, 114381. [Google Scholar] [CrossRef]
- Sonker, R.K.; Yadav, B.C. Development of Fe2O3–PANI nanocomposite thin film based sensor for NO2 detection. J. Taiwan Inst. Chem. Eng. 2017, 77, 276–281. [Google Scholar] [CrossRef]
- He, W.; Zhao, Y.; Xiong, Y. Bilayer polyaniline–WO3 thin-film sensors sensitive to NO2. ACS Omega 2020, 5, 9744–9751. [Google Scholar] [CrossRef]
- Guan, Y.; Wang, C.; Yu, H.; Zou, Z.; Zhou, Y.; Cao, G.; Yao, J. Flexible ZnO/PANI/nonwoven nanocomposite based high-sensitive NH3 gas sensor via vapor phase polymerization method. Mater. Sci. Energy Technol. 2020, 3, 862–867. [Google Scholar] [CrossRef]
- Lupan, O.; Ababii, N.; Santos-Carballal, D.; Terasa, M.I.; Magariu, N.; Zappa, D.; Comini, E.; Pauporte, T.; Siebert, L.; Faupel, F.; et al. Tailoring the selectivity of ultralow-power heterojunction gas sensors by noble metal nanoparticle functionalization. Nano Energy 2021, 88, 106241. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Brinzari, V.; Cho, B.K. Conductometric gas sensors based on metal oxides modified with gold nanoparticles: A review. Microchim. Acta 2016, 183, 1033–1054. [Google Scholar] [CrossRef]
- Zhao, C.; Shen, J.; Xu, S.; Wei, J.; Liu, H.; Xie, S.; Pan, Y.; Zhao, Y.; Zhu, Y. Ultra-efficient trimethylamine gas sensor based on au nanoparticles sensitized WO3 nanosheets for rapid assessment of seafood freshness. Food Chem. 2022, 392, 133318. [Google Scholar] [CrossRef]
- Lee, H.Y.; Bang, J.H.; Majhi, S.M.; Mirzaei, A.; Shin, K.Y.; Yu, D.J.; Oum, W.; Kang, S.; Lee, M.L.; Kim, S.S. Conductometric ppb-level acetone gas sensor based on one-pot synthesized Au@ Co3O4 core-shell nanoparticles. Sens. Actuators B Chem. 2022, 359, 131550. [Google Scholar] [CrossRef]
- Guo, L.; Shen, Z.; Ma, C.; Ma, C.; Wang, J.; Yuan, T. Gas sensor based on MOFs-derived Au-loaded SnO2 nanosheets for enhanced acetone detection. J. Alloys Compd. 2022, 906, 164375. [Google Scholar] [CrossRef]
- Hsueh, T.-J.; Wu, S.-S. Highly sensitive Co3O4 nanoparticles/MEMS NO2 gas sensor with the adsorption of the Au nanoparticles. Sens. Actuators B Chem. 2021, 329, 129201. [Google Scholar] [CrossRef]
- Fan, Y.Y.; Tu, H.L.; Pang, Y.; Wei, F.; Zhao, H.B.; Yang, Y.; Ren, T.L. Au-decorated porous structure graphene with enhanced sensing performance for low-concentration NO2 detection. Rare Metals 2020, 39, 651–658. [Google Scholar] [CrossRef]
- Ponnuvelu, D.V.; Dhakshinamoorthy, J.; Prasad, A.K.; Dhara, S.; Kamruddin, M.; Pullithadathil, B. Geometrically controlled Au-decorated ZnO heterojunction nanostructures for NO2 detection. ACS Appl. Nano Mater. 2020, 3, 5898–5909. [Google Scholar] [CrossRef]
- Liang, J.; Zhu, K.; Yang, R.; Hu, M. Room temperature NO2 sensing properties of Au-decorated vanadium oxide nanowires sensor. Ceram. Int. 2018, 44, 2261–2268. [Google Scholar] [CrossRef]
- Cai, Z.; Kim, K.K.; Park, S. Room temperature detection of NO2 gas under UV irradiation based on Au nanoparticle-decorated porous ZnO nanowires. J. Mater. Res. Technol. 2020, 9, 16289–16302. [Google Scholar] [CrossRef]
- Rattan, S.; Kumar, S.; Goswamy, J.K. Gold nanoparticle decorated graphene for efficient sensing of NO2 gas. Sens. Int. 2022, 3, 100147. [Google Scholar] [CrossRef]
- Muñoz-Fernandez, L.; Gomez-Villalba, L.S.; Milošević, O.; Rabanal, M.E. Influence of nanoscale defects on the improvement of photocatalytic activity of Ag/ZnO. Mater. Charact. 2022, 185, 111718. [Google Scholar] [CrossRef]
- Farooq, N.; ur Rehman, A.; Qureshi, A.M.; ur Rehman, Z.; Ahmad, A.; Aslam, M.K.; Javed, H.M.A.; Hussain, S.; Habila, M.A.; AlMasoud, N.; et al. Au@ GO@ g-C3N4 and Fe2O3 nanocomposite for efficient photocatalytic and electrochemical applications. Surf. Interfaces 2021, 26, 101399. [Google Scholar] [CrossRef]
- Lin, C.K.; Kuo, J.L. Anharmonic IR spectra of solvated ammonium and aminium ions: Resemblance between water and bisulfate solvations. Phys. Chem. Chem. Phys. 2022, 24, 20318–20325. [Google Scholar] [CrossRef]
- Zhu, C.; Xue, H.; Zhao, H.; Fei, T.; Liu, S.; Chen, Q.; Gao, B.; Zhang, T. A dual-functional polyaniline film-based flexible electrochemical sensor for the detection of pH and lactate in sweat of the human body. Talanta 2022, 242, 123289. [Google Scholar] [CrossRef]
- Yang, W.; Sun, J.; Liu, D.; Fu, W.; Dong, Y.; Fu, Y.; Zhu, Y. Rational design of hierarchical structure of carbon@ polyaniline composite with enhanced microwave absorption properties. Carbon 2022, 194, 114–126. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, A.; Mudila, H.; Kumar., V. Synthesis and thermal analysis of polyaniline (PANI). In Journal of Physics: Conference Series; IOP Publishing: Bristol, UK, 2020. [Google Scholar]
- Henaish, A.M.A.; Salem, B.I.; Meaz, T.M.; Alibwaini, Y.A.; Ajlouni, A.W.; Hemeda, O.M.; Arrasheed, E.A. Synthesize, characterization, dielectric, linear and nonlinear optical properties of Ni–Al Ferrite/PANI nanocomposite film. Opt. Mater. 2021, 119, 111397. [Google Scholar] [CrossRef]
- Pandimurugan, R.; Thambidurai, S. Synthesis of seaweed-ZnO-PANI hybrid composite for adsorption of methylene blue dye. J. Environ. Chem. Eng. 2016, 4, 1332–1347. [Google Scholar] [CrossRef]
- Khairy, M.; Gouda, M.E. Electrical and optical properties of nickel ferrite/polyaniline nanocomposite. J. Adv. Res. 2015, 6, 555–562. [Google Scholar] [CrossRef] [PubMed]
- Shaikh, S.K.; Ganbavale, V.V.; Mohite, S.V.; Patil, U.M.; Rajpure, K.Y. ZnO nanorod based highly selective visible blind ultra-violet photodetector and highly sensitive NO2 gas sensor. Superlattices Microstruct. 2018, 120, 170–186. [Google Scholar] [CrossRef]
- Gao, H.; Yu, Q.; Chen, K.; Sun, P.; Liu, F.; Yan, X.; Liu, F.; Lu, G. Ultrasensitive gas sensor based on hollow tungsten trioxide-nickel oxide (WO3-NiO) nanoflowers for fast and selective xylene detection. J. Colloid Interface Sci. 2019, 535, 458–468. [Google Scholar] [CrossRef] [PubMed]
- Tonezzer, M.; Hieu, N.V. Size-dependent response of single-nanowire gas sensors. Sens. Actuators B Chem. 2012, 163, 146–152. [Google Scholar] [CrossRef]
- Arifin, P.; Mustajab, M.A.; Haryono, S.; Adhika, D.R.; Nugraha, A.A. MOCVD growth and characterization of TiO2 thin films for hydrogen gas sensor application. Mater. Res. Express 2019, 6, 076313. [Google Scholar] [CrossRef]
- Bao, C.; Chen, M.; Jin, X.; Hu, D.; Huang, Q. Efficient and stable photocatalytic reduction of aqueous hexavalent chromium ions by polyaniline surface-hybridized ZnO nanosheets. J. Mol. Liq. 2019, 279, 133–145. [Google Scholar] [CrossRef]
- Korotcenkov, G.; Cho, B.K. Engineering approaches for the improvement of conductometric gas sensor parameters: Part 1. Improvement of sensor sensitivity and selectivity (short survey). Sens. Actuators B Chem. 2013, 188, 709–728. [Google Scholar] [CrossRef]
- Kim, J.Y.; Lee, J.H.; Kim, J.H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. Realization of H2S sensing by Pd-functionalized networked CuO nanowires in self-heating mode. Sens. Actuators B Chem. 2019, 299, 126965. [Google Scholar] [CrossRef]
- Romain, A.C.; Nicolas, J. Long term stability of metal oxide-based gas sensors for e-nose environmental applications: An overview. Sens. Actuators B Chem. 2010, 146, 502–506. [Google Scholar] [CrossRef]
- Mirzaei, A.; Park, S.; Sun, G.-J.; Kheel, H.; Lee, C. CO gas sensing properties of In4Sn3O12 and TeO2 composite nanoparticle sensors. J. Haz. Mater. 2016, 305, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Kim, S.S.; Kim, H.W. Resistance-based H2S gas sensors using metal oxide nanostructures: A review of recent advances. J. Haz. Mater. 2018, 357, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Mirzaei, A.; Janghorban, K.; Hashemi, B.; Bonyani, M.; Leonardi, S.G.; Neri, G. A novel gas sensor based on Ag/Fe2O3 core-shell nanocomposites. Ceram. Int. 2016, 42, 18974–18982. [Google Scholar] [CrossRef]
- Mirzaei, A.; Kim, J.-H.; Kim, H.W.; Kim, S.S. How shell thickness can affect the gas sensing properties of nanostructured materials: Survey of literature. Sens. Actuators B Chem. 2018, 258, 270–294. [Google Scholar] [CrossRef]
- Chen, D.; Yu, W.; Wei, L.; Ni, J.; Li, H.; Chen, Y.; Tian, Y.; Yan, S.; Mei, L.; Jiao, J. High sensitive room temperature NO2 gas sensor based on the avalanche breakdown induced by Schottky junction in TiO2-Sn3O4 nanoheterojunctions. J. Alloys Compd. 2022, 912, 165079. [Google Scholar] [CrossRef]
- Choi, M.S.; Bang, J.H.; Mirzaei, A.; Oum, W.; Na, H.G.; Jin, C.; Kim, S.S.; Kim, H.W. Promotional effects of ZnO-branching and Au-functionalization on the surface of SnO2 nanowires for NO2 sensing. J. Alloys Compd. 2019, 786, 27–39. [Google Scholar] [CrossRef]
- Xuan, J.; Zhao, G.; Sun, M.; Jia, F.; Wang, X.; Zhou, T.; Yin, G.; Liu, B. Low-temperature operating ZnO-based NO2 sensors: A review. RSC Adv. 2020, 10, 39786–39807. [Google Scholar] [CrossRef]
- An, S.; Park, S.; Ko, H.; Jin, C.; Lee, W.I.; Lee, C. Enhanced gas sensing properties of branched ZnO nanowires. Thin Solid Films 2013, 547, 241–245. [Google Scholar] [CrossRef]
- Singh, N.S.; Kumar, L.; Kumar, A.; Vaisakh, S.; Singh, S.D.; Sisodiya, K.; Srivastava, S.; Kansal, M.; Rawat, S.; Singh, T.A. Fabrication of zinc oxide/polyaniline (ZnO/PANI) heterojunction and its characterisation at room temperature. Mater. Sci. Semi. Proc. 2017, 60, 29–33. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, X.; Guo, C.; Cheng, X.; Zhu, C.; Xu, Y.; Major, Z.; Huo, L. Resistive room temperature DMA gas sensor based on the forest-like unusual n-type PANI/TiO2 nanocomposites. Sens. Actuators B Chem. 2021, 342, 130067. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, S.; Wu, Z.; Zhang, M.; Cao, Y.; Guo, J.; Zhong, F.; Duan, H.; Jia, D. High-performance gas sensor of polyaniline/carbon nanotube composites promoted by interface engineering. Sensors 2019, 20, 149. [Google Scholar] [CrossRef] [PubMed]
- Fratoddi, I.; Venditti, I.; Cametti, C.; Russo, M.V. Chemiresistive polyaniline-based gas sensors: A mini review. Sens. Actuators B Chem. 2015, 220, 534–548. [Google Scholar] [CrossRef]
- Saaedi, A.; Shabani, P.; Yousefi, R. High performance of methanol gas sensing of ZnO/PAni nanocomposites synthesized under different magnetic field. J. Alloys Compd. 2019, 802, 335–344. [Google Scholar] [CrossRef]
- Cao, P.; Cai, Y.; Pawar, D.; Han, S.; Xu, W.; Fang, M.; Liu, X.; Zeng, Y.; Liu, W.; Lu, Y. Au@ ZnO/rGO nanocomposite-based ultra-low detection limit highly sensitive and selective NO2 gas sensor. J. Mater. Chem. C 2022, 10, 4295–4305. [Google Scholar] [CrossRef]
- Yang, X.; Salles, V.; Kaneti, Y.V.; Liu, M.; Maillard, M.; Journet, C.; Jiang, X.; Brioude, A. Fabrication of highly sensitive gas sensor based on Au functionalized WO3 composite nanofibers by electrospinning. Sens. Actuators B Chem. 2015, 220, 1112–1119. [Google Scholar] [CrossRef]
- Kim, J.-H.; Lee, J.-H.; Mirzaei, A.; Kim, H.W.; Kim, S.S. SnO2 (n)-NiO (p) composite nanowebs: Gas sensing properties and sensing mechanisms. Sens. Actuators B Chem. 2018, 258, 204–214. [Google Scholar] [CrossRef]
- Mirzaei, A.; Bang, J.H.; Choi, M.S.; Han, S.; Lee, H.Y.; Kim, S.S.; Kim, H.W. Changes in characteristics of Pt-functionalized RGO nanocomposites by electron beam irradiation for room temperature NO2 sensing. Ceram. Int. 2020, 46, 21638–21646. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonyani, M.; Zebarjad, S.M.; Janghorban, K.; Kim, J.-Y.; Kim, H.W.; Kim, S.S. Au-Decorated Polyaniline-ZnO Electrospun Composite Nanofiber Gas Sensors with Enhanced Response to NO2 Gas. Chemosensors 2022, 10, 388. https://doi.org/10.3390/chemosensors10100388
Bonyani M, Zebarjad SM, Janghorban K, Kim J-Y, Kim HW, Kim SS. Au-Decorated Polyaniline-ZnO Electrospun Composite Nanofiber Gas Sensors with Enhanced Response to NO2 Gas. Chemosensors. 2022; 10(10):388. https://doi.org/10.3390/chemosensors10100388
Chicago/Turabian StyleBonyani, Maryam, Seyed Mojtaba Zebarjad, Kamal Janghorban, Jin-Young Kim, Hyoun Woo Kim, and Sang Sub Kim. 2022. "Au-Decorated Polyaniline-ZnO Electrospun Composite Nanofiber Gas Sensors with Enhanced Response to NO2 Gas" Chemosensors 10, no. 10: 388. https://doi.org/10.3390/chemosensors10100388
APA StyleBonyani, M., Zebarjad, S. M., Janghorban, K., Kim, J.-Y., Kim, H. W., & Kim, S. S. (2022). Au-Decorated Polyaniline-ZnO Electrospun Composite Nanofiber Gas Sensors with Enhanced Response to NO2 Gas. Chemosensors, 10(10), 388. https://doi.org/10.3390/chemosensors10100388







