Rational Design of Nanozymes Enables Advanced Biochemical Sensing
Abstract
:1. Introduction
2. Rational Design of Nanozymes for Enhancing Detection Sensitivity
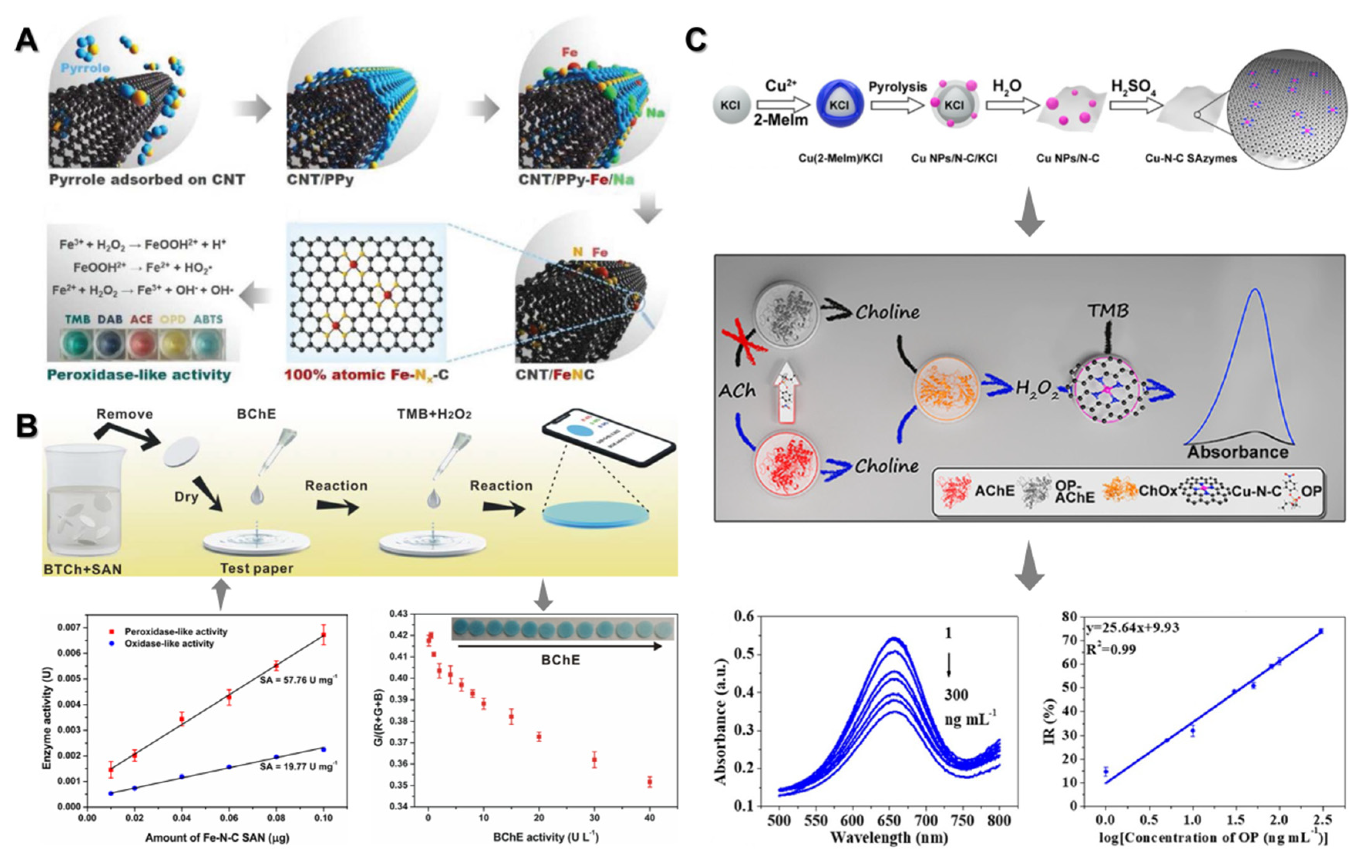
2.1. Single-Atom Nanozymes
2.2. Self-Cascade Nanozymes
3. Rational Design of Nanozymes to Improve Sensing Selectivity
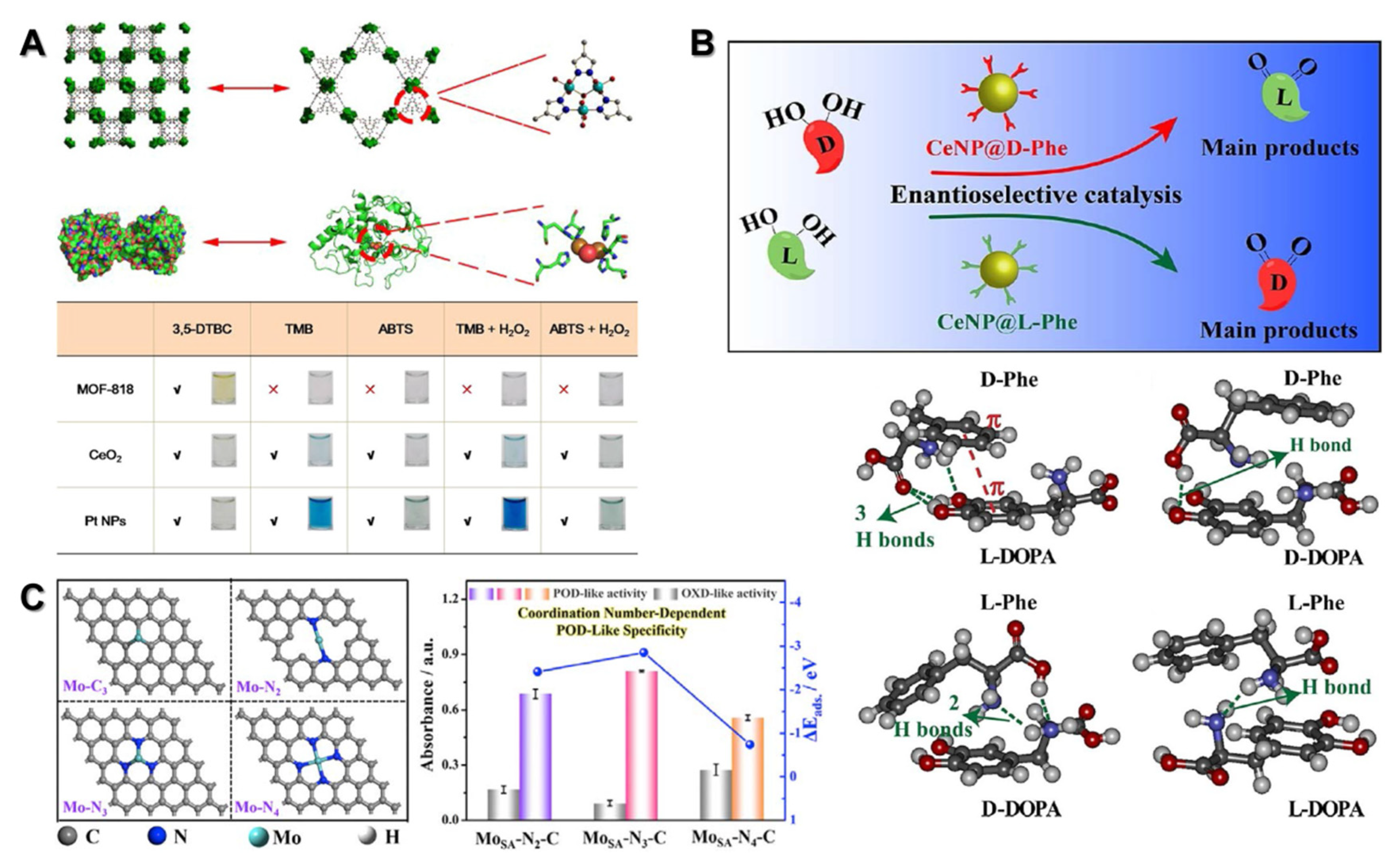
3.1. Structurally Biomimetic Nanozymes
3.2. Molecularly Imprinted Nanozymes
4. Rational Design of Nanozymes to Expand Application Scenarios
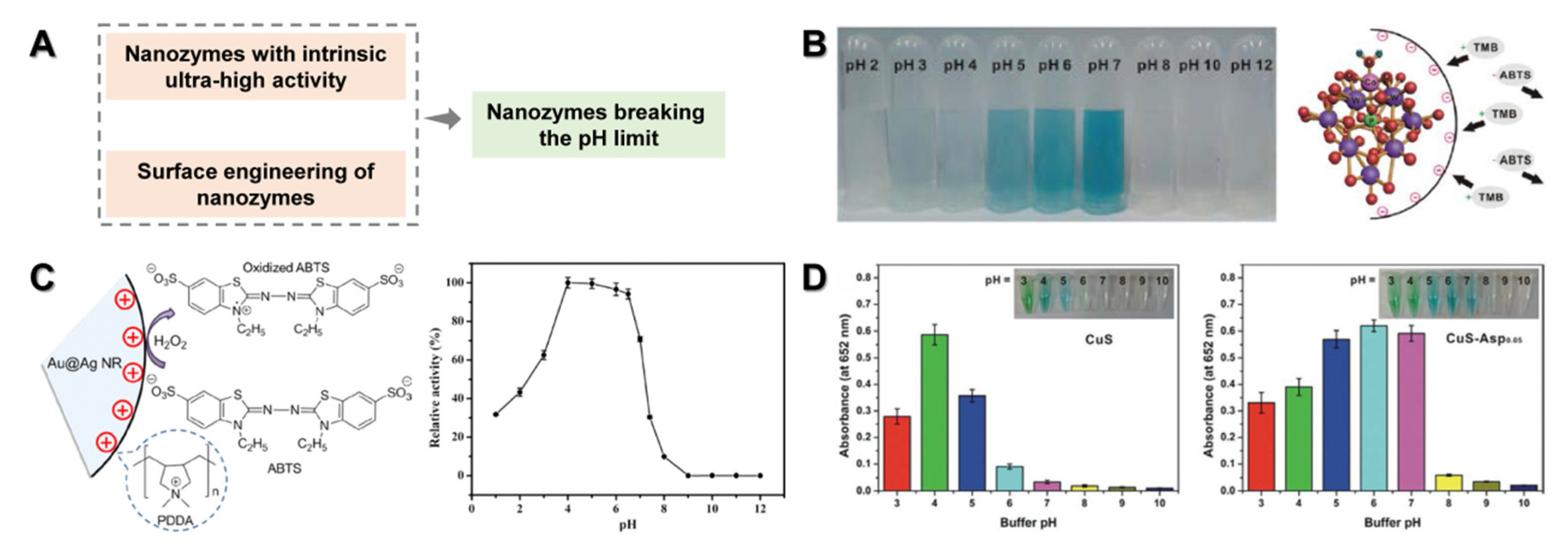
4.1. Nanozymes Breaking the pH Limit
4.2. Multifunctional Nanozymes
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AA | Ascorbic acid |
| AAP | Ascorbic acid 2-phosphate |
| ABTS | 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) |
| ACh | Acetylcholine |
| AChE | Acetylcholinesterase |
| ACP | Acid phosphatase |
| ALP | Alkaline phosphatase |
| Asp | Aspartic acid |
| BChE | Butyrylcholinesterase |
| CeNPs | Ceria nanoparticles |
| ChO | Choline oxidase |
| CNTs | Carbon nanotubes |
| CPNs(IV) | Ce-based coordination polymer nanoparticles |
| DA | Dopamine |
| DOPA | 3,4-Dihydroxyphenylalanine |
| GOx | Glucose oxidase |
| GSH | Glutathione |
| HRP | Horseradish peroxidase |
| LOD | Limit of detection |
| MIPs | Molecularly imprinted polymers |
| MOFs | Metal-organic frameworks |
| OP | Organophosphorus |
| OPD | o-Phenylenediamine |
| PDDA | Poly(diallyldimethylammonium) |
| Phe | Phenylalanine |
| Pi | Phosphate ion |
| PPy | Polypyrrole |
| SACs | Single atomic catalysts |
| SANs | Single-atom nanozymes |
| TA | Terephthalic acid |
| TAOH | 2-Hydroxyterephthalic acid |
| TC | Tetracycline |
| TMB | 3,3′,5,5′-Tetramethylbenzidine |
References
- Wei, H.; Wang, E. Nanomaterials with Enzyme-like Characteristics (Nanozymes): Next-Generation Artificial Enzymes. Chem. Soc. Rev. 2013, 42, 6060–6093. [Google Scholar] [CrossRef] [PubMed]
- Wei, H.; Gao, L.; Fan, K.; Liu, J.; He, J.; Qu, X.; Dong, S.; Wang, E.; Yan, X. Nanozymes: A clear definition with fuzzy edges. Nano Today 2021, 40, 101269. [Google Scholar] [CrossRef]
- Liang, M.; Yan, X. Nanozymes: From new concepts, mechanisms, and standards to applications. Acc. Chem. Res. 2019, 52, 2190–2200. [Google Scholar] [CrossRef] [PubMed]
- Jiao, J.; Fan, K.; Hu, Z.; Yan, X.; Du, P. Development trend and priority areas of nanozyme. Sci. Sin. Chim. 2019, 49, 1442–1453. [Google Scholar]
- Ragg, R.; Tahir, M.N.; Tremel, W. Solids Go Bio: Inorganic Nanoparticles as Enzyme Mimics. Eur. J. Inorg. Chem. 2015, 2016, 1906–1915. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, S.; Tang, Z. Recent progress in the design of analytical methods based on nanozymes. J. Mater. Chem. B 2021, 9, 8174–8184. [Google Scholar] [CrossRef]
- Niu, X.; Cheng, N.; Ruan, X.; Du, D.; Lin, Y. Review—Nanozyme-based immunosensors and immunoassays: Recent developments and future trends. J. Electrochem. Soc. 2020, 167, 037508. [Google Scholar] [CrossRef]
- Wang, Q.; Wei, H.; Zhang, Z.; Wang, E.; Dong, S. Nanozyme: An emerging alternative to natural enzyme for biosensing and immunoassay. TrAC Trends Anal. Chem. 2018, 105, 218–224. [Google Scholar] [CrossRef]
- Liu, B.; Liu, J. Surface modification of nanozymes. Nano Res. 2017, 10, 1125–1148. [Google Scholar] [CrossRef]
- Li, X.; Wang, L.; Du, D.; Ni, L.; Pan, J.; Niu, X. Emerging applications of nanozymes in environmental analysis: Opportunities and trends. TrAC Trends Anal. Chem. 2019, 120, 115653. [Google Scholar] [CrossRef]
- Song, W.; Zhao, B.; Wang, C.; Ozaki, Y.; Lu, X. Functional nanomaterials with unique enzyme-like characteristics for sensing applications. J. Mater. Chem. B 2019, 7, 850–875. [Google Scholar] [CrossRef]
- Huang, L.; Sun, D.; Pu, H.; Wei, Q. Development of Nanozymes for Food Quality and Safety Detection: Principles and Recent Applications. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1496–1513. [Google Scholar] [CrossRef]
- Li, S.; Zhang, Y.; Wang, Q.; Lin, A.; Wei, H. Nanozyme-Enabled Analytical Chemistry. Anal. Chem. 2021, 94, 312–323. [Google Scholar] [CrossRef]
- Li, X.; Zhu, H.; Liu, P.; Wang, M.; Pan, J.; Qiu, F.; Ni, L.; Niu, X. Realizing selective detection with nanozymes: Strategies and trends. TrAC Trends Anal. Chem. 2021, 143, 116379. [Google Scholar] [CrossRef]
- Wang, H.; Wan, K.; Shi, X. Recent Advances in Nanozyme Research. Adv. Mater. 2019, 31, 1805368. [Google Scholar] [CrossRef]
- Tian, Z.; Li, J.; Zhang, Z.; Gao, W.; Zhou, X.; Qu, Y. Highly sensitive and robust peroxidase-like activity of porous nanorods of ceria and their application for breast cancer detection. Biomaterials 2015, 59, 116–124. [Google Scholar] [CrossRef]
- Shu, Q.W.; Li, C.M.; Gao, P.F.; Gao, M.X.; Huang, C.Z. Porous hollow CuS nanospheres with prominent peroxidase-like activity prepared in large scale by a one-pot controllable hydrothermal step. RSC Adv. 2015, 5, 17458–17465. [Google Scholar] [CrossRef]
- Wu, H.; Liu, Y.; Li, M.; Chong, Y.; Zeng, M.; Lo, Y.M.; Yin, J.-J. Size-dependent tuning of horseradish peroxidase bioreactivity by gold nanoparticles. Nanoscale 2015, 7, 4505–4513. [Google Scholar] [CrossRef]
- Xi, Z.; Gao, W.; Xia, X. Size effect in Pd−Ir core-shell nanoparticles as nanozymes. ChemBioChem 2020, 21, 2440–2444. [Google Scholar] [CrossRef]
- Tang, G.; He, J.; Liu, J.; Yan, X.; Fan, K. Nanozyme for tumor therapy: Surface modification matters. Exploration 2021, 1, 75–89. [Google Scholar] [CrossRef]
- Jampaiah, D.; Reddy, T.S.; Kandjani, A.E.; Selvakannan, P.R.; Sabri, Y.M.; Coyle, V.E.; Shukla, R.; Bhargava, S.K. Fe-doped CeO2 nanorods for enhanced peroxidase-like activity and their application towards glucose detection. J. Mater. Chem. B 2016, 4, 3874–3885. [Google Scholar] [CrossRef]
- Gao, M.; Lu, X.; Chen, S.; Tian, D.; Zhu, Y.; Wang, C. Enhanced peroxidase-like activity of Mo6+-doped Co3O4 nanotubes for ultrasensitive and colorimetric L-cysteine detection. ACS Appl. Nano Mater. 2018, 1, 4703–4715. [Google Scholar] [CrossRef]
- Nagvenkar, A.P.; Gedanken, A. Cu0.89Zn0.11O, A New Peroxidase-Mimicking Nanozyme with High Sensitivity for Glucose and Antioxidant Detection. ACS Appl. Mater. Interfaces 2016, 8, 22301–22308. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Zhao, H.; Chen, S.; Yu, H.; Quan, X. Stimuli-responsive peroxidase mimicking at a smart graphene interface. Chem. Commun. 2012, 48, 7055–7057. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Ren, J.; Qu, X. Nanozymes: Classification, Catalytic Mechanisms, Activity Regulation, and Applications. Chem. Rev. 2019, 119, 4357–4412. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wang, X.; Wang, Q.; Lou, Z.; Li, S.; Zhu, Y.; Qin, L.; Wei, H. Nanomaterials with enzyme-like characteristics (nanozymes): Next-generation artificial enzymes (II). Chem. Soc. Rev. 2018, 48, 1004–1076. [Google Scholar] [CrossRef] [PubMed]
- Qiao, B.; Wang, A.; Yang, X.; Allard, L.F.; Jiang, Z.; Cui, Y.; Liu, J.; Li, J.; Zhang, T. Single-atom catalysis of CO oxidation using Pt1/FeOx. Nat. Chem. 2011, 3, 634–641. [Google Scholar] [CrossRef]
- Wang, A.; Li, J.; Zhang, T. Heterogeneous single-atom catalysis. Nat. Rev. Chem. 2018, 2, 65–81. [Google Scholar] [CrossRef]
- Kaiser, S.K.; Chen, Z.; Akl, D.F.; Mitchell, S.; Pérez-Ramírez, J. Single-Atom Catalysts across the Periodic Table. Chem. Rev. 2020, 120, 11703–11809. [Google Scholar] [CrossRef]
- Jiao, L.; Yan, H.; Wu, Y.; Gu, W.; Zhu, C.; Du, D.; Lin, Y. When Nanozymes Meet Single-Atom Catalysis. Angew. Chem. Int. Ed. 2019, 59, 2565–2576. [Google Scholar] [CrossRef]
- Ding, S.; Lyu, Z.; Fang, L.; Li, T.; Zhu, W.; Li, S.; Li, X.; Li, J.C.; Du, D.; Lin, Y. Single-atomic site catalyst with heme enzymes-like active sites for electrochemical sensing of hydrogen peroxide. Small 2021, 17, 2100664. [Google Scholar] [CrossRef]
- Wu, W.; Huang, L.; Wang, E.; Dong, S. Atomic engineering of single-atom nanozymes for enzyme-like catalysis. Chem. Sci. 2020, 11, 9741–9756. [Google Scholar] [CrossRef]
- Zhao, C.; Xiong, C.; Liu, X.; Qiao, M.; Li, Z.; Yuan, T.; Wang, J.; Qu, Y.; Wang, X.; Zhou, F.; et al. Unraveling the enzyme-like activity of heterogeneous single atom catalyst. Chem. Commun. 2019, 55, 2285–2288. [Google Scholar] [CrossRef]
- Ma, W.; Mao, J.; Yang, X.; Pan, C.; Chen, W.; Wang, M.; Yu, P.; Mao, L.; Li, Y. A single-atom Fe–N4 catalytic site mimicking bi-functional antioxidative enzymes for oxidative stress cytoprotection. Chem. Commun. 2019, 55, 159–162. [Google Scholar] [CrossRef]
- Li, X.; Liu, L.; Ren, X.; Gao, J.; Huang, Y.; Liu, B. Microenvironment modulation of single-atom catalysts and their roles in electrochemical energy conversion. Sci. Adv. 2020, 6, eabb6833. [Google Scholar] [CrossRef]
- Xu, B.; Wang, H.; Wang, W.; Gao, L.; Li, S.; Pan, X.; Wang, H.; Yang, H.; Meng, X.; Wu, Q.; et al. A Single-Atom Nanozyme for Wound Disinfection Applications. Angew. Chem. Int. Ed. 2019, 58, 4911–4916. [Google Scholar] [CrossRef]
- Huang, L.; Chen, J.; Gan, L.; Wang, J.; Dong, S. Single-atom nanozymes. Sci. Adv. 2019, 5, eaav5490. [Google Scholar] [CrossRef]
- Ji, S.; Jiang, B.; Hao, H.; Chen, Y.; Dong, J.; Mao, Y.; Zhang, Z.; Gao, R.; Chen, W.; Zhang, R.; et al. Matching the kinetics of natural enzymes with a single-atom iron nanozyme. Nat. Catal. 2021, 4, 407–417. [Google Scholar] [CrossRef]
- Lin, S.; Wei, H. Design of high performance nanozymes: A single-atom strategy. Sci. China Life Sci. 2019, 62, 710–712. [Google Scholar] [CrossRef]
- Jin, H.; Ye, D.; Shen, L.; Fu, R.; Tang, Y.; Jung, J.C.-Y.; Zhao, H.; Zhang, J. Perspective for Single Atom Nanozymes Based Sensors: Advanced Materials, Sensing Mechanism, Selectivity Regulation, and Applications. Anal. Chem. 2022, 94, 1499–1509. [Google Scholar] [CrossRef]
- Cheng, N.; Li, J.C.; Liu, D.; Lin, Y.; Du, D. Single-atom nanozyme based on nanoengineered Fe–N–C catalyst with superior peroxidase-like activity for ultrasensitive bioassays. Small 2019, 15, 1901485. [Google Scholar] [CrossRef]
- Wu, Y.; Jiao, L.; Luo, X.; Xu, W.; Wei, X.; Wang, H.; Yan, H.; Gu, W.; Xu, B.Z.; Du, D.; et al. Oxidase-like Fe-N-C single-atom nanozymes for the detection of acetylcholinesterase activity. Small 2019, 15, 1903108. [Google Scholar] [CrossRef]
- Lyu, Z.; Ding, S.; Zhang, N.; Zhou, Y.; Cheng, N.; Wang, M.; Xu, M.; Feng, Z.; Niu, X.; Cheng, Y.; et al. Single-atom nanozymes linked immunosorbent assay for sensitive detection of Aβ 1-40: A biomarker of Alzheimer’s disease. Research 2020, 2020, 4724505. [Google Scholar] [CrossRef]
- Niu, X.; Shi, Q.; Zhu, W.; Liu, D.; Tian, H.; Fu, S.; Cheng, N.; Li, S.; Smith, J.N.; Du, D.; et al. Unprecedented peroxidase-mimicking activity of single-atom nanozyme with atomically dispersed Fe–Nx moieties hosted by MOF derived porous carbon. Biosens. Bioelectron. 2019, 142, 111495. [Google Scholar] [CrossRef]
- Chen, Q.; Li, S.; Liu, Y.; Zhang, X.; Tang, Y.; Chai, H.; Huang, Y. Size-controllable Fe-N/C single-atom nanozyme with exceptional oxidase-like activity for sensitive detection of alkaline phosphatase. Sensors Actuators B Chem. 2019, 305, 127511. [Google Scholar] [CrossRef]
- Wu, Y.; Wu, J.; Jiao, L.; Xu, W.; Wang, H.; Wei, X.; Gu, W.; Ren, G.; Zhang, N.; Zhang, Q.; et al. Cascade Reaction System Integrating Single-Atom Nanozymes with Abundant Cu Sites for Enhanced Biosensing. Anal. Chem. 2020, 92, 3373–3379. [Google Scholar] [CrossRef]
- Wang, D.; Zhao, Y. Single-atom engineering of metal-organic frameworks toward healthcare. Chem 2021, 7, 2635–2671. [Google Scholar] [CrossRef]
- Jiang, B.; Duan, D.; Gao, L.; Zhou, M.; Fan, K.; Tang, Y.; Xi, J.; Bi, Y.; Tong, Z.; Gao, G.F.; et al. Standardized assays for determining the catalytic activity and kinetics of peroxidase-like nanozymes. Nat. Protoc. 2018, 13, 1506–1520. [Google Scholar] [CrossRef]
- Wu, J.; Xiong, L.; Zhao, B.; Liu, M.; Huang, L. Densely Populated Single Atom Catalysts. Small Methods 2019, 4, 2106139. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Chen, G.; Wu, D.; Wu, Y.; James, T.D. Enzyme Mimics for Engineered Biomimetic Cascade Nanoreactors: Mechanism, Applications, and Prospects. Adv. Funct. Mater. 2021, 31, 2106139. [Google Scholar] [CrossRef]
- Cai, X.; Jiao, L.; Yan, H.; Wu, Y.; Gu, W.; Du, D.; Lin, Y.; Zhu, C. Nanozyme-involved biomimetic cascade catalysis for biomedical applications. Mater. Today 2021, 44, 211–228. [Google Scholar] [CrossRef]
- Niu, X.; Liu, B.; Hu, P.; Zhu, H.; Wang, M. Nanozymes with Multiple Activities: Prospects in Analytical Sensing. Biosensors 2022, 12, 251. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Tian, Z.; Zhai, W.; Qu, Y. Insights on catalytic mechanism of CeO2 as multiple nanozymes. Nano Res. 2022, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; Guo, L. Unraveling the Multi-Enzyme-Like Activities of Iron Oxide Nanozyme via a First-Principles Microkinetic Study. J. Phys. Chem. C 2019, 123, 30318–30334. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, W.; Peng, M.; Ren, G.; Guan, L.; Li, K.; Lin, Y. ZIF-67 as a template generating and tuning “raisin pudding”-type nanozymes with multiple enzyme-like activities: Toward online electrochemical detection of 3,4-dihydroxyphenylacetic acid in living brains. ACS Appl. Mater. Interfaces 2020, 12, 29631–29640. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Xu, L.; Hu, P.; Liu, B.; Wang, M.; Yin, X.; Pan, J.; Niu, X. Smartphone-assisted bioenzyme-nanozyme-chromogen all-in-one test strip with enhanced cascade signal amplification for convenient paraoxon sensing. Biosens. Bioelectron. 2022, 215, 114583. [Google Scholar] [CrossRef]
- Sengupta, P.; Pramanik, K.; Datta, P.; Sarkar, P. Chemically modified carbon nitride-chitin-acetic acid hybrid as a metal-free bifunctional nanozyme cascade of glucose oxidase-peroxidase for “click off” colorimetric detection of peroxide and glucose. Biosens. Bioelectron. 2020, 154, 112072. [Google Scholar] [CrossRef]
- Lin, A.; Sun, Z.; Xu, X.; Zhao, S.; Li, J.; Sun, H.; Wang, Q.; Jiang, Q.; Wei, H.; Shi, D. Self-Cascade Uricase/Catalase Mimics Alleviate Acute Gout. Nano Lett. 2021, 22, 508–516. [Google Scholar] [CrossRef]
- Meng, X.; Li, D.; Chen, L.; He, H.; Wang, Q.; Hong, C.; He, J.; Gao, X.; Yang, Y.; Jiang, B.; et al. High-Performance Self-Cascade Pyrite Nanozymes for Apoptosis–Ferroptosis Synergistic Tumor Therapy. ACS Nano 2021, 15, 5735–5751. [Google Scholar] [CrossRef]
- Tao, N.; Li, H.; Deng, L.; Zhao, S.; Ouyang, J.; Wen, M.; Chen, W.; Zeng, K.; Wei, C.; Liu, Y.-N. A Cascade Nanozyme with Amplified Sonodynamic Therapeutic Effects through Comodulation of Hypoxia and Immunosuppression against Cancer. ACS Nano 2021, 16, 485–501. [Google Scholar] [CrossRef]
- Wang, Q.; Cheng, C.; Zhao, S.; Liu, Q.; Zhang, Y.; Liu, W.; Zhao, X.; Zhang, H.; Pu, J.; Zhang, S.; et al. A valence-engineered self-cascading antioxidant nanozyme for the therapy of inflammatory bowel disease. Angew. Chem. Int. Ed. 2022, 61, e202201101. [Google Scholar]
- Zhang, X.; Zhang, S.; Yang, Z.; Wang, Z.; Tian, X.; Zhou, R. Self-cascade MoS2 nanozymes for efficient intracellular antioxidation and hepatic fibrosis therapy. Nanoscale 2021, 13, 12613–12622. [Google Scholar] [CrossRef]
- Li, X.; Zhou, H.; Qi, F.; Niu, X.; Xu, X.; Qiu, F.; He, Y.; Pan, J.; Ni, L. Three hidden talents in one framework: A terephthalic acid-coordinated cupric metal–organic framework with cascade cysteine oxidase- and peroxidase-mimicking activities and stimulus-responsive fluorescence for cysteine sensing. J. Mater. Chem. B 2018, 6, 6207–6211. [Google Scholar] [CrossRef]
- He, L.; Lu, Y.; Gao, X.; Song, P.; Huang, Z.; Liu, S.; Liu, Y. Self-cascade system based on cupric oxide nanoparticles as dual-functional enzyme mimics for ultrasensitive detection of silver ions. ACS Sustain. Chem. Eng. 2018, 6, 12132–12139. [Google Scholar] [CrossRef]
- Chen, L.F.; Lin, M.T.; Noreldeen, H.A.A.; Peng, H.P.; Deng, H.H.; He, S.B.; Chen, W. Fructose oxidase-like activity of CuO nanoparticles supported by phosphate for a tandem catalysis-based fructose sensor. Anal. Chim. Acta 2022, 1220, 340064. [Google Scholar] [CrossRef]
- He, S.-B.; Balasubramanian, P.; Hu, A.-L.; Zheng, X.-Q.; Lin, M.-T.; Xiao, M.-X.; Peng, H.-P.; Deng, H.-H.; Chen, W. One-pot cascade catalysis at neutral pH driven by CuO tandem nanozyme for ascorbic acid and alkaline phosphatase detection. Sens. Actuators B Chem. 2020, 321, 128511. [Google Scholar] [CrossRef]
- Liu, Y.; Jin, H.; Zou, W.; Guo, R. Protein-mediated wool-ball-like copper sulfide as a multifunctional nanozyme for dual fluorescence “turn-on” sensors of cysteine and silver ions. J. Mater. Chem. B 2020, 8, 9075–9083. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, R.; Yan, X.; Fan, K. Structure and activity of nanozymes: Inspirations for de novo design of nanozymes. Mater. Today 2020, 41, 81–119. [Google Scholar] [CrossRef]
- Wei, H.; Wang, E. Fe3O4 magnetic nanoparticles as peroxidase mimetics and their applications in H2O2 and glucose detection. Anal. Chem. 2008, 80, 2250–2254. [Google Scholar] [CrossRef]
- Wang, C.; Tang, G.; Tan, H. Colorimetric determination of mercury(II) via the inhibition by ssDNA of the oxidase-like activity of a mixed valence state cerium-based metal-organic framework. Mikrochim. Acta 2018, 185, 475. [Google Scholar] [CrossRef]
- Weerathunge, P.; Ramanathan, R.; Shukla, R.; Sharma, T.K.; Bansal, V. Aptamer-Controlled Reversible Inhibition of Gold Nanozyme Activity for Pesticide Sensing. Anal. Chem. 2014, 86, 11937–11941. [Google Scholar] [CrossRef]
- Li, S.; Zhao, X.; Yu, X.; Wan, Y.; Yin, M.; Zhang, W.; Cao, B.; Wang, H. Fe3O4 nanozymes with aptamer-tuned catalysis for selective colorimetric analysis of ATP in blood. Anal. Chem. 2019, 91, 14737–14742. [Google Scholar] [CrossRef]
- Liu, B.; Huang, Z.; Liu, J. Boosting the oxidase mimicking activity of nanoceria by fluoride capping: Rivaling protein enzymes and ultrasensitive F− detection. Nanoscale 2016, 8, 13562–13567. [Google Scholar] [CrossRef] [PubMed]
- Long, Y.J.; Li, Y.F.; Liu, Y.; Zheng, J.J.; Tang, J.; Huang, C.Z. Visual observation of the mercury-stimulated peroxidase mimetic activity of gold nanoparticles. Chem. Commun. 2011, 47, 11939–11941. [Google Scholar] [CrossRef]
- Chen, J.; Huang, L.; Wang, Q.; Wu, W.; Zhang, H.; Fang, Y.; Dong, S. Bio-inspired nanozyme: A hydratase mimic in a zeolitic imidazolate framework. Nanoscale 2019, 11, 5960–5966. [Google Scholar] [CrossRef]
- Li, M.; Chen, J.; Wu, W.; Fang, Y.; Dong, S. Oxidase-like MOF-818 nanozyme with high specificity for catalysis of catechol oxidation. J. Am. Chem. Soc. 2020, 142, 15569–15574. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, C.; Gao, N.; Ren, J.; Qu, X. Stereoselective Nanozyme Based on Ceria Nanoparticles Engineered with Amino Acids. Chem.–A Eur. J. 2017, 23, 18146–18150. [Google Scholar] [CrossRef]
- Wang, Y.; Jia, G.; Cui, X.; Zhao, X.; Zhang, Q.; Gu, L.; Zheng, L.; Li, L.H.; Wu, Q.; Singh, D.J.; et al. Coordination Number Regulation of Molybdenum Single-Atom Nanozyme Peroxidase-like Specificity. Chem 2020, 7, 436–449. [Google Scholar] [CrossRef]
- Zhou, Y.; Wei, Y.; Ren, J.; Qu, X. A chiral covalent organic framework (COF) nanozyme with ultrahigh enzymatic activity. Mater. Horizons 2020, 7, 3291–3297. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Yan, X.; Fan, K. Advances in chiral nanozymes: A review. Microchim. Acta 2019, 186, 782. [Google Scholar] [CrossRef] [PubMed]
- Dong, K.; Xu, C.; Ren, J.; Qu, X. Chiral Nanozymes for Enantioselective Biological Catalysis. Angew. Chem. Int. Ed. 2022. [Google Scholar] [CrossRef]
- BelBruno, J.J. Molecularly imprinted polymers. Chem. Rev. 2019, 119, 94–119. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, X.; Liu, B.; Liu, J. Molecular Imprinting on Inorganic Nanozymes for Hundred-fold Enzyme Specificity. J. Am. Chem. Soc. 2017, 139, 5412–5419. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Liu, J. Intracellular delivery of a molecularly imprinted peroxidase mimicking DNAzyme for selective oxidation. Mater. Horizons 2018, 5, 738–744. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, B.; Liu, J. Molecular Imprinting for Substrate Selectivity and Enhanced Activity of Enzyme Mimics. Small 2016, 13, 1602730. [Google Scholar] [CrossRef]
- Zhang, Z.; Li, Y.; Zhang, X.; Liu, J. Molecularly imprinted nanozymes with faster catalytic activity and better specificity. Nanoscale 2019, 11, 4854–4863. [Google Scholar] [CrossRef]
- Zhang, Z.; Liu, Y.; Zhang, X.; Liu, J. A Cell-Mimicking Structure Converting Analog Volume Changes to Digital Colorimetric Output with Molecular Selectivity. Nano Lett. 2017, 17, 7926–7931. [Google Scholar] [CrossRef]
- Liu, B.; Zhu, H.; Feng, R.; Wang, M.; Hu, P.; Pan, J.; Niu, X. Facile Molecular Imprinting on Magnetic Nanozyme Surface for Highly Selective Colorimetric Detection of Tetracycline. Sens. Actuators B Chem. 2022, 370, 132451. [Google Scholar] [CrossRef]
- Fan, L.; Lou, D.; Wu, H.; Zhang, X.; Zhu, Y.; Gu, N.; Zhang, Y. A novel AuNP-based glucose oxidase mimic with enhanced activity and selectivity constructed by molecular imprinting and O2-containing nanoemulsion embedding. Adv. Mater. Interfaces 2018, 5, 1801070. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, Q.; Liu, S.; Xiao, H.; Zhang, M.; Zhang, X. Surface molecular imprinting on g-C3N4 photooxidative nanozyme for improved colorimetric biosensing. Chin. Chem. Lett. 2019, 30, 2186–2190. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, Y.S.; Ren, X.H.; He, X.W.; Li, W.Y.; Zhang, Y.K. Bimetallic molecularly imprinted nanozyme: Dual-mode detection platform. Biosens. Bioelectron. 2022, 196, 113718. [Google Scholar] [CrossRef]
- Cardoso, A.R.; Frasco, M.F.; Serrano, V.; Fortunato, E.; Sales, M.G.F. Molecular imprinting on nanozymes for sensing applications. Biosensors 2021, 11, 152. [Google Scholar] [CrossRef]
- Li, J.; Liu, W.; Wu, X.; Gao, X. Mechanism of pH-switchable peroxidase and catalase-like activities of gold, silver, platinum and palladium. Biomaterials 2015, 48, 37–44. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, J.; Liao, J.; Lin, Y.; Zheng, C.; Liu, J. In Situ Fabrication of Nanoceria with Oxidase-like Activity at Neutral pH: Mechanism and Boosted Bio-Nanozyme Cascades. ACS Appl. Mater. Interfaces 2021, 13, 50236–50245. [Google Scholar] [CrossRef]
- Zhang, J.; Wu, S.; Lu, X.; Wu, P.; Liu, J. Manganese as a Catalytic Mediator for Photo-oxidation and Breaking the pH Limitation of Nanozymes. Nano Lett. 2019, 19, 3214–3220. [Google Scholar] [CrossRef]
- He, Y.; Li, X.; Xu, X.; Pan, J.; Niu, X. A cobalt-based polyoxometalate nanozyme with high peroxidase-mimicking activity at neutral pH for one-pot colorimetric analysis of glucose. J. Mater. Chem. B 2018, 6, 5750–5755. [Google Scholar] [CrossRef]
- Li, D.; Guo, Q.; Ding, L.; Zhang, W.; Cheng, L.; Wang, Y.; Xu, Z.; Wang, H.; Gao, L. Bimetallic CuCo2S4 nanozymes with enhanced peroxidase activity at neutral pH for combating burn infections. ChemBioChem 2020, 21, 2620–2627. [Google Scholar] [CrossRef]
- Nguyen, P.T.; Lee, J.; Cho, A.; Kim, M.S.; Choi, D.; Han, J.W.; Kim, M.I.; Lee, J. Rational development of Co-doped mesoporous ceria with high peroxidase-mimicking activity at neutral pH for paper-based colorimetric detection of multiple biomarkers. Adv. Funct. Mater. 2022, 32, 2112428. [Google Scholar] [CrossRef]
- Zhu, Q.; Yang, J.; Peng, Z.; He, Z.; Chen, W.; Tang, H.; Li, Y. Selective detection of glutathione by flower-like NiV2O6 with only peroxidase-like activity at neutral pH. Talanta 2021, 234, 122645. [Google Scholar] [CrossRef]
- Niu, X.; Xu, X.; Li, X.; Pan, J.; Qiu, F.; Zhao, H.; Lan, M. Surface charge engineering of nanosized CuS via acidic amino acid modification enables high peroxidase-mimicking activity at neutral pH for one-pot detection of glucose. Chem. Commun. 2018, 54, 13443–13446. [Google Scholar] [CrossRef]
- Han, L.; Li, C.; Zhang, T.; Lang, Q.; Liu, A. Au@Ag Heterogeneous Nanorods as Nanozyme Interfaces with Peroxidase-Like Activity and Their Application for One-Pot Analysis of Glucose at Nearly Neutral pH. ACS Appl. Mater. Interfaces 2015, 7, 14463–14470. [Google Scholar] [CrossRef]
- Chen, Q.; Liu, Y.; Liu, J.; Liu, J. Liposome-boosted peroxidase-mimicking nanozymes breaking the pH limit. Chem. Eur. J. 2020, 26, 16659–16665. [Google Scholar] [CrossRef]
- Gu, H.; Huang, Q.; Zhang, J.; Li, W.; Fu, Y. Heparin as a bifunctional biotemplate for Pt nanocluster with exclusively peroxidase mimicking activity at near-neutral pH. Colloids Surf. A Physicochem. Eng. Asp. 2020, 606. [Google Scholar] [CrossRef]
- Yin, X.; Liu, P.; Xu, X.; Pan, J.; Li, X.; Niu, X. Breaking the pH limitation of peroxidase-like CoFe2O4 nanozyme via vitriolization for one-step glucose detection at physiological pH. Sens. Actuators B Chem. 2020, 328, 129033. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Y. Luminescence-Sensing Tb-MOF Nanozyme for the Detection and Degradation of Estrogen Endocrine Disruptors. ACS Appl. Mater. Interfaces 2020, 12, 8351–8358. [Google Scholar] [CrossRef]
- Wu, J.; Qin, K.; Yuan, D.; Tan, J.; Qin, L.; Zhang, X.; Wei, H. Rational design of Au@Pt multibranched nanostructures as bi-functional nanozymes. ACS Appl. Mater. Interfaces 2018, 10, 12954–12959. [Google Scholar] [CrossRef]
- Zhu, N.; Gu, L.; Wang, J.; Li, X.; Liang, G.-X.; Zhou, J.; Zhang, Z. Novel and Sensitive Chemiluminescence Sensors Based on 2D-MOF Nanosheets for One-Step Detection of Glucose in Human Urine. J. Phys. Chem. C 2019, 123, 9388–9393. [Google Scholar] [CrossRef]
- Lai, X.; Zhang, G.; Zeng, L.; Xiao, X.; Peng, J.; Guo, P.; Zhang, W.; Lai, W. Synthesis of PDA-Mediated Magnetic Bimetallic Nanozyme and Its Application in Immunochromatographic Assay. ACS Appl. Mater. Interfaces 2020, 13, 1413–1423. [Google Scholar] [CrossRef]
- Gökçal, B.; Kip, Ç.; Tuncel, A. One-pot, direct glucose detection in human whole blood without using a dilution factor by a magnetic nanozyme with dual enzymatic activity. J. Alloys Compd. 2020, 843, 156012. [Google Scholar] [CrossRef]
- Xu, X.; Wu, S.; Guo, D.; Niu, X. Construction of a recyclable oxidase-mimicking Fe3O4@MnOx-based colorimetric sensor array for quantifying and identifying chlorophenols. Anal. Chim. Acta 2020, 1107, 203–212. [Google Scholar] [CrossRef]
- Wen, S.H.; Zhong, X.L.; Wu, Y.D.; Liang, R.P.; Zhang, L.; Qiu, J.D. Colorimetric assay conversion to highly sensitive electro-chemical assay for bimodal detection of arsenate based on cobalt oxyhydroxide nanozyme via arsenate absorption. Anal. Chem. 2019, 91, 6487–6497. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhang, L.; He, J.; Guo, W.; Zhou, Z.; Zhang, X.; Nie, S.; Wei, H. Integrated Nanozymes with Nanoscale Proximity for in Vivo Neurochemical Monitoring in Living Brains. Anal. Chem. 2016, 88, 5489–5497. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Z.; Huang, Y.; Liu, W.; Ye, F.; Zhao, S. Immobilized Glucose Oxidase on Boronic Acid-Functionalized Hierarchically Porous MOF as an Integrated Nanozyme for One-Step Glucose Detection. ACS Sustain. Chem. Eng. 2020, 8, 4481–4488. [Google Scholar] [CrossRef]
- Shen, H.; Zhou, Z.; He, W.; Chao, H.; Su, P.; Song, J.; Yang, Y. Oligonucleotide-Functionalized Enzymes Chemisorbing on Magnetic Layered Double Hydroxides: A Multimodal Catalytic Platform with Boosted Activity for Ultrasensitive Glucose Detection. ACS Appl. Mater. Interfaces 2021, 13, 14995–15007. [Google Scholar] [CrossRef]
- Wu, J.; Li, S.; Wei, H. Multifunctional nanozymes: Enzyme-like catalytic activity combined with magnetism and surface plasmon resonance. Nanoscale Horiz. 2018, 3, 367–382. [Google Scholar] [CrossRef]
- Wang, N.; Shi, J.; Liu, Y.; Sun, W.; Su, X. Constructing bifunctional metaleorganic framework based nanozymes with fluorescence and oxidase activity for the dualchannel detection of butyrylcholinesterase. Anal. Chim. Acta 2022, 1205, 339717. [Google Scholar] [CrossRef]
- Lin, T.; Qin, Y.; Huang, Y.; Yang, R.; Hou, L.; Ye, F.; Zhao, S. A label-free fluorescence assay for hydrogen peroxide and glucose based on the bifunctional MIL-53(Fe) nanozyme. Chem. Commun. 2018, 54, 1762–1765. [Google Scholar] [CrossRef]
- Ye, K.; Wang, L.; Song, H.; Li, X.; Niu, X. Bifunctional MIL-53(Fe) with pyrophosphate-mediated peroxidase-like activity and oxidation-stimulated fluorescence switching for alkaline phosphatase detection. J. Mater. Chem. B 2019, 7, 4794–4800. [Google Scholar] [CrossRef]
- Li, X.; Liu, P.; Niu, X.; Ye, K.; Ni, L.; Du, D.; Pan, J.; Lin, Y. Tri-functional Fe–Zr bi-metal–organic frameworks enable high-performance phosphate ion ratiometric fluorescent detection. Nanoscale 2020, 12, 19383–19389. [Google Scholar] [CrossRef]
- Guo, J.; Liu, Y.; Mu, Z.; Wu, S.; Wang, J.; Yang, Y.; Zhao, M.; Wang, Y. Label-free fluorescence detection of hydrogen peroxide and glucose based on the Ni-MOF nanozyme–induced self-ligand emission. Mikrochim. Acta 2022, 189, 219. [Google Scholar] [CrossRef]
- Sun, Y.; Lin, T.; Zeng, C.; Jiang, G.; Zhang, X.; Ye, F.; Zhao, S. A self-correcting fluorescent assay of tyrosinase based on Fe-MIL-88B-NH2 nanozyme. Mikrochim. Acta 2021, 188, 158. [Google Scholar] [CrossRef]
- Liu, P.; Li, X.; Xu, X.; Ye, K.; Wang, L.; Zhu, H.; Wang, M.; Niu, X. Integrating peroxidase-mimicking activity with photoluminescence into one framework structure for high-performance ratiometric fluorescent pesticide sensing. Sens. Actuators B Chem. 2021, 328, 129024. [Google Scholar] [CrossRef]
- Xu, X.; Luo, Z.; Ye, K.; Zou, X.; Niu, X.; Pan, J. One-pot construction of acid phosphatase and hemin loaded multifunctional metal–organic framework nanosheets for ratiometric fluorescent arsenate sensing. J. Hazard. Mater. 2020, 412, 124407. [Google Scholar] [CrossRef]
- Guo, J.; Wu, S.; Wang, Y.; Zhao, M. A label-free fluorescence biosensor based on a bifunctional MIL-101(Fe) nanozyme for sensitive detection of choline and acetylcholine at nanomolar level. Sens. Actuators B Chem. 2020, 312, 128021. [Google Scholar] [CrossRef]
- Hou, L.; Qin, Y.; Lin, T.; Sun, Y.; Ye, F.; Zhao, S. Michael reaction-assisted fluorescent sensor for selective and one step de-termination of catechol via bifunctional Fe-MIL-88NH2 nanozyme. Sens. Actuators B Chem. 2020, 321, 128547. [Google Scholar] [CrossRef]
- Li, S.; Hu, X.; Chen, Q.; Zhang, X.; Chai, H.; Huang, Y. Introducing bifunctional metal-organic frameworks to the construction of a novel ratiometric fluorescence sensor for screening acid phosphatase activity. Biosens. Bioelectron. 2019, 137, 133–139. [Google Scholar] [CrossRef]
- Xia, Y.; Sun, K.; Zuo, Y.-N.; Zhu, S.; Zhao, X.-E. Fluorescent MOF-based nanozymes for discrimination of phenylenediamine isomers and ratiometric sensing of o-phenylenediamine. Chin. Chem. Lett. 2021, 33, 2081–2085. [Google Scholar] [CrossRef]
- Liu, P.; Zhao, M.; Zhu, H.; Zhang, M.; Li, X.; Wang, M.; Liu, B.; Pan, J.; Niu, X. Dual-mode fluorescence and colorimetric detection of pesticides realized by integrating stimulus-responsive luminescence with oxidase-mimetic activity into cerium-based coordination polymer nanoparticles. J. Hazard. Mater. 2022, 423, 127077. [Google Scholar] [CrossRef]
- Liu, P.; Zhu, H.; Wang, M.; Wei, M.; Liu, B.; Hu, P.; Lin, J.; Niu, X. Bimodal ratiometric fluorescence and colorimetric sensing of paraoxon based on trifunctional Ce,Tb co-coordinated polymers. Sens. Actuators B Chem. 2022, 360, 131616. [Google Scholar] [CrossRef]
- Wu, Y.; Gao, Y.; Du, J. Bifunctional gold nanoclusters enable ratiometric fluorescence nanosensing of hydrogen peroxide and glucose. Talanta 2019, 197, 599–604. [Google Scholar] [CrossRef]
- Wan, Y.; Zhao, J.; Deng, X.; Chen, J.; Xi, F.; Wang, X. Colorimetric and Fluorescent Dual-Modality Sensing Platform Based on Fluorescent Nanozyme. Front. Chem. 2021, 9, 774486. [Google Scholar] [CrossRef]
- Li, X.; Ding, S.; Lyu, Z.; Tieu, P.; Wang, M.; Feng, Z.; Pan, X.; Zhou, Y.; Niu, X.; Du, D.; et al. Single-Atomic Iron Doped Carbon Dots with Both Photoluminescence and Oxidase-Like Activity. Small 2022, 18, 2203001. [Google Scholar] [CrossRef]
- Zhang, W.; Li, X.; Xu, X.; He, Y.; Qiu, F.; Pan, J.; Niu, X. Pd nanoparticle-decorated graphitic C3N4 nanosheets with bifunctional peroxidase mimicking and ON–OFF fluorescence enable naked-eye and fluorescent dual-readout sensing of glucose. J. Mater. Chem. B 2018, 7, 233–239. [Google Scholar] [CrossRef]
- Gooding, J.J. Can Nanozymes Have an Impact on Sensing? ACS Sens. 2019, 4, 2213–2214. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, B.; Yang, R.; Liu, J. Filling in the Gaps between Nanozymes and Enzymes: Challenges and Opportunities. Bioconjugate Chem. 2017, 28, 2903–2909. [Google Scholar] [CrossRef]
- Ruan, X.; Hulubei, V.; Wang, Y.; Shi, Q.; Cheng, N.; Wang, L.; Lyu, Z.; Davis, W.C.; Smith, J.N.; Lin, Y.; et al. Au@PtPd enhanced immunoassay with 3D printed smartphone device for quantification of diaminochlorotriazine (DACT), the major atrazine biomarker. Biosens. Bioelectron. 2022, 208, 114190. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, J.; Niu, X. Rational Design of Nanozymes Enables Advanced Biochemical Sensing. Chemosensors 2022, 10, 386. https://doi.org/10.3390/chemosensors10100386
Liu J, Niu X. Rational Design of Nanozymes Enables Advanced Biochemical Sensing. Chemosensors. 2022; 10(10):386. https://doi.org/10.3390/chemosensors10100386
Chicago/Turabian StyleLiu, Jinjin, and Xiangheng Niu. 2022. "Rational Design of Nanozymes Enables Advanced Biochemical Sensing" Chemosensors 10, no. 10: 386. https://doi.org/10.3390/chemosensors10100386
APA StyleLiu, J., & Niu, X. (2022). Rational Design of Nanozymes Enables Advanced Biochemical Sensing. Chemosensors, 10(10), 386. https://doi.org/10.3390/chemosensors10100386






