3.1. Considerations in Computed Tomography
In X-ray CT, the scanning beam is arranged to penetrate the whole subject, from front to back, then detected as it emerges on the far side. Information about the scanned subject is contained within the characteristics of the detected signal. That form of propagation and detection is labelled S21 according to scattering parameters convention (S-parameters) [
17]. The extremely short wavelength of X-rays (0.01–10 nm) and the intensity of the beam ensure that the projection (i.e., shadow) cast by the subject on the detectors has a well-defined outline with little diffusion around the edges. An image of the scanned subject is reconstructed from the data delivered by the detectors using an algorithm based on the Inverse Radon Transform [
18], which is well suited to the sharply defined edges of the projection and the low level of diffusion.
Initially the authors adopted the S21 configuration in their new scanning modality in deference to the well-established convention in X-ray CT. However, it was found that the Inverse Radon Transform is not suited to the new implementation because the wavelength of the scanning beam is many orders of magnitude longer than that of X-rays. In addition, the beam undergoes significantly more attenuation, diffusion, and scatter during its passage through the scanned subject. Consequently, the outline of the projection is highly blurred and feint against the naturally occurring background noise. Reconstructing an image from the acquired data is therefore significantly more challenging than the case with X-rays.
Attention is now being given to the data acquired from the reflected portion of the scanning beam, labelled S11 in the S-parameters convention. Although the scanning beam is still subjected to attenuation, diffusion, and scatter, the typically shorter path length that the reflected portion undergoes ensures that data quality is improved, particularly if the stroke-affected region in the brain happens to be close to the surface. In addition, whereas S21 requires two antennas to translate around the subject multiple times in a co-ordinated pattern, S11 requires only one antenna to orbit the subject just once. Consequently, S11 facilitates a shorter scanning duration as well as a simpler construction of scanning chamber that surrounds the subject. Details are given in the next section.
It is important to note that S11 data, although derived from the reflected portion of the scanning beam, are not the same as pulsed radar in which discrete pulses are emitted from an antenna and the reflected signals are detected. S11 data in the context of the new scanning modality derive from a continuous-wave signal—not a pulsed signal, and S11 data characterise the dielectric properties of the static environment in close proximity to the antenna—not the round-trip propagation time of pulses.
S11 data are implicit in all of the experimental results reported in this paper.
3.2. Experimental Scanning Apparatus
To ensure that the experimental scanning apparatus affords maximum flexibility and ease of modification, its construction employs readily available materials and devices, and a simple mechanical movement.
Figure 2 shows the totality of the apparatus. It comprises a scanning chamber in which an antenna, labelled Tx, mechanically translates around the test subject, labelled phantom, under the action of a stepper motor. While the antenna is in motion, the phantom is stationary. This is the same convention used in CT. The antenna employs a compact Vivaldi design that is rated to operate across 5–18 GHz, although in reality, the operating range extends down to 1 GHz.
The antenna is connected to a Vector Network Analyser (VNA model P9374A, manufactured and supplied by Keysight Technologies, Santa Rosa, CA, USA), which measures S11 over a broad range of frequencies. The VNA, as well as the stepper motor, are under the control of a bespoke script running on a laptop PC, which also stores and processes the acquired data. It is evident that the scanning apparatus is minimalist, comprising only the scanning chamber, a VNA, and a controller. This supports the view that, in due course, a commercial version of the scanner could be compact, lightweight, and portable, as well as relatively low cost, particularly if the full-featured benchtop VNA in the photograph is replaced with a low-profile version that delivers only the required features. Preferably, the mechanically translated single antenna would also be replaced with a ring of stationary, electronically switched antennas.
During the scans reported in this paper, the antenna translates through 360 degrees in 100 equal steps (3.6 degrees per step), pausing for a brief moment at each step while the VNA measures S11 at 1601 spot frequencies between 1 GHz and 20 GHz. At each frequency, the S11 data include the magnitude and phase of the signal detected at the antenna. Consequently, each scan acquires 320,200 data points. These data and the frequency range are more than is needed for a reliable diagnosis; however, the current priority is to acquire as much data as are available to facilitate later work on refining the operation and performance of the scanner.
The use of a VNA ensures that the apparatus is highly immune to electromagnetic interference (EMI) in the surrounding environment, from sources such as Wi-Fi hubs, mobile phones, and masts, as well as other wireless services. This benefit stems from the fact that the detector side of the VNA is internally locked in frequency and phase to the transmitter side. Consequently, only the transmitted signal is recognised and accepted by the receiver. All other sources are effectively ignored. It is plausible that a future commercial development of this scanning apparatus would embed a low-profile VNA in its construction, thereby ensuring a high degree of EMI immunity. In addition, the scanning chamber that encloses the patient’s head and houses the antenna system would be designed to function as an electromagnetic screen.
A primary goal of the new scanning modality is that it must be fundamentally safe for the patient and operators and requires no specialist shielding or other safety precautions. To achieve that goal the intensity of the scanning beam must be very low. In the absence of formal regulatory guidance on the approved beam intensity for the kind of scanning modality being researched, the decision was taken early on to adopt a beam power of only 1 mW, 0 dBm. That is 100× lower than the radiated power of domestic Wi-Fi hubs (typically 100 mW, +20 dBm). At such low power levels, patients could be continuously scanned on a 24/7 basis with no safety concerns. There is no practical reason for that to be done, but it nevertheless serves to highlight the unparalleled safety margin that the new scanning modality affords compared with X-ray CT. In due course, when guidance for the new scanning modality is formally ratified by the regulatory authorities, it is reasonable to expect that the approved beam intensity will be at least 100× or even 1000× greater than the level being used because of the very short exposure period during a scan, while still remaining within the guidance limits for non-scanning wireless applications such as mobile telecommunications. However, for the time being, the authors’ research will continue with a conservative power level of 1 mW, 0 dBm. That level is implicit in all of the results reported in this paper.
3.3. Test Subjects (Phantoms)
The test subjects used in the scans, commonly referred to as phantoms, are constructed using fluids that closely replicate the dielectric properties of the anatomical constituents of a human head. These fluids are contained in the polycarbonate vessels shown in
Figure 3. That material is used because of its high transparency at the beam frequencies. The phantoms have a cylindrical geometry in order to maintain a constant gap of 3–4 mm between the antenna and the outer edge of the phantom while the antenna is in motion around the stationary phantom. The cylindrical geometry also simplifies data interpretation by limiting the acquired data to a single slice in the horizontal X-Y plane, located at the mid-point of the vertical Z axis. Future work will use ‘head-shaped’ phantoms and will acquire data at multiple X-Y planes along the Z axis.
The 175 mm diameter container in
Figure 3 represents an adult head, while the 150 mm diameter container represents an adolescent. Both containers have a 5–7 mm wide outer compartment that is filled with a proxy fluid for skull bone. The large inner compartment is filled with a proxy fluid for brain matter. Stroke-affected regions are implemented by placing one of the inclusion containers in the brain proxy fluid, anchored to the top lid of the outer container. The different diameters of the inclusion containers (11 mm, 21 mm, 30 mm, 44 mm) represent strokes of different severity and stage of progression, while their fluid contents are selected to represent an ischaemic or haemorrhagic stroke. By moving the top anchorage point of these containers along the slot in the top lid, strokes at different depths within the brain are represented. The use of nylon bungs and fixings with these containers ensures that they have minimal influence on the data acquired during scans. The photograph at the left in
Figure 3 shows an example fully populated 175 mm phantom with the 44 mm stroke inclusion installed and located close to the surface of the brain. The comprehensive program of scans that produced the results reported in this paper used both phantom sizes and all four stroke inclusions at a variety of locations between the surface of the brain and the centre.
The anatomical simplicity of these phantoms contrasts with the steps taken by some of the other researchers in this field in the construction of their phantoms. For example, the University of Queensland group elected to create discrete anatomical structures within their phantoms, each with a distinct set of dielectric properties for that particular anatomical element [
19]. Similarly, Micrima employed phantoms that contained a degree of anatomical geometry. Notwithstanding the undoubted validity of these approaches to phantom construction, the authors of this paper decided instead to favour an intrinsically simpler construction for several important reasons. Firstly, the wavelength of the scanning beam inside a phantom head (or a human head alike) ranges from several mm to several cm. Consequently, fine structural details within the phantom are inherently smeared out in the data, leaving just the macro-level details. It is therefore sufficient that the phantoms used in this study incorporate a simple geometry while still being a materially valid representation of a human subject. Secondly, for the purposes of a triage diagnosis at the site of the emergency, fine detail of the kind displayed in CT images is not required. The priority is firmly on determining whether the patient has or has not experienced a stroke. The simplicity of the authors’ phantoms is consistent with that priority. Thirdly, in order to physically assemble and sustain a detailed anatomical structure within a phantom, the proxy materials must have a solid, or at least a semi-solid consistency. Consequently, individual phantoms must be constructed from scratch for every different size/severity and location of the stroke-affected region that needs to be studied. That could amount to a great many phantoms if the study is wide ranging, as is the authors’ study reported herein. In contrast, the simpler anatomy favoured by the authors coupled with the use of fluid proxies enables a broad range of stroke size/severity and location to be represented with ease in just a single construction of a phantom for each head size: one for an adult and one for an adolescent.
Sourcing the correct proxy fluids is vital for the material validity of the phantoms. The dielectric properties of the fluids, and particularly their relative permittivity as a function of frequency, define how they interact with the scanning beam of the new modality. These parameters are therefore central to selecting fluids that have a relative permittivity that is closest to the human material(s) they represent. The proxy fluid selected for brain matter is produced by the National Physical Laboratory [
20] to an international standard and supplied to the telecoms industry for use in specific absorption rate (SAR) tests associated with the safety of mobile phones and the influence of their emissions on brain tissue [
21]. Its relative permittivity characterises that of grey and white matter and cranial fluids (blood, CSF, ECF, ISF, etc.) in a single unified medium. This off-white opaque fluid is evident in the fully populated phantom in
Figure 3.
Figure 4 shows plots of its relative permittivity against frequency (measured by the supplier) and compares those plots with measured and computed plots for the individual constituents of a human body that are widely available in the literature and frequently referenced by researchers in this field [
22,
23,
24,
25,
26,
27].
Figure 4 also includes a single data point for Ethylene Glycol, which serves as a proxy fluid for skull bone. It too is a single unified medium that characterises cancellous and cortical bone and marrow.
The proxy fluids for an ischaemic inclusion and a haemorrhagic inclusion are RS-I fluid [
28] and defibrinated sheep blood [
29], respectively. Given that 85% of all diagnosed strokes are ischaemic [
1], this paper focusses on the results obtained with RS-I fluid representing an ischaemic stroke. The results from defibrinated sheep blood representing a haemorrhagic stroke will be reported in due course, together with the authors’ investigation into whether the two types of stroke can be discriminated by the new scanning modality.
3.4. Data Processing
During a scan, the real and imaginary components of the detected S11 signal, denoted Sr[tx,k] and Si[tx,k], respectively, are acquired by the VNA at each of 100 stationary locations of the antenna tx as it steps around the phantom through 360 degrees. At each location, the VNA measures Sr[tx,k] and Si[tx,k] at up to 1601 spot frequencies between 1 GHz and 20 GHz, where tx = 1:100 is the antenna location index and k = 1:1601 is an index that corresponds to the spot frequencies actually used.
The signature of the stroke cannot be easily identified within this complex data for several reasons, but principally the following:
The beam intensity launched from the antenna is very low (1 mW, 0 dBm) for the reasons given earlier. In addition, the attenuation of the beam as it propagates through the phantom is significant, particularly towards the upper end of the range of frequencies. Consequently, the signal-to-noise ratio of the acquired data is low.
The beam undergoes significant scatter and diffusion during its passage through the phantom. This greatly reduces the definition of the signature of the stroke in the data against the naturally occurring background fluctuations and noise in the data.
To resolve these challenges several processes are performed on the dataset to facilitate a more effective search for features in the data that signify a stroke. The complex S11 signal
Sc[
tx,k] detected at the antenna at each measurement instant is thus expressed as follows:
Using this expression,
Figure 5a shows the magnitude of the totality of raw data acquired from the antenna during a scan of the 175 mm phantom containing a 44 mm stroke inclusion located close to the surface. The actual phantom is shown in the photo in
Figure 3 in the previous section. All of the results reported in this section derive from a scan of that particular phantom, which is henceforth referred to as ‘scan #1’ for brevity. The results from scans of a broad range of phantoms and inclusions of different sizes and locations are presented in the next section.
Interestingly, when the same scan is repeated with the stroke inclusion removed from the phantom vessel, the resulting raw data in
Figure 5b are superficially unchanged. This highlights the challenge faced in extracting the signature of the stroke inclusion from the raw data. It is at an extremely low level relative to the surrounding data. One potential solution explored by the authors involved subtracting the ‘no inclusion’ data from the ‘with inclusion’ data to accentuate information about the stroke inclusion and its location. This method was ultimately rejected for two primary reasons. Firstly, in a practical setting, it is all but impossible to envisage a scenario when clinicians will have two recent scans of the same patient: one taken shortly before the onset of their stroke and the other taken while their stroke is occurring. Consideration was given to utilising publicly accessible libraries of scans of healthy patients and developing a method to use those data as a generalised ‘no inclusion’ scan. However, the challenges in ensuring that these scans not only accurately represented a stroke patient prior to the onset of their stroke, but that they can also be formatted in a way that precisely replicates the output of the new scanning modality had it actually been used, were felt to be insurmountable. Secondly, subtracting the two scans from each other produces the highly complex data field in
Figure 5c. Reliably identifying and extracting the low-intensity signature of the stroke inclusion from within a data field containing such extreme variability is challenging, particularly for stroke inclusions that are small in size and deeply seated within the brain. The decision was therefore taken to develop the following robust and computationally efficient method that reliably extracts the signature of a stroke inclusion in the raw data from just a single scan of the patient while they are experiencing their stroke.
The Inverse Fast Fourier Transform (IFFT) is used to transform the dataset in
Figure 5a from the frequency domain to the time domain. Given that the S11 scanning modality that underpins this paper uses only one antenna, the data acquired at each stationary location of the antenna as it steps around the phantom are not influenced by a second nearby antenna, as was the case in the previous S21 scanning modality that was briefly alluded to earlier. It is therefore sufficient to perform a 1D IFFT on the complex signal
Sc[
tx,k] in Equation (1) at each antenna location, which produces:
where
N = 1:1601,
tx = 1:100, and
n = 1:1601. If only real data are applied to the transformation, the output data are reflected around its centre. However, for the purposes of this study, the real and imaginary components of the acquired data are applied to the transformation, which yields values in just the first half (i.e., left half) of the transform domain, as is evident in
Figure 6.
The output of the IFFT, denoted
s[
tx,n] in Equation (2), comprises
N complex 1D sequences that are computed independently in accordance with the structure of the data acquired during a scan. Therefore, at each antenna location as it steps around the phantom, the average of the transformed sequence over the temporal domain is calculated by averaging the sequence
s[
tx,n], and thus:
This 1D averaged data
sav[
tx] describes the S11 signal at the antenna for all frequencies that are the input to the IFFT at each antenna location. However, for each antenna location,
sav[
tx] does not yield a distinct unmistakable signature of the stroke inclusion because of the strong influence of unwanted values on it. This is evident in
Figure 7, which shows multiple peaks and troughs computed from Equation (3), rather than a single distinct signature.
Further study of the transformed series
s[
tx,n] in
Figure 6 using scans of several different phantoms in which the inclusion is present in some while is absent in others, reveals that the position of
n =
N/4 is dominant when the inclusion is present but not when the inclusion is absent. The data sequence around the
n =
N/4 index can therefore be summed to resolve a more distinct signature of the stroke inclusion. Equation (3) can then be rewritten as:
where
a ≥ 0 represents the width of the span centred on
n =
N/4. The value of
a is selected in accordance with the strength of the signature of the inclusion. For example, in instances when the strength is high, the value of
a is not critical, whereas when the strength is low, studies have found that
a = 2 returns optimum results. Throughout the results presented in this paper,
a is assumed to be 2. The real and imaginary components of the complex data sequence represented by Equation (4) are shown in
Figure 8.
Both components carry vital information about the presence and location of the stroke inclusion. It is therefore prudent to use both. They can be combined by computing the absolute value of the averaged data sequence in Equation (4), expressed thus as:
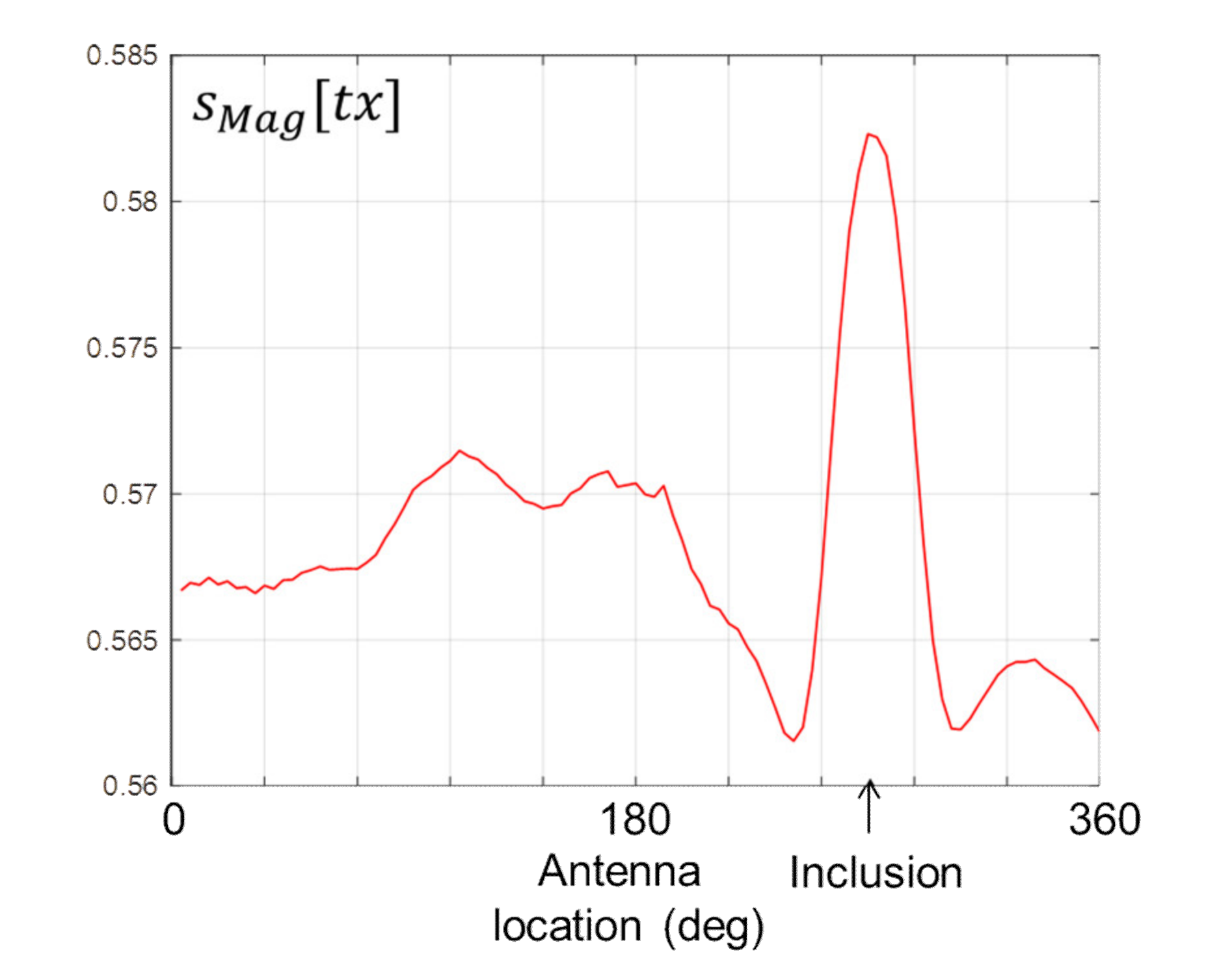
Figure 9 shows the data computed by Equation (5). The signature of the stroke inclusion is visible in the form of a distinctive peak, the location of which corresponds with the location of the inclusion on the horizontal axis.
To reduce the intensity of the data on either side of the peak in
Figure 9, and thereby increase the distinctiveness of the signature,
sMag[
tx] in Equation (5) can be differentiated as follows:
Figure 10 shows the differentiated data computed by Equation (6). The presence of the stroke inclusion is evidenced by the distinctive double peak, while the location of the inclusion on the horizontal axis coincides with the zero crossing between the two peaks.
The above results confirm that the method of data analysis devised for this study successfully extracts the signature of a stroke inclusion from the raw data acquired during a scan. The next section presents the results from a comprehensive programme of scans of phantoms of different sizes and inclusions of different sizes and locations. In this way, the results are representative of a population of adults and adolescents who are experiencing strokes of different severity and depth within the brain.