Somatic Symptoms and Sleep Disorders: A Literature Review of Their Relationship, Comorbidities and Treatment
Abstract
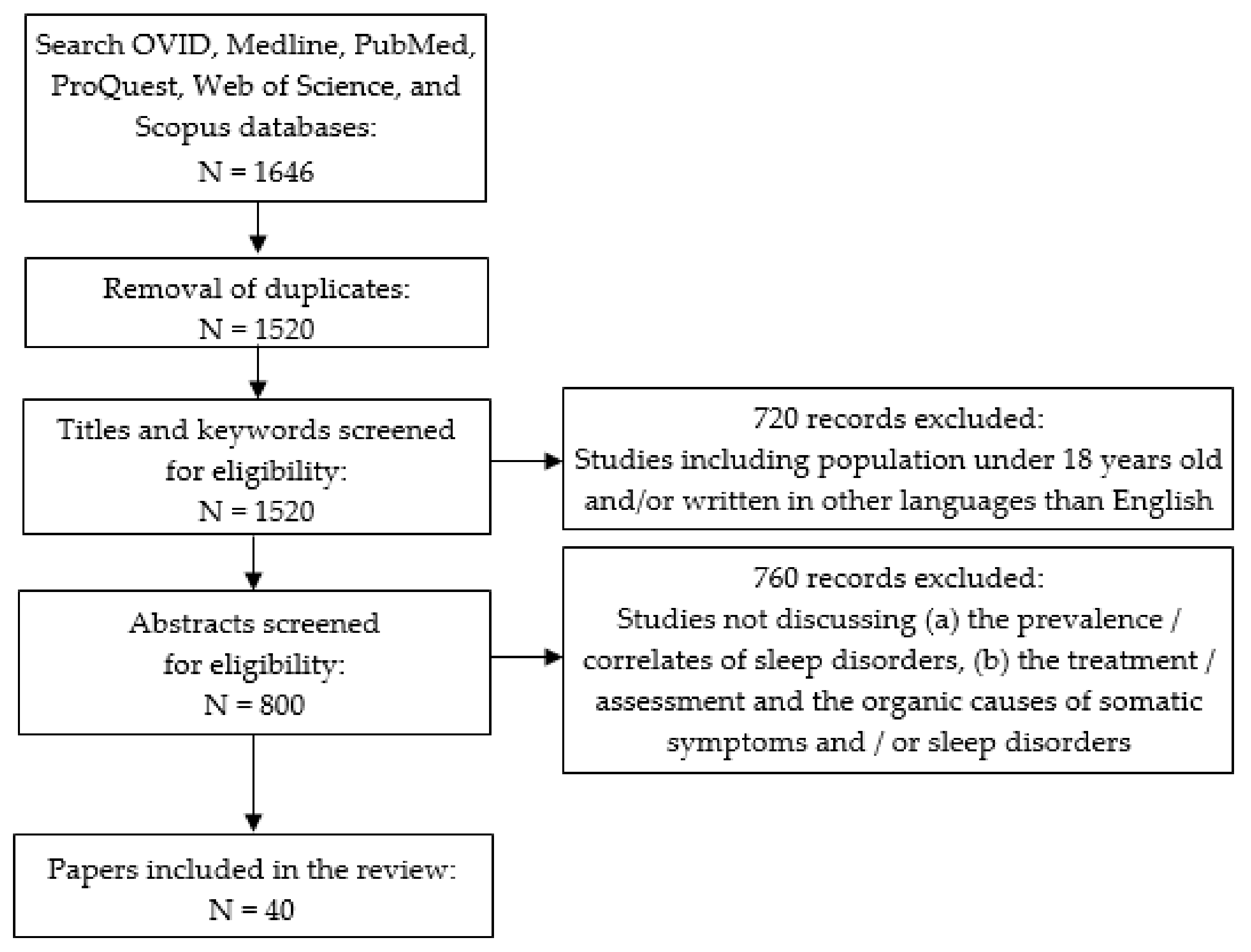
:1. Introduction
- -
- Definitions and classifications regarding SSD have been a subject of controversy over the past few years. For example, the new DSM-V definition of SSD [11], although being focused on abnormal and excessive thoughts, feelings, and behaviors associated with the burden of somatic symptoms, puts a lower emphasis on their medical explicability and sourcing [18,19,20];
- -
- Despite somatic patients often displaying sleep disorders, not all of them fully meet the DSM-V criteria for SSD. This is partially explained by the DSM-V requiring the symptoms to be persistent (more than six months) and to generate a significant disruption of daily life [11];
- -
- A further cause of bias is represented by the discrepancy between subjective and objective overall sleep quality measures [21]. Although subjective sleep measures have certain advantages (they are less expensive, able to be utilized in large population-based or community-based studies, and very easily standardized), they may over-report sleep duration length among elderly adults or adults in poor health. In contrast, objective measures, despite being more suitable for an adequate evaluation of sleep patterns, are generally costly, invasive, and less tolerated by patients. As a result, 25–50% of patients seek medical attention for sleep disorders [22], while about 20–30% of them develop persisting symptoms, and 71% display a significantly lower quality of life and/or extreme social burden [23,24]; and
- -
- Among SSD patients, a high number of them exhibit conditions, such as depression, anxiety, and fatigue, which themselves are correlated with sleep disorders [25].
2. Materials and Methods
3. Results
3.1. How Are Somatic Symptoms in SSD Correlated to Sleep Disorders?
3.2. What Are the Most Common Psychiatric Comorbidities Seen in Patients with SSD and Insomnia?
3.3. What Are the Potentially Effective Pharmacological and Non-Pharmacological Treatment Options for Both Somatic Symptoms and Insomnia?
4. Discussion
4.1. Limitations
4.2. Future Research
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Grossi, G.; Perski, A.; Osika, W.; Savic, I. Stress-related exhaustion disorder—Clinical manifestation of burnout? A review of assessment methods, sleep impairments, cognitive disturbances, and neuro-biological and physiological changes in clinical burnout. Scand. J. Psychol. 2015, 56, 626–636. [Google Scholar] [CrossRef]
- Popa-Velea, O.; Diaconescu, L.V.; Gheorghe, I.R.; Olariu, O.; Panaitiu, I.; Cerniţanu, M.; Goma, L.; Nicov, I.; Spinei, L. Factors associated with burnout in medical academia: An exploratory analysis of Romanian and Moldavian physicians. Int. J. Environ. Res. Public Health 2019, 16, 2382. [Google Scholar] [CrossRef] [Green Version]
- Popa-Velea, O.; Pamfile, D.; Popp, I. Psychosocial support and burnout at physicians attending advanced care patients: The impact of Balint training. Int. J. Behav. Med. 2014, 21, S95–S96. [Google Scholar]
- Faravelli, C.; Salvatori, S.; Galassi, F.; Aiazzi, L.; Drei, C.; Cabras, P. Epidemiology of somatoform disorders: A community survey in Florence. Soc. Psychiatry Psychiatr. Epidemiol. 1997, 32, 24–29. [Google Scholar] [CrossRef] [PubMed]
- Hilderink, P.H.; Collard, R.; Rosmalen, J.G.M.; Oude Voshaar, R.C. Prevalence of somatoform disorders and medically unexplained symptoms in old age populations in comparison with younger age groups: A systematic review. Ageing Res. Rev. 2013, 12, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Hiller, W.; Rief, W.; Brähler, E. Somatization in the population: From mild bodily misperceptions to disabling symptoms. Soc. Psychiatry Psychiatr. Epidemiol. 2006, 41, 704–712. [Google Scholar] [CrossRef]
- Creed, F.; Barsky, A. A systematic review of the epidemiology of somatisation disorder and hypochondriasis. J. Psychosom. Res. 2004, 56, 391–408. [Google Scholar] [CrossRef]
- Zheng, W.; Luo, X.N.; Li, H.Y.; Ke, X.Y.; Dai, Q.; Zhang, C.J.; Ng, C.H.; Ungvari, G.S.; Xiang, Y.T.; Ning, Y.P. Prevalence of insomnia symptoms and their associated factors in patients treated in outpatient clinics of four general hospitals in Guangzhou, China. BMC Psychiatry 2018, 18, 232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Popa-Velea, O.; Truţescu, C.; Ionescu, E.V.; Almăşan, E.R.; Bobîrnac, G. The usefulness of the Draw-a-Person (DAP) test in the diagnosis and assessment of domestic violence. Rom. J. Leg. Med. 2016, 24, 231–235. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Roth, T. Characteristics of insomnia in the United States: Results of the 1991 National Sleep Foundation Survey. I. Sleep 1999, 22 (Suppl. 2), S347–S353. [Google Scholar]
- American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, 5th ed.; American Psychiatric Association: Arlington, VA, USA, 2013; pp. 309–315. [Google Scholar]
- Power, J.D.; Perruccio, A.V.; Badley, E.M. Pain as a mediator of sleep problems in arthritis and other chronic conditions. Arthritis Rheum. 2005, 53, 911–919. [Google Scholar] [CrossRef] [PubMed]
- Roizenblatt, S.; Souza, A.L.; Palombini, L.; Godoy, L.M.; Tufik, S.; Bittencourt, L.R. Musculoskeletal pain as a marker of health quality. Findings from the Epidemiological Sleep Study among the adult population of Sao Paulo City. PLoS ONE 2015, 10, e0142726. [Google Scholar] [CrossRef]
- Tang, N.K.Y.; Wright, K.J.; Salkovskis, P.M. Prevalence and correlates of clinical insomnia co-occurring with chronic back pain. J. Sleep Res. 2007, 16, 85–95. [Google Scholar] [CrossRef]
- Artner, J.; Cakir, B.; Spiekermann, J.A.; Kurz, S.; Leucht, F.; Reichel, H.; Lattig, F. Prevalence of sleep deprivation in patients with chronic neck and back pain: A retrospective evaluation of 1016 patients. J. Pain Res. 2013, 6, 1–6. [Google Scholar] [CrossRef] [Green Version]
- de Waal, M.W.; Arnold, I.A.; Eekhof, J.A.; van Hemert, A.M. Somatoform disorders in general practice: Prevalence, functional impairment and comorbidity with anxiety and depressive disorders. Br. J. Psychiatry 2004, 184, 470–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieb, R.; Meinlschmidt, G.; Araya, R. Epidemiology of the association between somatoform disorders and anxiety and depressive disorders: An update. Psychosom. Med. 2007, 69, 860–863. [Google Scholar] [CrossRef]
- Dimsdale, J.E.; Levenson, J. What’s next for somatic symptom disorder? Am. J. Psychiatry 2013, 170, 1393–1395. [Google Scholar] [CrossRef] [PubMed]
- Löwe, B.; Mundt, C.; Herzog, W.; Brunner, R.; Backenstrass, M.; Kronmüller, K.; Henningsen, P. Validity of current somatoform disorder diagnoses: Perspectives for classification in DSM-V and ICD-11. Psychopathology 2008, 41, 4–9. [Google Scholar] [CrossRef]
- Toussaint, A.; Murray, A.M.; Voigt, K.; Herzog, A.; Gierk, B.; Kroenke, K.; Rief, W.; Henningsen, P.; Löwe, B. Development and validation of the Somatic Symptom Disorder–B Criteria Scale (SSD-12). Psychosom. Med. 2016, 78, 5–12. [Google Scholar] [CrossRef] [PubMed]
- Moul, D.E.; Hall, M.; Pilkonis, P.A.; Buysse, D.J. Self-report measures of insomnia in adults: Rationales, choices, and needs. Sleep Med. Rev. 2004, 8, 177–198. [Google Scholar] [CrossRef]
- Barsky, A.J.; Borus, J.F. Somatization and medicalization in the era of managed care. JAMA 1995, 274, 1931–1934. [Google Scholar] [CrossRef] [PubMed]
- Verhaak, P.F.M.; Meijer, S.A.; Visser, A.P.; Wolters, G. Persistent presentation of medically unexplained symptoms in general practice. Fam. Pract. 2006, 23, 414–420. [Google Scholar] [CrossRef]
- Jackson, J.L.; Passamonti, M. The outcomes among patients presenting in primary care with a physical symptom at 5 years. J. Gen. Intern. Med. 2005, 20, 1032–1037. [Google Scholar] [CrossRef] [Green Version]
- Roth, T. Insomnia: Definition, prevalence, etiology, and consequences. J. Clin. Sleep Med. 2007, 3 (Suppl. 5), S7–S10. [Google Scholar] [CrossRef] [Green Version]
- Oxford Centre for Evidence-Based Medicine—Levels of Evidence. Available online: http://www.cebm.net/index.aspx?o=1025 (accessed on 15 March 2020).
- Tricco, A.C.; Lillie, E.; Zarin, W.; O’Brien, K.K.; Colquhoun, H.; Levac, D.; Moher, D.; Peters, M.D.J.; Horsley, T.; Weeks, L.; et al. Preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (PRISMA-ScR) checklist. Ann. Intern. Med. 2018, 169, 467–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollrath, M.; Wicki, W.; Angst, J. The Zürich study. Eur. Arch. Psychiatry Neurol. Sci. 1989, 239, 113–124. [Google Scholar] [CrossRef]
- Kim, K.; Uchiyama, M.; Liu, X.; Shibui, K.; Ohida, T.; Ogihara, R.; Okawa, M. Somatic and psychological complaints and their correlates with insomnia in the Japanese general population. Psychosom. Med. 2001, 63, 441–446. [Google Scholar] [CrossRef] [Green Version]
- Aigner, M.; Graf, A.; Freidl, M.; Prause, W.; Weiss, M.; Kaup-Eder, B.; Saletu, B.; Bach, M. Sleep disturbances in somatoform pain disorder. Psychopathology 2003, 36, 324–328. [Google Scholar] [CrossRef] [PubMed]
- El-Anzi, F.O. Insomnia in relation to depression and somatic symptoms. Psychol. Rep. 2006, 99, 171–175. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Lam, S.-P.; Li, S.X.; Tang, N.L.; Yu, M.W.M.; Li, A.M.; Wing, Y.K. Insomnia, sleep quality, pain, and somatic symptoms: Sex differences and shared genetic components. Pain 2012, 153, 666–673. [Google Scholar] [CrossRef] [PubMed]
- Schlarb, A.A.; Claßen, M.; Hellmann, S.M.; Vögele, C.; Gulewitsch, M.D. Sleep and somatic complaints in university students. J. Pain Res. 2017, 10, 1189–1199. [Google Scholar] [CrossRef] [Green Version]
- Asai, T.; Kaneita, Y.; Uchiyama, M.; Takemura, S.; Asai, S.; Yokoyama, E.; Miyake, T.; Harano, S.; Suzuki, K.; Ibuka, E.; et al. Epidemiological study of the relationship between sleep disturbances and somatic and psychological complaints among the Japanese general population. Sleep Biol. Rhythm. 2006, 4, 55–62. [Google Scholar] [CrossRef]
- LeBlanc, M.; Mérette, C.; Savard, J.; Ivers, H.; Baillargeon, L.; Morin, C.M. Incidence and risk factors of insomnia in a population-based sample. Sleep 2009, 32, 1027–1037. [Google Scholar] [CrossRef]
- Zhang, J.; Lam, S.P.; Li, S.X.; Yu, M.W.; Li, A.M.; Ma, R.C.; Kong, A.P.; Wing, Y.K. Long-term outcomes and predictors of chronic insomnia: A prospective study in Hong Kong Chinese adults. Sleep Med. 2012, 13, 455–462. [Google Scholar] [CrossRef]
- Nagane, M.; Suge, R.; Watanabe, S.-I. Time or retiring and sleep quality may be predictors of academic performance and psychosomatic disorder in university students. Biol. Rhythm Res. 2016, 47, 329–337. [Google Scholar] [CrossRef]
- Tan, T.L.; Kales, J.D.; Kales, A.; Soldatos, C.R.; Bixler, E.O. Biopsychobehavioral correlates of insomnia. IV: Diagnosis based on DSM-III. Am. J. Psychiatry 1984, 141, 357–362. [Google Scholar] [CrossRef]
- Ohaeri, J.U.; Adeyemi, J.D. The pattern of somatization symptoms at the Ibadan Teaching Hospital Psychiatric Clinic. West. Afr. J. Med. 1990, 9, 26–34. [Google Scholar] [PubMed]
- Schneider-Helmert, D.; Whitehouse, I.; Kumar, A.; Lijzenga, C. Insomnia and alpha sleep in chronic non-organic pain as compared to primary insomnia. Neuropsychobiology 2001, 43, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Guo, J.; Liu, C.; Wang, X.; Qu, Z.; Zhang, W.; Zhang, X. Relationships between depression, pain and sleep quality with doctor visits among community-based adults in north-west China. Public Health 2017, 147, 30–38. [Google Scholar] [CrossRef]
- Moldofsky, H.; Scarisbrick, P. Induction of neurasthenic musculoskeletal pain syndrome by selective sleep stage deprivation. Psychosom. Med. 1976, 38, 35–44. [Google Scholar] [CrossRef]
- Hartz, A.; Ross, J.J.; Noyes, R.; Williams, P. Somatic symptoms and psychological characteristics associated with insomnia in postmenopausal women. Sleep Med. 2013, 14, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, N.; Kirk, K.M.; Heath, A.C.; Martin, N.G.; Hickie, I. Somatic distress as a distinct psychological dimension. Soc. Psychiatry Psychiatr. Epidemiol. 1999, 34, 451–458. [Google Scholar] [CrossRef] [PubMed]
- Stapleton, P.B.; Brunetti, M. The effects of somatisation, depression, and anxiety on eating habits among university students. Int. J. Heal. Caring 2013, 13, 1–16. [Google Scholar]
- Annagür, B.B.; Uguz, F.; Apiliogullari, S.; Kara, İ.; Gunduz, S. Psychiatric disorders and association with quality of sleep and quality of life in patients with chronic pain: A SCID-Based Study. Pain Med. 2014, 15, 772–781. [Google Scholar] [CrossRef] [Green Version]
- Bekhuis, E.; Schoevers, R.A.; van Borkulo, C.D.; Rosmalen, J.G.M.; Boschloo, L. The network structure of major depressive disorder, generalized anxiety disorder and somatic symptomatology. Psychol. Med. 2016, 46, 2989–2998. [Google Scholar] [CrossRef]
- Mol, A.J.J.; Gorgels, W.J.M.J.; Oude Voshaar, R.C.; Breteler, M.H.; van Balkom, A.J.; van de Lisdonk, E.H.; Kan, C.C.; Zitman, F.G. Associations of benzodiazepine craving with other clinical variables in a population of general practice patients. Compr. Psychiatry 2005, 46, 353–360. [Google Scholar] [CrossRef]
- Barthels, F.; Müller, R.; Schüth, T.; Friederich, H.-C.; Pietrowsky, R. Orthorexic eating behavior in patients with somatoform disorders. Eat Weight Disorders. 2021, 26, 135–143. [Google Scholar] [CrossRef]
- Lankes, F.; Schiekofer, S.; Eichhammer, P.; Busch, V. The effect of alexithymia and depressive feelings on pain perception in somatoform pain disorder. J. Psychosom. Res. 2020, 133, 110101. [Google Scholar] [CrossRef]
- Davidson, J.; Krishnan, R.; France, R.; Pelton, S. Neurovegetative symptoms in chronic pain and depression. J. Affect. Disord. 1985, 9, 213–218. [Google Scholar] [CrossRef]
- Yu, N.X.; Chan, J.S.M.; Ji, X.; Wan, A.H.Y.; Ng, S.M.; Yuen, L.P.; Chan, C.L.W.; Chan, C.H.Y. Stress and psychosomatic symptoms in Chinese adults with sleep complaints: Mediation effect of self-compassion. Psychol. Health Med. 2019, 24, 241–252. [Google Scholar] [CrossRef]
- Byers, H.D.; Lichstein, K.L.; Thorn, B.E. Cognitive processes in comorbid poor sleep and chronic pain. J. Behav. Med. 2016, 39, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.S.F.; Lee, D.T.F. Do medically unexplained somatic symptoms predict depression in older Chinese? Int. J. Geriatr. Psychiatry 2011, 27, 119–126. [Google Scholar] [CrossRef]
- Woud, M.L.; Zhang, X.C.; Becker, E.S.; Zlomuzica, A.; Margraf, J. Catastrophizing misinterpretations predict somatoform-related symptoms and new onsets of somatoform disorders. J. Psychosom. Res. 2016, 81, 31–37. [Google Scholar] [CrossRef] [PubMed]
- Lewis-Hall, F.C.; Wilson, M.G.; Tepner, R.G.; Koke, S.C. Fluoxetine vs. tricyclic antidepressants in women with major depressive disorder. J. Women’s Health 1997, 6, 337–343. [Google Scholar] [CrossRef]
- Saletu-Zyhlarz, G.; Anderer, P.; Brandstätter, N.; Dantendorfer, K.; Gruber, G.; Mandl, M.; Ritter, K.; Zoghlami, A.; Saletu, B. Placebo-controlled sleep laboratory studies on the acute effects of Zolpidem on objective and subjective sleep and awakening quality in nonorganic insomnia related to neurotic and stress-related disorder. Neuropsychobiology 2000, 41, 139–148. [Google Scholar] [CrossRef] [PubMed]
- Saletu, B.; Prause, W.; Anderer, P.; Mandl, M.; Aigner, M.; Mikova, O.; Saletu-Zyhlarz, G.M. Insomnia in somatoform pain disorder: Sleep laboratory studies on differences to controls and acute effects of Trazodone, evaluated by the Somnolyzer 24 × 7 and the Siesta Database. Neuropsychobiology 2005, 51, 148–163. [Google Scholar] [CrossRef] [PubMed]
- Han, C.; Pae, C.-U.; Lee, B.-H.; Ko, Y.-H.; Masand, P.S.; Patkar, A.A.; Joe, S.-H.; Jung, I.-K. Venlafaxine versus Mirtazapine in the treatment of undifferentiated somatoform disorder: A 12-week prospective, open-label, randomized, parallel-group trial. Clin. Drug. Investig. 2008, 28, 251–261. [Google Scholar] [CrossRef] [PubMed]
- Kleinstäuber, M.; Witthöft, M.; Steffanowski, A.; van Marwijk, H.; Hiller, W.; Lambert, M.J. Pharmacological interventions for somatoform disorders in adults. Cochrane Database Syst. 2014, 11, CD010628. [Google Scholar] [CrossRef] [Green Version]
- Kleinstäuber, M.; Witthöft, M.; Hiller, W. Efficacy of short-term psychotherapy for multiple medically unexplained physical symptoms: A meta-analysis. Clin. Psychol Rev. 2011, 31, 146–160. [Google Scholar] [CrossRef]
- Van Dessel, N.; den Boeft, M.; van der Wouden, J.C.; Kleinstäuber, M.; Leone, S.S.; Terluin, B.; Numans, M.E.; van der Horst, H.E.; van Marwijk, H. Non-pharmacological interventions for somatoform disorders and medically unexplained physical symptoms (MUPS) in adults. Cochrane Database Syst. Rev. 2014, 11, CD011142. [Google Scholar] [CrossRef]
- Abbass, A.; Town, J.; Holmes, H.; Luyten, P.; Cooper, A.; Russell, L.; Lumley, M.A.; Schubiner, H.; Allinson, J.; Bernier, D.; et al. Short-term psychodynamic psychotherapy for functional somatic disorders: A meta-analysis of randomized controlled trials. Psychother. Psychosom. 2020, 89, 363–370. [Google Scholar] [CrossRef]
- Jungquist, C.R.; O’Brien, C.; Matteson-Rusby, S.; Smith, M.T.; Pigeon, W.R.; Xia, Y.; Lu, N.; Perlis, M.L. The efficacy of cognitive-behavioral therapy for insomnia in patients with chronic pain. Sleep Med. 2010, 11, 302–309. [Google Scholar] [CrossRef] [Green Version]
- Schröder, A.; Rehfeld, E.; Ørnbøl, E.; Sharpe, M.; Licht, R.W.; Fink, P. Cognitive–behavioural group treatment for a range of functional somatic syndromes: Randomised trial. Br. J. Psychiatry 2012, 200, 499–507. [Google Scholar] [CrossRef] [Green Version]
- Tang, N.K.Y.; Goodchild, C.E.; Salkovskis, P.M. Hybrid cognitive-behaviour therapy for individuals with insomnia and chronic pain: A pilot randomised controlled trial. Behav. Res. Ther. 2012, 50, 814–821. [Google Scholar] [CrossRef]
- Pigeon, W.R.; Moynihan, J.; Matteson-Rusby, S.; Jungquist, C.R.; Xia, Y.; Tu, X.; Perlis, M.L. Comparative effectiveness of CBT interventions for co-morbid chronic pain & insomnia: A pilot study. Behav. Res. Ther. 2012, 50, 685–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fjorback, L.O.; Arendt, M.; Ornbøl, E.; Walach, H.; Rehfeld, E.; Schröder, A.; Fink, P. Mindfulness therapy for somatization disorder and functional somatic syndromes—Randomized trial with one-year follow-up. J. Psychosom. Res. 2013, 74, 31–40. [Google Scholar] [CrossRef]
- Sikandar, S.; Minett, M.S.; Millet, Q.; Santana-Varela, S.; Lau, J.; Wood, J.N.; Zhao, J. Brain-derived neurotrophic factor derived from sensory neurons plays a critical role in chronic pain. Brain 2018, 141, 1028–1039. [Google Scholar] [CrossRef]
- Sochal, M.; Małecka-Panas, E.; Gabryelska, A.; Fichna, J.; Talar-Wojnarowska, R.; Szmyd, B.; Białasiewicz, P. Brain-derived neurotrophic factor is elevated in the blood serum of Crohn’s disease patients, but is not influenced by anti-TNF-α treatment—A pilot study. Neurogastroenterol. Motil. 2020, 33, e13978. [Google Scholar] [CrossRef] [PubMed]
- Xue, J.; Li, H.; Xu, Z.; Ma, D.; Guo, R.; Yang, K.; Wang, Y. Paradoxical sleep deprivation aggravates and prolongs incision-induced pain hypersensitivity via BDNF signaling-mediated descending facilitation in rats. Neurochem. Res. 2018, 43, 2353–2361. [Google Scholar] [CrossRef] [PubMed]
- Boakye, P.A.; Olechowski, C.; Rashiq, S.; Verrier, M.J.; Kerr, B.; Witmans, M.; Baker, G.; Joyce, A.; Dick, B.D. A critical review of neurobiological factors involved in the interactions between chronic pain, depression, and sleep disruption. Clin. J. Pain. 2016, 32, 327–336. [Google Scholar] [CrossRef] [PubMed]
- Nijs, J.; Mairesse, O.; Neu, D.; Leysen, L.; Danneels, L.; Cagnie, B.; Meeus, M.; Moens, M.; Ickmans, K.; Goubert, D. Sleep disturbances in chronic pain: Neurobiology, assessment, and treatment in physical therapist practice. Phys. Ther. 2018, 98, 325–335. [Google Scholar] [CrossRef] [PubMed]
- Baglioni, C.; Nanovska, S.; Regen, W.; Spiegelhalder, K.; Feige, B.; Nissen, C.; Riemann, D. Sleep and mental disorders: A meta-analysis of polysomnographic research. Psychol. Bull. 2016, 142, 969–990. [Google Scholar] [CrossRef] [PubMed]
- Brasure, M.; Fuchs, E.; MacDonald, R.; Nelson, V.A.; Koffel, E.; Olson, C.M.; Khawaja, I.S.; Diem, S.; Carlyle, M.; Wilt, T.J.; et al. Psychological and behavioral interventions for managing insomnia disorder: An evidence report for a clinical practice guideline by the American College of Physicians. Ann. Intern. Med. 2016, 165, 113–124. [Google Scholar] [CrossRef] [PubMed]
- Kleinstäuber, M.; Rief, W. Cognitive-behavioral therapy for somatoform disorders and pain. In The Science of Cognitive Behavioral Therapy; Hoffman, S., Asmundson, G., Eds.; Academic Press: London, UK, 2017; pp. 405–427. [Google Scholar]
- Barsky, A.J.; Orav, E.J.; Bates, D.W. Somatization increases medical utilization and costs independent of psychiatric and medical comorbidity. Arch. Gen. Psychiatry 2005, 62, 903–910. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lehmann, M.; Pohontsch, N.J.; Zimmermann, T.; Scherer, M.; Löwe, B. Diagnostic and treatment barriers to persistent somatic symptoms in primary care—Representative survey with physicians. BMC Fam. Pract. 2021, 22, 60. [Google Scholar] [CrossRef] [PubMed]
- Cuijpers, P.; Sijbrandij, M.; Koole, S.L.; Andersson, G.; Beekman, A.T.; Reynolds, C.F., 3rd. Adding psychotherapy to antidepressant medication in depression and anxiety disorders: A meta-analysis. World Psychiatry 2014, 13, 56–67. [Google Scholar] [CrossRef]
- McCombie, A.; Gearry, R.; Andrews, J.; Mikocka-Walus, A.; Mulder, R. Computerised cognitive behavioural therapy for psychological distress in patients with physical illnesses: A systematic review. J. Clin. Psychol. Med. Settings 2015, 22, 20–44. [Google Scholar] [CrossRef]
| Study Authors | Country | Study Design | n | Study Aim | Outcomes |
|---|---|---|---|---|---|
| Vollrath et al. (1989) [28] | Switzerland | Cohort | 457 | The association of insomnia with functional syndromes | Insomnia was associated with functional somatic complaints |
| Kim et al. (2001) [29] | Japan | Cohort | 303 | The correlation between insomnia and somatic and psychological complaints | The prevalence of insomnia increased with the number of somatic complaints |
| Aigner et al. (2003) [30] | Austria | Cross- sectional | 147 | Pain intensity in patients with and without sleep disorders | Sleep disorders were correlated with higher pain in somatoform pain disorder patients |
| El-Anzi (2006) [31] | Kuwait | Cross- sectional | 358 | Results of scales regarding depression, anxiety, insomnia, and somatic symptoms | Somatic symptoms were correlated to insomnia |
| Zhang et al. (2012) [32] | Hong Kong | Cohort | 256 | Gender differences in patients presenting both sleep disorders and somatization | Insomnia and poor sleep quality was closely associated with pain and somatic symptoms |
| Schlarb et al. (2017) [33] | Germany | Cohort | 2443 | The association between somatic complaints and sleep disorders | Somatic complaints are positively associated with subjectively poor sleep quality |
| Asai et al. (2006) [34] | Japan | Cohort | 28,714 | The relationship between somatization and sleep disorders | The prevalence of sleep disorders increased with the number of somatic complaints |
| LeBlanc et al. (2009) [35] | Canada | Cohort | 464 | Factors related to new-onset insomnia | Higher bodily pain was associated with new-onset insomnia |
| Zhang et al. (2012) [36] | Hong Kong | Cohort | 2316 | The longitudinal course of insomnia in patients with mental disorders | Baseline insomnia was associated with chronic pain and poor mental health |
| Nagane et al. (2016) [37] | Japan | Cross- sectional | 135 | Sleep-wake patterns as predictors for somatic complaints | The sleep-wake pattern may predict somatic complaints |
| Tan et al. (1984) [38] | USA | Cross- sectional | 100 | The association between high emotional arousal and insomnia in SSD patients | SSD were much more common as an additional diagnosis in patients with primary insomnia |
| Ohaeri and Adeyemi (1990) [39] | Nigeria | Cross- sectional | 74 | Patterns of somatization symptoms | Insomnia was the most common symptom in patients with somatization |
| Schneider- Helmert et al. (2001) [40] | The Netherlands | Cross- sectional | 51 | The association between insomnia and chronic non-organic pain | Insomnia is more than a minor component of chronic non-organic pain |
| Guo et al. (2017) [41] | China | Cross- sectional | 7602 | The relationship between depression, pain, and sleep quality | Perceived pain and poor sleep quality were correlated with the number of doctor visits |
| Moldofsky et al. (1976) [42] | Canada | Cross- sectional | 13 | The relationship between sleep disorders and musculoskeletal symptoms | The emergence of somatic symptoms is induced by a disorder of non-REM sleep |
| Study Authors | Country | Study Design | n | Study Aim | Outcomes |
|---|---|---|---|---|---|
| Hartz et al. (2013) [43] | USA | Cross- sectional | 148,938 | The association between somatic symptoms and sleep disorders | Sleep disorders were positively correlated with somatic symptoms in depressive patients |
| Gillespie et al. (1999) [44] | Australia | Cohort | 3468 | Depression and anxiety symptoms in patients with somatization | Somatic symptoms were not genetically or biologically associated to anxiety/depression |
| Stapleton and Brunetti (2013) [45] | Australia | Cross- sectional | 167 | The association between depression, somatization, and anxiety and their effect on eating habits | Higher somatization scores were positively associated with depression, anxiety, and poor eating habits |
| Annagür et al. (2014) [46] | Turkey | Case- control | 187 | Self-esteem, depressive mood, and their impact on somatic symptoms | Depression and anxiety were linked to chronic pain and sleep disorders |
| Bekhuis et al. (2016) [47] | The Netherlands | Cohort | 2704 | The association of somatic symptoms and anxiety/depression | Depression and anxiety were associated with somatic complaints and insomnia |
| Mol et al. (2005) [48] | The Netherlands | Cross- sectional | 193 | The pattern of association between depression, somatization, and benzodiazepines craving | Self-reported negative mood and somatization were positively associated with craving |
| Barthels et al. (2021) [49] | Germany | Case- control | 61 | The association of orthorexia and depression in SSD patients | Orthorexia levels were elevated in patients with SSD |
| Lankes et al. (2020) [50] | Germany | Cross- sectional | 160 | The effect of alexithymia on SSD patients | Alexithymia mediated by negative affect was found in patients with somatoform pain |
| Davidson et al. (1985) [51] | USA | Cross- sectional | 52 | The pattern of neurovegetative symptoms in patients with depression | Major depression was linked with insomnia, anorexia, and weight loss in chronic pain patients |
| Yu et al. (2019) [52] | Hong Kong | Cross- sectional | 998 | The association of stress, depressive symptoms, and somatization | Stress was associated with depressive symptoms and somatic complaints |
| Byers et al. (2016) [53] | USA | Cross- sectional | 52 | The impact of cognitive pre-sleep arousal, catastrophizing on chronic pain, and insomnia | Cognitive pre-sleep arousal predicted insomnia severity in chronic pain patients |
| Yu et al. (2011) [54] | Hong Kong | Cohort | 1433 | The pattern of somatic presentation of depression | People with depression were more likely to have multiple medically unexplained symptoms, insomnia, and fatigue |
| Woud et al. (2016) [55] | Germany/ The Netherlands | Cohort | 1538 | The impact of catastrophic misinterpretations on SSD | Catastrophic misinterpretations were predictive for somatoform-related problems and new onset SSD |
| Study Authors | Country | n | Study Aim | Treatment(s) Duration | Outcomes |
|---|---|---|---|---|---|
| Lewis-Hall et al. (1997) [56] | USA | 854 | The outcome of MDD and somatization with Fluoxetine and TCAs | Fluoxetine vs TCAs, 5 weeks | Significant reductions in somatization and insomnia for both |
| Saletu-Zyhlarz et al. (2000) [57] | Austria | 30 | The effects of Zolpidem on insomnia and other psychiatric disorders | Zolpidem vs placebo, 1 week | Improvement in sleep efficiency and somatic complaints |
| Saletu et al. (2005) [58] | Austria/ Bulgaria | 11 | The effects of Trazodone on sleep disturbances | Trazodone, 1 week | Increased slow-wave sleep and reduced arousal index |
| Han et al. (2008) [59] | South Korea | 95 | The efficacy of Mirtazapine/Venlafaxine in SSD | Mirtazapine vs. Venlafaxine, 12 weeks | Somatization scores decreased from baseline to endpoint for both therapies, results in favor of Mirtazapine |
| Kleinstäuber et al. (2014) [60] | Germany | 2159 | The effects of pharmacological therapies on SSD | SSRI vs. SSRI and AP; variable, according to included studies | Low-quality evidence in favor of combined treatment for reducing the severity of somatic complaints |
| Study Authors | Country | n | Study Aim | Compared Interventions | Outcomes |
|---|---|---|---|---|---|
| Kleinstäuber et al. (2011) [61] | Germany | 1781 | The accuracy of STPP for somatization and depression | STPP vs. control | STPP significantly reduced somatic symptoms and depression |
| Van Dessel et al. (2014) [62] | The Netherlands | 2658 | The effectiveness of CBT on somatization patients | CBT vs. usual/enhanced care | CBT reduced somatic symptoms at 1-year follow-up but was not more effective compared with enhanced care |
| Abbas et al. (2020) [63] | United Kingdom | 2004 | STPP on patients with somatization | STPP vs. minimal treatment | STPP significantly outperformed minimal treatment |
| Jungquist et al. (2010) [64] | USA | 28 | The efficiency of CBT for insomnia and chronic pain | CBT vs. control | CBT patients exhibited decreases in sleep latency and increases in efficiency of sleep but no difference in pain severity |
| Schröder et al. (2012) [65] | Denmark | 66 | The efficiency of STreSS on patients with somatization | STreSS vs. enhanced care | STreSS group had a greater improvement of the primary outcome than enhanced care |
| Tang et al. (2012) [66] | United Kingdom | 20 | The hybrid CBT intervention on sleep and pain outcomes | CBT PI vs. symptom monitoring | Hybrid intervention was associated with greater improvement in sleep, although pain intensity did not change |
| Pigeon et al. (2012) [67] | USA | 21 | The efficiency of CBT PI on patients with co-occurring pain and insomnia | CBT PI vs. waiting list | CBT PI produced significant improvement in sleep and disability from pain |
| Fjorback et al. (2013) [68] | Denmark | 119 | The efficiency of mindfulness on somatization | Mindfulness therapy vs. enhanced care | Mindfulness therapy was comparable with enhanced care in improvingsomatic symptoms and insomnia |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, C.G.; Popa-Velea, O.; Mihăilescu, A.I.; Talaşman, A.A.; Bădărău, I.A. Somatic Symptoms and Sleep Disorders: A Literature Review of Their Relationship, Comorbidities and Treatment. Healthcare 2021, 9, 1128. https://doi.org/10.3390/healthcare9091128
Ionescu CG, Popa-Velea O, Mihăilescu AI, Talaşman AA, Bădărău IA. Somatic Symptoms and Sleep Disorders: A Literature Review of Their Relationship, Comorbidities and Treatment. Healthcare. 2021; 9(9):1128. https://doi.org/10.3390/healthcare9091128
Chicago/Turabian StyleIonescu, Claudiu Gabriel, Ovidiu Popa-Velea, Alexandra Ioana Mihăilescu, Ana Anca Talaşman, and Ioana Anca Bădărău. 2021. "Somatic Symptoms and Sleep Disorders: A Literature Review of Their Relationship, Comorbidities and Treatment" Healthcare 9, no. 9: 1128. https://doi.org/10.3390/healthcare9091128
APA StyleIonescu, C. G., Popa-Velea, O., Mihăilescu, A. I., Talaşman, A. A., & Bădărău, I. A. (2021). Somatic Symptoms and Sleep Disorders: A Literature Review of Their Relationship, Comorbidities and Treatment. Healthcare, 9(9), 1128. https://doi.org/10.3390/healthcare9091128






