Abstract
A novel topological index, the face index (), is proposed in this paper. For a molecular graph G, face index is defined as , where is the degree of the vertex v. The index is very easy to calculate and improved the previously discussed correlation models for energy and boiling point of benzenoid hydrocarbons. The study shows that the multiple linear regression involving the novel topological index can predict the -electron energy and boiling points of the benzenoid hydrocarbons with correlation coefficient . Moreover, the face indices of some planar molecular structures such as 2-dimensional graphene, triangular benzenoid, circumcoronene series of benzenoid are also investigated. The results suggest that the proposed index with good correlation ability and structural selectivity promised to be a useful parameter in QSPR/QSAR.
1. Introduction
In application of mathematical and statistical methods one of the most important purposes is to find a relationship between molecular structure and values of physical, chemical and biological properties. As a result, quantitative structure-property relationship (QSPR) and quantitative structure-activity relationship (QSAR) have been studied.
In QSPR/QSAR studies topological indices(molecular descriptors) are key tools [1,2,3,4]. A topological index is a graph invariant number calculated from a graph associated to a molecule.
Properties estimation can help to minimize the time and cost in producing new chemical materials with desired properties. For estimation some of the statistical tests are discussed in [5]. The physiochemical properties of molecular compounds are important in many fields. A lot of work has done on prediction of physiochemical properties of different compounds [6,7,8,9,10,11].
Molecules can be represented by molecular graphs, where vertices represent the atoms and edges represent the bonds between them. For a molecular graph , V and E represent the set of vertices and the set of edges, respectively. The graph theory-based structure descriptors can be determined by considering graph edges, vertices, or both. Rather simple arithmetic operations are carried out to get numerical indices. These indices are suppose to comprise information on properties/activities of the molecules.
The vertex-connectivity index of a (molecular) graph G was introduced by Randić in 1975 [12]:
where is the degree of the vertex u in G.
The edge-connectivity index of a (molecular) graph G was introduced by Estrada [11]:
where shows that the edges e and f are adjacent and for .
In [13] Nikolić and Trinajstić made a comparison between the vertex and edge connectivity indices for benzenoid hydrocarbons. They showed that the -electron energies (E) of benzenoids can be computed by means of either the vertex connectivity index or the edge connectivity index. Their best quadratic model based on the edge-connectivity index can predict the -electron energies of benzenoids within the error range of 0.8%–2%. While their best structure-boiling point () model was a quadratic model that can predict the boiling points of benzenoid hydrocarbons within the error range of 1.3%–4%. Their best models are as follows
where n is the number of benzenoids, r is the correlation coefficient, (adjusted) is the adjusted correlation coefficient, s is the standard error of estimate and F is the Fisher ratio.
We introduced the novel topological index to improve the efficiency of the above equations.
Definition of the Face Index
When a connected graph can be drawn on the plane without crossing any edges, it is called a planar graph. When a planar graph is drawn in this way, it divides the plane into regions called faces. The unbounded region is called the infinite face.
Let be a finite simple connected planar graph, where , and represent the vertex, edge and face sets, respectively. A face is incident to an edge if e is one of those edges which surrounds the face. Similarly, a face is incident to a vertex v in G if v is at the end of one of those incident edges.
The molecular graph of benzene ring is a cycle consist of six vertices and edges each and two faces. The molecular graph of benzenoid hydrocarbons contain considerable number of faces.
In the present study, we introduced a new topological index, the face index (), using the degree of the vertices incident with the faces of a graph. For a planar graph G, can be defined as
represents the incidency of the vertex v with the face f.
In benzenoid graphs (), vertices have either degree two or three; the vertices with degree two are incident with two faces and vertices with degree three are incident with three faces. Let and denote the number of vertices with degree two and three in , then the definition of for becomes
Hence, of any can be investigated by just knowing the number of vertices of degree two and three. In case of perylene (P) as shown in Figure 1 and , so . Authors in [14], discussed theoretical study of singlet fission in oligorylenes.
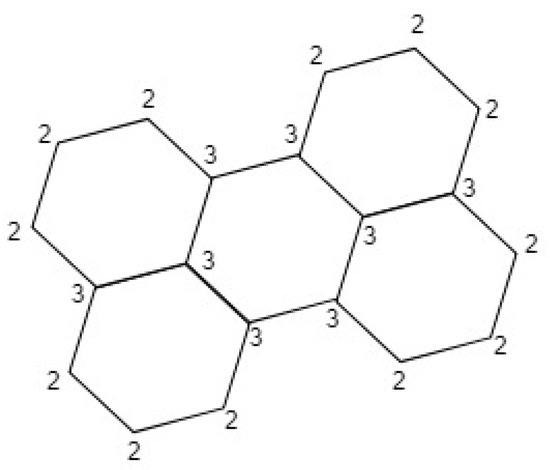
Figure 1.
Vertices degree of the perylene benzenoid graph (P).
2. Discussion
The novel topological index is used to construct the structure-energy and structure-boiling point models and the obtained results are comparable with the results obtained in [10,13,15]. We computed for 21 common benzenoid hydrocarbons. The values of for these hydrocarbons are given in Table 1 along with the -electron energies () and boiling points (°C). The -electron energies have been taken from the tabulation of Coulson and Streiwieser [16]. The experimental values of boiling points were taken from Basak et al. [17].

Table 1.
The face index (), Randić index (), edge-connectivity index (), -electron energies in and boiling points () in of studied benzenoid hydrocarbons.
2.1. Relationships between the Face Index and Vertex and Edge Connectivity Indices for Benzenoid Hydrocarbons
We first considered the relationships between the face index and the vertex-connectivity index and between the face index and the edge connectivity index for benzenoid hydrocarbons. Plots of versus and versus for benzenoids is given in Figure 2 and Figure 3 and the obtained mathematical models are:
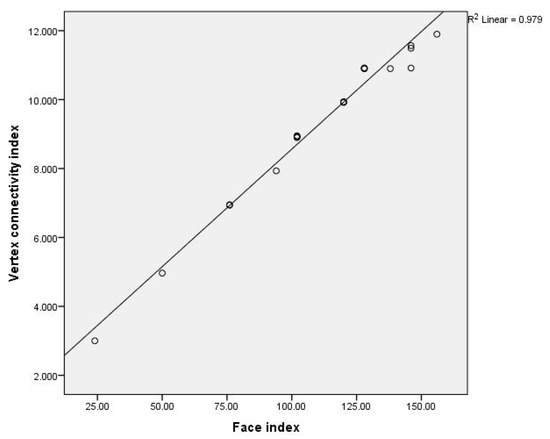
Figure 2.
Plot of the face index versus vertex connectivity index for 21 benzenoid hydrocarbons.
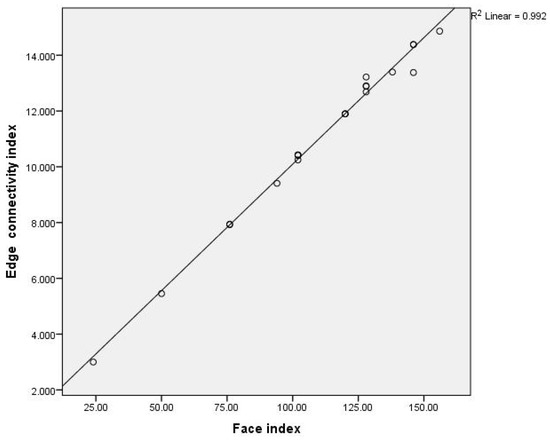
Figure 3.
Plot of the face index versus edge connectivity index for 21 benzenoid hydrocarbons.
From above equations we can see that the face index is more closely related with edge connectivity index rather the vertex connectivity index.
2.2. Linear Mathematical Models of the Face Index () for the -Electron Energy (E)
In this section, we constructed the linear and multiple linear relationships between the -electron energies of considered benzenoid hydrocarbons and the face index. The linear and multiple linear correlation plots are shown in Figure 4 and Figure 5.
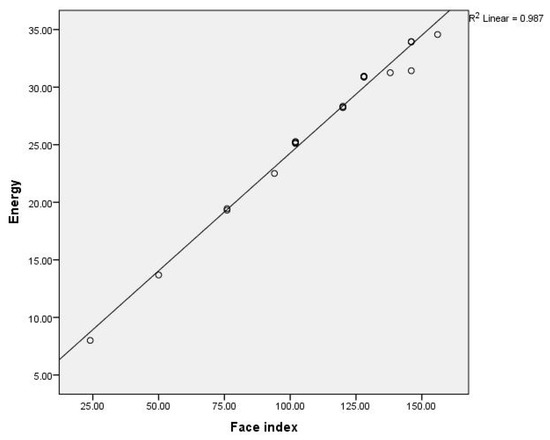
Figure 4.
The linear correlation between the first face index and the energy for benzenoid hydrocarbons.
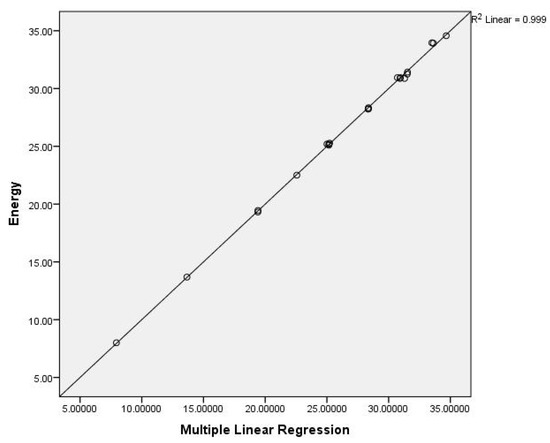
Figure 5.
The multiple linear correlation for the energy of benzenoid hydrocarbons.
The regression analysis gives the following relationships for -electron energy (E):
Linear Correlation:
Multivariate Correlation:
The Equation (6) gives the best correlation with energy.
2.3. Linear Mathematical Models of the Face Index () for the Boiling Points ().
We considered the linear and multiple linear relationships between the boiling points () of benzenoid hydrocarbons and the face index. The corresponding plots of linear and multiple linear correlation is shown in Figure 6 and Figure 7.
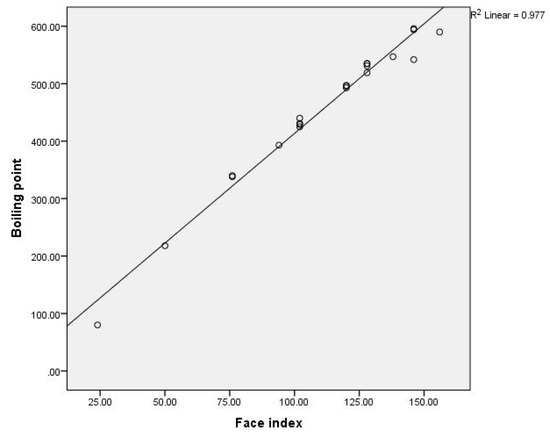
Figure 6.
The linear correlation between the first face index and the boiling points for benzenoid hydrocarbons.
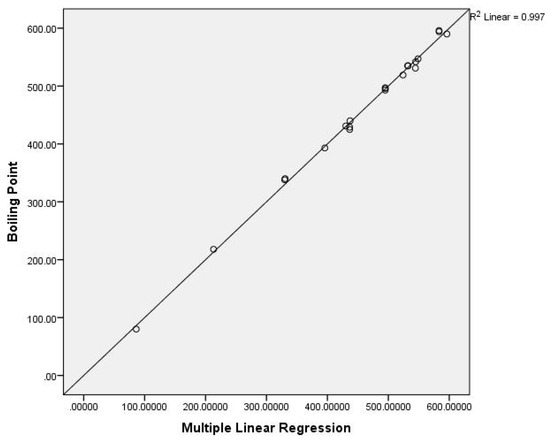
Figure 7.
The multiple linear correlation for the boiling points for benzenoid hydrocarbons.
The regression analysis gives the following relationships for the boiling points (°C)of benzenoid hydrocarbons:
Linear Correlation:
Multivariate Correlation:
Clearly, Equation (8) gives the best result.
3. Computational Techniques for the Face Index
In this section, we computed the face index of some planar molecular graphs. Method of partitioning of face set based on the degrees of the faces in the graph is used to find the face index. will denote the unbounded face of the graph. The face index of graphene, triangular benzenoid and circumcoronene series of benzenoid is computed in the following subsections. There are several papers in which authors used different techniques to calculate the certain topological indices of some special molecular graphs [18,19,20,21,22,23,24,25].
3.1. Face Index of Graphene
Graphene is a 2-dimensional planar sheet of carbon atoms which is densely packed in a honeycomb crystal lattice, and it is the major element of certain carbon allotropes including charcoal, fullerenes and graphite, see Figure 8. It is represented by where n is number of rows and s is number of benzene rings in each row. In the review [26], authors gave a brief introduction on the recent advances in the study of graphene edges, including edge formation energy, edge reconstruction, method of graphene edge synthesis and the recent progress on metal-passivated graphene edges.
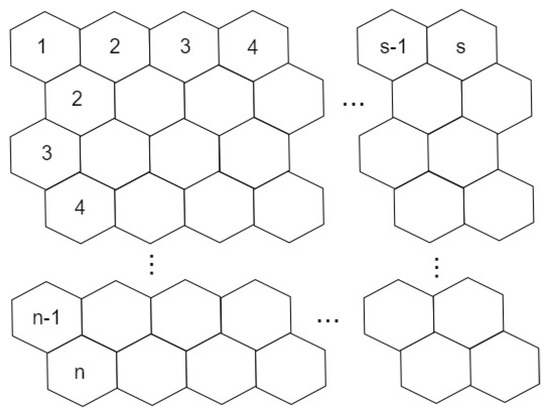
Figure 8.
2D graphene structure with ‘n’ rows and ‘s’ benzene rings in each row.
Theorem 1.
Let be a graphene structure, where ‘n’ is the number of rows and ‘s’ is the benzene rings in each row and and . The face index of is given as
Proof.

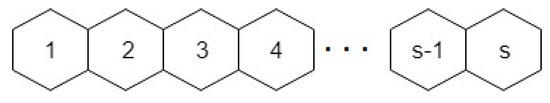
Consider the graphene structure, , with ‘n’ rows and each row has ‘s’ benzene rings. Let denotes a face having degree j, i.e., and denotes the number of faces with degree j. 2-dimensional structure of graphene (shown in Figure 8) contains five types of internal faces , , , , and an external face. For sum of degrees of vertices of external face is , For sum of degrees of vertices of external face is , for sum of degrees of vertices of external face is and similarly when graphene structure has n rows then sum of degrees of vertices of external face is . Table 2 shows the details of the number of faces with a degree in each row.

Table 2.
Numbers of , , , , in each row.
Case 1. From the definition of face index and Table 2 for , we have
Case 2. When 2-dimensional structure of graphene has just 1 row then it has two types of internal faces , and one external face whose sum of degrees is .
For , and as shown in Figure 9, and the face index is

Figure 9.
2-dimensional structure of graphene with one row having s benzene rings.
This completes the proof. □
3.2. Face index of Triangular Benzenoid
Now, we calculate the face index of a triangular benzenoid graph where n represents the number of rows depicted in Figure 10. For synthesis and characterization of -extended triangulene we refer [27].
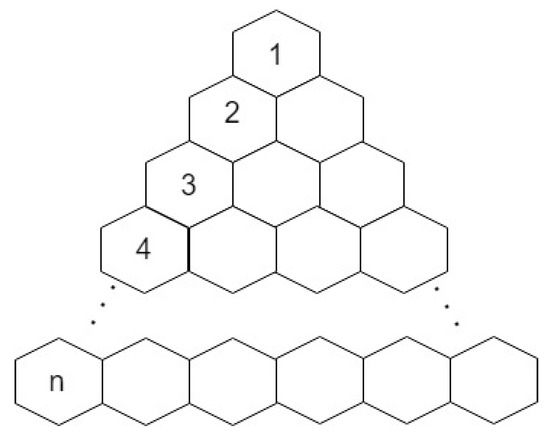
Figure 10.
Molecular graph of triangular benzenoid.
Theorem 2.
Let be a triangular benzenoid gragh where n represents the number of rows and . The face index of is equal to
Proof.

Let denotes the face having degree j, i.e., . Triangular benzenoid graph contains four types of internal faces , , and and an external face. When triangular benzenoid has just 1 row then sum of degrees of vertices of external face is 12, when triangular benzenoid has 2 rows then sum of degrees of vertices of external face is 27, when triangular benzenoid has 3 rows then sum of degrees of vertices of external face is 42, and similarly when triangular benzenoid has n rows then sum of degrees of vertices of external face is . The number of internal faces with the degree in each row is mentioned in Table 3.

Table 3.
Number of , , , in each row.
From Table 3 and according to the definition of face index, we have
Hence, this is our required result. □
3.3. Face Index of Circumcoronene Series of Benzeniod
A circumcoronene homologous series of benzenoid (k is the number of generations) consist of many copies of benzene C6 on circumference. First three molecule of this series is shown in Figure 11 and the generalized circumcoronene series is shown in Figure 12.
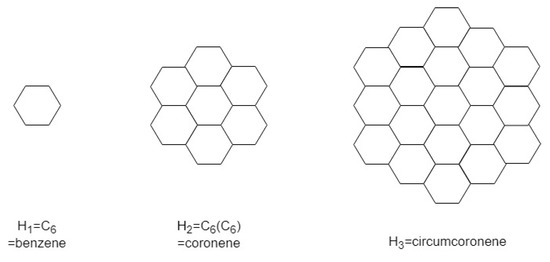
Figure 11.
The first, second and third molecular graphs , and from the circumcoronene series of benzenoid.
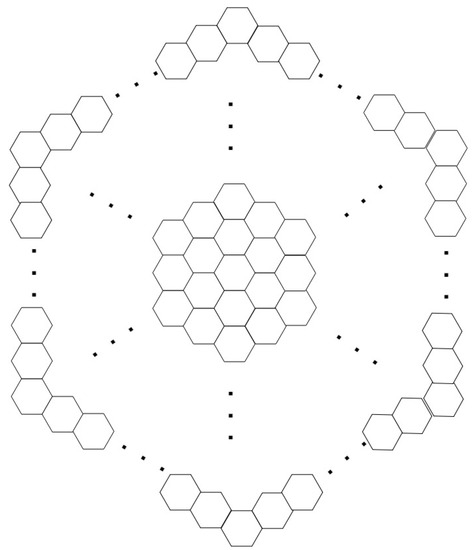
Figure 12.
The circumcoronene series of benzenoid for .
Theorem 3.
Let be a molecular graph of circumcoronene series of benzenoid, where . The face index of this graph is given by
Proof.
First we consider a circumcoronene series of benzeniod for presented in Figure 11 where k denotes the number of generations. Let denotes a face having and denotes the number of faces having degree j. In both internal and external faces have degree 12. In there is two types of internal faces , and 1 external face . In there are 3 types of internal faces , , and 1 external face . Figure 12 shown the circumcoronene series of benzenoid for .
Now we calculate the face index for , ... .
From above information we get a recurrence relation;
By solving this recurrence relation we get the following;
This is our required result. □
From above result we can obtain the face index of circumcoronene with a window which is . Where is the molecular structure of circumcoronene with a window as shown in Figure 13.
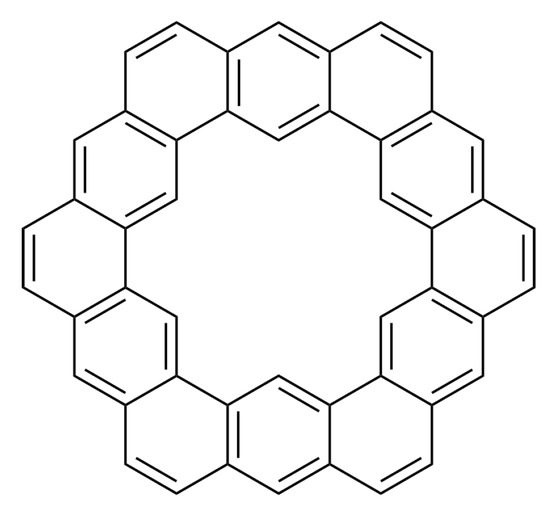
Figure 13.
Molecular structure of circumcoronene with a window.
4. Conclusions
In this paper a new topological index, the face index (), is proposed which is based on the degrees of the vertices incident to the face of a graph. We showed that novel face index can be computed by the vertex and edge connectivity indices within the error of 6%–9% and 2%–6%, respectively. Which shows that the novel index is more closely correlate with edge connectivity index. The model Equation (6) involve the face index that improved the Nikolić Equation (1) and can predict the energy of the benzenoid within the error range of 0.6%–1.5%. Equation (8) improved the Nikolić Equation (2) and can compute the boiling point within the range of 1.2%–3.5%. The results warrant further studies on the properties and uses of the face index. In the later section, we considered 2-dimensional graphene, triangular benzenoid and circumcoronene series of benzenoid to study the topological property using their faces. The analytical closed formulas of for these molecular graphs are determined.
Author Contributions
Conceptualization, M.K.J.; Formal analysis, K.A.S.; Funding acquisition, M.I.; Investigation, M.K.J. and M.I.; Software, M.K.J. and K.A.S.; Writing—original draft, K.A.S.; Writing—review and editing, M.K.J. and M.I. All authors have read and agreed to the published version of the manuscript.
Funding
This research is supported by the UPAR Grants of United Arab Emirates, Al-Ain, UAE via Grant No. G00002590 and G00003271.
Conflicts of Interest
Authors declare there is no conflict of interest in publishing the article.
References
- Balaban, A.T. Topological indices and their uses: A new approach for coding of alkanes. J. Mol. Struct. Theochem 1988, 165, 243–253. [Google Scholar] [CrossRef]
- Estrada, E.; Ivanciuc, O.; Gutman, I.; Gutierrez, A.; Rodrìguez, L. Extended Wiener indices. A new set of descriptors for quantitative structure-property studies. New J. Chem. 1998, 22, 819–823. [Google Scholar] [CrossRef]
- Taherpour, A.A.; Shafiei, F. The structural relationship between Randić indices, adjacency matrixes, distance matrixes and maximum wave length of linear simple conjugated polyene compounds. J. Mol. Struct. Theochem 2005, 726, 183–188. [Google Scholar] [CrossRef]
- Yao, X.J.; Fan, B.; Doucet, J.P.; Panaye, A.; Liu, M.; Zhang, R.; Zhang, X.; Hu, Z. Quantitative structure property relationship models for the prediction of liquid heat capacity. QSAR Comb. Sci. 2003, 22, 29–48. [Google Scholar] [CrossRef]
- Jäntschi, L. A test detecting the outliers for continuous distributions based on the cumulative distribution function of the data being tested. Symmetry 2019, 11, 835. [Google Scholar] [CrossRef]
- Cash, G.G. Correlation of physicochemical properties of alkylphenols with their graph-theoretical parameter. Chemosphere 1995, 31, 4307–4315. [Google Scholar] [CrossRef]
- Gupta, S.P.; Singh, P. The Relationship of π-binding energy with molecular connectivity in hydrocarbons. Bull. Chem. Soc. Jpn. 1979, 52, 2745–2746. [Google Scholar] [CrossRef]
- Gutman, I.; Trinajstić, N. Graph theory and molecular orbitals. Total π-electron energy of alternant hydrocarbons. Chem. Phys. Lett. 1972, 17, 535–538. [Google Scholar] [CrossRef]
- Hosseine, H.; Shafiei, F. Entropy prodection of benzene derivatives using topological indices. Stud. UBB Chem. 2017, LXII, 297–310. [Google Scholar] [CrossRef]
- Randić, M. Quantitative Structure-Property Relationship. Boiling Points of Planar Benzenoids. New J. Chem. 1996, 20, 1001–1009. [Google Scholar]
- Estrada, E. Edge Adjacency Relationships and a Novel Topological Index Related to Molecular Volume. J. Chem. Inf. Comput. Sci. 1995, 35, 31–33. [Google Scholar] [CrossRef]
- Randić, M. On Characterization of Molecular Branching. J. Am. Chem. Soc. 1975, 97, 6609–6615. [Google Scholar] [CrossRef]
- Nikolic, S.; Trinajstić, N. Comparison between the Vertex- and Edge-Connectivity Indices for Benzenoid Hydrocarbons. J. Chem. Inf. Comput. Sci. 1998, 38, 42–46. [Google Scholar] [CrossRef]
- Minami, T.; Ito, S.; Nakano, M. Theoretical study of singlet fission in oligorylenes. J. Phys. Chem. Lett. 2012, 3, 2719–2723. [Google Scholar] [CrossRef]
- Plavšić, D.; Trinajstić, N.; Amić, D.; Šoxsxkić, M. Comparison between the structure-boiling point relationships different descriptors for condensed benzenoids. New J. Chem. 1998, 22, 1075–1078. [Google Scholar] [CrossRef]
- Coulson, C.A.; Streitwieser, J. Dictionary of π-Electron Calculations; Freeman: San Francisco, CA, USA, 1965. [Google Scholar]
- Basak, S.C.; Grunwald, G.D.; Niemi, G.J. Use of Graph-Theoretic and Geometrical Molecular Descriptors in Structure-Activity Relationships. In From Chemical Topology to Three-Dimensional Geometry; Balaban, A.T., Ed.; Plenum: New York, NY, USA, 1997; pp. 73–116. [Google Scholar]
- Farahani, M.R.; Gao, W. Degree-based indices computation for special chemical molecular structures using edge dividing method. Appl. Math. Nonlinear Sci. 2016, 1, 94–117. [Google Scholar]
- Gao, W.; Farahani, M.R. Computing the reverse eccentric connectivity index for certain family of nanocone and fullerene structures. J. Nano. Tech. 2016. [Google Scholar] [CrossRef]
- Gao, W.; Siddiqui, M.K.; Imran, M.; Jamil, M.K.; Farahani, M.R. Forgotten topological index of chemical structure in drugs. Saudi Pharm. J. 2016. [Google Scholar] [CrossRef]
- Gao, W.; Shi, L. Szeged related indices of unilateral polyomino chain and unilateral hexagonal chain. IAENG Int. J. Appl. Math. 2015, 45, 138–150. [Google Scholar]
- Gao, W.; Wang, W.F.; Farahani, M.R. Topological indices study of molecular structure in anticancer drugs. J. Chem. 2016. [Google Scholar] [CrossRef]
- Wang, W.F.; Gao, W. Second atom-bond connectivity index of special chemical molecular structures. J. Chem. 2014. [Google Scholar] [CrossRef]
- Wang, W.F.; Gao, W. The vertex version of weighted wiener number for bicyclic molecular structures. Comput. Math. Meth. Med. 2015. [Google Scholar] [CrossRef]
- Yan, L.; Gao, W.; Li, J.S. General harmonic index and general sum connectivity index of polyomino chains and nanotubes. J. Comput. Theor. Nanosci. 2015, 12, 3940–3944. [Google Scholar] [CrossRef]
- Zhang, X.; Xin, J.; Ding, F. The edges of graphene. Nanoscale 2013, 5, 2556–2569. [Google Scholar] [CrossRef] [PubMed]
- Mishra, S.; Beyer, D.; Eimre, K.; Liu, J.; Berger, R.; Gröning, O.; Pignedoli, C.A.; Müllen, K.; Fasel, R.; Feng, X.; et al. Synthesis and Characterization of π-Extended Triangulene. J. Am. Chem. Soc. 2019, 141, 10621–10625. [Google Scholar] [CrossRef] [PubMed]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).